We published a comprehensive systematic review and meta-analysis evaluating the current evidence on the impact of 25‑hydroxy-cholecalciferol [25(OH)D] and its deficiency, on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and the severity and mortality of the coronavirus 19 disease (COVID-19).
Recently, we were informed that two studies included in our meta-analysis and published on pre-print platforms were withdrawn (original article references 39, 55). For this reason, in the attempt to understand whether the inclusion of these pre-prints could have affected the results of our meta-analysis, we decided to make an additional analysis after excluding not only the two withdrawn pre-prints but also a third one originally included in the analysis (45).
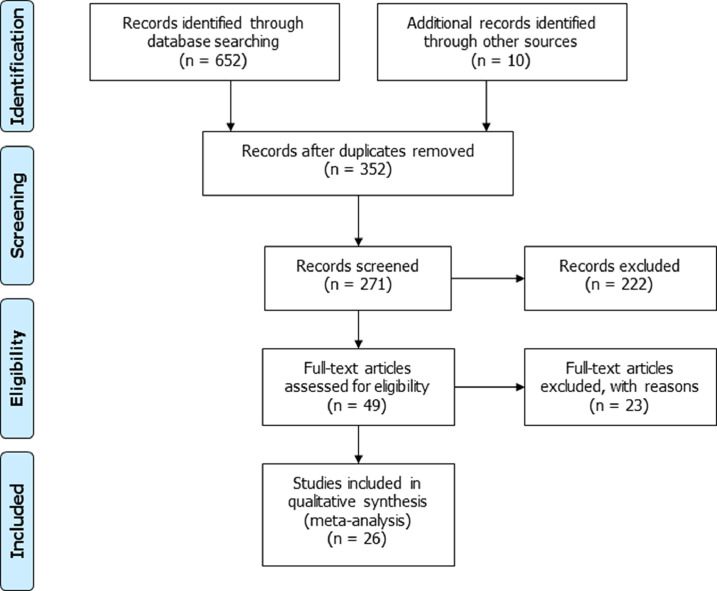
Because of the exclusion of the 3 pre-prints (original article references 39, 45, 55), the flowchart of the included studies was modified (Fig. 1). Table 1, showing the characteristics of the included studies, and Table 2, concerning the quality analysis of the studies, were also updated after exclusion of the 3 pre-prints (original article references 39, 45, 55).
Fig. 1.
Flowchart of the studies included in the meta-analysis.
Table 1.
Main characteristics of the studies included in this meta-analysis.
| First Author | Year | Country | Study design | Sample size | Mean Age | Gender Male/Female | Ethnicity | Outcome evaluated | Time at 25(OH)D levels assessment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdollahi | 2020 [27] | Iran | Case-control study | 402 | SARS-CoV-2 + | 48.0 ± 16.5 | SARS-CoV-2 + | 66/135 | NR | Difference in mean 25(OH)D levels between COVID-19 positive and controls | NR | |
| SARS-CoV-2 - | 46.34 ± 13.5 | SARS-CoV-2 - | 66/135 | |||||||||
| Abrishami | 2020 | Iran | Retrospective study | 73 | SARS-CoV-2 + | 55.2 ± 15.0 | SARS-CoV-2 + | 47/26 | NR | Difference in 25(OH)D levels between dead and discharged | Generally performed within 3 days of hospital admission | |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Arvinte | 2020 | USA | Pilot study | 21 | SARS-CoV-2 + | 60.2 ± 17.4 | SARS-CoV-2 + | 15/6 | SARS-CoV-2 + | Caucasian: 4 Hispanic: 17 | Difference in 25(OH)D levels between patients who died or were discharged from the hospital | Admission to hospital |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||
| Baktash | 2020 | UK | Prospective Cohort Study | 105 | SARS-CoV-2 + | 81 (SD NR) | SARS-CoV-2 + | 42/28 | SARS-CoV-2 + | Caucasian: 50 South Asian: 18 East Asian: 2 Afro-Caribbean: 1 | Difference in mean 25(OH)D levels between COVID-19 patients and controls. Assessment of the risk for COVID-19 related mortality in patients with VDD | Admission to hospital |
| SARS-CoV-2 - | 83.4 ± 8.1 | SARS-CoV-2 - | 15/20 | SARS-CoV-2 - | Caucasian: 30 South Asian: 3 East Asian: 0 Afro-Caribbean: 3 | |||||||
| Carpagnano | 2020 | Italy | Retrospective, observational single-center study | 42 | SARS-CoV-2 + | 65.0 ± 13.0 | SARS-CoV-2 + | 30/12 | NR | Assessment of the risk for mortality by COVID-19 in patients with VDD | Performed within 12 h of admission to RICU | |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Cereda | 2020 | Italy | Single-center cohort study | 129 | SARS-CoV-2 + | 73.6 ± 13.9 | SARS-CoV-2 + | 70/59 | SARS-CoV-2 + | / | Assessment of the risk for COVID-19 severity and related mortality in patients with VDD | Performed within 48 h of admission to hospital |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||
| Chodick | 2020 | Israel | Cross-sectional study | 14,520 | SARS-CoV-2 + | 40.6 (19.1) | SARS-CoV-2 + | 788/529 | NR | Difference in mean 25(OH)D levels between COVID-19 patients and controls | NR | |
| SARS-CoV-2 - | 37.0 (19.1) | SARS-CoV-2 - | 6092/7111 | |||||||||
| D'Avolio | 2020 | Swiss | Retrospective Cohort Study | 107 | SARS-CoV-2 + | 73.3 ± 12.5 | SARS-CoV-2 + | 19/8 | NR | Difference in mean 25(OH)D levels between COVID-19 patients and controls | Generally performed within 3 days of molecular testing for diagnosis of SARS-CoV-2 infection | |
| SARS-CoV-2 - | 72.0 ± 15.9 | SARS-CoV-2 - | 39/41 | |||||||||
| De Smet | 2020 | Belgium | Retrospective observational study | 186 | SARS-CoV-2 + | 67.0 ± 20.9 | SARS-CoV-2 + | 109/77 | NR | Difference in 25(OH)D levels between mild and severe cases and between dead or discharged patients. Assessment of the risk for COVID-19 severe forms in patients with VDD | Admission to hospital | |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Faul | 2020 [41] | Ireland | Observational study | 33 | SARS-CoV-2 + | NR | SARS-CoV-2 + | 33/0 | SARS-CoV-2 + | Caucasian: 33 | Difference in 25(OH)D levels between mild and severe COVID-19 patients | Admission to hospital |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||
| Hastie-Mackay | 2020 | UK | Retrospective cohort study | 348,598 | SARS-CoV-2 + | NR | SARS-CoV-2 + | 265/184 | SARS-CoV-2 + | White: 385 Black: 32 South Asian:19 Other: 13 | Difference in mean 25(OH)D levels between COVID-19 patients and controls | Pre-hospedalization (at least 10 years old dosages) |
| SARS-CoV-2 - | NR | SARS-CoV-2 - | 168,391/179,758 | SARS-CoV-2 - | White: 331,464 Black: 5022 South Asian:5917 Other: 5746 | |||||||
| Hernandez | 2020 | Spain | Case-control Study | 394 | SARS-CoV-2 + | 59.5 ± 16.8 | SARS-CoV-2 + | 123/74 | NR | Difference in mean 25(OH)D levels between COVID-19 patients and controls. Assessment of the risk for COVID-19 severity and related mortality in patients with VDD | Admission to hospital | |
| SARS-CoV-2 - | 61.0 ± 7.47 | SARS-CoV-2 - | 123/74 | |||||||||
| Im | 2020 [33] | South Korea | Case-control study | 200 | SARS-CoV-2 + | 52.2 ± 20.7 | SARS-CoV-2 + | 21/29 | NR | Difference in mean 25(OH)D levels between COVID-19 patients and controls | Dosing performed on average within 2 days of hospital admission and no later than 7 days | |
| SARS-CoV-2 - | 52.4 ± 20.2 | SARS-CoV-2 - | NR | |||||||||
| Jain | 2020 | India | Prospective observational study | 154 | SARS-CoV-2 + | NR | SARS-CoV-2 + | 95/69 | NR | Difference in 25(OH)D levels between mild and severe cases. Assessment of the risk for COVID-19 severe forms or mortality in patients with VDD | Admission to hospital | |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Karonova | 2020 | Russia | Observational cohort study | 80 | SARS-CoV-2 + | 53.2 ± 15.7 | SARS-CoV-2 + | 43/37 | NR | Difference in 25(OH)D levels between mild and severe COVID-19 forms and between dead or discharged patients | NE | |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Kerget | 2020 [44] | Turkey | Case-control Study | 88 | SARS-CoV-2 + | 49±21.1 | SARS-CoV-2 + | 41/47 | NR | Difference in 25(OH)D levels between mild and severe COVID-19 forms and between dead or discharged patients | Admission to hospital | |
| SARS-CoV-2 - | 35.2 ± 6.9 | SARS-CoV-2 - | 8/12 | |||||||||
| Luo | 2020 | China | Retrospective cross-sectional study | 895 | SARS-CoV-2 + | 54.3 ± 15.6 | SARS-CoV-2 + | 148/187 | NR | Difference in 25(OH)D levels between COVID-19 patients and controls. Difference in 25(OH)D levels between mild and severe COVID-19 forms and between dead or discharged patients. Assessment of the risk for COVID-19 severity and related mortality in patients with VDD | Admission to hospital | |
| SARS-CoV-2 - | 54.7 ± 8.2 | SARS-CoV-2 - | 257/303 | |||||||||
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Mardani | 2020 [35] | Iran | Case-control study | 123 | SARS-CoV-2 + | 43.3 ± 14.5 | SARS-CoV-2 + | 35/28 | NR | Difference in mean 25(OH)D levels between COVID-19 patients and controls and between dead or discharged patients | Admission to hospital | |
| SARS-CoV-2 - | 40.8 ± 15.8 | SARS-CoV-2 - | 30/30 | |||||||||
| Merzon | 2020 | Israel | Population based study | 7807 | SARS-CoV-2 + | 35.6 ± 15.6 | SARS-CoV-2 + | 385/397 | NR | Difference in mean 25(OH)D levels between COVID-19 patients and controls | Pre-hospedalization (not specified when) | |
| SARS-CoV-2 - | 47.4 ± 21.0 | SARS-CoV-2 - | 2849/4176 | |||||||||
| Panagiotou | 2020 | UK | Retrospective study | 134 | SARS-CoV-2 + | NR | SARS-CoV-2 + | 73/61 | SARS-CoV-2 + | Caucasian: 128 Asian: 4 Afro-Caribbean: 1 Other: 1 | Difference in 25(OH)D levels between mild and severe COVID-19 forms. Assessment of the risk for severe COVID-19 forms in patients with VDD | Admission to hospital |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||
| Pizzini | 2020 | Austria | Prospective Multicenter Observational Study | 109 | SARS-CoV-2 + | 58.0 ± 14.0 | SARS-CoV-2 + | 65/44 | NR | Difference in 25(OH)D levels between mild and severe COVID-19 forms | 25(OH)D assays performed 8 weeks after disease onset | |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Radujkovic | 2020 | Germany | Prospective Observational Study | 185 | SARS-CoV-2 + | 50.7 ± 15.7 | SARS-CoV-2 + | 95/90 | NR | Difference in 25(OH)D levels between mild and severe COVID-19 forms | Admission to hospital | |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Raisi-Estabragh | 2020 | UK | Prospective cohort study | 4510 | SARS-CoV-2 + | 68.1 ± 9.2 | SARS-CoV-2 + | 696/630 | SARS-CoV-2 + | White: 1.141 Black: 76 Asian: 60 Chinese: 6 Mixed: 9 Other: 34 | Difference in mean 25(OH)D levels between COVID-19 patients and controls | Pre-hospedalization (at least 10 years old dosages) |
| SARS-CoV-2 - | 68.91 ± 8.72 | SARS-CoV-2 - | 1505/1679 | SARS-CoV-2 - | White: 2927 Black: 91 Asian: 78 Chinese: 3 Mixed: 24 Other: 61 | |||||||
| Szeto | 2020 | USA | Retrospective cohort study | 93 | SARS-CoV-2 + | NR | SARS-CoV-2 + | 44/49 | SARS-CoV-2 + | Black: 27 | Assessment of the risk for COVID-19 severity and related mortality in patients with VDD | Prehospitalization (25(OH)D levels measured within the previous year and on average 136 days prior to hospital admission) |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||
| Vassiliou | 2020 | Greek | Prospective observational cohort study | 30 | SARS-CoV-2 + | 65.0 ± 11.0 | SARS-CoV-2 + | 24/6 | NR | Difference in 25(OH)D levels between dead and discharged COVID-19 patients and assessment of the risk for COVID-19 mortality in patients with VDD | Admission to ICU | |
| SARS-CoV-2 - | / | SARS-CoV-2 - | / | |||||||||
| Ye | 2020 [38] | China | Case-control study | 142 | SARS-CoV-2 + | 41.7 ± 15.9 | SARS-CoV-2 + | 32/48 | NR | Difference in mean 25(OH)D levels between COVID-19 patients and controls, and between patients with severe or non-severe forms of COVID-19. Assessment of the risk for severe COVID-19 forms in patients with VDD | Admission to hospital | |
| SARS-CoV-2 - | 44.7 ± 20.5 | SARS-CoV-2 - | 23/39 | |||||||||
Abbreviation: 25(OH)D, 25‑hydroxy-cholecalciferol; VDD, vitamin D deficiency; COVID-19, coronavirus disease 19; NR, Not Reported; SARS-CoV-2 +, patients positive for severe acute respiratory syndrome coronavirus 2 infection; SARS-CoV-2 -, patients negative for severe acute respiratory syndrome coronavirus 2 infection; SD, standard deviation; NE, Not evaluated; ICU, Intensive Care Unit; RICU, Respiratory Intermediate Care Unit.
Table 2.
Quality assessment tool for observational cohort and cross-sectional studies.
| Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrishami et al. (2020) [49] | + | + | + | + | – | NR | + | – | + | – | + | NA | + | + |
| Arvinte et al. (2020) [50] | + | + | + | – | – | NR | – | – | + | – | + | NA | + | – |
| Baktash et al. (2020) [28] | + | + | + | – | – | – | – | + | + | – | + | NA | + | – |
| Carpagnano et al. (2020) [54] | + | + | + | – | – | NR | – | + | + | – | + | NA | + | + |
| Cereda et al. (2020) | + | + | + | – | – | + | – | + | + | – | + | NA | + | + |
| Chodick et al. (2020) [29] | + | + | + | – | – | NR | – | – | + | – | + | NA | + | + |
| D'Avolio et al. (2020) [30] | + | + | + | + | – | – | – | – | + | – | + | NA | + | – |
| De Smet et al. (2020) [40] | + | + | + | – | – | – | – | + | + | – | + | NA | + | – |
| Faul et al. (2020) [41] | + | + | + | – | – | NR | NR | – | NR | NR | + | NA | + | – |
| Hastie-Mackay et al. (2020) [31] | + | + | + | + | – | + | + | + | + | NR | + | NA | + | + |
| Jain et al. (2020) | + | + | + | + | + | NR | – | + | + | – | + | NA | + | + |
| Karonova et al. (2020) [43] | not assessable because in Russian language | |||||||||||||
| Luo et al. (2020) | + | + | + | – | – | + | – | + | + | – | + | NA | + | + |
| Merzon et al. (2020) [36] | + | + | + | + | – | + | NA | + | + | NR | + | NA | + | + |
| Panagiotou et al. (2020) [46] | + | + | + | – | – | NR | – | + | + | – | + | NA | + | – |
| Pizzini et al. (2020) [47] | + | + | + | + | – | + | – | + | + | – | + | NA | + | – |
| Radujkovic et al. (2020) [48] | + | + | + | – | – | + | – | + | + | – | + | NA | + | + |
| Raisi-Estabragh et al. (2020) [37] | + | + | + | + | – | + | + | – | + | – | + | NA | + | + |
| Szeto et al. (2020) [53] | + | + | + | – | – | + | NR | + | + | + | + | NA | + | + |
| Vassiliou et al. (2020) [51] | + | + | + | + | – | + | – | + | + | – | + | NA | + | – |
1. Was the research question or objective in this paper clearly stated?
2. Was the study population clearly specified and defined?
3. Was the participation rate of eligible persons at least 50%?
4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study pre-specified and applied uniformly to all participants?
5. Was a sample size justification, power description, or variance and effect estimates provided?
6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured?
7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed?
8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)?
9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants?
10. Was the exposure(s) assessed more than once over time?
11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants?
12. Were the outcome assessors blinded to the exposure status of participants?
13. Was loss to follow-up after baseline 20% or less?
14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?
Analysis of serum 25(OH)D levels in SARS-CoV2-positive versus negative patients, and also analysis of patients with infection discharged versus those who died from the disease, were not performed, since pre-prints (original article references 39, 45, 55) were not included for these outcomes in the original meta-analysis.
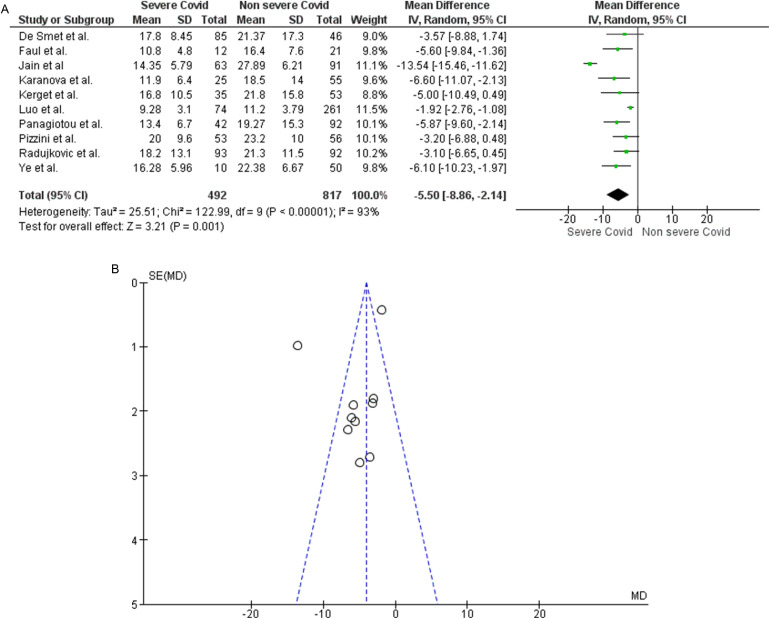
Regarding analysis related to 25(OH)D levels in patients with severe or non-severe COVID-19 (original article Fig. 3), after exclusion of the pre-prints referenced originally as 39 and 45, 10 studies assessing this outcome remained. Specifically, the new analysis confirmed that 25(OH)D levels were clearly lower in the 492 patients with severe disease compared to the 817 patients with a non-severe course of the disease [MD −5.50 (−8.86, −2.14); p = 0.001] (Fig. 3A). After exclusion of the two pre-prints mentioned above, high inter-study heterogeneity was still found (Chi2 P < 0.00001, I2=93%) (Fig. 3B). After the removal of the studies by Luo and colleagues (original article reference 34), and Jain and colleagues (original article reference 42), identified as a source of heterogeneity at the Funnel Plot, the analysis showed homogeneity of the remaining studies (Chi2 P = 0.86, I2=0%) maintaining the statistical significance [MD −4.80 (−6.27, −3.32); p < 0.00001].
Fig. 3.
Panel A. Forest plot of studies that assessed 25(OH)D levels as a continuous variable in patients with severe course of COVID-19 than those with mild course. Panel B. Funnel plot showing the source of heterogeneity of studies that evaluated 25(OH)D levels as a continuous variable in patients with severe course of COVID-19 than those with mild course. Serum 25(OH)D levels are expressed in ng/ml.
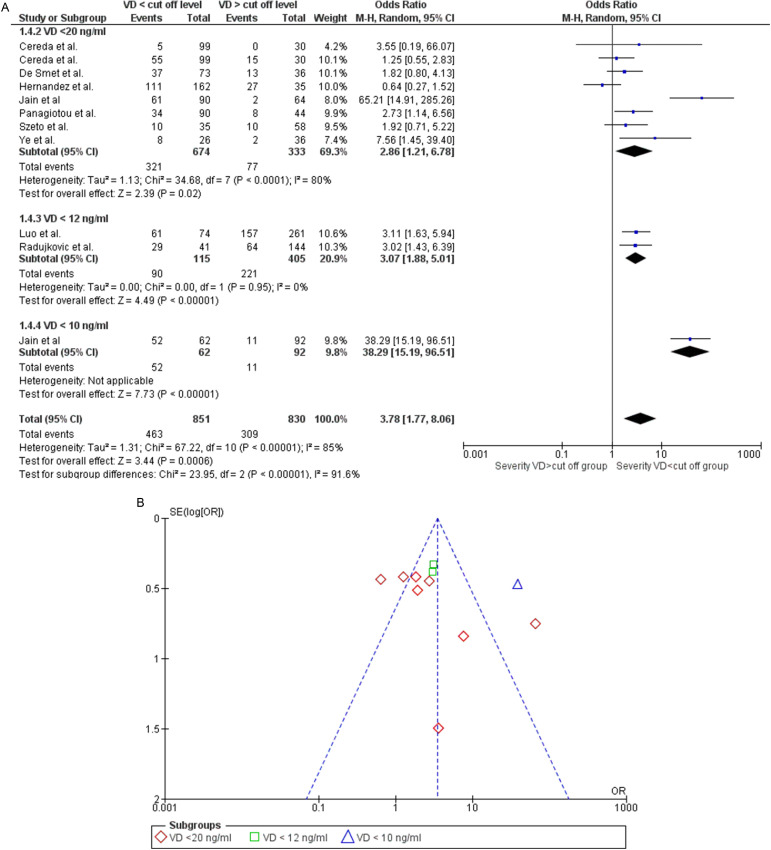
Also, the analysis of the risk of severe COVID-19 in patients with VDD (original article Fig. 5) did not change after the exclusion of pre-print reference 39. This outcome was assessed on data extracted from 10 studies. The study by Cereda and colleagues (original article reference 52) was considered twice since it evaluated both the percentage of patients with severe pneumonia and patients admitted to the intensive care units as an outcome of severity. The study by Jain and colleagues (original article reference 42) was also considered twice since they assessed the risk of infection severity both in patients with 25(OH)D<20 ng/ml and then in patients with levels below 10 ng/ml. The new statistical analysis confirmed that patients with VDD had a higher risk of a severe disease course than patients without deficiency [OR 3.78 (1.77, 8.06); p = 0.0006], regardless of the cut-off values considered to establish the efficiency (Fig. 5A). The Funnel plot showed that the heterogeneity found (Chi2 P < 0.00001, I2=85%) was attributable to the studies Jain and colleagues’ (original article reference 42) and Hernandez and coworkers’ (original article reference 32) (Fig. 5B). Once the data from these studies were excluded, heterogeneity was no longer observed (Chi2 P = 0.53, I2=0%) and the risk of developing a severe course of the disease in VDD patients remained significant [OR 2.47 (1.80, 3.37); p < 0.00001].
Fig. 5.
Panel A. Forest plot of studies that assessed the risk of a severe course of disease in subjects with 25(OH)D values below or above a specified cut-off. The different cut-offs used by the studies allowed for subgroup analysis. Studies using cut-off values higher than those established by the Endocrine Society for the diagnosis of Vitamin D Deficiency (<20 ng/ml) were not included. Panel B. Funnel plot showing the source of heterogeneity of studies that evaluated the risk of a severe course of disease in subjects with 25(OH)D below or above a specified cut-off.
Finally, the analysis of the risk of mortality in patients with VDD (original article supplementary Fig. 2) also remained unchanged after the exclusion of the pre-print reference 55. Indeed, the analysis of the remaining 8 studies confirmed the absence of a significant increase in mortality risk in patients with VDD compared to patients with adequate 25(OH)D levels [OR 1.74 [0.84, 3.59]; p = 0.14] regardless of the cut-off values considered for deficiency (supplementary Fig. 2A). Heterogeneity between studies was found (Chi2 P < 0.03, I2=55%), and its origin was due to the study by Jain and colleagues (42) (Supplementary Fig. 2B). When this was excluded from the analysis, the Funnel Plot showed homogeneity among the remaining studies (Chi2 P = 0.15, I2=36%), and the increased risk of COVID-19 mortality in the presence of VDD was confirmed to be non-significant [OR 1.30 (0.83, 2.03); p = 0.25].
In conclusion, the results of this new analysis showed no difference compared to the original one. Therefore, the inclusion of pre-prints did not affect the results of our meta-analysis. After the exclusion of pre-prints, we may still hypothesize a role for low 25(OH)D levels in the risk of SARS-CoV-2 infection and the development of more severe forms of COVID-19.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101168.
Appendix. Supplementary materials
References
- 27.Abdollahi A, KamaliSarvestani H, Rafat Z. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID-19 infection: A case-control study of hospitalized patients in Tehran, Iran. J Med Virol. 2021;93(4):2359–2364. doi: 10.1002/jmv.26726. [DOI] [PubMed] [Google Scholar]
- 28.Baktash V, Hosack T, Patel N. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2020 doi: 10.1136/postgradmedj-2020-138712. postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chodick G, Nutman A, Yiekutiel N, Shalev V. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are not associated with increased risk of SARS-CoV-2 infection. J Travel Med. 2020;27(5) doi: 10.1093/jtm/taaa069. taaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Avolio A, Avataneo V, Manca A. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastie CE, Mackay DF, Ho F. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes MetabSyndr. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mardani R, Alamdary A, Mousavi Nasab SD, Gholami R, Ahmadi N, Gholami A. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merzon E, Tworowski D, Gorohovski A. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raisi-Estabragh Z, McCracken C, Bethell MS. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf) 2020;42(3):451–460. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye K, Tang F, Liao X. Does Serum Vitamin D Level Affect COVID-19 Infection and Its Severity?-A Case-Control Study. J Am CollNutr. 2020:1–8. doi: 10.1080/07315724.2020.1826005. [DOI] [PubMed] [Google Scholar]
- 40.De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality. Am J ClinPathol. 2021;155(3):381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faul JL, Kerley CP, Love B. Vitamin D Deficiency and ARDS after SARS-CoV-2 Infection. Ir Med J. 2020;113(5):84. [PubMed] [Google Scholar]
- 43.Karonova TL, Andreeva АТ, Vashukova МА. Serum 25(OH)D level in patients with CoVID-19. Journal Infectology. 2020;12(3):21–27. [Google Scholar]
- 44.Kerget B, Kerget F, Kızıltunç A. Evaluation of the relationship of serum vitamin D levels in COVID-19 patients with clinical course and prognosis. TuberkToraks. 2020;68(3):227–235. doi: 10.5578/tt.70027. [DOI] [PubMed] [Google Scholar]
- 46.Panagiotou G, Tee SA, Ihsan Y. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. ClinEndocrinol (Oxf) 2020;93(4):508–511. doi: 10.1111/cen.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizzini A, Aichner M, Sahanic S. Impact of Vitamin D Deficiency on COVID-19-A Prospective Analysis from the CovILD Registry. Nutrients. 2020;12(9):2775. doi: 10.3390/nu12092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients. 2020;12(9):2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrishami A, Dalili N, Mohammadi Torbati P. Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: a retrospective study. Eur J Nutr. 2020:1–9. doi: 10.1007/s00394-020-02411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arvinte C, Singh M, Marik PE. Serum Levels of Vitamin C and Vitamin D in a Cohort of Critically Ill COVID-19 Patients of a North American Community Hospital Intensive Care Unit in May 2020: A Pilot Study. Med Drug Discov. 2020;8 doi: 10.1016/j.medidd.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vassiliou AG, Jahaj E, Pratikaki M, Orfanos SE, Dimopoulou I, Kotanidou A. Low 25-Hydroxyvitamin D Levels on Admission to the Intensive Care Unit May Predispose COVID-19 Pneumonia Patients to a Higher 28-Day Mortality Risk: A Pilot Study on a Greek ICU Cohort. Nutrients. 2020;12(12):3773. doi: 10.3390/nu12123773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szeto B, Zucker JE, LaSota ED. Vitamin D Status and COVID-19 Clinical Outcomes in Hospitalized Patients. Endocr Res. 2020:1–8. doi: 10.1080/07435800.2020.1867162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carpagnano GE, Di Lecce V, Quaranta VN. Vitamin D deficiencyas a predictor of poorprognosis in patients with acute respiratoryfailure due to COVID-19. J Endocrinol Invest. 2020:1–7. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.