Abstract
Introduction
Traditionally, estrogen receptor (ER)-positive breast cancer has been defined as tumors with ≥1% positive for ER. The updated American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines recommend that tumors with ER expression of 1%-10% should be classified as ER-low-positive, recognizing the limited clinical evidence on the prognostic and predictive role of low ER expression. We aimed to investigate the predictive role of ER-low expression to neoadjuvant chemotherapy (NeoCT) and the prognostic significance of ER-low expressing breast tumors compared with ER-positive or ER-negative breast tumors.
Methods
A meta-analysis was conducted using the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) guidelines and eligible articles were identified on PubMed and ISI Web of Science databases. The primary outcome was pathologic complete response and secondary outcomes were disease-free survival (DFS) and overall survival (OS). Twelve retrospective cohort studies were included in the meta-analysis. NeoCT resulted in higher pathologic complete response among patients with ER-low expression compared with ER-positive and comparable to ER-negative. Patients with ER-low breast cancer had a statistically significant worse DFS and OS compared with patients with ER-positive breast cancer, whereas no difference in DFS or OS was observed between ER-low and ER-negative subgroups.
Discussion
The current evidence suggests that ER-low breast cancer has a more similar outcome to ER-negative than to ER-positive breast cancer in terms of DFS and OS. ER-low expression seems also to have a predictive role regarding NeoCT. Considering the certainty of current evidence categorized as low to moderate, our results urge the need for well-designed prospective studies investigating the molecular background and the most appropriate treatment strategy for ER-low expressing breast cancer.
Key words: ER-low, neoadjuvant chemotherapy, adjuvant, prognosis, breast cancer, meta-analysis
Highlights
-
•
The predictive and prognostic roles of estrogen receptor (ER)-low breast cancer have been studied in this meta-analysis.
-
•
ER-low expression was associated with higher pathologic complete response after NeoCT compared with ER-positive.
-
•
Both disease-free and overall survival were comparable between ER-low breast cancer and ER-negative breast cancer.
Introduction
Breast cancer is the most common type of cancer in females with an incidence of 142.8 per 100 000 in the European Union and 148.8 per 100 000 in Sweden in 2020.1 Estrogen receptor (ER)-positive breast cancer is the most common breast cancer subtype, with nearly 70% of the cases considered ER-positive.2
Traditionally, ER-positive breast cancer has been defined as tumors with >1% of tumor nuclei positive for ER.3 The updated American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines recommend that tumors with ER expression of 1%-10% should be classified as ER-low-positive, recognizing the limited clinical evidence on the prognostic and predictive role of low ER expression and highlighting the need for more robust evidence.4 A similar approach has been adopted by the ABC5 international consensus guidelines for advanced breast cancer.5
Considering the different treatment strategies depending on ER status, where neoadjuvant chemotherapy (NeoCT) is the recommended treatment approach for triple negative breast cancer (TNBC)6 and adjuvant endocrine therapy is recommended in all luminal-like cancers,7 it is essential to investigate the predictive role of ER-low expression to NeoCT and the prognostic significance of ER-low expressing breast tumors compared with ER-positive (>10%) or ER-negative (<1%) breast tumors.
In the present systematic review and meta-analysis, we aimed to summarize the current evidence on ER-low-positive breast cancer in two clinical scenarios: (i) when NeoCT is given (compared with ER-negative or ER-positive breast cancer); (ii) in patients treated with adjuvant therapy including chemotherapy, endocrine therapy, or a combination (compared with ER-negative or ER-positive breast cancer).
Materials and methods
Study design
A systematic search in accordance with the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) guidelines was conducted.
Eligibility and exclusion criteria were prespecified according to the patient, intervention, control, and outcome (PICO) format.
Patient characteristics: breast cancer patients with information about quantitative ER status who received chemotherapy or endocrine therapy as neoadjuvant or adjuvant treatment; intervention: NeoCT or endocrine therapy for breast cancer with ER status 1%-10%; control: NeoCT for breast cancer with ER status <1% or ER >10%. Endocrine treatment of breast cancer with ER status >10%. Outcome: pathologic complete response (pCR) for neoadjuvant studies based on the definition of each study, disease-free survival (DFS) defined as the time from diagnosis until disease recurrence or death due to any cause, and overall survival (OS) defined as the time from diagnosis until death due to any cause. For DFS and OS, only results derived from multivariate analyses were used to limit the risk for confounding bias.
Search strategy
The electronic literature search was carried out using PubMed and ISI Web of Science without any year restrictions with the following algorithms: (neoadjuvant OR primary OR preoperative OR induction) AND (low OR poor OR low positiv∗) AND (estrogen OR progesterone OR hormone) AND (prognosis OR survival OR efficacy OR response OR remission) AND breast cancer or (adjuvant OR postoperative) AND (low OR poor OR low positiv∗) AND (estrogen OR progesterone OR hormone) AND (prognosis OR survival OR efficacy OR response OR remission) AND breast cancer. The last search date was on 8 August 2021.
The resulting abstracts and full texts were screened independently by two investigators (NP, AV). Consensus by discussion was achieved regarding eligible trials. Studies without a comparison group (ER >10% or ER <1%), studies without separate results on low ER expression group, studies that reported outcomes other than pCR, DFS, or OS, and studies without multivariate analyses for DFS or OS were excluded from the meta-analysis.
Quality assessment
The Newcastle-Ottawa Quality Assessment Scale (NOS) for cohort studies was used to judge the quality of the studies included in the systematic review and meta-analysis. Two investigators (NP, AV) assessed the quality of each trial independently and a consensus through discussion was reached regarding all eligible trials.
Data collection
Data were extracted independently by two investigators (NP, AV). Consensus by discussion was achieved in all extracted data. From each eligible trial, the following data were extracted: first author, journal, year of publication, country of origin, multicenter study, inclusion period, total number of patients, type of therapy, ER status, number of patients for each ER status, relevant outcomes as pCR (based on the definition of each study), hazard ratio (HR) for DFS, 95% low HR for DFS, 95% high HR for DFS, covariates in multivariate analysis for DFS, HR for OS, 95% low HR for OS, 95% high HR for OS, and covariates in multivariate analysis for OS. The results were divided into two subgroups according to neoadjuvant and adjuvant treatment.
Data synthesis
To carry out the meta-analysis for the neoadjuvant subgroup with pCR, a random-effects model was used to produce a pooled pCR and corresponding 95% confidence interval (CI) for each group (ER-low, ER-positive, ER-negative). An overall effect estimate among three comparisons was calculated using odds ratio (OR) with 95% CI through the DerSimonian and Laird method.
For the comparisons of DFS and OS for both neoadjuvant and adjuvant subgroups, a meta-analysis was carried out first by transforming the HRs and their errors into their log counterparts, and then using the inverse variance method for transforming back into the HR scale. If DFS or OS data were unavailable for direct extraction from the primary studies, data were extracted according to the method described by Tierney et al.8
The presence of statistical heterogeneity among the studies was addressed by using the Q statistics, and the magnitude of heterogeneity by using the I2 statistic. A P value <0.10 or an I2 value >50% was considered as substantial heterogeneity. All meta-analyses were carried out using the fixed- or random-effects model depending on the results of the statistical heterogeneity.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was applied to rate the certainty of current evidence in three research questions: the predictive role of ER-low to NeoCT (compared with ER-positive and ER-negative), the prognostic role of ER-low in terms of DFS, and the prognostic role in terms of OS.
Results
Literature search
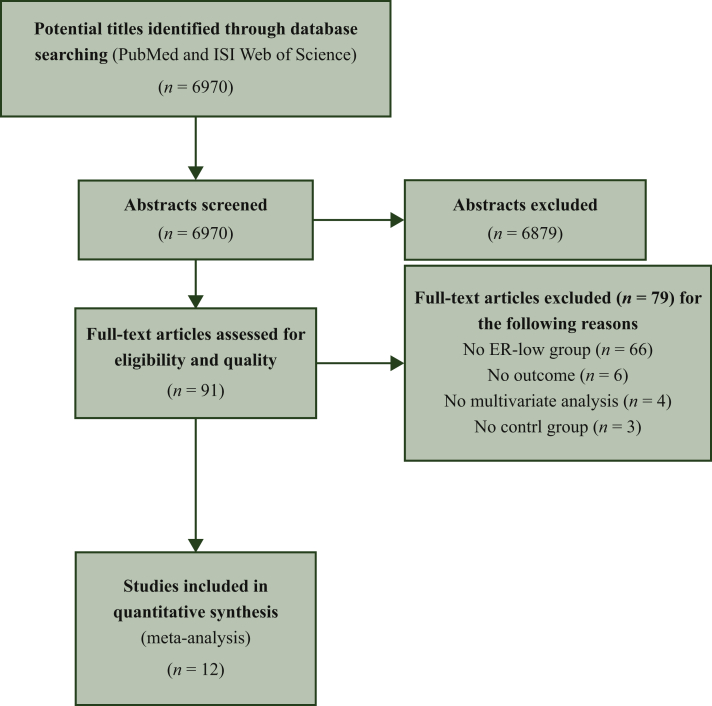
The search algorithm identified 6970 records. After reading the titles and abstracts, 91 studies were considered potentially eligible. The full texts of the potentially eligible articles were obtained and reviewed independently by two investigators (NP, AV) in further detail, and a consensus was reached on all studies. After excluding 79 studies due to various reasons (Figure 1), a total of 12 studies, 6 with data on NeoCT,9, 10, 11, 12, 13, 14 5 with adjuvant treatment,15, 16, 17, 18, 19 and 1 with data on both treatment settings,20 were considered eligible and included in the meta-analysis.
Figure 1.
Flowchart for study selection process.
Study characteristics
Table 1 presents the key characteristics of the eligible studies. The number of study participants ranged from 156 to 9639 and the majority of the studies were retrospective cohort studies. The median follow-up ranged between 29 and 89.3 months with three studies exceeding a median follow-up of >5 years.14,15,18
Table 1.
Characteristics of eligible studies
| Author, year | Country | Study design | Inclusion Period | Neoadjuvant CT | Type of neoadjuvant/adjuvant CT | Total number of patients | Number of patients according to ER status | % CT and HT as adjuvant |
|---|---|---|---|---|---|---|---|---|
| Balduzzi, 2012 | Italy | Retrospective analysis of prospectively collected data | 1995-2009 | No | Anthracycline only, anthracycline and CMF, taxane only, CMF only, others | 1424 | <1%: 1300 1%-10%: 124 |
HT 5; CT 89 HT 41; CT 59 |
| Colleoni, 2004 | Italy | Retrospective analysis of prospectively collected data | 1994-2002 | Yes | Anthracycline Anthracycline and taxane Other |
399 | <1%: 129 1%-9%: 94 ≥10%: 171 |
NR |
| Dieci, 2021 | Italy | Retrospective | 2000-2019 | Yes (41% of study cohort) | Anthracyclines and/or taxanes Other |
406 | <1%: 364 1%-9%: 42 |
HT 4; CT 100 HT 14; CT 100 |
| Ding, 2019 | China | Retrospective | 2007-2017 | Yes | Anthracycline, cyclophosphamide, and paclitaxel sequentially or concomitant | 570 | <1%: 209 1%-10%: 60 >10%: 301 |
NR |
| Fujii, 2017 | USA | Retrospective | 1982-2013 | Yes | Anthracyclines alone Taxanes alone Anthracycline and taxane |
3055 | <1%: 932 1%-9%: 171 ≥10%: 1952 |
HT 9; CT 17 HT 25; CT 9 HT 98; CT 15 |
| Landmann, 2018 | USA | Retrospective | 2010-2014 | Yes | Adriamycin-cyclophosphamide-taxane Other/unknown |
327 | <1%: 141 1%-10%: 41 >10%: 145 |
NR |
| Ohara, 2019 | Japan | Retrospective | 2004-2013 | Yes | Paclitaxel, followed by FEC | 156 | <1%: 32 1%-9%: 16 ≥10%: 108 |
NR |
| Prabhu, 2014 | India | Prospective | 2008-2013 | No | Anthracycline and taxane Anthracycline plus other Other |
235 | <1%: 74 1%-10%: 21 >10%: 140 |
HT 0; CT 84 HT 71; CT 76 HT 91; CT 59 |
| Raghav, 2012 | USA | Retrospective | 1990-2009 | No | Anthracycline-based, taxane-based, anthracycline and taxane, other | 1257 | <1%: 897 1%-5%: 241 6%-10%: 119 |
HT 4; CT 74 HT 14; CT 70 HT 40; CT 72 |
| Villegas, 2021 | Germany | Post hoc analysis of randomized data | NR | Yes | Anthacycline- and taxane-based | 2765 | <1%: 902 1%-9%: 94 ≥10%: 1769 |
NR |
| Yi, 2014 | USA | Retrospective | 1990-2011 | Yes (no separate data) | NR | 9639 | <1%: 1625 1%-9%: 250 ≥10%: 7764 |
HT 12.9; CT 49.7 HT 20.4; CT 49.2 HT 83.6; CT 35.5 |
| Zhang, 2014 | USA | Retrospective | 2000-2011 | No | NR | 1700 | <1%: 401 1%-10%: 32 >10%: 1267 |
HT 11; CT 78 HT 87; CT 81 HT 99; CT 86 |
CMF, cyclophosphamide, methotrexate, fluorouracil; CT, chemotherapy; ER, estrogen receptor; ET, endocrine therapy; FEC, fluorouracil, epirubicin, cyclophosphamide; HT, hormone therapy; NR, not reported.
Quality assessment
The quality assessment of eligible studies is summarized in Table 2. The median quality score was 7 (range: 5-9).
Table 2.
Quality assessment of eligible studies according to Newcastle-Ottawa Scale
| Included studies | Selection |
Comparability |
Outcome |
Total quality score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of interest not present at the start of study | For main factor (lymph node status) | For additional factor (tumor size) | Assessment of outcome | Sufficient follow-up (8 years) | Adequacy of follow-up | ||
| Balduzzi, 2014 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | ||
| Colleoni, 2004 | ∗ | ∗ | ∗ | ∗ | ∗ | 5 | ||||
| Dieci, 2021 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 | |
| Ding, 2019 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 | |
| Fujii, 2017 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | ||
| Landmann, 2018 | ∗ | ∗ | ∗ | ∗ | ∗ | 5 | ||||
| Ohara, 2019 | ∗ | ∗ | ∗ | ∗ | ∗ | 5 | ||||
| Prabhu, 2014 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | |||
| Raghav, 2012 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | ||
| Villegas, 2021 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 9 |
| Yi, 2014 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | ||
| Zhang, 2014 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | ||
Pooled pCR rates after neoadjuvant chemotherapy based on ER expression
Seven studies provided data on pCR in relation to ER status.8, 9, 10, 11,15,20 Overall, ER-low breast cancer reached a higher pooled pCR rate (24.8%) with neoadjuvant chemotherapy in comparison to ER-positive breast cancer (8.3%) with a pooled OR of 3.25 (95% CI 1.85-5.71). The pooled pCR for ER-negative breast cancer was 30.8% without a statistically significant difference compared with the pooled pCR rate for the ER-low patient group (OR: 1.37; 95% CI 0.83-2.22; Table 3).
Table 3.
pCR pooled rates and corresponding OR after neoadjuvant chemotherapy in ER-low breast cancer
| N patients | Pooled pCR (95% CI) | Odds ratio | 95% CI | Heterogeneity |
||
|---|---|---|---|---|---|---|
| I2 | P | |||||
| ER-positive breast cancer | 4446 | 8.3 (6.9-9.9) | – | – | – | – |
| ER-low breast cancer | 499 | 24.8 (16.0-34.7) | 3.25 (versus ER-positive) | 1.85-5.71 | 74 | 0.002 |
| ER-negative breast cancer | 2486 | 30.8 (25.9-35.7) | 1.37 (versus ER-low) 4.71 (versus ER-positive) |
0.83-2.22 3.69-6.02 |
74 49 |
<0.001 0.08 |
CI, confidence interval; ER, estrogen receptor; OR, odds ratio; pCR, pathologic complete response.
DFS based on ER expression
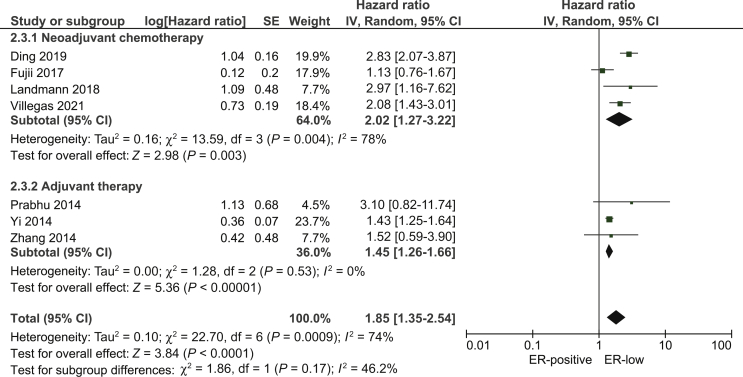
For comparison between ER-low and ER-positive breast cancer, four neoadjuvant10, 11, 12,14 and three adjuvant studies16,18,19 provided data on DFS. Fujii et al.11 provided data on time to recurrence (TTR) and Yi et al.18 on recurrence-free survival (RFS), but both studies were included in the pooled DFS analysis since TTR and RFS are part of the DFS definition.19 ER-low breast cancer was associated with worse DFS compared with ER-positive breast cancer (pooled HR: 1.85; 95% CI 1.35-2.54; Figure 2).
Figure 2.
Pooled hazard ratio for disease-free survival between patients with ER-low and ER-positive breast cancer.
CI, confidence interval; df, degrees of freedom; ER, estrogen receptor; SE, standard error.
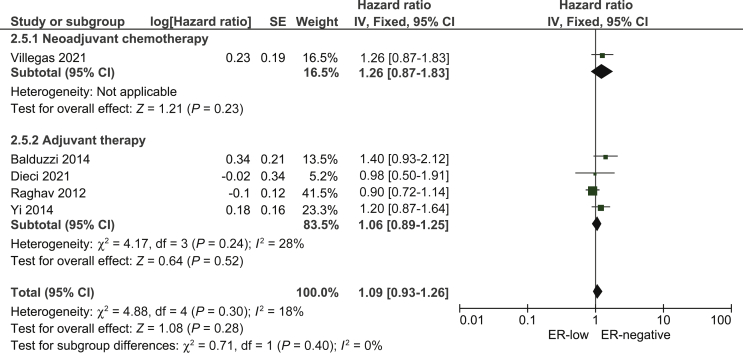
When ER-low breast cancer was compared with ER-negative breast cancer in terms of DFS, five studies14,15,17,18,20 were eligible, three of which presented data on RFS.17,18,20 We found no statistically significant difference between ER-low and ER-negative breast cancer in terms of DFS (pooled HR: 1.09; 95% CI 0.93-1.26; Figure 3).
Figure 3.
Pooled Hazard Ratio for disease-free survival between patients with ER-low and ER-negative breast cancer.
CI, confidence interval; df, degrees of freedom; ER, estrogen receptor; SE, standard error.
For the latter pooled analysis, we used data from the comparison between ER expression 0% and ER 1%-5% from Raghav et al.17 The authors also presented data on the ER 6%-10% group but we chose the ER 1%-5% group for the main analysis since it included more patients. When we carried out a sensitivity analysis by including the results from the ER expression 0% versus ER 6%-10% comparison from Raghav et al.,17 we found a similar pooled HR as in the main analysis (pooled HR: 1.17; 95% CI 0.97-1.35).
OS based on ER expression
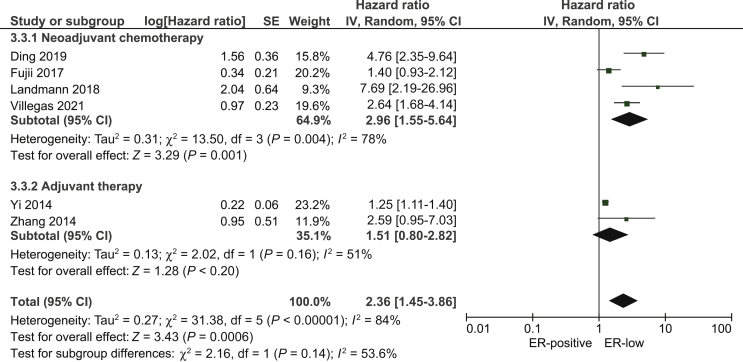
Six studies10, 11, 12,14,19,21 presented data on OS between ER-low and ER-positive breast cancer. ER-low breast cancer was associated with worse OS compared with ER-positive (pooled HR: 2.36; 95% CI 1.35-3.86; Figure 4).
Figure 4.
Pooled Hazard Ratio for overall survival between patients with ER-low and ER-positive breast cancer.
CI, confidence interval; df, degrees of freedom; ER, estrogen receptor; SE, standard error.
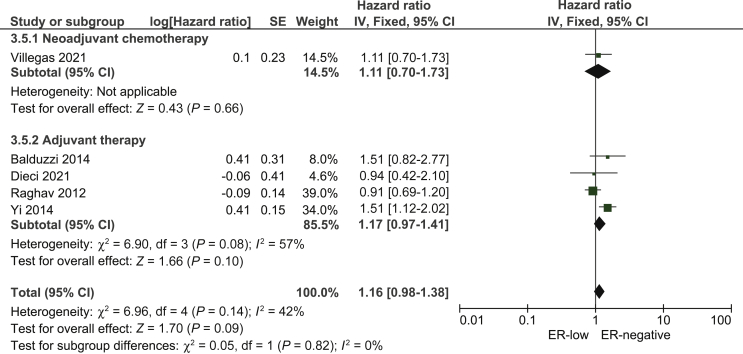
Five studies14,15,17,18,20 presented data on OS for the comparison between ER-low and ER-negative breast cancer. No statistically significant difference was observed between the two breast cancer patient groups in terms of OS (pooled HR: 1.16; 95% CI 0.98-1.38; Figure 4, Figure 5).
Figure 5.
Pooled Hazard Ratio for overall survival between patients with ER-low and ER-negative breast cancer.
CI, confidence interval; df, degrees of freedom; ER, estrogen receptor; SE, standard error.
OS data from Raghav et al.17 were addressed in the same way as described above and our sensitivity analysis when we included the comparison ER expression 0% and ER 6%-10% in the pooled analysis, we found similar results to the main analysis (pooled HR: 1.21; 95% CI 0.98-1.46).
Quality of evidence according to GRADE approach
The quality of evidence from the present meta-analysis was assessed by the GRADE approach for three research questions and six comparisons (Table 4).
Table 4.
Quality of evidence according to GRADE approach
| No. of studies | Certainty assessment |
Relative effect (95% confidence interval) | Certainty | |||||
|---|---|---|---|---|---|---|---|---|
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
| pCR in patients with ER-low compared with ER-positive breast cancer (assessed with: odds ratio) | ||||||||
| 6 | Observational studies | Serious | Not serious | Not serious | Not serious | None | 3.25 (1.85-5.71) | ⊕⊕⊕◯ MODERATE |
| pCR in patients with ER-low compared with ER-negative breast cancer (assessed with: odds ratio) | ||||||||
| 7 | Observational studies | Serious | Serious | Not serious | Not serious | None | 1.37 (0.83-2.22) | ⊕⊕◯◯ LOW |
| Disease-free survival ER-low versus ER-positive (assessed with: hazard ratio) | ||||||||
| 7 | Observational studies | Serious | Not serious | Not serious | Not serious | None | 1.85 (1.35-2.54) | ⊕⊕⊕◯ MODERATE |
| Disease-free survival ER-low versus ER-negative (assessed with: hazard ratio) | ||||||||
| 5 | Observational studies | Serious | Serious | Not serious | Not serious | None | 1.09 (0.93-1.26) | ⊕⊕◯◯ LOW |
| Overall survival ER-low versus ER-positive (assessed with: hazard ratio) | ||||||||
| 6 | Observational studies | Serious | Not serious | Not serious | Not serious | None | 2.36 (1.35-3.86) | ⊕⊕⊕◯ MODERATE |
| Overall survival ER-low versus ER-negative (assessed with: hazard ratio) | ||||||||
| 5 | Observational studies | Serious | Serious | Not serious | Not serious | None | 1.16 (0.98-1.38) | ⊕⊕◯◯ LOW |
ER, estrogen receptor; GRADE, Grading of Recommendations Assessment, Development and Evaluation; pCR, pathologic complete response.
All comparisons between ER-low and ER-positive breast cancer were categorized as moderate certainty of evidence, whereas the comparisons between ER-low and ER-negative were categorized as low certainty of evidence due to the observed inconsistency of the results from eligible studies.
Discussion
According to the pooled analyses based on current evidence, ER-low expression seems to be a predictive factor for NeoCT, with pCR rates similar to ER-negative breast cancer. Regarding the impact of ER-low expression on breast cancer prognosis, we found a worse prognosis in terms of DFS and OS compared with ER-positive breast cancer, whereas the prognoses of ER-low and ER-negative breast cancer were comparable. The quality of evidence for both the predictive and prognostic role of ER-low expression on breast cancer ranged between low (for the comparisons between ER-low and ER-negative breast cancer) and moderate (for the comparisons between ER-low and ER-positive breast cancer), highlighting the need for high-quality evidence on this topic.
Our findings on the similar efficacy of NeoCT and prognosis in patients with ER-low and ER-negative breast cancer are supported by prior studies on the molecular background of ER-low breast cancer. Iwamoto et al.22 and Deyarmin et al.23 analyzed the intrinsic subtype of ER-low expressing breast cancer and found that most ER-low breast cancers were molecularly primarily basal-like or secondarily human epidermal growth factor receptor 2 (HER2)-enriched, whereas only a small minority, 16% and 12%, respectively, had luminal-like molecular features. Similarly, Villegas et al.14 found that nearly 87% of ER-low breast cancer had a basal-like gene expression signature, whereas none was classified as luminal.
A meta-analysis by Chen et al.24 was published in 2016 and suggested an intermediate prognosis for ER-low breast cancer, with patients in this subgroup faring worse than the ER-positive subgroup but better than the ER-negative subgroup in DFS and OS. There are, however, some important methodological differences between the two meta-analyses that deserve attention. First, we included only studies with results on prognosis derived from multivariate analyses to mitigate the risk for confounding bias, whereas the prior meta-analysis included results from bivariate analyses as well. As confounding bias is a major source of bias in observational studies that can jeopardize the validity of the results and multivariate analysis is an analytic approach that can mitigate this risk, a meta-analysis based only on results from multivariate analyses is a more suitable approach when only observational studies are available. Second, our meta-analysis investigated an additional research question on the predictive role of NeoCT in patients with ER-low breast cancer. Since NeoCT is currently the recommended treatment strategy for ER-negative breast cancer, our meta-analysis provides evidence on a research question which is in line with current clinical practice. In addition, we used HR as a pooled effect measure for DFS and OS which is a more robust measure for time-to-event outcomes compared with OR, which was used in the prior meta-analysis. Finally, the pooled analyses from the present meta-analysis are accompanied by the level of evidence according to the GRADE approach, enabling the clinicians and policymakers to interpret the results following the principles of evidence-based medicine.
This meta-analysis has several limitations that need to be discussed. First, the eligible studies lack adequate analyses on the effectiveness of adjuvant endocrine therapy in patients with ER-low breast cancer, which made us unable to carry out a meta-analysis on this issue. Some evidence from observational studies, however, suggests that adjuvant endocrine therapy does not seem to improve DFS or OS in patients with ER-low breast cancer.15,17,25 This observation is also supported by randomized evidence from the latest Early Breast Cancer Trialists’ Collaborative Group meta-analysis on the benefit of adjuvant tamoxifen, where low ER expression was associated with nearly zero benefit.26 Second, most of the eligible studies had a median follow-up of <5 years which can be considered adequate for ER-negative but not for ER-positive breast cancer where there is a greater tendency for late recurrence not able to be captured with follow-up shorter than 8 years.27,28 Another potential limitation is the risk for variability in the immunohistochemical assessment of ER status throughout the years and among different laboratories and countries. This risk has been shown to be higher in low or medium ER expressions29 but considerably lower compared with other breast cancer biomarkers such as HER2 and Ki-67.30,31 Finally, this meta-analysis included only observational studies which negatively impact the certainty of evidence, as reflected by the grading of evidence according to the GRADE approach.
Based on current evidence, our findings suggest that ER-low expression in breast cancer is predictive for response to NeoCT with anticipated pCR comparable to ER-negative breast cancer. Furthermore, ER-low breast cancer appears to resemble ER-negative more than ER-positive breast cancer in terms of prognosis. Our results support the updated ASCO/CAP and ABC5 guidelines4,5 recommending that tumors with ER-low expression should be classified as ER-low-positive, namely separately from ER-positive tumors. Our results also raise reasonable clinical thoughts on whether new treatment strategies for TNBC such as immunotherapy and antibody–drug conjugates might be suitable for patients with low ER expression as well and emphasize the complexity of biological subtyping for breast cancer. Considering the low to moderate level of evidence for both the predictive and prognostic role of ER-low expression on breast cancer, our findings urge the need for high-quality, prospective studies investigating the molecular background and the most appropriate treatment strategy for this subgroup.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
The data presented in this study are available on request from the corresponding author.
References
- 1.European Cancer Information System. https://ecis.jrc.ec.europa.eu/explorer.php?$0-0 Available at.
- 2.Rosenberg P.S., Barker K.A., Anderson W.F. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J Natl Cancer Inst. 2015;107:djv159. doi: 10.1093/jnci/djv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond M.E.H., Hayes D.F., Dowsett M. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison K.H., Hammond M.E.H., Dowsett M. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F., Paluch-Shimon S., Senkus E. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korde L.A., Somerfield M.R., Carey L.A. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39:1485–1505. doi: 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso F., Kyriakides S., Ohno S. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 8.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colleoni M., Viale G., Zahrieh D. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10:6622–6628. doi: 10.1158/1078-0432.CCR-04-0380. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y., Ding K., Yu K. Prognosis and endocrine therapy selection for patients with low hormone receptor-positive breast cancer following neoadjuvant chemotherapy: a retrospective study of 570 patients in China. Oncol Lett. 2019;18:6690–6696. doi: 10.3892/ol.2019.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii T., Kogawa T., Dong W. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol. 2017;28:2420–2428. doi: 10.1093/annonc/mdx397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landmann A., Farrugia D.J., Zhu L. Low estrogen receptor (ER)-positive breast cancer and neoadjuvant systemic chemotherapy: is response similar to typical ER-positive or ER-negative disease? Am J Clin Pathol. 2018;150:34–42. doi: 10.1093/ajcp/aqy028. [DOI] [PubMed] [Google Scholar]
- 13.Ohara A.M., Naoi Y., Shimazu K. PAM50 for prediction of response to neoadjuvant chemotherapy for ER-positive breast cancer. Breast Cancer Res Treat. 2019;173:533–543. doi: 10.1007/s10549-018-5020-7. [DOI] [PubMed] [Google Scholar]
- 14.Villegas S.L., Nekljudova V., Pfarr N. Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors – an analysis of 2765 patients from neoadjuvant clinical trials. Eur J Cancer. 2021;148:159–170. doi: 10.1016/j.ejca.2021.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Balduzzi A., Bagnardi V., Rotmensz N. Survival outcomes in breast cancer patients with low estrogen/progesterone receptor expression. Clin Breast Cancer. 2014;14:258–264. doi: 10.1016/j.clbc.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Prabhu J.S., Korlimarla A., Desai K. A majority of low (1-10%) ER positive breast cancers behave like hormone receptor negative tumors. J Cancer. 2014;5:156–165. doi: 10.7150/jca.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghav K.P.S., Hernandez-Aya L.F., Lei X. Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer. 2012;118:1498–1506. doi: 10.1002/cncr.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi M., Huo L., Koenig K.B. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol. 2014;25:1004–1011. doi: 10.1093/annonc/mdu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z., Wang J., Skinner K.A. Pathological features and clinical outcomes of breast cancer according to levels of oestrogen receptor expression. Histopathology. 2014;65:508–516. doi: 10.1111/his.12412. [DOI] [PubMed] [Google Scholar]
- 20.Dieci M.V., Griguolo G., Bottosso M. Impact of estrogen receptor levels on outcome in non-metastatic triple negative breast cancer patients treated with neoadjuvant/adjuvant chemotherapy. NPJ Breast Cancer. 2021;7:101. doi: 10.1038/s41523-021-00308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gourgou-Bourgade S., Cameron D., Poortmans P. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials) Ann Oncol. 2015;26:873–879. doi: 10.1093/annonc/mdv478. [DOI] [PubMed] [Google Scholar]
- 22.Iwamoto T., Booser D., Valero V. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30:729–734. doi: 10.1200/JCO.2011.36.2574. [DOI] [PubMed] [Google Scholar]
- 23.Deyarmin B., Kane J.L., Valente A.L. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013;20:87–93. doi: 10.1245/s10434-012-2588-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen T., Zhang N., Moran M.S., Su P., Haffty B.G., Yang Q. Borderline ER-positive primary breast cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:1–8. doi: 10.1016/j.clbc.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Bouchard-Fortier A., Provencher L., Blanchette C., Diorio C. Prognostic and predictive value of low estrogen receptor expression in breast cancer. Curr Oncol. 2017;24:e106–e114. doi: 10.3747/co.24.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Davies C., Godwin J. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colleoni M., Sun Z., Price K.N. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Maaren M.C., de Munck L., Strobbe L.J.A. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: a large population-based study. Int J Cancer. 2019;144:263–272. doi: 10.1002/ijc.31914. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes A., Jasani B., Balaton A.J., Miller K.D. Immunohistochemical demonstration of oestrogen and progesterone receptors: correlation of standards achieved on in house tumours with that achieved on external quality assessment material in over 150 laboratories from 26 countries. J Clin Pathol. 2000;53:292–301. doi: 10.1136/jcp.53.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Schutter H., Van Damme N., Colpaert C. Quality of pathology reporting is crucial for cancer care and registration: a baseline assessment for breast cancers diagnosed in Belgium in 2008. Breast. 2015;24:143–152. doi: 10.1016/j.breast.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Acs B., Fredriksson I., Rönnlund C. Variability in breast cancer biomarker assessment and the effect on oncological treatment decisions: a nationwide 5-year population-based study. Cancers. 2021;13:1166. doi: 10.3390/cancers13051166. [DOI] [PMC free article] [PubMed] [Google Scholar]