Abstract
Bacteria-phage symbioses are ubiquitous in nature and serve as valuable biological models. Historically, the ecology and evolution of bacteria-phage systems have been studied in either very simple or very complex communities. Although both approaches provide insight, their shortcomings limit our understanding of bacteria and phages in multispecies contexts. To address this gap, here we synthesize the emerging body of bacteria-phage experiments in medium-complexity communities, specifically those that manipulate bacterial community presence. Generally, community presence suppresses both focal bacterial (phage host) and phage densities, while sometimes altering bacteria-phage ecological interactions in diverse ways. Simultaneously, community presence can have an array of evolutionary effects. Sometimes community presence has no effect on the coevolutionary dynamics of bacteria and their associated phages, whereas other times the presence of additional bacterial species constrains bacteria-phage coevolution. At the same time, community context can alter mechanisms of adaptation and interact with the pleiotropic consequences of (co)evolution. Ultimately, these experiments show that community context can have important ecological and evolutionary effects on bacteria-phage systems, but many questions still remain unanswered and ripe for additional investigation.
Subject terms: Microbial communities, Bacteriophages, Microbial ecology, Evolution, Bacterial evolution
Introduction
Bacteria and their viral symbionts, bacteriophages (phages), have long been important study systems in biology [1–3]. While early work focused on molecular and genetic details, the last few decades have seen growing interest in examining their ecological and evolutionary dynamics. The study of bacteria-phage ecology and evolution has largely been driven by two motivations: (1) to understand the microbial world per se, since bacteria and phages are ubiquitous, abundant, and diverse, with widespread importance, including for human health [4, 5]; and (2) as tractable models of generalized host-symbiont or predator-prey dynamics [6, 7].
However, studies on the ecology and evolution of bacteria and phages have predominately utilized one of two approaches: experiments with very simple communities (reductionist) or observations of very complex communities (holistic) [8]. For instance, reductionist work has described in-depth the ecology and coevolution of many bacteria-phage pairs (reviewed in [6, 9–11]). In parallel, holistic studies have examined semi-natural and natural systems to infer bacteria-phage ecology and (co)evolution [11–20]. Nonetheless, while both reductionist and holistic approaches have provided useful insights, they are limited in what we can learn about bacteria-phage ecology and evolution in multispecies contexts.
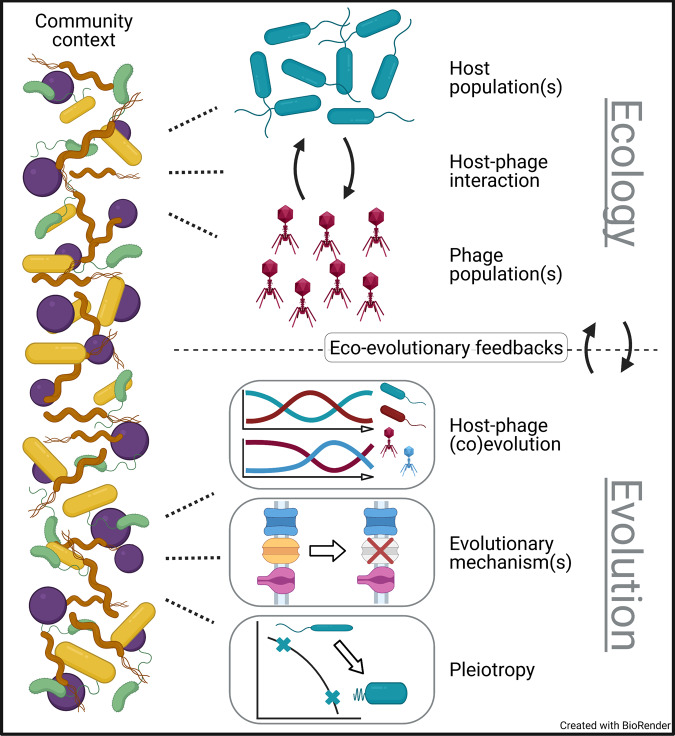
Here, we address this intellectual gap via synthesis and review of the recent and emerging body of phage-bacteria experiments in medium-complexity communities, something that has long been highlighted as a needed focus [11, 21–23]. Specifically, we discuss experiments that directly test how the ecology and evolution of “focal” bacteria and phages are altered by the presence vs absence of other bacterial species (“community context”, Fig. 1). We focus our discussion on experiments with three or more bacterial species (for two-species communities see Table S1, partially reviewed in [24]), and omit those lacking experimental manipulation of community context (Table S1, reviewed in [11–20]) or with eukaryotes (Table S1, communities with microbial eukaryotes reviewed in [25, 26]). The experiments we highlight bridge the gap between the historical holistic and reductionist approaches.
Fig. 1. Effects of community context on focal bacterial and phage ecology and evolution.
Bacterial community context (left) may affect the ecology and evolution of interacting phage and bacteria populations in various ways (dotted lines). Ecological effects include direct effects on the density of the focal bacterial and phage populations, as well as higher-order effects that alter the interaction between the focal bacteria and phage populations. Evolutionary effects can include: changes to (co)evolutionary dynamics, like fluctuating selection dynamics (depicted); mechanisms of evolution, like which receptor mutation bacteria acquire to evolve phage resistance (depicted); and pleiotropic consequences, like those involved in a trade-off between two traits (depicted). Moreover, ecological and evolutionary effects of community context may affect each other through eco-evolutionary feedbacks. Filled arrows denote abstract interactions, while unfilled arrows denote changes through time.
These experiments comprise part of a larger trend to better incorporate complexity in our ecological and evolutionary understanding of biological systems. Broadly, there is growing interest in how species richness alters the ecology and evolution of community members [27–31]. On the microbial side, for instance, there is an emerging focus on how the evolution of bacteria is altered by the presence of other bacterial species (Table S1, reviewed in [22, 31, 32]). In parallel, recent efforts have sought to incorporate more realism into laboratory bacteria-phage experiments, including the effects of abiotic environment (e.g., [33–35]) and spatial structure (e.g., [21, 24, 36]). Finally, studies are increasingly showing that biological communities can be affected by bacteria-phage ecology and evolution (reviewed in [11, 37]). While we do not discuss these topics in detail, they similarly constitute efforts to more deeply understand multispecies microbial communities.
Effects of community context on focal populations’ ecology
The presence of other bacterial species can directly alter the density of the focal bacterial population or the focal phage population as well as modifying the interaction between the focal bacteria and phage populations.
Ecological effects on focal host density
Much of the existing empirical work has shown that community presence suppresses the density of the focal host bacterial species (Table 1, Box 1). For instance, Gómez and Buckling carried out experiments with Pseudomonas fluorescens strain SBW25, its associated phage phi2 (Φ2), and natural soil microbial communities with undetermined species content. They found that community presence depressed the density of P. fluorescens in the presence of phage, relative to no-community treatments [36]. Similarly, Johnke et al. used a wastewater model community to investigate the dynamics of multitrophic predator-prey systems, including one bacteria-phage pair [38]. Their data show that community presence had a significant negative effect on the density of the focal host Klebsiella sp. relative to community-absent treatments (linear model community presence-absence two-tailed contrast, intercepts p = 0.049 in absence of phage, p = 0.36 in presence of phage, slopes p = 0.83 in absence of phage, p < 0.01 in presence of phage, Fig S1, [39]). In a final example, Mumford and Friman investigated the dynamics of Pseudomonas aeruginosa, its associated phage PT7, and a community modeling burn or cystic fibrosis lung infections that includes Staphylococcus aureus and Stenotrophomonas maltophilia [40]. Although they measured microbial densities only at the end of experimental evolution, they found that the presence of any or all community members suppressed the focal host density relative to community-absent treatments (Fig S2). Interestingly, they found that a quorum sensing-deficient mutant of the focal host was less sensitive to community suppression than the wild-type, suggesting that this suppression by community presence may be partially mediated by density-dependent behavioral changes of the focal bacteria. Across all of these studies, findings that community presence suppresses focal host density are consistent with longstanding ecological theory on competitive release, where removal of competitors can enable a focal species to increase in density [41]. Moreover, because community presence drives smaller population sizes, it may have secondary effects, including increased susceptibility to ecological drift and stochastic extinction [42] as well as weakened natural selection, stronger genetic drift, and decreased mutation supply.
Table 1.
Ecological effects of community presence on focal bacteria-phage populations.
| Reference | Focal host – Phage | Community | Effect on focal host densitya | Effect on focal phage densitya | Effect on focal host-phage interactiona |
|---|---|---|---|---|---|
| [43] | P. aeruginosa – DMS3vir | S. aureus, B. cenocepacia, A. baumannii | – | ↓ as initial focal frequency was manipulated; ↔ in experimental evolution conditions | – |
| [50, 51] | E. coli – P10 | Murine gut community | – | – | – |
| [36, 46, 49] | P. fluorescens – phi2 (Φ2) | Soil communities | ↓ | ↓ | Effect of phage on focal host was: ↑ in absence of community; ↓ in presence of community |
| [38] | Klebsiella sp. – Klebsiella-phage | P. putida, Staphylococcus sp. | ↓ in the absence of phage ↔/↓ in the presence of phage | ↑ | Synergistic suppression by community and focal phage led to focal host extinction |
| [48] |
Pseudoalteromonas sp. #1 –Pseudoalteromonas sp. #1 phage; Pseudoalteromonas sp. #2 –Pseudoalteromonas sp. #2 phage; Photobacterium sp. – Photobacterium sp. phage; Vibrio sp. – Vibrio sp. phage |
All four species pairs were co-cultured | – | – | – |
| [40] | P. aeruginosa – PT7 | S. aureus, S. maltophilia | ↓ (at end of experimental evolution) | ↓ or ↔ (depending on focal host genotype, at end of experimental evolution) | Effect of phage on focal host at end of experimental evolution was ↓/↔/↑ depending on community membership and focal host genotype |
aThe “↓”, “↔”, and “↑” symbols denote when community presence suppressed, had no effect, or elevated the density of the focal population, respectively. The “–” symbol denotes when the study did not measure the effect of community context.
Box 1 Alternative tests of the ecological effects of community presence.
All the studies which have tested for the ecological effect of community context have done so by directly contrasting focal densities between community-added and community-absent treatments. This is essentially a test for the presence of interspecies bacterial competition. However, other statistical comparisons may provide additional insights into the degree of effect of community presence.
For instance, one simple hypothesis is that all bacterial species undergo equal pairwise competition, and thus community presence should reduce the focal population density proportional to the total number of species in co-culture. To assess the utility of this comparison, we used data from two previous studies [38, 40] and a Markov chain Monte Carlo (MCMC) approach to generate Bayesian posterior likelihood distributions of the mean densities of focal bacterial populations in the presence or absence of other community members [39, 57–61] (see Supplementary Methods). In both studies, the suppressive effects of community presence were larger than would be expected from equal competition. In Johnke et al.’s experiments, community presence suppressed focal host density only in the absence of phage (Table S2). In Mumford & Friman’s experiments, community presence suppressed focal host density more strongly when the focal host was wildtype strain PAO1 than when it was a quorum sensing-deficient mutant strain (Table S2).
We can apply a similar approach to phage density measures. If phage density is bottom-up limited, we would expect equal pairwise competition among bacterial species to reduce phage density proportional to the total number of bacterial species in co-culture. Using the same methods, we find that all of the likely suppressive effects from [38, 40] were larger than would be expected from equal competition (Table S3). In contrast, in Alseth et al.’s experiment, phage density in the presence of B. cenocepacia was not likely different from the community-absent treatment, but was suppressed less than we would have predicted from equal competition (Table S3 [43]).
Overall, our analyses suggest that testing experimental data against additional hypotheses can augment the insights we gain. Indeed, the approach used here can be employed to implement a variety of hypothesis tests, including the absence of competition, equal competition, species identity-dependent competition, and others. Widespread adoption of such analysis approaches will accelerate our understanding of the ecological effects of community presence.
Ecological effects on focal phage density
Similar to the shared pattern of suppression of focal bacterial density, community presence generally suppresses the density of the focal phage population (Table 1, Box 1). For instance, Gómez and Buckling’s work with soil communities showed that community presence decreased phage densities, relative to no-community treatments [36]. Similarly, Alseth et al. investigated the evolution of resistance to phage DMS3vir by P. aeruginosa, using a model infection community where S. aureus, Burkholderia cenocepacia, and Acinetobacter baumannii were also present [43]. Although no effect of community presence on phage density was observed during experimental evolution (linear model two-tailed contrasts against community-absent treatment, intercepts p > 0.42 for all four treatments, Fig S3, [39]), experimental manipulation of the initial frequency of the non-focal community showed a clear suppressive effect of community presence on phage density. Finally, in Mumford and Friman’s experiments with a model infection community, community presence also decreased focal phage density (Fig S2, [40]). However, this suppression of phage density was eliminated when the focal host was quorum sensing-deficient, possibly because this mutant host strain was itself less strongly suppressed by community presence. In contrast to those three studies, when Johnke et al. carried out similar experiments with a wastewater community they found that community presence actually increased phage density (linear model community present-absent two-tailed contrast, p = 0.012, Fig S1, [38, 39]). Excepting the findings of Johnke et al., these studies are generally consistent with phage density being bottom-up limited by the density of their host bacteria. However, to our knowledge none of the studies have verified this ecological mechanism, leaving open the possibility that community presence suppresses phage density through other processes. Regardless of the mechanism, smaller population sizes of both phages and their hosts are likely to make phages more prone to extinction events, potentially favoring alternative reproduction strategies like lysogeny or environmental durability [44, 45].
Effects on the ecological interaction between focal populations
Community presence can also alter the interaction between the focal bacterial and phage populations (Table 1). For instance, in their wastewater system, Johnke et al. observed that the effects of community presence and phage presence synergize: while individually each suppresses focal host density, when both are present together they drive the focal host population extinct (Fig S1, [38]). In other cases, community presence can reverse the effect of phage presence. For example, Gómez and Buckling found that phage suppressed the density of their host P. fluorescens in the presence of other soil bacterial species, but in the absence of the community phage-presence actually increased the density of the focal host [36, 46]. The exact mechanism driving this effect remains unexplained. Similarly, Mumford and Friman found that community presence altered the effect of phage, with the direction of change dependent on the community membership (Fig S2, [40]). In their infection community they found that phage addition in monocultures depressed host density, while phage addition in the presence of other bacterial species suppressed, had no effect, or even elevated focal host density, depending on competitor identity and whether the focal host was wild type or quorum sensing-deficient. The conflicting findings of these three studies suggest that much further work is warranted before we can understand how community presence and phage presence interact with each other in a generalizable way.
Effects of community context on focal species’ evolution
The presence of other bacterial species can also alter the evolution of one or both of the focal bacteria and phage populations by: altering the progression and dynamics of (co)evolutionary change, changing the mechanisms by which evolution proceeds, or modifying the pleiotropic consequences of adaptation.
Bacteria-phage (co)evolution
In response to one another, bacteria and phages can (co)evolve adaptations conferring changes in resistance and infectivity, respectively [47]. However, existing evidence is conflicted over how community context affects the nature and rate of such antagonistic (co)evolution (Table 2).
Table 2.
Evolutionary effects of community presence on focal bacteria-phage species.
| Reference | Focal host – Phage | Community | (Co)evolutiona | Evolutionary mechanismsa | Pleiotropya |
|---|---|---|---|---|---|
| [43] | P. aeruginosa – DMS3vir | S. aureus, B. cenocepacia, A. baumannii | – | Favored CRISPR-based resistance over surface mutation-based resistance | Resistance was associated with loss of virulence, whose cost was elevated by community presence |
| [50, 51] | E. coli – P10 | Murine gut community | Enabled phage host range expansion | – | Community presence enabled phage to traverse trade-off front |
| [36, 46, 49] | P. fluorescens – phi2 (Φ2) | Soil communities | No effect on coevolutionary dynamics (FSD vs ARD)c, local adaptation, rate of genomic evolution, host adaptive radiation; resistance to contemporary phages was elevated | – | No effect on cost of phage resistance |
| [38] | Klebsiella sp. – Klebsiella-phage | P. putida, Staphylococcus sp. | Constrained host evolution: host went extinct | – | Adaptation to community or phage presence separately resulted in higher growth rates |
| [48] |
Pseudoalteromonas sp. #1 – Pseudoalteromonas sp. #1 phage; Pseudoalteromonas sp. #2 – Pseudoalteromonas sp. #2 phage; Photobacterium sp. – Photobacterium sp. phage; Vibrio sp. – Vibrio sp. phage |
All four species pairs were co-cultured | No effectb | – | – |
| [40] | P. aeruginosa – PT7 | S. aureus, S. maltophilia | No effect on coevolutionary dynamics (FSD vs ARD)c; constrained evolution of resistance to phage | – | Focal host paid genotype-dependent cost to adapt to community and/or phage presence, with no interaction between the two costs |
aThe “–” symbol denotes when the study did not measure the effect of community context.
bCulture differences between treatments limit the strength of conclusions that can be drawn.
cCoevolutionary dynamics are often characterized qualitatively as Arms Race Dynamics (ARD) or Fluctuating Selection Dynamics (FSD) [62].
For instance, some studies have found that community presence constrains the (co)evolution of one or both of the focal species (Table 2). In Mumford and Friman’s experiments with an infection community, although community presence did not alter the qualitative coevolutionary dynamics, it did reduce the frequency of evolved resistance to both contemporary and ancestral phages [40]. However, this effect only occurred when the host was wild type and not quorum sensing-deficient, a difference that may be, in part, driven by genotype-dependent ecological effects (see ecology section). Similarly, Johnke et al. observe that community presence prevents coevolution: in the absence of the community, bacteria and phages evolve in a fluctuating selection dynamics pattern; in the presence of the community, the host bacteria go extinct [38]. These two experiments are consistent with the idea that community presence constrains the evolution of focal species, possibly by limiting available niche space, imposing new selective pressures, or driving otherwise-unexperienced trade-offs between biotic and abiotic pressures [31].
In contrast, others have found that community presence has little effect on (co)evolutionary dynamics (Table 2). For instance, with four marine bacteria-phage pairs, Middelboe et al. observed the evolution of complete resistance to phage infection regardless of community context [48]. Similarly, Gómez and Buckling tested the effect of soil community presence on bacteria-phage (co)evolution. Although their resistance data were suggestive of community presence accelerating coevolution, they measured neither an effect of community presence on the rate of genomic evolution, the type of coevolutionary dynamics, the degree of local adaptation by phages, nor any interaction with phage presence in affecting focal host adaptive radiation [36, 46, 49]. These studies show that community presence can sometimes have little effect on focal bacteria-phage coevolution.
In one final example, community presence actually enabled greater evolution in the focal species. De Sordi et al. investigated the evolution of Escherichia coli phage P10 along with two strains of E. coli—one host and one nonhost [50, 51]. After inoculating all three in vitro, in germ-free mice guts, and in conventional mice guts (with an intact microbiome), they found that only the conventional mouse gut environment enabled the phage to evolve infectivity for the nonhost E. coli strain. They went on to show that a third strain of E. coli, present only in the conventional mouse gut, acted as an eco-evolutionary bridge enabling the phage to adapt to infect the nonhost E. coli strain. Thus, in this experiment, community presence accelerated the evolution of phage host-range shifts and expansion by enabling new coevolutionary trajectories.
Overall, community presence can retard, have no effect, or in rare cases accelerate bacteria-phage (co)evolution. Many factors could drive these differences, although experimental conditions like bottleneck size and evolutionary timescale appear not to drive the patterns observed here (Table 3). Future work is necessary to identify the determinants of community context-driven changes in (co)evolution.
Table 3.
Evolutionary timescale and bottleneck size in experimental studies.
| Reference | Focal host-Phage | Design | N0 (cfu) | N1 (cfu) | D | n (number of transfers) | t (duration) | Number of generations |
|---|---|---|---|---|---|---|---|---|
| [43] | P. aeruginosa – DMS3vir | Batch | 3.3 × 106 | ~2 × 107 | 100 | 2 | 3 days | ~15.9 |
| [50, 51] | E. coli – P10 (in vitro only) | Batch | 108 | – | 10 | 3 | 24 days | ~13.3 |
| [36, 46, 49] | P. fluorescens – phi2 (Φ2) | Batch | 106.75 | – | – | 0 | 48 days | – |
| [40] | P. aeruginosa – PT7 | Batch | 3.8 × 105 | – | 7 | 4 | 16 days | ~11.2 |
| [38] | Klebsiella sp. – Klebsiella-phage | Cont. | 8.7 × 106 | 3.0 × 108 | 0.1 | – | 3 days | ~5.4 |
| [48] | Pseudoalteromonas sp. #1 – Pseudoalteromonas sp. #1 phage; | Cont. | ~104.9 | ~106 | 1 | – | 9.375 days | ~13.0 |
| Pseudoalteromonas sp. #2 – Pseudoalteromonas sp. #2 phage; | ~104.5 | ~105.9 | ~14.0 | |||||
| Photobacterium sp. – Photobacterium sp. phage; | ~104.25 | ~104 | ~9.4 | |||||
| Vibrio sp. – Vibrio sp. phage | ~104.5 | ~105.8 | ~13.7 |
Details on reviewed experiments are listed here, including the type of experiment (batch or continuous), size of the initial inoculum population (N0), the size of the population before the first transfer (for batch culture) or at peak (for continuous culture) (N1), the dilution per transfer (for batch culture) or per day (for continuous culture) (D), the number of transfers (for batch culture only) (n), and the total duration of the experiment (t). N1 was estimated from data (Figs. S1, S3) or visually from published figures. The number of bacterial generations in each experiment was estimated as: batch culture generations = log2(N1/N0) + n × log2(D), or continuous culture generations = log2(N1/N0) + t × D. When N1 was unavailable for batch culture experiments, it was assumed growth from inoculum to first transfer was equivalent to subsequent dilutions, i.e., number of generations = (n + 1) × log2(D).
Evolutionary mechanisms
Community presence can also affect the mechanisms by which focal bacteria and phage populations tend to evolve. For instance, Alseth et al. showed that community presence can alter the ways that bacteria evolve resistance to phage infection [43]. In their model infection community, they found that the relative fitness of two resistance mechanisms was altered by community context: in the presence of other species, P. aeruginosa primarily evolved CRISPR-based resistance; in the absence of other species, P. aeruginosa primarily evolved surface mutation-based resistance. While the exact mechanism remains unknown, it may be related to prior work in the system finding cooperative phage anti-CRISPR systems [52] and nutrient-dependent evolution of resistance [53]. Regardless, to our knowledge, this is the first study to show how community context can alter the fitness landscape of bacterial resistance to phage, so more experiments are necessary to test how community presence can affect the evolution of other resistance mechanisms [54, 55].
Pleiotropy
In bacteria-phage communities, (co)evolution can drive changes beyond the resistance or infectivity phenotype. Such examples of pleiotropy may exist as an evolved trade-up, when the secondary effect is beneficial, or as an evolved trade-off, when the secondary effect is deleterious (a “cost” to evolution). Both trade-offs and trade-ups can have important effects, from competitive ability to virulence [56].
Some existing work has clearly documented community context interacting with the trade-offs and trade-ups faced by focal bacteria. For instance, Alseth et al. showed that community presence drove focal bacteria to escape a trade-off between phage resistance and virulence because community presence favored resistance mechanisms that did not trade-off with virulence (see evolutionary mechanisms section) [43]. On the other hand, Johnke et al. documented trade-ups associated with community and phage presence [38]. In their experiments, focal host Klebsiella sp. evolved a higher growth rate in treatments with phage or community presence alone, but was driven extinct in the treatment with both phage and community presence.
In contrast to these studies, others have failed to find evidence that community context interacts with the trade-offs and trade-ups faced by focal species. For instance, Gómez and Buckling found that the presence of a microbial soil community had no effect on the cost of evolved resistance to phage [36]. Similarly, Mumford and Friman found that focal P. aeruginosa paid a genotype-dependent cost to adapt to phages or bacterial competitors, but that these costs were independent and did not interact [40]. More work is needed to determine how the pleiotropic effects of adaptation to antagonists and community presence generally interact, especially across diverse systems whose molecular details differ.
Concluding remarks
There is a long history of studying the ecology and evolution of bacteria-phage communities, and the studies reviewed here have highlighted the importance of biotic community context as a key factor. Generally, these experiments have found community presence to have consistent ecological effects but divergent evolutionary effects. In part, this could arise from differences in how ecology and evolution have been measured: while ecological dynamics have often been measured similarly, evolutionary dynamics have been assessed with a diversity of metrics. At the same time, the molecular details specific to each experiment may be more important for evolution than ecology. Further work, especially using similar approaches across systems, is necessary to resolve this difference.
To our knowledge, the nine studies reviewed here are the only published experiments to manipulate bacterial community context while measuring the ecological or evolutionary dynamics of bacteria-phage populations. Given the dearth of studies, many questions remain unanswered (Box 2), but two limitations of the existing experiments particularly stand out. First, many of these studies have been conducted in communities with 3 or 4 bacterial species. More speciose communities are likely to more strongly suppress focal species densities and constrain focal coevolution, more closely resembling natural communities [31]. Thus, observing the ecological and evolutionary effects of species-rich communities is an important avenue for future work. Second, nearly all of these studies have observed dynamics over short timescales, typically 10–15 generations (Table 3). Given this, it’s likely that much of the observed evolutionary change is the result of selection for phage resistance in the focal host. It remains to be seen what ecological and evolutionary dynamics emerge over longer timescales in multispecies bacteria-phage communities.
Just as future experiments must expand the scope of approaches and ask novel questions, there is also a need for research to coalesce conceptually and methodologically (Fig S4). Ultimately, only by determining the effects of community context on bacteria-phage communities can we fully understand these important systems, both as laboratory models and for their central roles in the natural world and human health.
Box 2 Outstanding questions.
How does community presence interact with the ecological effects of phage presence? We might predict independent effects of community and phage presence, but existing findings have observed a variety of interactions.
How does community presence affect the evolution of bacterial resistance to phage? Prior work has shown that community presence can favor CRISPR-based resistance, but how community presence affects numerous other resistance mechanisms remains untested.
How does community presence affect phage host-range evolution? Phages can evolve to overcome host resistance or to shift their host range. Some experiments have found that community presence may facilitate host-range expansion.
Does community presence accelerate or decelerate (co)evolution of focal species? Community presence could accelerate evolution by creating ecological opportunities or decelerate evolution by imposing constraints. Existing evidence has often found no effect or a decelerating effect.
How does community presence affect coevolutionary dynamics? Coevolutionary dynamics are often qualitatively categorized (e.g., arms race; fluctuating selection). Existing work has found no effect of community presence on these dynamics.
What are the null expectations for how community presence should alter bacteria-phage evolution? Conceptual and theoretical advances are needed to establish null expectations for how community presence should affect the evolution of focal bacteria-phage species.
How do findings scale to communities with multiple phage hosts? Communities with multiple phage hosts, like multiple host-phage pairs or single phages with multiple hosts, could act as models of macro-parasite ecology.
How do existing findings scale to more species-rich communities? The results reviewed here are largely in the context of communities with 3–5 bacterial species, which may or may not follow the same patterns and rules as more (or less) diverse communities.
How do findings change over longer timescales? The results reviewed here are largely drawn from experiments lasting just 10–15 evolutionary generations. Over such short timescales ecological dynamics may still be stabilizing, and some evolutionary dynamics may have yet to emerge.
How do ecology and evolution interact in multispecies bacteria-phage communities? Ecological and evolutionary processes can occur on similar timescales. Future experiments should pursue approaches that enable characterization of eco-evolutionary feedbacks.
Supplementary information
Acknowledgements
We thank the authors of [38,43] for their willingness to share their data, and the authors of [40] for making their data publicly available. Three anonymous reviewers provided valuable comments on this study. Alita Burmeister provided feedback on drafts of the paper, and the members of the Turner lab provided feedback on the paper ideas. Our work was supported by NSF Cooperative Agreement DBI-0939454 through the BEACON Center for the Study of Evolution in Action, and by NIH Grant #R21AI144345 from the National Institute of Allergy and Infectious Diseases.
Compliance with ethical standards
Conflict of interest
MB declares no competing interests. PET is a co-founder of Felix Biotechnology Inc., and declares a financial interest in this company that seeks to commercially develop phages for use as therapeutics.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41396-021-01012-x.
References
- 1.Crick FHC, Barnett FRSL, Brenner S, Watts-Tobin RJ. General Nature of the Genetic Code for Proteins. Nature. 1961;192:1227–32. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- 2.Hershey AD, Chase M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952;36:39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luria S, Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kortright KE, Chan BK, Koff JL, Turner PE. Phage Therapy: a Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe. 2019;25:219–32. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Mushegian AR. Are there 10^31 virus particles on Earth, or more, or less? J Bacteriol. 2020;202:e00052–20. doi: 10.1128/JB.00052-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennehy JJ. What Can Phages Tell Us about Host-Pathogen Coevolution? Int J Evol Biol. 2012;2012:1–12. doi: 10.1155/2012/396165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jessup CM, Kassen R, Forde SE, Kerr B, Buckling A, Rainey PB, et al. Big questions, small worlds: microbial model systems in ecology. Trends Ecol Evol. 2004;19:189–97. doi: 10.1016/j.tree.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Tecon R, Mitri S, Ciccarese D, Or D, Meer JR, van der, Johnson DR. Bridging the Holistic-Reductionist Divide in Microbial Ecology. MSystems. 2019;4:e00265–18. doi: 10.1128/mSystems.00265-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannan BJM, Lenski RE. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol Lett. 2000;3:362–77. doi: 10.1046/j.1461-0248.2000.00161.x. [DOI] [Google Scholar]
- 10.Buckling A, Brockhurst MA. Bacteria-Virus Coevolution. In: Orkun S Soyer, editor. Evolutionary Systems Biology. 2012. New York, NY: Springer; 2012. p. 347–70. [DOI] [PubMed]
- 11.Koskella B, Brockhurst MA. Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol Rev. 2014;38:1–16. doi: 10.1111/1574-6976.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Sordi L, Lourenço M, Debarbieux L. The Battle Within: interactions of Bacteriophages and Bacteria in the Gastrointestinal Tract. Cell Host Microbe. 2019;25:210–8. doi: 10.1016/j.chom.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Scanlan PD. Bacteria–Bacteriophage Coevolution in the Human Gut: implications for Microbial Diversity and Functionality. Trends Microbiol. 2017;25:614–23. doi: 10.1016/j.tim.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Breitbart M. Marine viruses: truth or dare. Annu Rev Mar Sci. 2012;4:425–48. doi: 10.1146/annurev-marine-120709-142805. [DOI] [PubMed] [Google Scholar]
- 15.Pratama AA, van Elsas JD. The ‘neglected’ soil virome–potential role and impact. Trends Microbiol. 2018;26:649–62. doi: 10.1016/j.tim.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Lourenço M, De Sordi L, Debarbieux L. The diversity of bacterial lifestyles hampers bacteriophage tenacity. Viruses. 2018;10:1–11. doi: 10.3390/v10060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martiny JBH, Riemann L, Marston MF, Middelboe M. Antagonistic Coevolution of Marine Planktonic Viruses and Their Hosts. Annu Rev Mar Sci. 2014;6:393–414. doi: 10.1146/annurev-marine-010213-135108. [DOI] [PubMed] [Google Scholar]
- 18.Díaz-Muñoz SL, Koskella B. Bacteria–Phage Interactions in Natural Environments. In: Sariaslani S, Gadd GM, editors. Advances in Applied Microbiology. Cambridge, MA:Academic Press; 2014. p.135–83. [DOI] [PubMed]
- 19.Avrani S, Schwartz DA, Lindell D. Virus-host swinging party in the oceans. Mob Genet Elem. 2012;2:88–95. doi: 10.4161/mge.20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter C, Bouvier T, Weinbauer MG, Thingstad TF. Trade-Offs between Competition and Defense Specialists among Unicellular Planktonic Organisms: the “Killing the Winner” Hypothesis Revisited. Microbiol Mol Biol Rev. 2010;74:42–57. doi: 10.1128/MMBR.00034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen MF, Svenningsen SL, Røder HL, Middelboe M, Burmølle M. Big Impact of the Tiny: bacteriophage–bacteria Interactions in Biofilms. Trends Microbiol. 2019;27:739–52. doi: 10.1016/j.tim.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien S, Hodgson DJ, Buckling A. The interplay between microevolution and community structure in microbial populations. Curr Opin Biotechnol. 2013;24:821–5. doi: 10.1016/j.copbio.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Brockhurst MA, Koskella B. Experimental coevolution of species interactions. Trends Ecol Evol. 2013;28:367–75. doi: 10.1016/j.tree.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Geredew Kifelew L, Mitchell JG, Speck P. Mini-review: efficacy of lytic bacteriophages on multispecies biofilms. Biofouling. 2019;35:472–81. doi: 10.1080/08927014.2019.1613525. [DOI] [PubMed] [Google Scholar]
- 25.Miki T, Jacquet S. Complex interactions in the microbial world: Underexplored key links between viruses, bacteria and protozoan grazers in aquatic environments. Aquat Micro Ecol. 2008;51:195–208. doi: 10.3354/ame01190. [DOI] [Google Scholar]
- 26.Johnke J, Cohen Y, de Leeuw M, Kushmaro A, Jurkevitch E, Chatzinotas A. Multiple micro-predators controlling bacterial communities in the environment. Curr Opin Biotechnol. 2014;27:185–90. doi: 10.1016/j.copbio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Hall AR, Ashby B, Bascompte J, King KC. Measuring Coevolutionary Dynamics in Species-Rich Communities. Trends Ecol Evol. 2020;35:539–50. doi: 10.1016/j.tree.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Strauss SY. Ecological and evolutionary responses in complex communities: implications for invasions and eco-evolutionary feedbacks. Oikos. 2014;123:257–66. doi: 10.1111/j.1600-0706.2013.01093.x. [DOI] [Google Scholar]
- 29.Strauss SY, Irwin RE. Ecological and evolutionary consequences of multispecies plant-animal interactions. Annu Rev Ecol Evol Syst. 2004;35:435–66. doi: 10.1146/annurev.ecolsys.35.112202.130215. [DOI] [Google Scholar]
- 30.Inouye B, Stinchcombe JR. Relationships between ecological interaction modifications and diffuse coevolution: similarities, differences, and causal links. Oikos. 2011;95:353–60. doi: 10.1034/j.1600-0706.2001.950218.x. [DOI] [Google Scholar]
- 31.Barraclough TG. How Do Species Interactions Affect Evolutionary Dynamics Across Whole Communities? Annu Rev Ecol Evol Syst. 2015;46:25–48. doi: 10.1146/annurev-ecolsys-112414-054030. [DOI] [Google Scholar]
- 32.Bottery MJ, Pitchford JW, Friman V-P. Ecology and evolution of antimicrobial resistance in bacterial communities. ISME J. 2021;15:939–48. doi: 10.1038/s41396-020-00832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gómez P, Bennie J, Gaston KJ, Buckling A. The Impact of Resource Availability on Bacterial Resistance to Phages in Soil. PLoS ONE. 2015;10:e0123752. doi: 10.1371/journal.pone.0123752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorter FA, Scanlan PD, Buckling A. Adaptation to abiotic conditions drives local adaptation in bacteria and viruses coevolving in heterogeneous environments. Biol Lett. 2016;12:20150879. doi: 10.1098/rsbl.2015.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scanlan JG, Hall AR, Scanlan PD. Impact of bile salts on coevolutionary dynamics between the gut bacterium Escherichia coli and its lytic phage PP01. Infect Genet Evol. 2019;73:425–32. doi: 10.1016/j.meegid.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Gómez P, Buckling A. Bacteria-phage antagonistic coevolution in soil. Science. 2011;332:106–9. doi: 10.1126/science.1198767. [DOI] [PubMed] [Google Scholar]
- 37.Weinbauer MG, Rassoulzadegan F. Are viruses driving microbial diversification and diversity? Environ Microbiol. 2004;6:1–11. doi: 10.1046/j.1462-2920.2003.00539.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnke J, Baron M, de Leeuw M, Kushmaro A, Jurkevitch E, Harms H, et al. A generalist protist predator enables coexistence in multitrophic predator-prey systems containing a phage and the bacterial predator Bdellovibrio. Front Ecol Evol. 2017;5:1–12. doi: 10.3389/fevo.2017.00124. [DOI] [Google Scholar]
- 39.R Core Team. R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
- 40.Mumford R, Friman VP. Bacterial competition and quorum-sensing signalling shape the eco-evolutionary outcomes of model in vitro phage therapy. Evol Appl. 2017;10:161–9. doi: 10.1111/eva.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connell JH. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology. 1961;42:710–23. doi: 10.2307/1933500. [DOI] [Google Scholar]
- 42.Vellend M. Conceptual Synthesis in Community Ecology. Q Rev Biol. 2010;85:183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- 43.Alseth EO, Pursey E, Lujan AM, McLeod I, Rollie C, Westra ER. Bacterial biodiversity drives the evolution of CRISPR-based phage resistance in Pseudomonas aeruginosa. Nature. 2019;574:549–74. doi: 10.1038/s41586-019-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldhill DH, Turner PE. The evolution of life history trade-offs in viruses. Curr Opin Virol. 2014;8:79–84. doi: 10.1016/j.coviro.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Keen EC. Tradeoffs in bacteriophage life histories. Bacteriophage. 2014;4:e28365. doi: 10.4161/bact.28365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gómez P, Buckling A. Real-time microbial adaptive diversification in soil. Ecol Lett. 2013;16:650–5. doi: 10.1111/ele.12093. [DOI] [PubMed] [Google Scholar]
- 47.Houte S, van, Buckling A, Westra ER. Evolutionary Ecology of Prokaryotic Immune Mechanisms. Microbiol Mol Biol Rev. 2016;80:745–63. doi: 10.1128/MMBR.00011-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Middelboe M, Hagström A, Blackburn N, Sinn B, Fischer U, Borch NH, et al. Effects of bacteriophages on the population dynamics of four strains of pelagic marine bacteria. Micro Ecol. 2001;42:395–406. doi: 10.1007/s00248-001-0012-1. [DOI] [PubMed] [Google Scholar]
- 49.Gómez P, Buckling A. Coevolution with phages does not influence the evolution of bacterial mutation rates in soil. ISME J. 2013;7:2242–4. doi: 10.1038/ismej.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Sordi L, Khanna V, Debarbieux L. The Gut Microbiota Facilitates Drifts in the Genetic Diversity and Infectivity of Bacterial Viruses. Cell Host Microbe. 2017;22:801–8.e3. doi: 10.1016/j.chom.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 51.De Sordi L, Lourenço M, Debarbieux L. “I will survive”: A tale of bacteriophage-bacteria coevolution in the gut. Gut Microbes. 2019;10:92–9. doi: 10.1080/19490976.2018.1474322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landsberger M, Gandon S, Meaden S, Chabas H, Buckling A, Westra ER, et al. Anti-CRISPR phages cooperate to overcome CRISPR-Cas immunity. Cell. 2018;174:908–16. doi: 10.1016/j.cell.2018.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westra ER, van Houte S, Oyesiku-Blakemore S, Makin B, Broniewski JM, Best A, et al. Parasite exposure drives selective evolution of constitutive versus inducible defense. Curr Biol. 2015;25:1043–9. doi: 10.1016/j.cub.2015.01.065. [DOI] [PubMed] [Google Scholar]
- 54.Dy RL, Richter C, Salmond GP, Fineran PC. Remarkable mechanisms in microbes to resist phage infections. Annu Rev Virol. 2014;1:307–31. doi: 10.1146/annurev-virology-031413-085500. [DOI] [PubMed] [Google Scholar]
- 55.Rostøl JT, Marraffini L. (Ph)ighting phages: how bacteria resist their parasites. Cell Host Microbe. 2019;25:184–94. doi: 10.1016/j.chom.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burmeister AR, Turner PE. Trading-off and trading-up in the world of bacteria–phage evolution. Curr Biol. 2020;30:R1120–R1124. doi: 10.1016/j.cub.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plummer M. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. Vienna, Austria: Proc. 3rd Int. Workshop Distrib. Stat. Comput; 2003. p. 1–10.
- 58.Wickham H. ggplot2: elegant Graphics for Data Analysis. Verlag New York: Springer; 2016.
- 59.Wickham H. tidyr: Tidy Messy Data. 2020.
- 60.Plummer M. rjags: Bayesian Graphical Models using MCMC. 2019.
- 61.Wickham H, François R, Henry L, Müller K. dplyr: A Grammar of Data Manipulation. 2020.
- 62.GANDON S, BUCKLING A, DECAESTECKER E, DAY T. Host-parasite coevolution and patterns of adaptation across time and space. J Evol Biol. 2008;21:1861–6. doi: 10.1111/j.1420-9101.2008.01598.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.