Summary
Background
Over 1 year since the first reported case, the true COVID-19 burden in Ethiopia remains unknown due to insufficient surveillance. We aimed to investigate the seroepidemiology of SARS-CoV-2 among front-line hospital workers and communities in Ethiopia.
Methods
We did a population-based, longitudinal cohort study at two tertiary teaching hospitals involving hospital workers, rural residents, and urban communities in Jimma and Addis Ababa. Hospital workers were recruited at both hospitals, and community participants were recruited by convenience sampling including urban metropolitan settings, urban and semi-urban settings, and rural communities. Participants were eligible if they were aged 18 years or older, had provided written informed consent, and were willing to provide blood samples by venepuncture. Only one participant per household was recruited. Serology was done with Elecsys anti-SARS-CoV-2 anti-nucleocapsid assay in three consecutive rounds, with a mean interval of 6 weeks between tests, to obtain seroprevalence and incidence estimates within the cohorts.
Findings
Between Aug 5, 2020, and April 10, 2021, we did three survey rounds with a total of 1104 hospital workers and 1229 community residents participating. SARS-CoV-2 seroprevalence among hospital workers increased strongly during the study period: in Addis Ababa, it increased from 10·9% (95% credible interval [CrI] 8·3–13·8) in August, 2020, to 53·7% (44·8–62·5) in February, 2021, with an incidence rate of 2223 per 100 000 person-weeks (95% CI 1785–2696); in Jimma Town, it increased from 30·8% (95% CrI 26·9–34·8) in November, 2020, to 56·1% (51·1–61·1) in February, 2021, with an incidence rate of 3810 per 100 000 person-weeks (95% CI 3149–4540). Among urban communities, an almost 40% increase in seroprevalence was observed in early 2021, with incidence rates of 1622 per 100 000 person-weeks (1004–2429) in Jimma Town and 4646 per 100 000 person-weeks (2797–7255) in Addis Ababa. Seroprevalence in rural communities increased from 18·0% (95% CrI 13·5–23·2) in November, 2020, to 31·0% (22·3–40·3) in March, 2021.
Interpretation
SARS-CoV-2 spread in Ethiopia has been highly dynamic among hospital worker and urban communities. We can speculate that the greatest wave of SARS-CoV-2 infections is currently evolving in rural Ethiopia, and thus requires focused attention regarding health-care burden and disease prevention.
Funding
Bavarian State Ministry of Sciences, Research, and the Arts; Germany Ministry of Education and Research; EU Horizon 2020 programme; Deutsche Forschungsgemeinschaft; and Volkswagenstiftung.
Introduction
Despite the initial prediction that the COVID-19 pandemic would hit Africa hard, the feared humanitarian crisis from COVID-19 has so far largely been avoided.1, 2 The total reported numbers from Africa represent only 2·9% of COVID-19 cases and 3·7% of COVID-19 deaths reported globally.3, 4 However, the true number of cases and the impact of COVID-19 remains largely unknown due to insufficient testing and weak surveillance and reporting systems.5, 6, 7
Seroepidemiological evidence from various African countries showed high prevalence of SARS-CoV-2 antibodies among health-care workers: 41·2% in Democratic Republic of the Congo8 and 45·1% in Nigeria9 and 60% among blood donors in South Africa.10 These findings pose a serious question of the true extent to which Africa has been affected by COVID-19, and whether this is largely unknown due to underdiagnosis and under-reporting.
Ethiopia, which reported its first case on March 13, 2020,11 has implemented a targeted testing strategy that focuses on individuals who are symptomatic, contacts of confirmed cases, and high-risk groups.12 This approach neglects most cases with mild or no symptoms.13 With this strategy, fewer than 3% of the population has been tested, and only 273 175 cases have been detected.4, 14 As a result, the true number of SARS-CoV-2 infections in the larger community is completely unknown.
Research in context.
Evidence before this study
The burden of COVID-19 in Africa was not as overwhelming as in other regions of the world during the so-called first wave of the pandemic. However, an apparent second wave characterised by a greater impact on African health systems has been observed since the end of 2020. This observation was supported by a few cross-sectional serological studies indicating high infection rates, mainly among health-care workers. Ethiopia reported its first case in March, 2020, but the true burden of the pandemic remains unknown due to insufficient testing and weak surveillance. We searched PubMed from database inception to May 31, 2021, for peer-reviewed articles using the terms “COVID-19” OR “SARS-CoV-2” AND “Ethiopia”, with no language restrictions. Additionally, we searched bibliographies of identified studies and Google for manuscripts and unpublished reports. We identified three studies, one in preprint version, reporting seroprevalence of SARS-CoV-2 from Ethiopia. All three were cross-sectional studies with sample sizes ranging from 99 to 1856 individuals and focused mainly on the general population of Addis Ababa. Only one study additionally involved rural communities, and none involved health-care workers.
Added value of this study
To our knowledge, we provide the first report of prospective longitudinal SARS-CoV-2 transmission dynamics and incidence rates from an African country, derived from front-line health-care workers at major tertiary referral hospitals, urban residents, and rural communities. The sampling period of this repeated seroprevalence survey fell within the transmission period between the first and second COVID-19 wave in Africa and reveals a strong increase in SARS-CoV-2 transmission within different populations. Our data coincided with national reports of increased burden of critical patient care and PCR test positivity rates. On the basis of our seroprevalence data, we additionally provide a modelling analysis predicting possible SARS-CoV-2 herd immunity first for the wild-type virus, and then assuming introduction of variant strains.
Implications of all the available evidence
This study illustrates current COVID-19 disease dynamics in an African population, indicating a predominance of SARS-CoV-2 transmission in urban settings. It can be speculated that the greatest wave of SARS-CoV-2 infection in rural Ethiopia is currently evolving, and thus requires focused attention regarding health-care burden and outbreak control. Approaching peak herd immunity level, either through natural disease exposure or SARS-CoV-2 vaccination, is widely investigated regarding not only health-care burden, but also emerging viral escape variants. COVID-19 vaccinations are currently scaled out in Africa and will, for most individuals, represent a booster immunisation after previous SARS-CoV-2 exposure. Vaccination strategies should be adapted; for instance, assessing the serostatus before vaccination and providing boosting only with one dose. On the basis of our data on disease dynamics and modelling analysis, we expect valuable follow-up information on pandemic disease control strategies applicable for Africa.
Ethiopia lifted most of its COVID-19-related restrictions on Sept 8, 2020, and the daily testing capacity declined sharply due to insufficient laboratory infrastructure, supplies, and trained workforce.6 The country saw an increase in the number of cases, test positivity rate, severe disease, and COVID-19-related deaths from the second half of 2020 to March, 2021.14 As for most African countries, Ethiopia does not have routine death registration and baseline vital statistics. Therefore, it is extremely difficult to estimate excess deaths due to COVID-19 by use of mortality data. Nevertheless, more deaths compared with similar periods during previous years have been reported from cemeteries in Addis Ababa.15
Serological studies remain the only option to identify the burden of infection in the community and assess outbreak dynamics.16, 17 Therefore, in this study, we aimed to determine the seroprevalence and seroincidence of SARS-CoV-2 across time and model the COVID-19 epidemic among communities and health-care workers in Ethiopia.
Methods
Study design and settings
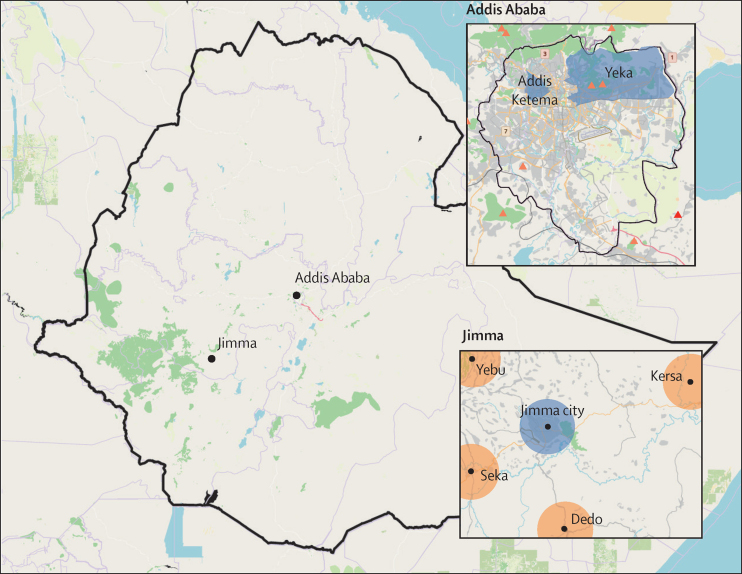
This population-based, longitudinal, exploratory cohort study was done at two tertiary teaching hospitals (Jimma Medical Center [JMC] and St Paul's Hospital), in Jimma Town and surrounding rural communities and Addis Ababa (figure 1).
Figure 1.
Map of Ethiopia showing the study sites
Base map reproduced from OpenStreetMap and OpenStreetMap Foundation, under the Creative Commons Attribution-ShareAlike 4.0 International License. Blue represents urban areas; orange represents rural areas.
JMC is the only tertiary referral centre in southwest Ethiopia, with a catchment population of more than 20 million, 800 inpatient beds, and about 3000 hospital workers. It is located in Jimma Town, the biggest city in southwest Ethiopia, with a population of 300 000.
St Paul's Hospital is one of several public tertiary teaching hospitals in Addis Ababa, with 700 beds and more than 2800 hospital workers. Addis Ababa is the capital and largest city of Ethiopia, with an estimated population of 5 million.
This research was approved by the Institutional Review Boards of Jimma University Institute of Health (RPGD/978/2020), St Paul's Hospital Millennium Medical College (PM23/239), and Ludwig Maximilian University of Munich (21–0293). Additional approvals were obtained from Addis Ababa and Oromia Regional Health Bureaus (BEFO/KBTFU/1-16/488). Written informed consent in local languages was obtained from all participants. For participants who could not read and write, an impartial witness was involved during the consenting process to ensure the provision of all necessary information before obtaining the participant's fingerprint for consent. Preliminary results were communicated to the Ethiopian Public Health Institute, Federal Ministry of Health of Ethiopia, and Ethiopian Medical Association.
Selection of study participants
Front-line hospital workers from outpatient and inpatient units—including clinical staff, medical interns, cleaners, guards, food handlers, and administrative personnel—were recruited at both hospitals. A sample size of 499 hospital workers per hospital was targeted on the basis of an estimated seroprevalence of 50% (95% CI, 5% margin of error) and a design effect of two clusters (JMC and St Paul's Hospital). A non-response rate of 10% for each round (a total of 30% for all three rounds) was assumed.
The recruitment of community participants was guided by convenience sampling and included urban metropolitan settings (Addis Ababa), urban and semi-urban settings (Jimma Town), and rural communities (four rural districts in Jimma Zone; figure 1). In Addis Ababa, we intentionally selected two subcities on the basis of their population density: Addis Ketema (most densely populated) and Yeka (sparsely populated). In Jimma Town, we recruited participants from all areas of the city. Rural residents were recruited from four rural districts located along four main roads connecting to Jimma Town. Households were selected randomly in a way that avoided frequent interaction from the next candidate household to prevent cross-contamination. The sample size calculation was done in July, 2020, when not much baseline data was available. At the time, we planned to include 664 households (332 in Jimma and 332 in Addis Ababa). However, we later became flexible as more data became available. Moreover, as the rate of dropout was more than 30% (our initial expectation), we recruited more participants to compensate for the dropouts. As a result, we included more participants than initially calculated. During data collection, data collectors included the next nearest household if the candidate household was closed or no eligible participant was available in the index household. Only one person from each household was recruited. Inclusion criteria were age 18 years or older, written informed consent, and willingness to provide blood samples by venepuncture.
This study was done between Aug 5, 2020, and April 10, 2021, and data collection was spaced with a minimum of 4 weeks between each round. All participants were enrolled before the introduction of COVID-19 vaccines in Ethiopia.
Data collection and laboratory procedures
We collected demographic data, COVID-19-related symptoms in the preceding 6 months, and prevention practices at the first round. During subsequent rounds, participants were asked about new onset of symptoms and contact with individuals with confirmed or suspected COVID-19. We collected about 3 mL of venous blood for serology at each round using standard serum tubes. Serum specimens were processed daily and stored at –20 °C in aliquots. To ensure best reproducibility and a cost-effective operation, one aliquot was subsequently thawed and serology testing was done in batches. We did measurements with Elecsys anti-SARS-CoV-2 antinucleocapsid assay using the Cobas 6000 module e601 system (Roche Diagnostics, Basel, Switzerland).18 This assay has an in-solution double-antigen sandwich format, with a reported specificity higher than 99·8% and sensitivity of 100%. It received emergency use authorisation from the US Food and Drug Administration in May, 2020.19 Results of the serology test were communicated to all participants during all rounds via text message containing a reminder to practice the recommended COVID-19 prevention methods regardless of the result.
Statistical analysis
Data were double entered into a study-specific database (EpiData Manager, version 4.6.0.0) and linked with serology data from analyser extracts. Data analysis was done in R and Python (details in appendix 3 p 1).
We calculated the seroprevalence of anti-SARS-CoV-2 antibodies as the number of positive cases divided by the total number of individuals tested per round. The incidence rate (IR) was calculated as the number of newly positive cases divided by those still at risk of infection. The IR is presented as rate per 100 000 person-weeks. Only participants with at least two timepoints were included in incidence calculations (appendix 3 pp 1–2). Prevalence and IR are given along with 95% credible interval (CrI). We used the national COVID-19 daily official report of the Federal Ministry of Health of Ethiopia for COVID-19 to compare the seroprevalence changes over time.
SEIR model
We developed compartment models using a SEIR (susceptible, exposed, infectious, and recovered) approach to analyse and predict the dynamics of the pandemic, encoded using the systems biology markup language format and simulated using the software toolbox AMICI. The model parameters were inferred with a Bayesian approach, integrating our seroprevalence data with previous knowledge from the literature on the rates of disease progression. We estimated model parameters using an adaptive Metropolis Hastings algorithm implemented in the Python Parameter Estimation Toolbox. For rounds with long recruitment periods, the seroprevalence datasets were split into an early and late phase. The resulting samples from the posterior distribution were used to derive prediction and prediction CrIs (more detail about modelling is provided in appendix 3 pp 3–5).
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to publish.
Results
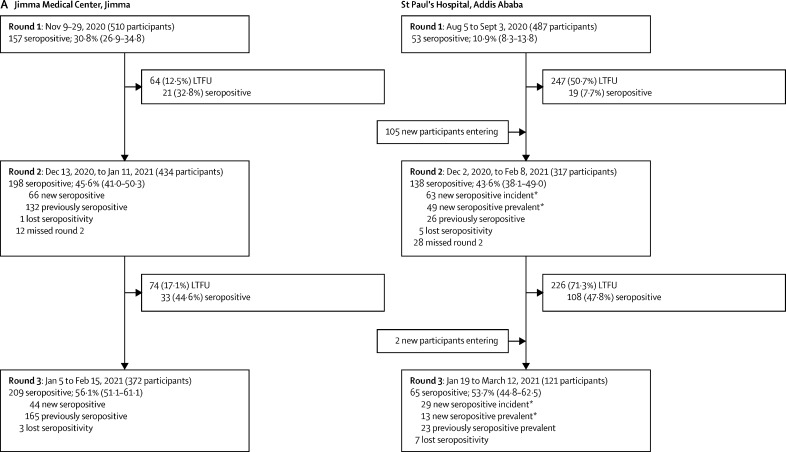
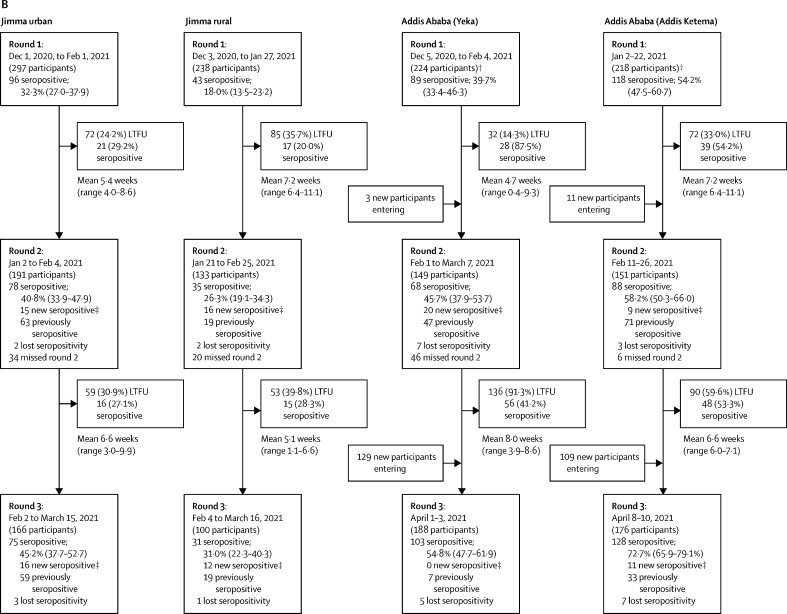
Between Aug 5, 2020, and April 10, 2021, we did three rounds of seroprevalence surveys. 1104 hospital workers and 1229 community residents participated in the study; demographic characteristics are provided in the table. Flow diagrams for recruitment and follow-up of participants are provided in figure 2.
Table.
Demographic characteristics of study participants
|
Hospital workers |
General population |
||||||
|---|---|---|---|---|---|---|---|
| Jimma Medical Center (n=510) | St Paul's Hospital (n=487) | Jimma urban (n=297) | Jimma rural (n=238) | Yeka subcity (n=224) | Addis Ketema subcity (n=218) | ||
| Age | 26 (24–29) | 28 (25–31) | 31 (25–45) | 30 (25–39) | 33 (28–40) | 38 (30–50) | |
| Sex | |||||||
| Men | 239 (46·9%) | 233 (47·8%) | 117 (39·4%) | 158 (66·4%) | 58 (25·9%) | 42 (19·3%) | |
| Women | 271 (53·1%) | 254 (52·2%) | 180 (60·6%) | 80 (33·6%) | 162 (72·3%) | 173 (79·4%) | |
| Missing | 0 | 3 (0·6%) | 0 | 0 | 4 (1·8%) | 3 (1·4%) | |
| Education | |||||||
| No formal education | 0 | 0 | 18 (6·1%) | 49 (20·6%) | 32 (14·3%) | 31 (14·2%) | |
| Primary school | 15 (2·9%) | 44 (9·0%) | 70 (23·6%) | 50 (21·0%) | 66 (29·5%) | 113 (51·8%) | |
| High school | 134 (26·3%) | 93 (19·1%) | 85 (28·6%) | 126 (52·9%) | 58 (25·9%) | 39 (17·9%) | |
| College graduate | 361 (70·8%) | 350 (71·9%) | 124 (41·8%) | 13 (5·5%) | 62 (27·7%) | 26 (11·9%) | |
| Missing | .. | .. | 0 | 0 | 6 (2·7%) | 9 (4·1%) | |
| Profession | |||||||
| Medical doctor | 230 (45·1%) | 199 (40·9%) | NA | NA | NA | NA | |
| Nurse or midwife | 164 (32·2%) | 112 (23·0%) | NA | NA | NA | NA | |
| Other health professionals | 38 (7·5%) | 62 (12·7%) | NA | NA | NA | NA | |
| Non-clinical staff | 78 (15·3%) | 113 (23·2%) | NA | NA | NA | NA | |
| Missing | 0 | 1 (0·2%) | NA | NA | NA | NA | |
| Routine PPE or mask use | |||||||
| PPE at work | 507 (99·4%) | ..* | NA | NA | NA | NA | |
| Mask use in public | 479 (93·9%) | ..* | 244 (82·2%) | 137 (57·6%) | 178 (79·5%) | 183 (83·9%) | |
| Missing | 0 | NA | NA | NA | 5 (2·2%) | 10 (4·6%) | |
Data are median (IQR) or n (%). NA=not applicable. PPE=personal protective equipment.
Such data were not collected at baseline for hospital workers at St Paul's Hospital.
Figure 2.
Study flow and point prevalence for SARS CoV-2 seropositivity in hospital workers recruited in Jimma and Addis Ababa (A) and in participants recruited from the general population in urban and rural Jimma and Addis Ababa (B)
Data are n, n (%), seroprevalence (% and 95% credible interval), or mean (range). LTFU=lost to follow-up. *New seropositive incident refers to seropositive cases with previous negative serology result during round 1; new seropositive prevalent refers to seropositive cases that entered the study without a preceding diagnosis. † Additional 13 participants from Addis Ababa, nine of whom participated in two rounds and four of whom only participated in one round, were included but did not have data available for subcity. ‡New seropositive refers only to participants who were negative in one or more previous rounds, but became seropositive in the subsequent round, excluding new participants entering; therefore, the sum of new and previously seropositive participants does not always equal the total number of seropositive participants in that round.
At St Paul's Hospital, serosurvey rounds were done in August and September, 2020; December, 2020, and January, 2021 (mean interval 17·7 weeks [SD 2·1] from round 1); and February and March, 2021 (mean interval 9·3 weeks [SD 1·9] from round 2). There was a high proportion of dropouts, potentially due to long survey intervals; only 51 (10·5%) of 487 individuals included in the first round completed all three rounds. Nevertheless, we did not observe significant differences in the proportions of dropouts regarding seropositivity across all cohorts and survey rounds, indicating that dropouts did not result in a sampling bias (more detail on missing data is provided in appendix 3, pp 5–7). At JMC, survey rounds were done in November, 2020; December, 2020, and January, 2021 (mean interval 5·2 weeks [SD 0·8] from round 1); and January and February, 2021 (mean interval 6·3 weeks [SD 0·9] from round 2). Dropout rates were lower than that in St Paul's Hospital—360 (70·6%) of 510 participants completed all three rounds.
Recruitment for the general population started with 297 participants from urban communities and 238 from rural communities in Jimma. The survey rounds were done in December, 2020; January and February, 2021 (mean interval 5·4 weeks [SD 0·6] in urban communities and 7·2 weeks [SD 0·9] in rural communities); and February and March, 2021 (mean interval 6·6 weeks [SD 0·9] in urban communities and 5·1 weeks [SD 1·0] in rural communities). General population survey rounds in Addis Ababa were done in December, 2020, and January, 2021; February, 2021 (mean interval 4·7 weeks [SD 1·9] in Addis Ketema and 5·1 weeks [SD 0·7] in Yeka); and April, 2021 (mean interval 8·0 weeks [SD 1·8] in Addis Ketema and 6·6 weeks [SD 0·5] in Yeka). At baseline, 224 participants from Yeka and 218 from Addis Ketema were included; however, new participants were added at later survey rounds (figure 2B).
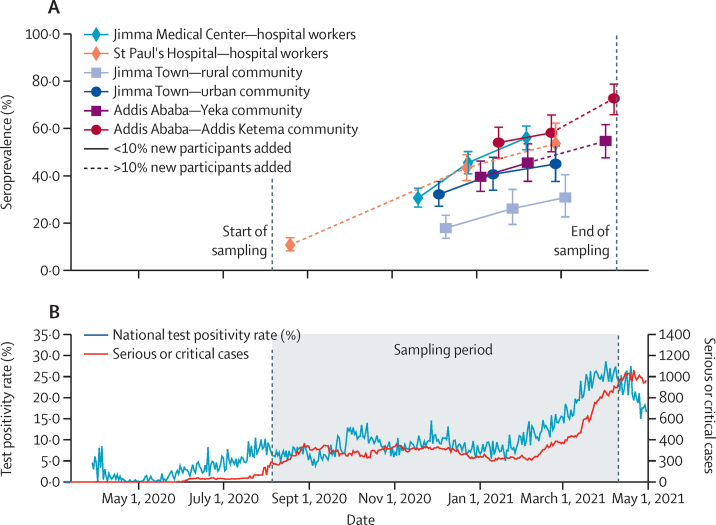
The evolution of seroprevalence over time in different cohorts is depicted in figure 2 and figure 3A, and the corresponding incidence data are reported in appendix 3 (p 1). Differences in seroprevalence for each survey period and for seroincidence are summarised in appendix 3 (p 2). In August, 2020, SARS-CoV-2 seroprevalence among hospital workers at St Paul's Hospital was 10·9% (95% CrI 8·3–13·8), increasing to 53·7% (44·8–62·5) by February, 2021 (figure 2A). The IR over this period was 2223 per 100 000 person-weeks (95% CI 1785–2696). At JMC, the seroprevalence increased from 30·8% (95% CrI 26·9–34·8) in November, 2020, to 56·1% (51·1–61·1) in February, 2021, with an IR of 3810 per 100 000 person-weeks (95% CI 3149–4540). The seroincidence in hospital workers from Addis Ababa was significantly lower than that in Jimma (risk ratio 0·6, 95% CrI 0·4–0·7).
Figure 3.
Seroprevalence over time for all six cohorts investigated in the study (A), and PCR test positivity rates and number of admissions to intensive care units due to COVID-19 in Ethiopia (B)
In the most populous area of the general population surveyed, Addis Ketema, an initial seroprevalence of 54·2% (47·5–60·7) in January, 2021, increased to 72·7% (65·9–79·1) in April, 2021 (figure 2B); in Yeka subcity, we observed an increase from 39·7% (33·4–46·3) to 54·8% (47·7–61·9) during the same timepoints. The seroprevalence in Addis Ketema was not only significantly higher than in Yeka during all rounds, but also higher than that of hospital workers at St Paul's Hospital, for example, during the December, 2020, to January, 2021 survey (odds ratio [OR] 1·5, 95% CI 1·1–2·1; appendix 3 p 2). The combined IR from both subcities was 4535 (95% CI 3372–5906) per 100 000 person-weeks, and the overall incidence was significantly higher compared with that of hospital workers at St Paul's Hospital (OR 2·0, 1·4–2·8). In Jimma Town, a seroprevalence of 32·3% (95% CrI 27·0–37·9) in December, 2020, increased to 45·2% (37·7–52·7) in February, 2021. The seroprevalence in rural communities was 18·0% (13·5–23·2) from November to December, 2020, and 31·0% (22·3–40·3) by March, 2021, which was significantly lower than in the city for the first two rounds and lower than that among hospital workers during all rounds. IRs were similar between urban and rural populations in Jimma, with a combined IR of 1720 (95% CI 1258–2258) per 100 000 person-weeks, and the overall Jimma community incidence was lower than that at JMC (OR 0·4, 95% CI 0·3–0·6). The seroincidence in communities from Addis Ababa was significantly higher than that in Jimma (2·6, 1·6–3·8; appendix 3 p 2).
When comparing the differences between rounds, we observed significant differences overall between round 2 and round 3 (OR 1·92, 95% CI 1·21–3·05). Differences between round 1 and round 2 were not significant except in St Paul's Hospital, where round 1 was done much earlier than in all other cohorts, and thus the finding is likely to result from a study design effect (appendix 3 p 2).
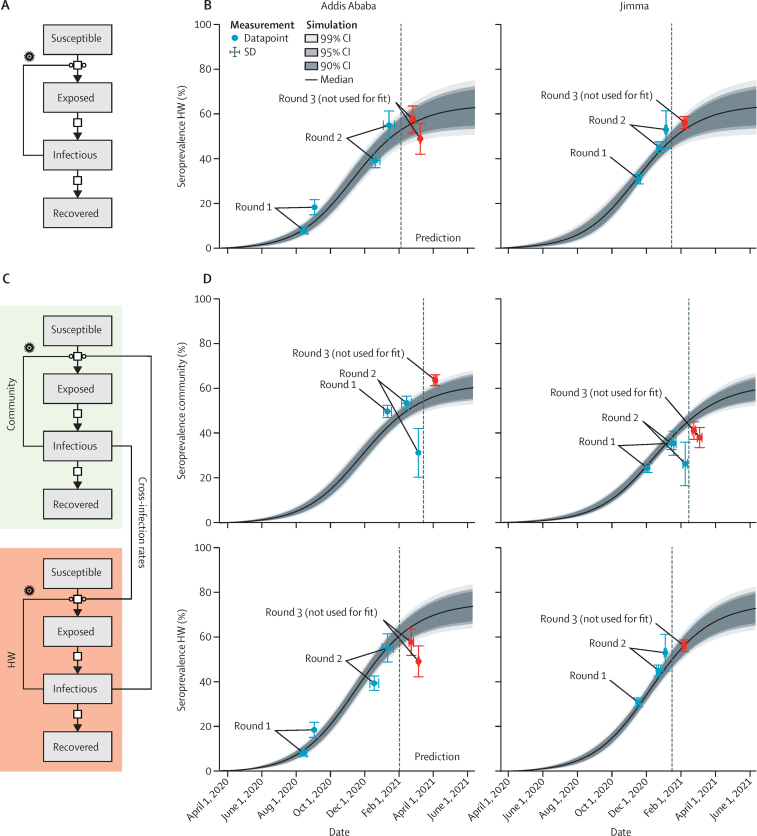
On the basis of the results for the first two rounds, we constructed an SEIR model for the progression of the SARS-CoV-2 epidemic in Ethiopia (figure 4A). We started estimating the model parameters with the data for the hospital workers because it provided better coverage for the early dynamics and more well determined parameter estimates. The resulting model for JMC and St Paul's Hospital provided a good description of the available data for round 1 and round 2 (figure 4B) and reliable parameter estimates (appendix 3 p 3). Particularly, we obtained a median exposure rate of 0·08 per day (IQR 0·06–0·13), a median incubation period of 5·6 days (2·2–13·6) and a median recovery time of 19·3 days (11·4–28·9).
Figure 4.
SEIR model of SARS-CoV-2 epidemic in Ethiopia
(A) Compartments of the SEIR models and possible transition. (B) Model simulation of SEIR model for HW in Jimma Medical Center and St Paul's Hospital; data from round 1 and 2 were used for model training; later points, including round 3, were predictions. (C) Compartments of the extended SEIR models and possible transition; data from round 1 and 2 were used for model training; later points, including round 3, were predictions. (D) Model simulation of extended SEIR model for HW in Jimma Medical Center and St Paul's Hospital and community members in Jimma (combined) and Addis Ababa (combined); data from round 1 and 2 were used for model training; later points, including round 3, were predictions. HW=hospital workers. SEIR=susceptible, exposed, infectious, and recovered.
The model showed a seroprevalence approaching a predicted saturation level of 50–70%. These predictions based on the first two rounds agreed with the findings in round 3, which was found to be a seroprevalence of 53·7% for hospital workers at St Paul's Hospital from mid-January to mid-March, 2021, and 56·1% at JMC from January to February, 2021.
In addition to the standard SEIR model, we constructed a combined model using data from hospital workers and the community (figure 4C). This model simultaneously described both groups and allowed for cross-infections. Infection of hospital workers by community members is considered more likely due to contact patterns. The extended model based on the first two rounds provided a good description of the joint datasets and also predicted saturation (figure 4D, appendix 3 p 3). As expected, the seroprevalences for the hospital workers were predicted to be higher than those in the community. Again, the model predictions of the extended model were supported by the observations in round 3. Moreover, we constructed a model considering the possible entry of a SARS-CoV-2 variant, which will be further debated in the Discussion section.
Because of the highly dynamic nature of the seroepidemiological change observed in this study, we sought to compare it with corresponding clinical effects of COVID-19 on the health-care system in Ethiopia. A strong increase in the test positivity rate for SARS-CoV-2 RT-PCR since February, 2021—reaching a high of 28·6% on April 1, 2021—was reported by the Ministry of Health. Similarly, numbers of admissions to intensive care units (ICUs) across Ethiopian hospitals passed 500 for the first time in March, 2021, and reached a peak of 1059 on April 21, 2021 (figure 3B). Clinical data for signs and symptoms of COVID-19 was collected from 1909 participants; however, only 721 (37·8%) of these participants reported having had COVID-19-related symptoms— 371 (45·8%) of 810 seropositive cases and 350 (31·8%) of 1099 seronegative individuals (p<0·0001) and none were admitted to hospital due to COVID-19.
Discussion
Here, we provide the first data from a seroepidemiological investigation for SARS-CoV-2 infection in a population-based, longitudinal, exploratory cohort study from Ethiopia. This study revealed a striking increase in seroprevalence of SARS-CoV-2 among front-line hospital workers and communities in Ethiopia over the last months of 2020 and the first quarter of 2021. A SEIR model predicted a seroprevalence approaching saturation for hospital workers and urban communities. Although no COVID-19-related severe disease (as defined by hospitalisation) was reported among our cohorts, the substantial change in seroepidemiology in our study aligns with increased caseloads and ICU admissions in Ethiopia during the same period (figure 3).
After detection of the first few cases, Ethiopia declared a state of emergency on April 8, 2020, to contain the COVID-19 outbreak and mitigate its impact.20 Various restrictions and prohibitions were imposed for 5 months to reinforce this, and the spread of infection appeared to be halted during that period. Two serosurveys done in April and May, 2020, among communities and outpatients in Addis Ababa reported seroprevalence of 8%21 and 3%.22 A seroprevalence survey done between July and September, 2020, among the general population indicated a seroprevalence lower than 1% in both Jimma Town and rural areas and 2–5% in Addis Ababa.23
Our first serosurvey, done from August to September, 2020, among hospital workers in Addis Ababa showed a seroprevalence of 10·9%, indicating a slow but steady spread of SARS-CoV-2 in Ethiopia even when restrictions were in place.
Ethiopia lifted the state of emergency and relaxed most restrictions on Sept 8, 2020.24 We subsequently observed a strong increase in seroprevalence among hospital workers to 53·7% in Addis Ababa and 56·1% in Jimma Town by March, 2021. Likewise, our community seroepidemiological data from two subcities in Addis Ababa indicated an increment of combined seroprevalence to 63·7% by April, 2021, and to 45·2% by March, 2021, in Jimma Town's urban community. Notably, a lower seroprevalence was observed among rural residents during all three rounds compared with that among urban communities. Seroprevalence among hospital workers and the surrounding urban communities were similar, except for the densely populated Addis Ketema, where seroprevalence was significantly higher than that for hospital workers.
It can be speculated that the Ethiopian Government's disease control restrictions during the first few months helped in slowing down the spread of the disease. It is widely believed that the COVID-19 burden was not as heavy in African countries as in other world regions because of a younger population being less susceptible to severe disease.25 However, the sheer increase of SARS-CoV-2 infections as observed in the second wave of the African COVID-19 pandemic probably inflicts greater health-care challenges.26 In this respect, our data are supported by the notification data obtained from the Ethiopian Government, showing great increases in SARS-CoV-2 RT-PCR test positivity rates and unprecedented numbers of ICU admissions (figure 3).
Despite a strong increment in seroprevalence, most individuals in our cohorts did not report COVID-19-related symptoms or hospital admissions. Therefore, silent transmission of SARS-CoV-2 infections in Ethiopia might be assumed for most of the population, considering also the younger age demographics compared with other world regions. However, the observed high seroincidence with no serious clinical impact can be a blessing in disguise because individuals who are asymptomatic but possibly infectious probably continue with their working, private, and social interactions, thus creating a risk for people with predisposing risk factors for COVID-19. Conversely, the observed high transmission of SARS-CoV-2 in the community with a low number of deaths, critical cases, and hospital admissions could lead to achieving herd immunity, given that the probability of repeated infection is low.27, 28
In this study, we were unable to determine the expected herd immunity threshold for COVID-19 in Ethiopia due to no data on the basic reproductive ratio. Instead, we did SEIR modelling to show the epidemic trajectories and indicate possible saturation points in time. The model assumed constant parameters because the intervention measures in Ethiopia were limited. Possible changes in behaviour of hospital workers and community members over the course of the pandemic and the seasonality component were not considered because they would have required additional data. However, despite these limitations, our model, based on the first two survey rounds, predicted disease saturation and assumed a related herd immunity by April, 2021. This finding was supported by the third-round serosurvey and will be further followed up in 2021 and 2022. However, the assumed herd immunity in Manaus, Brazil (suggested in September, 2020) did not prevent high COVID-19 disease burden during a subsequent wave from December, 2020, to January, 2021, possibly due to immune escape of the newly emerging gamma (P1) strain.29
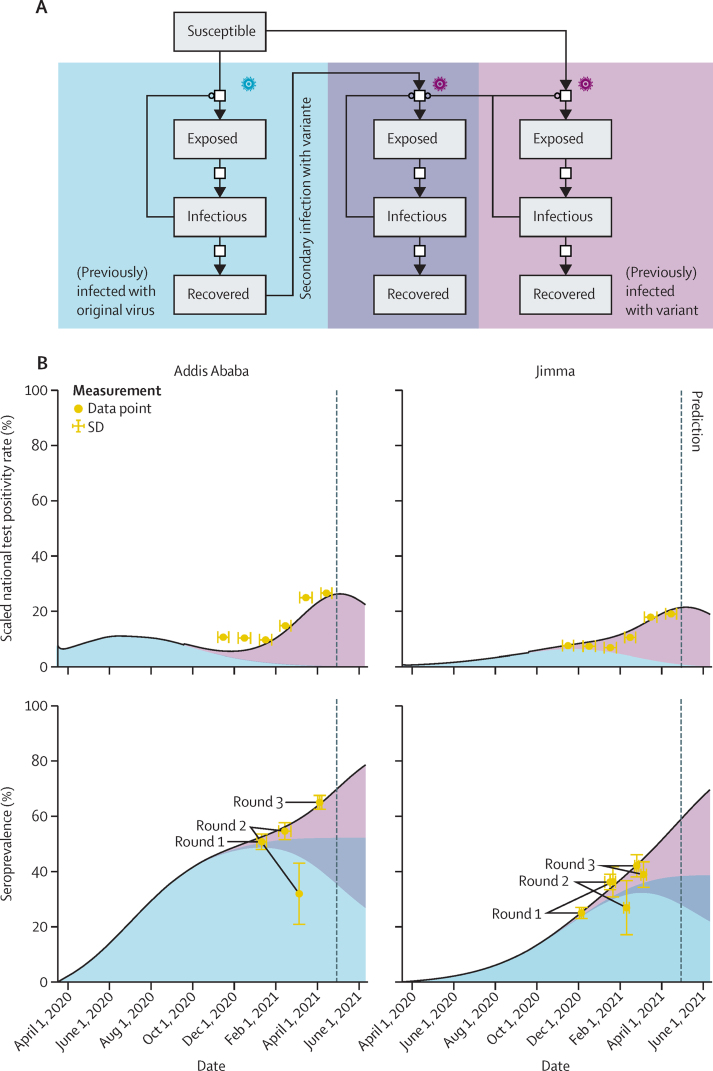
Although our SEIR model was able to describe and even predict seroprevalence observations, it did not explain the recent surge in the positivity rate of PCR tests. Because the test strategy did not change, we speculate that this fraction should, to some degree, reflect the current number of infectious individuals. We also considered the possible entry of a SARS-CoV-2 variant capable of re-infecting individuals who had recovered from COVID-19, and we developed a compartment model describing this scenario (figure 5, appendix 3, p 4). The model provided a good description of the seroprevalence data from rounds 1 to 3 for community members and hospital workers, as well as the test rates. By contrast with the basic SEIR model, it predicted a substantially higher number of infectious individuals over the months after round 3, as well as a final seroprevalence in the range of 80–90%. This prediction suggests that herd immunity is not easily reached if re-infections with possible secondary transmissions occur.
Figure 5.
SEIR model of SARS CoV-2 epidemic in Ethiopia integrating the potential effect of exposure to a SARS-CoV-2 variant with immune escape potential
(A) Topology of compartment model that allows for the infection with the variant of individuals who were exposed to the original virus. (B) Scaled test positivity rate (mapped from the complete country to the individual cities) and seroprevalence. The contribution of different variants is indicated, as well as the proportion of individuals exposed to both. SEIR=susceptible, exposed, infectious, and recovered.
Although infection-blocking immunity might wane rapidly or be challenged by immune escape variants, disease-reducing cross-immunity should be long lived, according to 2021 models.30 This effect would be even stronger when providing booster vaccinations to individuals previously exposed to SARS-CoV-2. Therefore, for individuals who have recovered from COVID-19, one booster vaccination dose might be sufficient to provide longer protection. Depending on availability of test systems or shortage of vaccines in some parts of the world, it might be cost-effective and reasonable to test the population serologically before administering vaccines.
Our study was based on hospital workers at major tertiary hospitals and on residents in typical metropolitan, semi-urban, and rural settings in Ethiopia. Because of the nature of the design, our study had significant dropout during round 3 among community participants. We recruited additional participants with similar characteristics to replace those who dropped out so that prevalence could be compared with the first two rounds. Furthermore, findings might not be generalisable to primary-level health facilities, which are the most common points of interaction, and to rural communities representing the majority of the Ethiopian population. Interpretation of our serosurvey data would have been more informative in the context of circulating viral variant characteristics. However, this information is not yet available due to the absence of tests for new variants in Ethiopia.
In conclusion, this study has shown that SARS-CoV-2 infection among hospital workers at tertiary hospitals and community residents in Ethiopia has been widespread and highly dynamic. Our SEIR model, fitted on the basis of the current trend of seroincidence and poor adherence to mitigation strategies, has shown that front-line hospital workers at tertiary hospitals were approaching a threshold for herd immunity, even before the start of the vaccination initiative. However, this pattern of disease spread poses a substantial risk to the community because silent spread among a mostly young population might ultimately put infectious pressure on highly vulnerable groups of society, leading to increased ICU admissions and deaths in the following few months. Hence, mitigation measures should target safeguarding the most vulnerable, including older people and those with underlying medical conditions.
Data sharing
All data used are publicly available, and sources are cited throughout. The data can be accessed on https://zenodo.org/record/4885064#.YU2tGbdCSyU.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We are grateful for research funding provided by the Bavarian State Ministry of Sciences, Research and the Arts (Bayerisches Staatsministerium, F.4-V0122.4/3/20); the Germany Ministry of Education and Research (MoKoCo19; 01KI20271); the EU Horizon 2020 programme (ORCHESTRA; 101016167); Deutsche Forschungsgemeinschaft (SEPAN; HA 7376/3-1); and Volkswagenstiftung (E2; 99 450). We thank participants, study teams, Jimma Medical Center, Oromia Regional Health Bureau, St Paul's Hospital, and Addis Ababa Health Bureau for the support provided during data collection. We would also like to thank Michel Pletschette from Ludwig Maximilian University for his support in the literature review and revision.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
EKG, SA, AK, AW, and MH conceived of and designed the study. EKG, SA, EG, AGi, BT, GBH, WTS, RA, AGe, and MB participated in data collection. KE, AB, and SM summarised, cleaned, and analysed the data. SM, LC, and JH did the modelling and parameter estimation. AGi, GBH, and BT did laboratory studies. EKG, SA, AK, AW, MH, and JH interpreted the results. EKG, SA, and AK drafted the Article. All authors contributed to the writing of the final version of the manuscript. EKG, SA, KE, AB, and AK have accessed and verified the data; all authors accepted responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Massinga Loembé M, Tshangela A, Salyer SJ, Varma JK, Ouma AEO, Nkengasong JN. COVID-19 in Africa: the spread and response. Nat Med. 2020;26:999–1003. doi: 10.1038/s41591-020-0961-x. [DOI] [PubMed] [Google Scholar]
- 2.Gudina EK, Gobena D, Debela T. COVID-19 in Oromia Region of Ethiopia: a review of the first 6 months’ surveillance data. BMJ Open. 2021;11:e046764. doi: 10.1136/bmjopen-2020-046764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.African Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) https://africacdc.org/covid-19/
- 4.Johns Hopkins University COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html
- 5.Gudina EK, Tesfaye M, Siraj D, Haileamilak A, Yilma D. COVID-19 in Ethiopia in the first 180 days: lessons learned and the way forward. Ethiop J Health Dev. 2020;34:6. [Google Scholar]
- 6.Mulu A, Bekele A, Abdissa A. The challenges of COVID-19 testing in Africa: the Ethiopian experience. Pan Afr Med J. 2021;38:6. doi: 10.11604/pamj.2021.38.6.26902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo S, Scuccato R, Fadda A, Cumbi AJ. COVID-19 in Africa: the little we know and the lot we ignore. Epidemiol Prev. 2020;44(suppl 2):408–422. doi: 10.19191/EP20.5-6.S2.146. [DOI] [PubMed] [Google Scholar]
- 8.Mukwege D, Byabene AK, Akonkwa EM. High SARS-CoV-2 seroprevalence in healthcare workers in Bukavu, eastern Democratic Republic of Congo. Am J Trop Med Hyg. 2021;104:1526–1530. doi: 10.4269/ajtmh.20-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olayanju O, Bamidele O, Edem F. SARS-CoV-2 seropositivity in asymptomatic frontline health workers in Ibadan, Nigeria. Am J Trop Med Hyg. 2021;104:91–94. doi: 10.4269/ajtmh.20-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sykes W, Mhlanga L, Swanevelder R. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021. Res Sq. 2021 doi: 10.21203/rs.3.rs-233375/v1. published online Feb 12. (preprint). [DOI] [Google Scholar]
- 11.WHO First case of COVID-19 confirmed in Ethiopia. 2020. https://www.afro.who.int/news/first-case-covid-19-confirmed-ethiopia
- 12.Ethiopian Public Health Institute . Interim national strategy and guidance for the laboratory diagnosis of COVID-19 in Ethiopia. Ethiopian Public Health Institute; Addis Ababa: 2020. [Google Scholar]
- 13.Alene M, Yismaw L, Assemie MA. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: a systematic review and meta-analysis. PLoS One. 2021;16:e0249090. doi: 10.1371/journal.pone.0249090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Public Health Emergency Operation Center of Ethiopia COVID-19 pandemic preparedness and response in Ethiopia. Ethiopian Public Health Institute. https://ephi.gov.et/download/pheoc/
- 15.Endris BS, Saje SM, Metaferia ZT. Excess mortality in the face of COVID-19: evidence from Addis Ababa Mortality Surveillance Program. SSRN. 2021 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3787447 published online Feb 17. (preprint). [Google Scholar]
- 16.Peeling RW, Wedderburn CJ, Garcia PJ. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20:e245–e249. doi: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter AK, Hegde ST. The important role of serology for COVID-19 control. Lancet Infect Dis. 2020;20:758–759. doi: 10.1016/S1473-3099(20)30322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche Diagnostics Elecsys® Anti-SARS-CoV-2: immunoassay for the qualitative detection of antibodies (incl IgG) against SARS-CoV-2. 2020. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html
- 19.Roche Diagnostics Roche's COVID-19 antibody test receives FDA Emergency Use Authorization and is available in markets accepting the CE mark. 2020. https://www.roche.com/media/releases/med-cor-2020-05-03.htm
- 20.Council of Minister of Ethiopia State of Emergency Proclamation No. 3/2020: Implementation Regulation No. 466/2020. A regulation issued to implement the state of emergency proclamation enacted to counter and control the spread of COVID-19 and mitigate its impacts. 2020. https://www.moh.gov.et/ejcc/sites/default/files/2020-04/negarit.pdf
- 21.Alemu BN, Addissie A, Mamo G. Sero-prevalence of anti-SARS-CoV-2 antibodies in Addis Ababa, Ethiopia. bioRxiv. 2020 doi: 10.1101/2020.10.13.337287. published online Oct 13. (preprint). [DOI] [Google Scholar]
- 22.Kempen JH, Abashawl A, Suga HK. SARS-CoV-2 serosurvey in Addis Ababa, Ethiopia. Am J Trop Med Hyg. 2020;103:2022–2023. doi: 10.4269/ajtmh.20-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdella S, Riou S, Tessema M. Prevalence of SARS-CoV-2 in urban and rural Ethiopia: randomized household serosurveys reveal level of spread during the first wave of the pandemic. EClinicalMedicine. 2021;35:100880. doi: 10.1016/j.eclinm.2021.100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ethiopian Public Health Institute A directive issued for the prevention and control of the COVID-19 pandemic No. 30/2020. 2020. https://www.ephi.gov.et/images/Registerd-COVID-19-Directive-2013_Final_051020.pdf
- 25.Diop BZ, Ngom M, Pougué Biyong C, Pougué Biyong JN. The relatively young and rural population may limit the spread and severity of COVID-19 in Africa: a modelling study. BMJ Glob Health. 2020;5:e002699. doi: 10.1136/bmjgh-2020-002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salyer SJ, Maeda J, Sembuche S. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. Lancet. 2021;397:1265–1275. doi: 10.1016/S0140-6736(21)00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumley SF, O’Donnell D, Stoesser NE. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabino EC, Buss LF, Carvalho MPS. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371:741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used are publicly available, and sources are cited throughout. The data can be accessed on https://zenodo.org/record/4885064#.YU2tGbdCSyU.