Abstract
Human pluripotent stem cells (hPSCs) are sources of several somatic cell types for human developmental studies, in vitro disease modeling, and cell transplantation therapy. Improving strategies of derivation of high-purity specific neural and glial lineages from hPSCs is critical for application to the study and therapy of the nervous system. Here, we will focus on the principles behind establishment of neuron and glia differentiation methods according to developmental studies. We will also highlight the limitations and challenges associated with the differentiation of several “difficult” neural lineages and delay in neuronal maturation and functional integration. To overcome these challenges, we will introduce strategies and novel technologies aimed at improving the differentiation of various neural lineages to expand the application potential of hPSCs to the study of the nervous system.
Keywords: human pluripotent stem cells (hPSCs), induced pluripotent stem cells (iPSCs), specific neural lineages, neuron and glia differentiation, central nerve system (CNS), peripheral nerve system (PNS)
Introduction
The human central nervous system (CNS) is majorly composed of the brain and spinal cord, which comprise several types of neurons and glial cells. These cells form complex circuits and provide organized nerve functions that support human behavior. Recently, imaging tools, single-cell analyses, and other technologies have helped researchers to demonstrate the existence of novel neural subtypes in the human nervous system, including types of neurons that do not exist in other animals 1 –4 . However, the coordination between different neuron types and formation of the complex nervous system remains largely unknown. Many neurological diseases are highly correlated with specific neural types, for example, motor neurons in amyotrophic lateral sclerosis (ALS) 5 , dopaminergic neurons (DA neurons) in Parkinson’s disease (PD) 6 , cortical and striatum neurons in Alzheimer’s disease (AD) 7 and Purkinje cells in spinocerebellar ataxia (SCA) 8,9 . Nevertheless, the major causes and processes of neuronal degeneration in these diseases are largely unknown. For example, mutation of the PTEN-induced kinase 1 (PINK1) gene alone causes DA neuron degeneration in PD 10,11 and superoxide dismutase 1 (SOD1) gene mutation is dominant for motor neuron cytopathies in ALS 5 . Why certain neuron types are specifically sensitive to these mutant proteins/cellular stresses remains a mystery. Recreation of specific disease cytopathies and progression of certain types of neurons outside the human body remains a challenge for studying neurological disease mechanisms and development of novel therapeutic methods.
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) 12 and induced pluripotent stem cells (iPSCs) 13,14 , have the potential to differentiate into cell types of the adult human body including neurons and glial cells. This powerful stem cell technology provides an in vitro source of cells for development studies, disease models, and cell transplantation therapies 15,16 .
hPSC differentiation into specific neuron types occurs via sequential steps according to the development of the human nervous system 17 . Here, we will introduce the common principles and strategies used for neuronal differentiation. Furthermore, we will address the novel single-cell transcriptome analysis, which has helped researchers to develop methods for differentiating challenging neuron types.
Pluripotent Stem Cells to Neuroepithelial (NE) Cells
NE cells are sources of neurons and glial cells. The neuroepithelium is derived from the ectoderm during human embryo gastrulation. In vitro, hPSCs can be driven into cell types of the three germ layers in the absence of pluripotency maintenance factors. For high-efficiency NE differentiation, hPSC inhibition toward the mesoderm and endoderm is essential. Bone morphogenic protein (BMP) and Wnt signaling pathways promote hPSC differentiation into the mesoendoderm and lead to the generation of their downstream cell types 18 –20 . Transforming growth factor β1 (TGFβ1) and activin A/Nodal signaling pathways maintain the pluripotent state of hPSCs in the presence of the basic fibroblast growth factor (FGF-basic, also named as FGF-2) 21 –26 , but promote endodermal differentiation in the present of BMPs or Wnt, which suggests the inhibitory role of TGFβ1 on ectodermal differentiation 27 .
Thus, the combination of SMAD1/5/8 and SMAD2/3 inhibition via BMP and activin A/Nodal signaling with the activation of FGF-2 signaling prevents hPSC self-renewal or differentiation toward the mesoderm/endoderm, followed by the differentiation of hPSCs toward NE cells 17,28 –31 .
To arrest cell cycle and transfer hPSCs to a state that is amenable to differentiation, hPSCs are dissociated and reaggregated into floating spheres termed embryoid bodies (EBs). In the presence of short-term induction, the major population of cells differentiate into NE cells or neural stem cells (NSCs), for further neuron subtype patterning 29,31 –33 . In this method, external ligands trigger the initial stage of neural induction exclusively. Subsequently, aggregated cells interact with each other via the secretion of factors and cell-cell adhesion communication. However, the EB methods share the common challenge of instability of experimental conditions. Cells in EBs are very sensitive to changes in the environment of the cell-cell communication networks, which leads to variations among neural induction cell batches. Moreover, NSCs located at different sites of the spheres may receive varying doses of morphogens and become heterogeneous cells. To avoid this, an induction method without the formation of EBs was developed. In the adherent culture condition, hPSCs are induced into NE cells or NSCs via the addition of dual SMAD inhibitors (dSMADi), which suppresses BMP and TGFβ1 signals (Fig. 1A) 34 . In this method, because of the reduction of cell-cell interactions and lack of a three-dimensional (3D) structure, external factors become more dominant and consistently generate a homogeneous NSC population for the next step of patterning. However, unlike that observed in EB methods, the lack of a uniform cell-cycle arresting step prolongs the essential neural induction period to about 1 week, to obtain NE cells. Most neural induction laboratories use these two methods. The adherent dSMADi method is a powerful tool for high-purity, robust, homogeneous, and specific neuron-type generation. To obtain spheres with functional layers or mini organoids, the EB method provides an efficient way to generate self-organized fetus-like nerve tissues. Alternatively, some researchers use mixed methods based on these two major approaches according to their research purpose.
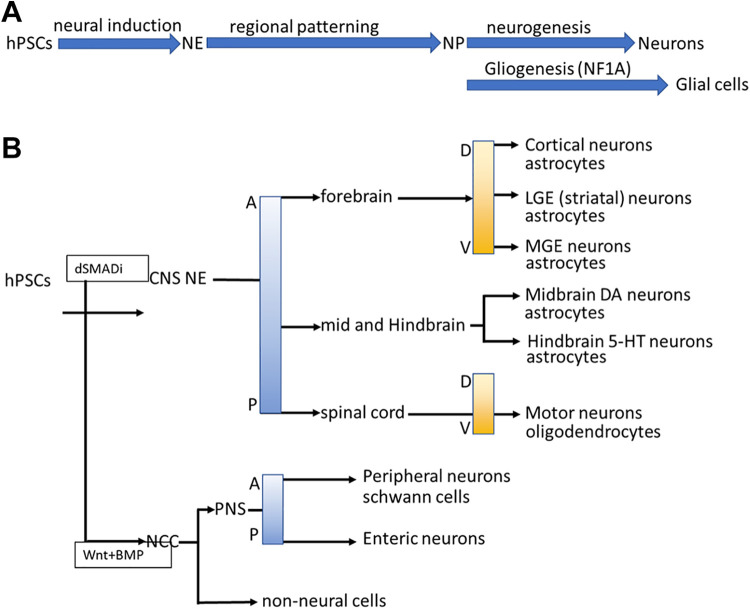
Figure 1.
Differentiation processes and guidelines of specific neuron and glial cell types from hPSCs. (A) The differentiation steps of specific neurons and glial cells, including NE differentiation, specific neural progenitor patterning, and neuro/glio-genesis. (B) Specific neuronal and glial types can be differentiated from hPSCs under the principles of neural tube development, including the CNS/PNS stem cell differentiation, D-V and A-P determination.
Wnt signaling promotes mesoendodermal differentiation of hPSCs. However, previously, we demonstrated that the combination of FGF-2, a TGFβ1 antagonist, and a Wnt agonist promoted a robust NE differentiation using the EB method 35 . These NE cells could pattern into several types of neurons, indicating that the coordination of several signals may promote an unexpected cell fate during differentiation.
Differentiation of Neural Stem Cells into Specific Neural Types of CNS According to A-P and D-V Axis
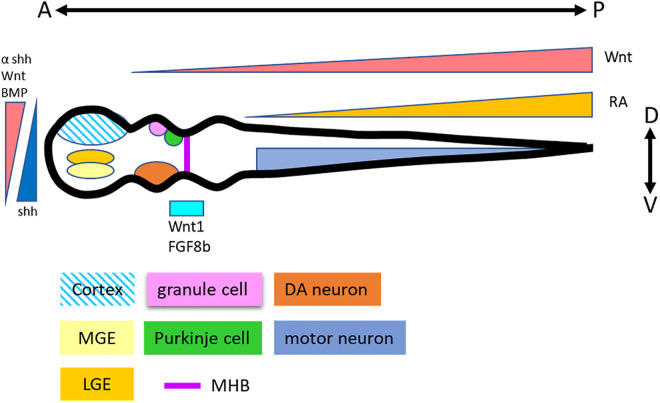
The developmental principle of the CNS basically follows the dosage gradient of developmental regulators called “morphogens.” Morphogens promote NSC differentiation into specific types of neurons according to their position on the neural tube (Fig. 2). sonic hedgehog (Shh), Wnt, and BMP signals regulate the positioning of the dorsal-ventral (D-V) axis, whereas Wnt and retinoic acid (RA) signals are involved in the formation of the anterior-posterior (A-P) axis of the neural tube 36,37 . Some morphogens directly promote NSC differentiation into specific regional neural types including cerebellar 38 and midbrain-hindbrain boundary (MHB) 39 –43 .
Figure 2.
Neural patterning principles in neural tube development. Morphogens Wnt, BMP, and Shh are involved in the D-V determination. Wnt and RA regulate the A-P development. Thus, specific neural types are patterned according to their positions in the neural tube.
Regardless of the differentiation protocol (SFEB/dSMADi), the naïve fate of hPSC-derived NE cells is the dorsal forebrain region, for further patterning to generate other region-specific neural types in the neural tube. Without morphogen addition, NE cells differentiate sporadically into the neural types of the forebrain 28,30,33,34 . Wnt signaling plays a dominant role in A-P determination. According to the dosage of Wnt signaling, NE cells are driven into the midbrain/hindbrain 37,44 . However, the Wnt protein is not an effective morphogen in vitro. The first Wnt agonist, BIO 45 , did not possess dosage dependence, via which it can only pattern neurons to the most posterior types. CHIR99021, a GSK3β inhibitor that can modulate Wnt signaling according to the treatment dosage, helped researchers perform in vitro A-P axis patterning 46 –49 . Consecutively, RA is the key morphogen in spinal cord patterning and coordinates with CHIR99021 to promote NE cell differentiation into spinal cord neurons 50,51 . Some morphogens, for example, Wnt1 and FGF-8b are highly expressed in the MHB region. However, Wnt1 and FGF8b exhibit very poor efficiency to drive the differentiation of NE cells into MHB neuron types in vitro 48,52 . A similar concept was also demonstrated by Prof. Muguruma’s work on Purkinje cell differentiation 53,54 . Exogenous Wnt and FGF-8b did not promote the differentiation of NE cells into Purkinje cells, whereas treatment with FGF-2 activated the endogenous expression of Wnt1 and FGF8b, thus promoting differentiation into Purkinje cells. However, the roles of endogenous and exogenous morphogens and how they cooperate to regulate neural fate warrant further clarification.
Shh, BMP, and Wnt signaling pathways are the key regulators of D-V patterning in the neural tube 55 . Shh secreted from the floor plate promotes the differentiation of neurons into ventral types, whereas the Wnt and BMP signals secreted from the root plate drive neurons toward a dorsal type fate and against each other 56 . Previous reports have suggested that Shh is the dominant morphogen for D-V decision and can influence the other two signals during embryonic neural tube specific neuron diffeentiation 47 . According to this principle, it is not difficult to generate the most ventral/dorsal neuron types using Shh or its antagonist, respectively. Exogenous Shh is essential for differentiation into ventral neural types, for example, interneurons, and motor neurons. The naïve fate of the hPSC-derived NSCs in the forebrain is the cortical-neuron type, which represent the most dorsal brain part. Patterning according to Shh dosage pushes NSCs toward a lateral ganglionic eminence and medial ganglionic eminence fate, which will become the striatum spiny neurons and GABA interneurons 57 –65 . In some cases, the inhibition of Shh signaling is essential for receiving dorsal neurons in the neural tube. However, because of the lack of a dosage-dependent antagonist of Shh, Shh inhibition for the generation of dorsal neural types is inefficient 66 .
Overall, A-P and D-V patterning principles provide guidelines (Fig. 1B) to generate specific neural types in vitro. However, several challenges remain regarding the identification of some neural types that may have more complex patterning principles during human CNS development.
Differentiation of Neural Stem Cells into CNS Glial Cells
Neurological diseases are not caused by the dysfunction of a single type of neuron; rather, most of these pathogenic processes are derived from the interactions among neurons, glial cells, and other cell types. The in vitro generation of glial cells via differentiation is extremely important for the exploration of disease processes. Glial cells are derived from NE cells via the transition of neurogenesis to gliogenesis. Astrocytes and oligodendrocytes are differentiated using different procedures. The astrocyte differentiation procedure includes three steps. The first step is hPSC differentiation into NE cells. The second step comprises NE cell transit from a neurogenic to a gliogenic state with the prolongation of the maintenance of NSCs to avoid neurogenesis. FGF-2 and epidermal growth factor treatment for 4 months can promote the transition of neurogenic NSCs into glial-competent NSCs. At the final stage, BMPs and ciliary neurotrophic factor promote the transition of astrocyte progenitors into mature astrocytes with functional activities 67,68 . As the transition of neurogenic NSCs into glial-competent progenitors takes an extremely long time, several modified protocols that accelerate this stage have been developed. However, most protocols accelerate this step via fetal bovine serum (FBS) addition, which may cause astrocyte activation to the A1 state, thus potentially altering the outcome of the in vitro modeling 69,70 . Recently, a key regulator of the neural-glial transition was discovered within a short time by three research groups. Overexpression of the nuclear factor 1 A-type (NFIA) in NE cells led to the successful shortening of the neural-glial transition stage to 1-2 months using a genetic manipulation method 71 –73 . Tchieu et al. further identified G1 phase arrest as the key modulator of the promotion of NF1A expression in NSCs, which become glial-competent astrocyte progenitors (Fig. 1A). These authors also discovered that the stepwise treatment of NSCs with TGFβ1 and leukemia inhibitory factor (LIF) promotes their differentiation toward a glial cell fate to become astrocytes 73 . However, the exact efficiency of astrocyte generation using this method was not evaluated. Thus, an improved approach may be warranted.
Oligodendrocytes are derived from the subventricular zone (SVZ), ventral part, and dorsal part of the spinal cord. In vitro differentiated oligodendrocyte progenitor cells (OPCs) are mostly driven toward a ventral spinal cord fate. The differentiation of oligodendrocytes is divided into three steps. The first step includes their differentiation into NE cells. The second step is NE cell patterning into motor-neuron-capable NSCs using Shh and RA. To prevent these OPCs from becoming motor neurons, the maintenance of Shh, platelet-derived growth factor, and insulin-like growth factor 1 treatment sustains the OPC fate and promotes OPCs to become mature oligodendrocytes with the sequential expression of O4 and MBP 74 –81 . The whole process takes about 6 months. The screening of potential mitogens is a major part of the promotion of OPC maturation and may shorten oligodendrocyte differentiation.
Differentiation of Pluripotent Stem Cells into Peripheral Nerve System Neurons and Glial Cells
Neural crest cells (NCCs) are derived from the borderline between the neural plate and non-neural ectoderm. During neural tube formation, NCCs from the roof plate of the neural tube differentiate into several cell types including melanocytes, craniofacial cartilage, bone, smooth muscle, peripheral, enteric neurons, and glia 82,83 . These NCCs provide neural types (including enteric neurons, sensory neurons, and Schwann cells) important to mimic neurological diseases such as Riley-Day syndrome and diabetes-related peripheral neuropathy 84,85 . Tchieu et al. demonstrated the principles underlying the generation of all major ectoderm cell types from hPSCs, including NCCs (Fig. 1B). Application of dSMADi for 2 days, followed by sequential activation by Wnt and low-dosage BMP signaling, can yield NCCs with >60% purity. At day 23-25 of differentiation, cells were purified by fluorescence-activated cell sorting, to obtain high-purity NCCs based on the SOX10 reporter and surface marker CD49d. To obtain Schwann cells, NCCs were patterned using CHIR99021 and gamma-Secretase Inhibitor IX (DAPT) to generate Schwann cell progenitors. Further cell culturing with FBS for >80 days is essential for Schwann cell maturation. A similar protocol was also applied to sensory neurons and enteric nervous system neurons 86 –92 . However, generation of high-purity NCCs using the current protocol is dependent on reporter-activated cell sorting. Moreover, the detailed procedure used to pattern NCCs into their derivate types warrants further fine tuning.
3D Culture to Generate CNS Organoids
There are currently few suitable simulations to identify the development and circuits of neurons within the human brain. In 2008, Eiraku et al. demonstrated that neural EB spheres can form forebrain-like structures containing neural layers 93 . With this concept, these authors established eye-cup-like 3D organoids for the first time 94,95 . In 2013, Lancaster et al. generated organoids with early forebrain structures and cortical layers and basically recapitulated the processes of human brain development using Matrigel-encapsulated, 3D stirring EBs 96 –98 . However, the long differentiation period and reproducibility problems have restricted the applications of brain organoids. Moreover, challenges remain regarding the generation of well-polarized and well-organized brain organoids. To overcome these, several approaches have been developed based on mini-bioreactors 99 , biomaterial scaffolding 100 , and air-liquid interface culturing 101 . For forebrain polarization, Cederquist et al. (2019) found that a Shh gradient promoted in vivo-like topographic organization of major forebrain subdivisions within organoids 102 . The organoid technology has also been applied to the generation of midbrain organoids, telencephalic tissue, and cerebellar tissue 53,103 –106 . To understand how neurons form circuits between different brain regions, fusion organoids have been generated 107 . This model was applied to the research of microcephaly, ZIKA virus infection and other brain diseases 98,99,108 –112 . To expand the applications of brain organoids, several challenges need to be addressed to enlarge organoids to contain mature neurons with functional circuits.
Challenges in Obtaining “Difficult” Neuronal Lineages
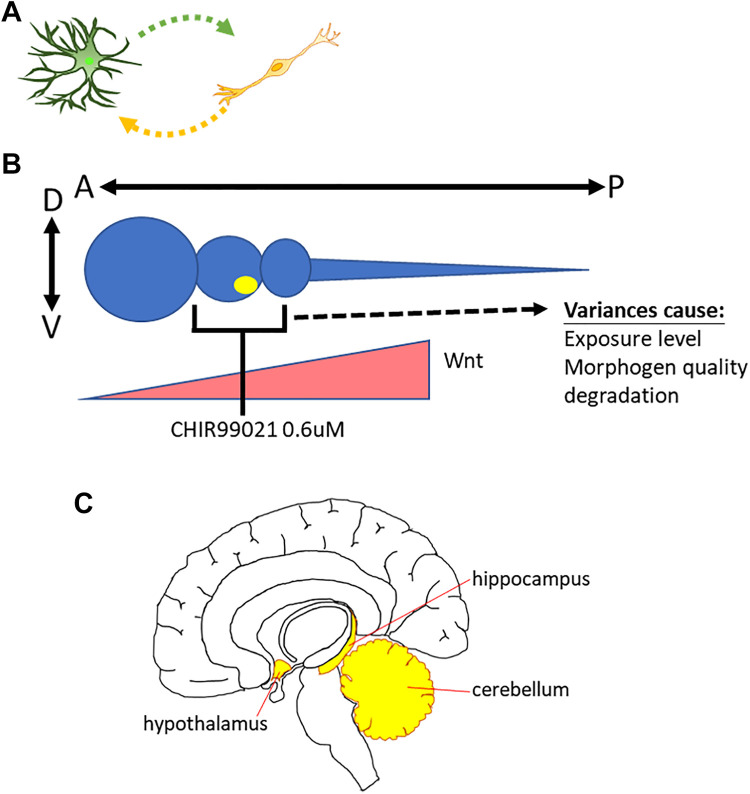
Methods that induce hPSCs to differentiate into several neural subtypes with high efficiency are available. However, efficient differentiation protocols remain insufficient for several “difficult” neural or glial cell types, such as DA neurons, Purkinje cells, and hypothalamus neurons. Moreover, several differentiation methods for neural lineages are undeveloped, such as CA1–CA4 specific neural subtypes in the hippocampus and specialized neural types in the cerebellum 53,54,61,113 .
The differentiation of specific neural types reflecting the development of the nervous system provides guidelines for the efficient generation of some neural lineages. Morphogens, signaling pathways, and cell–cell interactions have been explored during studies on CNS development. This basic information enables other investigators to establish guidelines for the development of specific neuron types; however, there are still several challenges for patterning specific neurons while following the developmental principles.
The first challenge is the difficulty in mimicking cell–cell direct interactions in vitro (Fig. 3A). Cell fate during CNS development is determined by not only the dosage of morphogens but also the cell–cell interactions that are not easy to recapitulate in the cell culture dishes. Moreover, mechanisms underlying cell fate determination by cell–cell interactions remain largely unknown.
Figure 3.
Challenges in obtaining “difficult” neuronal lineages. (A) The lack of in vivo like cell-cell interaction in culture dishes. (B) The major causes that influence the stability of dose dependent patterning protocol. (C) Specialized structures in the CNS.
The second challenge is the combination of morphogens (Fig. 3B). An example is the generation of DA neurons. Although the differentiation protocol for DA neurons was identified years ago, patterning efficiency is still insufficient. Most reports demonstrate 20%–30% efficiency for DA neuron differentiation, and the best efficiency to date is 50%–60% 46,48,49,114 –118 . The emerging position of DA neurons is in the most ventral midbrain, the middle part of the A-P axis. Thus, applying dosage-dependent or regional specific morphogens is a possible strategy to obtain high-purity DA neurons. CHIR99021 is a dose dependent GSK-3β inhibitor allow researchers to modulate Wnt signaling pathway as an A-P regulator. Low-dose CHIR99021 (0.4–0.8 μM) could promote NSCs into midbrain neurons 46,49 . However, delicate work is required to push only 50–60% of NSCs into DA neurons, based on the exposure level of every cell under the influence of morphogen. Several morphogens exhibit high-level expression at the midbrain–hindbrain boundary (MHB), and these molecules are termed MHB regional specific morphogens. Examples include FGF8b and Wnt1. However, these morphogens cannot induce NSCs to differentiate into midbrain neurons efficiently. Compared with “more challenging” neural type such as Purkinje cells, DA neurons are much easier because we only need to worry about the A-P patterning dosage. DA neurons are at the most ventral site of the D-V axis. However, for patterning of neural subpopulations that are not at the terminal points of A-P and D-V axes, the stability of morphogen combinations, dosage, and exposure level of every cell still needs further development.
The basic steps for inducing specific neural type include neural induction (promoting PSCs into NSCs) and neural type patterning (promoting NSCs into specific neural progenitors). However, patterning periods show a major impact on differentiation efficiency. Some neural types, such as motor neurons, are capable of late patterning 51 . The patterning process start late (start after >15 days of differentiation) can still yield high-purity motor neurons. Conversely, DA neurons need early-state patterning immediately after NSC induction, or DA neuron generation efficiency would be low 49 . Therefore, the timing of patterning should be considered during the development of methods for “difficult neural types.”
The A-P and D-V axis principle is not the only guideline. Some brain nuclei contain many distinct specialized subregions, such as the cerebellum, hippocampus, and hypothalamus (Fig. 3C). To date, developmental regulators of these regions are still not fully understood. Thus, neurons in these secondary structures pass through several stages that cannot be patterned with simple A-P/D-V principles. This process may be one issue that underlies challenges with Purkinje cells, CA1–CA4 specific, and hypothalamus neurons.
Strategies and Future Directions for Obtaining “Difficult” Neuronal Lineages
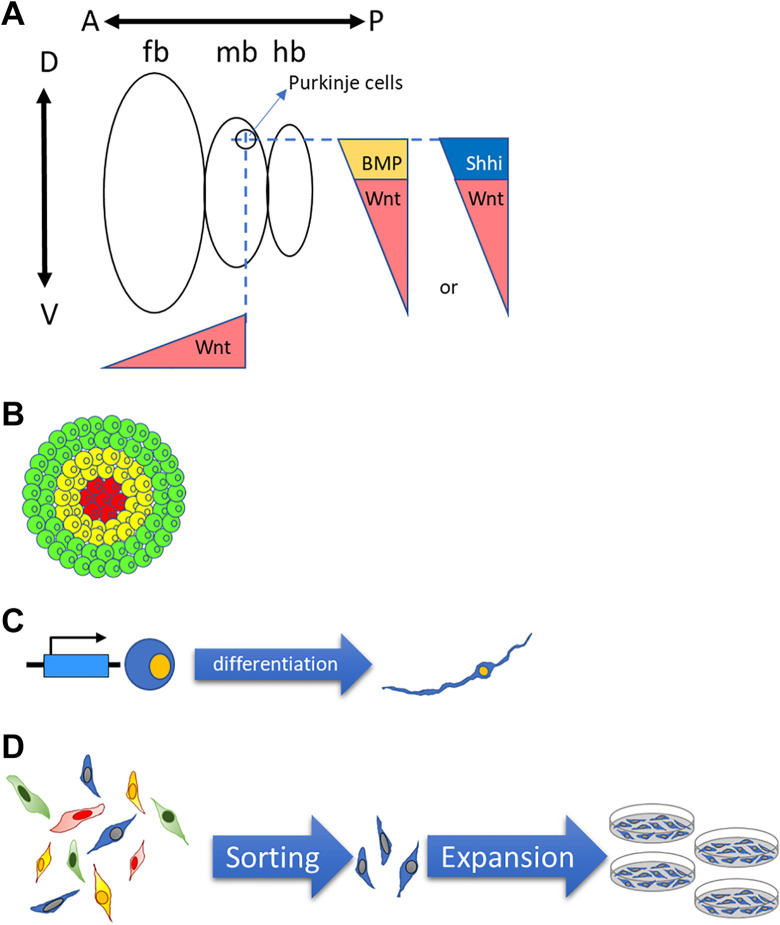
Several present and future approaches may be successful in addressing difficult neuronal types for cell transplantation and disease modeling. Advanced morphogen combinations may be workable for generating difficult neuronal lineages. Several morphogens can control the A-P and D-V axes simultaneously 57 . Patterning neural types between the most dorsal and ventral axes might yield the combinations of Shh and Wnt agonists CHIR99021 that provide ideal neural types based on the balance of D-V morphogen intensity 57 . Another morphogen, BMP, regulates the D-V axis exclusively and potentially participates in the precise patterning of some difficult neuron types that cannot be generated from the endpoint of D-V axis. For example, high-efficiency motor neuron differentiation protocols from embryonic stem cells are long since established. However, when we applied these protocols to our PSCs, we found that ventral spinal cord markers such as oligo2 and islet1 were expressed, but expression efficiency of the motor neuron-specific marker, HB9, varies batch to batch. Du et al. demonstrated that CHIR99021 (a dosage-dependent Wnt agonist) could drive the oligo2+ ventral spinal cord neural progenitors into nkx2.2− motor neuron progenitors instead of the p3 nkx2.2+ interneuron progenitors, thus stabilizing HB9+ motor neuron differentiation efficiency 51 . This concept of applying morphogen combinations with opposite functions for the specific neuronal type is a novel direction for investigating difficult neuronal types. Purkinje cells are located at the MHB, near the most dorsal granule cells. For A-P patterning, dosed CHIR99021 plays a key role in pushing NSCs into an MHB fate, and the coordination of Shh, CHIR99021, and BMP may provide the balance of D-V patterning necessary to generate Purkinje cells (Fig. 4B).
Figure 4.
Potential strategies in obtaining “difficult” neuronal lineages. (A) Combination of morphogens to target specific neural lineage. (B) Organoid method for obtaining specialized tissue like structures for specific neural lineages. (C) Sorting and expansion method for high purity interested neural types. (D) Over-expression of specific genes for obtaining interested neural lineages.
The second strategy is the co-culture or organoid approach (Fig. 4A). For some neuronal types that are in specialized structures, both the regional morphogens and cell–cell interactions are extremely important. Organoid approaches show promising effects in some cases. Muguruma et al. demonstrated that Purkinje cells could be retrieved from in vitro self-organized cerebellum organoids 53,54,113 . Another report suggested the differentiation of functional hippocampal neurons from the embryonic stem cell-derived dorsomedial telencephalic tissue 104 . These observations highlight the application potential of organoid approach to obtain specific neurons. A similar strategy is the co-culture of NSCs with stromal cells, glial cells, and specific primary neural types. In the early development of PSC differentiation protocols, co-culture methods were widely applied to induce neuronal types 119 . Some defined protocols were developed from these co-culture methods. Similarly, this approach could be a starting point for difficult neuronal types or for understanding control points for neuronal lineages.
The third approach is the overexpression of regional specific proteins by genetic modification. Zhang et al. demonstrated that overexpression of Ngn2 could promote PSCs into glutamate neurons, even bypassing the two-step differentiation principle 120 . This publication suggests the potential for specific gene overexpression methods to obtain specific types of neurons within a short time. Presently, genetic engineering should not be overly difficult. Transgenic methods, site-specific gene editing tools (zinc finger nuclease, TALENs, and CRISPR/Cas9), inducible expression systems, and fingerprint-free transposons provide ideal tools for a range of experimental purposes and lower the influences or risks of genetic modifications on stem cells.
Fluorescence-activated cell sorting (FACS) provides a promising tool for specific neuronal types. From the previous reports, Corin and LRTM1 are surface proteins that apply to DA progenitor FACS for higher-purity DA neurons 116,117 . Similarly, Corl2 was applied to Purkinje cell progenitor purification 113 . Thus, the discovery of neuronal specific surface markers from developmental knowledge or transcriptome analysis is a key step for specific neuronal types. The development of an expansion method for specific neuronal lineages is another major focus for amplifying ideal neuronal types. An established method to specifically amplify motor neural progenitors in defined factors 51 suggested the potential to expand specific neuronal lineages after or without FACS. These two strategies provide future directions for challenging neuronal type differentiation.
The shortage of knowledge of the nerve system development and gene expression patterns of specific neuron limits the development of neuron differentiation protocols. Thus, single-cell RNA sequence technology provides a powerful tool for exploring genet expression pattern of every cell during mammalian CNS development or stem cell differentiation. After transcriptome comparison, the identification of correlations between gene expression and neural populations is possible 121 –125 . Furthermore, correlation analysis will increase the possibility of defining novel surface markers, specific pathway activators, and miRNA regulators for the development of specific purification and differentiation strategies for specific neural precursors (Fig. 5).
Figure 5.
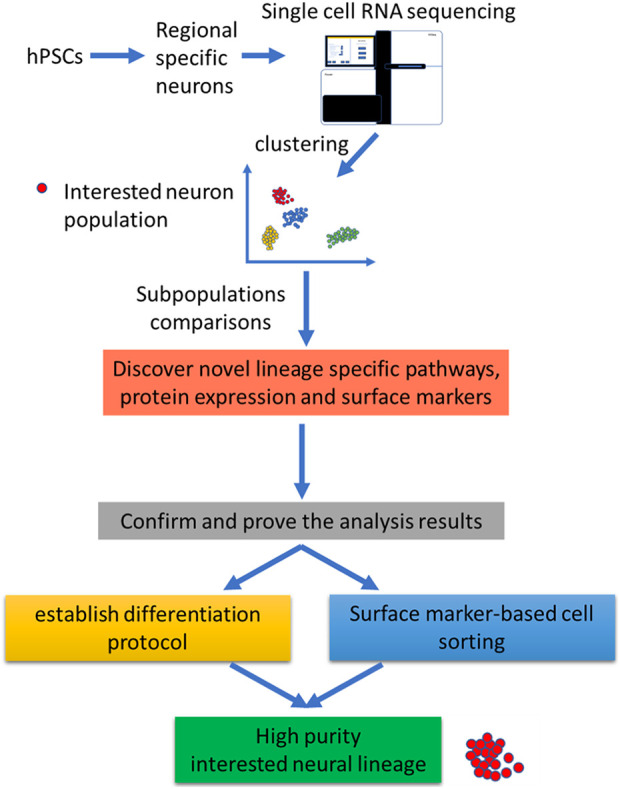
Application of single cell RNA sequencing on specific neural lineage differentiation from hPSCs. After differentiation of hPSCs into regional specific neurons, single cell RNA sequencing can be applied to cluster the neurons according to their transcriptome. Thus, after transcriptional properties comparison, novel differentiation protocol and surface markers can be identified.
Future Improvement of Neural Differentiation
Single-cell RNA sequencing might provide possibilities for novel differentiation methods for challenging neuronal types and identify currently unrecognized neuronal subpopulations. Kirkeby et al. (2017) discovered that ventral midbrain regional neural precursors might not benefit PD mice as much as expected. The most commonly used DA progenitor markers, that is, FOXA2, Lmx1a, Corin, and Nurr1, did not provide DA neurons at high yield or behavioral recovery after transplantation therapy. After the comparison of RNA sequencing with transplantation outcomes, authors found that key markers EN1 and Pax8 are directly linked to DA neuron yield after transplantation. According to these results, a protocol to generate caudal midbrain DA neurons was established for better therapeutic effects and add these factors into the differentiation system 126 . Recent years, studies showed novel DA neural subtypes and specific markers with single-cell RNA sequencing 121,124 . The realization of differentiation regulation and the major function of these DA subtypes might increase the therapeutic effects of iPSC derived DA neurons on PD therapy.
Protocols aimed at accelerating the maturation and functional integration of hPSC-derived neurons and glial cells are key for their application to in vitro nervous system modeling or transplantation therapy. The maturation of hPSC-derived neurons always takes >1 month. In some cases, such as Purkinje cells, >100 days are required for maturation. To develop drug screening platforms, researchers demonstrated that the overexpression of Ngn2 and other genes using genetic manipulation methods accelerate neuron maturation to only 1 week 120,127,128 . Without using artificial strategies, treatment with Notch inhibitor, compound E and DAPT, pushed neural progenitors into a functional neuron fate in 2–4 weeks 129,130 . The efficiency of this approach remains limited and varies according to neural type, and it highlights the possibility of screening for compounds that can benefit neuron maturation. For therapeutic transplantation, the rebuilding of nerve circuits between transplanted neurons and existing neurons is extremely important. Optogenetic and chemogenetic technologies provide tools to promote neural network formation. The light/chemical stimulation of optogenetic/chemogenetic receptors that express in human neurons triggers the expression of synaptic responses and the formation of circuits using mouse brain neurons in in vitro culture. Furthermore, optogenetics/chemogenetics were used to form neuromuscular junctions in vitro and motor circuits in a PD animal model after DA neuron transplantation 131 –134 .
Generally, the differentiation of hPSCs into functional neural types still faces challenges regarding differentiation methods, cell purity, neural maturation, circuit formation, quality stability, and large-scale preparation. Fortunately, biological techniques improve daily. Novel tools, such as next-generation sequencing, 3-D printing, and robotic screening systems, may shed light on the development of neural subtype differentiation strategies and expand the application of stem cells to the nervous system.
Acknowledgments
This work was supported by Bioinnovation Center and financially supported by the Ministry of Science and Technology, Taiwan (MOST 107-2314-B-303-003-MY3 and MOST 109-2314-B-303-023-), Buddhist Tzu Chi Medical Foundation and Hualien Tzu Chi Hospital (TCRD108-64 and TCMF-EP 108-03(109)), Hualien, Taiwan.
Footnotes
Author Contributions: T.J.H., S.Z.L. and H.J.H. initiated this project. C.Y.C. edited, organized and wrote the article. H.C.T. draw figures. C.Y.C. and C.A.L. wrote the paragraphs. H.L.S., T.W.C. and T.J.H. organized and proof the article. All authors reviewed this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ministry of Science and Technology, Taiwan (MOST 107-2314-B-303-003-MY3 and MOST 109-2314-B-303-023-), Buddhist Tzu Chi Medical Foundation and Hualien Tzu Chi Hospital (TCRD108-64and TCMF-EP 108-03(109)), Hualien, Taiwan.
ORCID iD: Chia-Yu Chang  https://orcid.org/0000-0002-3585-5944
https://orcid.org/0000-0002-3585-5944
Ching-Ann Liu  https://orcid.org/0000-0002-0392-7069
https://orcid.org/0000-0002-0392-7069
Horng-Jyh Harn  https://orcid.org/0000-0001-6777-3284
https://orcid.org/0000-0001-6777-3284
Shinn-Zong Lin  https://orcid.org/0000-0002-4601-9933
https://orcid.org/0000-0002-4601-9933
References
- 1. Boldog E, Bakken TE, Hodge RD, Novotny M, Aevermann BD, Baka J, Borde S, Close JL, Diez-Fuertes F, Ding SL, Faragó N, et al. Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat Neurosci. 2018;21(9):1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keefe MG, Nowakowski TJ. A recipe book for cell types in the human brain. Nature. 2019;573(7772):36–37. [DOI] [PubMed] [Google Scholar]
- 3. Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347(6226):1138–1142. [DOI] [PubMed] [Google Scholar]
- 4. Lake BB, Ai R, Kaeser GE, Salathia NS, Yung YC, Liu R, Wildberg A, Gao D, Fung HL, Chen S, Vijayaraghavan R, et al. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science. 2016;352(6293):1586–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. [DOI] [PubMed] [Google Scholar]
- 6. Barzilai A, Melamed E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol Med. 2003;9(3):126–132. [DOI] [PubMed] [Google Scholar]
- 7. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4(1):62–73. [DOI] [PubMed] [Google Scholar]
- 9. Xia G, McFarland KN, Wang K, Sarkar PS, Yachnis AT, Ashizawa T. Purkinje cell loss is the major brain pathology of spinocerebellar ataxia type 10. J Neurol Neurosurg Psychiatry. 2013;84(12):1409–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gandhi S, Muqit MM, Stanyer L, Healy DG, Abou-Sleiman PM, Hargreaves I, Heales S, Ganguly M, Parsons L, Lees AJ, Latchman DS, et al. PINK1 protein in normal human brain and Parkinson’s disease. Brain. 2006;129(Pt 7):1720–1731. [DOI] [PubMed] [Google Scholar]
- 11. Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85(2):257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 15. Chang CY, Ting HC, Liu CA, Su HL, Chiou TW, Harn HJ, Lin SZ. Induced pluripotent stem cells: a powerful neurodegenerative disease modeling tool for mechanism study and drug discovery. Cell Transplant. 2018;27(11):1588–1602. 963689718775406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang CY, Ting HC, Su HL, Jeng JR. Combining Induced Pluripotent Stem Cells and Genome Editing Technologies for Clinical Applications. Cell Transplant. 2018;27(3):379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107(9):4335–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engert S, Burtscher I, Liao WP, Dulev S, Schotta G, Lickert H. Wnt/beta-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development. 2013;140(15):3128–3138. [DOI] [PubMed] [Google Scholar]
- 19. Kurek D, Neagu A, Tastemel M, Tuysuz N, Lehmann J, van de Werken HJG, Philipsen S, van der Linden R, Maas A, van IWFJ, Drukker M, et al. Endogenous WNT signals mediate BMP-induced and spontaneous differentiation of epiblast stem cells and human embryonic stem cells. Stem Cell Reports. 2015;4(1):114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teo AK, Valdez IA, Dirice E, Kulkarni RN. Comparable generation of activin-induced definitive endoderm via additive Wnt or BMP signaling in absence of serum. Stem Cell Reports. 2014;3(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012;7(11):2029–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24(2):185–187. [DOI] [PubMed] [Google Scholar]
- 24. Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep 2014;4:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiao L, Yuan X, Sharkis SJ, Activin A.maintains self-renewal and regulates fibroblast growth factor, WNT, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24(6):1476–1486. [DOI] [PubMed] [Google Scholar]
- 26. Yoo YD, Huang CT, Zhang X, Lavaute TM, Zhang SC. Fibroblast growth factor regulates human neuroectoderm specification through ERK1/2-PARP-1 pathway. Stem Cells. 2011;29(12):1975–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313(1):107–117. [DOI] [PubMed] [Google Scholar]
- 28. Dhara SK, Stice SL. Neural differentiation of human embryonic stem cells. J Cell Biochem. 2008;105(3):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen MA, Itsykson P, Reubinoff BE. Neural differentiation of human ES cells. Curr Protoc Cell Biol. 2007;Chapter 23: Unit 23 7. [DOI] [PubMed] [Google Scholar]
- 31. Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–1133. [DOI] [PubMed] [Google Scholar]
- 32. Abranches E, Silva M, Pradier L, Schulz H, Hummel O, Henrique D, Bekman E. Neural differentiation of embryonic stem cells in vitro: a road map to neurogenesis in the embryo. PLoS One. 2009;4(7):e6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cai C, Grabel L. Directing the differentiation of embryonic stem cells to neural stem cells. Dev Dyn. 2007;236(12):3255–3266. [DOI] [PubMed] [Google Scholar]
- 34. Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen SM, Lee MS, Chang CY, Lin SZ, Cheng EH, Liu YH, Pan HC, Lee HC, Su HL. Prerequisite OCT4 maintenance potentiates the neural induction of differentiating human embryonic stem cells and induced pluripotent stem cells. Cell Transplant 2015;24(5):829–844. [DOI] [PubMed] [Google Scholar]
- 36. Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78. [DOI] [PubMed] [Google Scholar]
- 37. Maury Y, Come J, Piskorowski RA, Salah-Mohellibi N, Chevaleyre V, Peschanski M, Martinat C, Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat Biotechnol. 2015;33(1):89–96. [DOI] [PubMed] [Google Scholar]
- 38. Martinez S, Andreu A, Mecklenburg N, Echevarria D. Cellular and molecular basis of cerebellar development. Front Neuroanat. 2013;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lekven AC, Buckles GR, Kostakis N, Moon RT. Wnt1 and wnt10b function redundantly at the zebrafish midbrain-hindbrain boundary. Dev Biol 2003;254(2):172–187. [DOI] [PubMed] [Google Scholar]
- 40. Buckles GR, Thorpe CJ, Ramel MC, Lekven AC. Combinatorial Wnt control of zebrafish midbrain-hindbrain boundary formation. Mech Dev. 2004;121(5):437–447. [DOI] [PubMed] [Google Scholar]
- 41. Riou JF, Delarue M, Mendez AP, Boucaut JC. Role of fibroblast growth factor during early midbrain development in Xenopus. Mech Dev. 1998;78(1-2):3–15. [DOI] [PubMed] [Google Scholar]
- 42. Sunmonu NA, Li K, Guo Q, Li JY. Gbx2 and Fgf8 are sequentially required for formation of the midbrain-hindbrain compartment boundary. Development. 2011;138(4):725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu J, Liu Z, Ornitz DM. Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development. 2000;127(9):1833–1843. [DOI] [PubMed] [Google Scholar]
- 44. Brafman D, Willert K. Wnt/beta-catenin signaling during early vertebrate neural development. Dev Neurobiol. 2017;77(11):1239–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tran FH, Zheng JJ. Modulating the wnt signaling pathway with small molecules. Protein Sci. 2017;26(4):650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1(6):703–714. [DOI] [PubMed] [Google Scholar]
- 47. Lu J, Zhong X, Liu H, Hao L, Huang CT, Sherafat MA, Jones J, Ayala M, Li L, Zhang SC. Generation of serotonin neurons from human pluripotent stem cells. Nat Biotechnol. 2016;34(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011;480(7378):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang SC. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30(8):1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26(4):886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Du ZW, Chen H, Liu H, Lu J, Qian K, Huang CL, Zhong X, Fan F, Zhang SC. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun. 2015;6:6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23(6):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10(4):537–550. [DOI] [PubMed] [Google Scholar]
- 54. Muguruma K, Nishiyama A, Ono Y, Miyawaki H, Mizuhara E, Hori S, Kakizuka A, Obata K, Yanagawa Y, Hirano T, Sasai Y. Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat Neurosci. 2010;13(10):1171–1180. [DOI] [PubMed] [Google Scholar]
- 55. Le Dreau G, Marti E. Dorsal-ventral patterning of the neural tube: a tale of three signals. Dev Neurobiol. 2012;72(12):1471–1481. [DOI] [PubMed] [Google Scholar]
- 56. Ulloa F, Marti E. Wnt won the war: antagonistic role of Wnt over Shh controls dorso-ventral patterning of the vertebrate neural tube. Dev Dyn. 2010;239(1):69–76. [DOI] [PubMed] [Google Scholar]
- 57. Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136(23):4055–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Y, Liu H, Sauvey C, Yao L, Zarnowska ED, Zhang SC. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc. 2013;8(9):1670–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, Zhou GM, Ayala M, Zhang SC. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat Biotechnol. 2013;31(5):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10(4):455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tao Y, Zhang SC. Neural subtype specification from human pluripotent stem cells. Cell Stem Cell. 2016;19(5):573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, Sasai Y, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12(5):573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Merkle FT, Maroof A, Wataya T, Sasai Y, Studer L, Eggan K, Schier AF. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development. 2015;142(4):633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, Widmer HR, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12(5):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim TG, Yao R, Monnell T, Cho JH, Vasudevan A, Koh A, Peeyush KT, Moon M, Datta D, Bolshakov VY, Kim KS, et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cells. 2014;32(7):1789–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16(21):2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29(6):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Krencik R, Zhang SC. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc. 2011;6(11):1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tcw J, Wang M, Pimenova AA, Bowles KR, Hartley BJ, Lacin E, Machlovi SI, Abdelaal R, Karch CM, Phatnani H, Slesinger PA, et al. An efficient platform for astrocyte differentiation from human induced pluripotent stem cells. Stem Cell Reports. 2017;9(2):600–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shaltouki A, Peng J, Liu Q, Rao MS, Zeng X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells. 2013;31(5):941–952. [DOI] [PubMed] [Google Scholar]
- 71. Canals I, Ginisty A, Quist E, Timmerman R, Fritze J, Miskinyte G, Monni E, Hansen MG, Hidalgo I, Bryder D, Bengzon J, et al. Rapid and efficient induction of functional astrocytes from human pluripotent stem cells. Nat Methods. 2018;15(9):693–696. [DOI] [PubMed] [Google Scholar]
- 72. Li X, Tao Y, Bradley R, Du Z, Tao Y, Kong L, Dong Y, Jones J, Yan Y, Harder CRK, Friedman LM, et al. Fast generation of functional subtype astrocytes from human pluripotent stem cells. Stem Cell Reports. 2018;11(4):998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tchieu J, Calder EL, Guttikonda SR, Gutzwiller EM, Aromolaran KA, Steinbeck JA, Goldstein PA, Studer L. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat Biotechnol. 2019;37(3):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, Tabar V. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16(2):198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49(3):385–396. [DOI] [PubMed] [Google Scholar]
- 76. Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136(9):1443–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports. 2014;3(2):250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development. 2015;142(23):3983–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gorris R, Fischer J, Erwes KL, Kesavan J, Peterson DA, Alexander M, Nothen MM, Peitz M, Quandel T, Karus M, Brüstle O. Pluripotent stem cell-derived radial glia-like cells as stable intermediate for efficient generation of human oligodendrocytes. Glia. 2015;63(12):2152–2167. [DOI] [PubMed] [Google Scholar]
- 80. Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34(3):310–323. [DOI] [PubMed] [Google Scholar]
- 81. Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12(2):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol. 2004;275(1):1–11. [DOI] [PubMed] [Google Scholar]
- 83. Shakhova O, Sommer L. Neural crest-derived stem cells. StemBook; 2008. [PubMed] [Google Scholar]
- 84. Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, Zeltner N, Mica Y, El-Nachef W, Zhao H, de Stanchina E, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531(7592):105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zeltner N, Fattahi F, Dubois NC, Saurat N, Lafaille F, Shang L, Zimmer B, Tchieu J, Soliman MA, Lee G, Casanova JL, et al. Capturing the biology of disease severity in a PSC-based model of familial dysautonomia. Nat Med. 2016;22(12):1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tchieu J, Zimmer B, Fattahi F, Amin S, Zeltner N, Chen S, Studer L. A modular platform for differentiation of human PSCS into all major ectodermal lineages. Cell Stem Cell. 2017;21(3):399–410 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Clark AJ, Kaller MS, Galino J, Willison HJ, Rinaldi S, Bennett DLH. Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination. Brain. 2017;140(4):898–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cai S, Han L, Ao Q, Chan YS, Shum DK. Human induced pluripotent cell-derived sensory neurons for fate commitment of bone marrow-derived Schwann cells: implications for remyelination therapy. Stem Cells Transl Med. 2017;6(2):369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Okawa T, Kamiya H, Himeno T, Kato J, Seino Y, Fujiya A, Kondo M, Tsunekawa S, Naruse K, Hamada Y, Ozaki N, et al. Transplantation of neural crest-like cells derived from induced pluripotent stem cells improves diabetic polyneuropathy in mice. Cell Transplant. 2013;22(10):1767–1783. [DOI] [PubMed] [Google Scholar]
- 90. Ziegler L, Grigoryan S, Yang IH, Thakor NV, Goldstein RS. Efficient generation of schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev Rep. 2011;7(2):394–403. [DOI] [PubMed] [Google Scholar]
- 91. Kim HS, Lee J, Lee DY, Kim YD, Kim JY, Lim HJ, Lim S, Cho YS. Schwann cell precursors from human pluripotent stem cells as a potential therapeutic target for myelin repair. Stem Cell Reports. 2017;8(6):1714–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Barber K, Studer L, Fattahi F. Derivation of enteric neuron lineages from human pluripotent stem cells. Nat Protoc. 2019;14(4):1261–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3(5):519–532. [DOI] [PubMed] [Google Scholar]
- 94. Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–56. [DOI] [PubMed] [Google Scholar]
- 95. Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–785. [DOI] [PubMed] [Google Scholar]
- 96. Kelava I, Lancaster MA. Stem cell models of human brain development. Cell Stem Cell. 2016;18(6):736–748. [DOI] [PubMed] [Google Scholar]
- 97. Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014;9(10):2329–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 2017;35(7):659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, Paulsen O, et al. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 2019;22(4):669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cederquist GY, Asciolla JJ, Tchieu J, Walsh RM, Cornacchia D, Resh MD, Studer L. Specification of positional identity in forebrain organoids. Nat Biotechnol. 2019;37(4):436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Goke J, Tan ZY, Saw TY, Tan CP, Lokman H, Lee Y, et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell. 2016;19(2):248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, Sasai Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun. 2015;6:8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Monzel AS, Smits LM, Hemmer K, Hachi S, Moreno EL, van Wuellen T, Jarazo J, Walter J, Bruggemann I, Boussaad I, Berger E, et al. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Reports. 2017;8(5):1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16(11):1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, Zhong M, et al. Fusion of regionally specified hpsc-derived organoids models human brain development and interneuron migration. Cell Stem Cell. 2017;21(3):383–398 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhou T, Tan L, Cederquist GY, Fan Y, Hartley BJ, Mukherjee S, Tomishima M, Brennand KJ, Zhang Q, Schwartz RE, Evans T, et al. High-content screening in hpsc-neural progenitors identifies drug candidates that inhibit zika virus infection in fetal-like organoids and adult brain. Cell Stem Cell. 2017;21(2):274–283 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, Tsai LH. Self-organizing 3d human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS One. 2016;11(9):e0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, Wynshaw-Boris A, Kriegstein AR. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell. 2017;20(4):435–449 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, Khakh BS, Coppola G, Pearson CA, Yamauchi K, Gong D, Dai X, et al. Self-organized cerebral organoids with human-specific features predict effective drugs to combat zika virus infection. Cell Rep. 2017;21(2):517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mansour AA, Goncalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36(5):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ishida Y, Kawakami H, Kitajima H, Nishiyama A, Sasai Y, Inoue H, Muguruma K. Vulnerability of Purkinje cells generated from spinocerebellar ataxia type 6 patient-derived iPSCs. Cell Rep. 2017;18(4):1075–1076. [DOI] [PubMed] [Google Scholar]
- 114. Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, Park KJ, Lee IH, Lopes C, Feitosa M, Luna MJ, et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J Clin Invest. 2020;130(2):904–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, Jeon J, Cha Y, Kim K, Li Q, Henchcliffe C, et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N Engl J Med. 2020;382(20):1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports. 2014;2(3):337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Samata B, Doi D, Nishimura K, Kikuchi T, Watanabe A, Sakamoto Y, Kakuta J, Ono Y, Takahashi J. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nat Commun. 2016;7:13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017;548(7669):592–596. [DOI] [PubMed] [Google Scholar]
- 119. Hiragi T, Andoh M, Araki T, Shirakawa T, Ono T, Koyama R, Ikegaya Y. Differentiation of human induced pluripotent stem cell (hipsc)-derived neurons in mouse hippocampal slice cultures. Front Cell Neurosci. 2017;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78(5):785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kee N, Volakakis N, Kirkeby A, Dahl L, Storvall H, Nolbrant S, Lahti L, Bjorklund AK, Gillberg L, Joodmardi E, Sandberg R, et al. Single-cell analysis reveals a close relationship between differentiating dopamine and subthalamic nucleus neuronal lineages. Cell Stem Cell. 2017;20(1):29–40. [DOI] [PubMed] [Google Scholar]
- 122. Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–153. [DOI] [PubMed] [Google Scholar]
- 123. Yao Z, Mich JK, Ku S, Menon V, Krostag AR, Martinez RA, Furchtgott L, Mulholland H, Bort S, Fuqua MA, Gregor BW, et al. A single-cell roadmap of lineage bifurcation in human esc models of embryonic brain development. Cell Stem Cell. 2017;20(1):120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tiklova K, Bjorklund AK, Lahti L, Fiorenzano A, Nolbrant S, Gillberg L, Volakakis N, Yokota C, Hilscher MM, Hauling T, Holmström F, et al. Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat Commun. 2019;10(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Burke EE, Chenoweth JG, Shin JH, Collado-Torres L, Kim SK, Micali N, Wang Y, Colantuoni C, Straub RE, Hoeppner DJ, Chen HY, et al. Dissecting transcriptomic signatures of neuronal differentiation and maturation using iPSCs. Nat Commun. 2020;11(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kirkeby A, Nolbrant S, Tiklova K, Heuer A, Kee N, Cardoso T, Ottosson DR, Lelos MJ, Rifes P, Dunnett SB, Grealish S, et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hesc-based therapy for Parkinson’s disease. Cell Stem Cell. 2017;20(1):135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kondo T, Imamura K, Funayama M, Tsukita K, Miyake M, Ohta A, Woltjen K, Nakagawa M, Asada T, Arai T and others. iPSC-based compound screening and in vitro trials identify a synergistic anti-amyloid beta combination for Alzheimer’s disease. Cell Rep. 2017;21(8):2304–2312. [DOI] [PubMed] [Google Scholar]
- 128. Imamura K, Izumi Y, Watanabe A, Tsukita K, Woltjen K, Yamamoto T, Hotta A, Kondo T, Kitaoka S, Ohta A, Tanaka A, et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(391):eaaf3962. [DOI] [PubMed] [Google Scholar]
- 129. Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brustle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28(5):955–964. [DOI] [PubMed] [Google Scholar]
- 130. Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH, et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30(7):715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chen Y, Xiong M, Dong Y, Haberman A, Cao J, Liu H, Zhou W, Zhang SC. Chemical control of grafted human Psc-derived neurons in a mouse model of Parkinson’s disease. Cell Stem Cell. 2016;18(6):817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotechnol. 2015;33(2):204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Steinbeck JA, Jaiswal MK, Calder EL, Kishinevsky S, Weishaupt A, Toyka KV, Goldstein PA, Studer L. Functional connectivity under optogenetic control allows modeling of human neuromuscular disease. Cell Stem Cell. 2016;18(1):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Osaki T, Uzel SGM, Kamm RD. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci Adv. 2018;4(10):eaat5847. [DOI] [PMC free article] [PubMed] [Google Scholar]