Abstract
Dissemination of multidrug-resistant, particularly tigecycline-resistant, Acinetobacter baumannii is of critical importance, as tigecycline is considered a last-line antibiotic. Acquisition of tet(X), a tigecycline-inactivating enzyme mostly found in strains of animal origin, imparts tigecycline resistance to A. baumannii. Herein, we investigated the presence of tet(X) variants among 228 tigecycline-non-susceptible A. baumannii isolates from patients at a Taiwanese hospital via polymerase chain reaction using a newly designed universal primer pair. Seven strains (3%) carrying tet(X)-like genes were subjected to whole genome sequencing, revealing high DNA identity. Phylogenetic analysis based on the PFGE profile clustered the seven strains in a clade, which were thus considered outbreak strains. These strains, which were found to co-harbor the chromosome-encoded tet(X6) and the plasmid-encoded blaOXA-72 genes, showed a distinct genotype with an uncommon sequence type (Oxford ST793/Pasteur ST723) and a new capsular type (KL129). In conclusion, we identified an outbreak clone co-carrying tet(X6) and blaOXA-72 among a group of clinical A. baumannii isolates in Taiwan. To the best of our knowledge, this is the first description of tet(X6) in humans and the first report of a tet(X)-like gene in Taiwan. These findings identify the risk for the spread of tet(X6)-carrying tigecycline- and carbapenem-resistant A. baumannii in human healthcare settings.
Keywords: tigecycline, Acinetobacter baumannii, tet(X), tet(X6)
1. Introduction
The emergence of multidrug-resistant Gram-negative bacteria poses a serious threat to global health. Acinetobacter baumannii is a troublesome nosocomial pathogen that causes pneumonia, sepsis, and wound and urinary tract infections, and particularly leads to severe disease and death in intensive care unit (ICU) patients [1,2,3,4,5,6]. Tolerance to desiccation and evasion of host immunity, together with the notorious antimicrobial resistance of A. baumannii, confer an advantage for the environmental and in-host survival of this microorganism. The spread of multidrug-resistant A. baumannii (MDRAB) has increased rapidly, and A. baumannii strains resistant to carbapenem, which has been used to treat MDRAB infections, has emerged [7,8,9,10,11,12,13]. Colistin and tigecycline are the two last-resort antibiotic options for treatment of infections caused by carbapenem-resistant A. baumannii. However, cases of colistin- or tigecycline-resistant A. baumannii infections have been reported worldwide [14,15,16,17].
Tigecycline is a tetracycline family antibiotic that inhibits bacterial protein synthesis by interacting with the 30S ribosomal subunit and inhibiting tRNA entry [18]. Compared to classical tetracyclines, tigecycline exhibits a higher affinity for ribosomes. However, tigecycline-resistant bacteria have emerged with the increasing use of tigecycline [19].
The primary mechanisms of tigecycline resistance are associated with mutations in the ribosome that block drug binding or result from overexpression of efflux proteins that actively pump out the drug. For example, mutations in rpsJ, which encodes ribosomal protein S10, alter the tigecycline-binding site and thus contribute to tigecycline resistance [20]. Another resistance mechanism involves the increased expression of efflux pumps, such as OqxAB, AcrAB-TolC, and AdeABC [21,22,23]. In addition, Tet proteins, including the tigecycline-modifying enzyme tet(X), the ribosomal protective protein tet(M), and the mutated tet(A) efflux pump, have also been reported to decrease tigecycline susceptibility [24,25].
tet(X), a flavin-dependent monooxygenase, catalyzes the cleavage of tigecycline via an oxygen-dependent mechanism. The tet(X) gene was first identified in Tn4351 and Tn4400 transposons in Bacteroides fragilis [26,27]. Subsequently, tet(X) and its variants have been reported in other Bacteroides species, Enterobacteriaceae, and Acinetobacter strains from animals and humans. Two of these variants, tet(X3) and tet(X6), have been found on both chromosomes and plasmids. Three variants, tet(X), tet(X1), and tet(X2), are chromosomally encoded, whereas tet(X4) and tet(X5) are found in plasmids [28,29,30,31,32,33,34,35,36,37,38,39]. Additional variants, tet(X7) to tet(X13), have been detected in environmental and human gut metagenomes [40]. Recently, a tet(X) variant, tet(X14), was reported in the chromosome of an Empedobacter stercoris isolate from a pig fecal sample [41]. Although tet(X)-bearing bacteria have been reported in China, Africa, America, and Europe [42,43,44,45], indicating the widespread dissemination of these genes, the number of cases is low, and most of them are isolated from animals.
Since tet(X)-like genes could spread between species through horizontal gene transfer, surveillance of the prevalence and abundance of these genes is important. To the best of our knowledge, tet(X) variants have not been documented in Taiwan. Thus, we aimed to investigate 228 tigecycline-non-susceptible A. baumannii clinical isolates collected in Taiwan for the presence of tet(X) variants.
2. Results
2.1. Screening of tet(X) Variants
We screened 228 non-repetitive clinical tigecycline-non-susceptible A. baumannii isolates in Taiwan for the presence of tet(X) variants via polymerase chain reaction (PCR) using a universal primer pair designed in this study (described in the Materials and Methods section). The PCR and sequencing results indicated the presence of tet(X)-like genes in seven strains, with a positive rate of 3%. The sources of the seven strains were blood (n = 2), sputum (n = 2), tissue (n = 1), urine (n = 1), and pleural effusion (n = 1) samples (Table S1).
2.2. Antimicrobial Susceptibility of Strains Carrying tet(X) Variants
The seven tet(X) variant-harboring strains were not susceptible to ceftazidime, ciprofloxacin, cefoperazone/sulbactam, cefepime, imipenem, meropenem, ampicillin–sulbactam, tigecycline, and tazobactam, but were susceptible to amikacin and colistin (data not shown). We further determined that the minimum inhibitory concentration (MIC) of tigecycline for the seven strains was 4–8 mg/L (regarded as tigecycline-resistant) (Table 1).
Table 1.
MIC values of carbapenem-resistant Klebsiella pneumoniae 17CRE24, seven tigecycline-resistant A. baumannii strains, the recipient E. coli J53, and the transconjugants.
| Strain Name | Bacteria Species | Description | Carbapenem/Tigecycline Resistance Genes | MIC (mg/L) | |
|---|---|---|---|---|---|
| IMP | TIG | ||||
| J53 | E. coli | Recipient | - | 0.125 (S) | 0.125 (S) |
| 17CRE24 | K. pneumoniae | Donor | bla OXA-48 | >16 (R) | ND |
| J53-blaOXA-48 | E. coli | No. 1 transconjugant | bla OXA-48 | 8 (R) | ND |
| E. coli | No. 2 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 3 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 4 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 5 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 6 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 7 transconjugant | bla OXA-48 | >8 (R) | ND | |
| E. coli | No. 8 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 9 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 10 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 11 transconjugant | bla OXA-48 | 8 (R) | ND | |
| E. coli | No. 12 transconjugant | bla OXA-48 | 8 (R) | ND | |
| X4-65 | A. baumannii | Donor | tet(X6) | ND | 8 (R) |
| X4-107 | A. baumannii | Donor | tet(X6) | ND | 8 (R) |
| X4-136 | A. baumannii | Donor | tet(X6) | ND | 8 (R) |
| X4-201 | A. baumannii | Donor | tet(X6) | ND | 8 (R) |
| X4-300 | A. baumannii | Donor | tet(X6) | ND | 8 (R) |
| X4-584 | A. baumannii | Donor | tet(X6) | ND | 8 (R) |
| X4-705 | A. baumannii | Donor | tet(X6) | ND | 4 (R) |
Abbreviations: S, susceptible; R, resistant; ND, not determined; IMP, imipenem; TIG, tigecycline; MIC, minimum inhibitory concentration.
2.3. The Genomes of A. baumannii Isolates Carrying tet(X) Variants Are Highly Similar and Carry Two Plasmids
The genomes of the seven analyzed strains were almost identical (~100% identity and coverage) (BioProject ID: PRJNA672213; Accession Nos. CP064193–CP064204 and CP076736–CP076744), each comprising a circular chromosome and two plasmids of 112 kb and 9 kb. NCBI BLAST analysis showed that the chromosome shared high similarity with A. baumannii strain ab736 (Accession No. CP015121.1), which was isolated from a patient with bacteremia in the USA, and A. baumannii strain ZW85-1 (Accession No. CP006768) [46], which was isolated from the fecal sample of a patient with diarrhea in China (98.7% identity and 88% coverage for both isolates). Meanwhile, the best matches in the NCBI nucleotide database for the two plasmids were pCMCVTAb1-Ab59 [47] (Accession No. CP016299.1; 100% DNA identity and 98% coverage) and pAB-NCGM253 [48] (Accession No. AB823544; 100% DNA identity and coverage) for the 112 kb and 9 kb plasmids, which were obtained from clinical isolates in the USA and Japan, respectively.
2.4. Tigecycline-Resistant A. baumannii Isolates Carrying tet(X) Variants Also Carry Other Antimicrobial Resistance Genes
Antimicrobial resistance genes were identified using the Comprehensive Antibiotic Resistance Database (CARD). A total of 38 proteins exhibited >90% amino acid identity and coverage to proteins in the CARD database. We found a plasmid-encoded blaOXA-72 (blaOXA-24-like) carbapenemase gene (located in the 9 kb plasmid, which is almost identical to pAB-NCGM253, a common blaOXA-72-bearing plasmid found in several Acinetobacter spp. [49]) and a chromosome-encoded blaOXA-66 (blaOXA-51-like) carbapenemase gene (overexpression of blaOXA-51-like could confer carbapenem resistance), which may contribute to the carbapenem resistance of these isolates. The adc-56, a gene encoding extended-spectrum AmpC cephalosporinase, was found to confer cefepime resistance. In addition, genes encoding the aminoglycoside-modifying enzymes ANT(3′)-IIa, APH(3′)-Ib, APH(6)-Id, and APH(3′)-Ia; the chloramphenicol resistance gene floR; the sulfonamide resistance gene sul2; the tetracycline resistance genes tet(Y) and tet(X6); the abaQ gene encoding an efflux pump to mediate quinolone resistance; and the multidrug efflux pump-encoding genes such as ade were also identified.
2.5. The Tigecycline-Resistant A. baumannii Isolates Carry tet(X6) Genes
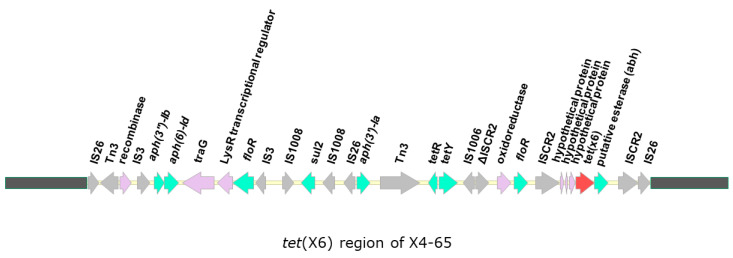
In all seven sequenced strains, a tet(X6) gene was identified in the chromosome and was located in a ~40 kb region that is absent in A. baumannii ab736 and ZW85-1 from the NCBI database, which shared high genome identity with the strains analyzed in this study (Figure 1). It is noteworthy that this 40 kb region flanked by two directly repeated IS26 sequences showed a higher G + C content (49.7%) than the rest of the chromosome (39%), indicating that this region may have originated from another source.
Figure 1.
Genetic organization of the region surrounding tet(X6). A ~40 kb region containing tet(X6) unique to the strains analyzed in this study but absent in ab736 and ZW85-1 are shown. The open reading frames are indicated by arrows. The tet(X6) is colored in red, and other genes are colored according to their annotated functions: Green, antimicrobial resistance; grey, mobile element; purple, other functions.
In addition to tet(X6), several other antibiotic resistance genes, including aph(3′)-Ib, aph(6)-Id, aph(3′)-Ia, floR, sul2, and tet(Y), were present in this region. Of note, several transposase-encoding genes were also identified, which suggests that transposition events occurred in this region and probably resulted in the accumulation of antimicrobial resistance genes. We further compared the genomic environments of previously reported tet(X6) genes in plasmids and chromosomes, including the sequences of plasmids from A. baumannii strain ABF9692 (plasmid pABF9692), Proteus cibarius strain ZN2, and the chromosomes of Proteus genospecies 6 strain T60, P. cibarius strain ZF1, P. cibarius strain 17SZRF8EW, P. mirabilis strain 18QD2AZ3W, A. lwoffii strain 18QD2AZ28W, Myroides phaeus strain 18QD1AZ29W, and A. baumannii strain X4-65 (this study) [35,38,50,51] (Figure 2). We found that, regardless of their location (plasmid or chromosome), tet(X6) were frequently associated with ISCR2, suggesting that ISCR2 may contribute to the transmission of tet(X6). In addition, tet(X6) was found on an SXT/R391 integrative and conjugative element (ICE) in the chromosome of Proteus genospecies 6 (T60), an isolate from retail pork (the authors named the novel ICE ICEPgs6Chn1) [38]. However, the genetic environment of tet(X6) in our current study was different from that of ICEPgs6Chn1, and we did not find a complete ICE in the strains analyzed in this study, as the ICE finder tool (https://db-mml.sjtu.edu.cn/ICEfinder/ICEfinder.html (accessed on 5 October 2021)) and oriTfinder tool (https://tool-mml.sjtu.edu.cn/oriTfinder/oriTfinder.html (accessed on 5 October 2021)) could not detect an integrase gene, relaxase gene, oriT, or type IV secretion system-encoding genes.
Figure 2.
Comparison of tet(X6)-containing regions in different strains. Open reading frames are shown as arrows. Comparative analysis of DNA identity was performed using Easyfig 2.2.2. The tet(X6) genes are colored in red, and the other genes are colored according to their annotated functions: Green, antimicrobial resistance genes; grey, ISCR2 or transposase-encoding genes; blue, other functions and hypothetical protein-encoding genes.
2.6. The A. baumannii Isolates Showed No Evidence of Conjugal Transfer of tet(X6)
Although we did not identify a complete ICE associated with tet(X6), we examined whether tet(X6) could be transferred to Escherichia coli by conjugation. To ensure that the conjugation experimental procedures were successful, we used a Klebsiella pneumoniae strain that was able to transfer the blaOXA-48 gene to E. coli as a control. The results showed that the control K. pneumoniae can successfully transfer the blaOXA-48 gene to E. coli, and the transconjugants (E. coli J53-blaOXA-48) exhibited a higher imipenem MIC of ≥8 mg/L compared to the recipient (E. coli J53), with an MIC of 0.125 mg/L. However, no transconjugant was obtained for the tet(X6)-harboring A. baumannii strains under the conditions used in this study (Table 1 and Figure S1).
2.7. K Type and Sequence-Tyype (ST) of the Tigecycline-Resistant A. baumannii Isolates Carrying tet(X6)
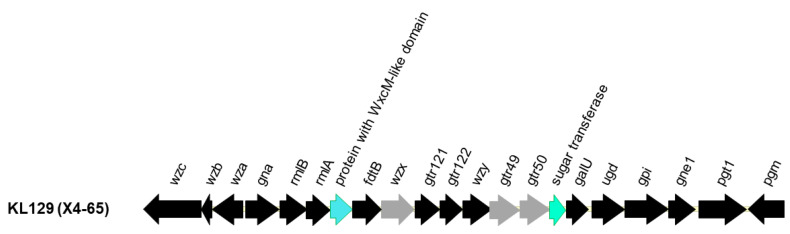
The capsular types (K-types) of the seven strains were determined using Kaptive, a tool that predicts the K-type of A. baumannii strains based on the sequences of the capsular polysaccharide synthesis (cps) locus [52]. The results showed that these strains belong to a new K type, which we designated as KL129 (Accession No. MW353360), that is related to KL60. Two genes differed between KL60 and KL129: A sugar transferase gene and a gene encoding a WxcM-like domain-containing protein (Figure 3). The sugar transferase ItrA2 in KL60 and the corresponding protein in KL129 shared 77% amino acid identity at 95% coverage, whereas the WxcM-like domain-containing protein FdtE in KL60 and the corresponding protein in KL129 shared 73% amino acid identity at 99% coverage. In addition, sequence variations were found in other proteins: Wzx shared 89% amino acid identity at 99% coverage, Gtr49 shared 94% amino acid identity at 99% coverage, and Gtr50 shared 92% amino acid identity at 99% coverage. We also determined the STs of the strains analyzed in this study based on the obtained genome sequences. The results showed that they belonged to Oxford ST793/CC208 (previously denoted as CC92) and Pasteur ST723/CC2, a clonal complex (CC) that corresponds to international clone II (Figure S2).
Figure 3.
Capsular polysaccharide synthesis (cps) gene clusters in KL129. Capsular polysaccharide synthesis (cps) locus of KL129, which was identified in this study, was compared with that of BAL_329, an A. baumannii strain with KL60 capsular type (Accession No. MN148382.1). Open reading frames are shown as arrows. Comparing the two cps loci, conserved genes that shared > 95% amino acid identity with KL60 are shown in black. Genes that share 80–95% amino acid identity with KL60 are shown in grey, and genes sharing < 80% amino acid identity with KL60 are shown in green or blue for the corresponding genes.
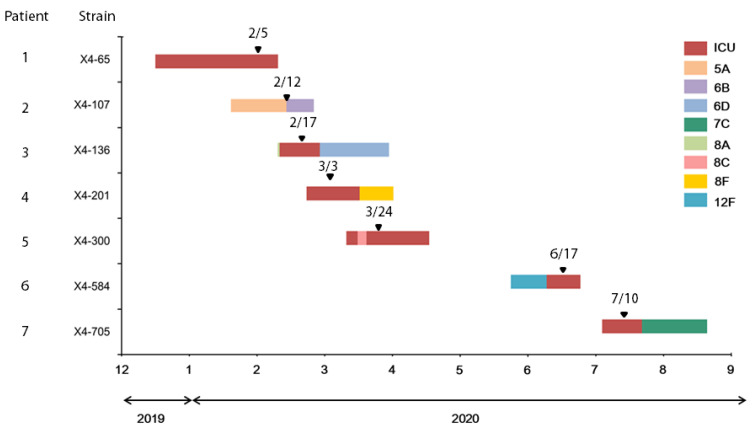
2.8. Nosocomial Spread of Tigecycline-Resistant A. baumannii Isolates Carrying tet(X6)
Six strains of tet(X6)-carrying A. baumannii were isolated from patients 1 and 3–7 in the same medical ICU; the last one (strain X4-107) was isolated from patient 2 in an orthopedic ward located on a separate floor of the same building (Figure 4 and Table S1). Patients 1, 3, and 5 had once been assigned to the same bed. The first strain (X4-65) was isolated (5 February 2020) from the sputum of patient 1 two months after admission to the ICU due to hepatic encephalopathy resulting from alcoholic liver cirrhosis. Patient 1 died of ventilator-associated pneumonia caused by tet(X6)-carrying A. baumannii three days later (8 February 2020). Strain X4-136 was isolated from patient 3, who was admitted on 10 February 2020, under the impression of pancreatitis with septic shock. The patient developed ventilator-associated pneumonia and central line-associated bloodstream infection caused by tet(X6)-carrying A. baumannii seven days later (17 February 2020). Approximately 52 days later, strain X4-300 was isolated from patient 5, who had hepatocellular carcinoma. The patient died of ventilator-associated pneumonia caused by tet(X6)-carrying A. baumannii. Strains from patients 4, 6, and 7, assigned to different beds in the same ICU, were isolated in March, June, and July, respectively. We presumed that this outbreak was caused by tet(X6)-carrying A. baumannii colonization in the environment of the medical ICU, where the healthcare worker(s) spread it to the orthopedic ward. We further performed in silico pulse field gel electrophoresis (PFGE) and constructed a phylogenetic tree based on the PFGE profile (Figure S4). The results showed that the seven strains collected in this study were clustered in a clade, indicating that these strains were clonally related.
Figure 4.
Medical history timeline of the patients with tet(X6)-carrying A. baumannii infection: Duration of hospital stay for each patient are shown. Intensive care unit is indicated in red, and general wards are shown in different colors representing different wards. The isolation time of tet(X6)-carrying A. baumannii was indicated by an arrowhead.
3. Discussion
Since the first tet(X) was found in B. fragilis [26,27], several other tet(X) variants have been reported in different bacterial species, including Acinetobacter spp. [28,29,30,31,32,33,34,35,36,37,38,39,40,41]. As previous studies have indicated, tet(X)-carrying bacteria were detected more frequently in animal sources than in human sources. In 2019, one study detected tet(X3)/tet(X4) in 6.9% (73/1060) of animal samples compared to 0.07% (4/5485) of human samples [37]. Among these tet(X3)/tet(X4)-harboring strains, one was A. baumannii. A tet(X4)-harboring A. baumannii strain was identified among a group of 76 tigecycline-resistant A. baumannii (~1.3% positive rate of tet(X)-like genes) in an analysis of 1273 A. baumannii isolates from humans [37]. Another clinical survey reported in 2020 detected two Acinetobacter spp. with tet(X3) or tet(X5) in a group of 103 tigecycline-resistant strains among 2591 Acinetobacter spp. [53], with a similar positive rate of ~1.9% in tigecycline-resistant Acinetobacter spp. In the current study, we designed a universal primer pair to detect tet(X)-like genes, including tet(X)–tet(X14), in 228 non-repetitive clinical isolates of tigecycline-non-susceptible A. baumannii, and the results showed that 3% of the collected strains have tet(X)-like genes. Interestingly, all seven strains carried tet(X6) genes and were the same clone (Oxford ST793/CC208, Pasteur ST723/CC2). The tet(X6)-carrying A. baumannii strains were identified as a new capsular type (designated as KL129). These strains belong to a clonal complex linked with international clone II, which is a widely distributed clone [54]. However, the tet(X6)-carrying A. baumannii strains in this study represent distinct genotypes with an uncommon ST and a new K-type compared to previously reported common STs of MDRAB: Pasteur ST2, Oxford ST208, common K-types KL2 and KL22, and other documented ST/K types [52,55,56]. Herein, we demonstrated the nosocomial dissemination of this clone and suggested that the main source of transmission is the ICU environment and healthcare workers.
The co-existence of tet(X)-like genes and other antibiotic resistance genes has been reported in different bacterial strains isolated from animals or their environments, posing a serious threat to the clinical treatment of humans. A study of E. coli strains from an animal source possessing both tet(X4) and the colistin resistance gene mcr-1 raised concerns, since tigecycline and colistin are regarded as last line drugs for the treatment of carbapenem-resistant bacteria [31]. A recent study documented an A. baumannii chicken isolate co-carrying a tet(X6) variant and the carbapenemase genes blaNDM-1 and blaOXA-58 [51]. Another study reported the co-occurrence of tet(X6) and the linezolid resistance gene cfr in Proteus spp. from swine farms [50]. In another study, Acinetobacter spp. harboring both tet(X) and blaOXA-58 were isolated from pigs [57]. In the current study, we found the co-carriage of carbapenemase gene blaOXA-72 and tet(X6) in A. baumannii strains isolated from patients. To the best of our knowledge, tet(X6) has been reported in the Myroides, Proteus, E. coli, Providencia rettgeri, and A. baumannii strains of animal origin [35,38,50,51,58], and this is the first description of tet(X6) in bacteria from human samples.
Although previous studies have reported that tet(X6) was associated with ICEs [38], we did not find a complete ICE in the region of the tet(X6) genes in the strains analyzed in this study. Concordantly, we failed to obtain tet(X)-containing transconjugants through conjugation, suggesting that other mechanisms, such as transformation, may be responsible for the transfer of tet(X)-containing DNA in the strains analyzed in this study. Of note, we found sequence similarity surrounding the genomic environments of reported tet(X6) genes in plasmids from A. baumannii and Proteus cibarius, and in the chromosomes of P. mirabilis, P. cibarius Proteus genospecies 6, A. lwoffii, Myroides phaeus, and A. baumannii (this study), suggesting that recombination events could occur between plasmids and chromosomes. Moreover, tet(X6) was associated with the presence of ISCR2, either at one or both ends, implying that ISCR2 could play a role in the transmission of tet(X6).
Taken together, we demonstrated the presence of tet(X6) together with the carbapenemase gene blaOXA-72 in clinical isolates of A. baumannii and reported an outbreak at a hospital in Taiwan. The findings revealed evidence of clonal spread of tet(X6)-carrying tigecycline- and carbapenem-resistant A. baumannii.
Even though it seems that tet(X6) is restricted to certain clones and has not widely spread to large numbers of A. baumannii clinical isolates, it poses a real threat to healthcare systems. To control its dissemination, further investigations on its prevalence and distribution should be undertaken at different hospitals.
4. Materials and Methods
4.1. Tigecycline-Non-Susceptible A. baumannii Isolate Collection
A total of 228 non-redundant (when repetitive samples from the same patient were isolated, only the first sample was included) tigecycline-non-susceptible A. baumannii isolates were collected at Chang Gung Memorial Hospital, Lin Kou branch, a 3700-bed medical center in northern Taiwan, from January to September 2020. The MIC of tigecycline was determined using the broth dilution method according to Clinical and Laboratory Standards Institute (CLSI) recommendations. Since CLSI does not suggest breakpoints for tigecycline against Acinetobacter spp., the results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) v8.1 criteria for Enterobacterales (strains with an MIC ≤ 1 mg/L were defined as susceptible; MIC >2 mg/L were defined as resistant) [59].
4.2. PCR Detection of tet(X) Variants
To detect tet(X) variants in the isolates, we analyzed the strains for 19 tet(X) variant sequences (Table S2 and Figure S3). A pair of universal primers was designed to detect the 15 tet(X) variants, i.e., tet(X)–tet(X14) (Table S3). The PCR cycling program consisted of 96 °C for 3 min, followed by 30 cycles of 96 °C for 30 s, 52 °C for 30 s, and 72 °C for 30 s. Products with an expected size of ~800 bp were subjected to Sanger sequencing.
4.3. Bacterial Genome Sequencing and Analysis
Bacterial genomic DNA was extracted with a commercial kit (QIAamp DNA Mini Kit, Qiagen, Valencia, CA, USA) and subjected to nanopore sequencing (Good Future BioMed Inc., Kwenshan, Taoyuan, Taiwan). The sequencing library was prepared with a Rapid Barcoding Sequencing Kit (SQK-RKK004; Oxford Nanopore Technologies, Oxford, UK) according to the manufacturer’s instructions. Sequencing was performed on the GridION platform, and FlowCell (R9.4.1 FLO-MIN106D; Oxford Nanopore) was used to generate raw signal data. Base calling of the raw signal data was performed by Guppy (v3.2.1) in the HAC mode. The adaptors remaining in the base-called reads were trimmed with Porechop (v0.2.4). The clean reads were assembled into chromosome or plasmid contigs using Flye (v2.7). Any sequencing errors in the genome and plasmid contigs were first polished by four runs of Racon (v1.4.3). The remaining errors were removed by Medaka (v1.0) and validated by in-house scripts searching against the EMBL-EBI cDNA database. The protein-coding genes and rRNAs in the chromosomes and plasmids were annotated using the Prokka pipeline (v1.14.6). To identify antibiotic-resistance genes, the annotated genes were searched against the CARD using Diamond (v0.9.36).
4.4. Multilocus Sequence Typing (MLST) Analysis
Two schemes for ST assignment were used. The Pasteur scheme of MLST relies on seven housekeeping genes (cpn60, gltA, recA, fusA, pyrG, rplB, and rpoB) [60], and the Oxford scheme relies on cpn60, gltA, recA, rpoD, gyrB, gdhB, and gpi [61]. The target genes were extracted from the genome and subjected to ST analysis (www.pasteur.fr/mlst (accessed on 5 October 2021).). The global optimal eBURST algorithm was used to define the major clonal complexes of the strains.
4.5. Conjugation Assay
The conjugation assay was performed as described previously [62]. To ensure that the conjugation experimental procedures were successful, we used a donor, carbapenem-resistant K. pneumoniae strain (17CRE24), which was able to transfer the blaOXA-48 gene to E. coli by conjugation, collected from Tung’s Taichung Metro Harbor Hospital, Taiwan, as a control [63]. Seven tigecycline-resistant A. baumannii strains were used as donors, and sodium azide-resistant E. coli J53 was used as the recipient. The donors and recipients were cultured overnight at 37 °C in LB broth supplemented with 2 mg/L of tigecycline (for the seven tigecycline-resistant A. baumannii strains), 4 mg/L of imipenem (for the control K. pneumoniae strain), or 100 mg/L of sodium azide (for the E. coli J53 recipient). The donor and recipient cells were mixed at a ratio of 1:10 (100 μL donor and 1 mL recipient) and centrifuged at 8000× g for 5 min. The small pellet was resuspended in ~3 μL of LB broth, dropped onto a nitrocellulose membrane on an LB agar plate, and incubated overnight. The nitrocellulose membrane was transferred to a tube containing fresh LB broth and incubated at 37 °C for 30 min with shaking. Transconjugants were selected on LB agar containing 100 mg/L of sodium azide and supplemented with 2 mg/L of tigecycline or 2 mg/L of imipenem. Transconjugants were further plated on eosin methylene blue (EMB) agar to confirm E. coli, which produces a green metallic sheen on EMB. Furthermore, the successful transfer of genes was confirmed via PCR using specific primers (Table S3). The MICs of the successful transconjugants were determined.
4.6. In Silico Analysis of PFGE
The complete genomes were explored using in silico PFGE patterns via AscI restriction digestion [64]. Phylogenetic trees were generated to compare genetic relatedness and clonal assignment using the Dice distance from band pattern and agglomeration using the ward.D2 method [65,66].
Acknowledgments
The authors thank Lii-Tzu Wu (Department of Microbiology and Immunology, School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan) for providing E. coli J53 and carbapenem-resistant K. pneumoniae 17CRE24.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10101239/s1, Table S1: the seven tet(X)-harboring strains and patients; Table S2: tet(X) variants included for sequence analysis, Table S3: Primer pairs used for PCR amplification, Figure S1: PCR confirmation of transconjugants, Figure S2: Clonal complexes of tigecycline-resistant A. baumannii isolates carrying tet(X6), Figure S3: Alignment of tet(X) variants and primer design, Figure S4: Phylogenetic tree of A. baumannii isolates based on PFGE profile.
Author Contributions
Conceptualization, Y.-C.H. and Y.-J.P.; methodology, Y.-J.P.; formal analysis, Y.-C.C., W.-C.L., S.-W.L., J.-W.W., Y.-Y.C. and T.L.T.Q.; data curation, Y.-Y.C. and T.L.T.Q.; writing—original draft preparation, Y.-C.H., Y.-J.P. and J.-W.W.; writing—review and editing, W.-C.L., S.-W.L., J.-W.W. and T.L.T.Q.; project administration, Y.-C.H. and Y.-J.P.; funding acquisition, Y.-C.H. and Y.-J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chang Gung Memorial Hospital, Taiwan (grant numbers CMRPG3K1011 and CMRPG3J0751-0752); the Ministry of Science and Technology, Taiwan (grant number MOST 110-2320-B-039-059); China Medical University, Taiwan (grant numbers CMU108-S-23, CMU109-MF-111, and CMU109-S-36).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Memorial Hospital (protocol code 201701896B0, Dec/24/2017).
Informed Consent Statement
Informed consent was obtained from all subjects who participated in the study.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ayobami O., Willrich N., Harder T., Okeke I.N., Eckmanns T., Markwart R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A systematic review and meta-analysis. Emerg. Microbes Infect. 2019;8:1747–1759. doi: 10.1080/22221751.2019.1698273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohd Sazlly Lim S., Zainal Abidin A., Liew S.M., Roberts J.A., Sime F.B. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: A systematic review and meta-analysis. J. Infect. 2019;79:593–600. doi: 10.1016/j.jinf.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Joly-Guillou M.L. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 2005;11:868–873. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 4.Jerassy Z., Yinnon A.M., Mazouz-Cohen S., Benenson S., Schlesinger Y., Rudensky B., Raveh D. Prospective hospital-wide studies of 505 patients with nosocomial bacteraemia in 1997 and 2002. J. Hosp. Infect. 2006;62:230–236. doi: 10.1016/j.jhin.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Wong D., Nielsen T.B., Bonomo R.A., Pantapalangkoor P., Luna B., Spellberg B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017;30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris F.C., Dexter C., Kostoulias X., Uddin M.I., Peleg A.Y. The Mechanisms of Disease Caused by Acinetobacter baumannii. Front. Microbiol. 2019;10:1601. doi: 10.3389/fmicb.2019.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathlouthi N., El Salabi A.A., Ben Jomaa-Jemili M., Bakour S., Al-Bayssari C., Zorgani A.A., Kraiema A., Elahmer O., Okdah L., Rolain J.M., et al. Early detection of metallo-beta-lactamase NDM-1- and OXA-23 carbapenemase-producing Acinetobacter baumannii in Libyan hospitals. Int. J. Antimicrob. Agents. 2016;48:46–50. doi: 10.1016/j.ijantimicag.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Munoz-Price L.S., Weinstein R.A. Acinetobacter infection. N. Engl. J. Med. 2008;358:1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 9.Su C.H., Wang J.T., Hsiung C.A., Chien L.J., Chi C.L., Yu H.T., Chang F.Y., Chang S.C. Increase of carbapenem-resistant Acinetobacter baumannii infection in acute care hospitals in Taiwan: Association with hospital antimicrobial usage. PLoS ONE. 2012;7:e37788. doi: 10.1371/journal.pone.0037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempf M., Rolain J.M. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: Clinical impact and therapeutic options. Int. J. Antimicrob. Agents. 2012;39:105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand X., Dowzicky M.J. Antimicrobial susceptibility among gram-negative isolates collected from intensive care units in North America, Europe, the Asia-Pacific Rim, Latin America, the Middle East, and Africa between 2004 and 2009 as part of the Tigecycline Evaluation and Surveillance Trial. Clin. Ther. 2012;34:124–137. doi: 10.1016/j.clinthera.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Kim U.J., Kim H.K., An J.H., Cho S.K., Park K.H., Jang H.C. Update on the Epidemiology, Treatment, and Outcomes of Carbapenem-resistant Acinetobacter infections. Chonnam Med. J. 2014;50:37–44. doi: 10.4068/cmj.2014.50.2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 14.Trebosc V., Gartenmann S., Totzl M., Lucchini V., Schellhorn B., Pieren M., Lociuro S., Gitzinger M., Tigges M., Bumann D., et al. Dissecting Colistin Resistance Mechanisms in Extensively Drug-Resistant Acinetobacter baumannii Clinical Isolates. mBio. 2019;10 doi: 10.1128/mBio.01083-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cafiso V., Stracquadanio S., Lo Verde F., Gabriele G., Mezzatesta M.L., Caio C., Pigola G., Ferro A., Stefani S. Colistin Resistant A. baumannii: Genomic and Transcriptomic Traits Acquired Under Colistin Therapy. Front. Microbiol. 2018;9:3195. doi: 10.3389/fmicb.2018.03195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakour S., Olaitan A.O., Ammari H., Touati A., Saoudi S., Saoudi K., Rolain J.M. Emergence of Colistin- and Carbapenem-Resistant Acinetobacter baumannii ST2 Clinical Isolate in Algeria: First Case Report. Microb. Drug Resist. 2015;21:279–285. doi: 10.1089/mdr.2014.0214. [DOI] [PubMed] [Google Scholar]
- 17.Costello S.E., Gales A.C., Morfin-Otero R., Jones R.N., Castanheira M. Mechanisms of Resistance, Clonal Expansion, and Increasing Prevalence of Acinetobacter baumannii Strains Displaying Elevated Tigecycline MIC Values in Latin America. Microb. Drug Resist. 2016;22:253–258. doi: 10.1089/mdr.2015.0168. [DOI] [PubMed] [Google Scholar]
- 18.Noskin G.A. Tigecycline: A new glycylcycline for treatment of serious infections. Clin. Infect. Dis. 2005;41((Suppl. 5)):S303–S314. doi: 10.1086/431672. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y., Cai Y., Liu X., Bai N., Liang B., Wang R. The emergence of clinical resistance to tigecycline. Int. J. Antimicrob. Agents. 2013;41:110–116. doi: 10.1016/j.ijantimicag.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Beabout K., Hammerstrom T.G., Perez A.M., Magalhaes B.F., Prater A.G., Clements T.P., Arias C.A., Saxer G., Shamoo Y. The ribosomal S10 protein is a general target for decreased tigecycline susceptibility. Antimicrob. Agents Chemother. 2015;59:5561–5566. doi: 10.1128/AAC.00547-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y., Hu D., Zhang Q., Liao X.P., Liu Y.H., Sun J. Efflux Pump Overexpression Contributes to Tigecycline Heteroresistance in Salmonella enterica serovar Typhimurium. Front. Cell Infect. Microbiol. 2017;7:37. doi: 10.3389/fcimb.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong X., Xu H., Chen D., Zhou H., Hu X., Cheng G. First emergence of acrAB and oqxAB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PLoS ONE. 2014;9:e115185. doi: 10.1371/journal.pone.0115185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruzin A., Keeney D., Bradford P.A. AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J. Antimicrob. Chemother. 2007;59:1001–1004. doi: 10.1093/jac/dkm058. [DOI] [PubMed] [Google Scholar]
- 24.Linkevicius M., Sandegren L., Andersson D.I. Potential of Tetracycline Resistance Proteins To Evolve Tigecycline Resistance. Antimicrob. Agents Chemother. 2016;60:789–796. doi: 10.1128/AAC.02465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore I.F., Hughes D.W., Wright G.D. Tigecycline is modified by the flavin-dependent monooxygenase TetX. Biochemistry. 2005;44:11829–11835. doi: 10.1021/bi0506066. [DOI] [PubMed] [Google Scholar]
- 26.Speer B.S., Salyers A.A. Characterization of a novel tetracycline resistance that functions only in aerobically grown Escherichia coli. J. Bacteriol. 1988;170:1423–1429. doi: 10.1128/jb.170.4.1423-1429.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park B.H., Levy S.B. The cryptic tetracycline resistance determinant on Tn4400 mediates tetracycline degradation as well as tetracycline efflux. Antimicrob. Agents Chemother. 1988;32:1797–1800. doi: 10.1128/AAC.32.12.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Y., Dong N., Zhang R., Liu C., Sun Q., Lu J., Shu L., Cheng Q., Chan E.W., Chen S. Emergence of an Empedobacter falsenii strain harbouring a tet(X)-variant-bearing novel plasmid conferring resistance to tigecycline. J. Antimicrob. Chemother. 2020;75:531–536. doi: 10.1093/jac/dkz489. [DOI] [PubMed] [Google Scholar]
- 29.Whittle G., Hund B.D., Shoemaker N.B., Salyers A.A. Characterization of the 13-kilobase ermF region of the Bacteroides conjugative transposon CTnDOT. Appl. Environ. Microbiol. 2001;67:3488–3495. doi: 10.1128/AEM.67.8.3488-3495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Liu D., Lv Y., Cui L., Li Y., Li T., Song H., Hao Y., Shen J., Wang Y., et al. Novel Plasmid-Mediated tet(X5) Gene Conferring Resistance to Tigecycline, Eravacycline, and Omadacycline in a Clinical Acinetobacter baumannii Isolate. Antimicrob. Agents Chemother. 2019;64 doi: 10.1128/AAC.01326-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J., Chen C., Cui C.Y., Zhang Y., Liu X., Cui Z.H., Ma X.Y., Feng Y., Fang L.X., Lian X.L., et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019;4:1457–1464. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun C., Cui M., Zhang S., Wang H., Song L., Zhang C., Zhao Q., Liu D., Wang Y., Shen J., et al. Plasmid-mediated tigecycline-resistant gene tet(X4) in Escherichia coli from food-producing animals, China, 2008-2018. Emerg. Microbes Infect. 2019;8:1524–1527. doi: 10.1080/22221751.2019.1678367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speer B.S., Bedzyk L., Salyers A.A. Evidence that a novel tetracycline resistance gene found on two Bacteroides transposons encodes an NADP-requiring oxidoreductase. J. Bacteriol. 1991;173:176–183. doi: 10.1128/jb.173.1.176-183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng K., Li R., He T., Liu Y., Wang Z. Characterization of a porcine Proteus cibarius strain co-harbouring tet(X6) and cfr. J. Antimicrob. Chemother. 2020;75:1652–1654. doi: 10.1093/jac/dkaa047. [DOI] [PubMed] [Google Scholar]
- 35.Liu D., Zhai W., Song H., Fu Y., Schwarz S., He T., Bai L., Wang Y., Walsh T.R., Shen J. Identification of the novel tigecycline resistance gene tet(X6) and its variants in Myroides, Acinetobacter and Proteus of food animal origin. J. Antimicrob. Chemother. 2020;75:1428–1431. doi: 10.1093/jac/dkaa037. [DOI] [PubMed] [Google Scholar]
- 36.Li R., Liu Z., Peng K., Liu Y., Xiao X., Wang Z. Co-occurrence of two tet(X) variants in an Empedobacter brevis of shrimp origin. Antimicrob. Agents Chemother. 2019 doi: 10.1128/AAC.01636-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He T., Wang R., Liu D., Walsh T.R., Zhang R., Lv Y., Ke Y., Ji Q., Wei R., Liu Z., et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019;4:1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 38.He D., Wang L., Zhao S., Liu L., Liu J., Hu G., Pan Y. A novel tigecycline resistance gene, tet(X6), on an SXT/R391 integrative and conjugative element in a Proteus genomospecies 6 isolate of retail meat origin. J. Antimicrob. Chemother. 2020;75:1159–1164. doi: 10.1093/jac/dkaa012. [DOI] [PubMed] [Google Scholar]
- 39.Cui C.Y., Chen C., Liu B.T., He Q., Wu X.T., Sun R.Y., Zhang Y., Cui Z.H., Guo W.Y., Jia Q.L., et al. Co-occurrence of Plasmid-Mediated Tigecycline and Carbapenem Resistance in Acinetobacter spp. from Waterfowls and Their Neighboring Environment. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.02502-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gasparrini A.J., Markley J.L., Kumar H., Wang B., Fang L., Irum S., Symister C.T., Wallace M., Burnham C.D., Andleeb S., et al. Tetracycline-inactivating enzymes from environmental, human commensal, and pathogenic bacteria cause broad-spectrum tetracycline resistance. Commun. Biol. 2020;3:241. doi: 10.1038/s42003-020-0966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng Y., Chen Y., Liu Y., Guo Y., Zhou Y., Xiao T., Zhang S., Xu H., Chen Y., Shan T., et al. Identification of novel tetracycline resistance gene tet(X14) and its co-occurrence with tet(X2) in a tigecycline-resistant and colistin-resistant Empedobacter stercoris. Emerg. Microbes Infect. 2020;9:1843–1852. doi: 10.1080/22221751.2020.1803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leski T.A., Bangura U., Jimmy D.H., Ansumana R., Lizewski S.E., Stenger D.A., Taitt C.R., Vora G.J. Multidrug-resistant tet(X)-containing hospital isolates in Sierra Leone. Int. J. Antimicrob. Agents. 2013;42:83–86. doi: 10.1016/j.ijantimicag.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Eitel Z., Soki J., Urban E., Nagy E., ESCMID Study Group on Anaerobic Infection The prevalence of antibiotic resistance genes in Bacteroides fragilis group strains isolated in different European countries. Anaerobe. 2013;21:43–49. doi: 10.1016/j.anaerobe.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Deng M., Zhu M.H., Li J.J., Bi S., Sheng Z.K., Hu F.S., Zhang J.J., Chen W., Xue X.W., Sheng J.F., et al. Molecular epidemiology and mechanisms of tigecycline resistance in clinical isolates of Acinetobacter baumannii from a Chinese university hospital. Antimicrob. Agents Chemother. 2014;58:297–303. doi: 10.1128/AAC.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bird K., Boopathy R., Nathaniel R., LaFleur G. Water pollution and observation of acquired antibiotic resistance in Bayou Lafourche, a major drinking water source in Southeast Louisiana, USA. Environ. Sci. Pollut Res. Int. 2019;26:34220–34232. doi: 10.1007/s11356-018-4008-5. [DOI] [PubMed] [Google Scholar]
- 46.Wang X., Zhang Z., Hao Q., Wu J., Xiao J., Jing H. Complete Genome Sequence of Acinetobacter baumannii ZW85-1. Genome Announc. 2014;2 doi: 10.1128/genomeA.01083-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao J., Susanti D., Childress J.C., Mitkos M.C., Brima J.K., Baffoe-Bonnie A.W., Pearce S.N., Grgurich D., Fernandez-Cotarelo M.J., Kerkering T.M., et al. Tn2008-driven carbapenem resistance in Acinetobacter baumannii isolates from a period of increased incidence of infections in a Southwest Virginia hospital (USA) J. Glob. Antimicrob. Resist. 2018;12:79–87. doi: 10.1016/j.jgar.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Tada T., Miyoshi-Akiyama T., Shimada K., Shimojima M., Kirikae T. Dissemination of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and emergence of OXA-72 carbapenemase coproducers in Japan. Antimicrob. Agents Chemother. 2014;58:2916–2920. doi: 10.1128/AAC.01212-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen F.J., Huang W.C., Liao Y.C., Wang H.Y., Lai J.F., Kuo S.C., Lauderdale T.L., Sytwu H.K. Molecular Epidemiology of Emerging Carbapenem Resistance in Acinetobacter nosocomialis and Acinetobacter pittii in Taiwan, 2010 to 2014. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02007-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li R., Peng K., Li Y., Liu Y., Wang Z. Exploring tet(X)-bearing tigecycline-resistant bacteria of swine farming environments. Sci. Total Environ. 2020;733:139306. doi: 10.1016/j.scitotenv.2020.139306. [DOI] [PubMed] [Google Scholar]
- 51.Zheng X.R., Zhu J.H., Zhang J., Cai P., Sun Y.H., Chang M.X., Fang L.X., Sun J., Jiang H.X. A novel plasmid-borne tet(X6) variant co-existing with blaNDM-1 and blaOXA-58 in a chicken Acinetobacter baumannii isolate. J. Antimicrob. Chemother. 2020;75:3397–3399. doi: 10.1093/jac/dkaa342. [DOI] [PubMed] [Google Scholar]
- 52.Wyres K.L., Cahill S.M., Holt K.E., Hall R.M., Kenyon J.J. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb. Genom. 2020;6 doi: 10.1099/mgen.0.000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang R., Dong N., Shen Z., Zeng Y., Lu J., Liu C., Zhou H., Hu Y., Sun Q., Cheng Q., et al. Epidemiological and phylogenetic analysis reveals Flavobacteriaceae as potential ancestral source of tigecycline resistance gene tet(X) Nat. Commun. 2020;11:4648. doi: 10.1038/s41467-020-18475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karah N., Sundsfjord A., Towner K., Samuelsen O. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist. Updatess Rev. Comment. Antimicrob. Anticancer. Chemother. 2012;15:237–247. doi: 10.1016/j.drup.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Adams M.D., Wright M.S., Karichu J.K., Venepally P., Fouts D.E., Chan A.P., Richter S.S., Jacobs M.R., Bonomo R.A. Rapid Replacement of Acinetobacter baumannii Strains Accompanied by Changes in Lipooligosaccharide Loci and Resistance Gene Repertoire. mBio. 2019;10 doi: 10.1128/mBio.00356-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsieh Y.C., Wang S.H., Chen Y.Y., Lin T.L., Shie S.S., Huang C.T., Lee C.H., Chen Y.C., Quyen T.L.T., Pan Y.J. Association of capsular types with carbapenem resistance, disease severity, and mortality in Acinetobacter baumannii. Emerg. Microbes Infect. 2020;9:2094–2104. doi: 10.1080/22221751.2020.1822757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J., Wang Y., Wu H., Wang Z.Y., Shen P.C., Tian Y.Q., Sun F., Pan Z.M., Jiao X. Coexistence of blaOXA-58 and tet(X) on a Novel Plasmid in Acinetobacter sp. From Pig in Shanghai, China. Front. Microbiol. 2020;11:578020. doi: 10.3389/fmicb.2020.578020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li R., Lu X., Peng K., Liu Z., Li Y., Liu Y., Xiao X., Wang Z. Deciphering the Structural Diversity and Classification of the Mobile Tigecycline Resistance Gene tet(X)-Bearing Plasmidome among Bacteria. mSystems. 2020;5 doi: 10.1128/mSystems.00134-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 8.1. 2018. [(accessed on 5 February 2019)]. Available online: http://www.eucast.org.
- 60.Diancourt L., Passet V., Nemec A., Dijkshoorn L., Brisse S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE. 2010;5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartual S.G., Seifert H., Hippler C., Luzon M.A., Wisplinghoff H., Rodríguez-Valera F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 2005;43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C.M., Guo M.K., Ke S.C., Lin Y.P., Li C.R., Vy Nguyen H.T., Wu L.T. Emergence and nosocomial spread of ST11 carbapenem-resistant Klebsiella pneumoniae co-producing OXA-48 and KPC-2 in a regional hospital in Taiwan. J. Med. Microbiol. 2018;67:957–964. doi: 10.1099/jmm.0.000771. [DOI] [PubMed] [Google Scholar]
- 63.Vy Nguyen H.T. Master’s Thesis. China Medical University Taichung; Taichung, Taiwan: 2019. Characteristics of Clinical Carbapenem—Resistant Enterobacteriaceae Isolates in Central Taiwan. [Google Scholar]
- 64.Vijayashree Priyadharsini J., Smiline Girija A.S., Paramasivam A. In silico analysis of virulence genes in an emerging dental pathogen A. baumannii and related species. Arch. Oral Biol. 2018;94:93–98. doi: 10.1016/j.archoralbio.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Murtagh F., Legendre P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014;31:274–295. doi: 10.1007/s00357-014-9161-z. [DOI] [Google Scholar]
- 66.Dice L.R. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. doi: 10.2307/1932409. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.