Abstract
In this update article, we present a review of the literature regarding the physiology of the articular cartilage, role of MR imaging in cartilage assessment, MRI sequences and protocols for cartilage imaging, brief overview of classifications and nomenclature for chondral and osteochondral lesions, MR imaging following cartilage repair and degenerative osteoarthritis.
Keywords: Cartilage imaging, MRI, Chondromalacia, Osteochondral lesions, Cartilage repair
1. Introduction
Articular cartilage is a smooth specialized connective tissue that provides lubrication, avoids friction and helps in load transmission of the joint. Due to the lack of a blood supply, lymphatic drainage and neural innervation, it lacks healing potential.1, 2, 3 Breakdown of the articular cartilage, due to any cause expedites the onset of degenerative joint disease. Conventional radiography helps indirectly evaluate the articular cartilage in advanced osteoarthritic cases as evident by progressive reduction in joint space, marginal osteophytes and changes in the subchondral bone.
2. Role of MRI in cartilage assessment
With the increase in the availability of the treatment options for cartilage repair and regrowth, magnetic resonance imaging (MRI) is the imaging modality of choice for primary chondral injury evaluation as well as for evaluation following cartilage repair with arthroscopy accepted as the gold standard.
MRI is useful for cartilage assessment in osteoarthritis, chronic or acute osteochondral injury, osteochondritis dissecans, chondromalacia, spontaneous osteonecrosis of the femoral condyle (SONK or Ahlbaeck's disease) and inflammatory arthropathies (in particular before invasive therapy).
MRI plays an important role in the pre-operative evaluation of the chondral injuries and guides further management. The choice of the reparative/reconstructive surgical techniques depends upon the site and size of the chondral lesion, associated bone involvement, presence of loose bodies and the stage of chondral injury. Distinguishing traumatic and degenerative cartilage injury and detection of the associated meniscal and ligamentous injuries can prognosticate and determine management choices for anterior cruciate ligament reconstruction surgery and partial meniscectomy. Standardized scoring system like MOCART scores are useful for post-surgical monitoring and early detection of post-operative complications. Newer MR imaging techniques can quantify and assess the biochemical composition of the articular cartilage. These can be useful in monitoring surgical responses as well as treatment responses following pharmacotherapies.
3. Articular cartilage structure
Articular cartilage is largely composed of water (75–80%), chondrocytes (5%), cellular substances (20–25%), non -collagenous proteins and glycoproteins. It is composed of a thin superficial, thick middle transitional and a deep zone comprising of various types of chondrocytes with variably packed collagen fibrils imparting the cartilage its characteristic tensile strength and resistance to compressive forces.4 These layers are separated by a ‘tide mark zone’ from the underlying calcified zone and subchondral bone that anchors the collagen fibrils to the underlying bone.5
4. MRI cartilage sequences
4.1. MR sequences for morphological assessment
The most widely clinically used MR sequences for cartilage imaging is 2D fast spin-echo (FSE) sequences with intermediate-weighted contrast.6
2D Intermediate-weighted FSE sequences with a mixed PD/T2 contrast and an echo time (TE) of 40–60 ms, has the highest sensitivity for intra-chondral signal alterations and provides excellent contrast between the cartilage-joint fluid interface.7 Besides it allows evaluation of menisci and ligaments. Subchondral bone marrow edema-like signal alteration is better appreciated on fat-suppressed sequences (Fig. 1a) whereas intra-chondral osteophytes, evaluation of subchondral bone plate and presence of osseous defects are better characterized on non-fat-saturated PDW TSE sequences.8 (Fig. 1b) However, FSE sequences have relatively higher slice thickness and slice-gap and can obscure small cartilage lesions secondary to partial volume averaging.
Fig. 1.
MR Sequences for evaluation of patellar cartilage (a) FS PDW axial image (b) Non-FS PDW axial image (c) 3D T2∗ GRE image showing normal patellar cartilage.
3D cartilage sequences with thin contiguous slices can reduce partial volume averaging artifacts and allow multi-planar reformatting of images (Fig. 1c). Limitations of 3D cartilage imaging include their long acquisition times; increased susceptibility to motion artifacts; and limited ability to evaluate the menisci, ligaments, and osseous structures of the knee joint when compared with 2D FSE sequences. Fat suppression is added to 3D cartilage sequences to reduce the chemical shift artifacts.
The 3D cartilage imaging sequences can be broadly divided into dark-fluid sequences and bright-fluid sequences on the basis of the signal intensity of synovial fluid.
-
•
Dark-fluid sequences consist of T1-weighted gradient-recalled echo (GRE) sequences such as spoiled gradient recalled-echo (SPGR) and fast low-angle shot (FLASH). Due to low signal of synovial fluid in dark fluid sequences, there is low cartilage –synovial fluid contrast and hence the superficial cartilage lesions are less conspicuous.
-
•
The bright-fluid sequences include dual-echo in the steady-state (DESS); driven equilibrium Fourier transform (DEFT); and T2∗-weighted gradient-echo sequences, such as gradient-recalled echo acquired in the steady-state (GRASS) and gradient-recalled two gradient echoes separated by a refocusing pulse into a single image. In bright fluid sequences, there is high contrast between the bright synovial fluid and dark cartilage, creating an arthrogram-like effect and increasing the conspicuity of superficial cartilage lesions.
3D SPGR and FLASH sequences are well suited to depict surface lesions. 3D double echo steady state sequence (3D DESS) which is a mixed T1/T2∗weighted sequence provides high spatial resolutions with the cartilage appearing more intermediate in signal. This sequence was found more accurate in diagnosing cartilage softening and lesser accurate in detecting cartilage surface abnormalities.
Balanced steady-state free precession (SSFP) sequences are additional 3D sequences that have been used to evaluate the articular cartilage of the knee joint and include commercially available sequences such as fast imaging employing steady-state acquisition (FIESTA) and true fast imaging with steady-state precession (true FISP) and variants such as fluctuating equilibrium MR (FEMR) and iso-tropic-projection steady-state free precession (VIPR-SSFP). These sequences have high cartilage SNR efficiency and produce images of the knee joint with T2-/T1-weighted contrast. These show bright synovial fluid and greater contrast between cartilage and adjacent joint structures than 2D FSE and fat-saturated SPGR sequences.
3D FSE sequences, such as FSE- Cube (GE Healthcare) and sampling perfection with application oriented contrast using different flip angle evolutions (SPACE, Siemens Healthcare), have been also been used recently to for articular cartilage assessment. These intermediate-weighted images have higher cartilage SNR, show bright synovial fluid but lower contrast between cartilage and synovial fluid as compared with 2D FSE sequences. The 3D FSE sequences holds advantages of 3D acquisition such as volumetric data acquisition that allows multi-planar reformatting of images. However, 3D FSE sequences have lower in-plane spatial resolution when compared with other 3D cartilage imaging sequences with similar acquisition times, that may reduce the conspicuity of superficial cartilage lesions.
4.2. Newer compositional MR imaging techniques
The newer compositional MR imaging techniques aim to detect biochemical and microstructural changes in the cartilage extra-cellular matrix even before gross morphologic changes occur.9 In addition to early diagnosis of cartilage degeneration before morphological changes are visible, biochemical MRI offers predictive markers for monitoring of disease-modifying drugs and monitoring the efficacy of different cartilage repair surgery techniques to develop hyaline-like cartilage.
T2 mapping can be easily implemented on clinical routine MR scanners. Measurement of the spatial distribution of the T2 reflects areas of increased and decreased water content and is used to quantify cartilage degeneration before morphologic changes are appreciated.10 Diffuse increase in T2 relaxation value of the articular cartilage is noted with aging whereas focally increased T2 is observed in damaged articular cartilage.11 (Fig. 2)
Fig. 2.
(a) PD non-FS axial image of patellar cartilage showing a full thickness chondral defect at lateral patellar cartilage. (b) T2 mapping of the patellar cartilage: increased T2 relaxation values (ranging from 5 to 60 ms) at the level of morphologically evident chondral defect (Zone B) as compared from T2 relaxation values (ranging from 31 to 41 ms) in adjacent normal appearing cartilage (zone A and C).
Current applications of other biochemical compositional MR techniques are limited in clinical practice but hold promising future. The T1rho method is technically more demanding and is not available on all MR scanners.12 Delayed gadolinium-enhanced MR imaging of cartilage (dGEMRIC), which can be performed with all field strengths, is now severely restricted due to the recent decision of the European Medical Agency (EMA) to withdraw linear gadolinium contrast agents from the market because of proven gadolinium deposition in the brain. Sodium imaging is the most sensitive MRI method for glycosaminoglycan (GAG), but is limited to 7 T.13 DTI (Diffusion tensor imaging) has reflected the macromolecular environment (like GAG concentration) as well as predominant alignment of the collagenous fiber network on a 9.4 T, but its use in human subjects at clinical high field strength systems is yet to be tested.
5. Cartilage injury
Cartilage appearances on MRI may vary depending upon traumatic or degenerative cause and at times may be difficult to differentiate the two. Besides long-standing cartilage injury due to any cause promotes early onset of degenerative osteoarthritic changes.
Usual characteristics for different lesions include:14
-
•
Acute traumatic lesions (Fig. 3f): Sharply demarcated chondral lesions with a narrow transition zone e.g. osteochondral fractures, chondral fissures and flaps following shearing forces secondary to transient patellar dislocation, osteochondral impaction injuries following tangential and rotational forces causing ACL tear
-
•
Degenerative lesions (Fig. 3g): Shallow chondral defects with a wide transition zone e.g. Diffuse chondral thinning with varying degree of bone marrow edema-like signal, subchondral sclerosis and cyst-like changes.
Fig. 3.
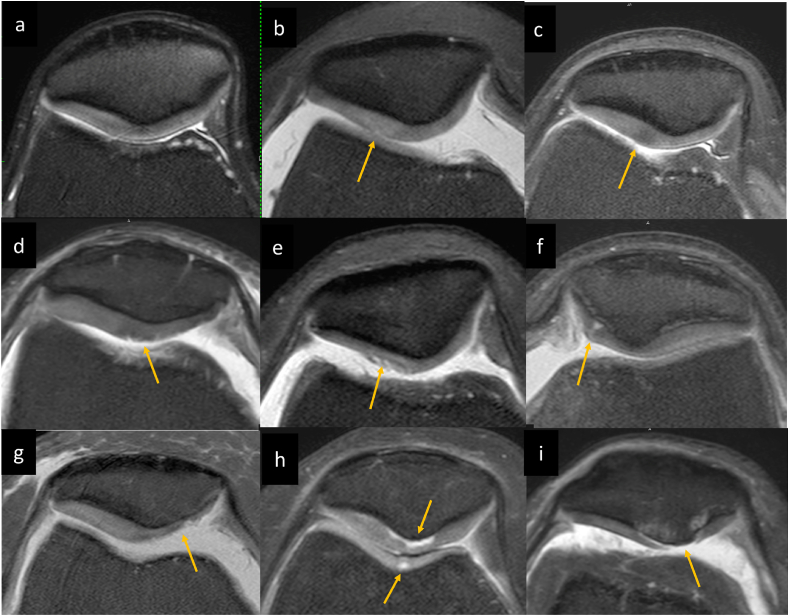
ICRS Grades of chondromalacia: (a) Normal patellar cartilage (b) Grade 1a intrinsic cartilage signal abnormality (hyperintense signal) without detectable morphologic changes (c) Grade 1a intrinsic cartilage signal abnormality (hypointense signal) without detectable morphologic changes (d) Grade 1b showing superficial fissures (e) Grade 2 focal chondral fissures involving <50% thickness of the cartilage. (f) Grade 3 focal cartilage fissure extending to > 50% of cartilage depth but not through the subchondral bone. (g) Grade 3 partial thickness cartilage loss >50% cartilage thickness without subchondral marrow changes in degenerative knee. (h) Chondral delamination is also included in grade 3 which are detected as fluid signal intensity subjacent to cortex without subchondral marrow changes, noted in patellar apex as well as trochlea in this case. (I) Grade 4 full thickness chondral lesions with subchondral marrow changes.
6. Chondral lesions
Currently, MR imaging adaptation of the arthroscopic classification for focal chondral lesions developed by a working group of International Cartilage Repair Society (ICRS), is the most accepted MR classification system to report articular cartilage lesions.
6.1. International cartilage repair society (ICRS) grading of focal chondral lesions
MR imaging adaptation of the International Cartilage Repair society (ICRS) Grades for the clinical and arthroscopic evaluation of the focal chondral lesions is as follows.15
ICRS grade 0 - Normal cartilage showing homogenous signals with a normal layered appearance of the articular cartilage throughout its thickness. No evident fissuring or surface irregularity (Fig. 3a).
ICRS grade 1 - Superficial lesions which is further divided into grade 1a featuring intrinsic cartilage signal abnormality without detectable morphologic changes (Fig. 3b and c) and grade 1b showing superficial fissures or lacerations (Fig. 3d).
ICRS grade 2 - Focal chondral lesions involving less than 50% thickness of the cartilage (Fig. 3e).
ICRS grade 3 - Cartilage defects extending to > 50% of cartilage depth but not through the subchondral bone (Fig. 3f and g). Chondral blisters, delamination (Fig. 3h) and chondral flap (Fig. 5) without associated subchondral marrow signal are also included in this grade.
Fig. 5.
Grade 3 chondral lesion: (a, b) Consecutive T2W FS coronal images (c) PD FS sagittal image shows a full thickness chondral fissure extending posteriorly parallel to the subchondral bone to form a chondral flap at weight bearing aspect of medial femoral condyle without subchondral marrow edema.
ICRS grade 4 – Full thickness chondral lesions extending to the subchondral bone plate with subchondral marrow edema-like signal or cystic changes (Fig. 3i).
ICRS grade 1 lesions can be missed on current MR protocols used in clinical practice due to their suboptimal spatial resolution.16 Superficial morphologic changes of articular cartilage such as fibrillation and pitting can only be consistently distinguished from the smooth surface of normal articular cartilage when using an in-plane spatial resolution of 0.3 mm, which is beyond the spatial resolution of most cartilage imaging sequences used in clinical practice.17 Occasionally these superficial lesions may be evident as subtle areas of increased signal intensity on T2W FSE sequences (Fig. 3b). Such non-specific findings can easily be confused with imaging artifacts such as truncation and magic angle effects.
Focal areas of heterogeneous change in chondral signal intensity, whether increased or decreased signal intensity (Fig. 3b and c) has been found to correlate with cartilage degeneration on arthroscopy and can progress to cartilage loss in future.18 “Cartilage black line” sign (Fig. 4) described as a linear low signal perpendicular to the subchondral bone on T2 weighted images, located in morphologically normal appearing cartilage on MRI have been found to correlate with cartilage fissuring on arthroscopy.19 T2 dark chondral lesions should be considered as a sign of occult cartilage degeneration.20
Fig. 4.
PD FS sagittal in a 48-year-old female shows a linear low signal in lateral femoral cartilage, perpendicular to the subchondral bone on T2 weighted images, located in morphologically normal appearing cartilage suggest” cartilage black line” sign. (b, c) PD FS images of another 54-year-old female with focal T2 hypointense chondral signal involving patellar cartilage with smooth overlying chondral surface could represent early chondral degeneration.
7. Cartilage imaging following repair
Knowledge about the preoperative MRI findings, details of the surgical technique performed and duration from surgery is crucial to interpret the post-operative MR findings. Follow-up MR imaging studies can be performed at 3–6 postoperative months to assess the volume and the integration of repair tissue and after 1 year to evaluate the maturation of the graft and identification of any complication. MR imaging is suggested when there is persistent or recurrent or development of new symptoms after cartilage repair surgery.
MOCART 2.0 knee score is a recent incremental update on the original MOCART score, that incorporates the progression made with regard to newer surgical treatment options for cartilage defects and their MRI appearances. “Adhesions” and “effusion” variables are no longer included in the new MOCART 2.0 evaluation. In the MOCART 2.0 knee score,21 the degree of defect filling is assessed in 25% increments and the severity of surface damage is determined in reference to cartilage repair length rather than depth. The integration of graft with adjacent cartilage, chondral surface and graft signal intensity, bony defect or overgrowth and subchondral marrow changes are also evaluated as a part of 7-parameter variable evaluation and total score ranging from 0 to 100 points is assigned. An atlas with MOCART 2.0 improves comparability of MR evaluation amongst observers.21
Most commonly performed cartilage repair technique - microfracture technique, promotes formation of reparative fibrocartilage which initially appears hypertintense to the native hyaline cartilage (Fig. 6) and as the repair tissue matures, it eventually appears hypointense to native cartilage and completely fills the defect with a smooth chondral surface at 1–2 year follow up.22
Fig. 6.
Post microfracture MR imaging (a, b) PD FS sagittal and coronal images of a 49-year-old male presenting with knee pain and instability following a fall 2 years ago shows a full thickness chondral loss with subchondral cystic changes and marrow edema at posterior weight bearing aspect of medial femoral condyle. Note grade III longitudinal tear at peripheral aspect of posterior horn of medial meniscus evident on sagittal images. He also had complete ACL tear (not shown in images) (c,d) 1.5 year follow up MR imaging following microfracture and ACL reconstruction shows re-growth of cartilage with near complete filling of previously noted full thickness chondral loss at posterior weight bearing aspect of medial femoral condyle and near complete resolution of subchondral marrow edema (at site of microfracture).
MR Assessment of the transplanted grafts and the donor sites is performed following osteochondral grafting procedures or mosaicplasty, if indicated.23 Most donor sites get filled with fibro-cartilage reparative tissue over a period of approx. 6–9 months (Fig. 7), usually to a level below the articular surface. Associated edema-like marrow signal intensities in the donor and repair sites usually regress at similar rates. Persistent edema-like marrow signal intensity at the donor site may be indicative of a complication. Recipient graft site ideally would show complete volume fill of the cartilage defect with uniform graft cartilage signal intensity and complete integration with the native cartilage and subchondral bone. Persistent significant edema-like marrow signal intensity within subchondral bone beyond 18 months, increase in edema-like signal, articular surface collapse and subchondral cyst formation are of concern and may be signs of poor tissue integration.
Fig. 7.
Post Cartilage repair MR imaging: 19-years-old male presenting with knee pain (a) AP radiograph of knee joint showing osteochondral lesion at weight bearing aspect of medial femoral condyle. (b) Sagittal non-FS PDW MR images shows a Stage 3 osteochondritis dissecans with undisplaced completely separated osteochondral fragment. (c) Coronal FS-PDW and (d) Sagittal FS-PDW MR images obtained 2.5 year following Autologous Chondrocyte implantation show complete integration of the chondral graft with the native cartilage and subchondral bone. Mildly proud chondral graft is noted with homogenous mildly hyperintense signal and subtle subchondral marrow edema-like signal that is not of much concern. Case Courtesy: Dr.Kalpesh Trivedi. Arthroscopy surgeon. Ahmedabad.
8. Osteochondral lesions
Osteochondral lesion refers to any lesion that involves the articular surface and the subchondral region of a joint, affecting both the cartilage and the bone. Recent recommendations for use of terms like osteochondral defects, osteochondritis dissecans and osteochondral fractures published by the Society of Skeletal Radiology (SSR) Subchondral Bone Nomenclature24 are as follows:
‘Osteochondral defect’ is a broad ‘umbrella’ term to describe a focal defect in the articular cartilage and the subchondral bone. A specific diagnosis or cause and chronicity of the osteochondral defect should be stated, wherever possible.
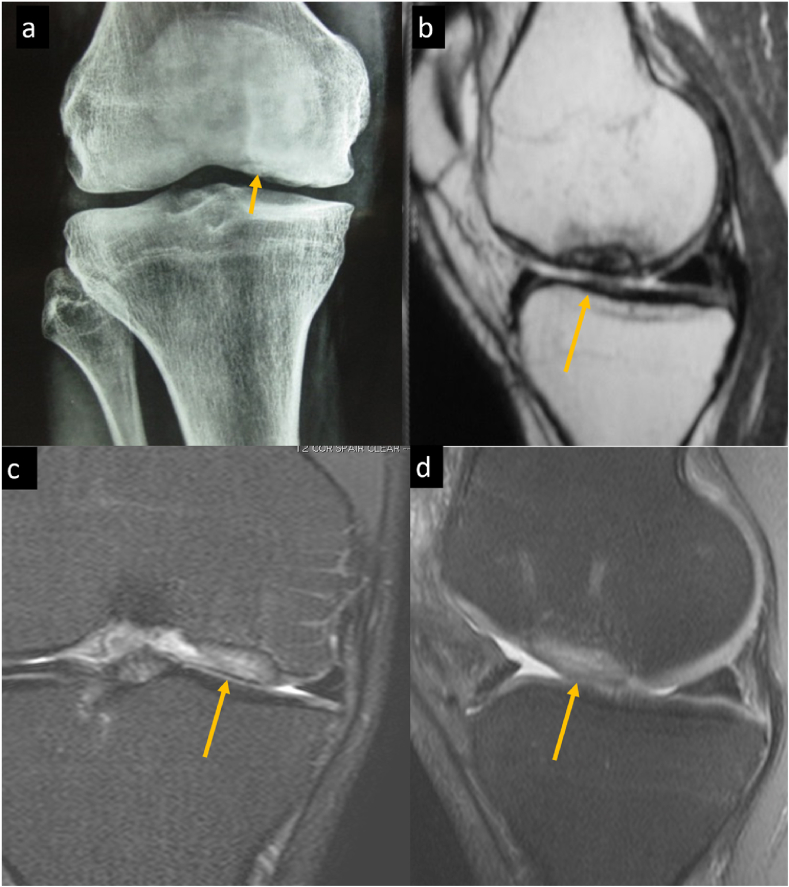
‘Osteochondritis dissecans’ (Fig. 8) is a chronic osteochondral lesion occurring in the young adults and children at a classic location that represents as a progressive separation of the articular cartilage and the underlying subchondral bone with the cleavage plane situated in the subchondral bone and eventually involving the full thickness of the overlying articular cartilage. The common sites of osteochondral dissecans are the femoral condyle (most common), the humeral head, the talus and the capitellum of the humerus. The term ‘Osteochondral fracture’ (Fig. 9, Fig. 10) is reserved for subchondral bone depression or fragmentation creating an osteochondral fragment, in the setting of an acute trauma.
Fig. 8.
Schematic presentation of magnetic resonance imaging-based adaptation of the ICRS (International Cartilage Repair Society) staging system of osteochondritis dissecans (OCD) (a) Stable lesion in continuity with the host bone and covered by an intact articular cartilage is categorized as stage 1 OCD. (b) Stable lesion showing partial discontinuity of the cartilage from the host bone is categorized as stage 2 OCD. (c) Stage 3 OCD is characterized when there is an unstable lesion showing a complete discontinuity of the “dead in situ” lesion without dislodgement of the osteochondral fragment. (d) Stage 4 OCD is characterized by the presence of an osteochondral defect with a dislocated fragment.
Fig. 9.
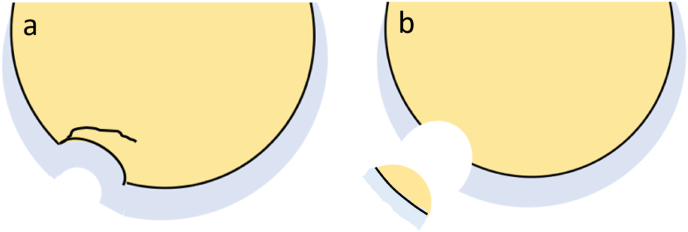
Schematic Presentation of Types of Osteochondral Fractures. (a) In the setting of an acute trauma, osteochondral fracture may present as an osteochondral lesion with a complete or incomplete fracture line through the articular cartilage and the sub-chondral bone with or without subchondral plate depression (b) The osteochondral fracture can also present as a localized defect involving the cartilage and the subchondral bone with a displaced osteochondral fracture fragment.
Fig. 10.
Osteochondral fracture (a, b) PD FS and non FS sagittal images of a 21-year-old male with history of twisting injury. These show an osteochondral fracture as evident by an incomplete fracture line at posterior (not shown in mages) aspect of lateral tibial plateau with adjacent marrow edema and subtle depression. He had associated ACL tear (Not shown in images). (c, d) PD FS axial and sagittal image of a 23-year-old male showing an osteochondral fracture at medial patellar facet with a displaced osteochondral fracture fragment at anterior aspect of knee joint in a case of transient patellar dislocation. Note bone marrow contusion at typical location at anterolateral aspect of lateral femoral condyle in (c).
Magnetic resonance imaging is the modality of choice for determining the extent and stability of the osteochondral lesions and acts as a guide for the further management.25,26
MR imaging adaptation of the International Cartilage Repair society (ICRS) Grades for the clinical and arthroscopic evaluation of the osteochondritis dessicans (OCD) is as follows.
ICRS (International Cartilage Repair Society) staging system of osteochondritis dissecans (OCD) (Fig. 8, Fig. 11).
Fig. 11.
ICRS Grades of osteochondritis Dessicans (OCD) (a, b) Stable lesion in continuity with the host bone and covered by an intact articular cartilage suggest Stage 1 OCD (c, d) Stable lesion showing partial discontinuity of the cartilage from the host bone (as marked by arrow) suggest Stage 2 OCD (e, f) Unstable lesion showing a complete discontinuity of the "dead in situ" lesion without dislocation of the fragment suggest Stage 3 OCD. (g, h) Presence of an osteochondral defect with a dislocated fragment suggest Stage 4 OCD.
Stage 1 OCD - Stable lesion in continuity with the host bone and covered by an intact articular cartilage.
Stage 2 OCD - Stable lesion showing partial discontinuity of the cartilage from the host bone.
Stage 3 OCD - Unstable lesion showing a complete discontinuity of the "dead in situ" lesion. However, the fragment is not dislocated.
Stage 4 OCD - Presence of an osteochondral defect with a dislocated fragment.
9. Degenerative cartilage
Degenerative osteoarthritic changes can be primary (idiopathic) or secondary to prior trauma, prior surgery, abnormal mechanical forces like obesity or deformities, crystal deposition diseases etc. It presents with varying degrees of chondral changes with diffuse ill-defined subchondral bone sclerosis and marrow edema on MRI. Advanced osteoarthritic changes are associated with larger volume of concomitant bone marrow edema-like signal and sub-chondral cysts at the sites of progressive cartilage damage which is best depicted on fat suppressed PD or T2 weighted images (Fig. 12). Subchondral sclerosis is better appreciated on non-fat suppressed PD weighted images as a low signal intensity area with apparent thickening of subchondral bone plate.27 Besides chondral damage, osteoarthritic changes are accompanied by bone remodeling and marginal osteophyte formation, ligamentous laxity, periarticular muscle weakness and synovitis, which can be evaluated on an MRI.
Fig. 12.
Degenerative osteoarthritic changes (a) T2 weighted coronal and (b) PD weighted axial fat suppressed image of a 72 year old male with knee pain. (a) Shows diffuse full thickness chondral loss involving the weight bearing aspect of medial femoral and tibial plateau and (b) patellar apex with subchondral marrow edema. Superficial chondral surface defect is also noted in medial trochlea in (b). Marginal osteophytes are noted along femoral condyles in (a). These findings suggest degenerative osteoarthritic changes. Note subtle degenerative fraying of the free edge of body of medial meniscus without significant tear in (a).
10. Role of arthrography in cartilage imaging
Arthrography is an invasive technique of joint evaluation following intra-articular injection of contrast agent or combination of saline &/or local anesthetic28 to achieve joint distension. Multiplanar fat suppressed T1 weighted image acquisition is performed for MR arthrographic evaluation, if gadolinium based contrast agents are administered. It is used most commonly for evaluation of shoulder partial thickness rotator cuff tears, gleno-humeral ligament tears, chondro-labral and labral lesions,29 hip labral and cartilage evaluation30 & in femoro-acetabular impingement, triangular fibrocartilage & scapho-lunate ligament tears of wrist joint,31 ulnar collateral ligament injury in elbow joint32 and repaired meniscal evaluation of knee joint.33 MR arthrography can be used for evaluation of superficial chondral lesions, where the contrast material may increase the conspicuity of chondral surface defects.34 It can be a useful adjunct for evaluation, following cartilage repair.35 Role of MR arthrography is supplemental to conventional MR evaluation, depending upon the clinical query that needs to be answered and guide further management.36 Conventional T2/PD weighted MR sequences with bright synovial fluid can create a similar arthrogram-like effect, particularly in presence of significant synovial effusion.
MR arthrography has also been reported to be superior in detection of acetabular cartilage defects as compared with conventional MRI.37 Similar to MR, CT arthrography can also be used for evaluation of intra-articular hip pathologies including cartilage injury.38 In elbow, there is no significant difference in sensitivity and specificity of CT and MR arthrography in evaluation of cartilage lesions.39 There is a very small likelihood to identify an additional abnormality on MR arthrogram, if conventional 3.0 T MRI shoulder is normal.40
11. Future avenues
Cartilage imaging has evolved as newer MR techniques provide better morphologic visualization and quantification as well as insights in the cartilage composition. Standard MR morphological cartilage assessment used in current clinical practice depicts cartilage damage at stages when cartilage is irreversibly lost. The future goal of MR imaging would be to implement the MR compositional techniques to detect early cartilage degeneration at a reversible stage as depicted by promising tools, such as T1-rho, T2-relaxation mapping etc. into clinical practice.
Declaration of competing interest
We hereby declare that we have no conflict of interest.
Contributor Information
Ankur J. Shah, Email: drankur203@gmail.com.
Drushi Patel, Email: drushi43@yahoo.com.
References
- 1.Buckwalter J.A., Hunzinker E., Rosenberg L. In: Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, IL: American Academy of Orthopaedic Surgeons. Woo S.L.Y., Buckwalter J.A., editors. 1988. Articular cartilage: composition and structure; pp. 405–425. [Google Scholar]
- 2.Buckwalter J.A., Mankin H.J. Articular cartilage, part 1: tissue design and chondrocyte-matrix interaction. J Bone Joint Surg Am. 1997;79:600–611. [Google Scholar]
- 3.Newman A. Articular cartilage repair. Am J Sports Med. 1998;26:309–324. doi: 10.1177/03635465980260022701. [PubMed: 9548130] [DOI] [PubMed] [Google Scholar]
- 4.Sophia Fox Alicej, Bedi Asheesh, Scott Rodeo. The basics of Articular cartilage structure, composition and function. SPORTS HEALTH. 2009;1(6):461–468. doi: 10.1177/1941738109350438
. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patil Anupama, Jadhav Aniket. Imaging for cartilage injuries. Asian Journal of Arthroscopy. 2019 Jan - Apr;4(1):4–8. [Google Scholar]
- 6.Crema M.D., Roemer F.W., Marra M.D. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011 Jan-Feb;31:37–62. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun H.J., Gold G.E. Advanced MRI of articular cartilage. Imaging Med. 2011;3(5):541–555. doi: 10.2217/iim.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin D.A., Harner C.D., Costello J.M. Treatable chondral injuries in the knee: frequency of associated focal subchondral edema. AJR Am J Roentgenology. 2000;174 doi: 10.2214/ajr.174.4.1741099. 1099-106. [DOI] [PubMed] [Google Scholar]
- 9.Wheaton A.J., Casey F.L., Gougoutas A.J. Correlation of T1r with fixed charge density in cartilage. J Magn Reson Imag. 2004;20:519–525. doi: 10.1002/jmri.20148. [PubMed: 15332262] [DOI] [PubMed] [Google Scholar]
- 10.Mosher T.J., Dardzinski B.J. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Muscoskel Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [PubMed: 15643574] [DOI] [PubMed] [Google Scholar]
- 11.Smith H.E., Mosher T.J., Dardzinski B.J. Spatial variation in cartilage T2 of the knee. J Magn Reson Imag. 2001 Jul;14(1):50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 12.Regatte R.R., Akella S.V., Lonner J.H., Kneeland J.B., Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imag. 2006;23(4):547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 13.Zbyn S., Mlynarik V., Juras V., Szomolanyi P., Trattnig S. Evaluation of cartilage repair and osteoarthritis with sodium MRI. NMR Biomed. 2016 Feb;29(2):206–215. doi: 10.1002/nbm.3280. [DOI] [PubMed] [Google Scholar]
- 14.Omoumi P., Teixeira P., Delgado G., Chung C.B. Imaging of lower limb cartilage. Top Magn Reson Imag : TMRI. 2009;20(3):189–201. doi: 10.1097/RMR.0b013e3181d4426d. Pubmed. [DOI] [PubMed] [Google Scholar]
- 15.Casula Association between quantitative MRI and ICRS arthroscopic grading of articular cartilage. Knee Surg Sports Traumatol Arthrosc. 2016;24:2046–2054. doi: 10.1007/s00167-014-3286-9. [DOI] [PubMed] [Google Scholar]
- 16.Brittberg M., Winalski C. Evaluation of cartilage injuries and repair. The journal of bone and joint surgery JBJS. 2003 doi: 10.2106/00004623-200300002-00008. 85-2 · 20. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein J.D., Li J.G., Majumdar S., Henkelman R.M. Image resolution and signal-to-noise ratio requirements for MR imaging of degenerative cartilage. AJR. 1997;169:1089–1096. doi: 10.2214/ajr.169.4.9308470. [DOI] [PubMed] [Google Scholar]
- 18.Biswal S., Hastie T., Andriacchi T.P., Bergman G.A., Dillingham M.F., Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46:2884–2892. doi: 10.1002/art.10573. [DOI] [PubMed] [Google Scholar]
- 19.Stephens T., Diduch D.R., Balin J.I., Gaskin C.M. The cartilage black line sign: an unexpected MRI appearance of deep cartilage fissuring in three patients. Skeletal Radiol. 2011;40:113–116. doi: 10.1007/s00256-010-0994-1. [DOI] [PubMed] [Google Scholar]
- 20.Keegan Markhardt B., Kijowski Richard. The clinical significance of dark cartilage lesions identified on MRI. AJR. 2015;205:1251–1259. doi: 10.2214/AJR.15.14409. [DOI] [PubMed] [Google Scholar]
- 21.Schreiner M.M., Raudner M., Marlovits S. 2019. The MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 2.0 Knee Score and Atlas. [published online ahead of print, 2019 Aug 17]. Cartilage. 1947603519865308, PMID: 31422674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mithoefer K., Williams R.J., 3rd, Warren R.F. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–1920. doi: 10.2106/JBJS.D.02846. PMID: 16140804. [DOI] [PubMed] [Google Scholar]
- 23.Link T.M., Mischung J., Wörtler K., Burkart A., Rummeny E.J., Imhoff A.B. Normal and pathological MR findings in osteochondral autografts with longitudinal follow-up. Eur Radiol. 2006;16(1):88–96. doi: 10.1007/s00330-005-2818-6. PMID : 16021456. [DOI] [PubMed] [Google Scholar]
- 24.Gorbachova T., Ian Amber, Beckmann N. Nomenclature of subchondral non neoplastic bone lesions. AJR. 2019;213:963–982. doi: 10.2214/AJR.19.21571. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor M.A., Palaniappan M., Khan N., Bruce C.E. Osteochondritis dissecans of the knee in children. A comparison of MRI and arthroscopic findings. J Bone Joint Surg Br. 2002;84 doi: 10.1302/0301-620x.84b2.11823. 258-62. [DOI] [PubMed] [Google Scholar]
- 26.Bohndorf K. Osteochondritis (osteochondrosis) dissecans: a review and new MRI classification. Eur Radiol. 1998;8:103–112. doi: 10.1007/s003300050348. [DOI] [PubMed] [Google Scholar]
- 27.Guangyi Li et al. Subchondral Bone in Osteoarthritis: Insights into Risk Factors and Microstructural Changes. [DOI] [PMC free article] [PubMed]
- 28.Kho J., Azzopardi C., Davies A.M. Direct MR arthrography of the shoulder: current practice in the UK. Radiol med. 2020;125:605–608. doi: 10.1007/s11547-020-01144-8. [DOI] [PubMed] [Google Scholar]
- 29.Czerny C., Hofmann S., Neuhold A. Lesions of the acetabular labrum: accuracy of MR imaging and MR arthrography in detection and staging. Radiology. 1996;200(1):225–230. doi: 10.1148/radiology.200.1.8657916. [DOI] [PubMed] [Google Scholar]
- 30.Jayakar R., Merz A., Plotkin B. Magnetic resonance arthrography and the prevalence of acetabular labral tears in patients 50 years of age and older. Skeletal Radiol. 2016;45(8):1061–1167. doi: 10.1007/s00256-016-2392-9. [DOI] [PubMed] [Google Scholar]
- 31.LiMarzi G.M., O'Dell M.C., Scherer K. Magnetic resonance arthrography of the wrist and elbow. Magn Reson Imag Clin N Am. 2015;23(3):441–455. doi: 10.1016/j.mric.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Delport A.G., Zoga A.C. MR and CT arthrography of the elbow. Semin Muscoskel Radiol. 2012;16(1):15–26. doi: 10.1055/s-0032-1304298. [DOI] [PubMed] [Google Scholar]
- 33.Cardello P., Gigli C., Ricci A. Retears of postoperative knee meniscus: findings on magnetic resonance imaging (MRI) and magnetic resonance arthrography (MRA) by using low and high field magnets. Skeletal Radiol. 2009;38(2):149–156. doi: 10.1007/s00256-008-0600-y. [DOI] [PubMed] [Google Scholar]
- 34.Paunipagar B.K., Rasalkar D. Imaging of articular cartilage. Indian J Radiol Imag. 2014 Jul;24(3):237–248. doi: 10.4103/0971-3026.137028. PMID: 25114386; PMCID: PMC4126138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvi M., Curti M., Ossola C. Knee articular cartilage injury treatment with matrix-induced autologous chondrocyte implantation (MACI): correlation at 24 and 120 months between clinical and radiological findings using MR arthrography. Skeletal Radiol. 2021 Oct;50(10):2079–2090. doi: 10.1007/s00256-021-03775-y. Epub 2021 Apr 15. PMID: 33855594; PMCID: PMC8364544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grainger A.J., Elliott J.M., Campbell R.S., Tirman P.F., Steinbach L.S., Genant H.K. Direct MR arthrography: a review of current use. Clin Radiol. 2000 Mar;55(3):163–176. doi: 10.1053/crad.1999.0374. PMID: 10708607. [DOI] [PubMed] [Google Scholar]
- 37.Sutter R., Zubler V., Hoffmann A. Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage. AJR Am J Roentgenol. 2014 Jan;202(1):160–169. doi: 10.2214/AJR.12.10266. [DOI] [PubMed] [Google Scholar]
- 38.Christie-Large M., Tapp M.J., Theivendran K., James S.L. The role of multidetector CT arthrography in the investigation of suspected intra-articular hip pathology. Br J Radiol. 2010 Oct;83(994):861–867. doi: 10.1259/bjr/76751715. Epub 2010 Aug 17. PMID: 20716653; PMCID: PMC3473755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldt S., Bruegel M., Ganter K. Comparison of multislice CT arthrography and MR arthrography for the detection of articular cartilage lesions of the elbow. Eur Radiol. 2005 Apr;15(4):784–791. doi: 10.1007/s00330-004-2585-9. Epub 2005 Feb 9. PMID: 15702339. [DOI] [PubMed] [Google Scholar]
- 40.Magee T. Utility of pre- and post-MR arthrogram imaging of the shoulder: effect on patient care. Br J Radiol. 2016;89(1062):20160028. doi: 10.1259/bjr.20160028. [DOI] [PMC free article] [PubMed] [Google Scholar]