Abstract
Notch proteins are transmembrane receptors that mediate intercell communication and direct individual cell fate decisions. The activated intracellular form of Notch, NotchIC, translocates to the nucleus, where it targets the DNA binding protein CBF1. CBF1 mediates transcriptional repression through the recruitment of an SMRT-histone deacetylase-containing corepressor complex. We have examined the mechanism whereby NotchIC overcomes CBF1-mediated transcriptional repression. We identified SKIP (Ski-interacting protein) as a CBF1 binding protein in a yeast two-hybrid screen. Both CBF1 and SKIP are highly conserved evolutionarily, and the SKIP-CBF1 interaction is also conserved in assays using the Caenorhabditis elegans and Drosophila melanogaster SKIP homologs. Protein-protein interaction assays demonstrated interaction between SKIP and the corepressor SMRT. More surprisingly, SKIP also interacted with NotchIC. The SMRT and NotchIC interactions were mutually exclusive. In competition binding experiments SMRT displaced NotchIC from CBF1 and from SKIP. Contact with SKIP is required for biological activity of NotchIC. A mutation in the fourth ankyrin repeat that abolished Notch signal transduction did not affect interaction with CBF1 but abolished interaction with SKIP. Further, NotchIC was unable to block muscle cell differentiation in myoblasts expressing antisense SKIP. The results suggest a model in which NotchIC activates responsive promoters by competing with the SMRT-corepressor complex for contacts on both CBF1 and SKIP.
Notch is a cell surface receptor that, when activated by ligand-expressing cells, influences a broad spectrum of developmental processes (2). Notch signal transduction regulates cell fate decisions by influencing differentiation and proliferative responses to developmental cues. The Notch signaling pathway is conserved among species, and the effects of Drosophila melanogaster Notch and the Caenorhabditis elegans homologs LIN-12 and GLP-1 on development have been the subjects of extensive genetic analyses (18, 29). Notch receptor proteins have characteristic structural features that include an extracellular ligand binding domain comprising multiple tandemly arrayed epidermal growth factor-like repeats followed by cysteine-rich Notch/Lin-12 repeats and an intracellular domain containing six tandem ankyrin repeats that are required for signal transduction. The extracellular domain of Notch is cleaved by the furin protease in the trans Golgi network as part of Notch maturation. The resulting cleavage fragments remain associated and are found at the surface as a heterodimeric receptor (6, 41). The Notch ligands are transmembrane proteins of the DSL (Delta, Serrate, and Lag-2) family that, like Notch, contain multiple epidermal growth factor-like repeats in the extracellular domain (59).
The mechanism by which Notch signaling activates downstream target genes has been the subject of controversy. The observation that the intracellular domain of Notch, NotchIC, functions as a constitutively activated Notch receptor (16, 31, 37, 56) formed the basis for a model in which ligand binding induces proteolytic cleavage and the release of NotchIC, which translocates to the nucleus. A proteolytic site that releases NotchIC has been identified (53), and NotchIC cleavage has recently been linked to presenilins (7, 12, 48, 57, 60).
Support for the nuclear translocation of NotchIC as a component of signaling came from experiments with Drosophila using chimeric constructions in which the Saccharomyces cerevisiae Gal4 DNA binding domain was fused to full-length Notch just C-terminal to the Notch transmembrane domain. In the presence of the Notch ligand Delta, this chimeric Notch-Gal4 protein was able to activate a nuclear reporter containing Gal4 DNA binding sites (35, 55). An important nuclear target of NotchIC is the CSL (CBF1 [also called RBPJk and RBP2N], Su(H), and Lag-1) family of DNA binding proteins. Direct interaction between NotchIC and CSL proteins was demonstrated first for Drosophila (15) and subsequently for mammalian cells (23, 26). Several genes containing CSL protein binding sites in their promoters have been shown to be responsive to Notch signaling. These include members of the mammalian Hairy/Enhancer of split family of genes (24, 45), the related Drosophila Enhancer of split genes (4, 36), and the C. elegans genes lin-12, glp-1, and lag-1 (9). The human CSL protein CBF1 binds to the sequence GTGGGAA (39, 58) and functions as a transcriptional repressor (13, 22). CBF1-mediated repression involves destabilization of transcription factor IID (TFIID)-TFIIA interactions (46) and recruitment of a histone deacetylase (HDAC) corepressor complex to the promoter (25, 28). CBF1 has been shown to interact with the corepressor complex proteins SMRT and HDAC1 (28) and CIR, SAP30, and HDAC2 (25). On binding to CBF1, NotchIC activates gene expression through a two-part mechanism, first, by overcoming CBF1-mediated repression and, second, by activating transcription through the presence of an endogenous activation domain (23, 32). Evidence that NotchIC abolishes CBF1 repression through displacement of the SMRT corepressor complex has been presented (28).
Disregulated expression of NotchIC not only affects cell fate responses but also is associated with hematopoietic and epithelial malignancies. Truncation of human Notch1 (TAN1) as a result of chromosomal translocation was described for T-cell-lymphoblastic leukemia (14); mice transplanted with bone marrow expressing activated Notch1IC developed T-cell neoplasms (47); Notch1 was the site of provirus insertional mutagenesis in T-cell tumors developing in a transgenic mouse model (17); T-cell lymphomas were associated with transduction of Notch2IC by feline leukemia virus (52), and the activated form of Notch4 causes adenocarcinomas in transgenic mice (27). The transcriptional reprogramming induced by NotchIC is a key aspect of Notch function in development and tumorigenesis. To gain insight into the mechanism by which NotchIC overcomes CBF1-mediated transcriptional repression, we undertook a yeast two-hybrid screen to identify additional CBF1-interacting proteins. This screen identified SKIP (Ski-interacting protein), a human homolog of the Drosophila Bx42 protein that was originally described as an interacting partner of the avian retroviral oncogene v-Ski (11). c-Ski has recently been found to be a component of the HDAC corepressor complex and to be required for transcriptional repression by the Mad and thyroid hormone receptor complexes (44). We present evidence that SKIP interacts with the CBF1 corepressor complex and that SKIP has a role in orchestrating the conversion of CBF1 from an SMRT-HDAC-tethered transcriptional repressor to a NotchIC-tethered activation complex. Analysis of the NotchIC interaction with SKIP provided insight into the nature of the functional defect that results from mutation of the fourth ankyrin repeat of Notch.
MATERIALS AND METHODS
Plasmids. (i) Yeast expression plasmids.
SKIP cDNA was isolated from a B-cell library (Clontech) in a yeast two-hybrid screen with CBF1 as the bait protein. The SKIP sequence is identical to that of GenBank accession no. U51432 and 1 base different from that of NCoA-62 (accession no. AF045184). The homolog C. elegans SKIP (accession no. Z74045) was isolated from the C. elegans clone YK117g2 obtained from The National Institute of Genetics, Mishima, Japan. The D. melanogaster SKIP homolog (accession no. X64536) cDNA was obtained from the Berkeley Drosophila Genome Project. Lag-1 cDNA was obtained from J. Kimble (9). SNF1 and SNF4 controls were obtained commercially (Clontech). Proteins were expressed in yeast as activation domain (ACT) fusions in the vector pACTII or as DNA binding domain (DBD) fusions in the vector pAS1-CYH2. Gal4ACT-EBNA2(252–476) (pYW163) and Gal4ACT-CBF1 (pJH346) plasmids have been previously described, and Gal4ACT-Notch1IC (pLC8) was generated from the Notch1 plasmid pJH142 (25). DBD-SKIP was expressed in pJH313, Gal4DBD-C. elegans SKIP was expressed in pLC2, Gal4DBD-D. melanogaster SKIP was expressed in pLC3, Lag1-Gal4ACT was expressed in pLC1, and DBD-EBNA2(252–425) was expressed in pJH357. Bacterially expressed glutathione S-transferase (GST) fusions were generated in the pGEX2T (Promega)-derived plasmid pGH413; GST-CBF1(1–500) was expressed in pJH163.
(ii) Eukaryotic expression plasmids.
Gal4DBD fusions were expressed from a simian virus 40 promoter in the pGH250 vector background. Gal4-CBF1(1–500) was expressed in pJH93, Gal4-SKIP(1–536) was expressed in pJH274, Gal4-C. elegans SKIP was expressed in pJH470, and Gal4-D. melanogaster SKIP was expressed in pJH364. The SG5 vector (Stratagene) was modified to incorporate either Flag (pJH253), hemagglutinin (HA) (pHYC66), or Myc (pJH363) epitopes. These vectors were used to generate Flag-SKIP (pJH281), Myc-CBF1 (pMF1), and HA-SKIP (pJH277). SG5-SKIP-Rta (pJH511) expresses SKIP fused to the ACT (amino acids [aa] 520 to 605) of Epstein-Barr virus (EBV) Rta (19). The rat Notch1IC (rNotch1IC) ankyrin repeat mutant pBOS-FCDN1ank contains Notch1 aa 1747 to 2531 with an AA-to-EF mutation in the fourth ankyrin repeat and is in pEF-BOS. The wild-type rNotch1IC (aa 1747 to 2531) control plasmid was pBOS-FCDN1. Gal4-SMRT (CMX-Gal-FSMRT) (8), Flag-SMRT (pCMX-PL2-SMRT Flag), and SMRT expressed as a fusion with the herpes simplex virus VP16 ACT (VP-SMRT) (pCMX-VP-FSMRT) (28) were obtained from R. Evans. Expression vectors for Flag-Notch1IC(1751–2294) (pJH279), HA-Notch1IC-E2TANLS (pJH208), Notch1ICRAM(1751–1864)-E2TANLS (pJH198), and Notch2IC ΔRAM-E2TANLS(1822–2241) (pJH374) have been described (23, 24, 38). The E2TANLS constructions are fused to the EBNA2 transcriptional activation domain and nuclear localization signal (aa 425 to 487) (40). The reporter plasmids 5× Gal4TK-CAT, thymidine kinase (TK)-luciferase, 8× wtCBF1-luciferase, 8× mtCBF1-luciferase, and 4× Cp-CAT have been previously described (23, 39, 40).
Yeast two-hybrid assays.
The yeast two-hybrid screen and yeast assays for SKIP interactions were performed with yeast strain Y190 as previously described (25). Beta-galactosidase activity was measured from three independent cotransformants using 2-nitrophenyl β-d-galactopyranoside as the substrate. The amount of 2-nitrophenol liberated after 2 to 4 h of incubation was measured by determining absorbance at 420 nm.
CAT and luciferase assays.
HeLa cells were maintained in Dulbecco modified Eagle medium (DMEM) plus 10% fetal calf serum and plated at 1.2 × 105 cells per well in six-well plates (Nunc) 1 day prior to transfection. Cells were transfected using the calcium phosphate procedure, and cultures received 0.8 μg of 5× Gal4TK-CAT, 4× Cp-CAT, 8× wtCBF1-luciferase or 8× mtCBF1-luciferase reporter, 0.5 μg of the Gal4 vector or Gal4 fusion plasmid, 0.5 μg of EBNA2 or the NotchIC effector plasmid, and 1 μg of TK-luciferase as an internal control for transfection efficiency. The total DNA was kept constant for each sample using the vector plasmid. Each experiment was repeated at least two times. Chloramphenicol acetyltransferase (CAT) and luciferase assays were performed as previously described (23).
Immunoprecipitation and Western blotting.
293T cells seeded at 106 per 10-cm-diameter culture dish were transfected with 8 μg of expression plasmid using the calcium phosphate method. Two days after transfection, the cells were washed and lysed in 2.5 ml of ice-cold lysis buffer (0.1% sodium dodecyl sulfate, 1% deoxycholic acid, 0.5% NP-40, 0.2 mM phenylmethylsulfonyl fluoride, and 2 μg of aprotinin in phosphate-buffered saline per ml). The cell suspension was passed five times through a 20-gauge syringe needle, and the extract was clarified by centrifugation for 10 min at 15,000 rpm in a Sorvall MC12V microcentrifuge. Anti-Flag or anti-Myc mouse monoclonal antibodies (Sigma) and anti-CBF1, anti-Notch, or anti-SKIP rabbit polyclonal antibodies were mixed with protein A-Sepharose 4B (20 μl; Pharmacia) in 60 μl of lysis buffer and incubated at 4°C for 2 h. The beads were blocked with 3% skim milk in lysis buffer for 15 min and washed three times in lysis buffer. One milliliter of cell extract was added to the beads and incubated for 2 h at 4°C. The beads were then washed six times with lysis buffer and mixed with 35 μl of sample buffer. Samples (5 to 25 μl) were subjected to electrophoresis using a 9% denaturing polyacrylamide gel. The amount of sample used for direct immunoprecipitations was one-quarter of the amount used for the coimmunoprecipitated sample. Western blot analysis was performed using peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G secondary antibodies and the enhanced-chemiluminescence system (Amersham). Rabbit anti-CBF1 polyclonal antisera were generated using the peptide Y-P-G-K-F-G-E-R-P-P-P-K-R-L-T-R-S-C as the immunogen, and rabbit anti-Notch1 antisera were generated using the peptide Y-G-D-E-D-L-E-T-K-K-F-R-F-E-E-P-S-C as the immunogen. Molecular mass standards were purchased from Gibco BRL.
GST-protein affinity assays.
293T cells were transfected in 10-cm-diameter dishes with 12 μg of each plasmid. Extracts were prepared 2 days after transfection by washing the cells with phosphate-buffered saline, followed by lysis in ice-cold lysis buffer (0.2% NP-40, 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 5% glycerol, 0.2 mM phenylmethylsulfonyl fluoride, 2 μg of aprotinin in Tris-HCl [pH 7.4] per ml). The suspension was sonicated for 15 s on ice and clarified by centrifugation for 10 min at 15,000 rpm.
Extracts from bacterial cells induced to express GST-CBF1 were prepared by standard procedures. These extracts were incubated for 2 h at 4°C with 20 μl of glutathione-Sepharose 4B beads (Pharmacia). After three washes in lysis buffer, the bound GST fusion proteins were incubated for 2 h at 4°C with transfected 293T cell extract. The beads were then washed six times in lysis buffer and added to 30 μl of sample buffer. Samples were electrophoresed through sodium dodecyl sulfate–9% polyacrylamide gels, the separated proteins were transferred to a nitrocellulose membrane, and proteins were detected by Western blotting as described above.
Muscle cell differentiation assays.
The C2C12 cell line is a clonal mouse cell population that proliferates as mononuclear myoblasts in growth medium (DMEM plus 10% fetal bovine serum and 5% cosmic calf serum). These cells undergo morphological and molecular changes that correlate with muscle cell differentiation when they are switched to differentiation medium (DMEM plus 10% horse serum). CDN2 cells are C2C12 cells selected for expression of the Notch2 intracellular domain. Muscle cell differentiation is blocked by NotchIC in these cells. The open reading frame of SKIP was cloned in the pREP4 vector in either the sense (pSZ20) or antisense (pSZ21) direction, and the plasmids were transfected into CDN2 cells. Stably transfected cell lines were generated using hygromycin selection (250 μg/ml). The muscle fusion assays were performed essentially as previously described (24). Briefly, C2C12, CDN2, CDN2-SKIP (CDN2 cells overexpressing SKIP), and CDN2-asSKIP (CDN2 cells expressing antisense SKIP) were plated in 100-mm-diameter dishes in growth medium. When the cells were 80% confluent, they were switched to differentiation medium and monitored daily. The differentiation medium was changed every two days. After 6 days of differentiation induction, the cells were photographed.
RESULTS
SKIP and SKIP homologs interact with CBF1.
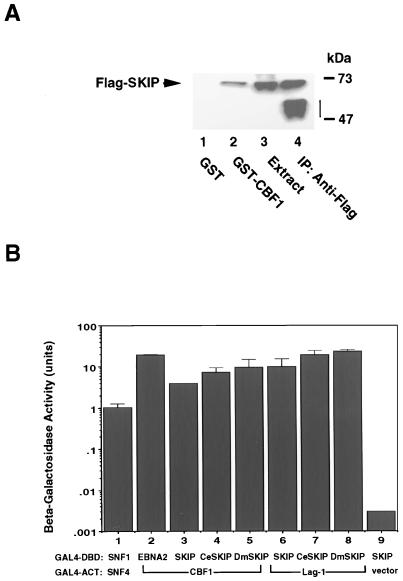
We identified SKIP as a CBF1-interacting protein in a yeast two-hybrid screen. Members of both the CBF1 and the Notch family of proteins are highly conserved across species. Database comparisons also indicate that SKIP has homologs in C. elegans and D. melanogaster that exhibit a high level of amino acid conservation (Fig. 1). We first sought to verify the SKIP-CBF1 interaction. A GST-affinity assay was performed (Fig. 2A) using extract from 293T cells transfected with Flag-SKIP. Binding of Flag-SKIP was detected by Western blot analysis of the bound proteins and probing with anti-Flag monoclonal antibody. Flag-SKIP did not bind to GST (Fig. 2A, lane 1) but did bind to GST-CBF1 (Fig. 2A, lane 2). The presence of Flag-SKIP in the transfected extract is shown in Fig. 2A, lane 3, and the identity of Flag-SKIP was further confirmed by direct immunoprecipitation of Flag-SKIP from the transfected cell extract using anti-Flag monoclonal antibody (Fig. 2A, lane 4).
FIG. 1.
Comparison of the human SKIP protein sequence with the C. elegans (CeSKIP) (accession no. Z74045) and D. melanogaster (DmSKIP) (accession no. [Dbx42] X64536) homologs illustrating the high degree of amino acid conservation. Shading within the outlined areas indicates amino acid identity, and no shading in the outlined areas indicates conservative changes. The alignment was generated using MacVector (Oxford Molecular Group).
FIG. 2.
SKIP and its homologs interact with CBF1. (A) GST-affinity assay showing binding of Flag-SKIP to GST-CBF1 (lane 2) but not to GST (lane 1). Positive control lanes contained transfected cell extract (10 μl) (lane 3) and Flag-SKIP that was immunoprecipitated (IP) with mouse anti-Flag monoclonal antibody (lane 4). The vertical bar indicates the position of the immunoglobulin heavy chain. (B) SKIP-CBF1 interactions are evolutionarily conserved. Yeast two-hybrid assay in which interaction is measured by induction of beta-galactosidase activity. SNF1-SNF4 (lane 1) and EBNA2-CBF1 (lane 2) formed positive controls, while the SKIP-ACT vector pairing (lane 9) formed the negative control. SKIP, C. elegans SKIP (CeSKIP), and D. melanogaster SKIP (DmSKIP) (lanes 3 to 5) each showed interaction with CBF1. Furthermore the SKIP, C. elegans SKIP, and Drosophila SKIP interactions could also be demonstrated using the C. elegans CBF1 homolog Lag-1 as the interacting partner (lanes 7 to 9). The results shown are averages of results of three experiments, with the standard deviations indicated.
To address the biological relevance of the CBF1-SKIP interaction, we performed a yeast two-hybrid assay to determine whether the SKIP-CBF1 interaction was conserved across species. Interaction between Gal4-DBD fusion proteins and Gal4-ACT fusion proteins in cotransformed yeast was measured by induction of beta-galactosidase activity. Two pairs of proteins known to be interacting partners, SNF1 plus SNF4 and CBF1 plus EBV EBNA2, were included in the assay (Fig. 2B, lanes 1 and 2). SKIP plus the Gal4-ACT vector formed the negative control (Fig. 2B, lane 9). In this assay, both C. elegans SKIP and Drosophila SKIP interacted with CBF1 as effectively as SKIP (Fig. 2B, lanes 3 to 5). The degree of conservation of the CBF1 and SKIP interaction domains across species was further emphasized by the demonstration that the C. elegans CBF1 homolog Lag-1 not only interacted with C. elegans SKIP but also retained interaction with SKIP and Drosophila SKIP (Fig. 2B, lanes 6 to 8).
SKIP mediates transcriptional repression.
To ascertain the role of SKIP in the CBF1-associated complex, we generated Gal4-SKIP fusion proteins and examined the properties of these fusion proteins in transient-expression assays. Cotransfection of the Gal4-SKIP expression plasmids into HeLa cells with a CAT reporter containing five upstream Gal4 binding sites, 5× Gal4TK-CAT, led to repression of reporter CAT expression (Fig. 3). The repression mediated by Gal4-SKIP was comparable to that induced by Gal4-CBF1. The Drosophila SKIP fusion also mediated transcriptional repression, indicating conservation of function as well as conservation of interaction with CBF1. Gal4-C. elegans SKIP induced a mild repression of reporter CAT expression. The C. elegans SKIP protein may be less effective than SKIP and Drosophila SKIP at mediating contacts with other mammalian proteins required for transcriptional repression.
FIG. 3.
SKIP mediates transcriptional repression. Shown are results of a transient-expression assay demonstrating repression of the 5× Gal4TK-CAT reporter by Gal4-SKIP fusion proteins. SKIP, C. elegans SKIP (CeSKIP), and Drosophila SKIP (DmSKIP) expressed as fusions with Gal4(1–147) were cotransfected into HeLa cells with the 5× Gal4TK-CAT reporter (0.8 μg) and 1 μg of TK-luciferase as a control for transfection efficiency.
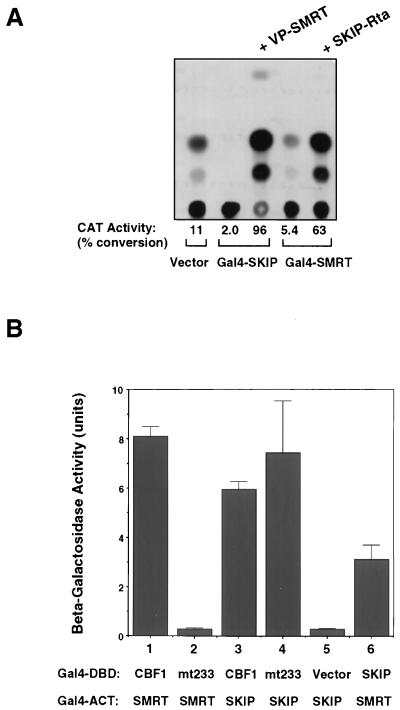
The ability of Gal4-SKIP to function as a transcriptional repressor suggested that SKIP might interact with proteins in the CBF1 corepressor complex. SMRT is a key component of the CBF1 corepressor complex (28). Interaction between SKIP and SMRT was tested in a mammalian two-hybrid assay in HeLa cells cotransfected with a 5× Gal4TK-CAT reporter, Gal4-SKIP, and VP-SMRT (28) (Fig. 4A). Gal4-SKIP repressed expression of the CAT reporter. However, reporter expression was activated by the addition of VP-SMRT. This activation is indicative of an interaction between SMRT and the promoter-bound Gal4-SKIP which results in bringing the VP16 activation domain of VP-SMRT to the promoter. The same activation was also observed when SMRT was expressed as the DNA binding partner (Gal4-SMRT), and SKIP was expressed as a fusion with the transcriptional ACT from the EBV Rta transactivator (SKIP-Rta) (Fig. 4A). Gal4-SMRT weakly repressed expression from 5× Gal4TK-CAT. Cotransfection of SKIP-Rta resulted in strong reporter activation, which is consistent with an interaction between SKIP and the promoter-bound Gal4-SMRT that brings the Rta ACT to the promoter. Thus, SKIP appears to mediate repression through either direct or indirect contacts with SMRT and the CBF1 corepressor complex.
FIG. 4.
SKIP interacts with SMRT in addition to CBF1. (A) Mammalian two-hybrid assay demonstrating interaction between SKIP and the corepressor SMRT. Cotransfection into HeLa cells of Gal4-SKIP or Gal4-SMRT repressed expression from the 5× Gal4TK-CAT reporter. Addition of SMRT fused to the ACT of herpes simplex virus VP16 (VP-SMRT) activated CAT expression, indicating interaction between SMRT and the reporter-bound Gal4-SKIP. This interaction was confirmed using the complementary pairing in which addition of SKIP fused to the ACT of the EBV Rta transactivator (SKIP-Rta) activated expression through reporter-bound Gal4-SMRT. TK-luciferase was cotransfected as a control for transfection efficiency. (B) Yeast two-hybrid assay demonstrating that the SKIP interaction site on CBF1 is separable from the SMRT interaction site. Induction of beta-galactosidase activity was used as a measure of interaction. As described by others (28), SMRT interacts with CBF1 (lane 1) but not with CBF1(EEF233AAA) (mt233). SKIP interacts with CBF1 (lane 3) and with mt233 (lane 4). The SKIP-DBD vector pairing formed the negative control (lane 5). The SKIP-SMRT interaction can also be demonstrated in the yeast assay (lane 6). The results shown are averages of results of three experiments, with the standard deviations indicated.
To address whether SKIP contacted the SMRT corepressor complex independently of SKIP contacts with CBF1, a yeast two-hybrid assay was performed to examine the effect of the CBF1(EEF233AAA) mutation (Fig. 4B). Beta-galactosidase activity was induced in the yeast assay when Gal4DBD-CBF1 was paired with Gal4ACT-SMRT, and in confirmation of previous results (28), the interaction with SMRT was abolished by a mutation at codon 233 of CBF1 (mt233) (Fig. 4B, lanes 1 and 2). In contrast to the result obtained with SMRT, the Gal4DBD-mt233 was just as effective as wild-type CBF1 in interacting with SKIP (Fig. 4B, lanes 3 and 4). This observation indicates that the SKIP interaction interface on CBF1 is distinguishable from the SMRT interaction site and that SKIP interacts with CBF1 independently of its interaction with SMRT. The SKIP-SMRT interaction shown by the mammalian two-hybrid assay in Fig. 4A could also be demonstrated in yeast (Fig. 4B, lane 6).
NotchIC interacts with SKIP.
NotchIC is known to interact with CBF1 (22, 23, 26, 28). To better understand how NotchIC overcomes CBF1-mediated transcriptional repression, we evaluated whether there were also interactions between NotchIC and SKIP. An immunoprecipitation assay was performed using extracts of 293T cells cotransfected with Flag-SKIP and HA-NotchIC (Fig. 5A). Immunoprecipitated proteins were analyzed by Western blotting, and Flag-SKIP was detected using anti-Flag monoclonal antibody. Flag-SKIP coprecipitated with HA-NotchIC in immunoprecipitates generated using anti-Notch rabbit antiserum (Fig. 5A, lane 3). The coprecipitated Flag-SKIP had the same mobility in the gel as Flag-SKIP directly precipitated with anti-SKIP rabbit antibody (Fig. 5A, lane 1) or directly precipitated with anti-Flag monoclonal antibody (Fig. 5A, lanes 2 and 6).
FIG. 5.
NotchIC interacts with SKIP. (A) Immunoprecipitation assay using extracts of cells cotransfected with Flag-SKIP and NotchIC. A Western blot of the immunoprecipitates was probed with anti-Flag antibody to detect Flag-SKIP. Flag-SKIP was directly precipitated by rabbit anti-SKIP antibody (lane 1) and by anti-Flag monoclonal antibody (lanes 2 and 6). Flag-SKIP also coprecipitated with NotchIC in immunoprecipitates formed by anti-Notch rabbit antibody (lane 3). Transfected cell extract (10 μl) was loaded in lane 4. Flag-SKIP was not coprecipitated by rabbit preimmune serum (lane 5) or by an irrelevant mouse monoclonal antibody (anti-CD23; lane 7). The amount of extract used for direct precipitations was one-quarter of that used for coprecipitations. The vertical bar indicates the position of the immunoglobulin heavy chain. Ab, antibody; cont., control. (B) The Notch-SKIP interaction is conserved across species. Shown are the results of a yeast two-hybrid assay in which protein-protein interaction is measured by induction of beta-galactosidase activity. The SKIP-plus-ACT vector interaction (lane 4) formed the negative control. NotchIC interacted equally with SKIP, C. elegans SKIP (CeSKIP), and Drosophila SKIP (DmSKIP) (lanes 1 to 3). The results shown are averages from three experiments, with the standard deviations indicated.
Again, we obtained evidence for a high degree of conservation of the NotchIC-SKIP interaction across species. A yeast two-hybrid assay in which yeast cells were cotransformed with Gal4ACT-NotchIC and Gal4DBD fusions with SKIP, C. elegans SKIP, or Drosophila SKIP revealed comparable levels of induction of beta-galactosidase enzyme activity with all three SKIP homologs (Fig. 5B, lanes 1 to 3). Cotransformation of yeast with Gal4DBD-SKIP and the Gal4ACT vector formed the negative control in this assay (Fig. 5B, lane 4). In summary, the protein-protein interaction assays indicated that NotchIC interacts with SKIP in addition to CBF1, as previously recognized.
The NotchIC-SKIP interaction is separable from the NotchIC-CBF1 interaction.
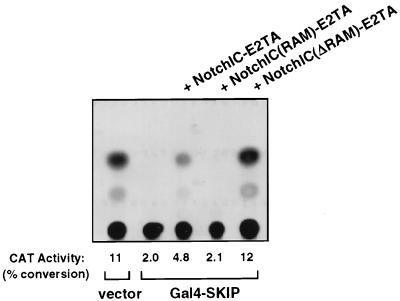
SKIP acted as a repressor when it was expressed as a Gal4 fusion protein (Fig. 3) and, consistent with that observation, interacted with the SMRT corepressor (Fig. 4A). Therefore, the finding that SKIP interacted with NotchIC was somewhat unexpected. Since NotchIC interacts with CBF1 and SKIP also interacts with CBF1, the possibility existed that the NotchIC-SKIP interaction that we detected was really indirect and was CBF1 mediated, i.e., that NotchIC interacted with CBF1 and CBF1 in turn interacted with SKIP but that NotchIC and SKIP did not make direct contacts. To determine whether CBF1 was a necessary intermediate for NotchIC-SKIP interaction, we performed a mammalian two-hybrid assay with NotchIC expressed as a fusion with the transactivation domain and nuclear localization signal of EBV EBNA2 (NIC-E2TA) and two NotchIC variants: one in which the CBF1 interaction domain (RAM domain) of NotchIC was expressed as a fusion with the E2TA domain [NIC(RAM)-E2TA] and the other in which a NotchIC-E2TA fusion was deleted for the RAM domain (NICΔRAM-E2TA) (Fig. 6). Expression from the 5× Gal4TK-CAT reporter was repressed by cotransfection of Gal4-SKIP. Expression was increased by cotransfection of NIC-E2TA, indicative of an interaction between NotchIC and the reporter-bound Gal4-SKIP. No increase in expression was seen on cotransfection of NIC(RAM)-E2TA, implying that the RAM domain was unable to mediate contacts with Gal4-SKIP. [The NIC(RAM)-E2TA construction has previously been shown to be able to activate expression of 5× Gal4TK-CAT in the presence of Gal4-CBF1 and therefore is capable of binding to CBF1 (23)]. Deletion of the RAM domain from NotchIC did not impair the ability of the NICΔRAM-E2TA construction to activate expression from 5× Gal4TK-CAT. Thus, the RAM domain is neither sufficient nor necessary for NotchIC interaction with SKIP and the domain on NotchIC that contacts SKIP is separable from the CBF1 interaction domain.
FIG. 6.
NotchIC interaction with SKIP is independent of the NotchIC-CBF1 interaction. The results of a mammalian two-hybrid assay show that the CBF1-interacting domain, NotchIC(RAM), is neither required nor sufficient to mediate interaction with SKIP. HeLa cells were transfected with the 5× Gal4TK-CAT reporter, Gal4-SKIP, and the indicated NotchIC-EBNA2 transactivation domain (E2TA) fusion constructions. TK-luciferase was included as a control for transfection efficiency. The NIC(RAM)-E2TA fusion protein expresses only the CBF1 interaction domain (RAM domain) of NotchIC and is unable to mediate activation of the 5× Gal4TK-CAT reporter. The NICΔRAM-E2TA fusion expresses a NotchIC that has the RAM domain deleted and is capable of mediating reporter activation.
SMRT displaces NotchIC from both CBF1 and SKIP.
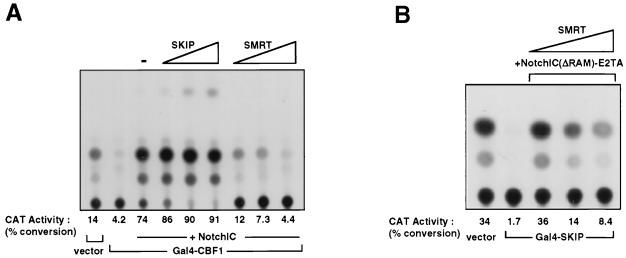
SMRT has been shown to compete with NotchIC for binding to CBF1 (28). To better understand the consequences of the SKIP interaction with CBF1, we compared the effects of the addition of SKIP with the effects of the addition of SMRT in a mammalian two-hybrid assay of NotchIC transactivation. HeLa cells were cotransfected with the 5× Gal4TK-CAT reporter, Gal4-CBF1, NotchIC, and increasing amounts of SKIP or SMRT expression vectors (Fig. 7A). As expected, cotransfection of Gal4-CBF1 repressed CAT expression from the 5× Gal4TK-CAT reporter and the addition of NotchIC led to reporter activation through NotchIC interaction with reporter-bound Gal4-CBF1. The addition of SMRT abolished NotchIC activation. This is consistent with the results of a previous report (28) of competition between SMRT and NotchIC for binding to CBF1. In contrast, the addition of SKIP had a small positive effect on NotchIC activation of the 5× Gal4TK-CAT reporter, suggesting that SKIP does not function like SMRT to compete for NotchIC binding to CBF1 but may instead facilitate the NotchIC-CBF1 interaction. This interpretation was strengthened by the observation that cotransfection of SKIP could partially restore the ability of RAM-deleted NotchIC to activate a luciferase reporter containing eight copies of the wild-type CBF1 binding site (8× wtCBF1 BS-Luc) (data not shown).
FIG. 7.
SMRT competes with NotchIC for contacts on both CBF1 and SKIP. (A) Competition two-hybrid assay of HeLa cells transfected with the 5x Gal4TK-CAT reporter, Gal4-CBF1, NotchIC, and increasing amounts (0.1, 0.5, and 2 μg) of SKIP or SMRT. TK-luciferase was included as an internal control. Introduction of SKIP had a mild positive effect on NotchIC activation of the Gal4-CBF1-bound reporter, whereas SMRT abolished NotchIC activation. (B) Competition two-hybrid assay of HeLa cells transfected with the 5× Gal4TK-CAT reporter, Gal4-SKIP, NotchIC(ΔRAM)-E2TA, and increasing amounts (0.1, 0.5, and 2 μg) of SMRT. TK-luciferase was included as an internal control. Activation of the Gal4-SKIP-bound reporter by the non-CBF1-interacting NotchIC(ΔRAM)-E2TA fusion protein was also abolished by SMRT.
The protein-protein interaction data indicated that SKIP contacted both the SMRT corepressor complex and the NotchIC activator. We next addressed whether there was competition between SMRT and NotchIC for binding to SKIP. A second mammalian two-hybrid assay was performed in which the effect of the addition of SMRT on NotchIC interaction with Gal4-SKIP was examined (Fig. 7B). HeLa cells were cotransfected with the 5× Gal4TK-CAT reporter, Gal4-SKIP, NotchIC, and increasing amounts of SMRT. The NotchIC(ΔRAM-E2TA) version of NotchIC was used in this assay to eliminate any CBF1 interactions that might occur through the RAM domain and complicate analysis of the results. In this assay, Gal4-SKIP repressed expression from the 5× Gal4TK-CAT reporter and the addition of NotchIC(ΔRAM-E2TA) activated expression through tethering of NotchIC(ΔRAM-E2TA) to reporter-bound Gal4-SKIP. Cotransfection of SMRT diminished the ability of NotchIC(ΔRAM-E2TA) to activate the 5× Gal4TK-CAT reporter. The SMRT-induced effect occurred in a dose-responsive manner. The competition data are consistent with a model in which binding of SMRT and NotchIC to SKIP are mutually exclusive events.
NotchIC mutated in the fourth ankyrin repeat is unable to interact with SKIP.
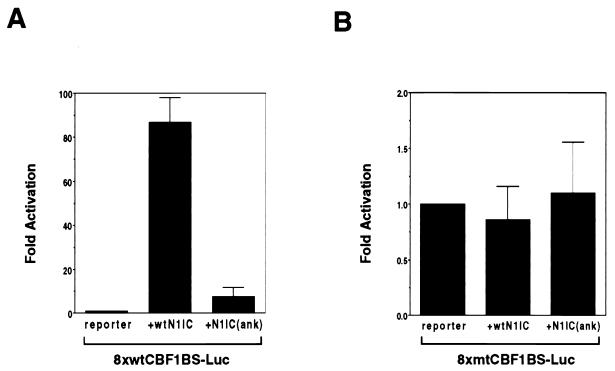
A Notch mutant that has been used in a number of studies carries a 2-aa change in the fourth ankyrin repeat. This M2 mutant is unable to block muscle cell differentiation (30) and unable to activate the HES-1 promoter (26). A version of rat NotchIC carrying this mutation was generated along with a wild-type rat NotchIC control. The NotchIC(ank) mutant was functionally impaired, as illustrated in a transient-expression assay in which the wild-type or mutant NotchIC expression vectors were cotransfected with 8× wtCBF1 BS-Luc (Fig. 8A). The reporter was activated approximately 85-fold by the parental NotchIC construction but only 7-fold by the ankyrin mutant NotchIC. To demonstrate the specificity of the activation, the assay was repeated using a luciferase reporter containing eight copies of a mutated CBF1 binding site (8× mtCBF1 BS-Luc) (Fig. 8B). Neither wild-type nor mutant NotchIC activated expression from this reporter.
FIG. 8.
NotchIC mutated in the fourth ankyrin repeat has impaired ability to activate a reporter containing CBF1 binding sites. (A) Transient-expression assay of HeLa cells cotransfected with 8× wtCBF1 BS-Luc and either wild-type NotchIC (wtN1IC) or an ankyrin repeat NotchIC mutant [N1IC(ank)] as indicated. (B) Transient-expression assay performed as described for panel A but with 8× mtCBF1 BS-Luc. The reporter carrying mutated CBF1 binding sites was not responsive to wild-type or mutant NotchIC.
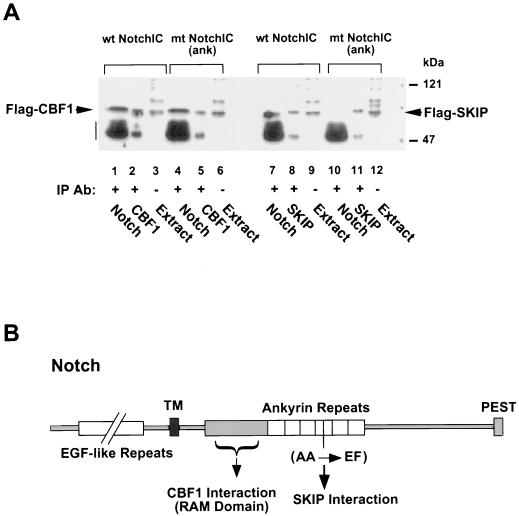
We next examined the ability of the NotchIC ankyrin repeat mutant to bind to CBF1 and SKIP. Coimmunoprecipitation assays were performed on extracts of 293T cells cotransfected with Flag-CBF1 plus wild-type or mutant NotchIC or cotransfected with Flag-SKIP plus wild-type or mutant NotchIC. The precipitated proteins were analyzed on Western blots which were probed with anti-Flag monoclonal antibody to detect Flag-CBF1 and Flag-SKIP. As illustrated in Fig. 9A, Flag-CBF1 coprecipitated with both wild-type and mutant NotchIC (lanes 1 and 4). Flag-CBF1 directly precipitated from the extract with anti-Flag antibody is shown in lanes 2 and 5. The ability of mutant NotchIC to coprecipitate with Flag-CBF1 indicates that the ankyrin repeat mutation does not interfere with interactions between NotchIC and CBF1.
FIG. 9.
Mutation of the fourth ankyrin repeat does not affect NotchIC interaction with CBF1 but abolishes NotchIC SKIP interaction. (A) Coimmunoprecipitation assay using extracts of cells cotransfected with the wild-type (wt) or ankyrin repeat mutant NotchIC [mt NotchIC(ank)] and either Flag-CBF1 or Flag-SKIP. Western blots of the immunoprecipitated proteins were probed with mouse anti-Flag monoclonal antibody to detect Flag-CBF1 (lanes 1 to 6) or Flag-SKIP (lanes 7 to 12). Flag-CBF1 coprecipitated with both wild-type and mutant NotchIC (lanes 1 and 4), whereas Flag-SKIP coprecipitated with wild-type but not mutant NotchIC (lanes 7 and 10, respectively). Lane 1, coprecipitation with anti-Notch rabbit antibody; lane 2, direct precipitation with anti-CBF1 rabbit antibody; lane 3, transfected cell extract (10 μl); lane 4, coprecipitation with anti-Notch rabbit antibody; lane 5, direct precipitation with anti-CBF1 rabbit antibody; lane 6, transfected cell extract (10 μl); lane 7, coprecipitation with anti-Notch rabbit antibody; lane 8, direct precipitation with anti-SKIP rabbit antibody; lane 9, transfected cell extract (10 μl); lane 10, coprecipitation with anti-Notch rabbit antibody; lane 11, direct precipitation with anti-SKIP rabbit antibody; lane 12, transfected cell extract (10 μl). The amount of extract used for direct precipitations was one-quarter of that used for coprecipitations. The vertical bar indicates the position of the immunoglobulin heavy chain. IP, immunoprecipitation; Ab, antibody. (B) Schematic of the Notch protein showing the relative locations of the CBF1 and SKIP binding domains and the location of the ankyrin repeat mutation. TM, transmembrane domain. EGF, epidermal growth factor; PEST, protein turnover motif.
Figure 9A (lanes 7 to 12) presents the analysis of SKIP interaction with the wild-type and mutant NotchIC proteins. Immunoprecipitation of wild-type NotchIC with anti-Notch rabbit antiserum resulted in coprecipitation of Flag-SKIP (lane 7). However, Flag-SKIP did not coprecipitate with the mutant NotchIC (lane 10). The anti-Notch rabbit antiserum used to precipitate NotchIC was raised against an epitope at the carboxy terminus of NotchIC, and this antiserum recognizes mutant NotchIC as effectively as wild-type NotchIC. The coprecipitation of Flag-CBF1 with mutant NotchIC in immunoprecipitates generated with the anti-NotchIC rabbit antiserum indicated that proteins that interact with mutant NotchIC are detectable by coimmunoprecipitation with this antiserum (Fig. 9A, lane 4). Direct immunoprecipitation from the transfected cell extracts of Flag-SKIP by anti-Flag monoclonal antibody is shown in lanes 8 and 11. To summarize, the NotchIC ankyrin repeat mutant retained the ability to interact with CBF1 but lost the ability to interact with SKIP. The relative locations of the CBF1 and SKIP interaction domains on Notch are illustrated in Fig. 9B.
The ability of NotchIC to block muscle cell differentiation is impaired in cells expressing antisense SKIP.
We have shown that mutation of the fourth ankyrin repeat of NotchIC abolished NotchIC interaction with SKIP and interfered with NotchIC transactivation function in a transient-expression assay. To strengthen the concept that SKIP is an important contributor to NotchIC activity, we examined the effect of constituitive expression of sense and antisense SKIP on NotchIC function in a muscle differentiation assay.
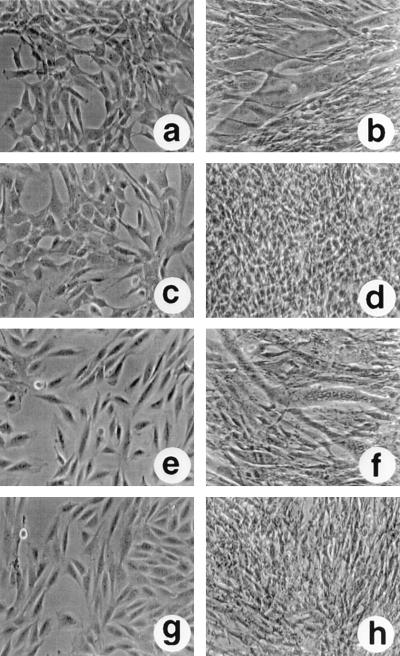
C2C12 myoblasts proliferate in an undifferentiated state when they are grown in medium containing fetal bovine serum, but the cells undergo growth arrest and differentiate to form myotubes when they are switched to medium containing horse serum (Fig. 10a and b). Constituitive expression of NotchIC in C2C12 cells blocks myoblast differentiation. This is illustrated in Fig. 10c and d by using the previously described C2C12-derived cell line CDN2 (24). In medium containing fetal bovine serum, CDN2 cells grow as undifferentiated myoblasts that are morphologically indistinguishable from the parental C2C12 cells (Fig. 10c). However, culturing in medium containing horse serum does not lead to growth arrest or myoblast formation. The CDN2 cells continue to proliferate in an undifferentiated state (Fig. 10d).
FIG. 10.
Muscle differentiation assay showing blockage of NotchIC function by antisense SKIP. Photomicrographs of C2C12 and C2C12-derived cell lines in growth medium (a, c, e, and g) or after 6 days in differentiation medium (b, d, f, and h). In differentiation medium, C2C12 cells fused to form myotubes (b) whereas the CDN2 cells, which constitutively express Notch2IC, continued to grow as undifferentiated myoblasts (d). In antisense-SKIP-expressing CDN2-asSKIP cells, myotubes formed that had the same morphology as those formed by C2C12 cells (f). This differentiation was not observed in sense-SKIP-expressing CDN2-SKIP cells (h).
We introduced a vector expressing either sense or antisense SKIP into CDN2 cells. The vector also contained a gene for hygromycin resistance, and drug-resistant cell lines were selected. The expression of sense and antisense SKIP transcripts in the CDN2-SKIP and CDN2-asSKIP cell lines was confirmed by reverse transcription-PCR (data not shown). Constituitive expression of SKIP in the CDN2 cells did not have a significant effect on their growth phenotype. In medium containing bovine serum, the cells grew as undifferentiated myoblasts (Fig. 10g), and on conversion to differentiation medium, the cells continued to proliferate in an undifferentiated state in a manner similar to that of the parental CDN2 cells (Fig. 10h). In contrast, expression of antisense SKIP had a marked effect. In medium containing bovine serum, the CDN2-asSKIP cells grew as undifferentiated myoblasts (Fig. 10e). However, when they were placed in differentiation medium, the CDN2-asSKIP cells, unlike the parental CDN2 cells, were able to differentiate and form myotubes (Fig. 10f).
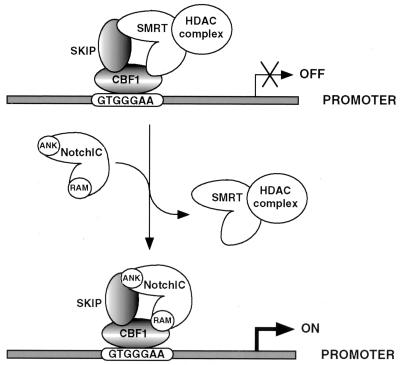
The combined protein-protein interaction and functional data indicate that interaction with CBF1 alone is not adequate for full NotchIC function and further suggest that binding to SKIP is required for NotchIC to efficiently activate CBF1-bound promoters. The model for NotchIC activation of CBF1-repressed promoters that derives from this study is presented in Fig. 11.
FIG. 11.
Model for Notch activation of CBF1-repressed promoters. CBF1 binds promoters carrying the sequence GTGGGAA. SKIP interacts with CBF1. SMRT contacts both SKIP and CBF1. SMRT is linked to a corepressor complex that includes Sin3A, SAP30, CIR, HDAC1, and HDAC2 (25, 28) and mediates promoter repression through chromatin remodeling. NotchIC competes with SMRT for contacts on both CBF1 and SKIP. Displacement of the corepressor complex relieves repression, and the promoter is further activated through the endogenous NotchIC transcriptional ACT. Loss of CBF1 interaction through mutation of the RAM domain or loss of interaction with SKIP through mutation of the fourth ankyrin repeat cripples the ability of NotchIC to activate CBF1-repressed promoters.
DISCUSSION
Evidence has accumulated that ligand-induced Notch signaling is associated with proteolytic cleavage events that result in the release of NotchIC and its translocation into the nucleus. The pathways by which intranuclear NotchIC modulates transcription are not fully understood, but a major intranuclear target for NotchIC is the CSL family of DNA binding proteins. The human CSL protein CBF1 acts as a transcriptional repressor through tethering of a corepressor complex that has been shown to include the proteins SMRT, CIR, SAP30, HDAC1, and HDAC2 (25, 28). The model of transcriptional repression that has emerged from studies of the nuclear hormone receptors and the Mad-Myc-Max family of proteins is one in which a DNA binding protein tethers the corepressor N-CoRs or the related SMRT, which in turn are participants in a complex that also contains Sin3A or -B, a variety of Sin3-associated proteins, including SAP30, SAP18, RbAp46, and RbAp48, and HDAC1 and HDAC2 (1, 20, 21, 33, 34, 42, 61, 62). The involvement of the corepressor complex in CBF1-mediated repression was originally substantiated through the use of a CBF1 repression-minus mutant, CBF1(EEF233), which was shown to have lost interaction with both the SMRT and CIR proteins of the corepressor complex (25, 28).
Our yeast two-hybrid screen identified SKIP as a CBF1-interacting protein, and we presented evidence for interaction between SKIP and the SMRT corepressor. SKIP was originally identified as a Ski-interacting protein and as a component of the vitamin D receptor (5, 10). Recently, c-Ski has been shown to be a component of the thyroid hormone receptor and Mad corepressor complexes and to bind both N-CoR and Sin3A (44). A trait shared by the nuclear hormone receptors and CBF1 is that these proteins mediate not only transcriptional repression but also transcriptional activation. In the absence of ligand, thyroid hormone and retinoic acid receptors interact with SMRT or N-CoR through the ligand binding domain to mediate repression. Binding of hormone causes dissociation of the corepressor complex and recruitment of a coactivator complex to produce transcriptional activation. DNA-bound CBF1 is converted from a transcriptional repressor into an activator in two known circumstances: in response to Notch signaling and in the presence of the EBV EBNA2 protein. Both NotchIC and EBNA2 bind to the repression domain of CBF1 (22, 23). SMRT competes with NotchIC for binding to CBF1 (28), and we have found that there is similar competition between SMRT and EBNA2 for binding to CBF1 (63).
The identification of SKIP as a CBF1-interacting protein provides additional insight into the mechanism by which NotchIC converts CBF1 into a mediator of transcriptional activation. SKIP appears to serve as a tether protein for both the SMRT corepressor and NotchIC. In contrast to SMRT, SKIP does not compete NotchIC from CBF1 but rather seems to facilitate NotchIC binding. Furthermore, the binding of SMRT and NotchIC to SKIP is mutually exclusive. SMRT competed with NotchIC for binding to SKIP. Thus, the conversion from transcriptional repression to activation involves both CBF1- and SKIP-exchanging partners from the SMRT-corepressor complex to a NotchIC activation complex.
The importance of SKIP contacts for NotchIC function was illustrated by the inability of NotchIC to block muscle cell differention when antisense SKIP was constituitively expressed in the cells and by the behavior of NotchIC carrying a mutation in the fourth ankyrin repeat, NotchIC(ank). The demonstration that NotchIC(ank) is unable to interact with SKIP sheds light on at least one function of the NotchIC ankyrin repeat domain. The integrity of the ankyrin repeat domain of NotchIC is important for biological activity (37, 49, 50). Mutation of two alanine residues in the fourth ankyrin repeat of Notch IC has previously been found to ablate the ability of NotchIC to block muscle cell differentiation and to impair the ability of NotchIC to activate reporters carrying CBF1 binding sites (26, 30). The ankyrin repeats have been assumed to directly contact CBF1. However, we found that NotchIC carrying a double alanine mutation in the fourth ankrin repeat retained the ability to bind CBF1 but had lost the ability to interact with SKIP. The C. elegans Notch homolog GLP-1 was shown to interact directly with Lag-1 through the subtransmembrane RAM domain, while interaction between the ankyrin repeat domain of GLP-1 and Lag-1 appeared to be indirect, since it could be observed only in colocalization assays with nematodes (50). This interpretation of an indirect interaction between the ankyrin repeats and Lag-1 is consistent with our demonstration that the ankyrin repeats directly interact with SKIP, which in turn interacts with CBF1.
NotchIC variants consisting solely of the ankyrin repeats are able to elicit some of the responses of activated NotchIC. For example, a C2C12 cell line that constituitively expressed the NotchIC ankyrin repeat domain did not form myotubes when cells were placed in differentiation medium nor did these cells express differentiation markers such as myogenin or myosin light chain (54). Similarly, nuclear expression of the Drosophila Notch ankyrin repeats was sufficient to block neuroblast segregations in Drosophila embryos (55) and expression of the ankyrin repeat domain of GLP-1 was able to regulate vulval cell fate in C. elegans (51). The ankyrin repeats also demonstrate transcriptional activation activity (3, 50). We have previously shown that transcriptional activation by NotchIC can be separated into two stages, loss of repression (i.e., displacement of the corepressor complex) and activation mediated through the endogenous NotchIC activation domain (23). In our model, SKIP acts as a tethering point for the CBF1 corepressor complex to mediate repression and as a tethering point for the ankyrin repeat domain of NotchIC during activation (Fig. 11). Thus, overexpression of the ankyrin repeat domain may result in displacement of the corepressor complex from CBF1 and relief of promoter repression. In some instances, relief of repression alone may be sufficient to alter transcriptional programming. However, NotchIC also mediates CBF1-independent effects (43) and the ankyrin repeats may also be involved in these activities.
The SKIP homologs in C. elegans and Drosophila are highly conserved in amino acid sequence. In yeast interaction assays we demonstrated that both the worm and fly SKIP homologs also interact with CBF1/Lag-1 and with NotchIC. This conservation of protein-protein interactions suggests that SKIP is likely to function in Notch signaling analagously across species. The presence of SKIP in the vitamin D complex and c-Ski, a protein with which SKIP interacts, in the Mad and thyroid hormone receptor complexes also suggests that SKIP may be a common tether for mammalian corepressor complexes. Furthermore, the role played by SKIP in orchestrating the corepressor-to-activator switch on DNA-bound CBF1 may be conserved and recapitulated in the nuclear hormone receptor and Mad complexes.
ACKNOWLEDGMENTS
We are grateful to R. Evans and J. Kimble for gifts of SMRT and Lag-1 plasmids. We thank M. Chiu and M. Poderycki for technical assistance and F. Chang for help with manuscript preparation.
This work was supported by National Institutes of Health grants RO1 CA42245 to S.D.H. and RO1 NS31885 to G.W. G.W. also received support from STOP cancer.
REFERENCES
- 1.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas S, Rand M D, Lake R J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 3.Aster J C, Robertson E S, Hasserjian R P, Turner J R, Kieff E, Sklar J. Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jkappa or nuclear localization sequences retain the ability to associate with RBP-Jkappa and activate transcription. J Biol Chem. 1997;272:11336–11343. doi: 10.1074/jbc.272.17.11336. [DOI] [PubMed] [Google Scholar]
- 4.Bailey A M, Posakony J W. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- 5.Baudino T A, Kraichely D M, Jefcoat S C, Jr, Winchester S K, Partridge N C, MacDonald P N. Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription. J Biol Chem. 1998;273:16434–16441. doi: 10.1074/jbc.273.26.16434. [DOI] [PubMed] [Google Scholar]
- 6.Blaumueller C M, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–291. doi: 10.1016/s0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 7.Chan Y-M, Jan Y N. Roles for proteolysis and trafficking in Notch maturation and signal transduction. Cell. 1998;94:423–426. doi: 10.1016/s0092-8674(00)81583-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen J D, Evans R M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 9.Christensen S, Kodoyianni V, Bosenberg M, Friedman L, Kimble J. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H) Development. 1996;122:1373–1383. doi: 10.1242/dev.122.5.1373. [DOI] [PubMed] [Google Scholar]
- 10.Dahl R, Kieslinger M, Beug H, Hayman M J. Transformation of hematopoietic cells by the Ski oncoprotein involves repression of retinoic acid receptor signaling. Proc Natl Acad Sci USA. 1998;95:11187–11192. doi: 10.1073/pnas.95.19.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl R, Wani B, Hayman M J. The Ski oncoprotein interacts with Skip, the human homolog of Drosophila Bx42. Oncogene. 1998;16:1579–1586. doi: 10.1038/sj.onc.1201687. [DOI] [PubMed] [Google Scholar]
- 12.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm J S, Schroeter E H, Schrijvers V, Wolfe M S, Ray W J, Goate A, Kopan R. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 13.Dou S, Zeng X, Cortes P, Erdjument-Bromage H, Tempst P, Honjo T, Vales L D. The recombination signal sequence-binding protein RBP-2N functions as a transcriptional repressor. Mol Cell Biol. 1994;14:3310–3319. doi: 10.1128/mcb.14.5.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellisen L W, Bird J, West D C, Soreng A L, Reynolds T C, Smith S D, Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 15.Fortini M E, Artavanis-Tsakonas S. The Suppressor of Hairless protein participates in Notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 16.Fortini M E, Rebay I, Caron L A, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993;365:555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- 17.Girard L, Hanna Z, Beaulieu N, Hoemann C D, Simard C, Kozak C A, Jolicoeur P. Frequent provirus insertional mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes Dev. 1996;10:1930–1944. doi: 10.1101/gad.10.15.1930. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- 19.Hardwick J M, Tse L, Applegren N, Nicholas J, Veliuona M A. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activation domain with properties different from those of VP16. J Virol. 1992;66:5500–5508. doi: 10.1128/jvi.66.9.5500-5508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassig C A, Fleischer T C, Billin A N, Schreiber S L, Ayer D E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 21.Heinzel T, Lavinsky R M, Mullen T M, Soderstrom M, Laherty C D, Torchia J, Yang W M, Brard G, Ngo S D, Davie J R, Seto E, Eisenman R N, Rose D W, Glass C K, Rosenfeld M G. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh J J-D, Hayward S D. Masking of the CBF1/RBPJk transcriptional repression domain by Epstein-Barr virus EBNA2. Science. 1995;268:560–563. doi: 10.1126/science.7725102. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh J J-D, Henkel T, Salmon P, Robey E, Peterson M G, Hayward S D. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol Cell Biol. 1996;16:952–959. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh J J-D, Nofziger D E, Weinmaster G, Hayward S D. EBV immortalization: Notch2 interacts with CBF1 and blocks differentiation. J Virol. 1997;71:1938–1945. doi: 10.1128/jvi.71.3.1938-1945.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh J J-D, Zhou S, Chen L, Young D B, Hayward S D. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc Natl Acad Sci USA. 1999;96:23–28. doi: 10.1073/pnas.96.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarriault S, Brou C, Logeat F, Schroeter E H, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 27.Jhappan C, Gallahan D, Stahle C, Chu E, Smith G H, Merlino G, Callahan R. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1992;6:345–355. doi: 10.1101/gad.6.3.345. [DOI] [PubMed] [Google Scholar]
- 28.Kao H-Y, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner C R, Evans R M, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimble J, Simpson P. The LIN-12/Notch signaling pathway and its regulation. Annu Rev Cell Dev Biol. 1997;13:333–361. doi: 10.1146/annurev.cellbio.13.1.333. [DOI] [PubMed] [Google Scholar]
- 30.Kopan R, Nye J S, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120:2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- 31.Kopan R, Schroeter E H, Weintraub H, Nye J S. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci USA. 1996;93:1683–1688. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurooka H, Kuroda K, Honjo T. Roles of the ankyrin repeats and C-terminal region of the mouse notch1 intracellular region. Nucleic Acids Res. 1998;26:5448–5455. doi: 10.1093/nar/26.23.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laherty C D, Billin A N, Lavinsky R M, Yochum G S, Bush A C, Sun J-M, Mullen T-M, Davie J R, Rose D W, Glass C K, Rosenfeld M G, Ayer D E, Eisenman R N. SAP30, a component of the mSin3 corepressor complex involved in N-CoR-mediated repression by specific transcription factors. Mol Cell. 1998;2:33–42. doi: 10.1016/s1097-2765(00)80111-2. [DOI] [PubMed] [Google Scholar]
- 34.Laherty C D, Yang W M, Sun J M, Davie J R, Seto E, Eisenman R N. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 35.Lecourtois M, Schweisguth F. Indirect evidence for Delta-dependent intracellular processing of Notch in Drosophila embryos. Curr Biol. 1998;8:771–774. doi: 10.1016/s0960-9822(98)70300-8. [DOI] [PubMed] [Google Scholar]
- 36.Lecourtois M, Schweisguth F. The neurogenic Suppressor of Hairless DNA-binding protein mediates the transcriptional activation of the Enhancer of split complex genes triggered by Notch signaling. Genes Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- 37.Lieber T, Kidd S, Alcamo E, Corbin V, Young M W. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 1993;7:1949–1965. doi: 10.1101/gad.7.10.1949. [DOI] [PubMed] [Google Scholar]
- 38.Ling P D, Hayward S D. Contribution of conserved amino acids in mediating interaction between EBNA2 and CBF1/RBPJk. J Virol. 1995;69:1944–1950. doi: 10.1128/jvi.69.3.1944-1950.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling P D, Hsieh J J-D, Ruf I K, Rawlins D R, Hayward S D. EBNA-2 upregulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J Virol. 1994;68:5375–5383. doi: 10.1128/jvi.68.9.5375-5383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling P D, Ryon J J, Hayward S D. EBNA-2 of herpesvirus papio diverges significantly from the type A and type B EBNA-2 proteins of Epstein-Barr virus but retains an efficient transactivation domain with a conserved hydrophobic motif. J Virol. 1993;67:2990–3003. doi: 10.1128/jvi.67.6.2990-3003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah N G, Israel A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy L, Kao H Y, Chakravarti D, Lin R J, Hassig C A, Ayer D E, Schreiber S L, Evans R M. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 43.Nofziger D, Miyamoto A, Lyons K M, Weinmaster G. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development. 1999;126:1689–1702. doi: 10.1242/dev.126.8.1689. [DOI] [PubMed] [Google Scholar]
- 44.Nomura T, Khan M M, Kaul S C, Dong H-D, Wadhwa R, Colmenares C, Kohno I, Ishii S. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev. 1999;13:412–423. doi: 10.1101/gad.13.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as Notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olave I, Reinberg D, Vales L D. The mammalian transcriptional repressor RBP (CBF1) targets TFIID and TFIIA to prevent activated transcription. Genes Dev. 1998;12:1621–1637. doi: 10.1101/gad.12.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pear W S, Aster J C, Scott M L, Hasserjian R P, Soffer B, Sklar J, Baltimore D. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183:2283–2291. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray W J, Yao M, Nowotny P, Mumm J, Zhang W, Wu J Y, Kopan R, Goate A M. Evidence for a physical interaction between presenilin and Notch. Proc Natl Acad Sci USA. 1999;96:3263–3268. doi: 10.1073/pnas.96.6.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rebay I, Fehon R G, Artavanis-Tsakonas S. Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 1993;74:319–329. doi: 10.1016/0092-8674(93)90423-n. [DOI] [PubMed] [Google Scholar]
- 50.Roehl H, Bosenberg M, Blelloch R, Kimble J. Roles of the RAM and ANK domains in signaling by the C. elegans GLP-1 receptor. EMBO J. 1996;15:7002–7012. [PMC free article] [PubMed] [Google Scholar]
- 51.Roehl H, Kimble J. Control of cell fate in C. elegans by a GLP-1 peptide consisting primarily of ankyrin repeats. Nature. 1993;364:632–635. doi: 10.1038/364632a0. [DOI] [PubMed] [Google Scholar]
- 52.Rohn J L, Lauring A S, Linenberger M L, Overbaugh J. Transduction of Notch2 in feline leukemia virus-induced thymic lymphoma. J Virol. 1996;70:8071–8080. doi: 10.1128/jvi.70.11.8071-8080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schroeter E H, Kisslinger J A, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 54.Shawber C, Nofziger D, Hsieh J J-D, Lindsell C, Bogler O, Hayward S D, Weinmaster G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–3773. doi: 10.1242/dev.122.12.3765. [DOI] [PubMed] [Google Scholar]
- 55.Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 56.Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993;74:331–345. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- 57.Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 58.Tun T, Hamaguchi Y, Matsunami N, Furukawa T, Honjo T, Kawaichi M. Recognition sequence of a highly conserved DNA binding protein RBP-J kappa. Nucleic Acids Res. 1994;22:965–971. doi: 10.1093/nar/22.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinmaster G. Review: the ins and outs of Notch signaling. Mol Cell Neurosci. 1997;9:91–102. doi: 10.1006/mcne.1997.0612. [DOI] [PubMed] [Google Scholar]
- 60.Ye Y, Lukinova N, Fortini M E. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature. 1999;398:525–529. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Sun Z-W, Iratni R, Erdjument-Bromage H, Tempst P, Hampsey M, Reinberg D. SAP30, a novel protein conserved between human and yeast, is a component of a histone deacetylase complex. Mol Cell. 1998;1:1021–1031. doi: 10.1016/s1097-2765(00)80102-1. [DOI] [PubMed] [Google Scholar]
- 63.Zhou S, Fujimuro M, Hsieh J J-D, Chen L, Hayward S D. A role for SKIP in EBNA2 activation of CBF1-repressed promoters. J Virol. 2000;74:1939–1947. doi: 10.1128/jvi.74.4.1939-1947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]