Abstract
The sympathetic nervous system prepares the body for ‘fight or flight’ responses and maintains homeostasis during daily activities such as exercise, eating a meal or regulation of body temperature. Sympathetic regulation of bodily functions requires the establishment and refinement of anatomically and functionally precise connections between postganglionic sympathetic neurons and peripheral organs distributed widely throughout the body. Mechanistic studies of key events in the formation of postganglionic sympathetic neurons during embryonic and early postnatal life, including axon growth, target innervation, neuron survival, and dendrite growth and synapse formation, have advanced the understanding of how neuronal development is shaped by interactions with peripheral tissues and organs. Recent progress has also been made in identifying how the cellular and molecular diversity of sympathetic neurons is established to meet the functional demands of peripheral organs. In this Review, we summarize current knowledge of signalling pathways underlying the development of the sympathetic nervous system. These findings have implications for unravelling the contribution of sympathetic dysfunction stemming, in part, from developmental perturbations to the pathophysiology of peripheral neuropathies and cardiovascular and metabolic disorders.
Although primarily recognized for its role in mediating the ‘fight or flight’ response, the sympathetic division of the autonomic nervous system is indispensable for body homeostasis. Sympathetic axons innervate peripheral organs and tissues throughout the body (Fig. 1a) to control diverse physiological processes, including cardiac output, body temperature, blood glucose levels and immune function under basal conditions and in response to external stressors such as cold or danger1. The sympathetic regulation of organ function relies on intimate contacts between neurons and targets established during embryonic and postnatal development2. Sympathetic dysfunction has been implicated in several human disorders, including peripheral neuropathies, heart failure, hypertension and diabetes, some of which may have a developmental origin3,4. There is also emerging evidence that sympathetic innervation regulates stem cell niches to promote tissue regeneration through deployment of developmental signalling pathways5. Thus, there is intense interest in understanding the mechanisms driving sympathetic neuron development and establishment of connections with peripheral tissues, with the potential for developing nerve-based therapies for disease.
Fig. 1 |. Organization of the mouse sympathetic nervous system and timeline of developmental events.
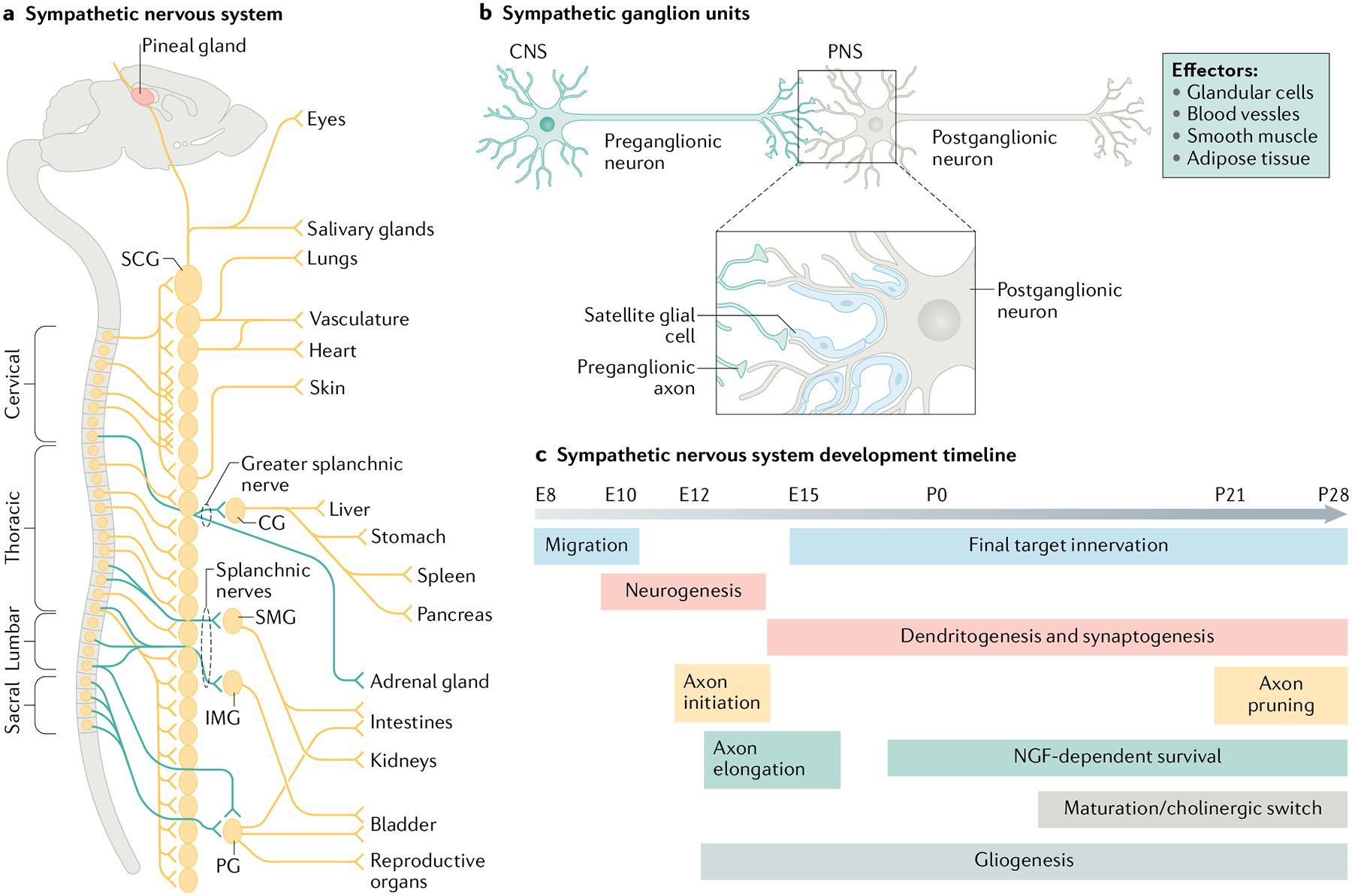
a | Sympathetic neuron cell bodies located in either paravertebral (for example, superior cervical ganglia (SCG)) or prevertebral sympathetic ganglia (for example, celiac ganglia (CG), superior mesenteric ganglia (SMG), inferior mesenteric ganglia (IMG), or pelvic ganglia (PG)) send axonal projections during development to innervate diverse organs and/or tissues in the periphery. b | Postganglionic sympathetic neurons serve as a bridge between the central nervous system (CNS) and diverse peripheral organs through inter-neuronal synapses with preganglionic sympathetic neurons whose cell bodies lie in the spinal cord. Sympathetic ganglia also contain satellite glia cells that enwrap cell bodies, dendrites and synapses of postganglionic neurons. c | Key events in the development of postganglionic sympathetic neurons are correlated with axon innervation of peripheral organs, preganglionic input and gliogenesis. The embryonic (E) and postnatal (P) time-points are based on studies done in mice and rats. NGF, nerve growth factor; PNS, peripheral nervous system. Part a adapted with permission from REF.6, AAAS. Part c adapted with permission from REF.2, Annual Reviews.
The sympathetic nervous system consists of two populations of neurons, namely preganglionic and post-ganglionic sympathetic neurons that are anatomically organized in series and connected synaptically (Fig. 1b). Preganglionic neurons reside in the intermediolateral column at thoracic and lumbar levels in the spinal cord and extend their axons to connect with postganglionic neurons that lie in paravertebral ganglia found in bilateral chains along the rostro-caudal axis or in prevertebral ganglia located between the chain and peripheral tissues. Paravertebral ganglia neurons send axonal projections to innervate the pupil, heart, respiratory system, blood vessels and exocrine glands of the face, trunk and limbs, whereas prevertebral neurons innervate abdominal, pelvic and perineal organs6.
The assembly of functional sympathetic neuron circuits, which encompasses axon innervation, neuron survival, dendrite elaboration and synaptogenesis during embryonic to postnatal stages, has been well characterized in mice (Fig. 1c) and relies on both cell-intrinsic as well as cell-extrinsic mechanisms, specifically, target-derived signals (summarized in Table 1 with details in sections below). Emerging evidence suggests that sympathetic nerves reciprocally influence the development and maturation of innervated targets7–9. Advances in single-cell transcriptomic analyses reveal that sympathetic neurons are remarkably heterogenous and that acquisition of the molecular and cellular diversity needed to meet functional demands of different peripheral organs occurs during development10. Sympathetic ganglia also contain satellite glia, a poorly understood glial population, which form a tight sheath around soma, dendrites, and synapses (Fig. 1b) and have proposed functions in neuronal morphogenesis and activity11. Thus, the establishment of functional sympathetic neuron circuits involves coordinated interactions between neurons, glial cells and peripheral tissues.
Table 1 |.
Summary of signals (ligands, receptors, cytoplasmic effectors and transcription factors) involved in sympathetic nervous system development
| Process | Signal | Type | Description | Refs |
|---|---|---|---|---|
| Axon initiation/elongation | HGF/c-Met | Ligand/receptor | Neurite outgrowth in vitro | 14,16 |
| Artemin/Ret/GFRα3 | Ligand/receptor | Axon growth in vitro; aberrant axon projections in knockout mice in vivo | 17,18 | |
| NT3/TrkA | Ligand/receptor | Axon growth in vitro; impaired projections along intermediate targets in knockout mice in vivo | 19,20 | |
| Netrin/DCC | Ligand/receptor | Arterial innervation | 21 | |
| Endothelin 1/EdnrA | Ligand/receptor | Axon guidance | 23 | |
| Endothelin 3/EdnrA | Ligand/receptor | Axon pathway selection | 25 | |
| Sema3F | Ligand | Promotes growth cone collapse by antagonizing TrkA signalling in vitro | 52 | |
| Target innervation | NGF/TrkA | Ligand/receptor | Axon growth, branching and growth cone responses in vitro; impaired target innervation in knockout mice in vivo | 28,152,189,190 |
| Calcineurin | Phosphatase | Promotes axon growth by regulating dynamin 1 phosphorylation and TrkA endocytosis | 34 | |
| Rac1 | Small GTPase | Axonal synthesis and prenylation promotes axon growth | 38 | |
| Tp53inp2 | Axonal untranslated mRNA | Essential for NGF-mediated growth and target innervation | 37 | |
| CREB | Transcription factor | Axon growth defects in knockout mice | 39 | |
| Egr3 | Transcription factor | Defects in target innervation in knockout mice | 40 | |
| Wnt5a/Ror1–2 | Ligand/receptor | Autocrine signalling promotes axon branching in vitro; innervation defects in knockout mice in vivo | 42,44,45 | |
| CD40/CD40L | Ligand/receptor | Autocrine signalling regulates innervation of low-NGF-expressing targets | 43 | |
| GDF5 | Ligand | Target-derived factor promotes innervation of select targets, independent of NGF | 46 | |
| TNFα/TNFR1 | Ligand/receptor | Reverse signalling promotes axon growth and branching | 47 | |
| Cistn3β/S100b | ER-resident protein/soluble factor | Tissue-specific innervation of brown adipose tissue | 48 | |
| Sema3a/Nrp1/Plexin A4 | Ligand/receptor/co-receptor | Defects in axon projections and cardiac innervation in knockout mice | 23,50,191,192 | |
| Axon pruning | BDNF/p75 | Ligand/receptor | Activity-dependent axon pruning via suppression of TrkA signalling | 54,55 |
| DR6 | Receptor | Axon pruning | 55,57,58 | |
| Dendrite growth | NGF/TrkA | Ligand/receptor | Dendrite elaboration and maintenance | 91,92 |
| Egr3 | Transcription factor | Impaired dendrite elongation and branching in knockout mice in vivo | 93 | |
| α3 nAChR | Ion channel subunit | Loss of primary dendrites in knockout mice | 100 | |
| CaMKII/MEK/ILK | Kinases | Downstream effectors in activity-dependent dendrite growth | 97,99 | |
| BMP5, BMP6 and BMP7/Bmpr1a | Ligand/receptor | Promotes dendrite growth in vitro; perturbed dendrite growth in Bmpr1a knockout mice | 101,102 | |
| p75 | Receptor | Necessary for BMP-induced dendrite maturation in vitro and in vivo | 107 | |
| Synapse formation | BDNF/TrkB | Ligand/receptor | Increases synapse density by influencing preganglionic axon innervation | 113 |
| NGF/TrkA | Ligand/receptor | Promotes synapse assembly in ganglia; increases synaptic contacts with peripheral targets and potentiates neurotransmitter release | 64,65,94,95,112,193 | |
| Agrin | Proteoglycan | Synapse stability | 114 | |
| α3 nAChR | Ion channel subunit | Excess synapse numbers, mis-localized synaptic contacts and impaired neurotransmission in knockout mice | 100,119 | |
| Neuron survival | NGF/TrkA | Ligand/receptor | Loss of sympathetic neurons in knockout mice in vivo; NGF on distal axons promotes survival by retrograde TrkA signalling in compartmentalized cultures | 27,71,72,194 |
| PI-3K/Akt | Kinases | Effector in retrograde TrkA trafficking and survival | 195 | |
| CREB | Transcription factor | Loss of sympathetic neurons in knockout mice in vivo and in vitro; promotes expression of pro-survival genes | 39,77,196 | |
| Egr3 | Transcription factor | Neuron loss in knockout mice | 40 | |
| JNK | Kinase | Retrograde transport of apoptotic signals | 83,85 | |
| c-Jun | Transcription factor | Pro-apoptotic nuclear signalling | 84 | |
| BDNF/p75 | Ligand/receptor | Pro-apoptotic signalling | 41,82,83 | |
| Pro-neurotrophins | Ligands | Pro-apoptotic signalling | 86,87 | |
| Neuronal diversity | Hmx1 | Transcription factor | Specification of adrenergic fate in precursors | 124 |
| TrkC/Ret | Receptors | Specification of cholinergic fate in precursors | 124 | |
| LIF/CNTF | Ligands | Exogenous application or overexpression induces neurotransmitter switch in target innervation; no phenotypes observed in knockout mice | 132,134–137 | |
| Artemin/neurturin/Ret/GFRα1/GFRα3 | Ligand/receptor/co-receptor | Postnatal specialization of noradrenergic subtypes upon target innervation | 10 |
BDNF, brain-derived neurotrophic factor; BMP, bone morphogenetic protein; Clstn3, calsyntenin 3α; CNTF, ciliary neurotrophic factor; Egr3, early growth response 3; ER, endoplasmic reticulum; GDF, growth differentiation factor 5; HGF, hepatocyte growth factor; ILK, integrin-linked kinase; JNK, Jun-N-terminal kinase; LIF, leukaemia inhibitory factor; nAChR, nicotinic acetylcholine receptor; NGF, nerve growth factor.
In this Review, we highlight recent advances in understanding the cellular and molecular mechanisms that govern the survival, axon and dendrite growth, synaptogenesis, and cellular/molecular diversity of postganglionic sympathetic neurons with a focus on neuronal interactions with target tissues as well as neighbouring satellite glia. Early aspects of neuron migration and specification are briefly discussed for the context of embryonic origin (BOX 1). Finally, we discuss emerging studies implicating the developing sympathetic nervous system in the pathogenesis of peripheral neuropathies and cardiovascular and metabolic diseases.
Box 1 |. Early development of sympathetic precursors.
Neurons and glia in sympathetic ganglia are generated from trunk neural crest cells (NCCs) that migrate ventrally to coalesce in the vicinity of the dorsal aorta197. Trunk NCCs appear to be restricted to specific fates prior to migration. For example, selective expression of the transcription factor neurogenin 1 biases trunk NCCs to become cell types in sensory ganglia but not in sympathetic ganglia198,199. Ventral migration of NCCs that give rise to sympathetic progenitors is guided by receptor tyrosine kinase signalling and axon guidance receptors197.
In mice, NCCs aggregate to form primordial sympathetic chain ganglia starting at approximately embryonic day 10.5 (E10.5)200. Early differentiation in the sympathetic lineage occurs in the vicinity of the dorsal aorta, guided by diffusible signals, specifically, bone morphogenetic proteins (BMPs), which activate a regulatory network of transcription factors, including Mash1, Phox2a/2b, Hand2 and Gata3, to drive expression of enzymes involved in norepinephrine biosynthesis and regulate the proliferation and survival of sympathetic progenitors197,201. Early sympathetic progenitors have mixed expression of noradrenergic and cholinergic biosynthetic enzymes124. Hybrid precursors segregate in a manner dependent on the selective expression of transcription factor 1, Hmx1 and the neurotrophic receptors TrkA, TrkC and c-Ret124. Onset of Hmx1 expression induces TrkA expression and consolidates the noradrenergic phenotype, whereas specification of cholinergic fate occurs through interactions between TrkC and c-ret that subsequently repress Hmx1 expression and noradrenergic markers124.
The majority of sympathetic neurons are generated by the proliferation of sympathetic progenitors over a period lasting several days (E12.5–E16.5) in rodents13,200. Sympathetic progenitors are unique compared to their counterparts elsewhere in the peripheral and central nervous systems in that they express mature neuron markers, including catecholamine synthetic and release machinery and neurofilament proteins, and even bear neuritic processes while undergoing proliferation202,203. Although mechanisms underlying neurogenesis are poorly defined, studies in gene knockout mice suggest that Ret17, Frizzled 3 (REF.204), artemin205 and insulin-like growth factors206 as well as several transcription factors required for neuronal specification197 are essential.
Axon growth and target innervation
In the growing embryo, axons of postganglionic sympathetic neurons navigate over long distances to make connections with diverse peripheral targets distributed throughout the body. Target innervation is a carefully orchestrated process that encompasses axon elongation and pathfinding, terminal branching to completely cover target fields upon reaching end-organs and refinement of connections (FIG. 2). The precision by which sympathetic axons select their navigation routes and connect with the correct peripheral targets is remarkable and arises from the combined actions of several secreted neurotrophic and guidance cues, transmembrane receptors, and adhesion proteins.
Fig. 2 |. Axon growth and innervation of peripheral targets.
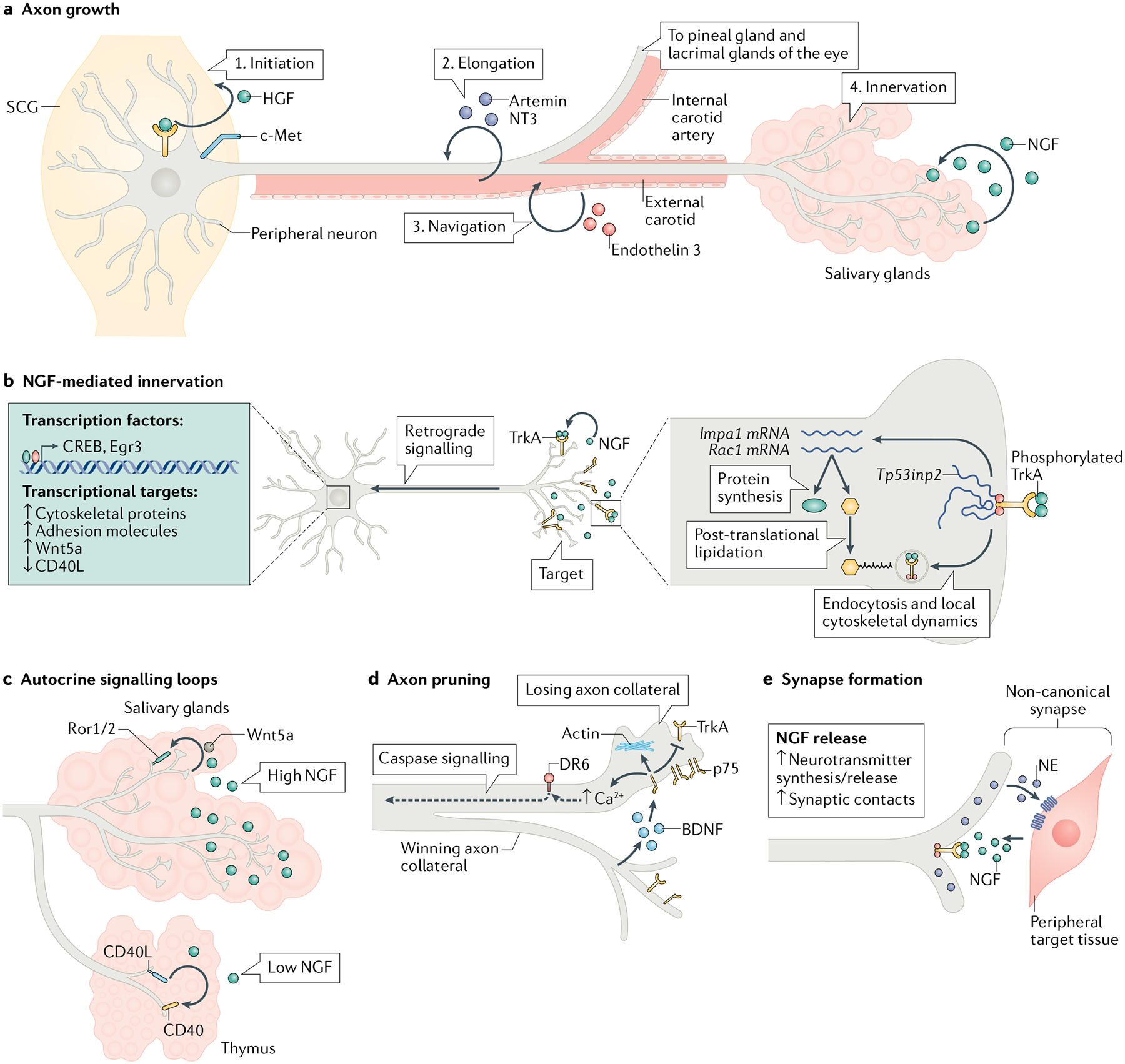
a | Target innervation is a carefully orchestrated process that encompasses axon specification, elongation, pathfinding, and branching in end-organs and involves the combined effects of autocrine and paracrine secreted factors. Autocrine hepatocyte growth factor (HGF) signalling initiates axon extension (1) and axons extend along blood vessels in response to vascular-derived guidance signals, artemin, neurotrophin 3 (NT3) and endothelin 3 (2, 3). Axons undergo terminal branching and growth to fully innervate NGF-expressing final targets (4). b | Target-derived nerve growth factor (NGF) promotes target innervation by activating local cytoskeletal and endocytic pathways in axons and retrogradely regulating transcriptional events in cell bodies and/or nuclei. NGF-mediated axon growth also relies on local protein synthesis of effectors, including Impa1 and Rac1, and also post-translational lipidation of newly synthesized proteins in distal axons. Tp53inp2 is an axonally abundant mRNA that mediates NGF-dependent growth in a non-coding manner. Retrograde NGF signalling via axonal transport of TrkA-signalling endosomes activates transcription factors and the expression of downstream target genes essential for long-term axon growth and target innervation. c | NGF signalling regulates autocrine signalling loops to promote innervation of peripheral organs. NGF induces Wnt5a synthesis in sympathetic neurons and autocrine Wnt signalling through Ror1/2 receptors promotes axon branching. In tissues with low NGF expression, such as the thymus, autocrine CD40L signalling via the CD40 receptor promotes axon branching and innervation. This pathway is downregulated when axons encounter high NGF levels in tissues such as the salivary glands. d | Axon pruning occurs by competitive collateral elimination mediated by the actions of the p75 and DR6 receptors. Activity-dependent brain-derived neurotrophic factor (BDNF) release by ‘winning’ axons activates p75 to antagonize TrkA trophic signalling in ‘losing’ axons. p75 also promotes actin remodelling and intracellular Ca2+ increase to induce degenerative signalling. The actions of DR6 and axonal caspase signalling are also required for axon loss. e | Postganglionic sympathetic neurons form non-canonical synapses with peripheral tissues. Sympathetic synapses resemble bouton like-structures loaded with norepinephrine (NE). The formation of synaptic contacts between sympathetic neurons and peripheral tissue as well as neurotransmitter synthesis and release are regulated by NGF signalling. Egr3, early growth response 3; SCG, superior cervical ganglia.
Axon initiation and elongation.
Axon specification is the earliest morphological representation of neuronal polarity. Although extensive in vitro studies using cultured hippocampal neurons have identified key roles for intrinsic cytoskeletal dynamics and polarized transport in axon specification12, the molecular mechanisms underlying this process in sympathetic neurons remain largely undefined. Sympathetic neurons extend a single axon as early as embryonic day (E) 12.5 in rodents, when the ganglia consist mainly of proliferating neuroblasts13. Autocrine signalling by hepatocyte growth factor (HGF) acting via its receptor, c-Met, may be involved in sympathetic axon outgrowth14,15 (FIG. 2a). HGF and Met transcripts are expressed in sympathetic ganglia throughout embryonic development14,16. HGF application induces neurite outgrowth in cultured neurons, whereas antibody-mediated neutralization of endogenous HGF prevents growth14,16. However, HGF exerts multiple effects on sympathetic neuroblasts, including enhancing their survival and differentiation16, and its influence on neurite outgrowth may reflect a general effect on viability.
Axons projecting from ganglia undergo rapid elongation and extend along an organized route largely following blood vessels13. Proximal axons grow alongside arteries, attracted by vascular-derived cues such as artemin and neurotrophin 3 (NT3)2 (FIG. 2a). Artemin and NT3 promote sympathetic axon growth in vitro and axon growth is stunted or misdirected in mice lacking artemin, Ret/GFRα3 receptors or NT3 (REFS17–20). However, some axons are still able to reach peripheral targets in mice deficient for artemin17,18 or NT3 (REFS19,20), suggesting a synergistic effect of these factors and/or the existence of additional signals. Arteries serve not only as paths for axons to reach end-organs but are themselves targets for innervation. Axon growth switches from axon extension along the arterial vasculature during embryonic development to innervation and formation of en passant synapses after birth in mice21. Arterial innervation is mediated by netrin, which is secreted by vascular smooth muscle cells and signals via DCC receptors in sympathetic axons21. In addition to the arterial vasculature, sympathetic axons navigate along other scaffolds to reach targets. For example, axons from stellate ganglia project along veins, instead of arteries, to reach the heart22,23. Sensory axons may also serve to guide sympathetic nerves from trunk ganglia to the mouse limbs24.
Recent findings suggest that axons take pre-selected trajectories to reach the correct end-organs. The superior cervical ganglia (SCG) is the rostral-most ganglia in the sympathetic chain and lies at the bifurcation of the internal and external carotid arteries. SCG axons project either along internal or external carotid arteries to innervate tissues in the head and face or the salivary glands. Recent work shows that these axons actively distinguish and choose between the two vascular trajectories to innervate their matching end-organs25. A subset of SCG axons expressing the EdnrA receptor for endothelin 3, which is selectively produced by external carotid arteries, preferentially use the external carotid arteries as a ‘highway’ to reach the salivary glands25 (FIG. 2a). These findings imply that molecularly distinct subtypes of neurons exist prior to target innervation and that they direct axon projections along predetermined routes to their matching tissues during development.
Target innervation.
Sympathetic innervation of final target tissues is initiated at embryonic stages (~E15.5) in mice, and continues for 3–4 weeks after birth13. The key signal controlling innervation of final targets is the neurotrophin nerve growth factor (NGF) (FIG. 2a). NGF is produced by sympathetic targets and innervation density corresponds to the amount of NGF produced by an organ26. The innervation of several peripheral tissues is either absent or incomplete in mice lacking NGF or its TrkA receptor27,28, whereas NGF overexpression in target tissues results in hyper-innervation29,30. The initial phases of axonal outgrowth from sympathetic ganglia and extension along proximal targets are independent of NGF28, suggesting that NGF is critical for axon elaboration and arborization only after axons have reached their final destinations. Interestingly, the two neurotrophins, NT3 and NGF, mediate sequential steps in sympathetic axon growth by acting through a common TrkA receptor. NT3 promotes axon elongation along intermediate vascular targets, whereas NGF controls final target innervation20. This difference in functional outcomes is related to the differential trafficking of TrkA by the two ligands20,31.
NGF-dependent growth of sympathetic axons has served as a prototype for understanding the molecular control of axonal development by target-derived cues (FIG. 2b). In distal axons, NGF binds TrkA receptors to regulate cytoskeletal dynamics through the activation of MAPK and PI-3K–Akt pathways32,33. Further, NGF-triggered endocytosis of TrkA receptors via a calcineurin–dynamin 1 signalling pathway promotes growth and target innervation34. Emerging evidence also supports a critical role for axonal protein synthesis in NGF-mediated sympathetic growth35–38 (FIG. 2b). In compartmentalized cultures of neurons, hundreds of mRNAs are targeted to sympathetic axons in response to NGF applied to distal axons in a process that is necessary for axonal viability36. Disrupting NGF-dependent transport and local synthesis of Impa1, which is involved in lipid metabolism, results in axon degeneration36. NGF also controls localized post-translational modifications of newly synthesized proteins. A recent study showed that NGF-dependent growth and target innervation relied on both the local synthesis and lipidation of newly synthesized Rac1 in sympathetic axons38. Intriguingly, axonal mRNAs do not always have to be translated to promote growth. Tp53inp2 is one of the most abundant transcripts in sympathetic axons yet it is not translated37. The deletion of Tp53inp2 impairs the NGF-dependent innervation of end-organs in mice. Strikingly, axonal defects are rescued by a translationally silent form of Tp53inp2, suggesting a non-coding role37. The precise mechanisms by which Tp53inp2 promotes growth remain to be fully elucidated, although this may involve the regulation of TrkA phosphorylation and endocytosis37.
Target-derived NGF also retrogradely activates transcriptional signalling to control axon growth and branching (FIG. 2b). Knockout mice lacking the transcription factors CREB or early growth response 3 (Egr3) exhibit abnormalities in NGF-mediated axon growth and target innervation39,40. Growth-promoting genes retrogradely activated by NGF include the TrkA receptor itself, adhesion proteins, cytoskeletal regulators and secreted autocrine factors41–43. In sympathetic neurons, retrograde NGF signalling induces the synthesis of Wnt5a, a member of the Wnt family of morphogens, which in turn promotes axon branching by signalling via Ror1/2 receptors42,44,45. Autocrine signalling by CD40L, a member of the tumour necrosis factor superfamily, specifically promotes the innervation of peripheral tissues that express low levels of NGF (thymus) but not of tissues with higher NGF expression (salivary glands)43. NGF negatively regulates CD40L activity, ensuring that this pathway is only operative in neurons that encounter low levels of NGF43. Together, these results support a hierarchical model of growth factor signalling where NGF regulates the expression of secreted signals that in turn exert an autocrine effect to promote and adjust innervation density based on NGF expression (FIG. 2c).
Although NGF has dominated as the leading factor controlling sympathetic innervation of targets, other factors are also involved. Analyses of NGF knockout mice reveal that several targets, that is, stomach, gastrointestinal tract and gonads, are still innervated, albeit reduced, whereas others, for example, the trachea, are completely unaffected by NGF loss28. Recent studies have pointed to promising candidates that either work synergistically with NGF or independently to promote target innervation, including growth differentiation factor 5 (GDF5), a member of the transforming growth factor-β (TGFβ) superfamily46, and tumour necrosis factor receptor 1 (TNFR1)47. Tissue-specific growth factors may also promote selective target innervation. For example, the enriched innervation of brown adipose tissue (relative to white adipose tissue) is due to selective expression of a novel endoplasmic reticulum membrane-bound protein, calsyntenin 3β (Clstn3β), which controls the secretion of a growth factor, S100b, to promote innervation and regulate thermogenesis48. Other cues may include mesenchymal-derived secreted factors such as angiopoietin 1 and vascular endothelial growth factor C49.
A fine balance between the activities of trophic factors and repulsive cues helps to coordinate the final axon innervation patterns in target fields. Sema3a is expressed in the developing heart and cardiac innervation patterning is disrupted in knockout mice50. Conversely, cardiac-specific Sema3a overexpression reduces sympathetic innervation50. Semaphorins function, in part, by antagonizing trophic signalling in axons as Sema3A inhibits NGF-induced nerve sprouting in vivo51 and Sema3F suppresses TrkA-induced MAPK and PI-3K signalling to trigger growth cone collapse in sympathetic axons52.
Axon pruning.
Final patterns of axon innervation are shaped by both pro-growth and regressive events. During late embryonic and neonatal stages, there is excessive axonal branching in final target fields and competition for target-derived NGF eliminates neurons that do not receive trophic support. At later postnatal stages, pruning of excessive axon collaterals in end-organs occurs independently of neuron loss to refine connectivity with targets. Similar to classical findings of terminal arbor pruning at the neuromuscular junction53, sympathetic axon pruning is a competitive process where winning axon terminals actively eliminate neighbouring axons54 (FIG. 2d). Studies of SCG projections to the eye in postnatal 20–50-day-old rats reveal that neurons initially extend collaterals to both eye chambers followed by pruning of projections to one compartment54. This refinement is an activity-dependent mechanism, where winning axons secrete brain-derived neurotrophic factor (BDNF) in response to neuronal activity, which binds the p75 receptor on proximal losing axons to cause retraction54 (FIG. 2d). p75 is a member of the TNF superfamily, which interacts with all neurotrophins and can either enhance or diminish Trk signalling depending on the cellular context. In sympathetic axons, p75-mediated pruning occurs, at least in part, by suppressing TrkA-dependent trophic signalling54. In addition, a p75-mediated increase in intracellular calcium and actin remodelling may underlie rupture of axonal membranes55. A powerful in vitro model to study molecular mechanisms underlying axon pruning has been the use of compartmentalized sympathetic neuron cultures where distal axons are selectively deprived of NGF while keeping NGF on soma and proximal axons56. In this system where neurons survive but distal axons undergo fragmentation, several groups have provided evidence for the role of death receptor 6 (DR6)57,58, a member of the TNF superfamily, as well as of axonal caspase signalling59 in pruning following NGF deprivation (FIG. 2d). The extent to which axon elimination in this in vitro paradigm resembles pruning during development, how p75 and DR6 receptors coordinate axon loss, and the involvement of caspases in vivo remain to be defined.
Synaptic connectivity with targets.
Compared to the abundant knowledge about synapse formation in the central nervous system (CNS) and neuromuscular junctions, relatively little is known about how sympathetic axons make contacts with peripheral targets. Limited insight has come from classical ultrastructural studies where nerve terminals are observed as far away as micrometre distances from target cells60,61, in contrast to the 20 nm-wide synaptic cleft in CNS synapses62. Neurotransmitter release is thought to occur from varicosities or large bouton-like structures that contain clusters of small clear and large dense-core vesicles distributed along the axonal shafts60,61 (FIG. 2e). Axonal varicosities are devoid of endoneurium or glial sheaths at the sites facing the target cells to facilitate effective neurotransmission5,63. There are no detectable postsynaptic specializations on peripheral cells63.
Classical studies using co-cultures of neonatal sympathetic neurons with cardiac myocytes also provide some insight into neurotransmitter release and regulation of synaptic strength during target innervation. Sympathetic neurons establish functional synaptic contacts with myocytes and influence their spontaneous beat rate in vitro64. NGF increases synaptic contacts with myocytes, enhances norepinephrine (NE) synthesis and potentiates NE release (FIG. 2e), leading to myocyte depolarization and increased beat rate64,65. Interestingly, individual neurons simultaneously release both NE and acetylcholine (ACh) in these co-cultures, which have opposing effects; in contrast to NE, ACh is inhibitory and results in myocyte hyperpolarization and slowing of contractility66. The neurotransmitter phenotype is plastic as neurons undergo a rapid switch from excitatory to inhibitory neurotransmission in response to BDNF signalling through p75 (REF.66). Thus, neurotrophin-mediated regulation of neurotransmitter release may underlie synaptic plasticity and could contribute to differential control of target functions under normal and maladaptive conditions.
Neuron survival
The best-characterized event in the development of the sympathetic nervous system is the apoptotic elimination of ~50% of postmitotic sympathetic neurons around the time when their axons innervate target tissues2,67. This mass-scale reduction of neuronal numbers shapes functional circuits by matching neuronal numbers to the size and demands of innervated end-organs. Pioneering work from Levi-Montalcini, Hamburger and colleagues identified a critical role for targets in regulating neuronal survival, laying the foundation for the ‘neurotrophic factor hypothesis’ that postulates that neurons are over-produced during development and later culled in a manner dictated by competition for limiting amounts of target-derived factors68,69. This hypothesis was consolidated by the discovery of NGF and other neurotrophins as target-derived factors essential for the survival of select populations of neurons during development, with profound loss of sympathetic neurons in knockout mice lacking NGF or its TrkA receptor27,70,71.
Extensive studies indicate that retrograde survival signalling is communicated via dynein-mediated vesicular transport of internalized NGF–TrkA receptor complexes72,73 (FIG. 3). More than one type of endosomal organelle may be responsible for retrograde transport, with some studies identifying Rab5-positive early endosomes74 or Rab7-positive late endosomes75 as being the long-distance carriers, while others have suggested a role for multi-vesicular bodies76. In cell bodies, axon-derived Trk endosomes promote survival by regulating cytoplasmic signalling or the expression of anti-apoptotic, endosomal and metabolism-related genes via the activation of downstream transcription factors, including CREB41,77. Trk endosomes are capable of persistent signalling in cell bodies, facilitated by the endosomal effector coronin 1 (REF.78).
Fig. 3 |. Axonal trafficking of survival and apoptotic signals underlie a precarious balance between neuronal survival and death.
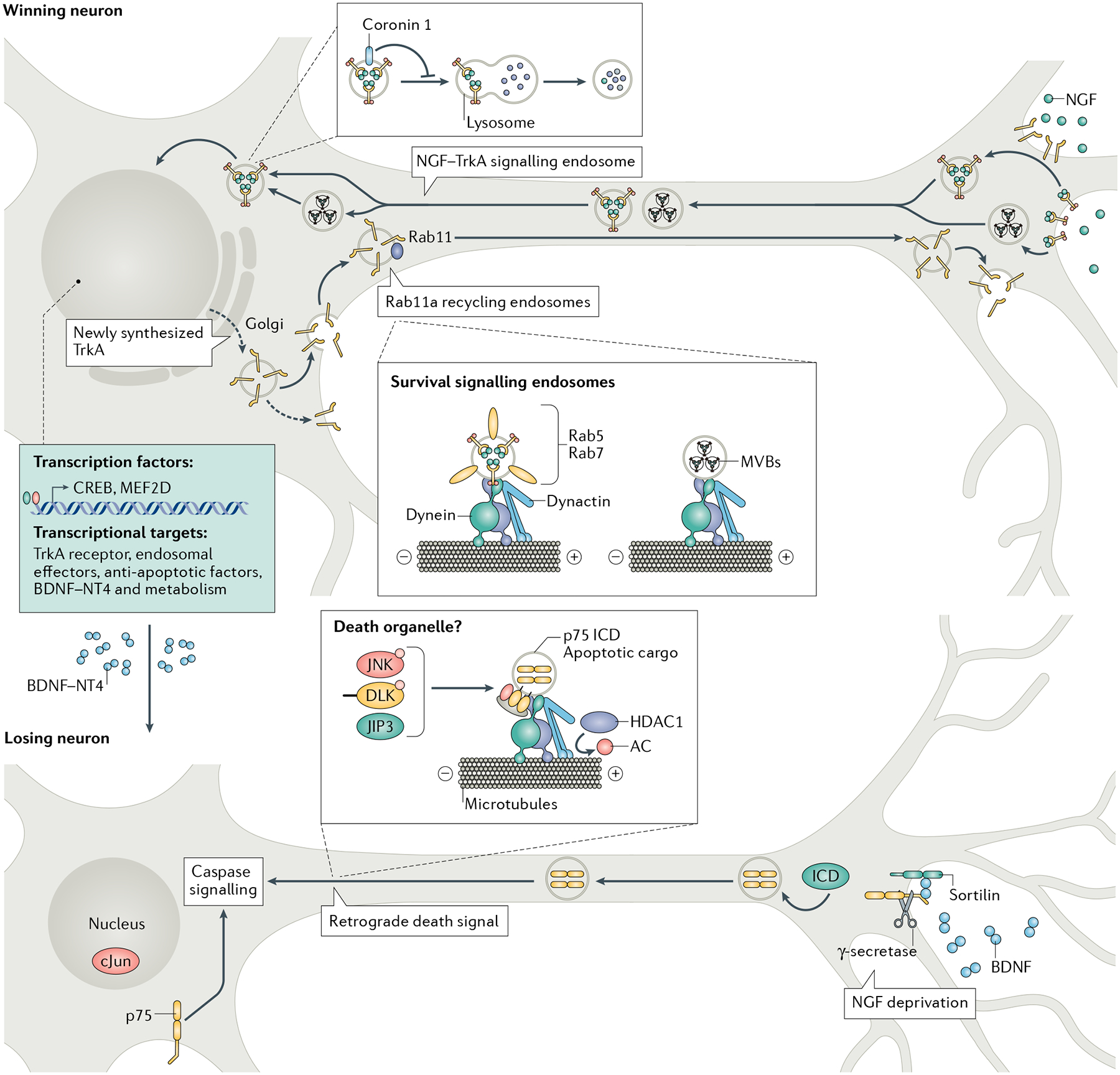
Activated TrkA receptors are retrogradely transported by dynein along axon microtubules in heterogenous organelles, including Rab5-positive and Rab7-positive endosomes and multi-vesicular bodies (MVBs). Axon-derived TrkA organelles activate pro-survival transcriptional signalling in cell bodies as well as the synthesis of paracrine factors that induce death in neighbouring losing neurons. Nerve growth factor (NGF)–TrkA signalling persists in cell bodies of winning neurons through the activity of coronin 1, which prevents TrkA degradation. Retrograde NGF signalling also regulates anterograde transcytosis of TrkA receptors from soma surfaces to axon terminals via Rab11-positive recycling endosomes in a positive feedback mechanism. Brain-derived neurotrophic factor (BDNF)–neurotrophin 4 (NT4)-dependent and BDNF–NT4-independent apoptosis is mediated by activation of p75 receptors on losing neurons. In the axons of losing neurons, pro-neurotrophins and neurotrophins may initiate a retrograde death signal by binding to a p75–sortilin receptor complex. Proteolytic cleavage of p75 releases the intracellular domain (ICD), which is transported in a death organelle along with apoptotic signals dual leucine zipper kinase (DLK) and c-Jun-N-terminal kinase (JNK), which are anchored to the vesicles by adaptor protein JIP3, to the cell body to activate death signalling. AC, acetyl group. Adapted with permission from REF.79, Elsevier.
A remarkable aspect of retrograde NGF signalling is the activation of positive feedback loops such as the transcription of its own TrkA receptor and endosomal effectors that help to distinguish the neurons that survive from those that undergo apoptosis41,78,79. Furthermore, NGF signalling augments the expression of pro-death signals that act in a paracrine manner to kill neighbouring neurons41. In another example of a positive feedback loop, newly synthesized TrkA receptors resident on soma surfaces are internalized and recycled to axons in a manner regulated by retrograde NGF signalling80,81. Ligand-triggered soma-to-axon transcytosis of TrkA is necessary for survival and growth responses to NGF80,81. Together, these feedback mechanisms enhance the competitive ability of the ‘winning’ neurons and weaken neighbouring neurons that do not gain sufficient access to NGF.
Retrograde transport of apoptotic signals is also part of the developmental competition for target-derived trophic support (FIG. 3). The activation of p75 in distal axons in response to BDNF or NGF withdrawal triggers a retrograde degenerative signal that involves proteolytic cleavage of p75 and long-distance transport of its intracellular domain to cell bodies82,83. Other studies have implicated axonal signalling of stress-induced kinases, dual leucine zipper kinase (DLK) and c-Jun-N-terminal kinase (JNK) in initiating apoptotic signals that result in retrograde phosphorylation of the pro-apoptotic transcription factor c-Jun in cell bodies of sympathetic neurons and cell death84,85. Retrograde trafficking of apoptotic signals during development may also be initiated by target-derived pro-neurotrophins, which are precursors of neurotrophins and act through p75 to mediate apoptosis86,87.
Thus, a tightly controlled balance between pro-survival and pro-death signalling events orchestrates the final complement of sympathetic neurons during development.
Dendrite formation and maintenance
The ability of postganglionic sympathetic neurons to integrate synaptic inputs from preganglionic neurons is influenced by the size and complexity of their dendrite arbors. Extension of sympathetic dendrites is initiated at E14 and continues through postnatal life13. Dendritic arbors dramatically increase in size and complexity during late embryonic to neonatal stages coincident with the axon innervation of peripheral target tissues and access to target-derived NGF88. Sympathetic dendrites grow in size through adulthood with the size and complexity of the dendrite arbors reliant on the size of the targets innervated by sympathetic axons89,90. Thus, the morphological complexity of dendrites likely reflects the functional demands of innervated peripheral tissues.
Target-derived NGF has been implicated in mediating dendrite elaboration and maintenance (FIG. 4). Exogenous NGF administration enhances dendrite complexity in vivo, whereas neutralizing NGF has the opposite effect in neonatal and adult mice91,92. The loss of Egr3, an NGF-induced transcription factor, in sympathetic neurons impairs dendrite elongation and branching in vivo93. Transcriptional profiling of Egr3-deficient sympathetic neurons revealed changes in genes with well-documented roles in dendritogenesis, including p75, Notch1, ephrin B1 and Slit2, suggesting a role for NGF signalling in regulating transcriptional programmes necessary for dendritic development93 (FIG. 4a). Furthermore, a subset of TrkA receptors, originating from distal axons, were found in dendrites94,95, suggesting a local role in regulating growth, possibly by impinging on the actin or microtubule cytoskeleton.
Fig. 4 |. Signalling mechanisms underlying dendrite growth and synaptogenesis in sympathetic ganglia.
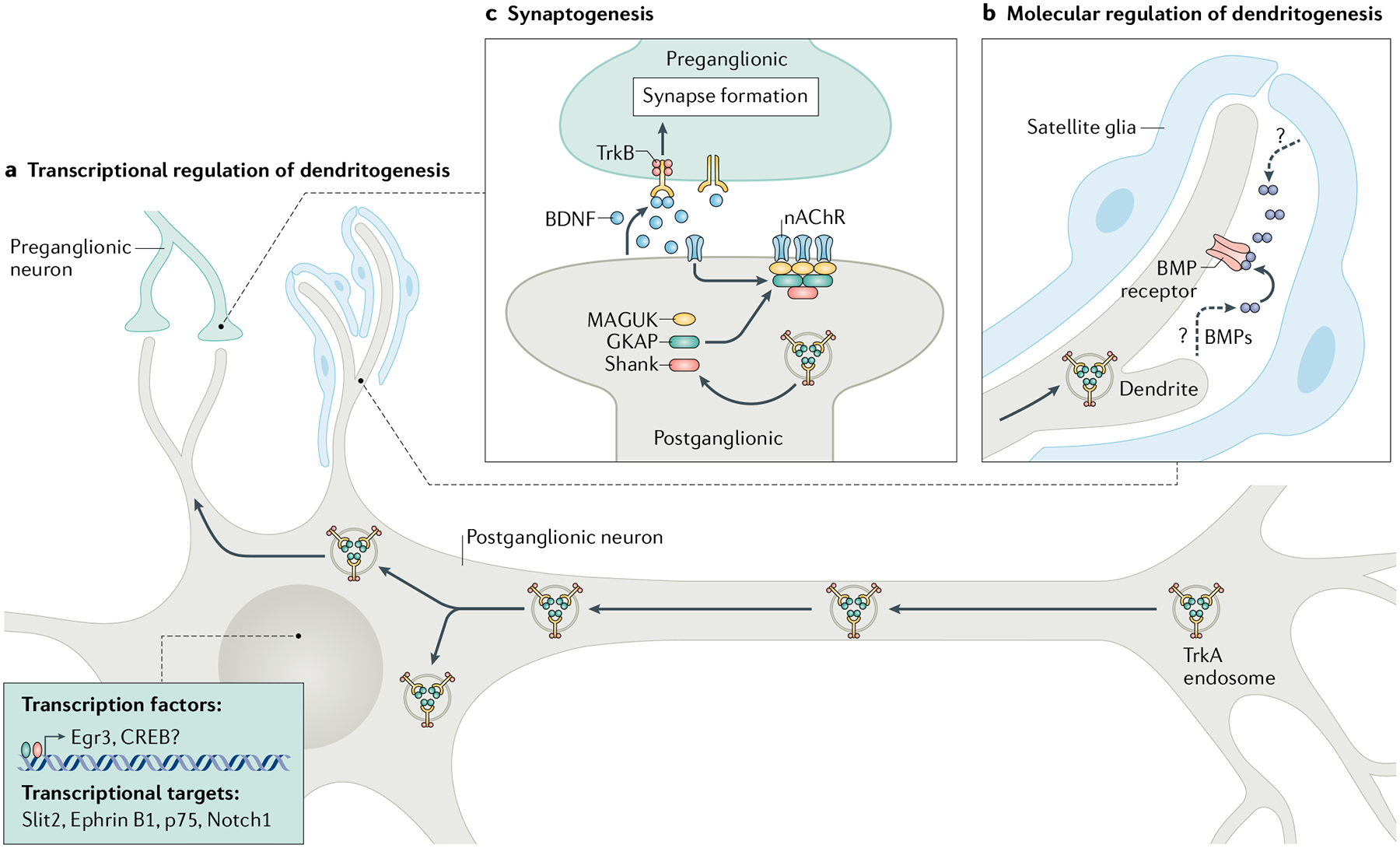
a | Axon-derived TrkA endosomes are transported to dendrites, where they signal locally to control dendrite growth, in conjunction with autocrine bone morphogenetic protein (BMP) signalling as well as BMPs derived from neighbouring satellite glia. b | Retrograde nerve growth factor (NGF)–TrkA signalling activates transcriptional programmes to promote dendritic growth and development. c | Axon-derived TrkA endosomes influence synaptogenesis by promoting the clustering of nicotinic acetylcholine receptors (nAChRs) and postsynaptic density components, including MAGUK, GKAP and Shank. Retrograde NGF signalling also regulates preganglionic innervation by promoting secretion of brain-derived neurotrophic factor (BDNF) from postganglionic neurons. Egr3, early growth response 3.
However, NGF alone is not sufficient to induce dendritic growth96,97 and in vivo, rudimentary dendritic arbors are present before axons reach NGF-expressing peripheral targets13. Neuronal activity, driven by preganglionic input, could be one factor responsible for dendrite initiation. Cholinergic preganglionic axon terminals enter sympathetic ganglia prior to dendrite formation98 and, in vitro, neuronal activity induces dendrite formation via the activation of kinase signalling pathways, including CaMKII, MEK and integrin-linked kinase (ILK) signalling97,99. In vivo, loss of excitatory synaptic transmission in sympathetic neurons through the deletion of functional nicotinic ACh receptors (nAChRs) results in a loss of primary dendrites in mice100. Together, these results suggest a scenario where preganglionic activity promotes initial dendrite outgrowth, whereas the final size and complexity of dendrite arbors is dictated by the size of the target territory and access to target-derived NGF.
Further dendritic elaboration is controlled by additional signals; members of the bone morphogenetic protein (BMP) family — BMP5, BMP6 and BMP7 — promote robust dendrite growth in sympathetic neurons in vitro101 (FIG. 4b) and conditional deletion of the BMP receptor BMPR1A perturbs dendrite growth and complexity during postnatal development in vivo102. BMPs are expressed in both sympathetic neurons and satellite glia, coincident with dendrite formation103,104. In vitro, satellite glial cells augment dendrite growth105, which is attenuated by the inhibition of BMP signalling104. The molecular mechanisms underlying BMP-induced dendritic development are unclear. Although in vitro evidence supports a role for canonical SMAD signalling via the regulation of microRNAs106 or p75 (REF.107), dendrite complexity was unaffected in Smad4-mutant mice102.
Synaptic connectivity in ganglia
Postganglionic sympathetic neurons connect the CNS to peripheral organs through synapses with preganglionic sympathetic neurons whose cell bodies lie in the spinal cord. Preganglionic axons enter embryonic sympathetic ganglia before dendrites are formed and establish nicotinic cholinergic synapses initially on cell bodies of postganglionic neurons108. Immediately after birth, there is a rapid increase in synapse number during dendritogenesis, with >90% of synapses formed being axo-dendritic108. There is a high degree of organization in connectivity from the outset because preganglionic axons from specific spinal segments enter matching sympathetic ganglia and form appropriate connections with specific subtypes of postganglionic neurons108. The overall process by which preganglionic axons find and form synapses with postganglionic neurons is poorly understood, although there is evidence to support the involvement of target-derived signalling. The proportion of preganglionic input to postganglionic neurons is commensurate with the size of innervated peripheral targets89,109,110 and input is lost upon axotomy of postganglionic axons110–112. Retrograde NGF signalling regulates preganglionic innervation by promoting the secretion of synaptogenic factors, for example, BDNF, from postganglionic neurons113 as well as the clustering of postsynaptic components, including nAChRs, in dendrites94,112 (FIG. 4c). In particular, a subset of axon-derived TrkA endosomes act locally in dendrites to maintain postsynaptic clusters94,95. In vivo studies suggest that the proteoglycan agrin, an essential regulator of synaptogenesis at neuromuscular junctions, also regulates the stability of nascent sympathetic synapses114. Cholinergic synapses in sympathetic ganglia are structurally similar to CNS glutamatergic synapses and contain similar postsynaptic machinery, including scaffolding proteins PSD93 and PSD95 as well as MAGUK, GKAP/SAPAP and Shank/ProSAP115.
Synaptic connections between preganglionic inputs and postsynaptic neurons undergo refinement during the first postnatal month116,117. Initially, 8–10 preganglionic axons poly-innervate individual sympathetic neurons at birth but excess synaptic contacts are eliminated so that only one to three inputs persist by 1 month100. Recent evidence suggests that postsynaptic activity is necessary for synapse elimination and for strengthening remaining inputs100. In α3 nAChR knockout mice lacking fast nicotinic transmission, the elimination of excess synaptic connections is impaired and synapses are mislocalized on cell bodies of postganglionic neurons100. Remarkably, in contrast to neuromuscular junctions and central synapses62,118, activity is dispensable for the long-term structural maintenance of sympathetic synapses119. However, postsynaptic activity is necessary to sustain synaptic transmission through regulating acetylcholine synthesis in preganglionic neurons119. Together, these results suggest that synaptic activity is essential for the refinement and functional maturation but not for the structural integrity of synaptic connections between preganglionic and postganglionic sympathetic neurons. The identity and cellular origin of activity-dependent factors that regulate synaptic connectivity remain to be fully defined. Further, silencing of postsynaptic activity could also contribute to synaptic defects through indirect effects on neighbouring satellite glia or target innervation.
Diversity of sympathetic neurons
Sympathetic neurons exhibit considerable differences in soma and dendrite morphology, expression of neurotransmitters and neuropeptides, and electrophysiological properties90,120–122. Single-cell RNA sequencing analyses are beginning to shed light on the cellular and molecular diversity of postganglionic sympathetic neurons10,123. A recent study identified seven neuronal populations in stellate and thoracic sympathetic ganglia, including five adrenergic and two cholinergic subtypes, defined by unique expression patterns of growth factor receptors, neurotransmitters and neuropeptides10.
How this specification of sympathetic neurons is established is poorly understood, though some insight has been gained by studying a subset of cholinergic sympathetic neurons. During early embryogenesis, sympathetic precursors exhibit both noradrenergic and cholinergic properties and then segregate into distinct fates prior to target innervation124. The early diversification is coordinated through an intrinsic programme that involves antagonistic interactions between pro-adrenergic and pro-cholinergic transcription factors124,125. The temporal order of neuronal birth and/or the role of embryonic patterning genes could be involved in fate specification. In a recent transcriptome profiling analysis, sympathetic neurons had a preferentially high expression of the HoxC cluster of homeodomain transcription factors, suggesting a HoxC-mediated specification of neurons along a spatial axis123.
In addition to early acquirement of cholinergic fate, some noradrenergic sympathetic neurons later switch to a cholinergic fate upon the postnatal innervation of target tissues such as sweat glands, periosteum and skeletal muscle vasculature126–129. The necessity and sufficiency of the target for the noradrenergic-to-cholinergic switch was demonstrated by the use of tabby mutant mice lacking sweat glands as well as transplantation and co-culture experiments with sweat glands129–131. The cytokines leukaemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF) have been proposed to be important for cholinergic specification due to their ability to induce cholinergic differentiation in vitro and in vivo132–134. However, deletion of LIF and/or CNTF in mice did not affect the cholinergic switch, suggesting that other factors may be involved135–137.
Differences in gene expression during neuronal maturation give rise to discrete circuitry for the regulation of autonomic functions. Piloerection (goosebumps) and nipple erection are distinct autonomic responses despite being activated by common stimuli such as hypothermia, emotional arousal or mechanical stimuli10,138,139. Using single-cell sequencing, lineage and retrograde tracing, and transgenic mouse models, Furlan et al. identified two distinct noradrenergic subpopulations innervating the nipple erector and piloerector muscles10. Both subtypes are embryonically derived from the same noradrenergic precursors and marked by the expression of TrkA and Ret receptors but undergo specialization during target innervation10. In particular, Ret is downregulated during the embryonic commitment to noradrenergic fate but is re-expressed in the two subtypes postnatally10. The Ret ligands artemin and neurturin are uniquely expressed by nipple erector or piloerector muscles, respectively, with the onset of expression correlated with target innervation10. The two neuronal subtypes express either GFRα2 or GFRα3, which are Ret co-receptors, to selectively innervate nipple erector or piloerector muscles and undergo differentiation in a target-dependent manner10.
These findings, together with the neurotransmitter switch in some sympathetic neuron populations upon target innervation129, emphasize the importance of target-dependent factors in regulating the postnatal specialization and diversification of sympathetic neurons necessary for establishing dedicated circuits. However, it is unlikely that neuronal diversity is generated exclusively via target-dependent differentiation given that differences in neuronal morphology, electrophysiological properties and neurotransmitter/neuropeptide expression are already evident prior to target innervation122. Some neuron subtypes also use pre-specified axonal trajectories to reach their targets25 and functionally distinct classes of sympathetic neurons are known to innervate common targets such as blood vessels122. Thus, target-derived cues likely work together with cell-autonomous programmes and/or local signals within the ganglia, such as preganglionic input or glia-derived factors, to fine-tune the final identity of sympathetic neuron populations.
The development, targets and functions of other noradrenergic subtypes identified by single-cell sequencing from stellate and thoracic ganglia10 remain undefined. Notably, it remains unclear whether the seven identified neuron subtypes10 exist across the entire sympathetic chain or if each ganglion has its own unique set of neuronal populations. Further, given the sex-specific differences in autonomic functions140,141, sexual dimorphism in sympathetic neuron diversity and circuits warrant further exploration.
The expression of different repertoires of neuropeptides adds to the diversity of sympathetic neurons. Noradrenergic neurons are marked by the co-expression of neuropeptide Y and galanin, whereas vasoactive intestinal peptide, calcitonin gene-related polypeptide and somatostatin largely colocalize with cholinergic neurons10,121,122. It has been proposed that combinatorial patterns of neurotransmitter/neuropeptide expression provide the basis for a ‘neurochemical code’ that allows the innervation of specific targets and regulation of distinct autonomic functions121. How neurotransmitter expression is coordinated with that of specific neuropeptides during development, the signals involved, the identity of targets innervated by specific neuropeptide-expressing sympathetic neurons and the modulatory roles of neuropeptides remain to be defined.
Development of satellite glia
A mature sympathetic nervous system requires the assembly of neurons and their associated glial cells, Schwann cells and satellite glial cells. The development of Schwann cells, which wrap around axons, has been extensively covered in several excellent reviews142,143. Here, we focus on the current, albeit limited, understanding of the development of the enigmatic satellite glial cells in sympathetic ganglia. Satellite glia form thin cytoplasmic sheaths around the cell bodies, dendrites and synapses of peripheral neurons, with only 20 nm of space (the same width of a synaptic cleft) between neuronal and glial membranes11,144,145. Multiple satellite glia (4–10 in mice) surround a single neuron soma and are connected with each other and the neurons via gap junctions, with the number of glial cells per neuron being positively correlated to soma size11,145. This unique arrangement of satellite glia is found in sympathetic, parasympathetic and sensory ganglia11,145, with each neuron and associated satellite glia thought to form discrete structural and functional units.
Like sympathetic neurons, satellite glial cells are derived from multipotent neural crest precursors142. One early determinant of glial cell fate is the transcription factor Sox10, which is expressed in all migrating neural crest cells but is downregulated in neuronal precursors and maintained in glial precursors146. Both Schwann cells and satellite glia are lost in Sox10-deficient mice147. The mechanisms underlying the decision to maintain or lose Sox10 expression are unclear, although it may, in part, be intrinsically determined as neural crest precursors are already committed to a glial or neuronal fate as they populate the nascent sympathetic chain around E11.5–E12.5 (REF.148). Another early marker of satellite glia in sympathetic ganglia is brain lipid binding protein (BLBP), which is expressed in glial precursors and maintained in mature satellite glia149,150.
Despite an early commitment to a glial fate148, precursors remain quiescent until about E16.5 in sympathetic ganglia, at which time robust gliogenesis is initiated coincident with the completion of neurogenesis151. The bulk of satellite glia are generated at the ganglion periphery during a rapid phase of proliferation between E16.5 and E18.5 (REF.151). By birth, glia migrate within the ganglia to contact and enwrap neuronal soma, where some limited proliferation continues for 2–3 postnatal weeks151. Thus, satellite glia undergo dramatic morphological changes to facilitate their migration and ensheathing of neuronal cell bodies during a narrow developmental window.
The timing of glial development is coincident with preganglionic input and target innervation13,108. Thus, an intriguing possibility is that neuronal activity and/or retrograde signalling from the periphery may be involved in satellite glia development. Indeed, satellite glia are lost in knockout mouse models of NGF and TrkA27,152; however, whether satellite glia are lost due to the loss of NGF–TrkA signalling and/or the loss of neurons in these mice is unclear. Further studies in NGF-knockout or TrkA-knockout mice with concomitant deletion of the pro-apoptotic factor Bax to prevent neuronal apoptosis28 are warranted to address if retrograde NGF signalling in neurons results in the production of signals that then influence glial proliferation, migration and morphogenesis.
The intimate physical association between satellite glia and sympathetic neurons place these cells in an ideal position to be critical regulators of neuronal connectivity, synaptic transmission and homeostasis. Morphologically, satellite glia in vertebrates are similar to soma-ensheathing glia identified in Caenorhabditis elegans and Drosophila that have important roles in neuronal survival, regulating soma size and positioning, dendrite branching, synaptic activity, and removal of dying cells153,154. In a key difference from sensory ganglia, satellite glia in sympathetic ganglia ensheathe the dendrites and synapses of neurons in addition to cell bodies145. In neuron–glia co-cultures, satellite glia selectively enhance dendrite but not axonal growth105, promote formation of cholinergic synapses and enhance spontaneous synaptic activity155. Satellite glia have also been proposed to modulate neuronal activity through the regulation of ion channel expression and controlling extracellular K+ concentrations156. In dorsal root ganglia, satellite glia precursors and not the canonical macrophages were found to be responsible for clearing neuronal corpses during naturally occurring cell death157, raising the possibility of a similar function in sympathetic ganglia.
Aberrant development in disease
Considering their physiological importance in body homeostasis and stress responses, it is not surprising that sympathetic dysfunction is linked to several human disorders, including peripheral neuropathies4,158, congestive heart failure3, hypertension159, diabetes160, immune dysfunction and epithelial cancers161. Although the majority of studies have focused on adults, recent evidence suggests that aberrant development of sympathetic neurons could contribute to the aetiology of several disorders, including familial dysautonomia (FD)162,163, Down syndrome164, and cardiac and metabolic dysfunctions3,7,8,165.
FD is the most prevalent form of a group of peripheral neuropathies collectively called hereditary sensory and autonomic neuropathies that result in defects in the development and survival of sympathetic and sensory neurons162. Patients with FD exhibit autonomic abnormalities, including cardiovascular, renal, gastrointestinal and pulmonary dysfunction, and have a shortened lifespan162. FD is caused by mutations in Elp1 (IKBKAP), which is best known as a component of the transcriptional elongator complex with functions in transcription, histone acetylation, tRNA modifications and translation4. In mice, loss of Elp1 or expression of a disease-associated variant largely recapitulates FD phenotypes with loss of sympathetic neurons and impaired target innervation during the period of NGF dependence166,167. In recent findings, Li et al. revealed a non-canonical role for Elp1 in sustaining NGF signalling during retrograde transport by associating with NGF–TrkA endosomes and suppressing the activity of the Shp1 phosphatase163.
Dysregulated TrkA trafficking has also been implicated in sympathetic abnormalities in Down syndrome164. Down syndrome is caused by trisomy of human chromosome 21 and children with Down syndrome exhibit autonomic abnormalities in heart rate and blood pressure168. Patel et al. observed a developmental loss of innervation in human Down syndrome organs and in a mouse model164. Using transgenic mice, they found that sympathetic neuron loss was due, in part, to an excess of regulator of calcineurin 1 (RCAN1), an endogenous inhibitor of calcineurin, which promotes TrkA internalization164. In a mouse model of Down syndrome, defects in TrkA trafficking and neuron loss were ameliorated by genetically reducing RCAN1 dosage164.
Changes in sympathetic innervation and activity have been implicated in many cardiac pathologies ranging from sudden infant death syndrome to prevalent disorders such as hypertension, myocardial ischaemia and cardiac arrhythmias3,165. Sympathetic tone is disturbed in preterm infants and is thought to underlie sudden infant death syndrome and to result in a greater risk of hypertension in adulthood169,170. In animal models of genetic hypertension, neonatal elevation in sympathetic activity precedes the development of hypertension in later life171. In humans, both sympathetic hyperinnervation and denervation can lead to cardiac arrhythmias3,165. In mice, disruptions in cardiac sympathetic innervation during development due to overexpression or loss of the axon guidance cues semaphorin 3A or endothelin 1 results in abnormal heart rate, cardiac failure and lethality23,50,172. Aberrantly high levels of NGF in the heart have been linked to sympathetic hyper-innervation, cardiac hypertrophy and sudden cardiac death in animal models30,173. In addition to altered innervation, the modulation of sympathetic activity by satellite glia could also be a contributing factor to cardiac disease. In transgenic mice, chemogenetic activation of satellite glia enhanced sympathetic output to the heart and accelerated heart rate and contraction174. Together, these findings suggest that pathways that govern cardiac sympathetic innervation during development or neuron–glia interactions could serve as important targets for prophylactic interventions in heart disease.
The adult sympathetic nervous system is well known to control hormone secretion, glucose production and glucose/lipid metabolism in the pancreas, liver and adipose tissue175. The developmental roles of innervation in these metabolic tissues is less well defined. However, there is growing evidence that early perturbations in innervation may be an instigating factor in the pathogenesis of diabetes. An early and selective loss of sympathetic innervation occurs in humans and animal models of type 1 diabetes prior to the onset of hyperglycaemia176,177. Further, diabetic mice show an early impairment in synaptic transmission in sympathetic ganglia due to hyperglycaemia-induced inactivation of acetylcholine receptors178. The developmental loss of sympathetic nerves in mice results in reduced insulin secretion and impaired glucose tolerance in adults7. Insulin secretion is also impaired in children with hereditary sensory and autonomic neuropathy type IV (HSAN4), a peripheral neuropathy caused by mutations in the TrkA gene179 and characterized by loss of sympathetic innervation180.
An emerging area of interest is the prominent role of sympathetic nerves in modulating immune functions. In the gastrointestinal tract, sympathetic nerves densely innervate enteric ganglia, intestinal vasculature and sphincter muscle to control gut motility, fluid exchange and blood flow. Sympathetic nerves exert both pro-inflammatory and anti-inflammatory effects in animal models of inflammatory bowel disease and gastric ulcer and, in turn, exhibit structural remodelling and increased activity in these pathological conditions181. The precise mechanisms by which sympathetic nerves influence intestinal immunity remain undefined but may involve direct interactions with gut-resident macrophages182. Macrophages in close proximity to sympathetic nerve endings activate tissue-protective programmes in response to bacterial infections dependent on sympathetic activity and β2-adrenergic signalling182. Sympathetic nerves are also capable of directly responding to the gut microbiome composition or perturbations in microbial-derived metabolites as part of gut–brain circuits that regulate digestive physiology and host defence183. Although these studies have been conducted in adult animals, outstanding questions include addressing if these neuro-immune or nerve–gut microbiota interactions are established during development, gaining insight into reciprocal signalling mechanisms and their dysregulation in pathological conditions.
In the bone marrow niche, sympathetic nerves are a critical regulator of haematopoietic stem and progenitor cells and play key roles in their mobilization into the bloodstream and their proliferation and self-renewal under basal conditions and in response to stress161. Sympathetic nerve loss and neuropathy is a feature in mouse models of acute myeloid leukaemia, a cancer affecting the bone marrow niche, where loss of nerve-derived adrenergic signalling contributes to aberrant expansion of haematopoietic stem and progenitor cells184. In contrast to their protective effects in leukaemia, sympathetic innervation is increased in epithelial cancers, such as prostate cancer, and promote tumour growth and metastasis, emphasizing the context-dependent roles of nerves in cancer. Increased nerve density during cancer initiation and progression in certain cancers has been attributed to a re-activation of developmental pathways, specifically the up-regulation of neurotrophin signalling in the tumour microenvironment184.
Together, these studies underscore the need for further investigation of the sympathetic nervous system during development as a target for early interventions. A better understanding of how sympathetic innervation governs the development of innervated targets is necessary to fully elucidate how early defects in innervation contribute to organ dysfunction (BOX 2).
Box 2 |. Role of sympathetic innervation in target development.
During development, sympathetic neurons extend axons in tandem with the development and morphogenesis of innervated tissues. Though the contribution of innervation to organogenesis is not well defined, emerging studies suggest that sympathetic innervation plays a broad role in instructing the growth, morphogenesis and maturation of innervated peripheral organs beyond regulating their adult functions.
Sympathetic innervation plays an important role in postnatal heart development via regulating cardiomyocyte cell cycle activity8,9. Innervation coincides with cardiomyocyte cell cycle arrest and a switch to growth by cellular hypertrophy9,207. Neonatal sympathectomy disrupts cardiomyocyte proliferation and results in a smaller heart size9. In in vitro co-cultures, sympathetic neurons reduce proliferation208, enhance expression of calcium channels209 necessary for functional maturation and improve contractility in neonatal cardiomyocytes64. Sympathetic effects on cardiomyocyte proliferation and maturation are mediated via β-adrenergic signalling210. Notably, blocking β-adrenergic signalling in neonatal mice increases cardiomyocyte numbers through regulation of cytokinesis and enhances regeneration after myocardial infarction in adults8.
A role for sympathetic innervation in pancreatic islet development has also been recognized in recent years. Pancreatic islets, which regulate blood glucose levels, are richly innervated by sympathetic nerves211. Denervation during embryonic development results in perturbed islet morphology with a loss of contacts between β-cells, suggesting that sympathetic nerves provide key organizational cues during islet formation7. Similar to heart development, the early effects of sympathetic innervation on islet architecture are mediated, in part, via norepinephrine signalling7. This inductive interaction between sympathetic axons and islets is critical for the functional maturation of islets, because developmental denervation results in loss of mature β-cell markers, impaired insulin secretion and glucose intolerance in adult mice7.
Elucidating the role of innervation in the growth, morphogenesis and differentiation of peripheral targets is necessary for a complete understanding of organogenesis. This knowledge has implications that extend beyond the development to tissue regeneration. Several sympathetic targets are capable of adult regeneration and recent exciting findings indicate that innervation regulates stem cell renewal and proliferation to promote normal homeostasis and tissue repair5.
Conclusions and future perspectives
The sympathetic nervous system has long served as a paradigm for neurobiologists to understand the molecular and cellular mechanisms governing neuron survival, axon growth and innervation of peripheral targets during development. However, the past decade has seen an exponentially growing interest in sympathetic nervous system development from the broader perspective of investigating the role of innervation in organogenesis, organ function and tissue regeneration. Aided by innovative techniques, such as single-cell sequencing, new insights have been gained into the unexpected diversity of sympathetic neurons and the sophisticated organization of discrete sympathetic circuits, acquired during development, that govern different physiological tasks. Given the increasing awareness of the contribution of sympathetic dysfunction to disease, we anticipate more in-depth studies of postganglionic neurons at the molecular, cellular and circuit levels. Below, we outline a few outstanding directions that should be prioritized for future investigations.
Emerging studies highlight the considerable heterogeneity of sympathetic neurons at cellular and molecular levels and are beginning to identify dedicated neuron subtypes and circuitry that underlie functional diversity10,123. Sympathetic neurons also show considerable heterogeneity in electrical properties that seem to be tightly linked to the ganglion of origin and less on their innervated targets120. Advances in single-cell sequencing, optogenetics, chemogenetic tools and animal models offer new opportunities to fully define the molecular logic underlying the establishment of target-specific circuits connecting preganglionic and postganglionic neurons with matching end-organs.
Although significant advances have been made in understanding the pathways underlying neuron survival and axon growth, the knowledge of dendrite growth and synaptogenesis in the sympathetic nervous system have lagged far behind. Much of the current understanding of these events has been gleaned from in vitro culture studies. Fundamental questions remain about in vivo mechanisms of dendrite initiation, growth, maintenance and plasticity. A fascinating question is to define how dendritic morphologies are coordinated with the specification of neuronal identity and innervation of select peripheral targets. Specific neuronal subtypes innervating different peripheral targets have different dendrite arborization patterns90. How these subtype-specific patterns are established, the relative contributions of preganglionic input versus target-derived factors to the establishment of dendrite geometries and the implications of subtype-specific dendrite geometries on distinct autonomic functions remain to be elucidated. Aberrant dendritic morphologies of sympathetic neurons have been linked to maladies, including spontaneous hypertension185, highlighting the importance of dendritic morphology in sympathetic regulation of organ function.
Similarly, synaptogenesis within sympathetic ganglia remains a poorly understood process. Outstanding questions for the future include elucidating mechanisms underlying the assembly and stabilization of presynaptic and postsynaptic machinery as well as synapse elimination. Additional studies are also needed to understand how preganglionic axons discriminate between different postganglionic neuron subtypes and the contributions of target-derived and glia-derived factors to synapse assembly, refinement and maturation. Compared to inter-neuronal synapse formation in the ganglia, even less is known about how postganglionic axons form and maintain synaptic contacts with peripheral target cell types. Advances in serial section electron microscopy have the potential to provide high-resolution insight into peripheral cell types that are directly connected by sympathetic nerves and morphological specializations of nerve–target contacts.
A key direction should be to focus on satellite glia in the sympathetic nervous system. It remains poorly understood how satellite glia are specified during development, how they develop intimate spatial relationships with their neuronal neighbours with precise neuron–glial cell matching, and how glia–neuron interactions influence the development, functions and maintenance of sympathetic circuits. The unique morphologies of satellite glia, the close physical association with neurons and the rapid changes in gene expression after dissociation have traditionally presented challenges to their study. However, recent advances in the isolation of satellite glia and single-cell RNA sequencing analyses now make it possible to elucidate the molecular diversity of these fascinating glial cells in peripheral ganglia11,186. Advances in techniques for the genetic labelling of satellite glia and live imaging in sympathetic ganglia will be critical to visualize their dynamic behaviour from birth to wrapping around neurons during development.
Finally, an area of growing attention is harnessing the fundamental knowledge of molecular and cellular mechanisms governing sympathetic nervous system development to translational applications. The manipulation of sympathetic innervation or activity during early life presents exciting new avenues in the prevention and treatment of autonomic neuropathies and cardiovascular and metabolic disorders. Given the emerging role of sympathetic nerves in immune functions and cancer progression161, there is intense interest in gaining a mechanistic understanding of nerve–target interactions for therapeutic interventions. Recent advances in deriving sympathetic neurons from human pluripotent stem cells, co-cultures with target tissues and modelling of disease phenotypes in vitro187,188 hold significant promise for studying neuron–target interactions in autonomic diseases to facilitate drug discovery and personalized medicine.
Acknowledgements
We thank Haiqing Zhao, Chris Deppmann, Raluca Pascalau and all members of the Kuruvilla laboratory for helpful comments. We apologize to authors whose work could not be cited due to space limitations. The authors’ work is supported by NIH R01 awards (NS114478 and NS107342) to R. Kuruvilla.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Goldstein DS Differential responses of components of the autonomic nervous system. Handb. Clin. Neurol 117, 13–22 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Glebova NO & Ginty DD Growth and survival signals controlling sympathetic nervous system development. Annu. Rev. Neurosci 28, 191–222 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Hasan W Autonomic cardiac innervation: development and adult plasticity. Organogenesis 9, 176–193 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tourtellotte WG Axon transport and neuropathy: relevant perspectives on the etiopathogenesis of familial dysautonomia. Am. J. Pathol 186, 489–499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shwartz Y et al. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182, 578–593.e19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinosa-Medina I et al. The sacral autonomic outflow is sympathetic. Science 354, 893–897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borden P, Houtz J, Leach SD & Kuruvilla R Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep. 4, 287–301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides the first evidence of a developmental role for sympathetic nerves in instructing islet morphology.
- 8.Liu H et al. Control of cytokinesis by beta-adrenergic receptors indicates an approach for regulating cardiomyocyte endowment. Sci. Transl. Med 11, eaaw6419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreipke RE & Birren SJ Innervating sympathetic neurons regulate heart size and the timing of cardiomyocyte cell cycle withdrawal. J. Physiol 593, 5057–5073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furlan A et al. Visceral motor neuron diversity delineates a cellular basis for nipple-and pilo-erection muscle control. Nat. Neurosci 19, 1331–1340 (2016). [DOI] [PubMed] [Google Scholar]; Using single-cell sequencing, lineage and retrograde tracing, and mouse models, this elegant study identifies seven neuronal subtypes in thoracic sympathetic ganglia and reveals target-dependent mechanisms underlying neuronal diversity.
- 11.Hanani M & Spray DC Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci 21, 485–498 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This comprehensive review discusses satellite glia morphology, association with neurons and newly identified functions in neuronal activity and regeneration.
- 12.Bradke F & Dotti CG Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr. Opin. Neurobiol 10, 574–581 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Rubin E Development of the rat superior cervical ganglion: ganglion cell maturation. J. Neurosci 5, 673–684 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XM et al. Autocrine hepatocyte growth factor provides a local mechanism for promoting axonal growth. J. Neurosci 18, 8369–8381 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maina F & Klein R Hepatocyte growth factor, a versatile signal for developing neurons. Nat. Neurosci 2, 213–217 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Maina F et al. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron 20, 835–846 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Enomoto H et al. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development 128, 3963–3974 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Honma Y et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron 35, 267–282 (2002). [DOI] [PubMed] [Google Scholar]
- 19.elshamy WM & Ernfors P Requirement of neurotrophin-3 for the survival of proliferating trigeminal ganglion progenitor cells. Development 122, 2405–2414 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Kuruvilla R et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118, 243–255 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Brunet I et al. Netrin-1 controls sympathetic arterial innervation. J. Clin. Invest 124, 3230–3240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nam J et al. Coronary veins determine the pattern of sympathetic innervation in the developing heart. Development 140, 1475–1485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manousiouthakis E, Mendez M, Garner MC, Exertier P & Makita T Venous endothelin guides sympathetic innervation of the developing mouse heart. Nat. Commun 5, 3918 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L et al. A conserved axon type hierarchy governing peripheral nerve assembly. Development 141, 1875–1883 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Makita T, Sucov HM, Gariepy CE, Yanagisawa M & Ginty DD Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature 452, 759–763 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that sympathetic axons use prespecified routes to reach targets, suggesting the existence of molecularly distinct neuronal subtypes prior to target innervation.
- 26.Shelton DL & Reichardt LF Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc. Natl Acad. Sci. USA 81, 7951–7955 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowley C et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76, 1001–1011 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Glebova NO & Ginty DD Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J. Neurosci 24, 743–751 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards RH, Rutter WJ & Hanahan D Directed expression of NGF to pancreatic beta cells in transgenic mice leads to selective hyperinnervation of the islets. Cell 58, 161–170 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Hassankhani A et al. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Dev. Biol 169, 309–321 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Harrington AW et al. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell 146, 421–434 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atwal JK, Massie B, Miller FD & Kaplan DR The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron 27, 265–277 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Spillane M et al. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J. Neurosci 32, 17671–17689 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bodmer D, Ascano M & Kuruvilla R Isoform-specific dephosphorylation of dynamin1 by calcineurin couples neurotrophin receptor endocytosis to axonal growth. Neuron 70, 1085–1099 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spillane M, Ketschek A, Merianda TT, Twiss JL & Gallo G Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 5, 1564–1575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreassi C et al. An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nat. Neurosci 13, 291–301 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Crerar H et al. Regulation of NGF signaling by an axonal untranslated mRNA. Neuron 102, 553–563. e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides the first evidence for a non-coding axonal mRNA in promoting sympathetic axon growth and target innervation.
- 38.Scott-Solomon E & Kuruvilla R Prenylation of axonally translated rac1 controls NGF-dependent axon growth. Dev. Cell 53, 691–705.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lonze BE, Riccio A, Cohen S & Ginty DD Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron 34, 371–385 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Eldredge LC et al. Abnormal sympathetic nervous system development and physiological dysautonomia in Egr3-deficient mice. Development 135, 2949–2957 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deppmann CD et al. A model for neuronal competition during development. Science 320, 369–373 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using gene expression profiling and computational modelling, this article shows that developmental competition for survival between sympathetic neurons is mediated by a series of target-initiated feedback loops.
- 42.Bodmer D, Levine-Wilkinson S, Richmond A, Hirsh S & Kuruvilla R Wnt5a mediates nerve growth factor-dependent axonal branching and growth in developing sympathetic neurons. J. Neurosci 29, 7569–7581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McWilliams TG, Howard L, Wyatt S & Davies AM Regulation of autocrine signaling in subsets of sympathetic neurons has regional effects on tissue innervation. Cell Rep. 10, 1443–1449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu YK, Collins SE, Ho HY, Zhao H & Kuruvilla R An autocrine Wnt5a-Ror signaling loop mediates sympathetic target innervation. Dev. Biol 377, 79–89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho HY et al. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc. Natl Acad. Sci. USA 109, 4044–4051 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Keeffe GW et al. Region-specific role of growth differentiation factor-5 in the establishment of sympathetic innervation. Neural Dev. 11, 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisiswa L et al. TNFα reverse signaling promotes sympathetic axon growth and target innervation. Nat. Neurosci 16, 865–873 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng X et al. Innervation of thermogenic adipose tissue via a calsyntenin 3beta-S100b axis. Nature 569, 229–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toma JS et al. Peripheral nerve single-cell analysis identifies mesenchymal ligands that promote axonal growth. eNeuro 10.1523/ENEURO.0066-20.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ieda M et al. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat. Med 13, 604–612 (2007). [DOI] [PubMed] [Google Scholar]; This important study shows that aberrant sympathetic innervation during heart development results in cardiac failure in mice.
- 51.Tang XQ, Tanelian DL & Smith GM Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J. Neurosci 24, 819–827 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atwal JK, Singh KK, Tessier-Lavigne M, Miller FD & Kaplan DR Semaphorin 3F antagonizes neurotrophin-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase kinase signaling: a mechanism for growth cone collapse. J. Neurosci 23, 7602–7609 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gan WB & Lichtman JW Synaptic segregation at the developing neuromuscular junction. Science 282, 1508–1511 (1998). [DOI] [PubMed] [Google Scholar]
- 54.Singh KK et al. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat. Neurosci 11, 649–658 (2008). [DOI] [PubMed] [Google Scholar]; This study identifies a role for p75 in activity-dependent pruning of axon collaterals during development.
- 55.Yong Y et al. p75NTR and DR6 regulate distinct phases of axon degeneration demarcated by spheroid rupture. J. Neurosci 39, 9503–9520 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campenot RB Independent control of local environment of somas and neurites. Meth. Enzymol 58, 302–307 (1979). [DOI] [PubMed] [Google Scholar]
- 57.Nikolaev A, McLaughlin T, O’Leary DD & Tessier-Lavigne M APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457, 981–989 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Gamage KK et al. Death receptor 6 promotes wallerian degeneration in peripheral axons. Curr. Biol 27, 890–896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J & Deshmukh M Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat. Commun 4, 1876 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smolen AJ Morphology of synapses in the autonomic nervous system. J. Electron. Microsc. Tech 10, 187–204 (1988). [DOI] [PubMed] [Google Scholar]
- 61.Hill CE, Phillips JK & Sandow SL Development of peripheral autonomic synapses: neurotransmitter receptors, neuroeffector associations and neural influences. Clin. Exp. Pharmacol. Physiol 26, 581–590 (1999). [DOI] [PubMed] [Google Scholar]
- 62.Waites CL, Craig AM & Garner CC Mechanisms of vertebrate synaptogenesis. Annu. Rev. Neurosci 28, 251–274 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Hirst GD, Choate JK, Cousins HM, Edwards FR & Klemm MF Transmission by post-ganglionic axons of the autonomic nervous system: the importance of the specialized neuroeffector junction. Neuroscience 73, 7–23 (1996). [DOI] [PubMed] [Google Scholar]
- 64.Luther JA & Birren SJ Neurotrophins and target interactions in the development and regulation of sympathetic neuron electrical and synaptic properties. Auton. Neurosci 151, 46–60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lockhart ST, Mead JN, Pisano JM, Slonimsky JD & Birren SJ Nerve growth factor collaborates with myocyte-derived factors to promote development of presynaptic sites in cultured sympathetic neurons. J. Neurobiol 42, 460–476 (2000). [PubMed] [Google Scholar]
- 66.Yang B, Slonimsky JD & Birren SJ A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat. Neurosci 5, 539–545 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Oppenheim RW Cell death during development of the nervous system. Annu. Rev. Neurosci 14, 453–501 (1991). [DOI] [PubMed] [Google Scholar]
- 68.Oppenheim RW The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 12, 252–255 (1989). [DOI] [PubMed] [Google Scholar]
- 69.Cowan WM Viktor Hamburger and Rita Levi-Montalcini: the path to the discovery of nerve growth factor. Annu. Rev. Neurosci 24, 551–600 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Snider WD Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77, 627–638 (1994). [DOI] [PubMed] [Google Scholar]
- 71.Smeyne RJ et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368, 246–249 (1994). [DOI] [PubMed] [Google Scholar]
- 72.Harrington AW & Ginty DD Long-distance retrograde neurotrophic factor signalling in neurons. Nat. Rev. Neurosci 14, 177–187 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Scott-Solomon E & Kuruvilla R Mechanisms of neurotrophin trafficking via Trk receptors. Mol. Cell. Neurosci 91, 25–33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delcroix JD et al. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 39, 69–84 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Deinhardt K et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 52, 293–305 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Ye M, Lehigh KM & Ginty DD Multivesicular bodies mediate long-range retrograde NGF-TrkA signaling. eLife 7, e33012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riccio A, Pierchala B, Ciarallo C & Ginty DD An NGF-TrkA-Mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science 227, 1097–1100 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Suo D et al. Coronin-1 is a neurotrophin endosomal effector that is required for developmental competition for survival. Nat. Neurosci 17, 36–45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ascano M, Bodmer D & Kuruvilla R Endocytic trafficking of neurotrophins in neural development. Trends Cell Biol. 22, 266–273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ascano M, Richmond A, Borden P & Kuruvilla R Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J. Neurosci 29, 11674–11685 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study defines a positive feedback mechanism where target-derived NGF recruits TrkA receptors to axons via receptor endocytosis in cell bodies and long-distance recycling to axons.
- 81.Yamashita N, Joshi R, Zhang S, Zhang ZY & Kuruvilla R Phospho-regulation of soma-to-axon transcytosis of neurotrophin receptors. Dev. Cell 42, 626–639.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pathak A et al. Retrograde degenerative signaling mediated by the p75 neurotrophin receptor requires p150(Glued) deacetylation by axonal HDAC1. Dev. Cell 46, 376–387.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies an apoptotic signalling pathway that relies on proteolytic cleavage of p75 in axons and retrograde transport of its intracellular domain.
- 83.Escudero CA et al. c-Jun N-terminal kinase (JNK)-dependent internalization and Rab5-dependent endocytic sorting mediate long-distance retrograde neuronal death induced by axonal BDNF-p75 signaling. Sci. Rep 9, 6070 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mok SA, Lund K & Campenot RB A retrograde apoptotic signal originating in NGF-deprived distal axons of rat sympathetic neurons in compartmented cultures. Cell Res. 19, 546–560 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Ghosh AS et al. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J. Cell Biol 194, 751–764 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee R, Kermani P, Teng KK & Hempstead BL Regulation of cell survival by secreted proneurotrophins. Science 294, 1945–1948 (2001). [DOI] [PubMed] [Google Scholar]
- 87.Nykjaer A et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 427, 843–848 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Voyvodic JT Development and regulation of dendrites in the rat superior cervical ganglion. J. Neurosci 7, 904–912 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voyvodic JT Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J. Neurosci 9, 1997–2010 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andrews TJ, Thrasivoulou C, Nesbit W & Cowen T Target-specific differences in the dendritic morphology and neuropeptide content of neurons in the rat SCG during development and aging. J. Comp. Neurol 368, 33–44 (1996). [DOI] [PubMed] [Google Scholar]
- 91.Snider WD Nerve growth factor enhances dendritic arborization of sympathetic ganglion cells in developing mammals. J. Neurosci 8, 2628–2634 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruit KG & Snider WD Administration or deprivation of nerve growth factor during development permanently alters neuronal geometry. J. Comp. Neurol 314, 106–113 (1991). [DOI] [PubMed] [Google Scholar]
- 93.Quach DH, Oliveira-Fernandes M, Gruner KA & Tourtellotte WG A sympathetic neuron autonomous role for Egr3-mediated gene regulation in dendrite morphogenesis and target tissue innervation. J. Neurosci 33, 4570–4583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma N et al. Long-distance control of synapse assembly by target-derived NGF. Neuron 67, 422–434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lehigh KM, West KM & Ginty DD Retrogradely transported TrkA endosomes signal locally within dendrites to maintain sympathetic neuron synapses. Cell Rep. 19, 86–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bruckenstein DA & Higgins D Morphological differentiation of embryonic rat sympathetic neurons in tissue culture. I. Conditions under which neurons form axons but not dendrites. Dev. Biol 128, 324–336 (1988). [DOI] [PubMed] [Google Scholar]
- 97.Vaillant AR et al. Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron 34, 985–998 (2002). [DOI] [PubMed] [Google Scholar]
- 98.Rubin E Development of the rat superior cervical ganglion: ingrowth of preganglionic axons. J. Neurosci 5, 685–696 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naska S et al. An essential role for the integrin-linked kinase-glycogen synthase kinase-3 beta pathway during dendrite initiation and growth. J. Neurosci 26, 13344–13356 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chong Y et al. Removing 4E-BP enables synapses to refine without postsynaptic activity. Cell Rep. 23, 11–22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lein P, Johnson M, Guo X, Rueger D & Higgins D Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron 15, 597–605 (1995). [DOI] [PubMed] [Google Scholar]
- 102.Majdazari A et al. Dendrite complexity of sympathetic neurons is controlled during postnatal development by BMP signaling. J. Neurosci 33, 15132–15144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beck HN, Drahushuk K, Jacoby DB, Higgins D & Lein PJ Bone morphogenetic protein-5 (BMP-5) promotes dendritic growth in cultured sympathetic neurons. BMC Neurosci. 2, 12 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lein PJ et al. Glia induce dendritic growth in cultured sympathetic neurons by modulating the balance between bone morphogenetic proteins (BMPs) and BMP antagonists. J. Neurosci 22, 10377–10387 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tropea M, Johnson MI & Higgins D Glial cells promote dendritic development in rat sympathetic neurons in vitro. Glia 1, 380–392 (1988). [DOI] [PubMed] [Google Scholar]
- 106.Pravoverov K et al. MicroRNAs are necessary for BMP-7-induced dendritic growth in cultured rat sympathetic neurons. Cell Mol. Neurobiol 39, 917–934 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Courter LA et al. BMP7-induced dendritic growth in sympathetic neurons requires p75(NTR) signaling. Dev. Neurobiol 76, 1003–1013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rubin E Development of the rat superior cervical ganglion: initial stages of synapse formation. J. Neurosci 5, 697–704 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Devay P, McGehee DS, Yu CR & Role LW Target-specific control of nicotinic receptor expression at developing interneuronal synapses in chick. Nat. Neurosci 2, 528–534 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosenberg MM, Blitzblau RC, Olsen DP & Jacob MH Regulatory mechanisms that govern nicotinic synapse formation in neurons. J. Neurobiol 53, 542–555 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Purves D & Nja A Effect of nerve growth factor on synaptic depression after axotomy. Nature 260, 535–536 (1976). [DOI] [PubMed] [Google Scholar]
- 112.Nja A & Purves D The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J. Physiol 277, 53–75 (1978). [PMC free article] [PubMed] [Google Scholar]
- 113.Causing CG et al. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron 18, 257–267 (1997). [DOI] [PubMed] [Google Scholar]
- 114.Gingras J, Rassadi S, Cooper E & Ferns M Agrin plays an organizing role in the formation of sympathetic synapses. J. Cell Biol 158, 1109–1118 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Conroy WG, Liu Z, Nai Q, Coggan JS & Berg DK PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons. Neuron 38, 759–771 (2003). [DOI] [PubMed] [Google Scholar]
- 116.Purves D & Lichtman JW Elimination of synapses in the developing nervous system. Science 210, 153–157 (1980). [DOI] [PubMed] [Google Scholar]
- 117.Lichtman JW & Purves D The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J. Physiol 301, 213–228 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanes JR & Lichtman JW Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci 2, 791–805 (2001). [DOI] [PubMed] [Google Scholar]
- 119.Krishnaswamy A & Cooper E An activity-dependent retrograde signal induces the expression of the high-affinity choline transporter in cholinergic neurons. Neuron 61, 272–286 (2009). [DOI] [PubMed] [Google Scholar]; This study provides rare insight into synapse maintenance in sympathetic ganglia by showing that synapses remain structurally intact without synaptic activity, although high-frequency transmission is impaired in α3 nAChR-knockout mice.
- 120.Jobling P & Gibbins IL Electrophysiological and morphological diversity of mouse sympathetic neurons. J. Neurophysiol 82, 2747–2764 (1999). [DOI] [PubMed] [Google Scholar]
- 121.Ernsberger U, Deller T & Rohrer H The diversity of neuronal phenotypes in rodent and human autonomic ganglia. Cell Tissue Res. 382, 201–231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cane KN & Anderson CR Generating diversity: mechanisms regulating the differentiation of autonomic neuron phenotypes. Auton. Neurosci 151, 17–29 (2009). [DOI] [PubMed] [Google Scholar]
- 123.Zeisel A et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Furlan A, Lubke M, Adameyko I, Lallemend F & Ernfors P The transcription factor Hmx1 and growth factor receptor activities control sympathetic neurons diversification. EMBO J. 32, 1613–1625 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Q et al. Temporal requirements for ISL1 in sympathetic neuron proliferation, differentiation, and diversification. Cell Death Dis. 9, 247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guidry G & Landis SC Target-dependent development of the vesicular acetylcholine transporter in rodent sweat gland innervation. Dev. Biol 199, 175–184 (1998). [DOI] [PubMed] [Google Scholar]
- 127.Ernsberger U & Rohrer H Development of the cholinergic neurotransmitter phenotype in postganglionic sympathetic neurons. Cell Tissue Res. 297, 339–361 (1999). [DOI] [PubMed] [Google Scholar]
- 128.Asmus SE, Parsons S & Landis SC Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J. Neurosci 20, 1495–1504 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Francis NJ & Landis SC Cellular and molecular determinants of sympathetic neuron development. Annu. Rev. Neurosci 22, 541–566 (1999). [DOI] [PubMed] [Google Scholar]
- 130.Schotzinger RJ & Landis SC Acquisition of cholinergic and peptidergic properties by sympathetic innervation of rat sweat glands requires interaction with normal target. Neuron 5, 91–100 (1990). [DOI] [PubMed] [Google Scholar]
- 131.Habecker BA & Landis SC Noradrenergic regulation of cholinergic differentiation. Science 264, 1602–1604 (1994). [DOI] [PubMed] [Google Scholar]
- 132.Saadat S, Sendtner M & Rohrer H Ciliary neurotrophic factor induces cholinergic differentiation of rat sympathetic neurons in culture. J. Cell Biol 108, 1807–1816 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamamori T et al. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science 246, 1412–1416 (1989). [DOI] [PubMed] [Google Scholar]
- 134.Bamber BA, Masters BA, Hoyle GW, Brinster RL & Palmiter RD Leukemia inhibitory factor induces neurotransmitter switching in transgenic mice. Proc. Natl Acad. Sci. USA 91, 7839–7843 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rao MS et al. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron 11, 1175–1185 (1993). [DOI] [PubMed] [Google Scholar]
- 136.Masu Y et al. Disruption of the CNTF gene results in motor neuron degeneration. Nature 365, 27–32 (1993). [DOI] [PubMed] [Google Scholar]
- 137.Francis NJ, Asmus SE & Landis SC CNTF and LIF are not required for the target-directed acquisition of cholinergic and peptidergic properties by sympathetic neurons in vivo. Dev. Biol 182, 76–87 (1997). [DOI] [PubMed] [Google Scholar]
- 138.Ootsuka Y & Blessing WW Inhibition of medullary raphe/parapyramidal neurons prevents cutaneous vasoconstriction elicited by alerting stimuli and by cold exposure in conscious rabbits. Brain Res. 1051, 189–193 (2005). [DOI] [PubMed] [Google Scholar]
- 139.Benedek M & Kaernbach C Physiological correlates and emotional specificity of human piloerection. Biol. Psychol 86, 320–329 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dart AM, Du XJ & Kingwell BA Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc. Res 53, 678–687 (2002). [DOI] [PubMed] [Google Scholar]
- 141.Hinojosa-Laborde C, Chapa I, Lange D & Haywood JR Gender differences in sympathetic nervous system regulation. Clin. Exp. Pharmacol. Physiol 26, 122–126 (1999). [DOI] [PubMed] [Google Scholar]
- 142.Jessen KR & Mirsky R The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci 6, 671–682 (2005). [DOI] [PubMed] [Google Scholar]
- 143.Monk KR, Feltri ML & Taveggia C New insights on Schwann cell development. Glia 63, 1376–1393 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pannese E The satellite cells of the sensory ganglia. Adv. Anat. Embryol. Cell Biol 65, 1–111 (1981). [DOI] [PubMed] [Google Scholar]
- 145.Hanani M Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res. Rev 64, 304–327 (2010). [DOI] [PubMed] [Google Scholar]
- 146.Paratore C, Goerich DE, Suter U, Wegner M & Sommer L Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development 128, 3949–3961 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Britsch S et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66–78 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hall AK & Landis SC Early commitment of precursor cells from the rat superior cervical ganglion to neuronal or nonneuronal fates. Neuron 6, 741–752 (1991). [DOI] [PubMed] [Google Scholar]
- 149.Kurtz A et al. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 120, 2637–2649 (1994). [DOI] [PubMed] [Google Scholar]
- 150.Shi H et al. Nestin expression defines both glial and neuronal progenitors in postnatal sympathetic ganglia. J. Comp. Neurol 508, 867–878 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hall AK & Landis SC Division and migration of satellite glia in the embryonic rat superior cervical ganglion. J. Neurocytol 21, 635–647 (1992). [DOI] [PubMed] [Google Scholar]
- 152.Fagan AM et al. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J. Neurosci 16, 6208–6218 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Freeman MR Drosophila central nervous system glia. Cold Spring Harb. Perspect. Biol 7, a020552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oikonomou G & Shaham S The glia of Caenorhabditis elegans. Glia 59, 1253–1263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Enes J et al. Satellite glial cells modulate cholinergic transmission between sympathetic neurons. PLoS One 15, e0218643 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McFarlane S & Cooper E Extrinsic factors influence the expression of voltage-gated K currents on neonatal rat sympathetic neurons. J. Neurosci 13, 2591–2600 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu HH et al. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat. Neurosci 12, 1534–1541 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Low PA Autonomic neuropathies. Curr. Opin. Neurol 15, 605–609 (2002). [DOI] [PubMed] [Google Scholar]
- 159.Goldstein DS, Robertson D, Esler M, Straus SE & Eisenhofer G Dysautonomias: clinical disorders of the autonomic nervous system. Ann. Intern. Med 137, 753–763 (2002). [DOI] [PubMed] [Google Scholar]
- 160.Saravia F & Homo-Delarche F Is innervation an early target in autoimmune diabetes? Trends Immunol. 24, 574–579 (2003). [DOI] [PubMed] [Google Scholar]
- 161.Hanoun M, Maryanovich M, Arnal-Estape A & Frenette PS Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 86, 360–373 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Axelrod FB, Nachtigal R & Dancis J Familial dysautonomia: diagnosis, pathogenesis and management. Adv. Pediatr 21, 75–96 (1974). [PubMed] [Google Scholar]
- 163.Li L, Gruner K & Tourtellotte WG Retrograde nerve growth factor signaling abnormalities in familial dysautonomia. J. Clin. Invest 130, 2478–2487 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a combination of mouse models and compartmentalized neuron cultures, this elegant study reveals aberrant TrkA phosphorylation during retrograde transport as the basis for neuronal loss in an autonomic neuropathy.
- 164.Patel A et al. RCAN1 links impaired neurotrophin trafficking to aberrant development of the sympathetic nervous system in Down syndrome. Nat. Commun 6, 10119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ieda M Heart development, diseases, and regeneration-new approaches from innervation, fibroblasts, and reprogramming. Circ. J 80, 2081–2088 (2016). [DOI] [PubMed] [Google Scholar]
- 166.Lefcort F, Mergy M, Ohlen SB, Ueki Y & George L Animal and cellular models of familial dysautonomia. Clin. Auton. Res 27, 235–243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jackson MZ, Gruner KA, Qin C & Tourtellotte WG A neuron autonomous role for the familial dysautonomia gene ELP1 in sympathetic and sensory target tissue innervation. Development 141, 2452–2461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O’Driscoll DM et al. Cardiac and sympathetic activation are reduced in children with Down syndrome and sleep disordered breathing. Sleep 35, 1269–1275 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yiallourou SR, Witcombe NB, Sands SA, Walker AM & Horne RS The development of autonomic cardiovascular control is altered by preterm birth. Early Hum. Dev 89, 145–152 (2013). [DOI] [PubMed] [Google Scholar]
- 170.Crump C, Winkleby MA, Sundquist K & Sundquist J Risk of hypertension among young adults who were born preterm: a Swedish national study of 636,000 births. Am. J. Epidemiol 173, 797–803 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tucker DC & Johnson AK Development of autonomic control of heart rate in genetically hypertensive and normotensive rats. Am. J. Physiol 246, R570–R577 (1984). [DOI] [PubMed] [Google Scholar]
- 172.Ieda M et al. Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression. J. Clin. Invest 113, 876–884 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cao JM et al. Nerve sprouting and sudden cardiac death. Circ. Res 86, 816–821 (2000). [DOI] [PubMed] [Google Scholar]
- 174.Xie AX, Lee JJ & McCarthy KD Ganglionic GFAP+ glial Gq-GPCR signaling enhances heart functions in vivo. JCI Insight 2, e90565 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin EE, Scott-Solomon E & Kuruvilla R Peripheral innervation in the regulation of glucose homeostasis. Trends Neurosci. 44, 189–202 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mei Q, Mundinger TO, Lernmark A & Taborsky GJ Jr Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 51, 2997–3002 (2002). [DOI] [PubMed] [Google Scholar]
- 177.Persson-Sjogren S, Holmberg D & Forsgren S Remodeling of the innervation of pancreatic islets accompanies insulitis preceding onset of diabetes in the NOD mouse. J. Neuroimmunol 158, 128–137 (2005). [DOI] [PubMed] [Google Scholar]
- 178.Campanucci V, Krishnaswamy A & Cooper E Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron 66, 827–834 (2010). [DOI] [PubMed] [Google Scholar]
- 179.Schreiber R, Levy J, Loewenthal N, Pinsk V & Hershkovitz E Decreased first phase insulin response in children with congenital insensitivity to pain with anhidrosis. J. Pediatr. Endocrinol. Metab 18, 873–877 (2005). [DOI] [PubMed] [Google Scholar]
- 180.Indo Y et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat. Genet 13, 485–488 (1996). [DOI] [PubMed] [Google Scholar]
- 181.Lomax AE, Sharkey KA & Furness JB The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol. Motil 22, 7–18 (2010). [DOI] [PubMed] [Google Scholar]
- 182.Gabanyi I et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Muller PA et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 583, 441–446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zahalka AH & Frenette PS Nerves in cancer. Nat. Rev. Cancer 20, 143–157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This timely review covers emerging evidence for contributions of sympathetic nerves to tumour growth and discusses nerve manipulation as a novel therapeutic opportunity in cancer.
- 185.Peruzzi D, Hendley ED & Forehand CJ Hypertrophy of stellate ganglion cells in hypertensive, but not hyperactive, rats. Am. J. Physiol 261, R979–R984 (1991). [DOI] [PubMed] [Google Scholar]
- 186.Avraham O et al. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun 11, 4891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Oh Y et al. Functional coupling with cardiac muscle promotes maturation of hPSC-derived sympathetic neurons. Cell Stem Cell 19, 95–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the derivation of functional sympathetic neurons from human pluripotent stem cells, facilitating the study of human sympathetic neuron development in normal and disease conditions.
- 188.Zeltner N et al. Capturing the biology of disease severity in a PSC-based model of familial dysautonomia. Nat. Med 22, 1421–1427 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Markus A, Patel TD & Snider WD Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol 12, 523–531 (2002). [DOI] [PubMed] [Google Scholar]
- 190.Gallo G & Letourneau PC Localized sources of neurotrophins initiate axon collateral sprouting. J. Neurosci 18, 5403–5414 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kawasaki T et al. Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development 129, 671–680 (2002). [DOI] [PubMed] [Google Scholar]
- 192.Maden CH et al. NRP1 and NRP2 cooperate to regulate gangliogenesis, axon guidance and target innervation in the sympathetic nervous system. Dev. Biol 369, 277–285 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schafer T, Schwab ME & Thoenen H Increased formation of preganglionic synapses and axons due to a retrograde trans-synaptic action of nerve growth factor in the rat sympathetic nervous system. J. Neurosci 3, 1501–1510 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tasdemir-Yilmaz OE et al. Diversity of developing peripheral glia revealed by single-cell RNA sequencing. Dev Cell. 56, P2516–2535.E8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kuruvilla R, Ye H & Ginty DD Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron 27, 499–512 (2000). [DOI] [PubMed] [Google Scholar]
- 196.Riccio A, Ahn S, Davenport CM, Blendy J & Ginty DD Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286, 2358–2361 (1999). [DOI] [PubMed] [Google Scholar]
- 197.Chan WH, Anderson CR & Gonsalvez DG From proliferation to target innervation: signaling molecules that direct sympathetic nervous system development. Cell Tissue Res. 372, 171–193 (2018). [DOI] [PubMed] [Google Scholar]
- 198.Ma Q, Kintner C & Anderson DJ Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87, 43–52 (1996). [DOI] [PubMed] [Google Scholar]
- 199.Perez SE, Rebelo S & Anderson DJ Early specification of sensory neuron fate revealed by expression and function of neurogenins in the chick embryo. Development 126, 1715–1728 (1999). [DOI] [PubMed] [Google Scholar]
- 200.Gonsalvez DG et al. Proliferation and cell cycle dynamics in the developing stellate ganglion. J. Neurosci 33, 5969–5979 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Goridis C & Rohrer H Specification of catecholaminergic and serotonergic neurons. Nat. Rev. Neurosci 3, 531–541 (2002). [DOI] [PubMed] [Google Scholar]
- 202.Rothman TP, Gershon MD & Holtzer H The relationship of cell division to the acquisition of adrenergic characteristics by developing sympathetic ganglion cell precursors. Dev. Biol 65, 322–341 (1978). [DOI] [PubMed] [Google Scholar]
- 203.DiCicco-Bloom E, Townes-Anderson E & Black IB Neuroblast mitosis in dissociated culture: regulation and relationship to differentiation. J. Cell Biol 110, 2073–2086 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Armstrong A, Ryu YK, Chieco D & Kuruvilla R Frizzled3 is required for neurogenesis and target innervation during sympathetic nervous system development. J. Neurosci 31, 2371–2381 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Andres R et al. Multiple effects of artemin on sympathetic neurone generation, survival and growth. Development 128, 3685–3695 (2001). [DOI] [PubMed] [Google Scholar]
- 206.Zackenfels K, Oppenheim RW & Rohrer H Evidence for an important role of IGF-I and IGF-II for the early development of chick sympathetic neurons. Neuron 14, 731–741 (1995). [DOI] [PubMed] [Google Scholar]
- 207.Hildreth V, Anderson RH & Henderson DJ Autonomic innervation of the developing heart: origins and function. Clin. Anat 22, 36–46 (2009). [DOI] [PubMed] [Google Scholar]
- 208.Kugler JD et al. Effect of chemical sympathectomy on myocardial cell division in the newborn rat. Pediatr. Res 14, 881–884 (1980). [DOI] [PubMed] [Google Scholar]
- 209.Ogawa S et al. Direct contact between sympathetic neurons and rat cardiac myocytes in vitro increases expression of functional calcium channels. J. Clin. Invest 89, 1085–1093 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Triposkiadis F et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol 54, 1747–1762 (2009). [DOI] [PubMed] [Google Scholar]
- 211.Woods SC & Porte D Jr Neural control of the endocrine pancreas. Physiol. Rev 54, 596–619 (1974). [DOI] [PubMed] [Google Scholar]


