Abstract
Introduction
Focal brain stimulation has potential as a treatment for posttraumatic stress disorder (PTSD). In this review, we aim to inform selection of focal brain stimulation targets for treating PTSD by examining studies of the functional neuroanatomy of PTSD and treatment response. We first briefly review data on brain stimulation interventions for PTSD. Although published data suggest good efficacy overall, the neurobiological rationale for each stimulation target is not always clear.
Methods
Therefore, we assess pre- and post-treatment (predominantly psychotherapy) functional neuroimaging studies in PTSD to determine which brain changes seem critical to treatment response. Results of these studies are presented within a previously proposed functional neural systems model of PTSD.
Results
While not completely consistent, research suggests that down-regulating the fear learning and threat and salience detection circuits (i.e., amygdala, dorsal anterior cingulate cortex and insula) and upregulating the emotion regulation and executive function and contextual processing circuits (i.e., prefrontal cortical regions and hippocampus) may mediate PTSD treatment response.
Conclusion
This literature review provides some justification for current focal brain stimulation targets. However, the examination of treatment effects on neural networks is limited, and studies that include the stimulation targets are lacking. Further, additional targets, such as the cingulate, medial prefrontal cortex, and inferior parietal lobe, may also be worth investigation, especially when considering how to achieve network level changes. Additional research combining PTSD treatment with functional neuroimaging will help move the field forward by identifying and validating novel targets, providing better rationale for specific treatment parameters and personalizing treatment for PTSD.
Keywords: Posttraumatic Stress Disorder (PTSD), functional Magnetic Resonance Imaging (fMRI), functional connectivity (FC), Treatment, Neuromodulation, Predictors
1. Introduction
Posttraumatic stress disorder (PTSD) is a serious psychiatric condition characterized by re-experiencing, avoidance, negative changes in thoughts and mood, and hyperarousal following exposure to a traumatic event (Association, 2013). Current clinical practice guidelines for PTSD recommend trauma-focused psychotherapy (TFP) as the first-line treatment for PTSD (for a review, see Hamblen et al., 2019). If TFP is not available or not preferred, non-trauma-focused psychotherapies or medications, such as specific selective serotonin reuptake inhibitors (SSRI’s), are then recommended (Hamblen et al., 2019). Although these treatments are beneficial for many patients, a large number of patients have an inadequate response (Jonas et al., 2013) or do not prefer conventional treatment, and additional treatment options for PTSD are needed (Fonzo et al., 2020; Sippel et al., 2018).
Focal brain stimulation has potential as a stand-alone or adjunctive treatment for individuals with PTSD. Focal brain stimulation applies energy to a specific brain region to alter activity of that region, as well as a network of brain areas connected to the target of stimulation (Deng et al., 2015). To advance the understanding of focal brain stimulation as an intervention for PTSD, it is necessary to know which neural circuits are central to the pathophysiology of the disorder, how brain regions are functionally interconnected, and how stimulation of specific nodes within these neural networks mediate recovery.
We review the evidence for focal brain stimulation modalities and discuss how each might be used or further developed as a treatment for PTSD. Then we review functional neuroimaging findings that have direct relevance to PTSD treatment response and non-response within a circuit-based neurobiological model with the goal of identifying “key nodes” for focal brain stimulation. After integrating these literatures, we discuss new target brain regions and best approaches to reach these regions with focal brain stimulation. Finally, we discuss limitations of the extant literature and suggest future directions for clinical application and research advancement of focal brain stimulation for PTSD.
2. Focal brain stimulation
Focal brain stimulation can be used to target precise brain regions within discrete neural networks. Several focal brain stimulation techniques are in varying stages of investigation and clinical use for PTSD. Most research is focused on three techniques, which differ in terms of their ability to deliver focal stimulation and their invasiveness. Deep brain stimulation is the most focal technique, but is also most invasive in that it requires neurological surgery. On the other side of the spectrum is transcranial direct current stimulation (tDCS), which is non-focal, but also noninvasive and easily applied. Transcranial magnetic stimulation (TMS) is more focal than tDCS, but less focal than DBS, and is also a noninvasive treatment that is easy to safely apply. Prior reviews have summarized the evidence for these in the treatment of PTSD (Gouveia et al., 2020; Kan et al., 2020). Below we briefly review key findings for TMS, tDCS, and DBS for PTSD. Other brain stimulation techniques include electroconvulsive therapy (ECT), which triggers a brief seizure, and different forms of vagus nerve stimulation (VNS) to indirectly stimulate neural targets via the vagal nerve, but their implications for PTSD have been reviewed elsewhere (Koek et al., 2019).
2.1. Transcranial magnetic stimulation
TMS is a noninvasive technique that uses a rapidly changing magnetic field, delivered at the scalp surface, to induce an electric current in the underlying cerebral cortex. TMS can only target a few centimeters below the scalp. The prefrontal cortex (PFC) is typically stimulated PTSD (Gouveia et al., 2020; Kan et al., 2020), and TMS is postulated to indirectly reach deeper brain regions that are functionally connected with the stimulated prefrontal area. TMS depolarizes cortical neurons and can have inhibitory or excitatory effects depending on stimulation location and parameters.
Data suggest that TMS may have efficacy for PTSD as a monotherapy or adjunctive treatment (Kan et al., 2020). However, the small sample sizes and high degree of variability across treatment protocols limit conclusions regarding the optimal use of TMS to treat PTSD. Studies differ in terms of treatment target location (left dorsolateral PFC (DLPFC) vs. right DLPFC vs. medial PFC (mPFC)), stimulation frequency (low vs. high vs. theta burst), stimulation intensity (ranging from 80% to 120% of the motor threshold), and dose (the number of TMS pulses delivered within a treatment session and across a treatment series). It is not clear which combination of treatment location and parameters are most likely to be efficacious for PTSD (Gouveia et al., 2020; Karsen et al., 2014).
2.2. Transcranial direct current stimulation
tDCS is a noninvasive technique that applies a low intensity electrical current to the brain via an anode and cathode. This approach is relatively nonfocal and does not directly depolarize neurons like TMS, but may alter the likelihood that groups of neurons will activate with subsequent provocation. tDCS for PTSD has been less well studied than TMS, but results are encouraging. Overall, clinical improvement has been demonstrated despite a large range in stimulation parameters and anatomical target (DLPFC vs ventromedial PFC vs right lateral temporal cortex), as well as the patient population (Gouveia et al., 2020).
2.3. Deep Brain Stimulation
DBS is an invasive technique involving neurosurgical placement of stimulation electrodes within the brain, with delivery of electrical stimulation to a specific deep brain region. DBS has the highest degree of focality, and can target essentially any brain region with great precision. DBS is an established intervention for patients with medication refractory movement disorders, such as Parkinson’s Disease and essential tremor. DBS is also approved by the Food and Drug Administration (FDA), under a Humanitarian Device Exemption, for the treatment of treatment-resistant obsessive compulsive disorder (OCD). DBS has been shown to have efficacy in treatment-resistant depression (Dandekar et al., 2018).
To date, there are no published randomized controlled trials (RCTs) of DBS in the treatment of PTSD. A preclinical study in an animal model of PTSD found that focal stimulation of the basolateral amygdala reduced fear and anxiety-like behavior in rats (Reznikov et al., 2018). A pilot study of DBS of the basolateral amygdala is currently underway, and notable benefit was seen in the first patient enrolled in this study (Koek et al., 2014; Langevin et al., 2016). Furthermore, a recent case report showed complete recovery from PTSD following DBS to the medial PFC and uncinate fasciculus, a white matter tract connecting the amygdala and hippocampus with frontal regions (Hamani et al., 2020).
2.4. Limitations of focal brain stimulation research in PTSD to date
Although the studies reviewed above suggest potential for focal brain stimulation as a treatment for PTSD, the results are clearly not definitive. One challenge in translating this work to clinical application is that the optimal targets for intervention have not been firmly established, and multiple targets using different treatment parameters have shown efficacy. Yet, efficacy rates could be significantly enhanced, presumably by improving target location. The targets that are currently being used are not always hypothesis-driven. A few reviews have been published which suggested targeting the fear neurocircuitry based on its overlap with PTSD symptoms (Marin et al., 2014) and reviewed different neuromodulation targets implicated in PTSD (Koek et al., 2019). Neither of these reviews used pre/post-treatment neuroimaging studies in PTSD to inform targets, which could help us understand the mechanisms of successful treatment and identify brain targets related to treatment non-response. Here propose that better understanding of the functionality of the neural circuitry of PTSD that mediates recovery may clarify which targets are the most promising for neuromodulation.
3. Functional brain circuits in PTSD treatment response
In this section, we review neuroimaging studies comparing PTSD treatment responders and non-responders or showing an association between brain measures and treatment outcome. Electroencephalogram/event-related potential studies were not included because the poor spatial resolution of these methods limits the contribution of these studies to our main goal of defining focal neurostimulation targets. The data are discussed within an existing model of the functional neural systems involved in the pathophysiology of PTSD, i.e., threat and salience detection, fear learning, executive function and emotion regulation, and contextual processing circuits (Shalev et al., 2017). While other models for the neurobiology of PTSD have been published (e.g. (Fenster et al., 2018; Williams, 2017) we chose the Shalev framework for its parsimony and direct relevance to treatment mechanisms. Details on these neuroimaging study results, including specific information on fMRI tasks, are provided in Table 1. More details on sample characteristics, treatment type and study design are provided in Supplementary Table 1. There is substantial heterogeneity in sample size and types of trauma, but most studies collected fMRI, used regions of interest (ROIs) for their analyses, and examined outcomes of TFP.
Table 1.
Neuroimaging predictors for PTSD treatment non-response and post-treatment changes
| Brain Region | Hemisphere | Predictors non-response | Post-treatment changes | Task/Stimuli Numbers indicate different contrasts being used |
Study | Notes | |
|---|---|---|---|---|---|---|---|
| Threat and Salience Circuit | Amygdala | Bilateral | Greater | - | Fearful faces (backward masking) | Bryant (2007) | |
| Bilateral | Greater | - | Fearful and neutral facial expressions | Cisler (2015) | |||
| Bilateral | Greater | Decrease in non-responders | Negative vs neutral trauma-unrelated pictures | van Rooij (2016a) | |||
| Left | Greater | - | Fearful vs neutral faces | Fonzo (2017) | Median split based on brain activation for treatment and waitlist group separately; Opposite pattern in waitlist; No effect in reapprasal task, in contrast to Bryant et al 2020. | ||
| - | - | 1. Greater increase = less reduction hyperarousal symptoms 2. Smaller increase = less reduction reexperience and avoidance symptoms |
1. BLA-vmPFC RSFC 2. CeA-OFC RSFC |
Zhu (2018) | Also described in mPFC section | ||
| 1. Left 2. Left |
1. Greater | 2. Greater change = less symptom reduction | 1. Amygdala - Superior Parietal Cortex FC during modulation by appraisal 2. DLPFC-Amygdala FC during modulation by appraisal |
Duval (2020) | |||
| - | Greater (trend-level) | - | Amygdala - PCC RSFC | Sheynin (2020) | Only low-responders showed a RSFC larger than zero. No post-treatment difference between low- and high responders | ||
| 1. Left 2. Bilateral |
1. Greater 2. Lower |
- | 1. Cognitive reappraisal of traumatic images 2. Emotional reactivity to traumatic images |
Bryant (2020a) | Correlation analyses performed for association with treatment response. | ||
| dACC | - | - | Smaller decrease, less symptom reduction | Emotional stroop, negative vs neutral words | Thomaes (2012) | ||
| Right | 1. Lower 2. Greater |
1. Increase in all participants (12/14 treatment responders) | 1. Anticipation of negative vs positive images 2. Negative vs positive trauma-unrelated pictures |
Aupperle (2013) | Opposite effect depending on contrast used | ||
| Bilateral | Greater | - | Negative vs neutral trauma-unrelated pictures | van Rooij (2016) | |||
| Bilateral | Greater | - | Negative vs neutral trauma-unrelated pictures | Kennis (2017) | |||
| - | 1. Lower 2. Lower |
- | 1. Fearful vs neutral faces 2. Emotional conflict regulation |
Fonzo (2017) | Median split based on brain activation for treatment and waitlist group separately Opposite pattern in waitlist |
||
| Insula | Right | - | Decrease in all participants (12/14 treatment responders) | Anticipation of negative vs positive images | Aupperle (2013) | Opposite effect depending on contrast used | |
| Left | - | 1. Decrease in responders 2. Increase in non-responders |
1. Anticipation of negative pictures 2. Anticipation of positive pictures |
Simmons (2013) | |||
| Bilateral | Greater | - | Negative vs neutral trauma-unrelated pictures | van Rooij (2016a) | |||
| Left | Lower | - | Fearful vs neutral faces | Fonzo (2017) | Median split based on brain activation for treatment and waitlist group separately Opposite pattern in waitlist |
||
| Right | Lower | - | Emotion modulation by appraisal (Shifted Attention Emotion Appraisal Task; SEAT) | Duval (2020) | Combined patterns of activation in 7 ROIs (bilateral dlPFC, bilateral amygdala, bilateral anterior insula, and mPFC) at pretreatment accounted for 29.7% of the variance in change in total CAPS scores; Significant for avoidance subscale symptoms; Pre-post-treatment decrease in insula activation was not associated with symptom change, and may indicate habituation or practice effect | ||
| Left | 1. Lower 2. Greater |
- | 1. Sad versus neutral faces for overall PTSD symptoms Sad or fearful (vs baseline) for PTSD fear symptoms 2. insula - pgACC FC during sad vs neutral faces for overall PTSD, fear and dysphoris symptoms |
Bryant (2020b) | Left insula activation to sad faces was strongest predictor, specifically for fear symptoms | ||
| Salience network/ventral attention network | - | Lower | - | Within VAN resting state functional connectivity in combination with impaired memory | Etkin (2019) | VAN RSFC was predictive in combination with impaired cognitive task performance on a verbal memory word list learning task | |
| - | Greater | - | FC during emotional reactivity between left amygdala to sgACC, pgACC, right Insula | Bryant (2020a) | Correlation analyses performed for association with treatment response. | ||
| PCC | Right Left |
1. Lower 2. Greater |
- | 1. Anticipation of negative vs positive images 2. Negative vs positive trauma-unrelated pictures |
Aupperle (2013) | Treatment response as continuous variable; Opposite effect depending on contrast used | |
|
| |||||||
| Executive Function and Emotion Regulation Circuit | mPFC | Left | - | Smaller decrease = less reduction | 99mTc-HMPAO SPECT, script-driven imagery | Seedat (2004) | mPFC is left paracingulate region |
| - | Greater | - | Interaction of successful memory and emotional expression (faces) | Dickie (2011) | Region within mPFC is sgACC | ||
| Right | - | Decrease rACC in all PTSD patients Smaller decrease sgACC = smaller symptom reduction |
Extinction recall | Helpman (2016) | PTSD patients showed decrease in rACC activation; Correlation between decrease in CAPS score and right sgACC | ||
| - | Lower | - | Emotional conflict regulation | Fonzo (2017) | Region within mPFC is vmPFC | ||
| Lower | - | Emotion modulation by appraisal (Shifted Attention Emotion Appraisal Task; SEAT) | Duval (2020) | see note Duval et al above | |||
| Greater | Emotion regulation (reappraise vs maintain) | Joshi (2020) | |||||
| - | - | 1. Greater increase = less reduction hyperarousal symptoms 2. Smaller increase = less reduction reexperience and avoidance symptoms |
1. BLA-vmPFC RSFC 2. CeA-OFC RSFC |
Zhu (2018) | Also described in amygdala section | ||
| DLPFC | - | - | Decrease in all participants (12/14 treatment responders) | Negative vs positive images | Aupperle (2013) | Findings interpreted as related to treatment response because 12/14 responders | |
| bilateral | Lower | - | Fearful vs neutral faces | Fonzo (2017) | Median split based on brain activation for treatment and waitlist group separately Opposite pattern in waitlist Use of TMS in subset of patients |
||
| 1. Left 2. Right 3. Right 4. Left |
1. Greater 2. Lower (trend-level) |
3. Smaller change = less reduction 4. Greater change = less reduction |
1. Emotion modulation by appraisal (Shifted Attention Emotion Appraisal Task; SEAT) 2/3. DLPFC - superior parietal cortex FC during attention modulation 4. DLPFC-left amygdala FC during modulation by appraisal |
Duval (2020) | see note Duval et al above | ||
| VLPFC | - | Greater | - | Fearful faces (backward masking) | Bryant (2007) | VLPFC described as ventral ACC | |
| right | Greater | - | Emotion Regulation | MacNamara (2015) | region: VLPFC/IFG | ||
| Executive control network | - | Lower | - | RSFC, within ECN connectivity | Zilcha-Mano (2020) | ||
| Frontostriatal network | - | Lower | - | Response inhibition | Falconer (2013) | ||
| Frontopolar network | - | Greater | - | RSFC, group difference observed in right superior frontal gyrus | Zhutovsky (2019) | Data-driven approach: Group-level resting state networks were determined based on rs-fMRI data; Univariate analysis to test group differences | |
| Pre-Supplementary Motor Area network | - | Greater | - | RSFC, individual prediction based on clusters in left inferior temporal gyrus, left superior frontal gyrus and right precentral gyrus. | Zhutovsky (2019) | ||
|
| |||||||
| Contextual Processing Circuit | Hippocampus | Right | Lower | - | Succesful memory encoding | Dickie (2011) | Precise nature of treatment not characterized |
| Left | - | Smaller decrease = less reduction | Extinction recall | Helpman (2016) | |||
| Lower CBF in responders post-treatment | 99mTc-HMPAO SPECT, script-driven imagery | Pagani (2007) | SPECT study | ||||
| Bilateral | 1. Lower 2a. Greater 2b. Lower |
- | 1. Emotional reactivity task 2. FC during emotional reactivity between a. left hippocampus and right amygdala b. right hippocampus and left amygdala |
Bryant (2020a) | Correlation analyses performed for association with treatment response. | ||
| Thalamus | - | - | - | - | - | ||
| Locus Ceruleus | - | - | - | - | - | ||
| IPL | Right | - | Increase in all participants | Negative vs positive images | Aupperle (2013) | Opposite effect depending on contrast used | |
| Left | Lower | - | Context processing during response inhibition | van Rooij (2015a) | |||
| Precuneus | - | - | Smaller decrease = less reduction | Amplitude of low-frequence fluctuation (ALFF) during resting state | Zhu (2014) | ||
| - | - | Precuneus, superior temporal area, insula, dmPFC, frontal ortibal cortex, supplementary motor area, lingual gyrus and cerebellum predict outcome | Amplitude of low-frequence fluctuation (ALFF) during resting state | Yuan (2018) | Regions represent the “most informative voxeLs for outcome prediction”. Accuracy of 72.5% | ||
Studies of both psychotherapy and medication are included. For each study, the treatment-related brain findings are organized within Shalev et al.’s (2017) model of the pathophysiology of PTSD. The bolded brain regions are part of one of the circuits as proposed by Shalev et al. and are discussed within this circuit regardless of fMRI task used to prope this response. For findings in brain regions not part of a circuit (e.g., the inferior parietal lobe, IPL), findings are discussed in the circuit that most closely matches the brain region’s function or the task used to probe this response. Finally, for brain regions that are part of multiple circuits (i.e., the amygdala and mPFC), findings are discussed in only one circuit to present results most parsimoniously.
3.1. Threat and salience detection circuit
The threat and salience detection circuit includes the amygdala, dorsal anterior cingulate cortex (dACC) and insula, which are functionally connected in the salience network (SN) (Shalev et al., 2017). This circuit is involved in detecting and orienting attention to salient stimuli. Specifically, the amygdala is the key region for emotional responses (particularly fear), the dACC is implicated in directing attention, and the insula is important for interoceptive awareness and subjective emotional experience (Shalev et al., 2017). PTSD patients have been found to exhibit vigilance to threat and amplified emotional and neural responses to valenced stimuli, which is reflected by heightened activation in response to emotional stimuli and increased functional connectivity (FC) of the nodes of the salience network (e.g., (Aupperle et al., 2012; Etkin & Wager, 2007; Rabinak et al., 2011; Rougemont-Bücking et al., 2011; van Rooij et al., 2014).
3.1.1. Amygdala
Decreased response to TFP has consistently been associated with greater pre-treatment amygdala activation during a range of emotion and fear-based tasks (Bryant et al., 2020a; Bryant et al., 2007; Cisler et al., 2015; Fonzo et al., 2017; van Rooij et al., 2016a) (Table 1). One study demonstrated decreased pre-treatment amygdala reactivity in relation to treatment non-response during cognitive reappraisal (Bryant et al., 2020a), but this was not seen in another study (Fonzo et al., 2018). Increased pre-treatment FC between the amygdala and superior parietal cortex (Duval et al., 2020) and posterior cingulate cortex (PCC) (Sheynin et al., 2020) has been related to non-response to Prolonged Exposure (PE). In another study, TFP non-responders exhibited a pre- to post-treatment decrease in amygdala activation to trauma-unrelated negative vs. neutral pictures (van Rooij et al., 2016a); this inconsistent finding could reflect a habituation effect at the level of the amygdala as suggested by the authors.
3.1.2. dACC
Decreased response to TFP has been associated with both higher and lower pre-treatment dACC activation. This is likely explained by the type of tasks that were used across studies. Lower pre-treatment dACC activation during emotion regulation or anticipation tasks (Aupperle et al., 2013; Fonzo et al., 2017), but higher pre-treatment dACC activation during passive viewing of trauma-unrelated negative emotional images (Aupperle et al., 2013; Kennis et al., 2017; van Rooij et al., 2016a) were related to treatment non-response. Furthermore, studies assessing pre- to post-treatment dACC changes observed both an increase in PTSD responders (Aupperle et al., 2013), as well as a smaller decrease in non-responders compared to responders (Thomaes et al., 2012).
The PCC is not formally part of Shalev’s model, but findings related to the PCC are discussed in this section because it has structural and FC with other critical regions including the dACC. The PCC is implicated in awareness and episodic memory retrieval. One study showed that treatment non-response was related to lower pre-treatment PCC activation during emotional anticipation, but higher PCC activation during passive viewing of emotional images (Aupperle et al., 2013).
3.1.3. Insula
Lower pre-treatment insula activation during emotion processing has been related to TFP non-response (Bryant et al., 2020b; Duval et al., 2020; Fonzo et al., 2017), though one study observed higher pre-treatment insula activation during emotion processing in non-responders (van Rooij et al., 2016a). Higher pre-treatment FC between the insula and the pregenual ACC during emotion processing was also associated with treatment non-response (Bryant et al., 2020b). When comparing pre- to post-treatment assessments, treatment responders showed a decrease in insula activation (Aupperle et al., 2013; Simmons et al., 2013), whereas treatment non-responders showed an increase in insula activation (Simmons et al., 2013).
3.1.4. Network-based predictors
Greater FC between nodes of the SN, specifically the left amygdala with the sgACC, pgACC and insula, was related to treatment non-response (Bryant et al., 2020a). Lower FC within the ventral attention network (VAN, consisting of the insula, dACC, anterior middle frontal gyrus and supramarginal gyrus) was related to treatment non-response in patients with impaired verbal memory, but not in patients with intact verbal memory (Etkin et al., 2019). A recent study, however, did not identify a subgroup of patients with combined memory impairment and lower VAN FC (Esterman et al., 2020).
3.2. Fear learning circuit
The fear learning circuit in Shalev’s model consists of different nuclei of the amygdala, which is the location for formation of fear-related memory. Neuroimaging studies have demonstrated an overactive amygdala in response to conditioned fear stimuli such as trauma-related and threat stimuli (e.g., (Rauch et al., 2000; Shin et al., 2004) and during rest (Koch et al., 2016) among patients with PTSD. Treatment studies targeting the amygdala did not assess fear learning and findings were therefore discussed above within the threat and salience detection circuit. Other studies using fear conditioning and extinction paradigms that observed findings related to the PFC and hippocampus are discussed in the executive function and emotion regulation and contextual processing circuits respectively.
3.3. Executive function and emotion regulation circuit
The executive function and emotion regulation circuit consists of the medial, dorsolateral and ventrolateral prefrontal cortices (PFC). This circuit is important for top-down regulation of emotions by inhibiting the amygdala. This allows for flexibility in emotional responding, which is necessary for controlling or inhibiting the fear response in safe circumstances. The dorsolateral PFC (DLPFC) is a key region for executive functioning, including working memory, cognitive flexibility and emotion regulation. The medial PFC (mPFC) is more specifically associated with regulation of fear responses, whereas the ventrolateral PFC (vlPFC) has mostly been associated with motor inhibition. Reduced prefrontal control over fear is considered a key deficit in PTSD, and neuroimaging studies have demonstrated reduced prefrontal activation and decreased PFC-amygdala connectivity (e.g., (Garfinkel et al., 2014; Milad et al., 2009; Stevens et al., 2013).
3.3.1. Dorsolateral prefrontal cortex
Greater pre-treatment left DLPFC activation during emotion modulation and a pre- to post-treatment increase in DLPFC - left amygdala FC was related to TFP non-response (Duval et al., 2020). Another study showed that lower pre-treatment bilateral DLPFC activation during passive emotional processing was related to treatment non-response, and enhancing DLPFC control over the amygdala using transcranial magnetic stimulation (TMS) to the right DLPFC improved treatment response (Fonzo et al., 2017). A pre- to post-treatment reduction in DLPFC activation was observed in treatment responders (Aupperle et al., 2013).
3.3.2. Medial prefrontal cortex
Both lesser and greater pre-treatment mPFC activation have been associated with treatment non-response and no clear pattern has emerged. Lower pre-treatment activation during emotional conflict regulation (Fonzo et al., 2017) and emotion modulation (Duval et al., 2020), but greater pre-treatment mPFC activation during the interaction of successful memory and emotional expression (Dickie et al., 2011) and emotion regulation (Joshi et al., 2020), were related to treatment non-response. Studies assessing pre- to post-treatment changes in mPFC activation showed that a smaller decrease was related to non-response to PE (Helpman et al., 2016) or 8 weeks of citalopram (Seedat et al., 2004). A greater increase from pre- to post-treatment in FC between the amygdala and the vmPFC (Zhu et al., 2018) was related to poorer treatment outcome, specifically for hyperarousal symptoms (Zhu et al., 2018). In contrast, a smaller increase in FC between the amygdala and orbitofrontal cortex (OFC) was related to poorer treatment outcome, specifically reexperiencing and avoidance symptoms (Zhu et al., 2018).
3.3.3. Ventrolateral prefrontal cortex
Greater pre-treatment VLPFC activation was associated with non-response to TFP (Bryant et al., 2007) and SSRI treatment (MacNamara et al., 2016).
3.3.4. Network approaches
Lower pre-treatment activation across a larger frontostriatal network (Falconer et al., 2013) as well as lower FC within the executive control network was related to non-response (Zilcha-Mano et al., 2020). A treatment study using a data-driven approach observed greater FC in the frontopolar network (specifically the right superior frontal gyrus) and the pre-supplementay motor area network (specifically left inferior temporal gyrus, left superior frontal gyrus and right precentral gyrus) in treatment non-responders (Zhutovsky et al., 2019).
3.4. Contextual processing circuit
The contextual processing circuit include the hippocampus, medial PFC (mPFC), thalamus, and locus coeruleus. The hippocampus is essential for contextual memory and the mPFC for the regulation of the fear response based on this contextual information. The thalamus relays sensory signals to the cerebral cortex and the locus coeruleus is involved in the physiological responses to stress. Appropriate processing of contextual information is essential for the regulation of fear in safe situations, and contextual processing is often impaired in PTSD (Liberzon & Abelson, 2016). Moreover, neuroimaging studies have demonstrated reduced hippocampal volume (Logue et al., 2018), as well as reduced hippocampal and vmPFC functioning and connectivity in PTSD (Garfinkel et al., 2014; Jovanovic et al., 2013; Sripada et al., 2012; van Rooij et al., 2018).
3.4.1. Hippocampus
Lower pre-treatment (right) hippocampal activation was related to treatment non-response in two studies (Bryant et al., 2020a; Dickie et al., 2011). Pre-treatment FC between the hippocampus and amygdala was linked to treatment outcome, but the effects were opposite for each hemisphere. Greater pre-treatment FC between the left hippocampus and right amygdala, but lower pre-treatment FC between right hippocampus and left amygdala were associated with treatment non-response (Bryant et al., 2020a). A pre- to post-treatment study showed a smaller reduction in left hippocampal activation in treatment non-responders (Helpman et al., 2016). Likewise, non-responders showed a higher hippocampal response to their trauma script post-treatment compared to responders (Pagani et al., 2007).
3.4.2. Left inferior parietal lobe
The inferior parietal lobe (IPL), though not part of Shalev’s model of PTSD, is involved in contextual cue processing and working memory updating, and is functionally connected with the default mode network (DMN), including the mPFC. Lower pre-treatment left IPL activation predicted non-response to TFP (van Rooij et al., 2015a). Correspondingly, an increase in right IPL activation from pre- to post-treatment was observed in treatment responders (Aupperle et al., 2013).
3.4.3. Precuneus
The precuneus, also not part of Shalev’s model, is a major component of the DMN, which is implicated in self-referential processing and episodic memory. A smaller decrease of precuneus amplitude of low-frequency fluctuation (ALFF) was related to poorer treatment response (Zhu et al., 2015), and the precuneus was one of the main regions that predicted treatment outcome in the second study (Yuan et al., 2018).
3.2. Neuroimaging studies on PTSD neuromodulation treatments
Recent studies have collected pre- and post-neuromodulation treatment neuroimaging data. Here we describe the findings in the direction of a positive treatment response instead of treatment non-response as in the previous section to facilitate identification of novel targets that could benefit from neuromodulation.
Philip et al (2018; 2019) performed two RCTs comparing active versus sham TMS. In their first study, 5Hz TMS to the left DLPFC was delivered to PTSD patients with comorbid depression. Resting state FC (RSFC) analyses showed that clinical improvement was predicted by positive amygdala-vmPFC connectivity and negative sgACC-default mode network (DMN) connectivity. Furthermore, post-TMS symptom reductions were associated with reduced sgACC-DMN, left DLPFC-insula, and hippocampus-SN connectivity (Philip et al., 2018). Their second study assessed PTSD-only patients and used theta burst stimulation to the right DLPFC. Better treatment outcome was predicted by greater positive within DMN connectivity (dmPFC-right temporoparietal junction (TPJ) and mPFC-left anterior temporal cortex) and greater negative cross-network connectivity (left DLPFC-TPJ and right lateral temporal cortex-left VLPFC/opercularis) (Noah S. Philip et al., 2019). These TMS studies similarly targeted the DLPFC and provide some initial evidence of network-level changes in TMS responders; however, general conclusions are again precluded by inconsistency across the treatment protocols.
A recent study assessed the effects of surgical removal of the right amygdala in epilepsy patients receiving standard care for their treatment-refractory epilepsy (Bijanki et al., 2020). Right amygdala ablation in two patients resulted in amelioration of PTSD, suggesting a critical role for the (right) amygdala in maintenance of PTSD symptoms. Furthermore, neuroimaging measures were collected before and after surgery in one patient, and clinical improvement was accompanied by a decrease in activation of the left amygdala, and an increase in vmPFC activation during a fearful faces task as well as improved fear learning in a fear conditioning startle paradigm (Bijanki et al., 2020).
3.3. Summary
The most consistent neural predictor of treatment non-response in PTSD is greater pre-treatment activation of the amygdala, which is key to the threat and salience and fear learning circuits. Moreover, the amygdala ablation and DBS studies support the critical role of amygdala hyperactivation in the maintenance of PTSD. The other regions of the threat and salience circuit have also been associated with treatment outcome. Most studies showed decreased insula activation at baseline as a predictor for TFP non-response. For the dACC, results were mixed depending on the type of task and stimuli presented: treatment non-response was predicted by lower pre-treatment dACC activation to fearful faces or emotional conflict regulation and anticipation of negative images, but also predicted by greater dACC activation for trauma-unrelated emotional processing. Resting state data showed greater within-SN connectivity as a predictor of treatment non-response, though lower VAN within network RSFC in combination with impaired memory was observed in treatment non-responders.
Results for the executive function and emotion regulation circuit are inconsistent. Greater mPFC, DLPFC or VLPFC activation and frontopolar network RSFC, but also lower mPFC and DLPFC activation, frontostriatal network activation and lower within ECN RSFC have been associated with poor treatment outcome. Increased inhibition of the amygdala by the DLPFC (as induced by TMS) improved PE treatment outcome, suggesting that activation of the DLPFC relative to the amygdala, rather than absolute activation patterns, may be most informative.
Finally, findings for the contextual processing circuit are more coherent. Lower hippocampal activation and lower right hippocampus-left amygdala task-based FC predicted treatment non-response. The IPL was implicated in two studies: one study showed reduced left IPL activation to be a predictor for TFP non-response, and one study showed an increase in right IPL activation among responders.
In sum, while not entirely consistent, extant research supports down-regulating overactivation of the threat and salience detection circuit and up-regulating the emotion regulation and executive function and contextual processing circuits to mediate PTSD recovery. PTSD patients who are not responsive to TFP may not be able to accurately assess the threat saliency of stimuli and not sufficiently orient attention to indicators of safety. In addition, they may fail to integrate new contextual information with already existing internal representations of threat, and/or may not be able to regulate emotion and behavior accordingly.
4. Evidence-based focal brain stimulation targets for PTSD
Here we evaluate brain stimulation targets for PTSD based on the prior discussion of the literature, consider the best stimulation technique to reach the targets, and discusss the challenges and limitations of each approach. Figure 1 summarizes existing and novel brain stimulation targets for PTSD and the suggested neurostimulation techniques for each target.
Figure 1. Novel brain stimulation targets for PTSD.
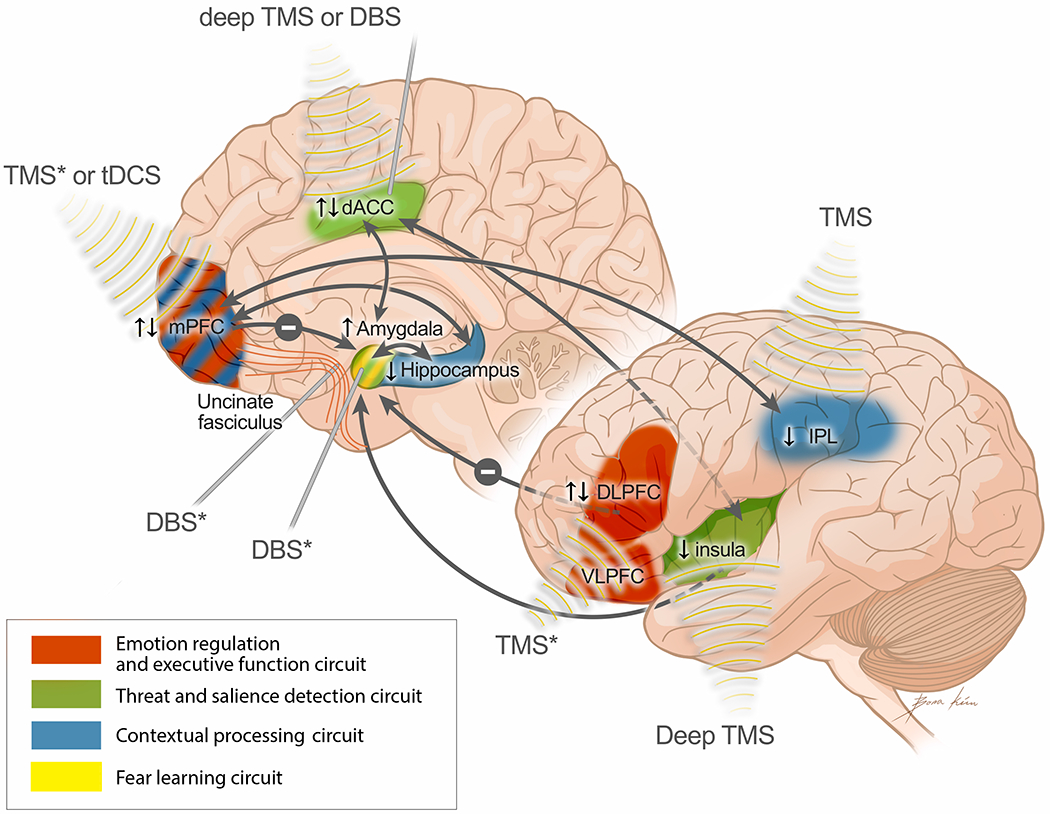
Figure 1 displays a model for novel brain stimulation targets for PTSD. The model is based on the PTSD pathophysiology model from Shalev et al. (2017), however, the regions that did not show a relation with treatment outcome as reviewed in this paper were excluded (i.e. LC and Thalamus). The IPL, which was found to be related to treatment outcome, was added to the model. Furthermore, connections between regions that are relevant to treatment outcome are now included in blue (i.e., PFC to amygdala, hippocampus to amygdala and IPL to mPFC). Finally, the orange text boxes and arrows display the brain stimulation approach that is suggested to target each brain region or connection. The asterisk (*) indicates that this specific method is under investigation. The arrows next to each brain region indicate direction of effect: ↑ indicates greater activation related to treatment non-response, ↓ indicates lower activation related to treatment non-response, ↑↓ indicates mixed (inconsistent) findings. TMS, transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; DBS, deep brain stimulation; mPFC, medial prefrontal cortex; dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; IPL, inferior parietal lobe.
Research showing that amygdala hyperactivation is associated with non-response to first-line PTSD treatments suggests that the amygdala is the most promising candidate for neuromodulation. However, an important challenge is that the amygdala cannot be reached with non-invasive stimulation, i.e., TMS or tDCS, because of its subcortical location. Two small studies directly targeting the amygdala with DBS (Langevin et al., 2016) and surgical ablation (Bijanki et al., 2020) both showed clinical improvement in PTSD symptoms. However, both techniques require surgical intervention with associated risks and restrictions, including eligibility for surgery. Therefore, neurostimulation studies have aimed to indirectly target the amygdala via its connections to prefrontal regions. The DLPFC is a popular target for TMS, and one TMS+fMRI study suggested increased TMS-induced prefrontal control over the amygdala as a potential mechanism of psychotherapy treatment response (Fonzo et al., 2018). This same study also demonstrated lower DLPFC activation as a predictor for TFP response, but this has been the only study to show this (and another study showed the opposite (Duval et al., 2020)). Greater VLPFC activation has been associated with treatment non-response in two studies; however, the function of the VLPFC in PTSD treatment is unclear as it has mostly been linked to non-emotional inhibition. Therefore, more neuroimaging treatment research focusing on the DLPFC, VLPFC and the executive function and emotion regulation circuit in general is needed.
Based on this review, some additional targets for brain stimulation could be considered. First, there is a clear role for the salience network in PTSD and PTSD treatment response. The dACC has been successfully targeted with deep TMS in OCD or addiction (Carmi et al., 2018; Martinez et al., 2018). As a different approach, previous cingulotomy and DBS for depression suggests a relatively safe profile and easier surgical accessibility for anterior cingulate targeting (Crowell et al., 2019; Steele et al., 2008). The insula could also be a noteworthy target with potential downstream effects on the amygdala, and might be reached with deep TMS (Malik et al., 2018).
Although results for the mPFC are mixed, the mPFC could be further explored as a candidate for neurostimulation. This frontal region has been associated with impaired fear inhibition, a hallmark feature of PTSD (Jovanovic et al., 2013) and shows impaired task-based FC with the amygdala in PTSD (Stevens et al., 2013). The mPFC can be targeted with TMS or tDCS. One study stimulating the mPFC with TMS has shown a reduction in intrusive symptoms in PTSD patients (Isserles et al., 2013). tDCS studies targeting the vmPFC have shown improved extinction learning in healthy controls (Dittert et al., 2018) and veterans with PTSD (van ‘t Wout et al., 2016) as well as improved extinction retention in veterans with PTSD (Van’t Wout et al., 2017). A pilot study applying tDCS to the vmPFC simultaneous with virtual reality exposure sessions showed a larger decrease in physiological arousal in active vs sham tDCS (van ‘t Wout-Frank et al., 2019). Together, these studies suggest the potential for clinical application of tDCS for PTSD by facilitating extinction.
Here we identified hippocampal activation as a rather consistent predictor of treatment non-response. The hippocampus has often and consistently been implicated in PTSD development both structurally (Gilbertson et al., 2002; Logue et al., 2018) and functionally (van Rooij et al., 2016b; van Rooij et al., 2018). Moreover, two structural MRI studies demonstrated that reduced hippocampal volume was a predictor for PTSD treatment non-response (Rubin et al., 2016; van Rooij et al., 2015b), further supporting the hippocampus as a potential target. However, because of its anatomical location, the hippocampus is difficult to stimulate. Targeting the mPFC, also part of the context processing circuit, could be an interesting approach to target both the context processing circuit and executive control and emotion regulation circuit. In support of this, the recent DBS study that targeted the mPFC and uncinate fasciculus (Hamani et al., 2020) yields great promise.
The IPL, involved in working memory updating and shown to mediate TFP response (van Rooij et al., 2015a), could be an compelling and feasible target for neurostimulation. Based on the finding that IPL is involved in context processing in treatment responders, this region could be a good candidate for stimulation prior to TFP to enhance the ability to process and integrate contextual safety information during exposure therapy. While this finding was observed in an unbiased whole brain analysis, replication is warranted.
Here we reviewed neuroimaging studies that predict treatment response as well as studies that show the effect of treatment on the brain. Both approaches contribute to the definition of potential targets of brain stimulation in different ways, and interestingly, most pre/post-treatment study designs allow for both types of analyses. Neuroimaging predictors of treatment response contribute to our understanding of the potential mechanism of TFT, for instance related to inability to extinguish trauma. They also contribute to the identification of likely non-responders and will be essential in advancing the personalized medicine approach. At the same time, changes in brain regions after successful treatment suggest these brain regions could be related to current PTSD symptoms, and modulation of these regions may mediate recovery, hence could be targets for neurostimulation. Furthermore, investigating the effects of treatment on the brain will elucidate mechanisms and time-course of recovery. This may be particularly relevant for neurostimulation treatments, which may not show immediate clinical improvements, but could show alterations in brain functioning that would either allow for a natural course of extinction or for individuals to successfully participate in TFT after neurostimulation.
5. Limitations and future directions
There are several limitations of the current state of research that restrict our ability to better define novel targets for neuromodulation at this moment. First, most studies have used ROI analyses; the predictors that were identified could reflect the bias in choice of these ROIs. Using hypothesis-free whole brain approaches would be less biased; yet, longitudinal treatment studies are time and resource intensive, explaining why most studies have relatively small sample sizes with limited power to identify predictors outside their ROIs using unbiased analyses. Cross-collaboration and imaging consortia could be helpful for overcoming the challenges of incorporating neuroimaging into longitudinal treatment studies.
Second, most studies have focused on single ROIs, but brain regions do not function independently; rather, they are part of larger networks. In this review, we discussed brain regions as part of circuits of an existing PTSD neurobiology model, and we suggest that the emphasis of future neuroimaging treatment studies should shift from single region to network analyses. Changing an individual region’s activity may not be sufficient to induce a therapeutic effect, and neuroimaging treatment studies should investigate network level activity and connectivity measures.
Third, there is also a clear need for more anatomical specificity or higher resolution images to define more specific focal brain stimulation targets. Most of the regions described in our review are rather sizeable and consist of several subregions with different functions. For example, the insula and dACC are very large regions, and many different anatomical definitions of the vmPFC exist. Moreover, rodent work has shown that even the smaller and more clearly defined subcortical regions such as the amygdala and hippocampus, are functionally and anatomically highly complex (e.g., (Ciocchi et al., 2010; Xu et al., 2016)). This is a particularly salient issue for the most focal brain stimulation technique, DBS, in which moving the target a few millimeters can impact treatment outcomes (Riva-Posse et al., 2018). But also TMS treatment efficacy could likely benefit from more precise treatment targets. Therefore, future studies should consider the use of high-resolution anatomical scans or tractography as well as performing more voxel-based functional analyses to improve specification of neurostimulation treatment targets.
Finally, there is a clear gap in knowledge of the exact mechanisms of different neurostimulation therapies, and we suggest that future neuromodulation studies collect pre- and post-treatment fMRI scans for three reasons. First, while, for example, DLPFC TMS has shown overall efficacy for PTSD, more information on mechanisms is needed to define optimal treatment parameters as these now vary widely between studies. Second, when mechanisms of different treatments are better understood, novel targets for neurostimulation therapy could be defined, either as a stand-alone treatment or in conjunction with TFP. Lastly, in line with the NIMH precision medicine initiative (Collins & Varmus, 2015), collecting pre- and post-treatment neuroimaging data could contribute to examining individual differences in treatment response and advance personalized medicine. Using pre-treatment MRI scans to individualize the target enhances precision and presumably improves treatment efficacy. While more research is needed to exactly understand the mechanisms of treatment response in order to optimize target selection, current research (in our group) is underway based on the findings of this review. Specifically, for our ongoing TMS treatment study, we use pre-TMS RSFC to define the area within the DLPFC that is most strongly connected with the amygdala and use neuronavigation for each individual to target this specific region (https://www.clinicaltrials.gov/ct2/show/NCT04563078). This approach would also eliminate interindividual structural differences related to age, sex, among other factors.
6. Conclusion
This review provides support for current focal brain stimulation targets and suggests novel candidates that could be stimulated using different intervention techniques. Neurostimulation can be used as a stand-alone treatment, or in conjunction with other treatments, such as psychotherapy and medication. This review focused on the role of neuroanatomic targets in non-response to TFT and provided evidence that appropriately activating or inhibiting neuroanatomic targets linked to PTSD could increase TFT efficacy.
The same underlying neuropathology that is associated with poor response to TF (e.g., over-engagement of threat and salience network, reduced context processing and emotion regulation) could also hamper a natural course of extinction of learned fear, the mechanism which serves as the basis for TFT. In support of this hypothesis, the regions implicated in PTSD treatment response generally overlap with those implicated in PTSD, and neurobiological profiles of treatment responders are comparable to trauma controls prior to treatment as shown in several studies (van Rooij et al., 2015b; van Rooij et al., 2016a). While some PTSD patients may have a full resolution of symptoms from stand-alone neurostimulation treatments, patients who have an incomplete response to TFT may benefit from supplemental neurostimulation to engage targets and augment the therapeutic process. Furthermore, different neurostimulation targets have different effects on the underlying neuropathology and can personalize the therapeutic approach to individual patients.
Major limitations in our ability to define novel targets are the lack of consistent findings across conclusive neuroimaging psychotherapy studies and a very small number of studies assessing neural correlates of neurostimulation treatments. Additional targets may also be worth investigation, especially when aiming to achieve network level changes. Large neuroimaging treatment studies on TFP and neurostimulation treatments, focusing on network-level changes or predictors, are needed to advance the field by providing better rationale for specific treatment parameters, identifying novel targets, and personalizing treatment for PTSD.
Supplementary Material
Acknowledgments
Funding and disclosures:
The authors report no conflict of interest.
Dr. van Rooij reports funding from the National Institute of Mental Health (NIMH) K01MH121653 and the Brain and Behavior Research Foundation (NARSAD Young Investigator Grant). Dr. McDonald reports research support by the National Institute of Mental Health, the National Institute of Aging, Wounded Warrior Project Warrior Care Network, GA Department of Behavioral Health and Developmental Disabilities, and GA Department of Human Services/Division of Aging services. He is a consultant for Signant Health and receives support from the JB Fuqua Foundation. Dr. Holtzheimer receives consulting fees from Abbott, royalties from Oxford University Press and UpToDate, and research funding from the Brain Behavior Research Foundation, NIMH (MH059282, UG3AT009758, MH117813, MH120126), and the Department of Veterans Affairs (I01CX002088).
The views expressed are those of the authors and do not necessarily reflect the position of theU.S. Veterans Health Administration or the government of the United States.
Footnotes
Data availability statement:
This review does not include original data and only discusses previously published data in original research papers. These papers were obtained through online searches using Pubmed and Google Scholar.
References
- Association AP (2013). Diagnostic and statistical manual of mental disorders (5th ed.). : Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, & et al. (2012). Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Archives of General Psychiatry, 69(4), 360–371. doi: 10.1001/archgenpsychiatry.2011.1539 [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Simmons AN, Flagan T, Thorp SR, Norman SB, … Stein MB (2013). Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Research: Neuroimaging, 214(1), 48–55. doi: 10.1016/j.pscychresns.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Bijanki KR, van Rooij SJH, Ely TD, Stevens JS, Inman CS, Fasano RE, … Willie JT (2020). Case Series: Unilateral Amygdala Ablation Ameliorates Post-Traumatic Stress Disorder Symptoms and Biomarkers. Neurosurgery, 87(4), 796–802. doi: 10.1093/neuros/nyaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Erlinger M, Felmingham K, Klimova A, Williams LM, Malhi G, … Korgaonkar MS (2020a). Reappraisal-related neural predictors of treatment response to cognitive behavior therapy for post-traumatic stress disorder. Psychol Med, 1–11. doi: 10.1017/s0033291720001129 [DOI] [PubMed] [Google Scholar]
- Bryant RA, Erlinger M, Felmingham K, Malhi GS, O’Donnell ML, Williams LM, & Korgaonkar MS (2020b). Differential neural predictors of treatment response for fear and dysphoric features of posttraumatic stress disorder. Depress Anxiety. doi: 10.1002/da.23061 [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, & Williams L (2007). Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychological Medicine, 38(4), 555–561. doi: 10.1017/S0033291707002231 [DOI] [PubMed] [Google Scholar]
- Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, & Zangen A (2018). Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul, 11(1), 158–165. doi: 10.1016/j.brs.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, … Lüthi A (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature, 468(7321), 277–282. doi: 10.1038/nature09559 [DOI] [PubMed] [Google Scholar]
- Cisler JM, Sigel BA, Kramer TL, Smitherman S, Vanderzee K, Pemberton J, & Kilts CD (2015). Amygdala Response Predicts Trajectory of Symptom Reduction During Trauma-Focused Cognitive-Behavioral Therapy among Adolescent Girls with PTSD. Journal of Psychiatric Research, 71, 33–40. doi: 10.1016/j.jpsychires.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, & Varmus H (2015). A New Initiative on Precision Medicine. New England Journal of Medicine, 372(9), 793–795. doi: 10.1056/NEJMp1500523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell AL, Riva-Posse P, Holtzheimer PE, Garlow SJ, Kelley ME, Gross RE, … Mayberg HS (2019). Long-Term Outcomes of Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression. Am J Psychiatry, 176(11), 949–956. doi: 10.1176/appi.ajp.2019.18121427 [DOI] [PubMed] [Google Scholar]
- Dandekar MP, Fenoy AJ, Carvalho AF, Soares JC, & Quevedo J (2018). Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry, 23(5), 1094–1112. doi: 10.1038/mp.2018.2 [DOI] [PubMed] [Google Scholar]
- Deng Z-D, McClintock SM, Oey NE, Luber B, & Lisanby SH (2015). Neuromodulation for mood and memory: from the engineering bench to the patient bedside. Current Opinion in Neurobiology, 30, 38–43. doi: 10.1016/j.conb.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, & Armony JL (2011). Neural correlates of recovery from post-traumatic stress disorder: A longitudinal fMRI investigation of memory encoding. Neuropsychologia, 49(7), 1771–1778. doi: 10.1016/j.neuropsychologia.2011.02.055 [DOI] [PubMed] [Google Scholar]
- Dittert N, Hüttner S, Polak T, & Herrmann MJ (2018). Augmentation of Fear Extinction by Transcranial Direct Current Stimulation (tDCS). Frontiers in Behavioral Neuroscience, 12, 76–76. doi: 10.3389/fnbeh.2018.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval ER, Sheynin J, King AP, Phan KL, Simon NM, Martis B, … Rauch SAM (2020). Neural function during emotion processing and modulation associated with treatment response in a randomized clinical trial for posttraumatic stress disorder. Depress Anxiety, 37(7), 670–681. doi: 10.1002/da.23022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Stumps A, Jagger-Rickels A, Rothlein D, DeGutis J, Fortenbaugh F, … McGlinchey R (2020). Evaluating the evidence for a neuroimaging subtype of posttraumatic stress disorder. Sci Transl Med, 12(568). doi: 10.1126/scitranslmed.aaz9343 [DOI] [PubMed] [Google Scholar]
- Etkin A, Maron-Katz A, Wu W, Fonzo GA, Huemer J, Vértes PE, … O’Hara R (2019). Using fMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Sci Transl Med, 11(486). doi: 10.1126/scitranslmed.aal3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. The American journal of psychiatry, 164(10), 1476–1488. doi: 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E, Allen A, Felmingham K, Williams L, & Bryant R (2013). Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. J Clin Psychiatry, 74(9), 895–901. [DOI] [PubMed] [Google Scholar]
- Fenster RJ, Lebois LAM, Ressler KJ, & Suh J (2018). Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nature Reviews Neuroscience, 19(9), 535–551. doi: 10.1038/s41583-018-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G, Goodkind M, Oathes D, Zaiko Y, Harvey M, Peng K, … Etkin A (2017). PTSD Psychotherapy Outcome Predicted by Brain Activation During Emotional Reactivity and Regulation. Am J Psychiatry. doi:doi: 10.1176/appi.ajp.2017.16091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Federchenco V, & Lara A (2020). Predicting and Managing Treatment Non-response in Posttraumatic Stress Disorder. Current Treatment Options in Psychiatry, 7(2), 70–87. doi: 10.1007/s40501-020-00203-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, … Etkin A (2018). PTSD Psychotherapy Outcome Predicted by Brain Activation During Emotional Reactivity and Regulation. American Journal of Psychiatry, 0(0), appi.ajp.2017.16091072. doi: 10.1176/appi.ajp.2017.16091072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, & Liberzon I (2014). Impaired Contextual Modulation of Memories in PTSD: An fMRI and Psychophysiological Study of Extinction Retention and Fear Renewal. The Journal of Neuroscience, 34(40), 13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, & Pitman RK (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience, 5(11), 1242–1247. doi: 10.1038/nn958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia FV, Davidson B, Meng Y, Gidyk DC, Rabin JS, Ng E, … Hamani C (2020). Treating Post-traumatic Stress Disorder with Neuromodulation Therapies: Transcranial Magnetic Stimulation, Transcranial Direct Current Stimulation, and Deep Brain Stimulation. Neurotherapeutics. doi: 10.1007/s13311-020-00871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Davidson B, Levitt A, Meng Y, Corchs F, Abrahao A, … Lipsman N (2020). Patient With Posttraumatic Stress Disorder Successfully Treated With Deep Brain Stimulation of the Medial Prefrontal Cortex and Uncinate Fasciculus. Biol Psychiatry. doi: 10.1016/j.biopsych.2020.05.018 [DOI] [PubMed] [Google Scholar]
- Hamblen JL, Norman SB, Sonis JH, Phelps AJ, Bisson JI, Nunes VD, … Schnurr PP (2019). A guide to guidelines for the treatment of posttraumatic stress disorder in adults: An update. Psychotherapy, 56(3), 359–373. doi: 10.1037/pst0000231 [DOI] [PubMed] [Google Scholar]
- Helpman L, Marin MF, Papini S, Zhu X, Sullivan GM, Schneier F, … Neria Y (2016). Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study. NeuroImage: Clinical, 12, 715–723. doi: 10.1016/j.nicl.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, & Zangen A (2013). Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder--a pilot study. Brain Stimul, 6(3), 377–383. doi: 10.1016/j.brs.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Jonas D,E, Cusack K, Forneris CA, Wilkins TM, Sonis J, Middleton JC, … Gaynes BN (2013). Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder (PTSD) [PubMed] [Google Scholar]
- Joshi SA, Duval ER, Sheynin J, King AP, Phan KL, Martis B, … Rauch SAM (2020). Neural correlates of emotional reactivity and regulation associated with treatment response in a randomized clinical trial for posttraumatic stress disorder. Psychiatry Res Neuroimaging, 299, 111062. doi: 10.1016/j.pscychresns.2020.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, … Ressler KJ (2013). Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex, 49(7), 1884–1891. doi: 10.1016/j.cortex.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan RLD, Zhang BBB, Zhang JJQ, & Kranz GS (2020). Non-invasive brain stimulation for posttraumatic stress disorder: a systematic review and meta-analysis. Transl Psychiatry, 10(1), 168. doi: 10.1038/s41398-020-0851-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsen EF, Watts BV, & Holtzheimer PE (2014). Review of the Effectiveness of Transcranial Magnetic Stimulation for Post-traumatic Stress Disorder. Brain Stimulation, 7, 151–157. [DOI] [PubMed] [Google Scholar]
- Kennis M, van Rooij SJH, Reijnen A, & Geuze E (2017). The predictive value of dorsal cingulate activity and fractional anisotropy on long-term PTSD symptom severity. Depression and Anxiety, 34(5), 410–418. doi: 10.1002/da.22605 [DOI] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, & Olff M (2016). ABERRANT RESTING-STATE BRAIN ACTIVITY IN POSTTRAUMATIC STRESS DISORDER: A META-ANALYSIS AND SYSTEMATIC REVIEW. Depression and Anxiety, 33(7), 592–605. doi: 10.1002/da.22478 [DOI] [PubMed] [Google Scholar]
- Koek RJ, Langevin JP, Krahl SE, Kosoyan HJ, Schwartz HN, Chen JW, … Sultzer D (2014). Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD): study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials, 15, 356. doi: 10.1186/1745-6215-15-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek RJ, Roach J, Athanasiou N, van ’t Wout-Frank M, & Philip NS (2019). Neuromodulatory treatments for post-traumatic stress disorder (PTSD). Progress in Neuro-Psychopharmacology and Biological Psychiatry, 92, 148–160. doi: 10.1016/j.pnpbp.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Langevin JP, Chen JW, Koek RJ, Sultzer DL, Mandelkern MA, Schwartz HN, & Krahl SE (2016). Deep Brain Stimulation of the Basolateral Amygdala: Targeting Technique and Electrodiagnostic Findings. Brain Sci, 6(3). doi: 10.3390/brainsci6030028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, & Abelson James L. (2016). Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron, 92(1), 14–30. doi: 10.1016/j.neuron.2016.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, … Morey RA (2018). Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biological Psychiatry, 83(3), 244–253. doi: 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB, & Phan KL (2016). Emotion Regulatory Brain Function and SSRI Treatment in PTSD: Neural Correlates and Predictors of Change. Neuropsychopharmacology, 41(2), 611–618. doi: 10.1038/npp.2015.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Jacobs M, Cho SS, Boileau I, Blumberger D, Heilig M, … Le Foll B (2018). Deep TMS of the insula using the H-coil modulates dopamine release: a crossover [(11)C] PHNO-PET pilot trial in healthy humans. Brain Imaging Behav, 12(5), 1306–1317. doi: 10.1007/s11682-017-9800-1 [DOI] [PubMed] [Google Scholar]
- Marin MF, Camprodon JA, Dougherty DD, & Milad MR (2014). Device-based brain stimulation to augment fear extinction: implications for PTSD treatment and beyond. Depress Anxiety, 31(4), 269–278. doi: 10.1002/da.22252 [DOI] [PubMed] [Google Scholar]
- Martinez D, Urban N, Grassetti A, Chang D, Hu MC, Zangen A, … Nunes EV (2018). Transcranial Magnetic Stimulation of Medial Prefrontal and Cingulate Cortices Reduces Cocaine Self-Administration: A Pilot Study. Front Psychiatry, 9, 80. doi: 10.3389/fpsyt.2018.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … Rauch SL (2009). Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological Psychiatry, 66(12), 1075–1082. doi: 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip Noah S., Barredo Jennifer, Aiken Emily, Larson Victoria, Jones Richard N., Tracie Shea M, … van ‘t Wout-Frank Mascha (2019). Theta-Burst Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder. American Journal of Psychiatry, 176(11), 939–948. doi: 10.1176/appi.ajp.2019.18101160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Högberg G, Salmaso D, Nardo D, Sundin Ö, Jonsson C, … Hällström T (2007). Effects of EMDR psychotherapy on 99mTc-HMPAO distribution in occupation-related post-traumatic stress disorder. Nuclear Medicine Communications, 28(10), 757–765. doi: 10.1097/MNM.0b013e3282742035 [DOI] [PubMed] [Google Scholar]
- Philip NS, Barredo J, van ‘t Wout-Frank M, Tyrka AR, Price LH, & Carpenter LL (2018). Network Mechanisms of Clinical Response; Transcranial Magnetic Stimulation in Posttraumatic Stress Disorder and Major Depressive Disorder. Biological Psychiatry, 83(3), 263–272. doi: 10.1016/j.biopsych.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, & Phan KL (2011). Altered Amygdala Resting-State Functional Connectivity in Post-Traumatic Stress Disorder. Frontiers in Psychiatry, 2, 62. doi: 10.3389/fpsyt.2011.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, MacKlin ML, Lasko NB, … Pitman RK (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry, 47(9), 769–776. doi: 10.1016/S0006-3223(00)00828-3 [DOI] [PubMed] [Google Scholar]
- Reznikov R, Bambico FR, Diwan M, Raymond RJ, Nashed MG, Nobrega JN, & Hamani C (2018). Prefrontal Cortex Deep Brain Stimulation Improves Fear and Anxiety-Like Behavior and Reduces Basolateral Amygdala Activity in a Preclinical Model of Posttraumatic Stress Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 43(5), 1099–1106. doi: 10.1038/npp.2017.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, … Mayberg HS (2018). A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Molecular Psychiatry, 23(4), 843–849. doi: 10.1038/mp.2017.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, … Milad MR (2011). Altered Processing of Contextual Information during Fear Extinction in PTSD: An fMRI Study. CNS Neuroscience & Therapeutics, 17(4), 227–236. doi: 10.1111/j.1755-5949.2010.00152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M, Shvil E, Papini S, Chhetry BT, Helpman L, Markowitz JC, … Neria Y (2016). Greater Hippocampal Volume is Associated with PTSD Treatment Response. Psychiatry Research, 252, 36–39. doi: 10.1016/j.pscychresns.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedat S, Warwick J, van Heerden B, Hugo C, Zungu-Dirwayi N, Van Kradenburg J, & Stein DJ (2004). Single photon emission computed tomography in posttraumatic stress disorder before and after treatment with a selective serotonin reuptake inhibitor. Journal of Affective Disorders, 80(1), 45–53. doi: 10.1016/S0165-0327(03)00047-8 [DOI] [PubMed] [Google Scholar]
- Shalev A, Liberzon I, & Marmar C (2017). Post-Traumatic Stress Disorder. New England Journal of Medicine, 376(25), 2459–2469. doi: 10.1056/NEJMra1612499 [DOI] [PubMed] [Google Scholar]
- Sheynin J, Duval ER, King AP, Angstadt M, Phan KL, Simon NM, … Liberzon I (2020). Associations between resting-state functional connectivity and treatment response in a randomized clinical trial for posttraumatic stress disorder. Depress Anxiety. doi: 10.1002/da.23075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, & et al. (2004). Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female vietnam veterans with ptsd. Archives of General Psychiatry, 61(2), 168–176. doi: 10.1001/archpsyc.61.2.168 [DOI] [PubMed] [Google Scholar]
- Simmons AN, Norman SB, Spadoni AD, & Strigo IA (2013). Neurosubstrates of Remission following Prolonged Exposure Therapy in Veterans with Posttraumatic Stress Disorder. Psychotherapy and Psychosomatics, 82(6), 382–389. Retrieved from https://www.karger.com/DOI/10.1159/000348867 [DOI] [PubMed] [Google Scholar]
- Sippel LM, Holtzheimer PE, Friedman MJ, & Schnurr PP (2018). Defining Treatment-Resistant Posttraumatic Stress Disorder: A Framework for Future Research. Biol Psychiatry, 84(5), e37–e41. doi: 10.1016/j.biopsych.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, & Liberzon I (2012). Neural Dysregulation in Posttraumatic Stress Disorder: Evidence for Disrupted Equilibrium between Salience and Default Mode Brain Networks. Psychosomatic medicine, 74(9), 904–911. doi: 10.1097/PSY.0b013e318273bf33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD, Christmas D, Eljamel MS, & Matthews K (2008). Anterior cingulotomy for major depression: clinical outcome and relationship to lesion characteristics. Biol Psychiatry, 63(7), 670–677. doi: 10.1016/j.biopsych.2007.07.019 [DOI] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, & Ressler KJ (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research, 47(10), 1469–1478. doi: 10.1016/j.jpsychires.2013.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, Elzinga BM, van Balkom AJ, … Veltman DJ (2012). Treatment effects on insular and anterior cingulate cortex activation during classic and emotional Stroop interference in child abuse-related complex post-traumatic stress disorder. Psychological Medicine, 42(11), 2337–2349. doi: 10.1017/S0033291712000499 [DOI] [PubMed] [Google Scholar]
- van ’t Wout M, Mariano TY, Garnaat SL, Reddy MK, Rasmussen SA, & Greenberg BD (2016). Can Transcranial Direct Current Stimulation Augment Extinction of Conditioned Fear? Brain Stimul, 9(4), 529–536. doi: 10.1016/j.brs.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Wout-Frank M, Shea MT, Larson VC, Greenberg BD, & Philip NS (2019). Combined transcranial direct current stimulation with virtual reality exposure for posttraumatic stress disorder: Feasibility and pilot results. Brain Stimul, 12(1), 41–43. doi: 10.1016/j.brs.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Geuze E, Kennis M, Rademaker AR, & Vink M (2015a). Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology, 40(3), 667–675. doi: 10.1038/npp.2014.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Kennis M, Sjouwerman R, Van Den Heuvel MP, Kahn RS, & Geuze E (2015b). Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychological Medicine, 45(13), 2737–2746. doi: 10.1017/S0033291715000707 [DOI] [PubMed] [Google Scholar]
- van Rooij SJH, Kennis M, Vink M, & Geuze E (2016a). Predicting Treatment Outcome in PTSD: A Longitudinal Functional MRI Study on Trauma-Unrelated Emotional Processing. Neuropsychopharmacology, 41(4), 1156–1165. doi: 10.1038/npp.2015.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Rademaker AR, Kennis M, Vink M, Kahn RS, & Geuze E (2014). Neural correlates of trauma-unrelated emotional processing in war veterans with PTSD. Psychological Medicine, 45(3), 575–587. doi: 10.1017/S0033291714001706 [DOI] [PubMed] [Google Scholar]
- van Rooij SJH, Stevens JS, Ely TD, Fani N, Smith AK, Kerley KA, … Jovanovic T (2016b). Childhood Trauma and COMT Genotype Interact to Increase Hippocampal Activation in Resilient Individuals. Frontiers in Psychiatry, 7(156). doi: 10.3389/fpsyt.2016.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, … Jovanovic T (2018). The Role of the Hippocampus in Predicting Future Posttraumatic Stress Disorder Symptoms in Recently Traumatized Civilians. Biological Psychiatry, 82(2), 106–115. doi: 10.1016/j.biopsych.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Wout M, Longo SM, Reddy MK, Philip NS, Bowker MT, & Greenberg BD (2017). Transcranial direct current stimulation may modulate extinction memory in posttraumatic stress disorder. Brain and behavior, 7(5), e00681. doi: 10.1002/brb3.681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM (2017). Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety, 34(1), 9–24. doi: 10.1002/da.22556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Krabbe S, Gründemann J, Botta P, Fadok JP, Osakada F, … Lüthi A (2016). Distinct Hippocampal Pathways Mediate Dissociable Roles of Context in Memory Retrieval. Cell, 167(4), 961–972.e916. doi: 10.1016/j.cell.2016.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Qiu C, Meng Y, Ren Z, Yuan C, Li Y, … Zhang W (2018). Pre-treatment Resting-State Functional MR Imaging Predicts the Long-Term Clinical Outcome After Short-Term Paroxtine Treatment in Post-traumatic Stress Disorder. Front Psychiatry, 9, 532. doi: 10.3389/fpsyt.2018.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Qiu C, Meng Y, Cui H, Zhang Y, Huang X, … Lui S (2015). Altered spontaneous neuronal activity in chronic posttraumatic stress disorder patients before and after a 12-week paroxetine treatment. J Affect Disord, 174, 257–264. doi: 10.1016/j.jad.2014.11.053 [DOI] [PubMed] [Google Scholar]
- Zhu X, Suarez-Jimenez B, Lazarov A, Helpman L, Papini S, Lowell A, … Neria Y (2018). Exposure-based therapy changes amygdala and hippocampus resting-state functional connectivity in patients with posttraumatic stress disorder. Depression and Anxiety, 35(10), 974–984. doi: 10.1002/da.22816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhutovsky P, Thomas RM, Olff M, van Rooij SJH, Kennis M, van Wingen GA, & Geuze E (2019). Individual prediction of psychotherapy outcome in posttraumatic stress disorder using neuroimaging data. Transl Psychiatry, 9(1), 326. doi: 10.1038/s41398-019-0663-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilcha-Mano S, Zhu X, Suarez-Jimenez B, Pickover A, Tal S, Such S, … Rutherford BR (2020). Diagnostic and Predictive Neuroimaging Biomarkers for Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging, 5(7), 688–696. doi: 10.1016/j.bpsc.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


