Abstract
A new species, Alliumnegianum (Amaryllidaceae), belongs to the genus Alliumsubg.Rhizirideum, sect. Eduardia is described here from the Uttarakhand Himalayan region of India. This taxon grows in Malari region of Niti valley in Chamoli district and Dharma valley of Pithoragarh district, Uttarakhand, India. It is a narrowly distributed species and morphologically more closer to A.przewalskianum Regel but differentiated by its tunic color of bulb, umbel with lax flowers, peduncle length, perigone colour, size and shape and leaf anatomy. Taxonomic delineation and relationship analysis based on nuclear ribosomal Internal Transcribed Spacers (ITS) region indicated that A.negianum is distinct and related to A.przewalskianum. This study provided a comprehensive description and comparison with A.przewalskianum, an identification key and notes on the distribution of the species.
Keywords: Alliumnegianum, India, Rhizirideum, Seasoning spice, Uttarakhand
Introduction
Allium L., one of the largest genera in the family Amaryllidaceae, has about 1,100 species distributed world-wide (Li et al. 2010; Govaerts et al. 2021). The genus Allium naturally occurs in dry seasons in the northern hemisphere and South Africa (Friesen et al. 2006; Nguyen et al. 2008; Neshati and Fritsch 2009). The primary centre of evolution for the genus extends across the Irano-Turanian bio-geographical region, and the Mediterranean basin and western North America are considered as the secondary centres of diversity (Friesen et al. 2006). The genus is characterized by bulbs that are enclosed within the membranous or fibrous tunics, free tepals, often a subgynobasic style and well-known characteristic plant odour and taste due to the presence of cysteine sulphoxides (Friesen et al. 2006). The classification of global species in the genus Allium is based on molecular phylogenetic analyses, which includes 15 subgenera and 56 sections (Friesen et al. 2006). The Indian Allium includes over 10 subgenera, 22 sections and 35–40 taxa excluding cultivated species distributed in different eco-geographical areas of the temperate and alpine regions of Himalayas sharing many taxa of Chinese origin (Pandey et al. 2008, 2017; Li et al. 2010). Indian Himalayan region has two distinct centres of diversity, the western Himalaya (over 85 per cent of total diversity) and the eastern Himalaya (6 per cent), covering the alpine-sub temperate region (2500–4500 m a.s.l.) (Gohil 1992; Pandey et al. 2008).
Globally Alliumsubg.Rhizirideum (G.Don ex Koch) Wendelbo s.str. has ca. 37 taxa that are included in four sections distributed mainly in Europe-East Asia, in China (Friesen et al. 2006; Choi et al. 2012; Jang et al. 2021) and also in Russia, Mongolia and Kazakstan (Sinitsyna et al. 2016; Friesen et al. 2020). Alliumsenescens L. of sect. Rhizirideum a species native to northern Europe and Asia from Siberia-Korea and also naturalized in parts of Europe, is an exception (Xu and Kamelin 2000; Li et al. 2010).
Taxa of the subg. Rhizirideum belong to the third and the most advanced evolutionary line, which is phylogenetically sister to taxa of the subg. Allium L., Cepa L., Reticulatobulbosa (Kamelin) N.Friesen and Polyprason Radic. (Friesen et al. 2006; Memariani et al. 2007; Li et al. 2010; Choi et al. 2012). The sect. Eduardia N.Friesen of the subg. Rhizirideum is mainly distributed in the western Himalaya with Pakistan on the west and Nepal and Tibet in the centre, and southwest China on the eastern side. Its habitat mainly comprises of mountainous, snow peak grassland, dry or rocky places in forests, subalpine meadows, steppes, sunny, saline areas, sandy deserts, stony and gravelly slopes, rocky crevices along the stream banks and damp places (Fritsch and Friesen 2002; Choi and Oh 2011; Choi et al. 2012).
Despite the importance of the genus Allium for the Indian region, meagre comprehensive studies have been attempted pertaining to molecular and taxonomic evaluation that led to gaps in the status of interspecific and infraspecific relationships among the taxa. Meagre taxonomic studies on the native taxa, unavailability of material for research, sporadic collections from under-explored/unexplored areas and lack of the published literature have led to the possibility of finding new taxonomic records from the Indian region (Pandey et al. 2008, 2017, 2021).
The subg. Rhizirideum is the smallest subgenus of Allium as per the flora of India, and it is represented only by the sect. Eduardia containing only one species, A.przewalskianum Regel. This taxon occurs in the scrub, drier slopes, ravines and rocky crevices (2000–4500 m a.s.l.) in Leh, Jammu and Kashmir and Spiti in Himachal Pradesh. The taxa under subg. Rhizirideum are characterized by the presence of several narrowly ovoid-cylindric bulbs, which borne on creeping rhizome usually covered with a common reticulate membrane, leaves shorter than scape, adaxially channeled and stamens slightly longer than perigone segments, spathe with a long beak, nearly 2 to 3 times longer than the base and hemispherical umbel. Most species share a basic chromosome number of x = 8 and 2n = 16 or 32. Occurrence of a polyploid complex in different sections of the subgenus Rhizirideum indicated recent origin of taxa as supported by phylogenetic and biogeographical evidences (Li et al. 2010). Areas with geographical isolation are the driving force of underestimated speciation (Seregin et al. 2015).
A new taxon, Alliumnegianum, was collected from the Indo-Tibetan border area of Malari village, Niti valley of Chamoli district in Uttarakhand (India) in 2019 and identity was confirmed by the authors. It is distinct from its closest relative, A.przewalskianum Regel (Table 2), the only taxon of subg. Rhizirideum, sect. Eduardia in India. It is characterized by finely reticulated red-brown outer tunics, hemispherical umbel having lax flowers, spathe with a very long beak, deep purple tepals, asynchronous flowering and inner stamen filaments having longer and sharp teeth. In the present work, A.negianum, is described and illustrated here. Authors have examined the evidences from morphology, eco-geography, leaf anatomy, molecular study, and taxonomic delineation from other related species.
Table 2.
Major morphological characters* (discriminating characters in bold) of Alliumnegianum in comparison with A.przewalskianum.
| Character | A.przewalskianum | A.negianum |
|---|---|---|
| Habitat | Carbonaceous slates-gravel; 3300–5200 m | Grassy meadows, open sandy slopes, along rivers/ streams; 3000–4800 m |
| Plant habit | Erect | Semi-erect |
| Plant growth (under experimental condition) | Robust, shorter | Taller, plants and leaves |
| Plant height (cm) | 20–45 | 27–50 |
| Bulbs no. in cluster | 2–4 | 2–7 |
| Bulb no., shape | Cluster 3–4; cylindrical-narrowly ovoid | Cluster 4–8; cylindrical-narrowly ovoid |
| Bulb length (cm) | 10.2–12.5 | 6.8–12 |
| Bulb diameter (cm) | 0.6–0.7 | 0.8–1.2 |
| Tunic outer** | Finely reticulate; reddish-orange-brown | Finely reticulate; reddish-dark brown |
| Tunic inner | Membranous, brown-red | Membranous, orange-red |
| Rhizome type; size (mm) | Vertical, short; 3–5 | Oblique; 7–12 |
| Leaf no., colour | 3–5, lighter brown-green | 4–6, dark green |
| Leaf vs. scape | Much shorter than scape | Slightly shorter than scape |
| Leaf blade shape; apex | Linear, not fistular; obtuse to subrounded | Linear, filiform; acute |
| Leaf length (cm) | 15–30 | 12–40 |
| Leaf width (mm) | 2.0–2.5 | 1–3.2 |
| Leaf erectness | Erect | Erect-semierect |
| Leaf waxiness | Non-waxy | Waxy |
| Leaf cross section | Circular | Circular |
| Spathe valve if persistent | 1(2)-valved, persistent | 1-valved, persistent |
| Spathe valve shape, size | Ovate | Ovate-oblong |
| Spathe size (cm) | 2–3 (two times the base; short, blunt) | 4–6 (long narrow beak; 3 times the base) |
| Scape type | Solid, terete, erect, central | Solid, terete, erect to semi-erect, lateral-central |
| Scape size (cm) | 30–40 × 0.2–0.35 | 20–50 × 0.36–0.48; 1/3-of the base |
| Pedicel vs. perigone | Subequal | 2–3 times longer |
| Umbel flower opening pattern | Synchronous (80 per cent) | Asynchronous (30–40 per cent) |
| Umbel shape | Spherical-hemispherical, densely flowered, compact | Hemispherical, lax, loosely flowered |
| Umbel diameter (mm) | 28.5–30.2 | 25.1–42.0 |
| Umbel flower (no.) | 25–40 | 30–40 |
| Peduncle size (cm) | 0.5–1.0 | 0.8–2.5 |
| Flower size (cm) | 0.4–0.5×0.3 | 0.5–0.5× 0.4 |
| Flower color | Pale red-purple pink (variable) | Dark purple (as recorded now) |
| Perigonium shape and color | Campanulate, pink-dark purple, tepal wide open | Campanulate, lilac, light to dark purple, tepal partly opened |
| Tepal shape | Ovate-lanceolate, apex obtuse | Elliptic, ovate-lanceolate; apex-acuminate-mucronate |
| Tepal inner size length × width (cm) | 0.3–0.4 ×0.2–0.3 | 0.5–0.6 × 0.3–0.4 |
| Tepal outer size length × width (cm) | 0.5–0.7 × 0.2–0.3 | 0.6–0.7 × 0.3–0.5 |
| Tepal apex shape | Acute-acuminate | Acute, mucronate |
| Tepal maturity | Curved outwards | Slightly inwardly curved/rolled |
| Tepal mid-vein | Non-conspicuous; purple green-dark purple | Very conspicuous; green-light green |
| Anther length (mm) | 6.1–9.3 | 6.8–8.5 |
| Anther lobe length (mm) | Oblong-ovate, 1–2 | Oblong, 1–2 |
| Anther lobe color | Yellow-purple | Yellow-purple |
| Filament color | Yellowish-purple | Greenish yellow-purplish green |
| Filament length, position | Double the size of tepal; exserted, | Half the size of tepal; slightly exerted |
| Filament inner and outer anther | Inner – two sharp teeth up to 1/2 to 1/4 length of filament with broader base; outer narrower base | Inner – two shallower-sharper teeth up to 1/2 to 2/3 length of filament with base as wide as tepal; outer narrower base |
| Ovary shape | Ovoid – globose, wrinkled | Obovoid – subglobose |
| Ovary color | Purple green, tinged with purple | Dark-pale purple |
| Ovary style vs. anther (after pollination) | Much exserted, longer than the ovary | Slightly exserted or equal |
| Ovary stigma tip | Acuminate-acute | Acuminate |
| Stigma vs. stamen | Sub-equal | Slightly longer |
| Capsule shape | Ovoid | Sub-globose |
| Seed length (mm) | 2.75–2.96 | 3.21–4.05 |
| Seed width (mm) | 1.55–1.59 | 1.92–1.97 |
| Seed color | Dull black | Shiny black |
| Seed no./locule | 2 | 2 |
| 1000 seed wt (g) | 2.12 | 2.73 |
| Odour when crushed# | Strong onion-light garlic | Strong onion-garlic |
*data recorded from a minimum of 30 specimens of each taxon; additional 43 e-images; **: recorded immediately after uprooting; #: data on feedback; also refer Pandey et al. 2021.
Materials and methods
Taxon sampling and morphological descriptor
A total of 110 plants representing 7 accessions of the new species were collected from the type locality and farmers’ fields in the Niti region of Uttarakhand, India. For delimitation of the taxon with other related species, plants were grown in the Field Gene Bank (FGB) at the ICAR-National Bureau of Plant Genetic Resources (ICAR-NBPGR), Regional Station Bhowali (Nainital), Uttarakhand for comparative study of morphological characters. Data were recorded using the Allium descriptor with modifications from the published literature. The floral characters were measured with separate parts to the nearest ten points of the decimal. The seeds having uniform size and maturity were recorded for ultra-features of the characters using the Stereozoom Microscope (LMI, England, model no. SZM167), and the images were captured as JPEG. Ten replicate voucher herbarium specimens of the new species were prepared as per standard procedure and deposited in the National Herbarium of Cultivated Plants (code-NHCP) (Holotype) and CAL (Isotype).
The new species was compared with its closest relative using data derived from the study of specimens preserved in the herbaria of CAL, DD, E, K and NHCP and available literature. Due to its closer affinity with A.przewalskianum, all the specimens from diverse sources were critically examined. Taxonomic description and identification key were provided for Alliumnegianum and affined species.
Leaf anatomy
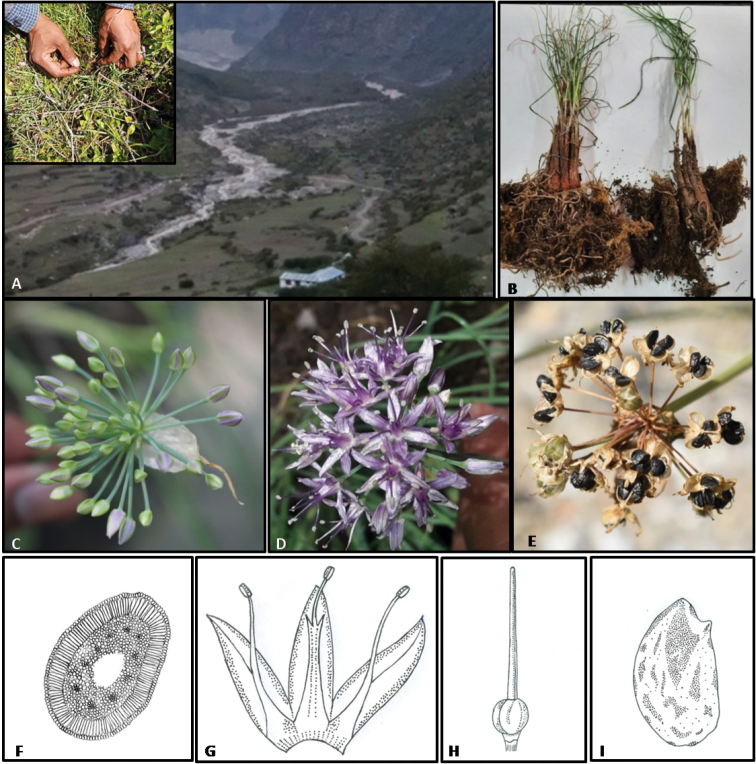
For leaf anatomy live plants were grown in the FGB at Regional Station Bhowali (Nainital), Uttarakhand. Leaf-blades were taken from a point 3–4 cm above the sheaths and fixed in 70% alcohol. Cross-sections were made at three different lengths of leaf and stained with Sartur solution (a mix of sudan III, aniline, chloral hydrate, lactic acid, iodine), the structure was studied, and analyzed with the help of a light microscope (Olympus BH-2) and line diagrammes drawn. The outlines of cells were diagrammatically depicted (Fig. 1F).
Figure 1.
AlliumnegianumA general habitat B bulb covered with reticulate fiber on bulbs of A.przewalskianum (orange-red) and A.negianum (red-brown) C inflorescence and spathe with a very long beak, persistent D inflorescence E capsule with mature seeds F line-illustrations of transverse section of leaf showing hollow channel G longitudinal section of flower with stamen with two sharp teeth H ovary I seed with prominent beak (C–I magnification × 30–40).
Taxonomic delineation and relationship analysis
DNA extraction, amplification and sequencing
Genomic DNA of nine known species and one new taxon (Table 1) which was collected from western Himalayan region and maintained as live material at Field Gene Bank (FGB), ICAR- National Bureau of Plant Genetic Resources, Regional Station, Bhowali, was isolated from fresh leaves using spin column-based Qiamp DNA kit according to the suppliers’ protocol. Selection of taxa for this study was mainly based on the fact that all taxa belong the third evolutionary line representing same eco-geographical areas, and were known by similar local names. This has resulted in confusion of their identity in Indian literature. The quantity and purity of the isolated genomic DNA was tested using the spectrophotometric method. The universal primers ITS1 and ITS4 (White et al. 1990) were used to amplify the ITS regions. The PCR protocol was run at 94 ° C for 5 minutes; 30 cycles of 94 °C for 45 seconds, 55 °C for 45 seconds and 74 °C for 45 seconds and 74 °C for 5 minutes. PCR products were purified using Zymo DNA concentrator kit following the supplier’s protocol. The purified PCR product was used in ABI 3730 DNA sequencer (Applied Biosystems) for generating sequences using PCR primers as sequencing primers. For remaining species from subgenus Rhizirideum and other related sub-genera, the ITS sequence were used from NCBI database.
Table 1.
List of Allium taxa used to generate nuclear ITS sequence in the study.
| S. no. | Taxon name | Subgenus | Section | NGB accession number | District; state |
|---|---|---|---|---|---|
| 1 | Alliumtuberosum Rottler ex Spreng. | Butomissa | Butomissa | IC353524 | Almora; Uttarakhand |
| 2 | Alliumstracheyi Baker | Polyprason | Orioprasum | IC567645 | Pithoragarh; Uttarakhand |
| 3 | Alliumprzewalskianum Regel | Rhizirideum | Eduardia | IC632207 | Leh; Jammu and Kashmir |
| 4 | Alliumnegianum sp. nov. | Rhizirideum | Eduardia | IC258493 | Chamoli; Uttarakhand |
| 5 | Alliumsativum L. | Allium | Allium | IC278243 | Chamoli; Uttarakhand |
| 6 | Alliumampeloprasumvar.ampeloprasum | Allium | Allium | IC353526 | Pithoragarh; Uttarakhand |
| 7 | Alliumcepavar.cepa L. | Cepa | Cepa | IC410711 | Uttarkashi; Uttarakhand |
| 8 | Alliumcepa L. var. aggregatum G.Don | Cepa | Cepa | AP/RP/2014 | Chamoli; Uttarakhand |
| 9 | Alliumoschaninii O.Fedtsch. | Cepa | Cepa | AP/2014 | Voucher; Uttarakhand |
| 10 | Alliumschoenoprasum L. | Cepa | Schoenoprasum | IC632213 | Kargil; Jammu and Kashmir |
Phylogenetic analysis based on the comparison of sequences
The generated DNA sequences from both the primers were checked for alignment using the BioEdit software. Multiple pairwise alignments of generated sequences and from NCBI database were made using ClustalW. The aligned sequences were used to generate the genetic distance between taxa and the evolutionary history, which was inferred by using the Maximum Likelihood method based on the Jukes-Cantor model using MEGA7.0 (Kumar et al. 2016).
Result and discussion
Taxonomic treatment
Allium negianum
A.Pandey, K.M.Rai, Malav & S.Rajkumar sp. nov.
040A06F2-2C03-5ECB-9E99-47717BA649B0
urn:lsid:ipni.org:names:77220799-1
Figure 2.
Holotype specimen of Alliumnegianum deposited in NHCP.
Type.
India, Uttarakhand: Chamoli, rocky areas (altitude 3000–4800 m), 22 Aug. 2019, KMR/AS/02/19 (Holotype: NHCP; Isotype: CAL; Seeds conserved in the National Genebank, New Delhi: IC258493).
Description.
Herbs, hermaphrodite, 27–50 cm tall. Rhizome condensed, 6.5–8.5 mm long, oblique. Bulb clustered, cylindric to narrowly ovoid, 0.8–1.2 cm in diameter, 6.8–12 cm long, outer tunic finely reticulate, reddish-dark brown, inner membranous, light-brick red. Leaves 4–6, slightly shorter than scape, 12–40 cm × 1.0–3.2 mm, erect, to semi-terete to terete, dark green; base slightly bulbous. Scape terete, semi-erect, covered with leaf sheaths at base only, stout, solid in cross-section (hollow in mature), 15–30 cm × 3.5–5.5 mm. Spathe 1-valved, persistent, beak very narrow-long, 2.5–4 mm. Inflorescence umbellate, hemispheric, 30–40 lax flowered. Peduncle subequal, 16–18 × 2–3 mm, without bulbils. Flowers bisexual, perigone campanulate, tepals dark purple with distinct green mid-line; inner tepals slightly longer than outer ones, oblong-lanceolate, apex acute, 6–8 × 3–4 mm; outer segments ovate to narrowly so, 5.5–6 × 2.5–3 mm. Stamens anthers oblong, yellow-purplish (on maturity), 2.3–2.6 mm long; filaments subequal, 6.8–8.5 mm, purple, slightly exserted, connate at base and adnate to perigone segments; outer ones subulate; inner ones broadened for 1/2–1/4 to their length, one sharp toothed on each side. Ovary sub-globose, purple-tinged, 3.6–4.8 × 1.8–3.5 mm. Style terete, exserted, stigma smooth, acute-acuminate, ovules 2 per locule. Capsules trigonous, 5–5.5 × 5.8–7.2 mm; seeds obovate with a prominent notch on one side, 3.2–4.0 × 1.9–1.9 mm, testa deep black. Plant has strong onion-garlic type aroma.
Habitat.
Slopes, sandy soils along rivers and streams along the alpine meadows (altitude 3000–4800 m asl) in Sumna valley (villages Gamsali, Niti, Tolma, Kailashpur and Farkya) in Chamoli district near Malari glacier of India.
Etymology.
The specific epithet, “negianum”, is named in honour of Late Dr. Kuldeep Singh Negi, an eminent explorer who has dedicated his life in collection of indigenous Allium species germplasm along with associated indigenous knowledge across the country. He was also instrumental in establishing the Allium Field Gene Bank (FGB) at the Regional Station, Bhowali, Uttarakhand. The entire germplasm of indigenous Allium species collected by him from remote areas of the country are characterized and successfully conserved at AlliumFGB, Bhowali, Uttarakhand.
Vernacular/local name.
Pharan, phran, jambu, sakua, sungdung, kacho, etc. (Pandey et al. 2021).
Phenology.
Flowering and fruiting is from June to middle September (altitude 3000–4800 m a.s.l.).
Leaf anatomy.
The transverse section of the leaf of A.negianum showed an elliptical outline. The epidermis has small cells covered with a thin cuticle layer, and stomata are narrowly distributed along the surface area. Single layered compactly arranged palisade tissue comprised of long cylindrical cells. The mesophyll cells are spongy tissue and compact in young leaf as well in the proximal ends of mature leaf while in the centre part of mature leaf, broken mesophyll cells are confused with fistulous leaf appearance; 10–12 vascular bundles are arranged along with the palisade tissue across the entire circumference (Fig. 1F).
Seed morphology.
Seed characters and testa sculptures represents a good taxonomic character in Allium (Neshati and Fritsch 2009; Celep et al. 2012; Lin and Tan 2017). Apparently, the seeds of the newly described species were marginally bigger than the related taxon, A.przewalskianum. Baasanmunkh et al. (2020) have discussed on the seed testa structure and its taxonomic implication for taxa of the subg. Rhizirideum. The seed size in A.negianum (Fig. 1I) measured 3.2–4.0 × 1.9–1.9 mm in contrast to 2.7–2.9 × 1.5–1.5 mm in the later taxon (Fig. 1I). The seeds of A.negianum are obovate in shape with a prominent notch on one side, gradually concave from edge to centre, with deep black and wrinkled testa.
The testa cell shape was irregularly hexagonal-pentagonal, loose with clear meshes of reticulated tissue. The anticlinal walls are usually raised, prominently small to intermediate granulose verrucae. The periclinal cells wall has several verrucae with irregular depressions. Study indicated that in subg. Rhizirideum testa cell shape varied from oval to irregular or oval to hemispherical; and seed length 1.30–2.35 mm, anticlinal wall were distinguished by nearly S type to straight and periclinal wall was flat to nearly convex with densely granulated verrucae (Baasanmunkh et al. 2020). A.przewalskianum was distinguished by irregular testa cells in a loose arrangement with reticulated tissue, straight to arched anticlinal walls, and concave periclinal walls with small to intermediate verrucae and granules (Lin and Tan 2017).
Distribution and ecology.
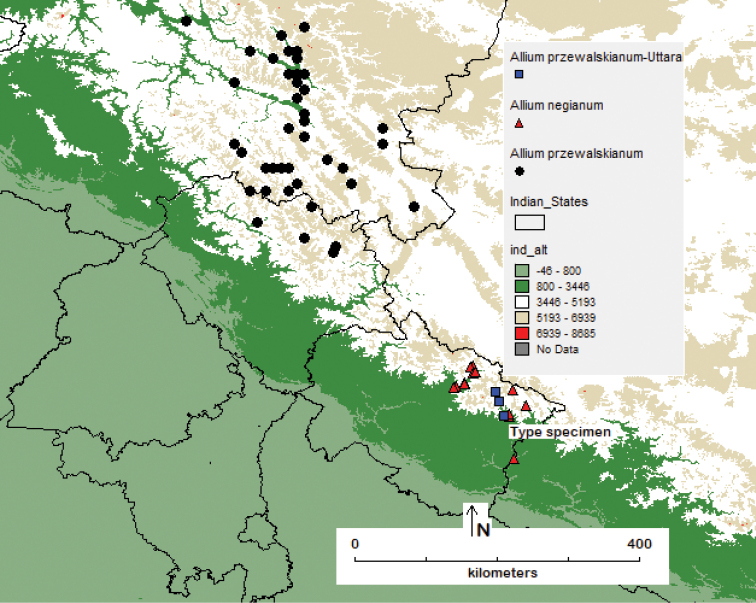
The sect. Eduardia of the subg. Rhizirideum is distributed in the southern most range of the Himalayan region of India extending to China which is the centre of diversification. Alliumnegianum is a species recorded from the southernmost transitional zone between India and China. The distribution of A.negianum is restricted to the phytogeographical region of western Himalaya from Sumna valley, Malari, Chamoli district of Uttarakhand, in western Himalaya, India where it commonly occurs along the open grassy meadows, sandy soils along rivers and streams occurring in the snow pasture lands along the alpine meadows (locally known as ‘bugyal’ or ‘bugial’) between 3000–4800 m a.s.l. (Fig. 1A; Fig. 3) in synanthropic habitats. It was reported growing as wild population in Darma valley of Pithoragarh, along Gori Ganga (also Gori Gad) river in the Munsiyari, Pithoragarh district, in Milam Glacier, in northeast of Nanda Devi, Uttarakhand, India. The seeds flowing with the melting snow led to its broader spread in the areas with good regeneration reported by the authors (Fig. 1A). Hence the taxon may be considered endemic in the area of study. Indiscriminate harvest of leaves and bulbs used for ‘seasoning’ purposes has threatened its wild population.
Figure 3.
Distribution of taxa of Alliumsubg.Rhizirideumsect.Eduardia in India: Alliumnegianum and A.przewalskianum shown by the red triangle and black circled dots respectively; blue rectangle showed the occurrence of A.przewalskianum as per the data from GBIF (records of occurrence from Uttarakhand).
The first report on large scale cultivation of this taxon in Niti valley, Uttarakhand, as ‘seasoning allium spice’ called ‘jambu’ and ‘phran’ has been published (Pandey et al. 2021). Though the taxon was reported commonly under cultivation, the authors have observed the wild populations primarily from the above ‘type’ locality. The authors could not trace large scale cultivation of another taxon, A.stracheyi (used for same purpose and known by same local name) in the described locality in Uttarakhand (Pandey et al. 2021). Considering that A.stracheyi was a rare species reported from wild habitats in Uttarakhand Himalaya, the authors assume that the reports by Kuniyal and Negi (2018) on large scale cultivation may be referring to this newly described taxon which is also known by the same local name. Unfortunately, earlier studies on A.stracheyi did not provide any locality details, nor were the voucher specimens deposited in any herbaria of the material used in their study. Therefore, validation of the taxonomic identity could not be ascertained. Also, there is no occurrence record of the taxa belonging to subg. Rhizirideum from Uttarakhand, India.
Specimens examined
(Paratypes).Alliumprzewalskianum: India. Himachal Pradesh. Spiti, Takcha 25 Jul. 1972 U.C. Bhattacharya 48815 (BSD); Tobo, Kinnaur, Lahul & Spity, 15 Sept. 2007, V.D. Verma & Ramchander (NHCP); Jammu & Kashmir. Ladakh, 25 July 1941, Ludlow & Sheriff8529 (BM); 8 Sep.1941, Ludlow & Sheriff8571 (BM); Ganglas, 1 Aug. 1988, H.J.Chowdhury & B.P.Uniyal 86043 (BSD); 1880, Aitchinson376 (CAL); Kashmir. Nubra, 24 July 1980, A.R. Naqshi & G.N. Dhar7370 under A.stracheyi; Leh (J&K), 8 Sept. 2014, K. Pradheep & P.S. Mehta1733 (NHCP); Leh (J&K), Nov. 2014, K.Pradheep HS21817 (NHCP); Pangu lake, Luthum village, Leh (4500 m), s.s. Malik & D. Gautam15298 (NHCP); Uttarakhand. Malari, Chamoli, 10 Sept. 2019, Badal Singh & K.Madhav RaiHS24013 (NHCP); Alliumauriculatum: Uttarakhand: Brahmmathya, district Chamoli, August 1988, K.S.Negi & M.N.Kopper 9387 (NHCP).
Online herbaria.
A.stoliczki: Ladakh, Khaedubgla, 18 Aug. 1982, P.K.Hazra98623(K), 1985, Jacquemont V. Type (K); T. Thomson, Type (K); China, 1 Jan. 1872, Przewalski N.M., #s.n., Type (P); 01 Jan. 1884, Przewalski N.M., Type (P, K); 1872–1873, Przewalski N.M., #s.n., Type (G).
There are no records on the availability of this new taxon from Uttarakhand (Dasgupta 2006). Shah (2014) has raised doubts on reported cultivation of A.przewalskianum in Uttarakhand by Negi (2006). Also recorded data on the occurrence of allied taxon under A.przewalskianum from Gori, Kumaon, Uttarakhand (dated 16 June 2005) and Gori, Martoli, Uttarakhand (7 Oct. 2004) during the study of a total of 413 specimens in the GBIF database need critical study.
Note.
Alliumnegianum was previously mistaken for identity as A.stracheyi as noted in the published records from India. Despite no morphological similarity with the latter taxon, Kuniyal and Negi (2018) referred ‘phran’ as A.stracheyi. In literature, it was also referred as A.auriculatum and A.przewalskianum due to morphologically similarity of the outer tunics (Pandey et al. 2021). However, the present study demonstrated that A.negianum is clearly distinguished from A.przewalskianum and A.stracheyi, particularly characters of the bulb tunic color when fresh, umbel, teeth in filament and perigone size and color (Fig. 1B–H; Table 1). Alliumnegianum is diploid (2n = 2x = 16) (data not produced), whereas A.przewalskianum is reported to be tetraploid (2n = 4x = 32) as well as diploid with no stated morphological variation except the stout habit. Authors noted that A.negianum has robust plant habit, stronger plant aroma in wild habitat as compared to plants growing under cultivation. In contrast, the related taxon of the subg. Rhizirideum is currently distributed in Jammu and Kashmir, Himachal Pradesh and adjoining parts in Nepal. A.negianum is reported from areas of Uttarakhand and only known from the type locality (altitude 3200–4800 m a.s.l.) and has never been collected from elsewhere in India and other parts of the world. Therefore A.negianum is said to be localized in distribution.
Upon critical examination of specimen of A.auriculatum deposited in the NHCP, all plant characters were found to be closer to A.negianum. Four specimens of this taxon were noted in label data as frequently growing on flat rocks in Brahmmathya, district Chamoli (3800 m asl.), Uttarakhand, used as leaves cooked as a vegetable.
Alliumnegianum is morphologically allied to a Chinese species A.eduardi Stearn that occurs on the dry slopes and plains in the adjoining regions of Mongolia and Russia and shares characters of spathe beak size, hemispherical umbel and perigone shape, but differs in having yellowish-brown bulb tunic color, tepal apex with a reflexed point and shorter stamen teeth length.
Taxnomic treatment
Two species, A.przewalskianum and A.negianum, of the subg. Rhizirideum, sect. Eduardia can be distinguished from A.stracheyi of the subg. Polyprason by using the following key.
Key to Alliumnegianum and related species
| 1 | Bulbs cylindrical-narrowly oblong-ovoid, outer tunic fibrous, with finely reticulate texture, reddish-dark brown, leaves semiterete-terete | 2 |
| – | Bulbs cylindric-narrowly ovoid, outer tunic fibrous scarious, brown-darkest brown, leaves narrow, fistulous | A.stracheyi |
| 2 | Bulbs outer tunic reticulate, reddish, inner tunic membranous, red-orange, rarely light brown; umbel compact globose, tepal pale-red to dark purple; filaments longer than perigone segments, inner ones broadened for 1/3–1/2 their length with shallow teeth; style very much exserted after anthesis | A.przewalskianum |
| – | Bulbs outer tunic reticulate, reddish-brown, inner tunic membranous red; umbel hemi-spherical, lax; tepals dark purple-pink purple; filaments equal to perigone segments, inner ones broadened at the base for 2/3–1/3 of length, sharply marked teeth; style slightly exserted after anthesis | A.negianum |
Taxonomic delineation and relationship analysis using nuclear ITS sequence
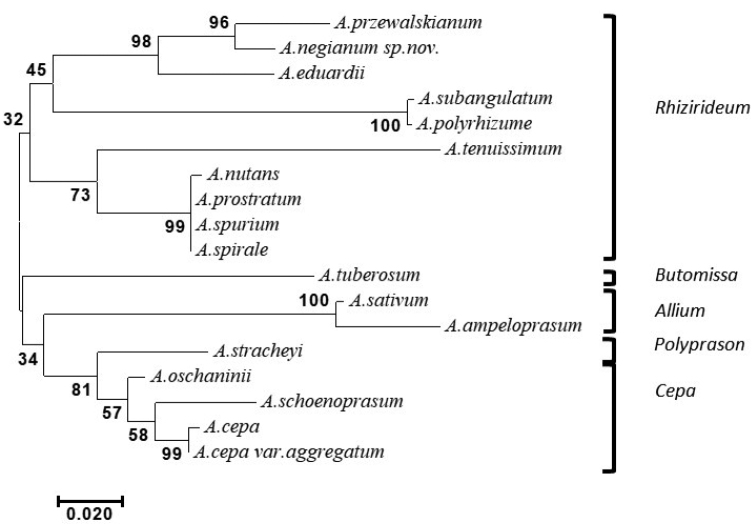
For taxonomic delineation and relationship analysis data set comprising 18 representative taxa from diverse subgenera were selected (Table 3; Fig. 4). The DNA sequence data set of nuclear Internal Transcribed Spacers (ITS) region used for phylogenetic analysis was generated for Alliumnegianum and other taxa used in the study. The generated ITS sequences and obtained ITS sequences from NCBI (Table 3) were used to construct the maximum likelihood tree. The tree with the highest log-likelihood is shown (Fig. 4). The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach and selecting the topology with superior log likelihood value. The branch lengths measured in the number of substitutions per site.
Table 3.
Details of nuclear ITS sequence used in present study.
| Sl. No. | Species | Genbank accession number |
|---|---|---|
| 1 | Alliumtuberosum Rottler ex Spreng. | MZ567234 (present study) |
| 2 | Alliumstracheyi Baker | MZ567226 (present study) |
| 3 | Alliumprzewalskianum Regel | MZ567224 (present study) |
| 4 | Alliumnegianum sp. nov. | MZ567225 (present study) |
| 5 | Alliumsativum L. | MZ567230 (present study) |
| 6 | Alliumampeloprasum L. var. ampeloprasum | MZ567231 (present study) |
| 7 | Alliumcepa L. var. cepa | MZ567228 (present study) |
| 8 | Alliumcepa L. var. aggregatum G.Don | MZ567232 (present study) |
| 9 | Alliumoschaninii O.Fedtsch. | MZ567229 (present study) |
| 10 | Alliumschoenoprasum L. | MZ567227(present study) |
| 11 | Alliumeduardii Stearn ex Airy Shaw | MK917745 |
| 12 | Alliumsubangulatum Regel. | AJ411870 |
| 13 | Alliumtenuissimum L. | AJ411846 |
| 14 | Alliumnutans L. | JN864787 |
| 15 | Alliumprostratusm Trevi. | LN867014 |
| 16 | Alliumspurium G.Don. | LN867017 |
| 17 | Alliumspirale Willd. | JN864784 |
| 18 | Alliumpolyrhizum Turcz. ex Regel | MK917742 |
Source: S. no. 1–10 refer table 1; 11–18: NCBI
Figure 4.
Maximum likelihood tree from nuclear ribosomal ITS sequence from Allium taxa showing distinctness of Alliumnegianum sp. nov.
Two major clades were found within Allium, comprising subgen. Rhizirideum, on one side and second cluster had four subg. Butomissa, Allium, Polyprason and Cepa. on the other side. This former group was divided in two sister clades, with first clade having Alliumprzewalskianum, Alliumnegianum sp. nov. A.eduardii (all from section Eduardia); Alliumsubangulatum, A.polyrhizum from sect. Caespitosoprason; and A.nutans, A.prostratusm, A.spurium and A.spirale in sect. Rhizirideum. One of the taxon A.tenuissimum from sect. Tenuissima grouped separately. Second clade was divided into subgenera, namely Butomissa with one taxon, Alliumtuberosum; subg. Allium, with Alliumsativum and Alliumampeloprasumvar.ampeloprasum; subg. Polyprason having Alliumstracheyi; subg. Cepa that was the largest having four taxa, Alliumcepavar.cepa, A.cepavar.aggregatum, A.oschaninii and A.schoenoprasum from distinct sections.
Based on the likelihood tree, the new Allium taxon was observed to be closely related to A.przewalskianum, both of Indian Himalayan origin along with a Chinese taxon, A.eduardii to form distinct cluster supporting the morphological resemblance of this taxa with section Eduardii under subg. Rhizirideum. The species from other sections under same genus were distantly placed in the phylogenetic tree. The species which are found in same geographical area belong to different subgenera viz. Allium, Cepa, Butomissa and Polyprason were distantly placed and used as outgroup in determining the integrity of newly described species Alliumnegianum.
The above findings indicated that the new taxon is a distinct species and is closely related to A.przewalskianum and belongs to sect. Eduardia under subg. Rhizideum. These findings supported the observations recorded using plant morphology, particularly the floral characters that were very distinct in both the taxa.
Recent advances in molecular phylogenetics have revolutionized our understanding of Allium taxonomy and evolution. However, the phylogenetic relationships in some Allium sections (such as the Alliumsect.Eduardia) and the genetic bases of adaptative evolution remain poorly understood for the Indian taxa (Pandey et al. 2021). Molecular phylogeny study of the wild Allium in different centers of diversity (Nguyen et al. 2008; Xie et al. 2019; Jang et al. 2021) has helped in unlocking many aspects of the taxon relationships. The present study uncovered a new species relationship with its closest allied species and suggested that the selective habitat pressure has played an important role in the adaptation and evolution of Allium in this habitat which will facilitate uncover more taxa in the genus.
Conclusions
Alliumnegianum, a new species under the subg. Rhizirideum, is described using live and herbarium specimens. With the inclusion of this taxon, in the subg. Rhizirideum of the sect. Eduardia there are two taxa in India, and the latter one A.negianum was reportedly restricted to the Uttarakhand flora. Samples of this taxon collected during earlier explorations that remained unidentified will be designated with this new name and conserved as seed in the National Gene Bank (NGB), New Delhi and vegetative material will be maintained in the Field Gene Bank (FGB) at Bhowali, Uttarakhand, India.
Supplementary Material
Acknowledgements
The authors are especially thankful to the Head, Division of Plant Exploration and Germplasm Collection and the Officer-in-charge, Regional Station Bhowali (Nainital), Uttarakhand to facilitate repeated surveys and field observations at the type locality under Global Environment Facility and National Exploration Plan (2019–20) programmes and for growing the germplasm. For the line diagrams help rendered by Mr. RK Pamarthi and Mr. Kunal Patil is greatly acknowledged.
Citation
Pandey A, Rai KM, Malav PK, Rajkumar S (2021) Allium negianum (Amaryllidaceae): a new species under subg. Rhizirideum from Uttarakhand Himalaya, India. PhytoKeys 183: 77–93. https://doi.org/10.3897/phytokeys.183.65433
Contributor Information
Anjula Pandey, Email: anjuravinder@yahoo.com.
Pavan Kumar Malav, Email: pavan.malav@icar.gov.in.
References
- Baasanmunkh S, Lee JK, Jang JE, Park MS, Friesen N, Chung S, Choi HJ. (2020) Seed morphology of Allium L. (Amaryllidaceae) from Central Asian Countries and its taxonomic implications. Plants 9(9): e1239. 10.3390/plants9091239 [DOI] [PMC free article] [PubMed]
- Celep F, Koyuncu M, Fritsch RM, Kahraman A, Dogan M. (2012) Taxonomic importance of seed morphology in Allium (Amaryllidaceae). Systematic Botany 37(4): 893–912. 10.1600/036364412X656563 [DOI] [Google Scholar]
- Choi HJ, Oh BU. (2011) A partial revision of Allium (Amaryllidaceae) in Korea and north-eastern China. Botanical Journal of the Linnean Society 167(2): 153–211. 10.1111/j.1095-8339.2011.01166.x [DOI] [Google Scholar]
- Choi HJ, Giussani LM, Jang CG, Oh BU, Cotaf-Sanchez JH. (2012) Systematics of disjunct north-eastern Asian and northern North American Allium (Amaryllidaceae). Botany 90(6): 491–508. 10.1139/b2012-031 [DOI] [Google Scholar]
- Dasgupta S. (2006) Alliaceae. In: Singh NP, Sanjappa M. (Eds) Fascicles of Flora of India, no.23. Botanical Survey of India, Calcutta, India, 18–19.
- Friesen N, Fritsch RM, Blattner FR. (2006) Phylogeny and new intrageneric classification of Allium (Alliaceae) based on nuclear ribosomal DNA ITS sequences. Aliso 22: 372–395. 10.5642/aliso.20062201.31 [DOI] [Google Scholar]
- Friesen N, Vesselova P, Osmonaly B, Sitpayeva G, Luferov A, Shmakov A. (2020) Alliumtoksanbaicum (Amaryllidaceae), a new species from Southeast Kazakhstan. Phytotaxa 494(3): 251–267. 10.11646/phytotaxa.494.3.1 [DOI] [Google Scholar]
- Fritsch RM, Friesen N. (2002) Evolution, domestication and taxonomy. In: Rabinowitch HD, Currah L. (Eds) Allium crop science: recent advances.CABI Publishing, Wallingford, 5–30. 10.1079/9780851995106.0005 [DOI]
- Gohil RN. (1992) Himalayan representatives of Alliums. In: Hanelt P, Hammer K, Knupffer H. (Eds) The genus Allium: taxonomic problems and genetic resources.Gatersleben, Germany, 335–340.
- Govaerts R, Kington S, Friesen N, Fritsch R, Snijman DA, Marcucci R, Silverstone-Sopkin PA, Brullo S. (2021) World checklist of Amaryllidaceae. Facilitated by the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/ [accessed 21 May 2021]
- Jang JE, Park J-S, Jung J-Y, Kim D-K, Yang S, Choi HJ. (2021) Notes on AlliumsectionRhizirideum (Amaryllidaceae) in South Korea and northeastern China: With a new species from Ulleungdo Island. PhytoKeys 176: 1–19. 10.3897/phytokeys.176.63378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7): 1870–1874. Epub2016Mar22. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed]
- Kuniyal CP, Negi BS. (2018) Cultivation of the Himalayan seasoning Allium in a remote village of Uttarakhand, India. Journal of Threatened Taxa 10(11): 12614–12617. 10.11609/jott.3807.10.11.12614-12617 [DOI] [Google Scholar]
- Li QQ, Zhou SD, He XJ, Yu Y, Zhang YC, Wei XQ. (2010) Phylogeny and biogeography of Allium (Amaryllidaceae: Allieae) based on nuclear ribosomal internal transcribed spacer and chloroplast rps16 sequences, focusing on the inclusion of species endemic to China. Annals of Botany 106(5): 709–733. 10.1093/aob/mcq177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Tan DY. (2017) Seed testa micromorphology of thirty-eight species of Allium (Amaryllidaceae) from central Asia and its taxonomic implications. Nordic Journal of Botany 35(2): 189–200. 10.1111/njb.01259 [DOI] [Google Scholar]
- Memariani F, Joharchi MR, Khassanov FO. (2007) Allium L. subgen. Rhizirideum sensu lato in Iran, two new records and a synopsis of taxonomy and phytogeography. Iranian Journal of Botany 13(1): 12–20. https://www.sid.ir/en/journal/ViewPaper.aspx?id=101166 [Google Scholar]
- Negi KS. (2006) Allium species in Himalayas and their uses with special reference to Uttaranchal. Ethnobotany 18: 53–66. [Google Scholar]
- Neshati F, Fritsch RM. (2009) Seed characters and testa sculptures of some Iranian Allium L. species (Alliaceae). Feddes Repertorium 120(5–6): 322–332. 10.1002/fedr.200911112 [DOI] [Google Scholar]
- Nguyen NH, Driscoll HE, Specht CD. (2008) A molecular phylogeny of the wild onions (Allium; Alliaceae) with a focus on the western North American center of diversity. Molecular Phylogenetics and Evolution 47(3): 1157–1172. 10.1016/j.ympev.2007.12.006 [DOI] [PubMed] [Google Scholar]
- Pandey A, Pandey R, Negi KS, Radhamani J. (2008) Realizing value of genetic resources of Allium in India. Genetic Resources and Crop Evolution 55(7): 985–994. 10.1007/s10722-008-9305-2 [DOI] [Google Scholar]
- Pandey A, Pradheep K, Negi KS. (2017) Onion and related taxa: ecogeographical distribution and genetic resources in Indian subcontinent. In: Ansari AA, Gill I, Singh, Abbas I, Naeem M. (Eds) Plant biodiversity: monitoring, assessment and conservation.Wallingford, Oxfordshire, Boston MA, CABI International, 429–442. 10.1079/9781780646947.0429 [DOI]
- Pandey A, Malav PK, Rai KM, Ahlawat SP. (2021) ‘Neodomesticates’ of the Himalayan allium spices (Allium species) in Uttarakhand, India and studies on morphology and eco-geography. Genetic Resources and Crop Evolution 68(5): 2167–2179. 10.1007/s10722-021-01164-x [DOI] [Google Scholar]
- Seregin AP, Anačkov G, Friesen N. (2015) Molecular and morphological revision of the Alliumsaxatile group (Amaryllidaceae): Geographical isolation as the driving force of underestimated speciation. Botanical Journal of the Linnean Society 178(1): 67–101. 10.1111/boj.12269 [DOI] [Google Scholar]
- Shah NC. (2014) Status of cultivated and wild Allium species in India: A review. Sciences et Techniques (Paris) 1(9): 28–36. [Google Scholar]
- Sinitsyna TA, Herden T, Friesen N. (2016) Dated phylogeny and biogeography of the Eurasian AlliumsectionRhizirideum (Amaryllidaceae). Plant Systematics and Evolution 302(9): 1311–1328. 10.1007/s00606-016-1333-3 [DOI] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR protocols: a guide to methods and applications.Academic Press Inc, New York, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Xie DF, Yu HX, Price M, Xie C, Deng YQ, Chen JP, Yu Y, Zhou SD, He XJ. (2019) Phylogeny of Chinese Allium species in section Daghestanica and adaptive evolution of Allium (Amaryllidaceae, Allioideae) species revealed by the chloroplast complete genome. Frontiers in Plant Science 10: e460. 10.3389/fpls.2019.00460 [DOI] [PMC free article] [PubMed]
- Xu JM, Kamelin RV. (2000) Allium L. In: Wu ZY, Raven PH. (Eds) Flora of China, vol.24. Flagellariaceae through Marantaceae. Science Press, Beijing and Missouri Botanical Garden Press, St. Louis, 165–202.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.