Abstract
Recent transcriptomic, histological and functional studies have begun to shine light on the fibroblasts present in the meninges, choroid plexus and perivascular spaces of the brain and spinal cord. Although the origins and functions of CNS fibroblasts are still being described, it is clear that they represent a distinct cell population, or populations, that have likely been confused with other cell types on the basis of the expression of overlapping cellular markers. Recent work has revealed that fibroblasts play crucial roles in fibrotic scar formation in the CNS after injury and inflammation, which have also been attributed to other perivascular cell types such as pericytes and vascular smooth muscle cells. In this Review, we describe the current knowledge of the location and identity of CNS perivascular cell types, with a particular focus on CNS fibroblasts, including their origin, subtypes, roles in health and disease, and future areas for study.
Subject terms: Blood-brain barrier, Neurodegeneration, Neuro-vascular interactions, Diseases of the nervous system
Fibroblasts in the CNS have been assigned a role in fibrotic scar formation in response to injury and inflammation but might perform additional roles attributed to other cell types. In this Review, Dorrier and colleagues discuss the available evidence regarding fibroblast functions in the CNS.
Introduction
With the advent of single-cell sequencing technologies, it has become increasingly recognized that fibroblasts are present in perivascular spaces, meninges, and choroid plexus of the brain and spinal cord1–3. Recent studies have revealed greater detail about the roles that these fibroblasts play in health and disease in the CNS. For example, in the meninges, fibroblasts secrete cytokines that are important for immune regulation and antigen recognition3. In addition, meningeal and perivascular fibroblasts are the main drivers of fibrotic scarring after neuroinflammation and ablating proliferating fibroblasts to reduce scarring leads to decreases in motor disability4.
Despite these recent advances, we still know very little about the roles of CNS fibroblasts in the development and maintenance of the healthy, adult CNS and how they contribute to disease aside from fibrotic scarring. While we focus mostly on rodent studies in this Review, perivascular fibroblasts have been detected in human tumour samples5 and associated with the vasculature of the human brain6,7, confirming that these cells are indeed present in the human CNS. Furthermore, perivascular fibroblasts have been shown to be dysfunctional in patients with amyotrophic lateral sclerosis (ALS)8. More in-depth analyses of human CNS fibroblasts will determine whether these cells are potential therapeutic targets for neurological diseases.
In this Review, we summarize what is known about fibroblasts in the CNS and discuss the gaps in our knowledge regarding their subtypes and functions. First, we compare the localizations and markers for many CNS perivascular cell types, including fibroblasts, as these cells are often mistaken for each other owing to a lack of specific molecular markers. Second, we provide an overview of the localization of CNS fibroblasts in perivascular spaces, meninges, and choroid plexus and discuss evidence regarding the developmental origins of fibroblasts in each of these regions. Third, we summarize the known roles of CNS fibroblasts in health and disease with a focus on reticular networks and fibrotic scarring. While perivascular and meningeal fibroblasts have been shown to be the main drivers of fibrotic scarring after experimental autoimmune encephalomyelitis (EAE; a model of the neuroinflammatory disease multiple sclerosis (MS)), rigorous experiments are needed to establish the involvement of these cells in other conditions with fibrotic scarring such as stroke and spinal cord injury (SCI). Finally, we discuss the main unanswered questions in CNS fibroblast biology and how future research studies can enhance our understanding of these important cells.
Localization of fibroblasts in the CNS
Perivascular spaces
Perivascular spaces in the CNS are home to various cell types that have important roles in communicating between the periphery and the brain and spinal cord parenchyma. These fluid-filled spaces are continuous with the subarachnoid space of the meninges, allowing for fluid transfer with the meninges, and are also a major route of solute clearance from the CNS9–11. Perivascular spaces are bordered by the vascular basement membrane produced by endothelial cells and mural cells and the glial basement membrane produced by astrocyte endfeet. In capillaries, these membranes are fused to form a composite basement membrane12. In penetrating arterioles, precapillary arterioles, postcapillary venules and venules, the glial and vascular basement membranes are separated by a fluid-filled perivascular space also referred to as the Virchow–Robin space9,13,14. Perivascular cells, which include mural cells (pericytes and vascular smooth muscle cells (vSMCs)), perivascular macrophages and perivascular fibroblasts, have been implicated in many different processes in the CNS, including fibrotic scarring. However, the lack of good markers to distinguish between these cell types has made it difficult to examine the specific roles of each cell type. Below, we describe the locations, functions and molecular markers for each of these cell types (Tables 1,2).
Table 1.
Histological markers of perivascular cell types and meningeal and choroid plexus fibroblasts
| Cell types | Markers | Refs |
|---|---|---|
| Pericytes | PDGFRβ, NG2, desmin, KCNJ8, ABCC9 and CD13 | 1,15–17 |
| Vascular smooth muscle cells | PDGFRβ, αSMA, CD13, NG2, CD146 and desmin | 1,17 |
| Macrophages | CD163, CD206, LYVE1 and F4/80 | 21,119 |
| Fibroblasts | PDGFRβ, PDGFRα, COL1A1, ERTR7, LAMA1, CD13 and FN | 1,2,4,23,24,26,28,29 |
| Dural fibroblasts | FXYD5, FOXP1 and SIX1 | 27 |
| Arachnoid fibroblasts | CRABP2, ALDH1A2 and SLC6A13 | 27 |
| Pial fibroblasts | S100A6 and NGFR | 27 |
Table 2.
Transgenic mice for lineage tracing of perivascular cell types and meningeal and choroid plexus fibroblasts
| Transgenic strain | Labelled cell types | Refs |
|---|---|---|
| Col1a2–CreERT | Fibroblasts and a small population (<5%) of pericytes and vSMCs | 4,120 |
| Pdgfrb–CreERT2 | Fibroblasts, pericytes, vSMCs and lymphatic endothelial cells | 121,122 |
| Slc1a3–CreER | Astrocytes, type A pericytes and fibroblasts | 1,87 |
| Cspg4–CreERTM | Pericytes, vSMCs and OPCs | 123–125 |
| Acta2–CreERT2 | Pericytes and vSMCs | 126 |
| Col1a1-GFP | Fibroblasts and a small population (<2%) of pericytes and vSMCs | 4,26 |
| Tbx18–CreERT | Fibroblasts, pericytes and vSMCs | 27,127 |
OPC, oligodendrocyte progenitor cell; vSMC, vascular smooth muscle cell.
Pericytes are located within the endothelial basement membrane of capillaries and extend finger-like processes that incompletely cover the endothelial tube (Fig. 1a). Commonly used markers of CNS pericytes include PDGFRβ and chondroitin sulfate proteoglycan NG2 (encoded by CSPG4), but neither marker is specific to pericytes as they are also expressed by fibroblasts and oligodendrocyte progenitor cells, respectively, and at lower levels in vSMCs15. Many of the proposed functions of pericytes have been determined in studies that relied on these markers (specifically PDGFRβ) for their identification. Consequently, many conclusions drawn from these studies may not be specific to pericytes. Sequencing studies have identified additional potential pericyte markers, including KCNJ8 and ABCC9, although these markers are rarely used to identify pericytes1,16.
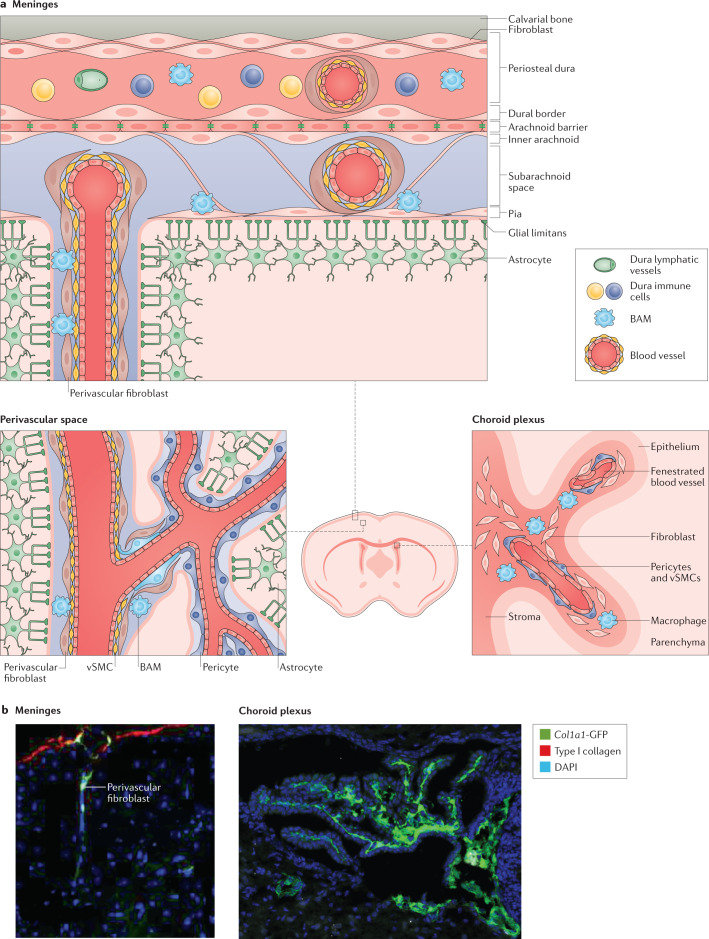
Fig. 1. Localization of fibroblasts in the adult mouse brain.
a | Fibroblasts are present in the meninges, choroid plexus and perivascular spaces. Fibroblasts are present in all three meningeal layers, the pia mater, arachnoid mater and dura mater9,10,30–34,37. Different immune cell populations and vasculature (barrier, non-barrier blood vasculature and lymphatic vessels) are distributed between the leptomeninges (that is, the pia and arachnoid) and dura mater20,36. Perivascular fibroblasts surround blood vessels in the dura, leptomeninges, penetrating arterioles and pre-capillary arterioles with ‘ensheathing’ pericytes but not capillaries1,9,10,22,41. Fibroblasts are located in the stroma (the inner region of the choroid plexus), which is surrounded by the epithelium, adjacent to non-barrier blood vasculature and macrophages43,45,46. b | In adult Col1a1-GFP mice, expression of GFP from the Col1a1 promoter is used to mark fibroblasts in the meninges and perivascular spaces4,26 (left) and within the stroma of the choroid plexus27 (right). BAM, border-associated macrophage; GFP, green fluorescent protein; vSMC, vascular smooth muscle cell. Left image in part b adapted from ref.4, Springer Nature Limited. Right image in part b adapted from ref.27, Elsevier.
vSMCs form continuous sheets around the walls of arteries, arterioles, and veins and have a major role in controlling blood flow of these larger vessels9 (Fig. 1a). Like other perivascular cell types, vSMCs express PDGFRβ, αSMA, CD13, NG2, CD146 and desmin17, although they show higher expression of αSMA and desmin but lower expression of NG2 (ref.17) and PDGFRβ18 than pericytes.
Perivascular macrophages (also known as border-associated macrophages) are part of the immune surveillance system in the brain and lie adjacent to parenchymal blood vessels19,20. CD163, CD206 and LYVE1 are the most widely used markers for this cell type, and these markers, along with their perivascular location, are used to distinguish these cells from microglia21.
Perivascular fibroblasts in the CNS are found immediately adjacent to vSMCs on arteries, arterioles, venules, and veins and are morphologically distinct from other perivascular cell types as they have flattened somata and ruffled, sheet-like membrane processes9,10,22 (Fig. 1a,b). Fibroblasts throughout the body (including those in the CNS) express type I collagen, a polymer of COL1A1 and COL1A2 proteins (encoded by COL1A1 and COL1A2, respectively), which is commonly used in sequencing and histological studies as a molecular marker of these cells23,24. A mouse Col1a1-GFP strain has been used to visualize fibroblasts, including perivascular fibroblasts, meningeal fibroblasts and choroid plexus fibroblasts4,25–27 (Fig. 1b). The ER-TR7 antibody is often used to label reticular fibroblasts and their adjoining extracellular network, although the molecular identity of this antigen is unknown28,29. All of these tools can be used to mark CNS fibroblasts in the perivascular spaces, meninges and choroid plexus. Perivascular fibroblasts in the CNS express both PDGFRα and PDGFRβ1,4 and it is likely that they have been confused with other PDGFRβ+ cells such as pericytes and vSMCs.
Meningeal fibroblasts form the distinct layers of the meninges
CNS fibroblasts are found throughout the three meningeal layers: the pia mater, arachnoid mater (collectively known as the leptomeninges) and dura mater (Fig. 1a,b). The dura mater is the strong outer layer of the meninges, consists primarily of collagen fibres and contains fenestrated blood vessels that connect this meningeal layer with the periphery9. Fibroblasts and collagen fibres attach the outer layer of the dura to the skull and a thin layer of fibroblasts (dural border cells) separates the dura from the arachnoid30,31. Below the dura is the arachnoid barrier layer, which consists of an outer layer of epithelial-like cells connected by tight junctions32–34. The arachnoid barrier layer is part of the blood–cerebrospinal fluid (CSF) barrier35 and functional studies using horseradish peroxidase show that this layer prevents the free movement of molecules from the dura into the subarachnoid space36 that lies beneath the arachnoid. The subarachnoid space contains a vascular network and immune cells and is traversed by arachnoid trabeculae that are formed by inner arachnoid fibroblasts and collagens, which connect this layer to the pia33,37. In the pia, a thin layer of fibroblasts and basement membrane separate the pia and the glia limitans, the layer between the parenchyma and meninges that is formed by astrocyte endfoot processes. The blood vessels in the leptomeninges are non-fenestrated, barrier vessels38,39 and are surrounded by a vSMC layer and an adventitial layer of fibroblasts13,22,40–42 that have been referred to as ‘pial’ or ‘leptomeningeal’ cells. The visualization of perivascular fibroblast morphology by two-photon live imaging in Col1a1-GFP mice revealed that perivascular fibroblasts along cerebral and leptomeningeal vessels have an essentially identical cell morphology22, suggesting that the perivascular fibroblasts in these two regions are likely the same cell type. However, it is currently unclear whether perivascular fibroblasts in the meninges or those associated with parenchymal vessels are a molecularly distinct cell type from fibroblasts in the pia or arachnoid layers.
Fibroblasts are present in the choroid plexus stroma
Each of the brain ventricles contain a choroid plexus that regulates cerebrospinal fluid composition and secretion43–45. The choroid plexus consists of an outer epithelial layer and an inner stromal compartment45 that contains various cell types (Fig. 1a,b). The cells of the epithelial layer are polarized and contain tight junctions, forming an essential component of the blood–CSF barrier separating the permeable blood vessels in the stromal layer and the CSF within the ventricles, which is secreted by the choroid plexus epithelial cells43,44. The stroma contains fibroblasts, distinct immune cell populations and a fenestrated vasculature with pericytes and vSMCs43,45,46 (Fig. 1a,b). A wide variety of immune cells, including macrophages and dendritic cells, reside in the stroma and it is thought that the choroid plexus along with the meninges serve as an important neuroimmunological gateway43,45. Stromal fibroblasts are thought to secrete extracellular matrix (ECM) and might thereby provide structural support in the choroid plexus43,47 in addition to having other, yet-to-be discovered roles.
CNS fibroblast origins and diversity
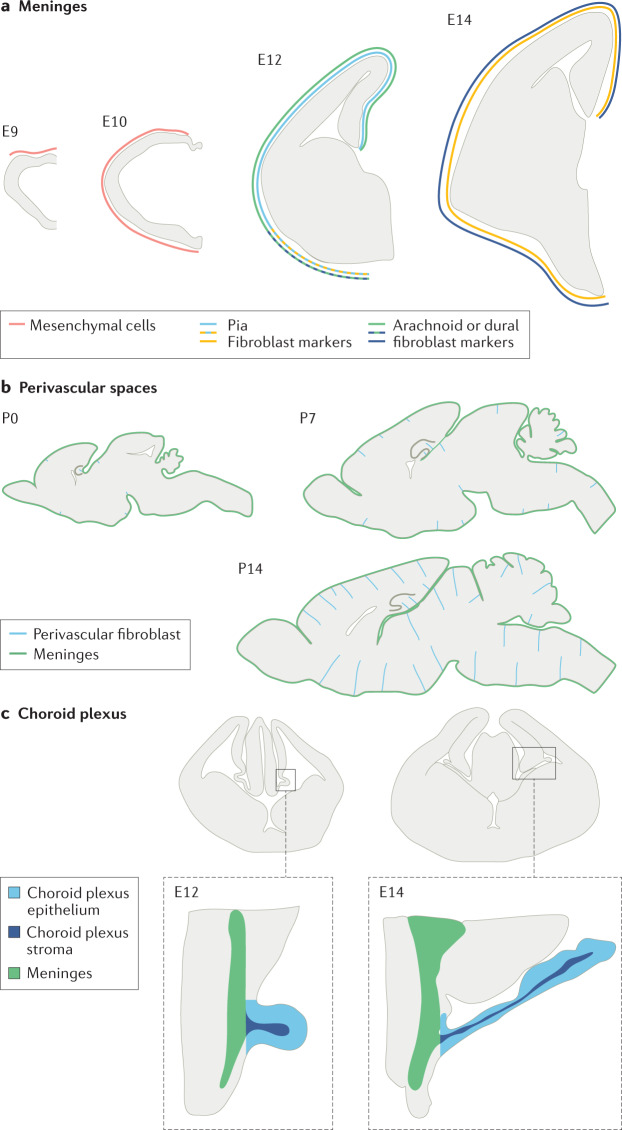
The development of CNS fibroblast populations has been most studied in the meninges. Lineage tracing studies in chick and mice show that meningeal fibroblasts in the midbrain, hindbrain and spinal cord are derived from the somatic and cephalic mesoderm, whereas those in the forebrain originate from the neural crest48–51. An immature meningeal primordium containing fibroblasts is detected by the fifth week of gestation in humans52 and between embryonic day 9 (E9) and E10 in mice37 (Fig. 2).
Fig. 2. Development of fibroblasts in different regions of the CNS.
a | Meningeal fibroblasts in the mouse forebrain first appear from the neural crest as undifferentiated mesenchymal cells at approximately embryonic day 9 (E9) and fully cover the forebrain by E10 (ref.37). Some layer-specific meningeal fibroblast markers first appear ventrally in the mouse forebrain at E12 (RALDH2 for arachnoid fibroblasts; the neurotrophin receptor p75 for pial fibroblasts) but are expressed over the entire forebrain by E14 (ref.27). b | Perivascular fibroblasts in the mouse brain parenchymal vasculature are infrequent at postnatal day 0 (P0) and located not far from the meninges53. Over the next 2 weeks of postnatal development, many more vessels have perivascular fibroblasts and the cells are present deeper in the brain. c | The choroid plexus stroma of the lateral ventricle contains fibroblasts and the blood vasculature is spatially continuous with the adjacent meninges and extends along with the growth of the choroid plexus epithelium43,56,57.
Recent single-cell transcriptional profiling has provided new insight into the molecular diversity of meningeal fibroblasts and their emergence during development27. This work showed that, in E14 mice, pial, arachnoid and dural fibroblasts have distinct gene expression profiles. Pial fibroblasts can be differentiated from dural and arachnoid fibroblasts by their expression of S100a6 and Ngfr, and they show enriched expression of genes involved in the production of ECM proteins, such as collagens, laminins and glycoproteins, consistent with their role in pial basement membrane maintenance. Arachnoid fibroblasts show enriched expression of various genes, including those encoding sulfate, magnesium and GABA transporters, and other ECM proteins implicated in regulation of ECM assembly. Dural fibroblasts have elevated expression of genes encoding small and large ribosomal subunits, the ion transport regulator FXYD5, and the transcription factors FOXP1 and SIX1. Markers of differentiating meningeal fibroblasts that are conserved across humans and mice include CRABP2 for arachnoid fibroblasts and S100a6 and p75NTR for pial fibroblasts27. Developmental studies show that many of the markers specific for the meningeal fibroblast layers are first detected between E12 and E14 in mice1, consistent with earlier electron microscopy studies describing distinct meningeal cell layers by E13 in rodents37 and the sixth week of gestation in humans52. In the forebrain, some markers specific for the meningeal fibroblast layer, such as RALDH2 (arachnoid fibroblasts), p75NTR (pial fibroblasts) and E-cadherin (arachnoid barrier cells), are first detected in ventral meninges before being detected in dorsal meninges27 (Fig. 2a). The expression of many, but not all, meningeal fibroblast markers identified in mouse embryos persists into adulthood, suggesting that meningeal fibroblast populations undergo additional, postnatal maturation in mice27.
In contrast to the meninges, the appearance of perivascular fibroblasts in the parenchymal vasculature in most brain regions occurs during mouse postnatal development. In mice, type I collagen-expressing perivascular fibroblasts gradually appear in all brain regions around the non-capillary vasculature in the first 3 weeks after birth53 (Fig. 2b). They first appear close to the pial surface and then later in deeper regions, suggesting that these cells originate from the meninges. Whether perivascular fibroblasts are pial fibroblasts that move into perivascular spaces, as inferred from electron and light microscopy studies of human and mouse brain tissue13,40,41, or are cellularly and molecularly distinct from pial fibroblasts remains to be resolved. The association of perivascular fibroblasts with the meningeal and parenchymal vasculature observed in electron microscopy studies and two-photon live imaging of fibroblasts in mice expressing Col1a1-GFP22 suggests that perivascular fibroblasts are morphologically distinct from pial fibroblasts. Perivascular fibroblasts wrap around large blood vessels and their cell bodies are aligned to vessels in the leptomeninges and brain, whereas pial fibroblast cell bodies and processes in the leptomeninges are aligned parallel to the brain surface13,27,42. Perivascular fibroblasts express many of the same markers as pial fibroblasts, including laminin α1 (refs1,41), PDGFRα1,53 and type I collagen, suggesting that these two fibroblast populations share some molecular characteristics. To better define the molecular characteristics of perivascular and pial fibroblasts, single-cell RNA sequencing of fibroblasts isolated separately from adult leptomeninges and brain will be required. However, a recent preprint study reporting vessel isolation and nuclei extraction for sequencing (VINE-seq) in humans used annotations from other sequencing studies to detect distinct clusters of perivascular and meningeal fibroblasts6 and found enriched expression of ECM components in perivascular fibroblasts whereas meningeal fibroblasts showed enriched expression of solute transporters6.
Although numerous studies have provided insights into the developmental origins, diversity and functions of choroid plexus epithelial cells46,54,55, investigation of the development of stromal cells, particularly choroid plexus fibroblasts, is limited. Ultrastructural studies in rodents and chick describe the choroid plexus stromal compartment as a connective tissue that emerges from the embryonic head mesenchyme and is thought to be developmentally related to leptomeningeal tissue, although a lack of lineage-tracing studies means that its precise developmental origin is unclear43,56,57 (Fig. 2c). A seminal study of human embryonic choroid plexus described an increasing abundance of fibroblasts and collagen fibrils in the stroma throughout development, suggesting the gradual differentiation of stromal fibroblasts43,47. However, the potential mechanisms controlling stromal fibroblast differentiation have not been uncovered, although it has been suggested that the stroma engages in cellular crosstalk with the developing choroid plexus epithelium and vascular plexus46.
Bulk and single-cell transcriptional profiling of fibroblasts in the meninges and perivascular spaces in healthy, adult mice revealed the presence of various subtypes of CNS fibroblasts. Comprehensive single-cell sequencing of brain vascular and perivascular cells in healthy mice found two clusters of perivascular fibroblasts, denoted F1 and F2 (ref.1). Both clusters express high levels of ECM proteins such as collagens and a comparison of CNS fibroblasts with lung fibroblasts found that 45 of the 50 transcripts most enriched in CNS fibroblasts compared with other CNS vascular cells were also found in lung fibroblasts1. Single-cell sequencing of spinal cord fibroblasts in healthy mice and in those with EAE also found two primary populations of fibroblasts as well as different states of activation of these cell populations in disease4. Further studies using techniques such as spatial single-cell sequencing and in situ hybridization are needed to better characterize these fibroblast subtypes and their localization within the CNS.
Similarly, a recent single-cell RNA sequencing analysis to molecularly profile the choroid plexus described fibroblasts that express Col1a1 and Pdgfra and show enriched expression of ECM protein transcripts in the choroid plexuses from each ventricle. Similar to the epithelium, choroid plexus fibroblasts in different ventricles (such as the lateral, third and fourth ventricles) displayed differential gene enrichment, suggesting that these fibroblasts may be patterned on the rostral–caudal axis and may have different functions in each ventricle2.
Several single-cell sequencing studies of the whole brain have also identified fibroblast cell clusters that represent different fibroblast subtypes, which likely contain a combination of fibroblasts from the meninges, perivascular spaces and choroid plexus58,59. In some of these studies, these cells were described as vascular leptomeningeal cells, although other names have been used to characterize the same populations58,60. Owing to the low abundance of fibroblasts in the brain, these whole brain analyses have only assessed a small number of cells for each cluster, which makes analysing diversity in these populations difficult using these datasets.
CNS fibroblast roles in health
The roles of CNS fibroblasts in healthy adults is just beginning to be studied. In the periphery, the foremost role of fibroblasts is to provide structural support to connective tissues through the secretion of ECM components, particularly type I and type III collagens61. Fibroblasts produce a wide variety of cytokines and growth factors and, when needed, can also differentiate into other cell types, such as fat cells or cartilage cells, in some tissues62. Peripheral fibroblasts are also crucial for angiogenesis as they secrete matrix proteins that facilitate tube formation and vascular endothelial growth factor (VEGF), which promotes angiogenesis63. They also sense and respond to mechanical stress by remodelling tissue as required and altering their membrane potential64,65.
The role of fibroblasts surrounding blood vessels in the perivascular space of the CNS is less clear. There is little evidence that these perivascular fibroblasts continually differentiate and angiogenesis is not widespread in the adult human CNS. Some researchers have proposed that fibroblasts covering pial vessels facilitate fluid exchange between the CSF and perivascular spaces9,11 and play roles in the development of the glymphatic system66 but more in-depth studies are needed to confirm these hypotheses.
Fibroblasts in the meninges provide structural support within and between the different meningeal layers. This role in maintaining the integrity and separation of the meningeal layers is vitally important as these layers have varying degrees of vascular leakage and therefore contact with the periphery9. As the meninges are home to a variety of immune cells20 and peripheral fibroblasts are known to recruit, maintain and interact with immune cells in various organs, including lymph nodes67,68, it is likely that there is signalling crosstalk between meningeal fibroblasts and neighbouring immune cells. Indeed, a recent study demonstrated that CXCL12 secretion by fibroblasts in the dura stroma contributes to the recruitment of T cells to dural sinuses, where these lymphocytes recognize antigens in the CSF3. More in depth experiments, both in vivo and in vitro, are needed to decode the full profile of signalling interactions of meningeal fibroblasts with immune cells.
The specific functions of choroid plexus fibroblasts in the healthy adult brain are also largely unknown, although some insights have been obtained from in vitro and transcriptomic studies of choroid plexus stromal cells. A study using cultured rat stroma found that a large proportion of intracellular adhesion molecule 1-positive (ICAM1+) fibroblast-like cells express the neural stem cell marker nestin and that these cells form clusters with choroid plexus myeloid cells, an interaction that was also observed in the choroid plexus in postnatal rats69. A recent single-cell analysis of choroid plexus cells also highlighted multiple potential functions of choroid plexus fibroblasts2. The enriched expression of genes encoding ECM components, such as collagen, fibronectin and prolyl-4-hydroxylase, indicates that choroid plexus fibroblasts might be an essential scaffold in the stroma2. The expression of nestin in a subset of choroid plexus fibroblasts suggests a degree of heterogeneity in this population of cells and that they might be progenitors of other stromal cell types2. Overall, the proximity of choroid plexus fibroblasts to the choroid plexus epithelium, vasculature and immune milieu could enable numerous cell–cell interactions that are important for choroid plexus maintenance and function.
CNS fibroblasts may also play a part in sensing changes in their environment, specifically changes in blood flow and tissue stiffness, via mechanotransduction. Cardiac fibroblasts are known to sense mechanical stress through membrane receptors such as integrins and respond by secreting ECM proteins, thereby remodelling local tissue64,65. Blood flow throughout the body is sensitive to environmental factors and fibroblasts in the CNS could respond to these changes over time by subtly remodelling their local tissue environment and signalling to nearby smooth muscle and endothelial cells. The exact responses of fibroblasts and the consequences of this mechanical sensing needs to be confirmed as these cells are present at crucial sites where they can monitor both the periphery and the CNS. Whether CNS fibroblasts are involved in maintaining the vascular basement membrane is not known but their high expression of ECM proteins suggests that this is likely1,4. The potential and confirmed roles of fibroblasts throughout the CNS are summarized in Table 3.
Table 3.
Confirmed and proposed roles of CNS fibroblasts in health and disease
| Location | Rolea | Refs |
|---|---|---|
| Health | ||
| Perivascular space | Development and function of the glymphatic system? | NA |
| Mechanosensation? | NA | |
| Maintenance of vascular basement membrane? | NA | |
| Meninges | Structural support | 9,32–34,37 |
| Separation of meningeal layers | 9,32–34,37 | |
| T cell trafficking and retention in the dura | 3 | |
| Choroid plexus | Progenitor cells? | 69 |
| Disease | ||
| Perivascular space | Spinal cord injury: fibrotic scarring blocks axon regeneration | 87,89–91 |
| EAE: fibrotic scarring blocks OPC migration | 4 | |
| Stroke: retinoic acid signalling that might induce axon regeneration | 53 | |
| Meninges | Fibrotic scarring following inflammation | 4 |
| Formation of reticular networks following infection and neuroinflammation | 71–73 | |
| Choroid plexus | Fibrotic scarring? | NA |
EAE, experimental autoimmune encephalitis; NA, not applicable; OPC, oligodendrocyte progenitor cell. aProposed functions of CNS fibroblasts are indicated as questions.
CNS fibroblasts in disease
Meningeal inflammation
Following peripheral inflammation, a fibroblastic reticular network forms in lymph nodes that serves as an immune cell niche67,68,70. A similar network forms in the meninges during neuroinflammation, most notably in MS71. These networks consist of T cells, B cells and other immune cells interacting with fibroblasts, all of which are held together by ECM secreted by the fibroblasts72. In mice with EAE, T helper 17 (TH17) cells support the formation of this network in the meninges — specifically, IL-17 and IL-22 secreted by TH17 cells upregulate the expression of fibrotic genes by CNS fibroblasts73.
The overall role of this meningeal network in disease progression has only recently been studied. In MS, the meningeal network is present near areas of grey matter demyelination, implicating this structure in lesion formation71,74. In mice with EAE, injecting blocking antibodies against IL-17 and IL-22 reduced the size of this meningeal network and led to a decrease in the EAE motor disability score73. However, the exact signalling contribution from the individual cells in this network, including fibroblasts, is unclear, as cell type-specific deletions have not been tested.
A similar fibroblastic reticular network in the meninges has also been described after CNS infections in mice, including coronavirus75 and lymphocytic choriomeningitis virus, in which the latter was shown to infect ER-TR7+ stromal cells76. Furthermore, this network also forms after infection with mouse hepatitis virus (MHV) strain A59 and stromal cells in the network produce the chemokine CCL19 that recruits anti-MHV CD8+ cells77. Many questions remain about the conditions that lead to the formation of this meningeal network, its overall role in disease progression (as it appears to be beneficial to recovery from infection but harmful in demyelination), and the exact role of fibroblasts in its formation and in signalling to maintain the immune cell niche. Transcriptional profiling of fibroblasts in this network could help to reveal the signalling mechanisms that are upregulated in fibroblasts in the network compared with those in the healthy meninges.
Fibrotic scarring
Scars in the CNS consist of two major parts: an outer glial scar consisting of reactive astrocytes that migrate and organize their processes around the injury border beginning 1–2 days after the injury and an inner fibrotic scar at the core of the injury or inflammation that seals the damage site and forms in the days and weeks following the injury or inflammation trigger78,79 (Fig. 3). The glial scar has been extensively characterized and can have an overall beneficial role in damage repair80–83. The inner fibrotic scar has been much less studied, although it has also been implicated in damage repair and recovery after injury or inflammation in the CNS. While these two scars occur in distinct areas after an injury to the CNS, they exhibit some overlap in inflammatory lesions with scarring4,79. In addition, the major fibrotic scar component type I collagen can induce glial scar formation following injury84; however, reducing fibrotic scar formation following inflammation does not prevent the formation of the glial scar4. Microglia have also been shown to be an integral component of the scar following injury and, in neonatal mice, these cells organize scar-free injury repair by secreting ECM proteins that form bridges to bind damaged tissue and peptidase inhibitors to prevent scar buildup85,86. Below, we summarize the main CNS injuries and diseases in which fibrotic scarring has been implicated and describe the cell types that have been proposed to form the scar, the role of the scar in recovery following specific triggers, and the mechanisms that lead to CNS fibroblast proliferation and collagen production. Many of these aspects remain incompletely understood; studies probing the cellular origin, role and mechanisms of fibrotic scarring in many CNS diseases would be useful to fully understand how scarring influences CNS damage repair.
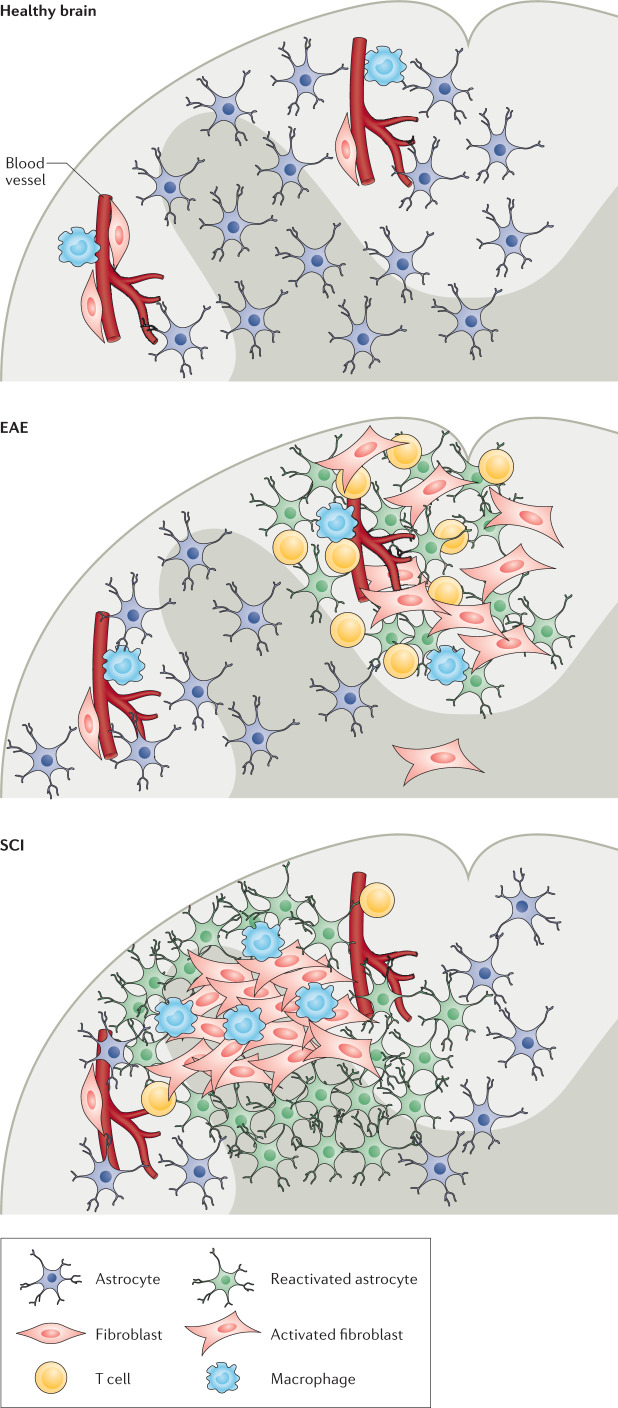
Fig. 3. Organization of the glial and fibrotic scars.
In a healthy spinal cord, perivascular fibroblasts and macrophages reside in perivascular spaces1,9. In experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis, neuroinflammatory lesions form in the white matter. These lesions include infiltrating immune cells, such as T cells, and a scar consisting of nearby fibroblasts and reactive astrocytes4,104. In spinal cord injury (SCI), a scar also forms in the area of the injury. However, the core of the injury site consists of an inner fibrotic scar containing extracellular matrix proteins, activated fibroblasts, microglia, and macrophages and an outer glial scar consisting of reactive astrocytes26,79,93.
Spinal cord injury
SCI is the most widely studied trigger for CNS fibrotic scarring in both humans and animal models. Several studies have suggested that this scar arises from type A pericytes that upregulate collagen expression following injury. For example, a study in Slc1a3–CreER mice, in which Cre expression driven by the promoter of Slc1a3 (the gene encoding GLAST, thought to be most active in astrocytes), leads to fluorescent labelling of scar-forming cells, these cells were classified as pericytes on the basis of their expression of PDGFRβ and perivascular localization87. Another study using Col1a1-GFP mice suggested that CNS fibroblasts form the fibrotic scar as the GFP+ collagen-producing cells do not express pericyte markers such as NG2 (ref.26). Light sheet microscopy images of an injured mouse spinal cord suggest that the GFP+ cells likely originate from the meninges although there are no definitive data regarding the proportion of the scar that arises from meningeal versus perivascular fibroblasts26. Single-cell sequencing studies revealed that, in addition to astrocytes and pericytes, fibroblasts also express Slc1a3 and, therefore, these two studies might have identified the same fibroblast cell population but named them differently1.
The fibrotic scar is thought to negatively affect disease progression in SCI as it blocks progenitor cells from entering the injury core to facilitate axon regeneration; consequently, therapies have been proposed to target this scar to stimulate recovery88. However, completely blocking fibrotic scar formation in mice leads to a failure to seal the injury site, resulting in an open lesion87. By contrast, reducing the size of the scar without completely blocking its formation (by combination knockout of HRas, NRas and a cell-specific deletion of KRas to prevent fibroblast proliferation) results in improved recovery from SCI in mice as shown in motor tests and functional assessment of optogenetically stimulated regenerating axons89. However, the Ras pathway is not specific to fibroblasts and is therefore unlikely to be used in a clinical setting. Other studies have shown that inhibiting microtubule formation prevents fibrotic scarring and dampening immune cell signalling can also be used to reduce scarring and enhance disease recovery88,90–92.
Traumatic brain injury
Traumatic brain injury (TBI), like spinal cord injury, is a physical injury to the CNS that often results in fibrotic scarring93–95. As in SCI, studies have reported different cellular origins of the fibrotic scar in TBI. For example, PDGFRβ+ cells accumulate in the injury core in rodents, with these cells identified as either pericytes or meningeal fibroblasts96,97. Inhibiting PDGFRβ signalling after TBI decreases scar formation in mice although how this manipulation affected overall tissue recovery was not studied98. Scar-forming cells also highly express TGFβ receptors following TBI99 and inhibiting TGFβ signalling after TBI in rodents reduces scar formation and promotes the regeneration of dopaminergic neurons95,100. Reducing scar formation by the pharmacological inhibition of type IV collagen deposition at the injury site also promotes axonal regeneration following brain injury94.
Stroke
Stroke is caused by loss of blood flow to specific areas of the CNS. The resulting hypoxia leads to blood–brain barrier breakdown, tissue damage and CNS scarring. In Col1a1-GFP mice, both PDGFRβ+ and GFP+ cells, which were referred to as stromal cells, increased in number in the lesion core surrounded by fibrotic proteins following middle cerebral artery occlusion, a model of stroke53,101. In Pdgfrβ+/– mice following middle cerebral artery occlusion, fibrotic scar formation is reduced but the overall infarct volume is larger, implicating PDGFR signalling in fibrotic scar formation and the fibrotic scarring process in managing infarct size in this model102. Whether this change in infarct sizes results in changes in disease recovery and tissue repair remains unknown. In a mouse model of meningeal cerebrovascular injury induced by transcranial ultrasound, blocking myeloid cell recruitment led to a lack of vascular repair and increased fibrosis in the injury site, suggesting that immune cell entry into vascular lesions is crucial for wound repair and that altering this process can lead to fibrosis103. The signals that lead to this fibrosis remain unknown.
Multiple sclerosis
MS is a neuroinflammatory disease characterized by concentrated areas of inflammation and demyelination. ECM depositions occur around blood vessels in MS and a recent study in Col1a1-GFP mice with EAE found an increase in the number of GFP+ cells in the spinal cord parenchyma following symptom onset104–107. In mice with EAE, lineage tracing studies demonstrated that the fibrotic scar arises from the proliferation of CNS fibroblasts and not that of pericytes or bone marrow-derived cells, although the relative contribution of meningeal and perivascular fibroblasts to the fibrosis remains unknown4. This is an especially intriguing question in inflammation, in which there may not be physical damage to the pial or glial barrier. Reducing fibroblast proliferation and the resulting scar formation in mice with EAE by fibroblast-specific expression of the herpes simplex virus thymidine kinase led to a modest improvement in motor symptoms and an increase in oligodendrocyte lineage cells in inflammatory, demyelinating lesions, suggesting that the fibrotic scar limits the ability of potential reparative cells to enter the lesion4. In addition, blocking IFNγ signalling in CNS fibroblasts reduced the area of fibrotic scar formation4.
Microbial infection
Infection leads to inflammation and, in severe cases, tissue damage and fibrotic scarring. Following Staphylococcus aureus infection in the mouse brain, an abscess forms that is surrounded by a fibrotic wall108. A small population of bone marrow-derived fibrocyte-like cells in the area of the fibrotic wall are positive for markers of fibrosis such as type I collagen. Whether and how bone marrow-derived cells contribute to fibrosis following other CNS triggers, such as injury or degeneration, remains unknown. However, even in cases where bone marrow-derived cells have been detected in areas of CNS scarring, their contribution to the scar seems to be minimal and most evidence strongly supports the conclusion that CNS fibrotic scarring arises from cells intrinsic to the CNS.
Retinopathies
Retinopathies are diseases that damage the retina usually as a result of altered retinal blood flow. Fibrotic scarring occurs in the retina in conditions such as diabetic retinopathy and retinopathy of prematurity and can lead to tractional retinal detachment and blindness109. The proposed origin of this fibrotic scar varies with the location of the fibrosis and the extent of tissue damage to specific layers of the retina. For example, when there is a physical injury to the retinal pigment epithelium, these pigmented epithelial cells have been proposed to migrate to the surface of the retina and deposit scar tissue110,111. In other retinal conditions, Müller glia (resident retinal glia) have been proposed to extend their processes to the surface of the retina and deposit scar tissue112. Although the retina contains an extensive vasculature, the presence of perivascular fibroblasts in the retina has not been reported. A single-cell transcriptomic study of the murine retina detected a few cells classified as fibroblasts, although these cells were reported to be contaminants109,113. More in-depth sequencing studies are needed to confirm whether fibroblasts are present in the retina of mice and humans and play a role in retinal scarring.
Neurodegenerative diseases
CNS fibroblasts and the presence and role of fibrotic scarring have not been extensively studied in neurodegenerative diseases. ECM components, such as proteoglycans, are deposited alongside amyloid plaques in Alzheimer disease and ECM remodelling occurs in ALS but has been attributed to astrocytes114. Even if fibroblasts are not engaged in scarring in these diseases, they could influence disease outcomes through signalling with nearby vascular cells. A preliminary study using VINE-seq to profile human vascular cells from the hippocampus and cortex of healthy individuals and patients with Alzheimer disease found dysregulation in IFNγ pathway and SMAD signalling genes in fibroblasts in Alzheimer disease6. In addition, in the pre-symptomatic stages of ALS, the fibroblast marker genes SPP1 and COLA6A1 are enriched and their protein products accumulate in perivascular spaces8. Increased expression levels of these genes predicted shorter survival times in patients with ALS, indicating that perivascular fibroblasts contribute to dysfunction early in disease progression8.
Choroid plexus stroma and immune cells
Choroid plexus stromal compartment dysfunction has been documented in cases of CNS injury, aging and disease. In a 1931 study of post-mortem choroid plexus from 61 head trauma cases, nearly every case had marked oedema of the choroid plexus stroma and the presence of phagocytic cells115. Given the close proximity of choroid plexus fibroblasts to the immune cells and vasculature of the stroma, it is likely that choroid plexus fibroblasts facilitate or contribute to oedema and immune cell activation following head trauma, although a potential mechanism has not been elucidated. Although this study found common brain injury pathologies, such as oedema, immune cell infiltration and choroid plexus epithelial vacuolization, it does not describe a common feature of choroid plexus dysfunction in many other diseases and disorders: fibrosis. For example, sclerosis of the choroid plexus stroma occurs in chronic hydrocephalus44,116 and extensive fibrosis, calcification and aberrant vasculature of the choroid plexus stroma occur with ageing, a fibrotic phenotype that is even more pronounced in patients with Alzheimer disease45. It is likely that choroid plexus fibroblasts become activated in these cases to contribute to choroid plexus fibrosis and scarring but the mechanisms of activation remain unknown. By contrast, in a mouse model of the human autoimmune disorder systemic lupus erythematosus, the basement membranes of the choroid plexus epithelium and stromal vasculature are ‘thickened’ and the stromal compartment seems to lack ‘interstitial material’ that typically separates the two basement membranes117. These observations could suggest a loss of choroid plexus fibroblasts in autoimmune disorders, although this has not been shown in other autoimmune diseases or models.
Conclusions
CNS fibroblasts contribute in important ways to CNS development and disease pathology, progression and recovery. Their role in contributing to CNS fibrosis makes them important drug targets for the treatment of neurological diseases and conditions. However, many unanswered questions remain about the functions of these cells in health and disease (Box 1). It is unknown whether there are significant transcriptional and functional differences between fibroblasts in the meninges, perivascular spaces and choroid plexus. Techniques such as spatial transcriptomics and two-photon microscopy can be utilized to further probe the location-specific properties of these cells. In addition, whether these cells have functions other than structural support in the healthy adult CNS remains unknown. Fibroblasts occur at interfaces between the CNS and the periphery and might thus monitor and respond to changes in blood flow, tissue stiffness and immune cell composition.
In the choroid plexus, comprehensive studies are needed to further our understanding of the identity and functions of choroid plexus fibroblasts, including using lineage tracing tools to elucidate the developmental origins of these cells and molecular tools, such as single-cell RNA sequencing, to further elucidate their functional diversity. Choroid plexus dysfunction, particularly fibrosis, has been extensively documented in CNS injury, ageing and disease but there has been very little focus on the mechanisms of fibrosis in the choroid plexus in these contexts. Understanding how fibroblasts become activated in these contexts will give greater insight into their pathogenesis and may explain other features of choroid plexus dysfunction such as aberrant vasculature and immune cell activation.
While the role of CNS fibroblasts in fibrotic scar formation has been the most widely studied function of these cells in disease, there are still many open questions about fibrosis such as whether fibrosis occurs following neurological injury in neurodegenerative diseases, the role of fibroblast proliferation and fibrosis in repair after stroke, and the full profile of signalling mechanisms that lead to scar formation. Although PDGFRβ, TGFβ and IFNγ signalling in fibroblasts have been shown to be involved in CNS fibrosis, manipulating one signalling pathway has only a partial effect on this process, suggesting that combinations of these pathways and/or other pathways likely contribute to scar formation. In addition, fibrosis can interfere with the function of neural implants, such as electrodes used for the treatment of epilepsy and Parkinson disease, and studies manipulating fibroblast activity by modulating these signalling pathways could identify ways to improve the longevity of these devices.
It is possible that the fibrotic scar is formed by different cell types in response to different triggers, which upregulate different signalling mechanisms in cells in the perivascular spaces. Genetic lineage tracing illustrated that fibroblasts are the predominant origin of the scar following neuroinflammation4, but a different cell type, such as pericytes, or a combination of fibroblasts and pericytes, could be activated to upregulate collagen production following hypoxia or injury. It is also possible that CNS fibroblasts are the primary cell type that forms the fibrotic scar after all triggers in tissues in which they are present. We hypothesize that this scenario is more likely, as the type A pericytes that have been identified as the scar-forming cells in SCI have not been clearly detected in single-cell sequencing studies in mice. These pericytes may actually be CNS fibroblasts as their defining characteristics (PDGFRβ expression, GLAST expression and perivascular localization) are shared by fibroblasts. Either way, repeating lineage tracing experiments in cases of CNS injury and hypoxia would resolve this question.
It is largely unknown how CNS fibroblasts contribute to diseases in ways other than secreting fibrotic matrices and supporting reticular networks. In the periphery, fibroblasts are key players in inflammatory signalling: they can recruit immune cells to sites of injury by releasing chemokines such as monocyte chemoattractant protein 1, influence leukocyte transendothelial migration and promote immune cell survival through reducing apoptosis118. RNA sequencing analysis showed that the expression of cytokines by spinal cord fibroblasts is upregulated in disease and fibroblasts are a source of retinoic acid following stroke4,53, indicating that they could play important roles in recruiting and eliciting damage responses from other cells in injury sites. In addition, as CNS fibroblasts express amyloid precursor protein (APP)4 and reside near the locations of amyloid deposition in cerebral amyloid angiopathy, these cells could be involved in the development and propagation of this disease. As current data show that fibrotic scarring impedes axon regeneration, it will be interesting to know whether other potential contributions of fibroblasts to disease have purely negative effects on recovery. Preventing collagen expression in fibroblasts without influencing their proliferation or signalling could delineate other roles for these intriguing cells in disease.
Finally, to determine the involvement of CNS fibroblasts in human disease and how the responses of CNS fibroblasts compare between humans and mice, further characterization of human tissue from different CNS pathologies is needed. While the presence of fibroblasts in the human meninges is well documented, less is known about whether perivascular fibroblasts are associated with parenchymal vessels. Fibrotic scarring in human pathologies such as spinal cord injury has been clearly demonstrated but whether the contribution of fibroblasts to this process occurs by similar or different mechanisms in humans than in mice remains to be discovered.
Box 1 Unanswered questions in CNS fibroblast research.
Development and heterogeneity
What are the transcriptional differences between fibroblasts in the meninges, perivascular spaces and choroid plexus?
Are there different subtypes of fibroblasts in these three regions?
What is the developmental origin of the choroid plexus stroma?
Functions in health
Do perivascular fibroblasts participate in mechanosensation?
How do fibroblasts influence vascular homeostasis and immune monitoring?
Can fibroblasts serve as progenitor cells?
How do fibroblasts influence cerebrospinal fluid solute clearance?
Roles in disease
What signalling pathways are sufficient for fibroblast activation in disease?
Are fibroblasts responsible for scarring in the choroid plexus?
Do fibroblasts have a role in amyloid deposition in neurodegenerative diseases?
How does fibroblast signalling affect disease progression other than through its effects on fibrosis?
Acknowledgements
R.D. is funded by NIH/NINDS grants R01 NS091281and R01 NS103844, National Multiple Sclerosis Society Grant and the UCSF Program for Breakthrough Biomedical Science. C.E.D. is funded by the UCSD Graduate Training Program in Cellular and Molecular Pharmacology through an institutional training grant (T32 GM007752) from the National Institute of General Medical Sciences and NIH/NINDS grant F31 NS108651. J.S. is funded by NIH/NINDS grant R01 NS098273.
Author contributions
All authors wrote the article. C.E.D., H.E.J., J.A.S. and R.D. reviewed and/or edited the manuscript before submission.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Neuroscience thanks A. Montagne, M. Sofroniew, who co-reviewed with T. O’Shea, and B. Engelhardt for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Julie A. Siegenthaler, Email: julie.siegenthaler@cuanschutz.edu
Richard Daneman, Email: rdaneman@ucsd.edu.
References
- 1.Vanlandewijck M, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 2.Dani N, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184:3056–3074.e21. doi: 10.1016/j.cell.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rustenhoven J, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184:1000–1016.e27. doi: 10.1016/j.cell.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorrier CE, et al. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 2021;24:234–244. doi: 10.1038/s41593-020-00770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, et al. A human cell type similar to murine central nervous system perivascular fibroblasts. Exp. Cell Res. 2021;402:112576. doi: 10.1016/j.yexcr.2021.112576. [DOI] [PubMed] [Google Scholar]
- 6.Yang AC, et al. A human brain vascular atlas reveals diverse cell mediators of Alzheimer’s disease risk. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.04.26.441262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia FJ, et al. Single-cell dissection of the human cerebrovasculature in health and disease. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.04.26.440975. [DOI] [Google Scholar]
- 8.Månberg A, et al. Altered perivascular fibroblast activity precedes ALS disease onset. Nat. Med. 2021;27:640–646. doi: 10.1038/s41591-021-01295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastorakos P, McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019;4:eaav0492. doi: 10.1126/sciimmunol.aav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam MA, et al. The ultrastructure of spinal cord perivascular spaces: implications for the circulation of cerebrospinal fluid. Sci. Rep. 2017;7:12924. doi: 10.1038/s41598-017-13455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen MK, Mestre H, Nedergaard M. Fluid transport in the brain. Physiol. Rev. 2021 doi: 10.1152/physrev.00031.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J. Anat. 1990;170:111–123. [PMC free article] [PubMed] [Google Scholar]
- 14.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bondjers C, et al. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rβ mutant mice identifies novel markers for brain pericytes. FASEB J. 2006;20:1703–1705. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- 17.Smyth LCD, et al. Markers for human brain pericytes and smooth muscle cells. J. Chem. Neuroanat. 2018;92:48–60. doi: 10.1016/j.jchemneu.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Sagare AP, Sweeney MD, Makshanoff J, Zlokovic BV. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci. Lett. 2015;607:97–101. doi: 10.1016/j.neulet.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldmann T, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Hove H, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 2019;22:1021–1035. doi: 10.1038/s41593-019-0393-4. [DOI] [PubMed] [Google Scholar]
- 21.Faraco G, Park L, Anrather J, Iadecola C. Brain perivascular macrophages: characterization and functional roles in health and disease. J. Mol. Med. 2017;95:1143–1152. doi: 10.1007/s00109-017-1573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonney SK, Sullivan LT, Cherry TJ, Daneman R, Shih AY. Distinct features of brain perivascular fibroblasts and mural cells revealed by in vivo two-photon imaging. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.05.14.444194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farbehi N, et al. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. eLife. 2019;8:e43882. doi: 10.7554/eLife.43882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallquist MD. Cardiac fibroblast diversity. Annu. Rev. Physiol. 2020;82:63–78. doi: 10.1146/annurev-physiol-021119-034527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yata Y, et al. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 26.Soderblom C, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 2013;33:13882–13887. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeSisto J, et al. Single-cell transcriptomic analyses of the developing meninges reveal meningeal fibroblast diversity and function. Dev. Cell. 2020;54:43–59.e4. doi: 10.1016/j.devcel.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Vliet E, Melis M, Van Ewijk W. Monoclonal antibodies to stromal cell types of the mouse thymus. Eur. J. Immunol. 1984;14:524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]
- 29.Van Vliet E, Melis M, Foidart JM, Van Ewijk W. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J. Histochem. Cytochem. 1986;34:883–890. doi: 10.1177/34.7.3519751. [DOI] [PubMed] [Google Scholar]
- 30.Haines DE, Harkey HL, Al-Mefty O. The “subdural” space: a new look at an outdated concept. Neurosurgery. 1993;32:111–120. doi: 10.1227/00006123-199301000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Kirmi O, Sheerin F, Patel N. Imaging of the meninges and the extra-axial spaces. Semin. Ultrasound CT MR. 2009;30:565–593. doi: 10.1053/j.sult.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Nabeshima S, Reese TS, Landis DM, Brightman MW. Junctions in the meninges and marginal glia. J. Comp. Neurol. 1975;164:127–169. doi: 10.1002/cne.901640202. [DOI] [PubMed] [Google Scholar]
- 33.Alcolado R, Weller RO, Parrish EP, Garrod D. The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol. Appl. Neurobiol. 1988;14:1–17. doi: 10.1111/j.1365-2990.1988.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 34.Vandenabeele F, Creemers J, Lambrichts I. Ultrastructure of the human spinal arachnoid mater and dura mater. J. Anat. 1996;189:417–430. [PMC free article] [PubMed] [Google Scholar]
- 35.Weller RO, Sharp MM, Christodoulides M, Carare RO, Møllgård K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018;135:363–385. doi: 10.1007/s00401-018-1809-z. [DOI] [PubMed] [Google Scholar]
- 36.Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J. Comp. Neurol. 1986;251:260–280. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- 37.McLone DG, Bondareff W. Developmental morphology of the subarachnoid space and contiguous structures in the mouse. Am. J. Anat. 1975;142:273–293. doi: 10.1002/aja.1001420302. [DOI] [PubMed] [Google Scholar]
- 38.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J. Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Revest PA, Jones HC, Abbott NJ. Transendothelial electrical potential across pial vessels in anaesthetised rats: a study of ion permeability and transport at the blood-brain barrier. Brain Res. 1994;652:76–82. doi: 10.1016/0006-8993(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 40.Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol. 2018;135:387–407. doi: 10.1007/s00401-018-1812-4. [DOI] [PubMed] [Google Scholar]
- 41.Hannocks M-J, et al. Molecular characterization of perivascular drainage pathways in the murine brain. J. Cereb. Blood Flow Metab. 2018;38:669–686. doi: 10.1177/0271678X17749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riew T-R, Jin X, Kim HL, Kim S, Lee M-Y. Ultrastructural and molecular characterization of platelet-derived growth factor beta-positive leptomeningeal cells in the adult rat brain. Mol. Neurobiol. 2020;57:1484–150. doi: 10.1007/s12035-019-01793-5. [DOI] [PubMed] [Google Scholar]
- 43.Ghersi-Egea J-F, et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018;135:337–361. doi: 10.1007/s00401-018-1807-1. [DOI] [PubMed] [Google Scholar]
- 44.Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 2013;93:1847–1892. doi: 10.1152/physrev.00004.2013. [DOI] [PubMed] [Google Scholar]
- 45.Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG. The choroid plexus in the rise, fall and repair of the brain. Bioessays. 2005;27:262–274. doi: 10.1002/bies.20193. [DOI] [PubMed] [Google Scholar]
- 46.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 2015;16:445–457. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shuangshoti S, Netsky MG. Histogenesis of choroid plexus in man. Am. J. Anat. 1966;118:283–316. doi: 10.1002/aja.1001180114. [DOI] [PubMed] [Google Scholar]
- 48.Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 49.Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev. Biol. 1987;120:198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 50.Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech. Dev. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 52.O’Rahilly R, Müller F. The meninges in human development. J. Neuropathol. Exp. Neurol. 1986;45:588–608. [PubMed] [Google Scholar]
- 53.Kelly KK, et al. Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke. BMC Neurosci. 2016;17:49–49. doi: 10.1186/s12868-016-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bill BR, Korzh V. Choroid plexus in developmental and evolutionary perspective. Front. Neurosci. 2014;8:363. doi: 10.3389/fnins.2014.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liddelow SA. Development of the choroid plexus and blood-CSF barrier. Front. Neurosci. 2015;9:32. doi: 10.3389/fnins.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilting J, Christ B. An experimental and ultrastructural study on the development of the avian choroid plexus. Cell Tissue Res. 1989;255:487–494. doi: 10.1007/BF00218783. [DOI] [PubMed] [Google Scholar]
- 57.Cancilla PA, Zimmerman HM, Becker NH. A histochemical and fine structure study of the developing rat choroid plexus. Acta Neuropathol. 1966;6:188–200. doi: 10.1007/BF00686764. [DOI] [PubMed] [Google Scholar]
- 58.Zeisel A, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.e22. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeisel A, et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 60.Marques S, et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352:1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alberts B. et al. Fibroblasts and Their Transformations: The Connective-Tissue Cell Family 4th edn (Garland Science, 2002).
- 63.Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CCW. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell. 2011;22:3791–3800. doi: 10.1091/mbc.E11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 65.Herum KM, Lunde IG, McCulloch AD, Christensen G. The soft- and hard-heartedness of cardiac fibroblasts: mechanotransduction signaling pathways in fibrosis of the heart. J. Clin. Med. 2017;6:53. doi: 10.3390/jcm6050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munk AS, et al. PDGF-B is required for development of the glymphatic system. Cell Rep. 2019;26:2955–2969.e3. doi: 10.1016/j.celrep.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang JE, Turley SJ. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol. 2015;36:30–39. doi: 10.1016/j.it.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat. Rev. Immunol. 2008;8:764–775. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]
- 69.Nataf S, et al. Rat choroid plexuses contain myeloid progenitors capable of differentiation toward macrophage or dendritic cell phenotypes. Glia. 2006;54:160–171. doi: 10.1002/glia.20373. [DOI] [PubMed] [Google Scholar]
- 70.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 71.Magliozzi R, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 72.Bajénoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pikor NB, et al. Integration of Th17- and lymphotoxin-derived signals initiates meningeal-resident stromal cell remodeling to propagate neuroinflammation. Immunity. 2015;43:1160–1173. doi: 10.1016/j.immuni.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 74.Magliozzi R, et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 2010;68:477–493. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe R, Kakizaki M, Ikehara Y, Togayachi A. Formation of fibroblastic reticular network in the brain after infection with neurovirulent murine coronavirus. Neuropathology. 2016;36:513–526. doi: 10.1111/neup.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cupovic J, et al. Central nervous system stromal cells control local CD8+ T cell responses during virus-induced neuroinflammation. Immunity. 2016;44:622–633. doi: 10.1016/j.immuni.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernández-Klett F, Priller J. The fibrotic scar in neurological disorders. Brain Pathol. 2014;24:404–413. doi: 10.1111/bpa.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J. Clin. Invest. 2017;127:3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson MA, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hara M, et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin–N-cadherin pathway after spinal cord injury. Nat. Med. 2017;23:818–828. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 85.Li Y, et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature. 2020;587:613–618. doi: 10.1038/s41586-020-2795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellver-Landete V, et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019;10:518. doi: 10.1038/s41467-019-08446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Göritz C, et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 88.Brazda N, Müller HW. Pharmacological modification of the extracellular matrix to promote regeneration of the injured brain and spinal cord. Prog. Brain Res. 2009;175:269–281. doi: 10.1016/S0079-6123(09)17518-0. [DOI] [PubMed] [Google Scholar]
- 89.Dias DO, et al. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165.e22. doi: 10.1016/j.cell.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hellal F, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klapka N, et al. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur. J. Neurosci. 2005;22:3047–3058. doi: 10.1111/j.1460-9568.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- 92.Vangansewinkel T, et al. Mouse mast cell protease 4 suppresses scar formation after traumatic spinal cord injury. Sci. Rep. 2019;9:3715–3715. doi: 10.1038/s41598-019-39551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawano H, et al. Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res. 2012;349:169–180. doi: 10.1007/s00441-012-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshioka N, Hisanaga S-I, Kawano H. Suppression of fibrotic scar formation promotes axonal regeneration without disturbing blood-brain barrier repair and withdrawal of leukocytes after traumatic brain injury. J. Comp. Neurol. 2010;518:3867–3881. doi: 10.1002/cne.22431. [DOI] [PubMed] [Google Scholar]
- 95.Logan A, et al. Effects of transforming growth factor β1, on Scar production in the injured central nervous system of the rat. Eur. J. Neurosci. 1994;6:355–363. doi: 10.1111/j.1460-9568.1994.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 96.Zehendner CM, et al. Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex. Sci. Rep. 2015;5:13497. doi: 10.1038/srep13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kyyriäinen J, Ekolle Ndode-Ekane X, Pitkänen A. Dynamics of PDGFRβ expression in different cell types after brain injury. Glia. 2017;65:322–341. doi: 10.1002/glia.23094. [DOI] [PubMed] [Google Scholar]
- 98.Pei D, et al. Inhibition of platelet-derived growth factor receptor β reduces reactive glia and scar formation after traumatic brain injury in mice. Brain Res. Bull. 2017;134:121–127. doi: 10.1016/j.brainresbull.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 99.Komuta Y, et al. Expression of transforming growth factor-β receptors in meningeal fibroblasts of the injured mouse brain. Cell. Mol. Neurobiol. 2010;30:101–111. doi: 10.1007/s10571-009-9435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshioka N, et al. Small molecule inhibitor of type I transforming growth factor-β receptor kinase ameliorates the inhibitory milieu in injured brain and promotes regeneration of nigrostriatal dopaminergic axons. J. Neurosci. Res. 2011;89:381–393. doi: 10.1002/jnr.22552. [DOI] [PubMed] [Google Scholar]
- 101.Fernández-Klett F, et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood Flow Metab. 2013;33:428–439. doi: 10.1038/jcbfm.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Makihara N, et al. Involvement of platelet-derived growth factor receptor β in fibrosis through extracellular matrix protein production after ischemic stroke. Exp. Neurol. 2015;264:127–134. doi: 10.1016/j.expneurol.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Mastorakos P, et al. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat. Neurosci. 2021;24:245–258. doi: 10.1038/s41593-020-00773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yahn SL, et al. Fibrotic scar after experimental autoimmune encephalomyelitis inhibits oligodendrocyte differentiation. Neurobiol. Dis. 2020;134:104674–104674. doi: 10.1016/j.nbd.2019.104674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Horssen J, Bö L, Dijkstra CD, de Vries HE. Extensive extracellular matrix depositions in active multiple sclerosis lesions. Neurobiol. Dis. 2006;24:484–491. doi: 10.1016/j.nbd.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 106.van Horssen J, Dijkstra CD, de Vries HE. The extracellular matrix in multiple sclerosis pathology. J. Neurochem. 2007;103:1293–1301. doi: 10.1111/j.1471-4159.2007.04897.x. [DOI] [PubMed] [Google Scholar]
- 107.Mohan H, et al. Extracellular matrix in multiple sclerosis lesions: fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain Pathol. 2010;20:966–975. doi: 10.1111/j.1750-3639.2010.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aldrich A, Kielian T. Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am. J. Pathol. 2011;179:2952–2962. doi: 10.1016/j.ajpath.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Friedlander M. Fibrosis and diseases of the eye. J. Clin. Invest. 2007;117:576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hiscott P, Sheridan C, Magee RM, Grierson I. Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog. Retin. Eye Res. 1999;18:167–190. doi: 10.1016/s1350-9462(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 111.Machemer R, van Horn D, Aaberg TM. Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. Am. J. Ophthalmol. 1978;85:181–191. doi: 10.1016/s0002-9394(14)75946-x. [DOI] [PubMed] [Google Scholar]
- 112.Bringmann A, et al. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 113.Macosko EZ, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D’Ambrosi N, Apolloni S. Fibrotic scar in neurodegenerative diseases. Front. Immunol. 2020;11:1394–1394. doi: 10.3389/fimmu.2020.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rand CW, Courville CB. Histologic studies of the brain in cases of fatal injury to the head: II. Changes in the choroid plexus and ependyma. Arch. Surg. 1931;23:357–425. [Google Scholar]
- 116.Shuangshoti S, Roberts MP, Netsky MG. Neuroepithelial (colloid) cysts: pathogenesis and relation to choroid plexus and ependyma. Arch. Pathol. 1965;80:214–224. [PubMed] [Google Scholar]
- 117.Dohrmann GJ, Herdson PB. Fine structural studies of capillaries in NZBNZW mice. Exp. Mol. Pathol. 1969;11:163–171. doi: 10.1016/0014-4800(69)90005-7. [DOI] [PubMed] [Google Scholar]
- 118.Van Linthout S, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014;102:258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- 119.Lapenna A, De Palma M, Lewis CE. Perivascular macrophages in health and disease. Nat. Rev. Immunol. 2018;18:689–702. doi: 10.1038/s41577-018-0056-9. [DOI] [PubMed] [Google Scholar]
- 120.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am. J. Pathol. 2002;160:1609–1617. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cuttler AS, et al. Characterization of Pdgfrb-Cre transgenic mice reveals reduction of ROSA26 reporter activity in remodeling arteries. Genesis. 2011;49:673–680. doi: 10.1002/dvg.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ulvmar MH, Martinez-Corral I, Stanczuk L, Mäkinen T. Pdgfrb-Cre targets lymphatic endothelial cells of both venous and non-venous origins. Genesis. 2016;54:350–358. doi: 10.1002/dvg.22939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu X, et al. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hartmann DA, et al. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics. 2015;2:041402. doi: 10.1117/1.NPh.2.4.041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grant RI, et al. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J. Cereb. Blood Flow Metab. 2019;39:411–425. doi: 10.1177/0271678X17732229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wendling O, Bornert JM, Chambon P, Metzger D. Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis. 2009;47:14–18. doi: 10.1002/dvg.20448. [DOI] [PubMed] [Google Scholar]
- 127.Guimarães-Camboa N, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–359.e5. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]