Abstract
Human pluripotent stem cells (PSCs), which have the capacity to self-renew and differentiate into multiple cell types, offer tremendous therapeutic potential and invaluable flexibility as research tools. Recently, remarkable progress has been made in directing myogenic differentiation of human PSCs. The differentiation strategies, which were inspired by our knowledge of myogenesis in vivo, have provided an important platform for the study of human muscle development and modelling of muscular diseases, as well as a promising source of cells for cell therapy to treat muscular dystrophies. In this review, we summarize the current state of skeletal muscle generation from human PSCs, including transgene-based and transgene-free differentiation protocols, and 3D muscle tissue production through bioengineering approaches. We also highlight their basic and clinical applications, which facilitate the study of human muscle biology and deliver new hope for muscular disease treatment.
Keywords: Human pluripotent stem cells (PSCs), Skeletal muscle, Tissue engineering, Muscular dystrophy, Myogenesis, Satellite cells
1. Introduction
Skeletal muscle, which makes up almost half of the human body mass, plays a critical role in movement and metabolism [1]. This tissue originates from the paraxial mesoderm, and forms from dermomyotome precursors through two main consecutive waves known as primary and secondary myogenesis (reviewed in [2,3]). Functional muscles are composed of contractile multinucleated myofibers, satellite cells (SCs), connective tissue, blood vessels, motor and sensory axons, Schwann cells and immune cells. Crosstalk between these different cell types is essential not only for muscle contraction, but also for muscle development, homeostasis and regeneration. Moreover, skeletal muscle possesses the capacity to regenerate after injury caused by overuse, disease, trauma or toxins. This process is primarily accomplished by SCs, the muscle stem cells lying under the basal lamina of myofibers [4-7].
Effective methods to restore or improve muscle function in patients suffering from muscle disorders are required for the alleviation of symptoms and improvement in their quality of life. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), collectively referred to as pluripotent stem cells (PSCs), have almost unlimited proliferative potential and the ability to differentiate into multiple lineages, and thus they constitute a promising source for cell-based therapies and drug screening [8]. Moreover, iPSCs allow the creation of patient-derived cells, which enables disease modelling in vitro. The production of myogenic cells from PSCs was first observed after differentiation of embryoid bodies derived from mouse ESCs [9]. The recent development of transgene-based and transgene-free strategies has made it possible to generate skeletal muscle in vitro more efficiently [2,3,10]. While transgene-based protocols can achieve direct myogenic differentiation with overexpression of transcription factors, transgene-free protocols manipulate signaling pathways using small molecules and growth factors to recapitulate the muscle development process in vitro [10]. Therefore, the stepwise transgene-free approach not only has immediate therapeutic implications, but also provides a platform for understanding the basic developmental biology of human myogenic lineage differentiation. In this review, we summarize the remarkable progress in generating myogenic cells in vitro from human PSCs and discuss both basic and clinical applications.
2. Making muscle in vitro
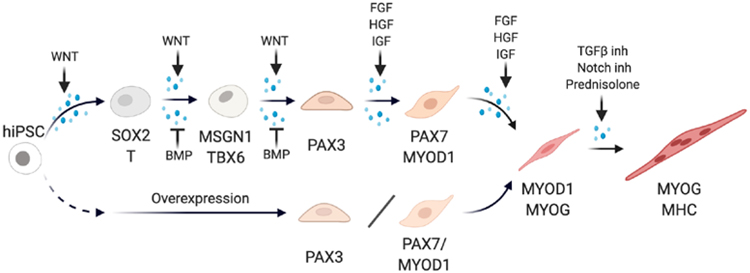
The road towards creating muscle in a dish started 30 years ago, when transfection of the transcription factor MyoD1 into fibroblasts was shown to induce their myogenic conversion [11]. In the last 10 years, there has been a surge of protocols for myogenic differentiation from human PSCs [12]. The two main strategies include (1) myogenic induction by overexpression of transcription factors and (2) stepwise induction of skeletal muscle by mimicking signaling pathways deployed during development (Fig. 1).
Fig. 1. Strategies of skeletal muscle production from hiPSCs.
The process of in vitro muscle generation by the stepwise differentiation protocol (top) or transgenic induction (bottom). The stepwise strategy uses small molecules and growth factors to manipulate critical signaling pathways in myogenesis while the transgenic approach induces myogenic differentiation by overexpression of core transcription factors. Key marker genes at different differentiation stages and key pathways manipulated in the stepwise protocol are indicated.
2.1. Myogenic induction by overexpression of transcription factors
Selective overexpression of myogenic transcription factors, such as MYOD1, was the first reported approach to induce myogenic differentiation [12,13]. Such transgenic approaches traditionally require the transduction of the myogenic construct via adenovirus [14], lentivirus [15,16] or piggyback transposons [17-19]. Additionally, the timing of overexpression can be controlled by inducible systems such as tetracycline/doxycycline [15,17-19] or tamoxifen [16].
Even though MYOD1 may seem the obvious candidate gene to induce the myogenic program in PSCs, it is expressed late in the differentiation of myogenic cells. Thus, overexpression of this transcription factor gives rise to terminally differentiated muscle cells but no muscle progenitors [10]. Moreover, human PSCs (hPSCs) are resistant to myogenic induction mediated by MYOD1 and require additional epigenetic cues to differentiate to skeletal muscle [13]. Protocols involving the use of PAX3 or PAX7, which are transcription factors expressed by myogenic progenitor cells (MPCs) and SCs, can give rise to MPCs able to differentiate into myotubes in vitro and endowed with the capacity to engraft in mouse muscles [15].
However, these protocols usually require a cell-sorting step in order to purify the precursor populations [20-22], thus complicating translational applications. Moreover, the required introduction of a transgene makes them less suitable for human cell-based therapies due to the risk of insertional mutagenesis [8,23]. This limitation was bypassed by Kim et al., who proposed an integration-free protocol using minicircle DNA vectors. While able to generate human iPSC (hiPSC)-derived PAX7+ MPCs, multiple transfections were required to maintain PAX7 expression and these MPCs showed limited engraftment potential [21]. On a similar note, Akiyama et al. proposed an integration-free protocol by introducing mRNA encoding MYOD1 together with siRNA-mediated knockdown of OCT4 [23]. This protocol reported efficient differentiation of PSCs into fetal-like myofibers, but no expression was reported for PAX7 or for MYH2, a marker of adult fast myofibers. Komatsu et al. proposed the use of an RNA virus-based episomal vector encoding MYOD1. This integration-free system induces persistent RNA transgene production and leads to the formation of Myosin Heavy Chain (MHC)-expressing myotubes. The novel advantage of this system is that the vector can be eliminated upon treatment with a small molecule, T-705, increasing the safety of this approach [24].
While still requiring integration, Kim et al. proposed using genomic safe harbor (GSH) loci for the integration of transgene components. The GSH-targeted PAX7 integration led to efficient generation of MPCs with lower proliferative capacity but similar engraftment potential in mice than lentivirus-induced MPCs [25].
Alternatively, Kwon et al. proposed to induce endogenous PAX7 activation using a CRISPR/Cas9-based transcriptional activator. MPCs obtained through endogenous PAX7 activation showed higher proliferation and engraftment capacity than by exogenous PAX7 overexpression [22].
Although transgenic-based methods do not recapitulate physiological development, they have become increasingly popular in the field. Research towards methodologies with higher security (e.g. integration-free approaches) and higher engraftment capacity (e.g. PAX7 overexpression) will progressively increase the suitability of these protocols towards human application.
2.2. Stepwise myogenic induction by mimicking development
Skeletal myogenesis can be recapitulated in vitro from PSCs by exposure to combinations of small molecules and growth factors, which activate the core signaling pathways deployed during development (reviewed in [2,3,10]). Most of these stepwise induction methods start with the formation of neuro-mesodermal progenitors (NMPs) which are characterized by the expression of SOX2 and Brachyury/T. This first step is achieved by treating PSCs with a Wnt activator such as the GSK3β inhibitor CHIR99021 (CHIR) [26-28]. Treating human PSC cultures with CHIR (in combination with the BMP inhibitor LDN) is sufficient to trigger Nodal activation, which is known to play a key role in mesoderm induction and patterning [27]. Thus, while some protocols include treatment with activin to activate Nodal at these early stages [29-31], this does not appear necessary as endogenous Nodal is activated following Wnt activation [32]. The same is true with FGF signaling, which is also found in several protocols aiming at differentiating paraxial mesoderm in vitro, but is spontaneously activated in cells downstream of Wnt activation [26,28]. Remarkably, Wnt activation in vitro appears sufficient to trigger an epithelium-to-mesenchyme transition (EMT) evoking cell ingression during gastrulation and resulting in the conversion to presomitic mesoderm (PSM)-like cells, indicated by MSGN1 and TBX6 expression [26].
Cell signaling in the culture also shows tight endogenous control which can be independent of the signaling activators added to the culture medium. For instance, both Wnt and FGF signaling are downregulated in vitro exactly as observed during PSM differentiation, where gradients of these two pathways control the segmentation process [33]. In vitro, their downregulation is observed despite the constant presence of the Wnt activator CHIR and of FGF, suggesting that it reflects an intrinsic regulation of the differentiation of the cells. This regulation involves a crosstalk between cell metabolism and signalling with FGF acting upstream of glycolysis to control Wnt activity [34]. Glycolysis acts by increasing the intracellular pH, thus promoting non-enzymatic β-catenin acetylation triggering Wnt activation required for paraxial mesoderm specification [35].
PSCs treated with CHIR in vitro differentiate towards a PSM fate but start to express BMP4 [36]. As BMP signaling promotes the lateral plate mesoderm fate, the induced paraxial mesoderm cells expressing BMP4 eventually lose their paraxial mesoderm identity to acquire a lateral plate fate [37]. BMP inhibition mediated by LDN-193189 in parallel with or after Wnt activation decreases BMP4 activation and stabilizes the paraxial mesoderm fate in vitro, leading to the recapitulation of a myogenic sequence very similar to that described for mouse in vivo [26]. Together, these treatments recapitulate the early stages of paraxial mesoderm induction resulting in the production of MPCs of the dermomyotome expressing PAX3 [26]. By comparing the transcriptional profiles of human PSM and somites, Xi et al. observed that both TGFβ and BMP signaling pathways are downregulated during human somite specification. Thus, they introduced treatment with a TGFβ inhibitor (SB-431542) along with BMP inhibition at the somite specification stage [38]. When further cultured with media containing the myogenic growth factors (FGF, HGF and IGF), PAX7+ MPCs and MHC+ myofibers are obtained [26,39].
These directed differentiation methods do not involve genetic modifications and thus may represent a more promising approach for cell therapy [26,38]. Moreover, the entire myogenic differentiation process in vitro closely resembles the in vivo myogenic sequence [26,40,41]. Therefore, it also provides a powerful tool to model human muscle development, which has been poorly understood due to limited access to human embryos.
3. Bioengineering approaches
A significant caveat of myogenic differentiation protocols in 2D is the non-physiological stiffness (~1 GPa) of the substrates employed, which do not mimic the physiological environment of myofibers (12-18 kPa). Traditional tissue culture substrates, such as plastic, do not support long-term spontaneous muscle contraction, leading to progressive myotube detachment and preventing maturation [42]. The growing knowledge of skeletal muscle microenvironment in vivo, as well as improvements in biomaterials, have allowed the development of 3D hiPSC-derived skeletal muscle tissues with greater biomimetic structure and function. Importantly, 3D engineered muscle tissues permit longer-term culture, leading to enhanced maturation of myotubes [42].
The field of tissue engineering has developed several techniques to mimic native skeletal muscle tissue, such as the creation of cell sheets [43], cell aggregates [44-47], fibrous scaffolds [48], hydrogels [20,49-51], and 3D bioprinting [52]. While initially these techniques were tested with primary myoblasts or immortalized myoblast cell lines, such as C2C12, recent studies have started to describe the use of hiPSC-derived skeletal muscle cells [20,44-47,51].
The first 3D systems supporting myogenic differentiation of PSCs were based on self-organization principles as observed in embryoid bodies (EBs), in which myogenesis can be spontaneously observed [9]. Hosoyama et al. proposed a method for the derivation of MPCs using a free-floating spherical culture (EZ spheres). Spheres containing PSCs were cultured in a medium rich in EGF and FGF2 for 6 weeks. Upon dissociation and culture, a portion of the resulting cells expressed PAX7, MYOD, and Myogenin [47]. A refined version of this protocol was presented by Jiwlawat et al., in which EZ spheres were dissociated and plated for a longer time resulting in more mature myofibers. Importantly, they also generated 3D muscle constructs with sphere-derived myogenic progenitors by using a mixture of collagen/Matrigel plated between two Velcro anchoring points [46]. This approach, based on the work of Van der Schaft et al. [53], gave rise to myofibers surrounded by basal lamina [46]. Chal et al. presented a similar muscle construct as a potential downstream application of their protocol to induce stepwise differentiation of hiPSCs towards the myogenic lineage [39]. These constructs, initially termed myooids by Dennis et al., consisted of fascicle-like muscle constructs ensembled around silk suture anchors [39,54].
Rao et al. combined the induction of paraxial mesoderm by GSK3β inhibition with a PAX7 inducible system to obtain MPCs. Then, they embedded those cells in a fibrin-based hydrogel and generated 3D induced-skeletal muscle (iSKM) bundles. Both PAX7+ cells and aligned contractile multinucleated myofibers were observed in the constructs. Although iSKM bundles still had a relatively immature expression pattern of MYH isoforms compared to adult muscle, they showed progressive maturation in their gene expression pattern, structure and function over time, while maintaining a PAX7+ cell pool. Importantly, skeletal muscle maturation in their 3D system exceeded that of their 2D system in molecular, structural and functional aspects, supporting the necessity of establishing 3D systems for successful maturation of the differentiating muscle [20]. Using the same 3D system, Khodabukus et al. showed that intermittent electrical stimulation significantly increased iSKM bundle growth, maturation and force generation independently of the frequency. Also, higher frequency stimulation resulted in greater myofiber hypertrophy and promoted a metabolic shift towards longer-chain fatty acid oxidation [55].
Recently, Maffioletti et al. used a MYOD1 overexpression protocol [16] to obtain hiPSC-derived myogenic cells. They embedded them in fibrin hydrogels polymerized between two silicone posts, which provided the tension necessary for myofiber alignment [51]. Moreover, in order to increase the complexity of these artificial tissues, the authors used established protocols to differentiate hiPSCs into vascular endothelial cells, pericytes and motor neurons [56,57]. Incorporation of ECs and pericytes into the artificial muscle resulted in the appearance of CD31+ vessel-like formations coexisting with myofibers. While no detailed characterization was made when all four cell types were added, the artificial constructs were stable and harbored cells with long axon-like processes resembling motor neurons. Hence, this supports the feasibility of more complex hiPSCs-based muscle scaffolds with the incorporation of supporting cell types [51].
Faustino Martins et al. described a protocol to derive hiPSC into NMPs and then in 3D organoids which contain both motoneurons and skeletal muscle fibers. NMP cells obtained from hiPSCs by activating Wnt and FGF signaling were induced to form 3D aggregates in the presence of FGF, HGF and IGF [44]. These structures progressively elongated and segregated into a neuroectodermal and a mesodermal part. Over time, maturation of these organoids gave rise to spinal cord motor neurons innervating skeletal muscle cells, with terminal Schwann cells capping the neuromuscular junction as observed in vivo. The authors reported the presence of PAX7+ cells and myofibers in the mesodermal part and showed that while the myofibers present were not aligned, these contained highly organized sarcomeric units [44].
The recent incorporation of 3D models might hold the key towards long-term culture and improved myofiber maturation, a current limitation of these systems.
4. Applications
4.1. Basic application: understanding human muscle development
The study of human muscle development has been a formidable task due to the limited access to human embryos and tissues [58]. However, the recent development of in vitro myogenic cell production strategies provides new tools for deciphering human myogenesis and the biology of skeletal muscle progenitors, SCs, and myofibers. These differentiating cells can be compared to mouse myogenic cells whose developmental programme has been well characterized. Thanks to the development of new imaging techniques such as light sheet fluorescence microscopy, which uses a plane of light to optically section the sample with high resolution [58], and to single cell omics [40,59], an increasing amount of data on human embryonic muscle is being generated. This data can be used to efficiently benchmark the data generated from human PSCs differentiated in vitro.
Stepwise myogenic induction protocols attempt to recapitulate skeletal muscle development in vivo including NMP induction, PSM and paraxial mesoderm formation, and primary and secondary myogenesis [2,3]. They can therefore be used to analyze in detail the roadmap of human myogenesis and to perform functional explorations of the differentiation process that would be impossible to carry out in vivo. Muscles of the trunk and neck are segmented structures periodically arrayed along the body axis. This repeated pattern is established during embryogenesis when the embryonic segments, the somites, are rhythmically produced from the PSM [60]. The rhythm of somite production is controlled by a molecular oscillator called segmentation clock, which has so far only been characterized in model organisms [61]. During the first two days of induction, following Wnt activator and BMP inhibitor treatment, hiPSCs acquire a posterior PSM fate [26,33]. Tracking the expression of oscillating genes of the segmentation clock such as HES7 in these hiPSC-derived PSM cells has allowed to identify the human segmentation clock, which ticks with a 5-hour period [33,62]. Further induction by manipulating core signaling pathways using small molecules and growth factors leads to the generation of skeletal muscle containing myofibers and PAX7+ myogenic precursors [26,38] which include the precursors of adult SCs [63,64]. Thus, hiPSC-derived PAX7+ cells provide an opportunity to investigate the development of the human SC lineage on which virtually nothing is known. Using iPSC reporter lines and single-cell RNA sequencing (scRNA-seq), the developmental trajectory of PAX7+ skeletal muscle progenitors has been characterized [40,41]. This also revealed their heterogeneity, which resembles that of mouse muscle progenitors and SCs in vivo [41].
In addition, this myogenic differentiation platform allows functional dissection of gene regulatory networks and signaling pathways during myogenesis. For instance, by comparing transcriptome differences between cells at different myogenic states, Choi et al. identified key factors and signaling pathways involved in myogenesis and discovered the role of the transcription factor TWIST1 in PAX7+ muscle progenitor cell maintenance [65]. Moreover, hiPSC-derived muscle production may permit the study of some important structures such as the neuromuscular (NMJ) and myotendinous junctions (MTJ) [44,66-68]. NMJs can be generated by co-culture of motor neurons and skeletal myotubes both in 2D and 3D culture systems [44,51,67,69].
Analyses of myogenic cells derived from mouse and human PSCs show that although they recapitulate muscle differentiation, these cells only reach the embryonic/fetal/perinatal stage [26,36,40,70,71]. Such is the case for most other lineages differentiated in vitro from PSCs, including cardiomyocytes, neurons or hepatocytes, for instance [72]. Using scRNA-seq, single-cell transcriptomic atlases were established for the hiPSC-derived skeletal muscle cells produced by three different transgene-free methods [26,38,73] and compared to human limb skeletal muscle cells at embryonic (week 5-8), fetal (week 9-18), juvenile (year 7-11) and adult (year 34-42) stages. By comparing these transcriptomes, Xi et al. concluded that the hiPSC-derived muscle progenitors produced in these conditions are at an embryonic-to-fetal transition stage [40].
Several studies have been conducted to promote the maturation of PSC-derived myotubes generated in vitro [70,74,75]. By comparing transcriptomic differences between myogenic progenitors and myotubes derived from both hiPSC and human fetal-stage muscles, TGFβ signaling was found to be reduced in differentiated myotubes. TGFβ inhibition of late stage PSC cultures with inhibitors of activin receptor like kinases ALK4/5/7 such as SB-431542 or A83-01, increases myotube fusion efficiency and leads to a cellular morphology that resembles late-stage fetal myotubes [70]. Specific myosin isoforms are expressed during skeletal muscle development [76]. Without TGFβ inhibition, hiPSC-derived myotubes mainly express embryonic MHC (MYH3) and fetal/neonatal MHC (MYH8) instead of late fetal/adult isoforms such as MYH1 or MYH2, indicating a fetal-like state. However, with TGFβ inhibition, expression of all myosin isoforms was elevated, including MYH1 [70,74]. Better-organized sarcomeres were also formed after TGFβ inhibition, as evidenced by transmission electron microscopy [70]. Another improvement of skeletal myofiber maturation was achieved by the addition of prednisolone, a synthetic glucocorticoid. The combination of prednisolone and TGFβ inhibition leads to the formation of better organized myofibrils and to an upregulated expression of the fast myosins MYH1/2/4 [74]. Moreover, a small molecule library screen identified the TGFβ inhibitor SB-431542, the Notch inhibitor DAPT, Dexamethasone and Forskolin as effective molecules regulating myotube maturation [75]. Notch inhibition with DAPT after the pervasive initial Wnt activation gave rise to robust myogenic induction, indicated by a large fraction of MYOG+ cell population [77]. However, treatment with DAPT was also shown to induce the differentiation of all PAX7+ cells, thus resulting in cultures depleted of SCs [41].
Taking advantage of the hiPSC-derived myogenic induction system, basic studies on human muscle specification, maturation and homeostasis can be conducted with an unlimited source of cells. However, as mentioned above, these myogenic cells generated from hiPSCs exhibit mainly fetal or embryonic features. Additionally, although the stepwise induction protocol mimics the in vivo muscle development, further studies are required to provide more detailed comparison between these cells and their in vivo counterparts. Nevertheless, the improved understanding of basic muscle biology and protocols of muscle generation will further contribute to the optimization of in vitro muscle generation and benefit the clinical applications.
4.2. Disease modelling
Human primary myogenic cells can be obtained from invasive biopsies, but their availability is very limited. Hence, the virtually infinite expansion capacity and controllable differentiation of PSCs into myogenic precursors hold great potential for muscle disease modelling and drug development [51]. Within musculoskeletal pathologies, muscular dystrophies are a group of heterogeneous genetic diseases characterized by progressive degeneration of skeletal muscle. The most common form of muscular dystrophy is Duchenne Muscular Dystrophy (DMD), a lethal X-linked muscular disease (affecting approximately 1 in 5000 live male births) caused by mutations in the dystrophin (DMD) gene [78,79]. This disease starts to manifest around 3 years of age, progressively leading to muscle weakness, respiratory insufficiency and cardiac failure that causes premature death in young adulthood [80]. While our knowledge of DMD etiology and pathogenesis has progressed, to date there is no cure, and current treatment is based on the chronic administration of corticosteroids, cardioprotective treatment, ventilatory support, physical therapy, and antisense-mediated exon-skipping treatment in a subset of patients [78,81].
The difficulty of accessing muscle material from human patients has resulted in much of the research on DMD to be carried out in the mdx mouse, a spontaneous dystrophin mutant [82]. However, a significant problem with the mdx mouse is that the pathology is much less severe and only partly phenocopies the human DMD [83]. Thus, progress in understanding the onset of the disease has suffered from the lack of an appropriate model, in which early stages of the pathology can be analyzed to identify the primary causes of DMD. This has led to considerable research efforts directed to model DMD using patient-derived hiPSCs. A summary of the recent publications modelling DMD with hiPSCs can be found in Table 1. Previous studies on biopsies from DMD patients and mdx mice have reported several specific phenotypes, such as branching/fusion defects [84], calcium signaling hyperactivation [85] and defective contractions of the myofibers [86]. These phenotypes are variably recapitulated in hiPSC-derived DMD myofibers depending on the differentiation protocol [19,74,77,87,88]. Interestingly, some studies have shown partial or complete defective myotube formation from DMD hiPSCs [77,89], while others document that DMD hiPSCs generate myotubes comparably to those of control hiPSCs [14,19,51,87,88,90] (See Table 1). This discrepancy may result from the use of different protocols for the differentiation of myotubes (e.g., transgene-free vs transgene-based). Alternatively, it might also result from the comparison of iPSC lines from unaffected and affected patients with different genetic backgrounds. Indeed, it is well known that different iPSC lines often exhibit different differentiation potential even with the same protocol. This problem has been circumvented in a recent study using isogenic lines in which common DMD mutations were introduced in a wild-type parental iPSC line or where a patient iPSC line was corrected using CRISPR-Cas9 to restore the dystrophin coding frame [74]. Isogenic mutant lines were able to differentiate to a myogenic fate, while showing reduced myogenic potential, and recapitulated the hallmarks of the DMD myofiber phenotype including branching, intrinsic defective contraction and calcium handling defects.
Table 1.
Overview of the current publications using hiPSC-derived DMD myogenic cultures.
| Reference | Cell line | Differentiation protocol |
Myogenic markers detected in DMD- hiPSC myogenic cultures (protein) |
Myogenic differentiation |
DMD Phenotype in vitro |
Engraftment |
|---|---|---|---|---|---|---|
| Goudenege et al., 2012 | DMD patient hiPSC and healthy controls | MYOD1 overexpression (Adenovirus) | MYOD1, MHC, Spectrin, Lamin A/C | Similar to control | Larger myotubes compared to healthy controls | Performed in rag/mdx mice with/without cardiotoxin. Documented presence of hybrid myofibers |
| Abujarour et al., 2014 | DMD patient hiPSCs and healthy controls | Doxycycline-dependent MYOD1 overexpression (Lentivirus) | MHC, MYOD1, MYOG, NCAM, CD44, CD29 | Similar to control | No | |
| Shoji et al., 2015 | DMD patient hiPSCs and healthy controls treated or not with exon-skipping oligonucle otides | Tetracycline-dependent MYOD1 overexpression (PiggyBac vector) | CKM, MHC, Skeletal Muscle Actin | Similar to control | Excess of calcium flux (Fluo-8 dye) upon electrical stimulation | No |
| Young et al., 2016 | DMD patient hiPSCs and CRISPR edited version rescued by deletion of exon 45-55 | Tamoxifen-dependent MYOD1 overexpression (Lentivirus) or Shelton et al. (2014) directed differentiation protocol | MHC, NCAM, Spectrin, Lamin A/C | Similar to control | β-dystroglycan downregulation and mislocalization | Performed in NOD-SCID-IL2rgnull (NSG)-mdx mice with cardiotoxin. Documented presence of engrafted myofibers by human Spectrin and Lamin A/C expression |
| Choi et al., 2016 | DMD patient hiPSCs, healthy controls and correction with human artificial chromoso me expressing DMD | CHIR/DAPT treatment in N2 medium, replating and FACS sorting of NCAM+/HNK1− cells | Desmin, Lamin A/C, Laminin | Defective | Decreased fusion and myogenic marker expression. Absence of spontaneous contractions. Increased branching. Increased levels of BMP4 and TGFβ signaling. Increased expression of interleukins 6 and 8 and collagen 3 | Performed in NOD-Rag1nu11 IL2rgnu11 (NRG) mice and NSG-mdx mice after cardiotoxin. Comparable levels of human myofiber formation in both models |
| Maffioletti et al., 2018 | DMD Patient hiPSC lines and healthy controls | Tamoxifen-dependent MYOD1 overexpression (Lentivirus) or directed differentiation (Caron et al., 2016) and 3D constructs | Laminin, Lamin A/C, MHC, Sarcomeric actin | Similar to control | Performed in NSG mice previously injured. Documented presence of engrafted myofibers by human Lamin A/C and embryonic MHC expression | |
| Caputo et al., 2020 | DMD patient hiPSC lines and healthy controls | Doxycycline-dependent MYOD1 and BAF60C overexpression (PiggyBac vector) | MYOD1, Desmin, MHC | Similar to control | DMD hiPSC-derived myotubes exhibit constitutive activation of TGFβ-SMAD2/3 signaling. Electrically paced DMD hiPSC-derived myotubes exhibit greater and persistent increase in the expression of pro-fibrotic genes (TGFβ1, TGFβ2, IL6, and CTGF) | No |
| Moretti et al., 2020 | DMD patient hiPSC line, CRISPR edited version rescued by deletion of exon 51 and healthy control | Commercial kit Amsbio; SKM-KITM | Not detected | Defective | No multinucleated myotubes | No |
| Al Tanoury et al., 2020 | DMD patient hiPSC, CRISPR edited version rescued, CRISPR-engineered DMD mutants and isogenic healthy parental control | Directed differentiation (Chal et al., 2015, 2016) | MYOG, Desmin, Titin, α-actinin, NCAM, nNOS, DAG1 and delta-Sarcoglycan | Defective | Increased branching and fusion. Mislocalization of proteins of the Dystrophin-associated Glycoprotein Complex. Force contraction defects and Ca2+ hyper-excitability | No |
Choi et al. also reported increased branching and fusion defects together with increased levels of BMP4 and TGFβ signaling in hiPSC-derived myofibers [77]. Treatment with dual SMAD inhibitors partially rescued the fusion defects, implying that aberrant TGFβ signaling might be responsible for the defective fusion. Similarly, Caputo et al. described that DMD hiPSC-derived myotubes exhibit a constitutive activation of TGFβ-SMAD2/3 signaling [88]. In contrast, Al Tanoury et al. reported the presence of increased branching accompanied by higher fusion of hiPSC-derived DMD myofibers, supporting the hypothesis that aberrant branching is due to increased fusion required for sustained regeneration in DMD patients [74,84].
Current studies have not been limited to the analysis of molecular mechanisms of DMD, but have also explored potential new treatments for DMD such as exon skipping [19], human artificial chromosome transfer [77], CRISPR/Cas9-mediated gene editing [87,89], and cell-based therapies [91].
Another potential application of hiPSC-derived DMD myofibers is the possibility to perform screenings of chemical libraries to identify drug candidates for skeletal muscle diseases. Sun et al. recently published the results of a screen performed on hiPSC-derived DMD myoblasts [92]. Using a high-content imaging approach, they evaluated fusion in hiPSC-derived DMD myoblasts after treatment with 1,524 compounds from the Johns Hopkins Clinical Compound Library. Two potential hits, ginsenoside Rd and fenofibrate, were identified as enhancers of myogenic fusion and were later validated in vivo in mdx mice. While ginsenoside Rd appears to interfere with FLT3 signaling, fenofibrate is a suppressor of TGFβ signaling [92]. The results of this study support the feasibility of using hiPSC-derived myogenic cultures for drug screening to identify new treatments for muscular dystrophies.
Additional studies have used hiPSCs to model other genetic diseases affecting muscles, such as carnitine palmitoyltransferase II deficiency [18], Pompe disease [93,94] and Miyoshi myopathy [17]. Recently, Maffioletti et al. generated fibrin-based artificial muscles using hiPSCs from healthy donors and patients diagnosed with several different muscular dystrophies such as Duchenne, limb-girdle type 2D, and LAMIN A/C (LMNA)-related muscular dystrophies. LMNA-mutant artificial muscles successfully recapitulated the nuclear abnormalities characteristic of this laminopathy, opening the door to more 3D-based muscle disease modelling studies [51].
Overall, there is a growing body of studies that are shifting towards the usage of PSC-derived muscle cultures. Further research is needed to understand the basis of the discrepancies found between differentiation protocols and genetic backgrounds. Moreover, the immature status of the myogenic populations generated in vitro poses a limitation for the study of adult stages of myogenic diseases. However, usage of isogenic lines, more standardized protocols and 3D bioengineered approaches will progressively increase the suitability of these systems. Nonetheless, their potential ability to recapitulate human pathophysiology without the limitation of sample obtainment provides a significant advantage for studying rare genetic disorders such as DMD.
4.3. Cell-based therapies
In recent years, stem cell-based technologies that can serve as the basis for cell-based therapies have been rapidly developing. The main purpose of cell therapy is to replenish lost cells or replace dysfunctional cells with new cells generated from transplanted progenitors. Several cell therapy clinical trials involving transplantation of primary myoblasts or pericytes into skeletal muscles have been carried out but they failed to show significant engraftment and restoration of muscle function [95-98]. However, research from the last two decades suggests that the ideal material for regenerating muscle by transplantation are the PAX7+ SCs [99-101]. This has led to the development of protocols aiming at differentiating human PSCs toward the PAX7-expressing SC fate with the long-term goal of developing cell therapy protocols for muscle repair.
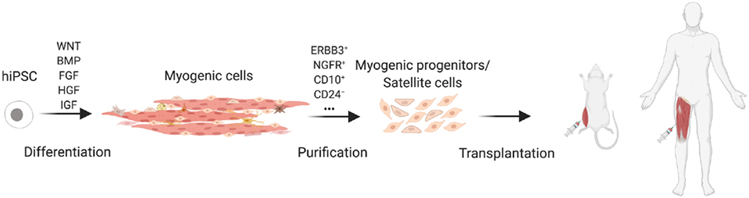
The production of human SC generated in vitro has been optimized using PSC reporter lines in which GFP is driven from the PAX7 or MYF5 locus. Their engraftment potential and regenerative capacity in vivo has been demonstrated in mice [26,41,91,102]. Purification of SCs before transplantation is required for safety to avoid the risk of teratoma from residual PSCs and to increase efficiency (Fig. 2). This requires identification of surface markers to purify the cell populations exhibiting the highest regeneration potential. ERBB3 and NGFR were identified as surface markers allowing enrichment of hiPSC-derived SCs, exhibiting in vivo myogenic regenerative capacity after engraftment [70]. Sorted cells have been used for transplantation experiments in rodent models to evaluate their myogenic potential [70,77,102-105]. Using a PAX7/MYF5 double-reporter line, a surface marker screen led to the identification of CD10 as a positive marker and CD24 as a negative marker for MPC purification. Successful engraftment was detected after CD10+CD24− cells injection [102,106]. Moreover, by comparing transcriptomic differences between early-stage (6 weeks) and late-stage (10 and 11 weeks) MYF5+ cells, a recent study revealed that late-stage MYF5+ cells resemble fetal SCs and possess high regeneration potential after injection in mice [91].
Fig. 2. Schematic model for cell therapy using hiPSC-derived myogenic cells.
Successful muscle cell therapy requires transplantation of sufficient myogenic progenitors with regenerating capacity. After myogenic differentiation of hiPSCs using the aforementioned protocols, the heterogeneous cell population obtained needs to undergo purification and enrichment through cell sorting based on specific surface markers.
Although significant progress has been made with the production of SC for cell therapy, some limitations still exist. Cell survival after transplantation needs to be improved, as the injected cells undergo necrosis due to the acute inflammatory response, which decreases the engraftment efficiency [107]. While immune rejection of the transplanted cells may be avoided by using autologous patient-specific hiPSCs, this approach is difficult to implement due to the cost and time associated with current good manufacturing practice (cGMP) requirements [108]. Alternative solutions involving allogeneic donor cells are more realistic and involve human leukocyte antigen (HLA)-matched donor cells or “universal” donor cells [109-111].
5. Conclusions and perspectives
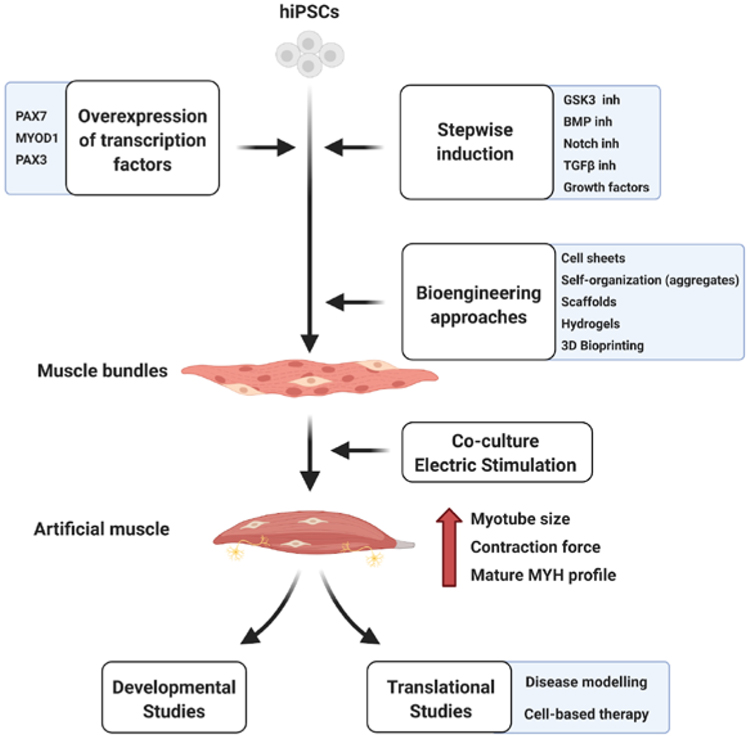
Our understanding of the development and physiopathology of human skeletal muscle has been limited until recently due to the limited access to human biopsies. The protocols to differentiate PSCs towards the myogenic lineage, while still new to the field of tissue engineering, are becoming progressively more efficient, generating increasingly mature cultures. So far, the two main strategies used are the overexpression of transcription factors and the stepwise induction of skeletal muscle by small molecules or growth factors treatments [8,12]. The combination of these two approaches with bioengineering techniques allows to mimic more faithfully the skeletal muscle 3D environment, hence promoting its maturation [42] (Fig. 3). The co-culture of PSC-derived muscle with other cell types present in physiological muscle, such as motor neurons or tenogenic cells, has started to be explored as a potential enhancer of maturation. During muscle development, innervation plays an important role in myofiber maturation [112], hence, the addition of this element into PSC-derived myogenic cultures might be a requirement for further development. Mechanical tension is another important element in the development and maturation of myofibers [113]. Tendon cells are key regulators of muscle fiber attachment, so their co-culture could also promote myofiber maturation. Another interesting approach is the electrical stimulation of hiPSC-derived muscles, which increases myofiber size, force generation and changes their metabolic profile [55]. Moreover, additional treatments including drugs such as prednisolone should be considered to promote maturation [74]. An improvement of the maturation of myofibers generated in vitro could contribute to recreate a more physiological muscle stem cell niche and lead to more mature SCs, which may improve cell-based therapies. The discovery of methods to improve maturation of these cultures towards an adult-like phenotype is therefore an important goal. While several challenges still lay ahead towards fully mimicking human muscle, hiPSC-derived myogenesis holds promise of becoming a gold standard methodology for developmental and disease modelling in muscle biology and for drug screening and cell-based therapies in pre-clinical studies.
Fig. 3. Overview of different techniques used in hiPSC-derived myogenesis.
Diagram depicting the different techniques and bioengineering approaches used towards the induction of skeletal muscle from hiPSCs. Further electrical stimulation or co-culture with other non-myogenic cell types present in adult muscle, such as motor neurons, might contribute towards increasing the maturation and function of the artificial muscles obtained, optimizing their suitability for basic and clinical applications.
Acknowledgements
We thank Emanuela Gussoni and Pourquie laboratory for the feedback and comments. This work was supported by the National Institutes of Health [grant number R01 AR074526]; the French Muscular Dystrophy Association (AFM) [grant number 20434]; the Human Frontier Science Program [grant numbers RGP0052/2018, LT000018/2020L]; and the "la Caixa" foundation (ID 100010434), under agreement LCF/BQ/AA18/11680032. Figures created with BioRender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Olivier Pourquié is a founder and shareholder of Anagenesis Biotechnologies. The other authors declare no conflict of interest.
References
- [1].Frontera WR, Ochala J, Skeletal muscle: a brief review of structure and function, Calcif. Tissue Int 96 (2015) 183–195. [DOI] [PubMed] [Google Scholar]
- [2].Chal J, Pourquié O, Making muscle: skeletal myogenesis in vivo and in vitro, Development. 144 (2017) 2104–2122. [DOI] [PubMed] [Google Scholar]
- [3].Pourquié O, Al Tanoury Z, Chal J, The long road to making muscle in vitro, Curr. Top. Dev. Biol 129 (2018) 123–142. [DOI] [PubMed] [Google Scholar]
- [4].Mauro A, Satellite cell of skeletal muscle fibers, J. Biophys. Biochem. Cytol 9 (1961) 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Konigsberg UR, Lipton BH, Konigsberg IR, The regenerative response of single mature muscle fibers isolated in vitro, Dev. Biol 45 (1975) 260–275. [DOI] [PubMed] [Google Scholar]
- [6].Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM, Self-renewal and expansion of single transplanted muscle stem cells, Nature. 456 (2008) 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Relaix F, Zammit PS, Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage, Development. 139 (2012) 2845–2856. [DOI] [PubMed] [Google Scholar]
- [8].Jiwlawat N, Lynch E, Jeffrey J, Van Dyke JM, Suzuki M, Current progress and challenges for skeletal muscle differentiation from human pluripotent stem cells using transgene-free approaches, Stem Cells Int. 2018 (2018) 6241681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rohwedel J, Maltsev V, Bober E, Arnold HH, Hescheler J, Wobus AM, Muscle cell differentiation of embryonic stem cells reflects myogenesis in vivo: developmentally regulated expression of myogenic determination genes and functional expression of ionic currents, Dev. Biol 164 (1994) 87–101. [DOI] [PubMed] [Google Scholar]
- [10].Magli A, Perlingeiro RRC, Myogenic progenitor specification from pluripotent stem cells, Semin. Cell Dev. Biol 72 (2017) 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tapscott S, Davis R, Thayer M, Cheng P, Weintraub H, Lassar A, MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts, Science. 242 (1988) 405–411. 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- [12].Kodaka Y, Rabu G, Asakura A, Skeletal muscle cell induction from pluripotent stem cells, Stem Cells Int. 2017 (2017) 1376151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Albini S, Coutinho P, Malecova B, Giordani L, Savchenko A, Forcales SV, Puri PL, Epigenetic reprogramming of human embryonic stem cells into skeletal muscle cells and generation of contractile myospheres, Cell Rep. 3 (2013) 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goudenege S, Lebel C, Huot NB, Dufour C, Fujii I, Gekas J, Rousseau J, Tremblay JP, Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation, Mol. Ther 20 (2012) 2153–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RCR, Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice, Cell Stem Cell. 10 (2012) 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maffioletti SM, Gerli MFM, Ragazzi M, Dastidar S, Benedetti S, Loperfido M, VandenDriessche T, Chuah MK, Tedesco FS, Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells, Nat. Protoc 10 (2015) 941–958. [DOI] [PubMed] [Google Scholar]
- [17].Tanaka A, Woltjen K, Miyake K, Hotta A, Ikeya M, Yamamoto T, Nishino T, Shoji E, Sehara-Fujisawa A, Manabe Y, Fujii N, Hanaoka K, Era T, Yamashita S, Isobe K-I, Kimura E, Sakurai H, Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro, PLoS One. 8 (2013) e61540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yasuno T, Osafune K, Sakurai H, Asaka I, Tanaka A, Yamaguchi S, Yamada K, Hitomi H, Arai S, Kurose Y, Higaki Y, Sudo M, Ando S, Nakashima H, Saito T, Kaneoka H, Functional analysis of iPSC-derived myocytes from a patient with carnitine palmitoyltransferase II deficiency, Biochem. Biophys. Res. Commun 448 (2014) 175–181. [DOI] [PubMed] [Google Scholar]
- [19].Shoji E, Sakurai H, Nishino T, Nakahata T, Heike T, Awaya T, Fujii N, Manabe Y, Matsuo M, Sehara-Fujisawa A, Early pathogenesis of Duchenne muscular dystrophy modelled in patient-derived human induced pluripotent stem cells, Sci. Rep 5 (2015) 12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rao L, Qian Y, Khodabukus A, Ribar T, Bursac N, Engineering human pluripotent stem cells into a functional skeletal muscle tissue, Nature Communications. 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim J, Oliveira VKP, Yamamoto A, Perlingeiro RCR, Generation of skeletal myogenic progenitors from human pluripotent stem cells using non-viral delivery of minicircle DNA, Stem Cell Res. 23 (2017) 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kwon JB, Vankara A, Ettyreddy AR, Bohning JD, Gersbach CA, Myogenic progenitor cell lineage specification by CRISPR/Cas9-based transcriptional activators, Stem Cell Reports. 14 (2020) 755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Akiyama T, Sato S, Chikazawa-Nohtomi N, Soma A, Kimura H, Wakabayashi S, Ko SBH, Ko MSH, Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing, Sci. Rep 8 (2018) 1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Komatsu Y, Takeuchi D, Tokunaga T, Sakurai H, Makino A, Honda T, Ikeda Y, Tomonaga K, RNA virus-based episomal vector with a fail-safe switch facilitating efficient genetic modification and differentiation of iPSCs, Mol Ther Methods Clin Dev. 14 (2019) 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim H, Selvaraj S, Kiley J, Azzag K, Garay BI, Perlingeiro RCR, Genomic safe harbor expression of PAX7 for the generation of engraftable myogenic progenitors, Stem Cell Reports. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bousson F, Zidouni Y, Mursch C, Moncuquet P, Tassy O, Vincent S, Miyanari A, Bera A, Garnier J-M, Guevara G, Hestin M, Kennedy L, Hayashi S, Drayton B, Cherrier T, Gayraud-Morel B, Gussoni E, Relaix F, Tajbakhsh S, Pourquié O, Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy, Nat. Biotechnol 33 (2015) 962–969. [DOI] [PubMed] [Google Scholar]
- [27].Henrique D, Abranches E, Verrier L, Storey KG, Neuromesodermal progenitors and the making of the spinal cord, Development. 142 (2015) 2864–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J, In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity, PLoS Biol. 12 (2014) e1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, Shyh-Chang N, Fernhoff NB, George BM, Wernig G, Salomon REA, Chen Z, Vogel H, Epstein JA, Kundaje A, Talbot WS, Beachy PA, Ang LT, Weissman IL, Mapping the pairwise choices leading from pluripotency to human bone, heart, and other mesoderm cell types, Cell. 166 (2016) 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sudheer S, Liu J, Marks M, Koch F, Anurin A, Scholze M, Senft AD, Wittler L, Macura K, Grote P, Herrmann BG, Different concentrations of FGF ligands, FGF2 or FGF8 determine distinct states of WNT-induced presomitic mesoderm, Stem Cells. 34 (2016) 1790–1800. [DOI] [PubMed] [Google Scholar]
- [31].Tsakiridis A, Huang Y, Blin G, Skylaki S, Wymeersch F, Osorno R, Economou C, Karagianni E, Zhao S, Lowell S, Wilson V, Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors, Development. 141 (2014) 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Robertson EJ, Dose-dependent Nodal/Smad signals pattern the early mouse embryo, Semin. Cell Dev. Biol 32 (2014) 73–79. [DOI] [PubMed] [Google Scholar]
- [33].Diaz-Cuadros M, Wagner DE, Budjan C, Hubaud A, Tarazona OA, Donelly S, Michaut A, Al Tanoury Z, Yoshioka-Kobayashi K, Niino Y, Kageyama R, Miyawaki A, Touboul J, Pourquié O, In vitro characterization of the human segmentation clock, Nature. 580 (2020) 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oginuma M, Moncuquet P, Xiong F, Karoly E, Chal J, Guevorkian K, Pourquié O, A gradient of glycolytic activity coordinates FGF and Wnt signaling during elongation of the body axis in amniote embryos, Dev. Cell 40 (2017) 342–353.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oginuma M, Harima Y, Tarazona OA, Diaz-Cuadros M, Michaut A, Ishitani T, Xiong F, Pourquié O, Intracellular pH controls WNT downstream of glycolysis in amniote embryos, Nature. 584 (2020) 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chal J, Al Tanoury Z, Oginuma M, Moncuquet P, Gobert B, Miyanari A, Tassy O, Guevara G, Hubaud A, Bera A, Sumara O, Garnier J-M, Kennedy L, Knockaert M, Gayraud-Morel B, Tajbakhsh S, Pourquié O, Recapitulating early development of mouse musculoskeletal precursors of the paraxial mesoderm, Development. 145 (2018). [DOI] [PubMed] [Google Scholar]
- [37].Tonegawa A, Funayama N, Ueno N, Takahashi Y, Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4, Development. 124 (1997) 1975–1984. [DOI] [PubMed] [Google Scholar]
- [38].Xi H, Fujiwara W, Gonzalez K, Jan M, Liebscher S, Van Handel B, Schenke-Layland K, Pyle AD, In vivo human somitogenesis guides somite development from hPSCs, Cell Rep. 18 (2017) 1573–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chal J, Al Tanoury Z, Hestin M, Gobert B, Aivio S, Hick A, Cherrier T, Nesmith AP, Parker KK, Pourquié O, Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro, Nat. Protoc 11 (2016) 1833–1850. [DOI] [PubMed] [Google Scholar]
- [40].Xi H, Langerman J, Sabri S, Chien P, Young CS, Younesi S, Hicks M, Gonzalez K, Fujiwara W, Marzi J, Liebscher S, Spencer M, Van Handel B, Evseenko D, Schenke-Layland K, Plath K, Pyle AD, A human skeletal muscle atlas identifies the trajectories of stem and progenitor cells across development and from human pluripotent stem cells, Cell Stem Cell. 27 (2020) 158–176.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Al Tanoury Z, Rao J, Tassy O, Gobert B, Gapon S, Garnier J-M, Wagner E, Hick A, Hall A, Gussoni E, Pourquié O, Differentiation of the human PAX7-positive myogenic precursors/satellite cell lineage, Development. 147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang J, Khodabukus A, Rao L, Vandusen K, Abutaleb N, Bursac N, Engineered skeletal muscles for disease modeling and drug discovery, Biomaterials. 221 (2019) 119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Takahashi H, Shimizu T, Okano T, Engineered human contractile myofiber sheets as a platform for studies of skeletal muscle physiology, Sci. Rep 8 (2018) 13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Faustino Martins JM, Fischer C, Urzi A, Vidal R, Kunz S, Ruffault P-L, Kabuss L, Hube I, Gazzerro E, Birchmeier C, Spuler S, Sauer S, Gouti M, Self-organizing 3D human trunk neuromuscular organoids, Cell Stem Cell. 26 (2020) 172–186.e6. [DOI] [PubMed] [Google Scholar]
- [45].Sakai-Takemura F, Narita A, Masuda S, Wakamatsu T, Watanabe N, Nishiyama T, Michiro Nogami K, Blanc M, Michi Takeda S, Miyagoe-Suzuki Y, Premyogenic progenitors derived from human pluripotent stem cells expand in floating culture and differentiate into transplantable myogenic progenitors, Sci. Rep 8 (2018) 6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiwlawat S, Lynch E, Glaser J, Smit-Oistad I, Jeffrey J, Van Dyke JM, Suzuki M, Differentiation and sarcomere formation in skeletal myocytes directly prepared from human induced pluripotent stem cells using a sphere-based culture, Differentiation. 96 (2017) 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hosoyama T, McGivern JV, Van Dyke JM, Ebert AD, Suzuki M, Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture, Stem Cells Transl. Med 3 (2014) 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].MacQueen LA, Alver CG, Chantre CO, Ahn S, Cera L, Gonzalez GM, O’Connor BB, Drennan DJ, Peters MM, Motta SE, Zimmerman JF, Parker KK, Muscle tissue engineering in fibrous gelatin: implications for meat analogs, Npj Science of Food. 3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dixon TA, Cohen E, Cairns DM, Rodriguez M, Mathews J, Jose RR, Kaplan DL, Bioinspired three-dimensional human neuromuscular junction development in suspended hydrogel arrays, Tissue Eng. Part C Methods 24 (2018) 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Afshar Bakooshli M, Lippmann ES, Mulcahy B, Iyer N, Nguyen CT, Tung K, Stewart BA, van den Dorpel H, Fuehrmann T, Shoichet M, Bigot A, Pegoraro E, Ahn H, Ginsberg H, Zhen M, Ashton RS, Gilbert PM, A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction, Elife. 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Maffioletti SM, Sarcar S, Henderson ABH, Mannhardt I, Pinton L, Moyle LA, Steele-Stallard H, Cappellari O, Wells KE, Ferrari G, Mitchell JS, Tyzack GE, Kotiadis VN, Khedr M, Ragazzi M, Wang W, Duchen MR, Patani R, Zammit PS, Wells DJ, Eschenhagen T, Tedesco FS, Three-dimensional human iPSC-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering, Cell Rep. 23 (2018) 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Russell CS, Mostafavi A, Quint JP, Panayi AC, Baldino K, Williams TJ, Daubendiek JG, Sánchez VH, Bonick Z, Trujillo-Miranda M, Shin SR, Pourquié O, Salehi S, Sinha I, Tamayol A, In situ printing of adhesive hydrogel scaffolds for the treatment of skeletal muscle injuries, ACS Applied Bio Materials. 3 (2020) 1568–1579. [DOI] [PubMed] [Google Scholar]
- [53].van der Schaft DWJ, van Spreeuwel ACC, Boonen KJM, Langelaan MLP, Bouten CVC, Baaijens FPT, Engineering skeletal muscle tissues from murine myoblast progenitor cells and application of electrical stimulation, J. Vis. Exp (2013) e4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dennis RG, Kosnik PE 2nd, Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro, In Vitro Cell. Dev. Biol. Anim 36 (2000)327–335. [DOI] [PubMed] [Google Scholar]
- [55].Khodabukus A, Madden L, Prabhu NK, Koves TR, Jackman CP, Muoio DM, Bursac N, Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle, Biomaterials. 198 (2019) 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Stacpoole SRL, Bilican B, Webber DJ, Luzhynskaya A, He XL, Compston A, Karadottir R, Franklin RJM, Chandran S, Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen, Nat. Protoc 6 (2011)1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Orlova VV, van den Hil FE, Petrus-Reurer S, Drabsch Y, ten Dijke P, Mummery CL, Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells, Nature Protocols. 9 (2014) 1514–1531. [DOI] [PubMed] [Google Scholar]
- [58].Belle M, Godefroy D, Couly G, Malone SA, Collier F, Giacobini P, Chédotal A, Tridimensional visualization and analysis of early human development, Cell. 169 (2017) 161–173.e12. [DOI] [PubMed] [Google Scholar]
- [59].Cao J, O’Day DR, Pliner HA, Kingsley PD, Deng M, Daza RM, Zager MA, Aldinger KA, Blecher-Gonen R, Zhang F, Spielmann M, Palis J, Doherty D, Steemers FJ, Glass IA, Trapnell C, Shendure J, A human cell atlas of fetal gene expression, Science. 370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bénazéraf B, Pourquié O, Formation and segmentation of the vertebrate body axis, Annu. Rev. Cell Dev. Biol 29 (2013) 1–26. [DOI] [PubMed] [Google Scholar]
- [61].Hubaud A, Pourquié O, Signalling dynamics in vertebrate segmentation, Nat. Rev. Mol. Cell Biol 15 (2014) 709–721. [DOI] [PubMed] [Google Scholar]
- [62].Matsuda M, Yamanaka Y, Uemura M, Osawa M, Saito MK, Nagahashi A, Nishio M, Guo L, Ikegawa S, Sakurai S, Kihara S, Maurissen TL, Nakamura M, Matsumoto T, Yoshitomi H, Ikeya M, Kawakami N, Yamamoto T, Woltjen K, Ebisuya M, Toguchida J, Alev C, Recapitulating the human segmentation clock with pluripotent stem cells, Nature. 580 (2020) 124–129. [DOI] [PubMed] [Google Scholar]
- [63].Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA, Pax7 is required for the specification of myogenic satellite cells, Cell. 102 (2000) 777–786. [DOI] [PubMed] [Google Scholar]
- [64].Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomès D, Tajbakhsh S, Pax3/Pax7 mark a novel population of primitive myogenic cells during development, Genes Dev. 19 (2005) 1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Choi IY, Lim H, Cho HJ, Oh Y, Chou B-K, Bai H, Cheng L, Kim YJ, Hyun S, Kim H, Shin JH, Lee G, Transcriptional landscape of myogenesis from human pluripotent stem cells reveals a key role of TWIST1 in maintenance of skeletal muscle progenitors, Elife. 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Puttonen KA, Ruponen M, Naumenko N, Hovatta OH, Tavi P, Koistinaho J, Generation of functional neuromuscular junctions from human pluripotent stem cell lines, Front. Cell. Neurosci 9 (2015) 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Demestre M, Orth M, Föhr KJ, Achberger K, Ludolph AC, Liebau S, Boeckers TM, Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes, Stem Cell Res. 15 (2015) 328–336. [DOI] [PubMed] [Google Scholar]
- [68].Kostrominova TY, Calve S, Arruda EM, Larkin LM, Ultrastructure of myotendinous junctions in tendon-skeletal muscle constructs engineered in vitro, Histol. Histopathol 24 (2009) 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Steinbeck JA, Jaiswal MK, Calder EL, Kishinevsky S, Weishaupt A, Toyka KV, Goldstein PA, Studer L, Functional connectivity under optogenetic control allows modeling of human neuromuscular disease, Cell Stem Cell. 18 (2016) 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hicks MR, Hiserodt J, Paras K, Fujiwara W, Eskin A, Jan M, Xi H, Young CS, Evseenko D, Nelson SF, Spencer MJ, Van Handel B, Pyle AD, ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs, Nat. Cell Biol 20 (2018) 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mavrommatis L, Jeong H-W, Gomez-Giro G, Stehling M, Kienitz M-C, Psathaki OE, Gabriele Bixel M, Morosan-Puopolo G, Gerovska D, Araúzo-Bravo MJ, Schwamborn JC, Schöler HR, Adams RH, Vorgerd M, Brand-Saberi B, Zaehres H, Human skeletal muscle organoids model fetal myogenesis and sustain uncommitted PAX7 myogenic progenitors, bioRxiv. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Robertson C, Tran DD, George SC, Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes, Stem Cells. 31 (2013) 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shelton M, Metz J, Liu J, Carpenedo RL, Demers S-P, Stanford WL, Skerjanc IS, Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells, Stem Cell Reports. 3 (2014) 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tanoury ZA, Zimmermann JF, Rao J, Sieiro D, McNamara H, Cherrier T, Hick A, Bousson F, Fugier C, Marchiano F, Habermann B, Chal J, Nesmith AP, Gapon S, Wagner E, Bassel-Duby R, Olson E, Cohen AE, Parker KK, Pourquié O, Prednisolone rescues Duchenne Muscular Dystrophy phenotypes in human pluripotent stem cells-derived skeletal muscle in vitro, bioRxiv. (2020) 1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Selvaraj S, Mondragon-Gonzalez R, Xu B, Magli A, Kim H, Lainé J, Kiley J, Mckee H, Rinaldi F, Aho J, Tabti N, Shen W, Perlingeiro RC, Screening identifies small molecules that enhance the maturation of human pluripotent stem cell-derived myotubes, Elife. 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schiaffino S, Rossi AC, Smerdu V, Leinwand LA, Reggiani C, Developmental myosins: expression patterns and functional significance, Skelet. Muscle 5 (2015) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Choi IY, Lim H, Estrellas K, Mula J, Cohen TV, Zhang Y, Donnelly CJ, Richard J-P, Kim YJ, Kim H, Kazuki Y, Oshimura M, Li HL, Hotta A, Rothstein J, Maragakis N, Wagner KR, Lee G, Concordant but varied phenotypes among Duchenne muscular dystrophy patient-specific myoblasts derived using a human iPSC-based model, Cell Rep. 15 (2016) 2301–2312. [DOI] [PubMed] [Google Scholar]
- [78].Mah JK, Current and emerging treatment strategies for Duchenne muscular dystrophy, Neuropsychiatr. Dis. Treat 12 (2016) 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sun C, Serra C, Lee G, Wagner KR, Stem cell-based therapies for Duchenne muscular dystrophy, Exp. Neurol 323 (2020) 113086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rahimov F, Kunkel LM, Cellular and molecular mechanisms underlying muscular dystrophy, Journal of Cell Biology. 201 (2013) 499–510. 10.1083/jcb.201212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dzierlega K, Yokota T, Optimization of antisense-mediated exon skipping for Duchenne muscular dystrophy, Gene Ther. 27 (2020) 407–416. [DOI] [PubMed] [Google Scholar]
- [82].Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ, The molecular basis of muscular dystrophy in the mdx mouse: a point mutation, Science. 244 (1989)1578–1580. [DOI] [PubMed] [Google Scholar]
- [83].Partridge TA, The mdx mouse model as a surrogate for Duchenne muscular dystrophy, FEBS J. 280 (2013) 4177–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chan S, Head SI, The role of branched fibres in the pathogenesis of Duchenne muscular dystrophy, Exp. Physiol 96 (2011) 564–571. [DOI] [PubMed] [Google Scholar]
- [85].Burr AR, Molkentin JD, Genetic evidence in the mouse solidifies the calcium hypothesis of myofiber death in muscular dystrophy, Cell Death Differ. 22 (2015) 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lowe DA, Williams BO, Thomas DD, Grange RW, Molecular and cellular contractile dysfunction of dystrophic muscle from young mice, Muscle Nerve. 34 (2006) 92–100. [DOI] [PubMed] [Google Scholar]
- [87].Young CS, Hicks MR, Ermolova NV, Nakano H, Jan M, Younesi S, Karumbayaram S, Kumagai-Cresse C, Wang D, Zack JA, Kohn DB, Nakano A, Nelson SF, Carrie Miceli M, Spencer MJ, Pyle AD, A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores Dystrophin function in hiPSC-derived muscle cells, Cell Stem Cell. 18 (2016) 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Caputo L, Granados A, Lenzi J, Rosa A, Ait-Si-Ali S, Puri PL, Albini S, Acute conversion of patient-derived Duchenne muscular dystrophy iPSC into myotubes reveals constitutive and inducible over-activation of TGFβ-dependent pro-fibrotic signaling, Skelet. Muscle 10 (2020) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moretti A, Fonteyne L, Giesert F, Hoppmann P, Meier AB, Bozoglu T, Baehr A, Schneider CM, Sinnecker D, Klett K, Fröhlich T, Rahman FA, Haufe T, Sun S, Jurisch V, Kessler B, Hinkel R, Dirschinger R, Martens E, Jilek C, Graf A, Krebs S, Santamaria G, Kurome M, Zakhartchenko V, Campbell B, Voelse K, Wolf A, Ziegler T, Reichert S, Lee S, Flenkenthaler F, Dorn T, Jeremias F, Blum H, Dendorfer A, Schnieke A, Krause S, Walter MC, Klymiuk N, Laugwitz KL, Wolf E, Wurst W, Kupatt C, Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy, Nat. Med 26 (2020) 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Abujarour R, Bennett M, Valamehr B, Lee TT, Robinson M, Robbins D, Le T, Lai K, Flynn P, Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery, Stem Cells Transl. Med 3 (2014) 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhao M, Tazumi A, Takayama S, Takenaka-Ninagawa N, Nalbandian M, Nagai M, Nakamura Y, Nakasa M, Watanabe A, Ikeya M, Hotta A, Ito Y, Sato T, Sakurai H, Induced fetal human muscle stem cells with high therapeutic potential in a mouse muscular dystrophy model, Stem Cell Reports. 15 (2020) 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sun C, Choi FY, Gonzalez YFR, Andersen P, Talbot CC Jr, Iyer SR, Lovering RM, Wagner KR, Lee G, Duchenne muscular dystrophy hiPSC-derived myoblast drug screen identifies compounds that ameliorate disease in mdx mice, JCI Insight. 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yoshida T, Awaya T, Jonouchi T, Kimura R, Kimura S, Era T, Heike T, Sakurai H, A skeletal muscle model of Infantile-onset Pompe disease with patient-specific iPS cells, Sci. Rep 7 (2017) 13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jiwlawat N, Lynch EM, Napiwocki BN, Stempien A, Ashton RS, Kamp TJ, Crone WC, Suzuki M, Micropatterned substrates with physiological stiffness promote cell maturation and Pompe disease phenotype in human induced pluripotent stem cell-derived skeletal myocytes, Biotechnol. Bioeng 116 (2019) 2377–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Torrente Y, Belicchi M, Marchesi C, D’Antona G, Cogiamanian F, Pisati F, Gavina M, Giordano R, Tonlorenzi R, Fagiolari G, Lamperti C, Porretti L, Lopa R, Sampaolesi M, Vicentini L, Grimoldi N, Tiberio F, Songa V, Baratta P, Prelle A, Forzenigo L, Guglieri M, Pansarasa O, Rinaldi C, Mouly V, Butler-Browne GS, Comi GP, Biondetti P, Moggio M, Gaini SM, Stocchetti N, Priori A, D’Angelo MG, Turconi A, Bottinelli R, Cossu G, Rebulla P, Bresolin N, Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients, Cell Transplant. 16 (2007) 563–577. [DOI] [PubMed] [Google Scholar]
- [96].Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, Noviello M, Roostalu U, Natali Sora MG, Scarlato M, De Pellegrin M, Godi C, Giuliani S, Ciotti F, Tonlorenzi R, Lorenzetti I, Rivellini C, Benedetti S, Gatti R, Marktel S, Mazzi B, Tettamanti A, Ragazzi M, Imro MA, Marano G, Ambrosi A, Fiori R, Sormani MP, Bonini C, Venturini M, Politi LS, Torrente Y, Ciceri F, Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy, EMBO Mol. Med 8 (2016) 1470–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gussoni E, Blau HM, Kunkel LM, The fate of individual myoblasts after transplantation into muscles of DMD patients, Nat. Med 3 (1997) 970–977. [DOI] [PubMed] [Google Scholar]
- [98].Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, Myoblast transfer in the treatment of Duchenne’s muscular dystrophy, N. Engl. J. Med 333 (1995) 832–838. [DOI] [PubMed] [Google Scholar]
- [99].Lorant J, Saury C, Schleder C, Robriquet F, Lieubeau B, Négroni E, Leroux I, Chabrand L, Viau S, Babarit C, Ledevin M, Dubreil L, Hamel A, Magot A, Thorin C, Guevel L, Delorme B, Péréon Y, Butler-Browne G, Mouly V, Rouger K, Skeletal muscle regenerative potential of human MuStem cells following transplantation into injured mice muscle, Mol. Ther 26 (2018) 618–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ, Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles, Cell. 134 (2008) 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE, Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche, Cell. 122 (2005) 289–301. [DOI] [PubMed] [Google Scholar]
- [102].Wu J, Matthias N, Lo J, Ortiz-Vitali JL, Shieh AW, Wang SH, Darabi R, A myogenic double-reporter human pluripotent stem cell line allows prospective isolation of skeletal muscle progenitors, Cell Rep. 25 (2018) 1966–1981.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Borchin B, Chen J, Barberi T, Derivation and FACS-mediated purification of PAX3+/PAX7+ skeletal muscle precursors from human pluripotent stem cells, Stem Cell Reports. 1 (2013) 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Magli A, Incitti T, Kiley J, Swanson SA, Darabi R, Rinaldi F, Selvaraj S, Yamamoto A, Tolar J, Yuan C, Stewart R, Thomson JA, Perlingeiro RCR, PAX7 targets, CD54, Integrin α9β1, and SDC2, allow isolation of human ESC/iPSC-derived myogenic progenitors, Cell Rep. 19 (2017) 2867–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tey SR, Robertson S, Lynch E, Suzuki M, Coding cell identity of human skeletal muscle progenitor cells using cell surface markers: current status and remaining challenges for characterization and isolation, Front Cell Dev Biol. 7 (2019) 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Arpke RW, Darabi R, Mader TL, Zhang Y, Toyama A, Lonetree C-L, Nash N, Lowe DA, Perlingeiro RCR, Kyba M, A new immuno-, dystrophin-deficient model, the NSG-mdx(4Cv) mouse, provides evidence for functional improvement following allogeneic satellite cell transplantation, Stem Cells. 31 (2013) 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Skuk D, Caron NJ, Goulet M, Roy B, Tremblay JP, Resetting the problem of cell death following muscle-derived cell transplantation: detection, dynamics and mechanisms, J. Neuropathol. Exp. Neurol 62 (2003) 951–967. [DOI] [PubMed] [Google Scholar]
- [108].Doss MX, Sachinidis A, Current challenges of iPSC-based disease modeling and therapeutic implications, Cells. 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Saetersmoen ML, Hammer Q, Valamehr B, Kaufman DS, Malmberg K-J, Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells, Semin. Immunopathol 41 (2019) 59–68. [DOI] [PubMed] [Google Scholar]
- [110].Riolobos L, Hirata RK, Turtle CJ, Wang P-R, Gomalusse GG, Zavajlevski M, Riddell SR, Russell DW, HLA engineering of human pluripotent stem cells, Mol. Ther 21 (2013)1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspumer H, Davis MM, Lanier LL, Schrepfer S, Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients, Nat. Biotechnol 37 (2019) 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Biressi S, Molinaro M, Cossu G, Cellular heterogeneity during vertebrate skeletal muscle development, Developmental Biology. 308 (2007) 281–293. [DOI] [PubMed] [Google Scholar]
- [113].Weitkunat M, Kaya-Çopur A, Grill SW, Schnorrer F, Tension and force-resistant attachment are essential for myofibrillogenesis in Drosophila flight muscle, Curr. Biol 24 (2014)705–716. [DOI] [PubMed] [Google Scholar]