Abstract
Yeast strains with a mutation in the MEC1 gene are deficient in the cellular checkpoint response to DNA-damaging agents and have short telomeres (K. B. Ritchie, J. C. Mallory, and T. D. Petes, Mol. Cell. Biol. 19:6065–6075, 1999; T. A. Weinert, G. L. Kiser, and L. H. Hartwell, Genes Dev. 8:652–665, 1994). In wild-type yeast cells, genes inserted near the telomeres are transcriptionally silenced (D. E. Gottschling, O. M. Aparichio, B. L. Billington, and V. A. Zakian, Cell 63:751–762, 1990). We show that mec1 strains have reduced ability to silence gene expression near the telomere. This deficiency was alleviated by the sml1 mutation. Overexpression of Mec1p also resulted in a silencing defect, although this overexpression did not affect the checkpoint function of Mec1p. Telomeric silencing was not affected by mutations in several other genes in the Mec1p checkpoint pathway (null mutations in RAD9 and CHK1 or in several hypomorphic rad53 alleles) but was reduced by a null mutation of DUN1. In addition, the loss of telomeric silencing in mec1 strains was not a consequence of the slightly shortened telomeres observed in these strains.
Our study concerns the relationship between two pathways in Saccharomyces cerevisiae: the pathway controlling reversible silencing of genes inserted near the telomeres, or telomere position effect (TPE), and the pathway regulating the response of the cell to DNA damage. TPE in S. cerevisiae was first reported by Gottschling et al. (14). Mutations in genes encoding telomere-binding proteins (Rap1p), Rap1p-interacting proteins (the Sir proteins, Rif1p and Rif2p), the histones H3 and H4, and proteins controlling posttranslational modifications of histones affect telomeric silencing (22); many of these mutations also affect the silencing of mating-type information at HML and HMR (2).
Silencing at the telomere is thought to involve interactions between the Rap1, Sir3p, and Sir4p proteins and the amino termini of histones H3 and H4 (17); subsequent “spreading” of silencing from the telomeric repeats to adjacent regions may involve posttranslational modifications of the histones (18). The net effect of these modifications is to reduce the availability of DNA in the silenced regions for the binding of transcription factors (3). Telomeric silencing also affects the timing of DNA replication. Telomeric sequences are replicated late in the S period in wild-type cells (27), and this replication delay is lost in strains in which TPE is eliminated (35).
In response to DNA damage, yeast cells induce various DNA repair enzymes and arrest the cell cycle in order to repair the damage (11, 40). Different gene products are involved in the early steps of recognizing DNA damage, in transmitting the DNA damage signal, and in responding to the DNA damage signal. The checkpoint proteins relevant to our study are Mec1p, Rad53p, Rad9p, Dun1p, and Chk1p (1, 39, 43). In current models of checkpoint pathways, Mec1p transduces signals from proteins that sense damaged DNA or delayed DNA replication to proteins that block the cell cycle or induce expression of DNA repair genes.
Mec1p is a very large protein with a protein/lipid kinase motif shared with the yeast Tel1p and the human ATM protein (19). Phosphorylation of Rad53p, Rad9p, and Dun1p requires Mec1p (12, 21, 38). Rad9p may be involved both in sensing DNA damage (23) and in activating functions downstream of Mec1p (12, 38). Both Rad53p and Dun1p are protein kinases that function in a damage response pathway downstream of Mec1p, with Rad53p functioning upstream of Dun1p (1, 13, 43). Both rad53 and mec1 strains are deficient in the transcriptional induction of various DNA repair genes, including RNR1-3 (43). Chk1p functions downstream of Mec1p in a G2-M checkpoint pathway different from that regulated by Rad53p and Dun1p (31).
Although the pathways controlling telomeric silencing and checkpoints appear to have separate functions, two recent results suggest overlaps. First, a mutation in the S. cerevisiae checkpoint gene MEC3 increases TPE (7). Second, a mutation in rad3+, a Schizosaccharomyces pombe gene homologous to MEC1, results in increased telomere length and reduced TPE (9, 25). Below, we show that mutations in MEC1 and in DUN1 lead to substantially reduced TPE.
MATERIALS AND METHODS
Plasmids.
Several plasmids containing URA3 adjacent to telomeric sequences derived from different chromosomes were used in our studies. The plasmid pAD-UCA (14) was used to insert URA3 near telomere VIIL (Fig. 1a). Plasmid pV-UCA (identical to pV-R URA3-TEL [14]) contained DNA derived from telomere VR on a HindIII fragment. In the construction of pPG70, this fragment was replaced by a fragment generated by PCR amplification of telomeric XVL sequences (primers 5′ GGATCCCAAGCTTGAATATTACGTACTTATG and 5′ GGATCCCAAGCTTCTCGAGGAGAACTTCTAG) followed by HindIII treatment.
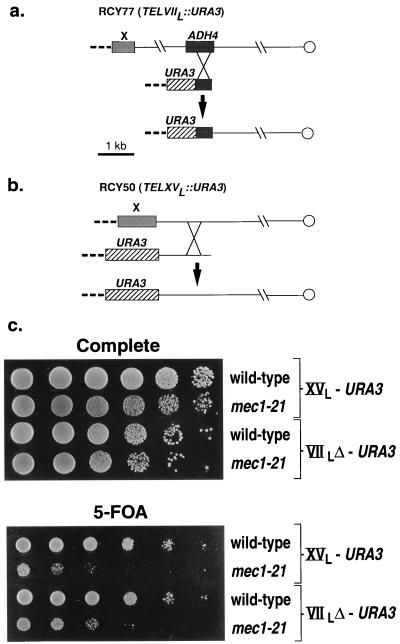
FIG. 1.
Construction of strains to monitor telomeric silencing and assay for telomeric silencing. As in previous studies (14), the URA3 gene was introduced at the telomere by transforming a ura3 mutant strain with a linear DNA fragment in which the URA3 gene was adjacent to telomeric sequences. Poly(TG1–3) repeats are indicated by dashed lines. (a) RCY77 (TELVIIL::URA3) was constructed by transforming W303a with an EcoRI/SalI restriction fragment derived from the plasmid pAD-UCA (14). (b) RCY50 (TELXVL::URA3) was derived from W303a by transformation with an EcoRI fragment of pPG70 (see Materials and Methods). (c) TPE was monitored by plating serial dilutions of various strains onto media lacking or containing 5-FOA. Since the URA3 insertion in TELVIIL::URA3 strains is more strongly silenced than the URA3 insertion in TELXVL::URA3 strains, 1:5 serial dilutions were used for strains shown in the top two rows, and 1:10 dilutions were used for strains shown in the bottom two rows. The effects of mec1-21 on TPE at two different telomeres are shown. Top rows, RCY109-2b; second rows, RCY109-1c; third rows, RCY110-5d; fourth rows, RCY110-1b.
Several plasmids with MEC1 were derived from YEp-MEC1 (MEC1 in the URA3-containing high-copy-number plasmid pRS426, provided by S. Elledge); since Escherichia coli transformed with MEC1-containing plasmids grows slowly, we generated these derivatives by recombination in yeast. We replaced URA3 with HIS3 (resulting in the plasmid pRC5) by transforming a strain containing YEp-MEC1 with a fragment generated by amplifying the HIS3 gene of pRS303 (33) with primers (5′ ATGTCGAAAGCTACATATAAGGAACGTGCTGCTACTCATCCTAGTCCTGTGATTGTACTGAGAGTGCACC and 5′ TTTTGC TGGCCGCATCTTCTCAAATATGCTTCCCAGCCTGCTTTTCTGTAACT GTGCGGTATTTCACACCG) that had 5′ homology to DNA sequences flanking URA3 in YEp-MEC1 and 3′ homology to sequences flanking HIS3.
To construct a plasmid with MEC1 on a CEN-containing plasmid (pRS4), we cotransformed (into the mec1-21 strain RCY109-1c) a 9-kb BsaI-NaeI fragment of YEp-MEC1 (containing the MEC1 gene and flanking vector sequences) and BamHI- and SalI-treated pR313 (CEN-containing vector with HIS3). The HIS3-containing high-copy-number vector pRS423 (6) was used as a control in some experiments. We also constructed a high-copy-number LEU2-containing plasmid (pRC11) with an insertion of RNR1. A PstI-KpnI fragment derived from the plasmid YEp24-(RNR1) (37) was ligated to the PstI- and KpnI-treated vector YEplac181 (15).
Yeast strains.
All strains used in this study were isogenic with W303a (a leu2-3,112 his3-11,15 ura3-1 ade2-1 trp1-1 can1-100 [36]) except for alterations introduced by transformation. To monitor TPE, we constructed derivatives of W303a with the URA3 gene inserted near the telomeres of different chromosomes (Fig. 1). The plasmids and restriction fragments used in the constructions were as follows: for RCY50, (TELXVL::URA3), an EcoRI fragment of pPG70, and for RCY77 (TELVIIL::URA3), an EcoRI/SalI fragment of pAD-UCA. Constructions were confirmed by Southern analysis.
The relevant genotypes for all haploid strains are shown in Table 1. Many of these haploids were spores derived from diploids. These diploids, constructed by crossing the strains listed in parentheses, are as follows: RCY61 (RCY28 [8] × RCY56 [8]), RCY78 (RCY61-1a × JMY303-15c [30]), RCY84 (RCY11 [8] × RCY28 [8]), RCY106 (RCY78-3b × RCY50), RCY109 (RCY50 × JMY303-2d), RCY110 (RCY77 × JMY303-2d), RCY112 (LPY253 × JMY303-15c), RCY123 (Y301 × RCY109-4d), RCY124 (RCY84-2c × LBY253), RCY126 (YS148 × RCY109-4d), RCY143 (DLY298 × RCY109-4d), RCY144 (Y286 × RCY109-4d), RCY155 (Y286 × RCY109-2b), RCY160 (DLY298 × RCY109-25c), RCY161 (DLY339 × RCY109-25c), RCY175 (RCY144-4b × JMY303-3b), RCY203 (W1973-6b × RCY109-9b), and RCY204 (W1974-6d × RCY109-9b).
TABLE 1.
Haploid strains used in this studya
| Strain | Relevant genotype | Progenitor strain or reference |
|---|---|---|
| W303a | Wild type | 36 |
| RCY50 | TELXVL::URA3 | W303a |
| JMY303-2d | α mec1-21 sml1::HIS3 | 30 |
| JMY303-3b | α sml1::HIS3 | 30 |
| RCY61-1a | rif1 rif2 | RCY61 |
| RCY78-3b | α rif1 mec1-21 | RCY78 |
| RCY106-1d | rif1 TELXVL::URA3 | RCY106 |
| RCY106-3d | mec1-21 TELXVL::URA3 | RCY106 |
| RCY106-4d | TELXVL::URA3 | RCY106 |
| RCY106-14a | rif1 mec1-21 TELXVL::URA3 | RCY106 |
| RCY109-1b | sml1::HIS3 TELXVL::URA3 | RCY109 |
| RCY109-1c | mec1-21 TELXVL::URA3 | RCY109 |
| RCY109-2b | TELXVL::URA3 | RCY109 |
| RCY109-3a | mec1-21 sml1::HIS3 TELXVL::URA3 | RCY109 |
| RCY109-4d | α TELXVL::URA3 | RCY109 |
| RCY109-9b | α TELXVL::URA3 | RCY109 |
| RCY109-25c | α mec1-21 TELXVL::URA3 | RCY109 |
| RCY77 | TELVIILΔ::URA3 | W303a |
| RCY110-1b | α mec1-21 TELVIILΔ::URA3 | RCY110 |
| RCY110-5d | α TELVIILΔ::URA3 | RCY110 |
| LPY253 | hml::TRP1 | 29 |
| JMY303-15c | α mec1-21 | 30 |
| RCY112-5c | hml::TRP1 mec1-21 | RCY112 |
| RCY112-13b | hml::TRP1 mec1-21 | RCY112 |
| RCY84-2c | α rap1-17 | RCY84 |
| RCY124-2a | hml::TRP1 rap1-17 | RCY124 |
| YS148 | chk1Δ::HIS3 rad53-21 | Y. Sanchez and S. Elledge |
| W1973-6b | rad53-1 | X. Zhao and R. Rothstein |
| W1974-6d | rad53-17 | X. Zhao and R. Rothstein |
| RCY126-3c | rad53-21 TELXVL::URA3 | RCY126 |
| RCY126-1d | chk1Δ::HIS3 TELXVL::URA3 | RCY126 |
| DLY298 | rad9Δ::HIS3 | T. Weinert |
| RCY143-2c | rad9Δ::HIS3 TELXVL::URA3 | RCY143 |
| Y286 | α dun1-Δ100::HIS3 | 43 |
| RCY144-4b | dun1-Δ100::HIS3 TELXVL::URA3 | RCY144 |
| DLY339 | mec3::HIS3 | T. Weinert |
| RCY160-1b | α rad9Δ::HIS3 TELXVL::URA3 | RCY160 |
| RCY160-3a | α mec1-21 rad9Δ::HIS3 TELXVL::URA3 | RCY160 |
| RCY160-4b | α mec1-21 TELXVL::URA3 | RCY160 |
| RCY160-4d | α TELXVL::URA3 | RCY160 |
| RCY175-4c | dun1-Δ100::HIS3 sml1::HIS3 TELXVL::URA3 | RCY175 |
All strains are isogenic with W303a (a leu2-3,112 his3-11,15 ura3-1 ade2-1 trp1-1 can1-100), except for the markers indicated. The construction of the diploids is described in Materials and Methods.
To determine the mutant substitutions in the mec1-21 and rad53-21 alleles, we sequenced PCR fragments derived from the strains JMY303-3c (30) and RCY123-1b (a rad53-21 spore derived from RCY123), respectively.
Measurement of telomere lengths.
Yeast DNA was isolated by standard methods (15) and treated with PstI. The resulting fragments were separated by agarose gel electrophoresis and transferred to Hybond N+ nylon membranes by standard procedures. The transferred fragments were hybridized to a probe derived from the Y′ element located centromere distal to the PstI site (30).
Genetic methods and silencing assays.
Methods of transformation, sporulation, tetrad analysis, and medium preparation were standard (15). Telomeric silencing assays were done as described by Gottschling et al. (14). Cells were grown on standard rich growth medium (yeast extract-peptone-dextrose) for 2 days at 30°C. Individual colonies were resuspended in water, and serial dilutions of 1:10 (TELVIILΔ::URA3 strains) or 1:5 (TELVIIL::URA3 strains) were performed. Five microliters of each dilution was plated onto yeast extract-peptone-dextrose (YPD) and synthetic complete medium containing 1 mg of 5-fluoro-orotate (5-FOA)/ml. In experiments involving strains with HIS3-containing plasmids, we used synthetic medium lacking histidine (SD−his). Sensitivity to hydroxyurea (HU) was monitored using medium containing 50 mM HU. To detect silencing of the TRP1 gene integrated at HML (hml::TRP1), we streaked cells onto medium lacking tryptophan (29).
RESULTS
Loss of TPE in mec1-21 strains.
In order to assay silencing of genes near the telomere, we inserted URA3 near the left end of chromosome XV or near the left telomere of chromosome VII (Fig. 1a and b). In strains with this insertion, inactivation of URA3 as a consequence of TPE can be detected by plating the cells onto medium containing 5-FOA (14). To examine the effects of Mec1p on TPE, we used the mec1-21 allele. This mutation results in a defect in the checkpoint pathway for DNA damage but, unlike null alleles of MEC1, is haploid viable (32). As shown in Fig. 1c, wild-type strains with URA3 inserted at the telomere of either chromosome XV or chromosome VII had high levels of 5-FOAr cells, indicating substantial TPE. Derivatives of these strains with the mec1-21 mutation had greatly reduced levels of TPE. Thus, the silencing of gene expression at two different chromosomal telomeres is greatly reduced by the mec1 mutation. We observed that the silencing of gene expression of a URA3 insertion at the right telomere of chromosome V is also Mec1p dependent (data not shown).
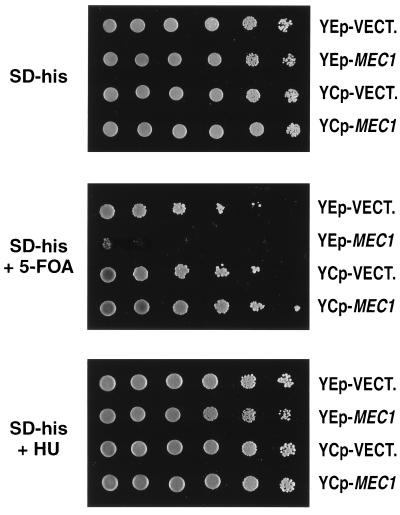
The silencing defect conferred by the mec1-21 mutation was complemented by the CEN-containing plasmid (YCp-MEC1; also called pRC4) that carries the wild-type MEC1 gene (Fig. 2, middle panel). This plasmid also complements the sensitivity of mec1 strains to the drug HU (Fig. 2, bottom panel). The phenotypes of the mec1-21 strain were unaffected by transformation with the vector plasmid (YCp-VECT.; also called pRS313). Transformation of the wild-type strain with pRC4 resulted in a slight increase in telomere silencing (compare top two rows in Fig. 2, middle panel).
FIG. 2.
Complementation of the mec1-21 silencing defect by the centromere-containing MEC1 plasmid. TPE was monitored by plating serial dilutions of various strains onto media lacking or containing 5-FOA; in some strains, sensitivity to HU, an inhibitor of ribonucleotide reductase, was also measured. Since the plasmids were marked with HIS3, the strains were grown in SD−his to force retention of the plasmid. All strains had the TELXVL::URA3 substitution. Top rows, RCY109-2b + pRS313 (YCp-VECT.); second rows, RCY109-2b + pRC4 (YCp-MEC1); third rows, RCY109-1c + pRS313; fourth rows, RCY109-1c + pRC4.
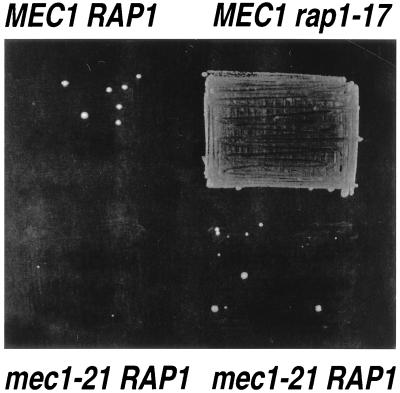
Although MEC1 is required for telomeric silencing, the mec1-21 mutation had no substantial effect on silencing at the HML locus. For this analysis, we used strains in which the TRP1 gene was integrated at HML (29). In wild-type (LPY253) and mec1-21 strains with this construction, expression of TRP1 was efficiently silenced (Fig. 3). In a strain with the rap1-17 mutation, shown previously to be defective in silencing at the telomere and at the silent mating-type loci (20), the same TRP1 gene was efficiently expressed.
FIG. 3.
Effects of mec1-21 and rap1-17 on silencing at HML. Four isogenic strains were constructed in which the TRP1 gene was inserted at HML: LPY253 (MEC1 RAP1 [29]), RCY112-5c and RCY112-13b (both strains are mec1-21 RAP1), and RCY124-2a (MEC1 rap1-17). These strains were then plated onto medium lacking tryptophan. The rap1-17 mutation, but not the mec1-21 mutation, resulted in a silencing defect.
Loss of TPE in strains overexpressing Mec1p.
Telomere silencing can be disrupted by the overproduction of several different classes of yeast gene products, including Sir4p and the RNA component of telomerase (34). We transformed the wild-type strain containing TELXVL::URA3 with plasmid pRS423 (YEp-VECT.), pRC5 (YEp-MEC1), pRS313 (YCp-VECT.), or pRC4 (YCp-MEC1). The strain with pRC5 lost telomeric silencing, and silencing was slightly enhanced in the strain with pRC4 (Fig. 4, middle panel). Interestingly, although pRC5 caused a loss of TPE, this plasmid did not increase the sensitivity of the strain to HU (Fig. 4, bottom panel). This result argues that the defective telomeric silencing observed in mec1 strains is unrelated to functions involved in the checkpoint defect associated with the mec1 mutation.
FIG. 4.
Loss of TPE by overproduction of MEC1. TPE was monitored by plating serial dilutions of various strains onto media lacking or containing 5-FOA; in some strains, sensitivity to HU, an inhibitor of ribonucleotide reductase, was also measured. Since the strains contained plasmids marked with HIS3, the strains were grown in SD−his to force retention of the plasmid. All strains had the TELXVL::URA3 substitution and the wild-type MEC1 gene. Top rows, RCY109-2b + pRS423 (YEp-VECT.); second rows, RCY109-2b + pRC5 (YEp-MEC1); third rows, RCY109-2b + pRS313 (YCp-VECT.); fourth rows, RCY109-2b + pRC4 (YCp-MEC1).
Roles of other checkpoint proteins in TPE.
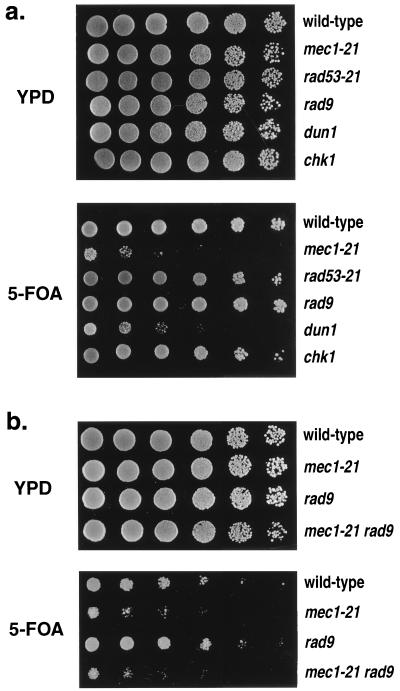
In response to DNA damage or delays in DNA replication, yeast cells arrest the cell cycle and induce the transcription of several genes involved in DNA repair (11, 40), as discussed in the introduction. We examined the effects of various checkpoint genes (RAD9, RAD53, DUN1, and CHK1) by crossing W303a-derived strains with mutations in these genes to an isogenic strain with the TELXVL::URA3 substitution. From each cross, we monitored TPE in at least four pairs of wild-type and mutant spores. Figure 5a shows examples of the results. Wild-type TPE was observed for strains with rad53-21 or null mutations of CHK1 and RAD9. In contrast, spores with a null mutation of DUN1 had greatly reduced TPE.
FIG. 5.
Effects of mec1-21, rad53-21, rad9, dun1, chk1, and sml1 on TPE. As for Fig. 1, TPE was monitored in a series of isogenic strains with TELXVL::URA3. (a) TPE in strains with single mutations affecting checkpoints. Top rows, RCY109-2b; second rows, RCY109-1c; third rows, RCY126-3c; fourth rows, RCY143-2c; fifth rows, RCY144-4b; sixth rows, RCY126-1d. (b) Epistasis analysis of mec1-21 and rad9 mutations. Top rows, RCY160-4d; second rows, RCY160-4b; third rows, RCY160-1b; fourth rows, RCY160-3a.
To determine the types of alterations in the hypomorphic mec1-21 and rad53-21 alleles, we PCR amplified and sequenced these alleles. The rad53-21 allele had a G-to-A alteration at position 1093, resulting in an E365K substitution. This alteration is within the kinase domain of the protein. The mec1-21 allele contained a change of G to A at position 2644 (G882S) (J. Mallory and T. Petes, unpublished data).
We also examined the effects of two additional hypomorphic alleles of RAD53, rad53-1 and rad53-17 (provided by X. Zhao and R. Rothstein). Diploids heterozygous for rad53-1 (RCY203) or rad53-17 (RCY204) and the TELXVL::URA3 substitution were sporulated and dissected. Neither rad53-1 nor rad53-17 affected telomeric silencing, confirming our observations with rad53-21 (data not shown). Since all of the rad53 alleles examined in our study are hypomorphic, we cannot exclude the possibility that other rad53 mutant alleles might affect TPE.
Since double-mutant rad53 chk1 strains are more sensitive to DNA-damaging agents than either single-mutant strain (31), we also examined telomeric silencing in the double-mutant strains. Such strains were constructed by sporulating a diploid (RCY126) that was heterozygous for rad53-21, chk1, and TELXVL::URA3. No telomere silencing defect was observed in the rad53-21 chk1 spores (data not shown).
Epistasis analysis of mec1 and other mutations affecting checkpoints.
In response to DNA damage, Rad9p exhibits Mec1p-dependent phosphorylation (12, 38), as discussed above. Rad9p is also required for the translocation of the silencing proteins Sir3p and Rap1p from the telomere following DNA damage (24, 26, 28). We found that mec1-21 rad9 double-mutant strains had the same silencing defect as the mec1-21 single-mutant strains (Fig. 5b).
We attempted to construct haploid strains with both mec1-21 and dun1Δ-100 mutations by sporulating a diploid strain (RCY155) that was heterozygous for both mutations. Of 18 tetrads examined, the numbers with four, three, and two viable spores were 3, 9, and 6, respectively. Of the spores analyzed, 19 were wild type, 16 were mec1-21, 16 were dun1Δ-100, and none contained the double mutation. Thus, mec1-21 and dun1Δ-100 are synthetically lethal. We also analyzed interactions between the mec3Δ mutation, which causes an increase in telomere length and telomeric silencing (7), and mec1-21. A diploid heterozygous for these mutations (RCY161) was sporulated and dissected. Of 15 tetrads examined, the numbers with four, three, and two viable spores were 1, 12, and 2, respectively. Of the spores analyzed, 20 were wild type, 12 were mec1-21, 12 were mec3Δ, and none contained the double mutation.
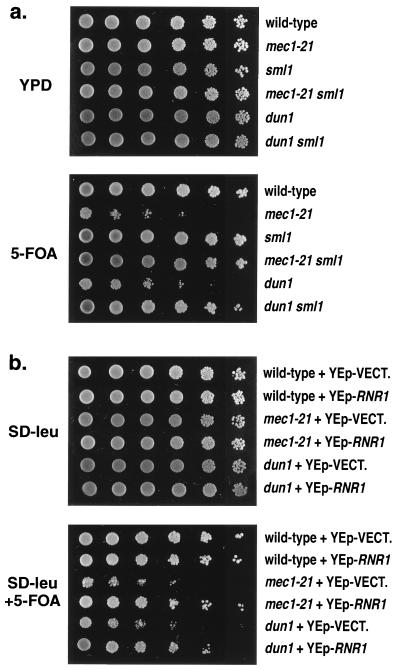
Although mec1-21 strains are haploid viable, null mutations of MEC1 result in haploid lethality. This lethality is suppressed in strains with an sml1 mutation (42) or in strains overexpressing RNR1 (10); both of these alterations are likely to result in elevated nucleotide pools. As shown in Fig. 6a, although the sml1 mutation has no obvious effect on TPE in otherwise wild-type strains, this mutation suppressed the silencing defects in both the mec1-21 and the dun1Δ-100 strains. In addition, overexpression of RNR1 suppressed the silencing defects of both strains (Fig. 6b).
FIG. 6.
Effects of sml1 and overproduction of RNR1 on TPE in mec1-21 and dun1 strains. As for Fig. 1, TPE was monitored in a series of isogenic strains with TELXVL::URA3. (a) Effect of sml1 on TPE. Top rows, RCY109-2b; second rows, RCY109-1c; third rows, RCY109-1b; fourth rows, RCY109-3a; fifth rows, RCY144-4b; sixth rows, RCY175-4c. (b) Effect of overproduction of RNR1 on TPE. Wild-type (RCY109-2b), mec1-21 (RCY109-1c), and dun1 (RCY144-44b) strains were transformed with either a high-copy-number vector plasmid (YEplac181 [YEp-VECT.]) or a high-copy-number plasmid containing the RNR1 gene (pRC11 [YEp-RNR1]).
The silencing defect caused by mec1-21 is not reverted by increased telomere length.
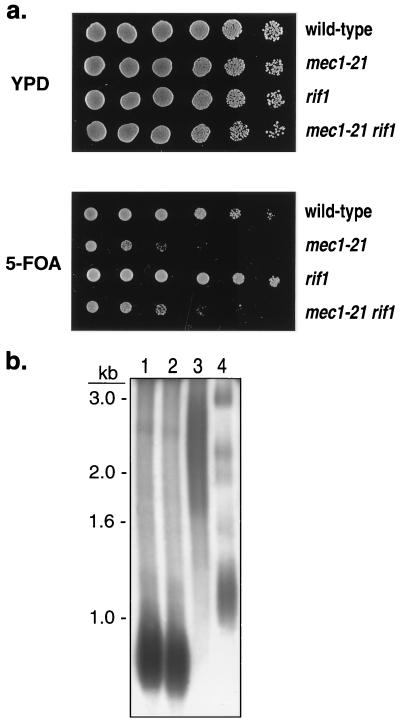
Cells harboring the mec1-21 mutation have slightly shortened telomeres, which elongate to wild-type lengths in the presence of the sml1 mutation (30). Telomere tract shortening is frequently correlated with loss of telomeric silencing, while elongated telomeres cause silencing to increase (20). Thus, it is possible that the loss of telomeric silencing in mec1-21 cells and the restoration of silencing in mec1-21 sml1 cells reflect changes in telomere length. To test this possibility, we constructed a strain (RCY106-11b) containing both the mec1-21 and rif1 mutations. Cells with rif1 mutations have increased levels of telomeric silencing and elongated telomeres (16, 20, 41). We found that strains with both mec1-21 and rif1 mutations had the same deficiency in TPE as that observed in mec1-21 strains (Fig. 7a). Telomeres in the double-mutant strain were longer than those in wild-type strains but shorter than those in rif1 strains (Fig. 7b). We conclude that telomeric tract size is not the sole determinant of silencing in mec1-21 cells.
FIG. 7.
Effect of telomere length on TPE. As for Fig. 1, TPE was monitored in a series of isogenic strains with TELXVL::URA3. (a) TPE in wild-type, mec1-21, rif1, and mec1-21 rif1 strains. Top rows, RCY106-4d; second rows, RCY106-3d; third rows, RCY106-1d; fourth rows, RCY106-14a. (b) Telomere lengths in wild-type, mec1-21, rif1, and mec1-21 rif1 strains. Purified genomic DNA was digested with PstI and examined by Southern analysis using a hybridization probe derived from the Y′ subtelomeric repeats (30). The positions of size standards are shown on the right. Lane 1, RCY106-4d (wild type); lane 2, RCY106-3d (mec1-21); lane 3, RCY106-1d (rif1); lane 4, RCY106-11b (mec1-21 rif1).
DISCUSSION
Our main conclusions are that (i) reduction or overexpression of Mec1p results in decreased telomeric silencing; (ii) the mec1-21 mutation does not substantially affect silencing at the HML locus; (iii) dun1 strains, but not strains with the rad53, chk1, or rad9 mutation, have a defect in TPE; (iv) the defects in TPE observed in mec1-21 and dun1 strains are alleviated by the sml1 mutation or overexpression of RNR1; and (v) the decreased telomeric silencing observed in mec1-21 strains is not a direct consequence of decreased telomere length.
Strains with mutations in most of the checkpoint genes have no obvious phenotype in the absence of DNA damage. Since null mutations of MEC1 and RAD53 are haploid lethal, these two genes are an exception to this generalization. It is likely that null mutations of MEC1 and RAD53 result in low nucleotide pools, since both MEC1 and RAD53 are required to activate Dun1p, and Dun1p is a positive activator of transcription of the RNR genes (11). The viability of strains with null mutations in MEC1 can be rescued by mutations in the sml1 gene (42), by overexpression of RNR1 (encoding ribonucleotide reductase) (10), and by mutations in the cyclin genes CLN1 and CLN2 (37). Strains with the sml1 mutation have elevated nucleotide pools (42), and it is likely that strains in which Rnr1p is elevated or Cln1 and Cln2 are reduced also have elevated nucleotide pools. Overproduction of RNR1 also suppresses the lethal effects of a null mutation in RAD53 (10).
The reasons for the lethality of null mutations of RAD53 and MEC1 and the suppression of this lethality by elevated nucleotide pools are not clear. One possibility is that strains with null mec1 and rad53 mutations die as a consequence of elevated levels of DNA damage (reflecting attempts to replicate DNA with low nucleotide pools) coupled with a defect in the DNA damage-sensitive checkpoint. Alternatively, the lethality observed in mec1 and rad53 strains may reflect an inability to complete chromosome replication (10, 42).
Why should the mec1-21 mutation lead to the loss of telomeric silencing? The most straightforward explanation of the loss of silencing is that one or more of the telomere-associated proteins involved in silencing (Sir2-4p, the Ku proteins, or Rap1p [2, 4, 20]) are depleted from the telomeres in mec1-21 strains. We will consider two models to explain this loss.
The first model is that Mec1p, acting as part of a protein complex, directly affects telomeric silencing by regulating the structure of the telomere. If Mec1p is part of a complex, then either mutations of MEC1 or overexpression of Mec1p could disrupt the function of this complex, leading to the loss of TPE; dominant negative effects caused by an overexpression of one component of a protein complex in yeast are quite common (15). The simplest form of this model is that the Mec1p complex phosphorylates one or more of the silencing proteins and that this phosphorylation is necessary for the telomeric silencing activity of these proteins. It should be pointed out that although the mec1-21 mutation is not located in the C-terminal putative kinase region, mutant substitutions located outside of the C-terminal region eliminate the kinase activity of the related Rad3 protein of S. pombe (5). An alternative form of this model is that the Mec1p complex acts to control the accessibility of telomeric DNA to the silencing proteins. In our previous study of the effects of Tel1p and Mec1p on telomere length, we suggested that Mec1p might affect the accessibility of the telomeric DNA to enzymes involved in telomere length regulation (30). This same activity could affect the binding of the silencing proteins.
One observation that is not explained by the simplest form of this model is that the elevation of nucleotide pools eliminates the telomeric silencing defect of mec1-21 strains. One possibility is that low levels of nucleotides in wild-type cells might affect the stability or enzymatic activity of the mutant Mec1-21p complex. Alternatively, low levels of nucleotides could affect the stability or function of the silencing proteins. At present, it is unclear whether the restoration of telomeric silencing caused by elevating nucleotide pools is directly related to the original defect or is caused by the superimposition of a different type of mechanism.
An alternative model is based on the observation that in yeast strains with double-stranded DNA breaks, several silencing proteins (Sir3p, Sir4p, yKu80p, and Rap1p) become redistributed from the telomeres to the sites of these DNA breaks (24, 26, 28). This redistribution is associated with the loss of telomeric silencing (26). One interpretation of our results is that a low level of DNA damage occurring in the mec1-21 and dun1 strains results in a redistribution of silencing proteins from the telomere, resulting in decreased TPE. Elevation of nucleotide pools by the sml1 mutation or by the overproduction of RNR1 would be expected to reduce the level of damage in mec1-21 strains, allowing the restoration of telomeric silencing. One argument against this model is that Mills et al. (28) and Martin et al. (24) reported that Mec1p and Rad9p were required for the redistribution of Sir3p. Although the protein encoded by mec1-21 could still be proficient in directing the redistribution of Sir3p, we found that a null mutation of RAD9 did not affect the silencing defect of mec1-21 (Fig. 5b). For this reason, we favor the first model. Finally, we point out that although the discussion above emphasizes the role of Mec1p in telomeric silencing, similar models could be proposed for the effects of the dun1 mutation.
Other studies support the conclusion that the pathways of telomeric silencing and DNA damage repair have functional overlaps. For example, the proteins yKu70p, Sir3p, and Mec3p affect both telomeric silencing and the repair of DNA damage (4, 7). Furthermore, the roles of Mec1p in telomere length regulation and telomeric silencing are evolutionarily conserved, because mutations of the rad3+ gene of S. pombe, a MEC1 homologue, result in short telomeres and the loss of TPE (9, 25). Thus, in two very different yeast species, similar proteins have multiple roles in checkpoint function, telomere length regulation, and TPE.
ACKNOWLEDGMENTS
We thank D. Gottschling, Y. Sanchez, S. Elledge, L. Pillus, A. Lustig, X. Zhao, R. Rothstein, E. Vallen, and T. Weinert for strains used in the study; K. Ritchie, J. Mallory, T. Weinert, R. Rothstein, X. Zhao, and S. Elledge for advice and/or comments on the manuscript; and P. Greenwell, L. Stefanovich, and M. Dominska for technical assistance.
The research was supported by NIH grants GM24110 and GM52319 to T.D.P. R.C. was supported by the American Cancer Society (PF-4435).
REFERENCES
- 1.Allen J B, Zhou Z, Siede W, Friedberg E C, Elledge S J. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 1994;8:2416–2428. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio O M, Billington B L, Gottschling D E. Modifiers of position effect are shared between telomeric and silent mating-type loci of S. cerevisiae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 3.Aparicio O M, Gottschling D E. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 1994;8:1133–1146. doi: 10.1101/gad.8.10.1133. [DOI] [PubMed] [Google Scholar]
- 4.Boulton S J, Jackson S P. Components of the Ku-dependent non-homologous end joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 1998;17:1819–1828. doi: 10.1093/emboj/17.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman C R, Evans S T, Carr A M, Enoch T. Requirement of sequences outside the conserved kinase domain of fission yeast Rad3p for checkpoint control. Mol Biol Cell. 1999;10:3223–3238. doi: 10.1091/mbc.10.10.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 7.Corda Y, Schramke V, Longhese M P, Smokvina T, Paciotti V, Brevet V, Gilson E, Geli V. Interactions between Set1p and checkpoint protein Mec3p in DNA repair and telomere functions. Nat Genet. 1999;21:204–208. doi: 10.1038/5991. [DOI] [PubMed] [Google Scholar]
- 8.Craven R J, Petes T D. Dependence of the regulation of telomere length on the type of subtelomeric repeat in the yeast Saccharomyces cerevisiae. Genetics. 1999;152:1531–1541. doi: 10.1093/genetics/152.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlen M, Olsson T, Kanter-Smoler B, Ramne A, Sunnerhagen P. Regulation of telomere length by checkpoint genes in Schizosaccharomyces pombe. Mol Biol Cell. 1998;9:611–621. doi: 10.1091/mbc.9.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desany B A, Alcasabas A A, Bachant J B, Elledge S J. Recovery from DNA replication stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 1998;12:2956–2970. doi: 10.1101/gad.12.18.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 12.Emili A. MEC1-dependent phosphorylation of Rad9p in response to DNA damage. Mol Cell. 1998;2:183–189. doi: 10.1016/s1097-2765(00)80128-8. [DOI] [PubMed] [Google Scholar]
- 13.Gardner R, Putnam C W, Weinert T. RAD53, DUN1, and PDS1 define two parallel G2/M checkpoint pathways in budding yeast. EMBO J. 1999;18:3173–3185. doi: 10.1093/emboj/18.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottschling D E, Aparicio O M, Billington B L, Zakian V A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. [Google Scholar]
- 16.Hardy C F J, Sussel L, Shore D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 17.Hecht A, Laroche T, Strahl-Bosinger S, Gasser S M, Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- 18.Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;383:92–95. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- 19.Jackson S P. The recognition of DNA damage. Curr Opin Genet Dev. 1996;6:19–25. doi: 10.1016/s0959-437x(96)90005-2. [DOI] [PubMed] [Google Scholar]
- 20.Kyrion G, Liu K, Liu C, Lustig A J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- 21.Longese M P, Foiani M, Muzi-Falconi M, Lucchini G, Plevani P. DNA damage checkpoint in budding yeast. EMBO J. 1998;17:5525–5528. doi: 10.1093/emboj/17.19.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lustig A J. Mechanisms of silencing in Saccharomyces cerevisiae. Curr Opin Genet Dev. 1998;8:233–239. doi: 10.1016/s0959-437x(98)80146-9. [DOI] [PubMed] [Google Scholar]
- 23.Lydall D, Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995;270:1488–1492. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- 24.Martin S G, Laroche T, Suka N, Grunstein M, Gasser S M. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97:621–633. doi: 10.1016/s0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura A, Naito T, Ishikawa F. Genetic control of telomere integrity in Schizosaccharomyces pombe: rad3+ and tel1+ are parts of two regulatory networks independent of the downstream protein kinases chk1+ and cds1+ Genetics. 1999;152:1501–1512. doi: 10.1093/genetics/152.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAinsh A D, Scott-Drew S, Murray J A H, Jackson S P. DNA damage triggers disruption of telomeric silencing and Mec1p-dependent relocation of Sir3p. Curr Biol. 1999;9:963–966. doi: 10.1016/s0960-9822(99)80424-2. [DOI] [PubMed] [Google Scholar]
- 27.McCarroll R M, Fangman W L. Time of replication of yeast centromeres and telomeres. Cell. 1988;54:505–513. doi: 10.1016/0092-8674(88)90072-4. [DOI] [PubMed] [Google Scholar]
- 28.Mills K D, Sinclair D A, Guarente L. MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell. 1999;97:609–620. doi: 10.1016/s0092-8674(00)80772-2. [DOI] [PubMed] [Google Scholar]
- 29.Nislow C, Ray E, Pillus L. SET1, a member of the Trithorax family, functions in transcriptional silencing and diverse cellular processes. Mol Biol Cell. 1997;8:2421–2436. doi: 10.1091/mbc.8.12.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie K B, Mallory J C, Petes T D. Interactions of TLC1 (which encodes the RNA subunit of telomerase), TEL1, and MEC1 in regulating telomere length in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:6065–6075. doi: 10.1128/mcb.19.9.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M, Elledge S J. Control of the DNA damage checkpoint by Chk1 and Rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–1171. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez Y, Desany B A, Jones W J, Liu Q, Wang B, Elledge S J. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- 33.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer M S, Kahana A, Wolf A J, Meisinger L L, Peterson S E, Goggin C, Mahowold M, Gottschling D E. Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics. 1998;150:613–632. doi: 10.1093/genetics/150.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson J B, Gottschling D E. Telomeric chromatin modulates replication timing near chromosome ends. Genes Dev. 1999;13:146–151. doi: 10.1101/gad.13.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas B J, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 37.Vallen E A, Cross F R. Interactions between the MEC1-dependent DNA synthesis checkpoint and G1 cyclin function in Saccharomyces cerevisiae. Genetics. 1999;151:459–471. doi: 10.1093/genetics/151.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vialard J E, Gilbert C S, Green C M, Lowndes N F. The budding yeast Rad9 checkpoint protein is subjected to Mec1/Tel1-dependent hyperphosphorylation and interacts with Rad53 after DNA damage. EMBO J. 1998;19:5679–5688. doi: 10.1093/emboj/17.19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinert T A, Kiser G L, Hartwell L H. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- 40.Weinert T A. DNA damage checkpoints update: getting molecular. Curr Opin Genet Dev. 1998;8:185–193. doi: 10.1016/s0959-437x(98)80140-8. [DOI] [PubMed] [Google Scholar]
- 41.Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 1997;11:748–760. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- 42.Zhao X, Muller E G D, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Elledge S J. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75:1119–1127. doi: 10.1016/0092-8674(93)90321-g. [DOI] [PubMed] [Google Scholar]