Abstract
Gastric cancer (GC) is the fifth most diagnosed cancer and the third leading cause of cancer-related death worldwide. Although progress has been made in diagnosis, surgical resection, systemic chemotherapy, and immunotherapy, patients with GC still have a poor prognosis. The overall 5-year survival rate in patients with advanced GC is less than 5%. The FOXO subfamily, of the forkhead box family of transcription factors, consists of four members, FOXO1, FOXO3, FOXO4, and FOXO6. This subfamily plays an important role in many cellular processes, such as cell cycle, cell growth, apoptosis, autophagy, stress resistance, protection from aggregate toxicity, DNA repair, tumor suppression, and metabolism, in both normal tissue and malignant tumors. Various studies support a role for FOXOs as tumor suppressors based on their ability to inhibit angiogenesis and metastasis, and promote apoptosis, yet several other studies have shown that FOXOs might also promote tumor progression in certain circumstances. To elucidate the diverse roles of FOXOs in GC, this article systematically reviews the cellular functions of FOXOs in GC to determine potential therapeutic targets and treatment strategies for patients with GC.
Keywords: FOXO, Gastric cancer, Regulation, Therapy, Expression
Core Tip: FOXOs perform diverse roles in the occurrence and development of gastric cancer, the fifth most diagnosed type of cancer and third leading cause of cancer-related death worldwide. This article reviews the cellular functions of FOXOs in gastric cancer and provides potential therapeutic targets for patients with gastric cancer.
INTRODUCTION
Gastric cancer (GC) is the fifth most diagnosed cancer and the third leading cause of cancer-related death worldwide[1]. Upper gastrointestinal series and endoscopy, which have been demonstrated to be effective for screening, have not been widely adopted worldwide because of their invasive nature and high cost. Moreover, the lack of universal guidelines for screening has increased the difficulty of early diagnosis of GC[2-4]. It is estimated that more than 700000 cancer-related deaths are caused by GC, which is primarily because the cancer is already at an advanced stage at initial diagnosis[5,6]. Unsurprisingly, although great progress has been made in diagnosis, surgical resection, systemic chemotherapy, and immunotherapy in recent decades, patients with advanced GC still exhibit a very poor prognosis, with a median overall survival (OS) of 10-12 mo and an overall 5-year survival rate of less than 5%[7-9]. To improve the availability of accurate diagnostic tests for the early detection of GC and to identify more specific therapeutic targets for GC patients, it is important to explore the molecular mechanism of GC. This will help overcome the critical limitations in diagnostics and therapeutics in patients with GC.
FOXOs, the O subfamily of the forkhead box (FOX) family of transcription factors, comprise four members, FOXO1, FOXO3, FOXO4, and FOXO6. This subfamily has been reported to be involved in the cell cycle, cell growth, apoptosis, autophagy, stress resistance, protection from aggregate toxicity, DNA repair, tumor suppression, and metabolism[10,11]. Importantly, FOXOs are involved in the pathological processes of malignant tumors, as well as in the physiological processes of development[12]. However, the functions of FOXOs in malignant tumors vary under different conditions. FOXOs function as tumor suppressors based on their ability to inhibit angiogenesis[13] and metastasis[14], and their ability to promote apoptosis[15]. However, other studies have indicated that FOXOs can also promote tumor progression under certain circumstances[10]. As transcription factors, FOXOs may affect different aspects of the occurrence and development of GC by regulating the expression of downstream target genes. This article focuses on the diverse cellular functions of FOXOs, in GCs, to identify potential early diagnostic biomarkers and therapeutic targets for patients with GC.
CHARACTERISTICS OF FOXO FAMILY MEMBERS
It is well known that transcription factors regulate the expression of target genes by identifying and binding to specific DNA sequences, after which they participate in the formation of a complex signaling network to maintain cell homeostasis[16]. Dysregulation of transcription factors leads to a variety of pathological changes in cells, results in the occurrence of various diseases, and determines the various behaviors of malignant tumors[17,18]. Among various transcription factors, FOX transcription factors are widely distributed in organisms from yeasts to humans. They are characterized by a forkhead domain (FHD) and a highly conserved DNA binding domain (DBD) that is composed of 100 amino acid residues folded into a helix-turn-helix motif with two characteristic large loops and three α helices[19].
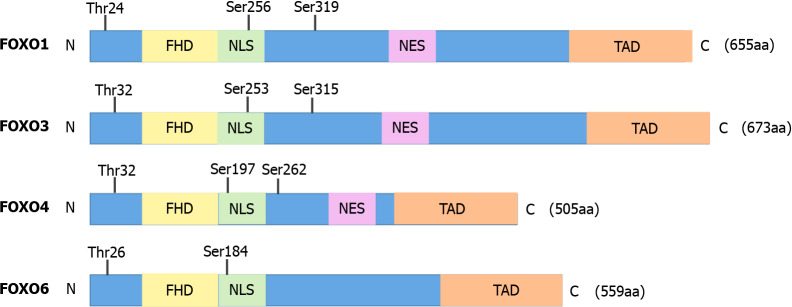
Among the different types of FOX transcription factors, the four FOXO isoforms, FOXO1, FOXO3, FOXO4, and FOXO6, in mammals belong to the O subfamily of the FOX family of transcription factors[20]. FOXOs have four common domains, including a FHD, a nuclear export sequence (NES) domain, a nuclear localization signal (NLS), and a C-terminal transactivation domain (TAD), although FOXO6 lacks the NES domain (Figure 1). All FOXOs can recognize and bind to two sequences: the Daf-16 family member-binding element (DEB), 5′-GTAAA(T/C)AA-3′, and the insulin-responsive sequence (IRE), 5′-(C/A)(A/C)AAA(C/T)AA-3′[21,22].
Figure 1.
Domains and AKT phosphorylation sites in FOXOs. FHD: Forkhead domain; NES: Nuclear export sequence; NLS: Nuclear localization signal.
Expression pattern of FOXOs
FOXO1, FOXO3, and FOXO4 are widely expressed in almost all tissues, and their transcriptional activity changes as they shuttle between different subcellular localizations[22,23]. FOXO6, a novel member of the FOXO class reported by Jacobs et al[24], was originally only observed in the central nervous system, but subsequent investigations have confirmed that FOXO6 is also expressed in peripheral tissues, including the lungs, liver, kidneys, intestine, muscle, and adipose tissue[25]. Interestingly, the expression pattern of FOXO6 is different from that of other FOXO isoforms in its evolution, and it is the least characterized member of the FOXO family. Due to the lack of an NES sequence, FOXO6 does not shuttle between the nucleus and cytoplasm and is located only in the nucleus[26].
Regulatory mechanism of FOXOs
FOXOs function as central transcription factors that regulate many cellular processes through transcriptional activity. Unsurprisingly, FOXOs are also regulated by multiple signaling pathways involving synthesis, phosphorylation, acetylation, and ubiquitination, which mainly determine subcellular localization, transcriptional activity, and protein stability[11,22,27]. As transcription factors, FOXOs usually exist in the nuclei of quiescent or growth factor (GF)-deficient cells. When GFs are absent, FOXOs shuttle into and accumulate in the nucleus to promote cell cycle arrest, stress resistance, and apoptosis, by upregulating the transcription of a series of target genes. However, in the presence of cell GFs, FOXOs relocate to the cytoplasm for degradation by the ubiquitin-proteasome pathway[23].
Phosphorylation via the classical PI3K-AKT pathway: Except for FOXO6, the regulation of FOXO-dependent transcription primarily depends on shuttling between the nucleus and cytoplasm. More specifically, negative regulation by the PI3K-AKT pathway is dependent on activation by GF receptor tyrosine kinases (RTKs)[28]. Under normal physiological conditions, RTKs are activated by autophosphorylation after binding GFs or insulin, which is followed by recruitment and activation of PI3K. Then, activated PI3K catalyzes phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), which serves as the docking site for AKT and PDK1. PIP3 facilitates the translocation of PDK1 and AKT to the cell membrane, where AKT is activated by phosphorylation on threonine 308 by PDK1. Activated AKT phosphorylates FOXOs at three sites to promote the binding of nuclear 14-3-3 protein to FOXO, which results in masking of the FOXO NLS; this causes the export of FOXO from the nucleus and prevents nuclear entry, thus preventing FOXO from binding to corresponding sites on DNA and inhibiting its transcriptional activity[11]. When the GF-PI3K-AKT pathway is constitutively activated, such as in cancer cells, the nuclear localization of FOXOs is negatively regulated, which results in the transfer of FOXOs to the cytoplasm and loss of their activity[22]. However, in the absence of GF signals, PIP3 will be dephosphorylated by PTEN (phosphatase and tensin homolog), thereby reducing PKB/AKT activity and concomitantly resulting in the loss of FOXO phosphorylation and nuclear accumulation.
According to a previously defined mechanism, FOXOs enter the nucleus, bind to a variety of transcription cofactors, and regulate the transcription of target genes related to the cell cycle, apoptosis, the antioxidant state, metabolism, and angiogenesis[28]. For FOXO6, phosphorylation of two residues (threonine 26 and serine 184) by AKT results in inactivation. Unlike other FOXOs, the PI3K-AKT pathway cannot affect the subcellular localization of FOXO6 due to the lack of carboxy-terminal AKT-dependent phosphorylation sites in FOXO6[11,25,29].
AKT-independent phosphorylation: Inhibition of FOXOs by the PI3K-AKT pathway is believed to enhance tumor development, while stress-activated kinases, such as c-Jun N-terminal kinase (JNK), mammalian sterile 20like kinase 1 (MST1), and protein kinase RNA-like endoplasmic reticulum kinase (PERK), play a tumor inhibitory role by promoting FOXO function in an AKT-independent manner[11].
Essers et al[30] illustrated that in contrast to insulin-mediated regulation, under oxidative stress, FOXO4 is phosphorylated by JNK on threonine 447 and threonine 451 in a GTPase-dependent manner, which leads to the nuclear translocation of p-FOXO4. Specifically, the regulatory effect of JNK on FOXO activity involves phosphorylation of 14-3-3 on serine 184 to block 14-3-3 proteins from binding to FOXOs[11,31].
Lehtinen et al[32] extended the molecular mechanism by which oxidative stress influences cell survival and homeostasis, by demonstrating the role of the protein kinase MST1 in oxidative stress-induced cell death. In the case of increased cellular oxidative stress, MST1 phosphorylates FOXO proteins at a conserved site to disrupt their interaction with 14-3-3 proteins, which results in FOXO nuclear translocation, and induces neuronal cell death[32]. Soon after, Yuan et al[33] also found that MST1-induced phosphorylation of FOXO1 at serine 212, which corresponds to serine 207 in FOXO3, disrupts the association between FOXO1 and 14-3-3 proteins. The above findings indicate that MST1-FOXO1 signaling is an important link to serum-deprivation-induced neuronal cell death.
Recently, PERK was found to be involved in endoplasmic reticulum (ER) stress related to the onset of type 2 diabetes[34]. Imbalances between protein synthesis and folding lead to ER stress, which partially enhances FOXO activity through the PERK pathway. Interestingly, although three target sites serine 298, serine 301, and serine 303 on FOXO1 can be phosphorylated by PERK, PERK-mediated phosphorylation preferentially occurs on serine 298, which is not a target site for AKT[34]. Phosphorylation by PERK enhances the transcriptional activity of FOXOs and counteracts the effect of Akt phosphorylation[34,35].
In addition, extracellular signalregulated kinase (ERK), p38, cyclin-dependent kinases (CDKs), adenosine monophosphate-activated protein kinase (AMPK), and IκB kinase (IκK) regulate FOXOs in an AKT-independent manner. For example, mitogen-activated protein kinases (MAPKs), ERK, and p38 jointly phosphorylate FOXO1, which results in p-FOXO1 serving as a coactivator for Ets-1[36]. Additionally, ERK mediates the phosphorylation of FOXO3 at serine 294, serine 344, and serine 425, which permits the association of p-FOXO3 with the E3 ubiquitin ligase MDM2 (murine double minute 2). This in turn results in the ubiquitination and degradation of p-FOXO3 to promote cell proliferation and tumorigenesis[37]. CDK2 binds to and phosphorylates FOXO1 at serine 249 in a glucose-dependent manner, and loss of CDK2 may mediate persistent insulin secretion defects through this pathway[38,39]. Lu et al[40] proposed FO1–6nls, a FOXO1-derived peptide inhibitor of CDK1/2-mediated phosphorylation of FOXO1 at serine 249, as a potential therapeutic for the treatment of prostate cancers. AMPK phosphorylates FOXO1 and forms the AMPK/ FOXO1 axis, which is involved in multiple pathological processes, such as liver fibrosis[41], cardiac hypertrophy[42], and epithelial-mesenchymal transition (EMT)[43]. The phosphorylation of FOXO3 at serine 644 by IκK normally leads to ubiquitin-dependent proteasomal degradation[44], but causes cytoplasmic retention in acute myeloid leukemia[45].
Acetylation: Histone acetylation is an epigenetic modification that regulates numerous genes essential for various biological processes, including development and stress responses[46]. It has been reported that calcium response element-binding protein (CBP)/p300 acetylates FOXOs to promote their phosphorylation by AKT and allows FOXOs to be retained in the cytoplasm[47]. However, stress-induced FOXO1 acetylation also arrests FOXO1 ubiquitination and prevents FOXO1 degradation through the ubiquitin-proteasome pathway[48]. Importantly, acetylation of FOXOs is a reversible process and can be eliminated by histone acetyltransferases and histone deacetylases (HDACs)[49,50]. For example, Sirt1, a class III HDAC, can deacetylate FOXOs and increase their transcription[47]. However, this increased effect is eliminated quickly because of the facilitated degradation of deacetylated FOXOs through the ubiquitin-proteasome pathway[51].
Other posttranslational modifications: In addition to phosphorylation, acetylation, and polyubiquitination, the activity of FOXOs is regulated by other posttranslational modifications, including mono-ubiquitination, methylation, and glycosylation.
In contrast to degradation induced by polyubiquitination, mono-ubiquitination enhances FOXO activity. Interestingly, under oxidative stress, MDM2, which promotes the degradation of p-FOXO3, can induce mono-ubiquitination of FOXO4 to increase FOXO4 nuclear entry and transcriptional activity[52]. Methylation of FOXO1 by protein arginine methyltransferase 1 (PRMT1) inhibits AKT-induced phosphorylation, and thus, promotes FOXO1 retention in the nucleus and increases the expression of downstream target genes[53]. However, methylation of FOXO3 by the Set9 methyltransferase reduces the DNA-binding and transcriptional activities of FOXO3[54]. O-glycosylation improves the transcriptional activity of FOXO1 without influencing its subcellular localization[55]. Recently, N6-methyladenosine modifications of FOXO1 mRNA, reported by Jian et al[56], were demonstrated to mediate METTL14-induced endothelial inflammation and atherosclerosis. Shin et al[57] identified a novel posttranslational modification of the FOXO family, O-GlcNAcylation of FOXO3 at serine 284, that impairs the ability of FOXO3 to induce subsequent cancer cell growth via abrogation of the p53 regulatory circuit.
Of course, other posttranslational modifications may exist and remain to be discovered. The transcriptional activities of FOXOs are involved in regulating the cell cycle, oxidative stress, apoptosis, and autophagy, as well as metabolic and immunoregulatory factors. Moreover, FOXO3 is closely related to longevity in humans[58-60]. The biological function of FOXO6 has not been well studied, and most research has indicated its participation in glucose and lipid metabolism[26]. Unsurprisingly, FOXOs are involved in many aspects of malignant tumors.
ROLES OF FOXOS IN CANCERS
It is well known that FOXOs are tumor suppressors in many types of malignant tumors[29]. Usually, in cancers, the PI3K-PKB/AKT signaling pathway is enhanced, and FOXOs are negatively regulated downstream molecules in the pathway. Specifically, activation of FOXOs leads to cell cycle arrest and apoptosis[28]. Therefore, reduction of PI3K/AKT phosphorylation via knockdown techniques or suppression with specific inhibitors enhances the transcriptional activity of FOXOs and induces cell cycle arrest and cell apoptosis in colorectal cancer (CRC) and pancreatic cancer cells[61,62].
In terms of cell cycle control, Baugh and Sternberg[63] found that the induced expression of cell cycle kinase inhibitors (CKIs) by FOXOs leads to the inhibition of cyclin/CDK complexes, which are responsible for cell cycle progression at different phases. This causes cell cycle arrest in G0/G1 and G2 phases and even senescence and promotes developmental arrest via transcriptional regulation of numerous target genes that control various aspects of development[63].
Moreover, in both normal and cancer cells, FOXOs are reported to induce the expression of proapoptotic genes, resulting in apoptosis. Wang et al[64] showed that activation of AMPK-FOXO is upstream of the KLF2 pathway and contributes to the induction of apoptosis and differentiation by DT-13 (Liriope muscari baily saponins C) in acute myelocytic leukemia. Laporte et al[65] revealed that HDAC inhibition-induced apoptosis and decreased tumor burden in synovial sarcoma are related to reactive oxygen species (ROS)-mediated FOXO activation and the subsequent increase in the expression of the proapoptotic factors BIK, BIM, and BMF. Interestingly, in the case of detachment from the extracellular matrix, FOXOs induce anoikis and prevent metastasis by promoting BMF expression, whereas under anchorage-independent conditions, cyclin D1 induces an antagonistic effect on FOXO-regulated anoikis[66].
It is widely accepted that ROS abnormally accumulate in cancer cells due to the reprogramming of redox metabolism, which plays opposite roles in various aspects of occurrence and development of malignant tumors[67]. Upon AKT activation, FOXOs become phosphorylated and translocate from the nucleus, which results in reduced expression of superoxide dismutase 2 (SOD2) and an increase in ROS and mitochondrial dysfunction[68]. Therefore, FOXOs promote detoxification of cells by inducing SOD2 and catalase expression, thus protecting cells from damage due to excessive accumulation of ROS and preventing cancer development.
Based on a previous mechanism, a series of investigations reported a significant relationship between FOXO expression and the clinical parameters of malignant tumors. Xu et al[69] found that a low level of FOXO4 expression in non-small cell lung cancer patients is significantly correlated with TNM stage and lymph node metastasis, which suggests an inhibition of FOXO4 function during the process of EMT. In CRC tissues, the expression of FOXO3 is also significantly lower than that in normal tissues, and interestingly, the progressive downregulation of FOXO3 is correlated with the progression of pathological stage in patients with CRC. Moreover, the mean disease-free survival (DFS) of CRC patients with low FOXO3 expression is significantly shorter compared with that of CRC patients with high FOXO3 expression[70]. Wu et al[71] conducted multivariate analyses and revealed that FOXO1 expression is an independent biomarker for predicting DFS in patients with breast cancer, with lower levels of FOXO2 predicting poorer OS. Not surprisingly, reduced FOXO1 levels were observed in prostate cancer and are responsible for promoting the migration and invasiveness of prostate cancer cells via Runx2 regulation[72]. Therefore, it is known that reduced FOXO levels play an important role in tumor metastasis.
For further study, knockout techniques have provided additional methods by which the function and molecular mechanism of FOXO in tumors can be investigated. Renault et al[73] revealed that FOXO3 is a direct target of the p53 tumor suppressor gene. However, no association was observed between FOXO3 loss and p53 loss in tumor development. Paik et al[74] established a FOXO1/FOXO3/FOXO4 triple knockout mouse model and observed common and severe vascular lesions and premature death, while the tumor spectrum following triple FOXO deletion was much more limited than that after PTEN/AKT misregulation.
However, every coin has two sides. The expression of FOXO3 has been found to be increased in glioblastoma (GBM), and a high level of FOXO3 is associated with a poor prognosis in GBM patients. In addition, FOXO3 knockout significantly reduces, whereas FOXO3 overexpression enhances, the proliferation and invasiveness of GBM cells[75]. Yu et al[76] demonstrated that the expression of FOXO3 can be upregulated by SP1, which promotes CRC cell progression in vitro and in vivo. FOXO3 was found to promote tumor growth, under hypoxic conditions, and angiogenesis in aggressive neuroblastoma, which predicts adverse clinical outcomes[77]. In addition, FOXO3 acts as a conditional chemoprotection factor in late-stage neuroblastoma, enhancing tumor cell survival under chemotherapy[78]. The above reports reveal the complicated roles of FOXOs in cancer. As Hornsveld et al[28] suggested, FOXOs may function to support resilience in both healthy and cancer cells, rather than as typical tumor suppressors.
EXPRESSION PATTERNS OF FOXOS IN GCS
Unsurprisingly, the expression level of FOXOs is often altered in GC. Decreased levels of FOXO1/FOXO3/FOXO4 and increased expression of FOXO6 in GC have been reported. By examining 50 pairs of samples, Zang et al[79] found that the mRNA level of FOXO1 is downregulated in GC tissues compared with corresponding noncancerous tissues. Lower levels of FOXO3 mRNA and protein have also been found in GC tissues compared with peritumoral tissues[80]. Similarly, FOXO4 expression is consistently lower in GC tissues than in adjacent normal tissues[81]. However, FOXO6 has been reported to be overexpressed in GC. Elevated FOXO6 expression was demonstrated to promote the proliferation, invasiveness, and migration of GC cells, and is associated with a poor prognosis in GC patients[82,83]. Although FOXO1/ FOXO3/FOXO4 are often downregulated in GC, and mainly play a tumor inhibitory role, FOXOs possess tumor-promoting functions in certain conditions, and these functions are associated with different underlying molecular mechanisms.
MOLECULAR MECHANISMS OF FOXOS IN GC
Tumor-suppressive roles of FOXOs
Tumorigenesis and proliferation: Tumorigenesis begins with one or more genetic or epigenetic changes in a single cell, followed by subsequent changes that promote tumor development and progression of the tumor to a more aggressive phenotype. Following the accumulation of multiple genetic and epigenetic changes, when a cell has adapted enough to escape cellular homeostasis, cancer processes are initiated[84]. The expression level of FOXO4 is controlled by methylation of its promoter, and Zhou et al[85] showed that hypermethylation of FOXO4, which is induced by ubiquitin-like containing PHD ring finger 1, is involved in GC carcinogenesis.
Regulating the tumorigenic ability of GC cells by FOXOs involves their ability to inhibit GC cell self-renewal. Negative crosstalk between FOXO1 and leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5) was found in GC, and downregulation of FOXO1 increases the self-renewal capacity of GC cells by increasing LGR5 levels[86]. The newly discovered oncogene lncRNA AK023391 was reported to promote the occurrence and progression of GC through activation of the PI3K/Akt pathway, which further regulates downstream signaling, including inactivation of FOXO3[87]. An in vitro analysis showed that the JNK inhibitor SP600125 decreases the expression of cyclin D1, enhances FOXO1 activity, and inhibits colony formation in GC[88]. As expected, silencing FOXO1 expression leads to the partial recovery of the colony forming ability of GC cells, which indicates that JNK activation is involved in GC initiation partly through FOXO1 inhibition[88].
After tumor formation, cancer cell proliferation controlled by FOXOs is related to cell cycle arrest and induction of autophagy. FOXO1 inhibition by activation of the upstream c-Myc/NAMPT/SIRT1 signaling pathway or upregulation of downstream HER2, which results from FOXO1 Loss, promotes GC cell growth[89,90]. Su et al[81] found that FOXO4 induces cell cycle arrest in G1 phase and also reported a shortened S phase in GC cells. Activation of FOXO1 induces the expression of CDKI, p21Cip1, and p27Kip1, which can suppress GC cell proliferation by triggering cell cycle arrest[91,92]. MiR-96–5p and miR-1274a directly target the 3’-untranslated regions of FOXO3 and FOXO4 mRNA, respectively, and promote GC cell proliferation[93,94]. Moreover, in an acidic microenvironment, FOXO3 enhances autophagy by increasing the expression of autophagy proteins, such as LC3I, LC3II, and Beclin-1, to inhibit GC cell growth[95].
Apoptosis: The ability to escape apoptosis is a hallmark of cancer cells[96]. Identification of the mechanism of apoptosis induction provides potential therapeutic strategies for malignant tumors. In an α-fetoprotein (AFP)-producing GC (AFPGC) model, miR-122-5p inhibited apoptosis and promoted tumor progression by directly targeting FOXO3[97]. The transcription factor RUNX3 binds to two RUNX binding elements (RBE1 and RBE2) in the promoter region of the Bim gene, which encodes a pro-apoptotic protein. FOXO3 binds upstream of RBE1, which triggers apoptosis by activating Bim transcription through a physical interaction with RUNX3[98,99]. The induced Bim protein promotes the release of cytochrome c into the cytoplasm to initiate the formation of the apoptosome, which activates caspase-3 and leads to the execution phase of apoptosis[100]. Shahbazi et al[101] revealed the molecular mechanism of apoptosis induction by the nitric oxide synthase inhibitor L-NMMA and showed that L-NMMA promotes the phosphorylation of FOXO3 at threonine 32 and activates signaling by the Rho-associated coiled-coil kinase (ROCK). ROCK has been widely shown to regulate apoptosis[101], and is expressed in GC cells independently of PI3K/AKT and caspase-3[102]. Fas-associated death domain (FADD) protein can be recruited by the intracellular death domain of death receptors. FADD participates in apoptosis induced by the extrinsic death receptor pathway[100], which can be promoted by FOXO3 by suppressing the expression of the FADD inhibitor miR-633 in GC cells[103]. These findings expand on previous reports of the underlying molecular mechanism of FOXOs in promoting apoptosis of GC cells.
Angiogenesis: Angiogenesis-dependent tumor growth is an important characteristic of cancers[96]. Vascular endothelial GF (VEGF) and hypoxia-inducible factor-1α (HIF-1α) are critical in promoting tumor angiogenesis[104]. Under hypoxic conditions, HIF-1α and HIF-1β subunits form heterodimers that activate the transcription of many target genes to adapt to the hypoxic environment of human cancer cells[105]. However, under anoxic conditions, inhibition of FOXO1 causes upregulated expression of HIF-1α and VEGF in GC cells and increases microvessel areas in GC tissue, thus promoting angiogenesis[106,107]. In GC cells, miR-135b can be delivered via exosomes to human umbilical vein endothelial cells (HUVECs) and can then directly bind to and downregulate FOXO1 mRNA in HUVECs, which promotes ring formation of HUVECs and angiogenesis[108].
Drugs can also affect angiogenesis in GC through FOXO-related signaling pathways. Zhang et al[109] showed that arsenic trioxide reduces FOXO3 phosphorylation by inhibiting p-AKT, which results in the increased localization of FOXO3 in the nucleus where it suppresses GC migration and angiogenesis.
Metastasis: Metastasis is the leading cause of cancer-related death[96], and is related to EMT, which is characterized by loss of polarity of epithelial cells, decreased expression of epithelial markers, such as E-cadherin and β-catenin, and increased expression of mesenchymal markers, such as N-cadherin and vimentin. These characteristics endow tumor cells with metastatic properties by enhancing cell motility, invasiveness, and resistance to apoptosis. In addition, EMT-associated transcription factors, including Snail and Zeb, are involved in core EMT programs[110,111].
FOXO1 silencing results in upregulation of HER2 expression, which induces a mesenchymal cell phenotype, including decreased E-cadherin levels, increased Snail levels, and the presence of many filamentous processes with abundant actin bundles in GC cells, thus promoting the migration and invasiveness of GC cells[89]. Human telomerase reverse transcriptase cooperates with MDM2 to enhance FOXO3 degradation through ubiquitination, thus attenuating the inhibition of integrin β1 (ITGB1) expression induced by FOXO3. Subsequent increased ITGB1 expression promotes degradation of the extracellular matrix and enhances invasiveness of GC cells[112]. In addition to cell cycle arrest, Su et al[81] also found that upregulation of FOXO4 reduces the metastatic ability of GCs by decreasing vimentin expression, which inhibits EMT.
Chemoresistance: Studies that have focused on the role of FOXOs in GC chemoresistance are limited. Park et al[113] investigated resistance of GC cells to lapatinib in GC cells and showed that FOXO1 serves as an important link between the HER2 and MET signaling pathways by negatively regulating HER2 and MET expression at the transcriptional level, which could reverse resistance to lapatinib. Moreover, rosmarinic acid (RA) was found to increase FOXO4 expression by downregulating miR-6785–5p and miR-642a–3p levels and enhancing the sensitivity of drug-resistant GC cells to 5-fluorouracil[114].
Tumor-promoting roles of FOXOs
Although many studies support the inhibitory effect of FOXOs in cancers, several recent studies have provided solid evidence of the opposite effect, whereby FOXOs can promote GC progression, including proliferation, invasion, migration, and chemoresistance.
Park et al[115] reported that treatment with cisplatin increases the mRNA level of FOXO1 and promotes the accumulation and activation of the FOXO1 protein to confer protection against cisplatin-induced cytotoxicity in GC cells. Interestingly, in addition to their findings of the suppressive role of FOXO1 in acquired lapatinib-resistance in HER2-positive GC cells, Park et al[115] also investigated the role of FOXO1 in cisplatin-resistant GC cells. They showed that constitutive activation of FOXO1 increases resistance to cisplatin, whereas FOXO1 silencing enhances cisplatin-induced cytotoxicity along with apoptotic features in GC cells. Yu et al[116] artificially overexpressed FOXO3, and found that increased FOXO3 levels enhance the migratory and invasive abilities of GC cells by directly activating the transcription of cathepsin L, which targets and cleaves E-cadherin, leading to EMT. In contrast, FOXO3 knockdown experiments produced different results in vitro and in vivo. Li et al[117] reported that FOXO3 promotes cell survival in colon cancer under serum-free conditions, which suggests that the role of FOXO3 in tumorigenesis might depend on the environment. In the initial stage of GC, the AKT pathway becomes constitutively activated, resulting in phosphorylation and inactivation of FOXO3, which is beneficial to tumor proliferation. However, in advanced stages of GC, hypoxia, oxidative stress, and restricted serum access promote activation of FOXO3 to help cell adapt to a stressed state and enhance cell survival[116,117].
High FOXO6 expression promotes the proliferation of GC cells by binding to the transcription factor hepatic nuclear factor 4, which mediates histone acetylation and leads to subsequent induction of c-Myc expression after removal of HDAC3 from the c-Myc gene promoter[82]. Noncoding RNA activated by DNA damage, an lncRNA with potential carcinogenic effects in bladder and colon cancers, was found to be downregulated in GC cells, which could reduce the targeted inhibition of FOXO6 by miR-608 through competitive inhibition[118].
Therefore, it can be inferred that the changing microenvironment of GC at different stages of development may be one of the reasons why studies on the role of FOXOs in GC have reached opposite conclusions as to whether FOXOs participate in tumor progression.
POTENTIAL CLINICAL SIGNIFICANCE OF FOXOS IN GCS
Prognostic value of FOXOs
As mentioned above, phosphorylation results in the translocation of FOXO1 to the cytoplasm, which prevents FOXO-dependent transcription and loss of FOXO-dependent regulation of downstream target genes. High levels of phosphorylated FOXO1 are associated with vascular invasion, lymph node metastasis, distant metastasis, and higher pTNM stage in colon cancer and are indicative of a poor prognosis in astrocytomas[119,120]. In prostate cancer, the traditional Chinese medicines CFF-1 (alcohol extract from an anticancer compound Chinese medicine) and ISO (isorhapontigenin) inhibit cell growth and induce cell apoptosis by decreasing p-FOXO1 and regulating the expression of apoptosis-related and cycle-related genes[121,122]. These findings are consistent with the antitumor effect of FOXO1 in GC. However, Kim et al[123] reported that p-FOXO1 is expressed in 84.6% of GC tissues and that its expression is higher in early stage GC and is correlated with better outcomes. These findings further confirm that the role of FOXO1 is dependent on cancer stage.
Yang et al[80] reported a significant correlation between low FOXO3 levels and large tumor size, poor histopathological classification, greater depth of invasion, local lymph node metastasis, distant metastasis, and high AJCC stage. Upregulation and activation of FOXO3 in GC are closely associated with a good outcome in GC patients[124], which suggests that FOXO3 is a potential prognostic marker as well as a therapeutic target in GC patients. Li et al[125] demonstrated that a low FOXO4 level is an independent prognostic factor for poor OS and DFS in GC patients, while a high FOXO6 level promotes tumor invasiveness and predicts a poor prognosis in GC patients[83].
Targeting FOXOs for GC therapeutics
Some potential GC chemotherapeutic agents antagonize tumors by targeting FOXOs and related proteins to inhibit cell growth and proliferation, and induce cell differentiation and apoptosis. Endogenous proteins, such as sphingosine kinase 1 (SPHK1) and PRMT1, microRNAs, and circular RNAs also affect FOXOs and their related signaling pathways, and change the biological characteristics of GC cells. All of these molecules are potential therapeutic targets for the treatment of GC (Table 1).
Table 1.
Molecules targeting FOXOs and related proteins for potential gastric cancer therapy
|
Molecule
|
Targets
|
Mechanism
|
Effects
|
Ref.
|
| Luteolin | FOXO1 | Increases FOXO1 expression | Represses GC cell growth | Ding et al[127] |
| Celecoxib | Akt, GSK3b, FOXO1, and caspase-9 | Downregulates Akt, GSK3b, and FOXO1 and upregulates caspase-9 in the mitochondrial apoptotic pathway | Represses GC cell growth | Kim et al[128] |
| 4-Amino-2-trifluoromethyl-phenyl retinate | 14-3-3ε | Downregulates expression of 14-3-3ε, resulting in increased expression of FOXO1 and P27kip1, decreased expression of CDK2 and cyclin E, and decreased activity of AKP and LDH. Blocks the cell cycle at G0/G1 phase | Inhibits cell proliferation and induces cell differentiation | Xia et al[129] |
| Gramicidin | FOXO1 | Decreases phosphorylation of FOXO1 and down-regulates the expression of cyclinD1 and Bcl-2, leading to G2/M cell cycle arrest | Inhibits cell proliferation | Chen et al[130] |
| Olaparib | PARP1 | Inhibits PARP1 and thus induces G2/M cell cycle arrest by activating FOXO3 | Inhibits cell proliferation | Park et al[126] |
| Bacillomycind-C16 | Akt and FOXO3 | Inhibits phosphorylation of Akt and increases the level of FOXO3 protein | Induces apoptosis | Lin et al[131] |
| Protein arginine methyltransferase 1 | FOXO1 and BAD | Activates FOXO1 and BAD | Induces chemosensitivity | Altan et al[132] |
| Sphingosine kinase 1 | FOXO1 and FOXO3 | Attenuates the transcriptional activity of FOXO1 and FOXO3 via promoting PI3K/Akt-mediated phosphorylation | Enhances proliferation (targeting FOXO1) and resistance to apoptosis (targeting FOXO3) | Xia et al[91] and Xiong et al[133] |
| Hsa_circ_0001368 | miR-6506-5p | Acts as a competing endogenous RNA for miR-6506-5p and inhibits the downregulation by miR-6506-5p on FOXO3 | Inhibits tumor growth | Lu et al[134] |
| miR-1274a | FOXO4 | Inhibits FOXO4 expression | Promotes tumor growth and migration | Wang et al[94] |
It is worth noting that the effect of some drugs is influenced by oncogene expression. Inhibition of PARP1 by olaparib can induce G2/M cell cycle arrest by activating FOXO3 in GC cells. Moreover, knockout of BRCA1 or BRCA2 increases the sensitivity of MKN28 GC cells to olaparib, which suggests that olaparib therapy may be particularly beneficial for patients with BRCA-deficient GC[126]. HER2 expression in GC tissues is reported to be higher than that in adjacent normal tissues. Luteolin, a natural flavonoid compound, can repress the growth of GC cells by increasing FOXO1 expression. Luteolin encapsulation by poly(lactic-co-glycolic acid) nanoparticles (NPs) with HER-2 antibody conjugation increases recognition and endocytosis of NPs by GC cells and significantly enhances the inhibitory effect of luteolin on GC cells[127].
Additionally, miR-633 enhances the chemoresistance of GC cells by downregulating FADD expression. Doxorubicin-induced nuclear accumulation of FOXO3 inhibits miR-633 transcription. Inhibition of miR-633 by an antagomir increases the FADD level and enhances doxorubicin/cisplatin-induced apoptosis. A miR-633 antagomir combined with doxorubicin significantly reduces GC cell growth[103].
Overall, FOXOs are promising prognostic markers and therapeutic targets in GC. However, recent studies have primarily focused on the molecular mechanism and are limited to the cell level, which indicates a huge gap between recent research findings and clinical applications. Therefore, the clinical implications of FOXOs still require clarification by additional studies.
CONCLUSION
FOXOs have historically been regarded as tumor suppressors, but recent studies have suggested that FOXOs support resiliency in healthy and cancer cells. In GC, the antitumor effect of FOXO4 and the tumor-promoting effect of FOXO6 are relatively clear. FOXO1 and FOXO3 play dual roles in many types of cancers, including GC. Whether they promote or inhibit GC may be related to changes in the tumor microenvironment caused by tumor progression and drug treatment. In advanced GC, the effect of changes in the expression level or activity of FOXOs on GC treatment has not been investigated. Therefore, caution should be exercised when FOXO1 and FOXO3 are used as targets for cancer treatment.
ACKNOWLEDGEMENTS
We are thankful to Professor Lin S for his English editing and Shen L for his valuable advice and English editing.
Footnotes
Conflict-of-interest statement: No conflict of interest is claimed by any author.
Manuscript source: Invited manuscript
Peer-review started: April 6, 2021
First decision: June 23, 2021
Article in press: August 13, 2021
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY
Contributor Information
Yu-Han Chen, Department of Clinical Medicine, Shantou University Medical College, Shantou 515041, Guangdong Province, China; Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Breast Cancer, Changjiang Scholar's Laboratory, Department of Physiology, Shantou University Medical College, Shantou 515041, Guangdong Province, China.
Chun-Lan Li, Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Breast Cancer, Changjiang Scholar's Laboratory, Department of Physiology, Shantou University Medical College, Shantou 515041, Guangdong Province, China.
Wen-Jia Chen, Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Breast Cancer, Changjiang Scholar's Laboratory, Department of Physiology, Shantou University Medical College, Shantou 515041, Guangdong Province, China.
Jing Liu, Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Breast Cancer, Changjiang Scholar's Laboratory, Department of Physiology, Shantou University Medical College, Shantou 515041, Guangdong Province, China. jliu12@stu.edu.cn.
Hua-Tao Wu, Department of General Surgery, The First Affiliated Hospital of Shantou University Medical College, Shantou 515041, Guangdong Province, China.
References
- 1.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 2.Hamashima C Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol. 2018;48:673–683. doi: 10.1093/jjco/hyy077. [DOI] [PubMed] [Google Scholar]
- 3.Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc. 2016;84:18–28. doi: 10.1016/j.gie.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, Bleotu C, Diaconu CC, Chivu-Economescu M. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25:2029–2044. doi: 10.3748/wjg.v25.i17.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 7.Johnston FM, Beckman M. Updates on Management of Gastric Cancer. Curr Oncol Rep. 2019;21:67. doi: 10.1007/s11912-019-0820-4. [DOI] [PubMed] [Google Scholar]
- 8.Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A. Medical management of gastric cancer: a 2017 update. Cancer Med. 2018;7:123–133. doi: 10.1002/cam4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 10.Murtaza G, Khan AK, Rashid R, Muneer S, Hasan SMF, Chen J. FOXO Transcriptional Factors and Long-Term Living. Oxid Med Cell Longev. 2017;2017:3494289. doi: 10.1155/2017/3494289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav RK, Chauhan AS, Zhuang L, Gan B. FoxO transcription factors in cancer metabolism. Semin Cancer Biol. 2018;50:65–76. doi: 10.1016/j.semcancer.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calissi G, Lam EW, Link W. Therapeutic strategies targeting FOXO transcription factors. Nat Rev Drug Discov. 2021;20:21–38. doi: 10.1038/s41573-020-0088-2. [DOI] [PubMed] [Google Scholar]
- 13.Dumitrascu GR, Bucur O. Critical physiological and pathological functions of Forkhead Box O tumor suppressors. Discoveries (Craiova) 2013;1:e5. doi: 10.15190/d.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong T, Zhang Y, Chen Y, Liu P, An T, Zhang J, Yang H, Zhu W, Yang X. FOXO1 inhibits the invasion and metastasis of hepatocellular carcinoma by reversing ZEB2-induced epithelial-mesenchymal transition. Oncotarget. 2017;8:1703–1713. doi: 10.18632/oncotarget.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Signal. 2011;14:579–592. doi: 10.1089/ars.2010.3419. [DOI] [PubMed] [Google Scholar]
- 16.Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR, Weirauch MT. The Human Transcription Factors. Cell. 2018;172:650–665. doi: 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Lambert M, Jambon S, Depauw S, David-Cordonnier MH. Targeting Transcription Factors for Cancer Treatment. Molecules. 2018;23 doi: 10.3390/molecules23061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulasov AV, Rosenkranz AA, Sobolev AS. Transcription factors: Time to deliver. J Control Release. 2018;269:24–35. doi: 10.1016/j.jconrel.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Liang Z, Lou H. The Emerging Roles of Fox Family Transcription Factors in Chromosome Replication, Organization, and Genome Stability. Cells. 2020;9 doi: 10.3390/cells9010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiramongkol Y, Lam EW. FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 2020;39:681–709. doi: 10.1007/s10555-020-09883-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CG, Lee H, Gupta N, Ramachandran S, Kaushik I, Srivastava S, Kim SH, Srivastava SK. Role of Forkhead Box Class O proteins in cancer progression and metastasis. Semin Cancer Biol. 2018;50:142–151. doi: 10.1016/j.semcancer.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Link W. Introduction to FOXO Biology. Methods Mol Biol. 2019;1890:1–9. doi: 10.1007/978-1-4939-8900-3_1. [DOI] [PubMed] [Google Scholar]
- 23.Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int J Biol Sci. 2017;13:815–827. doi: 10.7150/ijbs.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278:35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Dong HH. FoxO integration of insulin signaling with glucose and lipid metabolism. J Endocrinol. 2017;233:R67–R79. doi: 10.1530/JOE-17-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon KM, Lee B, Kim DH, Chung HY. FoxO6 inhibits melanogenesis partly by elevating intracellular antioxidant capacity. Redox Biol. 2020;36:101624. doi: 10.1016/j.redox.2020.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klotz LO, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornsveld M, Dansen TB, Derksen PW, Burgering BMT. Re-evaluating the role of FOXOs in cancer. Semin Cancer Biol. 2018;50:90–100. doi: 10.1016/j.semcancer.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Coomans de Brachène A, Demoulin JB. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci. 2016;73:1159–1172. doi: 10.1007/s00018-015-2112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Yu T, Huang P. Post-translational modifications of FOXO family proteins (Review) Mol Med Rep. 2016;14:4931–4941. doi: 10.3892/mmr.2016.5867. [DOI] [PubMed] [Google Scholar]
- 32.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Z, Lehtinen MK, Merlo P, Villén J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem. 2009;284:11285–11292. doi: 10.1074/jbc.M900461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Hietakangas V, Wee S, Lim SC, Gunaratne J, Cohen SM. ER stress potentiates insulin resistance through PERK-mediated FOXO phosphorylation. Genes Dev. 2013;27:441–449. doi: 10.1101/gad.201731.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alasiri G, Jiramongkol Y, Zona S, Fan LY, Mahmud Z, Gong G, Lee HJ, Lam EW. Regulation of PERK expression by FOXO3: a vulnerability of drug-resistant cancer cells. Oncogene. 2019;38:6382–6398. doi: 10.1038/s41388-019-0890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal. 2007;19:519–527. doi: 10.1016/j.cellsig.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, Lee JH, Merrins MJ, Gavrilova O, Bisteau X, Kaldis P, Satin LS, Rane SG. Loss of Cyclin-dependent Kinase 2 in the Pancreas Links Primary β-Cell Dysfunction to Progressive Depletion of β-Cell Mass and Diabetes. J Biol Chem. 2017;292:3841–3853. doi: 10.1074/jbc.M116.754077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu H, Liu P, Pan Y, Huang H. Inhibition of cyclin-dependent kinase phosphorylation of FOXO1 and prostate cancer cell growth by a peptide derived from FOXO1. Neoplasia. 2011;13:854–863. doi: 10.1593/neo.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohseni R, Alavian SM, Sadeghabadi ZA, Heiat M. Therapeutic effects of Chlorella vulgaris on carbon tetrachloride induced liver fibrosis by targeting Hippo signaling pathway and AMPK/FOXO1 axis. Mol Biol Rep. 2021;48:117–126. doi: 10.1007/s11033-020-05978-3. [DOI] [PubMed] [Google Scholar]
- 42.Qiu J, Xiao H, Zhou S, Du W, Mu X, Shi G, Tan X. Bone marrow mesenchymal stem cells inhibit cardiac hypertrophy by enhancing FoxO1 transcription. Cell Biol Int. 2021;45:188–197. doi: 10.1002/cbin.11482. [DOI] [PubMed] [Google Scholar]
- 43.Xiao Q, Liu H, Wang HS, Cao MT, Meng XJ, Xiang YL, Zhang YQ, Shu F, Zhang QG, Shan H, Jiang GM. Histone deacetylase inhibitors promote epithelial-mesenchymal transition in Hepatocellular Carcinoma via AMPK-FOXO1-ULK1 signaling axis-mediated autophagy. Theranostics. 2020;10:10245–10261. doi: 10.7150/thno.47045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 45.Chapuis N, Park S, Leotoing L, Tamburini J, Verdier F, Bardet V, Green AS, Willems L, Agou F, Ifrah N, Dreyfus F, Bismuth G, Baud V, Lacombe C, Mayeux P, Bouscary D. IκB kinase overcomes PI3K/Akt and ERK/MAPK to control FOXO3a activity in acute myeloid leukemia. Blood. 2010;116:4240–4250. doi: 10.1182/blood-2009-12-260711. [DOI] [PubMed] [Google Scholar]
- 46.Kumar V, Thakur JK, Prasad M. Histone acetylation dynamics regulating plant development and stress responses. Cell Mol Life Sci. 2021;78:4467–4486. doi: 10.1007/s00018-021-03794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8:e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pomiès P, Blaquière M, Maury J, Mercier J, Gouzi F, Hayot M. Involvement of the FoxO1/MuRF1/Atrogin-1 Signaling Pathway in the Oxidative Stress-Induced Atrophy of Cultured Chronic Obstructive Pulmonary Disease Myotubes. PLoS One. 2016;11:e0160092. doi: 10.1371/journal.pone.0160092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morshneva A, Gnedina O, Svetlikova S, Pospelov V, Igotti M. Time-dependent modulation of FoxO activity by HDAC inhibitor in oncogene-transformed E1A+Ras cells. AIMS Genet. 2018;5:41–52. doi: 10.3934/genet.2018.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morshneva A, Gnedina O, Marusova T, Igotti M. Expression of Adenoviral E1A in Transformed Cells as an Additional Factor of HDACi-Dependent FoxO Regulation. Cells. 2019;9 doi: 10.3390/cells9010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hui B, Hou X, Liu R, Liu XH, Hu Z. Gypenoside inhibits ox-LDL uptake and foam cell formation through enhancing Sirt1-FOXO1 mediated autophagy flux restoration. Life Sci. 2021;264:118721. doi: 10.1016/j.lfs.2020.118721. [DOI] [PubMed] [Google Scholar]
- 52.Brenkman AB, de Keizer PL, van den Broek NJ, Jochemsen AG, Burgering BM. Mdm2 induces mono-ubiquitination of FOXO4. PLoS One. 2008;3:e2819. doi: 10.1371/journal.pone.0002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bayen S, Saini S, Gaur P, Duraisamy AJ, Kumar Sharma A, Pal K, Vats P, Singh SB. PRMT1 promotes hyperglycemia in a FoxO1-dependent manner, affecting glucose metabolism, during hypobaric hypoxia exposure, in rat model. Endocrine. 2018;59:151–163. doi: 10.1007/s12020-017-1463-6. [DOI] [PubMed] [Google Scholar]
- 54.Calnan DR, Webb AE, White JL, Stowe TR, Goswami T, Shi X, Espejo A, Bedford MT, Gozani O, Gygi SP, Brunet A. Methylation by Set9 modulates FoxO3 stability and transcriptional activity. Aging (Albany NY) 2012;4:462–479. doi: 10.18632/aging.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J, Wang P, Yu Z, Lai W, Cao Y, Huang P, Xu Q, Yu M, Xu J, Huang Z, Zeng B. Advanced glycosylation end product promotes forkhead box O1 and inhibits Wnt pathway to suppress capacities of epidermal stem cells. Am J Transl Res. 2016;8:5569–5579. [PMC free article] [PubMed] [Google Scholar]
- 56.Jian D, Wang Y, Jian L, Tang H, Rao L, Chen K, Jia Z, Zhang W, Liu Y, Chen X, Shen X, Gao C, Wang S, Li M. METTL14 aggravates endothelial inflammation and atherosclerosis by increasing FOXO1 N6-methyladeosine modifications. Theranostics. 2020;10:8939–8956. doi: 10.7150/thno.45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin H, Cha HJ, Na K, Lee MJ, Cho JY, Kim CY, Kim EK, Kang CM, Kim H, Paik YK. O-GlcNAcylation of the Tumor Suppressor FOXO3 Triggers Aberrant Cancer Cell Growth. Cancer Res. 2018;78:1214–1224. doi: 10.1158/0008-5472.CAN-17-3512. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, Yu W, Wang Y, Li P, Wang J. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17:104. doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing YQ, Li A, Yang Y, Li XX, Zhang LN, Guo HC. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018;193:124–131. doi: 10.1016/j.lfs.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 60.Liu W, Li Y, Luo B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell Mol Life Sci. 2020;77:651–663. doi: 10.1007/s00018-019-03297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. doi: 10.1186/1750-2187-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung CY, Park YL, Song YA, Myung E, Kim KY, Lee GH, Ki HS, Park KJ, Cho SB, Lee WS, Jung YD, Kim KK, Joo YE. Knockdown of RON inhibits AP-1 activity and induces apoptosis and cell cycle arrest through the modulation of Akt/FoxO signaling in human colorectal cancer cells. Dig Dis Sci. 2012;57:371–380. doi: 10.1007/s10620-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 63.Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol. 2006;16:780–785. doi: 10.1016/j.cub.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Wang C, He H, Liu G, Ma H, Li L, Jiang M, Lu Q, Li P, Qi H. DT-13 induced apoptosis and promoted differentiation of acute myeloid leukemia cells by activating AMPK-KLF2 pathway. Pharmacol Res. 2020;158:104864. doi: 10.1016/j.phrs.2020.104864. [DOI] [PubMed] [Google Scholar]
- 65.Laporte AN, Poulin NM, Barrott JJ, Wang XQ, Lorzadeh A, Vander Werff R, Jones KB, Underhill TM, Nielsen TO. Death by HDAC Inhibition in Synovial Sarcoma Cells. Mol Cancer Ther. 2017;16:2656–2667. doi: 10.1158/1535-7163.MCT-17-0397. [DOI] [PubMed] [Google Scholar]
- 66.Gan L, Liu P, Lu H, Chen S, Yang J, McCarthy JB, Knudsen KE, Huang H. Cyclin D1 promotes anchorage-independent cell survival by inhibiting FOXO-mediated anoikis. Cell Death Differ. 2009;16:1408–1417. doi: 10.1038/cdd.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Qi H, Liu Y, Duan C, Liu X, Xia T, Chen D, Piao HL, Liu HX. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839–4857. doi: 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El Maï M, Marzullo M, de Castro IP, Ferreira MG. Opposing p53 and mTOR/AKT promote an in vivo switch from apoptosis to senescence upon telomere shortening in zebrafish. Elife. 2020;9 doi: 10.7554/eLife.54935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu MM, Mao GX, Liu J, Li JC, Huang H, Liu YF, Liu JH. Low expression of the FoxO4 gene may contribute to the phenomenon of EMT in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:4013–4018. doi: 10.7314/apjcp.2014.15.9.4013. [DOI] [PubMed] [Google Scholar]
- 70.Bullock MD, Bruce A, Sreekumar R, Curtis N, Cheung T, Reading I, Primrose JN, Ottensmeier C, Packham GK, Thomas G, Mirnezami AH. FOXO3 expression during colorectal cancer progression: biomarker potential reflects a tumour suppressor role. Br J Cancer. 2013;109:387–394. doi: 10.1038/bjc.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Y, Elshimali Y, Sarkissyan M, Mohamed H, Clayton S, Vadgama JV. Expression of FOXO1 is associated with GATA3 and Annexin-1 and predicts disease-free survival in breast cancer. Am J Cancer Res. 2012;2:104–115. [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, Pan Y, Zheng L, Choe C, Lindgren B, Jensen ED, Westendorf JJ, Cheng L, Huang H. FOXO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion. Cancer Res. 2011;71:3257–3267. doi: 10.1158/0008-5472.CAN-10-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Renault VM, Thekkat PU, Hoang KL, White JL, Brady CA, Kenzelmann Broz D, Venturelli OS, Johnson TM, Oskoui PR, Xuan Z, Santo EE, Zhang MQ, Vogel H, Attardi LD, Brunet A. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene. 2011;30:3207–3221. doi: 10.1038/onc.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qian Z, Ren L, Wu D, Yang X, Zhou Z, Nie Q, Jiang G, Xue S, Weng W, Qiu Y, Lin Y. Overexpression of FoxO3a is associated with glioblastoma progression and predicts poor patient prognosis. Int J Cancer. 2017;140:2792–2804. doi: 10.1002/ijc.30690. [DOI] [PubMed] [Google Scholar]
- 76.Yu Y, Peng K, Li H, Zhuang R, Wang Y, Li W, Yu S, Liang L, Xu X, Liu T. SP1 upregulated FoxO3a promotes tumor progression in colorectal cancer. Oncol Rep. 2018;39:2235–2242. doi: 10.3892/or.2018.6323. [DOI] [PubMed] [Google Scholar]
- 77.Hagenbuchner J, Rupp M, Salvador C, Meister B, Kiechl-Kohlendorfer U, Müller T, Geiger K, Sergi C, Obexer P, Ausserlechner MJ. Nuclear FOXO3 predicts adverse clinical outcome and promotes tumor angiogenesis in neuroblastoma. Oncotarget. 2016;7:77591–77606. doi: 10.18632/oncotarget.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rupp M, Hagenbuchner J, Rass B, Fiegl H, Kiechl-Kohlendorfer U, Obexer P, Ausserlechner MJ. FOXO3-mediated chemo-protection in high-stage neuroblastoma depends on wild-type TP53 and SESN3. Oncogene. 2017;36:6190–6203. doi: 10.1038/onc.2017.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zang Y, Wang T, Pan J, Gao F. miR-215 promotes cell migration and invasion of gastric cancer cell lines by targeting FOXO1. Neoplasma. 2017;64:579–587. doi: 10.4149/neo_2017_412. [DOI] [PubMed] [Google Scholar]
- 80.Yang XB, Zhao JJ, Huang CY, Wang QJ, Pan K, Wang DD, Pan QZ, Jiang SS, Lv L, Gao X, Chen HW, Yao JY, Zhi M, Xia JC. Decreased expression of the FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients. PLoS One. 2013;8:e78158. doi: 10.1371/journal.pone.0078158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su L, Liu X, Chai N, Lv L, Wang R, Li X, Nie Y, Shi Y, Fan D. The transcription factor FOXO4 is down-regulated and inhibits tumor proliferation and metastasis in gastric cancer. BMC Cancer. 2014;14:378. doi: 10.1186/1471-2407-14-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qinyu L, Long C, Zhen-dong D, Min-min S, Wei-ze W, Wei-ping Y, Cheng-hong P. FOXO6 promotes gastric cancer cell tumorigenicity via upregulation of C-myc. FEBS Lett. 2013;587:2105–2111. doi: 10.1016/j.febslet.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 83.Wang JH, Tang HS, Li XS, Zhang XL, Yang XZ, Zeng LS, Ruan Q, Huang YH, Liu GJ, Wang J, Cui SZ. Elevated FOXO6 expression correlates with progression and prognosis in gastric cancer. Oncotarget. 2017;8:31682–31691. doi: 10.18632/oncotarget.15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foo J, Leder K, Michor F. Stochastic dynamics of cancer initiation. Phys Biol. 2011;8:015002. doi: 10.1088/1478-3975/8/1/015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou L, Shang Y, Jin Z, Zhang W, Lv C, Zhao X, Liu Y, Li N, Liang J. UHRF1 promotes proliferation of gastric cancer via mediating tumor suppressor gene hypermethylation. Cancer Biol Ther. 2015;16:1241–1251. doi: 10.1080/15384047.2015.1056411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi Y, Park J, Ko YS, Kim Y, Pyo JS, Jang BG, Kim MA, Lee JS, Chang MS, Lee BL. FOXO1 reduces tumorsphere formation capacity and has crosstalk with LGR5 signaling in gastric cancer cells. Biochem Biophys Res Commun. 2017;493:1349–1355. doi: 10.1016/j.bbrc.2017.09.163. [DOI] [PubMed] [Google Scholar]
- 87.Huang Y, Zhang J, Hou L, Wang G, Liu H, Zhang R, Chen X, Zhu J. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36:194. doi: 10.1186/s13046-017-0666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi Y, Park J, Choi Y, Ko YS, Yu DA, Kim Y, Pyo JS, Jang BG, Kim MA, Kim WH, Lee BL. c-Jun N-terminal kinase activation has a prognostic implication and is negatively associated with FOXO1 activation in gastric cancer. BMC Gastroenterol. 2016;16:59. doi: 10.1186/s12876-016-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ko YS, Cho SJ, Park J, Kim Y, Choi YJ, Pyo JS, Jang BG, Park JW, Kim WH, Lee BL. Loss of FOXO1 promotes gastric tumour growth and metastasis through upregulation of human epidermal growth factor receptor 2/neu expression. Br J Cancer. 2015;113:1186–1196. doi: 10.1038/bjc.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu H, Liu N, Zhao Y, Zhu X, Wang C, Liu Q, Gao C, Zhao X, Li J. Oncogenic USP22 supports gastric cancer growth and metastasis by activating c-Myc/NAMPT/SIRT1-dependent FOXO1 and YAP signaling. Aging (Albany NY) 2019;11:9643–9660. doi: 10.18632/aging.102410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia J, Wu Z, Yu C, He W, Zheng H, He Y, Jian W, Chen L, Zhang L, Li W. miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J Pathol. 2012;227:470–480. doi: 10.1002/path.4030. [DOI] [PubMed] [Google Scholar]
- 92.Fan C, Liu S, Zhao Y, Han Y, Yang L, Tao G, Li Q, Zhang L. Upregulation of miR-370 contributes to the progression of gastric carcinoma via suppression of FOXO1. Biomed Pharmacother. 2013;67:521–526. doi: 10.1016/j.biopha.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 93.He X, Zou K. MiRNA-96-5p contributed to the proliferation of gastric cancer cells by targeting FOXO3. J Biochem. 2020;167:101–108. doi: 10.1093/jb/mvz080. [DOI] [PubMed] [Google Scholar]
- 94.Wang GJ, Liu GH, Ye YW, Fu Y, Zhang XF. The role of microRNA-1274a in the tumorigenesis of gastric cancer: accelerating cancer cell proliferation and migration via directly targeting FOXO4. Biochem Biophys Res Commun. 2015;459:629–635. doi: 10.1016/j.bbrc.2015.02.160. [DOI] [PubMed] [Google Scholar]
- 95.Gao Y, Qi W, Sun L, Lv J, Qiu W, Liu S. FOXO3 Inhibits Human Gastric Adenocarcinoma (AGS) Cell Growth by Promoting Autophagy in an Acidic Microenvironment. Cell Physiol Biochem. 2018;49:335–348. doi: 10.1159/000492884. [DOI] [PubMed] [Google Scholar]
- 96.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 97.Maruyama S, Furuya S, Shiraishi K, Shimizu H, Saito R, Akaike H, Hosomura N, Kawaguchi Y, Amemiya H, Kawaida H, Sudo M, Inoue S, Kono H, Ichikawa D. Inhibition of apoptosis by miR-22-p in α-rotein-ducing gastric cancer. Oncol Rep. 2019;41:2595–2600. doi: 10.3892/or.2019.7023. [DOI] [PubMed] [Google Scholar]
- 98.Vogiatzi P, De Falco G, Claudio PP, Giordano A. How does the human RUNX3 gene induce apoptosis in gastric cancer? Cancer Biol Ther. 2006;5:371–374. doi: 10.4161/cbt.5.4.2748. [DOI] [PubMed] [Google Scholar]
- 99.Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem. 2006;281:5267–5276. doi: 10.1074/jbc.M512151200. [DOI] [PubMed] [Google Scholar]
- 100.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shahbazi R, Baradaran B, Khordadmehr M, Safaei S, Baghbanzadeh A, Jigari F, Ezzati H. Targeting ROCK signaling in health, malignant and non-malignant diseases. Immunol Lett. 2020;219:15–26. doi: 10.1016/j.imlet.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 102.Wang YZ, Feng ZQ. Induction of apoptosis by L-NMMA, via FKHRL1/ROCK pathway in human gastric cancer cells. Biomed Environ Sci. 2006;19:285–291. [PubMed] [Google Scholar]
- 103.Pang X, Zhou Z, Yu Z, Han L, Lin Z, Ao X, Liu C, He Y, Ponnusamy M, Li P, Wang J. Foxo3a-dependent miR-633 regulates chemotherapeutic sensitivity in gastric cancer by targeting Fas-associated death domain. RNA Biol. 2019;16:233–248. doi: 10.1080/15476286.2019.1565665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang S, Fu R, Shi J, Wu H, Mai J, Hua X, Chen H, Liu J, Lu M, Li N. CircRNA-Mediated Regulation of Angiogenesis: A New Chapter in Cancer Biology. Front Oncol. 2021;11:553706. doi: 10.3389/fonc.2021.553706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nienhüser H, Schmidt T. Angiogenesis and Anti-Angiogenic Therapy in Gastric Cancer. Int J Mol Sci. 2017;19 doi: 10.3390/ijms19010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim SY, Yoon J, Ko YS, Chang MS, Park JW, Lee HE, Kim MA, Kim JH, Kim WH, Lee BL. Constitutive phosphorylation of the FOXO1 transcription factor in gastric cancer cells correlates with microvessel area and the expressions of angiogenesis-related molecules. BMC Cancer. 2011;11:264. doi: 10.1186/1471-2407-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim SY, Ko YS, Park J, Choi Y, Park JW, Kim Y, Pyo JS, Yoo YB, Lee JS, Lee BL. Forkhead Transcription Factor FOXO1 Inhibits Angiogenesis in Gastric Cancer in Relation to SIRT1. Cancer Res Treat. 2016;48:345–354. doi: 10.4143/crt.2014.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, Zhu K, Ning T, Fan Q, Ying G, Ba Y. miR-135b Delivered by Gastric Tumor Exosomes Inhibits FOXO1 Expression in Endothelial Cells and Promotes Angiogenesis. Mol Ther. 2019;27:1772–1783. doi: 10.1016/j.ymthe.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Zhang L, Liu L, Zhan S, Chen L, Wang Y, Zhang Y, Du J, Wu Y, Gu L. Arsenic Trioxide Suppressed Migration and Angiogenesis by Targeting FOXO3a in Gastric Cancer Cells. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9:317–324. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 112.Hu C, Ni Z, Li BS, Yong X, Yang X, Zhang JW, Zhang D, Qin Y, Jie MM, Dong H, Li S, He F, Yang SM. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut. 2017;66:31–42. doi: 10.1136/gutjnl-2015-309322. [DOI] [PubMed] [Google Scholar]
- 113.Park J, Choi Y, Ko YS, Kim Y, Pyo JS, Jang BG, Kim MA, Lee JS, Chang MS, Park JW, Lee BL. FOXO1 Suppression is a Determinant of Acquired Lapatinib-Resistance in HER2-Positive Gastric Cancer Cells Through MET Upregulation. Cancer Res Treat. 2018;50:239–254. doi: 10.4143/crt.2016.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu C, Chen DQ, Liu HX, Li WB, Lu JW, Feng JF. Rosmarinic acid reduces the resistance of gastric carcinoma cells to 5-fluorouracil by downregulating FOXO4-targeting miR-6785-5p. Biomed Pharmacother. 2019;109:2327–2334. doi: 10.1016/j.biopha.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 115.Park J, Ko YS, Yoon J, Kim MA, Park JW, Kim WH, Choi Y, Kim JH, Cheon Y, Lee BL. The forkhead transcription factor FOXO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric Cancer. 2014;17:423–430. doi: 10.1007/s10120-013-0314-2. [DOI] [PubMed] [Google Scholar]
- 116.Yu S, Yu Y, Zhang W, Yuan W, Zhao N, Li Q, Cui Y, Wang Y, Li W, Sun Y, Liu T. FOXO3a promotes gastric cancer cell migration and invasion through the induction of cathepsin L. Oncotarget. 2016;7:34773–34784. doi: 10.18632/oncotarget.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Z, Zhang H, Chen Y, Fan L, Fang J. Forkhead transcription factor FOXO3a protein activates nuclear factor κB through B-cell lymphoma/leukemia 10 (BCL10) protein and promotes tumor cell survival in serum deprivation. J Biol Chem. 2012;287:17737–17745. doi: 10.1074/jbc.M111.291708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miao Z, Guo X, Tian L. The long noncoding RNA NORAD promotes the growth of gastric cancer cells by sponging miR-608. Gene. 2019;687:116–124. doi: 10.1016/j.gene.2018.11.052. [DOI] [PubMed] [Google Scholar]
- 119.Chen C, Xu T, Zhou J, Yan Y, Li W, Yu H, Hu G, Ding X, Chen J, Lu Y. High cytoplasmic FOXO1 and pFOXO1 expression in astrocytomas are associated with worse surgical outcome. PLoS One. 2013;8:e69260. doi: 10.1371/journal.pone.0069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ko YS, Kim NY, Pyo JS. Clinicopathological significance and angiogenic role of the constitutive phosphorylation of the FOXO1 transcription factor in colorectal cancer. Pathol Res Pract. 2020;216:153150. doi: 10.1016/j.prp.2020.153150. [DOI] [PubMed] [Google Scholar]
- 121.Wu Z, Zhu Q, Yin Y, Kang D, Cao R, Tian Q, Zhang Y, Lu S, Liu P. Traditional Chinese Medicine CFF-1 induced cell growth inhibition, autophagy, and apoptosis via inhibiting EGFR-related pathways in prostate cancer. Cancer Med. 2018;7:1546–1559. doi: 10.1002/cam4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu C, Zhu Q, Wu Z, Yin Y, Kang D, Lu S, Liu P. Isorhapontigenin induced cell growth inhibition and apoptosis by targeting EGFR-related pathways in prostate cancer. J Cell Physiol. 2018;233:1104–1119. doi: 10.1002/jcp.25968. [DOI] [PubMed] [Google Scholar]
- 123.Kim JH, Kim MK, Lee HE, Cho SJ, Cho YJ, Lee BL, Lee HS, Nam SY, Lee JS, Kim WH. Constitutive phosphorylation of the FOXO1A transcription factor as a prognostic variable in gastric cancer. Mod Pathol. 2007;20:835–842. doi: 10.1038/modpathol.3800789. [DOI] [PubMed] [Google Scholar]
- 124.Yu S, Yu Y, Sun Y, Wang X, Luo R, Zhao N, Zhang W, Li Q, Cui Y, Wang Y, Li W, Liu T. Activation of FOXO3a suggests good prognosis of patients with radically resected gastric cancer. Int J Clin Exp Pathol. 2015;8:2963–2970. [PMC free article] [PubMed] [Google Scholar]
- 125.Li J, Jiang Z, Han F, Liu S, Yuan X, Tong J. FOXO4 and FOXD3 are predictive of prognosis in gastric carcinoma patients. Oncotarget. 2016;7:25585–25592. doi: 10.18632/oncotarget.8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park SH, Jang KY, Kim MJ, Yoon S, Jo Y, Kwon SM, Kim KM, Kwon KS, Kim CY, Woo HG. Tumor suppressive effect of PARP1 and FOXO3A in gastric cancers and its clinical implications. Oncotarget. 2015;6:44819–44831. doi: 10.18632/oncotarget.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ding J, Li Q, He S, Xie J, Liang X, Wu T, Li D. Luteolin-loading of Her-2-poly (lactic-co-glycolic acid) nanoparticles and proliferative inhibition of gastric cancer cells via targeted regulation of forkhead box protein O1. J Cancer Res Ther. 2020;16:263–268. doi: 10.4103/jcrt.JCRT_438_18. [DOI] [PubMed] [Google Scholar]
- 128.Kim N, Kim CH, Ahn DW, Lee KS, Cho SJ, Park JH, Lee MK, Kim JS, Jung HC, Song IS. Anti-gastric cancer effects of celecoxib, a selective COX-2 inhibitor, through inhibition of Akt signaling. J Gastroenterol Hepatol. 2009;24:480–487. doi: 10.1111/j.1440-1746.2008.05599.x. [DOI] [PubMed] [Google Scholar]
- 129.Xia Q, Zhao Y, Wang J, Qiao W, Zhang D, Yin H, Xu D, Chen F. Proteomic analysis of cell cycle arrest and differentiation induction caused by ATPR, a derivative of all-trans retinoic acid, in human gastric cancer SGC-7901 cells. Proteomics Clin Appl. 2017;11 doi: 10.1002/prca.201600099. [DOI] [PubMed] [Google Scholar]
- 130.Chen T, Wang Y, Yang Y, Yu K, Cao X, Su F, Xu H, Peng Y, Hu Y, Qian F, Wang Z. Gramicidin inhibits human gastric cancer cell proliferation, cell cycle and induced apoptosis. Biol Res. 2019;52:57. doi: 10.1186/s40659-019-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin F, Yang J, Muhammad U, Sun J, Huang Z, Li W, Lv F, Lu Z. Bacillomycin D-C16 triggers apoptosis of gastric cancer cells through the PI3K/Akt and FoxO3a signaling pathways. Anticancer Drugs. 2019;30:46–55. doi: 10.1097/CAD.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 132.Altan B, Yokobori T, Ide M, Mochiki E, Toyomasu Y, Kogure N, Kimura A, Hara K, Bai T, Bao P, Suzuki M, Ogata K, Asao T, Nishiyama M, Oyama T, Kuwano H. Nuclear PRMT1 expression is associated with poor prognosis and chemosensitivity in gastric cancer patients. Gastric Cancer. 2016;19:789–797. doi: 10.1007/s10120-015-0551-7. [DOI] [PubMed] [Google Scholar]
- 133.Xiong H, Wang J, Guan H, Wu J, Xu R, Wang M, Rong X, Huang K, Huang J, Liao Q, Fu Y, Yuan J. SphK1 confers resistance to apoptosis in gastric cancer cells by downregulating Bim via stimulating Akt/FoxO3a signaling. Oncol Rep. 2014;32:1369–1373. doi: 10.3892/or.2014.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu J, Zhang PY, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Huang CM, Zheng CH. Circular RNA hsa_circ_0001368 suppresses the progression of gastric cancer by regulating miR-6506-5p/FOXO3 axis. Biochem Biophys Res Commun. 2019;512:29–33. doi: 10.1016/j.bbrc.2019.02.111. [DOI] [PubMed] [Google Scholar]