ABSTRACT
Aim:
The aim of this article is to evaluate the topical effect of camel whey protein (CWP) on the healing of recurrent aphthous stomatitis (RAS) and the serum levels of interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α.
Materials and Methods:
Forty patients with minor RAS were randomly assigned into control and study groups. The control group applied placebo methylcellulose gel topically over the aphthous ulcer, whereas the study group used CWP dissolved in methylcellulose gel topically over the aphthous ulcer. Healing period, pain scale, and serum inflammatory biomarkers were evaluated before and after gel application. Collected data were analyzed statistically using the paired t-test or independent sample t-test.
Results:
Ulcer healing period, pain scale, and immunological biomarkers were statistically improved in both groups with significant shortening of the ulcer duration and significant regulation of immunological values related to the study group.
Conclusion:
Topical CWP gel is potentially effective in the treatment of RAS.
KEYWORDS: Antioxidant, camel whey protein, immune modulation, recurrent aphthous stomatitis
INTRODUCTION
Recurrent aphthous stomatitis (RAS) disorder is a popular common recurrent chronic inflammatory disease characterized by painful ulcerations affecting the oral mucous membrane usually at lingual, buccal, and labial mucosa.[1]
RAS is diagnosed clinically and characterized by the presence of single or multiple recurring, small, circular or oval ulcerations with clear confined boundaries. RAS has erythematous red halo, with gray or yellow floor. Aphthous ulcers are self-limiting and can heal automatically within 10 days.[2] Painful and recurrent characteristics of RAS cause difficulties in talking and eating and reduce the quality of life of patients.[3]
RAS has three clinical variants: major, minor, and herpetiform RAS; minor RAS is the commonest form.[2] RAS affects 10–20% of the general population, especially school students and females aged between 10 and 19 years.[4]
The etiology and pathogenesis of RAS remain unclear till present. Some predisposing risk factors include genetic susceptibilities, trauma, smoking, viral and bacterial infections, hormonal disorders, nutrition deficiencies disorders, hematological deficiencies, celiac disease, stress, and immune-alteration diseases.[5] Regarding RAS immunopathogenesis, serum immunoglobulin’s alterations, T-lymphocytes, circulating immune complex, and active natural killer cells have been detected in the peripheral circulation of RAS patients. Unstimulated monocytes and depleted leukocytes in active RAS patients secrete a huge amount of tumor necrosis factor (TNF)-α. Circulating TNF-α may stimulate interleukin (IL)-6 release from macrophages, endothelial cells, and keratinocytes, which in turn activate T cells and induce growth and differentiation of cytotoxic T cells leading to epithelial cell destruction and ulcer formation.[6,7]
In light of etiopathogenesis diversity in RAS, different therapy modalities were administered; however, the treatment was only symptomatic in most of the cases.[6] The goals of various therapies were to reduce the ulcer duration, cease pain, enhance functions, and minimize the ulceration recurrence.[8] To attain such objectives, different topically and systemically applied medications have been used such as corticosteroids, anti-inflammatories, immunomodulatories, and antiseptic medications.[9] Immunomodulating agents modulate and enhance T-cell-mediated immunity, restore the phagocytic activity of regular macrophages and neutrophils, and potentiate human interferon and interleukin-2 activity.[7]
Camel whey protein (CWP) has a potent natural antioxidative effect as it reduces the oxidative stress and strengthens the immune system functions. The beneficial effects of CWP dietary supplementation include the stimulation of adaptive and innate immunity and anti-inflammatory, antibacterial, anticancer, antiviral, and antioxidative effects.[10] In addition, it has been shown that CWP improves the treatment of impaired wound healing in diabetic patients.[11]
Studies have demonstrated the consequences of supplementary CWP on the reduction of inflammatory biomarkers, proinflammatory cytokines, modulation of the immune functions, and free radicals [reactive oxygen species (ROS)].[12] These effects could play a potential role in treating RAS.
No previous researches have examined the outcomes of CWP on the treatment of RAS. In our present study, CWP gel was produced and used for RAS patients to assess its topical effect on the healing of RAS and the serum levels of IL-1, IL-6, and TNF-α.
MATERIALS AND METHODS
PATIENT RECRUITMENT
The protocol of the present study was reviewed and approved by the Ethics Committee of Faculty of Dentistry, Kafrelsheikh University (No. DK/14/20) and was registered at ClinicalTrial.gov (NCT04842188). All participants had signed a written informed consent. Our study protocol followed the Helsinki Principles Declaration stated in 1975.
The study inclusion criteria were as follows: (a) clinical manifestations with confirmed history of minor recurrent intraoral ulcer; (b) oral ulceration recurrence for 6 months at least; (c) age ranging from 18 to 60 years; (d) patients in good medical condition without serious systemic disease; (e) voluntary involvement; and (f) good cooperation.
Patients were excluded if they had (a) other forms of RAS (major or herpetiform); (b) covid-related ulcerations; (c) positive history of antibiotic or glucocorticoid intake that may interfere with the results of the experimental drugs; (d) history of drugs that could induce oral ulcerations; (e) neuropsychiatric; (f) uncontrolled systemic disorder as diabetes, renal insufficiency, hepatic disease, blood dyscrasia, inflammatory bowel disease, and rheumatological diseases; (g) use of immunomodulatory drugs during the previous 2 weeks; (h) allergic or hypersensitivity reactions to substances used in the study; (i) smoking or alcohol drinking habits; (j) poor compliance; or (k) non-recurrent oral ulcerations.
SAMPLE SIZE CALCULATION
The current study sample size calculation was determined using PASS software 11.0 (NCSS LLT). Non-inferiority margin has been recorded at 1, and the significance level (α) of 0.05 and 80% power (β) was established a priori. Depending on the previous data, the sample size had to be 14 participants per group. Considering <20% dropout, 34 participants should be recruited.
MEDICAL HISTORY COLLECTION AND LABORATORY TEST
The detailed medical history for participants was recorded at the first appointment, including participant’s age, sex, past medical history, previous and current drug intake, family history, allergy history, and clinical evaluation of signs and symptoms.
RANDOMIZATION AND BLINDING
The present trial was a randomized, prospective, and positive controlled clinical trial. Between June 2020 and March 2021, randomization was conducted using a computer software program to generate a series of codes and then placed in sealed envelopes. The patients opened these envelopes in order. Participants with odd numbers were assigned randomly to our study group; while subjects with random even numbers were allotted to the placebo group.
To avoid subjective bias in the present study, double-blinded assessment was applied. Another independent blinded investigator registered all the study outcomes. All participants in our study were not aware of their medication.
DRUG PREPARATION
Preparation of CWP
The raw milk material was obtained from national female healthy camels from Matrouh, Egypt and followed by milk centrifugation for cream removal. The resulted skimmed milk was acidified by 1N HCl at room temperature till pH 4.3 and finally centrifuged for 10 min at 10,000g. Ammonium sulfate was mixed with the resulted whey and whey proteins leading to whey protein precipitation. Dialyze the precipitated CWP for 48 h against distilled water. Freeze-dry and refrigerate the undenatured dialyzed CWP until further use.[13]
Preparation of gel system
Sodium citrate added to distilled water is continuously stirred till a clear solution is obtained. Methylcellulose polymer (M7027—Methyl cellulose, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was then added with continuous stirring to the prepared solution and was allowed to hydrate overnight. About 1% (w/v) of CWP was added for the study group under constant stirring to the polymer solution, whereas the control group used the methylcellulose gel without any drugs.
TRIAL DESIGN
All patients were examined to record the baseline characteristics of minor RAS. For the control group, patients were informed to use placebo gel, whereas for the study group CWP gel was used. All participants were instructed to apply a layer of gel over the ulcerated mucosa (three times daily, after each meal) until the ulcer disappears. Eating and drinking were prohibited for 1 h to guarantee proper drug absorption.
The duration of recovery for all participants in both groups was recorded. If ulcerative lesions were completely healed, discontinue the gel application.
CLINICAL ASSESSMENT
The clinical symptoms include the duration of ulceration and the degree of pain. The degree of pain was evaluated using visual analog scale (VAS) (0 = no subjective symptoms to 10 = worst subjective symptoms imaginable).
BLOOD SAMPLES ASSESSMENT
One day before gel application and 7 days after ulcer complete healing, 3 mL of peripheral venous blood was collected from each participant. The levels of serum IL-1, TNF-α, and IL-6 were examined in the blood samples using flow cytometry analysis.
SAFETY ASSESSMENT
All basic vital signs (including body temperature, respiration, pulse, blood pressure, and heart rate) and routine investigations (including blood glucose level, complete blood cell count, renal and hepatic clinical biochemistry, urinalysis, chest radiographs, abdominal ultrasound, and electrocardiography) of all participants prior and after gel application were registered to detect any adverse reactions.
DATA ANALYSIS
IBM SPSS software version 20 (IBM Corp., Armonk, NY, USA) has been utilized for statistical analysis. Comparing the two groups, data were conducted using independent two-sample t-test or Wilcoxon–Mann–Whitney test. To compare changes before and after treatment, the paired-samples t-test or Wilcoxon signed-rank test was also used. A significance level of P≤0.001 was put in application to all statistical tests.
RESULTS
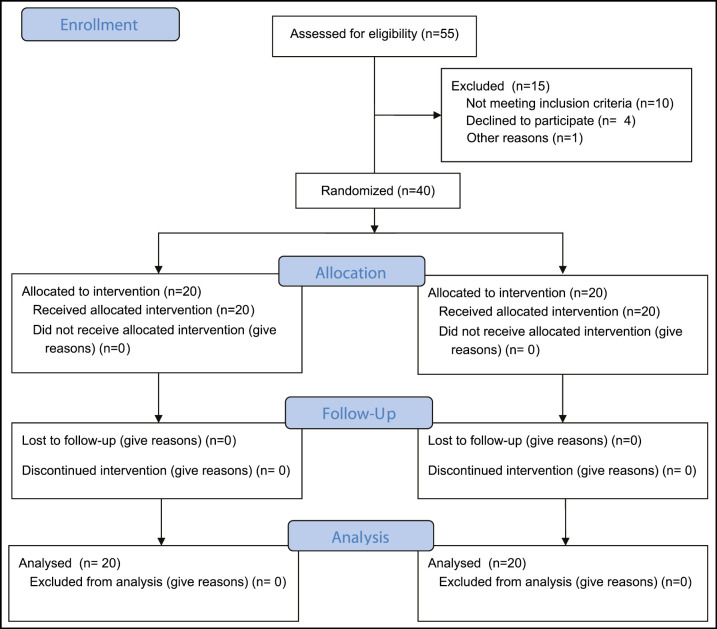
From 55 participants having RAS, 15 were omitted. Forty participants were recruited finally for the present study from January 2021 till April 2021. Remaining 40 patients were divided into two groups randomly (each group 20). The age of the control group (9 males and 11 females) was ranging from 18 to 42 years with mean 26.20 ± 8.64 years, and the study group age (7 males and 13 females) was ranging from 18 to 46 years with mean 27.15 ± 9.27 years. There was no statistically significant difference between the two groups regarding age and gender [Table 1 and Figure 1].
Table 1.
Demographic data of control and study groups
| Variable | Control group | Study group |
|---|---|---|
| Age (years) | ||
| Mean + SD | 26.20 ± 8.64 | 27.15 ± 9.27 |
| Median (range) | 26.2 | 27.15 |
| Minimum | 18 | 18 |
| Maximum | 42 | 46 |
| Gender [n (%)] | ||
| Male | 9 (45) | 7 (35) |
| Female | 11 (54) | 13 (65) |
Figure 1.
Flow chart diagram for the participants in the current study
Overall, clinical and immunological parameters for both groups were significantly improved. Intergroup comparison revealed significant reduction of the ulcer duration and significant improvement of immunological records in the study group when compared with the placebo group [Table 2 and Figure 2].
Table 2.
Paired t-test comparing the blood biomarkers before and after treatment
| Parameter | Control group, mean±SD | P | Study group, mean±SD | P |
|---|---|---|---|---|
| TNF-α | ||||
| Before treatment | 278.20±11.37 | <0.001* | 281.30±11.79 | <0.001* |
| After treatment | 178.20±5.69 | 135.35±6.46 | ||
| IL-1 | ||||
| Before treatment | 1.63±0.04 | <0.001* | 1.65±0.06 | <0.001* |
| After treatment | 0.79±0.70 | 0.36±0.02 | ||
| IL-6 | ||||
| Before treatment | 156.66±33.11 | <0.001* | 170.25±2.88 | <0.001* |
| After treatment | 106.44±22.07 | 63.75±0.96 |
Figure 2.
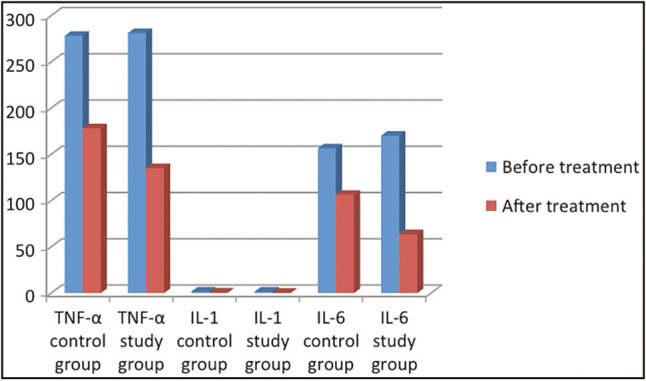
Mean levels of IL1, IL6, TNF-α before and after treatment in both the groups
The immunological results of our study using enzyme-linked immunosorbent assay test for blood samples demonstrated a statistical significant drop in TNF-α levels for the control group from 278.20±11.37 before treatment to 178.20±5.69 after gel application; in addition, the levels of TNF-α for the study group was significantly improved from baseline measurement 281.30±11.79 to post-treatment measurement 135.35±6.46. Upon comparing the reduction of TNF-α of both groups 1 weeks after ulcer resolution, there was a statistical significant reduction of TNF-α levels in the CWP group when compared with the placebo group [Table 2 and Figure 2].
The mean IL-1 level before gel application was 1.63±0.04 and 1.65±0.06 for the control and study groups, respectively; the mean IL-1values were significantly dropped after treatment to 0.79±0.70 and 0.36±0.02 for the control and study groups, respectively (P < 0.001) [Table 2 and Figure 2].
Comparing the mean IL-6 from baseline to 1 week after ulcer healing yielded a statistically significant reduction in IL-6 values from 156.66±33.11 to 106.44±22.07 for the control group and from 170.25±2.88 to 63.75±0.96 for the study group (P < 0.001) [Table 3 and Figure 3].
Table 3.
Independent sample test comparing the changes in chemical biomarkers between the two groups
| Parameter | Control group, mean±SD | Study group, mean±SD | P |
|---|---|---|---|
| TNF-α reduction | 100.00±7.86 | 145.95±5.98 | <0.001* |
| IL-1 drop | 0.83±0.03 | 1.29±0.04 | <0.001* |
| IL-6 reduction | 52.60±1.52 | 106.50±1.92 | <0.001* |
| VAS | 2.70±0.57 | 2.25±0.63 | >0.001 (NS) |
| Ulcer duration | 6.10±0.78 | 2.40±0.50 | <0.001* |
Figure 3.
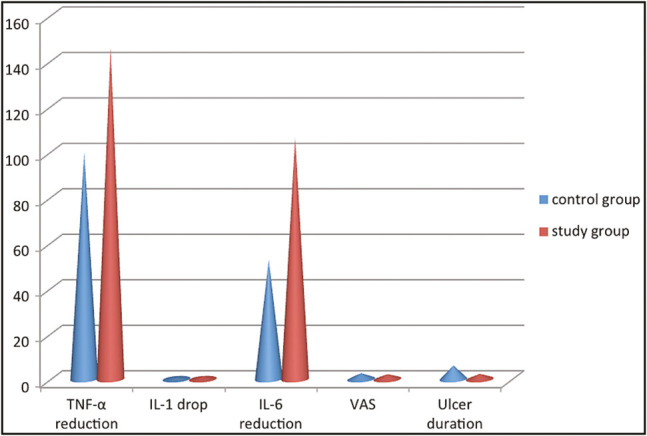
Comparison of changes in IL-1, IL-6, TNF-α, ulcer duration, and VAS of both the groups
After complete healing of the ulcer in the control and study groups, the mean TNF-α reduction was 100.00±7.86 and 145.95±5.98, respectively, and the mean drop of IL-1 was 0.83±0.03 and 1.29±0.04, respectively [Table 3]. Also, the mean decrease in IL-6 was 52.60±1.52 and 2.25±0.63 for the control and study groups, respectively [Table 3]. The mean reductions of TNF-α, IL-1, and IL-6 in the study group after gel application were statistically significantly different when compared with the control group (P < 0.001) [Table 3 and Figure 3].
Regarding pain measurement using VAS, the mean value of VAS was 2.70±0.57 in the control group and 2.25±0.63 in the study group. Upon comparing the mean VAS of both groups, there was no statistical significant difference (P >0.001) [Table 3 and Figure 3].
After application of the treatment protocol in both groups, the mean duration of the ulcer in the control group was 6.10±0.78 days and in the study group was 2.40±0.50 days. The study group yielded a statistical significant reduction of the ulcer duration when compared with the control group (P < 0.001) [Table 3 and Figure 3].
Throughout and after treatment, there were no observed inflammatory adverse reactions or complications in both groups secondary to topical gel application. In addition, all participants accepted the topical application of the used gel due to its muco-adhesive effect, ease of application, good viscosity, high spreadability, and acceptable taste or smell.
DISCUSSION
ROA is a chronic oral mucosal inflammatory disorder that causes feeding, drinking, and talking difficulties. The present study developed a sustained release CWP loaded in methylcellulose gel and evaluated its effects on RAS when compared with methylcellulose placebo gel in 40 patients from the Affiliated Periodontology Department, Faculty of Dentistry, Hospital of Kafrelsheikh University.
Methylcellulose gels were used in the current research as a placebo gel and as a base for CWP gel because of its characteristics such as being easily prepared, clear muco-adhesive gel, and its pH of 6.7, which is biocompatible with oral tissues. Methylcellulose gel is not irritant to the gingiva, safely applied intraorally, and raises the patient compliance.[14] Methylcellulose gel has constant stability at various temperatures and humidity degrees.[15]
For the authors’ knowledge, no other previous studies have evaluated the clinical and immunological effects obtained from the application of CWP gel in patients with RAS. Moreover, this is the first study that prepared CWP in a gel form for topical application. Hence, the present study results could not be correlated with any previous research.
For RAS, resolution of inflammation symptoms and wound healing are important for patients. Upon comparing the pain degree using VAS, results of the current study demonstrated non-significant reduction between both the groups. That could be attributed to the muco-adhesive effect of methylcellulose that prevents leakage after application and protects the ROA-affected mucosa from external irritants, thus reducing the degree of pain.[16]
Regarding the wound healing, the results suggested that the mean duration of oral RAS was 2.40±0.50 days for the study group and 6.10±0.78 days for the control group. The average ulcer duration in our treatment CWP group was shorter when compared with that observed by Liu et al.[17] upon using topical dexamethasone ointment for the treatment of minor RAS (average healing period of 6 days).
The significantly shorter ulcer duration observed in the study group could be referred to the effect of CWP on wound healing in addition to the sustained release and high binding effect obtained from methylcellulose gel base, which ensures a prolonged contact period of CWP to be consistently released and absorbed by tissues up to the end of 8 h.[14,18]
These results obtained from the current study, indicating that the CWP gel in the study group had beneficially potent wound healing than placebo gel used in the control group, go with the studies of Ebaid et al.,[19,20,21] who assessed the wound healing of CWP dietary supplement in diabetic rats. Ebaid et al. demonstrated that CWP was significantly effective by increasing the wound healing capacity in diabetic and non-diabetic rats through stimulation of collagen production, stimulation of antioxidant glutathione production, and cytokines regulation during inflammatory phase. Moreover, they confirmed the immunomodulatory effect of CWP through regulating the pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, thus stimulating wound healing.
TNF-α, IL-1, and IL-6 are produced by macrophage activation, highly expressed in case of inflammed oral mucosa, and oral ulcerations. Moreover, they can be used as evaluation indices to determine the occurrence of RAS and therapeutic effects.[22] In this study, TNF- α, IL-1, and IL-6 levels in the CWP group patients were reduced significantly than the placebo group, suggesting that the inflammatory reaction of topical CWP gel positively affects the healing of RAS.
The current study measured the serum levels of IL-1, IL-6, and TNF-α in RAS patients before and after topical application of CWP and placebo gel in the study and control groups, respectively. The choice of these pro-inflammatory cytokines was confirmed by many studies that investigated the clinical significance of serum IL-6, IL-1, and TNF-α in RAS.[6,23,24] Results of these studies revealed that the levels of serum IL-1, IL-6, and TNF-α in RAS patients were significantly increased.
The current study results yielded significantly reduced levels of serum IL-1, IL-6, and TNF-α after gel application in both the groups with statistically significant improvement in the study group after CWP gel application when compared with placebo gel application in the control group. This significant improvement in the study group could be attributed to the anti-inflammatory and antioxidant effects of CWP. The results of the present study go in agreement with Ebaid et al.[25] who evaluated dietary CWP supplement on the regulation of serum TNF-α, IL-1β, and IL-6 levels, hepatic lipid peroxidation, and the antioxidant defense system; the results revealed a significant drop of serum biomarkers, liver lipid peroxidation, and ROS with significant increase of cellular antioxidants activity in wounded diabetic rats. Moreover, CWP modulates various immune cell functions by activating lymphocytes, triggering chemotactic function and cytokines release, producing antibodies, activating phagocytosis, and granulocyte and NK cell activity.[26] WP also intensifies the regulation of IL-1β, IL-8, IL-6, macrophage inflammatory proteins (MIP-1α, MIP-1β), and TNF-α.[27]
CWP was found to reduce oxidative stress, magnify immune system functions, and raise glutathione levels. Badr et al.[28,29] reported the advantageous immunomodulatory characters of CWP as natural antioxidants. Badr et al.[30] explained the suppression of oxidative stresses of CWP by reducing hepatic lipid peroxidation and raising glutathione-stimulated hormone levels by activating glutathione-S-transferase.
The current study limitations include limited sample size, short evaluation period, the effect of topical CWP gel on the ulcer recurrence which was not evaluated, and the long-term drug efficacy and safety still not confirmed.
CONCLUSION
In conclusion, this clinical trial concluded that CWP gel successfully facilitated RAS healing, reduced healing period, and regulated the inflammatory biochemical markers.
This provides guidance for future researches and a possible drug for clinical applications. However, the effects of CWP gel were only assessed clinically and immunologically, so different mechanisms have to be tested to speed up this drug clinical implementation. Future studies with a longer evaluation period and a larger sample size are highly recommended to assess the potential actions of CWP gel. Also, further studies are required to evaluate the topical effect of CWP gel on different oral ulcers, lichen planus, and postsurgical wound healing.
FINANCIAL SUPPORT AND SPONSORSHIP
No financial support or sponsorship received, this study is self-funded.
CONFLICTS OF INTEREST
There are no conflicts of interest.
AUTHORS CONTRIBUTIONS
All authors contributed in the study design, data collection and analysis, study writing, and revision process.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
The protocol of the present study was reviewed and approved by the Ethics Committee of Faculty of Dentistry, Kafrelsheikh University (No. DK/14/20) and was registered at ClinicalTrial.gov (NCT04842188). Our study protocol followed the Helsinki Principles Declaration stated in 1975.
PATIENT DECLARATION OF CONSENT
All the patients had signed an informed consent and it is available upon request.
DATA AVAILABILITY STATEMENT
It is available upon request.
ACKNOWLEDGEMENTS
No acknowledgement to disclose.
REFERENCES
- 1.Gao Y, Gupta N, Abdalla M. Recurrent aphthous stomatitis improved after eradication therapy for Helicobacter pylori. Case Rep Gastrointest Med. 2021;2021:5543838. doi: 10.1155/2021/5543838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Z, He Z, Xie G, Fan Y, Shao T. Altered oral microbiota composition associated with recurrent aphthous stomatitis in young females. Medicine (Baltimore) 2021;100:e24742. doi: 10.1097/MD.0000000000024742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadian Z, Moghadamnia AA, Kazemi S, Shirzad A. Effect of omega-3 on recurrent aphthous stomatitis and improvement quality of life. Int J Dent. 2021;2021:6617575. doi: 10.1155/2021/6617575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ossama M, Lamie C, Tarek M, Wagdy HA, Attia DA, Elmazar MM. Management of recurrent aphthous ulcers exploiting polymer-based muco-adhesive sponges: In-vitro and in-vivo evaluation. Drug Deliv. 2021;28:87–99. doi: 10.1080/10717544.2020.1858999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altay DU, Korkmaz M, Ergun S, Korkmaz H, Noyan T. Salivary irisin: Potential inflammatory biomarker in recurrent apthous stomatitis patients. Eur Rev Med Pharmacol Sci. 2021;25:2252–9. doi: 10.26355/eurrev_202103_25257. [DOI] [PubMed] [Google Scholar]
- 6.Shen C, Ye W, Gong L, Lv K, Gao B, Yao H. Serum interleukin-6, interleukin-17A, and tumor necrosis factor-alpha in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2021;50:418–23. doi: 10.1111/jop.13158. [DOI] [PubMed] [Google Scholar]
- 7.Sun A, Chia JS, Chang YF, Chiang CP. Levamisole and Chinese medicinal herbs can modulate the serum interleukin-6 level in patients with recurrent aphthous ulcerations. J Oral Pathol Med. 2003;32:206–14. doi: 10.1034/j.1600-0714.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 8.Marya CM, Mehlawat J, Nagpal R, Kataria S, Taneja P. Comparative assessment of low-level laser therapy (LLLT) vs. topical application of amlexanox + lidocaine to treat recurrent aphthous ulcers (RAUs): A randomized controlled trial. J Dent Res Dent Clin Dent Prospects. 2021;15:11–5. doi: 10.34172/joddd.2021.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakeri M, Parsian H, Bijani A, Shirzad A, Neamati N. Serum levels of vitamin D in patients with recurrent aphthous stomatitis. Dent Med Probl. 2021;58:27–30. doi: 10.17219/dmp/126360. [DOI] [PubMed] [Google Scholar]
- 10.Ebaid H, Abdel-Salam B, Hassan I, Al-Tamimi J, Metwalli A, Alhazza I. Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids Health Dis. 2015;14:132. doi: 10.1186/s12944-015-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebaid H. Neutrophil depletion in the early inflammatory phase delayed cutaneous wound healing in older rats: Improvements due to the use of un-denatured camel whey protein. Diagn Pathol. 2014;9:46. doi: 10.1186/1746-1596-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Salam BK. Modulatory effect of whey proteins in some cytokines involved in wound healing in male diabetic albino rats. Inflammation. 2014;37:1616–22. doi: 10.1007/s10753-014-9888-z. [DOI] [PubMed] [Google Scholar]
- 13.Badr G. Supplementation with undenatured whey protein during diabetes mellitus improves the healing and closure of diabetic wounds through the rescue of functional long-lived wound macrophages. Cell Physiol Biochem. 2012;29:571–82. doi: 10.1159/000338511. [DOI] [PubMed] [Google Scholar]
- 14.Sanjana A, Ahmed MG, Gowda Bh J. Preparation and evaluation of in-situ gels containing hydrocortisone for the treatment of aphthous ulcer. J Oral Biol Craniofac Res. 2021;11:269–76. doi: 10.1016/j.jobcr.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal M, Mittal N, Yadav SK, Khan G, Gupta P, Mishra B, et al. Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: Preparation, in-vitro characterization and antimicrobial study. J Oral Biol Craniofac Res. 2018;8:126–33. doi: 10.1016/j.jobcr.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swain GP, Patel S, Gandhi J, Shah P. Development of moxifloxacin hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J Oral Biol Craniofac Res. 2019;9:190–200. doi: 10.1016/j.jobcr.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Zhou Z, Liu G, Wang Q, Chen J, Wang L, et al. Efficacy and safety of dexamethasone ointment on recurrent aphthous ulceration. Am J Med. 2012;125:292–301. doi: 10.1016/j.amjmed.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Itoh K, Hatakeyama T, Shimoyama T, Miyazaki S, D’Emanuele A, Attwood D. In situ gelling formulation based on methylcellulose/pectin system for oral-sustained drug delivery to dysphagic patients. Drug Dev Ind Pharm. 2011;37:790–7. doi: 10.3109/03639045.2010.541465. [DOI] [PubMed] [Google Scholar]
- 19.Ebaid H, Badr G, Metwalli A. Immunoenhancing property of dietary un-denatured whey protein derived from three camel breeds. Biologia. 2012;67:425–33. [Google Scholar]
- 20.Ebaid H, Hassanein KA, El-Feki MA. The undenatured whey protein enhanced wound healing in mice. J Egypt German Soc Zool. 2005;47:267–87. [Google Scholar]
- 21.Ebaid H, Salem A, Sayed A, Metwalli A. Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids Health Dis. 2011;10:235. doi: 10.1186/1476-511X-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soylu A, Yıldız G, Torun Bayram M, Kavukçu S. IL-1β blockade in periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: Case-based review. Rheumatol Int. 2021;41:183–8. doi: 10.1007/s00296-019-04389-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhu S, Shi Q, Lu J. Curative effect of oral ulcer powder on the treatment of recurrent aphthous ulcer. Pak J Pharm Sci. 2018;31(3(Special)):1175–8. PMID: 29735470. [PubMed] [Google Scholar]
- 24.Chen L, Ke Z, Zhou Z, Jiang X, Zhao Y, Zhang J. Associations of IL-1, 6, and 10 gene polymorphisms with susceptibility to recurrent aphthous stomatitis: Insights from a meta-analysis. Genet Test Mol Biomarkers. 2018;22:237–45. doi: 10.1089/gtmb.2017.0072. [DOI] [PubMed] [Google Scholar]
- 25.Ebaid H, Ahmed OM, Mahmoud AM, Ahmed RR. Limiting prolonged inflammation during proliferation and remodeling phases of wound healing in streptozotocin-induced diabetic rats supplemented with camel undenatured whey protein. BMC Immunol. 2013;14:31. doi: 10.1186/1471-2172-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauthier SF, Pouliot Y, Saint-Sauveur D. Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. Int Dairy J. 2006;16:1315–23. [Google Scholar]
- 27.Badr G, Ramadan NK, Sayed LH, Badr BM, Omar HM, Selamoglu Z. Why whey? Camel whey protein as a new dietary approach to the management of free radicals and for the treatment of different health disorders. Iran J Basic Med Sci. 2017;20:338–49. doi: 10.22038/IJBMS.2017.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badr G, Badr BM, Mahmoud MH, Mohany M, Rabah DM, Garraud O. Treatment of diabetic mice with undenatured whey protein accelerates the wound healing process by enhancing the expression of MIP-1α, MIP-2, KC, CX3CL1 and TGF-β in wounded tissue. BMC Immunol. 2012;13:32. doi: 10.1186/1471-2172-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badr G. Camel whey protein enhances diabetic wound healing in a streptozotocin-induced diabetic mouse model: The critical role of β-defensin-1, -2 and -3. Lipids Health Dis. 2013;12:46. doi: 10.1186/1476-511X-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badr G, Sayed LH, Omar HEM, Abd El-Rahim AM, Ahmed EA, Mahmoud MH. Camel whey protein protects B and T cells from apoptosis by suppressing activating transcription factor-3 (ATF-3)-mediated oxidative stress and enhancing phosphorylation of AKT and IκB-α in type I diabetic mice. Cell Physiol Biochem. 2017;41:41–54. doi: 10.1159/000455935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
It is available upon request.