Abstract
Contezolid 康替唑胺 (Youxitai 优喜泰®), an orally administered oxazolidinone antibacterial agent, is being developed by Shanghai MicuRx Pharmaceutical Co., Ltd. for the treatment of multidrug-resistant (MDR) Gram-positive bacterial infections, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci. In June 2021, it was approved by the National Medical Products Administration of China for the treatment of complicated skin and soft tissue infections (cSSTI), including, but not limited to, methicillin-susceptible S. aureus, MRSA, Streptococcus pyogenes and Streptococcus agalactiae. The recommended dosage of contezolid is 800 mg (i.e. two 400 mg tablets) every 12 h for 7–14 days. Contezolid is also undergoing clinical development for acute bacterial skin and skin structure infections (ABSSSI) in the USA, and for diabetic foot infections. This article summarizes the milestones in the development of contezolid leading to this first approval for the treatment of cSSTI.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-021-01576-0.
| Digital Features for this AdisInsight Report can be found at 10.6084/m9.figshare.15026013. |
Contezolid 康替唑胺 (Youxitai 优喜泰®): Key points
| An orally administered oxazolidinone antibacterial agent is being developed by Shanghai MicuRx Pharmaceutical Co., Ltd. for the treatment of MDR Gram-positive bacterial infections |
| Received its first approval on 1 June 2021 in China |
| Approved for use in cSSTI |
Introduction
Infections caused by multidrug-resistant (MDR) bacteria are associated with increased morbidity and mortality, and may render the use of current treatments ineffective for previously treatable infections [1]. Contezolid 康替唑胺 (Youxitai 优喜泰®) is an orally administered oxazolidinone antibacterial agent being developed by Shanghai MicuRx Pharmaceutical Co., Ltd. for the treatment of MDR Gram-positive bacterial infections, including methicillin-resistant (MR) Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci [2–5]. It was rationally designed to address the myelosuppression and monoamine oxidase (MAO) inhibition limitations associated with linezolid [6]. On 1 June 2021, contezolid was approved by the National Medical Products Administration of China for the treatment of complicated skin and soft tissue infections (cSSTI), including methicillin-susceptible S. aureus (MSSA), MRSA, Streptococcus pyogenes and Streptococcus agalactiae [2–4]. The recommended dosage of contezolid is 800 mg (i.e. two 400 mg tablets) every 12 h for 7–14 days; therapy can be extended if necessary [4]. Contezolid should be taken with food or within 30 min after a meal [4] (Sect. 2.2).
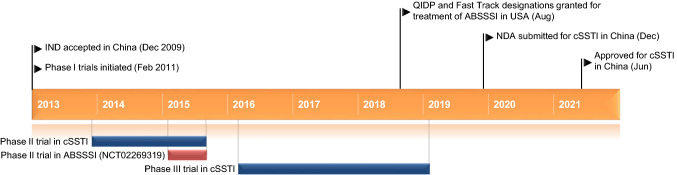
Key milestones in the development of contezolid. ABSSSI acute bacterial skin and skin structure infections, cSSTI complicated skin and soft tissue infections, IND Investigational New Drug, QIDP qualified infectious disease product, NDA New Drug Application
Contezolid is undergoing clinical development for acute bacterial skin and skin structure infections (ABSSSI) in the USA [7], and for diabetic foot infections.
Scientific Summary
Pharmacodynamics
Contezolid is an oxazolidinone antibacterial agent that prevents the formation of a functional 70S initiation complex, which is necessary for bacterial reproduction [4].
According to the Chinese prescribing information, the antibacterial spectrum of contezolid covers most aerobic Gram-positive bacteria, including MR staphylococci (e.g. MRSA, MR Staphylococcus epidermidis), penicillin-intermediate and -resistant Streptococcus pneumoniae, vancomycin-resistant Enterococcus and other MDR clinical isolates [4]. The susceptibility test interpretive criteria [in terms of minimum inhibitory concentration (MIC) values] for contezolid are ≤ 4 mg/L for Staphylococcus and Enterococcus species and ≤ 2 mg/L for S. pneumoniae, α-haemolytic streptococci and β-haemolytic streptococci [4]. Contezolid exhibited potent activity against Gram-positive clinical isolates collected in Europe and the USA as part of the 2015 SENTRY Antimicrobial Surveillance Program [8], including coagulase-negative staphylococci [MIC required to inhibit the growth of 90% of isolates (MIC90) 0.5 mg/L, 100 isolates], S. aureus (MIC90 1 mg/L, 606 isolates), MSSA (MIC90 1 mg/L, 398 isolates), S. pneumoniae (MIC90 1 mg/L, 201 isolates), Enterococcus species (MIC90 1 mg/L, 103 isolates), β-haemolytic streptococci (MIC90 1 mg/L, 102 isolates) and viridans group streptococci (MIC90 1 mg/L, 99 isolates). Moreover, it demonstrated excellent activity against 22 clinical isolates (8 of which were resistant to isoniazid) of Mycobacterium tuberculosis (MIC90 1 µg/mL) [9].
In vitro activity was observed with contezolid in isolates resistant to other antibacterial agents, including MRSA (MIC90 1 mg/L, 208 isolates) and vancomycin-resistant Enterococcus faecium (MIC90 1 mg/L, 26 isolates) [8].
In time-kill studies, contezolid demonstrated bactericidal activity against most streptococci and some staphylococci species, and bacteriostatic activity against Enterococcus faecium [4].
Contezolid demonstrated generally similar efficacy compared with linezolid in murine systemic infection models with S. aureus, S. pneumoniae, E. faecalis and S. pyogenes [10], murine neutropenic thigh infections models with S. aureus [10] and a murine model of tuberculosis [9].
In preclinical studies, contezolid had a low potential to develop resistance; pathogens resistant to other oxazolidinones (e.g. linezolid) may be generally cross-resistant to contezolid [4]. In vitro, there was additivity or indifference between contezolid and other antibacterial agents; no antagonistic effects were seen [4].
At the expected clinically therapeutic exposure level, contezolid did not significantly inhibit MAO in vitro or in vivo [4]. Moreover, compared with linezolid (which binds to and inhibits both the A and B isoforms of MAO and is thus associated with serotonin syndrome, particularly when coadministered with selective serotonin reuptake inhibitors, tricyclic antidepressants or other MAO inhibitors), contezolid exhibited an ≈ 2- and ≈ 148-fold reduction in MAO-A and -B isoform inhibition in vitro [6]. The considerable reduction in MAO-B isoform inhibition with contezolid versus linezolid may be clinically relevant, as MAO-B isoform inhibition with linezolid has been associated with neuropathic adverse events [6].
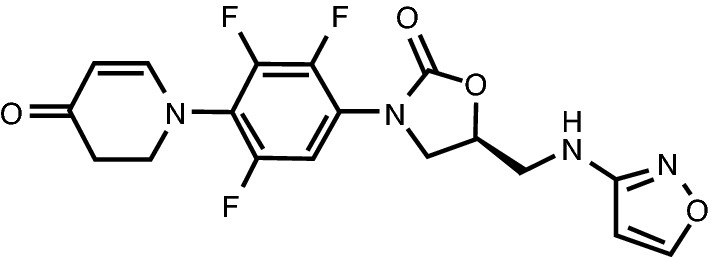
Chemical structure of contezolid
MAO inhibition, and thus a drug interaction associated with this inhibition is predicted to be very unlikely with the concomitant administration of contezolid and an MAO substrate (i.e. adrenergic or serotonergic agents) [4].
At the recommended dose (800 mg; administered under fed conditions), contezolid was not associated with a significant effect on cardiac repolarization or the corrected QT interval [4].
Pharmacokinetics
The pharmacokinetics of contezolid are best described by a two-compartment model with first order elimination, according to a population pharmacokinetic (PPK) analysis of data from five studies (three in healthy Chinese adults and two in Chinese adults with cSSTI) [11]. They are linear at doses < 600 mg and nonlinear at doses > 600 mg [12].
Contezolid was rapidly absorbed in healthy Chinese adults [12]; at steady state (after multiple oral doses of contezolid 800 mg every 12 h under fed conditions) in this healthy volunteer population, a maximum concentration was reached at a median of ≈ 2.5 h [4]. As food promotes the absorption of contezolid, it should be taken with meals or within 30 min after a meal (Sect. 1). No significant accumulation of either contezolid or its main metabolite (M2) was seen following 7–14 days’ therapy [4].
Features and properties of contezolid
| Alternative names | MRX-I; Youxitai |
| Class | Amines; anti-infectives; antibacterials; antituberculars; dihydropyridines; fluorinated hydrocarbons; oxazoles; oxazolidinones; skin disorder therapies |
| Mechanism of action | Protein synthesis inhibitors |
| Route of administration | Oral |
| Pharmacodynamics | Prevents the formation of a functional bacterial 70S initiation complex, disrupting reproduction |
| Minimum inhibitory concentration required to inhibit the growth of 90% of isolates of ≤ 2 mg/L against methicillin-susceptible Staphylococcus aureus (398 isolates), methicillin-resistant S. aureus (208 isolates), Streptococcus pneumoniae (201 isolates), Streptococcus pyogenes (12 isolates), Enterococcus species (103 isolates) and vancomycin-resistant Enterococcus faecium (26 isolates) | |
| Pharmacokinetics | Non-linear pharmacokinetics at doses > 600 mg; contezolid should be taken with meals or within 30 min after a meal (as food promotes its absorption) |
| Adverse events | Appears to be associated with less bone marrow suppression-associated toxicity than other oxazolidinone antibacterial agents; most treatment-emergent adverse events mild or moderate in severity |
| ATC codes | |
| WHO ATC code | J01 (antibacterials for systemic use) |
| EphMRA ATC code | J1 (systemic antibacterials) |
| Chemical name | (5S)-5-[(1,2-oxazol-3-ylamino)methyl]-3-[2,3,5-trifluoro-4-(4-oxo-2,3-dihydropyridin-1-yl)phenyl]-1,3-oxazolidin-2-one |
Contezolid is widely distributed in the body following oral administration; ≈ 90% of contezolid is bound to plasma proteins, with binding independent of the contezolid concentration [4]. It is primarily metabolized by oxidation of the dihydropyridinone ring [via flavin monooxygenase 5 (FMO5) and reductase in liver cytoplasm], which results in an inactive metabolite (M2) [4, 13]. Contezolid is predominately excreted as metabolites via the urine and faeces, with most metabolites excreted within 24 h of administration; the cumulative excretion of the parent drug via the urine and faeces was < 5% of the orally administered dose [4]. Following multiple oral doses of contezolid 800 mg every 12 h under fed conditions, the mean half-life of contezolid was 2.63 h [4].
Food intake, disease status and weight, but not age, sex, creatinine clearance, concomitant medication or baseline disease, were shown to affect contezolid pharmacokinetics [11]. Moreover, according to a PPK analysis of phase I–III data, contezolid dose adjustments are not required in patients with mild to moderate hepatic insufficiency or those with mild renal insufficiency [4]. However, the pharmacokinetic profile of contezolid in patients with moderate to severe renal insufficiency and those with severe hepatic insufficiency are not yet known [4].
Potential drug interactions involving FMO5 metabolism have not yet been investigated [4].
Therapeutic Trials
Complicated Skin and Soft Tissue Infections
Contezolid was noninferior to linezolid for the treatment of cSSTI in patients participating in a double-blind, multicentre, phase III study conducted in China [4]. In patients from the full-analysis set (FAS) population who were clinically evaluable, a clinical cure was achieved in 92.8% of 292 patients receiving contezolid (800 mg every 12 h for 7–14 days, administered orally under fed conditions) and 93.4% of 304 patients receiving linezolid (600 mg every 12 h for 7–14 days, administered orally under fed conditions) at the test-of-cure (TOC) visit (i.e. 7–14 days after the last dose of study medication). In patients from the modified FAS population who were microbiologically evaluable, there was no significant difference between contezolid and linezolid recipients (n = 124 and 132) in bacterial eradication rates (93.5% vs 93.9%) at the TOC visit [4].
Key clinical trials of contezolid (sponsored by MicuRx Pharmaceuticals, Inc.)
| Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
| Contezolid | Bacterial infections, skin and soft tissue infections | III | Planning | Multinational | |
| Contezolid | Acute bacterial skin and skin structure infections | III | Planning | USA | |
| Contezolid, linezolid | Complicated skin and soft tissue infections | III | Completed | China | |
| Contezolid, linezolid | Acute bacterial skin and skin structure infections | II | Completed | USA | NCT02269319 |
| Contezolid, linezolid | Complicated skin and soft tissue infections | II | Completed | China |
These data are supported by those from a double-blind, multicentre, phase II study conducted in China in patients with cSSTI [4]. In patients from the FAS population who were clinically evaluable, the clinical cure rate did not significantly differ between patients receiving contezolid 600 mg or 800 mg every 12 h and those receiving linezolid 600 mg every 12 h (n = 49, 51 and 62, respectively) [89.8% and 96.1% vs 95.2%] at the TOC visit (7–14 days after the last dose of study medication). In patients from the modified FAS population who were microbiologically evaluable, there was no significant difference between contezolid 800 mg and linezolid 600 mg recipients (n = 30 and 34) in bacterial eradication rates (96.7% vs 97.1%) at the TOC visit. The bacterial eradication rate in contezolid 600 mg recipients (n = 23) was 78.3% [4]. Patients in this study had a confirmed or suspected Gram-positive bacterial infection at the time of admission [14] and the study medication was administered under fed conditions [4].
According to pooled microbiologically evaluable population data from the two studies [4], bacterial eradication rates at the TOC visit were 91.3–100.0% for contezolid and 86.7–100.0% for linezolid against aerobic and anaerobic Gram-positive bacteria.
Acute Bacterial Skin and Skin Structure Infections
Contezolid showed promise as a treatment for ABSSSI in adults participating in a randomized, double-blind, active comparator-controlled, multicentre, phase II study (NCT02269319) conducted in the USA [7, 15]. At the early assessment visit (48–72 h), 90% of 80 contezolid recipients achieved a 20% reduction from baseline in ABSSSI lesion size (primary endpoint) compared with 87.5% of 40 linezolid recipients [7, 15]. In NCT02269319, patients received oral contezolid 800 mg twice daily (n = 80) or oral linezolid 600 mg twice daily (n = 40) for 10 days [15]. The study enrolled patients with systemic signs of infection who had been diagnosed with ABSSSI and cellulitis/erysipelas, major cutaneous abscess or wound infections. Those with ABSSSI exclusively due to Gram-negative pathogens, uncomplicated skin infections, severe sepsis or septic shock, or who had received systemic antibacterials within 96 h of randomization were excluded [15].
Adverse Events
The nature and incidence of adverse reactions in patients receiving contezolid 800 mg every 12 h were consistent across the two cSSTI studies [4]. Common (incidence ≥ 1/100 to < 1/10) adverse reactions were nausea, vomiting, abdominal discomfort, and increases in alanine aminotransferase, aspartate aminotransferase, blood uric acid and blood bilirubin levels [4].
In the phase III cSSTI study, the most frequently reported (incidence ≥ 1%) clinical adverse reactions were gastrointestinal in nature: nausea and vomiting, respectively, occurred in 3.4% and 3.4% of 354 contezolid 800 mg every 12 h recipients and 2.8% and 0.9% of 351 linezolid 600 mg every 12 h recipients [4]. The gastrointestinal reactions occurring in this study were mostly mild to moderate in severity, transient and resolved without treatment [4]. Treatment-emergent adverse events (TEAEs) considered by the investigators to be related to the study medication occurred in 23.4% of contezolid recipients and 26.8% of linezolid recipients [5]. The most frequently reported (incidence ≥ 1%) treatment-related laboratory abnormalities in this study were increased liver enzyme and serum uric acid levels in patients receiving contezolid and increased liver enzymes and significant changes in haematological parameters in those receiving linezolid [4]. Significant changes in haematological parameters associated with myelosuppression were not reported in contezolid 800 mg recipients [4, 5]. Specifically, treatment-related clinically significant abnormalities in white blood cell (0.3% vs 3.4%; p = 0.002) and platelet (0% vs 2.3%; p = 0.004) counts occurred in significantly fewer contezolid than linezolid recipients. Treatment-related clinically significant abnormalities in neutrophil and reticulocyte counts, respectively, occurred in 0.3% and 0.3% of contezolid recipients and 1.7% and 1.4% of linezolid recipients. Among patients who received > 10 days’ therapy, a significantly lower proportion of contezolid recipients (n = 204) than linezolid recipients (n = 201) experienced a > 30% reduction from baseline in platelet counts at the end-of-therapy (EOT) visit (2.5% vs 25.4%; p < 0.001) [4, 5].
In the phase II cSSTI study, among patients who received > 10 days of therapy, myelosuppression was not observed in the contezolid group, while one-third of patients in the linezolid group experienced a ≥ 30% reduction in the platelet count by the EOT [7]. No treatment-related serious TEAEs were reported in either contezolid or linezolid recipients [14].
In the phase II ABSSSI study, contezolid demonstrated a favorable safety and tolerability profile [7]. Its use did not cause myelosuppression, and no treatment-related serious TEAEs or treatment discontinuation due to adverse events were reported in patients receiving contezolid [7].
Ongoing Clinical Trials
MicuRx plans to conduct a multinational phase III study to accelerate the global approval of contezolid [2] and a phase III study in the USA assessing the efficacy and safety of intravenous contezolid acefosamil (MRX-4; a prodrug of contezolid) followed by oral contezolid in ABSSSI [16]. In addition, a study evaluating intravenous contezolid acefosamil and oral contezolid in diabetic foot infections is planned.
Current Status
Contezolid received its first approval on 1 June 2021 for the treatment of complicated skin and soft tissue infections in China [2–4].
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. S. M. Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Footnotes
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original version of this article was revised due to a retrospective Open Access order.
Change history
10/24/2021
Digital Features update
Change history
10/23/2021
A Correction to this paper has been published: 10.1007/s40265-021-01627-6
References
- 1.Koulenti D, Xu E, Song A, et al. Emerging treatment options for infections by multidrug-resistant Gram-positive microorganisms. Microorganisms. 2020;8(2):191. doi: 10.3390/microorganisms8020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MicuRx Pharmaceuticals. China NMPA approves MicuRx’s contezolid for treatment of drug-resistant bacterial infection [media release]. 2 Jun 2021. http://www.micurx.com/.
- 3.National Medical Products Administration. The State Food and Drug Administration approves the listing of contezolid tablets. 2021. http://www.nmpa.gov.cn/yaowen/ypjgyw/20210602083301144.html?type=pc&m=. Accessed 8 Jun 2021.
- 4.Shanghai MicuRx Pharmaceutical Co. Ltd. Youxitai 优喜泰® (contezolid 康替唑胺 tablets): Chinese prescribing information. Shanghai: Shanghai MicuRx Pharmaceutical Co., Ltd.; 2021.
- 5.MicuRx Pharmaceuticals. MicuRx reports positive top-line results of a China phase 3 clinical trial for novel antibiotic contezolid in complicated skin and soft tissue infections [media release]. 20 Sep 2019. http://www.micurx.com.
- 6.Wang W, Voss KM, Liu J, et al. Nonclinical evaluation of antibacterial oxazolidinones contezolid and contezolid acefosamil with low serotonergic neurotoxicity. Chem Res Toxicol. 2021;34(5):1348–1354. doi: 10.1021/acs.chemrestox.0c00524. [DOI] [PubMed] [Google Scholar]
- 7.MicuRx Pharmaceuticals. MicuRx reports positive top-line results from phase 2 US clinical trial for novel antibiotic MRX-I [media release]. 3 Nov 2015. http://www.micurx.com.
- 8.Carvalhaes CG, Duncan LR, Wang W, et al. In vitro activity and potency of the novel oxazolidinone contezolid (MRX-I) tested against Gram-positive clinical isolates from the United States and Europe. Antimicrob Agents Chemother. 2020;64(11):e01195-20. doi: 10.1128/AAC.01195-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoen C, DeStefano M, Hafkin B, et al. In vitro and in vivo activities of contezolid (MRX-I) against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2018;62(8):e00493-18. doi: 10.1128/AAC.00493-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CR, Zhai QQ, Wang XK, et al. In vivo antibacterial activity of MRX-I, a new oxazolidinone. Antimicrob Agents Chemother. 2014;58(4):2418–2421. doi: 10.1128/AAC.01526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Wu H, Chen Y, et al. Population pharmacokinetics study of contezolid (MRX-I), a novel oxazolidinone antibacterial agent, in Chinese patients. Clin Ther. 2020;42(5):818–829. doi: 10.1016/j.clinthera.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Li Y, Zhang J, et al. Short-term safety, tolerability, and pharmacokinetics of MRX-I, an oxazolidinone antibacterial agent, in healthy Chinese subjects. Clin Ther. 2018;40(2):322–32.e5. doi: 10.1016/j.clinthera.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Meng J, Zhong D, Li L, et al. Metabolism of MRX-I, a novel antibacterial oxazolidinone, in humans: the oxidative ring opening of 2,3-dihydropyridin-4-one catalyzed by non-P450 enzymes. Drug Metab Dispos. 2015;43(5):646–659. doi: 10.1124/dmd.114.061747. [DOI] [PubMed] [Google Scholar]
- 14.MicuRx Pharmaceuticals. MicuRX reports positive top-line results in phase 2 clinical trial for novel antibiotic MRX-I in complicated skin and soft tissue infections [media release]. 31 Aug 2015. http://www.micurx.com.
- 15.US National Institutes of Health. MRX-I versus linezolid for the treatment of acute bacterial skin and skin structure infection (ABSSSI). 2017. http://clinicaltrials.gov/. Accessed 18 Jun 2021.
- 16.MicuRx Pharmaceuticals. MicuRx closes $55 million Series C Financing to support development of next-generation antibiotic MRX-I [media release]. 27 Sep 2016. http://www.micurx.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.