Abstract
Objective
To provide a systematic review about the efficacy and safety of romosozumab and teriparatide for the treatment of postmenopausal osteoporosis.
Method
Randomized controlled trials (RCTs) were searched from electronic databases, including PubMed (1996 to June 2019), Embase (1980 to June 2019), Cochrane Library (CENTRAL, June 2019), Web of Science (1998 to June 2019), and others. The primary outcomes included the following: the percentage change in bone mineral density of lumbar spine and total hip from baseline at month 6 and month 12 in each group. The secondary outcomes included the following: the percentage change in bone mineral density of femoral neck from baseline at month 6 and month 12 in each group and the incidence of adverse events at month 12 in each group.
Results
Four studies containing 1304 patients met our selection criteria. The result of our analysis indicated that romosozumab showed better effects in improving BMD of lumbar spine (month 6: MD = 3.54, 95% CI [3.13, 3.94], P<0.001; month 12: MD = 4.93, 95% CI [4.21, 5.64], P<0.001), total hip (month 6: MD = 2.27, 95% CI [0.62, 3.91], P = 0.007; month 12: MD = 3.17, 95% CI [2.68, 3.65], P<0.001), and femoral neck (month 6: MD = 2.30, 95% CI [0.51, 4.08], P = 0.01; month 12: MD = 3.04, 95% CI [2.29, 3.78], P<0.001). Also, the injection‐site reaction was less (month 12: RR = 2.84, 95% CI [1.22, 6.59], P = 0.02), but there were no significant difference in the incidence of serious adverse events (month 12: RR = 0.78, 95% CI [0.46, 1.33], P = 0.37) and death (month 12: RR = 0.61, 95% CI [0.08, 4.62], P = 0.63).
Conclusion
Based on the available studies, our current results demonstrate that romosozumab was better than teriparatide both in terms of efficacy and side effects.
Keywords: Postmenopausal osteoporosis, Randomized controlled trials, Romosozumab, Systematic review, Teriparatide
To provide a systematic review about the efficacy and safety of romosozumab and teriparatide for the treatment of postmenopausal osteoporosis, Randomized controlled trials were searched from electronic database and then articles were screened according to inclusion and exclusion criteria. Risk of bias were assessmented, and statistical analysis and data synthesis were conducted with GRADE system to evaluate the level of the evidence. Our results demonstrate that romosozumab was better than teriparatide both in terms of efficacy and side effects.

Introduction
Postmenopausal osteoporosis is identified as a systemic skeletal disorder characterized by low bone mineral density (BMD) and qualitative changes in microarchitecture of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture 1 . In elderly patients, osteoporotic fracture (fragility fracture) is a catastrophic complication, which causes substantial morbidity and mortality 2 . This fracture often occurs in the spine, hip, and wrist, but also affects other bones, such as the humerus and radius 3 . Drugs for postmenopausal osteoporosis fall into two major categories, antiresorptive drugs and osteoanabolic drugs. Antiresorptive drugs for postmenopausal osteoporosis increase those for bone mineral density and prevent the progression of structural damage but may not restore bone structure 4 . However, osteoanabolic drugs can reverse microarchitectural deterioration of bone tissue and seem to be better. For postmenopausal osteoporosis treatment, the classic drug bisphosphonate represents the vast majority of prescriptions, and is a conventional drug. However, long‐term use of bisphosphonates may cause atypical fractures, and intravenous use of bisphosphonates may cause osteonecrosis of the jaw. Teriparatide (brand name FOTTEOTM), an N‐terminal (1–34) fragment of human parathyroid hormone, was the first osteoanabolic drug approved by the Food and Drug Administration in 2003 9 , 10 . It can significantly improve BMD. However, patients must inject this drug once each day in their thigh or abdomen. Besides, after teriparatide is discontinued, its benefits are quickly lost 11 . What's worse, a study of the Forteo Patient Registry (FPR) anticipated that they will be able to detect a fourfold increase in the risk of osteosarcoma if one exists by 2024 12 .
Sclerostin, a glycoprotein produced primarily by osteocytes, is encoded by the SOST gene, which can specifically block the canonical Wnt signaling 13 , 14 . This pathway plays a pivotal role in promoting bone formation and regulating bone homeostasis 15 . Sclerostin increases the expression of RANKL and decreases that of OPG, resulting in bone absorption 16 , 17 . Romosozumab, a humanized monoclonal anti‐sclerostin antibody, is a new osteoanabolic drug that inhibits sclerostin with a dual effect on bone, increasing bone formation and decreasing bone resorption 4 .
However, the efficacy and safety of this new drug are not well‐documented. Therefore, we conducted a systematic review and meta‐analysis of randomized controlled trials (RCTs) of romosozumab and teriparatide to fully evaluate their effects in postmenopausal osteoporosis patients.
Materials and Methods
Search Strategy
The electronic databases PubMed, Embase, the Cochrane Library, Web of Science, and the Cochrane Controlled Trials Register were searched up to June 2019. The search terms were as follows: ([AMG 785 OR evenity OR romosozumab OT CDP 7851] AND hPTH [1–34] OR Human Parathyroid Hormone [1–34] OR Parathar OR Forteo) AND (postmenopausal osteoporosis OR Postmenopausal Bone Loss). The Flow chart of the trial selection process was presented in Fig. 1. We also used the PRISMA guidelines 18 , GRADE system 19 , and Cochrane Handbook 20 to assess the quality of the included studies to make sure the data were reliable and veritable.
Fig. 1.
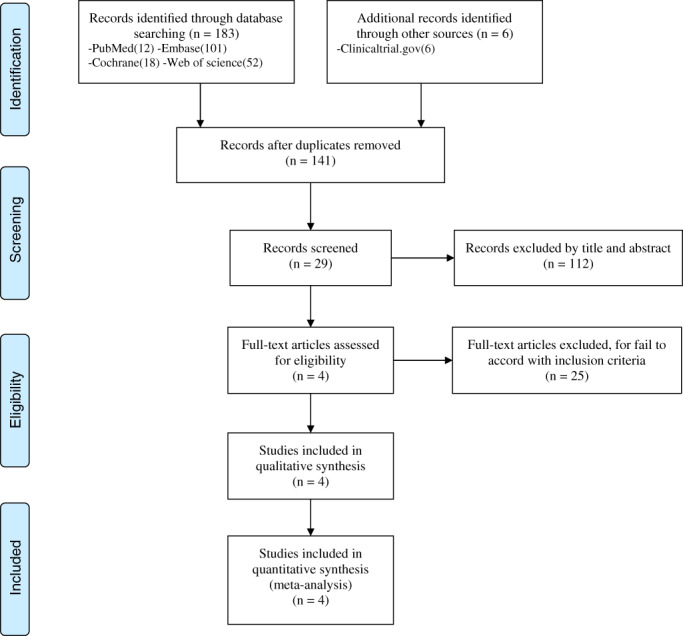
Flow chart of database searching, records screening, assessment of full‐text articles, and article inclusion.
Selection Criteria
Trials were included on conditions that they met the PICOS (population, intervention, comparator, outcome, study design) criteria.
-
(i)
Population: Female patients with postmenopausal osteoporosis.
-
(ii)
Intervention: Romosozumab.
-
(iii)
Comparator: Teriparatide.
-
(iv)
Outcomes: The primary outcomes included the following: the percentage change in bone mineral density of lumbar spine and total hip from baseline at month 6 and month 12 in each group. The secondary outcomes contained the following: the percentage change in bone mineral density of femoral neck from baseline at month 6 and month 12 in each group and the incidence of adverse events at month 12 in each group.
-
(v)
Study design: RCT.
Exclusion criteria were as follows: (i) non‐RCTs carried out in individuals with other disorders likely to affect bone and calcium metabolism (such as chronic kidney disease, pregnancy, and glucocorticoid use) or conducted in specific populations that might have a different risk of cardiovascular (CV) events (patients with cancer, transplant, or human immunodeficiency virus infection, or children); (ii) studies with duration less than 6 months; (iii) studies with zero CV events or without safety data published.
Data Extraction
A standard data extraction form was used to collect the relevant data from included studies. Two reviewers collected available data from included studies independently, and any disagreement between the two reviewers was judged by a third reviewer. The relevant data included authors, published dates, intervention types, age, sample size, outcomes, duration of follow‐up, and reference type. Baseline characteristics of included trials were presented in Table 1. Data on BMD (a T score of −2.0 or less at the lumbar spine, total hip, or femoral neck and −3.5 or more at each of the three sites) were obtained from the data presented in tables or figures if no direct data were available from the article text.
TABLE 1.
Baseline characteristics of included randomized controlled trials
| Study (year) | Intervention | Age (years, mean ± SD) | Number of patients with LS BMD | Number of patients with TH BMD | Number of patients with FN BMD | Outcomes | Follow‐up (months) | Reference type |
|---|---|---|---|---|---|---|---|---|
| Genant et al. 2017 21 |
Romosozumab 210 mg per month Teriparatide 20 μg per day |
64.3 ± 4.7 65.8 ± 5.7 |
24 30 |
9 19 |
— — |
BMD changes at LS, TH, FN; incidence of AEs |
12 | RCT |
| Keaveny et al. 2017 22 |
Romosozumab 210 mg per month Teriparatide 20 μg per day |
64.3 ± 4.7 65.8 ± 5.7 |
24 28 |
9 19 |
— — |
BMD changes at LS, TH, FN; incidence of AEs |
12 | RCT |
| Langdahl et al. 2017 23 |
Romosozumab 210 mg per month Teriparatide 20 μg per day |
71.8 ± 7.4 71.2 ± 7.7 |
206 209 |
206 209 |
206 209 |
BMD changes at LS, TH, FN; incidence of AEs |
12 | RCT |
| McClung et al. 2014 4 |
Romosozumab 210 mg per month Teriparatide 20 μg per day |
66.3 ± 6.5 66.8 ± 5.7 |
49 46 |
49 46 |
49 46 |
BMD changes at LS, TH, FN; incidence of AEs |
12 | RCT |
AEs, Adverse effect; BMD, Bone mineral density; FN, Femoral neck; LS, Lumber spine; RCT, randomized controlled trial; SD, Standard Deviation; TH, Total hip.
Risk of Bias Assessment
According to the Cochrane Handbook for Systematic Reviews of Interventions 20 , the methodological quality and basis of the included literature were assessed as follows: randomization, allocation concealment, blind method, selective reporting, incomplete outcome data, and other bias (Figs 2 and 3).
Fig. 2.
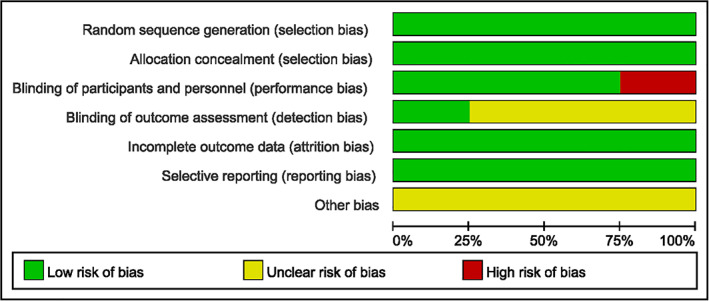
Risk of bias graph according to the Cochrane Handbook for Systematic Reviews of Interventions.
Fig. 3.
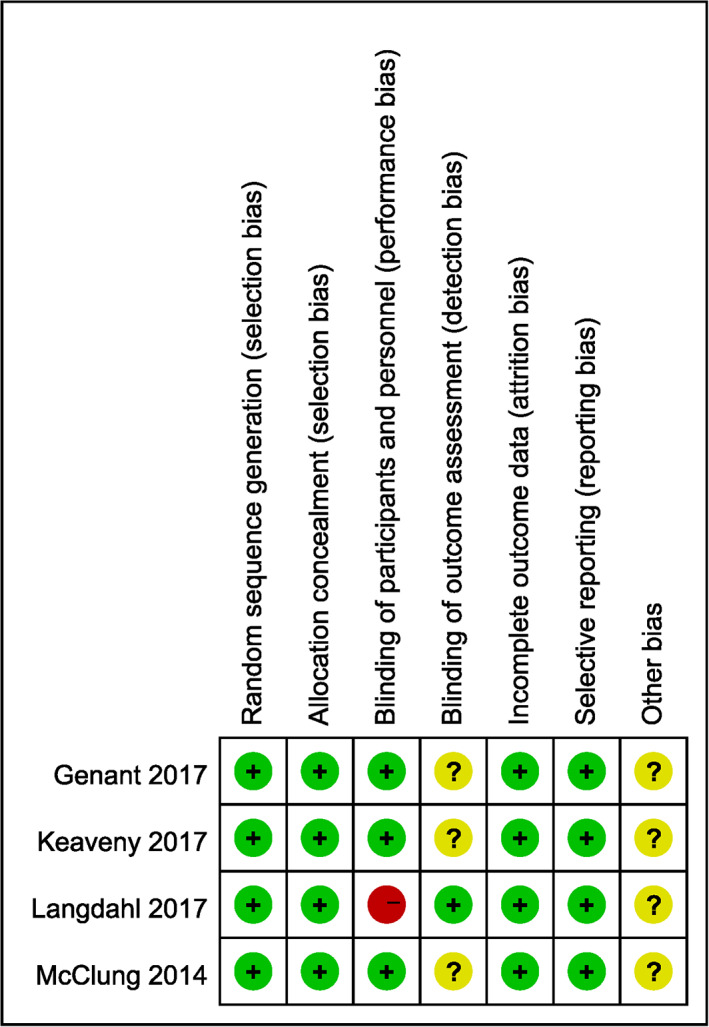
Risk of bias summary according to the Cochrane Handbook for Systematic Reviews of Interventions.
Grading Quality of Evidence
We used the GRADE system to evaluate the level of the evidence and strength of recommendations for included outcomes. GRADE software was used to evaluate the evidence of included outcomes. Initially, RCTs were considered as high confidence in an estimate of effect and cohort studies were considered as low confidence in an estimate of effect. Reasons that might decrease the level of confidence include limitations, inconsistency, indirectness, imprecision, and publication bias. Reasons that might raise the level of confidence include large effect, plausible confounding, dose‐response. The GRADE evidence was divided into the following categories: (i) high‐quality evidence, which indicated that further research was unlikely to change the confidence in an estimate of effect; (ii) moderate‐quality evidence, which indicated that further research was likely to have an important impact on confidence in an estimate of effect and may change the estimate; (iii) low‐quality evidence, which indicated that further research was likely to have an important impact on confidence in an estimate of effect and was likely to change the estimate; and (v) very low‐quality evidence, which indicated that we were very uncertain about the results. The results of the GRADE analysis were presented in Table 2.
TABLE 2.
The GRADE evidence quality for each outcome
| No of studies | Design | Decrease quality of evidence | Increase quality of evidence | Quality | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Large effect | Plausible confounding | Does‐response | ||||
|
Lumbar spine month 6 |
RCT | No | Serious | No | No | Likely | Large | No | No | Moderate | Critical |
|
Lumbar spine month 12 |
RCT | No | Serious | No | No | Likely | Large | No | No | Moderate | Critical |
| Total hip month 6 | RCT | No | Very serious | No | No | Likely | Large | No | No | Low | Important |
| Total hip month 12 | RCT | No | Serious | No | No | Likely | Large | No | No | Moderate | Critical |
| Femoral neck month 6 | RCT | No | Very serious | No | No | Likely | Large | No | No | Low | Important |
| Femoral neck month 12 | RCT | No | Very serious | No | No | Likely | Large | No | No |
Low |
Important |
| Incidence of SAEs | RCT | No | No | No | No | Likely | No | No | No | Moderate | Critical |
| Incidence of death | RCT | No | No | No | No | Likely | No | No | No | Moderate | Critical |
| Incidence of Injection‐site reaction | RCT | No | No | No | No | Likely | Large | No | No | High | Critical |
RCT, randomized controlled trial; High quality—further research is very unlikely to change our confidence in the estimate of effect; Moderate quality—further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality—further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality—we are very uncertain about the estimate.
Statistical Analysis and Data Synthesis
Meta‐analyses were performed with Review Manager Software for Windows (version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The mean difference (MD) was used to assess continuous outcomes in month 6 and 12, such as BMD of different parts, with a 95% confidence interval (CI). Relative risks (RR) with a 95% CI were used to assess dichotomous outcomes, such as AEs. The inverse variance and Mantel–Haenszel methods were used to combine separate statistics. If P values were < 0.05, the results were considered statistically significant.
Statistical heterogeneity of the included studies was evaluated using the chi‐square test in accordance with the values of P and I 2 . If the values of I 2 < 50%, the heterogeneity might not be important. A fixed‐effects model was used to assess these outcomes. If I 2 was between 50% and 100%, it could represent substantial heterogeneity. We used random effects model to evaluate these outcomes. Thresholds for the interpretation of I 2 can be misleading, since the importance of inconsistency depends on several factors. Therefore, subgroup analysis or sensitivity analysis was performed to interpret the potential source of heterogeneity. Because only four studies were included, publication bias test were not necessary.
Results
Characteristics of Included Studies
All patients were aged over 60 years. All follow‐up periods were 1 year. All postmenopausal women had a T score of −2.0 to −3.5 at the total hip or femoral neck. Patients were randomly assigned to receive subcutaneous injections of romosozumab (at a dose of 210 mg daily) or teriparatide (20 μg once daily) monthly for 12 months; thereafter, patients in each group received denosumab for 12 months, at a dose of 60 mg, administered subcutaneously every 6 months. The end points were the cumulative incidences of new vertebral fractures at 12 months and 24 months. Secondary end points included clinical (a composite of non‐vertebral and symptomatic vertebral) and non‐vertebral fractures.
Search Results
Initially, 198 citations were identified from electronic databases, of which 169 records were excluded by primary screening. After reading the full text of all remaining 29 studies in detail, 25 studies were also excluded according to the inclusion and exclusion criteria. Finally, four RCTs 4 , 21 , 22 , 23 were included. But only two studies 4 , 23 had the data measured in month 6 and the data of femoral neck BMD. The characteristics of the included studies were summarized in Table 1.
Primary Outcome
The BMD of lumbar spine and total hip were the primary outcome in our meta‐analysis, which were used to evaluate the therapeutic effect of postmenopausal osteoporosis. The treatment period was divided into two subgroups (month 6 and 12).
BMD of Lumbar Spine
Four studies assessed lumbar spine BMD of 620 patients through month 12 (I2 = 75%, P = 0.04) and two studies assessed 514 patients through month 6 (I2 = 86%, P < 0.001). Data were pooled according to the random effects model because of high heterogeneity. Compared with teriparatide, romosozumab significantly improved the BMD (month 6: MD = 3.54, 95% CI [3.13, 3.94], P<0.001; month 12: MD = 4.93, 95% CI [4.21, 5.64], P<0.001; Fig. 4).
Fig. 4.
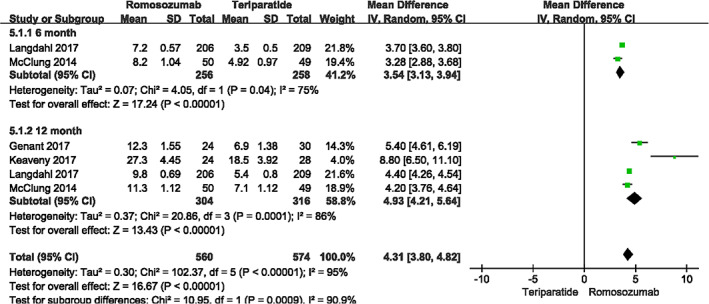
A forest plot diagram showing lumbar spine bone mineral density at month 12 and month 6.
BMD of Total Hip
Four studies assessed total hip BMD of 570 patients through month 12 (I2 = 84%, P < 0.001) and 514 patients through month 6 (I2 = 99%, P < 0.001). Data were pooled according to the random effects model because of high heterogeneity. Compared with teriparatide, romosozumab significantly improved the BMD (month 6: MD = 2.27, 95% CI [0.62, 3.91], P = 0.007; month 12: MD = 3.17, 95% CI [2.68, 3.65], P<0.001; Fig. 5).
Fig. 5.
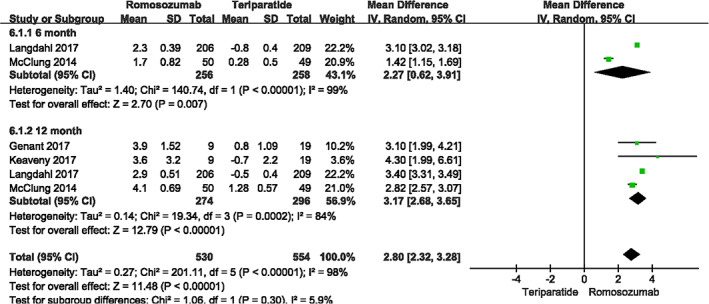
A forest plot diagram showing total hip bone mineral density at month 12 and month 6.
Secondary Outcome
BMD of Femoral Neck
The BMD of femoral neck was reported in two studies 4 , 23 , including 514 patients in month 6 (I2 = 99%, P < 0.001) and the same sample size in month 12 (I 2 = 94%, P < 0.001). Data were pooled according to the random effects model because of high heterogeneity. The percentage change from baseline in the romosozumab group was significantly improved (month 6: MD = 2.30, 95% CI [0.51, 4.08], P = 0.01; month 12: MD = 3.04, 95% CI [2.29, 3.78], P<.001; Fig. 6).
Fig. 6.
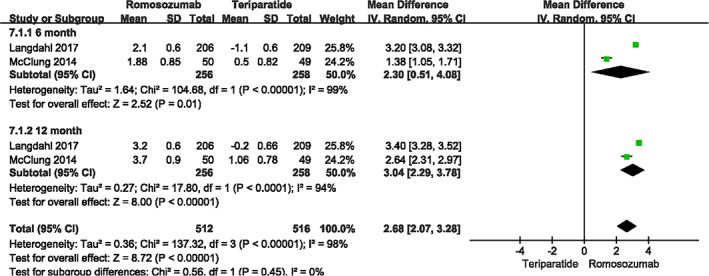
A forest plot diagram showing femoral neck bone mineral density at month 12 and month 6.
Adverse Events
There were also two studies 4 , 23 evaluating the incidence of adverse events. The common points of interest were serious adverse event (I2 = 0%, P = 0.58), death (I2 = 0%, P = 0.63), and injection‐site reaction (I2 = 0%, P = 0.91). No significant differences were found between the two groups in the incidence of serious adverse events (month 12: RR = 0.78, 95% CI [0.46, 1.33], P = 0.37) and death (month 12: RR = 0.61, 95% CI [0.08, 4.62], P = 0.63). However, romosozumab could significantly alleviate the local response (month 12: RR = 2.84, 95% CI [1.22, 6.59], P = 0.02; Fig. 7).
Fig. 7.
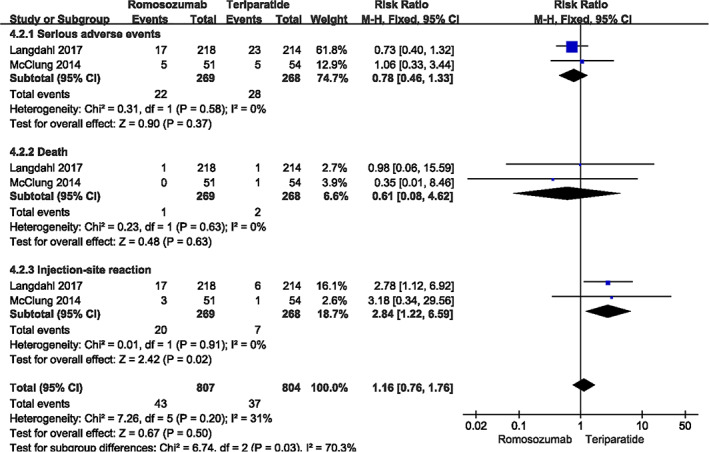
A forest plot diagram showing the incidence of adverse events in month 12.
Discussion
With an aging population, postmenopausal osteoporosis, especially the most common postmenopausal osteoporosis has brought great economic burden to global public health, and also seriously affected the quality of life of patients themselves 24 , 25 . In America, age‐related fractures are projected to increase nationally to over 3 mn fractures in 2025 26 . The process of aging in women is associated with an increase in the rate of bone remodeling in both cancellous and cortical bone, combined with a negative remodeling balance, resulting in bone loss and disruption of bone microarchitecture 1 . It is generally believed that the key to the treatment of postmenopausal osteoporosis is to restore the dynamic balance of bone metabolism, and the signal pathway between cells has become a key to research 27 . With the emergence of new signaling pathways, new avenues targeting them are also emerging, such as melatonin 28 .
The real‐life challenge, however, is rooted in the long therapeutic procedure for postmenopausal osteoporosis. Today, generally speaking, women still have a long life expectancy, possibly 30 years or more after menopause, and their fracture risk increases exponentially with age. There are few clinical extension trials for over 10 years for the treatment of postmenopausal osteoporosis, especially those of antiresorptive therapies. Additionally, serious adverse effects such as osteonecrosis of the jaw and atypical femoral fractures have been related to extended antiresorptive therapy, raising concerns of increased risks due to continuous inhibition of bone resorption. Osteoanabolic therapy is currently limited to 24 months of teriparatide treatment 2 .
There are two major categories of drugs for postmenopausal osteoporosis, antiresorptive drugs, and osteoanabolic drugs. The former inhibit the recruitment and activity of osteoclasts, and probably do not fully correct the negative remodeling balance. The latter have anabolic skeletal effects, which can be achieved through changes in bone remodeling, bone modeling, or a combination of the two 1 . Except teriparatide, there is another osteoanabolic drug, abaloparatide (brand name TymlosTM). It was approved by the FDA on 28 April 2017. Abaloparatide is a synthetic analogue of PTHrP, which can increase bone mass in animals 29 and in humans 30 . But patients still need daily subcutaneous injections like teriparatide. For a long time, abaloparatide injected subcutaneously in rats resulted in dose‐ and time‐dependent formation of osteosarcomas, with a comparable response to h‐PTH (1‐34) at similar exposure 31 . Although abaloparatide can reduce hypercalcaemia, its registration was denied in Europe on the grounds of concerns about its effectiveness in reducing nonvertebral fractures, and increases in heart rate and palpitations 1 . The EMA's Committee for Medicinal Products for Human Use (CHMP) thought some data from study sites of abaloparatide were not reliable and had to be excluded as the study had not been conducted in compliance with “good clinical practice” (GCP) at those sites 32 . After a number of clinical trials in postmenopausal women with osteoporosis 23 , 33 , 34 , 35 , 36 , 37 , romosozumab (EVENITYTM) has been proved to be safe and effective, and was approved by the FDA in 2019 38 .
There is a new avenue for the treatment of postmenopausal osteoporosis. Dating back to 1979, Frost first proposed the concept of sequential therapy for postmenopausal osteoporosis 39 . However, relevant DATA‐Switch studies have not attracted enough attention until now, and have shown better outcomes than monotherapy 40 , 41 , 42 , 43 . Similarly, there are few similar studies on romosozumab. Compared with monotherapy, the transition from romosozumab to other antiresorptive drugs may further increase BMD in postmenopausal osteoporotic women. Through bone‐targeting systems to deliver siRNA are also a new method for postmenopausal osteoporosis, this has already been examined in a preclinical study 44 .
In summary, all the current drugs for postmenopausal osteoporosis more or less have some side effects or lack efficacy, and a very ideal postmenopausal osteoporosis therapy has not yet been developed. Other than drugs, good nutrition, regular physical activity, avoiding harmful lifestyle habits, and fall prevention are recommended for all patients at risk of postmenopausal osteoporosis and should be considered of equal value as medical treatment 45 .
In our study, compared with teriparatide, romosozumab had better effectiveness for the treatment of postmenopausal osteoporosis, especially in increasing the BMD of lumbar spine, total hip, and femoral neck, decreasing the incidence of injection‐site reaction. But on the grounds of the concerns about small sample size, incomplete data, and heterogeneity for RCTs included, further studies are required to demonstrate our results.
Limitations
Our meta‐analysis has several limitations: (i) there were only four RCTs in our meta‐analysis, the sample size of included studies was small (N = 1304); (ii) in regard to the significant heterogeneity of LS BMD (I2 = 86%) and TH BMD (I2 = 84%), although we used a random effects model, we tried to find the source of heterogeneity. When we excluded the study of Keaveny et al., the heterogeneity of LS BMD (I2 = 71%) reduced at a level; when we excluded the study of Langdahl et al. and McClung et al., the heterogeneity of TH BMD (I2 = 0) reduced significantly. Therefore, we thought those excluded studies might be the source. The heterogeneity of FN BMD (I2 = 99% in month 6, I2 = 94% in month 12) could not be analyzed, because there were only two RCTs included; (iii) some original data could not be directly acquired; (iv) the follow‐up period was too short in included trials, so some AEs might not be revealed. The effectiveness and safety needed longer follow‐up time to be confirmed; and (v) only English publications were included in our meta‐analysis.
Conclusions
According to our study, romosozumab can significantly increase the BMD of lumbar spine, total hip, and femoral neck, with a lower incidence of injection‐site reaction. And those differences all have statistical significance (P < 0.05). There is no difference in the incidence of death and other serious adverse events. Fewer adverse events (P < 0.05) and longer half‐life may improve the compliance of patients and reduce the loss of benifits.
Author Contributions
Xinlong Ma and Jianxiong Ma contributed to the conception of the study. Shan Zhu contributed significantly to analysis and manuscript preparation. Aixian Tian and Haobo Jia performed the data analyses and wrote the manuscript. Bin Lu and Yan Li helped perform the analysis with constructive discussions.
Acknowledgements
This study was supported by grants from the Chinese National Natural Science Foundation (No. 81871777; 11772226), Tianjin Science and Technology Project in the Key Field of Traditional Chinese Medicine (No. 2017008), Tianjin Health Science and Technology Project (No. RC20204), Research Fund of Tianjin Institute of Orthopedics (No. 2019TJGYSKY03), Tianjin Natural Science Foundation (No. 17JCQNJC10900), and Tianjin Enterprise Postdoctoral Innovation Project (No. TJQYBSH2017013).
Disclosure: Each author certifies that they have no commercial associations that might pose a conflict of interest with the submitted article.
Contributor Information
Jianxiong Ma, Email: majianxiong2007@hotmail.com.
Xinlong Ma, Email: maxinlong2007@hotmail.com.
References
- 1. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet, 2019, 393: 364–376. [DOI] [PubMed] [Google Scholar]
- 2. Hofbauer LC, Rachner TD. More DATA to guide sequential osteoporosis therapy. Lancet., 2015, 386: 1116–1118. [DOI] [PubMed] [Google Scholar]
- 3. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. The Lancet., 2011, 377: 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med., 2014, 370: 412–420. [DOI] [PubMed] [Google Scholar]
- 5. Watts NB, Diab DL. Long‐term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab., 2010, 95: 1555–1565. [DOI] [PubMed] [Google Scholar]
- 6. Shane E. Evolving data about subtrochanteric fractures and bisphosphonates. N Engl J Med., 2010, 362: 1825–1827. [DOI] [PubMed] [Google Scholar]
- 7. Harvey NC, McCloskey E, Kanis JA, Compston J, Cooper C. Bisphosphonates in osteoporosis: NICE and easy? Lancet, 2017, 390: 2243–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis: where do we go from here? N Engl J Med., 2012, 366: 2048–2051. [DOI] [PubMed] [Google Scholar]
- 9. Takács I, Jókai E, Kováts DE, Aradi I. The first biosimilar approved for the treatment of osteoporosis: results of a comparative pharmacokinetic/pharmacodynamic study. Osteoporos Int., 2019, 30: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paik J, Scott LJ. Romosozumab: a review in postmenopausal osteoporosis. Drugs Aging., 2020, 37: 845–855. [DOI] [PubMed] [Google Scholar]
- 11. Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med., 2016, 374: 254–262. [DOI] [PubMed] [Google Scholar]
- 12. Gilsenan A, Harding A, Kellier‐Steele N, Harris D, Midkiff K, Andrews E. The Forteo patient registry linkage to multiple state cancer registries: study design and results from the first 8 years. Osteoporos Int., 2018, 29: 2335–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McClung MR. Romosozumab for the treatment of osteoporosis. Osteoporos Sarcopenia, 2018, 4: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem., 2005, 280: 26770–26775. [DOI] [PubMed] [Google Scholar]
- 15. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med., 2013, 19: 179–192. [DOI] [PubMed] [Google Scholar]
- 16. Tu X, Delgado‐Calle J, Condon KW, et al. Osteocytes mediate the anabolic actions of canonical Wnt/beta‐catenin signaling in bone. Proc Natl Acad Sci U S A, 2015, 112: E478–E486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fijalkowski I, Geets E, Steenackers E, et al. A novel domain‐specific mutation in a sclerosteosis patient suggests a role of LRP4 as an anchor for sclerostin in human bone. J Bone Miner Res., 2016, 31: 874–881. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg., 2010, 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 19. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ., 2008, 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delgado‐Rodríguez M, Sillero‐Arenas M. Systematic review and meta‐analysis. Med Intensiva (Engl Ed), 2018, 42: 444–453. [DOI] [PubMed] [Google Scholar]
- 21. Genant HK, Engelke K, Bolognese MA, et al. Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res., 2017, 32: 181–187. [DOI] [PubMed] [Google Scholar]
- 22. Keaveny TM, Crittenden DB, Bolognese MA, et al. Greater gains in spine and hip strength for Romosozumab compared with Teriparatide in postmenopausal women with low bone mass. J Bone Miner Res., 2017, 32: 1956–1962. [DOI] [PubMed] [Google Scholar]
- 23. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open‐label, phase 3 trial. Lancet., 2017, 390: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 24. Borgstrom F, Lekander I, Ivergard M, et al. The international costs and utilities related to osteoporotic fractures study (ICUROS)–quality of life during the first 4 months after fracture. Osteoporos Int., 2013, 24: 811–823. [DOI] [PubMed] [Google Scholar]
- 25. Marques A, Lourenco O, Da SJ. The burden of osteoporotic hip fractures in Portugal: costs, health related quality of life and mortality. Osteoporos Int., 2015, 26: 2623–2630. [DOI] [PubMed] [Google Scholar]
- 26. Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ III. Trends in fracture incidence: a population‐based study over 20 years. J Bone Miner Res., 2014, 29: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plotkin LI, Bellido T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat Rev Endocrinol., 2016, 12: 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li T, Jiang S, Lu C, et al. Melatonin: another avenue for treating osteoporosis? J Pineal Res., 2019, 66: 12548–12560. [DOI] [PubMed] [Google Scholar]
- 29. Bahar H, Gallacher K, Downall J, Nelson CA, Shomali M, Hattersley G. Six weeks of daily Abaloparatide treatment increased vertebral and femoral bone mineral density, microarchitecture and strength in Ovariectomized Osteopenic rats. Calcif Tissue Int., 2016, 99: 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leder BZ, O'Dea LS, Zanchetta JR, et al. Effects of abaloparatide, a human parathyroid hormone‐related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab., 2015, 100: 697–706. [DOI] [PubMed] [Google Scholar]
- 31. Jolette J, Attalla B, Varela A, et al. Comparing the incidence of bone tumors in rats chronically exposed to the selective PTH type 1 receptor agonist abaloparatide or PTH(1–34). Regul Toxicol Pharmacol., 2017, 86: 356–365. [DOI] [PubMed] [Google Scholar]
- 32. Erviti J, Gorricho J, Saiz LC, Perry T, Wright JM. Rethinking the appraisal and approval of drugs for fracture prevention. Front Pharmacol., 2017, 8: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cosman F, Crittenden DB, Ferrari S, et al. Romosozumab FRAME study: a post hoc analysis of the role of regional background fracture risk on nonvertebral fracture outcome. J Bone Miner Res., 2018, 33: 1407–1416. [DOI] [PubMed] [Google Scholar]
- 34. Lewiecki EM, Blicharski T, Goemaere S, et al. A phase III randomized placebo‐controlled trial to evaluate efficacy and safety of Romosozumab in men with osteoporosis. J Clin Endocrinol Metab., 2018, 103: 3183–3193. [DOI] [PubMed] [Google Scholar]
- 35. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med., 2017, 377: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 36. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med., 2016, 375: 1532–1543. [DOI] [PubMed] [Google Scholar]
- 37. Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single‐dose, placebo‐controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res., 2011, 26: 19–26. [DOI] [PubMed] [Google Scholar]
- 38. Markham A. Romosozumab: first global approval. Drugs, 2019, 79: 471–476. [DOI] [PubMed] [Google Scholar]
- 39. Frost HM. Treatment of osteoporoses by manipulation of coherent bone cell populations. Clin Orthop Relat Res., 1979. (143):227–244. [PubMed] [Google Scholar]
- 40. Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA‐switch study): extension of a randomised controlled trial. Lancet., 2015, 386: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsai JN, Nishiyama KK, Lin D, et al. Effects of Denosumab and Teriparatide transitions on bone microarchitecture and estimated strength: the DATA‐switch HR‐pQCT study. J Bone Miner Res., 2017, 32: 2001–2009. [DOI] [PubMed] [Google Scholar]
- 42. Leder BZ, Tsai JN, Jiang LA, Lee H. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: the Denosumab and Teriparatide follow‐up study (DATA‐follow‐up). Bone., 2017, 98: 54–58. [DOI] [PubMed] [Google Scholar]
- 43. Tsai JN, Jiang LA, Lee H, Hans D, Leder BZ. Effects of Teriparatide, Denosumab, or both on spine trabecular microarchitecture in DATA‐switch: a randomized controlled trial. J Clin Densitom., 2017, 20: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stapleton M, Sawamoto K, Almeciga‐Diaz CJ, et al. Development of bone targeting drugs. Int J Mol Sci., 2017, 18: 1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen LR, Ko NY, Chen KH. Medical treatment for osteoporosis: from molecular to clinical opinions. Int J Mol Sci., 2019, 20: 2213–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]


