Abstract
Objectives
To alleviate the overflow of coronavirus disease 2019 (COVID-19) patients in hospitals, less invasive and simple criteria are required to triage the patients. We evaluated the relationship between COVID-19 severity and fatty liver on plain computed tomography (CT) scan performed on admission.
Methods
In this retrospective cohort study, we considered all COVID-19 patients at a large tertiary care hospital between January 31 and August 31, 2020. COVID-19 severity was categorized into severe (moderate and severe) and non-severe (asymptomatic and mild) groups, based on the Japanese National COVID-19 guidelines. Fatty liver was detected on plain CT scan. Multivariate logistic regression analysis was performed to evaluate factors associated with severe COVID-19.
Results
Of 222 patients (median age: 52 years), 3.2%, 58.1%, 20.7%, and 18.0% presented with asymptomatic, mild, moderate, and severe COVID-19, respectively. Although 59.9% had no fatty liver on plain CT, mild, moderate, and severe fatty liver occurred in 13.1%, 18.9%, and 8.1%, respectively. Age and presence of fatty liver were significantly associated with severe COVID-19.
Conclusion
Our study showed that fatty liver on plain CT scan on admission can become a risk factor for severe COVID-19. This finding may help clinicians to easily triage COVID-19 patients.
Keywords: COVID-19, Pneumonia, Triage, Outcome
1. Introduction
Since its emergence in December 2019, coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected approximately 200 million people worldwide, with 4.2 million deaths as of July 21, 2021 [1,2]. COVID-19 is characterized by a unique appearance of pneumonia on computed tomography (CT) scan, with a spectrum of clinical severity [3]. While some COVID-19 patients are asymptomatic with no apparent appearance of pneumonia on CT scan, it could be fatal in others, requiring both hemodynamic and respiratory support.
Many studies have attempted to elucidate the risk factors for COVID-19 severity and fatality. In many case series, older patients [4], patients with hypertension, diabetes, and cardiovascular diseases [3] were more likely to develop acute respiratory distress syndrome (ARDS) and receive mechanical ventilation. One theory for the mechanism of its severe presentation is hyperinflammation and cytokine storm induced by SARS-CoV-2. In a study involving 1,484 patients in New York, high serum interleukin-6 (IL-6), IL-8, and tumor necrosis factor (TNF)-α levels at the time of hospitalization were strong predictors of patient survival, suggesting that hyperinflammatory response is a major cause of disease severity and death [5].
Interestingly, accumulating evidence has shown that obesity and metabolic-dysfunction-associated fatty liver disease (MAFLD), which are closely related to comorbidities, such as hypertension and diabetes, are other risk factors for severe COVID-19 [6,7]. This is plausible considering numerous reports indicating adipose tissue as a key player in immune function [8]. Adipocytes and triglycerides accumulation in the liver trigger the production of inflammatory cytokines. In fact, there have been several studies that reported the relationship between COVID-19 and fatty liver on CT scan [[9], [10], [11]]. For example, two studies have shown that low Hounsfield units (HU) of the liver on CT scan was significantly associated with severe COVID-19 [9,10]. However, none of these previous studies provided practical suggestion for the quick triage of COVID-19 patients by using CT scan in real life situations.
Even without the concomitant metabolic disorders or histopathological diagnosis by liver biopsy, fatty liver can be diagnosed on plain CT scan [12,13], which is one of the common radiological examinations required for patients with COVID-19 when they are admitted to the hospital. In this study, we hypothesized that a more robust classification of fatty liver on plain CT scan, instead of meticulously calculating liver HU, on admission may be able to predict severe COVID-19. We therefore evaluated the relationship between the clinical outcome of COVID-19 and fatty liver on plain CT scan.
2. Materials and methods
2.1. Ethics
This study was approved by the ethics committee of the National Center for Global Health and Medicine (NCGM) (approval no: NCGM-G-003665-00) and was implemented in accordance with the Declaration of Helsinki. Patient data were anonymized prior to the analysis. Due to the retrospective nature of the study, the requirement of patient consent was waived.
2.2. Study design and participants
This retrospective cohort study of all COVID-19 patients was conducted between January 31 and August 31, 2020, at the NCGM, Tokyo, Japan. NCGM has approximately 780 inpatient beds and serves as one of the four designated medical institutions for specific infectious diseases such as Ebola virus disease in Japan. All COVID-19 patients were diagnosed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) for SARS-CoV-2 using nasopharyngeal swabs, according to the recommended protocol by the Japanese National Institute of Infectious Disease [14]. Enrolled patients were over 20 years of age, and they underwent plain CT scan on admission.
2.3. Data collection
All COVID-19 patients’ data were extracted from the COVID-19 REGISTRY JAPAN (COVIREGI-JP) of the NCGM with permission. The study data were collected and managed using the Research Electronic Data Capture (REDCap), a secure, web-based data capture application hosted at the Japan Clinical Research Assist Center data center of the NCGM [15]. The parameters retrieved from the database included the following: (i) demographics including age, sex, height, weight, smoking status, drinking habit, and comorbidities; (ii) number of days from symptom onset to hospital admission and number of days from symptom onset to CT scan; (iii) clinical symptoms; (iv) laboratory findings; (v) radiological findings on CT scan including fatty liver on CT scan [10,13], and COVID-19 severity score [16,17]; (vi) COVID-19 severity (asymptomatic/mild or moderate/severe); and (vii) clinical outcomes (in-hospital mortality and total length-of-hospital stay).
2.4. Plain CT scan acquisition and interpretation
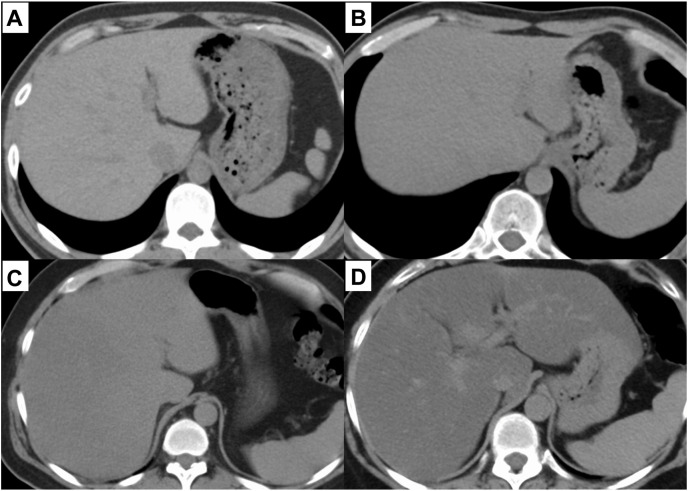
Plain CT examinations were performed from the chest to the upper abdomen, using one of two multidetector CT (MDCT) systems: a 320-MDCT system (Aquilion ONE, Canon Medical Systems) or a 128-dual-source MDCT system (SOMATOM Definition Flash, Siemens Healthcare) without administration of contrast medium. The section thickness was 5 mm, and the scanning parameters were 120 kVp, with automatically set mAs values. In the visual analysis, liver steatosis was evaluated as follows: (i) no fatty liver, hepatic vessels show lower attenuation than that for the liver parenchyma; (ii) mild, hepatic vessels show lower attenuation than that for the liver parenchyma, but the contours are blurred; (iii) moderate, hepatic vessels show the same attenuation as that for the liver parenchyma; and (iv) severe, hepatic vessels show higher attenuation than that for the liver parenchyma (Fig. 1 ) [12,13]. In the quantitative analysis, HUs of the liver parenchyma were calculated using a 1 cm2-circle region of interest. In addition, CT severity score, which represents the severity of COVID-19 pneumonia on CT scan, was calculated by a radiologist based on the extent of the lobar involvement (0:0%; 1: <5%; 2:5–25%; 3:26–50%; 4:51–75%; 5, >75%; range 0–5; total score 0–25) [16,17].
Fig. 1.
Fatty liver of each severity diagnosed by plane CT scan
Fig.1 shows the representative plain CT scan images of fatty liver for each severity category: (a) normal, (b) mild fatty liver, (c) moderate fatty liver, and (d) severe fatty liver.
2.5. Definitions of the variables
Based on the Japanese National COVID-19 guidelines [18], we divided the COVID-19 patients based on the four severity categories: (i) asymptomatic; (ii) mild (patients with symptoms but do not require oxygen therapy); (iii) moderate (patients require oxygen therapy either via nasal canula or by facial mask); and (iv) severe (patients require either high flow nasal canula, noninvasive ventilation, or tracheal intubation). The moderate and severe COVID-19 groups were further grouped into the severe COVID-19 group, while the asymptomatic and mild groups were the non-severe COVID-19 group. Mild to severe fatty liver on CT scan was defined as fatty liver on CT scan [12,13]. In-hospital mortality was defined as death occurring during the hospital stay.
2.6. Statistical analysis
Continuous variables are shown as median and interquartile range (IQR) and compared using the Mann-Whitney U test. Categorical variables are shown as absolute and relative frequencies and compared using the χ2 test or Fisher's exact test. Using logistic regression and univariate analyses reported as odds ratios (OR) and 95% confidence intervals (CI), the association between COVID-19 severity (non-severe and severe groups) and background factors including fatty liver on CT were evaluated. Multivariate logistic regression analysis was performed to calculate the adjusted odds ratio of fatty liver on CT scan for severe COVID-19. We considered potential risk factors with a p-value less than 0.05 in the univariate analysis, or a priori variables hypothesized to be clinically or epidemiologically important. Statistical significance was defined as a two-sided p-value of <0.05, and all statistical analyses were performed with R software version 3.4.0.
3. Results
3.1. Description of COVID-19 patients during the study period
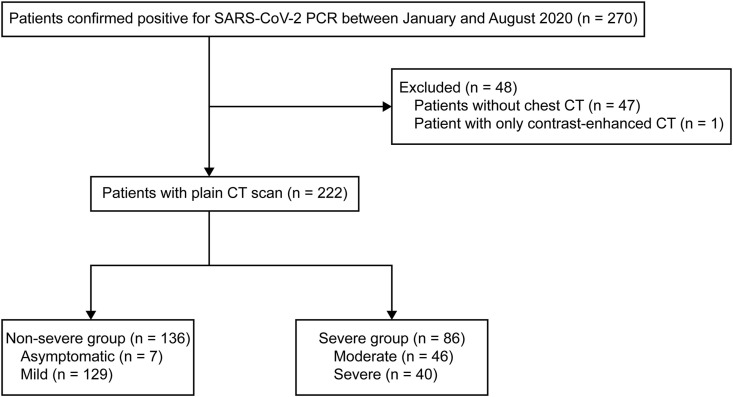
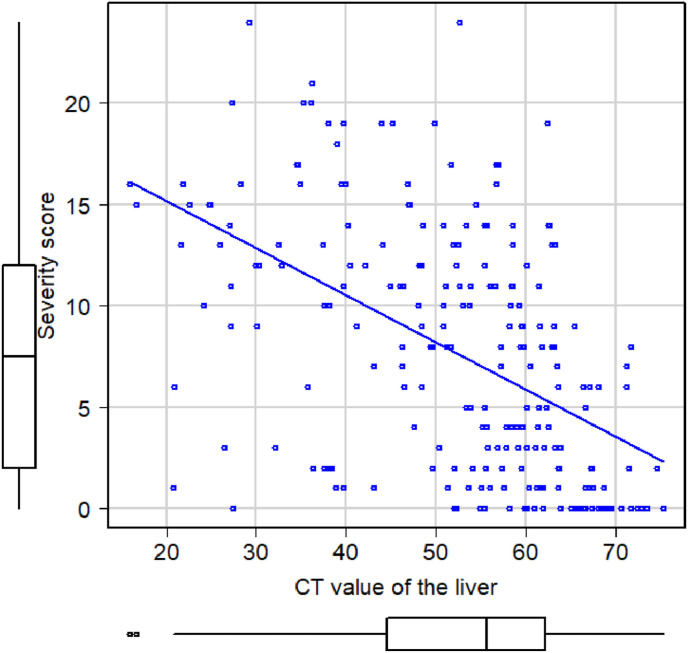
During the study period, 270 patients with laboratory-detected SARS-CoV-2 infection were hospitalized at the NCGM, Japan. We excluded 48 patients for having no plain CT scan: (i) no CT scan (n = 47) and (ii) only contrast-enhanced CT scan (n = 1). The remaining 222 patients were included in the final analysis (Fig. 2 ). The median age of this cohort was 52 (IQR 38.0–67.8) years, and 144 (65.2%) were males. In total, 108 patients (48.6%) had a history of smoking, and 44 patients (19.8%) had regular drinking habits. Major comorbidities were hypertension (n = 59, 26.6%), followed by diabetes (n = 38, 17.1%) and respiratory disorders (n = 26, 11.7%). The median durations from symptom onset to hospital admission and to undergoing plain CT scan were 6 (IQR 4–9) and 6 (IQR 4–8) days, respectively. The COVID-19 severity score among study population was significantly associated with lower prevalence of liver attenuation, with a dose-response relationship (r = −0.51; 95% CI = −0.60 to −0.41; p < 0.001) (Fig. 3 ). Although 133 patients (59.9%) had no fatty liver on plain CT, the proportions of those with mild, moderate, and severe fatty liver were 29 (13.1%), 42 (18.9%), and 18 (8.1%), respectively. In terms of COVID-19 severity, 7 patients (3.2%) were asymptomatic while 129 (58.1%), 46 (20.7%), and 40 (18.0%) had mild, moderate, and severe disease, respectively (Table 1 ). Table 2 shows the number of patients in each COVID-19 severity group stratified by the severity of fatty liver on CT scan. There were no patients with moderate or severe fatty liver among the asymptomatic COVID-19 patients.
Fig. 2.
Flow diagram of study enrollment.
Fig. 3.
The relationship the COVID-19 severity score and attenuation of the liver COVID-19 severity score among the study population was significantly associated with lower attenuation of the liver with a dose-response relationship (r = −0.51; 95% CI = −0.60 to −0.41; p < 0.001).
Table 1.
Baseline characteristics of COVID-19 patients (n=222).
| All patients (n = 222) | |
|---|---|
| Age, years | 52.00 (38.00–67.75) |
| Male | 144 (64.86) |
| Body mass index, kg/m2 | 23.73 (20.73–26.77) |
| Smoking | 108 (48.65) |
| Alcohol | 44 (19.82) |
| Comorbidities | |
| Hypertension | 59 (26.58) |
| Diabetes | 38 (17.12) |
| Respiratory disorders | 26 (11.71) |
| Cardiovascular diseases | 17 (7.68) |
| Malignancy | 7 (3) |
| Chronic renal failure | 4 (1.80) |
| Days from symptom onseta | |
| Days from onset to hospital admission | 6 (4–9) |
| Days from onset to CT scan | 6 (4–8) |
| Severity of fatty liver on CT scan | |
| No fatty liver | 133 (59.91) |
| Mild | 29 (13.06) |
| Moderate | 42 (18.92) |
| Severe | 18 (8.11) |
| Attenuation of the liver, HU | 55.65 (44.70–62.15) |
| COVID-19 severity | |
| Asymptomatic | 7 (3.15) |
| Mild | 129 (58.11) |
| Moderate | 46 (20.72) |
| Severe | 40 (18.02) |
Unless otherwise stated, data are presented as n (%).
Continuous variable data are presented as median (interquartile range).
COVID-19; coronavirus disease 2019, CT; computerized tomography, HU; Hounsfield unit.
Asymptomatic patients were excluded.
Table 2.
COVID-19 severity of the patients stratified by the severity of fatty liver on CT scan (n = 222).
| Severity of COVID-19 |
|||||
|---|---|---|---|---|---|
| Non-severe group |
Severe group |
||||
| Asymptomatic n = 7, 3.15% | Mild n = 129, 58.11% | Moderate n = 46, 20.72% | Severe n = 40, 18.02% | ||
| Severity of fatty liver on CT scan | No fatty liver n = 133, 59.91% | 6 (85.71) | 98 (75.97) | 17 (36.96) | 12 (30.00) |
| Mild n = 29, 13.06% | 1 (14.29) | 9 (6.98) | 8 (17.39) | 11 (27.50) | |
| Moderate n = 42, 18.92% | 0 (0.00) | 17 (13.18) | 14 (30.43) | 11 (27.50) | |
| Severe n = 18, 8.11% | 0 (0.00) | 5 (3.88) | 7 (15.22) | 6 (15.00) | |
Data are presented as n (%).
COVID-19; Coronavirus disease 2019, CT; computerized tomography.
3.2. Risk factors for severe COVID-19
In the univariate analysis, age (median 64.5 [IQR 47.5–74.0] vs. 46 [IQR 30.8–58.2] years; p < 0.001), male sex (OR = 2.23; 95% CI = 1.18–4.29; p = 0.009); higher body mass index (BMI) (median 25.0 [IQR 22.1–27.6] vs 22.9 [IQR 20.1–25.8] kg/m2; p < 0.001); and history of smoking (OR = 1.86; 95% CI = 1.04–3.35; p = 0.028) were significantly associated with severe (moderate and severe), compared to non-severe (asymptomatic and mild) COVID-19. Hypertension (OR = 5.31; 95% CI = 2.69–10.79; p < 0.001), diabetes (OR = 6.03; 95% CI = 2.63–14.88; p < 0.001), and cardiovascular disease (OR = 4.22; 95% CI = 1.32–15.90; p = 0.0081) were significantly associated with the severe COVID-19, although the prevalence of cardiovascular disease in the overall cohort was only 7.7%. Compared to the non-severe group, the severe group had a significantly longer duration from the onset of their symptoms to hospital admission and to undergoing plain CT scan (median 7 [IQR 5–9] vs. 5 [IQR 3–8] days; p = 0.015) and (median 7 [IQR 5–9] vs. 6 [IQR 3–8]; p = 0.039) days, respectively. Radiological findings on CT showed that the severe group had significantly higher COVID-19 severity score (median 13 [IQR 9–16] vs. 3 [IQR 1–8]; p < 0.001); higher prevalence of fatty liver (OR = 6.33; 95% CI = 3.37–12.14; p < 0.001); and lower prevalence of liver attenuation (median 47.4 [IQR 36.1–55.6] vs 59.8 [IQR 52.1–65.6]; p < 0.001). On the other hand, drinking habit was not significantly associated with COVID-19 severity (p = 0.73) (Table 3 ). Multivariate analysis showed that age (OR = 1.05; 95% CI = 1.02–1.07; p < 0.001) and fatty liver on CT scan (OR = 6.20; 95% CI = 2.82–13.62; p < 0.001) were significantly associated with severe COVID-19 (Table 4 ).
Table 3.
Comparison of the clinical factors between non-severe (asymptomatic/mild) and severe (moderate/severe) COVID-19 patient groups (n=222).
| Non-severe group n = 136, 61.26% | Severe group n = 86, 38.74% | OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Age, years | 46.0 (30.75–58.25) | 64.5 (47.50–74.00) | < 0.001 | ||
| Male | 79 (58.09) | 65 (75.58) | 2.23 | 1.18–4.29 | 0.0093 |
| Body mass index, kg/m2 | 22.86 (20.14–25.75) | 24.95 (22.15–27.62) | < 0.001 | ||
| Smoking | 58 (42.65) | 50 (58.14) | 1.86 | 1.04–3.35 | 0.028 |
| Alcohol | 26 (19.12) | 18 (20.93) | 1.12 | 0.54–2.30 | 0.73 |
| Comorbidities | |||||
| Hypertension | 19 (13.97) | 40 (46.51) | 5.31 | 2.69–10.79 | < 0.001 |
| Diabetes | 10 (7.35) | 28 (32.56) | 6.03 | 2.63–14.88 | < 0.001 |
| Respiratory disorders | 16 (11.76) | 10 (11.63) | 0.98 | 0.38–2.45 | 1.00 |
| Cardiovascular diseases | 5 (3.68) | 12 (13.95) | 4.22 | 1.32–15.90 | 0.0081 |
| Malignancy | 5 (3.68) | 2 (2.33) | 0.62 | 0.058–3.92 | 0.71 |
| Chronic renal failure | 2 (1.47) | 2 (2.33) | 1.59 | 0.11–22.35 | 0.64 |
| Days from symptoms onseta | |||||
| Days from onset to hospital admission | 5 (3–8) | 7 (5–9) | 0.015 | ||
| Days from onset to CT scan | 6 (3–8) | 7 (5–9) | 0.039 | ||
| Signs and Symptoms | |||||
| Fever | 81 (59.56) | 72 (83.72) | 3.98 | 1.91–8.83 | < 0.001 |
| Cough | 77 (56.62) | 56 (65.12) | 1.62 | 0.88–3.06 | 0.11 |
| Dyspnea | 32 (23.53) | 49 (57.98) | 5.08 | 2.62–10.1 | < 0.001 |
| Dysosmia | 29 (21.32) | 7 (8.14) | 0.42 | 0.14–1.10 | 0.068 |
| Radiological findings on CT scan | |||||
| COVID-19 severity score | 3 (1–8) | 13 (9–16) | < 0.001 | ||
| Fatty liver on CT scan | 32 (23.53) | 57 (66.28) | 6.33 | 3.37–12.14 | < 0.001 |
| Attenuation of the liver, HU | 59.75 (52.08–65.58) | 47.35 (36.13–55.63) | < 0.001 | ||
| Laboratory findings on admission | |||||
| AST, U/L | 26 (20–36) | 38 (27–71) | < 0.001 | ||
| ALT, U/L | 23 (15–36) | 33 (21–55) | < 0.001 | ||
| LDH, U/L | 200 (172–247) | 345 (251–442) | < 0.001 | ||
| C-reactive protein, mg/dL | 0.93 (0.22–3.5) | 8.42 (4.6–13) | < 0.001 | ||
| White blood cell count, × 103/μL |
4.5 (3.7–5.6) | 5.2 (4.3–7.2) | < 0.001 | ||
| Lymphocyte count, % | 26 (19–33) | 16 (9.3–23) | < 0.001 | ||
| Platelet count, × 103/μL | 195 (162–247) | 179 (144–259) | 0.30 | ||
| D-dimer, μg/mL | 0 (0–550) | 600 (0–1600) | < 0.001 | ||
| Outcome | |||||
| In-hospital mortality | 0 (0) | 7 (8.14) | < 0.001 | ||
| Total length of hospital stay, days | 10 (8–13) | 18 (11–30) | < 0.001 | ||
Unless otherwise stated, data are presented as n (%).
Continuous variable data are presented as median (IQR).
COVID-19; coronavirus disease 2019, OR; odds ratio, CI; confidence interval, CT; computerized tomography, HU; Hounsfield unit; AST; aspartate aminotransferase, ALT; alanine aminotransferase, LDH; lactate dehydrogenase, IQR; interquartile range.
Asymptomatic patients (n = 7) were excluded.
Table 4.
Risk factors for severe COVID-19 patients by multivariate analysis (n = 222).
| OR | 95% CI | p-value | |
|---|---|---|---|
| Age, years | 1.05 | 1.02–1.07 | <0.001 |
| Male | 1.50 | 0.65–3.46 | 0.34 |
| Body mass index, kg/m2 | 1.00 | 0.92–1.09 | 0.99 |
| Smoking | 1.70 | 0.82–3.50 | 0.15 |
| Hypertension | 1.56 | 0.66–3.72 | 0.32 |
| Diabetes | 2.06 | 0.78–5.41 | 0.14 |
| Fatty liver on CT scan | 6.20 | 2.82–13.62 | <0.001 |
COVID-19; coronavirus disease 2019, OR; odds ratio, CI; confidence interval, CT; computed tomography.
Overall, there was no in-hospital death among the non-severe group, while seven (8.1%) died during hospitalization among the severe group, and the severe group had significantly longer total length-of-hospital days (median 10 [IQR 8–13] vs. 18 [IQR 11–30] days; p < 0.001) (Table 3). Among the seven patients who died, three patients had no fatty liver, one patient had mild and moderate fatty liver, respectively, and two patients had severe fatty liver.
4. Discussion
Our study showed that fatty liver on CT scan performed on admission, along with age, was a risk factor for severe (moderate and severe) COVID-19 in patients that require oxygen therapy. Some studies have reported that older patients are at a high risk of severe COVID-19 [4,17]. Our study included fatty liver on CT scan, on admission, as a risk factor for severe COVID-19. Higher BMI values, hypertension prevalence, and diabetes prevalence were significantly associated with severe COVID-19 in univariate analysis in this study, but this association was lost in the multivariate analysis. In previous studies, obesity and MAFLD, which are closely related to comorbidities such as hypertension and diabetes, were reported as risk factors of severe COVID-19 [6,19]. Moreover, several studies have reported the relationship between low HU of the liver and the severity of COVID-19 [9,10]. However, these studies had several limitations. For example, the number of patients included in one study from Japan was only 35 [9]. In addition, this study did not adjust for other confounding factors such as hypertension and BMI. The other study required calculation of liver attenuation index, the subtraction of HU of the liver from that of the spleen, to assess the relationship between fatty liver and COVID-19 severity [10]. Compared with these previous studies, our study with a larger sample size (n = 222) showed that the simple criteria of fatty liver on CT, with no calculation required, remained associated with severe COVID-19 after adjusting for several confounding factors.
One hypothesis behind the association of fatty liver with COVID-19 pathophysiology is that inflammatory cytokine production triggered by adipose tissue or triglyceride accumulation in the liver exacerbates clinical severity. Dysfunctional hypertrophic adipocytes produce an excessive number of cytokines such as IL-6, IL-8, leptin, and plasminogen activator inhibitor-1 (PAI-1), which leads to increased recruitment of macrophages [8]. These macrophages, in turn, produce high amounts of proinflammatory molecules such as IL-1, IL-6, IL-8, and TNF-α. Kupffer cells, hepatic stellate cells, and sinusoidal endothelial cells also contribute to producing the proinflammatory status [20]. The presence of fatty liver might aggravate COVID-19 severity by producing this hyperinflammatory status.
In our study cohort, the overall in-hospital case fatality rate was 3.2% (7/222), with all deaths (8.1% [7/86]) occurring in the severe group. This is relatively higher than the case fatality rate of 1.5% (2,944/200,658) among the overall COVID-19 patients in Japan [21]. Because our hospital is one of the four designated hospitals for infectious diseases in Japan, we admitted several patients with severe COVID-19 during the earlier stages, when the treatment strategies were not well established globally. Moreover, the overall Japanese data included asymptomatic and mild COVID-19 patients who did not require hospitalization. This might have led to the relatively high case fatality rate in our study.
Although several studies have reported the association between MAFLD and severe COVID-19 [19], diagnosis of MAFLD requires that metabolic disorders, such as diabetes and plasma cholesterol levels, be diagnosed, in addition to the presence of fatty liver on imaging. Other risk factors of severe COVID-19, such as cytokines or biological markers [5], also require specific assays that are unavailable in most facilities worldwide. In contrast, our findings are less invasive and simple, and require nothing other than plain CT scan on admission, which is universally performed in most symptomatic COVID-19 patients. Among our severe groups (n = 86), 29 patients did not have fatty liver, and three patients without fatty liver died. Therefore, we concluded that it is difficult to establish a perfect, safe, and simple criteria. Recently, in many countries, medical staff and facilities are overwhelmed with the overflow of patients [22]. Thus, availability of a quick and simple criteria to identify those who are at a low risk of severe COVID-19 is imperative; such patients can then be followed up at the facilities for patients with less severe COVID-19 instead of hospitals equipped with ICU for severe patients. Our finding may be practical in identifying those patients and can aid in decision-making in clinical settings. In fact, in our cohort, there was no death reported in the non-severe group, which leads to the conclusion that our findings might be reliable in identifying patients who can be safely followed-up outside the hospitals.
This study has several limitations. First, the sample size of our cohort was relatively small. This might have led to overestimate the effect of fatty liver among other factors. Also, there might be some unmeasured confounding factors, such as time-dependent variables, due to the retrospective nature of the study. Because the COVIREGI-JP database used in this study did not include cholesterol and triglycerides levels on admission, we were also not able to include dyslipidemia as one of the confounding factors, which is another probable cause of fatty liver. We consider this as one of potential biases in this study. Second, we did not perform liver biopsy when diagnosing fatty liver. However, liver biopsy is highly invasive and impractical in terms of infection prevention and control. In addition, it was already demonstrated that CT scan reflects the histological diagnosis of fatty liver [13]. Therefore, we assume that fatty liver can be safely diagnosed on plain CT scan. Third, our study did not directly prove the impact of fatty liver on COVID-19 pathophysiology. Although we hypothesized that fatty liver aggravates the host immune response by producing inflammatory cytokines, we did not measure the cytokine levels in our patients. Lastly, our study did not consider the causality of COVID-19 inducing fatty liver. Several studies have shown that COVID-19 itself disrupts the mitochondrial activity, resulting in lipid accumulation in the liver [23]. Because our study focused on the CT scan on admission, it is not clear whether the fatty liver seen on admission is a pre-existing exacerbating factor or a result of the infection. However, as the aim of this study is simply to assess the relationship between fatty liver on admission and the outcome of COVID-19 regardless of the causality, we did not take that point into consideration.
In conclusion, our study showed that fatty liver on CT scan on admission may be one of the risk factors of being in the severe COVID-19 group of people that require oxygen therapy. With this ongoing COVID-19 pandemic, we need less invasive and simple criteria for a quick decision-making in managing the patients and in efficiently utilizing our limited medical resources. We believe that the presence of fatty liver on CT scan on admission may help the clinicians with decision-making. Further studies, adjusted for the treatment strategies, and with a larger number of patients, are required.
Authorship statement
MI was responsible for the organization and coordination of the study. AO was the chief investigator and responsible for the data analysis and writing the original manuscript. MH is also responsible for data analysis and reviewing the manuscript. AK, YM, and MT were responsible for data collection and reviewing the final manuscript. GY provided assistance in data analysis and reviewing the study design and the final manuscript. KK, MI, LS, MS, YA, TS, TN, HN, SI, KN, SS, NK, KY, SM, MU, KH, SK, YS, TT, KT, YF, MY, SI, MH, HS, and NO were all responsible for reviewing the study design and the manuscript. NO is also responsible for financial support. All authors contributed to the writing of the final manuscript. All members contributed to the management or administration of the trial.
Funding
This research was supported by the National Center for Global Health and Medicine (NCGM) Intramural Research Fund (grant number: 20A05).
Declaration of competing interest
All authors declare no conflicts of interest from this study.
CT; computed tomography.
Acknowledgement
We thank all the clinical staff at our hospital for their dedication to patients' care, and all the technical staff at COVID-19 REGISTRY JAPAN (COVIREGI-JP). Writing assistance for the manuscript was provided by Editage (www.editage.com).
References
- 1.WHO Coronavirus disease (COVID-19) dashboard. https://covid19.who.int/ accessed.
- 2.Hayakawa K., Kutsuna S., Kawamata T., Sugiki Y., Nonaka C., Tanaka K., et al. SARS-CoV-2 infection among returnees on charter flights to Japan from Hubei, China: a report from National Center for Global Health and Medicine. Glob Health Med. 2020;2:107–111. doi: 10.35772/ghm.2020.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahamid M., Nseir W., Khoury T., Mahamid B., Nubania A., Sub-Laban K., et al. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/meg.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida Y., Uemura H., Yamaba S., Hamada D., Tarumoto N., Maesaki S., et al. Significance of liver dysfunction associated with decreased hepatic CT attenuation values in Japanese patients with severe COVID-19. J Gastroenterol. 2020;55:1098–1106. doi: 10.1007/s00535-020-01717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parlak S., Çıvgın E., Beşler M.S., Kayıpmaz A.E. The effect of hepatic steatosis on COVID-19 severity: chest computed tomography findings. Saudi J Gastroenterol. 2021;27:105–110. doi: 10.4103/sjg.sjg_540_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medeiros A.K., Barbisan C.C., Cruz I.R., de Araújo E.M., Libânio B.B., et al. Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom Radiol (NY) 2020;45:2748–2754. doi: 10.1007/s00261-020-02648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S.W., Park S.H., Kim K.W., Choi E.K., Shin Y.M., Kim P.N., et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–485. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 13.Haberal K.M., Turnaoğlu H., Haberal Reyhan A.N. Is unenhanced computed tomography reliable in the assessment of macrovesicular steatosis in living liver donors? Exp Clin Transplant. 2019;17:749–752. doi: 10.6002/ect.2019.0326. [DOI] [PubMed] [Google Scholar]
- 14.Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., et al. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis. 2020;73:304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes at chest ct during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francone M., Iafrate F., Masci G.M., Coco S., Cilia F., Manganaro L., et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health, Labour and Welfare Japan COVID-19 management guideline. 2020. https://www.mhlw.go.jp/content/000712473.pdf
- 19.Gao F., Zheng K.I., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilan Y. Review article: novel methods for the treatment of non-alcoholic steatohepatitis - targeting the gut immune system to decrease the systemic inflammatory response without immune suppression. Aliment Pharmacol Ther. 2016;44:1168–1182. doi: 10.1111/apt.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins University of Medicine Johns. COVID-19 mortality analysis. 2021. https://coronavirus.jhu.edu/data/mortality
- 22.Matsuo T., Kobayashi D., Taki F., Sakamoto F., Uehara Y., Mori N., et al. Prevalence of health care worker burnout during the coronavirus disease 2019 (COVID-19) pandemic in Japan. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardo A.D., Schneeweiss-Gleixner M., Bakail M., Dixon E.D., Lax S.F., Trauner M., et al. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]