Abstract
Fungi are increasingly recognised to have a significant role in the progression of lung disease in Cystic fibrosis with Aspergillus fumigatus the most common fungus isolated during respiratory sampling. The emergence of novel CFTR modulators has, however, significantly changed the outlook of disease progression in CF. In this review we discuss what impact novel CFTR modulators will have on fungal lung disease and its management in CF. We discuss how CFTR modulators affect antifungal innate immunity and consider the impact of Ivacaftor on fungal disease in individuals with gating mutations. We further review the increasing complication of drug–drug interactions with concurrent use of azole antifungal medication and highlight key unknowns that require addressing to fully understand the impact of CFTR modulators on fungal disease.
Keywords: Cystic fibrosis, Aspergillus fumigatus, Fungi, Antifungal
Cystic Fibrosis Fungal Disease: An Introduction
Cystic fibrosis is an inherited multi-system disorder which manifests in the lungs as chronic airways infection and inflammation leading to bronchiectasis and, if left untreated, progressive respiratory failure and death. Although bacteria are the dominant airway pathogens in the majority of the CF population, fungi are increasingly being recognised as having a role in the pathophysiology of CF lung disease.
Aspergillus fumigatus is the most common and clinically significant fungus isolated from CF patients, but published prevalence rates for colonisation range widely from less than 5% to 60% [1]. This variability reflects differences in definitions of colonisation, patient demographics, treatment regimens, frequency and type of sampling as well as laboratory culture technique [2, 3]. Age, inhaled corticosteroids and antibiotic use (macrolide and inhaled antibiotics) have been identified as risk factors for Aspergillus colonisation. Although causality is unproven, it is hypothesised that earlier aggressive use of inhaled antibiotics as antimicrobial prophylaxis could potentially predispose to increased fungal colonisation rates [4, 4]
The clinical significance of Aspergillus fumigatus in CF depends on the host’s immunological response. Baxter et al. [6] proposed a classification for Aspergillus lung disease in CF based on microbiology (culture, sputum galactomannan, Aspergillus real-time polymerase chain reaction) and serology (A. fumigatus-specific IgE and IgG), which defined four clinical groups: allergic bronchopulmonary aspergillosis (ABPA), Aspergillus sensitisation, Aspergillus colonisation and Aspergillus bronchitis.
ABPA is a Th2- and IgE-mediated hypersensitivity response to Aspergillus with high prevalence (8.9%) in the CF population [7]. The US CF foundation and the International Society for Human and Animal Mycology (ISHAM) have published diagnostic criteria for ABPA in CF (Table 1) [8, 9]. In both criteria, diagnosis depends on elevated total and Aspergillus-specific IgE together with at least one (US CFF) or two (ISHAM) of the following: raised Aspergillus-specific IgG, consistent radiological changes or raised eosinophils (ISHAM criteria only). Evidence for the impact of ABPA on lung function in CF has been inconsistent. In an early retrospective study, Kraemer et al. [10] demonstrated a negative impact on several lung function parameters similar to the impact of chronic Pseudomonas infection. More recently, De Baets et al. [11] assessed Pseudomonas-negative CF patients in a retrospective case–control study and found that those with ABPA experienced a significant decline in lung function over 2 years leading up to diagnosis compared to control cases whose lung function remained stable. By contrast an ECFS registry study found only a modest ABPA-dependent difference in FEV1 at entry into the study that did not alter during the 3-year follow-up [12].
Table 1.
Table summarising proposed criteria for diagnosis for allergic bronchopulmonary aspergillosis and Aspergillus bronchitis in Cystic Fibrosis
| Classic case | Minimal diagnostic criteria |
|---|---|
| Criteria for diagnosis of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis | |
| Acute or subacute clinical deterioration that is not attributable to another aetiology (cough, wheeze, exercise intolerance, exercise-induced asthma, decline in pulmonary function, increased sputum) | Acute or subacute clinical deterioration that is not attributable to another aetiology (cough, wheeze, exercise intolerance, exercise-induced asthma, change in pulmonary function or increased sputum production) |
| A serum total IgE level of > 1,000 IU per ml (2,400 ng/ml) unless patient is receiving systemic steroids (requires retest when steroid treatment is discontinued) | A serum total IgE level of > 500 IU per ml (> 1,200 ng/ml). If ABPA is suspected and the total IgE level is 200–500 IU per mL, repeat testing one to three months is recommended. If patient is taking steroids, repeat when steroid treatment is discontinued |
| Presence of IgE antibodies to A. fumigatus in vitro or immediate cutaneous hypersensitivity to aspergillus | Immediate cutaneous reactivity to A. fumigatus (prick test wheal > 3 mm with surrounding erythema, off systemic antihistamines) or in vitro demonstration of IgE antibody to A. Fumigatus |
| Precipitating antibodies to A. fumigatus or serum IgG antibody to A. Fumigatus | One of the following: |
| Precipitating antibodies to A. fumigatus or serum IgG antibody to A. fumigatus | |
| New or recent infiltrates ( or mucus plugging) on chest radiology or computed tomography that do not respond to antibodies and standard physiotherapy | |
| New or recent abnormalities on chest radiography (infiltrates or mucus plugging) or computed tomography (bronchiectasis) that do not respond to antibiotics and standard physiotherapy | |
| Criteria for Aspergillus Bronchitis | |
| Microbiology | Repeat sputum culture for Aspergillus sp |
| Positive sputum galactomannan | |
| Symptoms | Chronic (> 4 weeks) pulmonary symptoms (chronic productive cough, tenacious mucus production, dyspnoea and difficult airway clearance) |
| Absence of semi-invasive disease | Absence of significant tissue invasion and lung parenchymal destruction (e.g. cavity formation) |
|
Serology (Bronchoscopy findings) |
Aspergillus IgG antibody detectable in serum |
| Negative IgE (lack of allergic response) | |
| Mucoid impaction, thick tenacious sputum with bronchial plugging, bronchial erythema (touch bleeding) and/or ulceration | |
| Superficial invasion of mucosa by Aspergillus hyphae | |
The proposed classification of Aspergillus bronchitis relates to microbiological colonisation with sputum galactomannan positivity and a positive Aspergillus-specific IgG but negative IgE response (Table 1). No formal diagnostic criteria have been published and the immunological host response in this subgroup, the prognostic implications and effect of antifungal therapy are as yet not well defined. Clinical presentation with deterioration despite ≥ 2 courses of antibiotics with exclusion of new bacterial growth and response to antifungal therapy has been proposed as confirmation of Aspergillus bronchitis [13].
The impact of non-ABPA manifestations of Aspergillus-related disease on lung function has been harder to determine with a wide variation in definitions used and a failure to distinguish between sub-groups. Studies that compared Aspergillus-sensitised to Aspergillus negative groups show a negative effect on lung function; however, most studies comparing colonised versus non-colonised populations have not identified Aspergillus as a risk factor for lung function decline [14–16].
As well as Aspergillus, individuals with CF are also susceptible to colonisation with other filamentous mould, in particular Scedosporium species and Exophiala dermatitidis, which can dominate the fungal airway community [17]. The susceptibility, implication and relevance of colonisation with these emerging pathogens are as yet unclear, with multicentre cross-sectional analyses recently not identifying a particular severity trait [18, 19]. Further longitudinal cohort studies are necessary to fully understand pathogenicity and implication on long-term outcome.
Fungal Immunity in Cystic Fibrosis
Cystic fibrosis is caused by mutations in the cystic fibrosis Transmembrane Conductance Regulator (CFTR) gene. CFTR encodes a cyclic AMP-activated chloride channel highly expressed in epithelial cells but also localised to the surface and endosomal membranes of immune cells [20, 21]. The absence of functional CFTR has a profound impact on innate and adaptive immune responses to inhaled fungal pathogens and it is this dysregulated host response which plays a key role in determining the pathogenicity of fungal exposure in CF.
Innate Immunity
The CFTR ion channel conducts Cl− and HCO3− ions across the cell membrane, with loss of function interfering with normal anion transport and, through its regulatory effect on the ENaC sodium channel, sodium reabsorption. This disruption across airway epithelial cell membranes results in abnormal hydration of airway surface liquid (ASL) and impaired mucociliary clearance, a key mechanism for preventing fungal colonisation [22]. Fungi retained within the airways are then liable to be sensed by epithelial cells and airway resident immune cells responsible for ingesting and clearing pathogens. In the CF airway, signalling pathways are triggered to recruit further inflammatory cells and activate adaptive immune responses. There is known to be variability in predisposition to fungal disease in CF, with genetic susceptibility and HLA-DRB1 alleles shown to be associated with ABPA [23].
CFTR-deficient epithelial cells show impaired phagocytosis and killing of Aspergillus conidia and increased rates of apoptosis in vitro and impaired fungal clearance in vivo [24]. IL-8 secretion, a key neutrophil chemoattractant, is increased as is NLRP3 inflammasome activation [25, 26]. Changes in TLR4 expression, cytokine production, phagosomal acidification, microbicidal activity, autophagy and inflammasome activation have all been demonstrated in CF macrophages [27–29]. Almost all of these findings, however, are based on work using bacterial stimulants, with little data on responses to fungal stimuli. A previous study has, however, shown impaired autophagy in lung macrophages isolated from A. fumigatus infected CFTR-deficient mice, alongside increased Aspergillus-dependent NLRP3 inflammasome activation and IL-1β release [25].
Neutrophils dominate the CF airway drawn by chronic microbial infection and augmented secretion of cytokines and chemokines including IL-1β, IL-8, TNF-α and LTB4 [30]. Once present, CF neutrophils show reduced rates of apoptosis, persisting in the airways [31]. Reactive oxygen species (ROS) release in response to Aspergillus infection is increased and has been linked to disease progression [32]. Aspergillus is also known to trigger formation of neutrophil extracellular traps (NETs), which are important for controlling hyphal growth [33]. Excess release of NETs have been identified as contributing to airway obstruction and diminished lung function in CF [34].
Adaptive Immunity
T lymphocytes play a key role in fungal immunity. Th1 CD4 + T cells enable inflammation and fungal clearance, whereas Th2 cells drive allergic inflammation with T regulatory cells having an immunomodulatory role [35–37]. A tendency to develop a Th2 response to Aspergillus has been demonstrated in CF murine models and in the peripheral blood of CF patients with ABPA [38, 39]. Impaired IFN-γ and IL-10 release by T helper cells from CF patients suggests skewing away from a protective Th1 response [40]. A recent study has additionally shown the importance of Aspergillus-specific Th17 cells in driving ABPA in CF with increased blood levels during exacerbations and a reduction following antifungal therapy [41]. Aspergillus-specific Th17 cells additionally recognised a restricted set of cross-reactive proteins including Candida albicans potentially driving pathogenicity although the full implications are as yet unclear.
CFTR Modulators
Over the past few years, a number of small molecule therapies capable of enhancing the functional expression of specific CFTR mutations have been released onto the market and still more are under development. They are known collectively as CFTR modulators but can be classified into five main groups: potentiators, correctors, amplifiers, read-through agents and stabilisers. The potentiator Ivacaftor (Kalydeco®) was the first to be approved by the FDA in 2012. Its mechanism of action is to increase the “open probability” (Po) of the CFTR ion channel at the cell surface. As such, Ivacaftor was originally licenced only for Class III gating mutations, which represent ~ 5% of the CF population, with subsequent expansion to a number of residual function mutations [42]. Several phase 3 trials demonstrated safety and efficacy with improvement in lung function and reduction in exacerbations [43].
Lumacaftor/ivacaftor (Orkambi®) and tezacaftor/ivacaftor (Symdeko®) are both combination corrector–potentiator drugs. The lumacaftor and tezacaftor entities work in synergy with ivacaftor to correct protein misfolding prior to its transport to the cell surface, where its resultant activity is enhanced by ivacaftor’s effect on anion channel function. Combination therapy enables efficacy in improving lung function (6.8% relative improvement in FEV1), quality of life and reducing exacerbations in a much larger cohort of patients, namely Phe508del homozygotes [44, 45], as well as Phe508del/residual function heterozygotes [46].
Finally, the most recently licenced modulator is elexacaftor/tezacaftor/ivacaftor (Trikafta® or Kaftrio®). This is a combination of two correctors and ivacaftor and has been licenced for use in Phe508del homozygotes and the 30% of patients who have one copy of Phe508del and one copy of a “minimal function” mutation [47]. For the former group, triple therapy was compared to tezacaftor/ivacaftor in a phase 3 trial and showed a relative 10% increase in FEV1, reduction in sweat chloride and improvement in quality of life scores [48]. For the Phe508del heterozygotes, triple therapy was compared to placebo and demonstrated an average 14% increase in FEV1 and 63% reduction in pulmonary exacerbations as well as improvements in sweat chloride and quality of life scores [49].
CFTR Modulator Impact on Fungal Colonisation and Disease
An important question in this new era of CFTR modulators is what impact these drugs will have on fungal lung disease and its management in CF. There is some early evidence from registry studies of a beneficial effect of Ivacaftor on Aspergillus colonisation rates [50–52]. Of note, these studies have identified a poly-microbial effect with significant reductions in Pseudomonas aeruginosa and Staphylococcus aureus prevalence rates as well, in part likely related to improved mucociliary clearance [53]. There is as yet no data on the impact of combination modulator therapies on microbial colonisation rates, though one might expect similar beneficial effects, with subsequent effects on sensitisation and ABPA.
Given the effects of CFTR dysfunction on the host immune cell response, it is likely that these modulators will also impact the dysregulated host response that drives fungal disease in CF, however the magnitude of response and clinical effect is as yet unknown. An early study by Pohl et al. showed that ivacaftor restores degranulation and improves bacterial killing by neutrophils [54]. Since then, others have demonstrated an ivacaftor-dependent normalisation of CF neutrophil phenotype with reduced expression of activation and adhesion markers and recovery of normal apoptosis rates [31, 55].
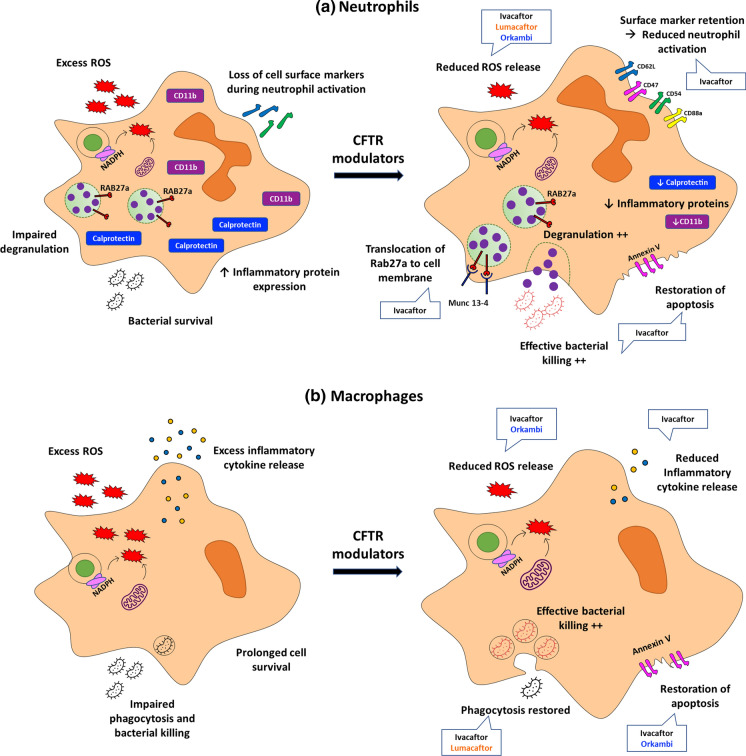
The impact of CFTR modulators on macrophage function is more variable and modulator-type dependent. Barnaby et al. [56] showed that lumacaftor alone, but not ivacaftor alone, restored defective CF macrophage phagocytosis and Pseudomonas killing. When ivacaftor was added to lumacaftor in the form of Orkambi®, however, this cancelled lumacaftor’s beneficial effects. By contrast, both Ivacaftor alone and Orkambi® reduced macrophage secretion of pro-inflammatory cytokines, whereas lumacaftor alone did not. In another study, both ivacaftor and Orkambi® normalised increased macrophage apoptosis rates, but only ivacaftor normalised phagocytosis and Pseudomonas killing and reduced inflammatory cytokine release [57]. Only one study has examined the effect of CFTR modulators on immune responses to fungal infection. All three modulators studied, ivacaftor, lumacaftor and Orkambi® reduced the exaggerated ROS production seen in CF mononuclear cells and neutrophils infected with Aspergillus [58]. Figure 1 provides an illustration of the impact of CFTR modulators on antimicrobial immunity.
Fig. 1.
a Ivacaftor-treated neutrophils demonstrate: reduced reactive oxygen species (ROS) release; restored degranulation and bacterial killing; reduced expression of inflammatory proteins and retention of surface markers normally lost during neutrophil activation and normalised rates of apoptosis. Lumacaftor and Orkambi also reduce ROS release. b Ivacaftor-treated macrophages demonstrate: reduced ROS and inflammatory cytokine release; restored phagocytosis and bacterial killing and normalised rates of apoptosis. Lumacaftor reduces ROS release and restores phagocytosis and bacterial killing. Orkambi reduces ROS release and restores normal rates of apoptosis
Any impact of CFTR modulators on antifungal immunity will, however, need to be balanced with susceptibility to pathogenic fungi as life expectancy increases, with resultant potential longer duration of inhaled antibiotic exposure in individuals with pre-existing structural lung disease, and additional effects of older age on the host immune response. Aspergillus colonisation rates have additionally been shown to be associated with non-tuberculous mycobacteria and bacteria such as Pseudomonas aeruginosa. As such, there may be ongoing susceptibility to fungi in CF individuals with pre-existing lung disease and Pseudomonas aeruginosa or NTM colonisation and requirement for inhaled antibiotic prophylaxis, but reduced susceptibility in individuals with minimal existing structural lung disease prior to CFTR modulator initiation. Further longitudinal prospective cohort studies will thus be needed to determine the long-term cumulative implications of CFTR modulator therapy on fungal colonisation, sensitisation and disease prevalence in CF.
CFTR Modulator–Antifungal Interactions
A complication of CFTR modulator therapy is the potential for drug–drug interactions with the most commonly used group of antifungal drugs, the triazoles. Ivacaftor, tezacaftor and elexacaftor are all primarily metabolised via CYP3A-mediated oxidation, whereas Lumacaftor is not extensively metabolised and is mostly excreted unchanged in the faeces. Lumacaftor is, however, a strong CYP3A inducer so drug–drug interactions with Orkambi® can be more difficult to predict.
The triazoles are all CYP3A inhibitors and so CFTR modulator dose adjustments have to be made [59, 60]. Itraconazole, posaconazole and voriconazole are all considered strong inhibitors and so a dose reduction to a single morning combination tablet twice a week for Symdeko® and Trikafta/Kaftrio® and a single ivacaftor tablet twice weekly for Kalydeco®, is advised. There is to date, however, limited available data on the bioavailability of CFTR modulator therapy. The novel azole isavuconazole is considered to be only a moderate CYP3A inducer and so less severe dose reductions can be made [61]. Alternate-day single doses of the combination and ivacaftor tablets are advised for Symdeko® and Trikafta/Kaftrio® and once daily ivacaftor for Kalydeco®.
The management of these drug–drug interactions, however, is significantly complicated by the variability of triazole pharmacokinetics [62–64]. Gastrointestinal absorption of itraconazole and posaconazole is strongly influenced by drug formulation, gastric pH and co-administration with food and frequency of administration [65]. Voriconazole meanwhile exhibits nonlinear and saturable pharmacokinetics, making drug exposure after dose adjustments unpredictable. The area under the curve (AUC) varies according to age, sex and CYP2C19 polymorphism [66]. Current first-line treatment for CF-related fungal disease is inconsistent, but more commonly itraconazole or voriconazole [67]. Therapeutic drug monitoring has a key role in improving patient outcomes, preventing increasing prevalence of antifungal azole resistance and minimising toxicity, but studies have shown widespread prevalence of subtherapeutic azole levels in CF, potentially resulting in ineffective CFTR modulator therapeutic dosing [68–71]. To date, there is little real-world data on the implication of azole interaction on CFTR modulator dosing and efficacy. Isavuconazole, a novel azole with more predictable pharmacokinetics and reduced drug–drug interactions, may present a better therapeutic choice, but there is as yet limited data on CFTR modulator interaction.
Alternative antifungal therapy such as the echinocandin (e.g. Caspofungin) and polyene (e.g. amphotericin) classes do not interact with CFTR modulator therapy but currently are only available in intravenous form, making them impractical in a chronic setting where long duration therapy is needed. Longer duration echinocandin therapy with weekly dosing (e.g. Rezafungin) and novel oral formulation glucan synthase inhibitor antifungal therapy with minimal cyp interaction (e.g. Ibrexafungerp) are, however, currently in phase 3 clinical trials in an invasive fungal infection setting [72, 73]. Whether these drugs would be effective in CF fungal disease and present an alternative attractive therapeutic option within a CFTR modulator setting in the future remains to be seen.
Conclusion
In conclusion, fungal disease in individuals with CF provides an evolving clinical challenge with new resistance and therapies developing. The advent of CFTR modulators represents a significant revolution in CF care and is likely to alter the phenotype of CF lung disease. The effect these drugs will have on the prevalence and presentation of fungal disease, and the impact of antifungal therapy in patients on CFTR modulators is not yet clear. Further research is required to systematically assess CF fungal disease and antifungal therapy as increasing numbers of patients gain access to these life-changing treatments.
Funding
AS is supported by a MRC Clinical Academic Research Partnership award MRC; MR/TOO5572/1 and by the MRC centre grant MRC; MR/R015600/1.
Ethics declarations
Conflict of interest
AS reports grants from Vertex pharmaceuticals and Gilead Sciences and speaker fees from Pfizer and Gilead Sciences.
Footnotes
Handling Editor: Sanjay Haresh Chotirmall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Warris A, Bercusson A, Armstrong-James D. Aspergillus colonization and antifungal immunity in cystic fibrosis patients. Med Mycol. 2019;57:S118. doi: 10.1093/mmy/myy074. [DOI] [PubMed] [Google Scholar]
- 2.Noni M, Katelari A, Dimopoulos G, Kourlaba G, Spoulou V, Athanassoulis HA, et al. Inhaled corticosteroids and Aspergillus fumigatus isolation in cystic fibrosis. Med Mycol. 2014;52:715. doi: 10.1093/mmy/myu038. [DOI] [PubMed] [Google Scholar]
- 3.de Vrankrijker AMM, van der Ent CK, van Berkhout FT, Stellato RK, Willems RJL, Bonten MJM, et al. Aspergillus fumigatus colonization in cystic fibrosis: Implications for lung function? Clin Microbiol Infect. 2011;17:1381. doi: 10.1111/j.1469-0691.2010.03429.x. [DOI] [PubMed] [Google Scholar]
- 4.Düesberg U, Wosniok J, Naehrlich L, Eschenhagen P, Schwarz C. Risk factors for respiratory Aspergillus fumigatus in German Cystic Fibrosis patients and impact on lung function. Sci Rep. 2020;75:75886. doi: 10.1038/s41598-020-75886-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong G, Psoter KJ, Jennings MT, Merlo CA, Boyle MP, Hadjiliadis D, et al. Risk factors for persistent Aspergillus respiratory isolation in cystic fibrosis. J Cyst Fibros. 2018;17:624. doi: 10.1016/j.jcf.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter CG, Dunn G, Jones AM, Webb K, Gore R, Richardson MD, et al. Novel immunologic classification of aspergillosis in adult cystic fibrosis. J Allergy Clin Immunol. 2013;132:560. doi: 10.1016/j.jaci.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Maturu VN, Agarwal R. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: Systematic review and meta-analysis. Clin Exp Allergy. 2015;45:1765. doi: 10.1111/cea.12595. [DOI] [PubMed] [Google Scholar]
- 8.Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis—state of the art: cystic fibrosis foundation consensus conference. Clin Infect Dis. 2003;37:S225. doi: 10.1086/376525. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 10.Kraemer R, Deloséa N, Ballinari P, Gallati S, Crameri R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2006;5:S53. doi: 10.1164/rccm.200603-423OC. [DOI] [PubMed] [Google Scholar]
- 11.De Baets F, De Keyzer L, Van daele S, Schelstraete P, Van Biervliet S, Van Braeckel E, , et al. Risk factors and impact of allergic bronchopulmonary aspergillosis in Pseudomonas aeruginosa-negative CF patients. Pediatr Allergy Immunol. 2018;29:726. doi: 10.1111/pai.12953. [DOI] [PubMed] [Google Scholar]
- 12.Kaditis AG, Miligkos M, Bossi A, Colombo C, Hatziagorou E, Kashirskaya N, et al. Effect of allergic bronchopulmonary aspergillosis on FEV 1 in children and adolescents with cystic fibrosis: a European cystic fibrosis society patient registry analysis. Arch Dis Child. 2017;102:742. doi: 10.1136/archdischild-2016-311132. [DOI] [PubMed] [Google Scholar]
- 13.Brandt C, Roehmel J, Rickerts V, Melichar V, Niemann N, Schwarz C. Aspergillus bronchitis in patients with cystic fibrosis. Mycopathologia. 2018;183:61. doi: 10.1007/s11046-017-0190-0. [DOI] [PubMed] [Google Scholar]
- 14.Fillaux J, Brémont F, Murris M, Cassaing S, Tétu L, Segonds C, et al. Aspergillus sensitization or carriage in cystic fibrosis patients. Pediatr Infect Dis J. 2014;33:680. doi: 10.1097/INF.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 15.Chotirmall SH, Branagan P, Gunaratnam C. McElvaney NG (2008) Aspergillus/allergic bronchopulmonary aspergillosis in an Irish cystic fibrosis population: a diagnostically challenging entity. Respir Care. 2008;53:1035. [PubMed] [Google Scholar]
- 16.Reece E, Segurado R, Jackson A, McClean S, Renwick J, Greally P. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: An Irish registry analysis. BMC Pulm Med. 2017;17:70. doi: 10.1186/s12890-017-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuthbertson L, Felton I, James P, Cox MJ, Bilton D, Schelenz S, et al. The fungal airway microbiome in cystic fibrosis and non-cystic fibrosis bronchiectasis. J Cyst Fibros. 2020;13:S1569. doi: 10.1016/j.jcf.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel TGP, Slabbers L, de Jong C, Melchers WJG, Hagen F, Verweij PE, et al. Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients—a Dutch, multicentre study. J Cyst Fibros. 2019;18:221. doi: 10.1016/j.jcf.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 19.de Jong CCM, Slabbers L, Engel TGP, Yntema JB, van Westreenen M, Croughs PD, et al. Clinical relevance of Scedosporium spp. and Exophiala dermatitidis in patients with cystic fibrosis: a nationwide study. Med Mycol. 2020;58:859. doi: 10.1093/mmy/myaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 21.Painter RG, Valentine VG, Lanson NA, Leidal K, Zhang Q, Lombard G, et al. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muro M, Mondejar-López P, Moya-Quiles MR, Salgado G, Pastor-Vivero MD, Lopez-Hernandez R, et al. HLA-DRB1 and HLA-DQB1 genes on susceptibility to and protection from allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Microbiol Immunol. 2013;57:193. doi: 10.1111/1348-0421.12020. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhary N, Datta K, Askin FB, Staab JF, Marr KA. Cystic fibrosis transmembrane conductance regulator regulates epithelial cell response to Aspergillus and resultant pulmonary inflammation. Am J Respir Crit Care Med. 2012;185:301. doi: 10.1164/rccm.201106-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iannitti RG, Napolioni V, Oikonomou V, De Luca A, Galosi C, Pariano M, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun. 2016;7:10791. doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reihill JA, Moore JE, Elborn JS, Ennis M. Effect of Aspergillus fumigatus and Candida albicans on pro-inflammatory response in cystic fibrosis epithelium. J Cyst Fibros. 2011;10:401. doi: 10.1016/j.jcf.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Assani K, Tazi MF, Amer AO, Kopp BT. IFN-γ stimulates autophagy-mediated clearance of Burkholderia cenocepacia in human cystic fibrosis macrophages. PLoS ONE. 2014;9:e96681. doi: 10.1371/journal.pone.0096681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopp BT, Abdulrahman BA, Khweek AA, Kumar SB, Akhter A, Montione R, et al. Exaggerated inflammatory responses mediated by Burkholderia cenocepacia in human macrophages derived from Cystic fibrosis patients. Biochem Biophys Res Commun. 2012;424:221. doi: 10.1016/j.bbrc.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assani K, Shrestha CL, Robledo-Avila F, Rajaram MV, Partida-Sanchez S, Schlesinger LS, et al. Human cystic fibrosis macrophages have defective calcium-dependent protein kinase C activation of the nadph oxidase, an effect augmented by Burkholderia cenocepacia. J Immunol. 2017;198:1985. doi: 10.4049/jimmunol.1502609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sagel SD, Kapsner R, Osberg I, Sontag MK, Accurso FJ. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164:1425. doi: 10.1164/ajrccm.164.8.2104075. [DOI] [PubMed] [Google Scholar]
- 31.Gray RD, Hardisty G, Regan KH, Smith M, Robb CT, Duffin R, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73:134. doi: 10.1136/thoraxjnl-2017-210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunel SF, Willment JA, Brown GD, Devereux G, Warris A. Aspergillus-induced superoxide production by cystic fibrosis phagocytes is associated with disease severity. ERJ Open Res. 2018;4:68. doi: 10.1183/23120541.00068-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, et al. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 2010;12:928. doi: 10.1016/j.micinf.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Marcos V, Zhou-Suckow Z, Önder Yildirim A, Bohla A, Hector A, Vitkov L, et al. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflamm. 2015;20:1. doi: 10.1155/2015/408935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedke T, Iannitti RG, De Luca A, Giovannini G, Fallarino F, Berges C, et al. Distinct and complementary roles for Aspergillus fumigatus-specific Tr1 and Foxp3+ regulatory T cells in humans and mice. Immunol Cell Biol. 2014;92:659. doi: 10.1038/icb.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker KL, Gresnigt MS, Smeekens SP, Jacobs CW, Magis-Escurra C, Jaeger M, et al. Pattern recognition pathways leading to a Th2 cytokine bias in allergic bronchopulmonary aspergillosis patients. Clin Exp Allergy. 2015;45:423. doi: 10.1111/cea.12354. [DOI] [PubMed] [Google Scholar]
- 37.Murdock BJ, Shreiner AB, McDonald RA, Osterholzer JJ, White ES, Toews GB, et al. Coevolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect Immun. 2011;79:125. doi: 10.1128/IAI.00508-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. 2011;44:922. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allard JB, Poynter ME, Marr KA, Cohn L, Rincon M, Whittaker LA. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol. 2006;177:5186. doi: 10.4049/jimmunol.177.8.5186. [DOI] [PubMed] [Google Scholar]
- 40.Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;120:518. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bacher P, Hohnstein T, Beerbaum E, Röcker M, Blango MG, Kaufmann S, et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell. 2019;176:1340. doi: 10.1016/j.cell.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 42.Nick JA, St. Clair C, Jones MC, Lan L, Higgins M. Ivacaftor in cystic fibrosis with residual function: lung function results from an N-of-1 study. J Cyst Fibros. 2020;19:91. doi: 10.1016/j.jcf.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 43.McKone EF, Borowitz D, Drevinek P, Griese M, Konstan MW, Wainwright C, et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST) Lancet Respir Med. 2014;2:904. doi: 10.1016/S2213-2600(14)70218-8. [DOI] [PubMed] [Google Scholar]
- 44.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377:2013. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 46.Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, et al. Tezacaftor–ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med. 2017;377:2024. doi: 10.1056/NEJMoa1709847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G, et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res. 2019;5:00082. doi: 10.1183/23120541.00082-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a Single Phe508del Allele. N Engl J Med. 2019;381:1809. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frost FJ, Nazareth DS, Charman SC, Winstanley C, Walshaw MJ. Ivacaftor is associated with reduced lung infection by key cystic fibrosis pathogens a cohort study using national registry data. Ann Am Thorac Soc. 2019;16:1375. doi: 10.1513/AnnalsATS.201902-122OC. [DOI] [PubMed] [Google Scholar]
- 51.Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, et al. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis. 2015;60:703. doi: 10.1093/cid/ciu944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bessonova L, Volkova N, Higgins M, Bengtsson L, Tian S, Simard C, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax. 2018;73:731. doi: 10.1136/thoraxjnl-2017-210394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdolrasouli A, Petrou MA, Park H, Rhodes JL, Rawson TM, Moore LSP, et al. Surveillance for azole-resistant Aspergillus fumigatusin a centralized diagnostic mycology service, London, United Kingdom, 1998–2017. Front Microbiol. 2018;9:2234. doi: 10.3389/fmicb.2018.02234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pohl K, Hayes E, Keenan J, Henry M, Meleady P, Molloy K, et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124:999. doi: 10.1182/blood-2014-02-555268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardisty GR, Law SM, Carter S, Grogan B, Singh PK, McKone EF, et al. Ivacaftor modifies cystic fibrosis neutrophil phenotype in subjects with R117H residual function CFTR mutations. Eur Respir J. 2020;57:2002161. doi: 10.1183/13993003.02161-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnaby R, Koeppen K, Nymon A, Hampton TH, Berwin B, Ashare A, et al. Lumacaftor (VX-809) restores the ability of CF macrophages to phagocytose and kill Pseudomonas aeruginosa. Am J Physiol Cell Mol Physiol. 2018;314:432. doi: 10.1152/ajplung.00461.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang S, Shrestha CL, Kopp BT. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci Rep. 2018;8:17066. doi: 10.1038/s41598-018-35151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Currie AJ, Main ET, Wilson HM, Armstrong-James D, Warris A. CFTR modulators dampen aspergillus-induced reactive oxygen species production by cystic fibrosis phagocytes. Front Cell Infect Microbiol. 2020;10:372. doi: 10.3389/fcimb.2020.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garg V, Shen J, Li C, Agarwal S, Gebre A, Robertson S, et al. Pharmacokinetic and drug–drug interaction profiles of the combination of Tezacaftor/Ivacaftor. Clin Transl Sci. 2019;12:267. doi: 10.1111/cts.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson SM, Luo X, Dubey N, Li C, Chavan AB, Gilmartin GS, et al. Clinical drug–drug interaction assessment of ivacaftor as a potential inhibitor of cytochrome P450 and P-glycoprotein. J Clin Pharmacol. 2015;55:56. doi: 10.1002/jcph.377. [DOI] [PubMed] [Google Scholar]
- 61.Rivosecchi RM, Samanta P, Demehin M, Nguyen MH. Pharmacokinetics of azole antifungals in cystic fibrosis. Mycopathologia. 2018;183:139. doi: 10.1007/s11046-017-0189-6. [DOI] [PubMed] [Google Scholar]
- 62.Berge M, Guillemain R, Boussaud V, Pham MH, Chevalier P, Batisse A, et al. Voriconazole pharmacokinetic variability in cystic fibrosis lung transplant patients. Transpl Infect Dis. 2009;11:211. doi: 10.1111/j.1399-3062.2009.00384.x. [DOI] [PubMed] [Google Scholar]
- 63.Hennig S, Wainwright CE, Bell SC, Miller H, Friberg LE, Charles BG. Population pharmacokinetics of itraconazole and its active metabolite hydroxy-itraconazole in paediatric cystic fibrosis and bone marrow transplant patients. Clin Pharmacokinet. 2006;45:1099. doi: 10.2165/00003088-200645110-00004. [DOI] [PubMed] [Google Scholar]
- 64.Hennig S, Waterhouse TH, Bell SC, France M, Wainwright CE, Miller H, et al. A D-optimal designed population pharmacokinetic study of oral itraconazole in adult cystic fibrosis patients. Br J Clin Pharmacol. 2007;63:438. doi: 10.1111/j.1365-2125.2006.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Theuretzbacher U, Clancy CJ, Nguyen MH, Derendorf H. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin Pharmacokinet. 2010;49:379. doi: 10.2165/11319340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 66.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45:649. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 67.Boyle M, Moore JE, Whitehouse JL, Bilton D, Downey DG. The diagnosis and management of respiratory tract fungal infection in cystic fibrosis: a UK survey of current practice. Med Mycol. 2019;57:155. doi: 10.1093/mmy/myy014. [DOI] [PubMed] [Google Scholar]
- 68.Periselneris J, Nwankwo L, Schelenz S, Shah A, Armstrong-James D. Posaconazole for the treatment of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. J Antimicrob Chemother. 2019;74:1701. doi: 10.1093/jac/dkz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D M, M N, R S, Y T, C C. Therapeutic drug monitoring among lung transplant recipients receiving voriconazole prophylaxis: Identification of preventive breakpoints. Clin Microbiol Infect. 2011;
- 70.Dave K, Di Paolo M, Vijayasingam A, Sheth R, Luke E, Scourfield A, et al. P075 Anti-fungal therapeutic drug monitoring in adults with cystic fibrosis. J Cyst Fibros. 2018;17:S80. doi: 10.1016/S1569-1993(18)30372-2. [DOI] [Google Scholar]
- 71.Abdolrasouli A, Scourfield A, Rhodes J, Shah A, Elborn JS, Fisher MC, et al. High prevalence of triazole resistance in clinical Aspergillus fumigatus isolates in a specialist cardiothoracic centre. Int J Antimicrob Agents. 2018;52:637. doi: 10.1016/j.ijantimicag.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Sofjan AK, Mitchell A, Shah DN, Nguyen T, Sim M, Trojcak A, et al. Rezafungin a next-generation echinocandin: A systematic literature review and assessment of possible place in therapy. J Glob Antimicrob Resist. 2018;14:58. doi: 10.1016/j.jgar.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Davis MR, Donnelley MA, Thompson GR. Ibrexafungerp: A novel oral glucan synthase inhibitor. Med Mycol. 2019;58:579. doi: 10.1093/mmy/myz083. [DOI] [PubMed] [Google Scholar]