Abstract
Purpose:
Disease recurrence after radical cystectomy generally occurs within 2 years and has a poor prognosis. Less well defined are the outcomes in patients who experience a late recurrence (>3 years after radical cystectomy). We report our institutional experience with late recurrences and describe the relationships between time to recurrence, management strategies, and survival.
Materials and Methods:
The study cohort comprised 2,315 patients who underwent radical cystectomy for urothelial carcinoma at our center between 2000 and 2014, of whom 617 had a recurrence. Median follow-up for survivors was 2.6 years post-recurrence (IQR 0.95–4.5). For the study, we considered disease recurrence as recurrences outside the urinary tract. We compared baseline characteristics and post-recurrence management between those with recurrence ≤3 and >3 years after radical cystectomy.
Results:
58 patients with late recurrence had significantly lower consensus T stage and lower frequency of nodal involvement. The average 1-year bladder cancer death rate from the time of recurrence declined from 66% to 50% to 33% for patients with recurrence times of 6 months, 2 years, and 5 years after radical cystectomy, respectively. For patients who survived at least 1 year after recurrence, the estimated survival at 5 years after recurrence was 45% for late recurring patients and 21% for patients who had an early recurrence. Local consolidative therapy (metastasectomy or radiation) was more common in patients with late recurrence (19% vs. 3.6%, p < 0.0001), and cancer-specific survival in early-recurring patients was significantly worse than in late-recurring patients in the subset receiving local consolidation (p = 0.02).
Conclusions:
The prolonged lifespan of patients experiencing a late recurrence after radical cystectomy can be leveraged to individualize management. There is strong rationale for investigating the role of metastasectomy in the management of late recurrences.
Keywords: disease management, cystectomy, recurrence, urinary bladder neoplasms
Introduction
Disease recurrence following RC generally occurs within 2 years.1,2 Recurrence is thought to confer a universally poor prognosis, as evidenced by a median post-recurrence survival ranging from 3–10 months.3–5 Predictors of survival following recurrence include pathological stage, lymph node density, site of recurrence, and post-recurrence chemotherapy administration.3,5 Recurrence can occur as new tumors within the remnant urinary tract or as soft-tissue recurrences outside of the urinary tract. These are distinct entities and our study focuses only on recurrences outside of the urinary tract.
The characteristics of patients who experience a late recurrence (>3 years after RC) are not well defined. Existing studies have grouped late soft-tissue recurrences together with late urinary tract recurrences,4,6–8 limiting our understanding of late soft-tissue recurrences as a distinct clinical entity. Nonetheless, 3.9–12.2% of patients will experience a late recurrence and this may be associated with an improved post-recurrence survival.3,4,6–8 Late recurrence is thought to occur in those with more favorable clinicopathologic characteristics following RC, such as those with non-muscle-invasive or lymph node-negative disease.6,8 The implications of timing of recurrence for salvage treatments have not been well described.
In this study, we report our institutional experience with late soft-tissue recurrences after RC with the goal of describing the relationship of recurrence timing with post-recurrence survival and characterizing our post-recurrence management strategy.
Materials and Methods
After obtaining institutional review board approval, we identified a cohort of 863 patients who had undergone RC for urothelial carcinoma of the bladder between January 2000 and November 2014 and had a subsequent recurrence. These recurrences were identified from a prospective institutional RC database with 2,315 consecutive cases during this period. Patients were excluded for the following reasons: if they had known unresectable disease or metastases prior to RC (n=81); if they experienced a urinary tract recurrence only (new tumor within the remaining urinary system) (n=80); if they had a history of upper tract urothelial carcinoma (n=49); if there was longer than 6 months between start of preoperative chemotherapy and RC, indicating a non-standard course of treatment (n=16); if they underwent salvage RC after radiotherapy (n=9); or if they had a prior partial cystectomy (n=3). Patients were also excluded if the history of recurrence was uncertain due to other primary malignancies (n=2), or if perforation of the bladder occurred prior to RC (n=1), leaving a total of 617 patients for analysis. These 617 patients were considered to have a soft-tissue recurrence, defined as all suspected recurrences of urothelial carcinoma of the bladder outside of the urinary tract.
We aimed to determine whether disease characteristics or prior treatments affected cancer-specific survival differently based on the time interval between RC and development of the recurrence. Disease characteristics studied were consensus T stage (≤pT1, pT2, pT3, pT4), and histology (not otherwise specified, multiple variants, squamous, adenocarcinoma, micropapillary, nested, sarcomatoid, plasmacytoid, neuroendocrine, and other histology). The treatment factors studied were use of intravesical therapy, use of preoperative systemic chemotherapy, response to preoperative chemotherapy at time of surgery, use of adjuvant chemotherapy, and use of salvage chemotherapy. We created logistic regression models for the outcome of cancer-specific death at 1 year after first recurrence, adjusting for time from RC to first recurrence, each disease characteristic or treatment, and the interaction between time from cystectomy to recurrence and that characteristic. We tested for the significance of this association on the probability scale. If a significant interaction was found, we plotted the risk of death from bladder cancer at one year after recurrence over the time from cystectomy to recurrence separately for each level of the disease characteristic or treatment using locally-weighted scatterplot smoothing (Lowess). Only the histology categories of not otherwise specified, multiple variants, adenocarcinoma, micropapillary, squamous and, nested were included in the interaction analysis for histology due to the limited number of events for other histology types. We also aimed to investigate the natural history of late-recurring patients and present disease and treatment characteristics for these patients. All analyses were performed using Stata 15 (StataCorp, College Station, TX).
Results
Among the 617 patients with recurrence after RC, 515 patients died of bladder cancer, with 344 of those deaths occurring within 1 year of recurrence. We identified 58 patients with recurrences occurring more than 3 years after RC. Median follow-up for survivors was 2.6 years (IQR 0.95–4.5). Patients who had late recurrences had lower consensus T stage (p <0.0001) and were less likely to have had lymph node involvement (p = 0.0003) (Table 1). Recurrences in the lymph nodes, at multiple sites, or those occurring locally were the most common. There was some evidence that patients with early recurrence were more likely to have multiple initial recurrence sites (37%) than patients with late recurrence (22%), although this did not meet conventional levels of statistical significance (p = 0.095) (Table 2).
Table 1.
Patient and treatment characteristics, by early recurrence (≤ 3 years) and late recurrence (> 3 years). Data are presented as frequency (%) or median (quartiles).
| Early recurrence (n=559) | Late recurrence (n=58) | p Value | |
|---|---|---|---|
| Age at radical cystectomy (years) | 70 (62–77) | 68 (60–74) | 0.11 |
| Male sex | 420 (75%) | 48 (83%) | 0.3 |
| Type of urinary diversion: | 0.4 | ||
| Ileal conduit | 389 (70%) | 35 (60%) | |
| Orthotopic neobladder | 139 (25%) | 19 (33%) | |
| Continent cutaneous diversion | 28 (5.0%) | 4 (6.9%) | |
| None | 3 (0.5%) | 0 (0%) | |
| Use of intravesical therapies (n=616) | 172 (31%) | 26 (45%) | 0.038 |
| Use of preoperative chemotherapy | 182 (33%) | 7 (12%) | 0.001 |
| Radical cystectomy pathologic T stage <= T1 among those who received preoperative chemotherapy | 24 (13%) | 3 (43%) | 0.028 |
| Use of adjuvant chemotherapy (n=615) | 76 (14%) | 7 (12%) | 0.8 |
| Use of salvage chemotherapy (n=581) | 357 (67%) | 37 (73%) | 0.5 |
| Use of local consolidative therapies | 20 (3.6%) | 11 (19%) | <0.0001 |
Table 2.
Pathologic characteristics, by early recurrence (≤ 3 years) and late recurrence (> 3 years). Data are presented as frequency (%) or median (quartiles).
| Early recurrence (n=559) | Late recurrence (n=58) | p Value | |
|---|---|---|---|
| Consensus T stage: | <0.0001 | ||
|
| |||
| pTa | 3 (0.5%) | 0 (0%) | |
|
| |||
| pTis | 1 (0.2%) | 1 (1.7%) | |
|
| |||
| pT1 | 37 (6.6%) | 15 (26%) | |
|
| |||
| pT2 | 121 (22%) | 19 (33%) | |
|
| |||
| pT3 | 241 (43%) | 15 (26%) | |
|
| |||
| pT4 | 143 (26%) | 5 (8.6%) | |
|
| |||
| Unknown | 13 (2.3%) | 3 (5.2%) | |
|
| |||
|
| |||
| Tumor grade: | >0.9 | ||
|
| |||
| Low grade | 2 (0.4%) | 0 (0%) | |
|
| |||
| High grade | 526 (94%) | 54 (93%) | |
|
| |||
| Unknown | 31 (5.5%) | 4 (6.9%) | |
|
| |||
| Variant histology: | 0.087 | ||
|
| |||
| Not otherwise specified | 288 (52%) | 38 (66%) | |
|
| |||
| Multiple variants | 92 (16%) | 5 (8.6%) | |
|
| |||
| Squamous | 59 (11%) | 2 (3.4%) | |
|
| |||
| Adeno | 25 (4.5%) | 6 (10%) | |
|
| |||
| Micropapillary | 25 (4.5%) | 4 (6.9%) | |
|
| |||
| Nested | 19 (3.4%) | 2 (3.4%) | |
|
| |||
| Other | 18 (3.2%) | 0 (0%) | |
|
| |||
| Sarcomatoid | 17 (3.0%) | 0 (0%) | |
|
| |||
| Plasmacytoid | 10 (1.8%) | 1 (1.7%) | |
|
| |||
| Neuroendocrine | 6 (1.1%) | 0 (0%) | |
|
| |||
| Secondary muscle-invasive bladder cancers (n=543) | 145 (29%) | 15 (38%) | 0.2 |
|
| |||
| Lymph node involvement (n=594) | 237 (44%) | 11 (20%) | 0.0003 |
|
| |||
| Initial site of soft-tissue recurrence: | 0.095 | ||
|
| |||
| Bone | 63 (11%) | 5 (8.6%) | |
|
| |||
| Brain | 9 (1.6%) | 2 (3.4%) | |
|
| |||
| Liver | 16 (2.9%) | 5 (8.6%) | |
|
| |||
| Local | 71 (13%) | 10 (17%) | |
|
| |||
| Lung | 53 (9.5%) | 6 (10%) | |
|
| |||
| Lymph Node | 114 (20%) | 14 (24%) | |
|
| |||
| Multiple | 209 (37%) | 13 (22%) | |
|
| |||
| Number of mets sites (N=221)* | |||
| 2 | 139 (67%) | 11 (85%) | |
| 3 | 49 (24%) | 0 (0%) | |
| 4 | 14 (6.7%) | 2 (15%) | |
| 5 | 6 (2.9%) | 0 (0%) | |
|
| |||
| Other | 24 (4.3%) | 3 (5.2%) | |
The observed association between time to recurrence and subsequent cancer-specific mortality over the next year reflects the fact that those with a longer disease-free interval harbor less aggressive disease and have a lower risk of dying from bladder cancer (fig. 1). For example, an average patient who recurs at 6 months after surgery has a risk of approximately 66% of dying from bladder cancer within the following year, as compared to a risk of approximately 50% or 33% for patients recurring after 2 or 5 years after RC, respectively. The estimated conditional survival for patients alive 1 year after a late recurrence was 65% at 2 years (95% CI, 45–79) and 45% at 5 years (95% CI, 23–64, fig. 2). This was higher than the 1-year conditional survival in the early recurrence group: 48% at 2 years (95% CI, 41–55) and 21% at 5 years (95% CI, 15– 28).
Figure 1.
Risk of death from bladder cancer at 1 year after recurrence vs. timing of soft-tissue recurrence (solid line). The 95% confidence interval is indicated by dashed lines and the distribution of times from cystectomy to recurrence is shaded in gray. An average patient who recurs at 6 months after surgery has a risk of approximately 66% of dying from bladder cancer within the following year, as compared to a risk of approximately 50% or 33% for patients recurring after 2 or 5 years after RC, respectively. As indicated by the area under the right-skewed gray curve, most recurrences occur within 3 years of radical cystectomy.
Figure 2.
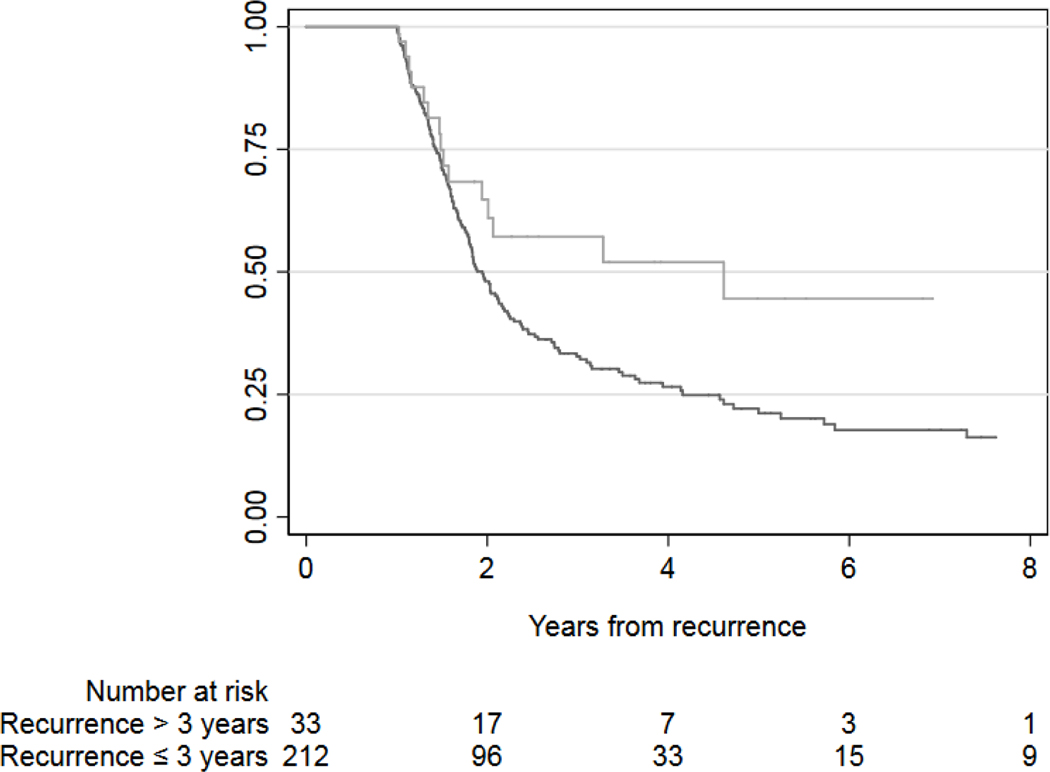
Kaplan-Meier survival estimates for death from bladder cancer among patients who survived at least one year after bladder cancer recurrence, separately for early-recurring (black line) and late-recurring (gray line) patients.
We examined whether the association between time from surgery to recurrence and risk of bladder cancer death at 1 year after recurrence differed based on disease characteristics. We found no evidence that this association differed by pathologic T stage (p = 0.2) or presence of variant histology (p = 0.5). We then tested whether type of treatment influenced the risk of bladder cancer death at 1 year. This association did not differ significantly between those who received intravesical therapy (p = 0.2) or preoperative (p >0.9) or adjuvant chemotherapy (p = 0.6). The association between time from surgery to recurrence and death from bladder cancer within 1 year of recurrence differed significantly between patients who received salvage chemotherapy and those who did not (p = 0.028). Patients who did not receive salvage chemotherapy had a higher risk of bladder cancer death, although this risk decreased as the time between surgery and recurrence increased. Patients who received salvage chemotherapy had a lower risk of bladder cancer death overall, with a small difference in risk between patients with early or late recurrences.
When comparing treatment modalities between early and late recurrences, patients with late recurrences were more likely to have received intravesical therapies (31% early recurrence vs. 45% late recurrence; p = 0.038) and less likely to have received preoperative chemotherapy (33% early recurrence vs. 12% late recurrence; p = 0.001). Among patients with late recurrences, 37 (73%) received salvage chemotherapy, which did not differ significantly from the 67% rate in early recurrences (p = 0.5). Most of these patients received one type of chemotherapy regimen (74%), although 14% received two types and 11% received 3 or more types. The median number of salvage chemotherapy cycles received over all regimens was 6 (quartiles 3–6). Sixteen patients with late recurrence (28%) received radiation therapy following recurrence. The purpose of radiation therapy was palliative in 13 patients (81%) and as part of local consolidation in 3 (19%).
A total of 11 (19%) patients received local consolidative therapy directed towards the recurrence in the late recurrence group. This was more frequent than in the early recurrence group where local consolidative therapy was used in 20 patients (3.6%, p <0.0001). All 11 patients with late recurrence undergoing local consolidation had a solitary site of recurrence and a Karnofsky performance status above 80% at the time of recurrence. Metastasectomy alone, radiation alone, and a combination of metastasectomy and radiation were utilized in 8, 2, and 1 patient(s) respectively. Salvage chemotherapy was also used in 6 patients receiving local consolidative therapy (55%). Similar to the overall cohort, those with a late recurrence receiving local consolidation had a longer median disease-specific survival time from recurrence than early recurrences receiving local consolidation (114 months vs. 57 months, p = 0.03, fig. 3). The 5-year cancer specific survival for patients with late recurrence receiving local consolidation was 89%, compared to 46% for patients with early recurrence.
Figure 3.
Kaplan-Meier survival estimates for death from bladder cancer among patients who had local consolidation, separately for early-recurring (black line) and late-recurring (gray line) patients.
Discussion
In our large single-institution cohort, we found that patients with a longer interval between RC and recurrence have improved post-recurrence survival compared to those who quickly relapse. We believe this observation reflects an inherently less-aggressive disease in those with a late recurrence but is also partly attributable to differences in post-recurrence management strategies. This lower-risk disease profile is evidenced by the lower pathologic stage and frequency of lymph node involvement at the time of RC in patients with late recurrences. This finding is expected, given that these conventional predictors of aggressive disease are based on survival analyses that estimate time to recurrence.1,2
Differences in treatment characteristics likely also contribute to the improved survival observed in those with late soft-tissue recurrences after radical cystectomy. Treatment differences were driven by our selective use of consolidative therapy to metastatic sites in those who experience a late recurrence, with one-fifth undergoing surgical resection or curative-intent radiation. Rates of salvage chemotherapy administration did not vary between groups.5,9 Metastasectomy has rarely been performed for early recurrences after radical cystectomy because of the poor prognosis of this disease state. Decision-making for local consolidation is highly individualized and our institutional selection criteria for metastasectomy reflect this: timing to recurrence, limited disease burden, complete resectability of the recurrence, acceptable morbidity of the proposed resection, response to systemic therapies, and an adequate performance status. These criteria are consistent with other reports on the subset of patients that enjoyed favorable outcomes after metastasectomy.10
Nearly one-fifth of patients in our late recurrence group received a metastasectomy or curative-intent radiotherapy. This was more commonly performed in this group because we consider late recurrence to be an indicator of a patient’s natural history of systemic disease progression. While representing a small and highly selected subgroup, certain patients who underwent consolidative therapy in our study had prolonged survival, with several patients surviving five years after therapy. Five-year cancer-specific survival following recurrence for patients receiving local consolidation was 89% for late recurrence vs. 46% early recurrence (fig. 3). Although underlying selection biases prevent us from concluding that consolidative therapy directly improved survival, the outcomes in this group warrant further study.
The favorable survival outcomes observed in our late recurrence group are consistent with prior series that have reported on predictors of post-recurrence survival. The University of Southern California group has reported a series of 447 non-urinary tract recurrences, focusing on predictors of recurrence and post-recurrence survival.3 Those with a time to recurrence of more than 1 year comprised 53% of their study cohort and had a median survival time of 7.82 months compared to 3.98 months for those recurring within 1 year. Conditional survival probabilities at other time points and the management of soft-tissue recurrences were not evaluated. Rink et al. reported a multi-institutional series of 1,545 patients who experienced disease recurrence after radical cystectomy.4 Due to a lack of data on anatomic site of recurrence, they were not able to look specifically at non-urinary tract recurrences, limiting comparison with our series. Nonetheless, the authors found that the adjusted risk of death within 1 year after disease recurrence decreased from 75% for a recurrence at 6 months to 57% for a recurrence at 36 months post-surgery.
Several series have also reported a more favorable clinicopathologic risk profile in late recurrences.6–8 Aimed at informing post-RC follow-up, these reports include secondary urinary tract tumors and provide neither a description of management strategies employed nor conditional survival following recurrence. Secondary urinary tract tumors commonly develop in the time period of late soft-tissue recurrences and comprise 12–59% of the study cohorts in prior studies.6–8 While consideration of these tumors may be relevant for postoperative surveillance, urinary tract recurrences do not inform clinical decision making for soft-tissue recurrences as these recurrences are managed distinctly and are associated with a favorable prognosis.11–13 As a result, we feel that the strength of our study is that it informs on the prognosis and management of a distinct clinical scenario that has not previously been well described.
Our study is limited by its retrospective nature, resulting in several biases. Firstly, we were unable to determine if late recurrences with a more unfavorable prognosis were less likely to seek follow-up at our center. Our median follow-up of 2.6 years in those free of recurrence suggests that most early recurrences are being captured in follow-up. Time to recurrence is influenced by several factors, most importantly the risk profile of an individual patient, and may not be an independent predictor of survival. Nonetheless, time to recurrence remains a clinically useful indicator that is readily available and can be leveraged for decision making. Finally, while we demonstrated favorable survival outcomes in those who underwent local consolidation, we are unable to make conclusions on the efficacy of this therapy. In clinical practice, the decision to proceed with consolidative surgery or radiotherapy is highly individualized and these factors are difficult to analyze in a retrospective study.
Future work will focus on clarifying the less aggressive disease biology of these late recurrences. Urothelial carcinoma has the 3rd highest mutation rate of any human cancer14, with 58 genes identified as significantly mutated.15 Our group has previously reported that PIK3CA mutations or loss of TP53 or CDKN2A predicted a better recurrence-free and cancer-specific survival after RC.16 Gene expression profiling has also revealed that there are distinct subtypes of bladder cancer that have a distinct prognosis15 and chemosensitivity.17 Building upon these data, we hypothesize that late recurrences are associated with distinct molecular characteristics which underlie their differences in disease biology. Understanding the molecular underpinnings of this disease state will be important in the eventual use of molecular biomarkers or genomically-directed treatment for these patients.
Conclusions
The favorable prognosis of a late recurrence after RC can be leveraged to individualize management in these patients. The favorable outcomes that we have observed among late soft-tissue recurrences treated with metastasectomy or curative-intent radiotherapy warrant further study.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Abbreviations and Acronyms
- NOS
not otherwise specified
- RC
radical cystectomy
References
- 1.Stein JP, Lieskovsky G, Cote R et al. : Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001; 19: 666. [DOI] [PubMed] [Google Scholar]
- 2.Bochner B, Kattan M, Vora K et al. : Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006; 24: 3967. [DOI] [PubMed] [Google Scholar]
- 3.Mitra AP, Quinn DI, Dorff TB et al. : Factors influencing post-recurrence survival in bladder cancer following radical cystectomy. BJU Int 2012; 109: 846. [DOI] [PubMed] [Google Scholar]
- 4.Rink M, Lee DJ, Kent M et al. : Predictors of cancer-specific mortality after disease recurrence following radical cystectomy. BJU Int 2013; 111. [DOI] [PubMed] [Google Scholar]
- 5.Bajorin DF, Dodd PM, Mazumdar M et al. : Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999; 17: 3173. [DOI] [PubMed] [Google Scholar]
- 6.Linder BJ, Boorjian SA, Hudolin T et al. : Late recurrence after radical cystectomy: patterns, risk factors and outcomes. J Urol 2014; 191: 1256. [DOI] [PubMed] [Google Scholar]
- 7.Solsona E, Iborra I, Rubio J et al. : Late oncological occurrences following radical cystectomy in patients with bladder cancer. Eur Urol 2003; 43: 489. [DOI] [PubMed] [Google Scholar]
- 8.Soria F, Moschini M, Wirth GJ et al. : Characterization of late recurrence after radical cystectomy in a large multicenter cohort of bladder cancer patients. Urology 2017. [DOI] [PubMed] [Google Scholar]
- 9.Apolo AB, Ostrovnaya I, Halabi S et al. : Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst 2013; 105: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abufaraj M, Dalbagni G, Daneshmand S et al. : The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gakis G, Black PC, Bochner BH et al. : Systematic review on the fate of the remnant urothelium after radical cystectomy. Eur Urol 2017; 71: 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitra AP, Alemozaffar M, Harris BN et al. : Outcomes after urothelial recurrence in bladder cancer patients undergoing radical cystectomy. Urology 2014; 84: 1420. [DOI] [PubMed] [Google Scholar]
- 13.Tran W, Serio AM, Raj GV et al. : Longitudinal risk of upper tract recurrence following radical cystectomy for urothelial cancer and the potential implications for long-term surveillance. J Urol 2008; 179: 96. [DOI] [PubMed] [Google Scholar]
- 14.Glaser AP, Fantini D, Shilatifard A et al. : The evolving genomic landscape of urothelial carcinoma. Nat Rev Urol 2017; 14: 215. [DOI] [PubMed] [Google Scholar]
- 15.Robertson AG, Kim J, Al-Ahmadie H et al. : Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017; 171: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim PH, Cha EK, Sfakianos JP et al. : Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. Eur Urol 2015; 67: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiler R, Ashab HAD, Erho N et al. : Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol 2017; 72: 544. [DOI] [PubMed] [Google Scholar]