Abstract
Chemosensory scientists have been skeptical that reports of COVID-19 taste loss are genuine, in part because before COVID-19, taste loss was rare and often confused with smell loss. Therefore, to establish the predicted prevalence rate of taste loss in COVID-19 patients, we conducted a systematic review and meta-analysis of 376 papers published in 2020–2021, with 241 meeting all inclusion criteria. Additionally, we explored how methodological differences (direct vs. self-report measures) may affect these estimates. We hypothesized that direct prevalence measures of taste loss would be the most valid because they avoid the taste/smell confusion of self-report. The meta-analysis showed that, among 138,897 COVID-19-positive patients, 39.2% reported taste dysfunction (95% CI: 35.34–43.12%), and the prevalence estimates were slightly but not significantly higher from studies using direct (n = 18) versus self-report (n = 223) methodologies (Q = 0.57, df = 1, p = 0.45). Generally, males reported lower rates of taste loss than did females and taste loss was highest in middle-aged groups. Thus, taste loss is a bona fide symptom COVID-19, meriting further research into the most appropriate direct methods to measure it and its underlying mechanisms.
Keywords: gustatory dysfunction, taste loss, ageusia, COVID-19, coronavirus
Introduction
The novel coronavirus (COVID-19), a respiratory infection caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first identified in Wuhan, China, and has since spread throughout the world. When the World Health Organization first declared this a pandemic in March 2020, researchers and clinicians were not yet aware that the virus affected individuals’ senses of smell and taste, but these symptoms soon became apparent via patient reports. As a result of COVID-19, affected people can experience chemosensory dysfunction in a variety of ways, including complete loss of smell or taste (anosmia or ageusia, respectively), partial loss of smell or taste (hyposmia or hypogeusia), and/or a distorted sense of smell or taste (e.g., parosmia, dysgeusia). These chemosensory dysfunctions can be distressing to the affected individuals and can last for extended times, with some patients experiencing resolution within a few weeks to a month (Lee et al., 2020b; Gerkin et al., 2021) and others with symptoms for 6 months or longer (Blomberg et al., 2021).
Previous meta-analyses examined smell and taste loss in COVID-19 patients, but often with a focus on onset and duration (Santos et al., 2021) or recovery (Boscutti et al., 2021) of chemosensory symptoms. Many focused only on smell loss (Hannum et al., 2020; Pang et al., 2020; Rocke et al., 2020) or general neurological symptoms (Abdullahi et al., 2020; Favas et al., 2020; Mair et al., 2021; Yassin et al., 2021). Very few continued to evaluate articles published in 2021 and often capped reviewing articles 6–10 months after March 2020, when the pandemic was declared, limiting the number of articles included (ranging from 5 to 59 articles total). Therefore, we decided to conduct a more comprehensive analysis, spanning a year and a half, to ensure a fuller coverage of the available research.
Additionally, taste loss is often neglected in research compared to smell loss, as there is a common notion that taste loss is not as “real” as smell loss. Some claim taste loss is indistinguishable from smell loss (Le Bon et al., 2021) or is confused with smell loss (Deems et al., 1991), specifically with retronasal smell perception (Hintschich et al., 2020a). For the general population, loss of taste can be difficult to distinguish from smell loss. Therefore, it may be difficult to know, based on self-report measures alone, whether or not a participant truly lost their sense of taste.
Thus, many chemosensory researchers may attribute the taste loss phenomena seen in the current reports of COVID-19-positive patients to deficiencies of self-report or subjective measures of taste loss. Therefore, we conducted a systematic review and meta-analysis to estimate the true prevalence of taste loss in COVID-19 patients across a wide sample of studies (n=241) and to evaluate effects of major methodological differences in data collection. In particular, we compared overall findings on taste loss as determined by individual taste tests (herein referred to as direct tests) with self-reports without direct sensory testing. We hypothesized that direct methodologies would support the presence of taste loss as a distinct symptom, and direct measures might even be higher than self-report despite the possible inflation of self-reported taste loss which is exacerbated by smell loss.
Currently scientists are using both direct and self-report measures to examine chemosensory dysfunction, with self-report far more common due to the pandemic restrictions, e.g., sensory laboratories where direct testing is often conducted are closed. For taste, direct tests include standardized and non-standardized tests that contain various sweet, salty, and sometimes bitter and sour stimuli given to participants via solutions, drops, strips, or sprays (Cao et al., 2021; Singer-Cornelius et al., 2021). Non-standardized direct taste measures created to study COVID-19-related taste dysfunction include solution-based tests, often prepared at home by participants (Vaira et al., 2020f). Self-report measures include interviews with researchers and clinicians, electronic health records as well as surveys administered over the phone, online, or in person.
To understand taste loss as a symptom of COVID-19, we conducted a large systematic review and meta-analysis, examining how it has been measured (direct vs. self-report) and how the measurement type can affect prevalence rates. We tested the hypothesis that direct measures are at least as sensitive as self-report measures and would confirm taste loss as a distinct symptom and not merely misattributed smell loss.
Methods
Article Selection
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Moher et al., 2009). Articles were selected via searches on Pubmed/Medline and Google Scholar, using the keyword “COVID-19” with “taste”, “smell”, and/or “olfaction”, as well as “gustatory”. Literature retrieval began on May 15, 2020, and concluded on June 1, 2021, resulting in 377 articles in total.
Initial screening of the articles included reading the titles and abstracts to assess their relevance. Articles with an abstract that reported chemosensory dysfunction in COVID-19-infected individuals were included in the systematic review (n = 376). Next, at least two authors read the articles initially deemed relevant, to evaluate whether they fit the inclusion criteria: reporting positive COVID-19 tests, written in the English language, and lack of population bias. COVID-19 must have been confirmed via nasopharyngeal swab, reverse transcription polymerase chain reaction (RT-PCR), or assessment by physician or other medical personnel. The articles were then evaluated on whether they reported taste loss data specifically. In total, 135 articles were excluded based on such criteria as not evaluating taste loss, recruiting participants with chemosensory dysfunction, not testing for COVID-19, and presenting overlapping data (see Figure 1). In total, 241 articles were included in the final meta-analysis (corresponding citations described in “Included Articles” section at the end of the paper).
Figure 1.
CONSORT flow diagram demonstrating the article selection process for this systematic review and meta-analysis.
Data Extraction
We extracted from each article either the number or percentage of patients with taste dysfunction due to a SARS-CoV-2 infection. The prevalence of taste loss reported in each article was calculated by dividing the reported number of participants with taste loss as a symptom by the total number of COVID-19-positive participants. Additionally, measures of taste loss were labeled as “self-report” or “direct” to identify the method used to evaluate participants. Self-report measures included reported loss of taste via surveys, interviews, and electronic medical health records. Most articles (n = 223) used self-report methods. Table 1 summarizes the studies that used direct measures (n = 18), comprising actual taste tests administered either at home (Adamczyk et al., 2020; Hintschich et al., 2020a; Petrocelli et al., 2020b; Cao et al., 2021; Singer-Cornelius et al., 2021), at a testing facility (Altin et al., 2020; Bidkar et al., 2020a; Mazzatenta et al., 2020; Ramteke et al., 2020; Vaira et al., 2020a; Le Bon et al., 2021; Niklassen et al., 2021a; Salcan et al., 2021) or both in home and in a hospital environment (Vaira et al., 2020b, 2020c, 2020d, 2020f). Vaira et al. [2020e] had an unknown testing location. Many of the measures consisted of solution-based tests measuring four basic taste sensations: sweet, sour, salty, and bitter.
Table 1.
Overview of the direct approaches to assess taste loss in participants.
| Taste Quality Measured | ||||||||
|---|---|---|---|---|---|---|---|---|
| Test | Article | Test Method | Test Objective | Salty | Sweet | Sour | Bitter | Umami |
| Four-solution test |
Vaira et al., 2020a–2020f Petrocelli et al., 2020A |
1 mL of each solution, plus deionized water as control, placed on the participant’s tongue via cotton swab. Quarantined patients prepared their own solutions. | Identification | ☑ | ☑ | ☑ | ☑ | ⨆ |
| Four-solution test & Taste Strips |
Altin et al., 2020
Salcan et al., 2021 |
Participants swallowed and identified the solution. Next, paper strips dipped into each solution and placed on the participant’s tongue. | Identification Duration | ☑ | ☑ | ☑ | ☑ | ☑ |
| Two-solution test (1) | Bidkar et al., 2020 | Two drops (2 mL) of each solution placed on the participant’s tongue via pipette. | Identification | ☑ | ☑ | ⨆ | ⨆ | ⨆ |
| Two-solution test (2) | Mazzatenta et al., 2020 | 200 μl of each solution dropped onto participant’s tongue. | Detection | ☑ | ☑ | ⨆ | ⨆ | ☑ |
| Taste Strips by BMGB | Hintschich et al., 2020 Singer-Cornelius et al., 2021 Le Bon et al., 2021 Niklassen et al., 2021C |
Taste strips (provided by Burghart Messtechnik GmbH) placed on the participant’s tongue. | Identification and threshold concentration | ☑ | ☑ | ☑ | ☑ | ⨅ |
| Taste sprays | Niklassen et al., 2021C | Each solution sprayed onto participant’s tongue. | Identification | ☑ | ☑ | ☑ | ☑ | ⨆ |
| Tastant capsules | Adamczyk et al., 2020 | Each of ten 0.33 mL gelatin capsules (one tasteless and nine with tastant) dissolved on participant’s tongue. | Description | ☑ | ☑ | ☑ | ☑ | ⨆ |
| Brief Self-Administered Waterless Empirical Taste Test (SA-WETT)B | Cao et al., 2021 | 27 disposable plastic strips that contain dried solutions of each taste (and some tasteless strips) self-placed on participant’s tongue. | Identification | ☑ | ☑ | ☑ | ☑ | ☑ |
| Chemosensory Test by India Protocol | Ramteke et al., 2020 | Authors used coconut oil, chocolates, and flavored milk to test smell and taste function. No further details are provided. | N/A | N/A | ||||
These studies used a sweet solution concentration that is double what was used in the other four-solution tests.
Validated test.
Niklassen et al. (2021) used both Taste Strips and taste sprays with participants.
Although some authors reported exclusively smell loss or taste loss, many reported on both senses. Thus, it was necessary to include additional coding options for when authors reported the symptoms in tandem (e.g., “loss of smell or taste”). Therefore, articles were labeled as “taste only”, “smell and/or taste”, “smell and taste”, “smell or taste”, and “smell and/or taste; taste only” (in the event both values were reported, the numbers were summed), depending on how these symptoms were phrased in the article.
We also extracted demographic characteristics of each study, including the population mean and/or median age, sex (expressed as percentage of males in the population), and country of origin (for the geographic distribution of the study populations, see Supplementary Figure 1 (S1)).
Four authors performed the initial reading of full texts and the data extraction from the studies (R.D.H., A.K.T., S.S.M., R.J.K.). Two authors confirmed this information and resolved any inconsistencies (M.E.H., D.R.R.). Differences were resolved through a discussion and renewed consensus on the proposed solution from all authors who read that specific article.
Risk-of-Bias Assessment
We used a risk-of-bias assessment from Hoy et al. (2012) to examine the articles selected for the meta-analysis. The assessment contained nine questions, outlined in Supplementary Materials (see Supplementary Table (S2)). Responses were scored as 1 (no) or 0 (yes), with summary scores of low (0–3), moderate (4–6), and high (7–9). Two authors completed the risk-of-bias assessment of each article using the checklist developed by Hoy et al. (2012), as described and adapted by Tong et al. (2020) (S.S.M. and A.K.T.). One author resolved any discrepancies (M.E.H.).
Statistical Analysis
The meta-analysis was conducted using the meta package in R (Schwarzer et al., 2019). Generalized linear mixed models were used for the meta-analysis, as recommended for the analysis of binary outcomes and proportions (Bakbergenuly and Kulinskaya, 2018; Schwarzer et al., 2019). Heterogeneity (e.g., between study variance) was assessed using Cochran’s Q, I2, and tau squared (τ2). We concluded there was evidence for heterogeneity when the p-value for Cochran’s Q was less than 0.05 and if the I2 was greater than 50% (Higgins and Thompson, 2002). Tau squared (τ2), a measure of between study variance, was estimated using the maximum likelihood approach and has no associated test-statistic.
An overall pooled prevalence estimates were computed and reported for a random-effects model with the parameters described for all 241 studies. While both fixed and random-effect models were computed, excess the heterogeneity among our studies (see the Results section) suggested that unmeasured effects contribute to variance in our data more than would be expected from sampling error. The extreme diversity of taste loss as reported in individual studies (0 to 93.4%) suggested that the random-effects model was more appropriate.
Subgroup analysis was performed for studied employing direct methods (n = 18) and self-report methods (n = 223) to assess taste function in COVID-19–positive individuals. Additionally, the average age of participants and their sex (percentage of male subjects in each study) were included as covariates in univariate mixed-regression models. Subgroup analysis for age was arbitrarily categorized into five groupings: adolescents (0–18 years old), young adults (19–35 years old), middle-aged adults (36–50 years old), older adults (51–65 years old), and elderly adults (65+ years old). Finally, studies that used direct tests were separated, and subgroup analysis was performed for each type of collection methodology: solution based (N=12), strip based (N=5), and other (N=1). For continuous variables (e.g., age), the transformed beta coefficients are reported. The transformation for the generalized linear mixed models uses a logit transformation for each proportion, so the models are interpreted as the log(odds) or log(p/1 – p), where p is equal to the prevalence for each study. Back-transformation into the prevalence estimates were done for subgroup analysis (e.g., age).
All statistical analyses were performed using R 4.0.5 (R Core, 2021) and RStudio 1.4.1106 (RStudio Team, 2020). Visualization of the meta-analysis is displayed as an orchard plot adapted from Nakagawa et al. (2020). The R scripts and compiled data used for this analysis are available without restriction at GitHub (https://github.com/vramirez4/COVID19-TasteLoss).
Results
Risk-of-Bias Assessment
Each of the 241 articles included in this meta-analysis were reviewed for risk of bias. Among these articles, none had a high risk of bias, 142 studies had a moderate risk, and 99 studies had a low risk (see Supplementary Table S2 for the full assessment).
Prevalence of taste loss in COVID-19-positive patients
Among the 241 studies, the sample sizes ranged from 11 to over 40,000 patients with COVID-19. The number of cases of taste loss per study ranged from 0 to 4,668, with raw prevalence estimates ranging from 0% to 93.4%. Collectively, the meta-analysis included 138,785 patients who tested positive for COVID-19. Of these, 32,918 patients had some form of taste loss after infection with SARS-CoV-2. Heterogeneity among the prevalence estimates across all studies (n = 241) yielded a significant Cochran’s Q (Q = 29388.75, degrees of freedom [df] = 240, P < 0.001), and an I2 estimate of 99.2%, and τ2 estimate of 1.5. The pooled estimate for taste loss prevalence in COVID-19-positive patients following meta-analysis for the overall cohort was 36.90% (95% CI: 33.27–40.69%).
Effect of methodology (direct vs. self-report) on prevalence estimate
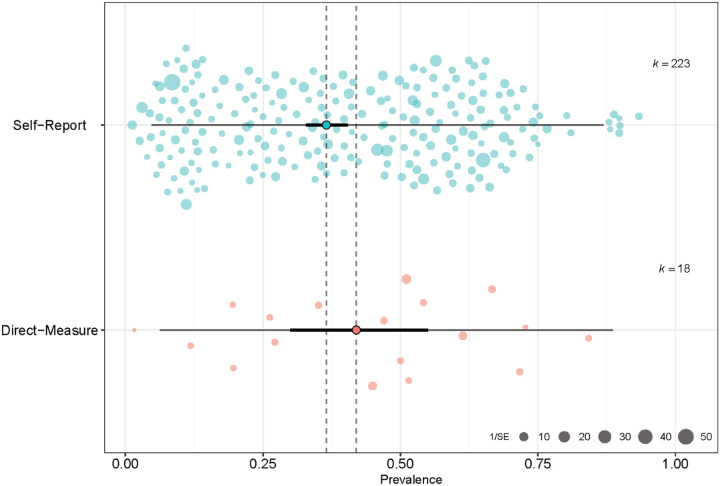
We employed a subgroup analysis to determine the effect of direct versus self-report approaches on taste loss prevalence (see Figure 2). Eighteen studies used direct methods to assess taste loss, comprising 2,240 COVID-19 patients, with 1,092 reported cases of taste loss. Per study, the prevalence of taste loss ranged from 0% to 84% among COVID-19-positive patients. For studies using direct approaches, the pooled estimate of the prevalence for the random-effect model was 42.0% (95% CI: 30.0–55.0%). Cochran’s Q was significant (Q = 187.93, df = 17, P < 0.001), and an I2 of 91.0% was obtained, confirming heterogeneity of the data collected via direct measures. The τ2 for the direct methodologies was 1.2.
Figure 2.
Orchard plot of taste loss and COVID-19. The point estimate of the pooled prevalence (trunk) is represented by the bold turquoise or pink dot. The confidence interval of the pooled prevalence estimate (branch) is represented by the bold black line, and the prediction interval (twig) is represented by the thin black line. Individual prevalence estimates from each study are represented by the scattered colored points (slightly transparent circles). Each point is scaled by the precision of the point estimate of prevalence for each study, i.e., inverse of the standard error.
A total of 223 studies used self-report methods (e.g., questionnaire, interview), comprising 136,545 subjects, with 31,826 cases of taste loss. The reported prevalence of taste loss ranged from 1% to 93% per study. The pooled estimate of the prevalence under the random-effect models was 36.53% (95% CI: 32.8%−40.5%). Similar to the direct subgroup, Cochran’s Q was significant (Q = 29188.14, df = 222, P < 0.001), and the I2 value was 99.2%, confirming that the studies differed in prevalence across the self-report studies. The τ2 for self-reported studies was 1.5.
A test of heterogeneity between groups was employed using Cochran’s Q, which revealed that the differences between methodologies were not statistically significant (Q = 0.66, df = 1, p = 0.4157) under the random-effects model. While our analysis showed that the prevalence of taste loss was higher when measured directly than by self-report, there was no significant effect of measurement method on the prevalence estimates of taste loss.
Effect of age and sex on taste loss prevalence
Additional analyses were undertaken to assess the effect of age and sex. Univariate mixed models for each covariate revealed that both age and sex had significant effects on the prevalence of taste loss. After categorizing each study by mean age group, we conducted the meta-analysis for those 210 studies that reported the ages of the participants (see Table 2). Among this subset of studied the pooled prevalence was 37.16% and demonstrated significant heterogeneity with (Q=33943.46, df = 209, p<0.001), I2 = 98.9, and τ2 =1.5. The prevalence estimates per age category ranged from 11% in studies with average ages younger than 18 years to 44% in studies with average ages between 36 and 50 years. Heterogeneity in these studies was high (I2 = 96.4–98.9%) both within groups (Q = 14106.77, df = 205, p<0.0001) and between groups (Q = 32.19, df = 4, p<0.0001). The results demonstrated that both the youngest and oldest age groups report the lowest prevalence of taste loss, while age groups between 18 and 65 years had pooled estimates ranging from 32% to 44%, with the highest in the middle-age (36–50 years) group. Similarly, it appeared that age accounted for some of the overall heterogeneity among some groups as measured by reductions in τ2 among studies who contained mostly adolescent and elderly individuals.
Table 2.
Random-effect estimate of age group on COVID-19 taste loss prevalence using generalized linear mixed models.
| Age Category | k | Proportion | 95%-CI | Q | I2 | Tau2 |
|---|---|---|---|---|---|---|
| Adolescent | 9 | 0.12 | 0.06–0.20 | 382.58 | 97.90% | 0.828 |
| Young Adults | 20 | 0.32 | 0.1984–0.4640 | 1482.59 | 98.70% | 1.961 |
| Middle Age | 118 | 0.44 | 0.3864–0.4954 | 10517.56 | 98.90% | 1.4649 |
| Eldery | 8 | 0.18 | 0.0803–0.3570 | 193.64 | 96.40% | 1.6031 |
| Older | 55 | 0.35 | 0.2882–0.4067 | 1522.64 | 96.50% | 0.9307 |
The effects of sex were similarly examined. A univariate mixed model using percentage of males in each study as a covariate found an effect of β = −0.0296 (p < 0.001) for each percent increase. Overall, the higher the percentage of males in a study, the lower the prevalence of taste loss.
Effect of type of direct approach on taste loss prevalence
When we compared the types of direct report test (e.g., solution-based test, taste strip-based test), we found a significant difference in prevalence rates (see Table 3). We classified studies into three categories: taste strip testing (n=5), taste solution testing (n = 12), and “other” (n = 1) for methods that do not employ either solution or strips. Pooled prevalence for solution-based tests was 54.25% (95% CI: 45.01–63.19%) and for taste strips was 24.63% (95% CI: 13.10–41.46%). There was reduced heterogeneity in the subgroups compared to the overall meta-analysis: solution-based testing, I2 = 88.8%; and strip-based testing, I2 = 76.8%. Measurements of τ2, were 0.3807 and 0.5991 for solution-based testing and strip-based testing respectively. There was significant heterogeneity between our pooled estimates (Q = 8.68, df = 2, p = 0.01). Together, our results demonstrate that studies using solution-based taste tests, on average, result in higher prevalence of taste loss in COVID-19 patients than do studies using strips or other methods.
Table 3.
Random-effect estimate of direct testing type on COVID-19 taste loss prevalence using generalized linear mixed models.
| Direct Testing Type | k | Proportion | 95%-CI | Q | I2 | Tau2 |
|---|---|---|---|---|---|---|
| Solution | 11 | 0.5602 | 0.4666–0.6497 | 87.62 | 88.60% | 0.3566 |
| Strip | 5 | 0.2183 | 0.1510–0.3048 | 14.45 | 72.30% | 0.1663 |
| Other | 2 | 0.0539 | 0.0000–0.9868 | 0 | 0.00% | 17.9895 |
Discussion
Despite the occurrence of true taste loss in a variety of diseases such as cancer (Nolden et al., 2019), as well as in the general population (Rawal et al., 2016), taste loss has often been confused with smell loss (Le Bon et al., 2021). However, the current coronavirus pandemic suggests that in reality, taste loss is its own unique feature of the illness. The present meta-analysis found an overall taste loss prevalence of 37 % among 138,897 COVID-19-positive participants, which aligns with other meta-analyses of taste loss prevalence, ranging from 38% (Agyeman et al., 2020) to 49% (Hajikhani et al., 2020). This high prevalence is not due to confusion with smell loss because direct taste measures yield similar (or even slightly higher) prevalence than self-report. Therefore self-reports of taste loss appear to be valid among people with COVID-19 as they are among other groups (Jang et al., 2021).
The COVID-19 pandemic created an urgent need for direct taste measures suitable for the pandemic research environment, e.g., home testing, and researchers were innovative, but each developed their own method which makes it difficult to compare results (see Table 1). However, despite the differences in methods, we can draw one general conclusion, which is that the form of the tastants matters (taste solutions are better than taste strips) but this general conclusion must be tentative given that the forms of delivery were not compared directly using the same approach, e.g., thresholds or identification.
The present meta-analysis found that around 4 in every 10 COVID-19 patients experience taste loss. We also found age and sex effects: females experienced higher rates of taste loss than males, aligning with other meta-analyses reporting a similar effect (von Bartheld et al., 2020; Amorim Dos Santos et al., 2021; Saniasiaya et al., 2021). Females may be more susceptible to taste loss because they are in general are more sensitive than males and have more sensory capacity to lose. Additionally, we found that COVID-19 associated taste loss peaks in middle age aligning with the general consensus across other COVID-19 meta-analyses (Agyeman et al., 2020; von Bartheld et al., 2020). Why the youngest and oldest groups report less taste loss than do middle-age adults is not currently known.
Although COVID-19 has intensified awareness of taste loss and furthered chemosensory research, scientists are still unsure of the biological mechanisms behind this symptom. The amount of SARS-CoV-2 virus in saliva is positively related to loss of taste: the more virus, the more taste loss (Huang et al., 2021; Taziki Balajelini et al., 2021), although this observation is controversial (Jain et al., 2020). Taste cells may be attacked directly by the virus because studies of expression patterns for ACE2, the receptor protein known to transport the SARS-CoV-2 virus into cells, and for TMPRSS2, the protein essential for processing the SARS-CoV-2 spike protein, showed both are expressed in the supporting cells of taste bud (Sakaguchi et al., 2020; Huang et al., 2021), including taste receptor cells themselves, at least in one patient (Doyle et al., 2021). There may also be direct effects on the brain that contribute to taste loss (Douaud et al., 2021).
Limitations and Future Research
In many of the included articles, clinicians and researchers collected self-reported taste loss information in tandem with smell loss (e.g., participants responding yes to “Loss of taste and smell” on a symptom screener), which can confound the results. Therefore, we explored any differences in how taste loss was collected, reflecting how it was reported in the articles (e.g., “smell and taste” vs. “taste only”), and found no significant impact on the prevalence rate (see Supplementary Materials S3). This result indicates that taste loss is a common and pervasive symptom of COVID-19. Additionally, far more articles in this meta-analysis used self-report tests (n = 223) than direct tests (n = 18). This disparity may have prevented us from capturing significant differences between the two methods.
Nearly all of the articles included in this meta-analysis were nonspecific to different tastes, instead summarizing scores across multiple stimuli (e.g., sweet and salty) and reporting taste loss as a whole (though in one study participants self-reported taste-specific dysfunction: salt taste loss, 29.3%; sweet taste, 25.9%; general taste, 34.5% [El Kady et al., 2021]). However, specific taste sensitivities can be difficult to assess via self-report. Members of the general population, untrained in chemosensory science, may have difficulties identifying whether or not they truly lost a specific taste. Therefore, it is important to use direct measures to distinguish dysfunctions of specific tastes.
There are clear opportunities for advancements of standardized direct taste tests to measure taste loss. Of the 18 studies that used direct tests in general, only five used standardized tests, representing just 2.06% of the 242 studies examined in this systematic review: Taste Strips by Burghart Messtechnik (Hintschich et al., 2020a; Le Bon et al., 2021; Niklassen et al., 2021a; Singer-Cornelius et al., 2021) and the Brief Self-Administered Waterless Empirical Taste Test (SA-WETT) by Sensonics International (Cao et al., 2021). Three other studies using direct tests were examined during the systematic review but were excluded for not meeting the inclusion criteria: reporting on a case study (Lee and Lee, 2020), recruiting patients with chemosensory dysfunction (Le Bon et al., 2020), and not reporting the required taste loss prevalence data (Huart et al., 2020). Among these three studies, Lee and Lee (2020) used a non-standardized direct test, and Huart et al. (2020) and Le Bon et al. (2020) used the Taste Strips – although this represents a missed opportunity to analyze more studies that used standardized tests, including these two articles would have increased the rate of standardized test use among all 241 studies to just 2.89% (N=7).
As of September 2021, 220 million individuals have been infected with SARS-CoV- 2 virus, with large number of recovered individuals with persistent symptoms included taste loss. It is well documented that disease-related or age-related or chemosensory loss have profound effects on an individual’s quality of life. Unlike other disorders such as vision and hearing for which preventative and screening guidelines exist (United States Preventive Services Taskforce), they are not available for taste and smell disorders. The COVID-19 pandemic further highlighted this existing gap, lack of standardized measures and clinical guidelines for screening, assessing, and monitoring the taste system making it difficult for clinicians to track progression of disease. Assessment of taste function in patients with and without confirmed COVID-19 needs to become standard of practice for clinicians. This is particularly important for at least two reasons: 1) having baseline measures help clinicians assess trends over time, and 2) given the interrelatedness between the sense of smell and taste, objective measures of taste collected during clinical assessments may help dissociate whether it is a smell or taste problem. For patients who report changes in taste function during screening questionnaires, full testing with standardized objective chemosensory tools should be performed. It is critical that clinicians are aware that most patients with chemosensory dysfunction complain of taste alterations, therefore, a closer inquiry of patient’s reports regarding the specific taste quality (i.e., sweet, bitter, sour, and salt, fat) affected is important to further distinguish between taste and perception of flavor.
Conclusion
The COVID-19 pandemic demanded an urgent response from scientists and clinicians, who have been working to understand the novel virus and the symptoms it inflicts. Of the many unique features of this virus, smell and taste dysfunctions are among the most prominent. Yet as taste loss joined smell loss as a more prolific topic in scientific literature, many initially speculated that taste loss rates were overestimated due to confusion between taste and smell in self-reports. However, our meta-analysis found a prevalence rate for taste loss of 36.9% among 138,897 COVID-19-positive individuals (95% CI: 33.27–40.69%), supported by direct methods, reflecting the validity of this distinct symptom. Dysfunction in the sense of taste was, and still is, a difficult reality for millions of people affected by the virus and merits further research to fully understand the mechanisms behind this phenomenon and how to properly assess and address it. Among the population of 138,897 COVID-19-positive individuals included in this meta-analysis, only 257 of them, across five separate studies, were assessed using standardized taste tests. Future research should include the development of fast and accurate taste tests, studies that measure taste and smell function separately to dissociate olfacto-gustatory interactions, as well as the employment of standardized taste tests in clinical settings to examine taste dysfunction. In addition, clinical trials are needed to elucidate frequency of screening and age at which to start and stop screening for chemosensory disorders. Finally, more mechanistic studies to understand taste and smell disorders associated with COVID-19 to aid in developing new therapeutic options for patients with long-lasting impairment of their chemical senses.
Included Articles
In total, 241 were included in the present systematic review and meta-analysis (Gamper et al., 2012; Liu et al., 2016; Adamczyk et al., 2020; Adedeji et al., 2020; Adorni et al., 2020; Aggarwal et al., 2020; Al-Ani and Acharya, 2020; Altin et al., 2020; Andrews et al., 2020; Anna et al., 2020; Asai et al., 2020; Bastiani et al., 2020; Beltran-Corbellini et al., 2020; Bergquist et al., 2020; Biadsee et al., 2020; Bidkar et al., 2020b; Boscolo-Rizzo et al., 2020a, 2020b, 2020c, 2021; Boudjema et al., 2020; Brandao Neto et al., 2020; Bulgurcu et al., 2020; Calica Utku et al., 2020; Carignan et al., 2020; Chary et al., 2020; Chen et al., 2020; Chiesa-Estomba et al., 2020; Cho et al., 2020; Chung et al., 2020; Cocco et al., 2020; Dawson et al., 2020; Dell’Era et al., 2020; De Maria et al., 2020; Durrani et al., 2020; Elimian et al., 2020; Farah Yusuf Mohamud et al., 2020; Fistera et al., 2020; Fontanet et al., 2020; Freni et al., 2020; Garg et al., 2020; Gelardi et al., 2020; Giacomelli et al., 2020; Gorzkowski et al., 2020; Gozen et al., 2020; GÜner et al., 2020; Gudbjartsson et al., 2020; Guillen Martinez et al., 2020; Haehner et al., 2020; Hintschich et al., 2020b; Horvath et al., 2020; Iversen et al., 2020; Izquierdo-Domínguez et al., 2020; Jain et al., 2020; Jalessi et al., 2020; Kacem et al., 2020; Karadas et al., 2020; Kempker et al., 2020; Kim et al., 2020; Klopfenstein et al., 2020; Konstantinidis et al., 2020; Krishnasamy et al., 2020; Kronborg et al., 2020; Kumar et al., 2020, 2021a, 2021b; Lagi et al., 2020; Lan et al., 2020; Lapostolle et al., 2020; La Torre et al., 2020; Lechien et al., 2020b, 2020c, 2020a, 2021, 2021, 2021; Lechner et al., 2020; Lee et al., 2020a, 2020b, 2021; Levinson et al., 2020; Liang et al., 2020; Liguori et al., 2020; Lindahl et al., 2020; Lombardi et al., 2020, 2021; Luers et al., 2020; Lv et al., 2020; Maechler et al., 2020; Magnavita et al., 2020; Makda et al., 2020; Mao et al., 2020; Martin-Sanz et al., 2020; Mazzatenta et al., 2020; Meini et al., 2020; Menni et al., 2020; Mercante et al., 2020; Merkely et al., 2020; Merza et al., 2020; Moein et al., 2020; Morshed et al., 2020; Murat et al., 2020; Nakagawa et al., 2020, 2020; Nakakubo et al., 2020; Nakanishi et al., 2020; Noh et al., 2020; Otte et al., 2020; Ozcelik Korkmaz et al., 2020; Paderno et al., 2020; Patel et al., 2020; Perlman et al., 2020; Petersen et al., 2020; Petrocelli et al., 2020b, 2020a; Pinna et al., 2020; Printza et al., 2020, 2021; Qiu et al., 2020; Rajkumar et al., 2020; Ramteke et al., 2020, 2020; Rojas-Lechuga et al., 2020; Roland et al., 2020; Romero-Sanchez et al., 2020; Rubel et al., 2020; Sakalli et al., 2020; Salepci et al., 2020; Sayin et al., 2020a, 2020b; Schirinzi et al., 2020; Schmithausen et al., 2020; Seo et al., 2020; Shoer et al., 2020; Sierpinski et al., 2020; Smith et al., 2020; Somekh et al., 2020; Speth et al., 2020; Spinato et al., 2020; Stavem et al., 2020; Sun et al., 2020; Teklu et al., 2020; Tham et al., 2020; Tsivgoulis et al., 2020; Tudrej et al., 2020; Vacchiano et al., 2020; Vaira et al., 2020e, 2020f, 2020b, 2020c, 2020d, 2020a, 2020b; Van Loon et al., 2020; Vena et al., 2020; Villarreal et al., 2020; Wagner et al., 2020; Waterfield et al., 2020; Wee et al., 2020; Weiss et al., 2020; Weng et al., 2020; Yan et al., 2020a, 2020b; Zayet et al., 2020a, 2020b; Zimmerman et al., 2020; Akinbami et al., 2021; Alharbi et al., 2021; AlShakhs et al., 2021; Amano et al., 2021; Arslan et al., 2021; Ashrafi et al., 2021; Bagnasco et al., 2021; Barillari et al., 2021; Besli et al., 2021; Bianco et al., 2021; Breyer et al., 2021; Callejon-Leblic et al., 2021; Cao et al., 2021; Carcamo Garcia et al., 2021; Concheiro-Guisan et al., 2021; Dini et al., 2021; Dixon et al., 2021; El Kady et al., 2021; Fisher et al., 2021; Fleischer et al., 2021; Galluzzi et al., 2021; Gianola et al., 2021; Gibbons et al., 2021; Gonzalez et al., 2021; Gupta et al., 2021a, 2021b; Gurrola et al., 2021; Hijazi et al., 2021; Ismail et al., 2021; Jethani et al., 2021; Kamel et al., 2021; Kandakure et al., 2021; Karaarslan et al., 2021; Karni et al., 2021; Kavaz et al., 2021; Ladoire et al., 2021; Lampl et al., 2021; Makaronidis et al., 2021; Mannan et al., 2021; Moeller et al., 2021; Monti et al., 2021; Morlock et al., 2021; Niklassen et al., 2021b; Ninchritz-Becerra et al., 2021; Nishanth et al., 2021; Noviello et al., 2021; Oda et al., 2021; Omezli and Torul, 2021; O’Keefe et al., 2021; O’Sullivan et al., 2021; Ozcan et al., 2021; Parcha et al., 2021; Perula de Torres et al., 2021; Polat et al., 2021; Rodebaugh et al., 2021; Rousseau et al., 2021; Sahoo et al., 2021; Salcan et al., 2021; Savtale et al., 2021; Sbrana et al., 2021; Schwab et al., 2021; Sehanobish et al., 2021; Singer-Cornelius et al., 2021; Soh et al., 2021; Song et al., 2021a, 2021b; Tarifi et al., 2021; Thakur et al., 2021; Trachootham et al., 2021; İşlek and Balcı, 2021; van den Besselaar et al., 2021; Wierdsma et al., 2021; Yadav et al., 2021; Zejda et al., 2021; Zifko et al., 2021; Zou et al., 2021)
Supplementary Material
Acknowledgements
We acknowledge Michael G. Tordoff for his assistance with the review of the literature, Sarah Lipson for her initial effort in reading the earlier articles, and Akane Kikuchi for ensuring no article was missed. Additionally, we thank Gary K. Beauchamp for his comments on the manuscript.
Funding
Dr. Mackenzie Hannum is supported by NIH T32 funding (DC000014). Dr. Joseph is supported by the National Institute of Alcohol Abuse and Alcoholism under award number, Z01AA000135 and the National Institute of Nursing Research, the NIH Office of Workforce Diversity, National Institutes of Health Distinguished Scholar, and the Rockefeller University Heilbrunn Nurse Scholar Award. Dr. Reed is supported in part through NIH U01DC019578.
References
- Abdullahi A., Candan S.A., Abba M.A., Bello A.H., Alshehri M.A., Afamefuna Victor E., Umar N.A., and Kundakci B. 2020. Neurological and Musculoskeletal Features of COVID-19: A Systematic Review and Meta-Analysis. Front Neurol. 11:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczyk K., Herman M., Frączek J., Piec R., Szykuła-Piec B., Zaczyński A., Wójtowicz R., Bojanowski K., Rusyan E., Król Z., et al. 2020. Sensitivity and specificity of prediction models based on gustatory disorders in diagnosing COVID-19 patients: a case-control study. Infectious Diseases (except HIV/AIDS). [Google Scholar]
- Adedeji I.A., Abdu Y.M., Bashir M.F., Adamu A.S., Gwarzo G.D., Yaro B.S., Musa A.A., Hassan Z.I., Maigoro A.M., and Jibrin Y.B. 2020. Profile of children with COVID-19 infection: a cross sectional study from North-East Nigeria. Pan Afr Med J. 35:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorni F., Prinelli F., Bianchi F., Giacomelli A., Pagani G., Bernacchia D., Rusconi S., Maggi S., Trevisan C., Noale M., et al. 2020. Self-reported symptoms of SARS-CoV-2 infection in a non-hospitalized population: results from the large Italian web-based EPICOVID19 cross-sectional survey. JMIR Public Health Surveill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C., Lippi G., and Henry B.M. 2020. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagn Berl. 7:91–96. [DOI] [PubMed] [Google Scholar]
- Agyeman A.A., Chin K.L., Landersdorfer C.B., Liew D., and Ofori-Asenso R. 2020. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin Proc. 95:1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinbami L.J., Petersen L.R., Sami S., Vuong N., Lukacs S.L., Mackey L., Atas J., and LaFleur B.J. 2021. COVID-19 symptoms and SARS-CoV-2 antibody positivity in a large survey of first responders and healthcare personnel, May-July 2020. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ani R.M., and Acharya D. 2020. Prevalence of Anosmia and Ageusia in Patients with COVID-19 at a Primary Health Center, Doha, Qatar. Indian J Otolaryngol Head Neck Surg. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi H., You S., and Katz J. 2021. Should anosmia and dysgeusia be a concern for oral and maxillofacial surgeons during the COVID-19 pandemic? Oral Maxillofac Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlShakhs A., Almomen A., AlYaeesh I., AlOmairin A., AlMutairi A.A., Alammar Z., Almomen H., and Almomen Z. 2021. The Association of Smell and Taste Dysfunction with COVID19, And Their Functional Impacts. Indian J Otolaryngol Head Neck Surg. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin F., Cingi C., Uzun T., and Bal C. 2020. Olfactory and gustatory abnormalities in COVID-19 cases. Eur Arch Otorhinolaryngol. 277:2775–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano Y., Kage H., Tanaka G., Gonoi W., Nakai Y., Kurokawa R., Inui S., Okamoto K., Harada S., Iwabu M., et al. 2021. Diagnostic prediction of COVID-19 based on clinical and radiological findings in a relatively low COVID-19 prevalence area. Respir Investig. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim Dos Santos J., Normando A.G.C., Carvalho da Silva R.L., Acevedo A.C., De Luca Canto G., Sugaya N., Santos-Silva A.R., and Guerra E.N.S. 2021. Oral Manifestations in Patients with COVID-19: A Living Systematic Review. J Dent Res. 100:141–154. [DOI] [PubMed] [Google Scholar]
- Andrews P.J., Pendolino A.L., Ottaviano G., Scarpa B., Grant J., Gaudioso P., Bordin A., Marchese-Ragona R., Leoni D., Cattelan A., et al. 2020. Olfactory and taste dysfunction among mild-to-moderate symptomatic COVID-19 positive health care workers: An international survey. Laryngoscope Investig Otolaryngol. 5:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anna F., Goyard S., Lalanne A.I., Nevo F., Gransagne M., Souque P., Louis D., Gillon V., Turbiez I., Bidard F.-C., et al. 2020. High seroprevalence but short-lived immune response to SARS-CoV-2 infection in Paris. Eur J Immunol. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan G., Aktürk H., and Duman M. 2021. Clinical Characteristics of Pediatric COVID-19 and Predictors of PCR Positivity. Pediatr Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai N., Sakanashi D., Nakamura A., Kishino T., Kato H., Hagihara M., Shiota A., Koizumi Y., Yamagishi Y., and Mikamo H. 2020. Clinical manifestations and radiological features by chest computed tomographic findings of a novel coronavirus disease-19 pneumonia among 92 patients in Japan. J Microbiol Immunol Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi F., Ommi D., Zali A., Khani S., Soheili A., Arab-Ahmadi M., Behnam B., Nohesara S., Semnani F., Fatemi A., et al. 2021. Neurological Manifestations and their Correlated Factors in COVID-19 Patients; a Cross-Sectional Study. Arch Acad Emerg Med. 9:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnasco D., Passalacqua G., Braido F., Tagliabue E., Cosini F., Filauro M., Ioppi A., Carobbio A., Mocellin D., Riccio A.M., et al. 2021. Quick Olfactory Sniffin’ Sticks Test (Q-Sticks) for the detection of smell disorders in COVID-19 patients. World Allergy Organ J. 14:100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakbergenuly I., and Kulinskaya E. 2018. Meta-analysis of binary outcomes via generalized linear mixed models: a simulation study. BMC Med Res Methodol. 18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillari M.R., Bastiani L., Lechien J.R., Mannelli G., Molteni G., Cantarella G., Coppola N., Costa G., Trecca E.M.C., Grillo C., et al. 2021. A structural equation model to examine the clinical features of mild-to-moderate COVID-19: A multicenter Italian study. J Med Virol. 93:983–994. [DOI] [PubMed] [Google Scholar]
- von Bartheld C.S., Hagen M.M., and Butowt R. 2020. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem Neurosci. 11:2944–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani L., Fortunato L., Pieroni S., Bianchi F., Adorni F., Prinelli F., Giacomelli A., Pagani G., Maggi S., Trevisan C., et al. 2020. EPICOVID19: Psychometric assessment and validation of a short diagnostic scale for a rapid Covid-19 screening based on reported symptoms. J Med Internet Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Corbellini A., Chico-Garcia J.L., Martinez-Poles J., Rodriguez-Jorge F., Natera-Villalba E., Gomez-Corral J., Gomez-Lopez A., Monreal E., Parra-Diaz P., Cortes-Cuevas J.L., et al. 2020. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist S.H., Partin C., Roberts D.L., O’Keefe J.B., Tong E.J., Zreloff J., Jarrett T.L., and Moore M.A. 2020. Non-hospitalized Adults with COVID-19 Differ Noticeably from Hospitalized Adults in Their Demographic, Clinical, and Social Characteristics. SN Compr Clin Med. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besli G.E., Öcal Demir S., Girit S., Arman T., Duyu M., and Arslanoglu S. 2021. COVID-19 in children: A single center experience from Istanbul, Turkey. Med J BAKIRKOY. 17:64–71. [Google Scholar]
- van den Besselaar J.H., Sikkema R.S., Koene F., van Buul L.W., Oude Munnink B.B., Frénay I., Te Witt R., Koopmans M.P.G., Hertogh C., and Buurman B.M. 2021. Are presymptomatic SARS-CoV-2 infections in nursing home residents unrecognized symptomatic infections? Sequence and metadata from weekly testing in an extensive nursing home outbreak. Age Ageing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biadsee A., Biadsee A., Kassem F., Dagan O., Masarwa S., and Ormianer Z. 2020. Olfactory and Oral Manifestations of COVID-19: Sex-Related Symptoms-A Potential Pathway to Early Diagnosis. Otolaryngol Head Neck Surg. 194599820934380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco M.R., Modica D.M., Drago G.D., Azzolina A., Mattina G., De Natale M., Rossi G., Amata M., Canzoneri G., Manganaro G., et al. 2021. Alteration of Smell and Taste in Asymptomatic and Symptomatic COVID-19 Patients in Sicily, Italy. Ear Nose Throat J. 100:182S–185S. [DOI] [PubMed] [Google Scholar]
- Bidkar V., Mishra M., Selvaraj K., Joshi P., H S.B., Dabhekar S., Prathipati K.K., Rathod B.S., Shendre P., and Gondode P. 2020a. Testing Olfactory and Gustatory Dysfunctions among Quarantine COVID-19 Suspects. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngol India. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidkar V., Mishra M., Selvaraj K., Joshi P., H S.B., Dabhekar S., Prathipati K.K., Rathod B.S., Shendre P., and Gondode P. 2020b. Testing Olfactory and Gustatory Dysfunctions among Quarantine COVID-19 Suspects. Indian J Otolaryngol Head Neck Surg. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Mohn K.G.-I., Brokstad K.A., Zhou F., Linchausen D.W., Hansen B.-A., Lartey S., Onyango T.B., Kuwelker K., Sævik M., et al. 2021. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P., Borsetto D., Fabbris C., Spinato G., Frezza D., Menegaldo A., Mularoni F., Gaudioso P., Cazzador D., Marciani S., et al. 2020a. Evolution of Altered Sense of Smell or Taste in Patients With Mildly Symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P., Borsetto D., Spinato G., Fabbris C., Menegaldo A., Gaudioso P., Nicolai P., Tirelli G., Da Mosto M.C., Rigoli R., et al. 2020b. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P., Guida F., Polesel J., Marcuzzo A.V., Antonucci P., Capriotti V., Sacchet E., Cragnolini F., D’Alessandro A., Zanelli E., et al. 2021. Self-reported smell and taste recovery in COVID-19 patients: a one-year prospective study. MedRxiv. 2021.03.18.21253862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P., Polesel J., Spinato G., Fabbris C., Calvanese L., Menegaldo A., Borsetto D., and Hopkins C. 2020c. Predominance of an altered sense of smell or taste among long-lasting symptoms in patients with mildly symptomatic COVID-19. Rhinology. [DOI] [PubMed] [Google Scholar]
- Boscutti A., Delvecchio G., Pigoni A., Cereda G., Ciappolino V., Bellani M., Fusar-Poli P., and Brambilla P. 2021. OLFACTORY AND GUSTATORY DYSFUNCTIONS IN SARS-CoV-2 INFECTION: Brain Behav Immun - Health. 100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudjema S., Finance J., Coulibaly F., Meddeb L., Tissot-Dupont H., Michel M., Lagier J.C., Million M., Radulesco T., Michel J., et al. 2020. Olfactory and gustative disorders for the diagnosis of COVID-19. Travel Med Infect Dis. 37:101875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao Neto D., Fornazieri M.A., Dib C., Di Francesco R.C., Doty R.L., Voegels R.L., and Pinna F.R. 2020. Chemosensory Dysfunction in COVID-19: Prevalences, Recovery Rates, and Clinical Associations on a Large Brazilian Sample. Otolaryngol Head Neck Surg. 194599820954825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer M.K., Breyer-Kohansal R., Hartl S., Kundi M., Weseslindtner L., Stiasny K., Puchhammer-Stockl E., Schrott A., Fodinger M., Binder M., et al. 2021. Low SARS-CoV-2 seroprevalence in the Austrian capital after an early governmental lockdown. Sci Rep. 11:10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgurcu S., Oztutgan T., Baz E., Yonem A., Koc N.G., Erkul E., and Cekin E. 2020. Assessment of Smell and Taste Disorders in COVID-19: A Cross-sectional Study. J Craniofac Surg. [DOI] [PubMed] [Google Scholar]
- Calica Utku A., Budak G., Karabay O., Guclu E., Okan H.D., and Vatan A. 2020. Main symptoms in patients presenting in the COVID-19 period. Scott Med J. 36933020949253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejon-Leblic M.A., Moreno-Luna R., Del Cuvillo A., Reyes-Tejero I.M., Garcia-Villaran M.A., Santos-Pena M., Maza-Solano J.M., Martin-Jimenez D.I., Palacios-Garcia J.M., Fernandez-Velez C., et al. 2021. Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach. J Clin Med. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao A.C., Nimmo Z.M., Mirza N., Cohen N.A., Brody R.M., and Doty R.L. 2021. Objective screening for olfactory and gustatory dysfunction during the COVID-19 pandemic: A prospective study in healthcare workers using self-administered testing. World J Otorhinolaryngol - Head Neck Surg. S2095881121000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcamo Garcia M.H., Garcia Choza D.D., Salazar Linares B.J., and Diaz M.M. 2021. Neurological manifestations of patients with mild-to-moderate COVID-19 attending a public hospital in Lima, Peru. ENeurologicalSci. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan A., Valiquette L., Grenier C., Musonera J.B., Nkengurutse D., Marcil-Heguy A., Vettese K., Marcoux D., Valiquette C., Xiong W.T., et al. 2020. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. CMAJ. 192:E702–E707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary E., Carsuzaa F., Trijolet J.P., Capitaine A.L., Roncato-Saberan M., Fouet K., Cazenave-Roblot F., Catroux M., Allix-Beguec C., and Dufour X. 2020. Prevalence and Recovery From Olfactory and Gustatory Dysfunctions in Covid-19 Infection: A Prospective Multicenter Study. Am J Rhinol Allergy. 34:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A., Agarwal A., Ravindran N., To C., Zhang T., and Thuluvath P.J. 2020. Are Gastrointestinal Symptoms Specific for COVID-19 Infection? A Prospective Case-Control Study from the United States. Gastroenterology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa-Estomba C.M., Lechien J.R., Portillo-Mazal P., Martinez F., Cuauro-Sanchez J., Calvo-Henriquez C., and Saussez S. 2020. Olfactory and gustatory dysfunctions in COVID-19. First reports of Latin-American ethnic patients. Am J Otolaryngol. 41:102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho R.H., To Z.W., Yeung Z.W., Tso E.Y., Fung K.S., Chau S.K., Leung E.Y., Hui T.S., Tsang S.W., Kung K.N., et al. 2020. COVID-19 Viral Load in the Severity of and Recovery from Olfactory and Gustatory Dysfunction. Laryngoscope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T.W., Sridhar S., Zhang A.J., Chan K.H., Li H.L., Wong F.K., Ng M.Y., Tsang R.K., Lee A.C., Fan Z., et al. 2020. Olfactory Dysfunction in Coronavirus Disease 2019 Patients: Observational Cohort Study and Systematic Review. Open Forum Infect Dis. 7:ofaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco A., Amami P., Desai A., Voza A., Ferreli F., and Albanese A. 2020. Neurological features in SARS-CoV-2-infected patients with smell and taste disorder. J Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concheiro-Guisan A., Fiel-Ozores A., Novoa-Carballal R., González-Duran M.L., Portugués de la Red M., Martínez-Reglero C., Fernández-Pinilla I., and González-Guijarro I. 2021. Subtle olfactory dysfunction after SARS-CoV-2 virus infection in children. Int J Pediatr Otorhinolaryngol. 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P., Rabold E.M., Laws R.L., Conners E.E., Gharpure R., Yin S., Buono S., Dasu T., Bhattacharyya S., Westergaard R.P., et al. 2020. Loss of Taste and Smell as Distinguishing Symptoms of COVID-19. MedRxiv. 2020.05.13.20101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria A., Varese P., Dentone C., Barisione E., and Bassetti M. 2020. High prevalence of olfactory and taste disorder during SARS-CoV-2 infection in outpatients. J Med Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deems D.A., Doty R.L., Settle R.G., Moore-Gillon V., Shaman P., Mester A.F., Kimmelman C.P., Brightman V.J., and Snow J.B. 1991. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 117:519–528. [DOI] [PubMed] [Google Scholar]
- Dell’Era V., Farri F., Garzaro G., Gatto M., Aluffi Valletti P., and Garzaro M. 2020. Smell and taste disorders during COVID-19 outbreak: A cross-sectional study on 355 patients. Head Neck. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini G., Montecucco A., Rahmani A., Barletta C., Pellegrini L., Debarbieri N., Orsi A., Caligiuri P., Varesano S., Manca A., et al. 2021. Clinical and epidemiological characteristics of COVID-19 during the early phase of the SARS-CoV-2 pandemic: a cross-sectional study among medical school physicians and residents employed in a regional reference teaching hospital in Northern Italy. Int J Occup Med Env Health. [DOI] [PubMed] [Google Scholar]
- Dixon B.E., Wools-Kaloustian K.K., Fadel W.F., Duszynski T.J., Yiannoutsos C., Halverson P.K., and Menachemi N. 2021. Symptoms and symptom clusters associated with SARS-CoV-2 infection in community-based populations: Results from a statewide epidemiological study. PLoS One. 16:e0241875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., et al. 2021. Brain imaging before and after COVID-19 in UK Biobank.
- Doyle M.E., Appleton A., Liu Q.-R., Yao Q., Mazucanti C.H., and Egan J.M. 2021. Human Type II Taste Cells Express Angiotensin-Converting Enzyme 2 and Are Infected by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Am J Pathol. 191:1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrani M., Haq I.U., Kalsoom U., and Yousaf A. 2020. Chest X-rays findings in COVID 19 patients at a University Teaching Hospital - A descriptive study. Pak J Med Sci. 36:S22–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kady D.M., Gomaa E.A., Abdella W.S., Ashraf Hussien R., Abd ElAziz R.H., and Khater A.G.A. 2021. Oral manifestations of COVID –19 patients: An online survey of the Egyptian population. Clin Exp Dent Res. cre2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elimian K.O., Ochu C.L., Ebhodaghe B., Myles P., Crawford E.E., Igumbor E., Ukponu W., Olayinka A., Aruna O., Dan-Nwafor C., et al. 2020. Patient characteristics associated with COVID-19 positivity and fatality in Nigeria: retrospective cohort study. BMJ Open. 10:e044079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah Yusuf Mohamud M., Garad Mohamed Y., Mohamed Ali A., and Ali Adam B. 2020. Loss of Taste and Smell are Common Clinical Characteristics of Patients with COVID-19 in Somalia: A Retrospective Double Centre Study. Infect Drug Resist. 13:2631–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favas T.T., Dev P., Chaurasia R.N., Chakravarty K., Mishra R., Joshi D., Mishra V.N., Kumar A., Singh V.K., Pandey M., et al. 2020. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol Sci. 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.L., Pavel A., and Malnick S. 2021. Rapid recovery of taste and smell in a patient with SARS-CoV-2 following convalescent plasma therapy. QJM Int J Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fistera D., Pabst D., Hartl A., Schaarschmidt B.M., Umutlu L., Dolff S., Holzner C., Kill C., and Risse J. 2020. Separating the wheat from the chaff-COVID-19 in a German emergency department: a case-control study. Int J Emerg Med. 13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer M., Kohrmann M., Dolff S., Szepanowski F., Schmidt K., Herbstreit F., Gungor C., Stolte B., Steiner K.M., Stadtler C., et al. 2021. Observational cohort study of neurological involvement among patients with SARS-CoV-2 infection. Ther Adv Neurol Disord. 14:1756286421993701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanet A., Tondeur L., Madec Y., Grant R., Besombes C., Jolly N., Fernandes Pellerin S., Ungeheuer M.-N., Cailleau I., Kuhmel L., et al. 2020. Cluster of COVID-19 in northern France: A retrospective closed cohort study. MedRxiv. 2020.04.18.20071134. [Google Scholar]
- Freni F., Meduri A., Gazia F., Nicastro V., Galletti C., Aragona P., Galletti C., Galletti B., and Galletti F. 2020. Symptomatology in head and neck district in coronavirus disease (COVID-19): A possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 41:102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi F., Rossi V., Bosetti C., and Garavello W. 2021. Risk Factors for Olfactory and Gustatory Dysfunctions in Patients with SARS-CoV-2 Infection. Neuroepidemiology. 1–8. [DOI] [PubMed] [Google Scholar]
- Gamper E.-M., Giesinger J.M., Oberguggenberger A., Kemmler G., Wintner L.M., Gattringer K., Sperner-Unterweger B., Holzner B., and Zabernigg A. 2012. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: Prevalence, course of severity, and quality of life correlates. Acta Oncol. 51:490–496. [DOI] [PubMed] [Google Scholar]
- Garg R., Jain R., Sodani A., Chouksey D., Dosi R., Athale S., Goyal N., Rathi P., Singh H., and Telang K. 2020. Neurological Symptoms as Initial Manifestation of Covid-19 - An Observational Study. Ann Indian Acad Neurol. 23:482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelardi M., Trecca E., Cassano M., and Ciprandi G. 2020. Smell and taste dysfunction during the COVID-19 outbreak: a preliminary report. Acta Biomed. 91:230–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkin R.C., Ohla K., Veldhuizen M.G., Joseph P.V., Kelly C.E., Bakke A.J., Steele K.E., Farruggia M.C., Pellegrino R., Pepino M.Y., et al. 2021. Recent Smell Loss Is the Best Predictor of COVID-19 Among Individuals With Recent Respiratory Symptoms. Chem Senses. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., et al. 2020. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianola S., Bargeri S., Campanini I., Corbetta D., Gambazza S., Innocenti T., Meroni R., Castellini G., and Turolla A. 2021. The Spread of COVID-19 Among 15,000 Physical Therapists in Italy: A Cross-Sectional Study. Phys Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons C., Hussain M., O’Keeffe D.T., and Simpkin A.J. 2021. An analysis of patient self-reported COVID-19 symptoms during the first wave of the pandemic in Ireland. Ir J Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Garcia-Huidobro F.G., Lagos A.E., Aliaga R., Fuentes-Lopez E., Diaz L.A., Garcia-Salum T., Salinas E., Toro A., Callejas C.A., et al. 2021. Prospective assessment of smell and taste impairment in a South-American coronavirus disease 2019 (COVID-19) cohort: Association with the need for hospitalization and reversibility of dysfunction. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzkowski V., Bevilacqua S., Charmillon A., Jankowski R., Gallet P., Rumeau C., and Nguyen D.T. 2020. Evolution of olfactory disorders in COVID-19 patients. Laryngoscope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozen E.D., Aliyeva C., Tevetoglu F., Karaali R., Balkan, Yener H.M., and Ozdogan H.A. 2020. Evaluation of Olfactory Function With Objective Tests in COVID-19-Positive Patients: A Cross-Sectional Study. Ear Nose Throat J. 145561320975510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson D.F., Helgason A., Jonsson H., Magnusson O.T., Melsted P., Norddahl G.L., Saemundsdottir J., Sigurdsson A., Sulem P., Agustsdottir A.B., et al. 2020. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. 382:2302–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen Martinez A., Andreu Galvez M., Rodriguez Sanz S., Hernandez Ruiz P., Garcia Morillas A., and Esteban Sanchez T. 2020. Incidence of smell and taste disorders and associated factors in patients with mild to moderate COVID-19. Otolaryngol Pol. 75:1–5. [DOI] [PubMed] [Google Scholar]
- GÜner H.R., HasanoĞlu İ., Kayaaslan B., Aypak A., Kaya Kalem A., Eser F., Özdemİr B., SaricaoĞlu E.M., Ayhan M., Aybar Bİlİr Y., et al. 2020. COVID-19 experience of the major pandemic response center in the capital: Results of the pandemic’s first month in Turkey. Turk J Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Kumbhat P., and Seervi M. 2021a. Olfactory and Gustatory Dysfunction in Covid-19: An Observational Study in a Tertiary Care Institute of Western Rajasthan. Indian J Otolaryngol Head Neck Surg. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Banavara Rajanna L., Upadhyay K., Bhatia R., Madhav Reddy N., Malik D., and Srivastava A. 2021b. Olfactory and Gustatory Dysfunction in COVID-19 Patients from Northern India: A Cross-Sectional Observational Study. Indian J Otolaryngol Head Neck Surg. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurrola J.G., Chang J.L., Roland L.T., Loftus P.A., and Cheung S.W. 2021. Short-term chemosensory distortions and phantoms in COVID-19. Laryngoscope Investig Otolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A., Draf J., Draeger S., With K. de, and Hummel T. 2020. Predictive value of sudden olfactory loss in the diagnosis of COVID-19. MedRxiv. 2020.04.27.20081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajikhani B., Calcagno T., Nasiri M.J., Jamshidi P., Dadashi M., Goudarzi M., Eshraghi A.A., and Mirsaeidi M. 2020. Olfactory and gustatory dysfunction in COVID-19 patients: A meta-analysis study. Physiol Rep. 8:e14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum M., E., Ramirez V., A., Lipson S., J., Herriman R., D., Toskala A., K., Lin C., Joseph P., V., and Reed D., R. 2020. Objective Sensory Testing Methods Reveal a Higher Prevalence of Olfactory Loss in COVID-19-Positive Patients Compared to Subjective Methods: A Systematic Review and Meta-Analysis. Chem Senses. 45:856–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., and Thompson S.G. 2002. Quantifying heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- Hijazi L.O., Alaraifi A.K., and Alsaab F. 2021. Otolaryngology manifestations of COVID-19 in pediatric patients. Int J Pediatr Otorhinolaryngol. 144:110701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintschich C.A., Wenzel J.J., Hummel T., Hankir M.K., Kühnel T., Vielsmeier V., and Bohr C. 2020a. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. 10:1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintschich C.A., Wenzel J.J., Hummel T., Hankir M.K., Kühnel T., Vielsmeier V., and Bohr C. 2020b. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath L., Lim J.W.J., Taylor J.W., Saief T., Stuart R., Rimmer J., and Michael P. 2020. Smell and taste loss in COVID-19 patients: assessment outcomes in a Victorian population. Acta Otolaryngol. 1–5. [DOI] [PubMed] [Google Scholar]
- Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., Baker P., Smith E., and Buchbinder R. 2012. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 65:934–939. [DOI] [PubMed] [Google Scholar]
- Huang N., Pérez P., Kato T., Mikami Y., Okuda K., Gilmore R.C., Conde C.D., Gasmi B., Stein S., Beach M., et al. 2021. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 27:892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huart C., Philpott C., Konstantinidis I., Altundag A., Whitcroft K.L., Trecca E.M.C., Cassano M., Rombaux Ph., and Hummel T. 2020. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology. 58:623–625. [DOI] [PubMed] [Google Scholar]
- İşlek A., and Balcı M.K. 2021. Diagnostic Value of Butanol Threshold Test in COVID-19 Related Olfactory Dysfunction. Indian J Otolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail F., Farag A., Haq S., and Kamal M.A. 2021. Clinical Characteristics of the First 100 Patients of COVID-19 in Tobruk, Libya: A Brief Report From Low-Resource Settings. Disaster Med Public Health Prep. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J.H., Nielsen P.B., Pries-Heje M., Knudsen A.D., Christensen C.E., Fogh K., Norsk J.B., et al. 2020. Risk of COVID-19 in healthcare workers in Denmark: an observational cohort study. Lancet Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Domínguez A., Rojas-Lechuga M.J., Chiesa-Estomba C., Calvo-Henríquez C., Ninchritz-Becerra E., Soriano-Reixach M., Poletti-Serafini D., Villarreal I.M., Maza-Solano J.M., Moreno-Luna R., et al. 2020. Smell and taste dysfunctions in COVID-19 are associated with younger age in ambulatory settings - a multicenter cross-sectional study. J Investig Allergol Clin Immunol. 0. [DOI] [PubMed] [Google Scholar]
- Jain A., Kumar L., Kaur J., Baisla T., Goyal P., Pandey A.K., Das A., and Parashar L. 2020. Olfactory and taste dysfunction in coronavirus disease 2019 patients: its prevalence and outcomes. J Laryngol Otol. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalessi M., Barati M., Rohani M., Amini E., Ourang A., Azad Z., Hosseinzadeh F., Cavallieri F., Ghadirpour R., Valzania F., et al. 2020. Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S.S., Choi J.S., Kim J.H., Kim N., and Ference E.H. 2021. Discordance Between Subjective and Objective Measures of Smell and Taste in US Adults. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 1945998211018386. [DOI] [PubMed] [Google Scholar]
- Jethani B., Gupta M., Wadhwani P., Thomas R., Balakrishnan T., Mathew G., Mathur M., Rao B.P., Shukla D., Khullar A., et al. 2021. Clinical Characteristics and Remedy Profiles of Patients with COVID-19: A Retrospective Cohort Study. Homeopathy. [DOI] [PubMed] [Google Scholar]
- Kacem I., Gharbi A., Harizi C., Souissi E., Safer M., Nasri A., Letaief H., Akkari M., Hechaichi A., Mrabet S., et al. 2020. Characteristics, onset, and evolution of neurological symptoms in patients with COVID-19. Neurol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel F.O., Magadmi R.M., Alqutub S.T., Badawi M., Al-Sayes F., Badawi M., Madni T.A., Alhothali A., Abozinadah E.A., and Adam S. 2021. Clinical and hematologic presentations of adults with COVID-19 patients in Jeddah: A case control study. J Infect Public Health. 14:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandakure V.T., Valvi H.R., Khokle P., More M.S., and Chouhan R. 2021. Prevalence and Recovery from Newly Onset Anosmia and Ageusia in Covid 19 Patients at our Teritary Care Centre. Indian J Otolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaarslan F., Demircioğlu Güneri F., and Kardeş S. 2021. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: prospective follow-up by phone interviews. Rheumatol Int. 41:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadas O., Ozturk B., and Sonkaya A.R. 2020. A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci. 41:1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni N., Klein H., Asseo K., Benjamini Y., Israel S., Nammary M., Olshtain-Pops K., Nir-Paz R., Hershko A., Muszkat M., et al. 2021. Self-Rated Smell Ability Enables Highly Specific Predictors of COVID-19 Status: A Case-Control Study in Israel. Open Forum Infect Dis. 8:ofaa589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaz E., Tahir E., Bilek H.C., Kemal O., Deveci A., and Aksakal Tanyel E. 2021. Clinical significance of smell and taste dysfunction and other related factors in COVID-19. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempker R.R., Kempker J.A., Peters M., Rebolledo P.A., Carroll K., Toomer L., Wang Y.F.W., Ray S.M., and Hunter M. 2020. Loss of Smell and Taste Among Healthcare Personnel Screened for Coronavirus 2019. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.U., Kim M.J., Ra S.H., Lee J., Bae S., Jung J., and Kim S.H. 2020. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 26:948 e1–948 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Zahra H., Kadiane-Oussou N.J., Lepiller Q., Royer P.Y., Toko L., Gendrin V., and Zayet S. 2020. New loss of smell and taste: Uncommon symptoms in COVID-19 patients on Nord Franche-Comte cluster, France. Int J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis I., Delides A., Tsakiropoulou E., Maragoudakis P., Sapounas S., and Tsiodras S. 2020. Short-Term Follow-Up of Self-Isolated COVID-19 Patients with Smell and Taste Dysfunction in Greece: Two Phenotypes of Recovery. ORL J Otorhinolaryngol Relat Spec. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnasamy N., Natarajan M., Ramachandran A., Vivian Thangaraj J.W., Etherajan T., Rengarajan J., Shanmugasundaram M., Kandasamy A., Ramamoorthy R., Velusamy A., et al. 2020. Clinical Outcomes among Asymptomatic or Mildly Symptomatic COVID-19 Patients in an Isolation Facility in Chennai, India. Am J Trop Med Hyg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronborg T.M., Kimer N., Junker A.E., Werge M.P., Gluud L.L., and Ytting H. 2020. Experience from a COVID-19 first-line referral clinic in Greater Copenhagen. Dan Med J. 67. [PubMed] [Google Scholar]
- Kumar A.A., Lee S.W.Y., Lock C., and Keong N.C.H. 2021a. Geographical Variations in Host Predisposition to COVID-19 Related Anosmia, Ageusia, and Neurological Syndromes. Front Med. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar L., Kahlon N., Jain A., Kaur J., Singh M., and Pandey A.K. 2021b. Loss of smell and taste in COVID-19 infection in adolescents. Int J Pediatr Otorhinolaryngol. 142:110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Bhartiya S., Desai S., Mutha A., Beldar A., and Singh T. 2020. Seroprevalence of Antibodies Against SARS-CoV-2 Among Health Care Workers in Mumbai, India. Asia Pac J Public Health. 1010539520977307. [DOI] [PubMed] [Google Scholar]
- La Torre G., Massetti A.P., Antonelli G., Fimiani C., Fantini M., Marte M., Faticoni A., Previte C.M., Turriziani O., Pugliese F., et al. 2020. Anosmia and Ageusia as Predictive Signs of COVID-19 in Healthcare Workers in Italy: A Prospective Case-Control Study. J Clin Med. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoire S., Goussot V., Redersdorff E., Cueff A., Ballot E., Truntzer C., Ayati S., Bengrine-Lefevre L., Bremaud N., Coudert B., et al. 2021. Seroprevalence of SARS-CoV-2 among the staff and patients of a French cancer centre after first lockdown: The canSEROcov study. Eur J Cancer. 148:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagi F., Piccica M., Graziani L., Vellere I., Botta A., Tilli M., Ottino L., Borchi B., Pozzi M., Bartalesi F., et al. 2020. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Euro Surveill. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl B.M.J., Buczovsky M., Martin G., Schmied H., Leitzmann M., and Salzberger B. 2021. Clinical and epidemiological data of COVID-19 from Regensburg, Germany: a retrospective analysis of 1084 consecutive cases. Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F.Y., Filler R., Mathew S., Buley J., Iliaki E., Bruno-Murtha L.A., Osgood R., Christophi C.A., Fernandez-Montero A., and Kales S.N. 2020. COVID-19 symptoms predictive of healthcare workers’ SARS-CoV-2 PCR results. PLoS One. 15:e0235460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapostolle F., Schneider E., Vianu I., Dollet G., Roche B., Berdah J., Michel J., Goix L., Chanzy E., Petrovic T., et al. 2020. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern Emerg Med. 15:813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon S., Payen L., Prunier L., Steffens Y., Horoi M., Vaira L.A., Hopkins C., Lechien J.R., and Saussez S. 2021. Making scents of loss of taste in COVID-19: Is self-reported loss of taste due to olfactory dysfunction? A prospective study using psychophysical testing. Int Forum Allergy Rhinol. alr.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon S.-D., Pisarski N., Verbeke J., Prunier L., Cavelier G., Thill M.-P., Rodriguez A., Dequanter D., Lechien J.R., Le Bon O., et al. 2020. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Cabaraux P., Chiesa-Estomba C.M., Khalife M., Plzak J., Hans S., Martiny D., Calvo-Henriquez C., Hopkins C., and Saussez S. 2020a. Objective olfactory testing in patients presenting with sudden onset olfactory dysfunction as the first manifestation of confirmed COVID-19 infection.
- Lechien J.R., Chiesa-Estomba C.M., Beckers E., Mustin V., Ducarme M., Journe F., Marchant A., Jouffe L., Barillari M.R., Cammaroto G., et al. 2021. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med. n/a. [DOI] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Hans S., Barillari M.R., Jouffe L., and Saussez S. 2020b. Loss of Smell and Taste in 2013 European Patients With Mild to Moderate COVID-19. Ann Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., Huet K., Plzak J., Horoi M., Hans S., et al. 2020c. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 288:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner M., Liu J., Counsell N., Ta N.H., Rocke J., Anmolsingh R., Eynon-Lewis N., Paun S., Hopkins C., Khwaja S., et al. 2020. Course of symptoms for loss of sense of smell and taste over time in one thousand forty-one healthcare workers during the Covid-19 pandemic: Our experience. Clin Otolaryngol. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.J., Daliyot D., Wang R., Lockwood J., Das P., Zimlichman E., and Lee J.M. 2021. Comparative Study of Chemosensory Dysfunction in COVID-19 in 2 Geographically Distinct Regions. Ear Nose Throat J. 1455613211000170. [DOI] [PubMed] [Google Scholar]
- Lee D.J., Lockwood J., Das P., Wang R., Grinspun E., and Lee J.M. 2020a. Self-reported anosmia and dysgeusia as key symptoms of coronavirus disease 2019. CJEM. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-M., and Lee S.J. 2020. Olfactory and Gustatory Dysfunction in a COVID-19 Patient with Ankylosing Spondylitis Treated with Etanercept: Case Report. J Korean Med Sci. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Min P., Lee S., and Kim S.W. 2020b. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci. 35:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson R., Elbaz M., Ben-Ami R., Shasha D., Levinson T., Choshen G., Petrov K., Gadoth A., and Paran Y. 2020. Anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. MedRxiv. 2020.04.11.20055483. [DOI] [PubMed] [Google Scholar]
- Liang Y., Xu J., Chu M., Mai J., Lai N., Tang W., Yang T., Zhang S., Guan C., Zhong F., et al. 2020. Neurosensory dysfunction: A diagnostic marker of early COVID-19. Int J Infect Dis. 98:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C., Pierantozzi M., Spanetta M., Sarmati L., Cesta N., Iannetta M., Ora J., Mina G.G., Puxeddu E., Balbi O., et al. 2020. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. 88:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl J.F., Hoffman T., Esmaeilzadeh M., Olsen B., Winter R., Amer S., Molnar C., Svalberg A., and Lundkvist A. 2020. High seroprevalence of SARS-CoV-2 in elderly care employees in Sweden. Infect Ecol Epidemiol. 10:1789036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Zong G., Doty R.L., and Sun Q. 2016. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open. 6:e013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A., Consonni D., Carugno M., Bozzi G., Mangioni D., Muscatello A., Castelli V., Palomba E., Cantu A.P., Ceriotti F., et al. 2020. Characteristics of 1,573 healthcare workers who underwent nasopharyngeal swab for SARS-CoV-2 in Milano, Lombardy, Italy. Clin Microbiol Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A., Mangioni D., Consonni D., Cariani L., Bono P., Cantu A.P., Tiso B., Carugno M., Muscatello A., Lunghi G., et al. 2021. Seroprevalence of anti-SARS-CoV-2 IgG among healthcare workers of a large university hospital in Milan, Lombardy, Italy: a cross-sectional study. BMJ Open. 11:e047216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luers J.C., Rokohl A.C., Loreck N., Wawer Matos P.A., Augustin M., Dewald F., Klein F., Lehmann C., and Heindl L.M. 2020. Olfactory and Gustatory Dysfunction in Coronavirus Disease 19 (COVID-19). Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Zhang W., Zhu Z., Xiong Q., Xiang R., Wang Y., Shi W., Deng Z., and Xu Y. 2020. Prevalence and recovery time of olfactory and gustatory dysfunctions of hospitalized patients with COVID19 in Wuhan, China. Int J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler F., Gertler M., Hermes J., van Loon W., Schwab F., Piening B., Rojansky S., Hommes F., Kausch F., Lindner A.K., et al. 2020. Epidemiological and clinical characteristics of SARS-CoV-2 infections at a testing site in Berlin, Germany, March and April 2020 - A cross-sectional study. Clin Microbiol Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnavita N., Tripepi G., and Di Prinzio R.R. 2020. Symptoms in Health Care Workers during the COVID-19 Epidemic. A Cross-Sectional Survey. Int J Env Res Public Health. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair M., Singhavi H., Pai A., Singhavi J., Gandhi P., Conboy P., Baker A., and Das S. 2021. A Meta-Analysis of 67 Studies with Presenting Symptoms and Laboratory Tests of COVID-19 Patients. The Laryngoscope. 131:1254–1265. [DOI] [PubMed] [Google Scholar]
- Makaronidis J., Firman C., Magee C.G., Mok J., Balogun N., Lechner M., Carnemolla A., and Batterham R.L. 2021. Distorted chemosensory perception and female sex associate with persistent smell and/or taste loss in people with SARS-CoV-2 antibodies: a community based cohort study investigating clinical course and resolution of acute smell and/or taste loss in people with and without SARS-CoV-2 antibodies in London, UK. BMC Infect Dis. 21:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makda A., Kumar S., Kumar A., Kumar V., and Rizwan A. 2020. The Frequency of Neurological Symptoms in COVID-19 Patients at a Tertiary Care Hospital in Pakistan. Cureus. 12:e10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan A., Mehedi H.M.H., Chy N., Qayum M.O., Akter F., Rob M.A., Biswas P., Hossain S., and Ayub M. 2021. A multi-centre, cross-sectional study on coronavirus disease 2019 in Bangladesh: clinical epidemiology and short-term outcomes in recovered individuals. New Microbes New Infect. 40:100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Wang M., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Li Y., Jin H., et al. 2020. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. MedRxiv. 2020.02.22.20026500. [Google Scholar]
- Martin-Sanz E., Riestra J., Yebra L., Larran A., Mancino F., Yanes-Diaz J., Garrote M., Colmenero M., Montiel E., Molina C., et al. 2020. Prospective study in 355 patients with suspected COVID-19 infection. Value of cough, subjective hyposmia, and hypogeusia. Laryngoscope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzatenta A., Neri G., D’Ardes D., De Luca C., Marinari S., Porreca E., Cipollone F., Vecchiet J., Falcicchia C., Panichi V., et al. 2020. Smell and Taste in Severe CoViD-19: Self-Reported vs. Testing. Front Med. 7:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meini S., Suardi L.R., Busoni M., Roberts A.T., and Fortini A. 2020. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., El-Sayed Moustafa J.S., et al. 2020. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercante G., Ferreli F., De Virgilio A., Gaino F., Di Bari M., Colombo G., Russo E., Costantino A., Pirola F., Cugini G., et al. 2020. Prevalence of Taste and Smell Dysfunction in Coronavirus Disease 2019. JAMA Otolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkely B., Szabo A.J., Kosztin A., Berenyi E., Sebestyen A., Lengyel C., Merkely G., Karady J., Varkonyi I., Papp C., et al. 2020. Novel coronavirus epidemic in the Hungarian population, a cross-sectional nationwide survey to support the exit policy in Hungary. Geroscience. 42:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merza M.A., Haleem Al Mezori A.A., Mohammed H.M., and Abdulah D.M. 2020. COVID-19 outbreak in Iraqi Kurdistan: The first report characterizing epidemiological, clinical, laboratory, and radiological findings of the disease. Diabetes Metab Syndr. 14:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., and Doty R.L. 2020. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller A.L., Mills E.H.A., Collatz Christensen H., Gnesin F., Blomberg S., Zylyftari N., Jensen B., Ringgren K.B., Broccia M.D., Boggild H., et al. 2021. Symptom presentation of SARS-CoV-2-positive and negative patients: a nested case-control study among patients calling the emergency medical service and medical helpline. BMJ Open. 11:e044208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., and Group T.P. 2009. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti G., Leggieri C., Fominskiy E., Scandroglio A.M., Colombo S., Tozzi M., Moizo E., Mucci M., Crivellari M., Pieri M., et al. 2021. Two months quality of life of COVID-19 invasively ventilated survivors; an Italian single-center study. Acta Anaesthesiol Scand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlock R., Morlock A., Downen M., and Shah S.N. 2021. COVID-19 prevalence and predictors in United States adults during peak stay-at-home orders. PLoS One. 16:e0245586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshed M.S., Mosabbir A.A., Chowdhury P., Ashadullah S.M., and Hossain M.S. 2020. Clinical manifestations of patients with Coronavirus Disease 2019 (COVID- 19) attending at hospitals in Bangladesh. MedRxiv. [Google Scholar]
- Murat S., Dogruoz Karatekin B., Icagasioglu A., Ulasoglu C., İçten S., and Incealtin O. 2020. Clinical presentations of pain in patients with COVID-19 infection. Ir J Med Sci 1971−. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Lagisz M., O’Dea R.E., Rutkowska J., Yang Y., Noble D.W., and Senior A.M. 2020. The Orchard Plot: Cultivating a Forest Plot for Use in Ecology, Evolution and Beyond. Research Synthesis Methods. 12:4–12. [DOI] [PubMed] [Google Scholar]
- Nakakubo S., Suzuki M., Kamada K., Yamashita Y., Nakamura J., Horii H., Sato K., Matsumoto M., Abe Y., Tsuji K., et al. 2020. Proposal of COVID-19 Clinical Risk Score for the management of suspected COVID-19 cases: a case control study. BMC Infect Dis. 20:858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Suzuki M., Maeda H., Nakamura Y., Ikegami Y., Takenaka Y., Mori Y., Hasuo T., and Hasegawa C. 2020. Differential Diagnosis of COVID-19: Importance of Measuring Blood Lymphocytes, Serum Electrolytes, and Olfactory and Taste Functions. Tohoku J Exp Med. 252:109–119. [DOI] [PubMed] [Google Scholar]
- Niklassen A.S., Draf J., Huart C., Hintschich C., Bocksberger S., Trecca E.M.C., Klimek L., Bon S.D.L., Altundag A., and Hummel T. 2021a. COVID-19: Recovery from Chemosensory Dysfunction. A Multicentre study on Smell and Taste. The Laryngoscope. 131:1095–1100. [DOI] [PubMed] [Google Scholar]
- Niklassen A.S., Draf J., Huart C., Hintschich C., Bocksberger S., Trecca E.M.C., Klimek L., Le Bon S.D., Altundag A., and Hummel T. 2021b. COVID-19: Recovery from chemosensory dysfunction. A multicentre study on smell and taste. Laryngoscope. [DOI] [PubMed] [Google Scholar]
- Ninchritz-Becerra E., Soriano-Reixach M.M., Mayo-Yánez M., Calvo-Henríquez C., Martínez-Ruiz de Apodaca P., Saga-Gutiérrez C., Parente-Arias P., Villareal I.M., Viera-Artiles J., Poletti-Serafini D., et al. 2021. Subjective evaluation of smell and taste dysfunction in patients with mild COVID-19 in Spain. Med Clínica Engl Ed. 156:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishanth D., Jhuma S., Nitesh G., Ramesh chand M., Charanjit S., Gupta D.K., and Sen M.K. 2021. COVID-19 with and without anosmia/dysgeusia: A case-control study. J Med Virol. n/a. [DOI] [PubMed] [Google Scholar]
- Noh J.Y., Yoon J.G., Seong H., Choi W.S., Sohn J.W., Cheong H.J., Kim W.J., and Song J.Y. 2020. Asymptomatic infection and atypical manifestations of COVID-19: Comparison of viral shedding duration. J Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolden A.A., Hwang L.-D., Boltong A., and Reed D.R. 2019. Chemosensory Changes from Cancer Treatment and Their Effects on Patients’ Food Behavior: A Scoping Review. Nutrients. 11:2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noviello D., Costantino A., Muscatello A., Bandera A., Consonni D., Vecchi M., and Basilisco G. 2021. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: A controlled cohort study. Neurogastroenterol Motil. e14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y., Shiraishi S., Shimada M., and Kurai O. 2021. Clinical profiles and outcome of patients with COVID-19 in a specialized hospital in Japan. J Anesth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J.B., Tong E.J., O’Keefe G.D., and Tong D.C. 2021. Description of symptom course in a telemedicine monitoring clinic for acute symptomatic COVID-19: a retrospective cohort study. BMJ Open. 11:e044154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omezli M.M., and Torul D. 2021. Evaluation of the xerostomia, taste and smell impairments after Covid-19. Med Oral Patol Oral Cir Bucal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan G., Jacob S., Barrett P.M., and Gallagher J. 2021. Covid-19 presentation among symptomatic healthcare workers in Ireland. Occup Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte M.S., Eckel H.N.C., Poluschkin L., Klussmann J.P., and Luers J.C. 2020. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol. 1–4. [DOI] [PubMed] [Google Scholar]
- Ozcan E., Yavuzer S., Borku Uysal B., Islamoglu M.S., Ikitimur H., Unal O.F., Akpinar Y.E., Seyhan S., Koc S., Yavuzer H., et al. 2021. The Relationship Between Positivity for COVID-19 RT-PCR and Symptoms, Clinical Findings, and Mortality in Turkey. Expert Rev Mol Diagn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcelik Korkmaz M., Egilmez O.K., Ozcelik M.A., and Guven M. 2020. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paderno A., Schreiber A., Grammatica A., Raffetti E., Tomasoni M., Gualtieri T., Taboni S., Zorzi S., Lombardi D., Deganello A., et al. 2020. Smell and taste alterations in Covid-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang K.W., Chee J., Subramaniam S., and Ng C.L. 2020. Frequency and Clinical Utility of Olfactory Dysfunction in COVID-19: a Systematic Review and Meta-analysis. Curr Allergy Asthma Rep. 20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcha V., Booker K.S., Kalra R., Kuranz S., Berra L., Arora G., and Arora P. 2021. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 11:10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Charani E., Ariyanayagam D., Abdulaal A., Denny S.J., Mughal N., and Moore L.S.P. 2020. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman A., Vodonos Zilberg A., Bak P., Dreyfuss M., Leventer-Roberts M., Vurembrand Y., Jeffries H.E., Fisher E., Steuerman Y., Namir Y., et al. 2020. Digital and Remote Care among 71,619 Individuals Seeking COVID-19 Health Information and Services: A Retrospective Cohort Study. J Med Internet Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perula de Torres L.A., Gonzalez-Lama J., Jimenez Garcia C., Sanchez Montero R., Rider Garrido F., Ortega Lopez Y., Pajares Conde D., Ramirez Baena M., Parraga Martinez I., Romero-Rodriguez E., et al. 2021. Frequency and predictive validity of olfactory and taste dysfunction in patients with SARS-CoV-2 infection. Med Clin Barc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.S., Kristiansen M.F., Hanusson K.D., Danielsen M.E., Steig B. á, Gaini S., Strøm M., and Weihe P. 2020. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocelli M., Ruggiero F., Baietti A.M., Pandolfi P., Salzano G., Salzano F.A., Lechien J.R., Saussez S., De Riu G., and Vaira L.A. 2020a. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J Laryngol Otol. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocelli M., Ruggiero F., Baietti A.M., Pandolfi P., Salzano G., Salzano F.A., Lechien J.R., Saussez S., Riu G.D., and Vaira L.A. 2020b. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J Laryngol Otol. 134:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna P., Grewal P., Hall J.P., Tavarez T., Dafer R.M., Garg R., Osteraas N.D., Pellack D.R., Asthana A., Fegan K., et al. 2020. Neurological manifestations and COVID-19: Experiences from a tertiary care center at the Frontline. J Neurol Sci. 415:116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat B., Yilmaz N.H., Altin G., Atakcan Z., and Mert A. 2021. Olfactory and Gustatory Dysfunctions in COVID-19 Patients: From a Different Perspective. J Craniofac Surg. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printza A., Katotomichelakis M., Metallidis S., Panagopoulos P., Sarafidou A., Petrakis V., and Constantinidis J. 2020. The clinical course of smell and taste loss in COVID-19 hospitalized patients. Hippokratia. 24:66–71. [PMC free article] [PubMed] [Google Scholar]
- Printza A., Katotomichelakis M., Valsamidis K., Metallidis S., Panagopoulos P., Panopoulou M., Petrakis V., and Constantinidis J. 2021. Smell and Taste Loss Recovery Time in COVID-19 Patients and Disease Severity. J Clin Med. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Cui C., Hautefort C., Haehner A., Zhao J., Yao Q., Zeng H., Nisenbaum E.J., Liu L., Zhao Y., et al. 2020. Olfactory and Gustatory Dysfunction as an Early Identifier of COVID-19 in Adults and Children: An International Multicenter Study. Otolaryngol Head Neck Surg. 194599820934376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core, T. 2021. R: A language and environment for statistical computing.
- Rajkumar I., Anand K.H., Revathishree K., Shoba K., and Srinivasan K. 2020. Contemporary Analysis of Olfactory Dysfunction in Mild to Moderate Covid 19 Patients in A Tertiary Health Care Centre. Indian J Otolaryngol Head Neck Surg. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramteke S., Tikkas R., Goel M., Mandraha S., and Shrivastava J. 2020. Paediatric COVID-19: Milder Presentation-A Silver Lining in Dark Cloud. J Trop Pediatr. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal S., Hoffman H.J., Bainbridge K.E., Huedo-Medina T.B., and Duffy V.B. 2016. Prevalence and Risk Factors of Self-Reported Smell and Taste Alterations: Results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses. 41:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocke J., Hopkins C., Philpott C., and Kumar N. 2020. Is loss of sense of smell a diagnostic marker in COVID-19: A systematic review and meta-analysis. Clin Otolaryngol. 45:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh T.L., Frumkin M.R., Reiersen A.M., Lenze E.J., Avidan M.S., Miller J.P., Piccirillo J.F., Zorumski C.F., and Mattar C. 2021. Acute Symptoms of Mild to Moderate COVID-19 Are Highly Heterogeneous Across Individuals and Over Time. Open Forum Infect Dis. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Lechuga M.J., Izquierdo-Dominguez A., Chiesa-Estomba C., Calvo-Henriquez C., Villarreal I.M., Cuesta-Chasco G., Bernal-Sprekelsen M., Mullol J., and Alobid I. 2020. Chemosensory dysfunction in COVID-19 out-patients. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland L.T., Gurrola J.G., Loftus P.A., Cheung S.W., and Chang J.L. 2020. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sanchez C.M., Diaz-Maroto I., Fernandez-Diaz E., Sanchez-Larsen A., Layos-Romero A., Garcia-Garcia J., Gonzalez E., Redondo-Penas I., Perona-Moratalla A.B., Del Valle-Perez J.A., et al. 2020. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 95:e1060–e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau M.C., Hully M., Milh M., Juzeau D., Pollez B., Peudenier S., Bahi Buisson N., Gautheron V., Chabrol B., and Billette de Villemeur T. 2021. Clinical characteristics of COVID-19 infection in polyhandicapped persons in France. Arch Pédiatrie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team. 2020. RStudio: Integrated Development for R. RStudio. Boston, MA: RStudio, PBC. [Google Scholar]
- Rubel K., Sharma D., Campiti V., Yedlicka G., Burgin S.J., Illing E.A., Kroenke K., and Ting J.Y. 2020. COVID-19 Status Differentially Affects Olfaction: A Prospective Case-Control Study. OTO Open. 4:2473974X20970176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo P.R., Sahu M., Surapaneni P.S., Maiti A., Vankamamidi R., Panda N., and Biswal R.N. 2021. Evolution of olfactory and gustatory dysfunctions in COVID-19 patients in India. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi W., Kubota N., Shimizu T., Saruta J., Fuchida S., Kawata A., Yamamoto Y., Sugimoto M., Yakeishi M., and Tsukinoki K. 2020. Existence of SARS-CoV-2 Entry Molecules in the Oral Cavity. Int J Mol Sci. 21:6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakalli E., Temirbekov D., Bayri E., Alis E.E., Erdurak S.C., and Bayraktaroglu M. 2020. Ear nose throat-related symptoms with a focus on loss of smell and/or taste in COVID-19 patients. Am J Otolaryngol. 41:102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcan İ., Karakeçili F., Salcan S., Ünver E., Akyüz S., Seçkin E., and Cingi C. 2021. Is taste and smell impairment irreversible in COVID-19 patients? Eur Arch Otorhinolaryngol. 278:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salepci E., Turk B., Ozcan S.N., Bektas M.E., Aybal A., Dokmetas I., and Turgut S. 2020. Symptomatology of COVID-19 from the otorhinolaryngology perspective: a survey of 223 SARS-CoV-2 RNA-positive patients. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saniasiaya J., Islam M.A., and Abdullah B. 2021. Prevalence and Characteristics of Taste Disorders in Cases of COVID-19: A Meta-analysis of 29,349 Patients. Otolaryngol Neck Surg. 165:33–42. [DOI] [PubMed] [Google Scholar]
- Santos R.E.A., da Silva M.G., Monte Silva M.C.B. do, Barbosa D.A.M., Gomes A.L. do V., Galindo L.C.M., Silva Aragão R. da, and Ferraz-Pereira K.N. 2021. Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: A systematic review. Am J Otolaryngol. 42:102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savtale S., Hippargekar P., Bhise S., and Kothule S. 2021. Prevalence of Otorhinolaryngological Symptoms in Covid 19 Patients. Indian J Otolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin I., Yasar K.K., and Yazici Z.M. 2020a. Taste and Smell Impairment in COVID-19: An AAO-HNS Anosmia Reporting Tool-Based Comparative Study. Otolaryngol Head Neck Surg. 194599820931820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin P., Altinay M., Cinar A.S., and Ozdemir H.M. 2020b. Taste and Smell Impairment in Critically Ill Patients With COVID-19: An Intensive Care Unit Study. Ear Nose Throat J. 145561320977464. [DOI] [PubMed] [Google Scholar]
- Sbrana M.F., Fornazieri M.A., Bruni-Cardoso A., Avelino-Silva V.I., Schechtman D., Voegels R.L., Malnic B., Glezer I., and de Rezende Pinna F.. 2021. Olfactory Dysfunction in Frontline Health Care Professionals During COVID-19 Pandemic in Brazil. Front Physiol. 12:622987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirinzi A., Cazzolla A.P., Lovero R., Lo Muzio L., Testa N.F., Ciavarella D., Palmieri G., Pozzessere P., Procacci V., Di Serio F., et al. 2020. New Insights in Laboratory Testing for COVID-19 Patients: Looking for the Role and Predictive Value of Human epididymis secretory protein 4 (HE4) and the Innate Immunity of the Oral Cavity and Respiratory Tract. Microorganisms. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithausen R.M., Dohla M., Schobetaler H., Diegmann C., Schulte B., Richter E., Eis-Hubinger A.M., and Streeck H. 2020. Characteristic Temporary Loss of Taste and Olfactory Senses in SARS-CoV-2-positive-Individuals with Mild Symptoms. Pathog Immun. 5:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J., Jensen C.D., and Fjaeldstad A.W. 2021. Sustained Chemosensory Dysfunction during the COVID-19 Pandemic. ORL J Otorhinolaryngol Relat Spec. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer G., Chemaitelly H., Abu-Raddad L.J., and Rücker G. 2019. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 10:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehanobish E., Barbi M., Fong V., Kravitz M., Sanchez Tejera D., Asad M., Matsumura C., Ferastraoaru D., O’Neill M., Karagic M., et al. 2021. COVID-19-Induced Anosmia and Ageusia Are Associated With Younger Age and Lower Blood Eosinophil Counts. Am J Rhinol Allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M.Y., Seok H., Hwang S.J., Choi H.K., Jeon J.H., Sohn J.W., Park D.W., Lee S.H., and Choi W.S. 2020. Trend of Olfactory and Gustatory Dysfunction in COVID-19 Patients in a Quarantine Facility. J Korean Med Sci. 35:e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoer S., Karady T., Keshet A., Shilo S., Rossman H., Gavrieli A., Meir T., Lavon A., Kolobkov D., Kalka I., et al. 2020. Who should we test for COVID-19? A triage model built from national symptom surveys. MedRxiv. 2020.05.18.20105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierpinski R., Pinkas J., Jankowski M., Zgliczynski W.S., Wierzba W., Gujski M., and Szumowski L. 2020. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders in 1942 nonhospitalized patients with coronavirus disease 2019 (COVID-19). Pol Arch Intern Med. 130:501–505. [DOI] [PubMed] [Google Scholar]
- Singer-Cornelius T., Cornelius J., Oberle M., Metternich F.U., and Brockmeier S.J. 2021. Objective gustatory and olfactory dysfunction in COVID-19 patients: a prospective cross-sectional study. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.S., Richey E.A., and Brunetto W.L. 2020. A Symptom-Based Rule for Diagnosis of COVID-19. SN Compr Clin Med. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh S.H.L., See A., Teo N.W.Y., Tan H.K., Palaniappan G., Lim M.L.A., Kadir H.B.A., and Toh S.T. 2021. Prevalence of olfactory and taste dysfunction in COVID-19 patients: a community care facility study. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somekh I., Yakub Hanna H., Heller E., Bibi H., and Somekh E. 2020. Age-Dependent Sensory Impairment in Covid-19 Infection and Its Correlation with Ace2 Expression. Pediatr Infect J. [DOI] [PubMed] [Google Scholar]
- Song J., Deng Y., Wang H., Wang Z., Liao B., Ma J., He C., Pan L., Liu Y., Alobid I., et al. 2021a. Self-reported Taste and Smell Disorders in Patients with COVID-19: Distinct Features in China. Curr Med Sci. 41:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.W., Kim D., Park J.Y., and Lee S. 2021b. Symptoms and Characteristics Which Require Attention During COVID-19 Screening at a Port of Entry. J Korean Med Sci. 36:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth M.M., Singer-Cornelius T., Obere M., Gengler I., Brockmeier S.J., and Sedaghat A.R. 2020. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol Head Neck Surg. 194599820929185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C., and Boscolo-Rizzo P. 2020. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavem K., Ghanima W., Olsen M.K., Gilboe H.M., and Einvik G. 2020. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., He W.-T., Wang L., Lai A., Ji X., Zhai X., Li G., Suchard M.A., Tian J., Zhou J., et al. 2020. COVID-19: Epidemiology, Evolution, and Cross-Disciplinary Perspectives. Trends Mol Med. 26:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarifi A., Al Shdaifat A.A., Al-Shudifat A.M., Azab M., Ismail J., Bashir R., Amro A., Altarifi A., and Khader Y. 2021. Clinical, SinoNasal, and Long-Term Smell and Taste Outcomes in Mildly Symptomatic COVID-19 Patients. Int J Clin Pr. e14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taziki Balajelini M.H., Rajabi A., Mohammadi M., Nikoo H.R., Tabarraei A., Mansouri M., and Hosseini S.M. 2021. Virus load and incidence of olfactory, gustatory, respiratory, gastrointestinal disorders in COVID-19 patients: A retrospective cohort study. Clin Otolaryngol Off J ENT-UK Off J Neth Soc Oto-Rhino-Laryngol Cervico-Facial Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teklu S., Sultan M., Azazh A., Worku A., Redae B., Walelegn M., Tefera M., Argaw R., Waganew W., Yifru S., et al. 2020. Clinical and Socio-demographic Profile of the First 33 COVID-19 Cases Treated at Dedicated Treatment Center in Ethiopia. Ethiop J Health Sci. 30:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur K., Sagayaraj A., Prasad K.C., and Gupta A. 2021. Olfactory Dysfunction in COVID-19 Patients: Findings from a Tertiary Rural Centre. Indian J Otolaryngol Head Neck Surg. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham A.C., Thein T.-L., Lee C.S., Tan G.S.E., Manauis C.M., Siow J.K., Leo Y.S., and Lim M.Y. 2020. Olfactory taste disorder as a presenting symptom of COVID-19: a large single-center Singapore study. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J.Y., Wong A., Zhu D., Fastenberg J.H., and Tham T. 2020. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol Neck Surg. 163:3–11. [DOI] [PubMed] [Google Scholar]
- Trachootham D., Thongyen S., Lam-Ubol A., Chotechuang N., Pongpirul W., and Prasithsirikul W. 2021. Simultaneously Complete but Not Partial Taste and Smell Losses were Associated with SAR-CoV-2 Infection. Int J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivgoulis G., Fragkou P.C., Delides A., Karofylakis E., Dimopoulou D., Sfikakis P.P., and Tsiodras S. 2020. Quantitative evaluation of olfactory dysfunction in hospitalized patients with Coronavirus [2] (COVID-19). J Neurol. 267:2193–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudrej B., Sebo P., Lourdaux J., Cuzin C., Floquet M., Haller D.M., and Maisonneuve H. 2020. Self-Reported Loss of Smell and Taste in SARS-CoV-2 Patients: Primary Care Data to Guide Future Early Detection Strategies. J Gen Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Preventive Services Taskforce. Recommended Topics for Vision and Hearing.
- Vacchiano V., Riguzzi P., Volpi L., Tappata M., Avoni P., Rizzo G., Guerra L., Zaccaroni S., Cortelli P., Michelucci R., et al. 2020. Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Deiana G., Fois A.G., Pirina P., Madeddu G., Vito A.D., Babudieri S., Petrocelli M., Serra A., Bussu F., et al. 2020a. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. 42:1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Chiesa-Estomba C.M., Salzano G., Cucurullo M., Salzano F.A., Saussez S., Boscolo-Rizzo P., et al. 2020b. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol. 134:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Soma D., Giovanditto F., Rizzo D., Salzano G., Piombino P., Saussez S., et al. 2020c. Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. J Otolaryngol - Head Neck Surg. 49:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Hopkins C., Salzano G., Petrocelli M., Melis A., Cucurullo M., Ferrari M., Gagliardini L., Pipolo C., Deiana G., et al. 2020d. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 42:1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Lechien J.R., Salzano G., Salzano F.A., Maglitto F., Saussez S., and De Riu G. 2020e. Gustatory Dysfunction: A Highly Specific and Smell-Independent Symptom of COVID-19. Indian J Otolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Salzano G., Petrocelli M., Deiana G., Salzano F.A., and Riu G.D. 2020f. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck. 42:1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon N., Verbrugghe M., Cartuyvels R., and Ramaekers D. 2020. Diagnosis of COVID-19 Based on Symptomatic Analysis of Hospital Healthcare Workers in Belgium: Observational Study in a Large Belgian Tertiary Care Center during Early COVID-19 Outbreak. J Occup Env Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vena A., Berruti M., Adessi A., Blumetti P., Brignole M., Colognato R., Gaggioli G., Giacobbe D.R., Bracci-Laudiero L., Magnasco L., et al. 2020. Prevalence of Antibodies to SARS-CoV-2 in Italian Adults and Associated Risk Factors. J Clin Med. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal I.M., Morato M., Martinez-RuizCoello M., Navarro A., Garcia-Chilleron R., Ruiz A., Almeida I.V. de, Mazon L., and Plaza G. 2020. Olfactory and taste disorders in healthcare workers with COVID-19 infection. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T., Shweta F.N.U., Murugadoss K., Awasthi S., Venkatakrishnan A.J., Bade S., Puranik A., Kang M., Pickering B.W., O’Horo J.C., et al. 2020. Augmented Curation of Clinical Notes from a Massive EHR System Reveals Symptoms of Impending COVID-19 Diagnosis. MedRxiv. 2020.04.19.20067660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield T., Watson C., Moore R., Ferris K., Tonry C., Watt A., McGinn C., Foster S., Evans J., Lyttle M.D., et al. 2020. Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch Dis Child. [DOI] [PubMed] [Google Scholar]
- Wee L.E., Chan Y.F.Z., Teo N.W.Y., Cherng B.P.Z., Thien S.Y., Wong H.M., Wijaya L., Toh S.T., and Tan T.T. 2020. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J.J., Attuquayefio T.N., White E.B., Li F., Herz R.S., White T.L., Campbell M., Geng B., Datta R., Wyllie A.L., et al. 2020. Tracking Smell Loss to Identify Healthcare Workers with SARS-CoV-2 Infection. MedRxiv. 2020.09.07.20188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng C.H., Saal A., Butt W.W.W., and Chan P.A. 2020. Characteristics and clinical outcomes of COVID-19 in Hispanic/Latino patients in a community setting: A retrospective cohort study. J Med Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierdsma N.J., Kruizenga H.M., Konings L.A., Krebbers D., Jorissen J.R., Joosten M.I., van Aken L.H., Tan F.M., van Bodegraven A.A., Soeters M.R., et al. 2021. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN. 43:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V., Bhagat S., Sharma D.K., Sibia R.P.S., Pandav R., and Sandhu V.P. 2021. Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Tertiary Care Institute Experience in India. Indian J Otolaryngol Head Neck Surg. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Boone C.E., and DeConde A.S. 2020a. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Ostrander B.T., and DeConde A.S. 2020b. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin A., Nawaiseh M., Shaban A., Alsherbini K., El-Salem K., Soudah O., and Abu-Rub M. 2021. Neurological manifestations and complications of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. BMC Neurol. 21:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayet S., Kadiane-Oussou N.J., Lepiller Q., Zahra H., Royer P.Y., Toko L., Gendrin V., and Klopfenstein T. 2020a. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect. 22:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayet S., Klopfenstein T., Mercier J., Kadiane-Oussou N.J., Lan Cheong Wah L., Royer P.Y., Toko L., and Gendrin V. 2020b. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zejda J.E., Brożek G.M., Kowalska M., Barański K., Kaleta-Pilarska A., Nowakowski A., Xia Y., and Buszman P. 2021. Seroprevalence of Anti-SARS-CoV-2 Antibodies in a Random Sample of Inhabitants of the Katowice Region, Poland. Int J Environ Res Public Health. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifko U., Schmiedlechner T., Saelens J., Zifko K., Wagner M., Assadian O., Grisold W., and Stingl H. 2021. Covid-19: Involvement of the nervous system. Identifying neurological predictors defining the course of the disease. J Neurol Sci. 425:117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman R.K., Nowalk M.P., Bear T., Taber R., Clarke K.S., Sax T.M., Eng H., Clarke L.G., and Balasubramani G.K. 2020. Proposed clinical indicators for efficient screening and testing for COVID-19 infection using Classification and Regression Trees (CART) analysis. Hum Vaccin Immunother. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Yu T., Zhang Y., Dai L., Zhang Z., and Zhang Z. 2021. Olfactory and Gustatory Dysfunctions in Patients With COVID-19 in Wuhan, China.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.