Abstract
Exercise intolerance (EI) is the primary manifestation of chronic heart failure with preserved ejection fraction (HFpEF), the most common form of HF among older individuals. The recent recognition that HFpEF is likely a systemic, multi-organ disorder that shares characteristics with other common, difficult-to-treat aging-related disorders suggest that novel insights may be gained from combining knowledge and concepts from aging and cardiovascular disease disciplines. This state-of-the-art review is based on the outcomes of an NIA-sponsored working group meeting on aging and EI in HFpEF. We discuss aging-related and extra-cardiac contributors to EI in HFpEF, and provide the rationale for a transdisciplinary, ‘gero-centric’ approach to advance our understanding of EI in HFpEF and identify promising new therapeutic targets. We also provide a framework for prioritizing future research, including developing a uniform, comprehensive approach to phenotypic characterization of HFpEF, elucidating key geroscience targets for treatment, and conducting proof-of-concept trials to modify these targets.
Keywords: Heart failure with preserved ejection fraction, aging, exercise intolerance, skeletal muscle, senescence
Condensed Abstract:
Exercise intolerance (EI) is the primary manifestation of heart failure with preserved ejection fraction (HFpEF) in older adults and is associated with poor outcomes. A better understanding of the pathophysiology of EI in HFpEF is needed for its optimal management. Here, we provide a comprehensive discussion of the evidence supporting key roles for extra-cardiac contributors to EI in HFpEF and biological mechanisms of aging to the development and progression of HFpEF. Future research leveraging a transdisciplinary approach that includes cardiovascular and geroscience experts are needed for better phenotypic characterization of HFpEF and the identification of key geroscience targets for treatment.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is the most common form of HF; its prevalence is increasing, its prognosis is worsening, and pharmaceutical trials in HFpEF have been neutral on their primary outcomes.(1,2) Thus, there is an urgent need for an enhanced understanding of HFpEF. The recognition that HFpEF is likely systemic and multi-organ in nature, and shares many characteristics with other common, difficult-to-treat aging disorders supports examining the condition from a gerontological viewpoint as well as from a cardiovascular perspective.(2,3) However, few efforts to date have capitalized on these new insights. This State-of-the-Art Review is based on an eponymous workshop sponsored by the National Institute of Aging (NIA) held September 12–13, 2019, to address this critical knowledge gap. The workshop brought together a diverse group of experts in aging and/or HFpEF, including geroscientists, geriatricians, cardiologists, exercise physiologists, skeletal muscle biologists, and metabolic and adipocyte experts. These disparate groups of investigators rarely interact, yet each holds complementary and potentially valuable knowledge and insights relevant to HFpEF. The goal was to maximize sharing of information and concepts that could enhance understanding of HFpEF pathophysiology and management to forge a framework for new transdisciplinary research discoveries.
The meeting focused on extra-cardiac mechanisms underlying development of exercise intolerance (EI) in HFpEF. EI is the primary manifestation of chronic, stable HFpEF, is severe and debilitating, and is associated with poor quality-of-life and clinical outcomes.(2) However, the pathophysiology and optimal management of EI in HFpEF are poorly understood. Although the role of cardiac contributors to the development of HFpEF and EI has been discussed extensively in prior reviews,(3–5) there has been far less focus on extracardiac determinants. This state-of-the-art review is unique in that it offers a new, comprehensive, evidence-based ‘road map’ of the under-recognized but critical contributions of extra-cardiac factors and the array of research opportunities to advance the diagnosis and treatment of this pervasive, debilitating condition in older adults.
New Paradigm for HFpEF as a Multi-system Geriatric Syndrome
HFpEF —originally described by a geriatrician, Robert Luchi, 38 years ago—is the most common form of HF in community-dwelling older persons.(1,6) Almost all HF cases encountered among nonagenarians are HFpEF. Aging is one of the strongest risk factors for HFpEF and its increasing prevalence is largely driven by the aging population. The impact of aging and circulating factors in promoting HFpEF is supported by heterochronic parabiosis studies, whereby animals of different ages are joined surgically leading to common circulation, demonstrating reversal of some HFpEF features in older mice after prolonged exposure to the circulating growth differentiation factor-11 in young mice.(7)
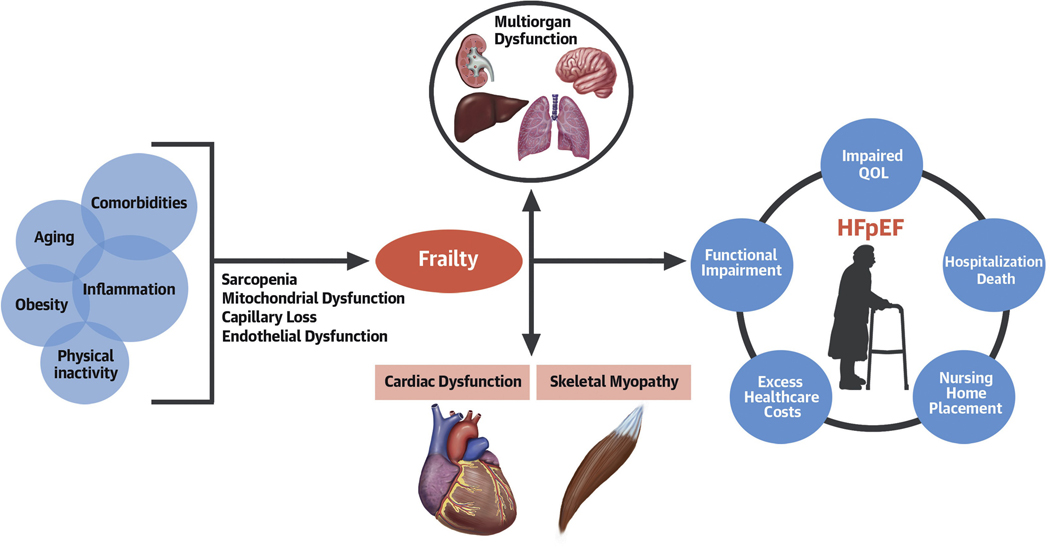
HFpEF was initially believed to be due solely to abnormal relaxation of the left ventricle and decreased LV compliance.(8) However, our understanding of the pathophysiology of HFpEF has evolved over time toward the concept of a systemic, multi-organ geriatric syndrome.(2) It is likely initiated by inflammation and other circulating factors that originate from increased adiposity, particularly excess intra-abdominal adipose tissue, in the setting of multi-morbidity, aging, and physical inactivity.(5) These factors foment loss of capillarity, sarcopenia, mitochondrial dysfunction, and endothelial dysfunction resulting in multiorgan dysfunction, frailty, cardiac and skeletal myopathy. Consistent with other geriatric syndromes, co-morbidities, frailty, and decline in functional capacity are central to the clinical manifestations of HFpEF and contribute to the high burden of mortality, hospitalizations, and poor quality-of-life (Central Illustration).
Central Illustration: Pathophysiology and outcomes in HFpEF.
Risk factors such as multimorbidity, aging, physical inactivity, and systemic inflammation lead to loss of capillarity, mitochondrial and endothelial dysfunction, and sarcopenia and result in downstream multi-organ dysfunction, frailty, and cardiac and skeletal muscle myopathy. This constellation of pathophysiologic abnormalities contributes to the clinical manifestation of HFpEF.
The implications of the concept of HFpEF as a systemic geriatric syndrome are profound and may help explain why pharmacological intervention trials to date have been neutral. They also redirect attention and resources from a purely cardio-centric approach to that of an integrated, whole-patient approach. Moreover, the new paradigm of HFpEF suggests a shift in treatment focus toward integrated patient-reported outcomes including quality-of-life and symptom burden, as well as functional assessments, i.e., exercise tolerance, physical performance, and cognition. They also point to opportunities for testing novel agents for treatment, including those that can affect anti-inflammatory; capillary-sparing, rejuvenating; and improved mitochondrial function. Thus, a geroscience approach, which focuses on biological mechanisms of aging to improve the treatment of age-related chronic conditions, may foster needed advances in the understanding and treatment of HFpEF.
EI: A Key Manifestation of HFpEF
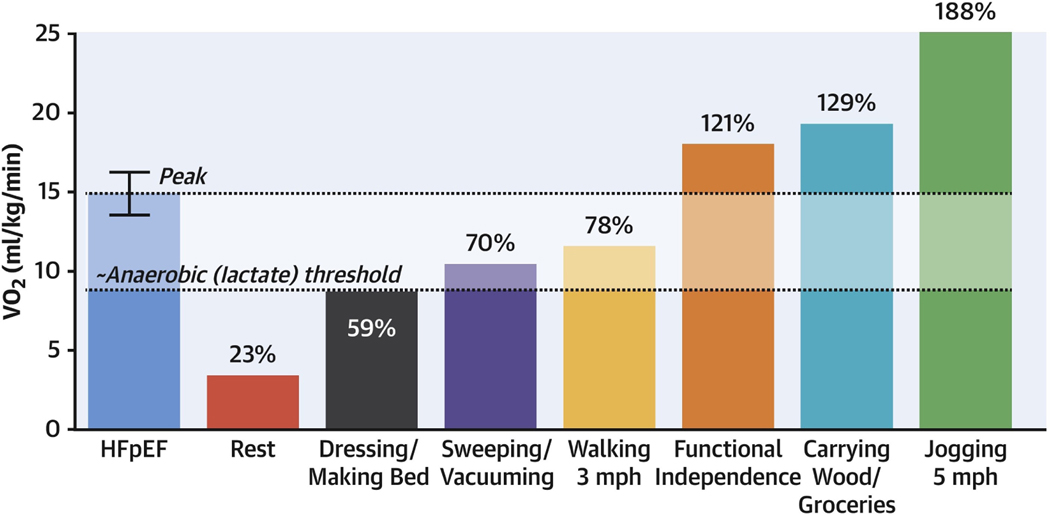
Low exercise capacity, measured objectively as peak oxygen uptake (VO2peak) during maximal exercise, is an independent predictor of HFpEF development among older adults.(9) On average VO2peak is reduced by 35% in HFpEF compared with healthy age- and sex-matched controls.(10) Among older HFpEF patients, VO2peak during upright exercise is on average 3–4 ml/kg/min below the VO2 threshold of functional independence.(11) Thus, many activities of daily living that are trivial for healthy adults to perform require near-maximal effort for patients with HFpEF (Figure-1).(4) VO2peak can be measured reproducibly with standardized cardiopulmonary exercise testing with modest participant burden and cost. VO2peak can be measured as an absolute value (ml/min) or relative to the body size (ml/kg/min), and their use depends on the setting. It is customary to index VO2peak to body size given its intimate relationship with a wide range of morphometric measures, including weight, body surface area, BMI, and skeletal muscle mass. Extremes of body size, obesity, and weight loss interventions may disproportionately influence body size indexed measures of VO2peak.In these settings, changes in VO2peak can be verified and compared with measures of exercise capacity that are not indexed to weight, including exercise time to exhaustion, six-minute walk distance, and maximal workload achieved.(12,13)
Figure 1. VO2peak required for ADLs relative to average VO2peak in HFpEF.
Among patients with HFpEF, the threshold of functional independence is 21% above the average peak VO2 (blue bar) of patients with HFpEF. Figure reproduced with permission from Nayor et al. JACC Heart Failure 2020.
Health aging is associated with declines in VO2peak but in the absence of disease does not cause EI, i.e., shortness of breath and fatigue with normal day-to-day activity. Development of HFpEF is associated with accelerated functional decline and lowering of the threshold for dyspnea and fatigue so that they occur even with usual normal daily activity. EI is a key, patient-centered outcome that is critical to understand HFpEF pathophysiology and optimal treatment, independent of clinical events. EI is an important outcome for observational, mechanistic, and interventional studies. In this section we have discussed the key attributes of HFpEF as a geriatric syndrome and their implications in the pathogenesis of EI and its management.
Aging, accelerated functional decline, and EI in HFpEF
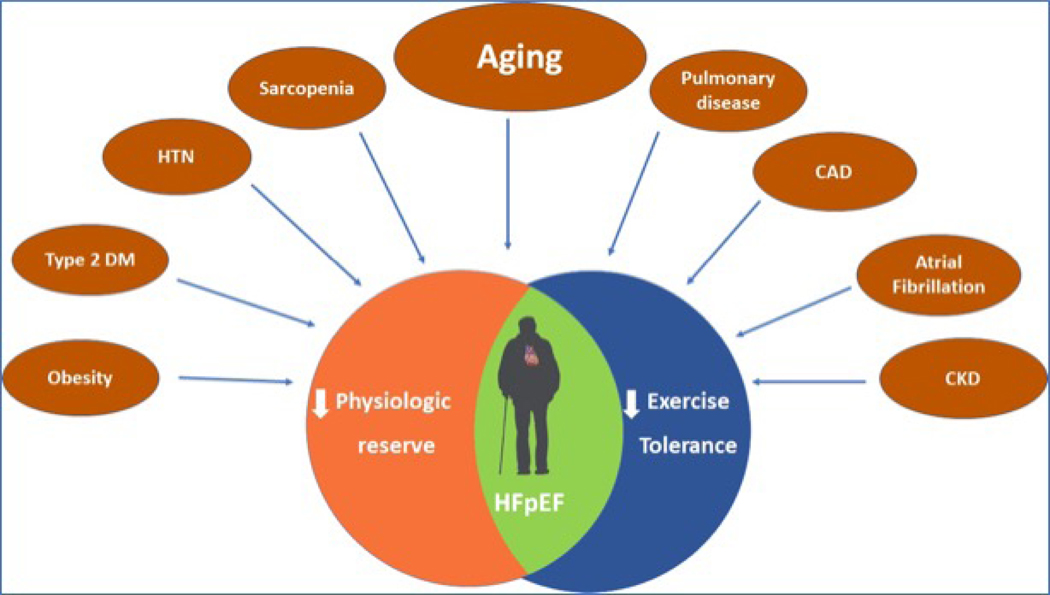
The strong predisposition of older adults to the development of HFpEF suggests potentially specific age-related vulnerabilities to HFpEF. Recently, molecular hallmarks of aging and shifts in subcellular biology have become recognized as fundamental determinants of age-related changes that can lead to chronic age-related diseases, including overt cardiovascular diseases (CVD).(14) Paneni et al. describe how progressive changes in geroscience-related subcellular mechanisms can lead to increased CVD vulnerability with aging that likely influence development of HFpEF.(15) This includes changes in sirtuins, autophagy, and cellular senescence which lead not only to changes in cardiac cells, but to broader changes in fat and skeletal muscle, autonomic regulation, hematology, endothelial function, and other wide-ranging effects that impact HFpEF and may require different approaches than those utilized for CVD in younger persons. The strong associations of HFpEF with multi-morbidity is predictable, since these changes related to the hallmarks of aging trigger multiple diseases, including frailty, cognitive changes, and other systemic effects. Thus, traditional single organ-focused care may not be optimal for understanding HFpEF that occurs in the context of change in aggregate aging, multiple comorbidities, and a decline in physiologic reserve (Figure-2).
Figure 2. The multimorbidity model of exercise intolerance in HFpEF.
Accumulation of cardiac and non-cardiac co-morbidities coupled with aging contributes to systemic inflammation, endothelial dysfunction, global reduction in physiologic reserve, and exercise intolerance in patients with HFpEF.
EI in HFpEF likely depends not only on cardiac changes specific to HFpEF but also on a broad range of factors related to aging that sets the stage for the development of EI in older HFpEF patients. Prior studies among healthy adults have showed that VO2peak declines significantly with age.(16,17) The rate of decline in VO2peak accelerates in older age from 3–6% per decade in the 20s to 30s to >20% per decade after the age of 70.(16) Age-related declines in strength and balance exacerbate decrements in VO2peak due to alterations in skeletal muscle including atrophy, loss of strength, and impaired mitochondrial respiration.(18)
Sex differences in EI in HFpEF
There are important sex differencesin the pathophysiology and clinical manifestations of HFpEF. Women have a two-fold higher lifetime risk of HFpEF vs. HFrEF and the prevalence of HFpEF is much higher than HFrEF among older women.(19) In the Cardiovascular Health Study, >80% of incident HF in older women was due to HFpEF.(1) Women with HFpEF have higher burden of symptoms, worse quality-of-life, and lower VO2peak (~20–30% lower VO2peak) compared with men(20); however percent predicted VO2peak appears similarly reduced among men (68%) and women (66%) with HFpEF.(21) Women have lower exercise cardiac reserve and reduced peripheral oxygen extraction.(22) The lower VO2peak among women may be related to sex-specific differences in cardiac remodeling patterns and adiposity distribution. Women (vs. men) have small LV cavity size, higher LV stiffness, higher LV filling pressure, and lower stroke volume at rest.(22) Furthermore, women have greater decline in LV contractility and greater concentric remodeling with aging.(23) Animal studies have also demonstrated sex differences in cardiac response to increased pressure overload (aortic banding) with greater LV dilation, loss of concentric remodeling, and loss of contractile reserve in male rats (vs. female rats).(24) Remodeling differences may be related, in part, due to ACE gene polymorphisms, sex-specific differences in mRNAs, and peri-menopausal adiposity changes in women.(25) Specifically, women with (vs. without) HFpEF have 34% higher visceral adipose tissue (VAT). Higher amount of VAT has been associated with more exaggerated increase in pulmonary capillary pressure with exercise in women but not in men.(26)
Role of extracardiac comorbidities in manifestations of EI in HFpEF
HFpEF is associated with, and partly driven by extra-cardiac comorbidities. On an average, patients with HFpEF have ≥5 co-existing comorbidities, with a significant increase in the comorbidity burden over time.(27) In older patients with HFpEF, noncardiac comorbidities are involved in adverse outcomes more frequently compared with HFrEF.(27,28) Higher burden of comorbidities contributes to lower VO2peak and EI in patients with HFpEF through extracardiac mechanisms detailed in Table-1. The importance of extracardiac contributors to EI in HFpEF is highlighted as they account for: 1) ~50% of the reduced VO2peak;(29) 2) ~85% of the improvement in VO2peak with exercise training;(30) and 3) skeletal myocytes, in contrast to cardiac myocytes which are terminally differentiated, have a rapid regenerative and repair capacity.(2,31) These observations may have important implications given that optimal management of comorbidities and their consequences may represent an important strategy for older patients with HFpEF. Consistent with this notion, lifestyle interventions such as exercise and weight loss, which have pleiotropic favorable effects on non-cardiac and cardiac co-morbidities, have been shown to improve exercise capacity and quality-of-life in HFpEF primarily through improvements in extracardiac factors such as systemic inflammation, adiposity, and peripheral oxygen utilization in skeletal muscle.(12,30,32)
Table 1:
Extracardiac contributors to exercise intolerance in older patients with heart failure with preserved ejection fraction.
|
Hallmarks of Aging - Cell senescence - Systemic inflammation - Oxidative stress - Accelerated exercise capacity decline - Reduced physiologic reserve |
|
Skeletal Muscle Abnormalities - Increased fatty deposition in skeletal muscle - Sarcopenia - Reduced microvascular density (capillarity) - Impaired skeletal muscle perfusion - Reduced conductive and diffusive oxygen transport - Impaired mitochondrial function and bioenergetics |
|
Excess Adiposity - Upregulation of inflammatory pathways - Increased metabolic and neurohormonal dysfunction - Insulin resistance - Natriuretic peptide deficiency - Plasma volume expansion - Systemic endothelial dysfunction - Reduced microvascular density (capillarity) - Impaired mitochondrial function |
|
Pulmonary Dysfunction - Altered ventilatory reserve - Impaired gas diffusion - Ventilation perfusion mismatch - Increased dead space ventilation - Restrictive and obstructive deficits - Obstructive and Central Sleep Apnea - Pulmonary vascular remodeling - Pulmonary hypertension - Right heart dysfunction |
|
Kidney dysfunction - Excess perinephric fat - Reduced glomerular filtration rate - Excess sodium retention and plasma volume expansion - Upregulation of renin-angiotensin-aldosterone pathway |
|
Liver dysfunction - Non-alcoholic fatty liver disease - Upregulation of proinflammatory pathways |
|
Vascular dysfunction - Increased aortic stiffness - Adverse pulsatile hemodynamics and late systolic load - Microvascular rarefaction - Impaired vasodilatory reserve |
Frailty and EI in HFpEF
Frailty is a biological syndrome reflecting decreased physiological reserve and vulnerability to stressors,(33) and represents the key factor underlying the heterogeneity in functional decline with aging.(34) The two most widely accepted measures of frailty, the Fried Frailty phenotype model and the Frailty Index, have advantages and disadvantages in the context of HF, as described in Table-2.(33) The Fried Frailty phenotype measures decline in physiologic reserve across 5 physical function domains: weight loss, weakness, poor endurance, slowness, and low physical activity level.(33) The Frailty Index quantifies frailty as cumulative deficit in multiple organ system resulting in decreased physiologic reserve.(35)
Table 2.
Measures of Physical Frailty
| Frailty measure | Description | Advantages | Disadvantages |
|---|---|---|---|
| Fried Frailty Phenotype Model |
• Quantifies the decline in physiological reserve across 5 domains: weight loss, weakness, poor endurance, slowness, and low physical activity levels | • Most commonly used tool to assess frailty in the literature | • Poor discriminatory power • Overlap in clinical features of HF and frailty • Time and resourceintensive |
| Frailty Index | • Based on a “multiple hit” model • Quantifies frailty as an accumulation of deficits across several healthrelated domains • Estimated as the ratio of the total deficits present to the number of deficits assessed across signs, symptoms, comorbidity index, laboratory values, and ADLs |
• Continuous nature of the estimate with a wide range of distribution • Ability to use preexisting data from medical records for estimating frailty • Ability to estimate cut-offs for specific clinical populations |
• The number of deficits assessed is not standardized and vary according to available data • Focuses more of the number of deficits and does not account for the severity of deficits |
The prevalence of frailty in HFpEF patients is reported to be up to 75%.(36,37) Frailty in older patients with HFpEF is associated with poor quality-of-life, greater functional impairment and reduced submaximal aerobic exercise capacity. Frailty predisposes to severe EI in HFpEF through several shared mechanisms including a higher burden of co-morbidities, chronic inflammation, sarcopenia, and a global decrease in functional reserve. Frailty is also associated with higher risk of hospitalization and mortality.(37,38)
Considering the prevalence and prognostic impact of frailty in HFpEF, it should be considered when designing treatment paradigms for older patients with HFpEF with the goal of reducing symptom burden, improving functional status and exercise capacity, and ultimately, improving clinical outcomes. Treatment approaches targeting frail patients with HFpEF are being evaluated; one study showed significant improvement with a novel physical rehabilitation intervention.(39)
Pathophysiology of EI in HFpEF
Both cardiac and extra-cardiac abnormalities contribute to EI in HFpEF by reducing physiologic reserve capacity for the key determinants of VO2peak: exercise cardiac output and peripheral oxygen extraction. An integrative approach to quantifying relative contributions to impaired VO2peak in HFpEF can be achieved by tracing oxygen pathway from “mouth to mitochondria”. Deficiencies in alveolar ventilation, lung diffusion capacity, cardiac output, hemoglobin, skeletal muscle diffusion capacity for O2, and mitochondrial respiration can contribute to impaired VO2peak associated with HFpEF.(11) There is heterogeneity of O2 pathway derangements in patients with HFpEF, the majority of whom have multiple abnormalities in the O2 pathway.(11) Given the serial nature of the steps in the O2 pathway, targeting a single step may not result in a significant overall enhancement of O2 utilization.(11) Deficiencies in specific steps of the O2 pathway can be assessed by invasive hemodynamic monitoring during exercise and via tests of peripheral skeletal muscle blood flow, O2 diffusion and utilization.
Hemodynamic measurements during exercise permit assessment of the relative contributions of cardiac versus peripheral impairments and confirm the presence HF and influence of extra-cardiac abnormalities. Anticipated changes in response to treatment interventions may then be modeled in the context of the compound O2 pathway deficits. Multiple benefits can be achieved through exercise training which thus serves as an example of an intervention that demonstrably improves EI in HFpEF by improving impairments in multiple steps in the O2 pathway in multiple organ systems.(40) In the following section, we have discussed in detail the underlying contribution of different cardiac and extra-cardiac abnormalities toward EI in older patients with HFpEF.
Cardiovascular contributors to EI
Impairment in left atrial and left ventricular reserve
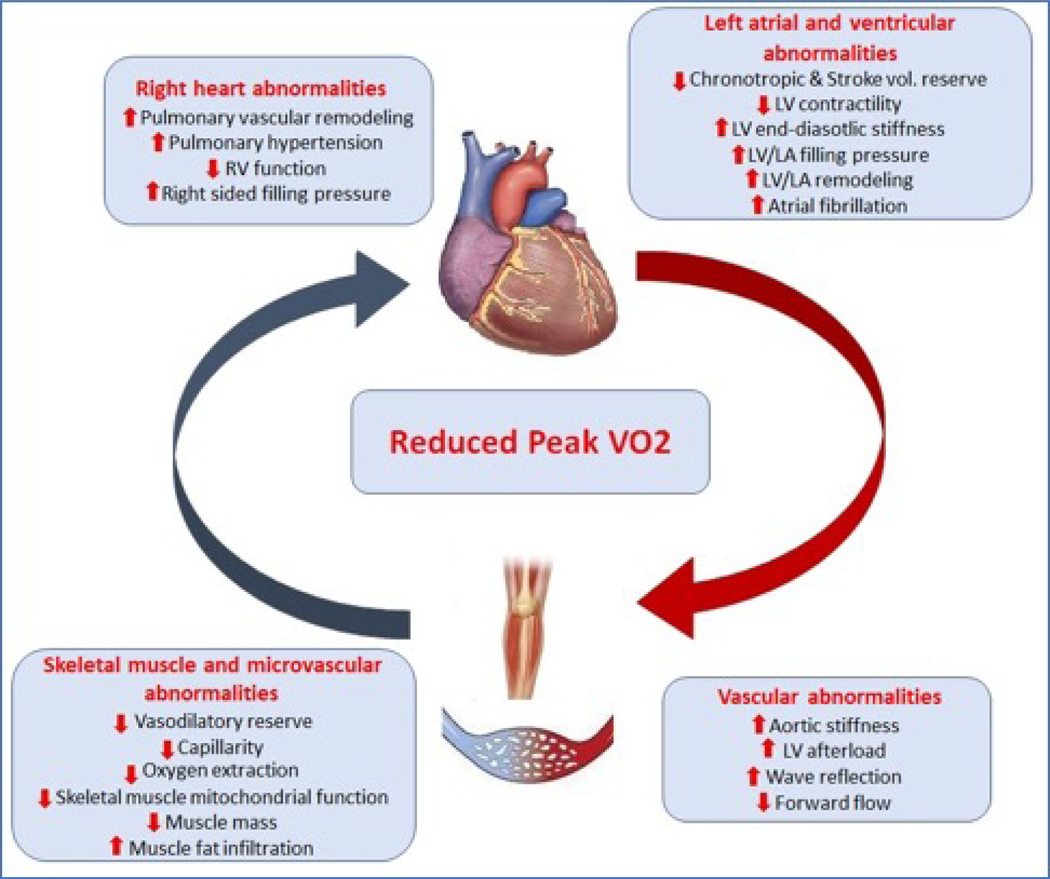
Abnormalities of left heart structure, function, and reserve play a pivotal role in the pathophysiology of EI in HFpEF. These components have been described in detail elsewhere(41) and are summarized in the online supplement and integrated into Figure-3.
Figure 3: Contribution of cardiovascular maladaptations to exercise intolerance in HFpEF.
Patients with HFpEF have impairment in stroke volume reserve and chronotropic reserve, and increased afterload, all of which contribute to reduced forward flow. This “central limitation’ coupled with impairment peripheral vasodilation and reduced oxygen extraction contributes to lower peak exercise oxygen uptake.
Right heart function and the pulmonary vasculature in HFpEF
Pulmonary vascular remodeling, both arterial and venous, are common and severe in HFpEF and is related to several factors including chronically elevated left-sided filling pressures, vasoconstriction, decreased nitric oxide availability, and metabolic and inflammatory dysregulation.(42) Pulmonary hypertension is prevalent in up to 80% of HFpEF patients and is associated with increased morbidity, mortality, and EI.(43)
Several age-related changes likely underlie the above findings, including decreased right ventricular (RV) systolic and diastolic function, which may exceed corresponding changes in the left heart.(44)Aging and associated cardiometabolic dysregulation may also contribute to impaired RV function (Figure-3).(45) Measures of pulmonary function and gas exchange deteriorate with aging; this may be related in part to aging-related kyphotic changes, declines in respiratory muscle function, and reduction in functional alveolar-capillary units.(46)Additionally, structural and dynamic compliance of the pulmonary vasculature likely is reduced with aging.(47) These abnormalities are amplified in patients with HFpEF.(48,49)
Systemic vascular contributions to HFpEF
The systemic arterial tree plays a central role in modulating LV afterload, ventricular-arterial coupling, systolic and diastolic function, and the distribution of the cardiac output at rest and during exercise.(50) Thus, it has an important mechanistic role at several steps of the oxygen consumption cascade and in the chronic maladaptive remodeling of the heart and other target organs (Figure-3). Due to widely varying characteristics, individual territories within the arterial tree are discussed separately below.
Aorta:
A healthy arterial system delivers relatively steady flow to the microvasculature, despite intermittent LV ejection, largely due to the cushioning (windkessel) function of the aorta. Aging results in stiffening of the aorta, and this is accelerated by comorbidities, particularly hypertension, obesity, and diabetes. Aortic stiffness is higher in patients with established HFpEF compared with healthy age-matched controls. Greater large-vessel arterial stiffness at rest is associated with lower Vo2peak in older HFpEF patients.(51) Moreover, central arterial stiffness worsens during exercise and contributes to EI in HF.(52,53) The changes in large arteries favors adverse patterns of pulse pressure with consequent changes in pulsatile LV afterload, which promotes LV remodeling, fibrosis, dysfunction, and failure.(54) Large artery changes also leads to excessive delivery of pulsatile energy to the microvasculature of target organs as a function of arteriolar resistance.(55) Therefore, in addition to its impact on the heart, large artery stiffening may be one of the factors contributing to clustering of HFpEF with various comorbidities in older persons, such as chronic kidney disease and dementia.
Muscular arteries:
LV contraction generates a pulse wave that travels forward in the arteries and is partially reflected at sites of impedance mismatch, such as points of branching or change in wall diameter or stiffness. Numerous peripheral reflections are summed into a larger reflected wave, which travels back to the LV. In older adults, lower vascular compliance results in a reduced wave transit time (pulse wave velocity) from the LV to reflection sites and back to the proximal aorta. In some cases, wave reflections return to the LV while the aortic valve is still open and ejecting blood in mid-to-late systole resulting in increased afterload. Thus, pulse wave reflections can increase the late systolic LV workload and adversely shift the LV loading sequence from early to late systole, which promotes LV remodeling and dysfunction.(54,55)
HFpEF patients have increased late systolic load from wave reflections during exercise, which contributes to exaggerated afterload, increased LV filling pressure, reduced exercise cardiac output, and reduced VO2peak. Furthemore, vasodilator therapies such as inorganic nitrites and beet-root juice have been shown to imporve arterial compliance and VO2peak in patients with HFpEF. Thus, wave reflections may represent a mechanistic therapeutic target for novel therapies such as inorganic nitrates/nitrites.(52,56)
Microvasculature:
In addition to its role in determining mean arterial pressure and resistive load to the LV, microvascular function has an important role in peripheral oxygen delivery and utilization. Vasodilatory responses during exercise mediate the peripheral redistribution of flow to working muscle, a key component of the normal hemodynamic response to exercise and depends on local vasodilatory mechanisms within muscle as well as neurovascular sympathetic control mechanisms.(57) Hypoxic vasodilation is an important local mechanism that ensures adequate local blood flow to oxygen to muscle tissue, and allows the local vasculature to overcome humoral and reflex-mediated vasoconstriction during exercise.(57) Microvascular dilatory reserve is abnormal in HFpEF, contributes to EI, and may be improved by exercise training.(58)
HFpEF patients also have abnormalities of the coronary microcirculation, impaired coronary flow reserve, and greater burden of myocardial injury with exercise due to oxygen supply-demand mismatch.(59,60) Large artery stiffness and wave reflections are key determinants of myocardial perfusion pressure through their effects on the diastolic pressure profile.(55) It is possible that the reduced coronary microvascular perfusion reserve in HFpEF is due to both abnormalities in coronary microvasculature such as myocardial microvascular rarefaction,(61) endothelial dysfunction, and abnormal central aortic hemodynamics.
Contribution of skeletal muscle myopathy to EI in HFpEF
Overview of skeletal muscle aging
Sarcopenia, the age-associated loss of skeletal muscle mass and function results in impaired mobility, reduced physical function, and increased disability and mortality.(62) The causes of sarcopenia are multi-factorial but include poor nutritional status, physical inactivity, inflammation, and chronic co-morbidities, i.e., the hallmarks of aging. The recognition of sarcopenia as a relevant clinical syndrome of advancing age has led to refinements in its identification, prevention, and treatment.(62–64)
Strength, power, and endurance each comprise unique physiological domains of muscle performance and are differentially related to the declines in physical functioning with aging (Table-3).
Table 3.
Measures of muscle performance relevant to older adults
| Muscle performance measure | Description |
|---|---|
| Strength | • Maximum capacity to generate force or tension • Can be assessed using isometric handgrip dynamometry or a more dynamic measure of the 1-repetition maximum |
| Power | • Rate of physical work performance • Has a component of velocity that may be have clinical relevance for older adults • Peak power is more closely associated with declines in physical function than muscle strength in older adults |
| Endurance | • Ability to maintain a specific force output • Related to functional capacity in older adults. |
The link between peripheral vascular function and sarcopenia
Aging is associated with reduced sensitivity of skeletal muscle proteins to all key anabolic stimuli: general nutrition; dietary amino acids; insulin; and resistance exercise.(65) This phenomenon, called anabolic resistance, is promoted by abnormal rapamycin (mTOR) complex 1 signaling (a key regulator of the anabolic response in skeletal muscle), and contributes to sarcopenia. Endothelial dysfunction may also play a fundamental role in the anabolic resistance of aging as shown in Figure-4.
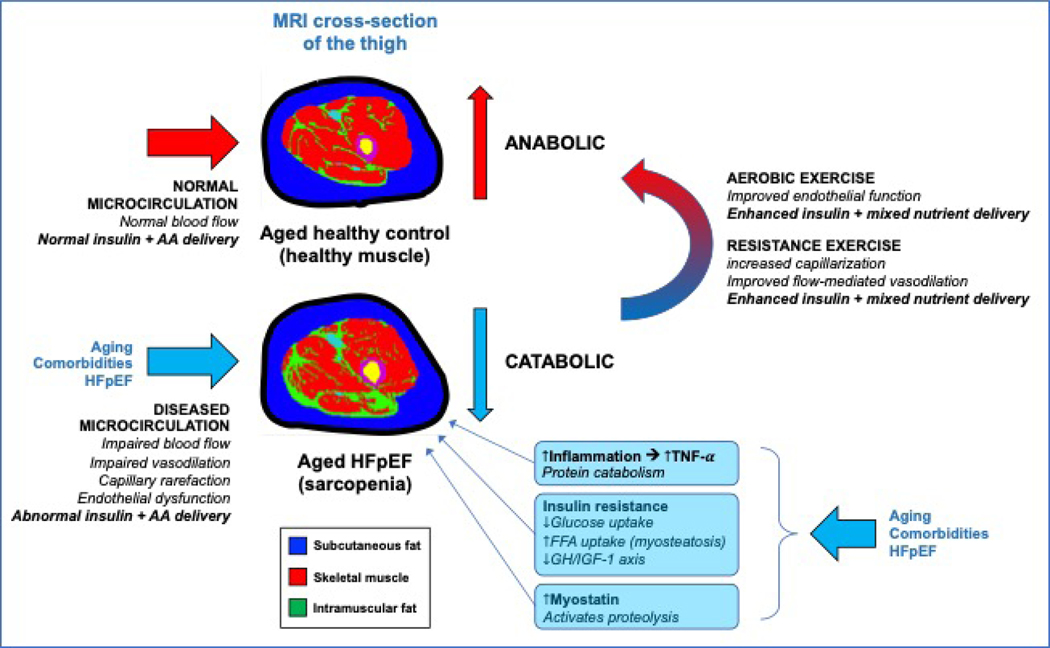
Figure 4: Microvascular function and sarcopenia.
Comparison of the thigh compartment fat and muscle composition in a control vs. HFpEF individual on MRI. HFpEF patients have greater intramuscular fat and reduced skeletal muscle mass. Impaired microcirculatory function leads to sarcopenia, which is magnified by the catabolic effects of inflammation, insulin resistance, and increased myostatin. AA=amino acids; FFA=free fatty acids; GH=growth hormone; IGF-1=insulin-like growth factor-1.
Because nutritive flow helps regulate skeletal muscle protein anabolism, the decrease in endothelial function with aging contributes to anabolic resistance and sarcopenia in older adults. HFpEF is associated with worse endothelial function, which is correlated with symptoms of EI and reduced VO2peak,(66) and could exacerbate aging-associated impaired muscle anabolism.
Impairment in skeletal muscle architecture and oxygen transport in HFpEF
HFpEF-mediated reduction in the peripheral O2 extraction reserve is also secondary to impaired skeletal muscle O2 diffusion capacity (i.e., transport of O2 from microcirculation to muscle mitochondria).(11) HFpEF patients with slower VO2 kinetics have more severely impaired peripheral oxygen utilization and higher cardiac output during submaximal exercise compared to HFpEF patients with faster VO2 kinetics as well as healthy controls suggesting that the limitation for oxygen during submaximal exercise is not related to impaired cardiac output.(67) Thus, evaluation of VO2 kinetics with brief, low-intensity exercise could help phenotype older HFpEF patients with peripheral limitations.
Since most of the O2 intake during exercise is consumed in the active skeletal muscles, the decline in VO2peak in older HFpEF patients may be partially due to a loss in muscle mass, which is reduced in HFpEF.(68) Haykowsky et al. demonstrated a strong association between lean mass (percent total as well as leg lean mass) and peak VO2peak. However, in addition to the muscle mass, Haykowsky et al. also implicated impaired muscle quality in the reduced VO2peak among patients with HFpEF such that VO2peak indexed to total and leg lean mass was significantly lower and the change in VO2peak with increasing percent leg lean mass was markedly reduced in HFpEF compared with healthy controls.(68) Older HFpEF patients also have significantly increased thigh intermuscular fat and intramuscular fat to skeletal muscle area ratio compared with healthy controls, both of which were inversely related to VO2peak.(25) Furthermore, skeletal muscle biopsy studies have shown that older patients with HFpEF have a shift in fiber type distribution with a reduction in percent type I (oxidative) fibers and reduced capillary to fiber ratio,(69) and both of these alterations were associated with reduced VO2peak.(69) This is in contrast with changes in skeletal muscle architecture noted with healthy aging whereby the loss of muscle mass in healthy old versus. young adults has been attributed to smaller type-II muscle fiber size without substantial reduction in muscle fiber numbers.(70) Taken together, abnormalities in skeletal muscle mass and quality contribute to EI in older HFpEF patients (Figure-4).(25,68)
Skeletal muscle mitochondrial bioenergetics and HFpEF
Older patients with HFpEF have abnormal skeletal muscle oxygen utilization that is related to reduced VO2peak.(11,29,71) By magnetic resonance spectroscopy, skeletal muscle oxidative metabolism is reduced in patients with HFpEF.(72) Using hemodynamic monitoring during exercise, the ability to extract O2 in the skeletal muscles and reduce venous O2 content is significantly impaired in HFpEF and is a major contributor to reduced VO2peak.(11,73) Mitochondrial content and oxidative capacity are significantly reduced in skeletal muscle biopsy samples from older patients with HFpEF vs. age-matched controls.(74) These mitochondrial alterations are related to measures of aerobic exercise capacity such as VO2peak and 6-minute walk distance. Improved mitochondrial function contributes to improvements in VO2peak associated with exercise training, further supporting that skeletal muscle mitochondrial abnormalities contribute to pathophysiology of EI in HFpEF patients.(2) Exercise training can increase mitochondrial electron transport chain (ETC) enzyme function 2-fold, accompanied by a 60% increase in mitochondrial mass(75) and has been shown to increase electron transport chain activity, mitochondrial mtDNA content, and citrate synthase activity.
Contribution of Myosteatosis to EI in HFpEF
Common risk factors associated with HFpEF such as obesity, aging, and physical inactivity contribute to deranged energy metabolism and metabolic inflexibility (76)– a loss in the capacity to switch effectively between fatty acids and carbohydrates as fuel for energy. These are associated with impaired skeletal muscle energetics and excess adipose accumulation in muscle tissue, or myosteatosis. Older HFpEF patients have significant myosteatosis and abnormal skeletal muscle energetics including impaired mitochondrial function, reduced mitochondrial density, and impaired peripheral O2 extraction with exercise compared with healthy, age-matched controls, and these are significantly correlated with their severe EI.(74,77,78) Furthermore, improvement in VO2peak in obese HFpEF patients in response to dietary weight loss and exercise training appear to be mediated, at least partly, by improved skeletal muscle mitochondrial function in tandem with reduction in adipose depots in the skeletal muscle tissue.(12)
Contribution of systemic metabolic abnormalities to EI in HFpEF
Obesity, Regional Adiposity, and their implications in senescence and HFpEF
Obesity, particularly central and visceral adiposity, have emerged as major risk factors for HFpEF.(9,25,79,80) Nearly half (~47%) of HFpEF patients are obese; and HFpEF patients with BMI in the “normal” range typically have significant visceral adiposity.(81) Visceral obesity is associated with abnormal cardiac mechanics prior to the development of HFpEF,(82) and the addition of hypertension to obesity accelerates the development of cardiac dysfunction and elevated filling pressures.(83)
Age-associated changes in body composition include redistribution of fat mass mainly to the visceral compartment. There is also a shift in lipid storage from the subcutaneous to the VAT depot,(84) which is thought to be due to a decline in progenitor cell function and an accumulation of senescent adipose tissue cells in subcutaneous fat.(85) There is also redistribution of adipose tissue to the abdominal compartment, which is presumed to be due to the decline in testosterone in men and estrogen in women following menopause. VAT is pro-inflammatory, vasoactive, and dysmetabolic, with many factors secreted by visceral adipocytes that may contribute to pathogenesis of HFpEF. Of these, plasminogen activator-inhibitor-1 (PAI-1), encoded by SERPINE1, is a leading candidate due to its known association with VAT, accelerated senescence, and risk of incident HFpEF but not HFrEF in population-based studies.(86–88) In animal models of diabetes and aging, PAI-1 levels are elevated and genetic deficiency or inhibition of PAI-1 prevents diabetes and extends lifespan.(86) These findings have been replicated in humans with genetic PAI-1 deficiency.(87)
Aging is also associated with changes in the cellularity and function of subcutaneous adipose tissue.(89) Brown adipose tissue declines with aging with a resultant predominance of white adipose tissue, which is proinflammatory and exacerbates aging-associated inflammation. In white adipose tissue, adipocytes undergo hypertrophy, adipocyte necrosis, adipose tissue inflammation, and a switch to proinflammatory classical cytokines and adipokines. In addition, there is recruitment of monocytes and other immune cells to remove necrotic adipocytes and to participate in tissue remodeling in an attempt to limiting lipid storage, but this eventually results in ectopic lipid accumulation (e.g., liver and skeletal muscle) and insulin resistance. Importantly immune cells have been shown to play a pivotal role in the pathogenesis of HFpEF.(90) The resulting state of chronic, low-grade inflammation and systemic metabolic inflammation,(90,91) and augmented nitrosative stress have been implicated in obesity-associated HFpEF and associated EI.(83) In a preclinical model of normal weight HFpEF, cardiac NP signaling causes alterations in energy expenditure and metabolism, and promotes brown adipose-like features in white adipose tissue depots.(92) The sympathetic nervous system and NP signaling increases metabolic activity in adipose tissue by activating lipolysis and modulating the brown-fat thermogenic program. NP signaling is an important regulator of overall NP activity in adipose tissue in HFpEF and its relevance in obese HFpEF and in aging requires further exploration.
Obesity, Regional Adiposity, and EI in HFpEF
Obese patients with HFpEF have reduced relative VO2peak (ml/kg/min) as compared with their non-obese counterparts(93). This is related to both peripheral and central limitations in exercise induced oxygen uptake. Obese individuals have exaggerated LV filling pressure and pulmonary artery pressure response to exercise which contributes to reduced exercise cardiac output. Obese individuals also have greater infiltration of fatty tissue in peripheral skeletal muscles leading to impairment in oxygen extraction.(25) Recent studies have also demonstrated that obese individuals have depletion of myocardial energetic source namely, the Creatine Kinase shuttle, which is the main transfer mechanism for maintaining ATP delivery in myocardial mitochondria.(94) As a result, the delivery of ATP cannot be maintained with exercise stress leading to reduced VO2peak. Furthermore, intentional weight loss is associated with restoration of the ATP delivery with exercise in the myocardium.(94)
Among regional adipose depots, increased VAT is a major contributor to reduced VO2peak in obese patients with HFpEF (25) and may partially explain the female-predominance of HFpEF. A recent study demonstrated that excess VAT is associated with more exaggerated increase in pulmonary capillary wedge pressure with exercise in women but not in men. VAT associated EI is modifiable and loss of visceral fat with caloric restriction has strongly associated with improvement in VO2peak among patients with HFpEF.(12) In tandem with VAT, pericardial fat also increases, which is associated with more adverse hemodynamics and lower VO2peak in HFpEF.(95) Taken together, obesity and excess regional adipose depot may be important targets for improvement in VO2peak in patients with HFpEF.
Contribution of diabetes and insulin resistance to EI in HFpEF
The risk of HF increases substantially with diabetes, and up to 40% of patients in HFpEF studies have diabetes.(96) HFpEF patients with (vs. without) diabetes have higher burden of co-morbitidies, lower VO2peak and submaximal exercise capacity, elevated levels of biomarkers of inflammation, and higher risk of adverse clinical events of follow up.(96) The lower VO2peak in HFpEF patients with diabetes may be related to higher left ventricular filling pressures, chronotropic incompetence, and impaired parasympathetic and sympathetic response to exercise due to underlying autonomic neuropathy.(97) Patients with diabetes also have reduced delivery and extraction of oxygen in the exercising skeletal muscles due to higher prevalence of anemia, reduced vasodilator reserve, higher peripheral vascular resistance from endothelial dysfunction and vasoconstriction, and impaired mitochondrial function.(96,98,99) Considering the higher burden of EI, HFpEF patients with diabetes are an important target for exercise training strategies which can improve VO2peak through peripheral adapations in the O2 pathway. Recent small RCTs have evaluated the role of novel pharamcotherapies such as Sodium Glucose Co-transpoter-2 inhibitors (SGLT2-i) in patients with HFpEF with variable effects (Table-4). Larger outcomes trials using these therapies in HFpEF are currently underway.
Table 4:
Therapeutic Strategies that have shown promise for improving exercise intolerance in patients with HFpEF via extra-cardiac factors
| Therapeutic Approach | Known and Potential Therapeutic Effects in HFpEF |
|---|---|
| Supervised Exercise | Improvement in peak VO2 Improvement in QOL Improvement in peripheral oxygen extraction at peak exercise |
|
Multidomain Physical
Function Intervention (REHAB-HF) |
Improved physical function Improvement in frailty burden Improvement in quality-of-life |
| Weight Loss | Improvement in VO2peak Improvement in quality-of-life Reduction in visceral adiposity and systemic inflammation burden Reduction in myostasis |
| IL-1 Receptor Blockers (Anti-inflammatory agents) | Reductions in CRP and NT-ProBNP levels Modest improvement in the treadmill exercise time from baseline to follow up |
| Senotherapeutic Agents | Reduction in senescent cell burden and the SASP in human tissues. In mouse model, alleviation of age- and senescence-related cardiovascular dysfunction, lung diseases, metabolic syndromes, frailty, and improvement in survival Therapeutic effects in animal models or patients with HFpEF are not available. |
| Novel pharmacotherapies for diabetes (SGLT-2 inhibitors/GLP-1 agonists | Improved in adverse cardiac remodeling patterns in animal models with use of liraglutide and dapagliflozin No significant effects on empagliflozin on exercise capacity or quality of life in patients with HFpEF in small RCT (EMPERIAL-PRESERVED) |
| Inorganic nitrates/nitrites | Single dose of Beet root juice (inorganic nitrate donor) has been shown to improve VO2peak and reduce systemic vascular resistance and arterial wave reflections with exercise 1-week of daily Beet root juice dosing has been shown to improve aerobic capacity by 24%. Inorganic sodium nitrite has been shown to improve VO2peak, skeletal muscle oxygen conductance, VO2 kinetics, alveolar capillary membrane O2 conductance, and O2 utilization during submaximal exercise. No effects of inhaled nitrite on VO2peak in HFpEF in the INDIE-HFpEF trial may be related to challenges with the drug delivery and short-acting nature of the therapy. Results from other ongoing studies awaited |
Abbreviations: VO2peak – Peak exercise oxygen uptake; HFpEF- heart failure with preserved ejection fraction; SASP – Senescence associated secretory phenotype; CRP- C-reactive protein; NT-ProBNP- N-terminal pro brain natriuretic peptide; RCT: Randomized controlled trial
Tenets of Aging and Geroscience: Implications for EI in HFpEF
Hallmarks of aging: Role in health and disease
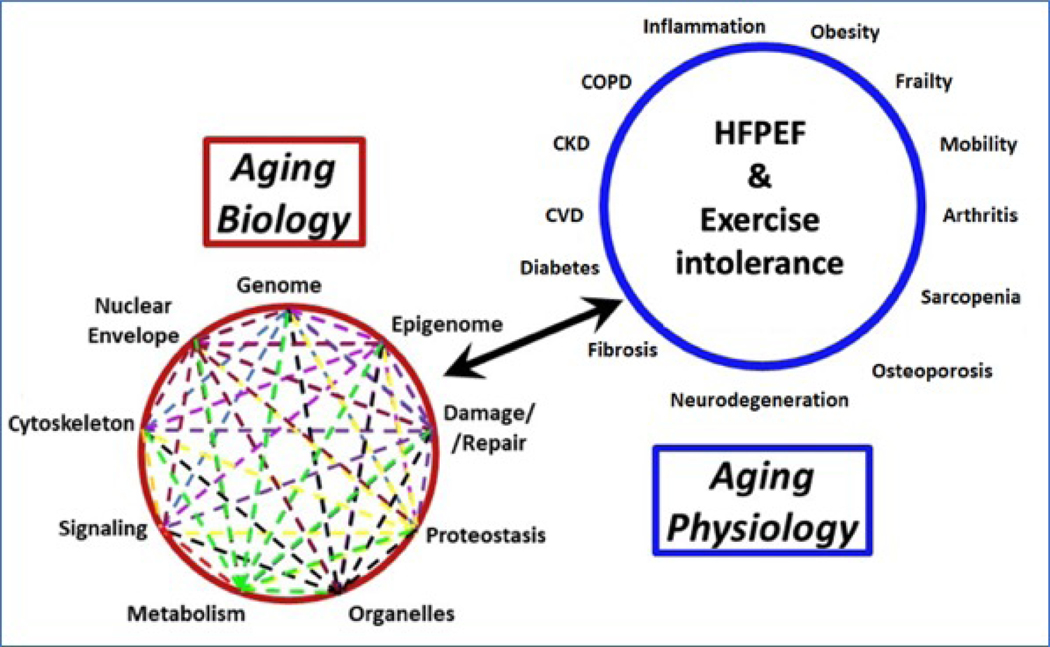
Two foundational reviews established the field of geroscience by identifying a short-list of biological processes implicated in aging and conserved across species which could serve as new therapeutic targets for many age-related chronic diseases and geriatric syndromes, including HFpEF. These curated lists of seven to nine biological processes, referred to “hallmarks” or “pillars” of aging provide a framework for understanding the role of conserved biological processes in health and disease (Figure-5).(14,100) These processes include macromolecular damage, metabolism, proteostasis, inflammation, adaptation to stress, epigenetics, cell senescence, stem cells/regeneration, and pleiotropic processes. As discussed earlier, aging is a universal biological phenomenon that results in a progressive decline in integrated function over time. These age-related declines ultimately lead to an increased probability of death.
Figure 5: Contribution of hallmarks of aging to HFpEF and exercise intolerance.
The hallmarks of aging refers to closely inter-related cellular and molecular processes implicated in aging and conserved across species which could serve as new therapeutic targets for many age-related chronic diseases and geriatric syndromes, including HFpEF.
Approximately 5–10 overlapping and highly intertwined molecular and cellular processes are evolutionally conserved ‘common denominators’ that are implicated in biological aging.(101) These biological processes are active during ‘normal aging’ and when ameliorated, the phenotype of aging and its sequelae are retarded. In contrast, when the activity of a hallmark is augmented, phenotypic aging processes are accelerated. The hallmarks are interdependent such that the effects of one biological process activates others and leads to increased dysregulation across multiple processes at progressively accelerating rates over time. Interventions to ameliorate the aging sequelae of each of the different hallmarks have been demonstrated in mice, suggesting that similar amelioration is possible in humans.(102)
The geroscience hypothesis posits that incident aging-related chronic disease can be prevented, delayed, or attenuated by therapeutically targeting these biological processes.(103) One example is mTOR, which has emerged as a key regulator of aging in laboratory studies, with the genetic or pharmacological inhibition of mTOR shown to delay aging in animal models.(102,104) Extant evidence suggests that mTOR antagonists may ameliorate nearly all identified biological aging processes to some extent, with consistent attenuation of multiple positive feedback interactions between hallmarks.(102) Another example is cellular senescence and the resulting generation of a senescence-associated secretory phenotype (SASP), which is hypothesized to be at the nexus of several age-related chronic diseases and geriatric syndromes.(100) The SASP is a consequence of altered gene expression that contributes to sterile inflammation and adverse tissue remodeling, and further accumulation of senescent cellsin vivo. Experimental therapies targeting cellular senescence, or senotherapeutics, include senolytic drugs (selective removal of senescent cells) and senomorphic drugs (modulate or reverse SASP) represent an emerging strategy for treatment of age-related disease(s), including HFpEF.
Contribution of proteostasis and autophagy to EI in HFpEF
Proteostasis is a homeostatic pathway involving the generation and removal of cellular proteins and organelles for regeneration purposes and for the provision of energy substrates.(105) The cellular machinery involved in proteostasis includes the proteasome and the autophagosome/lysosome. Functional proteostasis is required for tissue health and its impairment is associated with tissue dysfunction and disease. Aging brings a global reduction in cellular proteostasis, especially the autophagy process, in various tissues including skeletal muscle, cardiac tissue and liver.(106)
In cardiac tissue, aging is associated with reduction in autophagy and tissue proteostasis.(107) While relatively little is known regarding the role of cardiac proteostasis in HFpEF, mouse models of HFpEF have shown a decline in proteostasis and autophagy in skeletal muscle and liver tissue.(108) Exercise, which increases muscle autophagy in many other conditions, does not increase autophagy in a young rat model of HFpEF.(109) Taken together, both aging and HFpEF independently reduce proteostasis in tissues involved in the pathogenesis of HFpEF.
Inflammation, ‘omics of immune cells, and EI in HFpEF
Chronic inflammation, one of the hallmarks of aging, has been implicated as the key pathway unifying multifactorial and intertwined mechanisms leading to HFpEF.(110) Prior studies have shown high levels of circulating inflammatory biomarkers, including IL6 and CRP, are present in established HFpEF, and are associated with risk for development of HFpEF, and with severity and prognosis of HFpEF.(111) Furthermore, dietary weight loss in obese HFpEF results in improved symptoms, VO2peak, quality-of-life, and the improvement is associated with reduced inflammation biomarkers.(12) A causal role for inflammation in HF is further supported by results from a recent randomized clinical trial (RCT) showing that anti-inflammatory therapy targeting IL1β may reduce risk for HF.(112) Among patients with HFpEF, a 12-week, phase-II, placebo-controlled trial demonstrated a significant reductions in CRP and NT-ProBNP levels with IL-1 receptor blockers, and a modest improvement in the treadmill exercise time, a measure of aerobic exercise performance, within the treatment arm.(113)
Current understanding of mechanistic links between inflammation and HFpEF has mainly been derived from animal models.(114) The inflammatory response is a protective mechanism in which activated immune cells phagocytose and remove pathogens. However, during chronic inflammation, innate immune cells such as monocytes can be reprogrammed and promote exaggerated inflammatory responses after infiltrating the myocardium.(114,115) Therefore, ‘omics profiling of human immune cells, particularly of easily accessible circulating immune cells, holds great promise in the exploration of the cell-specific mechanisms leading to HFpEF and may facilitate the development of innovative interventions through modulation of these cells. Additionally, ‘omics approaches enable generation of a systemic view of fundamental mechanisms of the aging process and their interconnection. For example, aging-associated decline in several inter-correlated gene modules relevant to mitochondrial function has been reported in a transcriptomic study of human peripheral monocytes.(116) The down-regulation of mitochondrial function-related genes is a common feature of aging in various tissues across humans and other species,(117) and may contribute to EI in HFpEF.(14)
Approaches targeting aging-related contributions to EI in HFpEF
Exercise training and multidomain physical function interventions in HFpEF:
Short-term exercise training has been associated with significant improvement VO2peak (~20%) and quality-of-life in patients with HFpEF.(40) Exercise training can improve VO2peak by favorably affectingmultiple pathophysiologic impairments in the oxygen pathway as discussed earlier and summarized in Table-4. Despite the well-known benefits of exercise training, the optimal intensity and duration of exercise training for patients with HFpEF are uncertain. In a recent large multicenter exercise training trial, high intensity vs. moderate continuous exercise training for 3 months were associated with comparable improvements in VO2peak among patients with HFpEF.(32) Long-term adherence to exercise training in older patients with HFpEF is generally suboptimal. Older patients with HFpEF, particularly those recently hospitalized, are often frail and have physical function impairments in multiple domains including balance, mobility, and strength as well as endurance and may benefit from a novel, early, tailored, multidomain physical function intervention more than patients with HFrEF, as reported in the recent REHAB-HF trial.(39,118) However, such interventions need to be evaluated in larger RCTs of older patients with HFpEF to evaluate their efficacy in improving not only physical function and quality-of-life but also clinical outcomes (rehospitalization, death) which are important to ensure uptake and broad implementation of such an intervention by patients, healthcare systems, and payers.
Caloric restriction, weight loss, and nutrition
Dietary CR has strong potential as a strategy to prevent obese HFpEF and reduce EI when HFpEF develops. Intentional weight loss by CR and bariatric surgery in obese patients is associated with a lower risk of HFpEF.(119) In a 20-week, controlled trial of CR with and without exercise in 100 patients with obese HFpEF, CR produced significant weight loss, more than exercise alone, and both CR and exercise produced strong, additive improvements in VO2peak(ml/kg/min). Significant improvements in exercise capacity were confirmed by improvement in measures that are not indexed to body weight such as VO2 reserve, exercise time to exhaustion, workload, and leg power supporting the notion of true improvement in exercise capacity with weight loss. CR was also associated with greater improvements in quality-of-life than exercise. In the CR group versus exercise-only and control patients, VAT decreased by 15–20% and hs-CRP levels declined, and reductions in both were associated with improved VO2peak.(12)
CR studies in animal models have shown numerous, robust favorable impacts on aging-related pathways, such that the benefits of CR in HFpEF could extend beyond the pathways examined above.(120) In mice, these include mechanisms that have been directly implicated in the pathophysiology of HFpEF; CR from young adulthood prevents diastolic dysfunction, endothelial dysfunction and increased arterial stiffness, and even in older mice CR reduces oxidative stress, improves endothelial function, and increases nitric oxide bioavailability.(121,122) When compared with age-matched controls, humans voluntarily participating in moderate, long-term CR (while maintaining nutritional quality) have favorable alterations in aging-related pathways including PI3 K/AKT and AMPK/SIRT and up-regulation of antioxidant defense, DNA repair, proteostasis, and autophagy-related genes.(123)
In RCT of CR, 10–30% CR in young to middle-aged, non-obese participants produced decreased resting metabolic rate, improved insulin sensitivity, reduced VAT, decreased oxidative stress and associated DNA damage, and increased skeletal muscle mitochondrial DNA content, confirming feasibility, safety, and anti-aging potential of long-term CR.(123–125)
In the CR trials in non-obese adults as well as in the obese HFpEF patients, CR was associated with decreased skeletal muscle mass despite careful attention to adequate protein and micronutrient intake.(12,124,125) However, CR produced gains in endurance and strength despite decreased muscle mass. Future studies are needed to evaluate the long-term safety of CR in patients with obese and HFpEF. Among other dietary interventions, recent trials suggest that focusing on dietary quality among hospitalized patients with HF can improve quality-of-life, prevent readmissions, and reduce mortality.(126) Future studies are needed to evaluate the efficacy of such dietary interventions in patients with obesity and HFpEF.
Senotherapeutics: A novel potential treatment paradigm for EI in HFpEF
Senotherapeutics and senolytic drugs hold promise as therapeutic targets to improve function, geriatric syndromes, and age-associated chronic diseases including HFpEF. Several pro-survival senescent cell anti-apoptotic pathways (SCAPs) have been identified from bioinformatics analyses of senescent vs. non-senescent human cells and RNA interference studies. Dasatinib, a tyrosine kinase inhibitor used clinically to treat leukemia, and Quercetin, one of the first senolytic agents identified, is a natural product that targets B-cell lymphoma, insulin and insulin-like growth factor-1, and hypoxia-inducible factor-1α SCAP network components.(127) The combination of these two agents reduces senescent cell burden and the SASP in human tissues. In animal models, combination of Dasatinib and Quercetin alleviates a range of age- and senescence-related disorders in mice including cardiovascular dysfunction, , fibrosis-related lung diseases, lipotoxicity, hypercholesterolemia, metabolic syndromes, depression, cancer, frailty, and substantially increases survival.(127) Future animal and human studies are needed to test similar interventions that target biologic hallmarks of aging for management of HFpEF.
Knowledge gaps and recommendations for future research
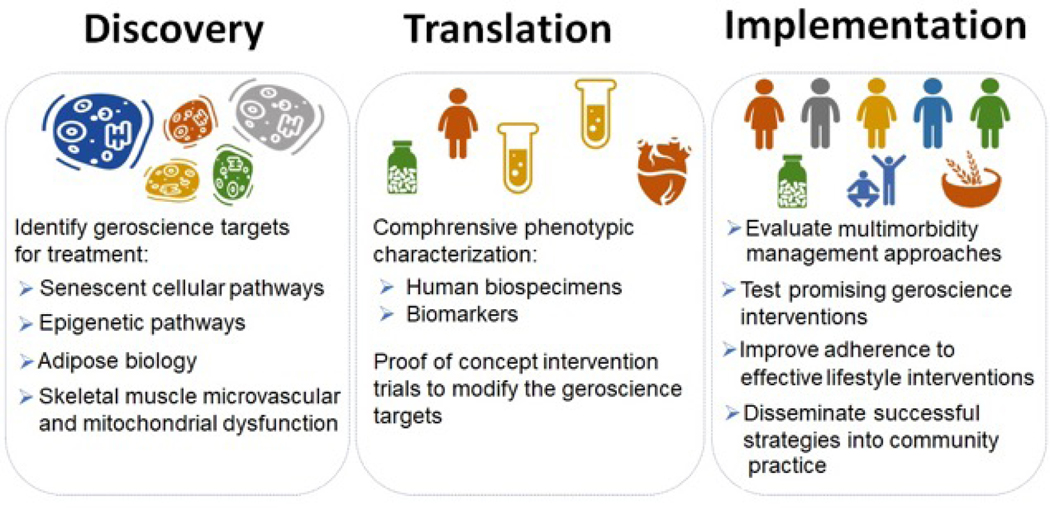
Several knowledge gaps pertaining to our understanding of the contribution of extracardiac factors toward EI in patients with HFpEF have been identified and summarized across different extra-cardiac domains in Table-5. Future research should focus on four key priorities in older patients with HFpEF: 1) better understand the pathophysiology of EI; 2) develop novel approaches to phenotypic classification of HFpEF based on underlying pathophysiology; 3) identify novel, promising targets for preventing and mitigating EI; 4) design and test transdisciplinary interventions to prevent and mitigate EI (Figure-6).
Table 5:
Key knowledge gaps in our understanding of the extracardiac contributors to exercise intolerance in HFpEF
| Organ System/Domain | Specific Pathyway/Target | Key knowledge Gaps for Future Research |
|---|---|---|
| Skeletal Muscle | Sarcopenia | 1. Integrating ‘pathophysiology of sarcopenia development’ in drug designing for HFpEF. 2. Examine structural and functional changes in muscle in HFpEF trials 3. Role of protein supplementation and / or resistance training in attenuating development of sarcopenia |
| Oxygen utilization | 1. Impact of exercise and weight loss intervention on skeletal muscle mitochondrial functional. 2. Role of VO2 kinetics during the rest to low-level exercise transition in phenotyping HFpEF patients with a ‘peripheral’ limitation to exercise 3. Evaluating the contribution of peripheral adaptations to the improvement in VO2peak with exercise training and/or CR in older HFpEF patients. |
|
| Endothelial Dysfunction | 1. The molecular mechanisms linking endothelial dysfunction and age-related muscle anabolic resistance 2. If treatment of endothelial dysfunction improves muscle bulk and function in older adults 3. Effects of endothelial function and nutrition on skeletal muscle anabolism and physical function in older patients with HFpEF. |
|
| Pulmonary and right ventricular system | Right heart and pulmonary function | 1. Delineate the interaction of other comorbidities with pulmonary hypertension in HFpEF 2. Examine the relative contributions of pulmonary venous versus arterial remodeling to EI 3. Improve understanding of the right ventricular response to aging and cardiopulmonary disease. |
| Metabolic disorders | Obesity | 1. Role of browning of white adipose tissue in development and outcome of HFpEF 2. Mechanisms whereby increased remote, inter- and intracellular adiposity contribute to EI in older HFpEF patients 3. Greater representation of obese patients in HFpEF trials |
| Diabetes and Insulin resistance | 1. Mechanisms underlying the increased risk of HFpEF in diabetes patients 2. Peripheral and central impairments in exercise reserve among HFpEF patients with diabetes or insulin resistance 3. Peripheral and central adaptations to exercise training in patients with HFpEF and diabetes 4. Effects of SGLT-2 inhibitors on exercise capacity in HFpEF |
|
| Geroscientific mechanism | Senescence | 1. Burden of senescence cells in HFpEF 2. Identify adipose, skeletal, and cardiac markers of senescence in patients with HFpEF. 1. Evaluate role of senolytic agent in managing patient with HFpEF. |
| Proteostasis | 1. Role of cardiac and skeletal muscle proteostasis in HFpEF development 3. Role of proteostatic function as a possible molecular marker of peripheral dysfunction in HFpEF and as a potential mechanism underlying EI. |
|
| Inflammation | 1. Inflammatory pathways specifically in HFpEF 2. Delineate ‘omics profiling of human immune cells, particularly of easily accessible circulating immune cells to better understand the role of aging hallmarks on HFpEF 3. Role of anti-inflammatory therapies and therapies targeting immune cells specific for HFpEF in improving EI and other outcomes in patients with HFpEF |
|
| Frailty | 1. Test interventions designed to reduce physical frailty and its adverse consequences in older patients with HFpEF 2. Develop and evaluate strategies to overcome barriers to frailty assessment in clinical care of patients with HFpEF 3. Develop novel screening tools to efficiently detect frailty |
|
| Lifestyle intervention | Calorie restriction, exercise, and multidomain physical function interventions | 1. Examine effects of dietary and exercise on clinical outcomes in patients with HFpEF 2. Evaluate efficacy of multidomain physical function intervention for improving functional status (balance, strength, mobility, and endurance) and clinical outcomes in HFpEF 3. Optimal dietary weight loss strategies in patients with HFpEF, particularly among patients with modestly elevated BMI 4. Optimal exercise training intensity (high intensity interval vs. moderate intensity continuous), modality (resistance vs. aerobic vs. combination) and duration for patients with HFpEF 5. Development of strategies to minimize loss of skeletal muscle during CR in patients with HFpEF 6. Development of strategies for enhancing long-term adherence to dietary and exercise regimens |
Figure 6: Proposed high priority extracardiac areas for future research in HFpEF.
Future research should focus on advancing the understanding of the pathophysiology of EI, development of novel approaches to pathophysiology-guided phenotypic classification of HFpEF, identification of novel targets for preventing and mitigating EI, and testing such interventions in animal and human studies.
A key step to advance understanding of the pathophysiology of EI in HFpEF would be the development of a uniform, comprehensive approach to phenotypic characterization of patients with HFpEF recognizing that the phenotypes may differ across the age distribution. This would entail baseline and follow up assessment of physical function, metabolic function, and cardiopulmonary exercise reserve testing using non-invasive and invasive approaches and uniform collection of biospecimen samples from cardiac, adipose tissue, skeletal muscle, endothelial, and blood cells. These efforts could be organized as inter-related studies, a registry, a precision biobank, or as ancillary studies to a clinical trial. The resultant data could be examined by computational modeling and machine learning approaches to elucidate the biological mechanisms and clinically meaningful phenotypes in older individuals with HFpEF.
Investigations should prioritize the assessment of the role of biological hallmarks of aging in the development of EI in HFpEF with the goal of identifying key geroscience therapeutic targets such as senescent cellular pathways, epigenetic pathways, adipose biology, skeletal muscle biology and mitochondrial dysfunction. Efforts should leverage approaches recently developed for use in contemporaneous NIA-sponsored efforts, including SOMMA, MoTrPac, LIFE, and the Geroscience network.(128–131) Aligning approaches with these efforts would also provide non-HFpEF comparison/control groups. Efforts should also leverage other NIA resources, including the Pepper Center Network, Nathan Shock Center, and relevant ongoing clinical studies.
Once promising targets are identified, proof-of-concept intervention trials can be designed to test whether they are modifiable, and if so, whether this improves EI and other key outcomes, including hallmarks of aging, in older patients with HFpEF. Positive proof-of-concept studies should then be scaled to community-based and larger trials to confirm proof-of-concept and to implement and disseminate the interventions.
Studies are critically needed to address long-term adherence, particularly to behavioral and lifestyle interventions such as exercise, CR, and nutrition. Since these interventions have been the most successful for improving EI in older patients with HFpEF, studies designed to understand their mechanisms of improvement could yield important insights that could be translated to other interventions. Studies should also carefully account for and address multi-morbidity, which is a key feature of HFpEF in older patients and strongly contributes to adverse outcomes and assess presence and impact of depression and cognitive dysfunction. The above research initiatives will require a broad range of approaches and funding mechanisms, including exploratory, pilot, developmental, and program projects, clinical conferences for developing consensus, infrastructure-building grants, and development of registries, precision biobanks, and large multi-center clinical trials.
Conclusions
EI is the primary manifestation of chronic HFpEF, the most common form of HF in the older population, its pathophysiology is not well understood, and there are few proven treatments. Progress can be optimized and accelerated with transdisciplinary teams that include experts in the fields of both aging and CVD, recognize key principles of both domains, and utilize a broad range of approaches.
Supplementary Material
Study Highlights.
In older patients with heart failure with preserved left ventricular ejection fraction (HFpEF), exercise intolerance is common and associated with adverse outcomes.
Several age-related extracardiac mechanisms contribute to the manifestation of exercise intolerance in older patients with HFpEF.
A multidisciplinary approach focused on age-related drivers of exercise intolerance are needed to improve outcomes among older patients with HFpEF
Acknowledgments:
The workshop titled, A Gero-centric Approach to Exercise Intolerance and Heart Failure with Preserved Ejection Fraction (HFpEF) in Older Adults: Elucidating and Targeting Extra-cardiac Contributors, was funded by the National Institute on Aging. Dr. Susan Zieman, Dr. Lyndon Johnson developed, organized, and co-lead this workshop with Dr. Kitzman. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding: Dr. Kitzman is supported in part by NIH grants R01AG18915, R01AG045551, P30AG021332, and U24AG059624, and the Kermit G. Phillips Endowed Chair in Cardiovascular Medicine. Dr. Hummel is supported by NIH grants R01AG062582 and R01HL139813, and VA grant I01CX001636. Dr. Sam is supported by NIH grant R01HL145985. Dr. Kellog Jr is supported by NIH grants P30 AG044271, K01AG059837). Dr. Borlaug is funded by NIH grant RO1 HL128526. Dr. Bertoni is partially supported by NIH grant 1R01HL127028-01. Dr. Forman is funded by NIH grants R01AG060499, R01AG058883, R01AG051376, and P30AG024827. Dr. Molina is supported by NIH grant R21 AG051077. Dr. Pipinos is supported by R01AG062198. Dr. Chirinosis supported by NIH grants R01-HL 121510, R33-HL-146390, R01-AG058969, 1R01-HL104106, P01-HL094307, R03-HL146874, and R56-HL136730. Dr. Justice has received NIH grant support from NIH K01AG059837, and P30AG021332. Dr. Pandey is supported by the Texas Health Resources Clinical Scholarship
Disclosures: Dr. Bertoni has consulted with Premier/Merck on a diabetes quality improvement project. Dr. Simon reports grant support from Aadi and consulting for Acceleron, Actelion, United Therapeutics. Dr. Chirinos has recently consulted for Bayer, Sanifit, Fukuda-Denshi, Bristol-Myers Squibb, JNJ, Edwards Life Sciences, Merck and the Galway-Mayo Institute of Technology. He is named as inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. He has received payments for editorial roles from the American Heart Association and the American College of Cardiology. Dr. Butler has been a Consultant to Abbott, Adrenomed, Arena Pharma, Array, Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Cardior, CVRx, Eli Lilly, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sequana Medical, V-Wave Limited, Vifor Dr. Mentz received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Medtronic, Merck, Novartis, Roche, Sanofi and Vifor. Dr. Pandey has served on the advisory board for Roche Diagnostics. Other authors report no disclosures relevant to this study. Dr. Kitzman reports receiving honoraria outside the present study as a consultant for AbbVie, Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer-Ingelheim, NovoNordisk, Astra Zeneca, and Novartis, and grant funding outside the present study from Novartis, Bayer, NovoNordisk, and Astra Zeneca, and stock ownership in Gilead Sciences.
Abbreviations:
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- EI
Exercise intolerance
- VO2peak
Peak aerobic power
- CVD
Cardiovascular diseases
- PH
Pulmonary hypertension
- BMI
Body mass index
- PAI-1
Plasminogen activator-inhibitor-1
- SCAP
Senescent cell anti-apoptotic pathways
- SASP
Senescence-associated secretory phenotype
- CRP
C-reactive protein
- CR
Caloric restriction
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitzman DW, Gardin JM, Gottdiener JS et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 2001;87:413–9. [DOI] [PubMed] [Google Scholar]
- 2.Upadhya B, Pisani B, Kitzman DW. Evolution of a Geriatric Syndrome: Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc 2017;65:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah SJ, Borlaug BA, Kitzman DW et al. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 2020;141:1001–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayor M, Houstis NE, Namasivayam M et al. Impaired Exercise Tolerance in Heart Failure With Preserved Ejection Fraction: Quantification of Multiorgan System Reserve Capacity. JACC Heart Fail 2020;8:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SJ, Kitzman DW, Borlaug BA et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction. Circulation 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luchi RJ, Snow E, Luchi JM, Nelson CL, Pircher FJ. Left ventricular function in hospitalized geriatric patients. J Am Geriatr Soc 1982;30:700–5. [DOI] [PubMed] [Google Scholar]
- 7.Loffredo FS, Steinhauser ML, Jay SM et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013;153:828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zile MR, Baicu CF, Gaasch WH. Diastolic Heart Failure — Abnormalities in Active Relaxation and Passive Stiffness of the Left Ventricle. New England Journal of Medicine 2004;350:1953–1959. [DOI] [PubMed] [Google Scholar]
- 9.Pandey A, Patel KV, Bahnson JL et al. Association of Intensive Lifestyle Intervention, Fitness, and Body Mass Index With Risk of Heart Failure in Overweight or Obese Adults With Type 2 Diabetes Mellitus: An Analysis From the Look AHEAD Trial. Circulation 2020;141:1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitzman DW, Little WC, Brubaker PH et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144–50. [DOI] [PubMed] [Google Scholar]
- 11.Houstis NE, Eisman AS, Pappagianopoulos PP et al. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzman DW, Brubaker P, Morgan T et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. Jama 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villareal DT, Chode S, Parimi N et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paneni F, Diaz Canestro C, Libby P, Luscher TF, Camici GG. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J Am Coll Cardiol 2017;69:1952–1967. [DOI] [PubMed] [Google Scholar]
- 16.Fleg JL, Morrell CH, Bos AG et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005;112:674–82. [DOI] [PubMed] [Google Scholar]
- 17.Pandey A, Kraus WE, Brubaker PH, Kitzman DW. Healthy Aging and Cardiovascular Function: Invasive Hemodynamics During Rest and Exercise in 104 Healthy Volunteers. JACC Heart Fail 2020;8:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Freire M, Scalzo P, D’Agostino J et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell 2018;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey A, Omar W, Ayers C et al. Sex and Race Differences in Lifetime Risk of Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Circulation 2018;137:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauricio R, Patel KV, Agusala V et al. Sex differences in cardiac function, biomarkers and exercise performance in heart failure with preserved ejection fraction: findings from the RELAX trial. European Journal of Heart Failure 2019;21:1476–1479. [DOI] [PubMed] [Google Scholar]
- 21.Lau ES, Cunningham T, Hardin KM et al. Sex Differences in Cardiometabolic Traits and Determinants of Exercise Capacity in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol 2020;5:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beale AL, Nanayakkara S, Segan L et al. Sex Differences in Heart Failure With Preserved Ejection Fraction Pathophysiology: A Detailed Invasive Hemodynamic and Echocardiographic Analysis. JACC Heart Fail 2019;7:239–249. [DOI] [PubMed] [Google Scholar]
- 23.Oneglia A, Nelson MD, Merz CNB. Sex Differences in Cardiovascular Aging and Heart Failure. Curr Heart Fail Rep 2020;17:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg EO, Thienelt CD, Katz SE et al. Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol 1999;34:264–73. [DOI] [PubMed] [Google Scholar]
- 25.Haykowsky MJ, Nicklas BJ, Brubaker PH et al. Regional Adipose Distribution and its Relationship to Exercise Intolerance in Older Obese Patients Who Have Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2018;6:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorimachi H, Obokata M, Takahashi N et al. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey A, Vaduganathan M, Arora S et al. Temporal Trends in Prevalence and Prognostic Implications of Comorbidities Among Patients With Acute Decompensated Heart Failure: The ARIC Study Community Surveillance. Circulation 2020;142:230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ather S, Chan W, Bozkurt B et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. Journal of the American College of Cardiology 2011;58:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol 2012;60:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitzman DW, Haykowsky MJ, Tomczak CR. Making the Case for Skeletal Muscle Myopathy and Its Contribution to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller S, Winzer EB, Duvinage A et al. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2021;325:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 34.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockwood K, Mitnitski A. Frailty in Relation to the Accumulation of Deficits. The Journals of Gerontology: Series A 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 36.Warraich HJ, Kitzman DW, Whellan DJ et al. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults ≥60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circ Heart Fail 2018;11:e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders NA, Supiano MA, Lewis EF et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 2018;20:1570–1577. [DOI] [PubMed] [Google Scholar]
- 38.Pandey A, Kitzman D, Whellan DJ et al. Frailty Among Older Decompensated Heart Failure Patients: Prevalence, Association With Patient-Centered Outcomes, and Efficient Detection Methods. JACC Heart Fail 2019;7:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitzman DW, Whellan DJ, Duncan P et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey A, Parashar A, Kumbhani D et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeffer MA, Shah AM, Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol 2018;3:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harhay MO, Kizer JR, Criqui MH et al. Adipokines and the Right Ventricle: The MESA-RV Study. PLoS One 2015;10:e0136818-e0136818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging 2013;8:1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs G, Olschewski A, Berghold A, Olschewski H. Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J 2012;39:319–28. [DOI] [PubMed] [Google Scholar]
- 48.Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olson TP, Johnson BD, Borlaug BA. Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart failure 2016;4:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitzman DW, Haykowsky MJ. Vascular Dysfunction in Heart Failure with Preserved Ejection Fraction. J Card Fail 2016;22:12–6. [DOI] [PubMed] [Google Scholar]
- 51.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension 2013;61:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddy YNV, Andersen MJ, Obokata M et al. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hundley WG, Kitzman DW, Morgan TM et al. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 2001;38:796–802. [DOI] [PubMed] [Google Scholar]
- 54.Chirinos JA. Deep Phenotyping of Systemic Arterial Hemodynamics in HFpEF (Part 2): Clinical and Therapeutic Considerations. J Cardiovasc Transl Res 2017;10:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chirinos JA, Segers P, Hughes T, Townsend R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;74:1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamani P, Rawat D, Shiva-Kumar P et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 2015;131:371–80; discussion 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 2012;302:H1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 2011;58:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obokata M, Reddy YNV, Melenovsky V et al. Myocardial Injury and Cardiac Reserve in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmad A, Corban MT, Toya T et al. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2020. [DOI] [PubMed] [Google Scholar]
- 61.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Studenski SA, Peters KW, Alley DE et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reginster JY, Cooper C, Rizzoli R et al. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res 2016;28:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fry CS, Drummond MJ, Glynn EL et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 2011;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borlaug BA, Olson TP, Lam CS et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hearon CM Jr., Sarma S, Dias KA, Hieda M, Levine BD. Impaired oxygen uptake kinetics in heart failure with preserved ejection fraction. Heart 2019. [DOI] [PubMed] [Google Scholar]
- 68.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci 2013;68:968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitzman DW, Nicklas B, Kraus WE et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nilwik R, Snijders T, Leenders M et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 2013;48:492–8. [DOI] [PubMed] [Google Scholar]
- 71.Pandey A, Khera R, Park B et al. Relative Impairments in Hemodynamic Exercise Reserve Parameters in Heart Failure With Preserved Ejection Fraction: A Study-Level Pooled Analysis. JACC Heart Fail 2018;6:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhella PS, Prasad A, Heinicke K et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 2011;13:1296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dhakal BP, Malhotra R, Murphy RM et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molina AJ, Bharadwaj MS, Van HC et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail 2016;4:636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 1967;242:2278–2282. [PubMed] [Google Scholar]
- 76.Goodpaster BH, Sparks LM. Metabolic Flexibility in Health and Disease. Cell Metab 2017;25:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985) 2015;119:739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014;113:1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao VN, Zhao D, Allison MA et al. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC: Heart Failure 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel KV, Bahnson JL, Gaussoin SA et al. Association of Baseline and Longitudinal Changes in Body Composition Measures With Risk of Heart Failure and Myocardial Infarction in Type 2 Diabetes: Findings From the Look AHEAD Trial. Circulation 2020;142:2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caughey MC, Vaduganathan M, Arora S et al. Racial Differences and Temporal Obesity Trends in Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc 2021;69:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kondamudi N, Thangada N, Patel KV et al. Regional adiposity, cardiorespiratory fitness, and left ventricular strain: an analysis from the Dallas Heart Study. J Cardiovasc Magn Reson 2021;23:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schiattarella GG, Altamirano F, Tong D et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz RS, Shuman WP, Bradbury VL et al. Body fat distribution in healthy young and older men. J Gerontol 1990;45:M181–5. [DOI] [PubMed] [Google Scholar]
- 85.Tchkonia T, Morbeck DE, Von Zglinicki T et al. Fat tissue, aging, and cellular senescence. Aging Cell 2010;9:667–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eren M, Boe AE, Klyachko EA, Vaughan DE. Role of plasminogen activator inhibitor-1 in senescence and aging. Semin Thromb Hemost 2014;40:645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vaughan DE, Rai R, Khan SS, Eren M, Ghosh AK. Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler Thromb Vasc Biol 2017;37:1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Boer RA, Nayor M, deFilippi CR et al. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol 2018;3:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 1983;72:1150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hulsmans M, Sager HB, Roh JD et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018;215:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oh A, Okazaki R, Sam F, Valero-Munoz M. Heart Failure With Preserved Ejection Fraction and Adipose Tissue: A Story of Two Tales. Front Cardiovasc Med 2019;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valero-Munoz M, Li S, Wilson RM et al. Heart Failure With Preserved Ejection Fraction Induces Beiging in Adipose Tissue. Circ Heart Fail 2016;9:e002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carbone S, Canada JM, Buckley LF et al. Obesity Contributes to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2016;68:2487–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rayner JJ, Peterzan MA, Watson WD et al. Myocardial Energetics in Obesity. Circulation 2020;141:1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2020;8:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindman BR, Dávila-Román VG, Mann DL et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 2014;64:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115:387–97. [DOI] [PubMed] [Google Scholar]
- 98.Wu YW, Hsu CL, Wang SS et al. Impaired exercise capacity in diabetic patients after coronary bypass surgery: effects of diastolic and endothelial function. Cardiology 2008;110:191–8. [DOI] [PubMed] [Google Scholar]
- 99.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 2007;30:2880–5. [DOI] [PubMed] [Google Scholar]
- 100.Kennedy BK, Berger SL, Brunet A et al. Geroscience: linking aging to chronic disease. Cell 2014;159:709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature 2013;493:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Franceschi C, Garagnani P, Morsiani C et al. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne) 2018;5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harrison DE, Strong R, Sharp ZD et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009;460:392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vilchez D, Simic MS, Dillin A. Proteostasis and aging of stem cells. Trends Cell Biol 2014;24:161–70. [DOI] [PubMed] [Google Scholar]
- 106.Klaips CL, Jayaraj GG, Hartl FU. Pathways of cellular proteostasis in aging and disease. J Cell Biol 2018;217:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miyamoto S. Autophagy and cardiac aging. Cell Death Differ 2019;26:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seiler M, Bowen TS, Rolim N et al. Skeletal Muscle Alterations Are Exacerbated in Heart Failure With Reduced Compared With Preserved Ejection Fraction: Mediated by Circulating Cytokines? Circ Heart Fail 2016;9. [DOI] [PubMed] [Google Scholar]
- 109.Bowen TS, Herz C, Rolim NPL et al. Effects of Endurance Training on Detrimental Structural, Cellular, and Functional Alterations in Skeletal Muscles of Heart Failure With Preserved Ejection Fraction. J Card Fail 2018;24:603–613. [DOI] [PubMed] [Google Scholar]
- 110.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 111.Kalogeropoulos A, Georgiopoulou V, Psaty BM et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010;55:2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Everett BM, Cornel JH, Lainscak M et al. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 113.Van Tassell BW, Trankle CR, Canada JM et al. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2018;11:e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Waddingham MT, Paulus WJ. Microvascular Paradigm in Heart Failure With Preserved Ejection Fraction: A Quest for Proof of Concept. Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]
- 115.Netea MG, Joosten LA, Latz E et al. Trained immunity: A program of innate immune memory in health and disease. Science 2016;352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reynolds LM, Ding J, Taylor JR et al. Transcriptomic profiles of aging in purified human immune cells. BMC Genomics 2015;16:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 2009;25:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mentz R WD MD, Reeves G et al. Rehabilitation Intervention in Older Patients with Acute Heart Failure with Preserved versus Reduced Ejection Fraction JACC: Heart Failure 2021;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Persson CE, Bjorck L, Lagergren J, Lappas G, Giang KW, Rosengren A. Risk of Heart Failure in Obese Patients With and Without Bariatric Surgery in Sweden-A RegistryBased Study. J Card Fail 2017;23:530–537. [DOI] [PubMed] [Google Scholar]
- 120.Fontana L, Partridge L, Longo VD. Extending Healthy Life Span—From Yeast to Humans. Science 2010;328:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Donato AJ, Walker AE, Magerko KA et al. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 2013;12:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 2010;9:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: An update. Ageing Res Rev 2017;39:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heilbronn LK, de Jonge L, Frisard MI et al. Effect of 6-Month Calorie Restriction on Biomarkers of Longevity, Metabolic Adaptation, and Oxidative Stress in Overweight IndividualsA Randomized Controlled Trial. JAMA 2006;295:1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Racette SB, Group TWUSoMC, Weiss EP et al. One Year of Caloric Restriction in Humans: Feasibility and Effects on Body Composition and Abdominal Adipose Tissue. The Journals of Gerontology: Series A 2006;61:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hummel SL, Karmally W, Gilespie BG et al. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization: the GOURMET-HF Pilot Study. Circulation: heart failure 2018;11:e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu Y, Tchkonia T, Pirtskhalava T et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015;14:644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.https://www.caph.pitt.edu/somma/. Date of Access: 6/8/2020.
- 129.https://www.motrpac.org/. Date of Access: 6/8/2020.
- 130.https://www.thelifestudy.org/public/index.cfm. Date of Access: 06/08/2020.
- 131.Pahor M, Guralnik JM, Ambrosius WT et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014;311:2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.