Abstract
Hypoxia is a common pathway to the progression of end-stage kidney disease. Retinoic acid-inducible gene I (RIG-I) encodes an RNA helicase that recognizes viruses including SARS-CoV2, which is responsible for the production of interferon (IFN)-α/β to prevent the spread of viral infection. Recently, RIG-I activation was found under hypoxic conditions, and klotho deficiency was shown to intensify the activation of RIG-I in mouse brains. However, the roles of these functions in renal inflammation remain elusive. Here, for in vitro study, the expression of RIG-I and IFN-α/β was examined in normal rat kidney (NRK)-52E cells incubated under hypoxic conditions (1% O2). Next, siRNA targeting RIG-I or scramble siRNA was transfected into NRK52E cells to examine the expression of RIG-I and IFN-α/β under hypoxic conditions. We also investigated the expression levels of RIG-I and IFN-α/β in 33 human kidney biopsy samples diagnosed with IgA nephropathy. For in vivo study, we induced renal hypoxia by clamping the renal artery for 10 min in wild-type mice (WT mice) and Klotho-knockout mice (Kl−/− mice). Incubation under hypoxic conditions increased the expression of RIG-I and IFN-α/β in NRK52E cells. Their upregulation was inhibited in NRK52E cells transfected with siRNA targeting RIG-I. In patients with IgA nephropathy, immunohistochemical staining of renal biopsy samples revealed that the expression of RIG-I was correlated with that of IFN-α/β (r = 0.57, P<0.001, and r = 0.81, P<0.001, respectively). The expression levels of RIG-I and IFN-α/β were upregulated in kidneys of hypoxic WT mice and further upregulation was observed in hypoxic Kl−/− mice. These findings suggest that hypoxia induces the expression of IFN-α/β through the upregulation of RIG-I, and that klotho deficiency intensifies this hypoxia-induced expression in kidneys.
Introduction
Chronic kidney disease (CKD) affected 697.5 million people globally in 2017 [1], so it is well recognized as a major health concern. To retard the progression of CKD, inhibitors of the renin–angiotensin–aldosterone system (RAAS) pathway are used in a clinical setting [2,3]. However, their beneficial effects are limited and many patients eventually require renal replacement therapy [4]. Pathologically, chronic inflammation and interstitial fibrosis are the common features of CKD, regardless of the primary disease [5]. Although transforming growth factor (TGF)-β1 plays a pivotal role in the development of interstitial fibrosis [6–8], previous studies demonstrated that the infiltration of inflammatory cells is responsible for the production of TGF-β1 [9]. These findings suggest that inflammation is the upstream event of interstitial fibrosis, and that inflammation is a candidate therapeutic target for CKD.
Hypoxia is a condition in which insufficient oxygen makes it into tissues. As a cellular response to hypoxia, hypoxia-inducible factors (HIF) play important roles in preventing tissue damage [10–12]. However, severe hypoxia leads to acute vascular diseases, such as stroke [13], angina pectoris [14], and peripheral artery disease [15]. Recently, it has been reported that chronic hypoxia, such as sleep apnea, also contributes to the development of various diseases [16]. Regarding the kidney, hypoxia reportedly intensifies with the progression of CKD stage and it is currently considered as a common pathophysiology leading to end-stage kidney disease [17]. As mentioned above, inflammation is considered as an upstream event, so the mechanism by which hypoxia induces inflammation should be clarified at the molecular level.
Inflammation is characterized by pain, heat, redness, swelling, and loss of function [18]. However, it basically functions to eliminate the initial cause of cell injury and to clear out necrotic cells and damaged tissue. In any case, inflammation is not a specific response, and innate immunity is considered to mainly participate in the initial process of inflammation through the production of interferon (IFN)-α/β [19]. Among the factors involved in innate immunity, retinoic acid-inducible gene I (RIG-I) is responsible for immune responses to viral infections [20]. Notably, recent studies have demonstrated that hypoxia upregulates RIG-I expression in brain and liver, resulting in the production of IFN-α/β [21,22]. However, the role of hypoxia in the RIG-I-mediated expression of IFN-α/β in kidney remains elusive.
Klotho was first reported as an anti-aging protein [23]. Indeed, klotho-deficient mice exhibit a shortened lifespan accompanied by phenotypes resembling human aging. In contrast, klotho transgenic mice show an extended lifespan [24]. In terms of renoprotective effects, klotho deficiency has been found to intensify renal damage in experimental models of renal diseases, whereas klotho overexpression or the administration of klotho protein ameliorates it [25]. Klotho protein exists in three forms: membrane, secreted, and intracellular klotho. Among these, intracellular klotho is reported to suppress RIG-I expression along with inflammation [26], raising the possibility that klotho deficiency exacerbates hypoxia-induced upregulation of RIG-I and IFN-α/β in kidneys. These findings led us to hypothesize that the expression of RIG-I and IFN-α/β increased under hypoxic conditions in a cell line of renal tubular cells, and that their hypoxia-induced upregulation was intensified in klotho-knockout mice (Kl−/− mice).
In this study, we show that hypoxia upregulates RIG-I as well as IFN-α/β in normal rat kidney cells (NRK-52E) and mouse kidneys. We also show that RIG-I is responsible for the hypoxia-induced upregulation of IFN-α/β. In actual human kidney samples from patients diagnosed with IgA nephropathy, RIG-I expression was found to be positively correlated with IFN-α/β. Lastly, hypoxia-induced upregulation of RIG-I and IFN-α/β was intensified in Kl−/− mice. These findings suggest that klotho deficiency intensifies hypoxia-induced expression of IFN-α/β through the upregulation of RIG-I in kidneys.
Materials and methods
Animals
Male C57BL/6J wild-type (WT) mice (aged 8 weeks and weighing 20–25 g) were obtained from Charles River Laboratories Japan (Yokohama, Japan). Male Kl−/− mice (aged 6 weeks and weighing approximately 15 g) were purchased from CLEA Japan, Inc. The mice were housed in the Institute of Laboratory Animal Science of Hiroshima University (Hiroshima, Japan), as previously described [27]. All experiments were approved by the Institutional Animal Care and Use Committee of Hiroshima University (permit numbers: A19-158 and 2019–156) and were performed in accordance with the National Institutes of Health (NIH) Guidelines on the Use of Laboratory Animals. The WT and Kl−/− mice were divided into a control group or a hypoxia group (n = 5 in each group). Hypoxic conditions were induced by clamping of the left renal artery for 10 min under general anesthesia (0.3 mg/kg medetomidine, 4 mg/kg midazolam, and 5 mg/kg butorphanol) [28]. Mice in the control group underwent a sham operation that was the same procedure as that in mice in the hypoxia group, except for the lack of artery ligation. After 10 min of hypoxia, the mice were euthanized by cardiac puncture and their kidneys were harvested. Hypoxia of the kidney was confirmed by western blotting for erythropoietin expression (S1 Fig).
Cell culture
The normal rat kidney (NRK)-52E cells were purchased from the American Type Culture Collection (Manassas, VA, USA). NRK-52E cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS) (Nichirei Bio Science, Tokyo, Japan) and penicillin/streptomycin (Nacalai Tesque, Kyoto, Japan). These cells were seeded into 10 cm culture dishes. After growing to subconfluence, the cells were incubated in a 1% O2 Modular Incubator Chamber (MIC 101; Billups-Rothenberg, San Diego, CA, USA) for 30, 60, 90, and 120 min. Whole-cell lysates were prepared and subjected to western blot analysis. Hypoxia of NRK-52E cells was confirmed by western blotting for HIF-1α expression (S2 Fig).
Transfection of RIG-I siRNA
NRK52E cells were transfected with 20 nM siRNA targeting RIG-I (RSS311418; Thermo Fisher Scientific, Waltham, MA, USA) or negative control siRNA (4390843; Applied Biosystems, Waltham, MA, USA) using Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific), in accordance with the manufacturer’s instructions. After 43–45 h, some of these cells were incubated under hypoxic conditions (1% O2) for 30 min to evaluate RIG-I. The other cells were changed to fresh medium. After 24 h, these cells were incubated under hypoxic conditions (1% O2) for 60 and 120 min to confirm the suppression of IFN-α/β. Whole-cell lysates were prepared and subjected to western blot analysis.
Western blot analysis
Sample collection and western blotting were performed in accordance with previously described methods [29]. Rabbit monoclonal anti-RIG-I antibody (#3743S; Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal anti-IFN-α 11 antibody (bs-7023R; Bioss Antibodies, Woburn, MA, USA), rabbit polyclonal anti-IFN-β antibody (GTX37658; GeneTex, Irvine, CA, USA), rabbit monoclonal anti-RIG-I antibody (#700366; Invitrogen, Carlsbad, CA, USA), rabbit polyclonal anti-IFN-α2 antibody (ab193055; Abcam, Cambridge, UK), rabbit polyclonal anti-IFN-β antibody (PA5-20390; Invitrogen), rat monoclonal anti-human klotho antibody (KO603; TransGenic, Fukuoka, Japan), mouse monoclonal anti-β-actin antibody (A5316; Sigma-Aldrich, St. Louis, MO, USA), and mouse monoclonal anti-α-tubulin antibody (T9026; Sigma-Aldrich) were used as primary antibodies. Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Dako, Glostrup, Denmark) and goat anti-mouse immunoglobulin G (Dako) were used as secondary antibodies. SuperSignal West Dura and the Pico system (Thermo Fisher Scientific) were used to detect signals. The intensity of each band was measured by ImageJ software (version 1.47v; National Institutes of Health, Bethesda, MD, USA) and normalized to the level of either β-actin or α-tubulin.
Immunohistochemical analysis of mouse kidney tissue
Immunohistochemical staining was performed in accordance with previously described methods [27]. As primary antibodies, the following products were applied: rabbit monoclonal anti-RIG-I antibody (#700366; Invitrogen), rabbit polyclonal anti-IFN-α 2 antibody (ab193055; Abcam), rabbit polyclonal anti-IFN-β antibody (PA5-20390; Invitrogen), and rat monoclonal anti-human klotho antibody (KO603; TransGenic). RIG-I-, IFN-α/β-, and klotho-positive areas were quantified as the average of 20 randomly selected fields with ImageJ software.
Clinical sample collection and ethics statement
Kidney specimens were obtained by renal biopsy at Hiroshima University Hospital between August 2014 and November 2016 from 33 patients who were diagnosed with IgA nephropathy. This study adhered to the principles embodied in the Declaration of Helsinki and was approved by the Ethics Committee of Hiroshima University (E-1718). Informed consent was obtained in the form of opt-out on a website (https://jinzounaika.hiroshima-u.ac.jp/research/opt_out.html).
Immunohistochemical analysis of human kidney tissue
Immunostaining was carried out in accordance with previously described methods [27]. The following primary antibodies were used: rabbit monoclonal anti-RIG-I antibody (#700366; Invitrogen), mouse monoclonal anti-IFN-α antibody (sc-373757; Santa Cruz Biotechnology, Dallas, TX, USA), and rabbit polyclonal anti-IFN-β antibody (PA5-20390; Invitrogen). RIG-I- and IFN-α/β-positive areas were quantified as the average of five randomly selected fields with ImageJ software.
Statistical analysis
Results are presented as mean ± standard deviation (SD). Correlations were calculated using univariate regression analysis. For multiple comparisons, we used one-way analysis of variance (ANOVA) followed by Student’s t-test with Bonferroni correction. Differences between two groups were analyzed by Student’s t-test. P < 0.05 was considered as statistically significant.
Results
Hypoxia upregulates RIG-I and IFN-α/β in a rat cell line of renal epithelial cells
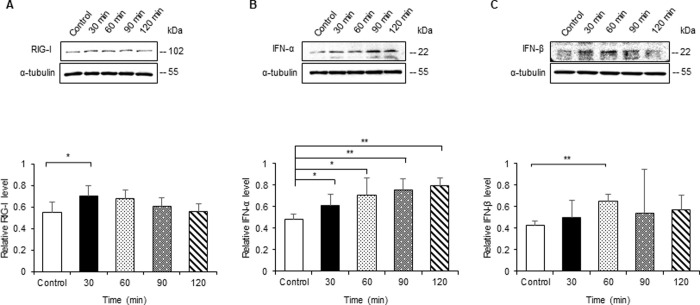
To identify the effect of hypoxia on the expression of RIG-I and IFN-α/β in vitro, we performed western blot analysis for these proteins in NRK-52E cells cultured under hypoxic conditions. The protein level of RIG-I peaked at 30 min and then gradually decreased in NRK-52E cells under hypoxic stimulation (Fig 1A). IFN-α/β levels increased and peaked at 120 and 60 min, respectively, in NRK-52E cells with hypoxic stimulation (Fig 1B and 1C).
Fig 1. Hypoxia enhances expression of RIG-I and IFN-α/β in a rat cell line of epithelial cells of renal tubules.
NRK-52E cells were incubated under hypoxic conditions (1.0% O2) for 30, 60, 90, and 120 min. Cell lysates were subjected to western blot analysis using antibodies against RIG-I and IFN-α/β. Typical western blot analysis demonstrated the expression levels of RIG-I (A) and IFN-α/β (B and C). Graphs show the expression levels quantified by densitometry and normalized to α-tubulin (n = 5 in each group). Values are expressed as the mean ± SD. Statistical analysis was performed using ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01.
Knockdown of RIG-I reduces the expression of IFN-α/β in NRK52E cells with hypoxia
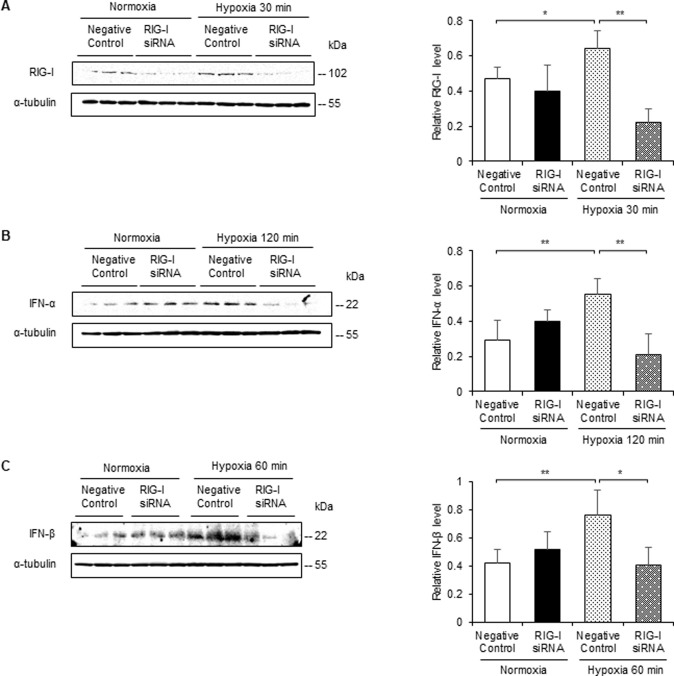
To assess whether RIG-I participates in the expression of IFN-α/β under hypoxic conditions, we investigated their expression in NRK-52E cells transfected with RIG-I siRNA or negative control siRNA. First, we confirmed the knockdown effect of RIG-I siRNA in NRK-52E cells. Western blotting for RIG-I revealed that RIG-I siRNA downregulated RIG-I expression in NRK-52E cells either with or without hypoxic stimulation (Fig 2A). We next investigated the role of hypoxia-induced upregulation of RIG-I in the expression of IFN-α/β in NRK-52E cells using RIG-I siRNA or negative control. RIG-I siRNA suppressed the expression of IFN-α/β in NRK-52E cells (Fig 2B and 2C).
Fig 2. RIG-I siRNA transfection attenuates expression of IFN-α/β in NRK-52E cells cultured under hypoxic conditions.
NRK-52E cells were transfected with RIG-I siRNA or negative control siRNA. Cell lysates were subjected to western blot analysis using antibodies against RIG-I and IFN-α/β. Typical western blot analysis demonstrated the expression levels of RIG-I (A) and IFN-α/β (B and C). Graphs show the expression levels quantified by densitometry and normalized to α-tubulin (n = 5 in each group). Values are expressed as the mean ± SD. Statistical analysis was performed using ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01.
RIG-I correlates with expression levels of IFN-α/β in human kidney specimens of IgA nephropathy
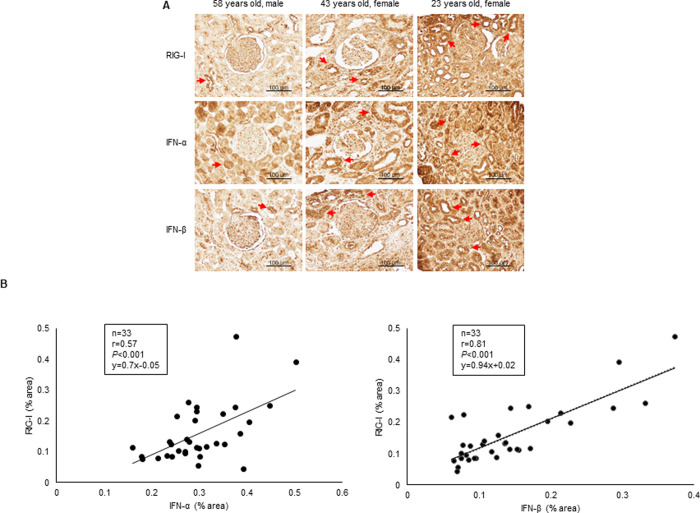
We examined whether the expression level of RIG-I is associated with IFN-α/β in renal biopsy samples obtained from patients with IgA nephropathy (n = 33). The detailed clinical characteristics of these patients are shown in S1 Table. Immunohistochemical staining for RIG-I and IFN-α/β revealed their expression in glomeruli and renal tubules (Fig 3A), and showed that RIG-I is positively correlated with IFN-α/β (r = 0.57, P<0.001, and r = 0.81, P<0.001, respectively) (Fig 3B).
Fig 3. RIG-I expression correlates with that of IFN-α/β in kidney biopsy specimens from IgA nephropathy patients.
Immunohistochemical staining for RIG-I and IFN-α/β was performed on 33 human kidney biopsy samples of IgA nephropathy. (A) Representative immunohistochemical staining images showing expression and localization of RIG-I and IFN-α/β in human kidney sections. (B) Expression levels of RIG-I are correlated with those of IFN-α/β (r = 0.57, P<0.001, and r = 0.81, P<0.001, respectively). Correlations were calculated using univariate correlation analysis (n = 33). Bar = 100 μm.
Hypoxia does not change expression level of klotho in WT and Kl−/− mice
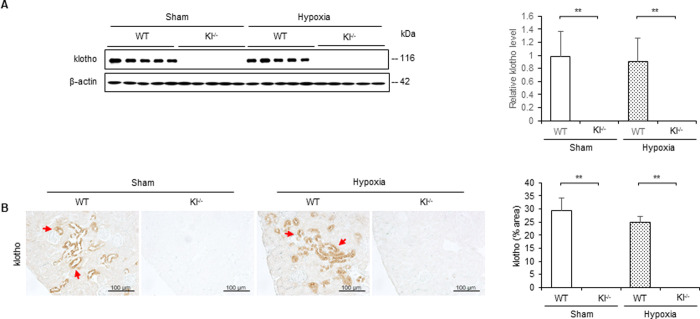
To determine the effect of hypoxic stimulation on klotho expression in vivo, we investigated its expression in WT and Kl−/− mice with or without 10 min of hypoxic stimulation. Western blotting and immunohistochemical staining revealed that klotho expression did not differ between WT mice with and without hypoxia, and that klotho expression was not observed in Kl−/− mice regardless of the presence or absence of hypoxic stimulation (Fig 4A and 4B). In the WT mice, klotho protein was stained in the cytoplasm of tubular cells (Fig 4B).
Fig 4. Expression level of klotho does not change during 10 min of hypoxic stimulation in WT and Kl−/− mice.
Renal hypoxia was induced by clamping the renal artery for 10 min in WT mice and Kl−/− mice. (A) Western blot analysis demonstrating klotho expression in WT and Kl−/− mice. Protein levels were normalized to β-actin levels (n = 5 in each group). (B) Representative immunohistochemical staining images showing expression and localization of klotho in WT and Kl−/− mice. Values are mean ± SD. *P < 0.05, **P < 0.01. Bar = 100 μm.
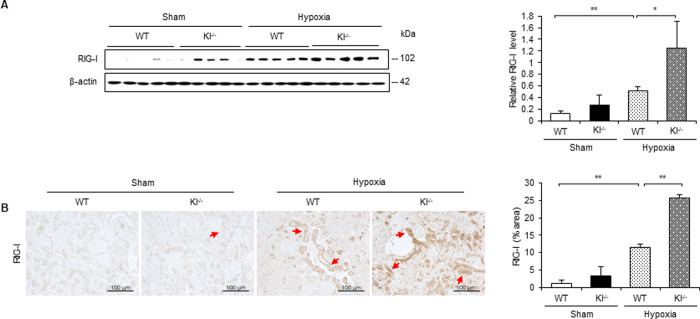
RIG-I expression is intensified under hypoxic conditions in Kl−/− mice
Intracellular klotho reportedly confers the ability to suppress RIG-I-mediated inflammation, so we examined the expression level and localization of RIG-I in hypoxic kidneys of WT and Kl−/− mice. Western blotting revealed that RIG-I expression increased in WT mice with hypoxic stimulation, and that it was intensified in Kl−/− mice (Fig 5A). Immunohistochemistry showed that the RIG-I-positive area increased in Kl−/− mice, which was similar to the results obtained from western blotting (Fig 5B).
Fig 5. RIG-I expression is intensified under hypoxic conditions in Kl−/− mice.
(A) Western blot analysis demonstrating RIG-I expression in WT and Kl−/− mice. Protein levels were normalized to β-actin levels (n = 5 in each group). (B) Representative immunohistochemical staining images showing expression and localization of RIG-I in WT and Kl−/− mice. Values are mean ± SD. *P < 0.05, **P < 0.01. Bar = 100 μm.
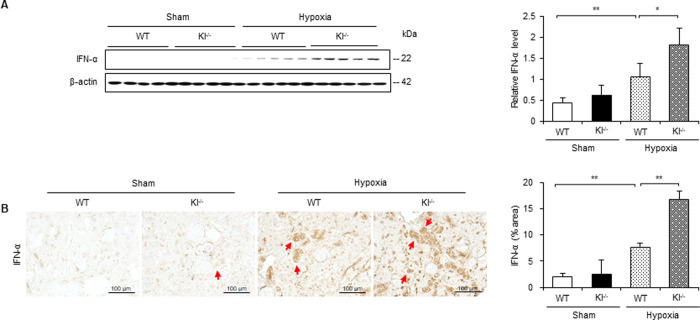
IFN-α is upregulated under hypoxic conditions in Kl−/− mice
To identify the effects of hypoxia on the expression of IFN-α in vivo, we examined its expression level and localization in hypoxic WT and Kl−/− mice. Western blotting revealed that IFN-α expression increased in WT mice with hypoxic stimulation, and that it was further upregulated in Kl−/− mice (Fig 6A). Immunohistochemical staining also revealed that the IFN-α-positive area was mainly observed in tubular cells, and that the expression level of IFN-α showed the same tendency as in the western blotting (Fig 6B).
Fig 6. IFN-α is upregulated under hypoxic conditions in Kl−/− mice.
(A) Western blot analysis demonstrating IFN-α expression in WT and Kl−/− mice. Protein levels were normalized to β-actin levels (n = 5 in each group). (B) Representative immunohistochemical staining images showing IFN-α expression in WT and Kl−/− mice. Values are mean ± SD. *P < 0.05, **P < 0.01. Bar = 100 μm.
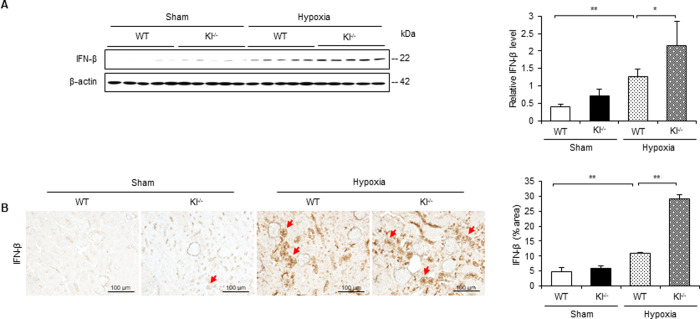
IFN-β is increased under hypoxic conditions in Kl−/− mice
In addition to IFN-α, we examined the expression level and localization of IFN-β in hypoxic WT and Kl−/− mice. From the results of western blotting, IFN-β expression increased in WT mice with hypoxic stimulation, while a further increase was observed in Kl−/− mice (Fig 7A). Upon quantifying the IFN-β-positive area, the expression level of IFN-β increased in Kl−/− mice compared with that in WT mice (Fig 7B).
Fig 7. IFN-β is increased under hypoxic conditions in Kl−/− mice.
(A) Western blot analysis demonstrating IFN-β expression in WT and Kl−/− mice. Protein levels were normalized to β-actin levels (n = 5 in each group). (B) Representative immunohistochemical staining images showing IFN-β expression in WT and Kl−/− mice. Values are mean ± SD. *P < 0.05, **P < 0.01. Bar = 100 μm.
Discussion
In this study, we demonstrated that the expression of RIG-I and IFN-α/β was upregulated in NRK-52E cells when these cells were cultured under hypoxic conditions. We also clarified that RIG-I was responsible for the expression of IFN-α/β in in vitro experiments. In human biopsy samples of IgA nephropathy, the expression level of RIG-I was shown to be positively correlated with that of IFN-α/β. In in vivo study, we found that the renal expression of klotho did not differ between WT mice with or without hypoxic stimulation. Klotho expression was not observed in Kl−/− mice regardless of the presence of hypoxic stimulation. We showed that renal hypoxia increased the expression of RIG-I and IFN-α/β, and that their expression was further intensified in Kl−/− mice. These findings suggested that klotho deficiency intensified the hypoxia-induced expression of RIG-I, resulting in the upregulation of IFN-α/β.
We identified that hypoxia induced the expression of RIG-I and IFN-α/β in both in vitro and in vivo studies. To adapt to hypoxic conditions, HIF is stabilized and functions as a transcription factor [30]. In a previous study, it was reported that RIG-I is involved in HIF-inducible molecules. However, we could not identify that RIG-I is a downstream effector of HIF. In addition to virus infection, RIG-I expression increases under hypoxic conditions [31]. Although previous studies reported that hypoxia affects tubular epithelial cells, endothelial cells, pericytes, fibroblasts, inflammatory cells, and progenitor cells in kidneys [32,33], we show that the upregulation of RIG-I was mainly observed in tubular epithelial cells in mice with hypoxic stimulation, as well as in patients with IgA nephropathy. Because renal tubules are considered as an entry site of transurethral pathogens [34], RIG-I may play a pivotal role in the immune response of tubular epithelial cells under hypoxic conditions.
The innate immune response is activated by not only microorganisms but also alarm signals from damaged, injured, or stressed cells [35]. In this study, we showed that the hypoxia-induced expression of RIG-I participated in the production of IFN-α/β in a rat cell line of renal tubules. The presented data suggest that RIG-I expression functions as the innate immune system of the cytosol in response to hypoxic stress as well as virus infection. Previous studies described that hypoxia enhances during the development of CKD regardless of the primary disease, and that hypoxia contributes to the progression of renal damage [17]. In a clinical study using blood oxygenation level-dependent magnetic resonance imaging, renal hypoxia not only intensified during the progression of CKD, but also predicted such progression [36]. Thus, hypoxia is currently considered as the common pathway to the progression of end-stage kidney disease, so hypoxia-induced inflammation should be a therapeutic target to suppress CKD progression.
We showed that hypoxia induces the production of IFN-α/β, which are classified as type 1 interferons. Type 1 interferons basically function to inhibit viral replication [37] and to resist hypoxia-induced immunosuppression [21,22]. However, studies performed to date have reported that type 1 interferons lead to the activation of natural killer cells, playing an important role in not only the removal of virus-infected cells but also tissue damage [38]. In fact, previous studies reported that RIG-I contributes to inflammation in various organs, such as lung [39], kidney [40], and the nervous system [21]. In this study, we also showed that hypoxia-induced upregulation of RIG-I is responsible for the production of IFN-α/β. Thus, type 1 interferons may function to protect against infection in a state of hypoxia, but they would be harmful under uninfected conditions. Moreover, a previous study reported that, in addition to type 1 interferons, RIG-I is involved in the production of various cytokines, such as IL-1β, IL-6, and TNF-α [41]. Because these cytokines cause tissue damage, hypoxia-induced upregulation of RIG-I may participate in the progression of CKD.
We showed that klotho deficiency enhances hypoxia-induced RIG-I expression, which is accompanied by the upregulation of IFN-α/β. Klotho expression reportedly decreases with aging and the progression of CKD [42,43]. Therefore, RIG-I expression may be upregulated more in the elderly and CKD patients under hypoxic conditions, leading to the production of IFN-α/β. As mentioned above, klotho exists in three forms—membrane, secreted, and intracellular klotho—with the latter being responsible for suppressing the RIG-I-mediated production of inflammatory cytokines [26]. In terms of localization, we showed that klotho expression mainly occurs at the renal tubular cells, with evidence that RIG-I expression is mainly intensified at the renal tubular cells under hypoxic conditions. These findings suggest that a decreased level of klotho expression contributes to enhanced RIG-I-mediated production of type 1 interferons under hypoxic conditions.
In summary, we showed that hypoxic stimulation induced RIG-I and IFN-α/β in mouse kidneys and a rat cell line of renal tubular cells, and that RIG-I was involved in hypoxia-induced upregulation of IFN-α/β in in vitro experiments. In renal biopsy samples from patients with IgA nephropathy, RIG-I expression correlated with the upregulation of IFN-α/β. Lastly, hypoxia-induced expression of RIG-I was intensified in klotho-knockout mice, along with the upregulation of IFN-α/β. RIG-I-mediated expression of type 1 interferon increased under hypoxic conditions, and further upregulation was observed in a state of klotho downregulation. According to clinical studies, hypoxia is exacerbated with advanced CKD stage [36] and is predictive of the long-term progression of CKD [36]. Additionally, klotho expression decreases with the decline of renal function, and reduction of klotho expression is associated with renal damage [44]. In this study, we show that klotho deficiency intensifies hypoxia-induced expression of IFN-α/β through the upregulation of RIG-I in kidneys, and that the expression of RIG-I is associated with that of IFN-α/β in actual patients with IgA nephropathy. Taking these findings together, both hypoxia and reduction of klotho are promoted during the progression of CKD, and therefore the activation of innate immunity is enhanced in CKD patients.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing the English text of a draft of this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020; 395: 709–733. doi: 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345: 861–869. doi: 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345: 851–860. doi: 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 4.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015; 385: 1975–1982. Epub 2015 Mar 13. doi: 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 5.Gu YY, Liu XS, Huang XR, Yu XQ, Lan HY. Diverse Role of TGF-β in Kidney Disease. Front Cell Dev Biol. 2020; 8: 123. doi: 10.3389/fcell.2020.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994; 331: 1286–1292. doi: 10.1056/NEJM199411103311907 [DOI] [PubMed] [Google Scholar]
- 7.Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, et al. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol. 2015; 26: 817–829. Epub 2014 Dec 22. doi: 10.1681/ASN.2013101137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Y, Kim Sl, Lee SY, Koo JK, Wang Z, Cho ME. Autophagy regulates TGF-β expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol. 2014; 25: 2835–2846. doi: 10.1681/ASN.2013101068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatachalam MA, Weinberg JM. Fibrosis without fibroblast TGF-β receptors? Kidney Int. 2015; 88: 434–7. doi: 10.1038/ki.2015.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004; 15: 2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Han CH, Chen XS, Zhang M, Xu LM, Zhang JJ, et al. Transient ureteral obstruction prevents against kidney ischemia/reperfusion injury via hypoxia-inducible factor (HIF)-2α activation. PLoS One. 2012; 7: e29876. Epub 2012 Jan 25. doi: 10.1371/journal.pone.0029876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, et al. Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol. 2007; 18: 1218–1226. Epub 2007 Mar 7. doi: 10.1681/ASN.2006060639 [DOI] [PubMed] [Google Scholar]
- 13.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011; 17: 796–808. doi: 10.1038/nm.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SM, Li YG, Wang DM, Zhang GH, Tan CJ. Expression of heme oxygenase-1, hypoxia inducible factor-1alpha, and ubiquitin in peripheral inflammatory cells from patients with coronary heart disease. Clin Chem Lab Med. 2009; 47: 327–333. doi: 10.1515/CCLM.2009.073 [DOI] [PubMed] [Google Scholar]
- 15.Bahadori B, Uitz E, Mayer A, Harauer J, Dam K, Truschnig-Wilders M, et al. Polymorphisms of the hypoxia-inducible factor 1 gene and peripheral artery disease. Vasc Med. 2010; 15: 371–374. doi: 10.1177/1358863X10379674 [DOI] [PubMed] [Google Scholar]
- 16.Mesarwi OA, Loomba R, Malhotra A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am J Respir Crit Care Med. 2019; 199: 830–841. doi: 10.1164/rccm.201806-1109TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006; 17: 17–25. Epub 2005 Nov 16. doi: 10.1681/ASN.2005070757 [DOI] [PubMed] [Google Scholar]
- 18.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014; 1843: 2563–2582. Epub 2014 Jun 2. doi: 10.1016/j.bbamcr.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 19.Paludan SR, Reinert LS, Hornung V. DNA-stimulated cell death: implications for host defence, inflammatory diseases and cancer. Nat Rev Immunol. 2019; 19: 141–153. doi: 10.1038/s41577-018-0117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loo YM, Gale M Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011; 34: 680–692. doi: 10.1016/j.immuni.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand FJ 3rd, de Rivero Vaccari JC, Mejias NH, Alonso OF, de Rivero Vaccari JP. Rig-I contributes to the innate immune response after cerebral ischemia. J Inflamm (Lond). 2015; 12: 52. eCollection 2015. doi: 10.1186/s12950-015-0101-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Wang G, Zhang L, Zhang J, Zhang J, Wang Q, et al. ADAR1 Suppresses the Activation of Cytosolic RNA-Sensing Signaling Pathways to Protect the Liver from Ischemia/Reperfusion Injury. Sci Rep. 2016; 6: 20248. doi: 10.1038/srep20248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997; 390: 45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 24.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005; 309: 1829–1833. doi: 10.1126/science.1112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011; 22: 124–136. Epub 2010 Nov 29. doi: 10.1681/ASN.2009121311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011; 13: 254–262. Epub 2011 Feb 20. doi: 10.1038/ncb2167 [DOI] [PubMed] [Google Scholar]
- 27.Shimoda H, Doi S, Nakashima A, Sasaki K, Doi T, Masaki T. Inhibition of the H3K4 methyltransferase MLL1/WDR5 complex attenuates renal senescence in ischemia reperfusion mice by reduction of p16 INK4a. Kidney Int. 2019; 96: 1162–1175. Epub 2019 Aug 1. doi: 10.1016/j.kint.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 28.Soji K, Doi S, Nakashima A, Sasaki K, Doi T, Masaki T. Deubiquitinase inhibitor PR-619 reduces Smad4 expression and suppresses renal fibrosis in mice with unilateral ureteral obstruction. PLoS One. 2018. 16; 13: e0202409. doi: 10.1371/journal.pone.0202409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi S, Zou Y, Togao O, Pastor J V., John GB, Wang L, et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011; 286: 8655–8665. doi: 10.1074/jbc.M110.174037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010; 40: 294–309. doi: 10.1016/j.molcel.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Luna N, Suárez-Calvet X, Lleixà C, Diaz-Manera J, Olivé M, Illa I, et al. Hypoxia triggers IFN-I production in muscle: Implications in dermatomyositis. Sci Rep. 2017; 7: 8595. doi: 10.1038/s41598-017-09309-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SF, Hosgood SA, Nicholson ML. Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int. 2019; 95: 50–56. doi: 10.1016/j.kint.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 33.Li ZL, Liu BC. Hypoxia and Renal Tubulointerstitial Fibrosis. Adv Exp Med Biol. 2019; 1165: 467–485. doi: 10.1007/978-981-13-8871-2_23 [DOI] [PubMed] [Google Scholar]
- 34.Lewis AJ, Richards AC, Mulvey MA. Invasion of Host Cells and Tissues by Uropathogenic Bacteria. Microbiol Spectr. 2016; 4: doi: 10.1128/microbiolspec.UTI-0026-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011; 11: 389–402. Epub 2011 May 20. doi: 10.1038/nri2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011; 22: 1429–34. Epub 2011 Jul 14. doi: 10.1681/ASN.2010111143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sainz B Jr, Mossel EC, Peters CJ, Garry RF. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology. 2004; 329: 11–7. doi: 10.1016/j.virol.2004.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 2007; 3: e123. doi: 10.1371/journal.ppat.0030123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pothlichet J, Meunier I, Davis BK, Ting JP, Skamene E, von Messling V, et al. Type I IFN Triggers RIG-I/TLR3/NLRP3-dependent Inflammasome Activation in Influenza A Virus Infected Cells. PLoS Pathog. 2013; 9: e1003256. Epub 2013 Apr 11. doi: 10.1371/journal.ppat.1003256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imaizumi T, Tanaka H, Tajima A, Tsuruga K, Oki E, Sashinami H, et al. Retinoic acid-inducible gene-I (RIG-I) is induced by IFN-{gamma} in human mesangial cells in culture: possible involvement of RIG-I in the inflammation in lupus nephritis. Lupus. 2010; 19: 830–836. Epub 2010 Feb 18. doi: 10.1177/0961203309360540 [DOI] [PubMed] [Google Scholar]
- 41.Li L, Yang R, Feng M, Guo Y, Wang Y, Guo J, et al. Rig-I is involved in inflammation through the IPS-1/TRAF 6 pathway in astrocytes under chemical hypoxia. Neurosci Lett. 2018; 672: 46–52. doi: 10.1016/j.neulet.2018.02.035 Epub 2018 Feb 21. [DOI] [PubMed] [Google Scholar]
- 42.Morii K, Yamasaki S, Doi S, Irifuku T, Sasaki K, Doi T, et al. microRNA-200c regulates KLOTHO expression in human kidney cells under oxidative stress. PLoS One. 2019; 14: e0218468. eCollection 2019. doi: 10.1371/journal.pone.0218468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchanan S, Combet E, Stenvinkel P, Shiels PG. Klotho, Aging, and the Failing Kidney. Front Endocrinol (Lausanne). 2020; 11: 560. doi: 10.3389/fendo.2020.00560 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada K, Doi S, Nakashima A, Kawaoka K, Ueno T, Doi T, et al. Expression of age-related factors during the development of renal damage in patients with IgA nephropathy. Clin Exp Nephrol. 2015; 19: 830–7. Epub 2014 Dec 11. doi: 10.1007/s10157-014-1070-2 [DOI] [PubMed] [Google Scholar]