Abstract
Anterior cruciate ligament (ACL) rupture alters knee kinematics and contributes to premature development of osteoarthritis. However, there is limited data regarding the in vivo biomechanical response of tibiofemoral cartilage to activities of daily living (ADLs) in ACL-deficient knees. In this study, eight otherwise healthy participants with chronic unilateral ACL deficiency completed a stress test to assess the effect of 20 minutes of level treadmill walking at a speed of 2.5 mph on tibiofemoral cartilage in their ACL-deficient and contralateral ACL-intact knees. Three-dimensional surface models developed from pre- and post-activity magnetic resonance (MR) images of the injured and uninjured knees were used to determine compressive strain across multiple regions of tibiofemoral cartilage (medial and lateral tibial plateaus, medial and lateral femoral condyles, medial aspect of femoral condyle adjacent to intercondylar notch of the femur). In the ACL-deficient knees, we observed significantly increased cartilage strain in the region of the medial femoral condyle adjacent to the intercondylar notch (6% in deficient vs. 2% in contralateral, p=0.01) as well as across the medial and lateral tibial plateaus (4% vs. 3%, p=0.01) relative to the contralateral ACL-intact knees. Increased compressive strain at the medial intercondylar notch and tibial plateau suggests alterations in mechanical loading or the response to load in these regions, presumably related to altered knee kinematics. These changes may disrupt cartilage homeostasis and contribute to subsequent development of osteoarthritis.
Keywords: anterior cruciate ligament, biomechanics, gait, MRI, osteoarthritis
INTRODUCTION
Anterior cruciate ligament (ACL) rupture is a common knee injury (Agel et al., 2016; Clayton and Court-Brown, 2008) with an increasing incidence estimated between 80,000 and 250,000 in the United States (Griffin et al., 2006; Sanders et al., 2016). Premature osteoarthritis (OA) is a long-term sequela of ACL rupture, frequently observed within 10–15 years of injury (Clayton and Court-Brown, 2008; Lohmander et al., 2004; Oiestad et al., 2009; Roos, 2005). OA is associated with the degeneration of articular cartilage, an aneural, avascular tissue that exhibits viscoelastic properties (Fox et al., 2009). Cyclic loading of cartilage has been shown to be critical to maintaining its homeostasis (Griffin and Guilak, 2005; Guilak et al., 1994a). However, altered mechanical loading of cartilage is thought to be a contributing factor to early cartilage degeneration in ACL-deficient knees (Andriacchi et al., 2015; Chaudhari et al., 2008; Chen et al., 2012; DeFrate, 2017; Haughom et al., 2012; Kaiser et al., 2017).
The ACL functions to resist anterior tibial translation, medial tibial translation, and internal tibial rotation (Andriacchi and Dyrby, 2005; DeFrate, 2017; Gao and Zheng, 2010; Kozanek et al., 2011). Therefore, ACL deficiency leads to altered knee biomechanics and potentially alters tibiofemoral cartilage loading (Sutter et al., 2019). However, there is limited data describing in vivo cartilage strain in response to activities of daily living (ADLs) such as walking with an ACL-deficient knee. Better understanding of cartilage biomechanics in the absence of ACL function may shed light on the subsequent development of OA in this population.
Previous work has shown elevated cartilage strain in the medial femoral condyle adjacent to the intercondylar notch of ACL-deficient knees after a single legged hopping exercise (Sutter et al., 2019). Other studies have utilized a treadmill walking stress test as a proxy for overground walking - a common ADL that cyclically loads cartilage - to better assess cartilage response to walking in healthy subjects (Lad et al., 2016; Paranjape et al., 2019). Therefore, the objective of this study was to investigate the influence of ACL deficiency on cartilage strain in response to a treadmill walking stress test. We hypothesized that ACL-deficient knees would exhibit elevated cartilage compressive strain compared with contralateral ACL-intact control knees.
METHODS
Eight participants with unilateral ACL rupture who had not undergone reconstruction at the time of the study (6M, 2F; mean age=35, range=22–48; mean BMI= 24.8 kg/m2, range=21.9–27.9 kg/m2; mean time since injury=3.67 years, range=87 days–11 years) were recruited for an IRB approved study at Duke University. Based on prior studies, the inclusion of eight subjects provides adequate power to evaluate cartilage strain (Collins et al., 2018; Lad et al., 2016; Paranjape et al., 2019; Sutter et al., 2019). Exclusion criteria included history of previous injury or surgery to the injured or contralateral knee, evidence of meniscal tears on magnetic resonance imaging (MRI), high grade or full thickness chondral defects, or symptomatic OA. ACL injuries were confirmed by both clinical exam and MRI. As a subjective measure of knee function, participants completed the International Knee Documentation Committee (IKDC) questionnaire at the time of the study (Irrgang et al., 2001) (mean score=70; range: 52–87).
Data were collected on two separate days, one for each knee. To minimize effects of diurnal loading and exercise on baseline cartilage thickness, participants arrived at 8am on the day of study and rested supine on a stretcher for 45 minutes (Coleman et al., 2013; Eckstein et al., 2006; Taylor et al., 2019) and were advised not to perform strenuous exercise for 24 hours prior to each study. After this rest period, participants were transported to the MRI suite via wheelchair for pre-activity imaging. Images were acquired using a 3.0T MR scanner (Trio Tim; Siemens) with an 8-channel knee coil while supine. Sagittal plane images (field of view, 16×16 cm; resolution, 512×512 pixels) of 1mm thickness were generated using a double-echo steady-state sequence (DESS; flip angle, 25°; repetition time, 17ms; echo time, 6ms). The total time of each DESS scan was approximately 9 minutes. After completion of pre-activity imaging, participants were transported via wheelchair to the treadmill where they walked for 20 minutes at a speed of 2.5 mph (Lad et al., 2016). Upon completion, participants immediately walked to the MRI suite for post-activity imaging. Time from walk completion to post-activity MRI initiation averaged 3.25 minutes (range: 2.75–4.0 minutes).
Prior to analysis, images were reviewed by a musculoskeletal radiologist. Cartilage thickness and strain were measured using 3D surface models created from MR images (Figures 1 and 2) (Lad et al., 2016; Sutter et al., 2019). Previous work has demonstrated that this methodology can measure cartilage thickness to within a resolution of 1% (Coleman et al., 2013, Cutcliffe et al., 2020). Pre- and post-activity images were registered to each other using an iterative closest point technique (Geomagic Studio 11, 3D Systems). Cartilage thickness was sampled at various sites evenly distributed across the cartilage surface (Figure 3) consistent with prior work (Sutter et al., 2019), including three sites on the medial femoral condyle adjacent to the intercondylar notch. Compressive strain was calculated as the percent change in cartilage thickness ((pre-activity – post-activity)/pre-activity*100%). Strain values were averaged across each region (medial and lateral tibial plateaus, medial and lateral femoral condyles, medial intercondylar notch of the femur).
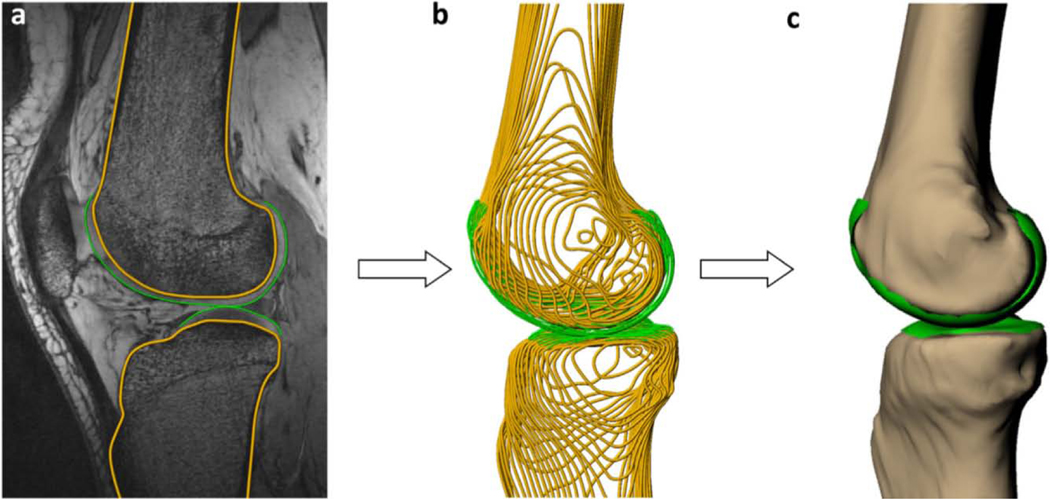
Figure 1.
Schematic demonstration of the process of converting MRI tracings (A) to wire frame (B) and 3D surface mesh models (C). Reprinted from Journal of Biomechanics, 49, Lad et al., 2016. Effect of normal gait on in vivo tibiofemoral cartilage strains, 2870–2876. (2016), with permission from Elsevier.
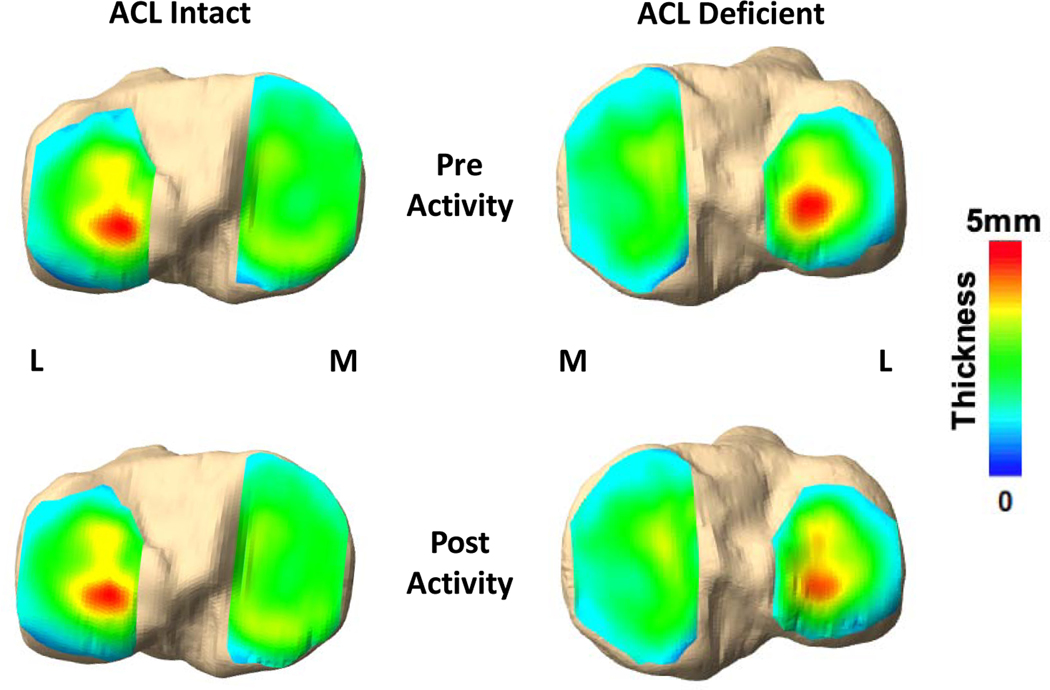
Figure 2.
Representative tibial cartilage thickness maps from one participant showing thickness changes (strain) in the ACL-deficient knee compared with the intact contralateral knee following a 20-minute treadmill walking activity. (L) refers to lateral side of the tibia, (M) refers to medial side of the tibia.
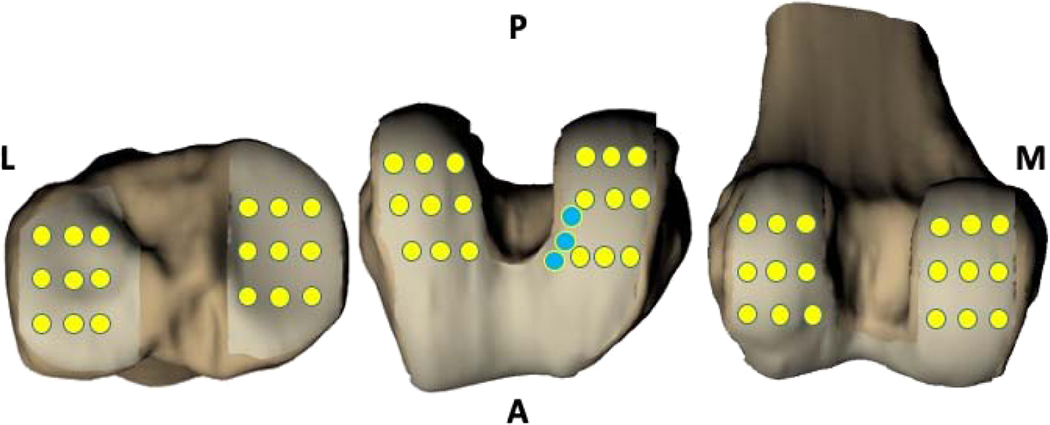
Figure 3.
Schematic representation of grid points used to sample cartilage thickness. 9 grid points were placed on the medial and lateral tibial plateau (yellow, left), and 18 grid points were placed on the medial and lateral femoral condyles (yellow, middle and right) in addition to 3 sites at the medial intercondylar notch of the femur (blue, middle).
Statistical analysis was performed using Statistica (Tibco Software Inc.). Shapiro-Wilk tests suggested that the data were normally distributed, and no outliers were identified using the inner and outer fences of the interquartile ranges. Therefore, data were presented as mean and 95% confidence interval. Two-way repeated measures ANOVA with knee state (ACL-deficient vs. ACL-intact) and region for the femur and tibia were used to detect differences in cartilage strain. Tukey’s post-hoc test was used to follow up significant (p<0.05) F-ratios.
RESULTS
Exercise-induced compressive strain was observed in cartilage of ACL-intact and ACL-deficient knees. Specifically, we observed a significant interaction between knee state and region across the femoral condyle (P=0.019) (Figure 4A). The greatest cartilage strain was found in the region of the medial femoral condyle adjacent to the intercondylar notch of ACL-deficient knees, which was 300% higher than in the ACL-intact knee (6% vs. 2%; p=0.010). No other significant differences in femoral cartilage strain were observed between ACL-intact and ACL-deficient knees. In the tibia, while no significant region (p=0.499) or interaction (p=0.076) effects were found, strain was significantly influenced by ACL state (p=0.010). Cartilage strain was 33% higher across the tibial plateaus of ACL-deficient knees compared with ACL-intact knees (4% versus 3%) (Figure 4B).
Figure 4.
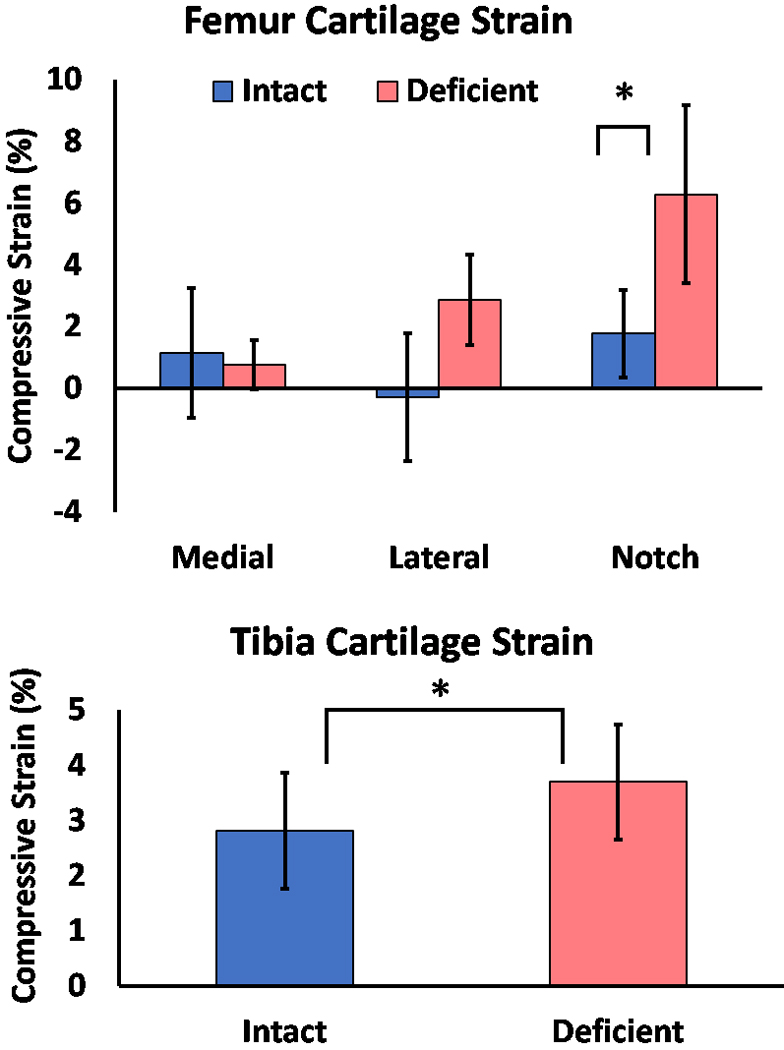
Mean cartilage strain values (±95% CI) by ACL condition and cartilage region. (A) femoral cartilage strain; (B) tibial cartilage strain. *P<0.05.
DISCUSSION
Cartilage health is influenced by multiple factors, including mechanical loading (Griffin and Guilak, 2005; Guilak, 2011; Guilak et al., 1994a). Walking is one of the principal ways tibiofemoral cartilage is cyclically loaded during ADLs (Andriacchi et al., 2009). Altered gait and joint instability in the setting of ACL injury have been proposed as risk factors in the development of OA (Andriacchi and Dyrby, 2005; Andriacchi et al., 2015; Andriacchi et al., 2009; Chaudhari et al., 2008; Georgoulis et al., 2003), possibly due to the disruption of normal cartilage loading. In this study, we observed statistically significant increases in the strain response of ACL-deficient knees compared with contralateral ACL-intact knees in the medial intercondylar notch and across the tibial plateau. These results confirm our hypothesis that ACL-deficient and intact knee cartilage exhibit different biomechanical responses to walking.
Our finding of increased strain adjacent to the intercondylar notch of ACL-deficient knees is consistent with previous work (Sutter et al., 2019) demonstrating increased strain in the notch region of ACL-deficient knees after a single-leg hopping exercise. In kinematic walking studies, ACL-deficient knees exhibit increased internal rotation throughout the gait cycle (Andriacchi and Dyrby, 2005). In addition, biplanar fluoroscopic studies of a quasi-static lunge in patients with ACL-deficient knees have shown that internal rotation plus anterior and medial translation of the tibia potentially shifts the medial tibial prominence into a position where it impinges on the medial intercondylar notch (DeFrate et al., 2006; Li et al., 2006). Prolonged cartilage compression at higher strain values in chronically ACL-deficient knees may lead to tissue damage (Guilak et al., 1994b) and may explain why the medial intercondylar notch is susceptible to degeneration and osteophytosis (Fairclough et al., 1990).
Previous studies have demonstrated that during quasi-static leg press (Scarvell et al. 2005) and lunge activities (Li et al., 2006; Scarvell et al., 2005), ACL deficiency leads to a posterior shift of the regions of contact between the femur on the tibia. Changing tibiofemoral contact from its native location to a location less adapted to withstand the cyclic loading associated with gait may cause the increase in tibial compressive strain we observed. Increased strain may alter cartilage homeostasis and ultimately propagate a degenerative cycle similar to that described above in the notch. Disruption of normal tibiofemoral contact may also change the type of forces experienced at localized regions of cartilage (Chaudhari et al., 2008). This may contribute to fibrillation of the superficial zone of cartilage that could increase friction at the cartilage surface and lead to increased shear force on the cartilage, thus causing further degeneration (Guilak et al., 1994b).
To date, uncertainty remains regarding the ability of ACL reconstruction to prevent premature OA (Chalmers et al., 2014; Delince and Ghafil, 2012; Lien-Iversen et al., 2020; Rothrauff et al., 2020). In studies of ACL-reconstructed knees, many patients continue to have kinematic differences such as altered anterior tibial translation, medial tibial translation, and tibial rotation when compared with uninjured knees (Abebe et al., 2011; Gao and Zheng, 2010; Papannagari et al., 2006). Alterations in ACL-reconstructed knee kinematics subsequently change tibiofemoral contact points at low flexion angles (Hosseini et al., 2012; Stergiou et al., 2007), which may contribute to cartilage degeneration in a manner similar to in ACL deficiency. Therefore, restoring native ACL function and knee kinematics may minimize cartilage thinning and degeneration (DeFrate, 2017; Haughom et al., 2012; Okafor et al., 2014).
In our study, strains in the uninjured contralateral knees were similar to a previous study of tibiofemoral cartilage strain after treadmill walking in healthy individuals (Lad et al., 2016). Similarity between the uninjured knee and previous work in healthy individuals suggests that the ACL-intact contralateral knee is a reasonable control in lieu of an uninjured cohort. Furthermore, studies of in vivo ACL function during gait show that the ACL is under greatest strain at low flexion angles (Englander et al., 2020). Given that the knee is loaded at low flexion angles during the stance phase of gait, ACL-deficient knee cartilage may be particularly susceptible to the sequelae of instability while walking, further underscoring the importance of this work.
Recent work has shown that cartilage strain increases with faster walking speeds and longer durations (Paranjape et al., 2019). It is possible that tibiofemoral cartilage strain differences could be amplified by optimizing the parameters (increasing speed, duration) of the stress test. Further, due to the recovery of cartilage, the strains measured here may be an underestimate of the strains occurring immediately after walking. Nonetheless, scans were initiated for both contralateral and deficient knees within 4 minutes after walking. Additionally, in this study we only measured cartilage strains in eight participants at a single time point after surgery. Future studies might follow a larger group of ACL-deficient and ACL-reconstructed individuals longitudinally to elucidate site-specific relationships between cartilage loading and degeneration. Finally, kinematic measurements during treadmill walking could relate variations in participant gait to differences observed in cartilage response to exercise.
In conclusion, this work examined the effect of ACL deficiency tibiofemoral cartilage compression in response to walking, a common ADL that cyclically loads cartilage. We found increased compressive strain in the medial femoral condyle adjacent to the intercondylar notch and the tibial plateau of ACL-deficient knees compared to contralateral ACL-intact knees. Our results are consistent with other work showing susceptibility of ACL-deficient knees to cartilage degeneration at the intercondylar notch and tibial plateau. The increased strain observed may contribute to early cartilage degeneration and predate symptomatic or radiographic evidence of OA.
ACKNOWLEDGEMENTS
The authors thank the Duke University Center for Advanced Magnetic Resonance Development for assistance in this project. The authors acknowledge Donald T. Kirkendall, ELS, a contracted medical editor for assistance in the preparation of this manuscript. The authors gratefully acknowledge the financial support of NIH Grants AR065527, AR074800, and AR075399.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors of this paper have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abebe ES, Utturkar GM, Taylor DC, Spritzer CE, Kim JP, Moorman CT 3rd, Garrett WE, DeFrate LE, 2011. The effects of femoral graft placement on in vivo knee kinematics after anterior cruciate ligament reconstruction. J Biomech 44, 924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agel J, Rockwood T, Klossner D, 2016. Collegiate ACL Injury Rates Across 15 Sports: National Collegiate Athletic Association Injury Surveillance System Data Update (2004–2005 Through 2012–2013). Clin J Sport Med 26, 518–523. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Dyrby CO, 2005. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech 38, 293–298. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Favre J, Erhart-Hledik J, Chu C, 2015. A Systems View of Risk Factors for Knee Osteoarthritis Reveals Insights into the Pathogenesis of the Disease. Annals of Biomedical Engineering 43, 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriacchi TP, Koo S, Scanlan SF, 2009. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am 91 Suppl 1, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers PN, Mall NA, Moric M, Sherman SL, Paletta GP, Cole BJ, Bach BR Jr., 2014. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. Journal of Bone adn Joint Surgery. American Volume 96, 292–300. [DOI] [PubMed] [Google Scholar]
- Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP, 2008. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc 40, 215–222. [DOI] [PubMed] [Google Scholar]
- Chen CH, Li JS, Hosseini A, Gadikota HR, Gill TJ, Li G, 2012. Anteroposterior stability of the knee during the stance phase of gait after anterior cruciate ligament deficiency. Gait Posture 35, 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton RA, Court-Brown CM, 2008. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 39, 1338–1344. [DOI] [PubMed] [Google Scholar]
- Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT 3rd, Guilak F, DeFrate LE, 2013. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech 46, 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Kulvaranon ML, Cutcliffe HC, Utturkar GM, Smith WAR, Spritzer CE, Guilak F, DeFrate LE, 2018. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Res Ther 20, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutcliffe HC, Davis KM, Spritzer CE, DeFrate LE, 2020. The Characteristic Recovery Time as a Novel, Noninvasive Metric for Assessing In Vivo Cartilage Mechanical Function. Annals of Biomedical Engineering 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrate LE, 2017. Effects of ACL Graft Placement on In Vivo Knee Function and Cartilage Thickness Distributions. Journal of Orthopaedic Research, 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G, 2006. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. American Journal of Sports Medicine 34, 1240–1246. [DOI] [PubMed] [Google Scholar]
- Delince P, Ghafil D, 2012. Anterior cruciate ligament tears: conservative or surgical treatment? A critical review of the literature. Knee Surg Sports Traumatol Arthrosc 20, 48–61. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Hudelmaier M, Putz R, 2006. The effects of exercise on human articular cartilage. J. anat. 208, 491–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander ZA, Garrett WE, Spritzer CE, DeFrate LE, 2020. In vivo attachment site to attachment site length and strain of the ACL and its bundles during the full gait cycle measured by MRI and high-speed biplanar radiography. J Biomech 98, 109443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough J, Graham G, Dent C, 1990. Radiological sign of chronic anterior cruciate ligament deficiency. Injury 21, 401–402. [DOI] [PubMed] [Google Scholar]
- Fox AJS, Bedi A, Rodeo SA, 2009. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 1, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Zheng NN, 2010. Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin Biomech (Bristol, Avon) 25, 222–229. [DOI] [PubMed] [Google Scholar]
- Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N, 2003. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med 31, 75–79. [DOI] [PubMed] [Google Scholar]
- Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE Jr., Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C Jr., Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B, 2006. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. American Journal of Sports Medicine 34, 1512–1532. [DOI] [PubMed] [Google Scholar]
- Griffin TM, Guilak F, 2005. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev 33, 195–200. [DOI] [PubMed] [Google Scholar]
- Guilak F, 2011. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol 25, 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Meyer BC, Ratcliffe A, Mow VC, 1994a. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2, 91–101. [DOI] [PubMed] [Google Scholar]
- Guilak F, Ratcliffe A, Lane N, Rosenwasser MP, Mow VC, 1994b. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res 12, 474–484. [DOI] [PubMed] [Google Scholar]
- Hatcher CC, Collins AT, Kim SY, Michel LC, Mostertz WC 3rd, Ziemian SN, Spritzer CE, Guilak F, DeFrate LE, McNulty AL, 2017. Relationship between T1rho magnetic resonance imaging, synovial fluid biomarkers, and the biochemical and biomechanical properties of cartilage. J Biomech 55, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughom B, Schairer W, Souza RB, Carpenter D, Ma CB, Li X, 2012. Abnormal tibiofemoral kinematics following ACL reconstruction are associated with early cartilage matrix degeneration measured by MRI T1rho. The Knee 19, 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Van de Velde S, Gill TJ, Li G, 2012. Tibiofemoral cartilage contact biomechanics in patients after reconstruction of a ruptured anterior cruciate ligament. J Orthop Res 30, 1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrgang JJ, Anderson AF, Boland AL, 2001. Development and Validation of the International Knee Documentation Committee Subjective Knee Form. THE AMERICAN JOURNAL OF SPORTS MEDICINE 29, 600–613. [DOI] [PubMed] [Google Scholar]
- Kaiser JM, Vignos MF, Kijowski R, Baer G, Thelen DG, 2017. Effect of Loading on In Vivo Tibiofemoral and Patellofemoral Kinematics of Healthy and ACL-Reconstructed Knees. Am J Sports Med 45, 3272–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozanek M, Hosseini A, de Velde SK, Moussa ME, Li JS, Gill TJ, Li G, 2011. Kinematic evaluation of the step-up exercise in anterior cruciate ligament deficiency. Clin Biomech (Bristol, Avon) 26, 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad NK, Liu B, Ganapathy PK, Utturkar GM, Sutter EG, Moorman CT 3rd, Garrett WE, Spritzer CE, DeFrate LE, 2016. Effect of normal gait on in vivo tibiofemoral cartilage strains. J Biomech 49, 2870–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ, 2006. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am 88, 1826–1834. [DOI] [PubMed] [Google Scholar]
- Lien-Iversen T, Morgan DB, Jensen D, Risberg MA, Engebretsen L, Viberg B, 2020. Does surgery reduce knee osteoarthritis, meniscal injury and subsequent complications compared with non-surgery after ACL rupture with at least 10 years follow-up? A systematic review and meta-analysis. British Journal Sports Medicine 54, 592–598. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Ostenberg A, Englund M, Roos H, 2004. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum 50, 3145–3152. [DOI] [PubMed] [Google Scholar]
- Oiestad BE, Engebretsen L, Storheim K, Risberg MA, 2009. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. American Journal of Sports Medicine 37, 1434–1443. [DOI] [PubMed] [Google Scholar]
- Okafor EC, Utturkar GM, Widmyer MR, Abebe ES, Collins AT, Taylor DC, Spritzer CE, Moorman CT 3rd, Garrett WE, DeFrate LE, 2014. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J Biomech 47, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G, 2006. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med 34, 2006–2012. [DOI] [PubMed] [Google Scholar]
- Paranjape CS, Cutcliffe HC, Grambow SC, Utturkar GM, Collins AT, Garrett WE, Spritzer CE, DeFrate LE, 2019. A new stress test for knee joint cartilage. Scientific reports 9, 2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos EM, 2005. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol 17, 195–200. [DOI] [PubMed] [Google Scholar]
- Rothrauff BB, Jorge A, de Sa D, Kay J, Fu FH, Musahl V, 2020. Anatomic ACL reconstruction reduces risk of post-traumatic osteoarthritis: a systematic review with minimum 10-year follow-up. Knee Surg Sports Traumatol Arthrosc. 28, 1072–1084. [DOI] [PubMed] [Google Scholar]
- Sanders TL, Maradit Kremers H, Bryan AJ, Larson DR, Dahm DL, Levy BA, Stuart MJ, Krych AJ, 2016. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am J Sports Med 44, 1502–1507. [DOI] [PubMed] [Google Scholar]
- Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR, 2005. Association between abnormal kinematics and degenerative change in knees of people with chronic anterior cruciate ligament deficiency: a magnetic resonance imaging study. The Australian journal of physiotherapy 51, 233–240. [DOI] [PubMed] [Google Scholar]
- Souza RB, Stehling C, Wyman BT, Hellio Le Graverand MP, Li X, Link TM, Majumdar S, 2010. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 18, 1557–1563. [DOI] [PubMed] [Google Scholar]
- Stergiou N, Ristanis S, Moraiti C, Georgoulis AD, 2007. Tibial rotation in anterior cruciate ligament (ACL)-deficient and ACL-reconstructed knees: a theoretical proposition for the development of osteoarthritis. Sports Med 37, 601–613. [DOI] [PubMed] [Google Scholar]
- Sutter EG, Liu B, Utturkar GM, Widmyer MR, Spritzer CE, Cutcliffe HC, Englander ZA, Goode AP, Garrett WE Jr., DeFrate LE, 2019. Effects of Anterior Cruciate Ligament Deficiency on Tibiofemoral Cartilage Thickness and Strains in Response to Hopping. American Journal of Sports Medicine 47, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Collins AT, Heckelman LN, Kim SY, Utturkar GM, Spritzer CE, Garrett WE, DeFrate LE, 2019. Activities of daily living influence tibial cartilage t1rho relaxation times. J Biomech 82, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]