Abstract
Objective:
To examine whether brain activity is associated with treatment response to cognitive behavioral therapy (CBT) in adolescents and adults with obsessive compulsive disorder (OCD), and whether associations are treatment-specific relative to an active control psychotherapy (stress management therapy; SMT).
Methods:
Eighty-seven patients with OCD (age range 12-45, 57 female, 39 medicated) were randomized to receive 12 weeks of CBT or SMT. Prior to treatment, functional magnetic resonance imaging was collected in patients performing an incentive flanker task, which probes brain activation to both cognitive control and reward processing. Data were collected between March 2015 and October 2018. Voxelwise linear-mixed effects models examined whether baseline brain activation was differentially associated with symptom change on the (Children’s) Yale-Brown Obsessive-Compulsive Scale ((C)Y-BOCS) scores over the course of CBT and SMT treatment.
Results:
Within the CBT group, a better treatment response was associated with greater pre-treatment activation within right temporal lobe and rostral anterior cingulate cortex during cognitive control and within ventromedial prefrontal, orbitofrontal, lateral prefrontal and amygdala regions during reward processing. In contrast, reduced pre-treatment activation within a largely overlapping set of regions was associated with a better treatment response to SMT (initial voxel threshold p<0.001, cluster corrected p<0.05).
Conclusions:
Findings of treatment-specific associations are important for the development of biomarkers to personalize treatment in OCD (ClinicalTrials.gov: NCT02437773).
Keywords: OCD, cognitive behavioral therapy, inhibitory control, reward-processing, fMRI
Introduction
Obsessive-compulsive disorder (OCD) affects 1-3% of children and adolescents and 2-3% of adults, and is characterized by recurrent and intrusive obsessive thoughts that patients attempt to neutralize with behavioral and/or mental compulsions (1). Psychological treatment for OCD consists primarily of cognitive behavioral therapy (CBT) incorporating in-vivo exposure and response prevention (ERP) (1). During ERP, patients interact with symptom-provoking stimuli while resisting compulsions, thereby learning that compulsive rituals are not necessary to prevent feared outcomes (2). Meta-analyses show large effect-sizes for symptom severity reductions following CBT, even when compared with active control psychotherapies (1, 3). However, approximately 30-50% of patients with OCD do not respond adequately to treatment, and reliable predictors of response to treatment have yet to be established (3).
The neural mechanisms underlying OCD remain poorly understood, but cingulo-opercular and orbito-striato-thalamic networks are commonly implicated in patients across the lifespan (4). OCD patients show impaired performance as well as hypoactivation within cingulo-opercular regions during cognitive control, identifying possible mechanisms of impaired control over obsessions and compulsions (4, 5). The orbito-striato-thalamic network appears hyperconnected at rest and hyperactive during symptom provocation and habit-driven responding in the disorder, whereas orbito-striatal regions are hypoactive during reward processing and decision-making, suggesting an imbalance of habit and goal-directed functions within these regions in OCD (6-8). Other work has linked cingulo-opercular and orbito-striatal activation during cognitive control and reward processing with treatment response to CBT (9, 10). Greater cingulo-opercular functioning during cognitive control may indicate a greater ability to engage this network during self-regulation to implement response-prevention strategies (10). Orbito-striatal and connected limbic regions are key for maintaining motivation as well as for learning new associations for environmental stimuli and behavioral actions, as required during ERP(11, 12).
Previous studies have examined whether individual differences in pre-treatment brain activation/structure are associated with treatment response to CBT in OCD, implicating cingulo-opercular, orbito-striatal and amygdalar regions (13-15). One study reported that adult patients with more pre-treatment activation during cognitive control within cingulo-opercular, dorsolateral prefrontal, posterior cingulate, striatal and temporal regions had a better response to CBT (10). However, the existing literature has limitations. First, published neuroimaging studies did not include a control psychotherapy group and could not separate findings associated with symptom change due to CBT from non-specific symptom reduction (10, 16). Findings of treatment-specific associations are critical in developing a mechanistic understanding of CBT, as well as individually-tailored treatment algorithms (14, 15). Second, neuroimaging studies of treatment response in patients with the disorder have focused primarily on adults. Since early intervention may well have advantages in disorders like OCD that are often early in onset, understanding whether treatment-specific predictors of recovery generalize beyond mature adulthood is important (1, 9).
Therefore, in the current study, we explored the associations between task-activation and treatment response to CBT compared to a control psychotherapy (stress management therapy; SMT) in adolescent and adult patients with OCD. Effective engagement with CBT likely places greater demands than control therapies on self-regulatory processes, which allow patients to control emotions, cognitions and behaviors in the face of symptom triggers (10). It also requires the capacity for maintaining the motivation to work towards long-term goals (e.g., symptom recovery) in the face of challenging exposures (9, 17). Consequently, we anticipated that more pre-treatment activation within cingulo-opercular regions during cognitive control and orbito-striatal regions during reward processing would be associated with larger symptom reductions in the CBT group, and that these associations would be treatment-specific relative to SMT. Secondary analyses tested whether associations remained in both adolescent and adult sub-groups.
Methods
Participants
Eighty-seven patients were included in the analysis. Of these, 42 (19 adolescents, 23 adults, 20 medicated, 28 females) were assigned to CBT and 45 (20 adolescents, 25 adults, 19 medicated, 29 females) were assigned to SMT. Linear mixed effects models allow for the inclusion of patients with missing data-points, and all patients providing usable scans and assessment data were included. Full demographic and clinical details are given in Table 1. Patients were recruited from outpatient programs at the University of Michigan Health System, from social media advertisements and via referrals from clinicians. We focused on two critical age periods – adolescence (13-17 years) and adulthood (25-45 years). Patients were required to have an early age of symptom onset (≤15 years) and moderate or greater levels of symptoms at baseline (≥16 on the (Child’s) Yale-Brown Obsessive Compulsive Scale ((C)Y-BOCS)(18, 19). Developed to measure OCD across the lifespan, the (C)Y-BOCS instruments use nearly identical wording and are comparable in terms of validity and reliability in adolescents and adults (18, 19). Full details on inclusion/exclusion criteria, characterization of participants and a CONSORT chart (Supplementary Figure 1) appear in the Supplement.
Table 1.
Patient Characteristics and Behavioral Performance
| Adolescent SMT | Adult SMT | All SMT | Adolescent CBT a | Adult CBT | All CBT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Week 1 | 20 | - | 25 | - | 45 | - | 19 | - | 23 | - | 42 | - |
| Week 6 | 20 | - | 21 | - | 41 | - | 16 | - | 21 | - | 37 | - |
| Week 12 | 19 | - | 21 | - | 40 | - | 16 | - | 20 | - | 36 | - |
| Female b | 13 | 65 | 16 | 64 | 29 | 64.4 | 14 | 73.68 | 14 | 60.86 | 28 | 66.67 |
| Medicated bc | 8 | 40 | 11 | 44 | 19 | 42.2 | 10 | 52.63 | 10 | 43.48 | 20 | 47.6 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age, y | 15.35 | 1.77 | 31.84 | 5.55 | 24.51 | 9.32 | 15.53 | 1.63 | 31.42 | 5.8 | 24.23 | 9.13 |
| No. of Interference errors | 27.45 | 12.51 | 27.2 | 13.99 | 27.31 | 13.21 | 30.37 | 11.62 | 30.74 | 14.01 | 30.57 | 12.83 |
| Interference RT (ms) | 17.18 | 10.02 | 19.94 | 11.25 | 18.72 | 10.69 | 15.42 | 9.63 | 22.46 | 12.86 | 19.28 | 11.92 |
| Incentive RT (ms) | −13.28 | 31.11 | −22.30 | 36.33 | −18.29 | 34.04 | −15.7 | 31.01 | −9.8 | 28.97 | −12.47 | 29.69 |
| (C)Y-BOCS | ||||||||||||
| Week 1 | 28.05 | 5.23 | 26.88 | 4.04 | 27.4 | 4.59 | 26.74 | 5.64 | 23.74 | 4.92 | 25.1 | 5.41 |
| Week 6 | 25.33 | 7.02 | 23 | 5 | 24.13 | 6.11 | 21.28 | 6.36 | 17.98 | 6.18 | 19.41 | 6.39 |
| Week 12 | 21.13 | 9.61 | 21.45 | 6.84 | 21.3 | 8.16 | 14.5 | 10.48 | 11.73 | 5.15 | 12.96 | 7.96 |
| QIDS | ||||||||||||
| Week 1 | 7.26 | 4.13 | 7.65 | 4.23 | 7.48 | 4.14 | 8.12 | 5.45 | 6.70 | 3.69 | 7.30 | 4.513 |
| Week 6 | 6.53 | 3.6 | 6.58 | 3.76 | 6.55 | 3.63 | 8.67 | 5.1 | 5.25 | 2.82 | 6.71 | 4.260 |
| Week 12 | 6.11 | 4.6 | 6.62 | 4.67 | 6.38 | 4.58 | 6.73 | 4.88 | 4.53 | 2.59 | 5.50 | 3.863 |
| HAM-A | ||||||||||||
| Week 1 | 10.1 | 6.95 | 12.76 | 7.06 | 11.58 | 7.06 | 14.32 | 11.88 | 9.17 | 6.99 | 11.5 | 9.74 |
| Week 6 | 8.85 | 7.42 | 11.57 | 6.34 | 10.24 | 6.94 | 12 | 9.4 | 10.05 | 8.38 | 10.89 | 8.76 |
| Week 12 | 7.11 | 5.82 | 10.76 | 6.22 | 9.03 | 6.24 | 10.88 | 12.76 | 7.9 | 6.74 | 9.22 | 9.84 |
| CGI: symptom severity | ||||||||||||
| Week 1 | 4.6 | 0.75 | 4.6 | 0.65 | 4.60 | 0.69 | 4.58 | 0.77 | 4.39 | 0.72 | 4.48 | 0.74 |
| Week 6 | 4.4 | 0.82 | 4.33 | 0.73 | 4.37 | 0.77 | 4.25 | 1.07 | 3.86 | 0.85 | 4.03 | 0.96 |
| Week 12 | 3.68 | 1.11 | 4.05 | 0.97 | 3.87 | 1.04 | 3.25 | 1.39 | 2.80 | 0.83 | 3.00 | 1.12 |
One CBT patient was excluded by the researchers during week 12 (see Supplement)
Number at week one.
Number receiving anti-depressant medication.
Abbreviations: CBT, cognitive behavioral therapy; CGI, Clinical Global Impression; (C)Y-BOCS, (Children’s) Yale-Brown Obsessive Compulsive Scale; HAM-A, Hamilton Anxiety Rating Scale; ms, milliseconds; RT, reaction time; QIDS, Quick Inventory of Depressive Symptomatology; SMT, stress management therapy; y, years.
Written informed consent and assent was obtained for all patients and/or their legal guardians, according to procedures reviewed and approved by the Institutional Review Board of the University of Michigan (IRBMED, IRB:HUM00091368). Data for included patients was collected between March 2015 and October 2018, as part of a larger clinical trial (ClinicalTrials.gov:NCT02437773). The current report is a planned interim analysis focused on the examination of pre-treatment neural associations with subsequent treatment response.
Study Design
Patients were randomized to receive either 12 weeks of individual CBT incorporating ERP, or 12 weeks of SMT (20, 21). Assignment was stratified based on medication, gender and age, using block randomization. SMT has been used in non-imaging studies of CBT, and shown to produce small to moderate reductions in OCD symptoms (2, 22, 23). SMT was included to control for potential, non-specific effects of time and weekly meetings with a therapist on symptom change. (C)Y-BOCS assessment of OCD severity occurred before, during and after treatment (weeks 1, 6 and 12) by an independent rater blind to treatment assignment. Patients were also assessed for anxiety and depression symptoms (See Supplement), and underwent scanning at the fMRI Laboratory, University of Michigan <6 weeks before treatment (CBT:mean number of weeks=1.52, SD=0.7;SMT:mean number of weeks=1.76,SD=1.19). Patients were assigned to treatment group following the scan. Details of treatment protocols and MRI acquisition are in the supplement.
Incentive Flanker Task:
In the Incentive Flanker Task (IFT), patients pressed one of two buttons to identify a target letter (S, K, H, and C) surrounded by four flankers, which either mapped to the same button response (low interference) or the opposite response (high interference) as the target. Target and flanker stimuli were preceded by cues (1.5 – 10 s) indicating how much money patients stood to lose (for an error) or gain (for a correct response) on the upcoming trial (0¢ -- 50% of the trials), 10¢ (25% of the trials), or 25¢ (25% of the trials). Patients’ responses lead to a feedback signal – asterisks in place of the target/flanker stimuli as white (correct) or red (incorrect). In total, participants completed 4 runs, each consisting of 48 trials (scan duration~25 min). Prior to the fMRI session, patients practiced the IFT to achieve an error rate of ~15%, titrated using a subject-specific response deadline (6). Previous studies indicate that reward and punishments on the IFT decrease reaction times in both OCD and healthy subjects, in line with a motivational effect (24). Performance measures include interference reaction time, incentivised reaction time and interference errors (See Supplement). Patients received approximately $10-20, based on amounts earned and lost for each incentive trial. See Supplementary Figure 2.
Statistical analysis of clinical and behavioral data
Linear mixed effect models were used to to test for differences in treatment response between CBT and SMT groups, as well as for relationships between task performance, treatment group and treatment outcome. See Supplement.
Statistical analysis of fMRI data
Standard preprocessing steps were performed in Statistical Parametric Mapping (SPM 12, http://www.fil.ion.ucl.ac.uk/spm; Wellcome Trust Centre for Neuroimaging, University College London, UK). First-level contrasts examined brain activation during cognitive control (“interference inhibition” -- correct high versus correct low interference trials), interference errors (incorrect versus correct high interference trials) and reward processing (rewarded correct trials versus non-rewarded correct trials, regardless of interference level). At the second level, voxelwise linear mixed effects analyses were performed in the nlme package (25) for R (http://www.r-project.org). These models examined voxel-activation by week by treatment group interactions on (C)Y-BOCS scores collected at weeks one, six and twelve of treatment while controlling for age group and medication status. A random intercept term for patient was included to account for the nonindependence of observations. T-scores for the interaction of interest were used to create whole-brain t-maps, which were then thresholded at a voxel-level threshold of p<0.001 and a familywise error cluster-level corrected threshold of p<0.05. The MarsBar toolbox (http://marsbar.sourceforge.net/) was used to extract mean Blood Oxygen Level Dependent (BOLD) signal for each patient in significant clusters. To determine which treatment group(s) drove significant interactions, extracted values were plotted and subjected to follow-up analyses examining mean cluster-activation by week interactions on (C)Y-BOCS performed within each level of treatment group (i.e., CBT, SMT). Voxelwise follow-up analyses were also performed within CBT and SMT sub-groups (see Supplement). Further details on fMRI preprocessing and analyses are given in the Supplement.
Results
Clinical outcomes
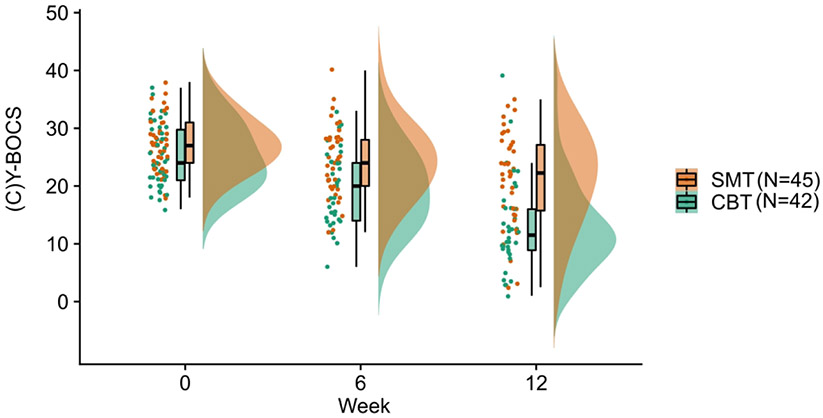
Both CBT (B=−6.13,t=−12.89, p< 0.001, 95% CI (−7.07, −5.18)) and SMT (B=−2.94, t=−5.64, p<0.001, 95% CI(−3.9, −1.9)) groups showed a significant decrease in symptoms over time. There was a significant treatment group by week interaction, such that CBT resulted in a steeper reduction in (C)Y-BOCS scores over time compared to SMT (B=−3.21,t=−4.52, p=0.001, 95% CI(−4.61, −1.81)). See Table 1 and Figure 1.
Figure 1. Raincloud plot of the change in OCD symptoms over the course of CBT and SMT treatment in patients with OCD.
Patients who underwent CBT (B=−6.13, t=−12.89, p< 0.001, 95% CI (−7.07, −5.18)) and SMT (B =−2.94, t=−5.64, p<0.001, 95% CI (−3.9, −1.9)) showed a significant decrease in symptoms over the course of treatment. There was a significant treatment group by week interaction. Patients who underwent CBT showed a steeper reduction in (C)Y-BOCS scores over time compared to patients who underwent SMT (B=−3.21, t=−4.52, p=0.001, 95% CI (−4.61, −1.81)).
Behavioral data
There were no significant task performance by treatment group by week interactions on (C)Y-BOCS (all p>0.05, see Supplement).
fMRI results
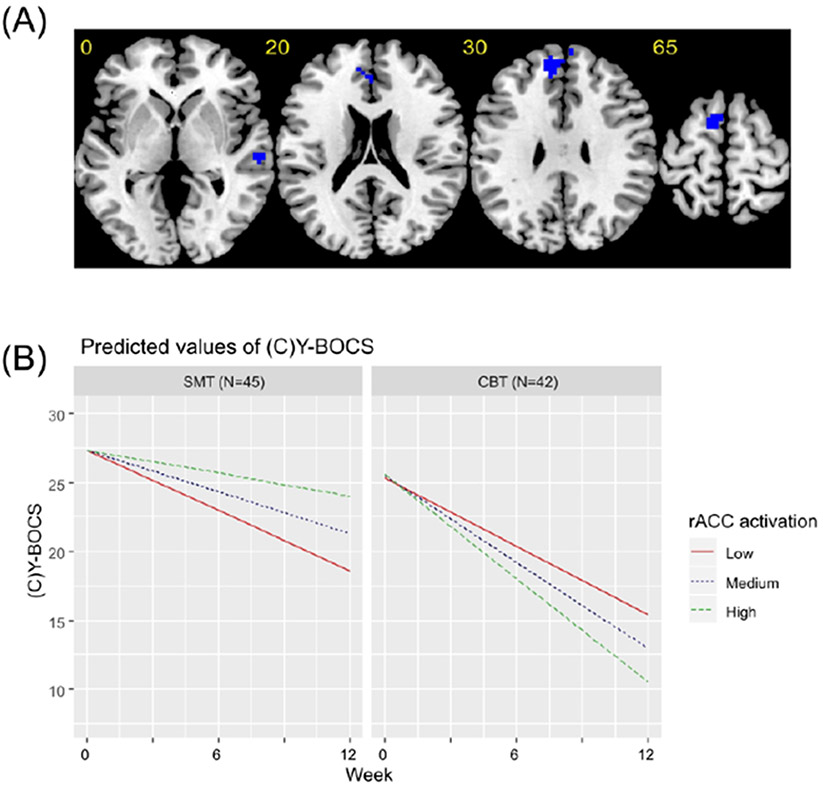
Brain activation maps are provided in Supplementary Figures 3-5. During cognitive control, activation within left premotor cortex and right temporal lobe showed a significant voxel-activation by treatment group by week interaction. At a relaxed cluster forming threshold of p<0.0025, a similar finding was observed within a hypothesized cingulo-opercular region of interest -- the rostral anterior cingulate cortex (rACC). Follow-up analyses revealed that while more pre-treatment activation within right temporal lobe (CBT:B=−3.96,p=0.003; SMT:B=5.09, p<0.001), rACC (CBT:B=−2.68, p=0.004; SMT: B=2.94, p=0.01) and left premotor cortex (CBT:B=−2.91, p=0.002; SMT:B=3.61, p=0.008) was associated with a better treatment response in patients undergoing CBT, less activation was associated with a better treatment response in the same regions in patients undergoing SMT (Table 2; Figure 2; Supplementary Figure 6).
Table 2.
Brain regions that were significant in the linear mixed effects model of the interactive effect of voxel-activation, treatment group and week on (Child’s) Yale-Brown Obsessive Compulsive Scale ((C)Y-BOCS) scores.
| Contrast | MNI x, y, z Coordinates |
Max-T | No. of Voxels |
Brodmann areas |
|---|---|---|---|---|
| Cognitive control | ||||
| L & R rACC a | −9 ,50 ,29 | −4.09 | 70 | 9,10,32 |
| L premotor cortex | −9 ,5,68 | −5.28 | 43 | 6 |
| R temporal lobe | 60 ,19, −4 | −4.68 | 52 | 21,22 |
| Reward Processing | ||||
| L & R vmPFC/OFC/amygdala/IFG/DLPFC | −6,65,−10 | −6.21 | 1502 | 10,11,8,47,6,32,25,9,45, |
| L temporal lobe | −57,−13,−16 | −6.07 | 283 | 21,22 |
| L inferior parietal lobe | −42,−70,41 | −4.12 | 108 | 39,40,19 |
| R premotor cortex/posterior insula | 51, −1, 5 | 4.82 | 100 | 6 |
| R inferior parietal lobe | 48,−34,32 | 5.15 | 75 | 40 |
Abbreviations: BA, Brodmann area; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; MNI, Montreal Neurological Institute; OFC, orbitofrontal cortex; rACC, rostral anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex;
Notes:
Significant at a relaxed uncorrected cluster-forming threshold of p<0.0025 and corrected cluster threshold of p<0.05. All other findings presented at an uncorrected cluster-forming threshold of p<0.001 and corrected cluster threshold of p<0.05
Figure 2. Brain regions significant in the linear mixed effects model for the cognitive control contrast.
(A) Axial slices showing brain regions that were significant in the linear mixed-effects model of the interactive effect of voxel-activation during cognitive control, treatment group and week on (Child’s) Yale-Brown Obsessive Compulsive Scale ((C)Y-BOCS) scores. All regions are presented at an uncorrected cluster forming threshold of p<0.001 and a familywise error corrected cluster threshold of p<0.05, except for the cluster in the rostral anterior cingulate (rACC), which is presented using an uncorrected cluster forming threshold of p<0.0025 and a familywise error corrected cluster threshold of p<0.05. Blue indicates regions where more pre-treatment activation was associated with greater symptom reduction over time in patients undergoing CBT, but a smaller reduction in symptoms over time in patients undergoing SMT. (B) Graphs showing predicted model estimates for cognitive behavioral therapy (CBT) and stress management therapy (SMT) groups. The y-axis represents the predicted (C)Y-BOCS based on model estimates, and separate lines indicate level of rACC activation (“Low” = one standard deviation below mean, “Medium” = mean, “High” = one standard deviation above the mean). Graphs for other regions are given in the Supplement.
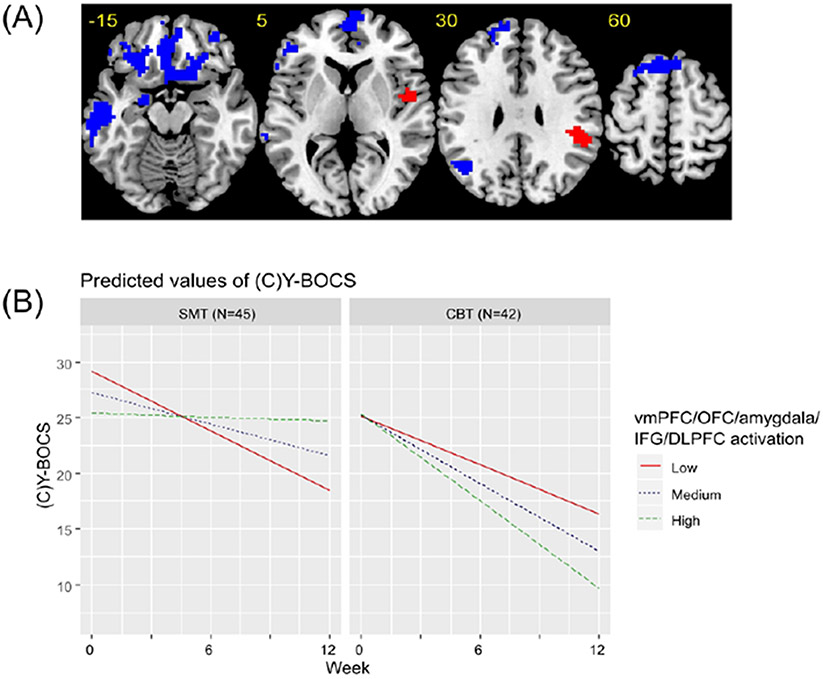
During reward processing, a significant voxel-activation by treatment group by week interaction was found within a large cluster incorporating bilateral ventromedial prefrontal cortex, orbitofrontal cortex, amygdala, inferior frontal gyrus and dorsolateral prefrontal cortex. More pre-treatment activation within these regions was associated with a better treatment response within the CBT group (B=−3.91, p<0.001) but a worse treatment response in patients who underwent SMT (B=5.62, p<0.001). A similar pattern of findings was found in left temporal lobe (CBT:B=−3.15, p<0.001; SMT:B=4.37, p<0.001) and parietal lobe (CBT:B=−1.22, p=0.045; SMT:B=2.53, p<0.001). Also significant in the interaction analysis were right posterior insula (CBT:B=5.35, p<0.001; SMT:B=−2.53, p=0.03) and parietal lobe (CBT:B=5.26, p<0.001; SMT:B=−2.75, p=0.015), in which relatively less activation was associated with a better response to CBT but more activation was associated with a better response to SMT (Table 2; Figure 3; Supplementary Figure 7).
Figure 3. Brain regions significant in the linear mixed effects model for the reward processing contrast.
(A) Axial slices showing brain regions that were significant in the linear mixed-effects model of the interactive effect of voxel-activation during reward processing, treatment group and week on (Child’s) Yale-Brown Obsessive Compulsive Scale ((C)Y-BOCS) scores. All regions are presented at an uncorrected cluster forming threshold of p<0.001 and a familywise error corrected cluster threshold of p<0.05. Blue indicates regions where more pre-treatment activation was associated with greater symptom reduction over time in patients undergoing CBT, but a smaller reduction in symptoms over time in patients undergoing SMT. Red indicates regions where more pre-treatment activation was associated with a smaller reduction in symptoms over time in patients undergoing CBT, but a greater reduction in symptoms over time in patients undergoing SMT. (B) Graphs showing predicted model estimates for cognitive behavioral therapy (CBT) and stress management therapy (SMT) groups. The y-axis represents the predicted (C)Y-BOCS based on model estimates, and separate lines indicate level of vmPFC/OFC/amygdala/IFG/DLPFC activation (“Low” = one standard deviation below mean, “Medium” = mean, “High” = one standard deviation above the mean). Graphs for other regions are given in the Supplement.
Voxelwise findings at each level of treatment group (CBT, SMT) are presented in Supplementary Figures 8-11.
Effects of age
Both adult and adolescent sub-groups showed similar symptom reductions during CBT (adult:B=−6.12, p<0.001; adolescent:B=−6.13,p<0.001) and SMT (adult:B=−2.74,p<0.001; adolescent:B=−3.21, p<0.001), as well as a group by time interaction, indicating greater efficacy of CBT relative to SMT (adult:B=−3.43, p< 0.001; adolescent:B=−2.93, p=0.01). Adding an additional interaction term for treatment group by week by age group to the model decreased model fit, as determined by the Bayesian information criterion (without interaction with age group=1543.81; including interaction with age group =1552.15). Moreover, this interaction was non-significant (B=−0.57, t=−0.39, p=0.69, 95% CI(−3.4, 2.27)). Adult and adolescent patients did not show any behavioral differences (all p>0.05), besides adolescents showing smaller interference reaction times (t(85)=2,p=0.046). Clusters found to be significant in the primary voxel-activation by treatment group by week interaction were extracted and subjected to robustness checks for the effects of age. Adolescents and adults did not differ on activation within any of the extracted clusters (all p>0.05). As shown in Supplementary Table 3, results remained significant for both adolescents and adults when analyses were repeated within each sub-group using extracted ROI data. Exploratory analyses performed on extracted data including age group in an additional interaction term were non-significant (all p>0.05). See Supplementary Figures 12-14.
Discussion
The current study sought to examine whether pre-treatment brain activation during cognitive control and reward processing is associated with treatment response to CBT in patients with OCD, and if so whether the associations are treatment-specific relative to an active control therapy (SMT) and/or vary with age. Patients in both treatment groups showed significant reductions in OCD symptoms following treatment, but symptom reduction following CBT was steeper than symptom reduction following SMT. In the CBT group, a pattern of greater pre-treatment activation during cognitive control and reward processing was associated with a better treatment response, while relatively less activation in these regions prior to treatment was associated with better outcomes after treatment in the SMT group. Follow-up analyses in adolescent and adult sub-groups indicated that findings were conserved across age.
Greater symptom reduction following CBT treatment was associated with more baseline brain activation within right temporal lobe and, at a relaxed cluster-forming threshold, within a hypothesized cingulo-opercular region, the rACC, shown in meta-analyses to be reduced in gray matter volume and hypoactive during cognitive control in patients with OCD (4, 5). These findings replicate a recent study of treatment response to CBT in OCD adults, and extend previous work by showing treatment-specificity relative to SMT (10). Interestingly, in the CBT subgroup analysis, greater activation within anterior insular, dorsolateral prefrontal and posterior cingulate regions was also associated with treatment response, providing further independent replication of this previous study, although these findings did not survive correction for multiple comparisons in the interaction analysis, leaving their treatment-specificity unclear (see Supplement). Meta-analytic studies have shown that reduced structure and function of the rACC is commonly implicated across multiple psychiatric disorders (5, 26, 27), and greater volume or activation within the rACC is arguably the most common predictor of a better treatment response to CBT (28, 29). The rACC is proposed to play important roles in flexible top-down control of emotions and self-regulation in response to potential symptom triggers, cognitiveaffective functions which are critically involved in CBT (30). These findings, together with a converging research literature, suggest that patients with relatively preserved functioning within brain regions supporting cognitive control may be better candidates for CBT (10).
During reward processing at pre-treatment, greater activation in bilateral vmPFC/OFC, lateral prefrontal and amygdalar regions predicted better response to CBT. Recent work has suggested parallels between reward processing and fear extinction, as experiencing the presentation of a conditioned stimulus without the aversive unconditioned stimulus is a better than expected and therefore quasi-rewarding outcome, and studies in humans and animals have demonstrated that functioning within mesolimbic dopaminergic circuitry during extinction learning mirrors that seen during reward-related tasks (11). Fear extinction learning is likely a key mechanism of ERP for OCD, and therefore patients with relatively robust orbito-striato-limbic brain activation may be better able to learn updated and less negative associations for their symptom triggers while undergoing ERP (2, 11). More broadly, robust functioning within reward-processing regions may protect against impairments in motivation (e.g., anhedonia) and positive affect, which have been shown to have a negative impact on response to treatments including CBT across multiple disorders (12, 31). Therefore, relatively greater pre-treatment activation in patients who respond strongly to CBT may also indicate a greater capacity for emotional resilience and the motivation to engage in challenging aspects of CBT therapy (12).
Interestingly, interaction analyses showed that while more brain activation within cognitive control and reward-processing regions was associated with a better treatment response to CBT, a better treatment response to SMT was associated with less activation in an overlapping set of brain regions. While these findings in the SMT group were unexpected, they are consistent with work comparing CBT with pharmacotherapy treatments, which reported increased vmPFC/OFC gray matter and resting-state metabolism to be associated with a better response to CBT and the opposite for pharmacotherapy (13, 15). One possibility is that CBT is most effective in patients already possessing the degree of cognitive control and reward responsiveness required for engaging with and learning from ERP (10, 11). SMT, on the other hand, may improve OCD symptoms indirectly by teaching patients how to relax and employ problem-solving techniques to reduce negative emotions in the face of common (i.e., non-OCD specific) life stressors; thus, SMT may bring about therapeutic change via improved self-regulation and feelings of self-efficacy (21). Consequently, SMT may be better able to meet the needs of patients with the most room for improvement in these domains. However, given that they were unanticipated, future work is needed to properly delineate the mechanisms driving these findings in the SMT group.
Findings of treatment-specific associations suggest that rather than being a general correlate of symptom reduction, greater activation during cognitive control and reward processing is likely linked in a more specific way to CBT response in patients with OCD. We have demonstrated in recent meta-analytic work that patients with OCD show impaired performance and reduced activation within rACC during cognitive control (4, 32). Moreover, hypoactivation during reward processing has been reported in patients with OCD in prefrontal and orbito-striato-limbic regions similar to those associated with treatment reponse in the present study (8, 33). Findings indicate that treatment response to CBT depends upon circuitry known to be dysfunctional in OCD, suggesting that engagement with CBT might be augmented by pharmacological or non-pharmacological treatments which target these underlying networks (26), and that it may be possible to identify good candidates for CBT based on relatively preserved functioning in these brain regions during cognitive control and reward processing (14).
In the present study, analyses in age-defined subgroups indicated that the same pattern of treatment-specific associations was present in both adolescents and adult patients. Findings provide initial evidence for a preservation of neural predictors of CBT response across the lifespan. However, all patients in our study had early onset forms of the disorder. OCD has a bimodal onset distribution, with peaks at around 10 and 20 years of age, and early and late onset forms of the disorder have been linked to distinct clinical, neuropsychological and neurobiological correlates, as well as distinct treatment outcome trajectories (34). Future research should examine whether there are differences in brain activation during cognitive control and reward processing between early and late onset forms of the disorder, and whether there are distinct neural predictors of treatment response in these patient subgroups. No differences in activation were found between adolescent and adult patients during cognitive control and reward processing, unlike in some studies in healthy subjects (35). However, interpreting this as evidence of altered development in the disorder would be problematic as findings in healthy subjects are mixed and no normative data on the development of activation during the IFT has been published (4, 35).
Limitations of the study include the fact that, although outcome assessments were made blinded to the treatment group, it was not possible to maintain blinded status for patients. This issue is common to previous clinical trials comparing CBT with SMT (2, 20). In addition, we excluded patients with common co-morbidities and findings may not generalize to patients with these co-morbid conditions. Furthermore, negative findings may be due to relatively lower power for the error processing contrast due to the smaller number of trials. Moreover, the sample size of the current study was moderate, and while the present findings provide initial evidence for the potential of using task-based fMRI in distinguishing good and poor responders to CBT and SMT, before translation to the clinic these findings must be shown to be robust, replicable and generalizable to other patient subgroups, and other treatment and imaging sites. Although similar findings were found for adolescents and adults, including two age groups added heterogeneity to the sample. While the current study findings reveal treatment-specific outcome predictors in patients with OCD undergoing CBT or SMT, it does not speak to whether these predictors are conserved across different disorders that are treated with similar therapies and in which similar cingulo-opercular and orbito-striatal regions have been implicated (26, 31).
In conclusion, the aim of this study was to examine whether treatment response to CBT was associated with treatment-specific brain activation during cognitive control and reward processing examined prior to therapy in adolescent and adult OCD. Findings show that greater activation within two networks commonly implicated in the disorder, the cingulo-opercular network during cognitive control and the orbito-striato-thalamic network during reward processing, was associated with better treatment responses to CBT but a worse treatment response to SMT. The present study advances the field by demonstrating that associations between brain activation and treatment response were treatment-specific to CBT relative to a control psychotherapy, and moreover that these associations were stable across the lifespan from adolescence to mature adulthood.
Supplementary Material
Acknowledgments
This work was funded by National Institute of Mental Health Grant No. R01 MH102242 (KDF, SFT). We thank all those who took part in or assisted with the clinical trial. We thank Dr Tim Johnson for his statistical assistance.
Footnotes
Conflict of Interest Disclosures
SFT has received research support from Neuronetics, St. Jude Medical (now Abbott), Boehringer–Ingelheim, and Otsuka Pharmaceutical. The remaining authors report no financial relationships with commercial interests.
Previous presentation: This work was presented as a poster presentation at the American College of Neuropsychopharmacology 57th annual meeting, The Diplomat Beach Resort, Hollywood, Florida, in December 2018 as well as at the Society of Biological Psychiatry 74rd annual meeting, Chicago, Illinois, in May 2019.
References
- 1.Hirschtritt ME, Bloch MH, Mathews CA: Obsessive-Compulsive Disorder: Advances in Diagnosis and Treatment. JAMA 2017; 317:1358–1367 [DOI] [PubMed] [Google Scholar]
- 2.Strauss AY, Huppert JD, Simpson HB, et al. : What matters more? Common or specific factors in cognitive behavioral therapy for OCD: Therapeutic alliance and expectations as predictors of treatment outcome. Behav Res Ther 2018; 105:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skapinakis P, Caldwell DM, Hollingworth W, et al. : Pharmacological and psychotherapeutic interventions for management of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry 2016; 3:730–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norman LJ, Taylor SF, Liu Y, et al. : Error Processing and Inhibitory Control in Obsessive-Compulsive Disorder: A Meta-analysis Using Statistical Parametric Maps. Biol Psychiatry 2019; 85:713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodkind M, Eickhoff SB, Oathes DJ, et al. : Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015; 72:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorsen AL, Hagland P, Radua J, et al. : Emotional Processing in Obsessive-Compulsive Disorder: A Systematic Review and Meta-analysis of 25 Functional Neuroimaging Studies. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillan CM, Apergis-Schoute AM, Morein-Zamir S, et al. : Functional neuroimaging of avoidance habits in obsessive-compulsive disorder. Am J Psychiatry 2015; 172:284–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remijnse PL, Nielen MMA, van Balkom AJLM, et al. : Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry 2006; 63:1225–1236 [DOI] [PubMed] [Google Scholar]
- 9.Forbes EE, Olino TM, Ryan ND, et al. : Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci 2010; 10:107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagliaccio D, Middleton R, Hezel D, et al. : Task-based fMRI predicts response and remission to exposure therapy in obsessive-compulsive disorder. Proc Natl Acad Sci U S A 2019; 116:20346–20353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalisch R, Gerlicher AMV, Duvarci S: A Dopaminergic Basis for Fear Extinction. Trends Cogn Sci 2019; 23:274–277 [DOI] [PubMed] [Google Scholar]
- 12.Taylor CT, Knapp SE, Bomyea JA, et al. : What good are positive emotions for treatment? Trait positive emotionality predicts response to Cognitive Behavioral Therapy for anxiety. Behav Res Ther 2017; 93:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brody AL, Saxena S, Schwartz JM, et al. : FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive compulsive disorder. Psychiatry Res 1998; 84:1–6 [DOI] [PubMed] [Google Scholar]
- 14.Reggente N, Moody TD, Morfini F, et al. : Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive-compulsive disorder. Proc Natl Acad Sci U S A 2018; 115:2222–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoexter MQ, Dougherty DD, Shavitt RG, et al. : Differential prefrontal gray matter correlates of treatment response to fluoxetine or cognitive-behavioral therapy in obsessive–compulsive disorder. Eur Neuropsychopharmacol 2013; 23:569–580 [DOI] [PubMed] [Google Scholar]
- 16.Fullana MA, Zhu X, Alonso P, et al. : Basolateral amygdala-ventromedial prefrontal cortex connectivity predicts cognitive behavioural therapy outcome in adults with obsessive-compulsive disorder. J Psychiatry Neurosci JPN 2017; 42:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor CT, Knapp SE, Bomyea JA, et al. : What good are positive emotions for treatment? Trait positive emotionality predicts response to Cognitive Behavioral Therapy for anxiety. Behav Res Ther 2017; 93:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. : Children’s Yale-Brown Obsessive Compulsive Scale: Reliability and Validity. J Am Acad Child Adolesc Psychiatry 1997; 36:844–852 [DOI] [PubMed] [Google Scholar]
- 19.Goodman WK, Price LH, Rasmussen SA, et al. : The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011 [DOI] [PubMed] [Google Scholar]
- 20.Simpson HB, Foa EB, Liebowitz MR, et al. : A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. Am J Psychiatry 2008; 165:621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay M, Crino R, Andrews G: Controlled trial of exposure and response prevention in obsessive-compulsive disorder. Br J Psychiatry J Ment Sci 1997; 171:135–139 [DOI] [PubMed] [Google Scholar]
- 22.Whittal ML, Woody SR, McLean PD, et al. : Treatment of obsessions: A randomized controlled trial. Behav Res Ther 2010; 48:295–303 [DOI] [PubMed] [Google Scholar]
- 23.Russell AJ, Jassi A, Fullana MA, et al. : Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30:697–708 [DOI] [PubMed] [Google Scholar]
- 24.Stern ER, Welsh RC, Fitzgerald KD, et al. : Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol Psychiatry 2011; 69:583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinheiro J, Bates D, DebRoy S, et al. : nlme: Linear and Nonlinear Mixed Effects Models [Internet]. 2020. Available from: https://CRAN.R-project.org/package=nlme [Google Scholar]
- 26.Downar J, Blumberger DM, Daskalakis ZJ: The Neural Crossroads of Psychiatric Illness: An Emerging Target for Brain Stimulation. Trends Cogn Sci 2016; 20:107–120 [DOI] [PubMed] [Google Scholar]
- 27.McTeague LM, Huemer J, Carreon DM, et al. : Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. Am J Psychiatry 2017; 174:676–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klumpp H, Keutmann MK, Fitzgerald DA, et al. : Resting state amygdala-prefrontal connectivity predicts symptom change after cognitive behavioral therapy in generalized social anxiety disorder. Biol Mood Anxiety Disord 2014; 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb CA, Olson EA, Killgore WDS, et al. : Rostral Anterior Cingulate Cortex Morphology Predicts Treatment Response to Internet-Based Cognitive Behavioral Therapy for Depression. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etkin A, Wager TD: Brain systems underlying anxiety disorders: a view from neuroimaging. Anxiety Disord Theory Res Clin Perspect 2010; 192–203 [Google Scholar]
- 31.Downar J, Geraci J, Salomons TV, et al. : Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 2014; 76:176–185 [DOI] [PubMed] [Google Scholar]
- 32.Carlisi CO, Norman LJ, Lukito SS, et al. : Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry 2017; 82:83–102 [DOI] [PubMed] [Google Scholar]
- 33.Koch K, Reess TJ, Rus OG, et al. : Increased Default Mode Network Connectivity in Obsessive-Compulsive Disorder During Reward Processing. Front Psychiatry 2018; 9:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontenelle LF, Mendlowicz MV, Marques C, et al. : Early- and late-onset obsessive–compulsive disorder in adult patients: an exploratory clinical and therapeutic study. J Psychiatr Res 2003; 37:127–133 [DOI] [PubMed] [Google Scholar]
- 35.Richards JM, Plate RC, Ernst M: A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev 2013; 37:976–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.