Key Points
Question
Does laparoscopic distal gastrectomy yield similar 5-year overall survival to open distal gastrectomy for patients with locally advanced gastric cancer?
Findings
In this randomized clinical trial of 1056 patients with clinically staged, locally advanced gastric cancer, laparoscopic distal gastrectomy resulted in a 5-year overall survival rate of 73% vs 76% for open distal gastrectomy, with no statistically significant difference between the 2 groups.
Meaning
The finding provides further evidence for the safety and efficacy of laparoscopic gastrectomy for patients assessed preoperatively as having locally advanced cancer.
Abstract
Importance
It is not clear whether laparoscopic and open distal gastrectomy produce similar outcomes among patients with locally advanced gastric cancer. Data from a multicenter, randomized clinical trial (Chinese Laparoscopic Gastrointestinal Surgical Study [CLASS]–01) showed that laparoscopic distal gastrectomy did not result in inferior disease-free survival at 3 years compared with open distal gastrectomy.
Objective
To report 5-year overall survival data from the CLASS-01 trial of laparoscopic vs open distal gastrectomy among patients with locally advanced gastric cancer.
Design, Setting, and Patients
This was a noninferiority, open-label, randomized clinical trial conducted at 14 centers in China. A total of 1056 eligible patients with clinical stage T2, T3, or T4a gastric cancer without bulky nodes or distant metastases were enrolled from September 12, 2012, to December 3, 2014. Final follow-up was on December 31, 2019.
Interventions
Participants were randomized in a 1:1 ratio after stratification by site, age, cancer stage, and histologic features to undergo either laparoscopic distal gastrectomy (n = 528) or open distal gastrectomy (n = 528) with D2 lymphadenectomy.
Main Outcomes and Measures
The 5-year overall survival rates were updated to compare laparoscopic distal gastrectomy with open distal gastrectomy. All analyses were performed on an intention-to-treat basis. In addition, per-protocol and as-treated analyses were performed for overall survival.
Results
Data from 1039 patients (726 men [69.9%]; mean [SD] age, 56.2 [10.7] years) who received curative therapy were analyzed. At 5 years, the overall survival rates were 72.6% in the laparoscopic distal gastrectomy group and 76.3% in the open distal gastrectomy group (log-rank P = .19; hazard ratio, 1.17; 95% CI, 0.93-1.48; P = .19). After comparison for competing risk events, gastric cancer–related deaths (hazard ratio, 1.14; 95% CI, 0.87-1.49; P = .34) and deaths from other causes (hazard ratio, 1.23; 95% CI, 0.74-2.05; P = .42) did not differ significantly between groups. Overall rates of survival did not differ significantly between groups with each tumor stage.
Conclusions and Relevance
This study found that laparoscopic distal gastrectomy with D2 lymphadenectomy performed by experienced surgeons in high-volume specialized institutions resulted in similar 5-year overall survival compared with open distal gastrectomy among patients with locally advanced gastric cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT01609309
This randomized clinical trial reports 5-year overall survival data from the CLASS-01 trial of laparoscopic vs open distal gastrectomy among patients with locally advanced gastric cancer.
Introduction
Gastric cancer is the fifth most common malignant neoplasm and the third leading cause of cancer-related death, causing an estimated 783 000 deaths worldwide in 2018, based on GLOBOCAN 2018 data.1 Patients without metastases are treated with surgery. There are 2 surgical options for patients with potentially curable gastric cancer. Kitano et al2 first reported treatment of early-stage distal gastric cancer with laparoscopic-assisted gastrectomy in 1991. After this report, the procedure rapidly achieved popularity in Eastern countries3,4 especially for treatment of early gastric cancer (T1, any N, and M0).
The Japanese Clinical Oncology Study Group provides a platform to launch randomized trials to evaluate treatment approaches, including surgery and laparoscopy, among patients with gastric cancer. The Korean Laparoscopic Gastrointestinal Surgical Study Group (KLASS) was founded in 2004 for the sharing of technical information and the effects of laparoscopic surgery on patients with gastric cancer. After an initial trial to investigate the effect of laparoscopic surgery on early gastric cancer (KLASS-01 trial), researchers initiated the KLASS-02 trial of patients with locally advanced gastric cancer (LAGC).5 Laparoscopic surgery is an option for treatment of patients in general practice with stage I cancer indicated for distal gastrectomy. In the 2014 version of the guidelines from the Japan Society for Endoscopic Surgery,6 distal gastrectomy via laparoscopy was recommended for patients with stage I cancer (rated recommendation B). This recommendation was made because the safety of the laparoscopic approach was proven in a prospective phase 2 study (JCOG0703)7 that involved only certified surgeons with sufficient experience. Data regarding the long-term outcome of pivotal phase 3 studies conducted in Japan (JCOG0912)8 and Korea (KLASS-01)9 were recently published, providing evidence for the efficacy of laparoscopic surgery in treatment of early gastric cancer.
For patients with more advanced cancer, there is currently insufficient evidence to recommend a laparoscopic approach, but randomized trials of its safety and long-term outcomes are underway. In China, more than 80% of all patients with gastric cancer have received a diagnosis of advanced-stage disease.10 The Chinese Laparoscopic Gastrointestinal Surgical Study (CLASS) Group was founded in 2009; it launched its first trial for patients with LAGC (the CLASS-01 trial) in 2012 and published primary end points from the study in May 2019.11 The CLASS-01 trial was the first completed multicenter, randomized clinical trial to compare long-term outcomes of laparoscopic distal gastrectomy (LDG) with open distal gastrectomy (ODG) for LAGC. The study found no significant difference in disease-free survival at 3 years among patients undergoing LDG (76.5%) vs those undergoing ODG (77.8%). A randomized clinical trial of patients in Korea with LAGC also recently reported that LDG was not inferior to ODG in disease-free survival at 3 years.12
However, overall survival is the standard end point for cancer trials. Although the CLASS-01 trial was designed with disease-free survival as its primary end point, it was also powered to determine effects on overall survival. At the time of the primary analysis of disease-free survival, overall survival data were immature; the outcomes of the CLASS-01 trial were updated, after a minimum 5 years of follow-up, at the end of 2019. We report the 5-year overall survival data from the CLASS-01 trial, along with data on the effects of LDG, among patients with tumors of different stages, especially in stage III tumors, and causes of death in the per-protocol, as-treated population.
Methods
Study Design
The CLASS-01 trial is an open-label, multicenter, randomized clinical noninferiority trial conducted at 14 centers in China. Patients were enrolled from September 12, 2012, through December 3, 2014. The final follow-up was on December 31, 2019. Participants were randomly assigned in a 1:1 ratio, after matching for study site, patient age, tumor stage, and histologic features, to groups that underwent LDG (n = 528) or ODG (n = 528) with D2 lymphadenectomy. The primary end point was 3-year disease-free survival. A detailed description of the study protocol and the main results have been published with clinical and demographic baseline characteristics (trial protocol and statistical analysis plan in Supplement 1).11,13 Each participating center (Nanfang Hospital, Southern Medical University, Guangzhou, China; Fujian Medical University Union Hospital, Fuzhou, China; Zhongshan Hospital, Fudan University, Shanghai, China; Peking University Cancer Hospital and Institute, Beijing, China; Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; West China Hospital, Sichuan University, Chengdu, China; the Cancer Hospital of Harbin Medical University, Harbin, China; the First Hospital of Jilin University, Changchun, China; Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Tangdu Hospital, Fourth Military Medical University, Xi’an, China; the Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China; Fujian Provincial Cancer Hospital, Fuzhou, China; Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; and General Hospital of PLA, Beijing, China) obtained institutional review board approval, according to local regulations. Patients provided written informed consent.
Study Population
The CLASS-01 trial enrolled patients with LAGC (cT1-4aN1-3M0) planned for elective surgery; the inclusion and exclusion criteria were published.11 Patients were included if they were aged 18 to 75 years; had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 (asymptomatic) or 1 (symptomatic but completely ambulatory); had histologically confirmed gastric adenocarcinoma, detected at a locally advanced stage, according to the Japanese Classification11 (and to T2-4aN0-3M0, corresponding to stages IB-IIIC excluding T1 or T4b tumors); had tumors located in the lower or middle third of the stomach by preoperative evaluation; or were expected to undergo distal gastrectomy with D2 lymphadenectomy for curative intent. Patients were excluded if they had enlarged or bulky regional lymph nodes larger than 3-cm maximum diameter, determined during preoperative imaging.
End Points
Patients were followed up for a minimum of 60 months after surgery. Overall survival was calculated from the day of randomization until the day of death (event) or the day of the last follow-up examination (censored). Data were censored for patients with no evidence of disease at the last follow-up examination or for patients who died of diseases other than gastric cancer without evidence of a recurrence.
Statistical Analysis
We used the Kaplan-Meier method to estimate the difference in overall survival between the groups at 5 years after the procedures. Cox proportional hazards regression analysis was used to determine the effect of covariates on time of death. Instead of median survival time, we used the area under the Kaplan-Meier curve within a specific time window as a reasonable summary to quantify the survival time, which is called restricted mean survival time (RMST). Laparoscopic distal gastrectomy was considered to be noninferior to ODG if the 1-sided 95% CI for the difference in overall survival excluded an absolute difference of 10 percentage points or more. We also conducted competing risk regression to eliminate the influence of different causes of death, and used the Gray test to assess differences between the LDG and ODG groups. All analyses were performed on an intention-to-treat basis. In addition, we performed per-protocol and as-treated analyses for overall survival. All analyses were performed using SPSS, version 25 (IBM Corp), SAS, version 9.4 (SAS Institute Inc), and R, version 3.6.2 (R Group for Statistical Computing). P < .05 was considered significant.
Results
Study Population
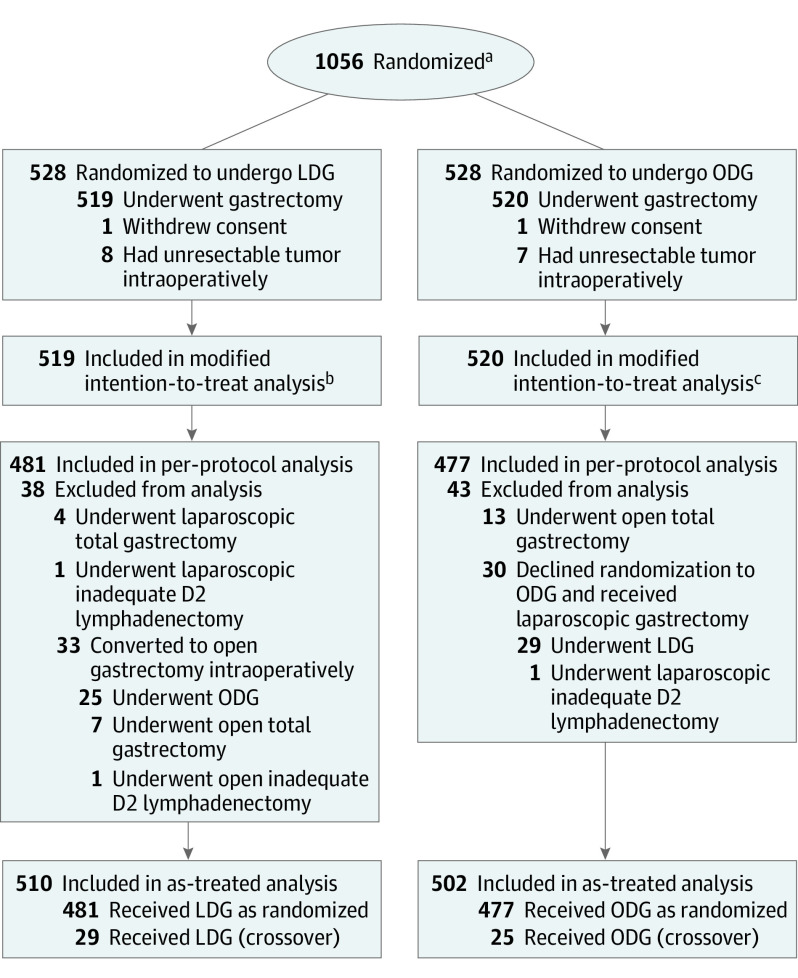
From September 12, 2012, through December 3, 2014, 528 patients were randomly assigned to the LDG group and 528 to the ODG group (Figure 1). In the LDG group, 1 patient withdrew informed consent and 8 had unresectable gastric cancer detected intraoperatively. In the ODG group, 1 patient withdrew informed consent and 7 had unresectable gastric cancer, detected intraoperatively. The primary analysis set comprised 1039 patients (519 in the LDG group and 520 in the ODG group). The per-protocol population comprised 958 patients, with 481 in the LDG group (519 patients in the primary analysis set minus 38 patients who did not adhere to their treatment plans) and 477 in the ODG group (520 patients in the primary analysis set minus 43 patients who did not adhere to their treatment plans). The as-treated population comprised 510 patients in the laparoscopic group (481 per-protocol patients plus 29 patients with protocol crossovers) and 502 patients in the ODG group (477 per-protocol patients plus 25 patients with protocol crossovers). The median follow-up period was 71 months (IQR, 43-77 months), with a total of 33 patients (3.2%) lost to follow-up (18 in the LDG group and 15 in the ODG group). A detailed description of the study protocol and the main results have been published previously with clinical and demographic baseline characteristics.11,13 Most patients in both groups were men (380 [73.2%] in the LDG group and 346 [66.5%] in the ODG group); the mean (SD) age was 56.5 (10.4) years in the LDG group and 55.8 (11.1) years in the ODG group (Table 1). Although all patients had a diagnosis of T2 stage or higher, 248 patients (23.9%) were found to have T1 tumors (116 [22.4%] in the LDG group and 132 [25.4%] in the ODG group). The number of patients who received adjuvant chemotherapy was 192 (37.0%) in the LDG group, and 217 (41.7%) in the ODG group, with no significant difference between the 2 groups.
Figure 1. CONSORT Flow Diagram of Patient Enrollment and Randomization.
CONSORT indicates Consolidated Standards of Reporting Trials; LDG, laparoscopic distal gastrectomy; and ODG, open distal gastrectomy.
aData for number screened for eligibility and reasons for exclusion were not available.
bIncludes 18 patients who were lost to follow-up and 2 patients who died within 30 days after the surgery (1 died of respiratory failure as a result of pneumonia and the other died of a cerebrovascular accident).
cIncludes 15 patients who were lost to follow-up.
Table 1. Demographic and Clinical Characteristics of the Patients at Baseline.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Laparoscopic surgery (n = 519) | Open surgery (n = 520) | |
| Sex | ||
| Female | 139 (26.8) | 174 (33.5) |
| Male | 380 (73.2) | 346 (66.5) |
| Age, mean (SD), y | 56.5 (10.4) | 55.8 (11.1) |
| BMI, mean (SD) | 22.7 (3.2) | 22.7 (3.2) |
| Eastern Cooperative Oncology Group performance status | ||
| 0 | 375 (72.3) | 391 (75.2) |
| 1 | 142 (27.4) | 127 (24.4) |
| Comorbidities | ||
| None | 359 (69.2) | 378 (72.7) |
| ≥1 | 159 (30.6) | 139 (26.7) |
| Tumor size, mean (SD), cm | 4.0 (2.0) | 4.0 (2.1) |
| Histologic features | ||
| Signet ring cell | 79 (15.2) | 99 (19.0) |
| Others | 440 (84.8) | 421 (81.0) |
| Retrieved lymph node, mean (SD), No. | 36.1 (16.7) | 36.9 (16.1) |
| Metastatic lymph node, mean (SD), No. | 4.9 (8.0) | 4.5 (6.9) |
| Received chemotherapy | ||
| Yes | 192 (37.0) | 217 (41.7) |
| No | 327 (63.0) | 303 (58.3) |
| Pathological T stage | ||
| <T2 | 116 (22.4) | 132 (25.4) |
| T2-T4a | 394 (76.1) | 383 (73.7) |
| T4b | 8 (1.5) | 4 (0.8) |
| Pathological N stage | ||
| N0 | 214 (41.2) | 216 (41.5) |
| N1 | 87 (16.8) | 79 (15.2) |
| N2 | 88 (17.0) | 98 (18.8) |
| N3 | 129 (24.9) | 126 (24.2) |
| Nx | 1 (0.2) | 1 (0.2) |
| Pathological M stage | ||
| M0 | 510 (98.3) | 511 (98.3) |
| M1 | 8 (1.5) | 8 (1.5) |
| Missing data | 1 (0.2) | 1 (0.2) |
| Pathological TNM stage | ||
| IA | 87 (16.8) | 99 (19.0) |
| IB | 64 (12.3) | 53 (10.2) |
| IIA | 66 (12.7) | 59 (11.3) |
| IIB | 71 (13.7) | 79 (15.2) |
| IIIA | 69 (13.3) | 77 (14.8) |
| IIIB | 73 (14.1) | 78 (15.0) |
| IIIC | 77 (14.8) | 66 (12.7) |
| IV | 11 (2.1) | 8 (1.5) |
| Missing data | 1 (0.2) | 1 (0.2) |
| Adjuvant chemotherapy | 192 (37.0) | 217 (41.7) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Overall Survival
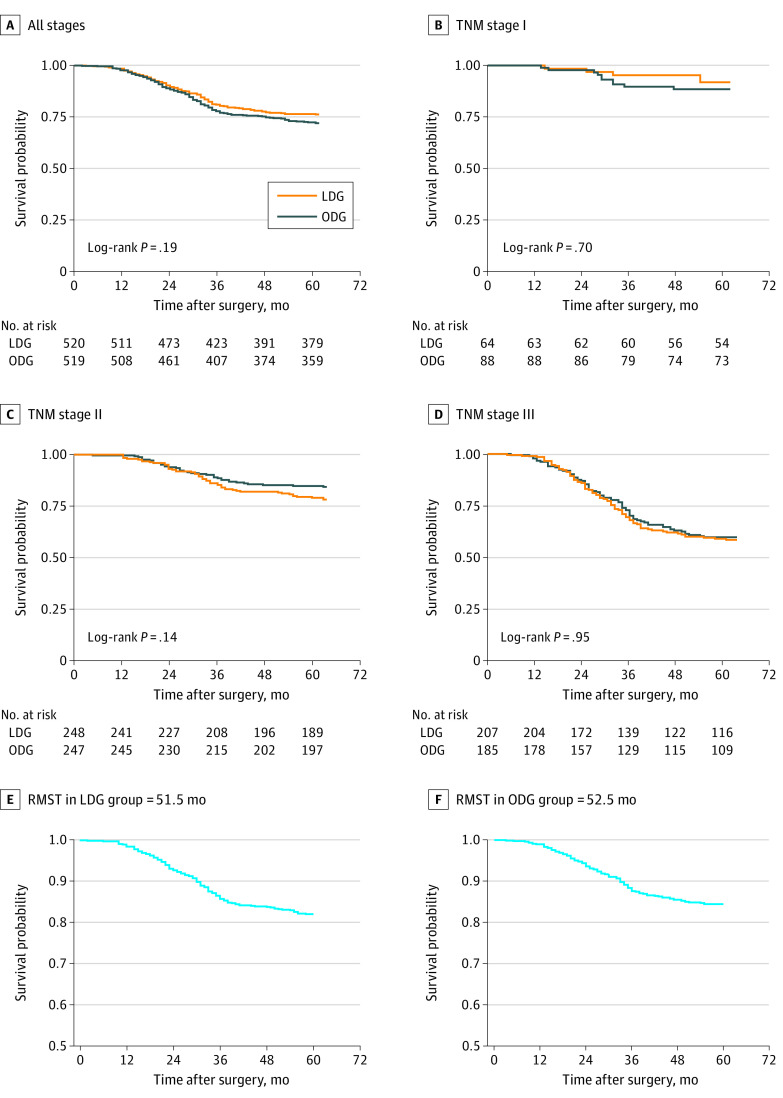
At the last follow-up, 286 patients (27.5%) had died (152 in the LDG group and 134 in the ODG group). The rates of 5-year overall survival were 72.6% in the LDG group and 76.3% in the ODG group (log-rank P = .19; hazard ratio [HR], 1.17; 95% CI, 0.93-1.48; P = .19) (Figure 2). The rates of 5-year overall survival were 90.0% in the LDG group vs 88.5% in the ODG group for patients with stage I tumors, 79.1% in the LDG group vs 84.5% in the ODG group for patients with stage II tumors, and 58.6% in the LDG group vs 59.5% in the ODG group for patients with stage III tumors. The HR for ODG vs LDG was 1.14 (95% CI, 0.89-1.46; P = .25) after we adjusted for age, sex, body mass index, ECOG performance status, comorbidity, tumor size, histologic features, TNM stage, and chemotherapy. For RMST, we set truncation at 60 months (5 years). The RMST in the LDG group was 51.5 months (95% CI, 50.2-52.8 months). Corresponding restricted mean times lost were 8.5 years (95% CI, 7.2-9.8 years) in the LDG group and 8.5 years in the ODG group (95% CI, 7.2-9.8 years). The RMST in the ODG group was 52.5 months (95% CI, 51.3-53.8 months) and the restricted mean time lost in the ODG group was 7.5 months (95% CI, 6.2-8.7 months). The RMST ratio for LDG to ODG was 0.98 (95% CI, 0.94-1.01), indicating that the LDG group had a median survival time that was 2% less than that of the ODG group (P = .27).
Figure 2. Overall Survival for Laparoscopic Distal Gastrectomy (LDG) vs Open Distal Gastrectomy (ODG) at 5 Years After Surgery.
A, Patients with all stages of cancer. B, Patients with TNM stage I cancer. C, Patients with TNM stage II cancer. D, Patients with TNM stage III cancer. E, Restricted mean survival time (RMST) in the LDG group. F, RMST in the ODG group.
In the LDG vs ODG groups, the rates of 5-year overall survival were 90.0% vs 88.5% for patients with stage I tumors (log-rank P = .70); 79.1% vs 84.5% for patients with stage II tumors (log-rank P = .14); and 58.6% vs 59.5% for patients with stage III tumors (log-rank P = .95). Crude HRs for 5-year overall survival with LDG vs ODG were 0.81 (95% CI, 0.29-2.25) for patients with stage I tumors, 1.34 (95% CI, 0.91-1.97) for patients with stage II tumors, and 1.01 (95% CI, 0.74-1.37) for patients with stage III tumors. After we adjusted for age, sex, body mass index, ECOG performance status, comorbidity, tumor size, histologic features, and chemotherapy, the adjusted HRs for 5-year overall survival with LDG vs ODG were 0.59 (95% CI, 0.16-2.13) for patients with stage I tumors, 1.40 (95% CI, 0.93-2.10) for patients with stage II tumors, and 1.03 (95% CI, 0.75-1.42) for patients with stage III tumors (Figure 2). Univariate analysis of overall survival at 5 years found no differences in outcomes of ODG vs LDG in terms of age, sex, or tumor stage (Table 2).
Table 2. Univariate Analysis of Overall Survival at 5 Years’ Follow-up.
| Variable | Patients, No. | LDG group, 5-y OS (95% CI), % | Patients, No. | ODG group, 5-y OS (95% CI), % | Hazard ratioa | Log-rank P value |
|---|---|---|---|---|---|---|
| Total | 519 | 73 (69-77) | 520 | 76 (73-80) | 1.17 (0.93-1.48) | .19 |
| Sex | ||||||
| Male | 380 | 72 (68-77) | 346 | 76 (72-81) | 1.12 (0.90-1.57) | .22 |
| Female | 139 | 73 (66-81) | 174 | 76 (69-82) | 1.12 (0.73-1.72) | .60 |
| Age, y | ||||||
| ≤65 | 419 | 75 (71-79) | 418 | 77.4 (73.5-81.6) | 1.13 (0.86-1.48) | .39 |
| >65 | 99 | 63 (55-74) | 102 | 69 (61-79) | 1.30 (0.83-2.06) | .25 |
| N stage | ||||||
| N0 | 214 | 90.5 (86.6-94.5) | 216 | 90.5 (86.7-94.6) | 0.92 (0.51-1.64) | .77 |
| N1 | 87 | 85 (78-93) | 79 | 83 (75-92) | 0.98 (0.47-2.03) | .96 |
| N2 | 88 | 66 (57-77) | 98 | 73 (65-83) | 1.31 (0.79-2.12) | .30 |
| N3 | 129 | 37(29-46) | 126 | 48 (40-58) | 1.37 (0.99-1.90) | .05 |
| TNM stage | ||||||
| IA | 87 | 94 (89-99) | 99 | 94 (89-99) | 1.31 (0.44-3.89) | .63 |
| IB | 64 | 95 (90-100) | 53 | 94 (88-100) | 0.51 (0.12-2.13) | .34 |
| IIA | 66 | 91 (84-98) | 59 | 90 (82-98) | 0.87 (0.28-2.71) | .81 |
| IIB | 71 | 81 (72-91) | 79 | 82 (73-92) | 0.99 (0.47-2.07) | .97 |
| IIIA | 69 | 73 (63-85) | 77 | 71 (61-82) | 1.02 (0.56-1.86) | .94 |
| IIIB | 73 | 47 (37-61) | 78 | 60 (50-72) | 1.31 (0.83-2.07) | .24 |
| IIIC | 77 | 28 (19-41) | 66 | 41 (33-55) | 1.35 (0.90-2.05) | .14 |
| IV | 11 | 27 (10-72) | 8 | 38 (15-92) | 1.20 (0.39-3.70) | .77 |
Abbreviations: LDG, laparoscopic distal gastrectomy; ODG, open distal gastrectomy; OS, overall survival.
Reference, ODG group.
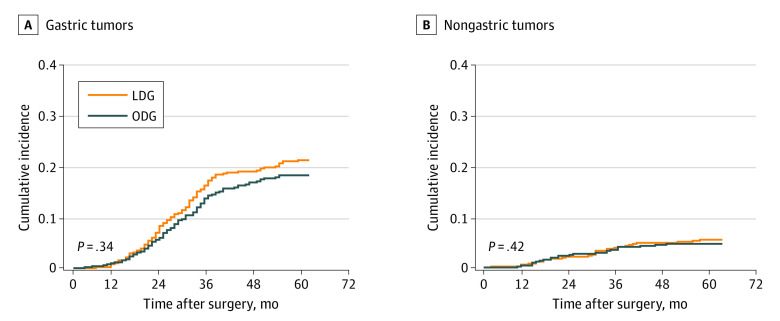
The cause of death was analyzed for all patients; the distribution for LDG and ODG group is presented in the eTable in Supplement 2. After we controlled for all other competing risk events, gastric cancer–related death (HR, 1.14; 95% CI, 0.87-1.49; P = .34) and death from other causes (HR, 1.23; 95% CI, 0.74-2.05; P = .42) did not differ significantly different between the LDG and ODG groups (Figure 3). In the post hoc sensitivity analysis, exclusion of the 248 patients with pathologic T1N0 to 3M0 tumors resulted in an HR for death of 1.13 for LDG vs ODG (95% CI, 0.89-1.44; P = .29) (eFigure 1 in Supplement 2).
Figure 3. Cumulative Risk of Death Due to Gastric Cancer or Other Causes.
A, Gastric tumors. B, Nongastric tumors. LDG indicates laparoscopic distal gastrectomy; and ODG, open distal gastrectomy.
Per-Protocol and As-Treated Populations
In the per-protocol analysis, 134 of 481 patients in the LDG group died within 5 years (72% survival rate), compared with 119 of 481 patients in the ODG group (74% survival rate); the HR for ODG vs LDG was 1.14 (95% CI, 0.89-1.45; P = .29). In the as-treated analysis, 143 of 506 patients in the LDG group died within 5 years (72% survival rate), compared with 127 of 506 of patients (75% survival rate) in the ODG group; the HR for ODG vs LDG was 1.10 (95% CI, 0.87-1.40; P = .40) (eFigure 2 in Supplement 2).
Discussion
In this multicenter randomized clinical trial to compare outcomes of LDG vs ODG among patients with LAGC (the CLASS-01 trial), we found 5-year overall survival not to differ significantly between patients who underwent LDG with D2 lymphadenectomy vs ODG performed by experienced surgeons at high-volume, specialized centers, regardless of tumor stage. This is the first multicenter, randomized study to our knowledge that compared effects of LDG vs ODG on long-term (5-year) survival. This finding provides further evidence for the safety and efficacy of LDG for treatment of gastric cancer.
The efficacy of LDG for early gastric cancer has been evaluated in a multicenter trial of 1416 patients in Korea with stage I gastric cancer.9 Rates of 5-year overall survival were 94.2% in the LDG group and 93.3% in the ODG group, indicating the noninferiority of LDG. However, this study did not have sufficient data on long-term oncologic outcomes of patients with advanced gastric cancer.
With accumulating surgical experience, indications for laparoscopic surgery have been gradually extended to LAGC, especially in Eastern countries.14,15 Because the completeness of lymphadenectomy becomes more important with increasing tumor stage, it is important to determine whether a minimally invasive approach is equivalent to ODG among patients with advanced gastric cancer. A recent meta-analysis of data from 6 randomized trials found that LDG with D2 lymphadenectomy does not differ significantly from ODG in short-term morbidity and mortality of patients with LAGC.16 Our safety analysis of data from the CLASS-01 trial found rates of postoperative morbidity to be 15.2% in the LDG group and 12.9% in the ODG group (not a significant difference).13 However, a multicenter randomized trial from Korea (the KLASS-02 trial) of 1050 patients reported that a significantly lower proportion of patients experienced morbidities in the early period after LDG (16.6%) compared with ODG (24.1%).17
The JLSSG0901 trial is underway in Japan to evaluate the efficacy of LDG in the treatment of LAGC.18 In the CLASS-01 trial, rates of 3-year disease-free survival (the primary end point) were 76.6% in the LDG group vs 77.8% in the ODG group; recurrence did not differ significantly between groups.11 The KLASS-02 trial reported the noninferiority of LDG to ODG in 3-year relapse-free survival (80.3% vs 81.3% in the ODG group).12 Although those trials set different noninferiority margins, their findings indicated that LDG is a safe and effective alternative treatment for LAGC.
Overall survival is the standard end point for cancer trials, although disease-free survival is another end point that might be used.19 Retrospective studies reported similar survival times between patients who underwent LDG vs ODG for LAGC,20,21 but there was insufficient evidence for effects on long-term survival from a large-scale, well-designed, randomized trial. In our trial, the 5-year overall survival for patients with any stage of gastric cancer was 72.6% in the LDG group vs 76.3% in the ODG group (90.0% vs 88.5% for patients with stage I tumors, 79.1% vs 84.5% for patients with stage II tumors, and 58.6% vs 59.5% for patients with stage III tumors). These findings support LDG with D2 lymphadenectomy as a treatment for LAGC.
In a large-scale, retrospective, multicenter study in Korea, rates of 5-year overall survival for patients with stage IA tumors were 94.0% in in the ODG group vs 95.6% in the LDG group; for patients with stage IB tumors these rates were 96.9% and 92.7%; for patients with stage IIA tumors these rates were 88.4% and 85.5%; for patients with stage IIB tumors these rates were 80.3% and 80.0%; for patients with stage IIIA tumors these rates were 70.0% and 61.9%; for patients with stage IIIB tumors these rates were 68.8% and 47.8%; and for patients with stage IIIC tumors these rates were 40.0% and 33.3%.22 There were no significant differences between the ODG and LDG groups among patients with any stage gastric cancer.
In a multicenter cohort study of 610 patients with stage II or III gastric cancer in Japan (the LOC-A study), rates of 5-year overall survival were 54.2% in the ODG group vs 53.0% in the LDG group, demonstrating noninferiority of LDG.23 A study of 17 449 patients in the National Cancer Database found that minimally invasive surgery for gastric cancer was associated with increased 5-year survival compared with open surgery (51.9% vs 47.7%), and found no difference between laparoscopic and robotic approaches.24 We analyzed data using the RMST method, which is increasingly recognized as a robust and clinically interpretable summary measure that is an alternative to HRs and median survival time, because it directly quantifies information of the entire observed survival curve.25,26,27,28 We found that RMSTs did not differ significantly between the LDG and ODG groups, indicating that mean survival time was 2% lower in the LDG group vs the ODG group.
Laparoscopic gastrectomy is underused in Western countries, with fewer trials, compared with Eastern countries. Analysis of data from the US National Cancer Databases showed that 73.4%, 23.1%, and 3.5% of all gastrectomies in patients with gastric cancer were performed using open, laparoscopic, and robotic procedures, respectively, from 2010 to 2012.29 It is not clear whether results from Eastern countries can be extrapolated to Western countries. Therefore, a prospective randomized multicenter trial (LOGICA trial) of LDG vs ODG for patients with surgically resectable gastric adenocarcinoma was launched in the Netherlands in 2014; final results are not expected until 2022.30 There is also evidence that patients in Eastern countries have better outcomes than patients in Western countries in stage by stage comparisons.31,32 However, there is no evidence yet to associate outcomes with technical procedures.
Laparoscopic gastrectomy with extended lymph node dissection has been used for more advanced gastric cancer, especially in serosa-positive gastric cancer that has a risk of cancer cell dissemination resulting from laparoscopic tumor handling. In our previous 3-year results, the Kaplan-Meier curves of disease-free survival between LDG and ODG (58.0% vs 63.8%; P = .23) for patients with stage III disease seem to diverge, suggesting the need for long-term follow-up.11 In present 5-year outcomes, the 5-year OS was 58.6% in the LDG group and 59.5% in the ODG group among patients with stage III tumors, and the Kaplan-Meier curve between the 2 groups was crossed, indicating that LDG could be a potential standard treatment option for more advanced gastric cancer.
Limitations
Our study has several limitations. First, 29 patients crossed over from the ODG to the LDG group and 25 patients crossed over from the LDG to the ODG group before the procedure. However, analyses of the per-protocol population after we excluded these crossover patients produced the same long-term results. Furthermore, the study was performed in China, so it is not clear whether the findings will be the same in Western countries, where total or proximal gastrectomy is more common. The CLASS-02 trial was launched to compare laparoscopic total gastrectomy with open surgery for patients with advanced cancer in the upper body of the stomach.33 Also, none of the patients received neoadjuvant therapy, which is typically recommended in Western countries and could affect surgical outcomes. The CLASS-03 trial is therefore being planned to evaluate the safety of laparoscopic gastrectomy after neoadjuvant therapy. Finally, it will be difficult to generalize our findings to surgeons with less-intensive training.
Conclusions
We found no significant difference in 5-year overall survival of patients with LAGC treated with LDG with D2 lymphadenectomy vs ODG performed by experienced surgeons at high-volume, specialized institutions.
Trial Protocol and Statistical Analysis Plan
eFigure 1. Sensitivity Analysis of ITT Population
eFigure 2. Per-Protocol and As-Treated Analyses
eTable. Cause of Death
Nonauthor Collaborators. The Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group nonauthor collaborators
Data Sharing Statement
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146-148. [PubMed] [Google Scholar]
- 3.Honda M, Hiki N, Kinoshita T, et al. Long-term outcomes of laparoscopic versus open surgery for clinical stage I gastric cancer: the LOC-1 Study. Ann Surg. 2016;264(2):214-222. doi: 10.1097/SLA.0000000000001654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim W, Kim HH, Han SU, et al. ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group . Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg. 2016;263(1):28-35. doi: 10.1097/SLA.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 5.Hur H, Lee HY, Lee HJ, et al. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015;15:355. doi: 10.1186/s12885-015-1365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1-19. doi: 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katai H, Sasako M, Fukuda H, et al. ; JCOG Gastric Cancer Surgical Study Group . Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13(4):238-244. doi: 10.1007/s10120-010-0565-0 [DOI] [PubMed] [Google Scholar]
- 8.Katai H, Mizusawa J, Katayama H, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(2):142-151. doi: 10.1016/S2468-1253(19)30332-2 [DOI] [PubMed] [Google Scholar]
- 9.Kim HH, Han SU, Kim MC, et al. ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group . Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5(4):506-513. doi: 10.1001/jamaoncol.2018.6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, Hu Y, Liu H. Current status of randomized controlled trials for laparoscopic gastric surgery for gastric cancer in China. Asian J Endosc Surg. 2015;8(3):263-267. doi: 10.1111/ases.12198 [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Huang C, Sun Y, et al. ; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group . Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321(20):1983-1992. doi: 10.1001/jama.2019.5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyung WJ, Yang HK, Park YK, et al. ; Korean Laparoendoscopic Gastrointestinal Surgery Study Group . Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial. J Clin Oncol. 2020;38(28):3304-3313. doi: 10.1200/JCO.20.01210 [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350-1357. doi: 10.1200/JCO.2015.63.7215 [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Ying M, Huang C, et al. ; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group . Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endosc. 2014;28(7):2048-2056. doi: 10.1007/s00464-014-3426-9 [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Hu J, Huang C, et al. ; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group . The impact of age and comorbidity on postoperative complications in patients with advanced gastric cancer after laparoscopic D2 gastrectomy: results from the Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) group. Eur J Surg Oncol. 2013;39(10):1144-1149. doi: 10.1016/j.ejso.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 16.Beyer K, Baukloh AK, Kamphues C, et al. Laparoscopic versus open gastrectomy for locally advanced gastric cancer: a systematic review and meta-analysis of randomized controlled studies. World J Surg Oncol. 2019;17(1):68. doi: 10.1186/s12957-019-1600-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Hyung WJ, Yang HK, et al. ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group . Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann Surg. 2019;270(6):983-991. doi: 10.1097/SLA.0000000000003217 [DOI] [PubMed] [Google Scholar]
- 18.Inaki N, Etoh T, Ohyama T, et al. A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg. 2015;39(11):2734-2741. doi: 10.1007/s00268-015-3160-z [DOI] [PubMed] [Google Scholar]
- 19.Oba K, Paoletti X, Alberts S, et al. ; GASTRIC group . Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: a meta-analysis. J Natl Cancer Inst. 2013;105(21):1600-1607. doi: 10.1093/jnci/djt270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara T, Satoh S, Kanaya S, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013;27(1):286-294. doi: 10.1007/s00464-012-2442-x [DOI] [PubMed] [Google Scholar]
- 21.Park DJ, Han SU, Hyung WJ, et al. ; Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group . Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26(6):1548-1553. doi: 10.1007/s00464-011-2065-7 [DOI] [PubMed] [Google Scholar]
- 22.Kim HH, Han SU, Kim MC, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32(7):627-633. doi: 10.1200/JCO.2013.48.8551 [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita T, Uyama I, Terashima M, et al. ; LOC-A Study Group . Long-term outcomes of laparoscopic versus open surgery for clinical stage II/III gastric cancer: a multicenter cohort study in Japan (LOC-A study). Ann Surg. 2019;269(5):887-894. doi: 10.1097/SLA.0000000000002768 [DOI] [PubMed] [Google Scholar]
- 24.Hendriksen BS, Brooks AJ, Hollenbeak CS, Taylor MD, Reed MF, Soybel DI. The impact of minimally invasive gastrectomy on survival in the USA. J Gastrointest Surg. 2020;24(5):1000-1009. doi: 10.1007/s11605-019-04263-4 [DOI] [PubMed] [Google Scholar]
- 25.Huang B, Kuan PF. Comparison of the restricted mean survival time with the hazard ratio in superiority trials with a time-to-event end point. Pharm Stat. 2018;17(3):202-213. doi: 10.1002/pst.1846 [DOI] [PubMed] [Google Scholar]
- 26.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pak K, Uno H, Kim DH, et al. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017;3(12):1692-1696. doi: 10.1001/jamaoncol.2017.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weir IR, Marshall GD, Schneider JI, et al. Interpretation of time-to-event outcomes in randomized trials: an online randomized experiment. Ann Oncol. 2019;30(1):96-102. doi: 10.1093/annonc/mdy462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenleaf EK, Sun SX, Hollenbeak CS, Wong J. Minimally invasive surgery for gastric cancer: the American experience. Gastric Cancer. 2017;20(2):368-378. doi: 10.1007/s10120-016-0605-5 [DOI] [PubMed] [Google Scholar]
- 30.Haverkamp L, Brenkman HJ, Seesing MF, et al. ; LOGICA study group . Laparoscopic versus open gastrectomy for gastric cancer, a multicenter prospectively randomized controlled trial (LOGICA-trial). BMC Cancer. 2015;15:556. doi: 10.1186/s12885-015-1551-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251(4):640-646. doi: 10.1097/SLA.0b013e3181d3d29b [DOI] [PubMed] [Google Scholar]
- 32.Strong VE, Song KY, Park CH, et al. Comparison of disease-specific survival in the United States and Korea after resection for early-stage node-negative gastric carcinoma. J Surg Oncol. 2013;107(6):634-640. doi: 10.1002/jso.23288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Huang C, Xu Z, et al. ; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group . Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: the CLASS02 multicenter randomized clinical trial. JAMA Oncol. 2020;6(10):1590-1597. doi: 10.1001/jamaoncol.2020.3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eFigure 1. Sensitivity Analysis of ITT Population
eFigure 2. Per-Protocol and As-Treated Analyses
eTable. Cause of Death
Nonauthor Collaborators. The Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group nonauthor collaborators
Data Sharing Statement