Abstract
Background
SARS-CoV-2 has been responsible for considerable mortality worldwide, owing in particular to pulmonary failures such as ARDS, but also to other visceral failures and secondary infections. Recent progress in the characterization of the immunological mechanisms that result in severe organ injury led to the emergence of two successive hypotheses simultaneously tested here: hyperinflammation with cytokine storm syndrome or dysregulation of protective immunity resulting in immunosuppression and unrestrained viral dissemination.
Methods
In a prospective observational monocentric study of 134 patients, we analysed a panel of plasma inflammatory and anti-inflammatory cytokines and measured monocyte dysregulation via their membrane expression of HLA-DR. We first compared the results of patients with moderate forms hospitalized in an infectious disease unit with those of patients with severe forms hospitalized in an intensive care unit. In the latter group of patients, we then analysed the differences between the surviving and non-surviving groups and between the groups with or without secondary infections.
Findings
Higher blood IL-6 levels, lower quantitative expression of HLA-DR on blood monocytes and higher IL-6/mHLA-DR ratios were statistically associated with the risk of severe forms of the disease and among the latter with death and the early onset of secondary infections.
Interpretation
The unique immunological profile in patients with severe COVID-19 corresponds to a moderate cytokine inflammation associated with severe monocyte dysregulation. Individuals with major CSS were rare in our cohort of hospitalized patients, especially since the use of corticosteroids, but formed a very severe subgroup of the disease.
Funding
None.
Keywords: SARS-CoV-2, Intensive care unit, Inflammation, Monocyte dysregulation, Immunosuppression, HLA-DR, Secondary infection
Research in Context.
Evidence before this study
SARS-CoV-2 infections are associated with significant mortality, mainly owing to lung damage such as ARDS, but also to damage to other organs or to secondary infections. Two opposing pathophysiological hypotheses have been successively put forward to explain these severe forms. In one, COVID-19 is considered to be a hyperinflammatory disease with cytokine release syndromes causing deleterious infiltration of immune cells into the lung. In the other, it is rather a disease with a complex deregulation of the immune system leading to uncontrolled viral spread and thus ARDS. Few studies have described the immunological characteristics of patients on both sides of the immune response simultaneously and did not provide a detailed description of the patient profile based on the severity of the disease or on the mortality induced. Furthermore, they did not at all describe whether these markers were associated or not with the occurrence of secondary infections. All these elements would however be necessary to guide therapeutic choices in a personalized medicine approach that would adapt treatment to the inflammatory or immunocompromised profile of the patients.
Added value of this study
Our data provide new evidence that patients with severe forms of COVID-19 most often exhibit moderate cytokine inflammation concurrently with severe monocytic dysregulation. We show that a quantitative decrease in mHLA-DR membrane expression and an increase in the IL-6/mHLA-DR ratio were associated with severe forms of the disease, the degree of pulmonary involvement, and the risk of death and onset of early infections. In addition, we show that patients with a major cytokine storm were rare in our cohort of hospitalized patients, especially since the systematic use of corticosteroids, but corresponded to a subgroup of patients with a very poor prognosis.
Implications of all the available evidence
The mHLA-DR expression and the IL-6/mHLA-DR ratio could be used in a routine care approach to identify patients at risk for severe forms, death and early secondary infections. This could make it possible to better adapt the therapies implemented in a personalized medicine approach, using treatments that target cytokine inflammation or, depending on the patient's profile, immunostimulant treatments.
Alt-text: Unlabelled box
1. Introduction
Since the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan in 2019, the disease has spread very rapidly to become a global pandemic currently responsible for more than 4.5 million deaths. About 15% of coronavirus disease 19 (COVID-19) patients over 60 years of age require hospitalization and 5% require intensive care unit (ICU) admission [1,2]. ICU mortality rate is high, ranging from 16 to 57%, and is mainly related to pulmonary complications of the acute respiratory distress syndrome (ARDS) [3], [4], [5], [6], [7]. Multiple organ failure (in 12 to 29% of cases) and secondary infections (in 10 to 57% of cases) can also frequently occur [3,8,9].
Understanding the pathophysiology of severe COVID-19 forms, especially of respiratory failure, is henceforth critical for determining the best management and treatment strategies. Recent progress in the characterization of the immunological mechanisms that result in organ injury, with particular regard to the determinants of severity, has shown the role of hyperinflammation, in particular elevated levels of proinflammatory cytokines or chemokines associated with anomalies of adaptive and innate immunity through disruption of the lymphoid and myeloid lineages [10], [11], [12].
The first related studies posed the hypothesis of the cytokine storm syndrome (CSS), suggesting that the increase in pro-inflammatory cytokines (IL-2, IL-6, IL-7, IP-10, TNFα…), which is marked in ICU patients and associated with impaired interferon type 1 response, induced the infiltration of inflammatory cells in the lung and thus injury to the organ [3,[12], [13], [14]]. However, this hypothesis has recently been called into question, in particular by comparing the level of increase in cytokines with that observed in other pathological situations that induce CSS, such as sepsis and CAR-T cell infusion, and those that do not, such as influenza [15], [16], [17].
A new explanation is gradually emerging that places emphasis on more complex cellular immune dysregulation. Severe forms of COVID-19 are associated with lymphopenia, including decreased numbers of circulating T, B, and NK cells, exhibit activation of CD4 T cells and decrease regulatory T cells associated with a skewing of CD8+ T cells towards a terminally differentiated/senescent phenotype [18], [19], [20], [21]. Deep analysis also revealed drastic changes within the myeloid cell compartments during severe COVID-19. With regard to neutrophils, multiple abnormalities have been described such as increased neutrophil/lymphocyte ratio, evidence of emergency myelopoiesis with release of immature neutrophils, transcriptional programs typical of dysfunction and immunosuppression, and lung recruitment with NETosis [22], [23], [24], [25]. Abnormalities of monocytes have been recently characterized, where expansion and early activation of classical monocytes (CD14+CD11c++HLA-DR++) in the blood are characteristics of mild COVID-19 which decrease during the natural course of the disease. In contrast, in severe forms the non-classical monocyte subpopulation (CD14loCD16++) appears depleted, probably due to its lung recruitment, and the intermediate (CD14+CD16+) and classical subsets (CD14++CD16−) become dysfunctional with the loss of HLA-DR membrane expression [11,[18], [19], [20], [21]].
Here we hypothesize that the two phenomena described above, hyperinflammation or dysregulation of protective immunity, probably took place at the same time in the severe forms and were negatively complementary rather than antagonistic. We therefore looked for simple biological tools that are routinely available in our university hospital centre to simultaneously test hyperinflammation, anti-inflammatory cytokines and monocyte dysregulation by a panel of plasma cytokines and by measuring the membrane expression of human leukocyte antigen–antigen D-related (HLA-DR) on the surface of monocytes. We initially chose this last marker because it is now a recognized diagnostic marker in the assessment of the severity of sepsis-induced immunosuppression [22]. We first sought to see if these markers were capable of distinguishing between mild forms observed in the hospital infectious disease unit (IDU) and ICU severe forms. Focusing on the severe forms, we then sought to assess the potential of these markers to 1) determine possible differences in the immune profiles between patients of the two successive COVID-19 waves in France, 2) identify patients according to whether they had fatal evolution or not, and 3) predict the risk of secondary infections in order to provide evidence of the real immunoparesis of the immune system.
2. Methods
2.1. Patients and data collection
All patients with confirmed COVID-19 admitted to the IDU medical wards and ICU of the Gabriel-Montpied Teaching Hospital in Clermont-Ferrand, France, from March to the end of November 2020 were eligible for enrolment. A total of 134 patients admitted to the ICU were thus included in a preliminary prospective monocentric observational study and followed up until ICU discharge and/or death. In our hospital, as in the rest of France, we experienced two distinctly separate waves of patient hospitalizations: the months of March to June (03/08/2020 to 06/02/2020), a period called Wave 1, during which 34 patients were enrolled, and from September to November (09/14/2020 – 11/23/2020), a period called Wave 2, during which 99 others were included. A single patient was hospitalized in August between the two waves, but for the purpose of the study was included in the Wave 1 group. We decided therefore a posteriori to analyse the data of ICU patients based on their period of hospitalization, especially since the results of the RECOVERY clinical trial, published in the summer of 2020, showed the clinical benefit of dexamethasone on 28-day mortality in patients requiring oxygen support. This led to a change in practice in the management of ICU COVID-19 patients with the introduction of dexamethasone as soon as the patient required oxygen [23].
The immunoactivation/immunosuppression balance was monitored in the patients at three different time points, on days 0-3, days 7-10 and at ICU discharge (endpoint).
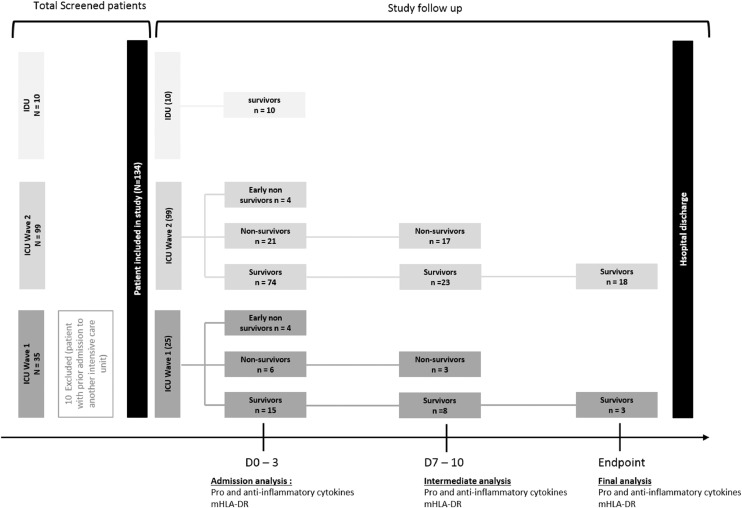
ICU admission data, ie demographic characteristics, comorbidities, severity score (SAPS II), nadir PaO2/FiO2 ratio, and laboratory findings were also collected (Table 1). During their ICU stay, patients were screened for nosocomial infections defined as microbiologically-proven pneumonia, complicated urinary tract infection (eg prostatitis), bacteremia, septic shock, catheter-associated infections and fungal infections. The survival status of included patients at discharge from the ICU was recorded. In terms of sample size, due to our pragmatic approach based on the interest in patients in daily care, we initially wanted to test all patients hospitalized in the IDU and ICU of our hospital without restrictive inclusion criteria to determine whether the markers chosen were predictive of the risk of worsening. However, this was not possible in practice for reasons of feasibility in a pandemic context that led to obstacles such as shortage of stocks of reagents and overwork of healthcare teams. Our aim at the outset was to include all patients hospitalized in the IDU as reference cases of mild-form COVID-19, which proved impossible because of organizational constraints related to the influx of patients into the department. We were therefore able to obtain only 10 IDU patients. To limit the heterogeneity of the ICU patient group and to be able to compare it with the IDU group, we decided to take into account for analysis patients who entered the ICU directly and patients who entered the ICU with prior admission to another department for a period < 3 days, but no patients from an ICU of another hospital. This reduced our Wave 1 ICU group to a total of 25 patients but did not impact the Wave 2 ICU group (see flow chart in Fig. 1).
Table 1.
Characteristics of patients admitted to the ICU or IDU for SARS-CoV-2 infection at the time of enrollment.
| Total ICU (n = 124) | ICU (n = 25) Wave 1 | ICU (n = 99) Wave 2 | P value Wave 1 vs Wave 2 | IDU (n = 10) | P value Total ICU vs IDU | Healthy controls (n = 11) | Reference range | |
|---|---|---|---|---|---|---|---|---|
| Demographic and clinical characteristics on admission | ||||||||
| Male – no. (%) | 89 (71.2) | 16 (64.0) | 73 (73.7) | 0.333 | 4 (40.0) | 0.030 | 4 (36.4) | - |
| Age – years | 68.0 (65.8 – 70.0) | 66.4 (62.3 – 70.5) | 68.4 (66.0 – 70.7) | 0,092 | 59.0 (49.2 – 68.8) | 0.010 | 34 (32 – 41) | - |
| BMI – kg/m² | 30.5 (29.2 – 31.7) | 30.0 (27.2 – 32.8) | 30.5 (29.2 – 31.9) | 0,407 | 28.4 (25.7 – 31.0) | 0.104 | 20.6 (19.6 – 22.9) | - |
| Hypertension – no. (%) | 70 (56.5) | 13 (52.0) | 57 (57.6) | 0.615 | 4 (40.0) | 0.314 | 0 (0) | - |
| Diabetes – no. (%) | 45 (36.3) | 8 (32.0) | 37 (37.3) | 0.618 | 4 (40.0) | 0.815 | 0 (0) | - |
| Cancer – no. (%) | 21 (16.9) | 5 (20.0) | 16 (16.1) | 0.648 | 1 (10.0) | 0.562 | 0 (0) | - |
| Laboratory findings | ||||||||
| Lymphocytes – /mm3 | 921 (750 – 1091) | 807 (689 – 956) | 931 (734 – 1128) | 0,363 | 1393 (1014 – 1771) | <0.001 | - | 1500 – 4000 |
| Monocytes – /mm3 | 506 (285 – 640) | 540 (220 – 900) | 516 (436 – 595) | 0.406 | 351 (273 – 430) | 0.103 | - | 200 – 800 |
| CRP – mg/L | 115.1 (100.3 – 129.8) | 146.8 (111.8 – 181.8) | 110.2 (93.8 – 126.7) | 0,276 | 67.8 (47 – 109) | 0.070 | - | < 5 |
| C3 – g/L | 1.3 (1.3 – 1.4) | 1.3 (1.2 – 1.4) | 1.3 (1.3 – 1.4) | 0,429 | - | - | - | 0.81 – 1.57 |
| C4 – g/L | 0.4 (0.3 – 0.4) | 0.3 (0.2 – 0.3) | 0.4 (0.4 – 0.5) | <0.001 | - | - | - | 0.13 – 0.39 |
| CH50 – UI/mL | 74.6 (71.6 – 77.5) | 75.2 (66.7 – 83.8) | 74.0 (71.8 – 76.2) | 0,202 | - | - | - | 41.7 – 95.1 |
| Serum ferritin – µg/mL | 1520.7 (1227.3 – 1814.2) | 3279.2 (1860.7 – 4697.8) | 1338.8 (1101.1 – 1576.6) | 0,023 | 590.7 (29.7 – 1151.6) | 0.070 | - | 8 – 252 |
| D-Dimers - ng/mL | 2024.6 (1609.7 – 2439.4) | 2787.3 (1899.9 – 3674.8) | 1828.0 (1365.6 – 2290.4) | 0,001 | 1388.8 (318.1 – 2459.4) | 0,081 | - | < 500 |
| IL-6 – pg/mL | 243.7 (3.3 – 484.0) | 837.3 (265.6 – 1940.1) | 93.8 (9.8 – 197.3) | <0.001 | 12.5 (9.7 – 15.3) | <0.001 | 1.6 (0.5 – 3.7) | <2.4 |
| CXCL8 – pg/mL | 46.8 (29.8 – 63.8) | 81.2 (27.6 – 134.7) | 38.1 (22.0 – 54.3) | <0.001 | 23.2 (15.4 – 31.0) | <0.001 | 3.7 (2.7 – 4.7) | <7.8 |
| IFN-α – pg/mL | 8.4 (2.9 – 13.8) | 17.7 (6.2 – 29.3) | 7.4 (1.3 – 13.5) | 0.020 | 8.8 (1.4 – 16.2) | 0.217 | 1.2 (0.1 – 2.2) | <4.4 |
| IL-10 – pg/mL | 6.1 (3.9 – 8.3) | 11.3 (1.6 – 21.0) | 4.8 (3.7 – 6.0) | 0.012 | 2.5 (1.5 – 3.6) | 0.004 | 0.8 (0.2 – 1.4) | <1.4 |
| IL1Ra – pg/mL | 552.0 (386.9 – 717.3) | 1284.1 (615.2 – 1953.1) | 492.3 (332.7 – 652.0) | 0.008 | 86.6 (66.1 – 107.1) | 0.011 | - | - |
| mHLA-DR – pg/mL | 11600 (10436 – 12764) | 5926 (5028 – 6824) | 11772 (10468 – 13076) | 0.033 | 21566 (18004 – 25128) | 0.010 | 44544 (26884 – 62203) | >15000 |
| During hospital stay | ||||||||
| Time from symptoms to unit admission day | 9.0 (4.7 – 13.4) | 10.6 (6.1 – 15.0) | 8.6 (4.3 – 13.0) | 0,428 | 7.9 (5.6 – 10.2) | <0.001 | - | - |
| SAPSII score | 36.6 (34.5 – 38.6) | 41.4 (36.2 – 46.5) | 35.3 (33.2 – 37.5) | 0,026 | - | - | - | - |
| Nosocomial infection – no. (%) | 38 (30.6) | 12 (48.0) | 26 (26.3) | 0.035 | 0 (0) | 0.039 | - | - |
| Including septic shock – no. (%) | 20 (16.1) | 5 (20.0) | 15 (15.2) | 0.556 | - | - | - | - |
| Length of stay – days | 9.6 (7.8 – 11.4) | 13.2 (6.3 – 20.1) | 8.6 (7.2 – 10.1) | 0.007 | 5.7 (2.1 – 9.3) | 0.030 | - | - |
| Death in hospital – no. (%) | 35 (28.2) | 10 (40.0) | 25 (25.3) | 0.090 | 0 (0.0) | 0.060 | - | - |
| Discharge from hospital – no. (%) | 91 (73.4) | 15 (60.0) | 76(76.8) | 0.090 | 10 (100.0) | 0.060 | - | - |
Data are expressed as mean [IC95] or percentages (%). ICU: Intensive care unit; IDU: Infectious disease unit; BMI: Body mass index; CRP: C-reactive protein; C3, C4, CH50: Complement fractions; ARDS: Acute respiratory distress syndrome; SAPS II: Simplified Acute Physiology Score II.
Fig. 1.
Flow chart of the study.
2.2. Ethics
All patients or their relatives received fair and relevant information. They gave written informed consent for the storage and research use of residual blood from samples collected as part of routine care (IRB n° 20.03.20.56342 from CPP-Ile-de-France VI Groupe Hospitalier Pitié-Salpêtrière). Blood samples from voluntary healthy donors were collected during the COVID-19 pandemic and then included as negative controls. Their demographics are given in Table 1.
2.3. Analysis of HLA-DR expression on monocytes
Monocyte HLA-DR (mHLA-DR) expression, in numbers of antibodies bound per monocyte (Ab/cell), was analysed at three time points, on days 0-3, days 7-10 and at ICU discharge (endpoint). EDTA-blood was drawn at the above time points after inclusion of patients in the study. The blood was stored at 4-8°C and processed within 4 h after withdrawal. The quantitative expression of mHLA-DR was determined with the anti-HLA-DR/anti-Monocyte QuantiBRITE assay (BD Biosciences, San Jose, CA, USA). Briefly, whole blood (25 μL) was stained with 10 μL of QuantiBRITE HLA‐DR/Monocyte mixture (QuantiBRITE anti‐HLA‐DR PE (clone L243)/anti‐monocytes (CD14) PerCP‐Cy5.5 (clone MϕP9), Becton Dickinson San Jose, CA, USA) at room temperature for 30 min in a dark chamber. Samples were then lysed using the FACS Lysing solution (Becton Dickinson San Jose, CA, USA) for 15 min. After a washing step, cells were acquired on a BD LSR II cytometer (BD Biosciences, San Jose, CA, USA) and flow data were analysed with FlowLogic software (version 7.3 software, Miltenyi Biotec, Germany). The total number of antibodies bound per monocyte (Ab/cell), defined as total CD14+ cells, were quantified by calibration with a standard curve determined with BD QuantiBRITE phycoerythrin (PE) beads (BD Biosciences, San Jose, CA, USA) (see Figure S1 for gating strategy). Antibodies, reagents and the respective suppliers are shown in Supplemental Table 1.
2.4. Plasma cytokine analysis
Plasma was obtained through centrifugation of EDTA samples within 4 h of phlebotomy at the indicated time points following inclusion of patients in the study. Plasma was placed in aliquots and stored at –20°C until use. Human pro-inflammatory cytometric bead array (CBA) and human IFN-α flex kits (BD Biosciences, San Jose, CA, USA) were used. The human pro-inflammatory cytokine kit simultaneously detects IL-6, IL-10 and CXCL8 cytokines in a single sample whereas the IFN-α flex kit detects IFN-α. The assays were performed according to the manufacturer's instructions. Samples and standards were acquired on the BD LSR II cytometer (BD Biosciences, San Jose, CA, USA) and the generated FSC files were analysed with FCAP Array version 3.0 software.
Plasma IL-1Ra levels were measured with a commercial enzyme-linked immunoassay (ELISA) kit (human IL-1Ra ELISA from Invitrogen, Villebon-sur-Yvette, France). Plasma samples were frozen and stored for batch analysis. In brief, to determine IL-1Ra levels, samples were thawed and 50 µL aliquots were incubated in microtitre wells coated with anti-human IL-1Ra antibody. The wells were then washed and detection achieved by adding biotin-conjugated anti-human IL-1Ra antibody followed by incubation with streptavidin-HRP, and finally by addition of horseradish peroxidase (HRP) substrate solution. A coloured product formed in proportion to the amount of human IL-1Ra present and absorbance was measured at 450 nm. Reagents and the respective suppliers are depicted in Supplemental Table 1.
2.5. Statistics
Statistical analyses and graphical presentations were conducted with GraphPad Prism version 8.0 software (Graph-Pad Software). The population was expressed as numbers and percentages for categorical variables. For quantitative variables, the results were expressed in terms of mean ± standard deviation in the Figures or mean and IC95 in the Tables. Categorical data were analysed with Chi-square test with a 5% risk of type 1 error. Statistical analysis of continuous variables, ie the different severity groups, mild versus severe forms, survivors versus non-survivors, was performed with one-way Mann–Whitney U test. For the longitudinal follow-up of patients, statistical analysis from baseline was performed with a Wilcoxon matched pairs test. Pearson's correlation coefficient was used to assess the relationship between the PaO2/FiO2 ratio and mHLA-DR expression or IL-6 and mHLA-DR expression. Analyses were performed with no correction for multiplicity. The receiver operating characteristics (ROC) curve analysis was performed using the “pROC” package on R software to identify the optimal cut-off values of baseline and D7-10 mHLA-DR, IL-6 or IL-6/mHLA-DR*103 parameters for prediction of fatal COVID-19 or secondary infections. The optimal cut‐off points to predict the severity of COVID‐19 were determined by Youden's index using R software. Sensitivity, specificity and the corresponding IC95 of optimal cut-off points were determined using the “pROC” package.
2.6. Role of funders
None. The study was based on routine care and received no financial sponsorship.
3. Results
3.1. Cohort of consecutive patients
A total of 134 patients who were laboratory-confirmed to be infected with SARS-CoV-2 in hospital were included. The immunological characteristics and their clinical and laboratory features were compared between 124 severe cases admitted to the ICU and 10 mild cases admitted to the IDU (Table 1). ICU patients were more often men and were older than IDU patients. They also had a more severe inflammatory profile induced by COVID-19 as evidenced by a much higher increase in serum ferritin and CRP and a statistically significant decrease in lymphocyte cell counts (Table 1). Thirty seven patients (30.6%) in the ICU as against none in the IDU developed a microbiologically-proven secondary infection, including 20 septic shocks (16.1%). Finally, 35 patients (28.2%) died in the ICU whereas all IDU patients recovered and were discharged (Table 1). Patient demographic characteristics on admission were not statistically different between the two waves of hospitalization. However, patients from Wave 1 were in a more severe condition than Wave 2 patients, as shown by higher CRP, D-Dimers and serum ferritin, associated with higher SAPSII scores, a longer length of stay in intensive care and the occurrence of statistically more secondary infections (Table 1). Medication and medical care administered to all ICU patients are given in Table S2. Overall mortality decreased in Wave 2, but not to a degree of significance.
3.2. COVID-19-induced inflammation at different times of ICU hospitalization
Upon admission, plasma concentrations of IL-6 and CXCL8 were statistically higher in patients who were primarily admitted to the ICU than in those admitted to a conventional IDU ward, whose concentrations were nevertheless statistically higher than those of the healthy controls (Table 1). In addition, changes in the management of ICU patients from June 2020 resulted in several modifications to pro-inflammatory cytokines. Specifically, Wave 1 patients, who were primarily admitted to the ICU up to March 2020, had a significant 8.9-fold increase in mean IL-6 values (837.3 pg/mL) compared to ICU patients from Wave 2 (93.8 pg/mL) (table 1). Also, mean CXCL8 concentrations were significantly 2.1-fold higher in Wave 1 ICU patients (81.2 pg/mL) than in Wave 2 ICU patients (38.1 pg/mL). No statistically significant difference in IFN-α values was observed between IDU and ICU patients, but the initial mean IFN-α level of Wave 1 ICU patients was statistically higher than in Wave 2 ICU patients (Table 1).
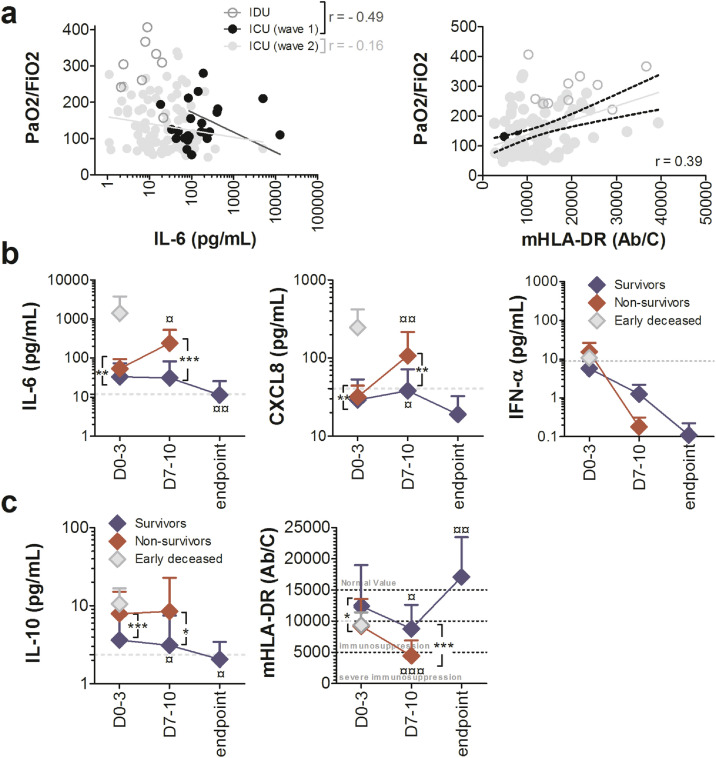
Interestingly, 3.2% (n = 4/124) of ICU patients developed a condition compatible with CSS, whose threshold was defined as the mean of plasma cytokine concentrations + 2 SD by Mudd et al. [24]. Of these patients, 3 were from Wave 1 (12%, n = 3/25) and only one from Wave 2 (1%, n = 1/99) (p = 0.01, Fisher's exact test) (Table S3). All but one of these patients died. None of these patients had a ferritin level greater than 4420 ng/mL (data not shown). The only survivor had been treated with tocilizumab, which produced a progressive decline in plasma IL-6 (data not shown). Finally, plasma IL-6 and the PaO2/FiO2 ratio at admission were well inversely associated in Wave 1 patients (r = -0.49) but weakly inversely correlated in Wave 2 patients (r = -0.16, Fig. 2a). Plasma IL-6 (p = 0.02, Mann–Whitney U test), but not CXCL8 (p = 0.2567, Mann–Whitney U test) values at admission were statistically higher in ICU COVID-19 patients with PaO2/FiO2 ratio less than 100 compared to others (Figure S2).
Fig. 2.
Plasma pro- and anti-inflammatory cytokine levels and monocyte dysregulation are associated in COVID-19 patients with pulmonary involvement, disease severity and mortality. (a) Spearman correlation of plasma levels of IL-6 or mHLA-DR and PaO2/FiO2 ratios on admission in COVID-19 individuals. The black full line and the grey full line represent the best fit linear relationship of data collected during Wave 1 and Wave 2, respectively. The black dotted lines represent the IC95. Evolution of plasma (b) pro-inflammatory cytokine levels or (c) immunosuppression markers over time during ICU hospitalization measured at baseline (D0-3), on D7-10 and until discharge of the patient for recovery (Endpoint) in alive (blue diamond) and deceased patients (red diamond). Grey diamonds represent patients deceased before D7 (n = 4). Grey dotted lines represent means of cytokines in IDU patients at baseline. Each value represents the mean ± SD. Only statistically significant results were indicated (¤p < 0.05, ¤¤p < 0.01, versus baseline, Wilcoxon matched pairs test, **p < 0.01, ***p < 0.001, alive patients vs deceased patients, Mann–Whitney U test). Number of cases for each time point, S: Survivors, NS: Non- survivors. Time D0-3 70(S), 21(NS); D7-10 23(S), 17(NS); Endpoint 18(S). PaO2: Patient's oxygen in arterial blood; FiO2: Fraction of the oxygen in the inspired air.
We re-analysed the cytokine results on admission no longer by grouping the patients by wave but depending on whether they had received corticosteroids or not. This confirmed that blood levels of pro-inflammatory cytokines were statistically lower in patients who had received corticosteroids (Figure S3).
We next focused on hospitalized ICU patients, monitoring their cytokine profile over time according to disease outcome, fatal or not. The mean IL-6 level of Wave 2 survivor patients hospitalized in the ICU dropped from 33.4 pg/mL at admission to 11.3 pg/mL at discharge, which represents a statistically significant decrease from baseline (p = 0.003, Mann–Whitney U test), and reached IDU values (dotted grey line in Fig. 2b). The mean CXCL8 concentration of survivor patients was 29.1 pg/mL at admission, 38.2 pg/mL at day 7-10, and 19.0 pg/mL at discharge: it decreased over time and was below IDU values at ICU discharge (dotted grey line in Fig. 2b). We chose to focus on the patients of Wave 2 because there were markedly more of them. Changes in CXCL8 levels over time had a fairly close profile in Wave 1 patients, but IL-6 levels remained stable over time (Figure S4). We divided non-survivors into two groups, one in which patients died less than 5 days after ICU entry and who therefore had no second sample taken on days 7-10, and the other for deaths occurring later. Remarkably, the 4 patients who died early had much higher plasma IL-6 and CXCL8 concentrations at baseline than other deceased ICU patients (Fig. 2b). The other non-survivor patients had statistically higher plasma IL-6 and CXCL8 concentrations (mean IL-6 = 52.8 pg/mL, mean CXCL8 = 33.0 pg/mL) at baseline (p = 0.008 and p = 0.009, respectively, Mann–Whitney U test) and on days 7-10 (p < 0.0001 and p = 0.006, respectively, Mann–Whitney U test) than survivor patients, with a statistically significant increase over time until death (p = 0.0108 for IL-6 and p = 0,0007 for CXCL8, Wilcoxon matched pairs test, Fig. 2b). The IFN-α levels of survivors decreased over time, starting at 5.6 pg/mL at admission and reaching 0.1 pg/mL at discharge, below IDU values (dotted grey line in Fig. 2b). The initial high IFN-α levels of non-survivor patients decreased over time to below-normal values at death (Fig. 2b). Finally, plasma IL-6 concentrations and PaO2/FiO2 ratio measured at all time points were significantly inversely correlated (r = -0.25, data not shown).
3.3. Severe COVID-19 diseases showed biological signs of immunosuppression
Upon admission, plasma concentrations of IL-10 were statistically higher in patients who were admitted to the ICU than those of patients admitted to conventional units, which in turn were statistically higher than those of healthy controls (Table 1). In addition, Wave 1 patients had a 2.4-fold significant increase in mean IL-10 concentrations (11.3 pg/mL) compared to ICU patients from Wave 2 (4.8 pg/mL) (Table 1). Notably, plasma IL-1Ra levels at admission were also statistically higher in ICU patients than in IDU patients, and Wave 1 ICU patients had a 2.6-fold significant increase in mean concentrations of this cytokine (1284.1 pg/mL) compared to ICU patients from Wave 2 (492.3 pg/mL) (Table 1).
In contrast, mHLA-DR expression was dramatically lower in COVID-19 patients upon admission than in healthy controls and was statistically decreased by two fold in ICU patients compared to IDU patients (Table 1). In addition, ICU patients from Wave 1 had a statistically lower expression of mHLA-DR than Wave 2 patients (Table 1). Finally, mHLA-DR expression and PaO2/FiO2 ratio measured at admission were significantly correlated (Fig. 2a). Plasma IL-10 values at admission were non-significantly higher (p = 0.1043, Mann–Whitney U test) but mHLA-DR expression significantly decreased (p < 0.001, Mann–Whitney U test) in ICU COVID-19 patients with PaO2/FiO2 ratio less than 100 than in other patients (Figure S2).
Among ICU hospitalized patients, IL-10 concentrations in survivors decreased over time (Fig. 2c), starting at 3.6 pg/mL at admission and reaching 2.0 pg/mL at discharge (p = 0,0407, Mann–Whitney U test), close to IDU values (dotted grey line in Fig. 2c). Conversely, non-survivors had statistically higher plasma IL-10 concentrations at baseline (mean IL-10 = 8.6 pg/mL) than did survivors, which remained higher on days 7-10 but without change over time (Fig. 2c). There was no difference in IL-10 values at admission between patients who died early and those who died later. Data from Wave 1 yielded the same observations (Figure S4).
The mean mHLA-DR expression of survivor patients hospitalized in the ICU was 12,414 Ab/monocyte at admission, 8,783 Ab/monocyte on days 7-10, and 17,069 Ab/monocyte at discharge, with an increase over time (p = 0.0081, Wilcoxon matched pairs test Fig. 2c). Non-survivors had significantly down-regulated mHLA-DR expression at baseline (mean = 9644 Ab/monocytes) compared to survivors (p = 0.0171, Mann–Whitney U test). There was no difference in mHLA-DR expression on admission between patients who died early and those who died later. The difference between survivors and non-survivors was significantly accentuated on days 7-10 (p < 0.0001, Mann–Whitney U test), with expression of mHLA-DR in the latter group decreasing to 4,325 Ab/monocyte on average (p = 0.0004, Wilcoxon matched pairs test Fig. 2c). Finally, expression of mHLA-DR and PaO2/FiO2 ratio measured at all time points were significantly correlated (r = 0.33, data not shown).
3.4. Severe COVID-19 patients had both cytokine inflammation and monocyte dysregulation
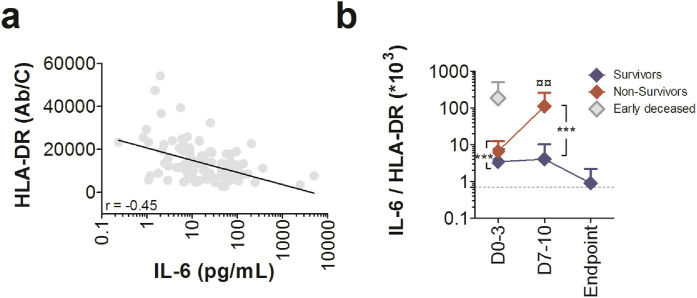
In Wave 2 COVID-19 ICU patients, blood mHLA-DR expression and plasma IL-6 levels were significantly inversely well correlated at all time points (Fig. 3a). To simultaneously analyse both facets of the immune response, we performed an IL-6/mHLA-DR*103 ratio analysis. Survivor ratio was 3.4 at admission, 4.1 on days 7-10, and 0.89 at discharge, with a decrease over time to reach IDU values at ICU discharge (dotted grey line in Fig. 3b). Remarkably, the 4 patients who died early had a much higher IL-6/mHLA-DR*103 ratio at baseline (ratio of 185.0) than other deceased ICU patients (Fig. 3b). The other non-survivor patients had a statistically higher IL-6/mHLA-DR*103 ratio at baseline (ratio of 6.6) and on days 7-10 (ratio of 110.5) than survivor patients, with a very significant increase over time until death (Fig. 3b).
Fig. 3.
Severe COVID-19 patients had both cytokine inflammation and monocyte dysregulation. (a) Spearman correlation of HLA-DR expression on monocytes and plasma IL-6 levels (D0-3, D7-10 and Endpoint) in Wave 2 ICU COVID-19 individuals. The full line represents the best fit linear relationship of data. (b) Evolution of IL-6/mHLA-DR ratio over time during ICU hospitalization measured at baseline (D0-3), at D7-10 and until discharge of the patient for recovery (Endpoint) in alive (blue diamond) and deceased patients (red diamond). Grey diamonds represent patients deceased before D7 (n = 4). Grey dotted lines represent the mean of IL-6/mHLA-DR ratio in IDU patients at baseline. Each value represents the mean ± SD. Only statistically significant results were indicated (¤¤p < 0.01 versus baseline, Wilcoxon matched pairs test, ***p < 0.001, alive patients vs deceased patients, Mann–Whitney U test). Number of cases for each time point, S: Survivors, NS: non survivors. Time D0-3 70(S), 20(NS); D7-10 23(S), 16(NS); Endpoint 18(S).
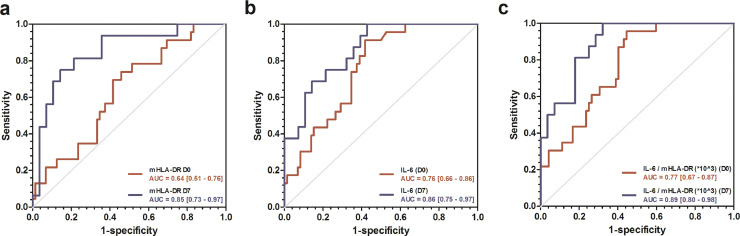
The ROC curve analysis identified the optimal cut-off values of baseline and D7 mHLA-DR, IL-6 or IL-6/mHLA-DR*103 parameters for prediction of fatal COVID-19 (Fig. 4, Table 2). These optimal cut‐off points showed high negative predictive values (NPV) for the different parameters ranging from 86% to 100%. We also determined for the different parameters the thresholds that yielded positive predictive values (PPV) of 100% in order to identify patients at high risk of death (Table 2).
Fig. 4.
Receiver operating characteristic (ROC) curves predicting unfavorable outcome of COVID-19 in ICU patients. Receiver operating characteristic (ROC) curves of (a) mHLA-DR, (b) plasma IL-6 and (c) IL-6/mHLA-DR ratio, obtained at baseline (red) and at D7-10 (blue), predicting unfavorable outcome (death) of COVID-19 in ICU patients. Area under the ROC curve with 95% CI are indicated for each parameter.
Table 2.
Cut-off values for mHLA-DR, plasma IL-6 and IL-6/mHLA-DR ratio analysed at D0 and D7-10 according to Youden's index or maximum PPV or NPV predicting unfavourable outcome (death).
| Laboratory tests | Optimal cut-off | Sp - % (CI95) | Se - % (CI95) | Npv - % | Ppv - % |
|---|---|---|---|---|---|
| Youden's index | |||||
| mHLA-DR (D0-3) – Ab/c | 11312.5 | 54 (43 – 65) | 74 (57 – 91) | 87 | 34 |
| mHLA-DR (D7-10) – Ab/c | 4672.5 | 86 (71 - 96) | 75 (50 – 94) | 86 | 75 |
| IL-6 (D0-3) – pg/mL | 19.1 | 58 (47 – 70) | 91 (78 – 100) | 95 | 41 |
| IL-6 (D7-10) - pg/mL | 14.3 | 57 (39 – 75) | 100 (100 – 100) | 100 | 57 |
| IL-6 / mHLA-DR *10^3 (D0-3) | 1.4 | 56 (44 – 66) | 96 (87 – 100) | 98 | 41 |
| IL-6 / mHLA-DR *10^3 (D7-10) | 2.7 | 68 (50 – 86) | 100 (100 – 100) | 100 | 64 |
| Maximum PPV = 100% | |||||
| mHLA-DR (D0-3) - Ab/c | 2795.5 | 100 | 4.3 (0 – 13) | 77 | 100 |
| mHLA-DR (D7-10) - Ab/c | 1361.0 | 100 | 6.3 (0 – 19) | 65 | 100 |
| IL-6 (D0-3) - pg/mL | 226.7 | 100 | 13 (0 – 30) | 78 | 100 |
| IL-6 (D7-10) - pg/mL | 236.1 | 100 | 38 (13 – 63) | 74 | 100 |
| IL-6 / mHLA-DR *10^3 (D0-3) | 18.1 | 100 | 22 (4 – 40) | 80 | 100 |
| IL-6 / mHLA-DR *10^3 (D7-10) | 49.6 | 100 | 38 (13 – 63) | 74 | 100 |
| Maximum NPV = 100% | |||||
| mHLA-DR (D0-3) – Ab/c | 19081.0 | 17 (8 – 26) | 100 | 100 | 28 |
| mHLA-DR (D7-10) – Ab/c | 12615.0 | 25 (10 – 43) | 100 | 100 | 43 |
Sp: Specificity; Se: Sensitivity; NPV: Negative predictive value; PPV: Positive predictive value, CI95: Confidence interval 95%
3.5. Inflammation and monocyte dysregulation in ICU COVID-19 patients with secondary infections
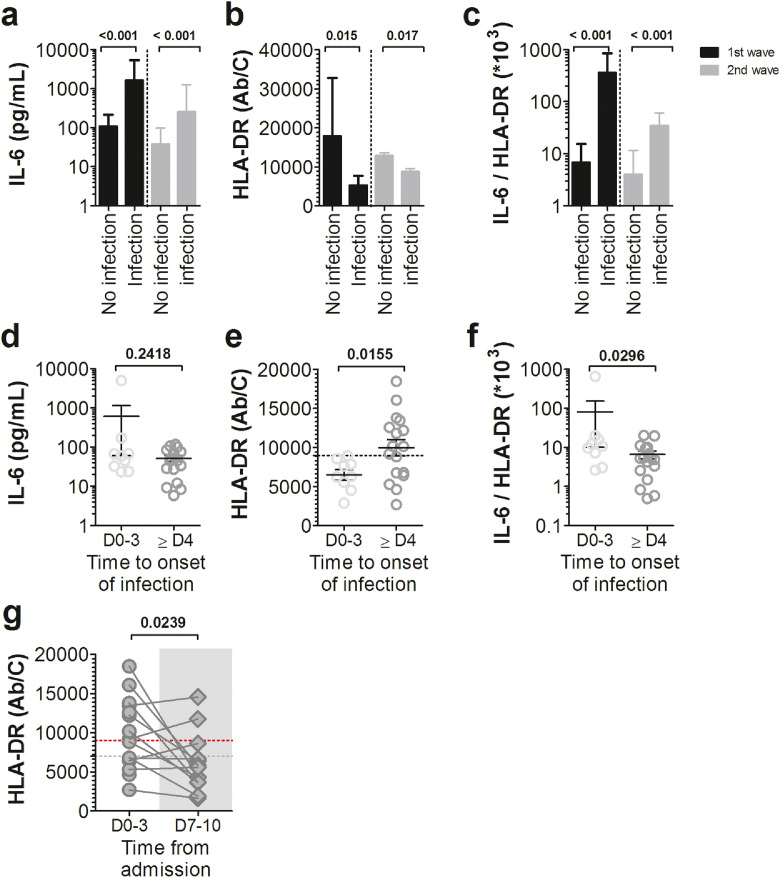
Secondary infections occurred in 26 out of 99 Wave 2-COVID-19 ICU patients. The microbiological data of those infections are given in supplemental Table S4. On admission, COVID-19 ICU patients who had experienced a secondary infection during their stay were significantly more inflammatory than those who had not, as evidenced by a 10-fold increase in plasma IL-6 during both waves (Fig. 5a). At the same time, these patients also had significantly down-regulated mHLA-DR expression during Wave 2, from 12,880 to 8,726 molecules per monocyte (Fig. 5b). Secondary infected patients had statistically increased IL-6/mHLA-DR*103 ratio values compared to non-secondary infected patients in both waves, resulting in 53-fold change and 9-fold change increases, respectively, for Wave 1 and Wave 2 (Fig. 5c). In Wave 2, 13 patients out of 26 who experienced a secondary infection developed a life-threatening infection and died (Table S4).
Fig. 5.
Cytokine inflammation and monocyte dysregulation at admission are more marked in COVID-19 ICU patients who will subsequently suffer from secondary infections. (a) Plasma IL-6 levels, (b) Monocyte HLA-DR membrane expression and (c) IL-6/mHLA-DR ratio were analysed at admission in ICU patients from Wave 1 (black bars) and Wave 2 (grey bars) according to the occurrence of secondary infections acquired during hospitalization. Bar graphs represent mean ± standard deviation (Statistical comparison by Mann-Whitney U test). Wave 2 ICU patients at baseline (d) plasma IL-6 levels, (e) monocyte HLA-DR membrane expression and (f) IL-6/mHLA-DR ratio were detailed according to the onset of secondary infection, defined as early infection (D0-3) or late infection (> D4). Each dot represents an individual value, and mean ± standard deviation are shown (Statistical comparison by Mann-Whitney U test). Dotted line in (e) represents mHLA-DR threshold predicting early secondary infection occurrence. (g) Monocyte membrane expression of human leucocyte antigen–antigen D-related (mHLA-DR) results from individual patients with late secondary infection, represented as dots in the figure, with lines connecting baseline mHLA-DR expression and D7-10 mHLA-DR expression. The time of onset of infection is shown by the grey area in the figure. Horizontal grey dotted lines represent the mean of mHLA-DR expression at D0-3 in ICU patients with early infection. Horizontal red dotted lines represents mHLA-DR threshold predicting early secondary infection occurrence (comparison versus baseline, Wilcoxon matched pairs test).
The ROC curve analysis identified the optimal cut-off values of mHLA-DR, IL-6 or IL-6/mHLA-DR*103 parameters for prediction of the occurrence of secondary infection (Figure S5, Table S5). These optimal cut‐off points showed PPV of 100% at baseline for the different parameters and PPV ranging from 64% to 100% on days 7-10.
We divided the ICU patients with a secondary infection into two groups, one with early infections occurring within less than 3 days after ICU admission and another for infections that occurred more than 4 days after admission. Remarkably, IL-6 plasma levels in patients with early secondary infections (mean IL-6 = 558.2 pg/mL) were not statistically different from those in patients with later infections (mean IL-6 = 53.3 pg/mL) (Fig. 5d). A notable exception was one Wave 2 patient who experienced a cytokine storm with IL-6 values at admission of 4981 pg/mL, which was 88 times higher than the median assay of the early infection group to which he belonged. Conversely, the levels of mHLA-DR were statistically different between the two groups of patients, with a two-fold downregulation in those with early secondary infections (Fig. 5e). Specifically, 26 ICU patients had at least one severe secondary infection in Wave 2. Of the 9 who developed early infections, all had an initial mHLA-DR lower than 9,000 Ab/monocyte (Table S6). Conversely, 10 out of 17 (59.0%) other patients who developed later infections after admission had initial mHLA-DR expression greater than this threshold. Likewise, we observed a difference in the IL-6/mHLA-DR ratio between the two groups, with about a 2-fold increase in the median in patients with early infection (Fig. 5f). In patients who developed infections 4 days after ICU admission, mHLA-DR expression recorded on days 7-10, ie near the onset of secondary infections, was statistically lower than that at admission and reached the mHLA-DR value obtained for ICU patients who presented early infections on days 0-3 (Fig. 5g). The lack of data on mHLA-DRs expression during Wave 1 prevented us from distinguishing between the relative impacts of IL-6 and mHLA-DR on the occurrence of secondary infections. However, it is important to emphasize that, as for the patient with a cytokine storm described above, all patients with pro-inflammatory cytokine storm syndrome (CSS) in Wave 1 had secondary infections. There is therefore an over-representation of infections of this type in the CSS ICU patient group compared to non-CSS ICU patients (3/3 = 100% vs 9/22 = 40.9%).
4. Discussion
Patients with severe forms of COVID-19 have mortality rates in the ICU which remain high to this day. They most often die of respiratory failure after the development of ARDS. However, other factors may be associated with an increased risk of death, including secondary infections. Based on what we knew and practised in the context of sepsis, we hypothesized that severe COVID-19 patients were likely to suffer from a complex immune dysfunction combining inflammation and immunosuppression.
In our series, the plasma IL-6 and CXCL8 levels in the different severity groups of COVID-19 patients were statistically different on admission. This is clear evidence that severe ICU COVID-19 patients were experiencing inflammation. The cytokine results were also inversely correlated with the PaO2/FiO2 ratio, attesting to a link between the degree of inflammation and the pulmonary involvement of ARDS. Thus, in our series, as in other previous studies, the degree of initial inflammation appears to be predictive of the severity of COVID-19, both in terms of the severity of the lung injury and of the risk of death [3,10,12,25]. However, in agreement with recent data from the literature and with our own (not shown), it is noteworthy that the mean values of these cytokine levels were much lower than in other pathological settings, such as bacterial septic shocks, other causes of ARDS or injections of CAR T cells, and quite close to levels induced by classical viral respiratory infections like influenza [[15], [16], [17],24]. In contrast to prevailing hypotheses about the pathophysiology of COVID-19 disease very few patients in our series presented cytokine profiles indicative of CSS. In the absence of any internationally recognized criterion we set the threshold according to the method recently described by Mudd et al., which indicated that only 4 of our COVID-19 patients had CSS [24], all of whom were hospitalized in the ICU and none in the IDU. Although cases of CSS were rare in our patient series they nevertheless made up a severe subgroup with a very poor prognosis, since 3 out of the 4 patients who met the criterion on admission died during hospitalization, a mortality rate of 75% compared to an overall mortality in ICU patients of 25%. Note that for the 3 patients for whom we had a ferritin assay, none reached the threshold of 4420 ng / mL, previously used for evoking a macrophage activation syndrome during COVID-19 [21].
Another interesting point among ICU patients was the dramatic decrease in proinflammatory cytokines measured during Wave 2 compared to Wave 1. In particular, 12% of patients met the criteria for CSS during Wave 1 as against 1% during Wave 2. These results show that CSS, and hence hyperinflammation, was not that rare during Wave 1 but much more so during Wave 2. They should be put in perspective with the significant change in management practices introduced in our teaching hospital between the two waves. Following the publication in July 2020 of the RECOVERY trial, the great majority of patients in Wave 2 received dexamethasone (89%) compared to a small minority in Wave 1 who had received corticosteroids (5/25, ie 20% of patients receiving between 10 and 60 mg of prednisone per day) [23]. One of the main known pharmacological mechanisms of the action of glucocorticoids is to inhibit the synthesis of pro-inflammatory cytokines [26]. It makes sense to think that the decrease in cytokine levels that we saw in Wave 2 was due to the almost systematic use of this molecule. However, to our knowledge, we are the first to demonstrate this in comparative data [27].
To assess whether COVID-19 induced monocyte dysregulation, we required a test that was both able to test a key functionality of the immune system and routinely available. Our choice quickly fell on the membrane expression of HLA-DR on blood monocytes. Low mHLA-DR has become one of the best markers of monocyte immunosuppressive phenotype in various diseases such as cancers and sepsis [22,[28], [29], [30], [31]]. The scientific rationale for these findings, from a fundamental immunological point of view, is that HLA-DR is the main antigen-presenting molecule for helper T cells on the surface of monocytes, macrophages and dendritic cells. Thus, it is assumed that the decrease in mHLA-DR on the surface of blood monocytes is a reflection of a reduced overall capacity for presenting antigens and therefore for inducing adaptative immune responses. The anomalies of the myeloid compartment induced by COVID-19 are being gradually documented, in particular those concerning monocytes, with early expansion and activation of blood classical CD14+ monocyte expressing HLA-DR++ being seen in moderate COVID-19 patients and conversely an accumulation of HLA-DRlo monocytes in severe patients [11,23,24,26,27]. All these data confirm the potential interest of the marker of monocyte immunosuppressive phenotype that we chose [32], [33], [34].
In our series, on the basis of recognized interpretation thresholds from the literature for the analysis of mHLA-DR expression, it can be considered that IDU patients with mild forms, despite having lower values than healthy controls, are not immunosuppressed on admission (mean HLA-DR = 21566 Ab/cells) unlike ICU patients, who clearly are [20,31,35,36]. Hence, as previously reported, decreased mHLA-DR was a significant predictor of COVID-19 disease severity [24,37,38]. In our study, levels of IL-10, one of the main anti-inflammatory cytokines, were statistically higher in ICU patients than in IDU patients and healthy controls. In addition, concentrations of IL-1Ra, a cytokine of the acute phase of inflammation which plays an anti-inflammatory role of counter-regulation, in particular at the level of monocytes (both target and producer of the molecule), were also statistically higher in ICU patients than in IDU patients. The profile of secretion of these two molecules therefore confirmed, in agreement with mHLA-DR data, the presence of immunosuppression in the most severe patients.
Comparison of the results of ICU patients between the two waves showed that patients in Wave 1 expressed monocyte mHLA-DR significantly less than those in Wave 2. On the basis of the same criteria as before, Wave 1 patients exhibited severe monocyte dysregulation, on the verge of immunoparalysis (mean HLA-DR = 5926 Ab/cells) [31,35], unlike Wave 2 patients, who were in a state of simple monocyte dysregulation (mean HLA-DR = 11772 Ab/cell). Here again, IL-10 and IL-1Ra levels were in close agreement with mHLA-DR results, with Wave 1 ICU patients showing higher concentrations of both immunosuppressive cytokines. One could suspect again that systematic treatment with dexamethasone played a role [39,40].
Comparison of the mHLA-DR data in the different groups of COVID-19 patients on ICU admission according to mortality showed that the expression of the marker was inversely proportional to the severity of the disease. The time-dependent evolution of mHLA-DR should be emphasized because in the two groups of patients, survivors and non-survivors, a statistically significant decrease in values was observed on days 7-10 compared to baseline. However, the difference in values between the two groups increased statistically, with surviving patients remaining in the zone of simple monocyte dysregulation while those in the non-survivor group became severely immunosuppressed [31,35]. Subsequently, in surviving patients, a statistically significant increase in measurements was observed before ICU discharge, with values returning to normal, thereby indicating the disappearance of monocyte dysregulation. It can thus be considered that the evolution of mHLA-DR follows a V trend curve, as recently reported, with a nadir probably between days 7 and 10 after ICU admission when immunosuppression is therefore probably at its greatest [41,42]. All these data show, for the first time to our knowledge, that the quantitative expression of HLA-DR on monocytes is a predictive marker of mortality in COVID-19 patients. They are to be compared with those of a previous study which found a decrease in the number of CD14+ HLA-DR+ monocytes in patients who died of COVID-19 compared to survivors [33]. In addition, mHLA-DR quantitative expression during hospitalization was also significantly correlated with the PaO2/FiO2 ratio thereby indicating, as previously described, a relation between the degree of monocyte dysregulation and the pulmonary involvement of ARDS [21]. Again, it is noteworthy that the IL-10 results are in agreement with those of mHLA-DR, notably with IL-10 levels being very significantly greater in the non-survivor group than in survivor patients. Thus, during severe COVID-19 infections, we found an immunosuppressive profile associating IL-10 production and a decrease in mHLA-DR the same as that already observed in septic shocks [43]. We could not evaluate it for reasons of simplicity and effectiveness contrary to our routine care approach, but we can hypothesize, given the data in the literature, that this result probably reflects a more overall myeloid (granulocytes, macrophages, dendritic cells) and lymphoid dysregulation which would be interesting to investigate in later studies.
Interestingly, we showed that IL-6 and mHLA-DR were well correlated in ICU patients, in an inversely proportional way. This enables us to assert that inflammation and monocyte dysregulation are indeed phenomena occurring concomitantly in patients with severe forms of COVID-19 and therefore confirm our initial hypothesis. Consequently, the use of the IL-6/mHLA-DR ratio could be of interest to simultaneously analyse the two aspects of the immune response in the same patient. In ICU patients, the ratio on admission was significantly higher in the non-survivor group than in the survivor group, and the difference was even more marked on days 7-10, confirming that the inflamed/immunocompromised dual state is associated with poor prognosis. The analysis of ROC curves for IL-6 concentrations, HLA-DR expression, and IL-6/mHLA-DR ratio on admission showed that IL-6/mHLA-DR ratio was the more reliable cut-off for predicting fatal outcome. Thus, we could propose the use of IL-6/mHLA-DR ratio in current clinical practice, with a threshold of 18, as in our series, to obtain a PPV of the risk of death of 100%. To our knowledge, this is the first report to demonstrate that IL-6/mHLA-DR ratio is a valuable marker for predicting death in COVID-19. This interesting result needs to be confirmed soon using the proposed threshold prospectively in studies involving a greater number of patients.
These results should be compared with those of recent studies suggesting that immune dysregulation during COVID-19 with early and prolonged immune system activation can result in cellular exhaustion [44]. More precisely, it has been proposed that severe COVID-19 patients suffered from a defective antigen-presentation, evidenced by a decrease in mHLA-DR and which, associated with lymphopenia, led to defective function of lymphoid cells, whereas monocytes remained potent for the production of TNFα and IL-6. Moreover, the involvement of IL-6 in the mHLA-DR decrease has been demonstrated in part by the fact that an IL-6 blocker, Tocilizumab, partially rescued this downregulation in vitro [21,45]. Our results could be considered as consistent with this observation. However, we cannot rule out a role for IL-10 as well since levels of the cytokine were also higher in our severe patients, and previous publications have shown its involvement in the internalization of HLA-DR molecules in monocytes in the context of septic shock [46]. Another factor that could contribute to the decrease in HLA-DR on monocytes was the involvement of neutrophilic granulocytes. The role of the activation of these cells in COVID-19, which has been reported by various teams, seems to be corroborated in our study by the increase in CXCL8 levels in severe forms. In particular, the secretion of neutrophilic elastase is associated with the severe forms of the disease [47,48]. However, once released this elastase has the property of lysing nearby HLA-DR molecules [49]. In our series, we observed an increase in this marker in our most severe patients and an inverse correlation with the expression of HLA-DR (data not shown). However, these preliminary results need to be confirmed in other studies.
To demonstrate that the changes in our markers in severe COVID-19 forms, especially the decrease in HLA-DR on monocytes, were relevant functional indicators of immunoparesis of the immune system, we decided to correlate the occurrence of serious secondary infections over time with the expression of the markers to see if they were predictive of a risk of secondary infections in the short term. Although relatively unknown, it is very common for COVID-19 patients to develop secondary infections during ICU hospitalizations with several studies reporting superinfection rates higher than 50% [8,9]. The occurrence of secondary infections is also recognized as a clinical predictor of fatal outcome in COVID-19 cases [50]. In our study, we can see that 52% of total deaths from Wave 2 were directly attributable to severe secondary infections, which makes these infections the main cause of death in our patients. On admission, IL-6 concentrations, mHLA-DR expression, and IL-6/HLA-DR ratio were all statistically higher in patients who later developed a secondary infection at some point during their hospitalization. Conversely, when we compared the two groups of patients with early or later infections, the IL-6 values were at levels not statistically different. Interestingly, mHLA-DR expression was on the other hand statistically lower in the early infections group than in the group with later infections with a threshold differentiating between the two groups set empirically (because unfortunately we did not have enough points to set a threshold using interpretable ROC curves) at around 9000 Ab/monocyte. Of note, the group of patients with later infections downregulated mHLA-DR expression to less than this threshold on day 7, the moment when they in turn developed secondary infections. These results clearly demonstrate that the decrease in mHLA-DR expression, but not the slight increase in IL-6 levels, corresponds to an immunosuppression state correlated with the risk of the rapid occurrence of secondary infections. Finally, we show, for the first time to our knowledge, that the downregulation of HLA-DR on monocytes is a predictive marker of early secondary infections in COVID-19 patients. With regard to IL-6, the only patient who presented a major CSS during Wave 2 developed a very early secondary infection. Likewise, the 3 patients who had a cytokine storm during Wave 1 also developed early secondary infections. Unfortunately, HLA-DR expression was measured in only one patient, who was severely immunocompromised (3422 Ab/monocyte). Thus, patients with COVID-19-induced CSS in our study seem to be a subgroup at high risk for secondary infections. Cytokine storms therefore seem to be occurrences that associate massive inflammation and severe immunosuppression.
4.1. Caveats and Limitations
Among the main limitations of this study was the relatively small population size, in particular the healthy controls, IDU patients and Wave 1 ICU groups. This was in part due to the health situation during the first wave, when the partial saturation of our hospital created difficulties in organizing the samples, and stocks of certain cytometry reagents were in short supply. It should be noted that we have recently analysed 18 additional IDU patients. Interestingly, they exhibited an immune profile identical to that of the patients presented in this study (Figure S6). In particular, there was no significant difference between the values obtained for the two IDU groups for all parameters tested. These new patients were included from 11/17/2020 to 05/02/2021, a period in France corresponding to the occurrence of the third epidemic wave (with the introduction into the territory of the Alpha variant), and so we preferred not to pool their results with those of the initial group to avoid introducing too much heterogeneity between the patients. The results nevertheless give reassuring arguments as to the validity of our results for IDU patients. Another limitation of our study, which is related to our decision to adopt a routine care approach, is that we assessed the degree of immunosuppression of patients on only three markers (mainly mHLA-DR) and did not perform complex multiparametric analyses to assess more extensively all the immune cell populations involved in the complex immune deregulation of COVID-19. In addition, direct conclusions on the causality between disease severity and immunological profiles and the use of dexamethasone cannot be drawn from our study due to its explorative nature. It would therefore be interesting to try a functional approach to test whether the monocytes of severe COVID-19 patients with downregulated mHLA-DR have altered properties, for example in terms of phagocytosis or cytokine synthesis in response to various stimuli, especially with regard to their possible mechanistic role in disease course and severity. We plan to test these last three points in an ancillary study that we will carry out soon of new patients. Patient assignment to our cohort was not random but exclusively based on the notion of hospitalization. The introduction of bias due to this approach cannot be excluded as it is possible that because of their profile certain patients were not hospitalized in teaching hospitals or that particularly severe patients died before they could be hospitalized. The fact that there are very few intensive care beds in our region other than those at our hospital appears to minimize this risk. We are unable to protect against the risk of death before hospitalization and this is why we made mention of it earlier in the text.
Finally, in the analysis of disease severity, we classified the patients according to admission to ICU versus IDU wards, which might differ from classifications used in other studies. In line with numerous previous works, we observed a relationship between the degree of increase in proinflammatory markers and disease severity. Plasma sampling was performed at pre-defined time points depending on the day of hospital admission but it could be argued that difference in severity is attributable to longer disease duration. Nevertheless, while this may have been theoretically a potential confounder, the time from disease onset to hospital admission was recorded and showed no significant difference between the two ICU groups. To limit the heterogeneity of the ICU patient group and to be able to compare it with the IDU group, we decided to include for analysis only patients admitted directly to the ICU and those who were admitted after prior admission to another department for a period < 3 days. As a consequence of our study's pragmatic design, we performed no correction for other potential confounders, such as comorbidity, medication or invasive mechanical ventilation use.
In the future, personalized medicine approaches to COVID-19 and complete immunomonitoring simultaneously investigating both sides of the immune response will undoubtedly be taken into account to choose the most appropriate therapeutic interventions since they can be completely opposite (for example anti-IL-6 antibodies versus immune stimulants such as GM-CSF or IL-7) according to the patient's profile [51,52]. Finally, owing to the use of simple routine care biological markers, our approach responds at least in part to the problem by making it possible to better characterize the profile of the immune response of patients with severe COVID-19 in terms of inflammation or immunosuppression. With the exception of a subgroup of very severe patients who experienced a major cytokine storm, the great majority of our hospitalized patients had moderate inflammation associated with severe monocyte dysregulation, which is predictive of the severity of the disease, its mortality and the risk of secondary infections.
Contributors
BE, BB and BS conceptualized and designed the study. BB, JC, LF, BE, EC, CD, LC, MA, MB, MV and LH conducted the investigations (recruiting patients or conducting laboratory tests). BB and BE conducted formal data analysis. BB and BE wrote the original first draft of manuscript. All authors reviewed the manuscript and gave significant input. BB, BE, BS, and CD had access to and verified all underlying data. BB ensures data curation. The final version of this paper was reviewed and approved by all authors.
Data Sharing Statement
Individual participant data cannot be made available due to EU Data Protection Regulations (GDPR). A limited and completely anonymized version of the dataset can be obtained upon request. Laboratory protocols will be available upon request. Enquiries should be directed to bevrard@chu-clermontferrand.fr.
Declaration of Competing Interest
Dr. Bonnet reports non-financial support from THE BINDING SITE GROUP LTD, non-financial support from DIASORIN SA, non-financial support from Werfen, outside the submitted work. Prof. SOUWEINE reports personal fees from MSD, non-financial support from TTM BARD, personal fees from SANOFI, personal fees from LABORATOIRE AGUETTANT, outside the submitted work. All other authors have no conflicts of interest to disclose.
Acknowledgements
We thank the promoters of the CORIMUNO-19 project (INSERM-Assistance Publique des Hôpitaux de Paris) for their help, the patients of our study for being part of this cohort (screening with signature of a consent) without being included in the associated trials (due to competition with other studies in progress). We thank Xavier Daudet, Estelle, Chapon, Charlène Pierson, Aurélie Briançon, Brigitte Goutte, Marlène Ravel, Julia Mercier, Marion Gaudard, Solène Revy, Maud Junda and Frèdéric Duée for their immense assistance in the laboratory during patient material collection. Thank you to the entire staff of the Department of Infectious Diseases and intensive care unit for their feedback and scientific discussions. We thank the AFRRI association, who kindly provided the additional doses of IL-1ra. This study was not supported by any grant.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103622.
Appendix. Supplementary materials
References
- 1.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‑20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669‑77. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‑506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheehan J, Ho KS, Poon J, Sarosky K, Fung JY. Palliative care in critically ill COVID-19 patients: the early New York City experience. BMJ Support Palliat Care. 2020 doi: 10.1136/bmjspcare-2020-002677. [DOI] [PubMed] [Google Scholar]
- 5.Socolovithc RL, Fumis RRL, Tomazini BM, Pastore L, Galas FRBG, de Azevedo LCP. Epidemiology, outcomes, and the use of intensive care unit resources of critically ill patients diagnosed with COVID-19 in Sao Paulo, Brazil: a cohort study. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052‑9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G. Risk factors associated with mortality among patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345‑55. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Zhang Y, Wu J, Li Y, Zhou X, Li X. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1958‑64. doi: 10.1080/22221751.2020.1812437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‑62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463‑9. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvin A, Chapuis N, Dunsmore G, Goubet A-G, Dubuisson A, Derosa L. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182(6) doi: 10.1016/j.cell.2020.08.002. 1401-1418.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910‑41. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020:eabc6027. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233‑44. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha P, Matthay MA, Calfee CS. Is a « Cytokine Storm » relevant to COVID-19? JAMA Intern Med. 2020;180(9):1152‑4. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 17.Monneret G, Benlyamani I, Gossez M, Bermejo-Martin JF, Martín-Fernandez M, Sesques P. COVID-19: what type of cytokine storm are we dealing with? J Med Virol. 2020 doi: 10.1002/jmv.26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell [Internet] 2020 doi: 10.1016/j.cell.2020.08.001. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7405822/ [cited on Sep18, 2020]; Available on: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol [Internet] 2020 doi: 10.1126/sciimmunol.abd7114. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7402634/ [cited on Sep 18, 2020];5(49). Available on: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peruzzi B, Bencini S, Capone M, Mazzoni A, Maggi L, Salvati L. Quantitative and qualitative alterations of circulating myeloid cells and plasmacytoid DC in SARS-CoV-2 infection. Immunology. 2020;161(4):345‑53. doi: 10.1111/imm.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6) doi: 10.1016/j.chom.2020.04.009. 992-1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venet F, Demaret J, Gossez M, Monneret G. Myeloid cells in sepsis-acquired immunodeficiency. Ann N Y Acad Sci. 2020 doi: 10.1111/nyas.14333. [DOI] [PubMed] [Google Scholar]
- 23.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mudd PA, Crawford JC, Turner JS, Souquette A, Reynolds D, Bender D. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv. 2020 doi: 10.1126/sciadv.abe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims JT, Krishnan V, Chang C-Y, Engle SM, Casalini G, Rodgers GH. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimba A, Ikuta K. Control of immunity by glucocorticoids in health and disease. Semin Immunopathol. 2020;42(6):669‑80. doi: 10.1007/s00281-020-00827-8. [DOI] [PubMed] [Google Scholar]
- 27.Andreakos E, Papadaki M, Serhan CN. Dexamethasone, pro-resolving lipid mediators and resolution of inflammation in COVID-19. Allergy. 2020 doi: 10.1111/all.14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862‑74. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monneret G, Venet F, Meisel C, Schefold JC. Assessment of monocytic HLA-DR expression in ICU patients: analytical issues for multicentric flow cytometry studies. Crit Care. 2010;14(4):432. doi: 10.1186/cc9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mengos AE, Gastineau DA, Gustafson MP. The CD14+HLA-DRlo/neg monocyte: an immunosuppressive phenotype that restrains responses to cancer immunotherapy. Front Immunol. 2019;10:1147. doi: 10.3389/fimmu.2019.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benlyamani I, Venet F, Coudereau R, Gossez M, Monneret G. Monocyte HLA-DR measurement by flow cytometry in COVID-19 patients: an interim review. Cytometry Part A [Internet]. [cited on Dec8, 2020] Available on: https://onlinelibrary.wiley.com/doi/abs/10.1002/cyto.a.24249 [DOI] [PubMed]
- 32.Jeannet R, Daix T, Formento R, Feuillard J, François B. Severe COVID-19 is associated with deep and sustained multifaceted cellular immunosuppression. Intensive Care Med sept. 2020;46(9):1769‑71. doi: 10.1007/s00134-020-06127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Hou H, Yao Y, Wu S, Huang M, Ran X. Systemically comparing host immunity between survived and deceased COVID-19 patients. Cell Mol Immunol. 2020;17(8):875‑7. doi: 10.1038/s41423-020-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kox M, Frenzel T, Schouten J, van de Veerdonk FL, Koenen HJPM, Pickkers P. COVID-19 patients exhibit less pronounced immune suppression compared with bacterial septic shock patients. Crit Care. 2020;24(1):263. doi: 10.1186/s13054-020-02896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Döcke W-D, Höflich C, Davis KA, Röttgers K, Meisel C, Kiefer P. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem. 2005;51(12):2341‑7. doi: 10.1373/clinchem.2005.052639. [DOI] [PubMed] [Google Scholar]
- 36.Moratto D, Chiarini M, Giustini V, Serana F, Magro P, Roccaro AM. Flow cytometry identifies risk factors and dynamic changes in patients with COVID-19. J Clin Immunol. 2020;40(7):970‑3. doi: 10.1007/s10875-020-00806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spinetti T, Hirzel C, Fux M, Walti LN, Schober P, Stueber F. Reduced monocytic human leukocyte antigen-dr expression indicates immunosuppression in critically Ill COVID-19 patients. Anesth Analg. 2020;131(4):993‑9. doi: 10.1213/ANE.0000000000005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatti A, Radrizzani D, Viganò P, Mazzone A, Brando B. Decrease of non-classical and intermediate monocyte subsets in severe acute SARS-CoV-2 infection. Cytometry A. 2020;97(9):887‑90. doi: 10.1002/cyto.a.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Tulzo Y, Pangault C, Amiot L, Guilloux V, Tribut O, Arvieux C. Monocyte human leukocyte antigen–DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med. 2004;169(10):1144‑51. doi: 10.1164/rccm.200309-1329OC. [DOI] [PubMed] [Google Scholar]
- 40.Schwiebert LM, Schleimer RP, Radka SF, Ono SJ. Modulation of MHC class II expression in human cells by dexamethasone. Cell Immunol. 1995;165(1):12‑9. doi: 10.1006/cimm.1995.1181. [DOI] [PubMed] [Google Scholar]
- 41.Payen D, Cravat M, Maadadi H, Didelot C, Prosic L, Dupuis C. A longitudinal study of immune cells in severe COVID-19 patients. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.580250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monneret G, Cour M, Viel S, Venet F, Argaud L. Coronavirus disease 2019 as a particular sepsis: a 2-week follow-up of standard immunological parameters in critically ill patients. Intensive Care Med. 2020;46(9):1764‑5. doi: 10.1007/s00134-020-06123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monneret G, Finck M-E, Venet F, Debard A-L, Bohé J, Bienvenu J. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 2004;95(2):193‑8. doi: 10.1016/j.imlet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Mudd PA, Remy KE. Prolonged adaptive immune activation in COVID-19: implications for maintenance of long-term immunity? J Clin Invest. 2020 doi: 10.1172/JCI143928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssen NAF, Grondman I, de Nooijer AH, Boahen CK, Koeken VACM, Matzaraki V. Dysregulated innate and adaptive immune responses discriminate disease severity in COVID-19. J Infect Dis. 2021;223(8):1322‑33. doi: 10.1093/infdis/jiab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fumeaux T, Pugin J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am J Respir Crit Care Med. 2002;166(11):1475‑82. doi: 10.1164/rccm.200203-217OC. [DOI] [PubMed] [Google Scholar]
- 47.Guéant J-L, Guéant-Rodriguez R-M, Fromonot J, Oussalah A, Louis H, Chery C. Elastase and exacerbation of neutrophil innate immunity are involved in multi-visceral manifestations of COVID-19. Allergy. 2021;76(6):1846‑58. doi: 10.1111/all.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Domon H, Maekawa T, Isono T, Furuta K, Kaito C, Terao Y. Proteolytic cleavage of HLA class II by human neutrophil elastase in pneumococcal pneumonia. Sci Rep. 2021;11(1):2432. doi: 10.1038/s41598-021-82212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‑8. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Remy KE, Mazer M, Striker DA, Ellebedy AH, Walton AH, Unsinger J. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrianopoulos I, Papathanasiou A, Papathanakos G, Chaidos A, Koulouras V. Tocilizumab’s efficacy in patients with Coronavirus Disease 2019 (COVID-19) is determined by the presence of cytokine storm. J Med Virol [Internet] 2020 doi: 10.1002/jmv.26209. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7361556/ [cited on Dec 9, 2020]; Available on: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.