ABSTRACT
Background
Increased maternal adiposity and inflammation have impacts on fetal growth.
Objectives
The purpose of this prospective study was to investigate the associations of 3 proinflammatory adipokines in pregnancy with neonatal anthropometry.
Methods
In a sample of 321 US pregnant women from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies-Singleton Cohort (NCT00912132), plasma IL-6, fatty acid binding protein-4 (FABP4), and chemerin were measured in plasma samples collected at 10–14, 15–26, 23–31, and 33–39 weeks of gestation. Generalized linear models were used to estimate associations of adipokines with neonatal weight, thigh, and crown-heel length, and skinfolds at birth. Models adjusted for age, race/ethnicity, education, nulliparity, prepregnancy BMI, and weeks of gestation at blood collection.
Results
At each time point, higher IL-6 was associated with lower neonatal birthweight and thigh length. At 15–26 weeks of gestation, a 1 SD pg/mL increase in IL-6 was associated with –84.46 g lower neonatal birthweight (95% CI: –150.70, –18.22), –0.17 cm shorter thigh length (95% CI: –0.27, –0.07), –0.43 cm shorter crown-heel length (95% CI: –0.75, –0.10), and –0.75 mm smaller sum of skinfolds (95% CI: –1.19, –0.31), with similar associations at 23–31 and 33–39 weeks of gestation. There were no associations of FABP4 and chemerin with neonatal anthropometry.
Conclusions
Starting as early as 15 weeks of gestation, higher maternal IL-6 concentrations in pregnancy were associated with lower neonatal birthweight, thigh and crown-heel length, and skinfolds. These data provide insight into the relevance of maternal inflammatory markers with neonatal anthropometry.
Keywords: pregnancy, adipokines, offspring body composition, interleukin 6, inflammation
Beginning in early pregnancy and throughout gestation, higher IL-6, a marker of inflammation, was associated with lower newborn weight, shorter length, and smaller skin folds.
Introduction
Both maternal adiposity and inflammation are associated with impaired placental function and pregnancy complications, with downstream impacts on fetal growth (1). Accumulating evidence supports that neonatal size and body composition at birth are related to future risk of adiposity, which in turn is associated with metabolic dysfunction (i.e. higher blood pressure, greater insulin resistance) (2–6). Identifying maternal factors associated with neonatal anthropometry could optimize risk-stratification approaches aimed at detecting offspring with elevated risk of adverse health outcomes, which could inform prevention strategies.
Adipokines involved in inflammatory processes such as IL-6 (7), fatty acid binding protein-4 (FABP4) (8, 9), and chemerin (10) have been associated with modulating cell proliferation and differentiation, local angiogenesis, immune tolerance, and inflammatory processes in maternal adipocytes, placenta, and endometrium (11). These pathways impact placental function and fetal nutrient supply, which suggests that these adipokines may be involved in fetal growth and subsequent neonatal size at birth (10–13). Previous studies on IL-6, FABP4, and chemerin during pregnancy have measured concentrations at delivery or in cord blood (14–21). In these studies, chemerin was positively associated with birth weight (14) and higher concentrations were found in cord blood of infants born large-for-gestational age (18); FABP4 was lower in cord blood of infants born small-for-gestational age (SGA) (19); and IL-6 was higher in cord blood of infants born SGA (15, 16). These studies examined adipokines with inflammatory potential in the context of the overall size of the neonate, which limits our understanding of the association with specific markers of neonatal body composition (14–16, 19, 21). Further, prior studies were among a racially/ethnically homogenous sample (22), or only included 1 or 2 time points during pregnancy (23–25).
Although the maternal inflammatory profile and adipose tissue metabolism change throughout pregnancy, prospective data capturing multiple measures of adipokines across gestation and their associations with neonatal size and body composition are lacking. Use of longitudinal exposure measures enables the assessment of sensitive time points during gestation which has the potential to support targeted intervention efforts during pregnancy. We have previously found that leptin and adiponectin, adipokines related to energy homeostasis, were associated with neonatal length and adiposity, and associations differed in timing across pregnancy and by prepregnancy obesity (26). For instance, adiponectin concentrations, in the latter half of pregnancy were associated with birthweight in women with and without obesity, but only neonatal length and skinfolds were associated with adiponectin in women with obesity.
The purpose of this study was to assess associations of inflammatory adipokines with neonatal size and body composition. The role of adipokines in inflammation are complementary to those classically considered to be regulators of energy homeostasis, as leptin and adiponectin play a role in regulating insulin-mediated glucose metabolism and are related to inflammatory responses in adipocytes (27–29). Given our previous findings with energy-related adipokines, and that within the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies-Singleton Cohort, fetal anthropometry differed by women's prepregnancy obesity status (30), we investigated whether prepregnancy obesity modified the associations of interest. We hypothesized that proinflammatory adipokines would be inversely associated with neonatal size and that prepregnancy obesity status would modify any observed associations.
Methods
Study population and design
This study used data collected from the prospective NICHD Fetal Growth Studies-Singleton Cohort (NCT00912132). This cohort only included 1 mother-offspring pair and did not include siblings from another pregnancy. A total of 2802 pregnant women without a history of chronic medical conditions (e.g. prepregnancy hypertension, autoimmune disorders) were enrolled between 8 and 13 weeks of gestation from 12 US clinical centers (2009–2013) and followed through to delivery. Extensive details on study design and participant characteristics have been previously published (31).
The current analysis used biospecimen data assayed from participants in a nested case-control study of gestational diabetes mellitus (GDM) within the NICHD Fetal Growth Studies-Singleton Cohort. The nested case-control study included 321 mother-offspring pairs – 107 GDM cases and 214 non-GDM controls matched at a ratio of 1:2 on maternal age (±2 y), race/ethnicity, and weeks of gestation (±2 wk) at blood collection.
The study was approved by the Institutional Review Boards (IRBs) of the data coordinating center, NICHD (09-CH-N152), and all participating institutions: Columbia University (NY), New York Hospital, Queens (NY), Christiana Care Health System (DE), Saint Peter's University Hospital (NJ), Medical University of South Carolina (SC), University of Alabama (AL), Northwestern University (IL), Long Beach Memorial Medical Center (CA), University of California, Irvine (CA), Fountain Valley Hospital (CA), Women and Infants Hospital of Rhode Island (RI), and Tufts University (MA). Written and informed consent was obtained from all participants to use the data included in the current analysis in perpetuity. Clinical sites and the data coordinating center, as well as investigators using these data are responsible for data security. All methods were performed in accordance with the rules of the Declaration of Helsinki.
Biomarker assessment
Following a standardized protocol, blood samples were collected at 4 study visits: 10–14 weeks of gestation, 15–26 weeks of gestation (fasting), 23–31 weeks of gestation, and 33–39 weeks of gestation. Only 1 fasting sample was collected during pregnancy to reduce participant burden. Biomarkers were measured at 10–14 and 15–26 weeks of gestation among all cases and both controls (n = 321), and at 23–31 and 33–39 weeks of gestation among all cases and 1 randomly selected control (n = 214). Blood samples were immediately processed into EDTA plasma and stored at −70°C until they were thawed immediately before analysis. Assays were performed by a certified clinical laboratory at the University of Minnesota (Minneapolis, MN, USA). Assay method and details on CVs of plasma adipokines (IL-6, FABP4, chemerin) can be found in Supplemental Table 1.
Assessment of neonatal body composition
Birthweight and gestational age at delivery were abstracted from medical records at each clinical site. Neonatal length and skin fold measurements were collected after delivery (median 1 d, IQR 1–2 d). Following a standard protocol, anthropometry was obtained in at least duplicate and the 2 closest measurements were averaged. Neonatal crown-heel length (cm) was measured using an infantometer, thigh and upper arm length (cm) were measured using a measuring tape, and skinfold thickness (mm) was measured using a Lange skinfold caliper. Abdominal flank, anterior thigh, subscapular, and triceps skinfolds were summed (sum of skinfolds) as a measure of neonatal adiposity (32). Following a standardized protocol, all clinical sites were instructed to use the same instruments to measure anthropometry. After the completion of the study, in the quality control process, we identified that 1 of the clinical sites used the incorrect calipers and, thus, participants from this site were excluded from skinfold analyses (n = 12).
Covariates
Maternal sociodemographic characteristics were collected from detailed questionnaires at enrollment. Maternal height and prepregnancy weight were self-reported and prepregnancy BMI (kg/m2) was calculated and categorized as normal (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0). Self-reported prepregnancy weight was highly correlated with weight measured by study personnel during the enrollment visit (8–13 weeks of gestation) (r = 0.97) (33). Women with GDM (34) were identified by medical record review conducted by study staff at each clinical site.
Statistical analyses
Sampling weights were applied to all analyses to represent the full NICHD fetal growth singletons cohort, and account for the oversampling of women with GDM in the case-control study, which allowed us to assess the association of adipokines with neonatal body composition irrespective of GDM (26, 35). Descriptive statistics of participant characteristics were presented as weighted mean ± SE for continuous variables, and frequency and weighted percent for categorical variables.
To inform the primary analysis we first plotted the median level of adipokines at each study visit during pregnancy and tested for significant differences over time using linear models with adipokine concentration as the outcome and study visit as the independent variable with 10–14 weeks of gestation as the reference group. We assessed the Pearson correlation within and among each log-transformed adipokine across pregnancy, and the correlation of adipokines at each study visit with neonatal outcomes. We used weighted generalized linear models with robust SE to examine associations between continuous levels (per 1 SD) of individual maternal adipokines at each visit and neonatal size. All models were adjusted for maternal matching factors. Additional covariates were based on a priori knowledge and bivariate analysis and included education (high school degree or less, associate degree, Bachelor's degree), prepregnancy BMI (continuous), and parity (nulliparous). Only multiparous women with obesity (8.4%), could have had GDM in a prior pregnancy due to exclusion criteria in the study design (31). Given that the prevalence of GDM among obese women in the USA is between 6 and 12% (36), only 1–3 women in our analytic sample would have had GDM in a prior pregnancy.
Models of neonatal body length, thigh length, arm length, and sum of skinfolds were further adjusted for the number of days between delivery and measurement date. Additionally, restricted cubic splines were used to test for nonlinear associations between maternal adipokines and neonatal anthropometry; however, a nonlinear relation was not found. To assess potential time-varying concentrations of adipokines, we used a latent-class model (a flexible data-driven semiparametric approach) to identify trajectories of maternal adipokine change throughout pregnancy and examine if trajectories were associated with neonatal size (37).
We assessed for effect modification by maternal prepregnancy obesity status (BMI ≥30.0), gestational weight gain up to each corresponding visit, offspring sex, and maternal race ethnicity by including an interaction term in separate regression models, and by performing stratified analysis as appropriate. In addition, we excluded women with a preterm delivery (<37 weeks of gestation) to ensure that associations were not a result of lower gestational age. As an additional ancillary analysis, we corrected for multiple comparisons by means of false discovery rate (FDR) estimation. In all models, we confirmed the assumptions of multivariate normality by examining model residuals. All analyses were implemented using SAS Version 9.4 (SAS Institute), with a 2-sided P value of <0.05 as the level of significance.
Results
Characteristics of study participants and adipokine profiles throughout pregnancy
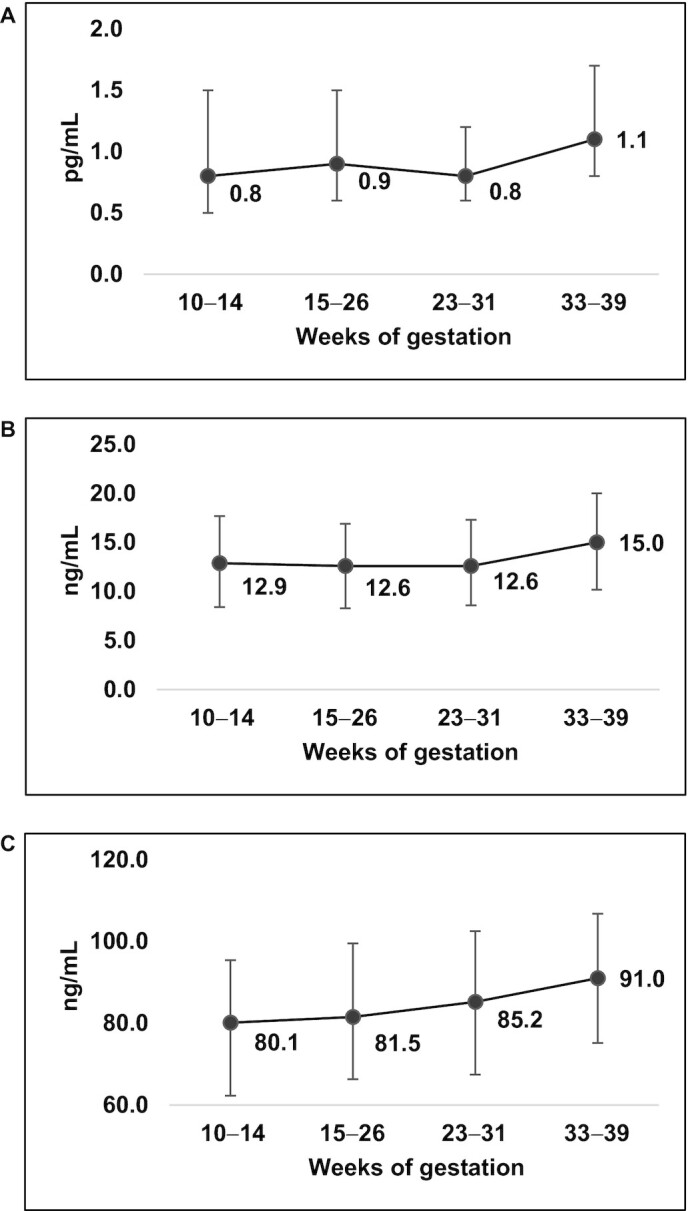
The characteristics of the study participants are presented in Table 1. The mean (SE) age of the participants was 28.2 y (0.46), and generally the weighted distribution across racial/ethnic groups was equal. Most participants had completed at least some college (75.0%), and most had a prepregnancy BMI in the normal weight range (51.7%). The mean (SE) neonatal weight, length, and sum of skinfolds was 3310.4 g (48.0), 50.3 cm (0.23), and 19.7 mm (0.4), respectively. The average gestational age at delivery was 39.0 (0.18) weeks. At 10–14 weeks of gestation all proinflammatory adipokines varied across race/ethnicity and prepregnancy BMI and chemerin was additionally associated with parity. For instance, non-Hispanic white women had higher concentrations of FABP4 and chemerin and lower concentrations of IL-6 compared with women of other races/ethnicities. At 10–14 weeks of gestation the median (IQR) concentrations of IL-6, FABP4, and chemerin were 0.8 pg/mL (0.5, 1.5), 12.9 ng/mL (8.4, 17.7), and 80.1 ng/mL (62.3, 95.4), respectively (Figure 1). In general, increases in all 3 adipokines were observed throughout pregnancy (10–14 compared with 33–39 weeks of gestation all Ps <0.05) and the strength of correlations within adipokines diminished as they became more temporarily separated (Figure 2).
TABLE 1.
Participant characteristics and associations with proinflammatory adipokines at 10–14 weeks of gestation (N = 321)
| Overall | IL-6 pg/mL | FABP4 ng/mL | Chemerin ng/mL | ||||
|---|---|---|---|---|---|---|---|
| n (weighted %) | Median (IQR) | P | Median (IQR) | P | Median (IQR) | P | |
| Age (y), mean (SE) | 28.2 (0.46) | — | — | — | |||
| Race-ethnicity | <0.001 | <0.001 | 0.03 | ||||
| Non-Hispanic White | 75 (30.9) | 0.71 (0.46, 1.32) | 15.08 (10.99, 21.03) | 83.42 (68.52, 97.61) | |||
| Non-Hispanic Black | 45 (23.3) | 1.13 (0.71, 1.96) | 13.09 (8.28, 17.66) | 80.06 (64.07, 96.13) | |||
| Hispanic | 123 (27.2) | 1.03 (0.57, 1.65) | 13.21 (8.68, 17.16) | 80.87 (64.95, 97.2) | |||
| Asian & Pacific Islander | 78 (18.5) | 0.72 (0.46, 1.10) | 9.12 (6.54, 12.02) | 65.56 (54.11, 84.33) | |||
| Education | 0.14 | 0.46 | 0.28 | ||||
| High school or less | 81 (25.1) | 0.93 (0.50, 1.39) | 12.08 (8.28, 16.3) | 76.91 (61.4, 93.21) | |||
| Some college/associate degree | 117 (35.2) | 0.96 (0.46, 1.67) | 14.1 (7.62, 20.23) | 81.46 (62.28, 97.26) | |||
| 4-y college degree or higher | 123 (39.8) | 0.76 (0.50, 1.43) | 12.75 (9.12, 16.16) | 77.71 (62.7, 91.12) | |||
| Parity | 0.37 | 0.06 | <0.001 | ||||
| Nulliparous | 144 (51.1) | 0.76 (0.47, 1.36) | 12.02 (8.28, 15.46) | 68.52 (56.47, 89.76) | |||
| Multiparous | 177 (48.9) | 1.04 (0.56, 1.58) | 14.4 (1.41, 70.14) | 83.56 (5.03, 248.91) | |||
| Smoked1 | 5 (0.7) | 1.18 (1.18, 1.18) | 0.65 | 20.23 (20.23, 20.23) | 0.17 | 159.84 (159.84, 159.84) | 0.25 |
| Prepregnancy BMI, kg/m2, mean (SE) | 25.7 (0.38) | — | — | — | |||
| 19.0–24.9 | 156 (51.7) | 0.72 (0.46, 1.26) | <0.001 | 10.0 (6.81, 13.2) | <0.001 | 72.5 (59.1, 91.7) | 0.003 |
| 25.0–29.9 | 99 (33.1) | 0.89 (0.50, 1.36) | 15.0 (12.1, 19.4) | 80.0 (66.0, 92.8) | |||
| 30.0–45.0 | 66 (15.2) | 1.85 (1.02, 3.20) | 21.7 (16.3, 28.2) | 92.0 (74.6, 113.0) | |||
| GA at delivery (wk), mean (SE) | 39.0 (0.18) | — | — | — | |||
| Birthweight (g), mean (SE) | 3310.4 (48.0) | — | — | — | |||
| Length (cm), mean (SE) | 50.3 (0.23) | — | — | — | |||
| Thigh length (cm), mean (SE) | 10.5 (0.11) | — | — | — | |||
| Upper arm length (cm), mean (SE) | 10.2 (0.09) | — | — | — | |||
| Sum of skinfolds (mm), mean (SE) | 19.7 (0.41) | — | — | — | |||
| Adipokine concentration at 10–14 GWs | — | 0.81 (0.50, 1.48) | 12.91 (8.44, 17.66) | 80.06 (62.28, 95.43) |
P value not based on robust variance estimates due to small cell size. FABP4, fatty acid binding protein-4; GA, gestational age; GWs, weeks of gestation.
FIGURE 1.
Median (IQR) of proinflammatory adipokine distributions throughout pregnancy. (A) IL-6, P values for significant differences in adipokine concentrations compared with concentrations at 10–14 GWs; 15–26 GWs, P = 0.97; 23–31 GWs, P = 0.73; 33–39 GWs, P = 0.01. (B) FABP4, P values for significant differences in adipokine concentrations compared with concentrations at 10–14 GWs; 15–26 GWs, P = 0.36; 23–31 GWs, P = 0.54; 33–39 GWs, P <0.0001. (C) Chemerin, P values for significant differences in adipokine concentrations compared with concentrations at 10–14 GWs; 15–26 GWs, P = 0.01; 23–31 GWs, P = 0.003; 33–39 GWs, P <0.0001. FABP4, fatty acid binding protein-4; GWs, weeks of gestation.
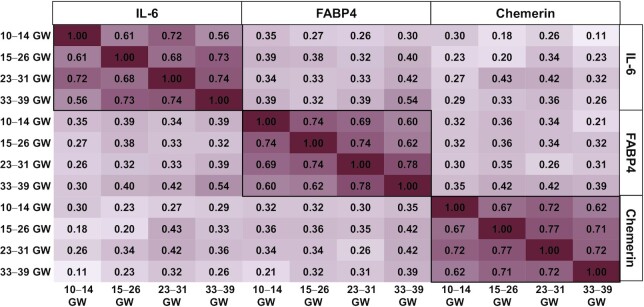
FIGURE 2.
Pearson correlation within log-transformed adipokines throughout pregnancy. Darker red indicates strong positive correlation. FABP4, fatty acid binding protein-4; GWs, weeks of gestation.
Associations of adipokines with neonatal size
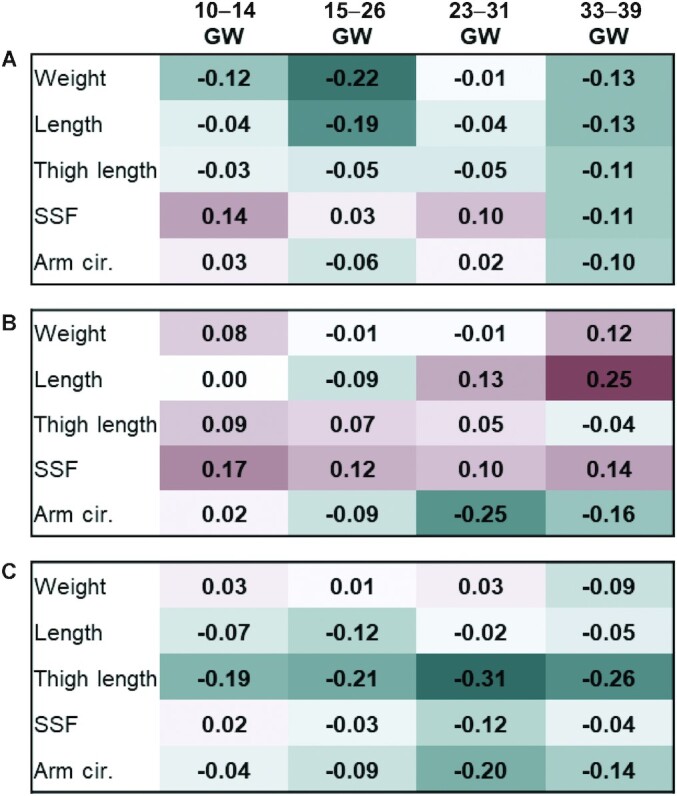
Throughout pregnancy, IL-6 showed the strongest inverse correlations at 15–26 weeks of gestation with neonatal weight and length, and the most consistent negative correlations with all anthropometric outcomes at 33–39 weeks of gestation (Figure 3). The strongest correlations of FABP4 and neonatal anthropometry was a positive correlation at 33–39 weeks of gestation with neonatal length, and for chemerin the strongest positive correlations were at 23–31 and 33–39 with thigh length.
FIGURE 3.
Pearson correlation between log-transformed adipokines and neonatal body composition measures. (A) IL-6, (B) FABP4, (C) chemerin. Darker green indicates stronger negative correlation, darker red indicates strong positive correlation. cir., circumference; FABP4, fatty acid binding protein-4; GWs, weeks of gestation; SSF, sum of skin folds.
The adjusted associations of IL-6, FABP4, and chemerin at 10–14, 15–26, 23–31, and 33–39 weeks of gestation with neonatal anthropometry are presented in Table 2. At each study visit an increase in IL-6 was associated with lower neonatal birthweight (g) and thigh length (cm), and starting at 15–26 weeks of gestation an increase in IL-6 was associated with decreases in measures of length (cm) and sum of skinfolds (mm). For instance, at 15–26 weeks of gestation a 1 SD increase in IL-6 was associated with –84.46 g lower neonatal birthweight (95% CI: –150.70, –18.22), –0.17 cm shorter thigh length (95% CI: –0.27, –0.07), –0.43 cm shorter crown-heel length (95% CI: –0.75, –0.10), and –0.75 mm smaller sum of skinfolds (95% CI: –1.19, –0.31). Similar associations were observed at 23–31 and 33–39 weeks of gestation, however, no significant associations were found with upper arm length. Following FDR correction, we found no association of FABP4 and chemerin with neonatal anthropometry, and therefore these adipokines were not explored further in additional analyses. There was no considerable variation in the concentration of IL-6 over time and thus the best fitting model for group-based trajectories resulted in 3 groups, 1 of which represented 92.8% of the participants. Since the remaining groups were comprised of so few participants, comparisons between the group-based trajectories and neonatal anthropometry were not explored further.
TABLE 2.
Separate multivariate models of maternal plasma adipokines at each individual study visit and the association with neonatal anthropometry adjusted for multiple comparisons (adjusted1 β [95% CI])
| Maternal biomarker | Weight, g | P | P 2 | Length, cm | P | P 2 | Thigh length, cm | P | P 2 | Upper arm length, cm | P | P 2 | Sum of skinfolds, mm | P | P 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 per SD | |||||||||||||||
| 10–14 wk | –64.05 (–113.58, –14.51) | 0.023 | 0.01 | –0.21 (–0.46, 0.05) | 0.16 | 0.12 | –0.16 (–0.27, –0.06) | 0.013 | <0.001 | –0.06 (–0.17, 0.05) | 0.34 | 0.31 | –0.59 (–1.41, 0.23) | 0.20 | 0.16 |
| 15–26 wk | –84.46 (–150.70, –18.22) | 0.023 | 0.01 | –0.43 (–0.75, –0.10) | 0.023 | 0.01 | –0.17 (–0.27, –0.07) | <0.0013 | <0.001 | –0.03 (–0.12, 0.06) | 0.59 | 0.56 | –0.75 (–1.19, –0.31) | <0.0013 | <0.001 |
| 23–31 wk | –59.92 (–113.93, –5.91) | 0.0463 | 0.03 | –0.31 (–0.54, –0.07) | 0.023 | 0.01 | –0.15 (–0.28, –0.01) | 0.0493 | 0.03 | 0.01 (–0.10, 0.12) | 0.84 | 0.84 | –0.70 (–1.34, –0.07) | 0.0493 | 0.03 |
| 33–39 wk | –104.35 (–140.77, –67.92) | <0.0013 | <0.001 | –0.45 (–0.62, –0.28) | <0.0013 | <0.001 | –0.20 (–0.28, –0.13) | <0.0013 | <0.001 | –0.05 (–0.12, 0.02) | 0.23 | 0.20 | –1.09 (–1.38, –0.81) | <0.0013 | <0.001 |
| FABP4 per SD | |||||||||||||||
| 10–14 wk | –35.56 (–161.88, 90.77) | 0.78 | 0.58 | –0.33 (–0.88, 0.23) | 0.72 | 0.25 | –0.06 (–0.35, 0.22) | 0.78 | 0.66 | –0.06 (–0.28, 0.16) | 0.78 | 0.59 | 0.58 (–0.44, 1.60) | 0.72 | 0.26 |
| 15–26 wk | –32.55 (–124.61, 59.50) | 0.78 | 0.49 | –0.39 (–0.81, 0.03) | 0.54 | 0.07 | 0.01 (–0.23, 0.26) | 0.92 | 0.92 | –0.09 (–0.22, 0.05) | 0.72 | 0.22 | 0.25 (–0.58, 1.07) | 0.78 | 0.56 |
| 23–31 wk | –17.28 (–108.99, 74.43) | 0.79 | 0.71 | 0.30 (–0.26, 0.87) | 0.72 | 0.29 | –0.08 (–0.40, 0.24) | 0.78 | 0.64 | –0.22 (–0.42, –0.01) | 0.54 | 0.04 | 0.74 (–0.41, 1.89) | 0.72 | 0.21 |
| 33–39 wk | –48.87 (–191.53, 93.79) | 0.78 | 0.50 | 0.35 (–0.55, 1.25) | 0.78 | 0.44 | –0.33 (–0.69, 0.04) | 0.54 | 0.08 | –0.08 (–0.34, 0.19) | 0.78 | 0.57 | 0.20 (–1.66, 2.06) | 0.88 | 0.83 |
| Chemerin per SD | |||||||||||||||
| 10–14 wk | 23.91 (–54.93, 102.76) | 0.99 | 0.55 | –0.06 (–0.47, 0.35) | 0.99 | 0.78 | –0.20 (–0.41, 0.02) | 0.46 | 0.07 | 0.04 (–0.10, 0.18) | 0.99 | 0.58 | –0.20 (–0.86, 0.47) | 0.99 | 0.56 |
| 15–26 wk | –19.61 (–108.42, 69.21) | 0.99 | 0.67 | –0.29 (–0.75, 0.17) | 0.54 | 0.21 | –0.21 (–0.44, 0.02) | 0.46 | 0.07 | –0.01 (–0.16, 0.14) | 0.99 | 0.91 | 0.02 (–0.66, 0.69) | 0.99 | 0.96 |
| 23–31 wk | –12.17 (–108.90, 84.56) | 0.99 | 0.81 | 0.00 (–0.61, 0.61) | 0.99 | 0.99 | –0.27 (–0.53, –0.01) | 0.46 | 0.04 | 0.15 (–0.06, 0.35) | 0.49 | 0.17 | –0.98 (–2.31, 0.35) | 0.49 | 0.15 |
| 33–39 wk | –11.27 (–110.68, 88.15) | 0.99 | 0.82 | 0.11 (–0.49, 0.70) | 0.99 | 0.72 | –0.24 (–0.53, 0.05) | 0.49 | 0.11 | 0.14 (–0.06, 0.33) | 0.49 | 0.17 | 0.03 (–1.18, 1.23) | 0.99 | 0.97 |
Adjusted for maternal age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian), education (high school or less, some college/associate degree, 4-y college degree or higher), nulliparity (yes/no), prepregnancy BMI (continuous), week of gestation at blood collection (continuous), and postnatal days at neonatal assessment (continuous; length, arm, and thigh length, and skinfolds model only). FABP4, fatty acid binding protein-4.
P value without multiple comparison correction
3 P <0.05 following false discovery rate estimation
In general, results stratified by prepregnancy obesity status were similar in direction, with evidence for a significant interaction between IL-6 and prepregnancy obesity at 10–14 weeks of gestation with sum of skinfolds (P-interaction = 0.01), and at 23–31 and 33–39 weeks of gestation with neonatal birthweight (P-interaction = 0.04 and 0.01, respectively) (Table 3). The magnitude of the association tended to be stronger among women with prepregnancy obesity. For instance, at 15–26 weeks of gestation a 1 SD increase in IL-6 was associated with a –167.10 g (95% CI: –243.86, –90.35) lower neonatal birthweight among women with obesity, and a –31.85 g (95% CI: –60.30, –3.40) lower neonatal birthweight among women without obesity. We found no statistical interactions between maternal IL-6 and gestational weight gain, offspring sex, or maternal race/ethnicity in association with neonatal body composition. Exclusion of neonates delivered preterm (n = 19) did not materially change the interpretation of the results between IL-6 and neonatal anthropometry (data not shown).
TABLE 3.
Plasma IL-6 concentrations and neonatal weight and sum of skinfolds among women with prepregnancy normal/overweight or obesity (Adjusted1 β [95% CI])
| Weight, g | Sum of skinfolds, mm | |||||
|---|---|---|---|---|---|---|
| Prepregnancy BMI | P for interaction | Prepregnancy BMI | P for interaction | |||
| <30.0 kg/m2 | ≥30.0 kg/m2 | <30.0 kg/m2 | ≥30.0 kg/m2 | |||
| IL-6, pg/mL | ||||||
| 10–14 wk | –21.5 (–45.53, 2.53) | –54.78 (–133.74, 24.18) | 0.21 | –0.16 (–0.57, 0.24) | –0.79 (–1.60, –0.01) 2 |
0.01 |
| 15–26 wk | –31.85 (–60.30, –3.40)2 | –167.10 (–243.86, –90.35)2 | 0.40 | –0.32 (–0.58, –0.06)2 | –1.70 (–2.30, –1.00)2 | 0.20 |
| 23–31 wk | –26.34 (–49.14, –3.55)2 | –119.59 (–210.20, –28.99)2 | 0.04 | –0.33 (–0.57, –0.08)2 | –0.27 (–1.50, 0.97) | 0.14 |
| 33–39 wk | –40.43 (–52.33, –28.53)2 | –214.99 (–344.06, –85.92)2 | 0.01 | –0.46 (–0.55, –0.36)2 | 1.31 (–0.47, 3.09) | 0.72 |
P value for interaction of prepregnancy BMI and IL-6.
Adjusted for maternal age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian) education (high school or less, some college/associate degree, 4-y college degree or higher), nulliparity (yes/no), prepregnancy BMI (continuous), week of gestation at blood collection (continuous), and postnatal days at neonatal assessment (continuous; skinfolds model only).
2 P <0.05.
Discussion
In the present study of maternal plasma IL-6, FABP4, and chemerin throughout gestation and associations with neonatal body size and composition, we observed that higher maternal IL-6 starting from 10 weeks of gestation and throughout pregnancy was associated with lower neonatal birthweight and thigh length. Beginning at 15 wk, higher IL-6 was also associated with shorter neonatal length and smaller sum of skinfolds. We found no association of FABP4 and chemerin with neonatal anthropometry after FRD correction.
In the current study, higher IL-6 was associated with lower neonatal birthweight and this effect was greater among women with prepregnancy obesity. Prior studies among Mexican and white European women have not reported significant associations of IL-6 with birthweight (22, 23). Differences in analytical strategy such as covariate adjustment may partially explain differences between the current and previous studies. However, higher IL-6 has been found in cord blood of infants born SGA (15, 16), implicating IL-6 in overall neonatal size.
In addition to an inverse association of IL-6 with neonatal thigh and crown-heel length, we found that higher IL-6 was associated with lower sum of skinfolds. This aligns with a recent study among lean Filipino women (BMI ∼ 21.5) where IL-6 measured at ∼30 wk was negatively associated with lower neonatal sum of skinfolds (25). Interestingly, in the current study, among women with obesity the inverse association of IL-6 and sum of skinfolds was only observed with IL-6 measured in early pregnancy (10–14 wk). These findings with sum of skin folds are in contrast to prior data indicating that higher maternal IL-6 at delivery is positively correlated with newborn adiposity (17). It has been hypothesized that in women with obesity – a condition often accompanied by greater inflammation – higher IL-6 activates placental nutrient transport and may explain fetal overgrowth (38). Taken together, these data highlight the importance of studies that consider potential effect modifiers such as obesity status, and measurement of IL-6 in early and late pregnancy.
IL-6 is a multifunctional adipokine/cytokine derived from numerous cell types in many tissues including adipose and placental tissue (39, 40). Although the exact metabolic mechanism whereby maternal circulating IL-6 is involved in fetal growth and subsequent neonatal body composition is unknown, there are several pathways by which IL-6 may impact fetal development. IL-6 has been suggested to partially modulate key hormonal pathways for pre- and postnatal growth such as downregulating placental and hepatic expression of insulin-like growth factor I axis (41, 42). In mouse and human studies conducted in the postnatal period, chronic IL-6 exposure was associated with uncoupling of osteoblasts and osteoclasts resulting in growth plate thinning (41). Additionally, IL-6 may directly and indirectly affect placental development and function through its role in trophoblast differentiation, placental vascularization, and T cell phenotype and response (39). Currently, it is unclear whether the association of IL-6 with neonatal size is primarily due to its role in placental function or maternal metabolism.
In the current study, we found associations of FABP4 and chemerin only at 23–31 weeks of gestation with neonatal upper arm length and thigh length, although the associations became statistically nonsignificant after FRD correction. Prior prospective data are lacking, and previous studies were cross-sectional in design and only investigated cord blood FABP4 or chemerin (14, 18, 19). Although some of these studies found positive correlations with birthweight, the use of cord blood addresses a different physiological question than use of maternal plasma.
Future directions
The findings from the current study provide insight into the potential role of maternal circulating IL-6, FABP4, and chemerin throughout pregnancy in neonatal body composition. Although the size of the estimates of the association between IL-6 and length and skinfolds were relatively small and the clinical relevance merits further investigation, these findings could inform hypotheses for long-term follow-up studies. Further, a recent systematic review of dietary patterns during pregnancy has shown that maternal diets characterized by a higher intake of animal protein and cholesterol and/or a lower intake of fiber are associated with proinflammatory markers such as IL-6 (43). Thus, future studies are needed to determine if modifying maternal diet during pregnancy modulates the maternal inflammatory status and neonatal size.
Limitations of the data
Our racially/ethnically diverse cohort increases the generalizability of our findings. However, given the relatively healthy women included in the present study, whether our findings can be generalized to women with chronic conditions prior to pregnancy warrants further investigation. Although we have controlled for known confounders, similar to other observational studies these do not represent causal effects, and we cannot completely exclude the possibility for residual confounding or measurement errors. There was a relatively small sample size, which precluded us from examining extreme phenotypes of fetal growth, such as SGA or large-for-gestational age. We did not have information on history of GDM; however, given that only multiparous women with obesity could have had GDM based on study inclusion criteria, we expect that only 1–3 women in the analytic sample would have had a history of GDM. We do not believe this would have strongly influenced the findings considering we accounted for differences in parity (a proxy for prior GDM). Lastly, although we assessed neonatal adiposity via sum of skinfolds based on standardized protocol, it may be subject to greater measurement error than measures such as DXA or air displacement plethysmography. Nonetheless, sum of skinfolds at birth has been significantly associated with body fat percentage measured by DXA in children (6).
Conclusion
In our prospective cohort with multiple measures of adipokines, greater IL-6 concentrations as early as 10–14 weeks of gestation and across pregnancy were associated with smaller neonatal body size. Future research is warranted to understand whether modifiable lifestyle factors, such as diet and physical activity in pregnancy can affect maternal IL-6 concentrations and subsequent neonatal size. Further, the long-term implications of exposure to greater concentrations of IL-6 during gestation on health outcomes in childhood and adolescence are needed to provide context for the overall clinical relevance of these findings for offspring health.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the NICHD Fetal Growth Study-Singleton participants.
The authors’ contribution were as follows—ZC: obtained the funding and supervised the study data collection and manuscript writing; ECF and ZC: conceived the research question; ECF and ZC: are the guarantors of the work; ECF: conducted the analysis, wrote the initial draft of the manuscript, and incorporated co-author comments; ML, SNH, JW, JC, YZ, HC, MYT, and LC: curated the data, provided feedback on the analysis, and participated in the writing of the manuscript; and all authors: read and approved the final manuscript.
Notes
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding and included American Recovery and Reinvestment Act funding via contract numbers: HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, and HHSN275201000001Z. ECF was a participant in the NIH Graduate Partnership Program and a graduate student at Clemson University. ECF is supported by the T32 fellowship from the National Institute of Child Health and Human Development (5T32HD007186-39). YZ was supported by a research grant funded by the National Institute of Diabetes and Digestive and Kidney Diseases (K01DK120807).
ECF was a participant in the National Institutes of Health Graduate Partnership Program and a graduate student at Clemson University.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: FABP4, fatty acid binding protein-4; FDR, false discovery rate; GDM, gestational diabetes mellitus; NICHD, National Institute of Child Health and Human Development; SGA, small-for-gestational age.
Contributor Information
Ellen C Francis, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Division of Intramural Population Health Research Epidemiology Branch, Bethesda, MD, USA; Lifecourse Epidemiology of Adiposity and Diabetes Center, University of Colorado - Anschutz Medical Campus, Aurora, CO, USA.
Mengying Li, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Division of Intramural Population Health Research Epidemiology Branch, Bethesda, MD, USA.
Stefanie N Hinkle, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Division of Intramural Population Health Research Epidemiology Branch, Bethesda, MD, USA.
Jinbo Chen, Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Jing Wu, Glotech Inc., Bethesda, MD, USA.
Yeyi Zhu, Kaiser Permanente Northern California, Division of Research, Oakland, CA, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA.
Haiming Cao, Cardiovascular Branch, National Heart, Lung and Blood Institute, NIH, Bethesda, MD, USA.
Michael Y Tsai, Department of Laboratory Medicine & Pathology, University of Minnesota, Minneapolis, MN, USA.
Liwei Chen, Department of Epidemiology, Fielding School of Public Health, University of California Los Angeles, Los Angeles, CA, USA.
Cuilin Zhang, Email: zhangcu@mail.nih.gov, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Division of Intramural Population Health Research Epidemiology Branch, Bethesda, MD, USA.
Data Availability
The data, along with a set of guidelines for researchers applying for the data, will be posted to a data-sharing site, the NICHD/DIPHR Biospecimen Repository Access and Data Sharing (https://brads.nichd.nih.gov) (BRADS).
References
- 1. Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30(7):441–6. [DOI] [PubMed] [Google Scholar]
- 2. Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50-60 years. BMJ. 1996;312(7028):406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tian JY, Cheng Q, Song XM, Li G, Jiang GX, Gu YY, Luo M. Birth weight and risk of type 2 diabetes, abdominal obesity and hypertension among Chinese adults. Eur J Endocrinol. 2006;155(4):601–7. [DOI] [PubMed] [Google Scholar]
- 4. Spiegel E, Shoham-Vardi I, Sergienko R, Landau D, Sheiner E. The association between birth weight at term and long-term endocrine morbidity of the offspring. J Matern Fetal Neonatal Med. 2019;32(16):2657–61. [DOI] [PubMed] [Google Scholar]
- 5. Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22(6):944–50. [DOI] [PubMed] [Google Scholar]
- 6. Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220(2):T47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cerezo LA, Kuklova M, Hulejova H, Vernerova Z, Pesakova V, Pecha O, Veigl D, Haluzik M, Pavelka K, Vencovsky J. The level of fatty acid-binding protein 4, a novel adipokine, is increased in rheumatoid arthritis and correlates with serum cholesterol levels. Cytokine. 2013;64(1):441–7. [DOI] [PubMed] [Google Scholar]
- 10. Carlino C, Trotta E, Stabile H, Morrone S, Bulla R, Soriani A, Iannitto ML, Agostinis C, Mocci C, Minozzi M. Chemerin regulates NK cell accumulation and endothelial cell morphogenesis in the decidua during early pregnancy. J Clin Endocrinol Metab. 2012;97(10):3603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Briana DD, Malamitsi-Puchner A. The role of adipocytokines in fetal growth. Ann N Y Acad Sci. 2010;1205(1):82–87. [DOI] [PubMed] [Google Scholar]
- 12. Wang P, Zhu Q, Peng H, Du M, Dong M, Wang H. Fatty acid-binding protein 4 in endometrial epithelium is involved in embryonic implantation. Cell Physiol Biochem. 2017;41(2):501–9. [DOI] [PubMed] [Google Scholar]
- 13. Li H, Cao G, Zhang N, Lou T, Wang Q, Zhang Z, Liu C. RBP4 regulates trophoblastic cell proliferation and invasion via the PI3K/AKT signaling pathway. Molecular Medicine Reports. 2018;18(3):2873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazaki-Tovi S, Kasher-Meron M, Hemi R, Haas J, Gat I, Lantsberg D, Hendler I, Kanety H. Chemerin is present in human cord blood and is positively correlated with birthweight. Am J Obstet Gynecol. 2012;207(5):412.e1–412.e10. [DOI] [PubMed] [Google Scholar]
- 15. Lausten-Thomsen U, Olsen M, Greisen G, Schmiegelow K. Inflammatory markers in umbilical cord blood from small-for-gestational-age newborns. Fetal Pediatr Pathol. 2014;33(2):114–8. [DOI] [PubMed] [Google Scholar]
- 16. Amarilyo G, Oren A, Mimouni FB, Ochshorn Y, Deutsch V, Mandel D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J Perinatol. 2011;31(1):30–32. [DOI] [PubMed] [Google Scholar]
- 17. Radaelli T, Uvena-Celebrezze J, Minium J, Huston-Presley L, Catalano P, Hauguel-de Mouzon S. Maternal interleukin-6: marker of fetal growth and adiposity. J Soc Gynecol Investig. 2006;13(1):53–57. [DOI] [PubMed] [Google Scholar]
- 18. Boutsikou T, Briana DD, Boutsikou M, Kafalidis G, Stamati L, Baka S, Hassiakos D, Gourgiotis D, Malamitsi-Puchner A. Cord blood chemerin and obestatin levels in large for gestational age infants. J Matern Fetal Neonatal Med. 2013;26(2):123–6. [DOI] [PubMed] [Google Scholar]
- 19. Joung KE, Cataltepe SU, Michael Z, Christou H, Mantzoros CS. Cord blood adipocyte fatty acid-binding protein levels correlate with gestational age and birth weight in neonates. J Clin Endocrinol Metab. 2017;102(5):1606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valsamakis G, Papatheodorou DC, Margeli A, Bakoulas V, Kapantais E, Papassotiriou I, Creatsas G, Kumar S, Mastorakos G. First trimester maternal BMI is a positive predictor of cord blood c-peptide levels while maternal visfatin levels is a negative predictor of birth weight. Hormones. 2014;13(1):87–94. [DOI] [PubMed] [Google Scholar]
- 21. Smerieri A, Petraroli M, Ziveri MA, Volta C, Bernasconi S, Street ME. Effects of cord serum insulin, IGF-II, IGFBP-2, IL-6 and cortisol concentrations on human birth weight and length: pilot study. PLoS One. 2011;6(12):e29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perichart-Perera O, Muñoz-Manrique C, Reyes-López A, Tolentino-Dolores M, Espino Y, Sosa S, Ramírez-González MC. Metabolic markers during pregnancy and their association with maternal and newborn weight status. PLoS One. 2017;12(7):e0180874-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farah N, Hogan AE, O'Connor N, Kennelly MM, O'Shea D, Turner MJ. Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine. 2012;60(1):96–99. [DOI] [PubMed] [Google Scholar]
- 24. Georgiou HM, Thio YS, Russell C, Permezel M, Heng YJ, Lee S, Tong S. Association between maternal serum cytokine profiles at 7-10 weeks' gestation and birthweight in small for gestational age infants. Am J Obstet Gynecol. 2011;204(5):415.e1–415.e12. [DOI] [PubMed] [Google Scholar]
- 25. Ragsdale HB, Kuzawa CW, Borja JB, Avila JL, McDade TW. Regulation of inflammation during gestation and birth outcomes: inflammatory cytokine balance predicts birth weight and length. Am J Hum Biol. 2019;31(3):e23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hinkle SN, Rawal S, Liu D, Chen J, Tsai MY, Zhang C. Maternal adipokines longitudinally measured across pregnancy and their associations with neonatal size, length, and adiposity. Int J Obes. 2019;43(7):1422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Y, Rui L. Leptin signaling and leptin resistance. Frontiers of Medicine. 2013;7(2):207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res Int. 2014;2014:658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–9. [DOI] [PubMed] [Google Scholar]
- 30. Zhang C, Hediger ML, Albert PS, Grewal J, Sciscione A, Grobman WA, Wing DA, Newman RB, Wapner R, D'Alton ME. Association of maternal obesity with longitudinal ultrasonographic measures of fetal growth: findings from the NICHD fetal growth studies-singletons. JAMA Pediatrics. 2018;172(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grewal J, Grantz KL, Zhang C, Sciscione A, Wing DA, Grobman WA, Newman RB, Wapner R, D'Alton ME, Skupski D. Cohort profile: NICHD fetal growth studies-singletons and twins. Int J Epidemiol. 2018;47(1):25–25l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91(7):612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang C, Hediger ML, Albert PS, Grewal J, Sciscione A, Grobman WA, Wing DA, Newman RB, Wapner R, D'Alton ME. Association of maternal obesity with longitudinal ultrasonographic measures of fetal growth: findings from the NICHD fetal growth studies-singletons. JAMA Pediatrics. 2018;172(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. ACOG Committee on Obstetric Practice . ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–64. [DOI] [PubMed] [Google Scholar]
- 35. Samuelsen SO. A psudolikelihood approach to analysis of nested case-control studies. Biometrika. 1997;84(2):379–94. [Google Scholar]
- 36. Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health. 2010;100(6):1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4(2):139. [DOI] [PubMed] [Google Scholar]
- 38. Omere C, Richardson L, Saade GR, Bonney EA, Kechichian T, Menon R. Interleukin (IL)-6: a friend or foe of pregnancy and parturition? Evidence from functional studies in fetal membrane cells. Frontiers in Physiology. 2020;11(891):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95(1-2):1–14. [DOI] [PubMed] [Google Scholar]
- 40. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50. [DOI] [PubMed] [Google Scholar]
- 41. Walters TD, Griffiths AM. Mechanisms of growth impairment in pediatric Crohn's disease. Nature Rev Gastroenterol Hepatol. 2009;6:513–23. [DOI] [PubMed] [Google Scholar]
- 42. Ashorn P, Hallamaa L, Allen LH, Ashorn U, Chandrasiri U, Deitchler M, Doyle R, Harjunmaa U, Jorgensen JM, Kamiza Set al. Co-causation of reduced newborn size by maternal undernutrition, infections, and inflammation. Matern Child Nutr. 2018;14(3):e12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeh K-L, Kautz A, Lohse B, Groth SW. Associations between dietary patterns and inflammatory markers during pregnancy: a systematic review. Nutrients. 2021;13(3):834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, along with a set of guidelines for researchers applying for the data, will be posted to a data-sharing site, the NICHD/DIPHR Biospecimen Repository Access and Data Sharing (https://brads.nichd.nih.gov) (BRADS).