Abstract
Portal vein thrombosis (PVT) is considered a relatively rare thrombotic complication in coronavirus disease 2019 (COVID-19). Most reported cases of PVT develop within 2 weeks from COVID-19 onset. We report a fatal case of extensive gastrointestinal necrosis due to portal and mesenteric vein thrombosis approximately 6 weeks after the onset of critical COVID-19. Excessive elevation of his plasma D-dimer level had continued for weeks during the hospitalization contrary with improvement of respiratory failure. Thrombotic complication should be cautiously paid attention even in the post-acute phase of COVID-19, especially in patients with persistent elevation of plasma D-dimer level.
Keywords: Coronavirus disease 2019, D-dimer, Gastrointestinal necrosis, Portal vein thrombosis, Post-acute phase, SARS-CoV-2
1. Introduction
Thrombosis complicated with coronavirus disease 2019 (COVID-19) through vascular endothelial damage is associated with poor prognosis, especially in patients requiring intensive care unit (ICU) admission [1]. Pulmonary thrombosis is the most common venous thrombotic event experienced by COVID-19 patients. However, portal vein thrombosis (PVT) has rarely been reported as a thrombotic event with COVID-19 [1,2]. Generally, the fatality rate is 50–70% for patients who develop intestinal infarction and extensive peritonitis induced by acute PVT with portal hypertension [3,4]. The anticoagulant therapy for PVT is recommended to prevent intestinal infarction due to portal vein hypertension [4].
Thrombotic events secondary to COVID-19 are usually developed within 2 weeks from ICU admission [1]. However, with a case report of late pulmonary embolism after COVID-19 pneumonia despite adequate anticoagulant therapy during the acute phase [5], the duration of high-risk period to develop thrombotic events after recovery from COVID-19 remains unknown. Herein, we report a fatal case of extensive gastrointestinal necrosis due to portal and mesenteric vein thrombosis in the post-acute phase of COVID-19.
2. Case
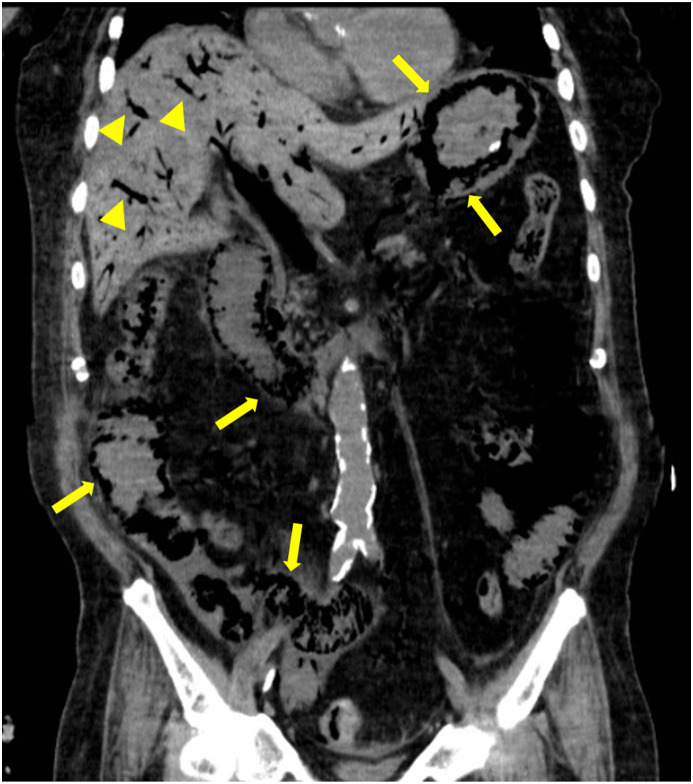
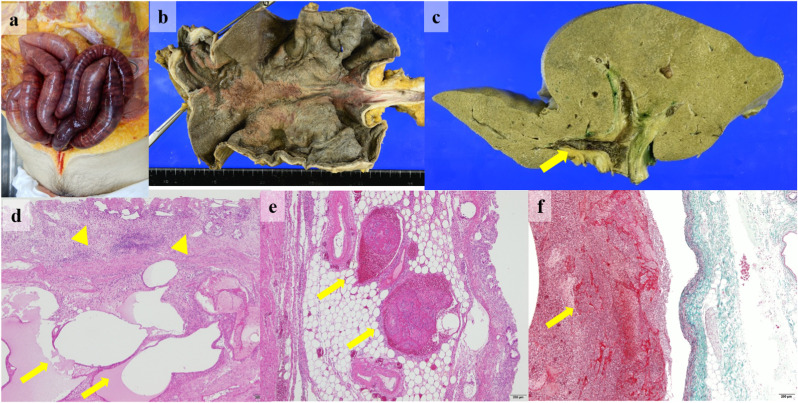
An 82-year-old Japanese man with a 3-day history of fever and fatigue was transferred to our hospital for the treatment of COVID-19. His medical history included coronary artery disease treated with aspirin and nicorandil, diabetes, and hypertension. He received favipiravir and subcutaneous unfractionated heparin (5000 units every 12 hours) for prophylaxis against deep vein thrombosis from the day of admission. On day 7 after disease onset, he developed acute respiratory distress syndrome and was admitted to the ICU for invasive mechanical ventilation. He received dexamethasone (6 mg per day). On the 17th day after disease onset, the negative result of real-time reverse transcription polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 was obtained four times from the nasopharyngeal sample. Temporary hemodynamic support with vasopressor was required for managing septic shock due to ventilator-associated pneumonia from days 18–26. Aspirin and subcutaneous unfractionated heparin were discontinued because of advancing anemia due to hemorrhage from the oral mucosa on days 22 and 28, respectively, although the patient's D-dimer level was consistently higher than 20 μg/mL from day 20. He was extubated after tracheostomy on day 30 and successfully withdrew from mechanical ventilation support on day 34. Non-contrast chest and abdominal computed tomography (CT) on day 36 revealed mild calcification of the aorta and superior mesenteric artery and absence of superior mesenteric vein diameter dilatation, bowel dilatation, and ascites. The patient had no abdominal pain but had constipation with nasogastric tube feeding for a week. Laboratory examination revealed elevation of aspartate aminotransferase and alanine transaminase levels within twofolds of upper limits of normal, no elevation of total bilirubin and alkaline phosphatase levels, and no prolonged prothrombin time for over 2 weeks. Intravenous unfractionated heparin was reinitiated from day 36. On day 42 from disease onset, he suddenly developed abdominal distension without tenderness. His blood pressure, heart rate, temperature, respiratory rate, and oxygen saturation were 81/42 mmHg, 120 beats per minutes, 37.2 °C, 30 beats per minutes, and 85% under oxygen administration of 2 L per minute, respectively. Results of arterial blood gas analysis under 10 L per minute of oxygen administration revealed the following: pH 7.18, partial pressure of oxygen 241.0 mmHg, partial pressure of carbon dioxide 49.9 mmHg, bicarbonate 16.9 mEq/L, and lactic acid 13.0 mmol/L. Abdominal CT revealed severe bowel dilatation, extensive pneumatosis in gastric intestinalis, and portal venous gas, consistent with acute mesenteric ischemia and massive intestinal necrosis (Fig. 1 ). He was unresponsive to treatment with fluid resuscitation and vasopressor for treating hypotension caused by lactic acidosis owing to extensive gastrointestinal necrosis; he died within 6 hours from the onset of the shock event. Autopsy was performed to clarify the etiology of extensive gastrointestinal necrosis. Macroscopic findings revealed hemorrhagic necrosis in the gastric and extensive small bowel mucosa (Fig. 2 a and b). Thrombosis was found in the bilateral portal vein (Fig. 2c), whereas no emboli or dissection was found in the superior mesenteric artery and the main branches. Except for the hemorrhagic necrosis in the gastro-intestinal mucosa, there was no evidence of liver cirrhosis, intra-abdominal infection, or any malignancy. Microscopic findings revealed ischemic degenerative changes with loss of the surface epithelium and pneumatosis of the gastrointestinal mucosa (Fig. 2d); neutrophilic infiltration or partially abscessed, several fibrin thrombi in the gastric submucosal vein and portal vein (Fig. 2e and f); and venous dilatation in the intestinal submucosal vein. Based on autopsy findings, the cause of his death was extensive gastrointestinal necrosis induced by excessive portal hypertension due to portal and mesenteric vein thrombosis.
Fig. 1.
Computed tomography (CT) of acute mesenteric ischemia on day 42 from the onset of coronavirus disease 2019. Portal venous gas (yellow arrow heads) and gastrointestinal pneumatocele (yellow arrows) consistent with extensive gastrointestinal ischemic change with necrosis are found in the coronal section of the abdominal CT scan.
Fig. 2.
Autopsy findings of gastrointestinal necrosis and portal and mesenteric vein thrombosis. The intestinal dilatation and the purple changing of the small bowel color consisted with ischemic necrosis (a). Macroscopic findings revealed hemorrhagic necrosis in the gastric mucosa (b). Massive thrombi were found in the bilateral portal vein (yellow arrow) (c). Microscopic findings revealed ischemic degenerative changes with loss of the surface epithelium (yellow arrow heads) and pneumatosis (yellow arrows) of the gastric mucosa (hematoxylin-eosin staining; the scale bar corresponds to 200 μm) (d), and several fibrin thrombi in the gastric submucosal vein (yellow arrows) (hematoxylin-eosin staining; the scale bar corresponds to 200 μm) (e) and portal vein (yellow arrow) (Elastica-Masson staining; the scale bar corresponds to 200 μm) (f).
3. Discussion
The present case report emphasizes two important clinical considerations. First, COVID-19-infected patients could develop fatal portal and mesenteric vein thrombosis more than 1 month after disease onset, despite administration of anticoagulant therapy. Second, persistent elevation of plasma D-dimer level may be a predictive marker of fatal thrombotic events in the post-acute phase of COVID-19.
PVT was reported as a rare complication among COVID-19 [1,2] and, similar with other thrombotic events, was developed within 2 weeks from admission in most previous case reports [[6], [7], [8]]. However, limited in COVID-19 patients with ischemic gastrointestinal complications, portal or mesenteric vein thrombosis was found in approximately one-fifth of patients [9]. Moreover, according to a previous report about liver autopsy in COVID-19 patients, PVT was found in approximately a half of COVID-19 patients without clinical symptoms of liver disease or signs of liver failure before and during hospitalization [10]. The radiological findings of portal venous gas and pneumatosis intestinalis and the pathological findings in the present case were consistent with intestinal ischemia, which was induced by acute portal hypertension due to portal and mesenteric vein thrombosis. The etiology of PVT in the present case was considered to be COVID-19 with no evidence of any other local risk factor on autopsy, although congenital and acquired coagulant disorders could not be excluded. Considering the asymptomatic cases of PVT among COVID-19 patients and lack of evaluation by contrast CT or ultrasonography with portal vein flow before the onset of the thrombotic event [10], the exact onset of the fatal thrombotic event in the present case was unclear, and chronic micro-thrombosis may have developed during the patient's hospitalization. However, we should note that the proportion of PVT among COVID-19 patients may be higher than that previously reported and a fatal portal and mesenteric vein thrombosis could develop or deteriorate in COVID-19 patients even in the post-acute phase.
Plasma D-dimer level, although nonspecific, is considered one of the inflammatory and thrombotic markers in COVID-19 patients, and the discrepancy between an increase in plasma D-dimer levels and a decrease in serum C-reactive protein levels may indicate the development of thrombotic events [11]. Yong Li et al. have shown the association between decreasing D-dimer levels and improvement of COVID-19 severity [12]. Persistent elevation of plasma D-dimer level indicates continuous vascular endothelial damage in COVID-19 patients [13]. The plasma D-dimer level elevated greater than 50 μg/mL in majority of patients with PVT diagnosed by autopsy in the previous study about liver histopathology in COVID-19 patients above [10]. Similar with the present case, COVID-19 patients with elevated plasma D-dimer level may have developed undiagnosed thrombotic events even without any clinical or laboratory evidence or may have persistent risk for thrombotic events through the continuous vascular endothelial damage.
Anticoagulant therapy could improve the prognosis of COVID-19 patients with elevated plasma D-dimer level. However, because breakthrough venous thrombotic events have often been reported with anticoagulant therapy in COVID-19 patients [14,15], the appropriate dose (i.e., for therapeutic or prophylactic purposes) and duration of anticoagulant therapy also remain to be established for hospitalized COVID-19 patients, especially in patients with hemorrhagic complication such as the present case [16]. Behnood B et al. have recommended extended prophylaxis for up to 45 days to prevent thrombotic events, especially in high-risk patients (e.g., reduced mobility, elevated D-dimer level) [17]. Some reports have recommended the D-dimer-driven-anticoagulation strategy to improve the outcome of hospitalized COVID-19 patients [18,19]. According to a questionnaire-based survey in Japan, some clinicians have decided to initiate and discontinue anticoagulant therapy depending on the COVID-19 patients’ plasma D-dimer level [20]. For the present case, we may have prevented the fatal portal and mesenteric vein thrombosis by restarting the therapeutic dose of the anticoagulant agent after the patient recovered of hemorrhage from the oral mucosa because persistent elevation of plasma D-dimer level may have indicated the development and worsening of micro-thrombosis in the portal and mesenteric vein.
4. Conclusion
Portal and mesenteric vein thrombosis could be sudden in onset or could deteriorate even in the post-acute phase of COVID-19. We should consider portal and mesenteric vein thrombosis for COVID-19 patients complicated with extended intestinal ischemia. Plasma D-dimer level may be a predicting marker of late thrombotic events, in addition to acting as a prognostic marker in the acute-phase of COVID-19. High-risk patients for thrombotic events, especially those with consistently high plasma D-dimer level, may receive prolonged anticoagulation therapy.
Authorship
Tomohiro Hosoda contributed to drafting the article, Hideki Orikasa performed autopsy and documented the autopsy findings.
Funding
None.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basit S.A., Stone C.D., Gish R. Portal vein thrombosis. Clin Liver Dis. 2015;19:199–221. doi: 10.1016/j.cld.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Parikh S., Shah R., Kapoor P. Portal vein thrombosis. Am J Med. 2010;123:111–119. doi: 10.1016/j.amjmed.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Di Tano G., Moschini L., Loffi M., Testa S., Danzi G.B. Late pulmonary embolism after COVID-19 pneumonia despite adequate Rivaroxaban treatment. Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan W., Ramadan H.K.A. COVID-19 as a novel etiology of portal vein thrombosis: change in the current management concepts. Infect Dis (Lond) 2021;53:148–150. doi: 10.1080/23744235.2020.1837943. [DOI] [PubMed] [Google Scholar]
- 7.Abeysekera K.W., Karteszi H., Clark A., Gordon F.H. Spontaneous portomesenteric thrombosis in a non-cirrhotic patient with SARS-CoV-2 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-238906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borazjani R., Seraj S.R., Fallahi M.J., Rahmanian Z. Acute portal vein thrombosis secondary to COVID-19: a case report. BMC Gastroenterol. 2020;20:386. doi: 10.1186/s12876-020-01518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keshavarz P., Rafiee F., Kavandi H., Goudarzi S., Heidari F., Gholamrezanezhad A. Ischemic gastrointestinal complications of COVID-19: a systematic review on imaging presentation. Clin Imag. 2021;73:86–95. doi: 10.1016/j.clinimag.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonzogni A., Previtali G., Seghezzi M., Alessio M.G., Gianatti A., Licini L., et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becher Y., Goldman L., Schacham N., Gringauz I., Justo D. D-dimer and C-reactive protein blood levels over time used to predict pulmonary embolism in two COVID-19 patients. Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Zhao K., Wei H., Chen W., Wang W., Jia L., et al. Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol. 2020;190:e24–e27. doi: 10.1111/bjh.16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan K.H., Lim S.L., Shaaban H., Guron G., Slim J. Persistent hypercoagulable state in COVID-19: a case series of COVID-19 associated pulmonary embolism. J Global Infect Dis. 2021;13:38–41. doi: 10.4103/jgid.jgid_180_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis P., Tharp J.L. Breakthrough venous thromboembolic events in five patients with COVID-19 on direct oral anticoagulants. J Clin Pharm Therapeut. 2021;46:519–523. doi: 10.1111/jcpt.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaczyk A., Rosovsky R.P., Reed C.T., Bankhead-Kendall B.K., Bittner E.A., Chang M.G. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit Care. 2020;24:559. doi: 10.1186/s13054-020-03273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stawiarski K., Loutoo A., Velardi L., Zarich S. D-dimer driven deep vein thrombosis prophylaxis strategy for hospitalized patients with COVID-19. Thromb Res. 2021;201:151–153. doi: 10.1016/j.thromres.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tassiopoulos A.K., Mofakham S., Rubano J.A., Labropoulos N., Bannazadeh M., Drakos P., et al. D-dimer-driven anticoagulation Reduces mortality in intubated COVID-19 patients: a cohort study with a propensity-matched analysis. Front Med (Lausanne) 2021;8:631335. doi: 10.3389/fmed.2021.631335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiuchi H., Morishita E., Urano T., Yokoyama K. Questionnaire-survey joint team on the COVID-19-related thrombosis. COVID-19-Related thrombosis in Japan: final report of a questionnaire-based survey in 2020. J Atherosclerosis Thromb. 2021;28:406–416. doi: 10.5551/jat.RPT001. [DOI] [PMC free article] [PubMed] [Google Scholar]