Summary.
The immunosuppressive tumor microenvironment in pancreatic cancer is comprised in part by various myeloid cells, including tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). We discuss the role of TAMs and MDSCs in promoting immune suppression and highlight current myeloid targeted therapies.
Pancreatic ductal adenocarcinoma (PDA), the most common pancreatic cancer, is a nearly universally lethal malignancy. PDA is characterized by extensive infiltration of immunosuppressive myeloid cells, including tumor-associated macrophages and myeloid-derived suppressor cells. Myeloid cells in the tumor microenvironment inhibit cytotoxic T-cell responses promoting carcinogenesis. Immune checkpoint therapy has not been effective in PDA, most likely because of this robust immune suppression, making it critical to elucidate mechanisms behind this phenomenon. Here, we review myeloid cell infiltration and cellular crosstalk in PDA progression and highlight current therapeutic approaches to target myeloid cell-driven immune suppression.
Pancreatic ductal adenocarcinoma (PDA) is one of the most lethal human malignancies, with a 5-year survival rate of only 10%.1 PDA is projected to become the second leading cause of cancer-related deaths by 2030.2 This poor prognosis is due in part to most patients presenting with metastatic disease and overwhelming resistance to chemotherapy and radiotherapy approaches. The only potential cure for PDA is surgical resection, for which only 20% of patients are eligible, and ultimately 80% of these patients will relapse with local recurrence or metastatic disease.3 Current frontline therapies are the chemotherapy regimens FOLFIRINOX or gemcitabine/nab-paclitaxel, which modestly extend survival.4, 5, 6 The main genetic drivers of PDA are mutations in the KRAS oncogene,7,8 along with loss of functional tumor suppressors (TP53, SMAD4, INK4A).9,10 Both acinar cells and ductal cells within the healthy pancreas can give rise to PDA, although acinar cells appear to have a higher propensity for transformation.11 Acinar cells go through a plastic transdifferentiation process called acinar to ductal metaplasia (ADM), which can progress to pancreatic intraepithelial neoplasia (PanINs) and ultimately adenocarcinoma.12 These stages of progression of human PDA have been recapitulated in genetically engineered mouse models that target oncogenic Kras expression to the pancreas, combined with inactivation of tumor suppressors.13, 14, 15
PDA is characterized by a dense fibroinflammatory stroma that consists of fibroblasts, vasculature, nerves, extracellular matrix components, and infiltrating immune cells.16 The immune cells within the tumor microenvironment (TME) are immunosuppressive in nature.17 Within the TME, there is an extensive infiltration of myeloid cells that directly promote tumor progression18 and prevent T-cell responses.19 Accordingly, myeloid cell abundance in tumors correlates with worse outcomes,20,21 whereas the abundance of tumor-infiltrating T cells correlates with longer survival.22
Immune therapy has revolutionized treatment for several malignancies.23,24 However, the benefit of single agent immunotherapy has not yet extended to PDA,25,26 with the exception of the 1% of PDA patients with microsatellite instability high tumors.27 Immune checkpoint therapy acts by reactivating T-cell effector functions most commonly through blockade of programmed cell death 1 (PD-1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4), unleashing anti-tumor T-cell responses that result in reduced tumor burden.28 Although single agent immunotherapy has not been effective in PDA, recent trials using combination of targeting of T cells and myeloid cells are ongoing, supported by robust preclinical data. In this review, we will describe the critical role myeloid cells play as mediators of immune suppression in PDA and highlight potential strategies to target these cells in the context of combination immunotherapy.
Multiple Myeloid Cell Populations Promote PDA
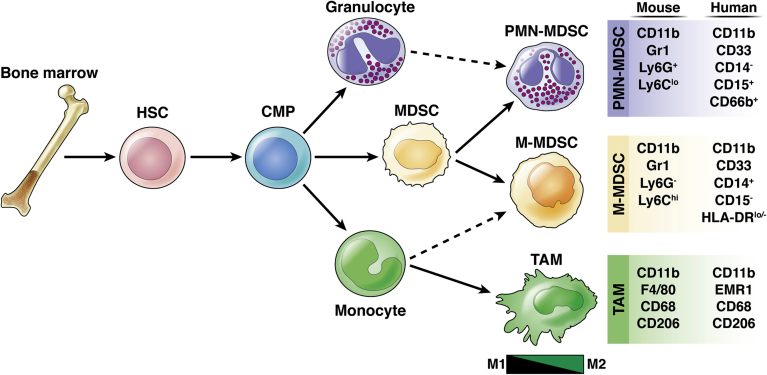
In normal physiology, myeloid cells develop from hematopoietic stem cells in the bone marrow in a process called myelopoiesis.29 Myeloid cells are defined as CD45+ CD11b+ cells but further differentiate into distinct populations: macrophages, granulocytes, mast cells, and dendritic cells, all components of the innate immune system. Macrophages within the tumor are referred to as tumor-associated macrophages (TAMs) and have distinct features compared with normal macrophages. Granulocytes can be further divided into eosinophils, basophils, and neutrophils. Within the TME, neutrophils and monocytes are often in an immature state referred to as immature myeloid cells/myeloid-derived suppressor cell (MDSC). In this review we will focus specifically on the role of TAMs and MDSCs in PDA progression (Figure 1).
Figure 1.
Myeloid cell lineage differentiation and markers. Schematic of myeloid cell differentiation from the bone marrow. Hematopoietic stem cells (HSC) from the bone marrow give rise to common myeloid progenitors (CMP), which give rise to monocytes, granulocytes, and immature myeloid cells, referred to as myeloid-derived suppressor cells (MDSCs). Monocytes in the circulation differentiate into tumor-associated macrophages (TAM) when they enter the tissue. TAMs exist on a spectrum of polarization, with M1 and M2 being at either extreme. MDSCs can be classified into 2 main subsets: PMN-MDSC and M-MDSC. PMN-MDSCs are phenotypically more similar to granulocytes, and M-MDSCs closely resemble monocytes (dashed arrow). Surface markers used to define each myeloid population in both mice and humans are listed on the right.
Tumor-Associated Macrophages
Within the PDA TME, macrophages are an abundant immune cell population.30,31 Macrophages derived from embryonic progenitors constitute the tissue-resident population; macrophages can also derive from infiltrating monocytes.32 Macrophages perform multiple physiological functions, including phagocytosis to eliminate debris, antigen presentation, and cytokine secretion to recruit other immune cells to the site of injury.33,34 Macrophages are defined by expression of CD11b+ CD68+ EMR1+ in humans and CD11b+ CD68+ F4/80+ in mice. Macrophages are plastic cells that exist on a spectrum of differentiation states. On the basis of in vitro assays, macrophages can be classified into 2 main subtypes on each extreme of the spectrum. M1, or classically activated, macrophages are generally considered to have anti-tumor activities and can be induced through interferon-gamma and toll-like receptor stimuli.35 M1 macrophages are characterized by high expression of interleukin 12 (IL12), tumor necrosis factor (TNF), and inducible nitric oxide synthase. M2, or alternatively activated, macrophages are considered to have pro-tumor activities36 and can be induced through the cytokines IL4 and IL13.37 M2 macrophages lose their antigen presentation abilities and act to instead suppress the immune response through a variety of mechanisms.
The M1/M2 classification is an oversimplification that is helpful for broad description but does not accurately describe the in vivo heterogeneity of TAMs. TAMs within the tumor are derived from either infiltrating monocytes or embryonically derived, tissue-resident macrophages.38 Furthermore, the heterogeneity of TAM origin has functional implications, where monocyte derived TAMs have increased antigen presentation abilities, and embryonically derived TAMs shape the fibrotic response.38 Within the TME, TAMs conform to neither the M1 nor the M2 phenotype but rather have traits of both polarization states.35 Their overall pro-tumor function explains the inverse correlation between TAMs and survival.39,40
TAMs have been extensively studied in PDA. Because of the plasticity of macrophages, TAM targeted therapy aims to reprogram them to their anti-tumor functions. The colony-stimulating factor 1/colony-stimulating factor 1 receptor (CSF1/CSF1R) axis recruits and polarizes immunosuppressive TAMs. CSF1R is the major lineage regulator for all macrophage subsets.35 PDA tumors are infiltrated by CSF1R+ macrophages.41,42 Inhibition of CSF1R in mice results in reduced tumor burden and an increase in T-cell infiltration, providing evidence that targeting TAMs relieves immune suppression in the TME.19,41 Furthermore, CSF1R inhibition in mice sensitizes PDA tumors to either PD-1 or CTLA-4 antagonists,42 suggesting that although single agent immunotherapy is not sufficient to reduce tumor burden, immune checkpoint blockade in combination with TAM modulating therapies can effectively reverse immune therapy resistance.
The CCL2/CCR2 chemokine axis is critical for the genesis of TAMs. CCL2 produced by tumor cells recruits CCR2+ monocytes from the bone marrow to the circulation that then differentiate into TAMs after entering the tumor tissue.43 PDA patients with high levels of circulating monocytes have worse overall survival rates.20 Monocytes in circulation do not possess the same immunosuppressive abilities as TAMs, suggesting the cellular crosstalk in the TME is critical for this function.20 CCR2 blockade in mice results in retention of CCR2+ monocytes in the bone marrow, impairing tumor growth.20 CCR2 blockade in combination with gemcitabine further impairs tumor growth.20 Similarly, in a PDA clinical trial, patients with borderline resectable and locally advanced disease were treated with a combination of FOLFIRINOX and CCR2 antagonist (PF-04136309).44 After treatment, patients had reduced circulating CCR2+ monocytes and subsequently fewer TAMs in the tumor, as well as increased CD8+ T cells.44 However, a recent phase 1b trial evaluated PF-04136309 in combination with gemcitabine/nab-paclitaxel in patients with metastatic PDA.45 Unlike the previous phase 1b trial, this study did not show that PF-04136309 added additional benefit to the prescribed chemotherapy regimen.45 Furthermore, in the setting of metastatic PDA, CCR2 inhibition in combination with gemcitabine/nab-paclitaxel was not tolerable in patients.45 Taken together, these reports suggest that the benefit of CCR2 inhibition may be limited to locally advanced disease that does not extend to metastatic patients.
In addition to an increase in macrophage frequency in PDA, a recent study used multiplex immunofluorescence to evaluate the spatial relationship of M1 and M2 macrophages in human PDA.46 M1 macrophages were more often found in close proximity to tumor cells, compared with M2 macrophages. Interestingly, when M2 macrophages resided near tumor cells, patients had worse survival outcomes, compared with patients with more distal M2 macrophages. This study provides evidence that both macrophage abundance and location are important factors for patient outcome.
TAMs within the PDA TME express less antigen presenting MHC II,47 suggesting that macrophages could be reprogrammed to perform their role as antigen presenting cells. CD40 is a member of the TNF receptor superfamily and is expressed broadly on immune cells including monocytes and macrophages.48,49 Activation of CD40 with an agonist (FGK45) in mice resulted in up-regulation of MHC II in macrophages from the tumor and spleen, suggesting CD40 activation in part reprograms TAMs to an anti-tumor phenotype.50,51 FGK45 in combination with gemcitabine resulted in reduced tumor burden in a cohort of patients.50 In addition, combination of gemcitabine and CD40 agonism resulted in increased tumoral T-cell infiltration in mice.52 Paralleling the human trials, mouse models of PDA are also resistant to single agent immune checkpoint blockade; however, combined chemotherapy and immunotherapy approaches have shown success. Combination therapy of gemcitabine/nab-paclitaxel and αCD40 agonist sensitizes tumors to αPD-1 and aCTLA-4 immunotherapy in murine models of PDA.53 This combined chemotherapy and immunotherapy approach (gemcitabine, nab-paclitaxel, αCD40 agonist, αPD-1) is currently under clinical trial for patients with metastatic PDA (NCT03214250). Furthermore, in mice, the effectiveness of the combined chemotherapy and immunotherapy regimen can be predicted on the basis of the amount of CD8+ T-cell infiltration, with tumors rich in CD8+ T cells correlating with increased therapeutic response.54
Taken together, these studies highlight the tumor promoting role of TAMs in the PDA TME. Macrophage targeted therapy is promising because it synergizes with frontline chemotherapy and immunotherapy regimens to reactivate effector T-cell responses and reduce tumor burden.
Myeloid-Derived Suppressor Cells
MDSCs are immature myeloid cells with immunosuppressive functions. MDSCs can be further classified into 2 main populations, polymorphonuclear (PMN)-MDSCs/granulocytic-MDSCs and mononuclear-MDSCs (M-MDSCs). These subsets are phenotypically distinct. PMN-MDSCs have more resemblance to granulocytes/neutrophils, whereas M-MDSCs closely resemble monocytes. In mice, MDSCs are broadly defined by CD11b+ Gr-1+, with Ly-6C and Ly-6G used to delineate MDSC populations.55 In mice, MDSCs are defined CD11b+ Ly6Clo Ly6G+ for PMN-MDSCs and CD11b+ Ly6Chi Ly6G- for M-MDSCs.55 Because of their phenotypic differences, human PMN-MDSCs, which closely mirror granulocytes/neutrophils, are defined by CD11b+ CD14- CD15+ or CD11b+ CD14- CD66b+, whereas human M-MDSCs, which are more similar to monocytes, are defined by CD11b+ CD14+ HLA-DR-/lo CD15- .55 Although PMN-MDSCs and M-MDSCs are the major MDSC populations, there are MDSCs that share markers of both and may represent a common progenitor. This third MDSC population is called early stage MDSCs and has yet to be functionally evaluated in PDA.55 Although MDSCs are unique from their mature myeloid counterparts, neutrophils and monocytes, controversy remains on separating PMN-MDSCs from neutrophils. Currently, there are no markers to distinguish the immature PMN-MDSCs from mature neutrophils, and the only possible method of separation is via density centrifugation.56 M-MDSCs differ from monocytes because they express low HLA-DR and differ from TAMs because they do not express F4/80.57 Distinction between neutrophils and PMN-MDSCs remains challenging, and distinctive markers are needed.
Importantly, MDSCs are ultimately defined by their functionality. MDSCs perform their immune suppressive functions through multiple mechanisms, with the main one being depletion of the essential amino acid L-arginine from the TME.58,59 MDSCs produce high levels of Arginase 1 (ARG1), an enzyme that metabolizes L-arginine, resulting in T-cell inhibition.60 When considering MDSC function, it is important to also consider that MDSCs exist in 2 main populations. PMN-MDSCs comprise the largest percentage of MDSCs found in the blood and the tumor, compared with M-MDSCs.61 Despite M-MDSCs making up a smaller portion of the tumor, they often have an increased immunosuppressive function than PMN-MDSCs.62 Both MDSC populations express high amounts of the enzyme ARG1, which depletes L-arginine, resulting in T-cell inhibition.63 However, PMN-MDSCs and M-MDSCs have additional and distinct immunosuppressive functions. PMN-MDSCs produce high amounts of reactive oxygen species and low nitric oxide.61 M-MDSCs produce high nitric oxide and low reactive oxygen species.61 Furthermore, M-MDSC immune suppression is in part due to tumor cell-derived prostaglandin E2 activating p50, a nuclear factor kappa B (NF-κB) subunit that results in increased inducible nitric oxide synthase production.64 These data show MDSC populations have distinct mechanisms to suppress T cells.
Because of the immunosuppressive nature of MDSCs, targeting these cells within the PDA TME is an attractive option for pancreatic cancer treatment. Early work in mouse models targeted MDSCs through administration of zoledronic acid, which acts to reduce MDSCs recruitment through inhibition of matrix metalloproteinase 9.65 Administration of zoledronic acid in a PDA mouse model results in delayed tumor growth, enhanced survival, and increased CD8+ T-cell infiltration.66 CXCR2 is a receptor found on neutrophils/MDSCs and regulates the recruitment of MDSCs to the TME.67 Inhibition of CXCR2 in a genetically engineered mouse model of pancreatic cancer resulted in extended survival, an increase in T-cell infiltration, and synergy with immunotherapy.68 MDSCs are also recruited to the tumor through tumor cell-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) secretion. Neutralization of GM-CSF in murine models of PDA results in a reduction in MDSC recruitment and subsequently reduced tumor growth.69,70 Depletion of the PMN-MDSC subset with an antibody against Ly-6G results in tumor cell death and increased CD8+ T-cell infiltration.71 Thus, MDSC-targeted therapies can partially reverse immune suppression.
Myeloid-Epithelial Crosstalk Promotes Immune Suppression
Myeloid cells do not act alone in establishing an immune suppressive TME. Rather, they act as a central hub in a complex cellular crosstalk that promotes tumor progression. Here we will explore mechanisms of cellular crosstalk between myeloid cells and cancer cells that activate signaling pathways that enhance immune suppression (Figure 2).
Figure 2.
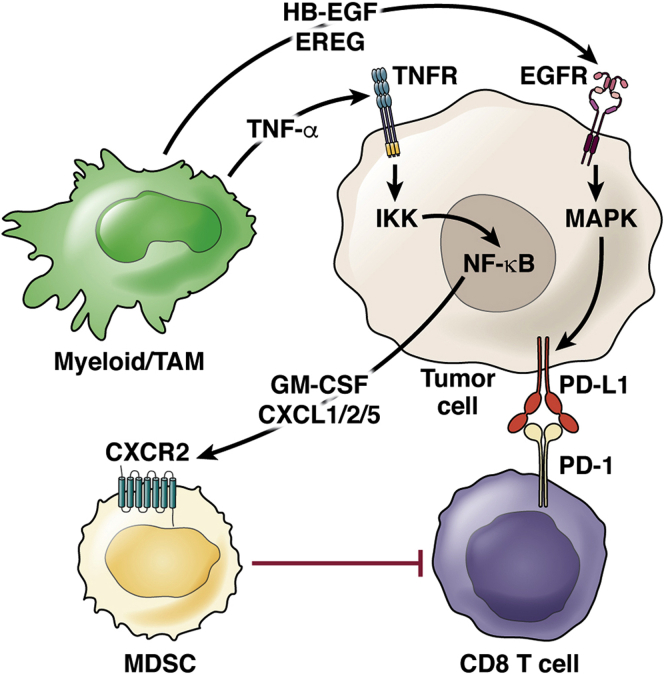
Myeloid-epithelial crosstalk promotes immune suppression. Schematic for cellular crosstalk and corresponding signaling pathways in the PDA TME that contribute to immune suppression. Myeloid cells secrete various ligands, HB-EGF, EREG, and TNF-α, that signal to their respective receptors, EGFR and TNFR, on tumor cells, thus activating EGFR/MAPK and NF-κB signaling, respectively. MAPK signaling in tumor cells results in elevation of PD-L1 expression, inhibiting CD8+ T cells through interaction with PD-1. NF-κB signaling in tumor cells results in secretion of GM-CSF and CXCL1, CXCL2, and CXCL5, which recruit MDSCs with the potential to suppress CD8+ T cells.
Beyond their role in establishing an immunosuppressive TME, myeloid cells play a critical role in promoting pancreatic carcinogenesis.18,72, 73, 74 In a PDA mouse model driven by inducible expression of oncogenic KrasG12D (iKras),75 myeloid cell ablation––using CD11b promoter driven expression of the diphtheria toxin receptor followed by diphtheria toxin treatment76–– causes regression of early PanIN lesions, preceded by reduced ERK activity in the neoplasia.18 Although oncogenic KRAS is the main genetic driver of PDA, it is not sufficient to induce carcinogenesis without additional activation of epidermal growth factor receptor (EGFR) to amplify mitogen-activated protein kinase (MAPK) signaling in the epithelium.77,78 Of note, myeloid cells in the neoplastic pancreas express high levels of the EGFR ligands, heparin-binding EGF-like growth factor (HB-EGF) and epiregulin, suggesting that they promote the initial stages of pancreatic carcinogenesis by stimulating epithelial EGFR. Conversely, oncogenic Kras expression in the epithelium also alters macrophage polarization.18 Extinguishing Kras expression in the iKras model results in decreased expression of Arginase 1 (Arg1) and the EGFR ligand HB-EGF (Hbegf) in the myeloid compartment, with subsequent loss of EGFR (Egfr) expression in the epithelial compartment. These data suggest that KRAS/EGFR/MAPK signaling regulates myeloid cell infiltration and polarization before PanIN formation, which in turn promotes epithelial transformation and progression of the neoplasia.
In addition to its early role in PDA formation, EGFR also regulates immune suppression in mouse models after carcinogenesis.74,79 Myeloid cell ablation from preexisting tumors results in reduced tumor burden, providing evidence that myeloid cells drive carcinogenesis in both early and late stages of disease.74 Myeloid cells secrete HB-EGF, an EGFR ligand, which activates EGFR/MAPK signaling in tumor cells leading to increased PD-L1 expression.74 Furthermore, ablation of EGFR in PDA sensitized tumors to chemotherapy and immunotherapy.79 Treatment with the EGFR inhibitor erlotinib reduced tumoral myeloid cells, increased CD8+ T cells, and enhanced response to immunotherapy.79 These studies suggest a role for EGFR/MAPK in promoting carcinogenesis and myeloid-mediated immune suppression.
NF-κB is a transcription factor with known diverse function in regulation of the immune system.80 Dysregulated NF-κB signaling can lead to inflammatory conditions such as cancer.81 Along with KRAS, NF-κB is constitutively active in PDA patients.82,83 NF-κB is held inactive in the cytoplasm in a complex with inhibitory κB proteins. Extracellular signals, such as TNFR ligation, activate inhibitory κB kinase (IKK), phosphorylate inhibitory κB, targeting it for degradation and resulting in the nuclear translocation of NF-κB complexes to activate transcription of target genes. The IKK complex is made up of 2 kinases, IKKα and IKKβ, and an additional subunit, NEMO/IKKγ.84 Inactivation of IKKβ in PDA tumors reduced infiltration of macrophages and MDSCs and blocked carcinogenesis, extending survival.82 Having established that both macrophages and NF-κB are important for initial transformation, it is interesting to note that one study linked an enhancement of ADM, the initial step of transformation, to macrophage production of TNF and subsequent activation of NF-κB.73 These data suggest NF-κB is not only critical for PDA formation but also mediates myeloid cell infiltration in the tumor.
NF-κB signaling also activates GM-CSF secretion.85 GM-CSF is a cytokine that functions to recruit MDSCs.69,70 Human PDA tumor cells treated with chemotherapy (gemcitabine or 5-FU) have increased levels of GM-CSF.86 Coincidentally, human tumor cells treated with gemcitabine have increased NF-κB activity. Monocytes cultured with chemotherapy treated tumor cells promote differentiation into immunosuppressive MDSCs.86 Taken together, these data suggest one possible mechanism for chemoresistance in PDA is active NF-κB signaling in tumor cells, which promotes an immunosuppressive myeloid phenotype, exacerbating disease.
NF-κB activates the expression of the chemokines CXCL1, CXCL2, and CXCL5, which in turn recruit CXCR2+ MDSCs, resulting in T-cell suppression.87, 88, 89 PDA patients have a heterogenous infiltration of T cells.90,91 Recent work identified CXCL1 as one mediator for T-cell heterogeneity in the PDA TME.54 Overexpression of tumor cell-derived Cxcl1 increases myeloid infiltration, specifically the granulocytic MDSCs, and fewer infiltrating CD8+ T cells, providing further evidence on the immunosuppressive role of CXCL1 in the TME.54 Furthermore, ablation of Cxcl1 in tumor cells results in fewer granulocytic MDSCs and a subsequent increase in CD8+ T cells, allowing the tumors to be sensitized to immunotherapy.54
Clearly, there is a complex cellular crosstalk between tumor cells and myeloid cells that suppresses T-cell infiltration and function in the TME. Multiple pathways are implicated in this immune suppressive phenotype. Work thus far targeting this tumor-myeloid interaction is compelling because it sensitizes tumors to immunotherapy approaches, highlighting the translational implications for PDA patients.
Myeloid Cells Establish the Pre-Metastatic Niche and Promote Metastatic Disease
The majority of PDA patients present with metastatic disease, and for those patients, limited therapeutic options are available. The liver is the most common site for metastatic dissemination in PDA. Pancreatic tumor cells disseminate early in carcinogenesis before progression to carcinoma.92 Despite the severity of metastatic disease, the process of metastasis is inefficient.93 A key barrier to tumor cell dissemination and survival in distal organs is the requirement of support from stromal cells.94 Inflammation is critical for progression of the primary tumor95 but is also critical for tumor cell dissemination.92 Myeloid cells colonize these distal sites before the arrival of the tumor cells in principle to create a hospitable environment for tumor cell growth96, 97, 98, 99 in a concept termed the pre-metastatic niche.
Currently, few studies have been performed evaluating the pre-metastatic niche in PDA. One study showed macrophages that are recruited to the liver secrete granulin, which in turn activates myofibroblasts, creating a permissive environment for tumor cell survival.94 Exosomes from tumor cells were identified as another mediator that promotes formation of the liver pre-metastatic niche in PDA.100 Tumor derived exosomes are taken up by Kupffer cells, resident liver macrophages, resulting in increased fibrosis in the liver and increased macrophage accumulation.100 This stromal accumulation prepares the liver for ultimate tumor cell survival. Macrophage migration inhibitory factor was determined to be the primary exosome cargo driving the pre-metastatic niche formation. As such, macrophage migration inhibitory factor ablation prevented formation of the pre-metastatic niche and subsequently reduced liver metastasis.100
IL6/signal transducer and activator of transcription 3/serum amyloid A signaling is another critical mechanism for the formation of the liver pre-metastatic niche.97 Rather than tumor cell-mediated formation of the pre-metastatic niche, this study identifies hepatocytes as an additional driver of the pre-metastatic niche.97 Genetic ablation of individual components of IL6/signal transducer and activator of transcription 3/serum amyloid A signaling resulted in fewer macrophages and PMN-MDSCs (Ly-6G+), preventing metastatic dissemination. The concept of the pre-metastatic niche is an important question that is relatively unexplored in PDA. Each of these studies provides a framework to explain the role myeloid cells play in pre-metastatic formation. Thus, identifying methods to interfere with myeloid function has the potential to mitigate metastasis of this highly aggressive cancer.
In addition to their role in tumorigenesis and pre-metastatic niche preparation, myeloid cells have been implicated in migration and invasion of metastatic disease in many cancer types.35,101,102 CCR220 and CXCR268 inhibition reduces metastatic dissemination in PDA through ablation of monocytes/macrophages and MDSCs, respectively. MDSC depletion in mouse PDA tumors converts the tumor from the highly invasive basal subtype to the less aggressive classical subtype and extended survival.68,103 Furthermore, pharmacologic depletion of macrophages with liposomal clodronate impairs angiogenesis and reduces metastasis formation in mice with PDA.104 Myeloid cells appear to be critical for both the formation of the pre-metastatic niche and metastatic dissemination.
Macrophages Drive Resistance to Chemotherapy
Because immune therapy has been ineffective in treating PDA, frontline therapy remains chemotherapy regimens, although they have only marginal efficacy.4,6,105,106 Current standard-of-care chemotherapy regimens for PDA patients include gemcitabine/nab-paclitaxel and FOLFIRINOX. However, PDA tumors are highly chemoresistant. A broad approach of depleting all myeloid cells using CD11b-DTR mice treated with diphtheria toxin results in tumors being sensitized to gemcitabine,107 suggesting myeloid cells can be targeted to reverse chemoresistance. Furthermore, dual inhibition of TAMs (CCR2+) and MDSCs (CXCR2+) resulted in increased efficacy of FOLFIRINOX.108
Myeloid Cell Compensatory Responses
Throughout this review we have highlighted a myriad of reports targeting monocytes/macrophages and MDSCs in PDA. It has become clear that these approaches, while beneficial, often result in a compensatory response of the other myeloid cell subsets. Two studies in PDA report a compensatory increase in monocyte and macrophage subsets when MDSCs are depleted.71,108 To prevent compensatory myeloid infiltration, another approach is to target all myeloid cells via integrin CD11b on their surface. Although antagonists for CD11b exist,109,110 they have not been well-tolerated in patients because of toxicity.111 Instead, an alternative approach to activate CD11b rather than antagonize has shown promise in preventing inflammation.112 The small molecule CD11b agonist reduces inflammation in a mouse model of PDA.113 CD11b agonism reduces myeloid infiltration, increases T-cell infiltration, and sensitizes tumors to both chemotherapy and immunotherapy.113 Although the total number of myeloid cells was reduced with CD11b agonism, macrophages that remained were reprogrammed, reducing the expression of a number of immunosuppressive genes (expressing Arginase 1, IL10, transforming growth factor beta) and increasing antigen presentation abilities, leading to activation of classical dendritic cells and subsequent T-cell infiltration.113 CD11b agonism is one potential avenue to avoid myeloid cell compensation when targeting a select myeloid cell subset.
Myeloid cells compensate for depletion of regulatory T cells, another immunosuppressive cell type in the PDA TME.114 In one study, depletion of regulatory T cells did not reverse immune suppression as hypothesized but rather accelerated tumor progression, in part because of a compensatory infiltration of immunosuppressive myeloid cells (Arginase 1, Chitinase3-like-3/YM1). This sustained immunosuppression was reduced through inhibition of the myeloid receptor CCR1, providing further indication that myeloid cells promote tumor progression and have complex and compensatory roles in the PDA TME.
Myeloid Single Cell Transcriptomics
Recent single cell RNA sequencing efforts in PDA have revealed significant heterogeneity within myeloid cell subsets that confirm the M1/M2 designation is an oversimplification. Analysis of human PDA tumor samples compared with adjacent normal pancreas tissue identified populations of neutrophils, classical monocytes/macrophages, resident macrophages, and alternatively activated macrophages.115 MARCO, APOE, SPP1, and C1QA emerged as novel macrophage markers that warrant further evaluation in PDA.115 Another study identified similar myeloid populations in human PDA compared with adjacent normal pancreas tissue with similar gene expression profiles.116 Myeloid cells are shown to have heterogenous expression of immune checkpoint receptors (LGALS9, CD274, PVR, CSF1R, SIRPA, HLA-DQA1).116 Putative immune checkpoint interactions were up-regulated in PDA compared with adjacent normal samples, and these interactions were heterogenous across patients.116 Because of the overwhelming lack of response to immunotherapy approaches, these data suggest the heterogeneity of immune checkpoints across patients is a contributing factor, and we should consider the possibility of precision medicine in immunomodulatory approaches.
Two studies used single cell transcriptomics analysis to evaluate the immune response during mouse PDA progression.117,118 Consistent with previous reports, macrophages were identified as one of the major immune cells infiltrating early lesions. Through unbiased clustering, 3 macrophage populations were identified in early lesions, whereas only 2 macrophage populations were identified in late/tumor samples.118 The macrophage population only found in early lesion samples had expression of Fn1, Lyz1, and Ear1, suggesting this population is involved in wound repair.118 There was not an equivalent macrophage population to this one seen in the late-stage tumor samples, suggesting macrophage populations change over the course of disease progression. In a separate study, macrophages from late lesions compared with early lesion samples had an increase in the chemokines, Cxcl1, Cxcl2, and Ccl8, which have known roles in recruitment of MDSCs (Cxcl1, Cxcl2) and macrophages (Ccl8), suggesting sustained infiltration of myeloid cells as carcinogenesis progresses.117 These macrophages up-regulated markers of alternative activation (Mrc1), further supporting the concept that macrophage polarization changes in later stages of PDA. Importantly, these combined efforts have revealed novel myeloid cells markers with potential functional importance in PDA.
Conclusions and Future Directions
In this review we have defined myeloid cell subsets in the PDA TME and discussed their role in myeloid cell-mediated immune suppression. We highlight the importance of myeloid cells through disease progression from initial formation of ADM to carcinogenesis to the formation of the pre-metastatic niche leading to ultimate tumor cell dissemination. Current myeloid targeted approaches in combination with chemotherapy and immunotherapy regimens relieve this robust immune suppression and activate T-cell effector responses.
However, many questions remain unanswered. The mechanisms behind the inverse correlation of myeloid cell and T cells have yet to be fully elucidated. Although we have some understanding of the pathways involved, we are lacking the complete picture, especially with respect to the complex compensatory networks that appear to overcome monolithic approaches. A better understanding of the mechanisms behind myeloid-mediated immune suppression will uncover novel and hopefully targetable components. With the large influx of single cell transcriptomics data, it has become even more evident that the M1/M2 designation is a gross oversimplification and does not accurately mirror the in vivo heterogeneity of macrophages. These reports have uncovered novel macrophage markers that may have functional implications and should be evaluated. Most of the MDSC work in PDA has targeted the PMN-MDSC subset. Because the M-MDSCs are more immunosuppressive in nature, selectively targeting this cell population is of interest. Myeloid cells comprise the largest part of the TME and are ideal targets to reverse immune suppression.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by NIH/NCI grants R01CA151588, R01CA198074 and the American Cancer Society to MPdM. This work was also supported by the NIH U01CA224145 and University of Michigan Cancer Center Support Grant (P30CA046592), including an Administrative Supplement to HCC and MPdM. SBK was supported by NIH T32-GM113900 and NCI F31-CA247076.
Contributor Information
Marina Pasca di Magliano, Email: marinapa@umich.edu.
Howard C. Crawford, Email: hcrawfo1@hfhs.org.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., Neoptolemos J.P. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y., Adenis A., Raoul J.L., Gourgou-Bourgade S., de la Fouchardiere C., Bennouna J., Bachet J.B., Khemissa-Akouz F., Pere-Verge D., Delbaldo C., Assenat E., Chauffert B., Michel P., Montoto-Grillot C., Ducreux M., Groupe Tumeurs Digestives of U., Intergroup P. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., Chone L., Francois E., Artru P., Biagi J.J., Lecomte T., Assenat E., Faroux R., Ychou M., Volet J., Sauvanet A., Breysacher G., Di Fiore F., Cripps C., Kavan P., Texereau P., Bouhier-Leporrier K., Khemissa-Akouz F., Legoux J.L., Juzyna B., Gourgou S., O’Callaghan C.J., Jouffroy-Zeller C., Rat P., Malka D., Castan F., Bachet J.B. Canadian Cancer Trials G, the Unicancer GIPG. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., Harris M., Reni M., Dowden S., Laheru D., Bahary N., Ramanathan R.K., Tabernero J., Hidalgo M., Goldstein D., Van Cutsem E., Wei X., Iglesias J., Renschler M.F. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 8.Hata T., Suenaga M., Marchionni L., Macgregor-Das A., Yu J., Shindo K., Tamura K., Hruban R.H., Goggins M. Genome-wide somatic copy number alterations and mutations in high-grade pancreatic intraepithelial neoplasia. Am J Pathol. 2018;188:1723–1733. doi: 10.1016/j.ajpath.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maitra A., Hruban R.H. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hezel A.F., Kimmelman A.C., Stanger B.Z., Bardeesy N., Depinho R.A. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 11.Kopp J.L., von Figura G., Mayes E., Liu F.F., Dubois C.L., Morris J.P.t., Pan F.C., Akiyama H., Wright C.V., Jensen K., Hebrok M., Sander M. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storz P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2017;14:296–304. doi: 10.1038/nrgastro.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguirre A.J., Bardeesy N., Sinha M., Lopez L., Tuveson D.A., Horner J., Redston M.S., DePinho R.A. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A., Ross S., Conrads T.P., Veenstra T.D., Hitt B.A., Kawaguchi Y., Johann D., Liotta L.A., Crawford H.C., Putt M.E., Jacks T., Wright C.V., Hruban R.H., Lowy A.M., Tuveson D.A. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 15.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H., Rustgi A.K., Chang S., Tuveson D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Chu G.C., Kimmelman A.C., Hezel A.F., DePinho R.A. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 17.Clark C.E., Hingorani S.R., Mick R., Combs C., Tuveson D.A., Vonderheide R.H. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Yan W., Mathew E., Kane K.T., Brannon A., 3rd, Adoumie M., Vinta A., Crawford H.C., Pasca di Magliano M. Epithelial-myeloid cell crosstalk regulates acinar cell plasticity and pancreatic remodeling in mice. Elife. 2017;6 doi: 10.7554/eLife.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchem J.B., Brennan D.J., Knolhoff B.L., Belt B.A., Zhu Y., Sanford D.E., Belaygorod L., Carpenter D., Collins L., Piwnica-Worms D., Hewitt S., Udupi G.M., Gallagher W.M., Wegner C., West B.L., Wang-Gillam A., Goedegebuure P., Linehan D.C., DeNardo D.G. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanford D.E., Belt B.A., Panni R.Z., Mayer A., Deshpande A.D., Carpenter D., Mitchem J.B., Plambeck-Suess S.M., Worley L.A., Goetz B.D., Wang-Gillam A., Eberlein T.J., Denardo D.G., Goedegebuure S.P., Linehan D.C. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsujikawa T., Kumar S., Borkar R.N., Azimi V., Thibault G., Chang Y.H., Balter A., Kawashima R., Choe G., Sauer D., El Rassi E., Clayburgh D.R., Kulesz-Martin M.F., Lutz E.R., Zheng L., Jaffee E.M., Leyshock P., Margolin A.A., Mori M., Gray J.W., Flint P.W., Coussens L.M. Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell Rep. 2017;19:203–217. doi: 10.1016/j.celrep.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balachandran V.P., Luksza M., Zhao J.N., Makarov V., Moral J.A., Remark R., Herbst B., Askan G., Bhanot U., Senbabaoglu Y., Wells D.K., Cary C.I.O., Grbovic-Huezo O., Attiyeh M., Medina B., Zhang J., Loo J., Saglimbeni J., Abu-Akeel M., Zappasodi R., Riaz N., Smoragiewicz M., Kelley Z.L., Basturk O., Australian Pancreatic Cancer Genome I. Garvan Institute of Medical R. Prince of Wales H. Royal North Shore H. University of G, St Vincent's H. Institute QBMR. University of Melbourne CfCR. University of Queensland IfMB. Bankstown H, Liverpool H, Royal Prince Alfred Hospital COBL. Westmead H., Fremantle H., St John of God H., Royal Adelaide H., Flinders Medical C., Envoi P., Princess Alexandria H., Austin H., Hopkins Johns, Medical I, Cancer AR-NCfARo. Gonen M., Levine A.J., Allen P.J., Fearon D.T., Merad M., Gnjatic S., Iacobuzio-Donahue C.A., Wolchok J.D., DeMatteo R.P., Chan T.A., Greenbaum B.D., Merghoub T., Leach S.D. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss S.A., Wolchok J.D., Sznol M. Immunotherapy of melanoma: facts and hopes. Clin Cancer Res. 2019;25:5191–5201. doi: 10.1158/1078-0432.CCR-18-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doroshow D.B., Sanmamed M.F., Hastings K., Politi K., Rimm D.L., Chen L., Melero I., Schalper K.A., Herbst R.S. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. 2019;25:4592–4602. doi: 10.1158/1078-0432.CCR-18-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., Pitot H.C., Hamid O., Bhatia S., Martins R., Eaton K., Chen S., Salay T.M., Alaparthy S., Grosso J.F., Korman A.J., Parker S.M., Agrawal S., Goldberg S.M., Pardoll D.M., Gupta A., Wigginton J.M. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S., Sherry R.M., Topalian S.L., Yang J.C., Lowy I., Rosenberg S.A. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., Wong F., Azad N.S., Rucki A.A., Laheru D., Donehower R., Zaheer A., Fisher G.A., Crocenzi T.S., Lee J.J., Greten T.F., Duffy A.G., Ciombor K.K., Eyring A.D., Lam B.H., Joe A., Kang S.P., Holdhoff M., Danilova L., Cope L., Meyer C., Zhou S., Goldberg R.M., Armstrong D.K., Bever K.M., Fader A.N., Taube J., Housseau F., Spetzler D., Xiao N., Pardoll D.M., Papadopoulos N., Kinzler K.W., Eshleman J.R., Vogelstein B., Anders R.A., Diaz L.A., Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messmer M.N., Netherby C.S., Banik D., Abrams S.I. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol Immunother. 2015;64:1–13. doi: 10.1007/s00262-014-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long K.B., Collier A.I., Beatty G.L. Macrophages: key orchestrators of a tumor microenvironment defined by therapeutic resistance. Mol Immunol. 2019;110:3–12. doi: 10.1016/j.molimm.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeNardo D.G., Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe S., Alexander M., Misharin A.V., Budinger G.R.S. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129:2619–2628. doi: 10.1172/JCI124615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 37.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y., Herndon J.M., Sojka D.K., Kim K.W., Knolhoff B.L., Zuo C., Cullinan D.R., Luo J., Bearden A.R., Lavine K.J., Yokoyama W.M., Hawkins W.G., Fields R.C., Randolph G.J., DeNardo D.G. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity. 2017;47:323–338 e6. doi: 10.1016/j.immuni.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurahara H., Shinchi H., Mataki Y., Maemura K., Noma H., Kubo F., Sakoda M., Ueno S., Natsugoe S., Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Ino Y., Yamazaki-Itoh R., Shimada K., Iwasaki M., Kosuge T., Kanai Y., Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Candido J.B., Morton J.P., Bailey P., Campbell A.D., Karim S.A., Jamieson T., Lapienyte L., Gopinathan A., Clark W., McGhee E.J., Wang J., Escorcio-Correia M., Zollinger R., Roshani R., Drew L., Rishi L., Arkell R., Evans T.R.J., Nixon C., Jodrell D.I., Wilkinson R.W., Biankin A.V., Barry S.T., Balkwill F.R., Sansom O.J. CSF1R(+) macrophages sustain pancreatic tumor growth through T cell suppression and maintenance of key gene programs that define the squamous subtype. Cell Rep. 2018;23:1448–1460. doi: 10.1016/j.celrep.2018.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y., Knolhoff B.L., Meyer M.A., Nywening T.M., West B.L., Luo J., Wang-Gillam A., Goedegebuure S.P., Linehan D.C., DeNardo D.G. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nywening T.M., Wang-Gillam A., Sanford D.E., Belt B.A., Panni R.Z., Cusworth B.M., Toriola A.T., Nieman R.K., Worley L.A., Yano M., Fowler K.J., Lockhart A.C., Suresh R., Tan B.R., Lim K.H., Fields R.C., Strasberg S.M., Hawkins W.G., DeNardo D.G., Goedegebuure S.P., Linehan D.C. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noel M., O’Reilly E.M., Wolpin B.M., Ryan D.P., Bullock A.J., Britten C.D., Linehan D.C., Belt B.A., Gamelin E.C., Ganguly B., Yin D., Joh T., Jacobs I.A., Taylor C.T., Lowery M.A. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest New Drugs. 2020;38:800–811. doi: 10.1007/s10637-019-00830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vayrynen S.A., Zhang J., Yuan C., Vayrynen J.P., Dias Costa A., Williams H., Morales-Oyarvide V., Lau M.C., Rubinson D.A., Dunne R.F., Kozak M.M., Wang W., Agostini-Vulaj D., Drage M.G., Brais L., Reilly E., Rahma O., Clancy T., Wang J., Linehan D.C., Aguirre A.J., Fuchs C.S., Coussens L.M., Chang D.T., Koong A.C., Hezel A.F., Ogino S., Nowak J.A., Wolpin B.M. Composition, spatial characteristics, and prognostic significance of myeloid cell infiltration in pancreatic cancer. Clin Cancer Res. 2021;27:1069–1081. doi: 10.1158/1078-0432.CCR-20-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 48.Vonderheide R.H. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083–1088. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 49.Vonderheide R.H., Bajor D.L., Winograd R., Evans R.A., Bayne L.J., Beatty G.L. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother. 2013;62:949–954. doi: 10.1007/s00262-013-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beatty G.L., Chiorean E.G., Fishman M.P., Saboury B., Teitelbaum U.R., Sun W., Huhn R.D., Song W., Li D., Sharp L.L., Torigian D.A., O’Dwyer P.J., Vonderheide R.H. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beatty G.L. Macrophage-based immunotherapy for the treatment of pancreatic ductal adenocarcinoma. Oncoimmunology. 2013;2 doi: 10.4161/onci.26837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beatty G.L., Winograd R., Evans R.A., Long K.B., Luque S.L., Lee J.W., Clendenin C., Gladney W.L., Knoblock D.M., Guirnalda P.D., Vonderheide R.H. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6C(low) F4/80(+) extratumoral macrophages. Gastroenterology. 2015;149:201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winograd R., Byrne K.T., Evans R.A., Odorizzi P.M., Meyer A.R., Bajor D.L., Clendenin C., Stanger B.Z., Furth E.E., Wherry E.J., Vonderheide R.H. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J., Byrne K.T., Yan F., Yamazoe T., Chen Z., Baslan T., Richman L.P., Lin J.H., Sun Y.H., Rech A.J., Balli D., Hay C.A., Sela Y., Merrell A.J., Liudahl S.M., Gordon N., Norgard R.J., Yuan S., Yu S., Chao T., Ye S., Eisinger-Mathason T.S.K., Faryabi R.B., Tobias J.W., Lowe S.W., Coussens L.M., Wherry E.J., Vonderheide R.H., Stanger B.Z. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity. 2018;49:178–193 e7. doi: 10.1016/j.immuni.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bronte V., Brandau S., Chen S.H., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-Rosenberg S., Rodriguez P.C., Sica A., Umansky V., Vonderheide R.H., Gabrilovich D.I. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marvel D., Gabrilovich D.I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bronte V., Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez P.C., Ochoa A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Youn J.I., Nagaraj S., Collazo M., Gabrilovich D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trovato R., Fiore A., Sartori S., Cane S., Giugno R., Cascione L., Paiella S., Salvia R., De Sanctis F., Poffe O., Anselmi C., Hofer F., Sartoris S., Piro G., Carbone C., Corbo V., Lawlor R., Solito S., Pinton L., Mandruzzato S., Bassi C., Scarpa A., Bronte V., Ugel S. Immunosuppression by monocytic myeloid-derived suppressor cells in patients with pancreatic ductal carcinoma is orchestrated by STAT3. J Immunother Cancer. 2019;7:255. doi: 10.1186/s40425-019-0734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raber P., Ochoa A.C., Rodriguez P.C. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest. 2012;41:614–634. doi: 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Porta C., Consonni F.M., Morlacchi S., Sangaletti S., Bleve A., Totaro M.G., Larghi P., Rimoldi M., Tripodo C., Strauss L., Banfi S., Storto M., Pressiani T., Rimassa L., Tartari S., Ippolito A., Doni A., Solda G., Duga S., Piccolo V., Ostuni R., Natoli G., Bronte V., Balzac F., Turco E., Hirsch E., Colombo M.P., Sica A. Tumor-derived prostaglandin E2 promotes p50 NF-kappaB-dependent differentiation of monocytic MDSCs. Cancer Res. 2020;80:2874–2888. doi: 10.1158/0008-5472.CAN-19-2843. [DOI] [PubMed] [Google Scholar]
- 65.Melani C., Sangaletti S., Barazzetta F.M., Werb Z., Colombo M.P. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porembka M.R., Mitchem J.B., Belt B.A., Hsieh C.S., Lee H.M., Herndon J., Gillanders W.E., Linehan D.C., Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Highfill S.L., Cui Y., Giles A.J., Smith J.P., Zhang H., Morse E., Kaplan R.N., Mackall C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steele C.W., Karim S.A., Leach J.D.G., Bailey P., Upstill-Goddard R., Rishi L., Foth M., Bryson S., McDaid K., Wilson Z., Eberlein C., Candido J.B., Clarke M., Nixon C., Connelly J., Jamieson N., Carter C.R., Balkwill F., Chang D.K., Evans T.R.J., Strathdee D., Biankin A.V., Nibbs R.J.B., Barry S.T., Sansom O.J., Morton J.P. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29:832–845. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayne L.J., Beatty G.L., Jhala N., Clark C.E., Rhim A.D., Stanger B.Z., Vonderheide R.H. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pylayeva-Gupta Y., Lee K.E., Hajdu C.H., Miller G., Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stromnes I.M., Brockenbrough J.S., Izeradjene K., Carlson M.A., Cuevas C., Simmons R.M., Greenberg P.D., Hingorani S.R. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014;63:1769–1781. doi: 10.1136/gutjnl-2013-306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liou G.Y., Doppler H., Necela B., Edenfield B., Zhang L., Dawson D.W., Storz P. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov. 2015;5:52–63. doi: 10.1158/2159-8290.CD-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liou G.Y., Doppler H., Necela B., Krishna M., Crawford H.C., Raimondo M., Storz P. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. J Cell Biol. 2013;202:563–577. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y., Velez-Delgado A., Mathew E., Li D., Mendez F.M., Flannagan K., Rhim A.D., Simeone D.M., Beatty G.L., Pasca di Magliano M. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut. 2017;66:124–136. doi: 10.1136/gutjnl-2016-312078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collins M.A., Bednar F., Zhang Y., Brisset J.C., Galban S., Galban C.J., Rakshit S., Flannagan K.S., Adsay N.V., Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duffield J.S., Forbes S.J., Constandinou C.M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ardito C.M., Gruner B.M., Takeuchi K.K., Lubeseder-Martellato C., Teichmann N., Mazur P.K., Delgiorno K.E., Carpenter E.S., Halbrook C.J., Hall J.C., Pal D., Briel T., Herner A., Trajkovic-Arsic M., Sipos B., Liou G.Y., Storz P., Murray N.R., Threadgill D.W., Sibilia M., Washington M.K., Wilson C.L., Schmid R.M., Raines E.W., Crawford H.C., Siveke J.T. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collins M.A., Yan W., Sebolt-Leopold J.S., Pasca di Magliano M. MAPK signaling is required for dedifferentiation of acinar cells and development of pancreatic intraepithelial neoplasia in mice. Gastroenterology. 2014;146:822–834 e7. doi: 10.1053/j.gastro.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J., Yuan S., Norgard R.J., Yan F., Sun Y.H., Kim I.K., Merrell A.J., Sela Y., Jiang Y., Bhanu N.V., Garcia B.A., Vonderheide R.H., Blanco A., Stanger B.Z. Epigenetic and transcriptional control of the epidermal growth factor receptor (EGFR) regulates the tumor immune microenvironment in pancreatic cancer. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Q., Lenardo M.J., Baltimore D. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilmore T.D. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 82.Ling J., Kang Y., Zhao R., Xia Q., Lee D.F., Chang Z., Li J., Peng B., Fleming J.B., Wang H., Liu J., Lemischka I.R., Hung M.C., Chiao P.J. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maier H.J., Wagner M., Schips T.G., Salem H.H., Baumann B., Wirth T. Requirement of NEMO/IKKgamma for effective expansion of KRAS-induced precancerous lesions in the pancreas. Oncogene. 2013;32:2690–2695. doi: 10.1038/onc.2012.272. [DOI] [PubMed] [Google Scholar]
- 84.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schreck R., Baeuerle P.A. NF-kappa B as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol Cell Biol. 1990;10:1281–1286. doi: 10.1128/mcb.10.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeuchi S., Baghdadi M., Tsuchikawa T., Wada H., Nakamura T., Abe H., Nakanishi S., Usui Y., Higuchi K., Takahashi M., Inoko K., Sato S., Takano H., Shichinohe T., Seino K., Hirano S. Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res. 2015;75:2629–2640. doi: 10.1158/0008-5472.CAN-14-2921. [DOI] [PubMed] [Google Scholar]
- 87.Chao T., Furth E.E., Vonderheide R.H. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2016;4:968–982. doi: 10.1158/2326-6066.CIR-16-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burke S.J., Lu D., Sparer T.E., Masi T., Goff M.R., Karlstad M.D., Collier J.J. NF-kappaB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab. 2014;306:E131–E149. doi: 10.1152/ajpendo.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ijichi H., Chytil A., Gorska A.E., Aakre M.E., Bierie B., Tada M., Mohri D., Miyabayashi K., Asaoka Y., Maeda S., Ikenoue T., Tateishi K., Wright C.V., Koike K., Omata M., Moses H.L. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carstens J.L., Correa de Sampaio P., Yang D., Barua S., Wang H., Rao A., Allison J.P., LeBleu V.S., Kalluri R. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun. 2017;8:15095. doi: 10.1038/ncomms15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stromnes I.M., Hulbert A., Pierce R.H., Greenberg P.D., Hingorani S.R. T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2017;5:978–991. doi: 10.1158/2326-6066.CIR-16-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhim A.D., Mirek E.T., Aiello N.M., Maitra A., Bailey J.M., McAllister F., Reichert M., Beatty G.L., Rustgi A.K., Vonderheide R.H., Leach S.D., Stanger B.Z. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malanchi I., Santamaria-Martinez A., Susanto E., Peng H., Lehr H.A., Delaloye J.F., Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 94.Nielsen S.R., Quaranta V., Linford A., Emeagi P., Rainer C., Santos A., Ireland L., Sakai T., Sakai K., Kim Y.S., Engle D., Campbell F., Palmer D., Ko J.H., Tuveson D.A., Hirsch E., Mielgo A., Schmid M.C. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–560. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaplan R.N., Riba R.D., Zacharoulis S., Bramley A.H., Vincent L., Costa C., MacDonald D.D., Jin D.K., Shido K., Kerns S.A., Zhu Z., Hicklin D., Wu Y., Port J.L., Altorki N., Port E.R., Ruggero D., Shmelkov S.V., Jensen K.K., Rafii S., Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee J.W., Stone M.L., Porrett P.M., Thomas S.K., Komar C.A., Li J.H., Delman D., Graham K., Gladney W.L., Hua X., Black T.A., Chien A.L., Majmundar K.S., Thompson J.C., Yee S.S., O’Hara M.H., Aggarwal C., Xin D., Shaked A., Gao M., Liu D., Borad M.J., Ramanathan R.K., Carpenter E.L., Ji A., de Beer M.C., de Beer F.C., Webb N.R., Beatty G.L. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567:249–252. doi: 10.1038/s41586-019-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hiratsuka S., Nakamura K., Iwai S., Murakami M., Itoh T., Kijima H., Shipley J.M., Senior R.M., Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 99.Hiratsuka S., Watanabe A., Aburatani H., Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 100.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., Xiang J., Zhang T., Theilen T.M., Garcia-Santos G., Williams C., Ararso Y., Huang Y., Rodrigues G., Shen T.L., Labori K.J., Lothe I.M., Kure E.H., Hernandez J., Doussot A., Ebbesen S.H., Grandgenett P.M., Hollingsworth M.A., Jain M., Mallya K., Batra S.K., Jarnagin W.R., Schwartz R.E., Matei I., Peinado H., Stanger B.Z., Bromberg J., Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 102.Pollard J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 103.Bailey P., Chang D.K., Nones K., Johns A.L., Patch A.M., Gingras M.C., Miller D.K., Christ A.N., Bruxner T.J., Quinn M.C., Nourse C., Murtaugh L.C., Harliwong I., Idrisoglu S., Manning S., Nourbakhsh E., Wani S., Fink L., Holmes O., Chin V., Anderson M.J., Kazakoff S., Leonard C., Newell F., Waddell N., Wood S., Xu Q., Wilson P.J., Cloonan N., Kassahn K.S., Taylor D., Quek K., Robertson A., Pantano L., Mincarelli L., Sanchez L.N., Evers L., Wu J., Pinese M., Cowley M.J., Jones M.D., Colvin E.K., Nagrial A.M., Humphrey E.S., Chantrill L.A., Mawson A., Humphris J., Chou A., Pajic M., Scarlett C.J., Pinho A.V., Giry-Laterriere M., Rooman I., Samra J.S., Kench J.G., Lovell J.A., Merrett N.D., Toon C.W., Epari K., Nguyen N.Q., Barbour A., Zeps N., Moran-Jones K., Jamieson N.B., Graham J.S., Duthie F., Oien K., Hair J., Grutzmann R., Maitra A., Iacobuzio-Donahue C.A., Wolfgang C.L., Morgan R.A., Lawlor R.T., Corbo V., Bassi C., Rusev B., Capelli P., Salvia R., Tortora G., Mukhopadhyay D., Petersen G.M., Australian Pancreatic Cancer Genome I., Munzy D.M., Fisher W.E., Karim S.A., Eshleman J.R., Hruban R.H., Pilarsky C., Morton J.P., Sansom O.J., Scarpa A., Musgrove E.A., Bailey U.M., Hofmann O., Sutherland R.L., Wheeler D.A., Gill A.J., Gibbs R.A., Pearson J.V., Waddell N., Biankin A.V., Grimmond S.M. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 104.Griesmann H., Drexel C., Milosevic N., Sipos B., Rosendahl J., Gress T.M., Michl P. Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut. 2017;66:1278–1285. doi: 10.1136/gutjnl-2015-310049. [DOI] [PubMed] [Google Scholar]
- 105.Burris H.A., 3rd, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., Nelson R., Dorr F.A., Stephens C.D., Von Hoff D.D. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 106.Goldstein D., El-Maraghi R.H., Hammel P., Heinemann V., Kunzmann V., Sastre J., Scheithauer W., Siena S., Tabernero J., Teixeira L., Tortora G., Van Laethem J.L., Young R., Penenberg D.N., Lu B., Romano A., Von Hoff D.D. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju413. [DOI] [PubMed] [Google Scholar]
- 107.Halbrook C.J., Pontious C., Kovalenko I., Lapienyte L., Dreyer S., Lee H.J., Thurston G., Zhang Y., Lazarus J., Sajjakulnukit P., Hong H.S., Kremer D.M., Nelson B.S., Kemp S., Zhang L., Chang D., Biankin A., Shi J., Frankel T.L., Crawford H.C., Morton J.P., Pasca di Magliano M., Lyssiotis C.A. Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metab. 2019;29:1390–1399 e6. doi: 10.1016/j.cmet.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nywening T.M., Belt B.A., Cullinan D.R., Panni R.Z., Han B.J., Sanford D.E., Jacobs R.C., Ye J., Patel A.A., Gillanders W.E., Fields R.C., DeNardo D.G., Hawkins W.G., Goedegebuure P., Linehan D.C. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67:1112–1123. doi: 10.1136/gutjnl-2017-313738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaeschke H., Farhood A., Bautista A.P., Spolarics Z., Spitzer J.J., Smith C.W. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17:915–923. [PubMed] [Google Scholar]
- 110.Rogers C., Edelman E.R., Simon D.I. A mAb to the beta2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci U S A. 1998;95:10134–10139. doi: 10.1073/pnas.95.17.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dove A. CD18 trials disappoint again. Nat Biotechnol. 2000;18:817–818. doi: 10.1038/78412. [DOI] [PubMed] [Google Scholar]
- 112.Maiguel D., Faridi M.H., Wei C., Kuwano Y., Balla K.M., Hernandez D., Barth C.J., Lugo G., Donnelly M., Nayer A., Moita L.F., Schurer S., Traver D., Ruiz P., Vazquez-Padron R.I., Ley K., Reiser J., Gupta V. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci Signal. 2011;4:ra57. doi: 10.1126/scisignal.2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Panni R.Z., Herndon J.M., Zuo C., Hegde S., Hogg G.D., Knolhoff B.L., Breden M.A., Li X., Krisnawan V.E., Khan S.Q., Schwarz J.K., Rogers B.E., Fields R.C., Hawkins W.G., Gupta V., DeNardo D.G. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y., Lazarus J., Steele N.G., Yan W., Lee H.J., Nwosu Z.C., Halbrook C.J., Menjivar R.E., Kemp S.B., Sirihorachai V.R., Velez-Delgado A., Donahue K., Carpenter E.S., Brown K.L., Irizarry-Negron V., Nevison A.C., Vinta A., Anderson M.A., Crawford H.C., Lyssiotis C.A., Frankel T.L., Bednar F., Pasca di Magliano M. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov. 2020;10:422–439. doi: 10.1158/2159-8290.CD-19-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Elyada E., Bolisetty M., Laise P., Flynn W.F., Courtois E.T., Burkhart R.A., Teinor J.A., Belleau P., Biffi G., Lucito M.S., Sivajothi S., Armstrong T.D., Engle D.D., Yu K.H., Hao Y., Wolfgang C.L., Park Y., Preall J., Jaffee E.M., Califano A., Robson P., Tuveson D.A. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steele N.G., Carpenter E.S., Kemp S.B., Sirihorachai V.R., The S., Delrosario L., Lazarus J., Amir E.-a.D., Gunchick V., Espinoza C., Bell S., Harris L., Lima F., Irizarry-Negron V., Paglia D., Macchia J., Chu A.K.Y., Schofield H., Wamsteker E.-J., Kwon R., Schulman A., Prabhu A., Law R., Sondhi A., Yu J., Patel A., Donahue K., Nathan H., Cho C., Anderson M.A., Sahai V., Lyssiotis C.A., Zou W., Allen B.L., Rao A., Crawford H.C., Bednar F., Frankel T.L., Pasca di Magliano M. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nature Cancer. 2020;1:1097–1112. doi: 10.1038/s43018-020-00121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schlesinger Y., Yosefov-Levi O., Kolodkin-Gal D., Granit R.Z., Peters L., Kalifa R., Xia L., Nasereddin A., Shiff I., Amran O., Nevo Y., Elgavish S., Atlan K., Zamir G., Parnas O. Single-cell transcriptomes of pancreatic preinvasive lesions and cancer reveal acinar metaplastic cells’ heterogeneity. Nat Commun. 2020;11:4516. doi: 10.1038/s41467-020-18207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hosein A.N., Huang H., Wang Z., Parmar K., Du W., Huang J., Maitra A., Olson E., Verma U., Brekken R.A. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight. 2019;5 doi: 10.1172/jci.insight.129212. [DOI] [PMC free article] [PubMed] [Google Scholar]