Abstract
Background: The COVID-19 pandemic urges for cheap, reliable, and rapid technologies for disinfection and decontamination. One frequently proposed method is ultraviolet (UV)-C irradiation. UV-C doses necessary to achieve inactivation of high-titre SARS-CoV-2 are poorly defined.
Aim: We investigated whether short exposure of SARS-CoV-2 to UV-C irradiation sufficiently reduces viral infectivity and doses necessary to achieve an at least 6-log reduction in viral titres.
Methods: Using a box and two handheld systems designed to decontaminate objects and surfaces, we evaluated the efficacy of 254 nm UV-C treatment to inactivate surface dried high-titre SARS-CoV-2.
Results: Drying for 2 hours did not have a major impact on the infectivity of SARS-CoV-2, indicating that exhaled virus in droplets or aerosols stays infectious on surfaces for at least a certain amount of time. Short exposure of high titre surface dried virus (3–5*10^6 IU/ml) with UV-C light (16 mJ/cm2) resulted in a total inactivation of SARS-CoV-2. Dose-dependency experiments revealed that 3.5 mJ/cm2 were still effective to achieve a > 6-log reduction in viral titres, whereas 1.75 mJ/cm2 lowered infectivity only by one order of magnitude.
Conclusions: SARS-CoV-2 is rapidly inactivated by relatively low doses of UV-C irradiation and the relationship between UV-C dose and log-viral titre reduction of surface residing SARS-CoV-2 is nonlinear. Our findings emphasize that it is necessary to assure sufficient and complete exposure of all relevant areas by integrated UV-C doses of at least 3.5 mJ/cm2 at 254 nm. Altogether, UV-C treatment is an effective non-chemical option to decontaminate surfaces from high-titre infectious SARS-CoV-2.
Keywords: SARS-CoV-2, UV-C irradiation, decontamination, disinfection, infection control, COVID-19
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has spread globally since January 2020 and there is an urgent need for rapid, highly efficient, environmentally friendly, and non-chemical disinfection procedures. Application of ultraviolet (UV)-C light is an established technology for decontamination of surfaces and aerosols [1-3]. This procedure has proven effective to inactivate SARS-CoV-1 [4-6], several other enveloped and non-enveloped viruses as well as bacteria [7]. UV-C-based disinfection could be applied in operating rooms and healthcare facilities and it also proved useful in the business sector, where it is necessary to sterilise surfaces frequently touched by multiple individuals. Some examples discussed in the context of public health are escalators, public transportation, rental cars, door handles and waiting rooms.
Recently, it has also been shown that SARS-CoV-2 is sensitive to inactivation by UV-C irradiation [8-12]. However, some of the studies used high UV-C doses from 108 mJ/cm2 to more than 1 J/cm2 at exposure times from 50 s to several minutes for total inactivation of SARS-CoV-2 [10-12]. These parameters are in a range complicating efficient application of UV-based methods for large-scale decontamination of surfaces and aerosols. Other studies used innovative 222 nm or 280 nm UV-C light-emitting diode (LED) technologies [8,9] which are not yet implemented in most established 254 nm UV-C-based decontamination devices and needed relatively high doses of UV-C irradiation for inactivation. Another recent study by Storm et al. established 254 nm UV-C dose-dependency inactivation kinetics of SARS-CoV-2 and reported doses necessary for complete sterilisation of dry and wet virus preparations between 4 s and 9 s at 0.85 mW/cm2 in a test box [13]. While these data are promising, a limitation of the study design was the use of a test box and relatively low viral titres which allowed for only 2- to 3- log titre reductions by the treatment.
The exact knowledge about dose-dependent inactivation kinetics is essential to design UV-C-based decontamination procedures that allow definite disinfection of SARS-CoV-2. We hence conducted an approach simulating the inactivation of dried surface residing high-titre infectious SARS-CoV-2 by two mobile handheld (HH) UV-C emitting devices and an UV-C box designed to decontaminate medium-size objects. We investigated whether short exposure of SARS-CoV-2 to UV-C irradiation is sufficient to reduce viral infectivity and which UV-C doses are necessary to achieve an at least 6-log reduction in viral titres.
Methods
Cell culture
Caco-2 (human colorectal adenocarcinoma) cells were cultured at 37 °C with 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal calf serum (FCS), with 2 mM l-glutamine, 100 μg/ml penicillin-streptomycin and 1% non-essential amino acids (NEAA).
Viruses
The recombinant SARS-CoV-2 expressing mNeonGreen (icSARS-CoV-2-mNG) [14] was obtained from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) at the University of Texas Medical Branch (UTMB, Galveston, United States (US)). To generate icSARS-CoV-2-mNG stocks, 200,000 Caco-2 cells were infected with 50 μl of virus stock in a 6-well plate, the supernatant was harvested 48 hours post infection (hpi), centrifuged, and stored at -80 °C. For multiplicity of infection (MOI) determination, a titration using serial dilutions of the virus stock was conducted. The number of infectious virus particles per ml was calculated as the (MOI × cell number)∕(infection volume), where MOI = − ln(1 − infection rate).
Ultraviolet-C light inactivation treatment
A total of 35 μl of virus stock, corresponding to ca 4–6*106 infectious units (IU) of icSARS-CoV-2-mNG were spotted (in triplicates) in 6-well plates and dried for 2 hours at room temperature (RT). This setup was chosen to mimic the situation in which an infected person exhales droplets that dry on surfaces and potentially stay infectious and hazardous over a prolonged period of time. Six-well plates spotted with dried virus were treated with UV-C-light (254 nm) using the Soluva pro UV Disinfection Chamber (Heraeus, Hanau, Germany) for 60 s or the Soluva Zone HP Disinfection Handheld (Heraeus) for 2 s in a fixed regime at 5 and 20 cm plate distance. In addition, a moving regime using slow (3.75 cm/s) and fast (12 cm/s) speed at 20 cm distance was tested.
We also employed a second-generation Disinfection Handheld Soluva Zone H (Heraeus) which is less powerful than the Soluva pro UV but works autonomously with a rechargeable battery. The spectrum of UV-C lamps employed in these devices are shown in Supplemental figure 1. The lower UV-C intensity emitted by this device allowed us to perform a dose-dependency experiment exposing dried virus with different UV-C intensities. The time-dependent UV-C intensity emitted by the Soluva Zone H at various distances is detailed and depicted in Supplemental figure 2. UV-C exposure was carried out after 10 min of pre-heating the device at a distance of 50 cm for 20 s, 10 s, 5 s, 2.5 s, 20 s + 97% UV-filter, 10 s + 97% UV-filter corresponding to 14 mJ/cm2, 7 mJ/cm2, 3.5 mJ/cm2, 1.75 mJ/cm2, 0.42 mJ/cm2 and 0.21 mJ/cm2. These values are based on an on-site and parallel measurement of UV-C intensity emitted by the device via an UV-C dosimeter (Dr Gröbel UV electronic GmbH, Ettlingen, Germany), which corresponds to 0.7 mJ/cm2 when the UV-C light is applied at 50 cm distance, and fits well to the previously company-measured value of 0.84 mJ/cm2 (Supplemental figure 2).
As control, 6-well plates were spotted with the virus and dried, but not UV-C treated. After UV-C treatment, the spotted virus was reconstituted using 1 ml of infection media (culture media with 5% FCS) and viral titres determined as explained below. As additional control, 35 μl of the original virus stock were diluted to 1 ml with infection media and used as virus stock infection control. All UV-C treatments were done at RT.
Evaluation of ultraviolet-C treatment
For infection experiments and titre determination, 1 x 104 Caco-2 cells/well were seeded in 96-well plates the day before infection. Cells were incubated with the SARS-CoV-2 strain icSARS-CoV-2-mNG at a MOI = 1.1 (stock) or the UV-C treated and reconstituted virus in serial twofold dilutions from 1:200 up to 1:51,200 and in one experiment up to 1:102,400. At 48 hpi, cells were fixed with 2% paraformaldehyde (PFA) and stained with Hoechst 33342 (Thermo Fisher, Waltham, US) (1 µg/ml final concentration) for 10 min at 37 °C. The staining solution was removed and exchanged for phosphate-buffered saline (PBS). For quantification of infection rates, images were taken with the Cytation3 (Biotek, Winooski, US) and Hoechst + and mNG + cells were automatically counted by the Gen5 Software (Biotek). Viral titres i.e. the number of infectious virus particles per ml, were calculated as the (MOI × cell number)∕(infection volume), where MOI = − ln(1 − infection rate). Infection rates lower than 0.01 were used as a cut-off and set to 0 in order to avoid false positive calculations.
Software and statistical analysis
Experiments were repeated two to four times each, using duplicate or triplicate infections. GraphPad Prism 8.0 was used for statistical analyses (one-way ANOVA with multiple comparison and Fishers least significant difference (LSD)-test) and to generate graphs, as well as CorelDrawX7. Other software used included Gen5 v.3.10.
Ethical statement
This study does not include any data obtained with primary patient cells or data. Hence, there was no necessity to obtain ethical approval by the internal review board.
Results
Inactivation of high-titre SARS-CoV-2 by ultraviolet-C treatment
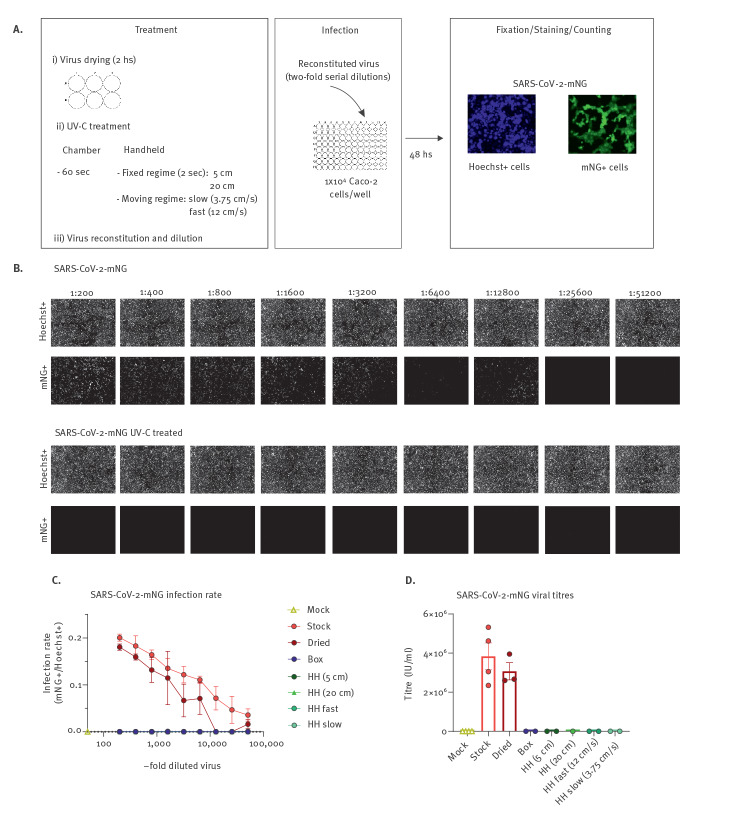
Simulating the situation in which exhaled droplets or aerosols from infected individuals contaminate surfaces, we produced a high-titre SARS-CoV-2 infectious stock and dried 35µl of this stock corresponding to ca 4–6*106 IU/ml in each well of a 6-well plate. The plates were then either non-treated or exposed to five UV-C regimens at 254 nm (Figure 1a). These include inactivation for 60 s in a box designed to disinfect medium-size objects, 2 s exposure at 5 cm or 20 cm distance with a HH UV-C disinfection device and an approach simulating decontamination of surfaces via the HH UV-C device (Zone HP). We performed this simulated HH decontamination in slow- and fast-moving speeds at a distance of ca 20 cm (Supplemental movie 1 and 2). UV-C irradiance (254 nm) in the box with an exposure time of 60 s corresponds to an irradiation dose of 600 mJ/cm2; for the HH device, at 5 cm the UV-C dose at 2 s irradiation time is 80 mJ/cm2 and at 20 cm is 16 mJ/cm2. From the speed of the slow and fast moving regimens we calculate a UV-C dose of 2.13 mJ/cm2 and 0.66 mJ/cm2, respectively, assuming a focused intensity beam. However, taking into consideration the UV-C light distribution underneath the HH device, the integrated UV-C dose accumulates to 20 mJ/cm2 for the fast regimen.
Figure 1.
Inactivation of SARS-CoV-2 by UV-C light treatment
HH: handheld; mNG: mNeonGreen; SARS-CoV-2: severe acute respiratory syndrome coronavirus; SEM: Standard error of the mean; UV: ultraviolet; IU: infectious units.
A. Experimental layout of the different UV-C treatments and the infection assay employed using the green-fluorescent virus SARS-CoV-2-mNG. B. Primary data showing the results of the infection assay using the non-treated stock virus as a positive control and the UV-C treated virus (HH, fast-moving regime). In the upper row, the total amount of cells for each well of the twofold serial dilution of virus is shown as Hoechst +. In the lower, infected cells are visualised indicated as mNG + cells. C. Infection rate curves for UV-C irradiated SARS-CoV-2-mNG using different UV-C treatments. The graph shows the infection rate at each twofold serial dilution, calculated as the number of infected cells (mNG +) over the total number of cells (Hoechst +) for the non-treated viral stock (n = 4), dried viral stock (n = 3), and dried and UV-C irradiated virus using five different UV-C treatments (n = 2). Data are presented as mean +/ − SEM with the number of biological replicates indicated above. D. SARS-CoV-2-mNG viral titres after UV-C treatment and reconstitution. The graph shows the viral titres calculated in IU/mL for the mock-infected, non-treated, and dried stock as well as the dried and UV-C irradiated virus under the different treatments. The number of biological replicates (n = 2–4) is directly plotted and indicated in 1c. Data are presented as mean +/ − SEM.
In the context of the moving fast regimen, even short UV-C treatment of the dried virus completely inactivated SARS-CoV-2. This was evident, as no infected cells were detected, when the dried virus was reconstituted in media and used to inoculate the naïve Caco-2 cells (Figure 1b). Titration of twofold series dilutions of the UV-C treated and non-treated control samples, as well as the freshly thawed strain as reference, revealed that (i) drying for 2 hours does not have a major impact on the infectivity of SARS-CoV-2 and (ii) all five UV-C treatment regimens effectively inactivate SARS-CoV-2 (Figure 1c). Calculation of viral titres based on the titration of the reconstituted virus stocks revealed a loss of titre because of drying from ca 4*106 to ca 3*106 IU/ml in this set of experiments and effective 6-log titre reduction of SARS-CoV-2 by all employed UV-C treatment regimens down to 16 mJ/cm2 (Figure 1d).
Dose-dependent ultraviolet-C mediated inactivation of SARS-CoV-2
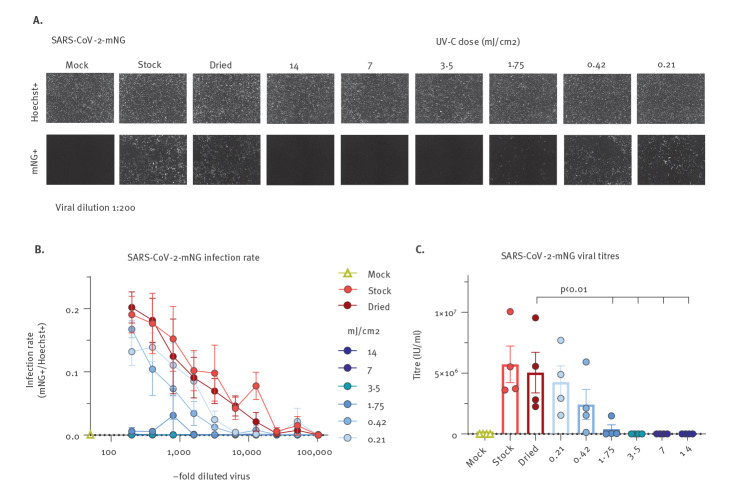
We next aimed to determine the UV-C doses at 254 nm that are sufficient to achieve complete disinfection with an at least 6-log reduction in viral titres. For this, we employed a battery-driven UV-C HH device (Zone H) emitting 254 nm UV-C light at 0.7 mJ/cm2 at a distance of 50 cm. This allowed us to treat surface dried SARS-CoV-2 with different UV-C doses by variation of the exposure time and additional use of a 97% UV-C filter. In agreement with our previous measurement, drying for 2 hours did not considerably affect SARS-CoV-2 infectivity and relatively high doses of 254 nm UV-C treatment (14 mJ/cm2) inactivated SARS-CoV-2 (Figure 2a exemplary images at 1:200 dilution and Figure 2b quantitative analyses). Furthermore, there was a dose-dependent reduction in SARS-CoV-2 infectivity with total inactivation down to 3.5 mJ/cm2 while partial inactivation was still observed at 1.75 mJ/cm2 (Figure 2a and b). Careful evaluation of viral titres post UV-C exposure revealed that > 6-log titre reduction was achieved by 3.5 mJ/cm2 254 nm UV-C treatment (Figure 2c). Of note, mean titres were only reduced by slightly more than one order of magnitude from 5.04*106 IU/ml of the dried and reconstituted SARS-CoV-2 to 3.5*105 IU/ml when the virus was exposed to 1.75 mJ/cm2, corresponding to 93% inactivation. Therefore, the relationship between inactivation of surface dried SARS-CoV-2 and UV-C treatment is nonlinear, at least in our system, and 3.5 mJ/cm2 are necessary to achieve a 6-log titre reduction.
Figure 2.
UV-C dose required for SARS-CoV-2 inactivation
ANOVA: analysis of variance; HH: handheld; LSD: least significant difference; mNG: mNeonGreen; SARS-CoV-2: severe acute respiratory syndrome coronavirus; SEM: Standard error of the mean; UV: ultraviolet; IU: infectious units.
A. Primary data showing the results of the infection assay using mock-infected cells, non-treated stock virus as a positive control, and virus treated with the 6 UV-C doses as indicated. In the upper row, the total amount of cells is shown as Hoechst +. In the lower, infected cells at a viral dilution of 1:200 are visualised indicated as mNG + cells. B. Infection rate curves for UV-C irradiated SARS-CoV-2-mNG using different UV-C doses. The graph shows the infection rate at each twofold serial dilution, calculated as the number of infected cells (mNG +) over the total number of cells (Hoechst +) for the non-treated viral stock, dried viral stock, and dried and UV-C irradiated virus using different UV-C-doses (n = 4). Data are presented as mean +/ − SEM with the number of biological replicates indicated above. C. SARS-CoV-2-mNG viral titres after UV-C treatment. The graph shows the viral titres calculated in IU/mL for the mock-infected, non-treated, and dried stock as well as the dried and UV-C irradiated virus under the different UV-C-doses. The number of biological replicates is n = 4. Data are presented as mean +/ − SEM. For analysis of statistical significance, we used a one-way ANOVA with multiple comparison and Fishers LSD-test.
Discussion
Disinfection of surfaces and aerosols by UV-C irradiation is an established, safe and non-chemical procedure used for the environmental control of pathogens [1-3,15]. UV-C treatment has proven effective against several viruses including SARS-CoV-1 [4-6] and other coronaviruses, i.e. canine coronaviruses [16]. Hence, as recently demonstrated by others [8-13] and now confirmed by our study, it was expected that SARS-CoV-2 can be inactivated by UV-C treatment.
One critical question is the suitability of this technology in a setting in which the exposure time of surfaces or aerosols should be kept as short as possible to allow for a realistic application, such as in rooms that need to be used frequently as operating rooms or lecture halls. In such settings, we assume that the virus is exhaled from an infected person by droplets and/or aerosols, dries on surfaces and hence represents a threat to non-infected individuals. We mimicked such a situation and first evaluated if surface dried SARS-CoV-2 is infectious. Drying for 2 hours, in agreement with previous work [13,17], did not result in a significant reduction of viral infectivity, indicating smear-infections could indeed play a role in the transmission of SARS-CoV-2. On the other hand, our virus-preparations are dried in cell culture pH-buffered medium containing FCS, which might stabilise viral particles. Hence, even though this is not the scope of the current study, it will be interesting to evaluate if longer drying or virus-preparations in PBS affect the environmental stability of SARS-CoV-2. Irrespective of the latter, UV-C-exposure of dried high-titre SARS-CoV-2 preparations containing ca 3–5*106 IU/ml after reconstitution resulted in a complete reduction of viral infectivity. In this context, it is noteworthy that we achieved a 6-log virus-titre reduction in a setting simulating surface disinfection with a moving handheld device. With our fast-moving protocol, the calculated integrated UV-C dose of 20 mJ/cm2 at 254 nm, was substantially lower than the previously reported 1,048 mJ/cm2 necessary to achieve a 6-log reduction in virus titres when exposing aqueous SARS-CoV-2 to UV-C [10]. In another study using a 222 nm UV-LED source, 3 mJ/cm2 lead to a 2.51-log (99.7%) reduction of infectious SARS-CoV-2 when irradiating for 30 s; however, inactivation did not increase with extended irradiation regimens up to 300 s [9]. In addition, 20 s deep-ultraviolet treatment at 280 nm corresponding to a dose of 75 mJ/cm2 reduced SARS-CoV-2 titre up to 3-logs [8]. Finally, Storm and colleagues reported a 2-log reduction of dried SARS-CoV-2 at 4 s with 0.85 mW/cm2 corresponding to 3.4 mJ/cm2 [13]. Of note, this value is highly similar to the dose of 3.5 mJ/cm2 calculated by us to be sufficient to achieve a > 6-log SARS-CoV-2 titre reduction when the virus is in a dried surface residing state (Figure 2). Comparing these values to other pathogens, SARS-CoV-2 seems particularly sensitive towards UV-C light. To achieve a 3-log titre reduction, 75–130 mJ/cm2 are necessary for adenovirus, 11–28 mJ/cm2 for poliovirus, and bacteria such as Bacillus subtilis require 18–61 mJ/cm2 [7].
Important limitations of UV-C-based disinfection procedures exist. First and most importantly, UV-C irradiation is harmful to humans because of the high energy of the germicidal lamps and exposure of skin or eyes must be avoided. This excludes decontamination of populated public spaces by UV-C. Furthermore, UV-C does not penetrate surfaces, hence for efficient disinfection, equal direct irradiation of all surfaces with a sufficient dose has to be assured. Our work highlights this aspect, as due to the nonlinear decay kinetic of the dose-response relationship, 3.5 mJ/cm2 will totally inactivate high viral titres, whereas a slightly reduced dose of 1.75 mJ/cm2 only achieves roughly one-log reduction (Figure 2c).
Apart from that, our study, as well as the research done by others [13], emphasises UV-C-based disinfection technologies as highly efficient to rapidly sterilise surfaces in different settings such as operating rooms, less-frequently populated areas in healthcare facilities and public transportation, as well as in research facilities. Ideally, applications should be performed in closed containers, precluding exposure of persons to UV-C radiation when sterilising small to medium-size objects. The use of UV-C lamps in air sterilisers would have a strong impact on public health and prevent exposure of the public to infectious aerosols. Currently, we do not know if SARS-CoV-2 in aerosols is inactivated by similar doses and the transferability of our results to viral aerosols might be limited. Nevertheless, our results may give a first indication on further use, even though dynamics and inactivation kinetics of virus in aerosols might differ. Hence, it is highly relevant and warranted to conduct studies to carefully determine UV-C doses necessary and sufficient for inactivation of SARS-CoV-2 in aerosols.
Conclusions
We established the effectiveness of UV-C treatment against SARS-CoV-2 in a setting designed to simulate close-to-reality conditions of decontamination. The easy, rapid and chemical-free application of UV-C treatment to inactivate SARS-CoV-2 and its high efficacy demonstrates the potential of this technology in a broad range of possible settings.
Acknowledgements
We are thankful to Jan Winderlich, Jasmin Zahn, Christoph Söller, und Anika Hofmann (Heraeus) for fruitful discussions and for providing UV-C lamp spectra and UV-C dose emission measurements on the Soluva Zone H.
Supplementary Data
Supplementary Data
Supplementary Data
Conflict of interest: Heraeus provided the devices and gave financial support for laboratory consumables. None of the authors received any remuneration or other financial gains. Heraeus was not involved in data interpretation or any decision to publish.
Authors’ contributions: NR and MS designed the experiments; NR performed the experiments with support from RB; NR, RB and MS analysed the data; NR and MS drafted the figures and wrote the manuscript; MS developed the manuscript to its final form; MS planned and supervised the study; all authors read, edited, and approved the final manuscript.
References
- 1. Walker CM, Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ Sci Technol. 2007;41(15):5460-5. 10.1021/es070056u [DOI] [PubMed] [Google Scholar]
- 2. Qureshi Z, Yassin MH. Role of ultraviolet (UV) disinfection in infection control and environmental cleaning. Infect Disord Drug Targets. 2013;13(3):191-5. 10.2174/1871526511313030007 [DOI] [PubMed] [Google Scholar]
- 3. Simmons S, Dale C, Holt J, Velasquez K, Stibich M. Role of Ultraviolet Disinfection in the Prevention of Surgical Site Infections. Adv Exp Med Biol. 2017;996:255-66. 10.1007/978-3-319-56017-5_21 [DOI] [PubMed] [Google Scholar]
- 4. Duan SM, Zhao XS, Wen RF, Huang JJ, Pi GH, Zhang SX, et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed Environ Sci. 2003;16(3):246-55. [PubMed] [Google Scholar]
- 5. Tsunetsugu-Yokota Y. Large-scale preparation of UV-inactivated SARS coronavirus virions for vaccine antigen. Methods Mol Biol. 2008;454:119-26. 10.1007/978-1-59745-181-9_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darnell ME, Subbarao K, Feinstone SM, Taylor DR. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. 2004;121(1):85-91. 10.1016/j.jviromet.2004.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malayeri AH, Mohseni M, Cairns B, Bolton J. Fluence (UV Dose) Required to Achieve Incremental Log Inactivation of Bacteria, Protozoa, Viruses and Algae. IUVA News. 2016;18:4-6. [Google Scholar]
- 8. Inagaki H, Saito A, Sugiyama H, Okabayashi T, Fujimoto S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerg Microbes Infect. 2020;9(1):1744-7. 10.1080/22221751.2020.1796529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitagawa H, Nomura T, Nazmul T, Omori K, Shigemoto N, Sakaguchi T, et al. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am J Infect Control. 2021;49(3):299-301. 10.1016/j.ajic.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heilingloh CS, Aufderhorst UW, Schipper L, Dittmer U, Witzke O, Yang D, et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am J Infect Control. 2020;48(10):1273-5. 10.1016/j.ajic.2020.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Criscuolo E, Diotti RA, Ferrarese R, Alippi C, Viscardi G, Signorelli C, et al. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg Microbes Infect. 2021;10(1):206-10. 10.1080/22221751.2021.1872354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabino CP, Sellera FP, Sales-Medina DF, Machado RRG, Durigon EL, Freitas-Junior LH, et al. UV-C (254 nm) lethal doses for SARS-CoV-2. Photodiagn Photodyn Ther. 2020;32:101995. 10.1016/j.pdpdt.2020.101995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Storm N, McKay LGA, Downs SN, Johnson RI, Birru D, de Samber M, et al. Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation. Sci Rep. 2020;10(1):22421. 10.1038/s41598-020-79600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xie X, Muruato A, Lokugamage KG, Narayanan K, Zhang X, Zou J, et al. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe. 2020;27(5):841-848.e3. 10.1016/j.chom.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weber DJ, Kanamori H, Rutala WA. ‘No touch’ technologies for environmental decontamination: focus on ultraviolet devices and hydrogen peroxide systems. Curr Opin Infect Dis. 2016;29(4):424-31. 10.1097/QCO.0000000000000284 [DOI] [PubMed] [Google Scholar]
- 16. Pratelli A. Canine coronavirus inactivation with physical and chemical agents. Vet J. 2008;177(1):71-9. 10.1016/j.tvjl.2007.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-7. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.