Abstract
Background
Stroke is the third leading cause of early death worldwide. Most ischaemic strokes are caused by a blood clot blocking an artery in the brain. Patient outcomes might be improved if they are offered anticoagulants that reduce their risk of developing new blood clots and do not increase the risk of bleeding. This is an update of a Cochrane Review first published in 1995, with updates in 2004, 2008, and 2015.
Objectives
To assess the effectiveness and safety of early anticoagulation (within the first 14 days of onset) for people with acute presumed or confirmed ischaemic stroke.
Our hypotheses were that, compared with a policy of avoiding their use, early anticoagulation would be associated with:
• reduced risk of death or dependence in activities of daily living a few months after stroke onset;
• reduced risk of early recurrent ischaemic stroke;
• increased risk of symptomatic intracranial and extracranial haemorrhage; and
• reduced risk of deep vein thrombosis and pulmonary embolism.
Search methods
We searched the Cochrane Stroke Group Trials Register (August 2021); the Cochrane Database of Systematic Reviews (CDSR); the Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 7), in the Cochrane Library (searched 5 August 2021); MEDLINE (2014 to 5 August 2021); and Embase (2014 to 5 August 2021). In addition, we searched ongoing trials registries and reference lists of relevant papers. For previous versions of this review, we searched the register of the Antithrombotic Trialists' (ATT) Collaboration, consulted MedStrategy (1995), and contacted relevant drug companies.
Selection criteria
Randomised trials comparing early anticoagulant therapy (started within two weeks of stroke onset) with control in people with acute presumed or confirmed ischaemic stroke.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed trial quality, and extracted data. We assessed the overall certainty of the evidence for each outcome using RoB1 and GRADE methods.
Main results
We included 28 trials involving 24,025 participants. Quality of the trials varied considerably. We considered some studies to be at unclear or high risk of selection, performance, detection, attrition, or reporting bias. Anticoagulants tested were standard unfractionated heparin, low‐molecular‐weight heparins, heparinoids, oral anticoagulants, and thrombin inhibitors. Over 90% of the evidence is related to effects of anticoagulant therapy initiated within the first 48 hours of onset. No evidence suggests that early anticoagulation reduced the odds of death or dependence at the end of follow‐up (odds ratio (OR) 0.98, 95% confidence interval (CI) 0.92 to 1.03; 12 RCTs, 22,428 participants; high‐certainty evidence). Similarly, we found no evidence suggesting that anticoagulant therapy started within the first 14 days of stroke onset reduced the odds of death from all causes (OR 0.99, 95% CI 0.90 to 1.09; 22 RCTs, 22,602 participants; low‐certainty evidence) during the treatment period. Although early anticoagulant therapy was associated with fewer recurrent ischaemic strokes (OR 0.75, 95% CI 0.65 to 0.88; 12 RCTs, 21,665 participants; moderate‐certainty evidence), it was also associated with an increase in symptomatic intracranial haemorrhage (OR 2.47; 95% CI 1.90 to 3.21; 20 RCTs, 23,221 participants; moderate‐certainty evidence). Similarly, early anticoagulation reduced the frequency of symptomatic pulmonary emboli (OR 0.60, 95% CI 0.44 to 0.81; 14 RCTs, 22,544 participants; high‐certainty evidence), but this benefit was offset by an increase in extracranial haemorrhage (OR 2.99, 95% CI 2.24 to 3.99; 18 RCTs, 22,255 participants; moderate‐certainty evidence).
Authors' conclusions
Since the last version of this review, four new relevant studies have been published, and conclusions remain consistent. People who have early anticoagulant therapy after acute ischaemic stroke do not demonstrate any net short‐ or long‐term benefit. Treatment with anticoagulants reduced recurrent stroke, deep vein thrombosis, and pulmonary embolism but increased bleeding risk. Data do not support the routine use of any of the currently available anticoagulants for acute ischaemic stroke.
Plain language summary
Early treatment with blood‐thinning drugs for people who have had a stroke
Review question We wanted to know whether people treated with anticoagulants (blood‐thinning drugs) soon after having a stroke got better or not, and whether they had problems with bleeding.
Background Millions of people around the world have a stroke every year. Most strokes take place when a blood clot blocks a blood vessel leading to the brain. When the blood supply to the brain is restricted or blocked, brain cells begin to die. This can lead to brain injury, which can be permanent, causing disability and possibly death. Damage from a stroke can cause arm or leg weakness, or difficulties with language or vision. Strokes are sometimes fatal but more often leave survivors unable to do the things they used to do. Because strokes are common and cause such damage, researchers are looking at ways to get rid of the blood clot soon after the stroke happens. One way to do this is to use blood‐thinning drugs called anticoagulants. If patients respond well to anticoagulants, they might be able to avoid the bad effects of a stroke. The main problem with anticoagulants is that if they cause bleeding, the patient can have very serious outcomes from this.
Search date The evidence is current to August 2021.
Study characteristics To find the best answer, we looked for studies in which investigators compared any anticoagulant to another medicine, to a dummy medicine that does not contain any active ingredients (placebo), or to normal care. To make the comparison fair, all patients in these studies must have had the same random chance (like the flip of a coin) to receive the anticoagulant, the other treatment, or normal care. We included in this updated review 28 studies involving 24,025 people with stroke. Two studies enrolled participants within 12 hours of stroke onset, four within 24 hours, and 10 within 48 hours.
Key results People treated with anticoagulants did not have less long‐term disability, and they experienced more bleeding. Anticoagulant‐treated patients had a lesser chance of developing blood clots in their legs and in their lungs following their stroke, but these benefits were offset by an increased number of bleeds.
Certainty of the evidence We used standard methods to assess the certainty of the evidence. We rated our confidence in the evidence based on factors such as study methods, numbers of participants included in the studies, and consistency of findings across studies. Low‐certainty evidence means we are uncertain about the results. In the same way, high‐certainty evidence means we are very certain about the results of this review.
Conclusion This review did not provide any evidence to suggest that early use of anticoagulants is beneficial overall for people with stroke caused by blood clots. More research is needed to find out if there are ways to select people with stroke who will benefit most from anticoagulants without suffering the bleeding complications.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Anticoagulant compared to control in acute presumed ischaemic stroke.
| Anticoagulant compared to control in acute presumed ischaemic stroke | ||||||
| Patient or population: acute presumed ischaemic stroke Setting: hospital Intervention: anticoagulant Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with anticoagulant | |||||
| Dead or dependent at end of follow‐up (if > 1 month) | 598 per 1000 | 593 per 1000 (578 to 605) | OR 0.98 (0.92 to 1.03) | 22428 (13 RCTs) | ⊕⊕⊕⊕ High | ‐ |
| Death from all causes during treatment period | 86 per 1000 | 85 per 1000 (78 to 93) | OR 0.99 (0.90 to 1.09) | 22602 (22 RCTs) | ⊕⊕⊝⊝ Low | Only 3 studies included in this analysis had low risk in each domain; only 2 out of 22 studies enrolled more than 500 patients, clearly underpowered |
| Deep vein thrombosis during treatment period | 443 per 1000 | 143 per 1000 (107 to 188) | OR 0.21 (0.15 to 0.29) | 916 (10 RCTs) | ⊕⊝⊝⊝ Very low | No studies included in this analysis had low risk in each domain; all the studies were too underpowered to draw a firm conclusion; publication bias has been detected |
| Recurrent ischaemic or unknown stroke during treatment period | 36 per 1000 | 27 per 1000 (24 to 32) | OR 0.75 (0.65 to 0.88) | 21665 (12 RCTs) | ⊕⊕⊕⊝ Moderate | Only 2 studies included in this analysis had low risk in each domain |
| Symptomatic intracranial haemorrhage during treatment period | 5 per 1000 | 12 per 1000 (9 to 16) | OR 2.47 (1.90 to 3.21) | 23221 (21 RCTs) | ⊕⊕⊕⊝ Moderate | Publication bias has been detected |
| Symptomatic pulmonary embolism during treatment period | 9 per 1000 | 6 per 1000 (4 to 8) | OR 0.60 (0.44 to 0.81) | 22544 (14 RCTs) | ⊕⊕⊕⊕ High | ‐ |
| Major extracranial haemorrhage during treatment period | 4 per 1000 | 11 per 1000 (9 to 15) | OR 2.99 (2.24 to 3.99) | 22255 (18 RCTs) | ⊕⊕⊕⊝ Moderate | Publication bias has been detected |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423948318800395805. | ||||||
Background
Description of the condition
Stroke was the third leading cause of disability‐adjusted life‐years and early death among people of all ages worldwide in 2017 (Kyu‐2018). Due to demographic (aging population) and lifestyle changes, the global burden of stroke is increasing, with the greatest burden noted in low‐ and middle‐income countries (Feigin 2014). In Western societies, ischaemic stroke is the most frequent pathological subtype of stroke (~80%) and is usually caused by a blood clot blocking flow in an artery supplying parts of the brain (Feigin 2009).
Description of the intervention
Anticoagulants are agents that act on the coagulation cascade to reduce fibrin polymerisation and thrombus formation; they are distinct from thrombolytic and defibrinogenating agents. Agents included in this review include unfractionated heparin, low‐molecular‐weight heparins, heparinoids, oral vitamin K antagonists, and specific thrombin inhibitors. The control was an inactive intervention ‐ placebo or no treatment ‐ delivered along with standard interventions of the respective healthcare systems.
Heparins are administered parenterally (intravenously or subcutaneously) and so have sufficiently rapid onset to be used in the acute phase of ischaemic stroke, whereas oral anticoagulants, such as vitamin K antagonists and direct thrombin inhibitors, have slower onset of effect and may be of less use. Unfractionated heparin, a sulphated polysaccharide, acts by binding to antithrombin to inhibit factor Xa and deactivate thrombin. Important side effects include thrombocytopenia and osteopenia. Low‐molecular‐weight heparins are depolymerised heparin fragments approximately one‐third the size of unfractionated heparin that act primarily to inhibit factor Xa. They have a longer half‐life, greater bioavailability, and more predictable anticoagulant effect than unfractionated heparin. Heparinoids are glycosaminoglycans whose components catalyse the effect of heparin co‐factor 2 to inhibit thrombin. All heparins ultimately prevent fibrin formation and subsequent thrombosis.
How the intervention might work
Theoretically, early use of anticoagulants, by reducing the propagation of a thrombus in an intracerebral artery, may decrease the volume of infarcted cerebral tissue and so decrease the neurological deficit and risks of disability and death. Additionally, anticoagulants might inhibit the formation of new arterial and venous thromboses and so reduce the risk of early recurrent thromboembolic stroke, deep vein thrombosis, and pulmonary embolism. However, these benefits could be offset by the possibility that anticoagulant therapy may increase the risk of intracranial and extracranial haemorrhage.
Why it is important to do this review
This is an update of a Cochrane Review first published in 1995, and most recently updated in 2020. We included all randomised trials of anticoagulants versus control for people with acute presumed or confirmed ischaemic stroke. The aim is to establish the balance of risk and benefit of early anticoagulation for acute ischaemic stroke.
Objectives
To assess the effectiveness and safety of early anticoagulation (within the first 14 days of onset) for people with acute presumed or confirmed ischaemic stroke.
Our hypotheses were that, compared with a policy of avoiding their use, early anticoagulation would be associated with:
reduced risk of death or dependence in activities of daily living a few months after stroke onset;
reduced risk of early recurrent ischaemic stroke;
increased risk of symptomatic intracranial and extracranial haemorrhage; and
reduced risk of deep vein thrombosis and pulmonary embolism.
Methods
Criteria for considering studies for this review
Types of studies
We sought all unconfounded, truly randomised trials in which early treatment with anticoagulants was compared with control for people with acute presumed or confirmed ischaemic stroke. People with ischaemic stroke due to cerebral venous thrombosis were not specifically included in these trials, and so are not represented in this review. People with transient ischaemic attacks (TIAs) also are not included in this review. We did not include trials in which allocation to treatment or control group was not truly random, or in which allocation was not adequately concealed (e.g. allocation by alternation, date of birth, hospital number, day of the week, open random number list), because foreknowledge of treatment allocation could lead to biased treatment allocation, leading to overestimation of the treatment effect by up to 30% (Odgaard‐Jensen 2011). We included trials in which it is unclear whether the method of randomisation provided adequate concealment of allocation.
Types of participants
This review was confined to early treatment of acute ischaemic stroke; therefore we excluded the following types of trials: those that randomised participants more than 14 days after stroke onset, those that included only people with TIAs, and those that included only people with intracerebral haemorrhage, confirmed by appropriate brain imaging before entry. We included trials in which the pathological type of stroke was not confirmed by scanning before entry, as a majority of such strokes are ischaemic (Bamford 1990).
Types of interventions
Anticoagulants are broadly defined as agents that act on the coagulation cascade to exert an anticoagulant effect, excluding thrombolytic agents (such as alteplase ) and defibrinogenating agents (such as ancrod). Use of thrombolytic agents for acute ischaemic stroke is the topic of a separate Cochrane Review (Wardlaw 2014), as is use of fibrinogen‐depleting agents (Hao 2012). We included the following anticoagulants in this review: subcutaneous and intravenous standard unfractionated heparin, low‐molecular‐weight heparins, subcutaneous and intravenous heparinoids, oral vitamin K antagonists, factor Xa inhibitors, and specific thrombin inhibitors.
Types of outcome measures
For each trial, we identified the number of participants originally randomly allocated to each treatment and control group. For both groups, we sought outcome information regarding the numbers of participants who met the following outcomes.
Primary outcomes
Death or dependency (i.e. people who were dead or were dependent on help from other people for their activities of daily living), at least one month after stroke. This minimum interval was used to allow time for recovery from the initial stroke. Comparable definitions of dependency were used in all of the trials assessed in this review
Secondary outcomes
Participants who died from any cause during the scheduled treatment period (generally shorter than the scheduled follow‐up period)
Participants who died from any cause during the scheduled follow‐up period (greater than one month after stroke)
Participants with objective evidence of deep vein thrombosis detected by the systematic use of imaging techniques such as iodine 125 fibrinogen scanning (I‐125 scan), ultrasound of the leg, plethysmography, or X‐ray contrast venography during the scheduled treatment period and during scheduled follow‐up. These methods detected clinically silent deep vein thrombosis while confirming or refuting the diagnosis for participants with clinical features suggestive of deep vein thrombosis. The outcome was therefore 'symptomatic or asymptomatic deep vein thrombosis'. Screening of participants by clinical observation alone was not considered adequate
Particpants with recurrent stroke during the treatment period and during follow‐up that was either definitively ischaemic (haemorrhage excluded by brain imaging or autopsy) or of unknown type (no brain imaging or autopsy performed)
Participants with symptomatic intracranial (intracerebral or extracerebral) haemorrhage, including symptomatic haemorrhagic transformation of the cerebral infarct, during the scheduled treatment period and during follow‐up. Haemorrhage must have been confirmed by appropriate brain imaging after clinical deterioration, or by autopsy
Participants with any recurrent stroke or symptomatic intracranial haemorrhage during the treatment period or during long‐term follow up (as previously defined)
Participants with at least one confirmed symptomatic pulmonary embolus diagnosed during life or at autopsy (symptomatic or not) within the scheduled treatment period and during scheduled follow‐up
Participants with any major extracranial haemorrhage during the scheduled treatment period. The definition of major haemorrhage was usually taken from the original article, but if none was given, major haemorrhage was defined as any fatal bleed, or bleeding severe enough to require transfusion or operation
Although we sought trials that reported the primary outcome (dead or dependent at least one month after stroke), we also included data from trials that reported only data on our secondary outcomes.
Search methods for identification of studies
See the 'Specialized register' section at the Cochrane Stroke Group website. We searched for trials published in all languages and arranged translation of relevant papers published in languages other than English .
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched April 2020) and the following bibliographic databases and trials registers.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 7), in the Cochrane Library (Appendix 1).
Cochrane Database of Systematic Reviews (CDSR; 2021, Issue 7), in the Cochrane Library (Appendix 1).
Database of Reviews of Effects (DARE; 2014, Issue 6), in the Cochrane Library (Appendix 1).
Health Technology Assessment Database (HTA; 2014, Issue 6), in the Cochrane Library (Appendix 1).
MEDLINE (Ovid; 2014 to August 2021) (Appendix 2).
Embase (Ovid; 2014 to 14 August 2021) (Appendix 3).
Using a comprehensive search strategy, the Cochrane Stroke Group Trials Search Co‐ordinator had already completed a retrospective search of MEDLINE and Embase for all stroke trials to January 2014 and added all relevant trials to the Cochrane Stroke Group Trials Register. To avoid duplication of effort, we have limited the search of these two databases from January 2014 onwards.
Searching other resources
ClinicalTrials.gov (https://clinicaltrials.gov; searched 5 August 2021) (Appendix 4)
International Standard Randomized Controlled Trials Number (ISRCTN) Registry (http://www.isrctn.com/; searched 5 August 2021) (Appendix 4)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 5 August 2021) (Appendix 4)
We scanned the reference lists of all relevant papers
-
For previous versions of this review:
we contacted the following anticoagulant manufacturers in an effort to identify unpublished trials (last contact 1999): Alfa Wasserman (parnaparin and dermatan sulphate), Kabi (dalteparin), Knoll (reviparin), Leo (tinzaparin), Mediolanum (dermatan sulphate), Mitsubishi Chemical (argatroban/MD‐805), Novo (tinzaparin), Organon (danaparoid), Rhone‐Poulenc Rorer (enoxaparin), Sandoz (Sandoz LMWH), and Sanofi Winthrop (nadroparin and CY 222);
we consulted a comprehensive guide to pharmaceutical development in the field of stroke (MedStrategy 1995) but have not updated the search, as relevant trials contained within it are included in the Cochrane Stroke Group Trials Register; and
in August 1998, we searched the trials register held by the Antithrombotic Trialists' (ATT) Collaboration, but this is no longer available and relevant trials from the register are now contained in the Cochrane Stroke Group Trials Register
Data collection and analysis
We followed standard Cochrane methodological procedures. For this update, Joshua Cheyne (Cochrane Stroke Group Information Specialist) performed the searches. XW and MO then independently screened all titles and abstracts of identified references and excluded obviously irrelevant studies. XW obtained full‐text articles of the remaining studies, and both XW and MO independently assessed these for inclusion or exclusion. We resolved any disagreements by discussion with LS, JY, and CA.
Selection of studies
Two review authors (PS and CC, for trials included in the first version of this review; PS and Gordon Gubitz for the proceeding two updates following the original review; Ayeesha Kamal and PS for the 2008 update; PS and EK for the 2015 update; XW and MO for this version) independently selected trials for inclusion in the review. We resolved disagreements through discussion. The same two review authors assessed the methodological quality of each trial.
Data extraction and management
Two review authors independently extracted and cross‐checked the data. We sought data on the number of participants with each outcome event, by allocated treatment group, irrespective of compliance, and whether or not participants were subsequently deemed ineligible or were otherwise excluded from treatment or follow‐up, to allow an intention‐to‐treat (ITT) analysis. We also sought data on use of brain imaging prior to randomisation, delay from stroke onset to trial entry, types of patients included, and types of anticoagulant regimens used. If any of the above data were not available in the publications, we sought further information by corresponding with the trialists.
Assessment of risk of bias in included studies
We assessed the risk of bias of each of the included trials using the following criteria of internal validity: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, adequate reporting and handling of missing outcome data, selective outcome reporting, and other risks of bias. We followed the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (MO, XW) independently assessed risk of bias for the included studies using RoB1. We followed GRADE recommendations to determine the certainty of the evidence (Guyatt 2008). This involved considering risk of bias together with inconsistency, indirectness, imprecision, and publication bias. Two review authors (MO, XW) completed this assessment.
Measures of treatment effect
Results reported in the text are odds ratios (ORs; i.e. ratios of the odds of an unfavourable outcome among treatment‐allocated participants to the corresponding odds amongst controls), which we calculated using the Peto fixed‐effect method (APT 1994). We calculated the significance of any differences between ORs (in relation to subgroup analyses) by using a standard method (Altman 1996). When relevant, we expressed the absolute effects of treatment on each outcome as the number needed to treat to benefit (NNTB) (i.e. to avoid one bad outcome event). For events that are adverse (such as intracranial haemorrhage), this was calculated as the number needed to treat to harm (NNTH). To calculate NNTBs or NNTHs, we used the NNT calculator at https://www2.ccrb.cuhk.edu.hk/stat/confidence%20interval/CI%20for%20ratio.htm. This applies the point estimate of relative effect and its 95% confidence interval (CI), then calculates NNTB or NNTH for a specified control event rate.
Unit of analysis issues
All included studies were trials in which individuals were randomised and follow‐up was generally provided to a prespecified and fixed time point; all analyses were by ITT where possible (see Dealing with missing data). For outcomes for which more than one event could occur during follow‐up, such as non‐fatal recurrent stroke, we counted only the first event.
Dealing with missing data
For some outcomes (such as deep vein thrombosis and any intracranial haemorrhage), ITT analyses were not possible because all participants did not have the relevant investigation performed to detect the event. For these analyses, we used the number of participants in each group who had the appropriate investigation as the denominator for the main analyses. However, if we found statistically significant results, we also analysed best‐ and worst‐case scenarios: the best‐case scenario (with regards to treatment) assumed that none of the participants excluded from the analysis in the treatment group had an adverse outcome, whilst all those excluded from the control group did, and vice versa for the worst‐case analysis.
Assessment of heterogeneity
We identified and measured statistical and clinical heterogeneity as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). We estimated heterogeneity between trial results using the I² statistic (Deeks 2021). We defined thresholds for interpreting heterogeneity (I²) as follows.
0% to 30%: no heterogeneity.
30% to 50%: moderate heterogeneity.
50% to 80%: substantial heterogeneity.
80% to 100%: considerable heterogeneity.
Evaluation of heterogeneity was not based on I² alone, as the importance of consistency depends on several factors, but rather included an overall evaluation of the data. We also considered the P value, noting that with P < 0.05, there was likely to be heterogeneity.
Assessment of reporting biases
We attempted to minimise publication bias by using a comprehensive search strategy that included searching for unpublished studies and searching trials registers. We examined the funnel plot for any evidence of asymmetry for three outcomes. Analyses including the greatest numbers of trials (and hence with the greatest statistical power) examined effects of treatment on death during the treatment period, death from all causes at final follow‐up, and deep vein thrombosis.
Data synthesis
We used RevMan 2014 for the analyses, in which we grouped together trials of each type of anticoagulant (e.g. unfractionated heparin, low‐molecular‐weight heparins, heparinoids, oral vitamin K antagonists, thrombin inhibitors) to assess whether there were any significant differences between classes of anticoagulant agents. It should be noted that this was an indirect rather than a direct randomised comparison.
We also specified the following classification of anticoagulant dosing regimens.
Low fixed‐dose anticoagulant ‐ a dose intended to be sufficient for prevention of deep vein thrombosis and pulmonary embolism.
Medium fixed‐dose anticoagulant ‐ a dose intended to have effects on arterial circulation, but not enough to require monitoring.
Adjusted‐dose anticoagulant ‐ a dose adjusted by blood testing or by body weight to meet a specific target.
Subgroup analysis and investigation of heterogeneity
For this update, we performed subgroup analyses for the primary outcome by:
type of anticoagulant agent used; and
dose of anticoagulant used, applying the classification above.
Sensitivity analysis
For this update, we performed the following sensitivity analyses for the primary outcome, restricting analyses to:
trials in which the method of randomisation ensured adequate concealment of treatment allocation;
trials in which all participants were recruited within 48 hours of stroke onset; and
trials except IST 1997, as this trial contained most of the data relevant to the review.
In the previous version of this review, we performed numerous sensitivity analyses to investigate whether exclusion or inclusion of trials with particular characteristics would alter the overall conclusions. These characteristics included trials that had intracerebral haemorrhages excluded by neuroimaging prior to trial entry, time from stroke onset (less than 48 hours versus more than 48 hours) to randomisation, or concomitant unconfounded treatment with antiplatelet agents; trials in which stroke was of suspected cardioembolic origin versus non‐cardioembolic origin; and trials that evaluated different anticoagulant doses. These analyses were not informative, and we have excluded them from this updated review for brevity and clarity. For future updates of the review, we do not plan to repeat these analyses unless substantial new trial data have been added.
Summary of findings and assessment of the certainty of the evidence
We used 'Summary of findings' tables to summarise the data comparing control and anticoagulation on (1) death or dependency at the end of follow‐up (if > 1 month), (2) death from all causes during the treatment period, (3) deep vein thrombosis during the treatment period, (4) recurrent ischaemic or unknown stroke during the treatment period, (5) symptomatic intracranial haemorrhage during the treatment period, (6) symptomatic pulmonary embolism during the treatment period, and (7) major extracranial haemorrhage during the treatment period. We used GRADE methods to assess the overall certainty of evidence for each outcome (Guyatt 2008).
Results
Description of studies
See Characteristics of ongoing studies and Characteristics of excluded studies.
Results of the search
For this update, we searched the Cochrane Stroke Group Trials Register and performed additional new comprehensive searches of the Cochrane Library databases, MEDLINE, and Embase. After we removed duplicate records, we screened the titles and abstracts of 9508 records from these electronic bibliographic databases and obtained the full text of 90 studies, of which we excluded 86 studies, leaving four new trials for inclusion. The total number of included studies in this review is 28. See Figure 1.
1.
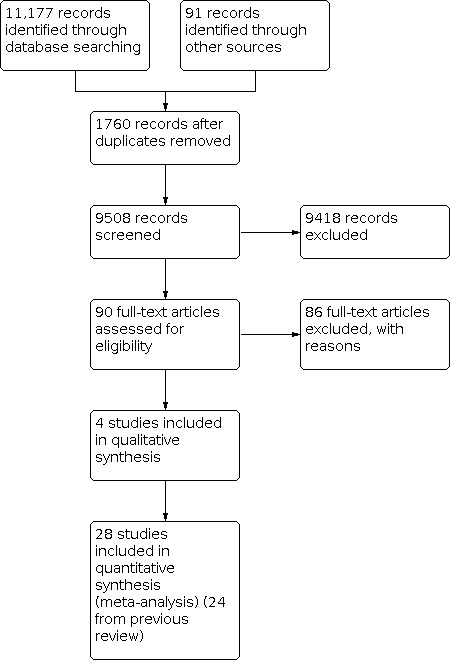
Study flow diagram for 2020 update.
We identified six new ongoing trials from searches of trials registers (see Characteristics of ongoing studies).
Included studies
We included in this review 28 trials with a total of 24,025 participants. Summary details for these trials are given in the Characteristics of included studies table. Of the 28 included studies, two enrolled participants within 12 hours of stroke onset (ARGIS‐1 2004; ARTSS‐2 2017), four enrolled participants within 24 hours of stroke onset (Dluha 2016; FISS‐bis 1998; Sarma 2003; TOAST 1998), 11 enrolled participants within 48 hours of stroke onset (Cazzato 1989; CESG 1983; Duke 1983; Duke 1986; Elias 1990; FISS 1995; IST 1997; Kwiecinski 1995; Liu 2020; McCarthy 1977; McCarthy 1986), and the rest enrolled participants within 14 days. The age of participants in the included studies ranged from 28 to 92 years. A significant proportion of participants were over 70 years old. For example, 61% of participants enrolled in IST 1997 were aged 70 or older. Most trials included slightly more men than women and excluded people thought to be at high risk of bleeding (e.g. clotting disorders, hepatic failure, renal failure). In addition, 13 trials excluded people with a significant degree of hypertension (generally diastolic pressure > 120 mmHg or systolic pressure > 180 mmHg), and 10 trials excluded comatose people.
The scheduled period of anticoagulant treatment in included trials was one to two weeks in 24 trials, and one month in four trials. The anticoagulants used were (one trial used two types of anticoagulants (Dluha 2016) and therefore was considered as two trials):
standard unfractionated subcutaneous heparin (six trials);
standard unfractionated intravenous heparin (three trials);
low‐molecular‐weight heparins (10 trials: two dalteparin, four nadroparin, one tinzaparin, one fraxiparin, one parnaparin, and one CY 222);
subcutaneous heparinoid (two trials: one danaparoid and one mesoglycan);
intravenous heparinoid (one danaparoid trial);
oral vitamin K antagonists (two trials); and
thrombin inhibitors (five trials: two MD805 trials, three argatroban).
In trials using oral vitamin K antagonists, heparin was given intravenously for the first few days to provide rapid anticoagulation (Marshall 1960; NAT‐COOP 1962). Five trials randomised between two doses of anticoagulant as well as control (ARTSS‐2 2017; Dluha 2016; FISS 1995; FISS‐bis 1998; IST 1997); for the main analyses in this review, we combined the two anticoagulant groups for these trials.
Eighteen trials routinely performed a CT head scan for all patients to rule out haemorrhage before randomisation (ARGIS‐1 2004; ARTSS‐2 2017; Cazzato 1989; CESG 1983; Dluha 2016; Duke 1986; Elias 1990; FISS 1995; FISS‐bis 1998; Kwiecinski 1995; Pambianco 1995; Prins 1989; Sarma 2003; Sandset 1990; Tazaki 1986; Tazaki 1992; TOAST 1998; Turpie 1987). Three trials performed CT for most patients (Duke 1983; IST 1997; Vissinger 1995): 81% of participants in Duke 1983 were scanned; in IST 1997, 67% were scanned before randomisation, and 29% after randomisation, so that overall, 96% of participants were scanned; in Vissinger 1995, 66% of participants were scanned, and the remainder had cerebral scintigraphy to exclude haemorrhage. Three trials performed almost no CT scans (McCarthy 1977; McCarthy 1986; Pince 1981), and two trials were undertaken before CT scanning was introduced (Marshall 1960; NAT‐COOP 1962). It is therefore likely that some people with intracerebral haemorrhage were inadvertently included in the main analyses of this review. This may have biased the results against anticoagulation if risks of anticoagulation are greater in those with intracerebral haemorrhage, although such bias is unlikely given the relatively small numbers of people with intracerebral haemorrhage involved in these trials, and because IST 1997 provided well over 80% of the overall data.
Two trials included only participants with presumed cardioembolic stroke (CESG 1983; NAT‐COOP 1962). One trial enrolled a subset of people with atrial fibrillation (IST 1997), and detailed information on the effects of heparin in this subgroup was reported in a paper published in 2001 (Saxena 2001).
The duration of follow‐up in included trials was generally short, although this was mainly a characteristic of the smaller trials, which contributed less to the overall analysis. Four trials in which the primary outcome of interest was deep vein thrombosis did not follow participants beyond 14 days (Elias 1990; McCarthy 1977; Pince 1981; Prins 1989), and only 14 trials followed participants longer than one month (ARGIS‐1 2004; ARTSS‐2 2017; Chaudhary 2002; Dluha 2016; Duke 1986; FISS 1995; FISS‐bis 1998; IST 1997; Kwiecinski 1995; Liu 2020; Marshall 1960; McCarthy 1986; TOAST 1998; Turpie 1987). This lack of long‐term follow‐up is a weakness of many of the smaller studies, as a significant proportion of deaths after one month could have been due to stroke‐related thromboembolic events and might therefore have been prevented by early anticoagulation. Similarly, disability is best assessed when most of the recovery has taken place (i.e. between three and six months), rather than in the first week or so.
Relatively few trials assessed the clinically most important outcome of long‐term functional status. Treatments that prevent death from stroke may lead to survival in a disabled state ‐ an outcome considered by many to be worse than death. The composite outcome of 'dead or dependent at follow‐up' is therefore the most important outcome in acute stroke trials. Eleven trials assessed this composite outcome. These trials contain data from well over 90% of the participants included in this review and evaluated the outcomes of death and dependency adequately (ARGIS‐1 2004; ARTSS‐2 2017; Cazzato 1989; Chaudhary 2002; Dluha 2016; FISS 1995; FISS‐bis 1998; IST 1997; Kwiecinski 1995; Liu 2020; TOAST 1998). Other important outcomes, including recurrent stroke or intracranial haemorrhage, were assessed but, once again, only by the more recent trials, which included large numbers of participants. Quality of life assessments were undertaken only in ARTSS‐2 2017.
Excluded studies
We excluded 103 studies for a variety of reasons (see Characteristics of excluded studies).
Risk of bias in included studies
Two review authors independently assessed risk of bias in all included studies across the following domains: random sequence generation and allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); and other potential sources of bias.
See 'Risk of bias' tables in Characteristics of included studies, in the overall 'Risk of bias' graph (Figure 2), and in the risk of bias summary (Figure 3).
2.

3.

Randomisation
There was marked variation in the quality of included trials. In 12 trials, the method of randomisation adequately prevented foreknowledge of treatment allocation; therefore they were at low risk. The remaining 16 trials had unclear risk. IST 1997 used a central telephone randomisation service. TOAST 1998 used permuted blocks to generate a randomisation list controlled by the hospital pharmacy. Eight trials utilised numbered or coded containers administered sequentially to enrolled participants (FISS 1995; FISS‐bis 1998; Prins 1989; Sandset 1990; Tazaki 1986; Tazaki 1992; Turpie 1987; Vissinger 1995). Three trials used random‐number tables controlled by an independent party (Cazzato 1989; Duke 1983; Duke 1986). The 2:1 treatment‐to‐control allocation ratio in Turpie 1987, Tazaki 1986, FISS 1995, and FISS‐bis 1998 was deliberate. CESG 1983 used opaque sequentially numbered envelopes. The method of randomisation was unclear in 10 trials. Six trials stated that sealed envelopes were used, but in five of these, it is not clear whether or not the envelopes were opaque and sequentially numbered (Elias 1990; McCarthy 1977; McCarthy 1986; NAT‐COOP 1962; Pince 1981). In Pambianco 1995, the envelopes were not numbered. The exact method of randomisation was unknown in ARGIS‐1 2004, Chaudhary 2002, Kwiecinski 1995, and Marshall 1960.
Allocation
Twelve trials had low risk of bias, and the remaining 16 trials had unclear risk. Allocation concealment for the following small trials was unclear: ARGIS‐1 2004, Chaudhary 2002, Elias 1990, Kwiecinski 1995, Marshall 1960, McCarthy 1977, McCarthy 1986, Pambianco 1995, Pince 1981, and Sarma 2003.
Blinding
Thirteen trials had low risk, six trials had high risk, and the remaining nine trials had unclear risk. Adequate blinding may be important to reduce bias in detection of deep vein thrombosis, pulmonary embolism, symptomatic intracranial haemorrhage, recurrent stroke, and functional outcome. Fifteen trials were double‐blind, that is, treatment allocation was concealed from participants, physicians, and outcome assessors (ARGIS‐1 2004; ARTSS‐2 2017; Dluha 2016; Duke 1983; Duke 1986; FISS 1995; FISS‐bis 1998; Liu 2020; Prins 1989; Sandset 1990; Tazaki 1986; Tazaki 1992; TOAST 1998; Turpie 1987; Vissinger 1995), and in two other trials, deep vein thrombosis was assessed by radiologists blinded to treatment allocation (McCarthy 1977; McCarthy 1986). Cazzato 1989 had a blinded outcome assessor only. IST 1997 was not designed as a blinded study. However, an analysis of 207 participants from the UK enrolled in the IST pilot study showed that at six‐month follow‐up, a majority of participants could not remember whether or not they had been treated, and so these participants were effectively 'blinded' (Lindley 1993). In IST 1997, follow‐up data were collected by self‐completed questionnaire mailed to the participant six months after randomisation, or by telephone interview by a person blinded to treatment allocation. The remainder of the trials did not appear to use any form of blinded assessment.
Incomplete outcome data
Twelve trials had low risk, nine trials had high risk, and the remaining seven trials had unclear risk. In total, only 218 participants (0.9% overall) were reported to be excluded from analysis after randomisation or were lost to follow‐up, with the vast majority of participants enrolled in studies in which an ITT analysis was performed. However, a number of participants in the smaller trials that did not report an ITT analysis may have been omitted from the analysis.
Selective reporting
Twenty trials had low risk, one trial had high risk, and the remaining seven trials had unclear risk. We assessed risk of bias due to missing results in syntheses of primary outcomes most important to patients and health professionals (Boutron 2021). There was no evidence of reporting bias.
Other potential sources of bias
Long‐term use of antiplatelet agents
In trials with follow‐up, differences in long‐term use of antiplatelet treatment between anticoagulant and control groups after hospital discharge may have biased the results, as antiplatelet treatment has been shown to reduce the risk of further vascular events by about 25% (ATC 2002). Aspirin was given to all survivors in FISS 1995. Aspirin was given to all patients without intracranial haemorrhage after 24 hours in Dluha 2016. Long‐term treatment with aspirin was encouraged but was optional in several other trials, including IST 1997, FISS‐bis 1998, and TOAST 1998. Aspirin (81 mg to 325 mg) was also used in both arms of the ARGIS‐1 2004 trial of the direct thrombin inhibitor argatroban.
Imbalance at baseline
None of the trials reported significant imbalances in important baseline prognostic variables, although the small size of many suggests that they might be ruling out only substantial differences.
Effects of interventions
See: Table 1
Outcome 1.1: Dead or dependent at end of follow‐up more than one month after randomisation
Twelve trials including randomised data from 22,428 participants (93.3% of participants included in the overall review) evaluated death and long‐term disability. The degree of dependence was determined by noting whether participants required help from other people for their activities of daily living at the time of final follow‐up. Treatment with early anticoagulation was not associated with a significant reduction in the odds of death or dependence at final follow up (odds ratio (OR) 0.98, 95% confidence interval (CI) 0.92 to 1.03; P = 0.37; 12 RCTs, 22,428 participants; high‐certainty evidence; Analysis 1.1). However, substantial heterogeneity of treatment effect (I² = 64%) was evident between the different regimens included.
1.1. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 1: Dead or dependent at end of follow‐up (if > 1 month)
Subgroup analyses
By type of anticoagulant agent used
There were no significant differences in death or dependence between subgroups of different anticoagulant agents (P = 0.12; moderate‐certainty evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 10: Subgroup analysis by type of anticoagulant agent used: effect on death or dependency
By anticoagulant dose
There was no statistically significant difference in death or dependence at final follow‐up between trials (P = 0.65; moderate‐certainty evidence; Analysis 1.11).
1.11. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 11: Subgroup analysis by anticoagulant dose: effect on death or dependency
Sensitivity analyses
Sensitivity analyses were restricted to:
trials in which the method of randomisation ensured adequate concealment of treatment allocation, which showed that all trials evaluating death and dependence at final follow‐up had adequate concealment of the randomisation process (Analysis 1.12);
trials that restricted entry to the study to within 48 hours of stroke onset, which showed that all trials evaluating death or dependence at final follow‐up enrolled participants within 48 hours of stroke onset (Analysis 1.13). Within IST 1997, there was no evidence that the effect of treatment was increased or decreased with increasing delay to randomisation up to 48 hours;
trials other than IST 1997 (since it contained most of the data important for the review), which showed an apparent difference in effects of treatment on death or dependence at final follow‐up if data from IST were included (OR 0.98, 95% CI 0.93 to 1.03; P = 0.41; 11 RCTs, 22,368 participants; high‐certainty evidence; Analysis 1.1)) or excluded (OR 0.82, 95% CI.70 to 0.96; P = 0.01; 10 RCTs, 2933 participants; moderate‐certainty evidence; Analysis 1.14); and
a post‐hoc sensitivity analysis to assess the impact of duration of follow‐up on the estimate of effect for the primary outcome, with exclusion of the trial with assessment of the primary outcome after only one month, which had no impact on overall estimate of effect (OR 0.98, 95% CI 0.92 to 1.03; P = 0.42; 11 RCTs, 22,371 participants; high‐certainty evidence; Analysis 1.15) nor on degree of heterogeneity (I² = 65%) (Cazzato 1989).
1.12. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 12: Sensitivity analysis: dead or dependent at end of follow‐up (if > 1 month) in trials with adequate concealment of treatment allocation
1.13. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 13: Sensitivity analysis: dead or dependent at end of follow‐up (if > 1 month) for patients within 48 hours of stroke onset
1.14. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 14: Sensitivity analysis: dead or dependent at end of follow‐up (if > 1 month) excluding IST3 trial
1.15. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 15: Sensitivity analysis: dead or dependent at end of follow‐up (if > 1 month) excluding trial with assessment of primary outcome after only 1 month
Outcome 1.2: Death from all causes during the scheduled treatment period
Data from 22 trials, which included randomised data from 22,602 participants (94.3% of participants included in the review), were available for this outcome. Anticoagulants were not associated with a significant reduction in death at the end of the treatment period (OR 0.99, 95% CI 0.90 to 1.09; P = 0.88; 22 RCTs, 22,602 participants; low‐certainty evidence; Analysis 1.2). There was no significant heterogeneity (I² = 16%).
1.2. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 2: Death from all causes during treatment period
Outcome 1.3: Death from all causes at final follow‐up more than one month after randomisation
Data were available for 15 trials, which included 23,079 participants (96.1 % of participants included in the overall review). Anticoagulants were not associated with any significant reduction in the odds of death at final follow‐up greater than one month (OR 1.05, 95% CI 0.98 to 1.12; P = 0.17; I² = 15%; 15 RCTs, 23,079 participants; moderate‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 3: Death from all causes at final follow‐up (if > 1 month)
Outcome 1.4: Deep vein thrombosis during the treatment period
Ten trials, which included randomised data from 916 participants (only 3.8% of participants included in the overall review), sought to systematically determine the effect of anticoagulants on occurrence of 'symptomatic or asymptomatic deep vein thrombosis' at the end of the treatment period, as detected by:
I‐125 fibrinogen scanning (Duke 1983; Elias 1990; McCarthy 1977; McCarthy 1986; Pince 1981; Prins 1989; Turpie 1987);
B‐mode and Doppler ultrasound (Pambianco 1995); or
X‐ray contrast venography (Sandset 1990; Vissinger 1995).
Despite the small numbers of participants studied, anticoagulation was associated with a highly significant reduction in the odds of deep vein thrombosis (OR 0.21, 95% CI 0.15 to 0.29; P < 0.00001; 10 RCTs, 916 participants; low‐certainty evidence; Analysis 1.4), although a majority of deep vein thromboses detected were subclinical and asymptomatic.
1.4. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 4: Deep vein thrombosis during treatment period
There was substantial heterogeneity between trial results (I² = 72%), which appeared to be due to three trials that did not show any clear effect of anticoagulation on the odds of deep vein thrombosis (Pambianco 1995; Sandset 1990; Vissinger 1995), as well as two trials that did (Elias 1990; McCarthy 1986). The three negative trials were the only ones that did not use I‐125 fibrinogen scanning. One used ultrasound assessment (Pambianco 1995), and the other two used venography (Sandset 1990; Vissinger 1995). In addition, in one of these trials, participants were randomised up to 14 days after their initial stroke (Pambianco 1995), whereas all other trials randomised participants within seven days. The two most positive trials had very small numbers of participants, with the resultant possibility that the results may have been due to chance. In addition, McCarthy 1986 (the most positive trial) was poorly concealed, introducing another potential source of bias.
Sensitivity analyses showed there was no significant difference in the reduction in deep vein thrombosis from the above result if the analysis was restricted to trials in which concealment of allocation was secure (OR 0.32, 95% CI 0.15 to 0.68; I² = 53%; P = 0.003; 3 RCTs, 282 participants; low‐certainty evidence; Analysis 1.16) or trials in which radiographic assessment was blinded (OR 0.44, 95% CI 0.27 to 0.72; I² = 51%; P = 0.0007; 6 RCTs, 499 participants; low‐certainty evidence; Analysis 1.17). One of the trials excluded from this review did provide data on the numbers of deep vein thromboses in participants by allocated treatment group (1/19 heparin, 3/27 placebo), but inclusion of these results did not significantly alter the analysis (Dahan 1986). No trials systematically sought to assess deep vein thrombosis after completion of treatment.
1.16. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 16: Sensitivity analysis: deep vein thrombosis during treatment period restricted to trials where concealment of allocation was secure
1.17. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 17: Sensitivity analysis: deep vein thrombosis during treatment period restricted to trials where radiographic assessment was blinded
Outcome 1.5: Recurrent ischaemic stroke or recurrent stroke of unknown pathological type during the treatment period
Twelve trials, which included 21,665 participants (90.2% of participants included in the overall review), systematically sought to record early recurrent strokes that were definitely ischaemic (CT scan excluded haemorrhage) or probably ischaemic, that is, in which the cerebral pathology was unknown because a CT scan had not been performed. Anticoagulation was associated with a statistically significant reduction in recurrent ischaemic stroke (OR 0.75, 95% CI 0.65 to 0.88; P =0.0003; 12 RCTs, 21,605 participants; moderate‐certainty evidence; I² = 0, Analysis 1.5). Most data (95%) were obtained from IST 1997.
1.5. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 5: Recurrent ischaemic or unknown stroke during treatment period
Outcome 1.6: Symptomatic intracranial haemorrhage during the treatment period
Twenty trials, which included randomised data from 23,221 participants (96.7% of participants included in the overall review), reported data on symptomatic (fatal and non‐fatal) intracranial haemorrhage confirmed by CT scanning or autopsy. Early anticoagulation significantly increased symptomatic intracranial haemorrhage more than twofold (OR 2.47, 95% CI 1.90 to 3.21; P < 0.00001; 20 RCTs, 23,221 participants; high‐certainty evidence; I² = 0, Analysis 1.6). Most data (76%) were contributed by IST 1997.
1.6. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 6: Symptomatic intracranial haemorrhage during treatment period
No significant heterogeneity was apparent in the excess of haemorrhage with different types of heparin. However, within IST 1997, intracranial haemorrhage significantly increased with increasing heparin dose. Participants allocated to avoid heparin, low‐dose heparin, and medium‐dose heparin had rates of intracranial haemorrhage of 0.3%, 0.7%, and 1.8%, respectively.
There is the possibility of some bias within these data, as there may have been a lower threshold for re‐scanning participants who had deteriorated clinically if they were known to be receiving anticoagulants (e.g. in IST 1997, which was not blinded). In addition, even in blinded trials, a physician is likely to be unblinded if bruising is observed at heparin injection sites. An unbiased assessment of the effect of anticoagulants on the occurrence of intracranial haemorrhage would come from systematic studies in which all participants undergo a CT scan before the start of treatment to exclude haemorrhage, and all survivors have a repeat CT scan at the end of the scheduled treatment period, regardless of their clinical status. In such an unbiased assessment, all participants who died during the study would have to undergo an autopsy. Unfortunately, it is rarely possible to achieve repeat CT scans in all survivors, or autopsies in all deaths. Five trials in this review made a systematic attempt to detect both symptomatic and asymptomatic intracranial haemorrhage in this way (ARGIS‐1 2004; CESG 1983; FISS 1995; Prins 1989; Sandset 1990). All of the confirmed intracranial haemorrhages were intracerebral. In FISS 1995, the use of systematic CT scanning was introduced during the trial, and so not all participants were eligible for this analysis. However, the numbers of participants and events in this analysis were small (symptomatic plus asymptomatic haemorrhages occurring in 20/266 participants (7.5%) allocated to anticoagulant versus 27/264 control participants (10.2%)), so the estimate of risk of 'symptomatic plus asymptomatic' haemorrhage is imprecise (OR 0.76, 95% CI 0.38 to 1.52; FISS 1995). In this trial, 25 participants (5% overall, 15 treated versus 10 control) did not have a repeat CT scan or autopsy (FISS 1995). Including these 25 participants in hypothetical best‐ and worst‐case analyses changed the odds ratio significantly (OR 0.44 and 1.44 respectively), which suggests that the results are compatible with substantial reductions or increases in the risk of 'symptomatic plus asymptomatic' intracranial haemorrhage with treatment.
Outcome 1.7: Any recurrent stroke or symptomatic intracranial haemorrhage during the treatment period and during long‐term follow‐up
Early anticoagulation reduces the odds of ischaemic stroke but also increases the odds of symptomatic intracranial haemorrhage. An outcome that combines these two (without double counting ‐ i.e. each participant is counted only once, even if both events occurred, with the first event being the one that is included) is useful for assessing the net short‐term effects of anticoagulants. Twelve trials, which included randomised data from 21,665 participants (90.2% of participants included in the overall review), evaluated the occurrence of 'any recurrent stroke or symptomatic intracranial haemorrhage' during the treatment period. Anticoagulation was not associated with a net reduction in the odds of this outcome (OR 0.97, 95% CI 0.84 to 1.11; P = 0.64; 12 RCTs, 21,665 participants; moderate‐certainty evidence; I² = 28%; Analysis 1.7). Most of the data (93.6%) were obtained from IST 1997. An analysis of recurrent stroke or intracranial haemorrhage during the follow‐up period could only include data from three small studies (FISS 1995; Marshall 1960; Turpie 1987). Events were far too few for a reliable analysis.
1.7. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 7: Any recurrent stroke or symptomatic intracranial haemorrhage during treatment period or follow‐up (> 1 month)
Outcome 1.8: Symptomatic pulmonary embolism during the treatment period
Fourteen trials, which included data from 22,544 participants (95.7% of participants included in the overall review), assessed reported fatal and non‐fatal symptomatic pulmonary embolism, but no trial had systematically sought asymptomatic pulmonary embolism by performing ventilation‐perfusion scans in all participants at the end of the treatment period. Anticoagulation was associated with a significant reduction in the odds of pulmonary embolism (OR 0.60, 95% CI 0.44 to 0.81; P = 0.0009; 14 RCTs, 22,544 participants; moderate‐certainty evidence; I² = 13.7%; Analysis 1.8).
1.8. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 8: Symptomatic pulmonary embolism during treatment period
In the trials described, the frequency of pulmonary embolism during the treatment period was variable but was quite low (1% in IST 1997 versus 7% in Elias 1990 and Prins 1989). Although not reported systematically, and thereby potentially under‐reported, the rate of pulmonary embolism in IST 1997 among participants not receiving heparin was only 0.8%. This observation is supported by data from prospective hospital‐based studies that have reported symptomatic pulmonary embolism as a complication in between 1% and 3% of patients with acute stroke (Davenport 1996).
Patients may continue to be at risk of pulmonary embolism after the early treatment period. This was suggested by data from three trials that continued to seek events systematically during the follow‐up period (FISS 1995; TOAST 1998; Turpie 1987). Eight further pulmonary emboli were recorded, six of which occurred in the control group. The potential use of antiplatelet or anticoagulant agents after the trial period may have influenced the results of several trials (FISS 1995; TOAST 1998; Turpie 1987). One trial, with an 80% autopsy rate, did show a significant reduction in the risk of symptomatic and asymptomatic pulmonary embolism detected at autopsy in the anticoagulation group (7/24 versus 33/47; OR 0.19, 95% CI 0.07 to 0.52) (McCarthy 1986).
Outcome 1.9: Major extracranial haemorrhage during the treatment period
Eighteen trials, which included randomised data from 22,255 participants (93.7 % of participants included in the overall review), reported data on major extracranial haemorrhage (defined as bleeding serious enough to cause death or to require hospitalisation or transfusion). Anticoagulation was associated with a significant threefold increase in major extracranial haemorrhage (OR 2.99, 95% CI 2.24 to 3.99; P < 0.00001; 18 RCTs, 22,255 participants; moderate‐certainty evidence; I² = 4%; Analysis 1.9).
1.9. Analysis.

Comparison 1: Anticoagulant vs control in acute presumed ischaemic stroke, Outcome 9: Major extracranial haemorrhage during treatment period
Publication bias
To determine whether or not we might have missed an important number of small negative trials (these are the trials most likely to remain unpublished), we undertook a funnel plot analysis (Egger 1997). Analyses with the greatest number of trials included (and hence the greatest statistical power) examined effects of treatment on death during the treatment period (Figure 4), death from all causes at final follow‐up (Figure 5), and deep vein thrombosis (Figure 6). For these outcomes, a plot of the sample size for each trial versus the odds ratio for that trial showed an approximate 'funnel distribution' with 'tails' in both positive and negative treatment effect directions (except for the outcome of deep vein thrombosis), indicating that we were unlikely to have missed a substantial number of negative trials.
4.

5.

6.

Discussion
The evidence provided in this updated systematic review has not changed any of the conclusions of the previous review published in 2015, and can be summarised as follows.
Summary of main results
Net effect of early anticoagulants in acute ischaemic stroke
Acute stroke treatment should aim to prevent disability as well as death, lest patients survive their acute stroke only to remain severely disabled. Currently available evidence from randomised trials indicates that routine early anticoagulation does not provide any significant net short‐term or long‐term reduction in death or disability. Although early anticoagulation leads to fewer recurrent ischaemic strokes, this benefit is entirely offset by a similarly sized increase in intracranial haemorrhage. The net result is no short‐term or long‐term benefit.
Hazards of early anticoagulants in acute ischaemic stroke
To be useful, a medical therapy must be safe. Current evidence from randomised trials demonstrates a clinically and statistically significant risk of major intracranial and extracranial haemorrhage with early use of anticoagulants for people with acute ischaemic stroke.
Different anticoagulant agents ‐ doses and routes of administration
Low‐molecular‐weight heparin and unfractionated intravenous heparin are not effective in reducing the risk of death or dependency, nor death, during the treatment period, or after follow‐up greater than one month. Other types of agents of unfractionated subcutaneous heparin, heparinoids, and specific thrombin inhibitors have shown no significant net benefit in terms of reducing death during the treatment period, nor death or dependency after follow‐up greater than one month. Direct comparisons of different anticoagulants show no clear benefit of heparinoids versus unfractionated heparin (Sandercock 2008). Available evidence does not support the routine use of adjusted‐dose intravenous heparin (or heparinoid) regimens, or of more intensive fixed‐dose regimens.
Prevention of deep vein thrombosis and pulmonary embolism in acute ischaemic stroke with anticoagulants
For participants with presumed or confirmed ischaemic stroke, allocation to early anticoagulation was associated with a highly significant 79% reduction in the odds of deep vein thrombosis during the treatment period ‐ similar to that seen with the use of prophylactic heparin for people undergoing different types of surgery (Collins 1988). In this review, the reductions in deep vein thrombosis with acute anticoagulation were statistically significant, although this estimate is based on relatively small numbers of participants, and most of the deep vein thromboses detected were asymptomatic. Heterogeneity in the effects of treatment is significant, which renders the overall estimate less reliable.
The clinical significance of this reduction depends critically on the control event rate. In included studies, there was substantial variation in the control event rate, from 13% in Vissinger 1995 to 73% in McCarthy 1986. The rate will depend on many factors such as severity of stroke, presence of leg paralysis, and history of previous deep vein thrombosis (Warlow 2008). The odds of pulmonary embolism were reduced significantly (by 40%) with the use of anticoagulants. In the included trials, pulmonary embolism was uncommon, so assuming the 0.8% control rate seen in the largest trial with the most representative sample of participants (IST 1997), the number needed to treat to benefit (NNTB) was 315, although with a perhaps more realistic control event rate of 2% it would be 127. The overall risk of pulmonary embolism appeared to be low, and the absolute benefit was small, so the apparent reduction in deep vein thrombosis may have little clinical relevance if there is not a correspondingly large reduction in pulmonary embolism. However, there may well have been under‐ascertainment of pulmonary embolism in all trials, given that data on pulmonary emboli were not sought systematically. In addition, deep vein thrombosis can lead to morbidity (such as post‐phlebitic leg and varicose ulcers), but data on these outcomes were not available from the included trials. Finally, it is possible that once anticoagulants are stopped, rebound thrombosis could occur, and deep vein thromboses may begin to develop. We were unable to exclude this possibility because no trials sought data on deep vein thrombosis systematically after the end of the treatment period.
If anticoagulants result in no net increase or decrease in long‐term death or disability but do lead to a reduction in the numbers of deep vein thromboses and pulmonary emboli (albeit in immobile patients at higher risk), the benefit of fixed heparin regimens associated with low risk of bleeding (e.g. low fixed‐dose unfractionated heparin, low‐molecular weight heparin) may yet outweigh the increased risk of haemorrhage. A low deep vein thrombosis risk reduces the justification for unselective thromboprophylaxis with heparin. In IST 1997, the frequency of fatal and non‐fatal symptomatic pulmonary embolism (perhaps a surrogate for the occurrence of deep vein thrombosis) was very similar among participants allocated to low‐dose subcutaneous heparin alone (0.8%) and aspirin alone (0.7%). Aspirin alone may therefore be an adequate antithrombotic agent to be used for routine deep vein thrombosis prophylaxis in some people with acute ischaemic stroke, as antiplatelet drugs, when used for prophylaxis of deep vein thrombosis and pulmonary embolism prophylaxis in other categories of high‐risk patients are of modest benefit (ATC 2002). The CLOTS‐3 2013 study demonstrated that for people with ischaemic and haemorrhagic stroke, intermittent pneumatic compression reduces the risk of deep vein thrombosis after stroke, without increased bleeding risk, and is effective in the presence and in the absence of background heparin therapy.
Effects on deep vein thrombosis and pulmonary embolism presented in the present review indicate there might be a net benefit of low‐dose heparin regimens among patients who are at high risk of venous thromboembolism but at relatively low risk of intracranial or major extracranial bleeding. PREVAIL 2007 illustrates some of the difficulty involved in identifying subgroups who might have a favourable balance of risk and benefit. Although the risk of venous thromboembolism (VTE) (symptomatic plus asymptomatic) was significantly lower among participants allocated to enoxaparin, this study could not exclude a 69% increase in risk of death up to Day 14 and a 134% increase in risk of intracranial haemorrhage with enoxaparin. This is so because event rates for the more major clinical outcomes were low: among participants allocated unfractionated heparin, pulmonary embolism occurred in 1% and intracerebral haemorrhage in 1%. With such low event rates, conducting randomised trials large enough to reliably determine the balance of risk and benefit for these major clinical outcomes is challenging. In conclusion, data from the present review were insufficient to reliably identify a subgroup that might benefit from use of heparin for thromboprophylaxis.
Overall completeness and applicability of evidence
This systematic review provides information about the use of anticoagulants for unselected people with ischaemic stroke, as well as limited information about various subgroups. Given that many of the trials were conducted more than 20 years ago, are these results still relevant to clinical practice in the 21st century? In the sense that they represent the totality of evidence comparing treatment with control, they remain relevant to current practice in many parts of the world and continue to be cited in stroke treatment guidelines. The pattern of background treatment has changed, with many patients now treated within organised stroke units (in the developed world at least). This might have an impact on the absolute risks and benefit of anticoagulation but is less likely to influence the estimates of relative effect, hence ‐ in our view ‐ the results remain relevant. Anticoagulants are also sometimes advocated for the treatment of acute carotid dissection and cerebral venous thrombosis. Separate Cochrane Reviews have been prepared for these topics (Coutinho 2011; Lyrer 2010). We were reluctant to pursue further subgroup analysis because it is hazardous to explore subgroup effects when there is no significant overall effect of an intervention on major outcomes, and we had access only to major outcomes. A more detailed assessment of the effects of anticoagulants for other categories of patients (e.g. patients treated within three hours, patients with large‐artery strokes, patients with carotid stenosis) would be possible with an individual patient data meta‐analysis.
Stroke patients included in trials of anticoagulants to prevent deep vein thrombosis generally had quite severe strokes, and paralysis of one leg (with attendant high risk of deep vein thrombosis) was almost invariably present at randomisation. If, however, one accepts the estimate of treatment effect from these trials in the 1980s and 1990s, it is difficult to assess the extent to which it may be generalisable to clinical practice from 2000 onwards. In current practice, the risk of deep vein thrombosis may well be low because many patients are admitted to stroke units, receive aspirin, maintain good hydration, and are mobilised early.
With that qualification in mind, the small quantities of (randomised) subgroup data evaluated here do not provide any evidence to support the routine use of anticoagulants in stroke patients of any specific category.
Quality of the evidence
The bulk of the evidence in this review comes from trials with adequate allocation concealment, which is a strength. However, IST 1997 ‐ by far the largest trial ‐ was unblinded ‐ which may well have led to ascertainment bias, especially for early outcomes. The final outcome, however, was assessed at a time when patients could not accurately recall their treatment allocation, so in all likelihood, assessment of the primary outcome probably was not materially biased.
Potential biases in the review process
This review is based on an analysis of tabular data, which limits the extent to which effects in subgroups can be explored. However, an individual patient data meta‐analysis has been performed. Included trials measured the primary outcome at differing times after randomisation, ranging from one to six months after randomisation, as we did not have access to individual patient level data to calculate person‐days or hazard ratios. In considering the impact of differing lengths of follow‐up on the primary outcome, a post‐hoc sensitivity analysis excluding the study with less than three months of follow‐up did not alter the overall estimate of effect nor reduce heterogeneity.
Agreements and disagreements with other studies or reviews
Lederle 2011 is a systematic review of venous thromboembolism prophylaxis in hospitalised medical patients and those with stroke, and review authors concluded, "Heparin prophylaxis had no significant effect on mortality, may have reduced PE (pulmonary embolism) in medical patients and all patients combined, and led to more bleeding and major bleeding events, thus resulting in little or no net benefit. No differences in benefits or harms were found according to type of heparin used." Similarly, there was no clear difference in effects between people with stroke and with other non‐surgical causes for hospital admission. A number of guideline statements have since been developed; the most recent American Stroke Association Guidelines include two specific recommendations that are supported by the evidence provided in this systematic review (AHA Guidelines 2019). These are as follows.
Urgent anticoagulation, with the goal of preventing early recurrent stroke, halting neurological worsening, or improving outcomes after acute ischaemic stroke, is not recommended for treatment of patients with acute ischaemic stroke.
At present, the usefulness of argatroban or other thrombin inhibitors for treatment of patients with acute ischaemic stroke is not well established.
An individual patient data meta‐analysis of the large trials of heparin in acute ischaemic stroke was able to explore subgroup effects in greater detail, with the aim of identifying the subgroup of patients most likely to derive net benefit from heparin. However, the review authors concluded, "There was no evidence that patients with ischaemic stroke who were at higher risk of thrombotic events or lower risk of haemorrhagic events benefited from heparins. We were, therefore, unable to define a targeted approach to select the patients who would benefit from treatment with early anticoagulant therapy" (Whiteley 2013).
Authors' conclusions
Implications for practice.
Evidence from this systematic review indicates that, compared with control, the types of anticoagulants tested in people with acute ischaemic stroke have no effect in terms of death in the short term, or death or dependency after follow‐up of at least one month. A reduction in recurrent ischaemic stroke during the treatment period is exactly offset by an increase in intracranial haemorrhage. Although anticoagulants decrease deep vein thrombosis and pulmonary embolus, these benefits are once again offset by similarly sized increases in extracranial haemorrhage.
Data do not support the routine use of early high‐dose intravenous or subcutaneous anticoagulants in any form for people with acute ischaemic stroke. Low‐dose subcutaneous regimens will prevent deep vein thrombosis, but with a small but definitely increased risk of major haemorrhage. Therefore, it may be advisable to consider safer alternatives for immobile patients (such as aspirin, pneumatic compression stockings, or early mobilisation).
The data reviewed from trials comparing these agents with control do not support the use of low‐molecular‐weight heparins, heparinoids, or thrombin inhibitors for the treatment of acute ischaemic stroke.
The analysis performed did not identify any category of patients who derived a clear net benefit. Clinicians who feel compelled to use early anticoagulants for specific categories of patients following acute ischaemic stroke should weigh any potential theoretical benefits versus the known risk of bleeding. Evidence of benefit and safety favors aspirin as an effective antithrombotic alternative to anticoagulation when used in the acute phase of ischaemic stroke.
Implications for research.
This review has not provided reliable evidence on a number of important categories of patients with acute cerebrovascular disease who might plausibly derive net benefit from early anticoagulation (very recent transient ischaemic attacks (within hours or days of onset), crescendo transient ischaemic attacks, and progressing ischaemic stroke are a few examples), and further trials targeted at these groups (perhaps with new agents) may be warranted. The choice of comparator agent against which to test any anticoagulant will depend on a number of factors, but further trials comparing anticoagulants against control seem unlikely.
Clinicians who wish to continue to use intensive intravenous dose‐adjusted heparin regimens routinely to treat specific categories of stroke patients should provide convincing evidence from new randomised controlled trials to support such practices.
This review has not provided clear evidence about the optimum antithrombotic regimen for prevention of deep vein thrombosis and pulmonary embolism in stroke patients. Aspirin alone, low‐dose subcutaneous heparin, and use of an intermittent pneumatic compression device are all promising possibilities, but a very large‐scale randomised trial with several tens of thousands of participants would be required to determine which treatment (or which combination) offers the most favourable balance of risk and benefit for overall clinical outcomes.
Feedback
Conclusions too weak, June 2007
Summary
This review of anticoagulant trials, including over 22,000 patients with acute ischaemic stroke, found no net benefit with the use of any anticoagulant. Consequently, the implications for practice and the implications for research are too timid. The implications for practice should say that anticoagulants should be contraindicated in patients with acute ischaemic stroke. The implications for research should say that further trials of anticoagulants in acute ischaemic stroke would be unethical.
Reply
This comment was submitted before the 2008 update was performed. The wording of the Authors' conclusions section has been modified to be more in keeping with this comment. Note: the response to this feedback was delayed by a number of unavoidable administrative factors.
Contributors
Commenter: David A Cundiff, MD Reply: Peter Sandercock
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2021 | Amended | Amendment to text of the plain language summary to improve clarity |
History
Protocol first published: Issue 1, 1995 Review first published: Issue 1, 1995
| Date | Event | Description |
|---|---|---|
| 22 October 2021 | New citation required but conclusions have not changed | Results and conclusions are unchanged |
| 22 October 2021 | New search has been performed | We have searched the literature for new relevant studies to April 2020. We have identified 4 new studies for inclusion. The review now includes 28 studies, involving 24,025 participants. We have reformatted and updated the text throughout. |
| 9 January 2009 | Feedback has been incorporated | Feedback on the previous version of this review had not been incorporated. This omission has now been rectified in this small revision to the 2008 update. |
| 7 November 2008 | Amended | Duplicate text in 'Updated' event of 11 January 2008 has been deleted. |
| 17 March 2008 | Amended | Converted to new review format. |
| 11 January 2008 | New citation required but conclusions have not changed | New co‐author: Ayeesha Kamal has replaced Gordon Gubitz. |
| 11 January 2008 | New search has been performed | The searches have been updated to October 2007. Two trials (ARGIS‐1 2004; Chaudhary 2002), with 201 participants, were included in this update, bringing the total number of trials to 24 involving 23,547 participants. The text has been extensively revised and updated. |
Acknowledgements
We thank Emiliana Tonini for 'Risk of bias' assessments for the two French papers.
We thank individual trialists for supplying additional information: Professor B Boneu for providing us with a copy of Dr Pince's thesis; Dr Duke and Dr R Kay (FISS 1995); Dr G Pambianco and Dr H Magnani from Organon International for supplying additional information on the trial using danaparoid (Turpie 1987); Dr L Antonutti and Dr M Zorzon (Cazzato 1989); Dr Ewa Lindenstrom from Leo Pharmaceuticals for data on tinzaparin (Vissinger 1995); Dr M Lamonte who provided additional data on the ARGIS study (ARGIS‐1 2004); H Willems who helped identify many of the early trials; Dr E Dick for supplying us with a copy of the MedStrategy document "Stroke: A focus on opportunity"; Dr AGM van den Belt and Dr RI Lindley who helped produce the previously published version of this review (Sandercock 1993); and Josh Cheyne for supplying regular lists of trials identified by the Cochrane Stroke Group's search strategy. David Signorini was a co‐author of an earlier version of the review. Dr Gord Gubitz contributed to earlier versions of this review. We also acknowledge the help given by the secretariat of the Antithrombotic Trialists' Collaboration (Dr C Baigent, Dr C Sudlow). We acknowledge Brenda Thomas, Steff Lewis, and Hazel Fraser of the Cochrane Stroke Group for their input and expertise in completing the update of this review.
We acknowledge Stella O’Brien and Maria Isabel Costa for reviewing the manuscript as consumers.
Ongoing trials
Anyone who knows of additional trials that we have omitted, please write to Dr Jie Yang.
Appendices
Appendix 1. Cochrane Library Databases (CENTRAL, CDSR, DARE, HTA)
#1 [mh ^"cerebrovascular disorders"] or [mh ^"basal ganglia cerebrovascular disease"] or [mh ^"brain ischemia"] or [mh "brain infarction"] or [mh ^"hypoxia‐ischemia, brain"] or [mh ^"carotid artery diseases"] or [mh ^"carotid artery thrombosis"] or [mh ^"carotid artery, internal, dissection"] or [mh ^"intracranial arterial diseases"] or [mh ^"cerebral arterial diseases"] or [mh ^"infarction, anterior cerebral artery"] or [mh ^"infarction, middle cerebral artery"] or [mh ^"infarction, posterior cerebral artery"] or [mh "intracranial embolism and thrombosis"] or [mh stroke] or [mh ^"vertebral artery dissection"] #2 isch*mi* near/5 (stroke* or apoplex* or cerebral next vasc* or cerebrovasc* or cva):ti,ab #3 (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle next cerebr* or mca* or "anterior circulation" or "basilar artery" or "vertebral artery") near/5 (isch*mi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*):ti,ab #4 #1 or #2 or #3 #5 [mh anticoagulants] #6 [mh "Blood coagulation factors"/AI,DE] or [mh "Blood coagulation"/AI,DE] #7 (anticoagul* or antithromb*):ti,ab #8 [mh ^Warfarin] or [mh ^4‐hydroxycoumarins] or [mh ^acenocoumarol] or [mh ^coumarins] or [mh ^dicumarol] or [mh ^"ethyl biscoumacetate"] or [mh ^phenindione] or [mh ^phenprocoumon] #9 [mh "Vitamin K"/AI] #10 (warfarin* or coumadin* or coumarin* or cumarin* or phenprocoum* or phenprocum* or dicoumar* or dicumar* or acenocoumar* or acenocumar* or fluindione or phenindione or clorindione or diphenadione or "ethyl biscoumacetate"):ti,ab #11 (Vitamin next K next antagonist* or VKA or VKAs or "antivitamin K"):ti,ab #12 [mh antithrombins] or [mh ^"hirudin therapy"] or [mh ^thrombin/AI] #13 ((direct* near/5 thrombin near/5 inhib*) or DTI or DTIs):ti,ab #14 (argatroban or MD805 or "MD‐805" or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin* or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil):ti,ab #15 [mh ^"factor Xa"/AI] #16 (("factor Xa" or "factor 10a" or fXa or "autoprothrombin c" or thrombokinase) near/5 inhib*):ti,ab #17 (activated near/5 ("factor X" or "factor 10") near/5 inhib*):ti,ab #18 xabans:ti,ab #19 (antistasin or apixaban or betrixaban or "du 176b" or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or "ym 150" or ym150 or LY517717 or darexaban or edoxaban or SSR126517E or fidexaban or idrabiotaparinux or letaxaban or tanogitran or taxexaban):ti,ab #20 [mh ^heparin] or [mh "heparin, low‐molecular‐weight"] or [mh ^heparinoids] #21 (heparin* or lmwh* or enoxaparin* or glycosaminoglycan* or nadroparin* or mesoglycan* or tedelparin* or certoparin or tinzaparin or parnaparin or dalteparin or reviparin or fraxiparin* or danaparoid or lomoparan or "org 10172" or mesoglycan or pentosan next polysul* or sp54 or "sp‐54" or cy222 or "cy‐222" or cy216 or "cy‐216" or dermatan next sul* or heparan next sul*):ti,ab #22 {or #5‐#21} #23 #4 and #22 #24 atrial fibrillation:ti #25 #23 not #24
Appendix 2. MEDLINE (Ovid)
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or exp brain infarction/ or hypoxia‐ischemia, brain/ or carotid artery diseases/ or carotid artery thrombosis/ or carotid artery, internal, dissection/ or intracranial arterial diseases/ or cerebral arterial diseases/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or exp "intracranial embolism and thrombosis"/ or exp stroke/ or vertebral artery dissection/ 2. (isch?emi$ adj5 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. exp anticoagulants/ 6. exp Blood coagulation factors/ai, de or exp Blood coagulation/ai, de 7. (anticoagul$ or antithromb$).tw. 8. Warfarin/ or 4‐hydroxycoumarins/ or acenocoumarol/ or coumarins/ or dicumarol/ or ethyl biscoumacetate/ or phenindione/ or phenprocoumon/ 9. exp Vitamin K/ai 10. (warfarin$ or coumadin$ or coumarin$ or cumarin$ or phenprocoum$ or phenprocum$ or dicoumar$ or dicumar$ or acenocoumar$ or acenocumar$ or fluindione or phenindione or clorindione or diphenadione or ethyl biscoumacetate).tw,nm. 11. (Vitamin K antagonist$ or VKA or VKAs or antivitamin K).tw. 12. exp antithrombins/ or hirudin therapy/ or thrombin/ai 13. ((direct$ adj5 thrombin adj5 inhib$) or DTI$1).tw. 14. (argatroban or MD805 or MD‐805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).tw,nm. 15. factor Xa/ai 16. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj5 inhib$).tw. 17. (activated adj5 (factor X or factor 10) adj5 inhib$).tw. 18. xabans.tw. 19. (antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717 or darexaban or edoxaban or SSR126517E or fidexaban or idrabiotaparinux or letaxaban or tanogitran or taxexaban).tw,nm. 20. heparin/ or exp heparin, low‐molecular‐weight/ or heparinoids/ 21. (heparin$ or lmwh$ or enoxaparin$ or glycosaminoglycan$ or nadroparin$ or mesoglycan$ or tedelparin$ or certoparin or tinzaparin or parnaparin or dalteparin or reviparin or fraxiparin$ or danaparoid or lomoparan or org 10172 or mesoglycan or pentosan polysul$ or sp54 or sp‐54 or cy222 or cy‐222 or cy216 or cy‐216 or dermatan sul$ or heparan sul$).tw,nm. 22. or/5‐21 23. Randomized Controlled Trials as Topic/ 24. random allocation/ 25. Controlled Clinical Trials as Topic/ 26. control groups/ 27. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 28. double‐blind method/ 29. single‐blind method/ 30. Placebos/ 31. placebo effect/ 32. Research Design/ 33. randomized controlled trial.pt. 34. controlled clinical trial.pt. 35. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 36. (random$ or RCT or RCTs).tw. 37. (controlled adj5 (trial$ or stud$)).tw. 38. (clinical$ adj5 trial$).tw. 39. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 40. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 41. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 42. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 43. (placebo$ or sham).tw. 44. trial.ti. 45. (assign$ or allocat$).tw. 46. controls.tw. 47. or/23‐46 48. 4 and 22 and 47 49. exp animals/ not humans.sh. 50. 48 not 49 51. atrial fibrillation.ti. 52. 50 not 51
Appendix 3. Embase (Ovid)
1. brain infarction/ or brain stem infarction/ or cerebellum infarction/ or exp brain ischemia/ or carotid artery disease/ or exp carotid artery obstruction/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp occlusive cerebrovascular disease/ or stroke patient/ 2. (isch?emi$ adj5 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. exp anticoagulant agent/ 6. anticoagul$.tw. 7. antithromb$.tw. 8. coumarin derivative/ or 4 hydroxycoumarin/ or 4 hydroxycoumarin derivative/ or acenocoumarol/ or coumarin/ or dicoumarol/ or ethyl biscoumacetate/ or phenprocoumon/ or warfarin/ or phenindione/ 9. antivitamin k/ 10. (warfarin$ or coumadin$ or coumarin$ or cumarin$ or phenprocoum$ or phenprocum$ or dicoumar$ or dicumar$ or acenocoumar$ or acenocumar$ or fluindione or phenindione or clorindione or diphenadione or ethyl biscoumacetate).tw. 11. (Vitamin K antagonist$ or VKA or VKAs or antivitamin K).tw. 12. exp thrombin inhibitor/ 13. ((direct$ adj5 thrombin adj5 inhib$) or DTI$1).tw. 14. (argatroban or MD805 or MD‐805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).tw. 15. blood clotting factor 10a inhibitor/ 16. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj5 inhib$).tw. 17. (activated adj5 (factor X or factor 10) adj5 inhib$).tw. 18. xabans.tw. 19. (antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717 or darexaban or edoxaban or SSR126517E or fidexaban or idrabiotaparinux or letaxaban or tanogitran or taxexaban).tw. 20. heparin derivative/ or heparin/ or heparinoid/ or exp low molecular weight heparin/ 21. (heparin$ or lmwh$ or enoxaparin$ or glycosaminoglycan$ or nadroparin$ or mesoglycan$ or tedelparin$ or certoparin or tinzaparin or parnaparin or dalteparin or reviparin or fraxiparin$ or danaparoid or lomoparan or org 10172 or mesoglycan or pentosan polysul$ or sp54 or sp‐54 or cy222 or cy‐222 or cy216 or cy‐216 or dermatan sul$ or heparan sul$).tw. 22. or/5‐21 23. Randomized Controlled Trial/ 24. Randomization/ 25. Controlled Study/ 26. control group/ 27. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/ 28. Double Blind Procedure/ 29. Single Blind Procedure/ or triple blind procedure/ 30. placebo/ 31. (random$ or RCT or RCTs).tw. 32. (controlled adj5 (trial$ or stud$)).tw. 33. (clinical$ adj5 trial$).tw. 34. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 35. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 36. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 37. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 38. placebo$.tw. 39. trial.ti. 40. (assign$ or allocat$).tw. 41. controls.tw. 42. or/23‐41 43. 4 and 22 and 42 44. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/) 45. 43 not 44 46. (atrial fibrillation or myocardial or coronary or cardiac or heart or renal or subarachnoid or arteritis or hypertens$ or aortic or cancer or pregnan$ or dementia or diabetes or sickle cell or aneurysm$ or cardiopulmonary or migrain$).ti. 47. 45 not 46
Appendix 4. Ongoing trials registries search
ClinicalTrials.gov "stroke" AND "anticoagulants" "stroke" AND "heparin" Internet Stroke Center Stroke Trials Registry "stroke" AND "anticoagulants" "stroke" AND "heparin" ISRCTN Registry "stroke" AND "anticoagulants" "stroke" AND "heparin"
Data and analyses
Comparison 1. Anticoagulant vs control in acute presumed ischaemic stroke.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Dead or dependent at end of follow‐up (if > 1 month) | 12 | 22428 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.92, 1.03] |
| 1.2 Death from all causes during treatment period | 22 | 22602 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.90, 1.09] |
| 1.3 Death from all causes at final follow‐up (if > 1 month) | 15 | 23079 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.98, 1.12] |
| 1.4 Deep vein thrombosis during treatment period | 10 | 916 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.15, 0.29] |
| 1.5 Recurrent ischaemic or unknown stroke during treatment period | 12 | 21665 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.65, 0.88] |
| 1.6 Symptomatic intracranial haemorrhage during treatment period | 20 | 23221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.47 [1.90, 3.21] |
| 1.7 Any recurrent stroke or symptomatic intracranial haemorrhage during treatment period or follow‐up (> 1 month) | 12 | 21665 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.84, 1.11] |
| 1.8 Symptomatic pulmonary embolism during treatment period | 14 | 22544 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.44, 0.81] |
| 1.9 Major extracranial haemorrhage during treatment period | 18 | 22255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.99 [2.24, 3.99] |
| 1.10 Subgroup analysis by type of anticoagulant agent used: effect on death or dependency | 12 | 22428 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.03] |
| 1.10.1 Unfractionated heparin (subcutaneous) versus control | 1 | 19435 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.06] |
| 1.10.2 Low‐molecular‐weight heparin versus control | 6 | 1328 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 1.10.3 Heparinoid (subcutaneous) versus control | 1 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.29] |
| 1.10.4 Heparinoid (intravenous) versus control | 2 | 1329 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 1.10.5 Direct thrombin inhibitor versus control (intravenous) | 3 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.55, 1.48] |
| 1.11 Subgroup analysis by anticoagulant dose: effect on death or dependency | 11 | 22339 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.93, 1.03] |
| 1.11.1 Adjusted full‐dose intravenous anticoagulant | 4 | 1522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 1.11.2 Medium fixed‐dose anticoagulant | 4 | 10361 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 1.11.3 Low fixed‐dose anticoagulant | 7 | 10456 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| 1.12 Sensitivity analysis: dead or dependent at end of follow‐up (if > 1 month) in trials with adequate concealment of treatment allocation | 6 | 21286 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.03] |
| 1.13 Sensitivity analysis: dead or dependent at end of follow‐up (if > 1 month) for patients within 48 hours of stroke onset | 11 | 22298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.89, 1.00] |
| 1.14 Sensitivity analysis: dead or dependent at end of follow‐up (if > 1 month) excluding IST3 trial | 11 | 2993 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.69, 0.95] |
| 1.15 Sensitivity analysis: dead or dependent at end of follow‐up (if > 1 month) excluding trial with assessment of primary outcome after only 1 month | 11 | 22371 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.03] |
| 1.16 Sensitivity analysis: deep vein thrombosis during treatment period restricted to trials where concealment of allocation was secure | 3 | 282 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.16, 0.71] |
| 1.17 Sensitivity analysis: deep vein thrombosis during treatment period restricted to trials where radiographic assessment was blinded | 6 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.27, 0.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ARGIS‐1 2004.
| Study characteristics | ||
| Methods | C: randomisation not described Patient, doctor, and assessments blinded Ex during trial: none Losses to FU: 5 participants | |
| Participants | North America 171 participants Mean age: 65 years 100% CT before entry Mild to moderate ischaemic stroke NIHSS 7 to 22 Less than 12 hours since onset | |
| Interventions | Rx: argatroban 100 mcg/kg bolus, followed by low‐dose arm 1 mcg/kg/min or high‐dose arm 3 mcg/kg/min for 5 days vs placebo Control: aspirin used in both arms | |
| Outcomes | Death Symptomatic ICH Major extracranial ICH Barthel Index, NIHSS, mRS at 90 days | |
| Notes | Ex: age > 85 BP: > 220/130 FU: 30 days, 90 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not clearly reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel and patients blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes apparently reported in the study |
ARTSS‐2 2017.
| Study characteristics | ||
| Methods | C: web‐based and stratified using sequential minimisation Open‐label trial, outcome assessor blinded Ex during trial: none Losses to FU: 1 participant | |
| Participants | USA and UK 90 randomised participants Mean age: 70 years, 44% female 100% CT or transcranial Doppler before entry Acute ischaemic stroke receiving IV rtPA within 4.5 hours of onset NIHSS ≥ 10 or any proximal intracranial arterial occlusion | |
| Interventions | Rx: low‐dose argatroban (infusion of 1.0 μg/kg per minute) + rtPA or high‐dose argatroban (infusion of 3.0 μg/kg per minute) + rtPA Control: standard dose rtPA alone Duration: 48 hours | |
| Outcomes | Efficacy: mRS score at day 90 Safety: sICH within 48 hours, parenchymal haematoma, haemorrhagic transformation, major systemic bleeding NIHSS neurological improvement Quality of life (EQ‐5D) Economic evaluation | |
| Notes | Ex: planned endovascular therapy FU: 90 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described that randomisation was web‐based and stratified using sequential minimisation methods |
| Allocation concealment (selection bias) | Low risk | Web‐based randomisation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Performance bias, because personnel were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neurologists not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At Day 90, 6/30, 6/31, 12/61 missing values (more than 20%), respectively, in 3 groups |
| Selective reporting (reporting bias) | High risk | All mentioned outcomes reported in the study |
Cazzato 1989.
| Study characteristics | ||
| Methods | C: random number table controlled by doctor not involved in treatment Outcome assessor blinded Ex during trial: none Losses to FU: none | |
| Participants | Italy 57 participants Mean age: 68 years 49% male 100% CT before entry Ischaemic stroke Less than 48 hours since stroke onset | |
| Interventions | Rx: mesoglycan (heparinoid) 50 mg 8‐hourly IM for 5 days, then 144 mg/day orally for 25 days Control: no treatment All participants received dexamethasone IV Duration: 30 days | |
| Outcomes | Death plus cause of death Functional outcome (OHS 3, 4, 5 = dependent) PE (symptomatic plus autopsy in 9/10 deaths) ICH (symptomatic CT) Recurrent stroke Major extracranial haemorrhage | |
| Notes | Ex: none FU: 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used for random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients/personnel not blinded and no report on how outcome was assessed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported if this study is blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detailed report on loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information given |
CESG 1983.
| Study characteristics | ||
| Methods | C: sealed envelopes (opaque and sequentially numbered) Non‐blinded Ex during trial: none Losses to FU: none | |
| Participants | North America 45 participants Age range: 42 to 83 years 58% male 100% CT before entry Cardioembolic stroke Less than 48 hours since stroke onset | |
| Interventions | Rx: heparin IV (continuous, APTT 1.5 to 2.5) ± coumadin Control: no treatment Duration: 10 days | |
| Outcomes | Death plus cause of death ICH (systematic CT on Day 4 to 10) Recurrent stroke Major extracranial haemorrhage | |
| Notes | Ex: BP > 180/115, bleeding risk FU: 14 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the method used for random sequence generation |
| Allocation concealment (selection bias) | Low risk | Treatment randomised |
| Blinding (performance bias and detection bias) All outcomes | High risk | Treatment randomised but patients/personnel not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment randomised but patients/personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome of review independently evaluated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported that only 98% completed follow‐up for the initial week, but no report on the following 14 days |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes apparently reported in the study |
Chaudhary 2002.
| Study characteristics | ||
| Methods | C: randomisation not described Assessment not blinded Ex during trial: none Losses to FU: none | |
| Participants | India 30 participants Mean age: 65 years 100% CT before entry Acute stroke ‐ time not otherwise defined | |
| Interventions | Rx: parnaparin 0.3 mL subcutaneous bid for 10 days vs placebo | |
| Outcomes | Death and Barthel Index at 3 months | |
| Notes | Ex: CT with ICH, coagulopathy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported only "prospectively randomised" with no description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinded design reported for patients/personnel or outcome assessment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinded design reported for patients/personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinded design reported for outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rate of loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes reported in the study |
Dluha 2016.
| Study characteristics | ||
| Methods | C: secure EDGAR II computer programme; computer‐generated list of numbers controlled by nurses Single centre, patients and outcome assessor blinded Ex during trial: none Losses to FU: none | |
| Participants | Slovak Republic 87 participants Mean age: 70 years, 42.5% female 100% CT before entry Acute ischaemic stroke with symptom duration at least 30 minutes, with mRS 0 to 1 and NIHSS 6 to 25 Treatment initiated within 4.5 to 24 hours since onset | |
| Interventions | Rx: initial bolus of heparin 2500 UI and further continuous application of 1000 UI/h (18 to 20 UI/kg/h) within 24 hours, then subcutaneous nadroparin calcium Control: placebo (saline solution) within first 24 hours All patients received aspirin 100 mg daily | |
| Outcomes | Safety: major bleeding (i.e. gastrointestinal bleeding), ICH (intracerebral, subarachnoid), death Efficacy: mRS, NIHSS, and Barthel Index were performed on 3rd, 7th, 30th, and 90th days | |
| Notes | Ex: ICH; spontaneous rapid improvement of stroke symptoms before initiation of treatment; large ischaemia visible on initial CT covering more than 2/3 of the territory of the affected artery; epileptic seizure at onset of stroke; acute ischaemic stroke on initial CT; recent ischaemic stroke treated with IVT or planned mechanical recanalisation; stroke, myocardial infarction, or serious trauma within previous 3 months; platelet count < 100,000/mm³; refractory hypertension; systolic pressure > 185 mmHg or diastolic pressure > 110 mmHg despite therapy; blood glucose < 2.77 or > 22.15 mmol/L; bleeding diathesis; severe liver or renal lesion; oral or parenteral anticoagulant treatment at time of stroke onset; current or previous life‐threatening bleeding; major surgery within previous 3 months; malignancy; active tuberculosis; pregnancy; known heparin or nadroparin allergy; alcohol and/or drug abuse; participation in another clinical study FU: 90 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of randomised numbers (1, 2, 3) with distribution ratio 1:1:1 created by the EDGAR II computer programme |
| Allocation concealment (selection bias) | Low risk | Computer‐based randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients not aware of type of medication they received. Clinical‐state evaluator also blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical‐state evaluator also blinded to therapy and results of APTT |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | Baseline characteristics tables not provided |
Duke 1983.
| Study characteristics | ||
| Methods | C: random number list controlled by pharmacy Doctor, patient, and assessor blinded Ex during trial: none Losses to FU: none | |
| Participants | Canada 65 participants ? age ? sex 81% CT before entry 'Partial stable' ischaemic stroke < 48 hours since stroke onset | |
| Interventions | Rx: heparin 5000 IU subcutaneous 8‐hourly Control: placebo Duration: 7 days | |
| Outcomes | Death DVT (systematic I‐125 scan) ICH (symptomatic) Extracranial haemorrhage | |
| Notes | Ex: BP > 120 diastolic, bleeding risk FU: 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of random sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported, randomised only mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment blinded to patients/personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment blinded to outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/78 lost to follow‐up at 1 year |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes reported |
Duke 1986.
| Study characteristics | ||
| Methods | C: random number list controlled by pharmacy Doctor, patient, and assessor blinded Ex during trial: none Losses to FU: 3 participants (number in each group unknown) | |
| Participants | Canada 225 participants Mean age: 67 years 63% male 100% CT before entry 'Partial stable' non‐cardioembolic ischaemic stroke < 48 hours since stroke onset | |
| Interventions | Rx: heparin IV (continuous, APTT 50 to 70 seconds) Control: placebo Duration: 7 days Antiplatelet therapy during follow‐up not reported | |
| Outcomes | Death but not cause of death Functional outcome (measured but not reported) ICH | |
| Notes | Ex: BP > 110 diastolic, coma FU: 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random tables prepared according to sex, age, and hospital |
| Allocation concealment (selection bias) | Low risk | Investigator doing randomisation not involved in patient evaluations |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and doctors blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor unaware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3/225 patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes reported |
Elias 1990.
| Study characteristics | ||
| Methods | C: sealed envelopes (? opaque and sequentially numbered) Non‐blinded Ex during trial: none Losses to FU: 2 in Rx group | |
| Participants | Europe 30 participants Mean age: 68 years 57% male 100% CT before entry Ischaemic stroke with immobility Less than 48 hours since stroke onset | |
| Interventions | Rx: CY 222 (LMWH) 15,000 anti‐Xa units subcutaneous 24‐hourly Control: no treatment Duration: 14 days | |
| Outcomes | Death plus cause of death DVT (systematic I‐125 scan, venography 50%) Major extracranial haemorrhage | |
| Notes | Ex: BP > 220/120, coma FU: 14 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "cette étude randomisée …"; "par randomisation, ils ont été repartis en 2 groupes ... " Translation: "this randomised study..." (p.96); "by randomisation, they [patients] have been divided in 2 groups …" (p.96) |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "… un groupe de sujets traité par une dose de CY222…, et un groupe de sujets témoins non‐traités..." Translation: "… a group of patients treated with a dosage of CY222 …, and a group of patients not treated …" (p.96) Probably no blinding of patient or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study duration: 14 days. 2/15 missing from intervention group; 1 removed on Day 4 – extensive thrombus of external carotid, 1 removed on Day 14 following diagnosis of cerebral infarct |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes including TVP incidence and mortality reported |
FISS 1995.
| Study characteristics | ||
| Methods | C: sequentially numbered boxes containing identical syringes Doctor, patient, and assessor blinded Ex during trial: 4 in Rx group (survival status known for all except 1) Losses to FU: 2 in Rx group | |
| Participants | Hong Kong 312 participants Mean age: 67 years 58% male 100% CT before entry Ischaemic stroke with motor deficit Less than 48 hours since stroke onset | |
| Interventions | Rx: nadroparin (LMWH) subcutaneous (randomised between 4100 anti‐Xa units 12‐hourly vs 24‐hourly) Control: placebo Duration: 10 days All surviving participants received aspirin 100 mg/d after 10 days | |
| Outcomes | Death plus cause of death Functional outcome (dependency assessed by International Stroke Trial simple questions) PE (symptomatic) ICH (symptomatic and systematic CT) Recurrent stroke Major extracranial haemorrhage Myocardial infarction | |
| Notes | Ex: over 80 years, BP > 180/120 mmHg, previous disabling stroke, bleeding risk, imminent death FU: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Syringes contained in sequentially numbered boxes assigned to patients consecutively |
| Allocation concealment (selection bias) | Low risk | Patients randomly assigned to 1 of 3 treatments by individual investigators at each hospital |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel unaware of treatment assignment for follow‐up; reviewed by another blinded observer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/312 lost to follow‐up for outcomes assessment |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes reported |
FISS‐bis 1998.
| Study characteristics | ||
| Methods | C: sequentially numbered boxes Doctor, patient, and assessor blind Ex during trial: unknown Losses to FU: unknown | |
| Participants | International 766 participants Mean age: unknown % male: unknown 100% CT before entry Ischaemic stroke with motor deficit < 24 hours since stroke onset | |
| Interventions | Rx: nadroparin (LMWH) 86 units/kg sc once daily vs 86 units/kg subcutaneous 12‐hourly Control: placebo Duration: 10 days | |
| Outcomes | Death Functional outcome (Barthel Index score < 85 = dependent) Intracerebral haemorrhage (symptomatic CT) | |
| Notes | Ex: mild stroke, coma FU: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods used for random generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods used for allocation concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded treatment allocation to participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded treatment allocation to outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes reported |
IST 1997.
| Study characteristics | ||
| Methods | C: telephone randomisation Unblinded; dependency assessment mainly blinded Ex during trial: none Losses to FU: data 99.99% complete for 14‐day outcome and 99.2% complete for 6‐month outcome | |
| Participants | International 19,435 participants 61% > 70 years 54% male 67% CT prior to randomisation, 29% CT after randomisation Ischaemic stroke < 48 hours since stroke onset | |
| Interventions | Rx: subcutaneous heparin (5000 IU or 12,500 IU 12‐hourly), aspirin 300 mg, both, or neither (factorial design) Duration: 14 days or until discharge from hospital | |
| Outcomes | Death Functional outcome (validated simple questions) Recurrent stroke PE ICH (symptomatic CT) Extracranial haemorrhage | |
| Notes | Ex: small likelihood of worthwhile benefit; high risk of adverse effect (e.g. hypersensitivity of aspirin, recent GI bleed or peptic ulcer disease, already on long‐term anticoagulation) FU: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐allocated study treatment(s) by a minimisation algorithm used to reduce any imbalance in recorded prognostic features between treatment groups |
| Allocation concealment (selection bias) | Low risk | Computer‐allocated study treatment(s) by a minimisation algorithm used to reduce any imbalance in recorded prognostic features between treatment groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome completed by 99.99% at Day 14 and by 99.2% at 6 months |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes reported |
Kwiecinski 1995.
| Study characteristics | ||
| Methods | C: unknown Blinding not stated Ex during trial: unknown Losses to FU: unknown | |
| Participants | Poland 120 participants Mean age: 57 years 65% male 100% CT before entry Enrolled less than 48 hours after ischaemic stroke | |
| Interventions | Rx: fraxiparin (LMWH) 0.6 mL 12‐hourly for 1 week, then 0.3 mL 12‐hourly for 1 week Control: placebo Duration: 14 days | |
| Outcomes | Death Functional outcome Intracerebral haemorrhage (symptomatic CT) Symptomatic DVT and PE during treatment period | |
| Notes | Ex: > 65 years, comatose, severe comorbidity, uncontrolled hypertension FU: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No methods reported for random generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinded design not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinded design not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinded design not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported during treatment period |
| Selective reporting (reporting bias) | Unclear risk | Abstract without full details of reporting outcomes |
Liu 2020.
| Study characteristics | ||
| Methods | C: a centralised web‐based randomisation system Doctor unblinded, assessor blinded Ex during trial: none Losses to FU: none | |
| Participants | China 60 participants Mean age: 57 years 78% male 100% MRI before entry 4.5 to 48 hours since stroke onset | |
| Interventions | Rx: 10 mg argatroban twice a day + standard treatment Control: standard treatment Duration: 7 days | |
| Outcomes | Recurrent stroke Death Infection Haemorrhage of digestive tract NIHSS, mBI at 7 days and 90 days, mRS at 90 days | |
| Notes | Ex: haemorrhagic stroke, central nervous disease, diabetes, tumour or haematological systemic disease, infection prior to stroke, antineoplastic or immune‐modulating therapies FU:7 days, 90 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolled patients allocated by computer in a 1: 1 ratio to control or argatroban group in a random way |
| Allocation concealment (selection bias) | Low risk | Centralised web‐based randomisation system used for allocation concealment, with participant identifier entered before allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatment assignment known only to clinicians ‐ not to evaluators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment assignment known only to clinicians ‐ not to evaluators. Each patient was clinically assessed upon enrolment (baseline), at Day 7, and day 90 after start of treatment, which was blinded to evaluators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Each patient clinically assessed upon enrolment (baseline), at Day 7, and at Day 90 after start of treatment, which was blinded to evaluators |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data on loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Data on all proposed outcomes reported |
Marshall 1960.
| Study characteristics | ||
| Methods | C: unknown Non‐blinded Ex during trial: none Losses to FU: none | |
| Participants | UK 51 participants Age range: 30 to 70 years 47% male No CT, 100% LP plus angiography Non‐cardioembolic ischaemic stroke < 72 hours since stroke onset | |
| Interventions | Rx: heparin IV (3 doses of 12,500 IU), then phenindione orally 12‐hourly (PT 2 to 3) Control: no treatment Duration: 21 days No antiplatelet therapy during FU | |
| Outcomes | Death but not cause of death Fatal ICH (autopsy in 8/9 deaths) Recurrent stroke Major extracranial haemorrhage | |
| Notes | Ex: bleeding risk FU: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods used for random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No detailed description of allocation concealment provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No detailed report provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detailed report on blinded design provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed report provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detailed report provided |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes reported |
McCarthy 1977.
| Study characteristics | ||
| Methods | C: sealed envelopes (? opaque and sequentially numbered) DVT assessment blinded Ex during trial: none Losses to FU: none | |
| Participants | UK 32 participants Mean age: 78 years 34% male No CT, 100% LP before entry Any stroke with no blood in CSF Less than 48 hours since stroke onset | |
| Interventions | Rx: heparin 5000 IU subcutaneous 8‐hourly Control: no treatment Duration: 14 days | |
| Outcomes | Death but not cause of death DVT (systematic I‐125 scan) | |
| Notes | Ex: BP > 120 diastolic, bleeding risk FU: 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods used for random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No clear description provided, reported only "randomly" assigned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel not blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Results done by a blinded second doctor who did not know the patients |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detailed number of patients who completed follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Mentioned outcomes reported |
McCarthy 1986.
| Study characteristics | ||
| Methods | C: sealed envelopes (? opaque and sequentially numbered) DVT assessment blinded Ex during trial: none Losses to FU: none | |
| Participants | UK 305 participants Mean age: 76 years 43% male No CT before entry All strokes (10% haemorrhagic on autopsy) Less than 48 hours since stroke onset | |
| Interventions | Rx: heparin 5000 IU subcutaneous 8‐hourly Control: no treatment Duration: 14 days Antiplatelet therapy during follow‐up not reported | |
| Outcomes | Death but not cause of death DVT (systematic I‐125 scan) | |
| Notes | Ex: BP > 120 diastolic, bleeding risk FU: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used for random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No detailed description provided, only mentioned "randomised" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No report on whether blinded design was used in this study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No report on whether patients were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No report on whether outcome evaluators were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rate of loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Mentioned outcomes reported |
NAT‐COOP 1962.
| Study characteristics | ||
| Methods | C: sealed envelopes (? opaque and sequentially numbered) Only patients blinded Ex during trial: none Losses to FU: none | |
| Participants | USA 30 participants 26 older than 55 years 53% male No CT, 100% LP before entry Cardioembolic stroke Less than 2 weeks since stroke onset | |
| Interventions | Rx: heparin 50 mg IV 4‐hourly, then dicumarol (oral anticoagulant; PT 15% to 20%) Control: placebo Duration: 1 month | |
| Outcomes | Death plus cause of death Recurrent stroke Extracranial haemorrhage | |
| Notes | Ex: bleeding risk FU: 1 year but FU completed for all patients up to 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes used |
| Allocation concealment (selection bias) | Low risk | Patients assigned to anticoagulant or non‐anticoagulant therapeutic groups on a statistically random basis by means of sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported whether this study was double‐blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Whether participants/personnel were blinded not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whether outcome assessors were blinded not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No accurate number of patients who completed the study reported |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in the methods reported |
Pambianco 1995.
| Study characteristics | ||
| Methods | C: sealed, opaque but not sequentially numbered envelopes Non‐blinded Ex during trial: none Losses to FU: none | |
| Participants | USA 131 participants Mean age: 71 years 50% male 100% CT before entry Ischaemic stroke with severe leg weakness Less than 14 days since stroke onset | |
| Interventions | Rx: heparin 5000 IU subcutaneous 8‐hourly, with dose adjustment to maintain PT 30 to 40 seconds Control: no treatment Duration: 28 days | |
| Outcomes | Death plus cause of death DVT (systematic B mode and Doppler ultrasound) PE Major extracranial haemorrhage | |
| Notes | Ex: bleeding risk, active cancer FU: 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelope used for randomisation |
| Allocation concealment (selection bias) | Low risk | Patients randomly assigned to groups in 3 clusters of 22; i.e. 22 As, 22 Bs, and 22 Cs were placed in a sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinded design of the study not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of treatment allocation to participants/personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of treatment allocation to outcome assessor not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 175/259 completed the study; no detailed reasons provided why patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in methods reported |
Pince 1981.
| Study characteristics | ||
| Methods | C: sealed envelopes (? opaque and sequentially numbered) Non‐blinded Ex during trial: Rx 0, control 4 participants (2 with ICH) Losses to FU: none | |
| Participants | France 80 participants Age range: 30 to 92 years 62% male No CT, 100% LP before entry Ischaemic stroke affecting the leg Less than 7 days since stroke onset (89% < 48 hours) | |
| Interventions | Rx: heparin 5000 IU subcutaneous 8‐hourly Control: no treatment Duration: 10 days | |
| Outcomes | Death but not cause of death DVT (systematic I‐125 scan) PE Major extracranial haemorrhage | |
| Notes | Ex: bleeding risk FU: 10 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "l’attribution du type de traitement est faite d’après la randomisation sous enveloppe …" Translation: "attribution of treatment type is done after randomisation with sealed envelope …" |
| Allocation concealment (selection bias) | Low risk | Treatment and placebo groups have the same conditioning and the same posology |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo et antiagrégants sont pour nous indiscernables ..." Translation: "placebo and antiaggregant are indistinguishable for us [the personnel] …" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given on blinded assessment of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/120 patients excluded |
| Selective reporting (reporting bias) | Low risk | Mentioned outcomes of frequency of phlebitis, side effects of treatments, and clinical evolution in function of treatment reported |
Prins 1989.
| Study characteristics | ||
| Methods | C: sequentially numbered identical syringes Double‐blinded Ex during trial: none Losses to FU: none | |
| Participants | The Netherlands 60 participants 78% > 70 years 52% male 100% CT before entry Ischaemic stroke less than 72 hours since stroke onset | |
| Interventions | Rx: dalteparin (LMWH, Kabi 2165) 2500 anti‐Xa units subcutaneous 12‐ hourly Control: placebo Duration: 14 days | |
| Outcomes | Death plus cause of death DVT (systematic I‐125 scan with venography) PE ICH (symptomatic plus systematic CT) Major extracranial haemorrhage | |
| Notes | Ex: coma FU: 14 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No report on method used for randomisation generation |
| Allocation concealment (selection bias) | Unclear risk | No report on method used for allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Tested using a double‐blind, placebo‐controlled, randomised trial design. with 30 patients allocated to each group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Mentioned double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mentioned double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No report of loss to follow‐up during 14 days |
| Selective reporting (reporting bias) | Unclear risk | Abstract with no detailed information |
Sandset 1990.
| Study characteristics | ||
| Methods | C: sequentially numbered identical ampoules Doctor, patient, and assessor blinded Ex during trial: none Losses to FU: none | |
| Participants | Norway 103 participants Age range: 47 to 90 years 45% male 100% CT before entry Non‐cardioembolic ischaemic stroke Less than 72 hours since stroke onset | |
| Interventions | Rx: dalteparin (LMWH, Kabi 2165) 3000 to 5500 anti‐Xa U subcutaneous 24‐hourly Control: placebo Duration: 14 days | |
| Outcomes | Death plus cause of death DVT (systematic venography or B‐mode ultrasound) PE (symptomatic plus autopsy in 5/6 deaths) ICH (systematic CT) Major extracranial haemorrhage | |
| Notes | Ex: BP > 120 diastolic, coma, bleeding risk FU: 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentioned 'randomised' without description of methods used |
| Allocation concealment (selection bias) | Unclear risk | Only mentioned 'randomised' without description of methods used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 42/52 patients in the intervention group being followed up for DVT |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in methods reported |
Sarma 2003.
| Study characteristics | ||
| Methods | C: computer‐generated randomisation table Blinding not clear Ex during trial: none Losses to FU: none | |
| Participants | India 40 participants Mean age: 63 years 32% female 100% CT before entry Acute ischaemic stroke Less than 24 hours since onset | |
| Interventions | Rx: combination of subcutaneous nadroparin 4100 units and aspirin 325 mg per day Control: aspirin 325 mg per day alone Nadroparin stopped after 10 days of therapy and aspirin continued for 4 weeks | |
| Outcomes | Neurological status (using the Mathew Scale) Haemorrhagic transformation | |
| Notes | Ex: haemorrhagic infarction, active peptic ulcer, recent head trauma or haemorrhagic stroke in preceding 3 months, GI or urinary tract haemorrhage in preceding 3 weeks or blood pressure > 185/120 mmHg FU: 4 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on method used for random generation reported |
| Allocation concealment (selection bias) | Unclear risk | No detailed description of randomisation provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details on blinding design reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details on blinding design reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details on blinding design reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not reported |
| Selective reporting (reporting bias) | High risk | Selective adverse events reported |
Tazaki 1986.
| Study characteristics | ||
| Methods | C: numbered or coded containers administered sequentially to enrolled participants Doctor, patient, and assessor blinded Ex during trial: none Losses to FU: none | |
| Participants | Japan 156 participants 68% > 60 years 66% male 100% CT before entry Ischaemic stroke within 7 to 10 days of stroke onset | |
| Interventions | Rx: MD805 (thrombin inhibitor) 60 mg/d vs 30 mg/d continuous iv for 2 days; then 20 mg 12 hourly IV for 5 days Control: placebo All patients received glycerol 500 mg/d Duration: 7 days | |
| Outcomes | Death Functional outcome (graded on ADL‐type scale) | |
| Notes | Ex: decreased level of consciousness, pregnancy, 'bleeding tendency', severe hepatic or renal damage FU: 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods used for randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods used for allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'Double‐blinded' mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Double‐blinded' design used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Double‐blinded' design used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 42/156 not analysed at 28 days; no reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Abstract: lack of information |
Tazaki 1992.
| Study characteristics | ||
| Methods | C: numbered or coded containers administered sequentially to enrolled patients Doctor, patient, and assessor blinded Ex during trial: none Losses to FU: none | |
| Participants | Japan 138 participants Mean age: 68 years 64% male 100% CT before entry Ischaemic stroke within 5 to 7 days of stroke onset | |
| Interventions | Rx: MD805 (thrombin inhibitor) 60 mg/d for 2 days (continuous IV), then MD805 20 mg 12‐hourly for 5 days (IV) Control: placebo All groups received glycerol 500 mL/d for 7 days Duration: 7 days | |
| Outcomes | Death Functional outcome ICH (symptomatic CT) | |
| Notes | Ex: decreased level of consciousness, bleeding risk, pregnancy, age > 85 years, significant medical disease (cardiac, hepatic, renal) FU: 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used for random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method used for allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'Double‐blinded' design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Double‐blinded' design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Double‐blinded' design |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 118/138 analysed at the end; no reasons provided for the 20 patients excluded |
| Selective reporting (reporting bias) | Unclear risk | Abstract in translation: lack of information |
TOAST 1998.
| Study characteristics | ||
| Methods | C: permuted blocks with randomly ordered sizes of 6, 6, and 4; randomisation lists pharmacy controlled Doctor, patient, and assessor blinded Ex during trial: none Losses to FU: 25 participants (11 treatment, 14 control) | |
| Participants | USA 1281 participants Mean age: 65 years 61% male 100% CT before entry Ischaemic stroke > 1 hour and < 24 hours from symptom onset with estimated pre‐stroke Barthel Index ≥ 12 | |
| Interventions | Rx: danaparoid (heparinoid Org 10172) bolus followed by continuous infusion to maintain blood anti‐Xa levels of 0.6 to 0.8 Control: placebo Duration: 7 days | |
| Outcomes | Functional outcome: Barthel Index < 85, NIHSS, Glasgow Outcome Scale Recurrent stroke ICH (symptomatic CT) Extracranial haemorrhage | |
| Notes | Ex: age < 18 or > 85, women of childbearing potential, severe stroke (NIHSS score > 15), weight < 125 pounds FU: 7 days and 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised 1:1 to treatment with ORG 10172 or placebo using permuted blocks with randomly ordered sizes and balanced for every 16 consecutive patients |
| Allocation concealment (selection bias) | Low risk | Randomised using permuted blocks |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/646 in treatment and 5/635 in control lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in methods reported |
Turpie 1987.
| Study characteristics | ||
| Methods | C: sequentially numbered identical containers Double‐blind Ex during trial: none Losses to FU: none | |
| Participants | Canada 75 participants Age range: 28 to 90 years 53% male 100% CT before entry Non‐cardioembolic ischaemic stroke with immobility Less than 7 days since stroke onset | |
| Interventions | Rx: danaparoid (heparinoid Org 10172) 750 anti‐Xa units subcutaneous 12‐hourly Control: placebo Duration: 14 days or prior to discharge Antiplatelet therapy during follow‐up not reported | |
| Outcomes | Death plus cause of death DVT (systematic I‐125 scan + plethysmography with venography) PE (symptomatic) ICH (symptomatic CT) Extracranial haemorrhage Recurrent stroke | |
| Notes | Ex: bleeding risk FU: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how randomisation was generated |
| Allocation concealment (selection bias) | Low risk | Patients allocated according to a prescribed randomised arrangement to Org 10172 prophylaxis or saline placebo |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, trial nurses, and attending physicians unaware of treatment regimen for each patient |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, trial nurses, and attending physicians unaware of treatment regimen for each patient |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors unaware of treatment regimen for each patient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/75 did not complete the study; reasons provided |
| Selective reporting (reporting bias) | Low risk | Mentioned outcomes reported |
Vissinger 1995.
| Study characteristics | ||
| Methods | C: coded containers administered sequentially to enrolled participants Doctor, patient, and assessor blinded Ex during trial: none Losses to FU: 31/50 participants lost to 6‐month follow‐up | |
| Participants | Denmark 50 participants Mean age: 71.8 years 58% male 66% CT before entry ‐ haemorrhage excluded in remainder by cerebral scintigraphy Non‐embolic ischaemic stroke with motor deficit < 24 hours since stroke onset | |
| Interventions | Rx: tinzaparin (LMWH) 3500 anti‐Xa IU sc once daily Control: placebo Duration: 14 days or until full mobilisation | |
| Outcomes | Death DVT (venography) PE (symptomatic) | |
| Notes | Ex criteria: < 50 years old, hypertension (BP > 200/120 mmHg), coma, aphasia, bleeding risk, oral anticoagulant treatment, severe hepatic or renal disease, clinical suspicion of DVT or PE FU: 12 to 14 days and 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation held by pharmacy |
| Allocation concealment (selection bias) | Unclear risk | Not provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Double‐blind design not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Identical pre‐packed injections; unblinded to personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded assessment for outcome not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/20 lost to follow‐up at the end |
| Selective reporting (reporting bias) | Unclear risk | No information given |
ADL: activities of daily living
APTT: activated partial thromboplastin time
BP: blood pressure
C: concealment
CSF: cerebrospinal fluid
CT: computed tomography
DVT: deep vein thrombosis
EQ‐5D: EuroQol ‐ 5 Dimension
Ex: exclusion
FU: follow‐up
GI: gastrointestinal
ICH: intracranial haemorrhage
IM: intramuscular
IV: intravenous
IVT: intravenous thrombolysis
LMWH: low‐molecular‐weight heparin
LP: lumbar puncture
mBI: modified Barthel Index
MRI: magnetic resonance imaging
mRS: modified Rankin Scale
NIHSS: National Institutes of Health Stroke Scale
OHS: Oxford Handicap Scale
PE: pulmonary embolism
PT: prothrombin time
rtPA: recombinant tissue plasminogen activator
Rx: treatment
sICH: symptomatic intracranial haemorrhage
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afshari 2015 | Not an RCT |
| Amarenco 2014 | Participants randomised within 14 days of stroke onset |
| Asberg 2018 | Comparison of 2 anticoagulant regimens |
| Bergmann 1996 | Not all participants had AIS |
| Bradshaw 1975 | Non‐random: allocation by odd/even year of birth |
| Camerlingo 2005 | Confounded by the use of low‐dose unfractionated heparin in control group |
| Carter 1961 | Non‐random: alternate allocation |
| Chang 2002 | No published data available |
| Chen 2019 | Comparison of 2 anticoagulant regimens |
| ChiCTR‐OIN‐17013510 | Not an RCT |
| COU 9116 | Participants randomised within 14 days of stroke onset |
| Coutinho 2015 | No anticoagulant |
| Crepin‐leblond 1994 | Comparison of 2 anticoagulant regimens |
| Czechanowski 1981 | Confounded with dihydroergotamine |
| Dahan 1986 | Not all participants were stroke patients: individual patient data for stroke patients not available |
| Dan 2001 | Methods of randomisation unclear; no clinically relevant outcomes reported |
| DATAS II 2020 | Comparison of anticoagulant and antiplatelet |
| Dehen 1994 | Control group received low‐molecular‐weight heparin plus placebo |
| Diener 2005 | Comparison of 2 anticoagulant regimens |
| Dong 2003 | No published data available |
| Donnan 1996 | No anticoagulant |
| Dumas 1994 | Comparison of 2 anticoagulant regimens |
| Egan 1999 | Not an RCT |
| Egberts 1996 | No published data available |
| ELAN 2017 | Does not compare effects of anticoagulants |
| EMT 1992 | Not an RCT |
| Enger 1965 | Time from stroke to treatment unclear |
| England 2010 | Not an RCT |
| Eriksson 1983 | Non‐random: allocation by month of admission |
| Fang 2015 | Study not conducted |
| Fassbender 1998 | Comparison of 2 anticoagulant regimens |
| Field 2017 | No published data available |
| Fradley 2014 | Comparison of 2 anticoagulant regimens |
| Geisler 2017 | Anticoagulant vs antiplatelet regimen |
| Gelmers 1980 | Non‐random: alternate allocation |
| Grond 2000 | Not an RCT |
| HAEST 2000 | Anticoagulant vs antiplatelet regimen |
| Hillbom 1999 | Comparison of 2 anticoagulant regimens |
| Hillbom 2002 | Comparison of 2 anticoagulant regimens |
| Hossman 1986 | No published data available |
| Hui 2016 | No published data available |
| IRCT2014020213698N1 | Comparison of 2 anticoagulant regimens |
| IRCT201407213106N18 | No anticoagulant |
| ISRCTN25644448 | Participant randomisation within 14 days of stroke onset |
| Ji 2017 | Comparison of 2 anticoagulant regimens |
| Jin 2017 | Comparison of 2 anticoagulant regimens |
| JPRN‐UMIN000014405 | Did not explore the effect of anticoagulant |
| JPRN‐UMIN000025392 | Randomisation over 14 days |
| Kamel 2018 | Participants randomised within 14 days of stroke onset |
| Kang 2010 | No clinical outcome data provided |
| Kario 1995 | No clinically relevant outcomes reported |
| Kataoka 2000 | Not an RCT |
| Kim 2003 | Comparison of 2 anticoagulant regimens |
| Kobayashi 1997 | No clinically relevant outcomes reported |
| Lee 1994 | Confounded with urokinase |
| Lee 2008 | Comparison of 2 anticoagulant regimens |
| Lee 2014 | Did not involve anticoagulant as an intervention |
| Liu 2015 | Anticoagulant vs antiplatelet regimen |
| Lu 2001 | No published data available |
| Luo 2013 | Comparison of 2 anticoagulant regimens |
| McCarthy 1993 | Data not available despite repeated contact with study authors |
| McDevitt 1959 | Time from stroke to treatment unclear |
| Melero 2007 | Comparison of 2 anticoagulant regimens |
| Meredit 2009 | Comparison of 2 anticoagulant regimens |
| Moulin 1994 | Comparison of 2 anticoagulant regimens |
| Muck 1997 | Comparison of 2 anticoagulant regimens |
| NAVIGATE ESUS 2014 | Randomised between 7 days and 6 months after stroke onset |
| NCT01924325 | Anticoagulant vs antiplatelet regimen |
| NCT02221102 | Study not conducted |
| NCT02283294 | Anticoagulant vs antiplatelet regimen |
| NCT03961334 | Anticoagulant vs antiplatelet regimen |
| OPTIMAS 2018 | Does not compare effect of anticoagulant but time of commencing anticoagulant |
| PREVAIL 2004 | Comparison of 2 anticoagulant regimens |
| RAPID 2005 | Anticoagulant vs antiplatelet regimen |
| RESPECT CVT 2018 | Randomisation 5 to 15 days after initial acute treatment |
| RE‐SPECT ESUS 2019 | Recruitment within 6 months of embolic stroke onset |
| Rha 2001 | No published data available |
| Rose 2019 | Comparison of 2 anticoagulant regimens |
| Rosin 1994 | Data not available despite repeated contact with study authors |
| Shi 2004 | No clinically relevant outcomes reported |
| SOCRATES 2016 | Anticoagulant not an intervention |
| Stiekema 1988 | No control group: dose‐finding trial |
| TAIST 2001 | Anticoagulant vs antiplatelet regimen |
| Tan 1998 | Not randomised; no clinically relevant outcomes reported |
| Thygesen 1964 | Non‐random allocation |
| Tomek 2011 | Comparison of 2 anticoagulant regimens |
| Toni 2000 | Not an RCT |
| Trencev 2008 | No published data available |
| Triple AXEL 2015 | Comparison of 2 anticoagulant regimens |
| Tsuchiya 1989 | Confounded with urokinase |
| Tsuchiya 1990 | Confounded with urokinase |
| Turpie 1991 | Comparison of 2 anticoagulant regimens |
| Turpie 1992 | Comparison of 2 anticoagulant regimens |
| VA Study 1961 | Treatment started acutely in only 50%; unable to get individual patient data for just these participants |
| Warach 2003 | No published data available |
| Wellington 2002 | Comparison of 2 anticoagulant regimens |
| Wong 2000 | Comparison of 2 anticoagulant regimens |
| Yamaguchi 2004 | Comparison of 2 anticoagulant regimens |
| Yang 2016 | Anticoagulant not an intervention |
| Yi 2015 | Anticoagulant vs antiplatelet regimen |
| Zhang 2005 | Not clearly randomised |
| Zhu 2014 | No published data available |
| Zuo 2017 | Anticoagulant not an intervention |
AIS: acute ischaemic stroke
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800018313.
| Study name | A multicenter trial for revascularization pretreated with argatroban for acute ischemic stroke |
| Methods | Randomised parallel controlled trial |
| Participants | Age 18 to 80 years, NIHSS < 30, ASPECTS > 6, CTA/DSA/MRA confirmed large vascular (internal carotid artery, vascular anomalies, basilar artery, the second segments of the middle cerebral artery) Time from attack to randomisation < 8 hours Estimated 300 participants China |
| Interventions | Argatroban treatment vs placebo |
| Outcomes | Functional outcomes: mRS at 90 days |
| Starting date | 10 October 2018 |
| Contact information | Junhui Chen; chenjunhui101@163.com |
| Notes | http://www.chictr.org.cn/showproj.aspx?proj=30871 |
ISRCTN76741621.
| Study name | Multicentre randomised clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: the effect of periprocedural medication: heparin, antiplatelet agents, both or neither |
| Methods | Multi‐centre phase III clinical trial with randomised treatment allocation, open‐label treatment, and blinded endpoint assessment (PROBE), with a 2 × 3 factorial design (treatment) |
| Participants | Age ≥ 18 years, acute ischaemic stroke caused by intracranial large vessel occlusion of anterior circulation with intra‐arterial treatment (groin puncture) possible within 0 to 6 hours Estimated 1500 participants The Netherlands |
| Interventions | Acetylsalicylic acid, unfractionated heparin, both or none during intra‐arterial treatment Acetylsalicylic acid will be administered intravenously, at a loading dose of 300 mg Unfractionated heparin will be administered IV at a low dose (loading dose of 5000 IU followed by 500 IU/hour × 6 hours) or a moderate dose (loading dose of 5000 IU followed by 1250 IU/hour × 6 hours) Both IV acetylsalicylic acid and heparin treatment should be started prior to groin puncture when no IVT is administered, or directly after/when IV alteplase has been stopped Duration of anticoagulation 6 hours |
| Outcomes | mRS at 90 days |
| Starting date | 01 November 2017 |
| Contact information | Rob van de Graaf, University Medical Center's Gravendijkwal 230, Departments of Neurology and Radiology, Room Ee 2240, 3000 CA, Rotterdam, The Netherlands |
| Notes | http://www.isrctn.com/ISRCTN76741621 |
NCT03506009.
| Study name | Argatroban Plus R‐tPA for Acute Posterior Circulation Infarction (AR‐PCI): a prospective, random, blinded assessment of outcome and open label multi‐center study |
| Methods | Prospective, random, blinded assessment of outcome and open‐label multi‐centre study |
| Participants | 18 to 80 years of age, posterior circulation, ischaemic stroke, NIHSS 4 to 25, time from onset to treatment ≤ 6 hours |
| Interventions | Rx: argotroban + rt‐PA vs rt‐PA only; acute ischaemic stroke |
| Outcomes | mRS at 90 days Early neurological deterioration, NIHSS Occurrence of stroke Symptomatic intracranial hemorrhage within 36 hours |
| Starting date | 11 July 2018 |
| Contact information | Hui‐Sheng Chen, General Hospital of Shenyang Military Region |
| Notes | https://clinicaltrials.gov/ct2/show/NCT03506009 |
NCT03570281.
| Study name | Edoxaban for the treatment of coagulopathy in patients with active cancer and acute ischemic stroke: a pilot study (ENCHASE) |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: none (open‐label) Primary purpose: treatment |
| Participants | 40 |
| Interventions | Edoxaban vs enoxaparin |
| Outcomes | Primary outcome: interval change of serum D‐dimer level between days 0 and 7 Secondary outcomes: number of microembolic signals detected by transcranial doppler, mRS at 90 days from 0 to 6 (higher is worse), symptomatic intracerebral haemorrhage, major bleeding, all‐cause death |
| Starting date | 26 June 2018 |
| Contact information | neuroboy50@naver.com |
| Notes |
NCT03735979.
| Study name | Multi‐arm Optimization of Stroke Thrombolysis (MOST): a single blinded, randomized controlled adaptive, multi‐arm, adjunctive‐thrombolysis efficacy trial in ischemic stroke |
| Methods | Double‐blinded (participant, outcomes assessor blinded) |
| Participants | 18 years of age and older, acute ischaemic stroke treated with 0.9 mg/kg IV rt‐PA within 3 hours of stroke onset or time last known well, NIHSS score ≥ 6 prior to IV rt‐PA, able to receive assigned study drug within 60 minutes of initiation of IV rt‐PA Estimated 1200 participants USA |
| Interventions | Argatroban: 100 µg/kg bolus followed by 3 µg/kg per minute for 12 hours Eptifibatide: 135 µg/kg bolus followed by 0.75 µg/kg per minute infusion for 2 hours Control: placebo |
| Outcomes | Efficacy: mRS at 90 days |
| Starting date | 15 October 2019 |
| Contact information | Opeolu Adeoye; adeoyeo@ucmail.uc.edu Andrew Barreto Andrew; d.barreto@uth.tmc.edu University of Cincinnati, National Institute of Neurological Disorders and Stroke |
| Notes | https://clinicaltrials.gov/ct2/show/NCT03735979 |
NCT03740958.
| Study name | Argatroban plus R‐tPA for acute ischemic stroke: a prospective, random, open label, blinded assessment of outcome multi‐center study |
| Methods | Multi‐centre, prospective, randomised, open‐label, blind‐endpoint trial |
| Participants | Patients with acute ischaemic stroke, NIHSS score ≥ 6 at time of randomisation, within 4.5 hours of symptom onset |
| Interventions | Argatroban (100 μg/kg bolus followed by infusion of 1.0 μg/kg per minute for 48 hours) plus r‐tPA or r‐tPA alone |
| Outcomes | Proportion of patients with excellent outcome of no clinically significant residual stroke deficits (mRS 0 to 1) at 90 days |
| Starting date | 21 December 2018 |
| Contact information | Contact: Xinhong Wang; Doctor15309885658 ext 024‐28897512450341972@qq.com Contact: Yu Cui; Master18842398646 ext 024‐28897512314486939@qq.com |
| Notes |
NCT04275180.
| Study name | Clinical study of argatroban in the treatment of acute progressive ischemic stroke |
| Methods | Randomised parallel open‐label |
| Participants | Progress of ischaemic stroke with neurological function deteriorated from 6 hours to 48 hours after onset of the disease, NIHSS score increased by ≥ 2 points Estimated 628 participants China |
| Interventions | Argatroban on the basis of standard medical treatment Control: standard medical treatment, including routine antiplatelet, blood pressure control, statins to stabilise plaque, etc |
| Outcomes | Efficacy: mRS at 3 months; NIHSS at 7 days, 1 month, and 3 months Safety: SICH at 7 days, adverse events at 3 months |
| Starting date | 21 March 2020 |
| Contact information | Min Lou, Loumingxc@vip.sina.com, Second Affiliated Hospital, School of Medicine, Zhejiang University |
| Notes | https://clinicaltrials.gov/ct2/show/study/NCT04275180 |
NCT04304508.
| Study name | NCT04304508 |
| Methods | Double‐blinded (participant, care provider, investigator, outcomes assessor blinded) |
| Participants | 45 years of age and older Non‐cardioembolic ischaemic stroke Estimated 1800 participants: Australia, Austria, Belgium, Bulgaria, Canada, China, Czechia, Denmark, Finland, Germany, Hungary, Italy, Japan, Netherlands, Poland, Portugal, Russian Ferderation, Slovakia, Spain, Sweden, Switzerland, UK, USA |
| Interventions | Oral FXIa inhibitor: BAY2433334 compared to placebo Tablet, taken orally once a day |
| Outcomes | Safety: major bleeding and clinically relevant non‐major (CRNM) bleeding; symptomatic ischaemic stroke or covert brain infarcts; death; adverse events |
| Starting date | 15 June 2020 |
| Contact information | Bayer Clinical Trials Contact clinical‐trials‐contact@bayer.com |
| Notes | https://clinicaltrials.gov/ct2/show/study/NCT04304508 |
ASPECTS: Alberta Stroke Programme Early CT Score CTA: computed tomography angiography DSA: digital subtraction angiography IV: intravenous MRA: magnetic resonance angiography MRI: magnetic resonance imaging mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale rt‐PA: recombinant tissue plasminogen activator Rx: treatment sc: subcutaneous SICH: symptomatic intracerebral hemorrhage
Differences between protocol and review
None
Contributions of authors
Peter Sandercock designed the original 1993 review of anticoagulant versus control, double‐checked the data, and supervised the analysis and writing of this report. Edward Kane did the new literature searches and helped reformat and rewrite this update. Ayeesha Kamal did the new literature searches, extracted the new data, performed the analysis, and helped rewrite the text of the 2008 update. Carl Counsell prepared the first version of the review and helped with the analysis and preparation of text for the 2015 update.
Xia Wang and Jie Yang rewrote the text of the 2020 update. Xia Wang, Menglu Ouyang, Jie Yang, and Ming Yang screened the new literature, extracted new data, and performed the analysis. Lili Song helped rewrite the text. Craig Anderson and Jie Yang supervised the analysis and writing of this report.
Sources of support
Internal sources
-
University of Edinburgh, UK
n/a
External sources
-
Wellcome Trust, UK
n/a
-
Medical Research Council, UK
n/a
-
New South Wales Ministry of Health, Australia
Investigator development funding
-
National Heart Foundation, Australia
Post‐doctoral fellowship 102117
-
National Health and Medical Research Council, Australia
Investigator grant APP1195237
-
National Natural Science Foundation of China, China
National Natural Science Foundation of China, 81870940
Declarations of interest
Xia Wang: Grants and contracts: investigator grant, post‐doc fellowship (NHMRC National Heart Foundation Australia), "Those grants are for my salary support".
Menglu Ouyang: none known.
Jie Yang: Grants and contracts: funding support (National Natural Science Foundation of China), Grant No. 81870940.
Lili Song: none known.
Min Yang: none known.
Craig Anderson: Grants and contracts: research grants (Takeda China), approximately Aust$1m over 4 grants in as many studies. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: lecture fees and travel reimbursement (Takeda China), total approximately Aust$10k over 2 years. Payment for a fellowship: research fellowship (National Health and Medical Research Council (NHMRC) of Australia), "pays half my salary". Work as a health professional: "I am a consultant neurologist who manages patients with acute stroke" (Royal Prince Alfred Hospital, Sydney, Australia), clinical academic appointment.
Edited (no change to conclusions)
References
References to studies included in this review
ARGIS‐1 2004 {published and unpublished data}
- LaMonte MP, Nash ML, Wang DZ, Woolfenden AR, Schulz J, Hursting MJ, et al. Argatroban anticoagulation in patients with acute ischemic stroke (ARGIS-1). Stroke 2004;35:1677-82. [DOI] [PubMed] [Google Scholar]
ARTSS‐2 2017 {published data only}
- Barreto AD, Ford GA, Shen L, Pedroza C, Tyson J, Cai C, et al. Randomized, multicenter trial of ARTSS-2 (Argatroban with Recombinant Tissue Plasminogen Activator for Acute Stroke). Stroke 2017;48:1608-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cazzato 1989 {published and unpublished data}
- Cazzato G, Zorzon M, Mase G, Antonutto L, Iona LG. Mesoglycan in the treatment of acute cerebral infarction [Il mesoglicano nelle ischemie cerebrali acute a focolaio]. Rivista di Neurologia 1989;59:121-6. [PubMed] [Google Scholar]
CESG 1983 {published and unpublished data}
- Hakim AM, Furlan AJ, Hart RG. Immediate anticoagulation of embolic stroke: a randomized trial. The Cerebral Embolism Study Group. Stroke 1983;14:668-76. [DOI] [PubMed] [Google Scholar]
- Hakim AM, Ryder-Cooke A, Melanson D. Sequential computerized tomographic appearances of strokes. Stroke 1983;14:893-7. [DOI] [PubMed] [Google Scholar]
Chaudhary 2002 {published data only}
- Chaudhry HR, Arora H, Yadav K, Dubey S, Gupta R, Jain R. Low molecular weight heparin In acute ischemic stroke. The Antiseptic 2002;99:31-2. [Google Scholar]
Dluha 2016 {published data only}
- Dluha J, Sivak S, Kurca E, Dusenka R, Kalmarova K, Koprusakova MT, et al. The safety and efficacy of heparin and nadroparin compared to placebo in acute ischemic stroke - pilot study. Biomedical Papers of the Medical Faculty of the University Palacký, Olomouc, Czechoslovakia 2016;160:543-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Duke 1983 {published and unpublished data}
- Duke RJ, Turpie AGG, Bloch RF, Derby IR, Kronby MH, Bayer NH. Clinical trial of low-dose heparin for the prevention of stroke progression. Circulation 1980;Suppl III:21. [Google Scholar]
- Duke RJ, Turpie AGG, Bloch RF, Trebilcock RG. Clinical trial of low-dose subcutaneous heparin for the prevention of stroke progression: natural history of acute partial stroke and stroke-in-evolution. In: Reivich M, Hurtig HI, editors(s). Cerebrovascular Disease. New York: Raven Press, 1983:399-405. [Google Scholar]
Duke 1986 {published and unpublished data}
- Duke RJ, Bloch RF, Turpie AGG, Trebilcock RG, Bayer N. Intravenous heparin for the prevention of stroke progression in acute partial stable stroke. Annals of Internal Medicine 1986;105:825-8. [DOI] [PubMed] [Google Scholar]
- Duke RJ, Bloch RF, Turpie AGG. Heparin in acute partial stable stroke. Annals of Internal Medicine 1987;106:782. [DOI] [PubMed] [Google Scholar]
- Easton JD, Sherman DG, Hart RG. Heparin in acute partial stable stroke. Annals of Internal Medicine 1987;106:781. [DOI] [PubMed] [Google Scholar]
- Loeliger EA. Heparin in acute partial stable stroke. Annals of Internal Medicine 1987;106:781-2. [DOI] [PubMed] [Google Scholar]
Elias 1990 {published and unpublished data}
- Elias A, Milandre L, Lagrange G, Aillaud MF, Alonzo B, Toulemonde F, et al. Prevention of deep venous thrombosis of the leg by a very low molecular weight heparin fraction (CY 222) in patients with hemiplegia following cerebral infarction: a randomized pilot study (30 patients). Revue de Medecine Interne 1990;11:95-8. [DOI] [PubMed] [Google Scholar]
FISS 1995 {published and unpublished data}
- Kay R, Wong KS, Lu YL, Chan YW, Tsoi TH, Ahuja AT, et al. Low-molecular-weight heparin for the treatment of acute ischemic stroke. New England Journal of Medicine 1995;333:1588-93. [DOI] [PubMed] [Google Scholar]
- Kay R. Fraxiparin in stroke study. Stroke 1994;25:253. [Google Scholar]
FISS‐bis 1998 {published data only}
- Hommel M, for the FISS-bis Investigators Group. Fraxiparine in Ischaemic Stroke Study (FISS bis). Cerebrovascular Diseases 1998;8 Suppl 4:19. [Google Scholar]
IST 1997 {published data only}
- International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet 1997;349:1569-81. [PubMed] [Google Scholar]
- International Stroke Trial Pilot Study Collaborative Group. Study design of the International Stroke Trial (IST), baseline data, and outcome in 984 randomised patients in the pilot study. Journal of Neurology Neurosurgery and Psychiatry 1996;60:371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Lewis S, Berge E, Sandercock P, Koudstaal P, for the International Stroke Trial Collaborative Group. Risk of early death and recurrent stroke and effect of heparin in 3169 patients with acute ischaemic stroke and atrial fibrillation in the International Stroke Trial. Stroke 2001;32:2333-7. [DOI] [PubMed] [Google Scholar]
Kwiecinski 1995 {published and unpublished data}
- Kwiecinski H, Pniewski J, Kaminska A, Szyluk B. A randomized trial of fraxiparine in acute ischaemic stroke. Cerebrovascular Diseases 1995;5:234. [Google Scholar]
Liu 2020 {published data only}
- Liu S, Liu P, Wang P, Zhang F, Wang l, Wang Y, et al. Argatroban increased the basal vein drainage and improved outcomes in acute paraventricular ischemic stroke patients. Medical Science Monitor 2020;26:e924593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 1960 {published data only}
- Marshall J, Shaw DA. Anticoagulant therapy in acute cerebrovascular accidents: a controlled trial. Lancet 1960;1:995-8. [DOI] [PubMed] [Google Scholar]
- Marshall J, Shaw DA. Anticoagulant therapy in cerebrovascular disease. In: Proceedings of the Royal Society of Medicine. 1960:547-9. [DOI] [PMC free article] [PubMed]
McCarthy 1977 {published and unpublished data}
- McCarthy ST, Turner JJ, Robertson D, Hawkey CJ, Macey DJ. Low dose heparin as a prophylaxis against deep-vein thrombosis after acute stroke. Lancet 1977;ii:800-1. [DOI] [PubMed] [Google Scholar]
McCarthy 1986 {published and unpublished data}
- McCarthy ST, Turner J. Low-dose subcutaneous heparin in the prevention of deep-vein thrombosis and pulmonary emboli following acute stroke. Age and Ageing 1986;15:84-8. [DOI] [PubMed] [Google Scholar]
NAT‐COOP 1962 {published data only}
- Baker RN, Broward JA, Fang HC, Fisher CM, Groch SN, Heyman A, et al. Anticoagulant therapy in cerebral infarction. Report on co-operative study. Neurology 1962;12:823-9. [Google Scholar]
- Miller Fisher C. Anticoagulant therapy in cerebral thrombosis and cerebral embolism. A national cooperative study, interim report. Neurology 1961;11:119-31. [DOI] [PubMed] [Google Scholar]
Pambianco 1995 {published and unpublished data}
- Desmukh M, Bisignani M, Landau P, Orchard TJ. Deep vein thrombosis in rehabilitating stroke patients. Incidence, risk factors and prophylaxis. American Journal of Physical Medicine and Rehabilitation 1991;70:313-6. [PubMed] [Google Scholar]
- Pambianco G, Orchard T, Landau P. Deep vein thrombosis: prevention in stroke patients during rehabilitation. Archives of Physical Medicine and Rehabilitation 1995;76:324-30. [DOI] [PubMed] [Google Scholar]
Pince 1981 {unpublished data only}
- Pince J. Thromboses veineuses des membres inferieurs et embolies pulmonaires au cours des accidents vasculaires cerebraux. A propos d'un essai comparitif de traitement preventif (These pour le doctorat d'etat en medecine). Toulouse: Universite Paul Sabatier, 1981. [Google Scholar]
Prins 1989 {published and unpublished data}
- Prins MH, den Ottolander GJH, Gelsema R, Woerkom TCM, Sing AK, Heller I. Deep venous thrombosis prophylaxis with a LMW heparin (KABI 2165) in stroke patients. Thrombosis and Haemostasis 1987;58:117. [DOI] [PubMed] [Google Scholar]
- Prins MH, Gelsema R, Sing AK, Heerde LR, den Ottolander GJ. Prophylaxis of deep venous thrombosis with a low-molecular-weight heparin (Kabi 2165/Fragmin) in stroke patients. Haemostasis 1989;19:245-50. [DOI] [PubMed] [Google Scholar]
Sandset 1990 {published and unpublished data}
- Sandset PM, Dahl T, Abildgaard U. Venous thromboembolic complications in acute thrombotic stroke. Progress report from a randomized trial. Acta Neurologia Scandinavica 1987;76:392. [Google Scholar]
- Sandset PM, Dahl T, Stiris M, Rostad B, Scheel B, Abildgaard U. A double-blind and randomized placebo-controlled trial of low molecular weight heparin once daily to prevent deep-vein thrombosis in acute ischemic stroke. Seminars in Thrombosis and Hemostasis 1990;16 Suppl:25-33. [PubMed] [Google Scholar]
Sarma 2003 {published data only}
- Sarma GR, Roy AK. Nadroparin plus aspirin versus aspirin alone in the treatment of acute ischemic stroke. Neurology India 2003;51:208-10. [PMID: ] [PubMed] [Google Scholar]
Tazaki 1986 {published data only}
- Tazaki Y, Kobayashi S, Togi H, Ohtomo E, Goto F, Araki G, et al. Therapeutic effect of thrombin inhibitor MD-805 in acute phase of cerebral thrombosis - Phase II double-blinded clinical trial (English translation). Rinsho to Kenkyu (Japanese Journal of Clinical and Experimental Medicine) 1986;63:3047-57. [Google Scholar]
Tazaki 1992 {published data only}
- Tazaki Y, Kobayashi S, Togi H, Ohtomo E, Goto F, Araki G, et al. Clinical usefulness of thrombin inhibitor MD-805 in acute phase of cerebral thrombosis - double blinded comparative study using placebo as a control (English translation). Igaku no Ayumi (Journal of Clinical and Experimental Medicine) 1992;161:887-907. [Google Scholar]
TOAST 1998 {unpublished data only}
- Adams JHP. Trial of Org 10172 in Acute Stroke Treatment (TOAST). Stroke 1994;25:545. [Google Scholar]
- TOAST Investigators. Low molecular weight heparinoid, ORG 10172 (Danaparoid), and outcome after acute ischaemic stroke. JAMA 1998;279:1265-72. [PubMed] [Google Scholar]
Turpie 1987 {published and unpublished data}
- Turpie AGG, Levine MN, Hirsh J, Carter CJ, Jay RM, Powers PJ, et al. A double-blind randomized trial of Org 10172 low molecular weight heparinoid in the prevention of deep venous thrombosis in patients with thrombotic stroke. Thrombosis and Haemostasis 1987;58:123. [DOI] [PubMed] [Google Scholar]
- Turpie AGG, Levine MN, Hirsh J, Carter CJ, Jay RM, Powers PJ, et al. Double-blind randomised trial of Org 10172 low-molecular-weight heparinoid in prevention of deep-vein thrombosis in thrombotic stroke. Lancet 1987;1:523-6. [DOI] [PubMed] [Google Scholar]
- Turpie AGG. Low molecular weight heparins: deep vein thrombosis prophylaxis in elective hip surgery and thrombotic stroke. Acta Chirurgica Scandinavica Supplementum 1988;543:85-6. [PubMed] [Google Scholar]
Vissinger 1995 {unpublished data only}
- Vissinger H, Husted S. Trial of tinzaparin versus placebo in ischaemic stroke. Unpublished work.
References to studies excluded from this review
Afshari 2015 {published data only}
- Afshari D, Moradian N, Nasiri F, Razazian N, Bostani A, Sariaslani P. The efficacy and safety of low-molecular-weight heparin and unfractionated heparin in the treatment of cerebral venous sinus thrombosis. Neurosciences (Riyadh, Saudi Arabia) 2015;20(4):357-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amarenco 2014 {published data only}
- Amarenco P, Davis S, Jones EF, Cohen AA, Heiss WD, Kaste M, et al. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke 2014;45(5):1248-57. [DOI] [PubMed] [Google Scholar]
Asberg 2018 {published data only}
- Asberg S, Hijazi Z, Norrving B, Wester P, Ohagen P, Oldgren J. Timing of oral anticoagulant therapy in acute ischemic stroke with atrial fibrillation: the first registry-based randomised controlled stroke trial. European Stroke Journal 2018;3(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergmann 1996 {published data only}
- Bergmann JF, Neuhart E. A multicenter randomized double-blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in-patients bedridden for an acute medical illness. Thrombosis and Haemostasis 1996;76:529-34. [PubMed] [Google Scholar]
Bradshaw 1975 {published data only}
- Bradshaw P, Brennan S. Trial of long-term anticoagulant therapy in the treatment of small stroke associated with a normal carotid angiogram. Journal of Neurology, Neurosurgery and Psychiatry 1975;38:642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Camerlingo 2005 {published data only}
- Camerlingo M, Salvi P, Belloni G, Gamba T, Cesana BM, Mamoli A. Intravenous heparin started within the first 3 hours after onset of symptoms as a treatment for acute non-lacunar hemispheric cerebral infarctions. Stroke 2005;36:2415-20. [DOI] [PubMed] [Google Scholar]
Carter 1961 {published data only}
- Carter AB. Anticoagulant treatment in progressing stroke. BMJ 1961;II:70-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AB. Use of anticoagulants in patients with progressive cerebral infarction. Neurology 1961;11:601-9. [DOI] [PubMed] [Google Scholar]
Chang 2002 {published data only}
- Chang KC, Tan TY, Liou CW, Chen SD, Gung C. Nadroparin in moderate acute ischemic stroke trial. Stroke 2002;33:386. [Google Scholar]
Chen 2019 {published data only}
- Chen JH, Zhang C, Zhang CX. Optimal timing to administer non-vitamin K oral anticoagulants (NOACS) and rivaroxaban in patients with acute ischemic stroke and nonvalvular atrial fibrillation. Journal of the American Geriatrics Society 2019;67:S629-S630. [Google Scholar]
ChiCTR‐OIN‐17013510 {published data only}
COU 9116 {unpublished data only}
- Oczkowski WJ. A randomised trial of fixed low dose warfarin in the prevention of deep vein thrombosis in patients undergoing rehabilitation after stroke (Cou 9116). Unpublished protocol.
Coutinho 2015 {published data only}
- Coutinho JM, Ferro JM, Zuurbier SM, Canhao P, Crassard I, Majoie CB, et al. Thrombolysis or anticoagulation for cerebral venous thrombosis (TO-ACT trial). International Journal of Stroke 2015;10:434-5. [DOI] [PubMed] [Google Scholar]
Crepin‐leblond 1994 {published data only}
- Crepin-leblond T, Moulin T, Ziegler F, Bataillard M, Chopard JL, Chavot D, et al. A randomised trial of heparin therapy in acute ischemic stroke: first results. Cerebrovascular Diseases 1994;4:259. [Google Scholar]
Czechanowski 1981 {published data only}
- Czechanowski B, Heinrich F. Prevention of venous thrombosis in recent ischaemic cerebrovascular accident: double-blind study with heparin-dihydroergotamine. Deutsche Medizinische Wochenschrift 1981;106:1254-60. [DOI] [PubMed] [Google Scholar]
Dahan 1986 {published data only}
- Dahan R, Houlbert D, Caulin C, Cuzin E, Viltart C, Woler M, et al. Prevention of deep vein thrombosis in elderly medical in-patients by a low molecular weight heparin: a randomized double-blind trial. Haemostasis 1986;16:159-64. [DOI] [PubMed] [Google Scholar]
Dan 2001 {published data only}
- Dan G, Gonta A, Serbanescu A, Ionete C. Improvement of outcome in patients with acute ischaemic stroke treated with fragmin. Haemostasis 2001;30 Suppl 2:168-9. [Google Scholar]
DATAS II 2020 {published data only}
- Butcher K, Ng K, Field T, Coutts S, Gioia L, Siddiqui M, et al. The dabigatran following acute transient ischemic attack and minor stroke trial: final results. European Stroke Journal 2018;3(1):3. [Google Scholar]
- Butcher K, Ng K, Sheridan P, Field T, Coutts S, Siddiqui M, et al. Dabigatran treatment for acute noncardioembolic ischemic stroke. Stroke 2020;51:1190-8. [DOI] [PubMed] [Google Scholar]
- Ng KH, Sharma M, Benavente O, Gioia L, Field TS, Hill MD, et al. Dabigatran following acute transient ischemic attack and minor stroke (DATAS II). International Journal of Stroke 2017;12:910-4. [DOI] [PubMed] [Google Scholar]
Dehen 1994 {unpublished data only}
- Dehen H, on behalf of Laboratoire Houde. Double blind placebo controlled study of dermatan sulphate in acute middle cerebral artery infarct [Etude en double aveugle, controlee contre placebo, du dermatane sulfate (RU 54 701) dans le traitement precoce de l'infarctus sylvien]. Unpublished work.
Diener 2005 {published data only}
- Diener HC, Ringelstein EB, Kummer R, Harenberg J, Koppenhagen K, Landgraf H, et al. Prevention of thromboembolic complications in acute ischemic stroke by the low-molecular-weight heparin certoparin (PROTECT). Stroke 2005;36:420. [Google Scholar]
Dong 2003 {published data only}
- Dong Q, Lu CZ, Zhen HM. Low-molecular-weight heparin for the treatment of acute ischemic stroke: a randomized controlled trial. Neurology 2003;60:A228. [Google Scholar]
Donnan 1996 {published data only}
- Donnan GA, Davis SM, Gerraty RP, Mitchell PJ, Fitt G, Bladin CF, et al. Australian urokinase stroke trial (AUST). Cerebrovascular Diseases 1996;6 Suppl 2:25. [Google Scholar]
Dumas 1994 {published data only}
- Dumas R, Woitinas F, Kutnowski M, Nikolic I, Berberich R, Adeninpour F, et al. A multicentre, double-blind, randomized study to compare the safety and efficacy of once-daily org 10172 and twice-daily low dose heparin in preventing deep-vein thrombosis in patients with acute ischemic stroke. Age and Ageing 1994;23:512-6. [DOI] [PubMed] [Google Scholar]
Egan 1999 {published data only}
- Egan R, Clark W, Lutsep H, Nesbit G, Barnwell S, Kellogg J. Efficacy of intraarterial thrombolysis of basilar artery stroke. Journal of Stroke and Cerebrovascular Diseases 1999;8:22-7. [DOI] [PubMed] [Google Scholar]
Egberts 1996 {unpublished data only}
- Egberts JFM, Sommer W. Multicentre, randomised, assessor-blind, dose-comparative study of org 10172 (orgaron (r)) in the treatment of acute ischaemic stroke. Unpublished data 1996.
ELAN 2017 {published data only}
- Fischer U, Paciaroni M, Strbian D, Seiler S, Ntaios G, Nedeltchev K, et al. Early versus late initiation of direct oral anticoagulation in postischaemic stroke patients with atrial fibrillation (ELAN). European Stroke Journal 2017;2:492-3. [Google Scholar]
- Fischer U, Strbian D, Paciaroni M, Thomalla G, Ntaios G, Bonati L, et al. Early versus late initiation of direct oral anticoagulants in postischaemic stroke patients with atrial fibrillation (ELAN): an international multicentre, randomised-controlled, two-arm, assessor-blinded trial. European Stroke Journal 2019;4:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMT 1992 {unpublished data only}
- European Multicenter Team. Randomized pilot study of org 10172 vs. heparin in acute stroke. Unpublished data 1992.
Enger 1965 {published data only}
- Enger E, Boyesen S. Long term anticoagulant therapy in patients with cerebral infarction: a controlled clinical study. Acta Medica Scandinavica 1965;178 Suppl 438:7-61. [PubMed] [Google Scholar]
England 2010 {published data only}
- England TJ, Bath PMW, Sare GM, Geeganage C, Moulin T, O'Neill D, et al. Asymptomatic hemorrhagic transformation of infarction and its relationship with functional outcome and stroke subtype: assessment from the Tinzaparin in Acute Ischaemic Stroke Trial. Stroke 2010;41:2834-9. [DOI] [PubMed] [Google Scholar]
Eriksson 1983 {published data only}
- Eriksson S-E, Link H. Evaluation of anticoagulants in patients with cerebral infarction with slight to moderate neurological deficit. Acta Neurologica Scandinavica 1983;68:96-106. [DOI] [PubMed] [Google Scholar]
Fang 2015 {published data only}
- Fang Y, Wenrui J, Ya B, Junliang H, Xueedong L, Guangyun Z, et al. Treatment of rivaroxaban versus aspirin for non-disabling cerebrovascular events (TRACE): study protocol for a randomized controlled trial. BMC Neurology 2015;15:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fassbender 1998 {published data only}
- Fassbender K, Eschenfelder C, Hennerici M. Effects of rt-pa thrombolysis and heparin treatment on fibrinolysis and coagulation in acute ischemic stroke. Cerebrovascular Diseases 1998;8 Suppl 4:16. [Google Scholar]
Field 2017 {published data only}
- Field TS, Hill M, Benavente O, Demchuck A, Dowlatshahi D, Honig A. SECRET: safety of rivaroxaban for cerebral venous thrombosis. International Journal of Stroke 2017;12:22. [Google Scholar]
Fradley 2014 {unpublished data only}
- Fradley M. Apixaban for early prevention of recurrent embolic stroke and hemorrhagic transformation. Unpublished data 2014. [DOI] [PMC free article] [PubMed]
Geisler 2017 {published data only}
- Geisler T, Poli S, Meisner C, Schreieck J, Zuern CS, Nagele T, et al. Apixaban for treatment of embolic stroke of undetermined source (atticus randomized trial): rationale and study design. International Journal of Stroke 2017;12:985-90. [DOI] [PubMed] [Google Scholar]
Gelmers 1980 {published data only}
- Gelmers HJ. Effects of low-dose subcutaneous heparin on the occurrence of deep vein thrombosis in patients with ischemic stroke. Acta Neurologica Scandinavica 1980;61:313-8. [DOI] [PubMed] [Google Scholar]
Grond 2000 {published data only}
- Grond M, Schneweis S, Heiss WD. Risk of concomitant heparin treatment in systemic thrombolysis of acute ischemic stroke. Stroke 2000;31:2884. [Google Scholar]
HAEST 2000 {published data only}
- Berge E, Abdelnoor M, Nakstad PH, Sandset PM, on behalf of the HSG. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. Lancet 2000;355:1205-10. [DOI] [PubMed] [Google Scholar]
Hillbom 1999 {published data only}
- Hillbom M, Erila T, Sotaniemi K, Floschbach CW, Tatlisumak T, Sarna S, et al. Comparison of the efficacy and safety of the low-molecular-weight heparin enoxaparin with unfractionated heparin in the prevention of deep vein thrombosis in patients with acute ischemic stroke. Blood 1999;94:183a. [Google Scholar]
Hillbom 2002 {published data only}
- Hillbom M, Erila T, Sotaniemi K, Tatlisumak T, Sarna S, Kaste M. Enoxaparin vs heparin for prevention of deep-vein thrombosis in acute ischaemic stroke: a randomized, double-blind study. Acta Neurologica Scandinavica 2002;106:84-92. [DOI] [PubMed] [Google Scholar]
Hossman 1986 {published data only}
- Hossman V, Loettgen J, Auel H, Bewermeyer H, Heiss WD. Prophylaxis of deep vein thrombosis in acute stroke. A prospective, randomised double blind study. Haemostasis 1986;16 Suppl 5:54. [Google Scholar]
Hui 2016 {published data only}
- Hui Y. The clinical efficacy research for the treatment among intravenous unfractionated heparin, two antiplatelet agents (clopidogrel and aspirin) and single antiplatelet agent (aspirin) to acute cerebral infarction and transient ischemic attack (TIA). Unpublished data 2016.
IRCT2014020213698N1 {unpublished data only}
- IRCT2014020213698N1. The study of the efficacy, safety and tolerability of low molecular weight heparin vs. unfractionated heparin in patients with embolic stroke due to atrial fibrillation. https://www.irct.ir/trial/13492 (first received 2 February 2014). [PMC free article] [PubMed]
- NCT02159287. Study of the efficacy, safety and tolerability of low molecular weight heparin vs. unfractionated heparin in stroke. https://clinicaltrials.gov/ct2/show/NCT02159287 (first received 9 June 2014).
IRCT201407213106N18 {published data only}
- IRCT201407213106N18. Evaluation of different doses administration of clopidogrel in acute ischemic stroke. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201407213106N18 (first received 5 March 2015).
ISRCTN25644448 {unpublished data only}
- Ferro JM, Miranda B. Extending oral anticoagulant treatment after acute cerebral vein thrombosis (EXCOA-CVT): a cluster randomised study. European Stroke Conference 2014.
- ISRCTN25644448. The benefit of extending oral blood thinning (anticoagulant) treatment after acute blockage of cerebral veins (cerebral venous thrombosis-CVT) (EX-COA CVT). https://www.isrctn.com/search?q=25644448 (first received 10 April 2014).
Ji 2017 {published data only}
- Jin F. A randomized controlled study on the second-level prevention of dabigatran and warfarin in atrial fibrillation associated ischemic stroke/TIA acute phase. Unpublished data 2017.
Jin 2017 {unpublished data only}
- Jin F. A randomized controlled study on the second-level prevention of dabigatran and warfarin in atrial fibrillation associated ischemic stroke/TIA acute phase. Unpublsihed data 2017.
JPRN‐UMIN000014405 {published data only}
- JPRN-UMIN000014405. Argatroban, aspirin and clopidgrel combination therapy for non-cardioembolic acute ischemic stroke - a prospective, randomized, argatroban and aspirin-controlled study. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000014405 (first received 27 June 2014).
JPRN‐UMIN000025392 {published data only}
- JPRN-UMIN000025392. Optimal antithrombotic therapy in ischemic stroke patients with non-valvular atrial fibrillation and atherothrombosis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000025392 (first received 1 February 2017).
Kamel 2018 {published data only}
- Kamel H. Atrial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke. International Journal of Stroke 2019;14:207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2010 {published data only}
- Kang K, Kim DW, Park H-K, Yoon B-W. Optimal dosing of intravenous unfractionated heparin bolus in transient ischaemic attack or stroke. Clinical and Applied Thrombosis/Haemostasis 2010;16(2):126-31. [DOI] [PubMed] [Google Scholar]
Kario 1995 {published data only}
- Kario K, Kodama K, Koide M, Matsuo T. Thrombin inhibition in the acute phase of ischaemic stroke using argatroban. Blood Coagulation and Fibrinolysis 1995;6:423-7. [DOI] [PubMed] [Google Scholar]
Kataoka 2000 {published data only}
- Kataoka S, Hirose G, Hori A, Shirakawa T. Early anticoagulation with heparin in vertebrobasilar occlusive disease. Journal of Stroke and Cerebrovascular Diseases 2000;9(2 Suppl 1):185-6. [Google Scholar]
Kim 2003 {published data only}
- Kim WJ, Lee KM, Park KW, Kim SH, Kim JW, Jeong MH, et al. Changes of platelet activation and clinical outcome in acute atherosclerotic cerebral infarction treated with clopidogrel loading dose (300 mg). Journal of the Korean Neurological Association 2003;21:128-33. [Google Scholar]
Kobayashi 1997 {published data only}
- Kobayashi S, Tazaki Y. Effect of the thrombin inhibitor argatroban in acute cerebral thrombosis. Seminars in Thrombosis and Hemostasis 1997;23:531-4. [DOI] [PubMed] [Google Scholar]
Lee 1994 {published data only}
- Lee JH, Seo DC, Kim JS, Lee MC. Therapeutic efficacy of urokinase and heparin in acute ischemic stroke. Stroke 1994;25:268. [Google Scholar]
Lee 2008 {published data only}
- Lee SH, Ahn YM, Ahn SY, Doo HK, Lee BC. Interaction between warfarin and panax ginseng in ischaemic stroke patients. Journal of Alternative and Complementary Medicine 2008;14(6):715-21. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee SH, Kim HY, Bae HJ, Yu KH, Kim GM, Kim DE, et al. Clopidogrel and aspirin versus aspirin alone for prevention of recurrent ischemic lesion in acute atherothrombotic stroke: a randomized, double-blind, placebo-controlled trial. Stroke 2014;45:ATP334. [Google Scholar]
Liu 2015 {published data only}
- Liu MC, Li FP, Han YL, Chen HS. Argatroban versus aspirin plus clopidogrel in the treatment of acute ischemic stroke: a pilot, randomised, open-label study. Medical Journal of Chinese People's Liberation Army 2015;40(6):433-9. [Google Scholar]
Lu 2001 {published data only}
- Lu CZ. Low-molecular-weight heparin for the treatment of acute ischemic stroke: a randomized controlled trial. Hong Kong Medical Journal 2001;7:9-10. [Google Scholar]
Luo 2013 {published data only}
- Luo W-L, He Y, Lee M, Ceng W. Safety and effects of using warfarin early in adult patients with cerebral venous and sinus thrombosis. Cerebrovascular Diseases 2013;36 Suppl 1:51. [Google Scholar]
McCarthy 1993 {unpublished data only}
- McCarthy S, McWalter R, Durkin CJ, Hallawi AH, Magnani HN, Pearson J, et al. Org 10172 for the prophylaxis of deep venous thrombosis in the legs (A protocol to establish the efficacy and safety of once or twice daily subcutaneous Org 10172 injections versus placebo in three groups of 60 non-haemorrhagic stroke victims). Unpublished protocol.
McDevitt 1959 {published data only}
- Groch SN, McDevitt E, Wright IS. A long-term study of cerebral vascular disease. Annals of Internal Medicine 1961;55:358-67. [DOI] [PubMed] [Google Scholar]
- McDevitt E, Groch SN, Wright IS. A cooperative study of cerebrovascular disease. Circulation 1959;20:215-23. [DOI] [PubMed] [Google Scholar]
- McDowell F, McDevitt E, Wright IS. Anticoagulant therapy: five years' experience with the patient with an established cerebrovascular accident. Archives of Neurology 1963;8:209-14. [Google Scholar]
- McDowell F, McDevitt E. Treatment of the completed stroke with long-term anticoagulant: six and one-half years experience. In: Siekert RG, Whisnant JP, editors(s). Cerebral Vascular Diseases. New York: Grune & Stratton Inc, 1965:185-99. [Google Scholar]
Melero 2007 {published data only}
- Melero MJ, Bergroth B, Contardo DM, Mazzei MM, Ricci S, Celani MG, et al. Prevention of venous thromboembolism after acute ischaemic stroke. Lancet 2007;370:735-7. [DOI] [PubMed] [Google Scholar]
Meredit 2009 {published data only}
- Meredit T, Ahmad O, Harvey I, Hughes A, Lindley R, Neeman T, et al. The Acute Cardioembolic Stroke Trial. International Journal of Stroke 2009;4 Suppl 1:20-1. [Google Scholar]
Moulin 1994 {published data only}
- Moulin T, Crepin-Leblond T. Protocol: Phase III trial using heparin full dose versus low weight heparinoid (preventive dose) in patients with carotid ischemia (< 24 hours). Unpublished data 1994.
Muck 1997 {published data only}
- Muck AOHH, Heinrich F. How effective is low molecular weight heparin combined with dihydroergotamine? Prevention of thromboembolism in acute ischemic stroke. Krankenhausarzt 1997;70:359-63. [Google Scholar]
NAVIGATE ESUS 2014 {published data only}
- Ameriso S, Lavados P, Arauz A, Gagliardi RJ, Shoamanesh A, Pater C, et al. NAVIGATE ESUS: phase-III RCT assessing prevention of stroke and systemic embolism in patients with embolic stroke of undetermined source. Journal of the Neurological Sciences 2015;357 Suppl 1:e364-5. [Google Scholar]
- Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. New England Journal of Medicine 2018;378:2191-201. [DOI] [PubMed] [Google Scholar]
- Hart RG, Sharma M, Mundl H, Shoamanesh A, Kasner SE, Berkowitz SD, et al. Rivaroxaban for secondary stroke prevention in patients with embolic strokes of undetermined source: design of the NAVIGATE ESUS randomized trial. European Stroke Journal 2016;1:146-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Figueroa C, Chen H, Pudov E, Yang Y, Brola W, et al. Rivaroxaban for secondary prevention in patients with embolic strokes of undetermined source: design of the NAVIGATE ESUS randomized trial. European Stroke Journal 2017;2:334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Field T, Teal P, Coutts S, Shuaib A, Stotts G, et al. Navigate ESUS: multicenter, randomized, double-blind, phase III study of prevention of recurrent stroke and systemic embolism in patients with recent embolic stroke of undetermined source. International Journal of Stroke 2015;10:36-7. [Google Scholar]
- Veltkamp R, Davalos A, Toni D, Cunha L, Brouns R, Muir K, et al. Navigate ESUS: multicenter, randomized, double-blind, phase III study of prevention of recurrent stroke and systemic embolism in patients with recent embolic stroke of undetermined source. International Journal of Stroke 2015;10:431-2. [Google Scholar]
NCT01924325 {published data only}
- NCT01924325. Apixaban versus Dual-antiplatelet therapy (clopidogrel and aspirin) in Acute Non-disabling Cerebrovascular Events (ADANCE). https://clinicaltrials.gov/ct2/show/NCT01924325 (first received 16 August 2013).
NCT02221102 {published data only}
- NCT02221102. Edoxaban for TIA and acute minor stroke. https://clinicaltrials.gov/show/NCT02221102 (first received 20 August 2014).
NCT02283294 {published data only}
- NCT02283294. A randomized controlled study on the second-level prevention of dabigatran and warfarin in atrial fibrillation associated ischemic stroke/TIA acute phase. https://clinicaltrials.gov/ct2/show/NCT02283294 (first received 5 November 2014).
NCT03961334 {published data only}
- NCT03961334. MidregiOn proatrial natriuretic peptide to guide SEconadry Stroke prevention: the MOSES-study. An international, multicentre, randomised-controlled, two-arm, assessor-blinded trial. https://clinicaltrials.gov/ct2/show/NCT03961334 (first received 23 May 2019).
OPTIMAS 2018 {published data only}
- Best J, Bennett K, Caverly E, Cohen H, Dore C, Fenner R, et al. Optimas: a randomised controlled trial to establish the optimal timing of anticoagulation after acute ischaemic stroke. European Stroke Journal 2019;4:810. [Google Scholar]
PREVAIL 2004 {published data only}
- Sherman DG, Albers GW, Bladin C, Fieschi C, Gabbai AA, Kase CS, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL study): an open-label randomised comparison. Lancet 2007;369:1347-55. [DOI] [PubMed] [Google Scholar]
RAPID 2005 {published data only}
- Chamorro A, Busse O, Obach V, Toni D, Sandercock P, Reverter JC, et al. The Rapid Anticoagulation Prevents Ischemic Damage study in acute stroke - final results from the Writing Committee. Cerebrovascular Diseases 2005;19:402-4. [DOI] [PubMed] [Google Scholar]
RESPECT CVT 2018 {published data only}
- Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhao P, et al. Safety and efficacy of dabigatran etexilate vs dose-adjusted warfarin in patients with cerebral venous thrombosis: a randomized clinical trial. JAMA Neurology 2019;76:1457-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
RE‐SPECT ESUS 2019 {published data only}
- Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. New England Journal of Medicine 2019;380:1906-17. [DOI] [PubMed] [Google Scholar]
- Diener HC, Sacco RL, Easton JD, Granger CB, Cronin L, Fraessdorf M, et al. RE-SPECT ESUS, a study of dabigatran versus acetylsalicylic acid for stroke prevention in patients with embolic stroke of undetermined source: baseline predictors of recurrent stroke. European Stroke Journal 2019;4:262-3. [Google Scholar]
Rha 2001 {published data only}
- Rha JH. Acute ischemic stroke trial - oral aspirin vs intravenous. Heparin on stroke progression. Aist-ash 2001.
Rose 2019 {published data only}
- Rose DZ, Meriwether JN, Fradley MG, Renati S, Martin RC, Kasprowicz T, et al. Protocol for AREST: apixaban for early prevention of recurrent embolic stroke and hemorrhagic transformation: a randomized controlled trial of early anticoagulation after acute ischemic stroke in atrial fibrillation. Frontiers in Neurology 2019;10:https://doi.org/10.3389/fneur.2019.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosin 1994 {unpublished data only}
- Rosin A, Abramovitz Z. Preventing deep venous thrombosis following acute strokes. Unpublished protocol.
Shi 2004 {published data only}
- Shi J, Zhang Y. The clinical observation of low molecular weight heparin calcium in treating patients with acute cerebral infarction. Pharmaceutical Care and Research 2004;4:258-9. [Google Scholar]
SOCRATES 2016 {published data only}
- Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans S, et al. Ticagrelor vs. aspirin in acute stroke or transient ischemic attack: primary results of the socrates randomized trial. European Stroke Journal 2016;1:708-9. [Google Scholar]
- Wong KSL, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Efficacy and safety of ticagrelor in relation to prior aspirin usage in the Socrates trial. Stroke 2018;49:1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stiekema 1988 {unpublished data only}
- Stiekema JCJ, Egberts J, Voerman J. An open, randomised, rising dose, pilot two-centre study of Org 10172 administered for the purpose of deep vein thrombosis prophylaxis in patients with a non-haemorrhagic stroke on recent onset. Organon International 1988 (SDG Release Report 2244).
TAIST 2001 {published data only}
- Investigators Team, Bath PM, editors. Tinzaparin in acute ischaemic stroke trial (TAIST). Unpublished data 2000.
Tan 1998 {published data only}
- Tan L, Li TS, Zhao RL. Nardoparin in treating cerebral embolism and thrombosis. Chinese Journal of New Drugs and Clinical Remedies 1998;17:325-6. [Google Scholar]
Thygesen 1964 {published data only}
- Thygesen P, Christensen E, Dyrbye M, Eiken M, Franzen E, Gormsen J, et al. Cerebral apoplexy: a clinical, radiological, electroencephalographic and pathological study with special reference to the prognosis of cerebral infarction and the result of long-term anticoagulant therapy. Danish Medical Bulletin 1964;11:233-57. [PubMed] [Google Scholar]
Tomek 2011 {published data only}
- Tomek A, Matoska V, Kumstyrova T, Sramek M, Sarbochova I, Stovickova K, et al. Warfarin loading dose guided by pharmacogenetics is effective and safe in cardioembolic stroke patients. Cerebrovascular Diseases 2011;31 Suppl 2:31. [Google Scholar]
Toni 2000 {published data only}
- Toni D, Argentino C, Fieschi C. Heparin versus aspirin in ischaemic stroke. Lancet 2000;356:504-5. [DOI] [PubMed] [Google Scholar]
Trencev 2008 {published data only}
- Trencev R, Stojanov M, Petrovska-Cvetkovska D, Georgievska A, Baneva N, Radulovic-Bekarovska S. Low-molecular-weight heparin vs aspirin in treatment of cardioembolic stroke. International Journal of Stroke 2008;3:315-6. [Google Scholar]
Triple AXEL 2015 {published data only}
- Hong KS, Choi YJ, Kwon SU. Rationale and design of triple axel: trial for early anticoagulation in acute ischemic stroke patients with nonvalvular atrial fibrillation. International Journal of Stroke 2015;10:128-33. [DOI] [PubMed] [Google Scholar]
- Hong KS, Kwon SU, Lee SH, Lee JS, Kim YJ, Song TJ, et al. Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: a randomized clinical trial. JAMA Neurology 2017;74:1206-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KS, Lee JS, Kim YJ, Kim YD, Kang HG, Park MS, et al. Trial of early anticoagulation with rivaroxaban versus warfarin in patients with and acute cerebral ischemia. European Stroke Journal 2016;1:699-700. [Google Scholar]
Tsuchiya 1989 {published data only}
- Tsuchiya T, Fujikake K, Oku K. Effects of urokinase and heparin on haemorrhagic infarction, recanalisation, recurrence - analysis of 111 cases with middle cerebral artery occlusion on a prospective controlled trial [author's translation]. Japanese Journal of Stroke 1989;11:500-10. [Google Scholar]
Tsuchiya 1990 {published data only}
- Tsuchiya T, Fujikake K, Oku K. A study on clinical effects of urokinase and heparin for acute lacunar infarcts in a prospective controlled trial. Japanese Journal of Stroke 1990;11:177-84. [Google Scholar]
Turpie 1991 {published data only}
- Turpie AGG, Levine MN, Powers PJ, Ginsberg JG, Jay RM, Klimek M, et al. A double blind randomized trial of org 10172 low molecular weight heparinoid versus unfractionated heparin in the prevention of deep venous thrombosis in patients with thrombotic stroke. Thrombosis and Haemostasis 1991;65(6):753. [Google Scholar]
Turpie 1992 {published data only}
- Turpie AGG, Gent M, Cote R, Levine MN, Ginsberg JS, Powers PJ, et al. A low molecular weight heparinoid compared with unfractionated heparin in the prevention of deep vein thrombosis in patients with acute ischemic stroke. Annals of Internal Medicine 1992;117:353-7. [DOI] [PubMed] [Google Scholar]
VA Study 1961 {published data only}
- Baker RN. An evaluation of anticoagulant therapy in the treatment of cerebrovascular disease. Report of the Veterans Administration Cooperative Study of Atherosclerosis, Neurology Section. Neurology 1961;11:132-8. [DOI] [PubMed] [Google Scholar]
Warach 2003 {published data only}
- Warach S. Combination anti-platelet and anti-coagulation treatment after lysis of ischemic stroke trial. Unpublished data 2003.
Wellington 2002 {published data only}
- Wellington S. An open-label randomised parallel-group multi-centre study to evaluate the efficacy and safety of enoxaparin versus unfractionated heparin in the prevention of venous thromboembolism in patients following acute ischaemic stroke. Unpublished data 2002.
Wong 2000 {published data only}
- Wong WJ, Lo YK, Sheng WY, Hu HH. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in progressive ischemic stroke. Journal of Stroke and Cerebrovascular Diseases 2000;9(2 Suppl 1):183-4. [Google Scholar]
Yamaguchi 2004 {published data only}
- Yamaguchi T. Edaravone and argatroban stroke therapy study for acute ischemic stroke (EAST). Unpublished data 2004.
Yang 2016 {published data only}
- Yang L, Zhao P, Zhao J, Wang J, Shi L, Wang X. Effects of ezetimibe and anticoagulant combined therapy on progressing stroke: a randomized, placebo-controlled study. Journal of Neurology 2016;263:2438-45. [DOI] [PubMed] [Google Scholar]
Yi 2015 {published data only}
- Yi X, Chi W, Wang C, Zhang B, Lin J. Low-molecular-weight heparin or dual antiplatelet therapy is more effective than aspirin alone in preventing early neurological deterioration and improving the 6-month outcome in ischemic stroke patients. Journal of Clinical Neurology 2015;11(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2005 {published data only}
- Zhang DJ, Zhu SW, Cui QX, Li YZ, Zhang HM, Xia ZL, et al. Clinical study of low molecular weight heparin calcium and aspirin therapy on acute cerebral infarction. Clinical Pharmacology 2005;40:634-6. [Google Scholar]
Zhu 2014 {published data only}
- Zhu X, Wang J, Cheng Y. Argatroban in Chinese patients with acute cerebral infarction: a randomized, double-blind, positive-controlled study. Latin American Journal of Pharmacy 2014;33:903-7. [Google Scholar]
Zuo 2017 {published data only}
- Zuo FT, Liu H, Wu HJ, Su N, Liu JQ, Dong AQ. The effectiveness and safety of dual antiplatelet therapy in ischemic cerebrovascular disease with intracranial and extracranial arteriostenosis in chinese patients. Medicine 2017;96:e5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800018313 {published data only}
- ChiCTR1800018313. A multicenter trial for revascularization pretreated with argatroban for acute ischemic stroke. https://www.chictr.org.cn/showproj.aspx?proj=30871 (first received 11 September 2018).
ISRCTN76741621 {published data only}
- ISRCTN76741621. Endovascular treatment for acute ischemic stroke; the use of periprocedural heparin or antiplatelet agents. https://www.isrctn.com/search?q=ISRCTN76741621 (first received 6 December 2017).
NCT03506009 {published data only}
- NCT03506009. Argatroban Plus r-tPA for Posterior Circulation Infarction (AR-PCI). https://clinicaltrials.gov/ct2/show/NCT03506009 (first received 23 April 2018).
NCT03570281 {published data only}
- NCT03570281. Edoxaban for the treatment of coagulopathy in patients with active cancer and acute ischemic stroke: a pilot study (ENCHASE study). https://clinicaltrials.gov/ct2/show/NCT03570281 (first received 26 June 2018).
NCT03735979 {published data only}
- NCT03735979. Multi-arm optimization of stroke thrombolysis (MOST). https://clinicaltrials.gov/ct2/show/NCT03735979 (first received 8 November 2018).
NCT03740958 {published data only}
- NCT03740958. Argatroban plus R-tPA for acute ischemic stroke. https://clinicaltrials.gov/ct2/show/NCT03740958 (first received 14 November 2018).
NCT04275180 {published data only}
- NCT04275180. Clinical study of argatroban in the treatment of acute progressive ischemic stroke. https://clinicaltrials.gov/ct2/show/NCT04275180 (first received 19 February 2020).
NCT04304508 {published data only}
- NCT04304508. Study to gather information about proper dosing and safety of the oral fxia inhibitor bay 2433334 in patients following a recent non cardioembolic ischemic stroke which occurs when a blood clot has formed somewhere in the human body (but not in the heart) travelled to the brain. https://clinicaltrials.gov/ct2/show/NCT04304508 (first received 11 March 2020).
Additional references
AHA Guidelines 2019
- William P, Alejandro AR, Teri A, Opeolu MA, Nicholas CB, Kyra B, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019;50:e344-e418. [DOI: ] [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Matthews JNS. Interaction 1: heterogeneity of effects. BMJ 1996;313:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
APT 1994
- Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy. I. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81-106. [PMC free article] [PubMed] [Google Scholar]
ATC 2002
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bamford 1990
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: The Oxfordshire Community Stroke Project 1981-1986. 2. Incidence, case fatality and overall outcome at one year of cerebral infarction, primary intracerebral haemorrhage and subarachnoid haemorrhage. Journal of Neurology, Neurosurgery and Psychiatry 1990;53:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2021
- Boutron I, Page MJ, Higgins JT, Altman DG, Lundh A, Hróbjartsson A. Chapter 7. Considering bias and conflicts of interest among the included studies. In: Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
CLOTS‐3 2013
- CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet 2013;382:516-24. [DOI] [PubMed] [Google Scholar]
Collins 1988
- Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. New England Journal of Medicine 1988;318:1162-73. [DOI] [PubMed] [Google Scholar]
Coutinho 2011
- Coutinho J, Bruijn SFTM, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: CD002005. [DOI: 10.1002/14651858.CD002005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davenport 1996
- Davenport R, Dennis M, Wellwood I, Warlow C. Complications after acute stroke. Stroke 1996;27:415-20. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JT, Altman DG (editors). Chapter 10. Analysing data and undertaking meta-analyses. In: Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feigin 2009
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009 Feb 21;8:355-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Feigin 2014
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383(9913):245-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hao 2012
- Hao Z, Liu M, Counsell C, Wardlaw JM, Lin S, Zhao X. Fibrinogen depleting agents for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD000091. [DOI: 10.1002/14651858.CD000091] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. [COCHRANE: www.training.cochrane.org/handbook.]
Kyu‐2018
- Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018;392:1859–922. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lederle 2011
- Lederle FA, Zylla D, MacDonald R, Wilt T. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Annals of Internal Medicine 2011;155:602-15. [DOI] [PubMed] [Google Scholar]
Lindley 1993
- Lindley RI. Are very large trials of promising treatments for acute stroke feasible?. Newcastle Upon Tyne: University of Newcastle Upon Tyne, 1993. [Google Scholar]
Lyrer 2010
- Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD000255. [DOI: 10.1002/14651858.CD000255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Odgaard‐Jensen 2011
- Odgaard-Jensen J, Vist GE, Timmer A, Kunz R, Akl EA, Schünemann H, et al. Randomisation to protect against selection bias in healthcare trials. Cochrane Database of Systematic Reviews 2011, Issue Issue 4. Art. No: MR000012. [DOI: 10.1002/14651858.MR000012.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
PREVAIL 2007
- Sherman DG, Albers GW, Bladin C, Fieschi C, Gabbai AA, Kase CS, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL study): an open-label randomised comparison. Lancet 2007;369:1347-55. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sandercock 2008
- Sandercock PAG, Counsell C, Tseng MC. Low molecular weight heparin or heparinoids versus standard unfractionated heparin for acute ischemic stroke. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD000119. [DOI: 10.1002/14651858.CD000119.pub3] [DOI] [PubMed] [Google Scholar]
Saxena 2001
- Saxena R, Lewis S, Berge E, Sandercock P, Koudstaal, P, the International Stroke Trial Collaborative Group. Risk of early death and recurrent stroke and effect of heparin in 3169 patients with acute ischemic stroke and atrial fibrillation in the International Stroke Trial. Stroke 2001;32:2333-7. [DOI] [PubMed] [Google Scholar]
Wardlaw 2014
- Wardlaw JM, Murray V, Berge E, Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD000213. [DOI: 10.1002/14651858.CD000213] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warlow 2008
- Warlow C, Bamford J, Dennis M, Rothwell P, Rinkel G, Sandercock P, et al. Stroke: Practical Management. 3rd edition. Blackwells Scientific, 2008. [Google Scholar]
Whiteley 2013
- Whiteley W, Adams HP, Bath PMW, Berge E, Sandset PM, Dennis M, et al. Targeted use of heparin, heparinoids, or low-molecular weight heparin to improve outcome after acute ischaemic stroke: an individual patient data meta-analysis of randomised controlled trials. Lancet Neurology 2013;12:539-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gubitz 2004
- Gubitz G, Sandercock P, Counsell C. Anticoagulants for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD000024. [DOI: 10.1002/14651858.CD000024.pub2] [DOI] [PubMed] [Google Scholar]
Sandercock 1993
- Sandercock PAG, den Belt AGM, Lindley RI, Slattery J. Antithrombotic therapy in acute ischaemic stroke: an overview of the completed randomised trials. Journal of Neurology, Neurosurgery and Psychiatry 1993;56:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]


