Summary
We used the recombinant trimeric spike (S) glycoprotein in the prefusion conformation to immunize horses for the production of hyperimmune globulins against SARS-CoV-2. Serum antibody titers measured by ELISA were above 1:106, and the neutralizing antibody titer against authentic virus (WT) was 1:14,604 (average PRNT90). Plasma from immunized animals was pepsin digested to remove the Fc portion and purified, yielding an F(ab’)2 preparation with PRNT90 titers 150-fold higher than the neutralizing titers in human convalescent plasma. Challenge studies were carried out in hamsters and showed the in vivo ability of equine F(ab’)2 to reduce viral load in the pulmonary tissues and significant clinical improvement determined by weight gain. The neutralization curve by F(ab’)2 was similar against the WT and P.2 variants, but displaced to higher concentrations by 0.39 log units against the P.1 (Gamma) variant. These results support the possibility of using equine F(ab’)2 preparation for the clinical treatment of COVID patients.
Subject areas: Immunology, Equine immunology, Biological sciences
Graphical abstract
Highlights
-
•
Trimeric spike glycoprotein induces high level of horse neutralizing antibodies
-
•
The F(ab’)2 Ab has neutralizing titer 150-fold higher than human convalescent plasma
-
•
The neutralizing activity of F(ab’)2 was high against the P.1 (Gamma) and P.2 variants
-
•
Anti-spike F(ab’)2 protected hamsters against SARS-CoV-2 challenge
Immunology; Equine immunology; Biological sciences
Introduction
The pandemic caused by SARS-CoV-2, the etiological agent of COVID-19, is an urgent health problem worldwide, especially in the Americas (https://covid19.who.int/). Consequences for human health and for the global economy have been devastating. Over the last 18 months (until September 2021), several vaccines have been developed, and 20 vaccines have been approved for human use in different countries (Klasse et al., 2021; Sette and Crotty, 2021). However, the COVID-19 scenario seems to still be far from a solution, especially because of the lack of antiviral treatments for individuals infected by SARS-CoV-2 (Long et al., 2020; Seow et al., 2020; Xu et al., 2020). Passive immunization using plasma from COVID-19 convalescent patients has been used as an alternative therapy (Agarwal et al., 2020; Li et al., 2020; Zeng et al., 2020; Libster et al., 2021; Simonovich et al., 2021). However, the heterogeneous neutralizing titers in different convalescent donors and the simultaneous transfer of other plasma components are drawbacks that hinder its wide use. Another strategy using passive immunotherapy has been the development of monoclonal antibodies (mAbs), and some have been approved for the early treatment of infected patients (Sun and Ho, 2020; Pinto et al., 2020).
The development of virus-neutralizing purified hyperimmune globulins produced in horses or llamas may be an approach to treat SARS-CoV-2 infection. The use of llamas (Huo et al., 2020) to develop passive immunization therapies is still experimental and limited by animal availability. On the other hand, hyperimmune serum, immunoglobulins or IgG fragments produced in horses have been used to treat many diseases, such as rabies, tetanus, and snake envenomation, among others (Waghmare et al., 2009; Zheng et al., 2016).
Brazil, like many other countries, has a large established capacity to produce equine hyperimmune globulin preparations for a range of indications, which makes the production of such products against SARS-CoV-2 highly feasible. Previous works on other related betacoronaviruses reported that equine hyperimmune sera resulted in neutralizing antibodies against SARS-CoV (Lu et al., 2005) and MERS-CoV (Zhao et al., 2017). Recently, the recombinant receptor-binding domain (RBD) of the SARS-CoV-2 S protein was shown to stimulate antibody production in mice and equines (Pan et al., 2020; Zylberman et al., 2020). Another recent work showed high neutralizing titers against SARS-CoV-2 for two formulations of whole-IgG from plasma of horses immunized with either the recombinant S1 domain or with a mixtures of SARS-CoV-2 recombinant proteins (León et al., 2021). A recent clinical study reported very positive results for the efficacy and safety of equine antibodies developed against the RBD (Lopardo et al., 2021). The study showed that the treatment led to clinical improvement of hospitalized patients with SARS-CoV-2 pneumonia, particularly those with severe disease (Lopardo et al., 2021).
In the present work, we also used a recombinant antigen to immunize horses. However, the antigen chosen was the trimeric version of the complete ectodomain of the spike protein, comprising both the S1 (responsible for receptor binding) and the S2 (responsible for fusion to the cell membrane) domains. Moreover, the chosen antigen was produced in house using a gene construct that yields the protein in a stabilized, trimeric prefusion conformation (Wrapp et al., 2020), with the aim of maximizing the formation of high-quality neutralizing antibodies. Our strategy may be easily reproduced in any part of the world and could be rapidly tested as a therapy for COVID-19. Equine immunization is a well-known and easily scalable technology proven to generate high titers of neutralizing antibodies, thus showing advantages over other strategies, such as using convalescent human plasma. In this study, we demonstrated extremely high neutralizing titers obtained by means of equine immunization. The final purified F(ab’)2 preparation had an average PRNT90 neutralizing titer 150-fold higher than that of human convalescent plasma from three patients in Brazil. We also evaluated the neutralizing activity of F(ab’) 2 against the P.1 (Gamma) and P.2 variants from Brazil, which have become prevalent in Brazil in 2021 (Faria et al., 2021; Naveca, 2021; Voloch et al., 2021). The P.1 strain has raised great concern because it has three mutations in the receptor binding domain (RBD): K417T, E484K and N501Y (Faria et al., 2021; Naveca, 2021). The P.2 variant has only one mutation in the RBD (E484K) (Voloch et al., 2021). The neutralization curve by F(ab’)2 was similar against WT and P.2 but slightly displaced to higher concentrations against the P.1 strain. The use of the recombinant full-length spike protein in a trimeric prefusion conformation combines the advantages of using an antigen that closely resembles its state in the native virus with the biosafety advantages related to using a recombinant immunogen.
Results
Immunization with the trimeric S protein induces high titers of specific equine IgG
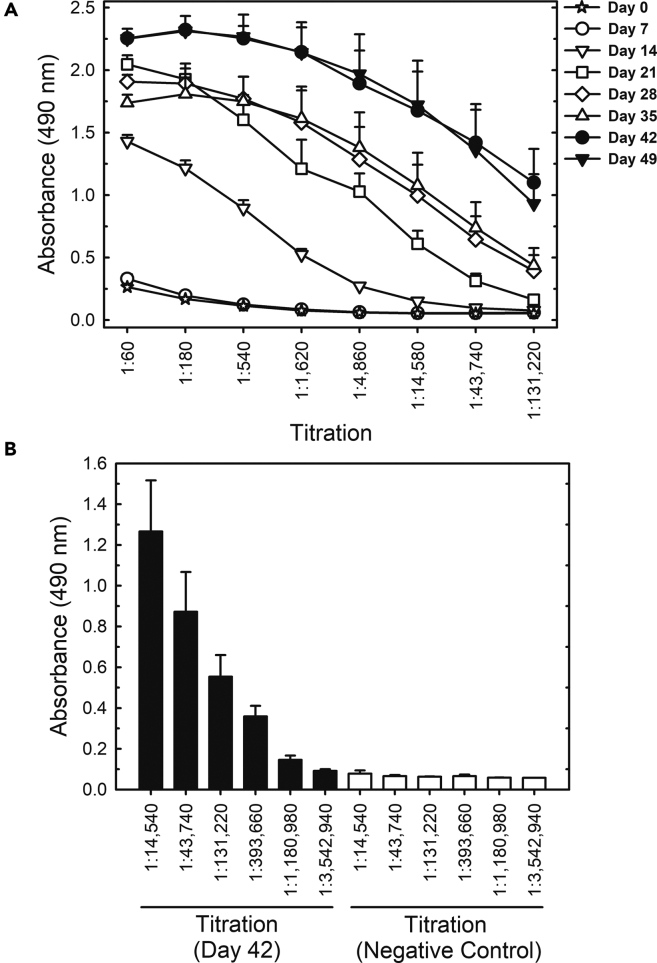
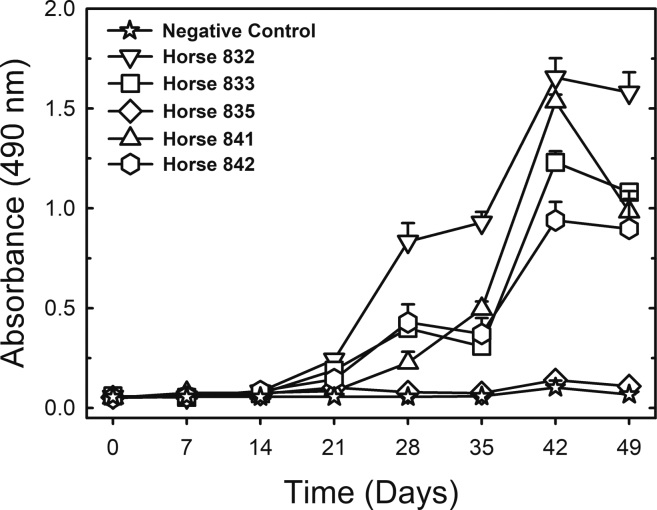
We produced trimers of the spike protein in the prefusion conformation by stably expressing the gene construct in mammalian cells as described previously (Alvim et al., 2020). Quality checks on purified trimeric spike were assessed by silver-stained SDS-PAGE, analytic size-exclusion chromatography, peptide mapping by electron-spray ionization mass spectrometry, and western-blot against host cell protein contaminants (Figures S1A–S1F). Its trimeric prefusion conformation was verified by size exclusion chromatography (Figure S1). Initially, five horses were immunized with six subcutaneous injections of S protein, with an interval of one week between inoculations. No adverse effects or animal suffering were observed for any of the five horses that received the protein injections. Anti-spike IgG measured by enzyme-linked immunosorbent assay (ELISA) in weekly serum samples showed that 1 week after the first immunization, anti-SARS-CoV-2 IgG was not yet detectable but that IgG titers increased progressively after successive immunizations (Figure 1A). Four out of the five immunized horses produced similar amounts of specific antibodies, but one did not show a strong response (Figure 2). Despite the low-responding horse (#835), the average IgG titer for all five horses reached 1,180,980 after 42 days, i.e., 1 week after the sixth immunization (Figure 1B), indicating that the trimeric S protein is a good immunogen to induce the production of specific anti-SARS-CoV-2 antibodies.
Figure 1.
Titration of horse antibodies by S protein ELISA in samples from the first group of animals
(A) Titration up to 131,200-fold dilution of serum samples collected from immunized horses on different days after initial immunization.
(B) Titration up to a 3,542,940-fold dilution of samples collected on day 42 after the first immunization. All results shown are the mean ± standard error for all 5 horses. The negative control was a pool of preimmune sera collected from all 5 horses of Group 1.
Figure 2.
Anti-S antibodies measured by ELISA in serum samples collected over time (first group of animals)
All sera were diluted 1:131,220. The results are shown as the mean ± standard error for analytical duplicates. The negative control was a pool of preimmune sera collected from all 5 horses of Group 1.
Equine sera and F(ab’)2 fragments developed against the trimeric S protein have potent neutralizing titers against SARS-CoV-2
We first evaluated the in vitro neutralizing activity against SARS-CoV-2 of equine sera collected after four to six immunizations (days 28–49 after the first immunization) using a microneutralization assay (Fintelman-Rodrigues et al., 2021). PRNT50 titers (average values for all five horses) seemed to achieve a plateau of approximately 1:23,000 after the fourth immunization, whereas the more stringent PRNT90 titers (average values for all five horses) still showed an ascendant trend over time, reaching an average PRNT90 titer of 1:14,604 on day 49. Owing to the low-responding horse (#835), the standard deviations were high (Figure 3; Table 1).
Figure 3.
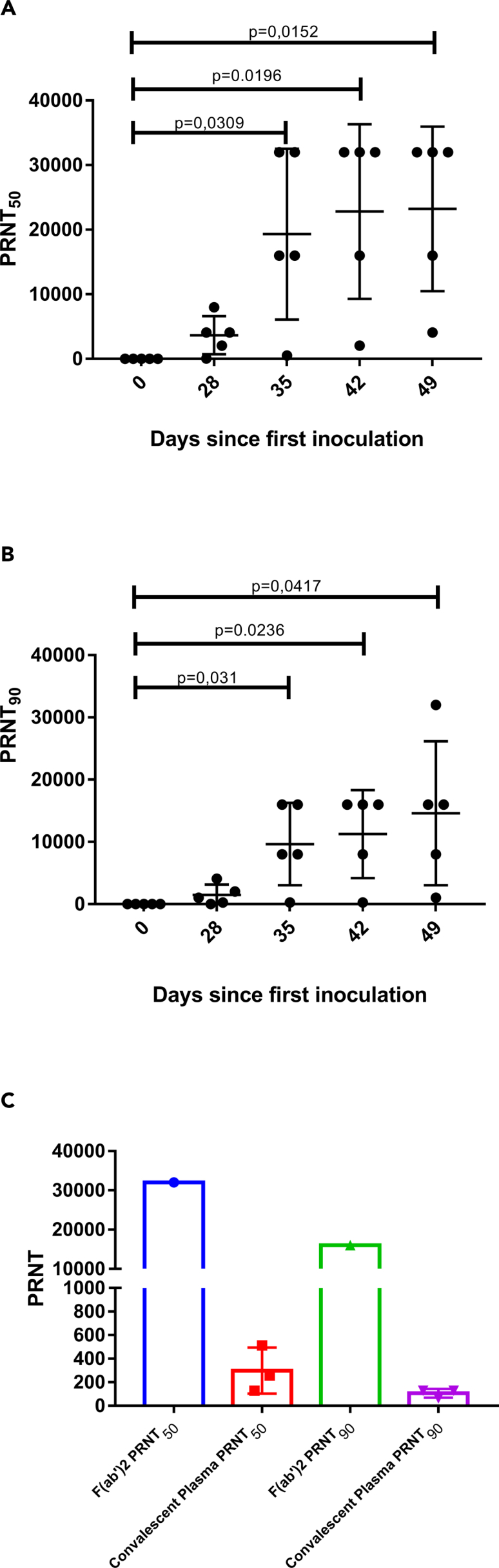
Microneutralization assays
(A and B) (A) PRNT50 and (B) PRNT90 for equine plasma collected on days 28–49 after the first immunization. Data are shown as the mean ± SD; p < 0.05 vs plasma collected on day 0 according to a t test for paired samples.
(C) Comparison of PRNT50 and PRNT90 for equine F(ab’)2 concentrate and human convalescent plasma. Data are shown as the mean ± standard deviation.
Table 1.
Microneutralization data for equine sera collected during the immunization of the animal group, for F(ab’)2 concentrates and for human convalescent plasma
| Sample ID | PRNT50 | Mean ± SD | PRNT90 | Mean ± SD | |
|---|---|---|---|---|---|
| 832 | <16 | <16 | |||
| Equine | 833 | <16 | <16 | ||
| Preimmune | 835 | <16 | 16 ± 0 | <16 | 16 ± 0 |
| Serum | 841 | <16 | <16 | ||
| 842 | <16 | <16 | |||
| 832 | 8,000 | 4,096 | |||
| Equine serum | 833 | 4,096 | 2,048 | ||
| On day 28 | 835 | 64 | 3,660 ± 2,947 | 32 | 1,491 ± 1,655 |
| 841 | 2,048 | 256 | |||
| 842 | 4,096 | 1,024 | |||
| 832 | 16,000 | 8,000 | |||
| Equine serum | 833 | 32,000 | 16,000 | ||
| On day 35 | 835 | 512 | 19,302 ± 13,203 | 256 | 9,651 ± 6,601 |
| 841 | 32,000 | 16,000 | |||
| 842 | 16,000 | 8,000 | |||
| 832 | 32,000 | 16,000 | |||
| Equine serum | 833 | 16,000 | 8,000 | ||
| On day 42 | 835 | 2,048 | 22,809 ± 13,516 | 256 | 11,251 ± 7,055 |
| 841 | 32,000 | 16,000 | |||
| 842 | 32,000 | 16,000 | |||
| 832 | 32,000 | 32,000 | |||
| Equine serum | 833 | 16,000 | 8,000 | ||
| On day 49 | 835 | 4,096 | 23,219 ± 12,738 | 1,024 | 14,604 ± 11,560 |
| 841 | 32,000 | 16,000 | |||
| 842 | 32,000 | 16,000 | |||
| Equine F(ab’)2 concentrate | Pilot lot | 32,000 | 16,000 | ||
| Plasma pool used in GMP production (1st immunization of Groups 1 and 2) | Pool of 2 bleedings (160 L) | 16,384 | 4,096 | ||
| GMP equine F(ab’)2 concentrate | Industrial lot (16 L API) | 65,556 | 16,384 | ||
| Human | 1 | 512 | 128 | ||
| Convalescent | 2 | 128 | 298 ± 195 | 64 | 106 ± 36 |
| plasma | 3 | 256 | 128 |
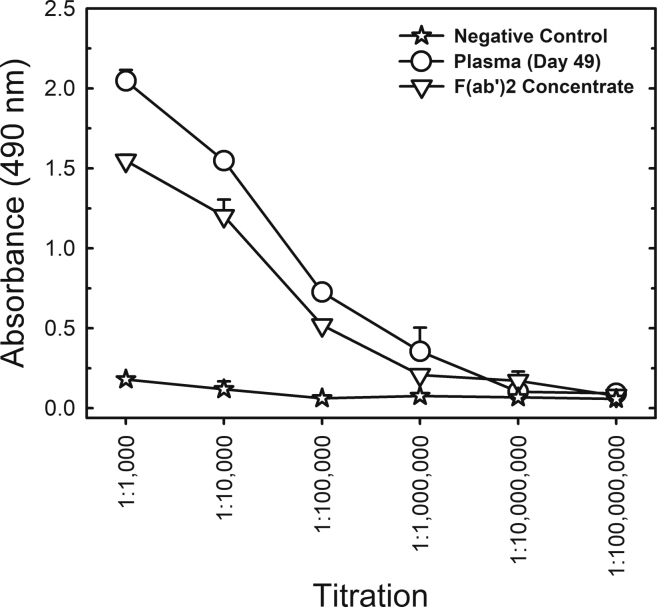
Plasma from all five horses was then pooled, digested with pepsin to cleave off the Fc portion and precipitated with ammonium sulfate for purification, resulting in a concentrate of F(ab’)2 fragments containing approximately 90 mg/mL total protein. The F(ab’)2 preparation has a high degree of purity as determined by polyacrylamide gel electrophoresis (PAGE), gel filtration (Figures S2) and dynamic light scattering (Table S1), showing no aggregates. The F(ab’)2 concentrate maintained the capacity to recognize the trimeric S protein, displaying an ELISA titer of 1:1,000,000 (Figure 4). It is noteworthy that even after cleavage of the Fc fragment and purification of F(ab’)2, the ELISA worked well.
Figure 4.
Comparison of S protein ELISA titration of equine F(ab’)2 concentrate and horse plasma collected on day 49 after the first immunization of the first group of animals
The sample from day 49 and the preimmune negative control were pools of samples collected from all 5 horses of Group 1. The results are shown as the mean ± standard error for analytical duplicates.
To confirm whether F(ab’)2 fragments also maintained their capacity to recognize SARS-CoV-2 proteins in cell culture, we prepared an F(ab’)2-FITC conjugate, which was shown to specifically bind to SARS-CoV-2-infected Vero E6 cells (Figures S3). F(ab’)2 neutralizing titers were also very high, achieving a PRNT50 of 1:32,000 and a PRNT90 of 1:16,000 (Figure 3; Table 1).
We further compared the neutralizing titers of the equine samples to the neutralizing titers determined for plasma from three convalescent COVID-19 patients. Interestingly, the average neutralizing titers of equine serum (from day 49) were 78-fold and 138-fold higher than the average human convalescent plasma titers in terms of PRNT50 and PRNT90, respectively. Regarding the F(ab’)2 concentrate, the neutralizing titers were 107-fold (for PRNT50) and 151-fold (for PRNT90) higher than the average human convalescent plasma titer. Although we are comparing a purified globulin fragment with plasma, it is important to show the great potential of using equine immunoglobulins in the treatment of COVID-19. We are also aware of the limitations of the comparison because convalescent people have been exposed to virus without adjuvant and have not been boosted several times to generate a hyperimmune response.
Sustainable production of potent antibodies against SARS-CoV-2
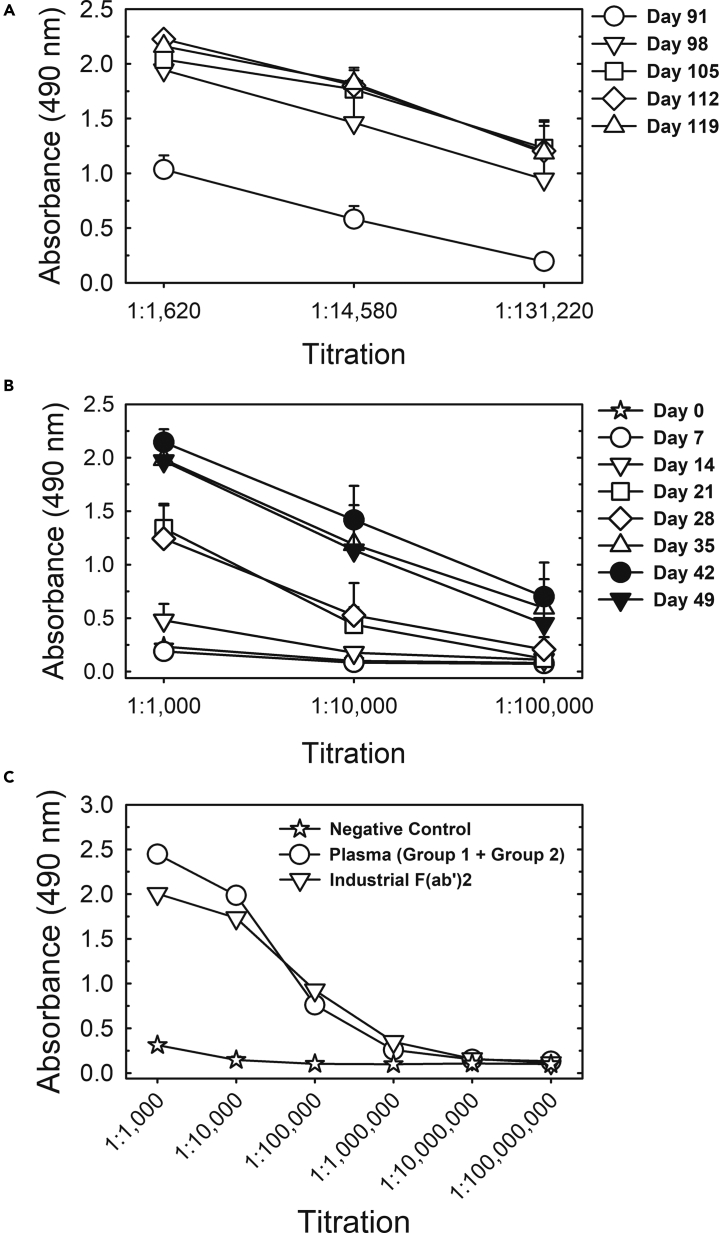
To evaluate the sustainability of equine antibody production and to enable scale-up to industrial manufacturing, the first group of five horses was reimmunized, and a second group of five horses was immunized using the same protocol as the first group (Figure 5). Reimmunization of the first group occurred by means of three spike protein injections on weeks 13, 14, and 15, i.e., 6 weeks after plasma collection on day 49 (week 7). The titer of antibodies, which had decreased since the first bleeding, was boosted and reached levels slightly higher than after the first series of immunizations (Figure 5A). The time interval to boost and the decreased number of immunizations to boost is a standard protocol applied to the production of other equine hyperimmune sera produced at IVB.
Figure 5.
S protein ELISA titration of Group 1 reimmunization, Group 2 (first immunization) and industrial F(ab’)2 concentrate
(A) Titration of pooled sera from all 5 horses of Group 1 upon reimmunization (injections on days 91, 98 and 105).
(B) Titration of pooled sera from all 5 horses of Group 2 (first immunization: 6 weekly injections on days 0–35).
(C) Titration of the pool of plasma collected from Groups 1 and 2 on day 49 and the industrial F(ab’)2 concentrate manufactured under GMP conditions from this pool of plasma. Data are shown as the mean ± standard error of analytical duplicates. The negative control was a pool of preimmune sera of Group 1 animals.
The immunization scheme applied to the second group of five horses was the same as that for the first immunization of the first group of animals (6 weekly spike protein injections) and equally resulted in increasing anti-S antibody titers (Figure 5B).
The plasma collected in the first bleeding of the first group of horses and in the first bleeding of the second group of horses was pooled and resulted in 160 L of plasma that was processed in the industrial facility of Vital Brazil Institute (IVB), which is certified for good manufacturing practices (GMP), resulting in a GMP-compliant batch of anti-COVID-19 equine F(ab’)2 active pharmaceutical ingredient (API) with high degree of purity (Figure S2). Figure 5C shows a comparison of ELISA titers of this GMP F(ab’)2 concentrate batch and of the plasma pool used as raw material for its production. Antibody titers were in the range of 1:1,000,000, as obtained for the pilot batch produced from the first bleeding of the first horse group (Figure 4).
Neutralizing activity in the GMP API (Table 1) was 1:65,556 as PRNT50 and 1:16,384 as PRNT90, showing that the well-established routine GMP process implemented at IVB leads to higher F(ab’)2 yields than the pilot process using laboratory equipment. This API was further processed (dilution in water-for-injection, fill and finish) for use in phase I/II clinical trials of the anti-COVID-19 F(ab’)2 concentrate.
Persistence of neutralizing activity after F(ab’)2 injection in mice
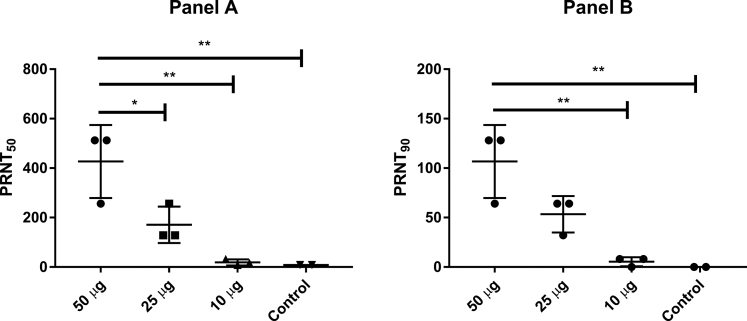
To test the clearance of the neutralizing activity, mice were injected with different doses (10, 25 or 50 μg) of the pilot batch of equine F(ab’)2 concentrate. After 72 h, blood was collected, and neutralizing activity in mouse plasma was determined (Figure 6). The data clearly show that high neutralizing titers are maintained for at least 3 days after injection, with PRNT50 and PRNT90 titers for the highest dose (50 μg) being comparable to human convalescent plasma (Table 1). This result indicates that the F(ab’)2 fragments would have a sufficient half-life to reach different organs, including the lungs, to exert their antiviral activity. Our study corroborates plentiful information on the pharmacokinetics of equine F(ab’)2 and whole-antibody preparations of antivenoms (Gutiérrez et al., 2011; León et al., 2013).
Figure 6.
Mice injected with equine F(ab’)2 still presented a neutralizing response in plasma 3 days post injection
Mice were injected via intraperitoneal inoculation with equine F(ab’)2 (50 μg, 25 μg or 10 μg total protein concentration), and blood was collected 72 h after injection. Neutralizing titers were determined by PRNT assay. The PRNT50 and PRNT90 results are shown in panels A and B, respectively. Data are shown as the mean ± SD; ∗p < 0.05; ∗∗p < 0.01 according to one-way ANOVA and Tukey’s post hoc test.
Neutralizing activity of F(ab’)2 against the P.1 and P.2 strains
SARS-CoV-2 P.1 (also named Gamma) has become a dominant variant in Brazil (Faria et al., 2021; Naveca, 2021). P.1 contains several mutations in the spike protein: K417T, E484K, and N501Y in the receptor-binding domain (RBD); L18F, T20N, P26S, D138Y, and R190S in the N-terminal domain (NTD); and H655Y near the furin cleavage site. This new strain can potentially affect the efficacy of vaccines and monoclonal antibody (mAb) therapies. The P.1 variant shares mutations in the RBD domain, which were present in a variant that first emerged from South Africa (Tegally et al., 2021). The Brazilian P.2 variant has only one mutation in the RBD (E484K) (Voloch et al., 2021).
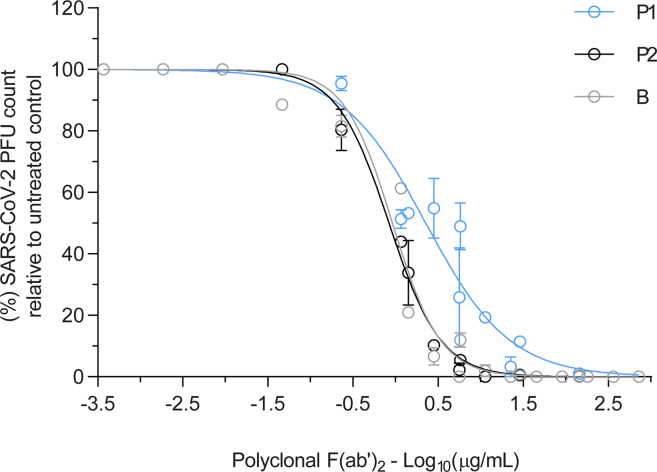
To compare the neutralizing activity of F(ab’)2 against the P.1 and P.2 antibodies, quantitative neutralization assays against the authentic virus strains were performed (Figure 7). The neutralization curve by F(ab’)2 was similar when tested against WT and P.2 but displaced to higher concentrations by 0.39 log units against P.1, considering the half maximal inhibitory concentration (IC50) (Figure 7). The neutralizing activity against P.1 can be explained by the polyclonal nature of the raised antibody against the whole spike protein. Recent studies showed that several monoclonal antibodies lost neutralizing activity against P.1 (Dejnirattisai et al., 2021; Wang et al., 2021). Altogether, these results demonstrated that the F(ab)’2 remained effective for all tested variants despite a small decrease in the P.1 titer.
Figure 7.
Dose-response curves of the effect of purified F(ab’)2 on SARS-CoV-2 infectivity analyzed by PRNT
Data are expressed as the mean ± standard error of the mean (s.e.m.).
Equine F(ab’)2 against the spike protein protected elderly hamsters against weight loss and reduced viral load in the respiratory tract during the acute phase of B.1.33 SARS-CoV-2 variant infection.
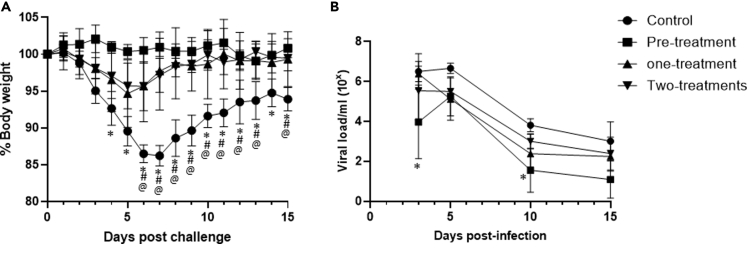
We evaluated the pretreatment and treatment with equine F(ab’)2 during SARS-CoV-2 infection. In general, the equine hyperimmune therapy improved the clinical parameters of the experimental SAR-CoV-2 infection. In our study, the Golden Syrian hamster model presented two clearly defined phases. The first phase was characterized by progressive weight loss (>10%), and this acute phase of SARS-CoV-2 infection was evident between 2 and 7 days postinfection (dpi), after which the recovery phase (between 7 and 15 dpi) was characterized by weight gain. All schedules adopted with hyperimmune serum treatment protected the animals against the weight loss observed in the acute phase of infection (Figure 8A). Non-treated animals (Control) showed more than 14% weight loss at 7 dpi, whereas pre-treatment (treatment at −1 dpi) resulted in no body weight reduction. One-treatment (treated at +1 dpi) presented 4% weight loss at 5 dpi, and two-treatments (treated at +2 dpi and +4 dpi) showed 7% at 6 dpi.
Figure 8.
Treatment with equine F(ab’)2 in SARS-CoV-2 experimental infection in elderly Golden Syrian hamsters
(A) The antiviral activity of equine F(ab’)2 therapy is expressed as a reduction of body weight (N = 4). ∗p < 0.05 in comparison of pretreatment with control, #p < 0.05 in comparison of one-treatment with control and @ p < 0.05 in comparison of two-treatments with control.
(B) The antiviral activity is expressed as a reduction of viral RNA load in the lung. N = 12 (control; 3 mice/time point) and N = 15 (pre-treatment, one-treatment and two-treatments; 4 mice/time point). ∗p < 0.05 in comparison of pre-treatment to control. The data were statistically analyzed by two-way ANOVA following Tukey’s post hoc test.
We also evaluated the impact of pretreatment and treatment on viral replication in the lung (Figure 8B). Comparison between control with pre-treatment revealed a significant difference at 3 and 10 dpi (Figure 8B). One-treatment and two-treatments reduced viral load at 5 dpi and at 10 dpi without significant difference. In a comparison between pre-treatment and one-treatment, pre-treatment showed more effectiveness for reducing the RNA load at 3 dpi. Altogether, the treatment using F(ab’)2 reduced clinical signs and viral load in treated hamsters and could be effective against SARS-CoV-2 infection.
Discussion
In the setting of a pandemic, in addition to vaccines, passive immunotherapies using monoclonal antibodies, convalescent human plasma or animal-derived hyperimmune globulins usually represent the first specific antiviral therapies to become available. In previous outbreaks caused by other viruses, such as SARS-CoV, MERS-CoV, Ebola, and avian influenza virus, horse immunization to produce hyperimmune globulins was evaluated (Lu et al., 2005; Bal et al., 2015; Pyankov et al., 2017; Zhao et al., 2017). In addition to their application in emerging and reemerging infectious diseases, heterologous polyclonal antibody therapies are also useful to treat long-standing neglected tropical diseases (Dixit et al., 2016). Equine antivenom products are routinely produced in both high-income and low-income countries (Pyankov et al., 2017) and WHO standardized guidelines are available for this class of products (www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguide/en), potentially allowing equine antibody products to be largely available worldwide within a relatively short period of time. In the last decades, horse hyperimmune sera shifted in most cases to be produced as purified antibody fragments or full-length antibodies, and since then the incidence of adverse effects has declined significantly in frequency and intensity. A small incidence of mild and moderate adverse effects for both purified equine antibody fragments (F(ab’)2) and full-length equine antibodies has been reported (León et al., 2013).
Motivated by the SARS-CoV outbreak in Asia in 2002–2003, equine F(ab’)2 was developed by immunizing horses using whole virus and CFA/IFA, resulting in high ELISA titers (Lu et al., 2005). In the case of MERS-CoV, equine immunoglobulin products were generated by immunizing horses with virus-like particles (VLPs) formed by three viral structural proteins (the spike, membrane and nucleocapsid proteins) (Zhao et al., 2017), also resulting in antibody preparations with high ELISA titers. The binding and neutralizing titers obtained in the present work were also high (1:1,000,000 and 1:23,219, respectively) and indicated high immunogenicity of the trimeric S protein used herein.
Recently, three reports have described the production of equine antibodies by immunizing horses with the recombinant SARS-CoV-2 RBD (Pan et al., 2020; Zylberman et al., 2020;; León et al., 2021), which comprises approximately 16% of the spike protomer. These works reached titers of binding antibodies similar to the titers obtained in the present work. In a recent study, León et al. (2021) clearly showed high neutralizing titers against SARS-CoV-2 for two formulations of whole-IgG from plasma of horses immunized with either the recombinant S1 domain or with a mixtures of SARS-CoV-2 recombinant proteins. In the present work, although our immunization strategy used only 1.2 mg of the S protein per of the 5 horses (6 weekly doses of only 200 μg), we achieved binding titers over 1:1,000,000 (Figure 1B), and more importantly, average titers for 90% neutralization were 1:14,604 when the low-responding horse was included or 1:18,000 when the low-responding animal was not included (Table 1). The higher ratio of neutralizing to binding antibodies obtained in the present work indicates a high quality of the antibodies generated by using the prefusion trimeric spike protein as the immunogen. Our results are in agreement with a previous comparison of mice immunized with the trimeric spike protein and the RBD, which showed higher neutralizing titers for the S protein (Mandolesi et al., 2021). This finding is probably related to the fact that other domains of the spike protein in addition to the RBD are also targets for neutralizing antibodies (Liu et al., 2020). Not surprisingly, most COVID vaccines developed worldwide focus on the whole spike protein, either as nucleic acids encoding the complete S protein, such as mRNA or vectored vaccines, or as the recombinant protein itself (Klasse et al., 2021; Mandolesi et al., 2021).
In our study, we used Montanide ISA 50V as an adjuvant. The Montanide adjuvant enhances the immune response by warranting a depot effect and promoting slow delivery of the protein to maintain recognition, stimulation and phagocytosis by dendritic cells and B cells for long time periods (Coffman et al., 2010). The present results not only indicate the high immunogenicity of the trimeric prefusion spike protein but also demonstrate the feasibility of using Montanide, which is a less reactogenic adjuvant than CFA/IFA (Oreskovic et al., 2019).
To reduce the risk of antibody-dependent enhancement (ADE) of infection, the pooled plasma obtained from the horses was processed to remove the Fc portion of IgG by pepsin digestion, followed by partial purification of the F(ab’)2 fragment by ammonium sulfate fractionation. This method is a proven technology used worldwide for the production of equine hyperimmune products, because the use of F(ab’)2 instead of whole IgG eliminates nonspecific binding between the Fc portion of antibodies and Fc receptors on cells, thus reducing the possibility of ADE, although there is no report of ADE with SARS-CoV-2. This technology is regularly used for GMP manufacturing of anti-rabies, anti-tetanus, and anti-venom F(ab’)2 products at Vital Brazil Institute (IVB), with no records of hypersensitivity issues and decades of excellent safety records. In addition, safety trials of an analogous equine F(ab’)2 product against avian influenza (H5N1) performed by Bal et al. confirmed that it is a well-tolerated product, causing just a few mild adverse events (Bal et al., 2015). Importantly, the anti-SARS-CoV-2 F(ab’)2 concentrate developed herein maintained the very high binding and neutralizing titers found in unprocessed sera (Figures 3, 4, and 5; Table 1).
As mentioned above, convalescent plasma is another therapeutic alternative that quickly becomes available in the setting of outbreaks. For comparison, we evaluated the neutralization titer of three human convalescent plasma samples. We found that the neutralization titers of the equine F(ab’)2 concentrates were two orders of magnitude higher than those of human convalescent plasma (107- and 151-fold higher for PRNT50 and PRNT90, respectively). Plasma from the low-responding horse, #835, which started to show relevant neutralizing activities just after the fourth inoculation, was included in the plasma pool used to produce the pilot-scale F(ab’)2 concentrate. Thus, if horses were preselected for their neutralizing activity prior to F(ab’)2 manufacture, the neutralizing ability of the antibody concentrate could be even higher, reducing infusion volumes in patients. Zylberman et al. (2020), who used SARS-CoV-2 RBD to immunize horses, reported that their F(ab’)2 product had an approximately 50-fold higher neutralizing capacity than convalescent plasma reported in the literature (Zylberman et al., 2020). In another work using the RBD to immunize horses, samples from 11 COVID-19 convalescent patients were evaluated, and at a 1:640 dilution, three human samples showed 50% neutralization, and another two showed 80–90% neutralization ability (Pan et al., 2020). León et al. (2021) also showed high neutralizing titers, about 80 times more potent than a pool of human convalescent plasma. Overall, these data confirm the promising potential of equine hyperimmune products as COVID-19 countermeasures over human convalescent plasma, considering the high potency and safety record of F(ab’)2 products as well as eventual limitations related to human plasma availability and eventual risks of adventitious agent transmission by human plasma products.
The advantage of using the fully folded trimeric S protein in the prefusion conformation to elicit equine polyclonal antibodies against SARS-CoV-2 is the main novelty of our work. Highly neutralizing monoclonal antibodies discovered from B cells of convalescent COVID-19 patients targeted epitopes in the RBD and in the N-terminal domain (NTD), as well as quaternary epitopes of the trimeric spike protein (Liu et al., 2020; Voss et al., 2021) Thus, the use of the trimeric spike protein combines the advantages of higher immunogenicity than smaller protein fragments (such as the RBD used in other works (Pan et al., 2020; Zylberman et al., 2020)) with low biosafety concerns compared to those that would apply in case of inoculating the whole virus (as used for SARS-CoV (Lu et al., 2005)).
Therapies utilizing hyperimmune sera produced in horses have been evaluated in experimental models against SARS and MERS infections. In 2007, Zhou et al. (2007) demonstrated that treatment with hyperimmune serum (anti-SARS-CoV F(ab')2 equine) protected elderly BALB/c mice that were susceptible to SARS-CoV infection. Using 40 mg/kg, Zhou et al. observed a significant reduction in viral load in the lungs, and this result was subsequently confirmed in other studies (Zhao et al., 2017; Luo et al., 2007). In 2017, Zhao and colleagues demonstrated that the hyperimmune serum also reduced the viral load because of MERS infection in the lungs when administered 24 h after infection (Zhao et al., 2017). For SARS-CoV-2 infection, we used 50 mg/kg for pretreatment (24 h before or −1 dpi), one treatment (48 h or 2 dpi), or two treatments (+2 dpi and +4 dpi) using elderly hamsters (Figure 8). As a proof of concept, we used the pretreatment that clearly demonstrated a reduction of clinical signs and viral load. We opted to perform treatment 48 h after infection, and we observed a reduction of clinical signals and viral load. When we administered two treatments at +2 dpi and +4 dpi, we did not observe any increase in protection in relation to one treatment, demonstrating that effective treatment was achieved when administered upon an increase in viral load. qRT-PCR showed a very high sensibility and could also detect the mRNA of the “destroyed” virus. The results with hamsters support the possibility of using F(ab')2 to treat SARS-CoV-2 infection.
The emergence of strains with mutations in the spike protein has brought much concern for the use of vaccines and passive immunotherapy. The Brazilian variant P.1 (also named Gamma variant) is of great concern because it has three mutations in the receptor binding domain (RBD): K417T, E484K, and N501Y (Faria et al., 2021). Another Brazilian variant, P.2, has only one mutation in the RBD (E484K) (Voloch et al., 2021). Our results revealed that the F(ab’)2 globulin fragment developed against the S protein construct without the mutations had high neutralizing activity against these strains (Figure 7). Although the F(ab’) 2 globulin lost 0.39 log units of potency when compared to WT and P.2, the neutralizing titer was still high to support potential use. Recent studies have shown that several monoclonal antibodies lose neutralizing activity against P.1 (Dejnirattisai et al., 2021; Wang et al., 2021). Using both pseudovirus and authentic P.1 virus, Wang et al. (2021) showed that the P.1 strain was resistant to several neutralizing monoclonal antibodies, convalescent plasma and vaccine sera. The degree of resistance was greater for monoclonal antibodies than vaccine sera. The P.1 variant partially compromises currently approved monoclonal antibody therapies (Dejnirattisai et al., 2021; Wang et al., 2021). The maintenance of neutralizing activity of equine F(ab’)2 against P.2 and only marginally reduced activity against P.1 can be explained by the polyclonal nature of the raised antibody against the whole spike protein. The preservation of neutralizing activity against P.2 and P.1 can be explained by the polyclonal nature of equine F(ab’)2, which was raised against the whole spike protein. The use of the recombinant full-length spike protein in a stabilized trimeric prefusion conformation allows one to easily tailor the process by replacing the WT construct with one or more mutant constructs (such as gamma or delta variants) and use a mixed antigen to immunize the horses.
Figure 9 illustrates our whole strategy and emphasizes the potential binding of F(ab’)2 fragments to the S protein on the viral surface. Because multiple organs can be affected by SARS-CoV-2, resulting in a worse prognosis, the relatively long half-life of F(ab’)2 is likely to prevent disease progression, supported by our studies in mice (Figure 6) and hamsters (Figure 8). In summary, the use of an anti-COVID-19 hyperimmune F(ab’)2 concentrate produced by using a potent antigen (the prefusion trimeric spike glycoprotein) is a prompt alternative for passive immunization therapy, especially (Figure 8). Even with the current availability of efficacious vaccines, the possibility of having equine antibody products is highly important, as has been proven in the case of the rabies vaccine, where both passive and active immunizations are used to save lives.
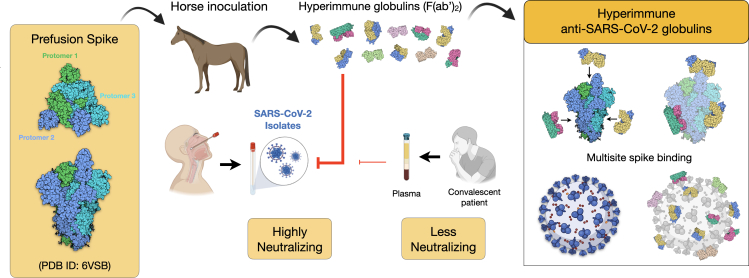
Figure 9.
Scheme of the immunization strategy and anti-SARS-CoV-2 hyperimmune globulin production
Recombinant prefusion trimeric S protein is used to inoculate horses and to produce hyperimmune F(ab’)2 concentrate. The equine antibody preparation presented a much higher capacity to neutralize a SARS-CoV-2 isolate than human convalescent plasma. One advantage of using the full-length recombinant spike trimer is the production of antibodies against different antigenic segments of the viral protein. This strategy may result in more efficient neutralizing capacity than that of antibodies produced against isolated fragments of the spike protein, such as the RBD.
Our results using the trimeric spike protein as antigen, as well the use of other immunogens, such as the RBD domain (Lopardo et al., 2021; Zylberman et al., 2020) and combinations of a spike domain with other structural proteins or protein epitopes (León et al., 2021) showed that neutralizing polyclonal equine full-length antibodies or F(ab')2 preparations seem to be good candidates for becoming well-established products, as observed for anti-snake or anti-scorpion F(ab’)2 therapies that have been used worldwide for decades. Our results with different groups of immunized horses and their repeated reimmunizations demonstrated good reproducibility. In the industrial production of purified equine antibody fragments, the final drug product (DP) is routinely formulated by diluting the concentrated drug substance (DS, which is the purified concentrated API). This dilution is intended to warrant that the specifications on the label and on the product insert are met for each batch, ensuring reproducibility.
Hyperimmune F(ab’)2 concentrates are manufactured in existing facilities available worldwide, in both high-income and low-income countries, using a long-proven platform technology, which could help accelerate the regulatory pathway to product approval for human use. We have started the regulatory submission to perform a phase I/II clinical trial for safety and efficacy evaluation of treatment with anti-SARS-CoV-2 equine immunoglobulin (F(ab’)2) in hospitalized patients with COVID-19 in early stages of the disease, i.e., not requiring invasive ventilation support (ClinicalTrials.gov identifier: NCT04573855). Taking as reference the production of other registered F(ab’)2 concentrates regularly produced by IVB in Brazil (e.g., anti-rabies immunoglobulin), each horse can supply at least 10 L of plasma for processing, resulting in at least 200 ampoules of F(ab’)2 concentrate. Because one horse can be bled up to six times per year without any animal suffering, this would mean that producing 100,000 ampoules (doses) per year would require approximately 80 horses per year. This is still a rough order-of-magnitude estimate, because the precise dose size still needs further studies to be defined. The use of the recombinant full-length spike protein in a stabilized trimeric prefusion conformation combines the advantages of using an antigen that closely mimics the native virus with the biosafety advantages related to using a recombinant immunogen. If needed, the process can be easily tailored by using mutant spike protein as antigen.
Limitations of the study
The hyperimmune equine F(ab’)2 developed against the trimeric spike glycoprotein provides high neutralization titers against different strains of SARS-CoV-2, including the P.1 (Gamma) and P.2. However, the efficacy of the equine F(ab’)2 has not been yet determined against other recent mutants such as the Delta variant.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-HCP | Antibodies-online.com | RRID: AB_1113182 |
| anti-rabbit IgG HRP | Sigma | Cat# A9169; RRID:AB_258434 |
| equine F(ab’)2 fragment | This paper | N/A |
| Horseradish peroxidase-conjugated rabbit anti-horse IgG | Sigma, USA | Cat# A6917; RRID:AB_258319 |
| Bacterial and virus strains | ||
| SARS-CoV-2 isolate (SARS-CoV-2/human/BRA/RJ01/2020) - IRB approval 30650420.4.1001.0008 | GenBank #MT710714 | |
| SARS-CoV-2 isolate from the B lineage (HIAE-02: SARS-CoV-2/SP02/human/2020/BRA) | Dr. Edison L. Durigon (Laboratory of Clinical and Molecular Virology, Institute of Biomedical Sciences, University of São Paulo) | GenBank #MT126808.1 |
| P.1 strain (EPI_ISL_1060902 – GISAID) | Dr. Lucy S. Villas-Boas and Prof .Maria Cassia Mendes-Correa (Laboratory of Medical Investigation in Virology from University of Sao Paulo) - Faria et al. (2021) | EPI_ISL_1060902 – GISAID |
| P.2 variant | Voloch et al. (2021) | N/A |
| SARS-CoV-2/B.1.33 variant | hCoV-19/Brazil/RJ-MD63-1a/202 | Accession ID: EPI_ISL_528637 |
| Biological samples | ||
| Serum of convalescent patients | Alvim et al., (2020) | Sample code numbers: #8942, #1639, #3065 |
| Chemicals, peptides, and recombinant proteins | ||
| Recombinant SARS-CoV-2 spike protein | Alvim et al., (2020) | N/A |
| HEK-GM medium | Xell AG, Germany | N/A |
| Superose 6 10/300 GL column | Cytiva | #29091596 |
| MW standards standardized molecules | Bio-rad, | #151-1901 |
| Rapigest SF | (Waters, Milford, MA) | N/A |
| PNGase F | New England BioLabs | #P0704S |
| Montanide ISA 50V adjuvant | Seppic, France | N/A |
| Pepsin | Sigma, USA | N/A |
| Tween 20 | Sigma, USA | N/A |
| OPD substrate | Sigma, USA | N/A |
| fetal bovine serum | HyClone/Cytiva, USA | N/A |
| Streptomycin | Thermo Fisher, USA | N/A |
| ketamine hydrochloride | Vetanarcol, König, Argentina | N/A |
| xylazine hydrochloride | Syntec Brazil, São Paulo, Brazil | N/A |
| sodium thiopental | Thiopentax, Cristalia, São Paulo, Brazil | N/A |
| Chemagic Viral DNA/RNA 300 kit H96 | Perkin-Elmer, Waltham, USA | N/A |
| RT-qPCR - Bio-Manguinhos SARS-CoV-2 molecular kit | Bio-Manguinhos, Rio de Janeiro, Brazil | N/A |
| Experimental models: Cell lines | ||
| African green monkey kidney (Vero, subtype E6) | ATCC | CRL-1586 |
| HEK293-COV2-S | Alvim et al. (2020) | N/A |
| Experimental models: Organisms/strains | ||
| Female BALB/c mice, 6–8 weeks old | Central Bioterium (Centro de Ciências da Saúde – Universidade Federal do Rio de Janeiro, Brazil) | N/A |
| Golden Syrian hamsters Mesocricetus auratus, males and females, age between 1.5–2.0 years |
Institute of Science and Technology in Biomodels (ICTB) of the Oswaldo Cruz Foundation (Fiocruz) | N/A |
| Ten 3- to 5-year-old healthy horses (6 males and 4 females) approximately 350 kg each | IVB farm, Rio de Janeiro, Brazil | N/A |
| Software and algorithms | ||
| GraphPad Prism 7® | GraphPad Software | N/A |
| GraphPad Prism® software (8.01 version) | GraphPad Software | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jerson L. Silva, PhD, MD (jerson@bioqmed.ufrj.br).
Materials availability
All requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jerson L. Silva, PhD, MD (jerson@bioqmed.ufrj.br). The resources and reagents include selective antibodies, viruses, serum and proteins. All reagents will be made available on request after completion of a Materials Transfer Agreement.
Additional information
Supplementary information is available for this paper.
Experimental model and subject details
Ethics statement
All procedures involving animals were performed in accordance with the animal research ethical principles determined by National Brazilian Law 11.794/08 of the National Council for the Control of Animal Experimentation (CONCEA, Brazil). The protocol for the horses was approved by the Animal Care and Use Committee from IVB under permission no. 003. For BALB/c mice, the protocol was approved by the Committee for Animal Use of the Universidade Federal do Rio de Janeiro (Permit Number: 074/20). For Golden Syrian hamsters the study was conducted at Animal Biosafety Level 3 facilities from Bio Manguinhos/Fiocruz The study protocol was approved (LW-9/20) by the Institutional Animal Care and Use Committee (CEUA-Fiocruz).
Human convalescent plasma was collected at Instituto Estadual de Hematologia Arthur de Siqueira Cavalcanti (HEMORIO) according to a protocol submitted to the local ethics committee (Comitê de Ética em Pesquisa do Instituto Estadual de Hematologia Arthur de Siqueira Cavalcanti - CEP HEMORIO) and approved under #4008095, as described in Alvim et al. (2020). Written and signed informed consent was obtained from all plasma donors. All research was performed in accordance with all applicable relevant guidelines and regulations.
Animals
Female BALB/c mice, 6–8 weeks old, from the Central Bioterium (Centro de Ciências da Saúde – Universidade Federal do Rio de Janeiro, Brazil), were housed in ventilated mini-isolators (Alesco, Brazil) and kept under controlled temperature and light conditions.
Sixty clinically healthy Golden Syrian hamsters were used in this study (Mesocricetus auratus, males and females, age between 1.5–2.0 years, weighing from 150 to 250 grams). All animals were considered old. They were obtained from a breeding colony at the Institute of Science and Technology in Biomodels (ICTB) of the Oswaldo Cruz Foundation (Fiocruz), Rio de Janeiro, Brazil. The study was conducted at Animal Biosafety Level 3 facilities from Bio Manguinhos/Fiocruz during the animal adaptation period and throughout the study. Animals were individually housed in cages (68,7 cm × 18.5 cm) in a climate-controlled room (temperature of 22 ± 1°C and humidity of 55 ± 5%) with a 12-h light/dark cycle and daily feeding with a commercial rodent diet. Water was provided ad libitum. The study protocol was approved (LW-9/20) by the Institutional Animal Care and Use Committee (CEUA-Fiocruz) and conducted in strict accordance with the recommendations from the Guide for the Care and Use of Laboratory Animals of the Brazilian Society of Science in Laboratory Animals (SBCAL) and the National Council for the Control of Animal Experimentation (CONCEA, MCT, Brazil). The single housing hamster approach was adopted in our study to prevent cross contamination, since SARS-CoV-2 is transmitted via the respiratory route. Our study adhered to all recommendations for hamsters, in accordance with Brazilian Normative Resolution CONCEA n.28 from November 13, 2015 (http://www.mct.gov.br/upd_blob/0240/240230.pdf). Clinical procedures were performed under anesthesia, and all efforts were made to minimize painful procedures.
Plasma collection from human donors
Human convalescent plasma was collected at Instituto Estadual de Hematologia Arthur de Siqueira Cavalcanti (HEMORIO) according to a protocol submitted to the local ethics committee (Comitê de Ética em Pesquisa do Instituto Estadual de Hematologia Arthur de Siqueira Cavalcanti - CEP HEMORIO) and approved under #4008095, as described in Alvim et al. (2020). Written and signed informed consent was obtained from all plasma donors. All research was performed in accordance with all applicable relevant guidelines and regulations.
Cells and viruses
The original isolate of SARS-CoV-2 was obtained from a nasopharyngeal swab from a confirmed case in Rio de Janeiro, Brazil (IRB approval 30650420.4.1001.0008). All procedures related to virus culture were handled in a biosafety level 3 (BSL3) multiuser facility according to WHO guidelines. Virus titers were determined as plaque-forming units (PFU)/mL. The virus strain was sequenced to confirm identity, and the complete genome is available in GenBank (SARS-CoV-2/human/BRA/RJ01/2020, #MT710714). The virus stocks were kept at −80°C.
The SARS-CoV-2 isolate from the B lineage (HIAE-02: SARS-CoV-2/SP02/human/2020/BRA (GenBank accession number MT126808.1) was also used. The P.1 strain (EPI_ISL_1060902 – GISAID) was donated by the Laboratory of Medical Investigation in Virology from University of Sao Paulo (Faria et al., 2021). The P.2 variant is described in Voloch et al. (2021).
For the experiments with the hamsters, SARS-CoV-2 B.1.33 variant was utilized.
The cell line HEK293-COV2-S was generated by Alvim et al. (2020) and stably expresses the soluble ectodomain of the spike protein of SARS-CoV-2 in the prefusion trimeric conformation (Wrapp et al., 2020). The cells were cultivated in HEK-GM medium (Xell AG, Germany), either at a 300-mL scale at 37°C and 5% CO2 in Erlenmeyer flasks under orbital agitation (180 rpm, 5-cm stroke) or in 1.5-L stirred-tank bioreactors operated at a pH setpoint of 7.1 and a dissolved oxygen setpoint of 40% air saturation.
Method details
Production and purification of recombinant SARS-CoV-2 spike (S) glycoprotein
The cell line HEK293-COV2-S was generated by Alvim et al. (2020) and stably expresses the soluble ectodomain of the spike protein of SARS-CoV-2 in the prefusion trimeric conformation (Wrapp et al., 2020). The cells were cultivated in HEK-GM medium (Xell AG, Germany), either at a 300-mL scale at 37°C and 5% CO2 in Erlenmeyer flasks under orbital agitation (180 rpm, 5-cm stroke) or in 1.5-L stirred-tank bioreactors operated at a pH setpoint of 7.1 and a dissolved oxygen setpoint of 40% air saturation. Cell-free supernatant was obtained by microfiltration with 0.45-μm PVDF membranes and injected into a 5-mL StrepTrap XT affinity chromatography column (Cytiva) following the manufacturer's instructions. Protein concentration, purity and identity in the eluted fractions were confirmed by NanoDrop (Thermo Fisher), SDS-PAGE and Western blot analyses, respectively. The purified protein obtained in the affinity chromatography eluate was used to immunize horses and as an antigen in the ELISA to detect anti-SARS-CoV-2 antibodies in samples.
Analytical size exclusion chromatography (A-SEC)
Spike preparations (100 μg/mL) were injected into a Superose 6 10/300 GL column (Cytiva, #29091596) that was previously equilibrated in phosphate buffer saline (140 mM PBS, 2.8 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4, pH 7.4). The flow rate was set to 0.7 mL/min and the RF-20A XS fluorescence detector used to track the protein’s exclusion volume at 350 nm upon excitation at 280 nm using a high-performance liquid chromatograph system (Shiumadzu). The column was calibrated using standardized molecules (Bio-rad, #151-1901).
Electron-spray ionization mass spectrometry (ESI-MS)
50 μg of purified spike was mixed with 0.5% w/v Rapigest SF (Waters, Milford, MA) and left for 15 min at 90°C. Spike deglycosylation was performed using 10 U/μL of PNGase F (New England BioLabs, #P0704S) at 50°C for 30 min. Next, the sample was subjected to tryptic and chymotryptic digestion as follows: disulfide reduction was done with 5 mM of DTT at 56°C for 30 min followed by cysteine alkylation with 15 mM iodoacetamine at 25°C, 60 min (protected from light). Digestion was performed with 1 μg of trypsin and chymotrypsin at 37°C for 20 h. Rapigest SF was precipitated after 0.5% v/v trifluoracetic acid incubation at 37°C, 45 min. The sample was centrifuged at 13,000 rpm at 4°C, 15 min and the supernatant subjected to LC-MS.
The LC-MS system consisted of Waters Nano Acquity connected to a Synapt HDMS spectrometer (Waters, Milford, NA). The dried sample was resuspended in 50 μL of 3% acetonitrile, 0.1% formic acid and injected into a C18 Waters Symmetry pre-column for desalting (180 μm × 20 mm, 5 μm). Two microliters were injected into a HSS T3 130 C18 column (100 μm × 100 μm, 1.7 μm) and the peptides eluted by a 10–40% acetonitrile gradient containing 0.1% formic acid at a flow rat of 0.5 μL/min. The Nano spray ion source was set at 3.5 kV.
The raw data file was processed using PLGS 3.0.2 against a database of revised human proteome, spike glycoprotein Sars-CoV-2 and their variants, porcine trypsin and E. miricola PNGase F. The search parameters were fragment masses 0.1 Da, oxidation Met, carbamidomethyl Cys, and deamination Asn and Gln.
Western-blot analysis
Samples were loaded onto SDS-PAGE gels (7%), transferred to nitrocellulose membranes using the Semidry system (Bio-rad, #170-3940), blocked for 1 h at 25°C with 20 mM Tris-Cl (pH 7.4) and 137 mM NaCl in the presence of dried milk 0.5% w/v and Tween-20 0.05% v/v and incubated overnight with specific primary antibodies. The following antibodies and dilutions were used: anti-HCP (Antibodies-online.com, # ABIN1113182) at 1:1,000 and anti-rabbit IgG HRP (Sigma, #A9169) at 1:20,000 for detection.
Dynamic light scattering
The hydrodynamic radius (Rh) and size distribution of F(ab)’2 samples were determined using the DynaPro Nanostar system (Wyatt) equipped with a 630-nm laser at 25°C (Table S1).
Fluorescence microscope
F(ab’)2 was labeled with FITC. Vero E6 cells were infected with SARS-CoV-2 (MOI = 0.05) for 48 h and immunolabeled with F(ab’)2-FITC conjugate and observed with 20× or 40× objective (Figure S3). The images were acquired using a Leica TCS-SPE confocal microscope.
Animal immunization
All procedures involving animals were performed in accordance with the animal research ethical principles determined by National Brazilian Law 11.794/08. The protocol was approved by the Animal Care and Use Committee from IVB under permission no. 003.
All animals were subjected to prophylactic vaccination and deworming programs routinely utilized at the IVB farm and were tested for equine infectious anemia and Burkholderia mallei, as determined by the Brazilian Ministry of Agriculture regulations.
Ten 3- to 5-year-old healthy horses (6 males and 3 females) from the IVB farm, weighing approximately 350 kg each, were used for the production of polyvalent sera. Before immunization, blood samples were drawn from the jugular vein, and sera were stored at −20°C for use as negative controls in the binding and neutralizing antibody determinations. Each horse was subcutaneously immunized six times at 7-day intervals (on days 0, 7, 14, 21, 28 and 35) in different positions of the dorsal region. Each immunization of each horse consisted of 200 μg of recombinant SARS-CoV-2 trimeric S protein mixed with Montanide ISA 50V adjuvant (Seppic, France) to form an emulsion (one part immunogen in sterile saline to one-part sterile adjuvant). The horses were checked daily, and food and water intakes were monitored. Blood samples were collected every 7 days just before the next inoculation and 7 and 14 days after the last inoculation (up to day 49 after the first inoculation). Sera were stored at −20°C until the measurement of binding and neutralizing antibody titers.
Production of bench-scale and industrial (GMP) F(ab’)2 batch
For the production of the bench-scale pilot batch, plasma processing to produce F(ab’)2 was initiated by adding 3 L of water and 15 mL of 90% phenol solution to 2 L of horse plasma in a reactor. The solution was homogenized for 10 min, and the pH was adjusted to 4.3. Under agitation, 1.25 g/L pepsin was added, the pH was adjusted to 3.2, and the sample remained under agitation for 10 min. The sample was then stirred at 37°C, and the pH was adjusted to 4.2 with sodium hydroxide. Under constant agitation, sodium pyrophosphate decahydrate (12.6 mM) and toluene (10 μM) were added. Later, ammonium sulfate was added at 12% (m/v), and the solution was incubated at 55°C for 1 h.
To separate the F(ab’)2 fragments, the Fc portion was precipitated and subsequently filtered under pressure at constant agitation. F(ab’)2 was recovered from the liquid phase. To subsequently precipitate F(ab’)2 from the liquid phase, ammonium sulfate was added at 19% (m/v), and a second precipitation was performed under constant agitation and alkaline pH. Subsequently, the solution was diafiltered using a 30-kDa tangential ultrafiltration system until ammonium sulfate became undetectable in the retentate. The samples were isotonized with 15 mM NaCl, and 90% phenol solution was added to a final concentration of 0.3% (v/v). After sterile filtration, the F(ab’)2 product was stored at 4°C.
The industrial scale GMP-compliant batch was produced according to an analogous process (but scaled up to start from 160 L of plasma) using the industrial GMP facility of IVB in Rio de Janeiro state, Brazil.
Enzyme-linked immunosorbent assay (ELISA)
In brief, polystyrene high-adsorption 96-well microplates (Thermo Fisher, USA) were coated with 500 ng/well recombinant SARS-CoV-2 S protein (100 μL/well at 5 μg/mL) in carbonate-bicarbonate buffer (pH 9.6) overnight at room temperature and then blocked with 3% BSA (Sigma, USA) in PBS for 2 h at 37°C. Serially diluted serum samples of the 5 horses were added to the plate and incubated at 37°C for 1 h. Horseradish peroxidase-conjugated rabbit anti-horse IgG (Sigma A6917, USA) diluted 1:10,000 in PBS was incubated at 37°C for 1 h. After each step, the plates were washed 3 times with PBS containing 0.05% Tween 20 (PBST). OPD substrate (100 μL/well - Sigma, USA) was added to the wells and incubated in the dark for 10 min at room temperature. The reaction was stopped by adding 50 μL of 30% H2SO4 (v/v) to each well, and the absorbance value was measured at 490 nm in a microplate reader (Epoch/2 microplate spectrophotometer, Biotek). The IgG antibody titer was defined as the highest dilution of serum yielding an absorbance ratio greater than 2 in the same dilution (λ490 of sample/λ490 of negative control). The analyses were carried out in duplicate.
Cells and virus used in neutralization assays
African green monkey kidney (Vero, subtype E6) cells were cultured at 37°C in high-glucose DMEM with 10% fetal bovine serum (HyClone/Cytiva, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Thermo Fisher, USA) in a humidified atmosphere with 5% CO2.
SARS-CoV-2 was prepared in Vero E6 cells. Originally, the isolate was obtained from a nasopharyngeal swab from a confirmed case in Rio de Janeiro, Brazil (IRB approval 30650420.4.1001.0008). All procedures related to virus culture were handled in a biosafety level 3 (BSL3) multiuser facility according to WHO guidelines. Virus titers were determined as plaque-forming units (PFU)/mL. The virus strain was sequenced to confirm identity, and the complete genome is available in GenBank (SARS-CoV-2/human/BRA/RJ01/2020, #MT710714). The virus stocks were kept at −80°C.
Microneutralization assay
To assess the neutralization titer (Fintelman-Rodrigues et al., 2021), the samples were incubated with 100 PFU of SARS-CoV-2 for 1 h at 37°C. Then, the samples were transferred to 96-well plates with monolayers of Vero cells (2 × 104 cells/well) with serial dilutions of sample for 1 h at 37°C. Cells were washed, and fresh medium with 2% FBS and 2.4% CMC was added. On day 3 postinfection, the cytopathic effect was scored in at least 2 replicates per dilution by independent readers. The readers were blind with respect to the sample ID.
Statistical analyses were performed with GraphPad Prism 7®. Neutralization assay data are shown as the mean ± SD; p < 0.05 vs. plasma collected on day 0 according to Student's t-test for paired samples.
Neutralizing titers in the plasma of mice injected with equine F(ab’)2
To investigate whether neutralizing activity against SARS-CoV-2 can be observed in animals treated with the fragments, equine F(ab’)2 (pilot batch) was injected by the intraperitoneal route, and neutralizing titers were measured in the plasma of mice. Eleven BALB/c mice were divided into 4 groups, which received different doses of the antibody fragments: 50 μg (n = 3); 25 μg (n = 3); 10 μg (n = 3) and negative control (saline) (n = 2). The total volume injected was 200 μL per mouse. Blood was collected from the retro-orbital plexus 72 hours after inoculation, and then the animals were euthanized by saturation with isoflurane. The neutralization titer in mouse plasma was determined by the plaque reduction neutralization test (PRNT). Statistical significance was calculated using GraphPad Prism® 7 software by one-way ANOVA and Tukey’s post hoc test to the confidence levels indicated in Figure S2.
Plaque reduction neutralization test (PRNT) and dose response curve
The plaque reduction neutralization test (PRNT) was used to compare titers of neutralizing antibodies in the purified F(ab’)2 solution against different SARS-CoV-2 variants, namely, P.1, P.2, and an isolate of the ancestral B lineage (named WT). The SARS-CoV-2 isolate from the B lineage (HIAE-02: SARS-CoV-2/SP02/human/2020/BRA (GenBank accession number MT126808.1) was used in this work. The P.1 strain (EPI_ISL_1060902 – GISAID) was donated by the Laboratory of Medical Investigation in Virology from University of Sao Paulo (Faria et al., 2021). The P.2 variant is described in Voloch et al. (2021). Serial dilutions of the purified F(ab’)2 solution were incubated with 50 PFU of SARS-CoV-2 for 1 hour at 37°C. The virus- F(ab’)2 mixture was inoculated into confluent monolayers of VERO cells. After 1 hour, the inoculum was removed, and semisolid medium (1.4% carboxymethylcellulose in alpha-MEM supplemented with 1.5% fetal bovine serum) was added. The cells were further incubated for 72 hours and then fixed with 4% formaldehyde solution. The cells were stained with crystal violet dye solution, and the concentrations of the F(ab’)2 solutions that inhibited 50% (IC50) and 90% (IC90) of input plaque counts (normalized to the untreated control) were determined by a non-linear regression model with variable slope using GraphPad Prism® software (8.01 version).
Study design with hamsters
Sixty hamsters were divided into four groups of fifteen animals each. Group 1 (G1) consisted of the SARS-CoV-2 6.0 log10 inoculum (B.1.33 variant). In group 2 (G2), the animals were treated with the equine hyperimmune group against SARS-CoV-2 at −1 dpi. Group 3 (G3) hamsters were treated with a single dose of equine hyperimmune serum against SARS-CoV-2 at +2 dpi. Group 4 (G4) animals were treated twice, at + 2 and +4 dpi. Cage side observation of hamsters for signs of illness were performed daily during quarantine and throughout the study period (15 days). The physical examinations for signs of respiratory distress and animal weighing were performed daily while the animals were awake. Hematological, biochemical, and serological basal parameters were established for each animal individually during the preinoculation period. Euthanasia was performed by full exsanguination via heart puncture under deep anesthesia. Necropsies were subsequently performed for three animals at −1, +3, +5, +10, and +15 dpi, at which time the blood samples were drained and the pulmonary and oropharyngeal samples were sectioned and stored at −70°C until further analysis. The anesthesia protocol used at all collection dates was performed with ketamine hydrochloride at 80 mg/kg (Vetanarcol, König, Argentina) and xylazine hydrochloride at 0.1 mg/kg (Syntec Brazil, São Paulo, Brazil). At 15 dpi, the last three animals were euthanized under deep barbiturate anesthesia with 5% sodium thiopental at 100 mg/kg (Thiopentax, Cristalia, São Paulo, Brazil) delivered intravenously. Subsequently, the animals were subjected to cardiac puncture and euthanized by exsanguination.
Equine hyperimmune serum treatment and SAR-CoV-2 inoculation in hamsters
The neutralizing titer of equine hyperimmune serum was 1:6000. All animals (G1, G2, and G3) received 1 mL via the intraperitoneal route at −1 dpi, +1 dpi, and +2 dpi as described above. Fifty microliters of SARS-CoV-2 (B.1.33 variant) was administered via the intranasal route at 0 dpi in all animals. The inoculum contained a load of 6.0 log10 RNA copies per milliliter, as determined by qRT-PCR.
Quantitative SARS-CoV-2 RNA detection in the respiratory tract (qRT-PCR)
The oropharyngeal swab and fragments of lung tissue were collected at the time of euthanasia. Nucleic acids from all the samples were purified using the Janus G3 and Chemagic Platform (Perkin-Elmer, Waltham, USA) for viral DNA/RNA purification with the Chemagic Viral DNA/RNA 300 kit H96 (Perkin-Elmer, Waltham, USA). A magnetic-bead-based method for extraction of viral DNA/RNA from 300 μL of the sample was applied according to the manufacturer’s instructions.
The SARS-CoV-2 detection and quantification in the purification eluate was performed by RT-qPCR using the Bio-Manguinhos SARS-CoV-2 molecular kit (Bio-Manguinhos, Rio de Janeiro, BR), which includes a FAM-probe for the E region of SARS-CoV-2 and a VIC-probe for the RP human gene, the internal positive control in the assay. All reactions included positive and negative controls. The RT-qPCR setup on the plate was performed by Janus G3 (Perkin-Elmer, Waltham, USA). The samples were considered positive for the E region when the cycle threshold (CT) was <38.0 and negative when the CT was >38.0. For validation of the internal control, the CT for RP gene amplification had to be <35. The positive control CT had to be <37.
Quantification and statistical analysis
For in vitro experiments, results are expressed as mean ± Standard Desviation (SD) or mean ± standard error of the mean (SEM). Where statistics are quoted, two experimental groups were compared via the Student’s t test. A p value of <0.05 was considered statistically significant. ∗P < 0.05; for indicated comparisons, error bars represent standard error of means.
For in vivo experiments, the results are expressed as mean ± SD. Where statistics are quoted, three or more experimental groups were compared with one-way or two-way ANOVA, with Turkey’s post-test. A p value of <0.05 was considered statistically significant. ∗ or # or @ P < 0.05; ∗∗ P < 0.01; for indicated comparisons, error bars represent standard error of means.
Acknowledgments
The authors gratefully acknowledge B. S. Graham and K. S. Corbett (Vaccine Research Center, NIAID/NIH) for sharing the plasmid (BEI Resources # NR-52563) that enabled the generation of the cell line producing SARS-CoV-2 spike protein. We thank Dr. Edison L. Durigon (Laboratory of Clinical and Molecular Virology, Institute of Biomedical Sciences, University of São Paulo) for kindly providing the SARS-CoV-2 isolate from the B lineage (HIAE-02: SARS-CoV-2/SP02/human/2020/BRA (GenBank accession number MT126808.1). We thank Dr. Lucy S. Villas-Boas and Prof.Maria Cassia Mendes-Correa (Laboratory of Medical Investigation in Virology, University of Sao Paulo) for kindly providing the SARS-CoV-2 P.1 variant. We also thank Dr. A. M. Vale from UFRJ and Dr. S. Garcia and Dr. L. Amorim from Hemorio for human convalescent plasma samples. Financial support was obtained from Senai, CTG, Embrapii, Sebrae and from the Brazilian research funding agencies Fundação Carlos Chagas Filho de Amparo à Pesquisa do Rio de Janeiro (FAPERJ), the Brazilian Ministry of Science, Technology, and Innovation for Virus Network, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Ensino Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP).
Author contributions
Overall design of the study and conceptualization, J.L.S., L.R.C., L.E.R.C., A.A.S., M.A.S., A.M.O.G., A.T., H.L.M.G; Funding acquisition: J.L.S., L.R.C., L.E.R.C; Experiments and analyses, M.A.S., V.A.R.P., C.H.D., L.E.R.C, A.M.O.G., A.A.S., F.L.M., L.M.H., P.N.C.S., J.G.F., F.E.P., L.G.R.M., J.W.M.A., C.Q.S., N.F.R., T.M.L., R.G.F.A., F.F.M., M.M.C., R.B.Z., G.A.P.O., T.M.L.S., A.S.S., R.M., D.R.F.R., L.J.C., A.D.R.A., M.A.P., A.C.O, H.L.M.G., A.T., L.R.C., J.L.S.; Writing – Original Draft, J.L.S., L.R.C., A.C.O., L.E.R.C., M.A.S., A.M.O.G., A.T., H.L.M.G; Writing – Review & Editing, All authors.
Declaration of interests
The authors declare no competing interest.
Published: November 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103315.
Contributor Information
Amilcar Tanuri, Email: atanuri1@gmail.com.
Leda R. Castilho, Email: leda@peq.coppe.ufrj.br.
Jerson L. Silva, Email: jerson@bioqmed.ufrj.br.
Supplemental information
Data and code availability
This study did not generate/analyze any datasets/code. The SARS-CoV-2 isolates have been deposited in GenBank (#MT710714; #MT126808.1) and GISAID (EPI_ISL_1060902; EPI_ISL_528637) as listed in the Key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P., PLACID Trial Collaborators Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvim R.G.F., Lima T.M., Rodrigues D.A.S., Marsili F.F., Bozza V.B.T., Higa L.M., Monteiro F.L., Leitão I.C., Carvalho R.S., Galliez R.M., et al. An affordable anti-SARS-COV-2 spike ELISA test for early detection of IgG seroconversion suited for large-scale surveillance studies in low-income countries. Preprint. 2020 doi: 10.1101/2020.07.13.20152884. https://www.medrxiv.org/content/10.1101/2020.07.13.20152884v3 [DOI] [Google Scholar]
- Bal C., Herbreteau C.H., Buchy P., Rith S., Zaid M., Kristanto W., Han V., Reynaud C., Granjard P., Lépine B., et al. Safety, potential efficacy, and pharmacokinetics of specific polyclonal immunoglobulin F(ab')₂ fragments against avian influenza A (H5N1) in healthy volunteers: a single-centre, randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2015;15:285–292. doi: 10.1016/S1473-3099(14)71072-2. [DOI] [PubMed] [Google Scholar]
- Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R., Herz J., Dalton R., Booy R. Benefits of using heterologous polyclonal antibodies and potential applications to new and undertreated infectious pathogens. Vaccine. 2016;34:1152–1161. doi: 10.1016/j.vaccine.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Zhou D., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;30:2939–2954.e9.. doi: 10.1016/j.cell.2021.03.055. Epub ahead of print. PMID: 33852911; PMCID: PMC8008340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria N.R., Mellan T.A., Whittaker C., Claro I.M., Candido D.D.S., Mishra S., Crispim M.A.E., Sales F.C.S., Hawryluk I., McCrone J.T., et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;2021:eabh2644. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fintelman-Rodrigues N., da Silva A.P.D., Dos Santos M.C., Saraiva F.B., Ferreira M.A., Gesto J., Rodrigues D.A.S., Vale A.M., de Azevedo I.G., Soares V.C., et al. Genetic evidence and host immune response in persons reinfected with SARS-CoV-2, Brazil. Emerg. Infect. Dis. 2021;27:1446–1453. doi: 10.3201/eid2705.204912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., León G., Lomonte B., Angulo Y. Antivenoms for snakebite envenomings. Inflamm. Allergy Drug Targets. 2011;10:369–380. doi: 10.2174/187152811797200669. PMID: 21745181. [DOI] [PubMed] [Google Scholar]
- Huo J., Le Bas A., Ruza R.R., Duyvesteyn H.M.E., Mikolajek H., Malinauskas T., Tan T.K., Rijal P., Dumoux M., Ward P.N., et al. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020;27:846–854. doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- Klasse P.J., Nixon D.F., Moore J.P. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci. Adv. 2021;7:eabe8065. doi: 10.1126/sciadv.abe8065. PMID: 33608249; PMCID: PMC7978427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León G., Herrera M., Vargas M., Arguedas M., Sánchez A., Segura Á., Gómez A., Solano G., Corrales-Aguilar E., Risner K., et al. Development and characterization of two equine formulations towards SARS-CoV-2 proteins for the potential treatment of COVID-19. Sci. Rep. 2021;11:9825. doi: 10.1038/s41598-021-89242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León G., Herrera M., Segura Á., Villalta M., Vargas M., Gutiérrez J.M. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. 2013;76:63–76. doi: 10.1016/j.toxicon.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial [published online ahead of print, 2020 Jun 3] [published correction appears in JAMA. 2020 Aug 4;324(5):519] JAMA. 2020;324:1–11. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., et al. Fundación INFANT–COVID-19 Group. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F.–W., Sahi V., Figueroa A., et al. Potent neutralizing antibodies directed to multiple epitopes on SARS-CoV-2 spike [published online ahead of print, 2020 Jul 22] Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- Long Q., Tang X., Shi Q., Li Q., Deng H., Yuan J., Hu J., Xu W., Zhang Y., Lv F., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. Epub 2020 Jun 18. [DOI] [PubMed] [Google Scholar]
- Lopardo G., Belloso W.H., Nannini E., Colonna M., Sanguineti S., Zylberman V., Muñoz L., Dobarro M., Lebersztein G., Farina J., et al. RBD-specific polyclonal F(ab')2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: a randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial. EClinicalMedicine. 2021;34:100843. doi: 10.1016/j.eclinm.2021.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.H., Guo Z.M., Han W.Y., Wang G.L., Zhang D.M., Wang Y.F., Sun S.Y., Yang Q.H., Zheng H.Y., Wong B.L., et al. Preparation and development of equine hyperimmune globulin F(ab')2 against severe acute respiratory syndrome coronavirus. Acta Pharmacol. Sin. 2005;26:1479–1484. doi: 10.1111/j.1745-7254.2005.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Ni B., Zhao G., Jia Z., Zhou L., Pacal M., Zhang L., Zhang S., Xing L., Lin Z., et al. Protection from infection with severe acute respiratory syndrome coronavirus in a Chinese hamster model by equine neutralizing F(ab')2. Viral Immunol. 2007;20:495–502. doi: 10.1089/vim.2007.0038. [DOI] [PubMed] [Google Scholar]
- Mandolesi M., Sheward D.J., Hanke L., Ma J., Pushparaj P., Perez Vidakovics L., Kim C., Àdori M., Lenart K., Loré K., et al. SARS-CoV-2 protein subunit vaccination of mice and rhesus macaques elicits potent and durable neutralizing antibody responses. Cell Rep. Med. 2021;2:100252. doi: 10.1016/j.xcrm.2021.100252. Epub 2021 Apr 5. PMID: 33842900; PMCID: PMC8020888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveca F. Phylogenetic relationship of SARS-CoV-2 sequences from Amazonas with emerging Brazilian variants harboring mutations E484K and N501Y in the Spike protein. 2021. https://virological.org/t/phylogenetic-relationship-of-sars-cov-2-sequences-fromamazonas-with-emerging-brazilian-variants-harboring-mutations-e484k-andn501y-in-the-spike-protein/585
- Oreskovic Z., Nechvatalova K., Krejci J., Kummer V., Faldyna M. Aspects of intradermal immunization with different adjuvants: the role of dendritic cells and Th1/Th2 response. PLoS One. 2019;14:e0211896. doi: 10.1371/journal.pone.0211896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zhou P., Fan T., Wu Y., Zhang J., Shi X., Shang W., Fang L., Jiang X., Shi J., et al. Immunoglobulin fragment F(ab’)2 against RBD potently neutralizes SARS-CoV-2 in vitro. Antiviral Res. 2020;182:104868. doi: 10.1016/j.antiviral.2020.104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Pyankov O.V., Setoh Y.X., Bodnev S.A., Edmonds J.H., Pyankova O.G., Pyankov S.A., Pali G., Belford S., Lu L., La M., et al. Successful post-exposure prophylaxis of Ebola infected non-human primates using Ebola glycoprotein-specific equine IgG. Sci. Rep. 2017;7:41537. doi: 10.1038/srep41537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow J., Graham C., Merrick B., Acors S., Steel K.J.A., Hemmings O., O’Bryne A., Kouphou N., Pickering S., Galao R.P., et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. Preprint. 2020 doi: 10.1101/2020.07.09.20148429. https://www.medrxiv.org/content/10.1101/2020.07.09.20148429v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy N., Giunta D.H., Pérez L.G., Sánchez M.D.L., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N. Engl. J. Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Ho M. Emerging antibody-based therapeutics against SARS-CoV-2 during the global pandemic. Antib Ther. 2020;3:246–256. doi: 10.1093/abt/tbaa025. PMID: 33912795; PMCID: PMC7717131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- Voloch C.M., da Silva F.R., Jr., de Almeida L.G.P., Cardoso C.C., Brustolini O.J., Gerber A.L., Guimarães A.P.D.C., Mariani D., da Costa R.M., Ferreira O.C., Jr., et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J. Virol. 2021;95:e00119–e00121. doi: 10.1128/JVI.00119-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss W.N., Hou Y.J., Johnson N.V., Delidakis G., Kim J.E., Javanmardi K., Horton A.P., Bartzoka F., Paresi C.J., Tanno Y., et al. Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes in COVID-19 convalescent plasma. Science. 2021;372:1108–1112. doi: 10.1126/science.abg5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare A., Deopurkar R.L., Salvi N., Khadilkar M., Kalolikar M., Gade S.K. Comparison of Montanide adjuvants, IMS 3012 (Nanoparticle), ISA 206 and ISA 35 (emulsion based) along with incomplete Freund's adjuvant for hyperimmunization of equines used for production of polyvalent snake antivenom. Vaccine. 2009;27:1067–1072. doi: 10.1016/j.vaccine.2008.11.103. [DOI] [PubMed] [Google Scholar]
- Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., Liu L., Kwong P.D., Huang Y., Shapiro L., et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–751.e4. doi: 10.1016/j.chom.2021.04.007. Epub ahead of print. PMID: 33887205; PMCID: PMC8053237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Sun J., Nie S., Li H., Kong Y., Liang M., Hou J., Huang X., Li D., Ma T., et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat. Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. Epub 2020 Jun 5. [DOI] [PubMed] [Google Scholar]
- Zeng F., Chen X., Deng G. Convalescent plasma for patients with COVID-19. Proc. Natl. Acad. Sci. U S A. 2020;117:12528. doi: 10.1073/pnas.2006961117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang C., Qiu B., Li C., Wang H., Jin H., Gai W., Zheng X., Wang T., Sun W., et al. Passive immunotherapy for Middle East Respiratory Syndrome coronavirus infection with equine immunoglobulin or immunoglobulin fragments in a mouse model. Antivir. Res. 2017;137:125–130. doi: 10.1016/j.antiviral.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Wong G., Zhao Y., Wang H., He S., Bi Y., Chen W., Jin H., Gai W., Chu D., et al. Treatment with hyperimmune equine immunoglobulin or immunoglobulin fragments completely protects rodents from Ebola virus infection. Sci. Rep. 2016;6:24179. doi: 10.1038/srep24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Ni B., Luo D., Zhao G., Jia Z., Zhang L., Lin Z., Wang L., Zhang S., Xing L., et al. Inhibition of infection caused by severe acute respiratory syndrome-associated coronavirus by equine neutralizing antibody in aged mice. Int Immunopharmacol. 2007;7:392–400. doi: 10.1016/j.intimp.2006.10.009. Epub 2006 Nov 27. PMID: 17276898; PMCID: PMC7106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylberman V., Sanguineti S., Pontoriero A.V., Higa S.V., Cerutti M.L., Seijo S.M.M., Pardo R., Muñoz L., Intrieri M.E.A., Alzogaray V.A., et al. Development of a hyperimmune equine serum therapy for COVID-19 in Argentina. Desarrollo de un suero equino hiperinmune para el tratamiento de COVID-19 en Argentina. Medicina (B Aires) 2020;80:1–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze any datasets/code. The SARS-CoV-2 isolates have been deposited in GenBank (#MT710714; #MT126808.1) and GISAID (EPI_ISL_1060902; EPI_ISL_528637) as listed in the Key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.