Abstract
Background
The rapid increase in coronavirus disease 2019 (COVID-19) cases during the subsequent waves in Saudi Arabia and other countries prompted the Saudi Critical Care Society (SCCS) to put together a panel of experts to issue evidence-based recommendations for the management of COVID-19 in the intensive care unit (ICU).
Methods
The SCCS COVID-19 panel included 51 experts with expertise in critical care, respirology, infectious disease, epidemiology, emergency medicine, clinical pharmacy, nursing, respiratory therapy, methodology, and health policy. All members completed an electronic conflict of interest disclosure form. The panel addressed 9 questions that are related to the therapy of COVID-19 in the ICU. We identified relevant systematic reviews and clinical trials, then used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach as well as the evidence-to-decision framework (EtD) to assess the quality of evidence and generate recommendations.
Results
The SCCS COVID-19 panel issued 12 recommendations on pharmacotherapeutic interventions (immunomodulators, antiviral agents, and anticoagulants) for severe and critical COVID-19, of which 3 were strong recommendations and 9 were weak recommendations.
Conclusion
The SCCS COVID-19 panel used the GRADE approach to formulate recommendations on therapy for COVID-19 in the ICU. The EtD framework allows adaptation of these recommendations in different contexts. The SCCS guideline committee will update recommendations as new evidence becomes available.
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; CrI, credible interval; CRP, C-reactive protein; EtD, evidenced to decision; GDT, guideline development tool; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; GUIDE, guidelines in intensive care, development, and evaluation; HR, hazard ratio; HCQ, hydroxychloroquine; HFNC, high flow nasal cannula; ICU, intensive care unit; IDSA, Infectious Disease Society of America; MI, myocardial infarction; NIH, National Institute of Health; NIV, noninvasive ventilation; OR, odds ratio; PE, pulmonary embolism; RCT, randomized controlled trials; RdRP, RNA-dependent RNA polymerase; RR, relative risk; SCCS, Saudi Critical Care Society; WHO, World Health Organization
Keywords: COVID-19, Therapy, Practice guideline, Intensive care unit
Background
Since the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) pandemic in 2020, over 158 million people worldwide were infected with severe acute respiratory syndrome corona virus-2 (SARS-CoV-2), causing over 3 million deaths. As of May 10th, 2021, over 427,370 COVID-19 cases causing 7085 deaths were reported in Saudi Arabia (https://covid19.moh.gov.sa/).
The SCCS issued the first guidelines on the management of COVID-19 in the intensive care unit (ICU) in 2020 [1]. Given the rapid evolution of evidence in this field, updating the guidelines is crucial. Therefore, the SCCS, in collaboration with the Ministry of Health in Saudi Arabia, undertook this initiative aiming to produce trustworthy evidence-based practice guidelines focusing on therapy for COVID-19.
Guideline scope
The scope of the current guideline is to provide recommendations to critical care teams on the management of adults with severe or critical COVID-19 in the ICU. We used simplified definition of severe and critical COVID-19 (Supplementary Table).
Target users
The target users of this guideline are healthcare workers caring for COVID-19 patients in the ICU, including intensivists, emergency physicians, infectious diseases physicians, clinical pharmacists, nurses, respiratory therapists, and policymakers.
Guideline structure
The chairs of the practice guidelines chapter under the SCCS selected panel members based on expertise with the aim to maintain gender and geographic balance. The panel included 51 members with collective expertise in critical care, infectious diseases, emergency medicine, clinical pharmacy, epidemiology, respiratory therapy, nursing, public health, clinical research and health policy and administration. We engaged representatives from different governmental and private healthcare sectors across the Kingdom of Saudi Arabia, including the Ministry of Health.
Conflict of interest
All authors completed an electronic WHO conflict of interest form, as previously outlined by Alhazzani et al. for the management of potential conflict of interest [2].
Methods
Systematic review and meta-analysis
With support from the GUIDE (Guidelines in Intensive Care, Development, and Evaluation) group, a professional medical librarian designed a search strategy of Cochrane Central and MEDLINE databases. The search is being updated on a monthly basis, and a dedicated systematic review team reviews titles and abstracts to identify relevant systematic reviews and randomized controlled trials (RCTs). For this guideline, we restricted the eligibility to only include relevant studies published in English prior to April 25, 2021.
When there are more than one RCT but no published meta-analysis on a specific question, we used a random-effects model meta-analysis to pool effect sizes across RCTs. We presented the results as relative risk (RR) and 95% confidence interval (CI) for binary outcomes and mean difference (MD) and 95% CI for continuous outcomes. For recommendations informed by data from published systematic reviews, we used the estimates reported by study authors (Supplement).
Quality of evidence
We used the GRADE approach to assess the quality of the body of evidence [3,4]. Methodologists rated the quality of evidence as high, moderate, low, or very low [5], and used the GRADEpro GDT (guideline development tool) online software (https://gradepro.org/) to produce the evidence summaries [6]. We only used direct evidence from COVID-19 population to inform our recommendations.
Moving from evidence to recommendation
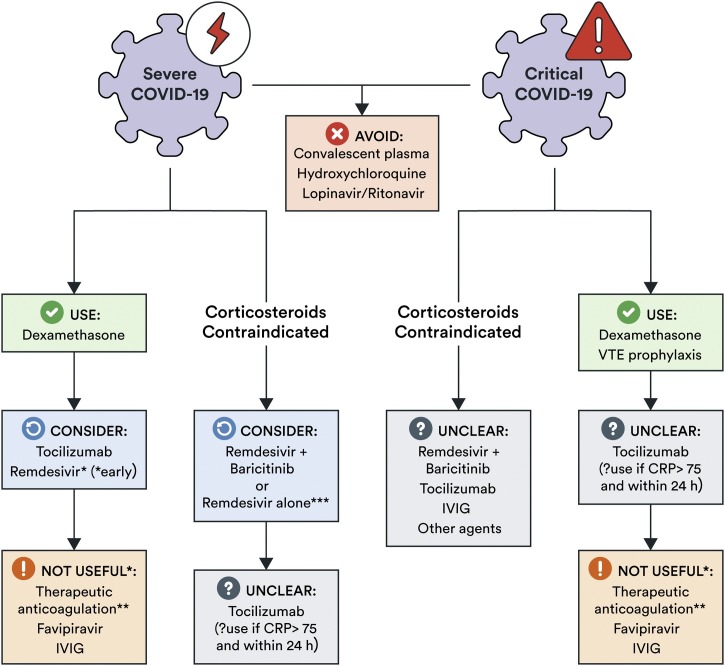
For every recommendation, we used the EtD framework to craft the recommendations. We included all recommendations-specific EtDs in the supplement. Panel members completed the EtD forms for each recommendation online. The methodologist used feedback and input from panel members to refine the judgment on EtD forms and craft the recommendations. In accordance with the GRADE taxonomy, we used the wording “we recommend” to express strong recommendations and “we suggest” to express weak recommendations. We present a summary of the guideline’s recommendations in Table 1 and Fig. 1 .
Table 1.
Summary of recommendations.
| Agent | Clinical question | Recommendation(s) | Strength of recommendation and quality of evidence |
|---|---|---|---|
| I. Immunomodulation therapy | |||
| Corticosteroids | Should corticosteroids vs. no corticosteroids be used for severe or critical COVID-19? | 1. For adults with severe or critical COVID-19 in the ICU, the panel recommends using systemic corticosteroids | Strong recommendation, high quality evidence. |
| 2. The panel suggests using dexamethasone 6 mg daily for 10 days over other corticosteroids | Weak recommendation, low quality evidence. | ||
| Tocilizumab | Should tocilizumab vs. no tocilizumab be used for severe or critical COVID-19? | 3. For adults with COVID-19 who are receiving high-flow nasal cannula or noninvasive ventilation, the panel suggests using tocilizumab over not using it | Weak recommendation, low quality evidence. |
| Remark: | |||
| Studies shown benefit of tocilizumab when used concomitantly with corticosteroids in patients with elevated C-reactive protein (CRP) >75 mg/l, or when tocilizumab therapy was initiated within 24 h of ICU admission. | |||
| 4. Due to insufficient evidence, we were unable to issue a recommendation for or against the use of tocilizumab in invasively ventilated COVID-19 patients. If clinicians decide to use tocilizumab in this context, it is probably better to use it early (within 24 h of admission) and/or in patients with CRP level >75 mg/l. | No recommendation | ||
| Baricitinib | Should baricitinib and remdesivir vs. remdesivir alone be used for severe or critical COVID-19? | 5. For adults with critical COVID-19; the panel suggests against the routine use of baricitinib in combination with remdesivir | Weak recommendation, low quality evidence. |
| 6. For selected cases with severe COVID-19 on NIV or HFNC who cannot receive corticosteroids or tocilizumab, we suggest using a combination of baricitinib and remdesivir over remdesivir alone | Weak recommendation, low quality evidence. | ||
| Convalescent plasma | Should convalescent plasma vs. no convalescent plasma be used for severe or critical COVID-19? | 7. For adults with severe or critical COVID-19 in the ICU, the panel recommends against using convalescent plasma | Strong recommendation, moderate quality evidence. |
| Immunoglobulins | Should intravenous immunoglobulin (IVIG) vs. no IVIG be used for severe or critical COVID-19? | 8. For adults with severe or critical COVID-19 in the ICU, the panel suggests against the routine use of IVIG outside the context of clinical trials | Weak recommendation, low quality evidence. |
| II. Antiviral therapy | |||
| Remdesivir | Should remdesivir vs. no remdesivir be used for critical COVID-19? | 9. For mechanically ventilated adults with critical COVID-19, we suggest against using remdesivir outside the context of clinical trials | Weak recommendation, low quality evidence. |
| Favipiravir | Should favipiravir vs. no favipiravir be used for severe or critical COVID-19? | 10. For adults with severe or critical COVID-19 in the ICU, the panel suggests against the routine use of favipiravir outside the context of clinical trials | Weak recommendation, very low quality evidence. |
| III. Hydroxychloroquine (HCQ) | |||
| HCQ | Should HCQ vs. no HCQ be used for severe or critical COVID-19? | 11. For adults with severe or critical COVID-19 in the ICU, the panel recommends against using HCQ | Strong recommendation, moderate quality evidence. |
| VI. Anticoagulation | |||
| Therapeutic anticoagulation | Should therapeutic anticoagulation vs. prophylactic dose anticoagulation be used for critical COVID-19? | 12. For adults with critical COVID-19 and no clinical suspicion of venous thromboembolism (VTE), we suggest using prophylactic dosing anticoagulation over therapeutic anticoagulation | Weak recommendation, low quality evidence. |
| Remark: | |||
| This recommendation does not apply to patients with high suspicion of acute VTE or those with other indications for therapeutic anticoagulation. | |||
Fig. 1.
Approach to pharmacotherapy for COVID-19 in the ICU.
Results
-
I
Immunomodulation therapy
Corticosteroids
Question: Should systemic corticosteroids vs. no corticosteroids be used for severe or critical COVID-19?
Recommendations
-
1
For adults with severe or critical COVID-19 in the ICU, the panel recommends using systemic corticosteroids (strong recommendation, high quality evidence).
-
2
The panel suggests using dexamethasone 6 mg daily for 10 days over other corticosteroids (weak recommendation, low quality evidence).
Rationale
Critically ill patients with COVID-19 can experience a systemic inflammatory response resulting in lung injury and/or multisystem organ failure, which is associated with high mortality [7]. A plausible hypothesis is that corticosteroids may help mitigate those effects, particularly in patients with severe disease. COVID-19 is also associated with impaired hypothalamic-pituitary-adrenal axis and with inflammation-induced microthrombi and coagulopathy; all could be potentially improved by corticosteroids therapy [8].
The RECOVERY trial demonstrated that dexamethasone (6 mg daily up to 10 days) in hospitalized patients with COVID-19 (n = 2104 received dexamethasone and 4321 received usual care) reduced 28-day mortality (relative risk [RR] 0.83; 95% confidence interval [CI] 0.75 to 0.93), duration of hospitalization and progression to invasive mechanical ventilation [9]. The mortality benefit was observed only in patients who were mechanically ventilated or needed supplementary oxygen. A meta-analysis of 7 RCTs (n = 1073) found reduction in mortality with corticosteroid use (odds ratio [OR] 0.66; 95% CI 0.53 to 0.82; high quality) [10].
Corticosteroid therapy in critically ill patients appears to have an acceptable safety profile. A large cohort study found a small increase in adverse events like gastrointestinal bleeding, sepsis, and heart failure with a short course of corticosteroids in an outpatient setting [11]. Additionally, the meta-analysis of COVID-19 corticosteroid trials showed no increase in the risk of adverse events with corticosteroid therapy [10]. Given that corticosteroids are inexpensive and widely available, this recommendation would increase equity and likely be an acceptable intervention to key stakeholders (Supplementary Table). Hence, the panel issued a strong recommendation for using corticosteroids in ICU COVID-19 patients.
There are several questions that remained unanswered. Some panelists felt that higher doses of corticosteroids might be warranted in selected patients with features of acute respiratory distress syndrome or those with organizing pneumonia features on radiological imaging, however; current data demonstrate no definitive subgroup effect suggesting that further studies are needed.
Our recommendation is consistent with the Surviving Sepsis Campaign guidelines and the WHO guidelines [12,13]. The National Institute of Health (NIH) guidelines recommend dexamethasone plus remdesivir (based on theoretical considerations) for those who require an increased amount of oxygen (within 24 h of admission) without mechanical ventilation and recommend dexamethasone alone for those who require invasive mechanical ventilation or extracorporeal membrane oxygenation [14]. The Infectious Disease Society of America (IDSA) guidelines recommend dexamethasone for critically ill patients and suggest dexamethasone for severe COVID-19 [15].
There is limited evidence regarding the optimal dosing, timing, duration, and steroids type. The effect of all these variables on mortality and other outcomes needs to be further assessed in clinical trials. However, the panel issued a weak recommendation favoring the use of dexamethasone over other corticosteroids as it was the only agent studied in the largest trial to date.
Tocilizumab
Question: Should tocilizumab vs. no tocilizumab be used for severe or critical COVID-19?
Recommendations
-
1
For adults with COVID-19 who are receiving high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) in the ICU, the panel suggests using tocilizumab over not using it (weak recommendation, low quality evidence).
Remarks:
Studies shown benefit of tocilizumab when used concomitantly with corticosteroids in patients with elevated C-reactive protein (CRP) >75 mg/l, or when tocilizumab therapy was initiated within 24 h of ICU admission.
-
2
Due to insufficient evidence, we were unable to issue a recommendation for or against the use of tocilizumab in invasively ventilated COVID-19 patients. If clinicians decide to use tocilizumab in this context, it is probably best to use it within 24 h of admission and/or in patients with CRP level >75 mg/l.
Rationale
Interleukin-6 (IL-6) is a pleiotropic cytokine with both proinflammatory and anti-inflammatory effects, and IL-6–related cytokine storm has been proposed as a major target for the treatment of COVID-19. Researchers hypothesize that inhibition of IL-6 may attenuate the cytokine release in patients with severe COVID-19 [16]. Tocilizumab is a humanized monoclonal antibody that blocks IL-6 receptors. It is commonly used in cytokine release syndrome and other inflammatory conditions such as rheumatoid arthritis [[17], [18], [19], [20]].
Our search identified 9 RCTs (n = 6494) that investigate the use of tocilizumab in hospitalized COVID-19 patients [[21], [22], [23], [24], [25], [26], [27], [28], [29]]. The pooled estimate of available data on non-invasively ventilated patients with COVID-19 showed that tocilizumab reduced risk of death compared to usual care (RR 0.87; 95% CI 0.79 to 0.96; moderate quality) (Supplementary Table). However, tocilizumab did not reduce risk of death in the subgroup of patients undergoing invasive mechanical ventilation (RR 0.93; 95% CI 0.80–1.08; low quality). Tocilizumab use did not increase serious adverse events or infection compared to usual care (Supplementary Table). However, these results need to be interpreted with caution as tocilizumab is often used with other medications potentially increasing the risk of infection such as corticosteroids. In addition, higher quality evidence from the rheumatoid arthritis literature showed an increased risk of infection [30,31].
Considering the moderate quality evidence of mortality reduction in non-intubated COVID-19 patients and possible reduction in serious adverse effects, the panel issued a weak recommendation for the use of tocilizumab to treat severe COVID-19 (Supplementary Table). However, given the lack of clear evidence in patients with respiratory failure requiring invasive ventilation, combined with the associated high cost of tocilizumab, the panel did not issue a recommendation in this specific subgroup pending more evidence. If clinicians choose to use tocilizumab in invasively ventilated patients, it is best used in patients who are receiving systemic corticosteroids with elevated CRP level and as early as possible.
Baricitinib
Question: Should baricitinib and remdesivir vs. remdesivir alone be used for severe or critical COVID-19?
Recommendations
-
1
For adults with critical COVID-19 in the ICU, the panel suggests against the routine use of baricitinib in combination with remdesivir (weak recommendation, low quality evidence).
-
2
For selected cases with severe COVID-19 on NIV or HFNC who cannot receive corticosteroids or tocilizumab, we suggest using a combination of baricitinib and remdesivir over remdesivir alone (weak recommendation, low quality evidence).
Rationale:
Emerging data suggest that COVID-19 severity may be related to dysregulated inflammatory response [32]. Baricitinib, an orally administered selective inhibitor of Janus kinase 1 and 2, that inhibits the intracellular signaling pathway of cytokines, which are known to be elevated in severe COVID-19. It acts against SARS-CoV-2 through AP2-associated protein kinase 1 impairment and consequently, prevents SARS-CoV-2 cellular entry and infectivity [[33], [34], [35]].
We identified one RCT that enrolled 1033 hospitalized adults with COVID-19 and randomized them to receive either baricitinib and remdesivir or remdesivir and placebo [36]. Patients received remdesivir intravenously as a 200 mg loading dose on day 1, followed by a 100 mg daily on days 2 through 10 or until hospital discharge. Baricitinib was administered as a 4 mg daily dose for 14 days. In the subgroup of patients receiving HFNC or NIV (n = 216), combination of baricitinib and remdesivir did not reduce risk of death compared to remdesivir alone (hazard ratio [HR] 0.55; 95% 0.22–1.38; low quality). Similarly, in the subgroup of patients undergoing invasive ventilation or extracorporeal membrane oxygenation, combination therapy did not reduce the risk of death (HR 1.00; 95% 0.45–2.22; low quality) (Supplementary Table). However, combination therapy reduced the risk of invasive ventilation or death (HR 0.69; 95% 0.50 to 0.95; low quality). Overall, patients receiving combination therapy were quicker to recover compared to controls (6 days vs. 7 days, respectively). Adverse events were lower in the combination group than in the control group.
Considering the low quality evidence, unclear effect on mortality, and the associated large cost of the intervention (Supplementary Table), the panel issued a weak recommendation against the routine use of baricitinib in combination with remdesivir in adults with critical or severe COVID-19. However, the panel felt that the use of combination therapy in selected cases requiring HFNC or NIV who are unable receive corticosteroids or tocilizumab is reasonable.
The NIH suggested using combination therapy of baricitinib and remdesivir when corticosteroids cannot be used in non-intubated patients requiring oxygen supplementation [14]. A similar conditional recommendation was issued by the IDSA in their most recent updated guidelines [15]. The addition of baricitinib to corticosteroids remains controversial and needs to be studied further in randomized trials.
Convalescent plasma
Question: Should convalescent plasma vs. no convalescent plasma be used for severe or critical COVID-19?
Recommendation
For adults with severe or critical COVID-19 in the ICU, the panel recommends against using convalescent plasma (strong recommendation, moderate quality evidence).
Rationale
Convalescent plasma (obtained from patients who recovered from COVID-19) has been used to treat severe and critical COVID-19 patients despite the lack of RCTs confirming its benefit. Recently, several RCTs have been published [37,38], the results of which have been summarized in a systematic review and meta-analysis including 1060 patients with COVID-19 [39]. The use of convalescent plasma did not reduce risk of death compared to usual care (RR 0.93; 95% CI 0.63–1.38). When six additional unpublished RCTs were included in the meta-analysis (10 RCTs, n = 10 722), the results were more precise confirming no benefit of convalescent plasma over usual care (RR 1.02; 95% CI 0.92–1.12; moderate quality). In a subgroup of patients receiving invasive mechanical ventilation, convalescent plasma did not reduce risk of death (RR 1.19; 95% CI 0.95–1.50; moderate quality). Further, convalescent plasma had no effect on the need for invasive mechanical ventilation (RR 0.81; 95% CI 0.42–1.58, low quality). Overall, convalescent plasma was not associated with improvement in any of the measured clinical outcomes. The quality of the evidence was moderate for mortality outcomes and low for other outcomes (Supplementary Table).
Post-infusion adverse events, such as transfusion-related circulatory overload and acute lung injury were not consistently captured in randomized trials. Additionally, convalescent plasma requires plasmapheresis to collect samples from donors, which is of significant cost and not widely available.
Considering the lack of benefit found in randomized trials, overall moderate quality evidence, associated costs, feasibility issues, and the availability of other effective alternatives, we issued a strong recommendation against using convalescent plasma in patients with COVID-19 in the ICU (Supplementary Table). Our recommendation is consistent with other published guidelines [[13], [14], [15]].
Immunoglobulins
Question: Should intravenous immunoglobulin (IVIG) vs. no IVIG be used for severe or critical COVID-19?
Recommendation
For adults with severe or critical COVID-19 in the ICU, the panel suggests against the routine use of IVIG outside the context of clinical trials (weak recommendation, low quality evidence).
Rationale
Intravenous immunoglobulin, a human blood product containing polyclonal immunoglobulin gamma, may improve passive immunity and modulate the hyperinflammatory response in COVID-19 patients through several proposed mechanisms including blocking proinflammatory cytokines as well as neutrophil activation [40,41].
We identified 3 relevant RCTs (n = 176) that compared the use of IVIG to placebo in patients with severe COVID-19 [[42], [43], [44]]. Doses of IVIG and standard of care varied between trials as follows: the first study enrolled 59 patients, comparing 20 g IVIG daily for 3 days to a placebo [42]. The second study enrolled 84 patients and used 400 mg/kg of IVIG daily for 3 days, in addition to using HCQ and lopinavir/ritonavir in both groups [44]. The third study enrolled 33 patients and used 0.5 g/kg of IVIG daily for 3 days in addition to methylprednisolone [43].
We identified a phase 2 RCT (n = 100) comparing IVIG to standard of care; however, this study focused on mild-to-moderate disease and only a few patients were admitted to the ICU, hence, it was not included in the quantitative analysis [45].
We undertook a meta-analysis pooling the effect sizes across the eligible studies (Supplementary Figure). Immunoglobulin use did not reduce risks of death (RR 0.66, 95% CI 0.31–1.42; low quality) or need for mechanical ventilation (RR 0.73, 95% CI 0.18–2.91; very low quality). The effect on ICU and hospital length of stay was unclear (MD 0.93, 95% CI −0.58 to 2.44; very low quality) and (MD 2.96 days, 95% CI 1.39–4.53; very low quality), respectively.
Only one trial reported on the safety of IVIG in COVID-19 [45]. However, indirect evidence from use in other conditions showed a good safety profile, with few adverse effects (e.g., chills, headache, low-grade fever, nausea, vomiting, self-limited aseptic meningitis) [46]. IVIG is expensive and is not widely available. Furthermore, we could not find data on cost-effectiveness.
Considering the low quality evidence, unclear effect on patient-important outcomes, associated cost, and availability and feasibility issues, the panel issued a weak recommendation against the routine use of IVIG in critical COVID-19 outside clinical trials. Our recommendation should not be applied to patients on IVIG for other medical conditions. Additionally, IVIG may have some role in neurological complications such as COVID-19-related encephalomyelitis, neuropathy, and demyelinating neuropathies. Our recommendation is consistent with NIH guidelines [14]. More clinical trials are needed to assess the safety and efficacy of IVIG in critical COVID-19.
-
II
Antiviral therapy
Remdesivir
Question: Should remdesivir vs. no remdesivir be used for invasively ventilated adults with COVID-19?
Recommendation
For mechanically ventilated adults with critical COVID-19, we suggest against using remdesivir outside the context of clinical trials (weak recommendation, low quality evidence).
Rationale
Remdesivir (GS-5734) is a broad-spectrum antiviral agent and a prodrug of its parent adenosine analog, GS-441524 [47]. Since it is a viral RNA-dependent RNA polymerase inhibitor, it can be used against various RNA viruses [48]. Remdesivir was initially developed for the treatment of the Ebola virus; however, clinical trials failed to show survival benefit [49]. Additionally, remdesivir may have antiviral activity against SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV) and Nipah virus [50]. Therefore, remdesivir gained worldwide attention because of its anti-SARS-CoV-2 activity in in vitro and animal models.
The SOLIDARITY trial investigators undertook a meta-analysis including other major trials, they performed a subgroup analysis comparing the efficacy of remdesivir with placebo in mechanically ventilated patients (3 RCTs, 1014 patients) [51]. In this meta-analysis, the effect of remdesivir on risk of death in invasively ventilated patients was unclear (RR 1.16; 95% CI 0.85–1.6; low quality).
Although remdesivir use maybe associated with hepatotoxicity and other adverse events such as cardiac toxicity, our meta-analysis (Supplementary Table) of 3 RCTs showed that remdesivir was associated with lower risk of serious adverse events compared to placebo (RR 0.76; 95% CI 0.62–0.92; moderate quality).
Given the uncertainty about the desirable effects of remdesivir in invasively ventilated patients, the associated high costs, and limited drug availability in Saudi Arabia, the panel issued a weak recommendation against its routine use outside the context of clinical research (Supplementary Table). If clinicians decide to use remdesivir in selected patients, they should avoid using it in patients with acute or chronic kidney disease and in those with acute liver failure or elevated liver enzymes (>5 times upper normal limit). Similarly, other guidelines, including those from WHO, NIH, and the Surviving Sepsis Campaign, suggested against using remdesivir in critical COVID-19 [13,14,52].
Favipiravir
Question: Should favipiravir vs. no favipiravir be used for severe or critical COVID-19?
Recommendation
For adults with severe or critical COVID-19, the panel suggests against the routine use of favipiravir outside the context of clinical trials (weak recommendation, very low quality evidence).
Rationale
Favipiravir is a selective RNA polymerase enzyme inhibitor which can prevent replication of the viral genome and has shown wide range of antiviral activity against different influenza viruses [[53], [54], [55]].
Our search identified two systematic reviews and meta-analyses that summarized the results of RCTs on favipiravir in hospitalized COVID-19 patients [56,57]. The most recent preprint systematic review and meta-analysis included 9 RCTs (n = 827) comparing favipiravir to any other intervention; out of those, only 3 studies compared favipiravir to usual care or placebo [[58], [59], [60]].
Only 3 RCTs reported on mortality [[59], [60], [61]]. Favipiravir did not reduce risk of death compared to other interventions (RR 0.70; 95% CI 0.26–1.28; very low quality). At 14 days, favipiravir did not result in clinical improvement (RR 1.10; 95% CI 0.97–1.25; very low quality) [[59], [60], [61], [62], [63], [64]]. Two studies reported no effect of favipiravir on the need for ICU transfer compared to controls [60,63]. Nausea, vomiting, diarrhea, and elevated uric acid and liver transaminases were the most commonly reported adverse events of favipiravir [59,[61], [62], [63], [64], [65]].
Studies were at high risk of bias. Moreover, the studies included different comparative arms, had small sample sizes, and used different dosing and duration of favipiravir therapy. Therefore, the overall quality of this indirect evidence is very low. There are however, several registered RCTs examining the efficacy and safety of favipiravir in hospitalized COVID-19 (NCT04373733, NCT04345419, NCT04351295, NCT04349241, NCT04356495, NCT04310228, NCT04319900, NCT04333589, NCT04358549, NCT04346628).
Considering the availability of other effective therapies, the lack of direct evidence, and associated adverse effects, the panel issued a weak recommendation against the use of favipiravir in critical COVID-19 outside clinical trials. Future clinical trials are needed to assess the efficacy and safety of favipiravir in critical COVID-19.
-
III
Hydroxychloroquine (HCQ)
Question: Should HCQ vs. no HCQ be used for severe or critical COVID-19?
Recommendation
For adults with severe or critical COVID-19 in the ICU, the panel recommends against using HCQ (strong recommendation, moderate quality evidence).
Rationale
Few studies showed in vitro activity of HCQ against SARS-CoV-2. The long clinical experience, its wide availability, low cost, and relative safety compared to chloroquine encouraged the use of HCQ for COVID-19 therapy early in the pandemic [15,66].
We identified a systematic review and meta-analysis summarizing 26 RCTs (n = 10,012) on HCQ in COVID-19 [67]. While most trials were small, the evidence from cumulative meta-analysis was dominated by the RECOVERY and the SOLIDARITY trials [51,68]. Both trials used HCQ in higher doses than all other trials except REMAP-CAP [67]. The meta-analysis revealed no mortality benefit of hospitalized patients with confirmed COVID-19. Of the 5696 patients treated with HCQ, 960 (16.9%) died compared to 606 (14.0%) of 4316 patients in the control groups (OR 1.11; 95% CI 1.02–1.20, moderate quality, [Supplement]). The effect was less clear in the subgroup of ICU patients (OR 1.04; 95% CI 0.49–2.18, very low quality).
Serious adverse events were reported in 3 RCTs. The pooled analysis showed higher risk of serious adverse events with HCQ use (RR 2.63; CI 1.36–5.09, low quality), the results are summarized in the Supplementary Table.
Considering the moderate quality evidence of no mortality benefit (and possible harm), and the associated serious adverse events, the panel issued a strong recommendation against using HCQ to treat critical COVID-19 cases (Supplementary Table). Our recommendation is consistent with most prominent international guidelines [[13], [14], [15]]. Additional studies on the role of HCQ in critical COVID-19 are probably not needed and future research should be focused on other therapeutic options.
-
IV
Anticoagulation
Question: Should therapeutic anticoagulation vs. prophylactic dose anticoagulation be used for critical COVID-19?
Recommendation
For adults with critical COVID-19 and no clinical suspicion of venous thromboembolism (VTE), we suggest using prophylactic dosing anticoagulation over therapeutic anticoagulation (weak recommendation, low quality evidence).
Remarks:
This recommendation does not apply to patients with high suspicion of (or confirmed) acute VTE or those with other indications for therapeutic anticoagulation.
Rationale
The rates of arterial thrombosis and VTE in COVID-19 patients are variable but reported to be higher than in non-COVID-19 patients. A systematic review and meta-analysis of 11 observational studies showed VTE rates around 23.9% (95% CI 16.2%–33.7%) despite prophylactic anticoagulation [69]. The rate of pulmonary embolism is relatively high in ICU COVID-19 patients (15%; 95% CI 9–25%) [69]. Similarly, the rates of arterial thrombosis such as myocardial infarction and stroke are high in ICU COVID-19 patients (13.9% and 3.7%, respectively) [70].
Until recently, there were no peer-reviewed RCTs addressing therapeutic anticoagulation compared to prophylactic anticoagulation in COVID-19 patients. Three open-label platform trials (REMAP-CAP, ATTACC, and ACTIV-4a as preprint) examined the effect of therapeutic anticoagulation, versus prophylactic or intermediate-intensity VTE prophylaxis in ICU COVID-19 patients [71]. Recruitment was terminated for futility after an interim analysis of 1074 patients. The combined analysis of these trials showed no difference in hospital mortality (OR 1.05, 95% CI 0.82–1.35, low quality [Supplement]) or days without organ support (adjusted OR 0.87, 95% credible interval [CrI] 0.70–1.08). In addition, the composite outcome of death or major thrombotic event did not differ between the two groups (adjusted OR 1.05, 95% CrI 0.79–1.40). However, therapeutic anticoagulation reduced major thrombotic events (5.7% versus 10.3%, low quality) and resulted in a small increase in the risk of major bleeding (3.1% versus 2.4%, low quality) [71].
These trials have several limitations: the quality of evidence is tempered by lack of blinding, high dropout rate (>10%), and early termination of recruitment. Therefore, the overall quality of evidence was judged to be low (Supplementary Table). In addition, the trials did not focus on high-risk patients such as those with elevated D-dimer levels [70,72].
Considering the lack of clear benefit, the potential for harm, and the low quality of evidence, the panel issued a weak recommendation favoring the use of prophylaxtic dosing VTE prophylaxis over therapeutic anticoagulation in COVID-19 patients. Our recommendation is similar to that of the American Society of Hematology [73]. In patients with D-dimer above 10,000 ng/mL [70], clinicians might need to rule out VTE, however, the optimal approach to address this problem is unclear. There remains uncertainty about using D-dimer levels to guide therapeutic decisions; that could be further studied in future research.
Future updates
The SCCS Guidelines Chapter members will meet monthly to discuss new evidence on relevant topics to this guideline. We plan to update recommendations in view of new evidence that would likely result in changing the recommendation strength and/or direction.
Discussion
The SCCS COVID-19 panel included 51 experts with expertise in critical care, respirology, infectious disease, epidemiology, emergency medicine, clinical pharmacy, nursing, respiratory therapy, methodology, and health policy. The panel addressed 9 questions that are related to the therapy of COVID-19 in the ICU. We used the GRADE approach and the evidence-to-decision framework (EtD) to generate recommendations.
The SCCS COVID-19 panel issued 12 recommendations on pharmacotherapeutic interventions for severe and critical COVID-19, of which 3 were strong recommendations and 9 were weak recommendations.
There are several strengths of these guidelines. The guidelines were developed using rigorous methodology, including comprehensive evidence synthesis and assessment, application of robust tools to allow reproducibility of the guidelines, and selecting panel members with attention to clinical, gender, and geographic diversity.
Although some members of the panel have suffered from COVID-19 and recovered, we did not formally include patient representatives in the guideline development process.
Conclusion
The SCCS COVID-19 recommendations can be used to facilitate evidence-based clinical decisions at the bedside. Additionally, the EtD framework allows adaptation of these recommendations to different contexts. The SCCS guideline committee will update recommendations as new evidence becomes available.
Author’s contributions
All authors confirm substantial contribution and provided content expertise on the production and final revision of this guideline. The corresponding author attests that all listed authors meet authorship criteria and that none have been intentionally omitted.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Copyright is transferred to the publisher in case of acceptance. The corresponding author had full access to all data presented and the final responsibility of deciding to submit the study for publication.
Availability of supporting data
All data produced and analyzed in this study are included in this article in Table 1, Fig. 1 and Supplement material.
Competing interest
All panel members completed the WHO declaration forms; none of the panel members declared any financial conflicts relevant to this guideline. Dr Waleed Alhazzani is a co-chair of the surviving sepsis campaign COVID-19 guidelines and member of the GRADE Working Group, Dr Yaseen Arabi is a co-investigator on REMAP-CAP trial.
Funding
The production of this guideline did not receive grant from any funding agency in the public, commercial or nonprofit sectors.
Acknowledgements
Authors would like to express gratitude to Drs. Kimberly Lewis and Manoj Mammen for helping with the meta-analysis and Ms. Laila Perlas Asonto for clerical assistance.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2021.10.005.
Includes evidence profiles and evidence to decision tables for each of the recommendations.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Alhazzani W., Al-Suwaidan F., Al Aseri Z., Al Mutair A., Alghamdi G., Rabaan A., et al. The Saudi critical care society clinical practice guidelines on the management of COVID-19 patients in the intensive care unit. Saudi Crit Care J. 2020;4(2):27–44. [Google Scholar]
- 2.Alhazzani W., Lewis K., Jaeschke R., Rochwerg B., Moller M.H., Evans L., et al. Conflicts of interest disclosure forms and management in critical care clinical practice guidelines. Intensive Care Med. 2018;44(10):1691–1698. doi: 10.1007/s00134-018-5367-6. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Andrews J., Guyatt G., Oxman A.D., Alderson P., Dahm P., Falck-Ytter Y., et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Guyatt G.H., Oxman A.D., Santesso N., Helfand M., Vist G., Kunz R., et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66(2):158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Al Sulaiman K.A., Aljuhani O., Eljaaly K., Alharbi A.A., Al Shabasy A.M., Alsaeedi A.S., et al. Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study. Int J Infect Dis. 2021;105:180–187. doi: 10.1016/j.ijid.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arabi Y.M., Chrousos G.P., Meduri G.U. The ten reasons why corticosteroid therapy reduces mortality in severe COVID-19. Intensive Care Med. 2020;46(11):2067–2070. doi: 10.1007/s00134-020-06223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group WHOREAfC-TW, Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao T.C., Huang Y.W., Chang S.M., Tsai S.Y., Wu A.C., Tsai H.J. Association between oral corticosteroid bursts and severe adverse events : a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325–330. doi: 10.7326/M20-0432. [DOI] [PubMed] [Google Scholar]
- 12.Lamontagne F., Agoritsas T., Macdonald H., Leo Y.S., Diaz J., Agarwal A., et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 13.Alhazzani W., Evans L., Alshamsi F., Moller M.H., Ostermann M., Prescott H.C., et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 14.COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2021. Coronavirus disease 2019 (COVID-19) treatment guidelines. [PubMed] [Google Scholar]
- 15.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C., et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically Ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. Accession Number: 32301997 PMCID: PMC7184354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner H.I., Ruperto N., Zuber Z., Keane C., Harari O., Kenwright A., et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis. 2015;74(6):1110–1117. doi: 10.1136/annrheumdis-2014-205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genovese M.C., van Adelsberg J., Fan C., Graham N.M.H., van Hoogstraten H., Parrino J., et al. Two years of sarilumab in patients with rheumatoid arthritis and an inadequate response to MTX: safety, efficacy and radiographic outcomes. Rheumatology (Oxford) 2018;57(8):1423–1431. doi: 10.1093/rheumatology/key121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokota S., Imagawa T., Mori M., Miyamae T., Aihara Y., Takei S., et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371(9617):998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 20.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A., et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. Oncologist. 2018;23(8):943. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P., et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Investigators R.-C., Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosas I.O., Brau N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soin A.S., Kumar K., Choudhary N.S., Sharma P., Mehta Y., Kataria S., et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9(5):511–521. doi: 10.1016/S2213-2600(21)00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veiga V.C., Prats J., Farias D.L.C., Rosa R.G., Dourado L.K., Zampieri F.G., et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell L., Chen C., Bhagat S.S., Parker R.A., Ostor A.J. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2011;50(3):552–562. doi: 10.1093/rheumatology/keq343. [DOI] [PubMed] [Google Scholar]
- 31.Geng Z., Yu Y., Hu S., Dong L., Ye C. Tocilizumab and the risk of respiratory adverse events in patients with rheumatoid arthritis: a systematic review and meta-analysis of randomised controlled trials. Clin Exp Rheumatol. 2019;37(2):318–323. [PubMed] [Google Scholar]
- 32.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sims J.T., Krishnan V., Chang C.Y., Engle S.M., Casalini G., Rodgers G.H., et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol. 2021;147(1):107–111. doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vazquez C., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janiaud P., Axfors C., Schmitt A.M., Gloy V., Ebrahimi F., Hepprich M., et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185–1195. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung I.F.N., To K.K.W., Lee C.K., Lee K.L., Yan W.W., Chan K., et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144(2):464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y., Yu X., Zhang F., Xia Y. Evaluation of the effect of intravenous immunoglobulin dosing on mortality in patients with sepsis: a network meta-analysis. Clin Ther. 2019;41(9):1823–1838. doi: 10.1016/j.clinthera.2019.06.010. e1824. [DOI] [PubMed] [Google Scholar]
- 42.Gharebaghi N., Nejadrahim R., Mousavi S.J., Sadat-Ebrahimi S.R., Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi: 10.1186/s12879-020-05507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakoulas G., Geriak M., Kullar R., Greenwood K.L., Habib M., Vyas A., et al. Intravenous Immunoglobulin Plus Methylprednisolone Mitigate Respiratory Morbidity in Coronavirus Disease 2019. Crit Care Explor. 2020;2(11):e0280. doi: 10.1097/CCE.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabarsi P., Barati S., Jamaati H., Haseli S., Marjani M., Moniri A., et al. Evaluating the effects of Intravenous Immunoglobulin (IVIg) on the management of severe COVID-19 cases: a randomized controlled trial. Int Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R SR, Barge V.B., Darivenula A.K., Dandu H., Kartha R.R., Bafna V., et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in COVID-19 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538–1543. doi: 10.1093/infdis/jiab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delforge M., Farber C.M., Spath P., Kaveri S., Witte T., Misbah S.A., et al. Recommended indications for the administration of polyclonal immunoglobulin preparations. Acta Clin Belg. 2011;66(5):346–360. doi: 10.2143/ACB.66.5.2062586. [DOI] [PubMed] [Google Scholar]
- 47.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pokhrel R., Chapagain P., Siltberg-Liberles J. Potential RNA-dependent RNA polymerase inhibitors as prospective therapeutics against SARS-CoV-2. J Med Microbiol. 2020;69(6):864–873. doi: 10.1099/jmm.0.001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardo J., Shukla A.M., Chamarthi G., Gupte A. The journey of remdesivir: from Ebola to COVID-19. Drugs Context. 2020;9 doi: 10.7573/dic.2020-4-14. 2020-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saqrane S., El Mhammedi M.A., Lahrich S., Laghrib F., El Bouabi Y., Farahi A., et al. Recent knowledge in favor of remdesivir (GS-5734) as a therapeutic option for the COVID-19 infections. J Infect Public Health. 2021;14(5):655–660. doi: 10.1016/j.jiph.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Consortium WHOST, Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rochwerg B., Agarwal A., Zeng L., Leo Y.S., Appiah J.A., Agoritsas T., et al. Remdesivir for severe covid-19: a clinical practice guideline. BMJ. 2020;370:m2924. doi: 10.1136/bmj.m2924. [DOI] [PubMed] [Google Scholar]
- 53.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smee D.F., Hurst B.L., Wong M.H., Bailey K.W., Tarbet E.B., Morrey J.D., et al. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice. Antimicrob Agents Chemother. 2010;54(1):126–133. doi: 10.1128/AAC.00933-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sleeman K., Mishin V.P., Deyde V.M., Furuta Y., Klimov A.I., Gubareva L.V. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses. Antimicrob Agents Chemother. 2010;54(6):2517–2524. doi: 10.1128/AAC.01739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shrestha D.B., Budhathoki P., Khadka S., Shah P.B., Pokharel N., Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. 2020;17(1):141. doi: 10.1186/s12985-020-01412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassanipour S., Arab-Zozani M., Amani B., Heidarzad F., Fathalipour M., Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11(1):11022. doi: 10.1038/s41598-021-90551-6. Accession Number: 34040117 PMCID: PMC8155021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivashchenko A.A., Dmitriev K.A., Vostokova N.V., Azarova V.N., Blinow A.A., Egorova A.N., et al. AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73(3):531–534. doi: 10.1093/cid/ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Udwadia Z.F., Singh P., Barkate H., Patil S., Rangwala S., Pendse A., et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. doi: 10.1016/j.ijid.2020.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khamis F., Al Naabi H., Al Lawati A., Ambusaidi Z., Al Sharji M., Al Barwani U., et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis. 2021;102:538–543. doi: 10.1016/j.ijid.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dabbous H.M., Abd-Elsalam S., El-Sayed M.H., Sherief A.F., Ebeid F.F.S., El Ghafar M.S.A., et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch Virol. 2021;166(3):949–954. doi: 10.1007/s00705-021-04956-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Zhao H., Zhu Q., Zhang C., Li J., Wei M., Qin Y., et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: a multicenter trial in a small sample size. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lou Y., Liu L., Yao H., Hu X., Su J., Xu K., et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 2021;157 doi: 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prakash A., Singh H., Kaur H., Semwal A., Sarma P., Bhattacharyya A., et al. Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (COVID-19) patients. Indian J Pharmacol. 2020;52(5):414–421. doi: 10.4103/ijp.ijp_998_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eljaaly K., Alireza K.H., Alshehri S., Al-Tawfiq J.A. Hydroxychloroquine safety: a meta-analysis of randomized controlled trials. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Axfors C., Schmitt A.M., Janiaud P., Van’t Hooft J., Abd-Elsalam S., Abdo E.F., et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-22446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Group R.C., Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., et al. Effect of Hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chi G., Lee J.J., Jamil A., Gunnam V., Najafi H., Memar Montazerin S., et al. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. J Clin Med. 2020;9(8) doi: 10.3390/jcm9082489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarychanski R. Therapeutic anticoagulation in critically ill patients with Covid-19 – preliminary report. medRxiv. 2021 2021.2003.2010.21252749. [Google Scholar]
- 72.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cuker A., Tseng E.K., Nieuwlaat R., Angchaisuksiri P., Blair C., Dane K., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data produced and analyzed in this study are included in this article in Table 1, Fig. 1 and Supplement material.