ABSTRACT
Background
We have developed a diet quality metric intended for global use. To assess its utility in high-income settings, an evaluation of its ability to predict chronic disease is needed.
Objectives
We aimed to prospectively examine the ability of the Global Diet Quality Score (GDQS) to predict the risk of type 2 diabetes in the United States, examine potential differences of association by age, and compare the GDQS with other diet quality scores.
Methods
Health, lifestyle, and diet information was collected from women (n = 88,520) in the Nurses’ Health Study II aged 27–44 y at baseline through repeated questionnaires between 1991 and 2017. The overall GDQS consists of 25 food groups. Points are awarded for higher intake of healthy groups and lower intake of unhealthy groups (maximum of 49 points). Multivariable HRs were computed for confirmed type 2 diabetes using proportional hazards models. We also compared the GDQS with the Minimum Diet Diversity score for Women (MDD-W) and the Alternate Healthy Eating Index-2010 (AHEI-2010).
Results
We ascertained 6305 incident cases of type 2 diabetes during follow-up. We observed a lower risk of diabetes with higher GDQS; the multivariable HR comparing extreme quintiles of the GDQS was 0.83 (95% CI: 0.76, 0.91; P-trend < 0.001). The magnitude of association was similar between women aged <50 y and those aged ≥50 y. An inverse association was observed with lower intake of unhealthy components (HR comparing extreme quintiles of the unhealthy submetric: 0.76; 95% CI: 0.69, 0.84; P-trend < 0.001) but was not with the healthy submetric. The inverse association for each 1-SD increase in the GDQS (HR: 0.93; 95% CI: 0.91, 0.96) was stronger (P < 0.001) than for the MDD-W (HR: 1.00; 95% CI: 0.94, 1.04) but was slightly weaker (P = 0.03) than for the AHEI-2010 (HR: 0.91; 95% CI: 0.88, 0.94).
Conclusions
A higher GDQS was inversely associated with type 2 diabetes risk in US women of reproductive age or older, mainly from lower intake of unhealthy foods. The GDQS performed nearly as well as the AHEI-2010.
Keywords: diet quality, diabetes, epidemiology, women, nutrition
Introduction
Several diet quality indices have been developed and evaluated for their association with risk of chronic diseases (1). These indices typically were based on recommendations for a healthy diet (2–4) or reflections of regional dietary habits (5–7). Many include nutrient components and therefore require the use of a food composition database (2–4), or a scoring algorithm that is based on population-specific intake levels (5, 8). Evidence from prospective studies is consistent that adherence to these diet quality indices is associated with a lower risk of several chronic diseases, including cardiovascular disease and diabetes (1).
To apply these diet quality indices in clinical and public health settings to guide individual dietary choices and public health surveillance, the metric must be simple and quick to administer. In addition, a metric that is valid and practical for use across different parts of the world and different economic development levels would have the additional advantage of enabling global comparisons. Therefore, indices that involve a food composition database or use population-specific scoring would be difficult to implement across regions. To circumvent these limitations, we previously developed the Prime Diet Quality Score (PDQS) that only consists of food groups. It is inversely associated with cardiovascular disease and gestational diabetes in US men and women (9, 10).
To provide a metric that is usable in regions where nutritional adequacy is a concern, we have further modified the PDQS and tested it for association with nutritional markers relevant to middle- and lower-income countries. Our final metric, the Global Diet Quality Score (GDQS), uses a combination of healthy and unhealthy food groups. It has reasonable correlation with measures of nutrient adequacy (11).
Because the GDQS has several differences from the PDQS, we assessed its utility in a higher-income setting by testing its ability to predict the risk of type 2 diabetes in US women. We chose type 2 diabetes because the incidence is increasing globally, with a projected increase from >400 million affected in 2019 to ∼700 million by 2045 (12). In the United States, it was estimated that 12% of adult women and 14% of adult men were living with diabetes in 2013–2016 (13). Although a plethora of medications are available (14), there is no cure in most cases and successful management requires adequate compliance and regular access to health care (15). Therefore, prevention through lifestyle, and especially diet, continues to be an important approach. In this analysis, we prospectively examined the association between the GDQS and the risk of type 2 diabetes among US women, and explored potential differences in association by age. To understand the function of the GDQS, we also explored how the healthy and unhealthy components would drive any observed association. We hypothesized that the overall GDQS and the healthy components (GDQS+ submetric) would be inversely associated with diabetes risk, whereas lower intake of the unhealthy components (GDQS− submetric) would have an inverse association. For the GDQS to be a useful nutrition metric to predict noncommunicable diseases, it must also perform at least similarly as other established diet quality indices. Therefore, we also compared it with the Minimum Diet Diversity score for Women (MDD-W) and the Alternate Healthy Eating Index-2010 (AHEI-2010) for prediction of type 2 diabetes.
Methods
Participants
The Nurses’ Health Study II (NHS II) is an ongoing prospective cohort study that is comprised of 116,430 US female Registered Nurses between 25 and 42 y old at inception in 1989 (16). Information on lifestyle practices and incidence of type 2 diabetes was collected every 2 y by self-reported questionnaires. Diet was assessed every 4 y beginning in 1991 using a validated FFQ. Women with diabetes, gestational diabetes, cancer, or cardiovascular disease or who died before the first dietary assessment were excluded. We also excluded those who did not complete additional questionnaires beyond baseline and those who reported implausible energy intakes (<500 or >3500 kcal/d) at baseline. If a participant reported being pregnant in a questionnaire period, person-time during that 2-y period was excluded. A total of 88,520 women were included in this analysis and loss to follow-up was ∼10% during the study period. This study was approved by the institutional review boards of Brigham and Women's Hospital and the Harvard TH Chan School of Public Health.
Diet assessment
A validated semiquantitative FFQ was self-administered every 4 y, each containing ∼135 items (17). For each food item, a standard portion size was provided with 9 intake frequency choices ranging from “never or less than once per month” to “≥6 times per day.” The GDQS was modified based on the PDQS (9) to capture food groups that would reflect nutrient adequacy and predict major noncommunicable diseases in both lower- and high-income countries globally. It consists of 16 healthy food groups (dark green leafy vegetables, cruciferous vegetables, deep orange vegetables, other vegetables, deep orange fruits, deep orange tubers, citrus fruits, other fruits, legumes, nuts and seeds, poultry and game meats, fish and shellfish, whole grains, liquid oils, low fat dairy, eggs) and 7 unhealthy food groups (white roots and tubers, processed meats, refined grains and baked goods, sugar-sweetened beverages, sweets and ice cream, juices, purchased deep fried foods) (Supplemental Table 1). Intake of each food group was classified into <1/wk, 1 to <4/wk, and ≥4/wk. For healthy food groups, points between 0 and 4 were given to each level of intake depending on the food group. For unhealthy food groups, 2, 1, and 0 points were given for the same 3 intake levels so lower intake would receive more points. In addition to the aforementioned food groups, the GDQS also has a red meat group and a full-fat dairy group with different scoring to account for their contribution to nutrient adequacy in low- to middle-income countries. Red meat was given 0, 1, and 0 points for intake of the same 3 intake levels as for the other unhealthy food groups, and full-fat dairy was given 0, 1, 2, and 0 points for intake of <1/wk, 1 to <4/wk, ≥4/wk to <3/d, and ≥3/d, respectively. The full GDQS has 25 food groups and a score range of 0–49 points, with higher points representing a healthier diet. The healthy portion of the GDQS (GDQS+) has a range of 0–32. For the purpose of this analysis, we included red meat and full-fat dairy as part of the unhealthy portion (GDQS−), which has a range of 0–17, with a higher score representing lower intake of unhealthy foods and hence healthier food choices.
To compare the GDQS with other established diet quality indices, we also computed the AHEI-2010 (2) and the MDD-W (18) for each participant. The AHEI-2010 consists of 11 food and nutrient groups. High points are given for higher intakes of healthy groups (vegetables, whole fruits, nuts and legumes, whole grains, polyunsaturated fat, and long-chain n–3 fatty acids) and lower intakes of unhealthy groups (red and processed meats, sugar-sweetened beverages and fruit juice, trans fat, and sodium). Points are also given for moderate intake of alcohol. Each component ranges from 0 to 10 points with the total possible score ranging from 0 to 110 points. It has previously been shown to be inversely associated with diabetes risk in women (2).
The MDD-W, originally developed as a proxy indicator for nutrient adequacy, consists of 10 food groups: grains and starchy vegetables, pulses, nuts and seeds, dairy, animal flesh, eggs, dark green leafy vegetables, vitamin A–rich vegetables and fruits, other vegetables, and other fruits (18). The scoring method for the original MDD-W is based on intake collected by 24-h recall. To adapt it for the FFQ, we assigned 1 point for each food group with intake ≥1 serving/d and 0 for less (9). The MDD-W has a range of 0–10 points.
Outcome assessment
Incident type 2 diabetes was first reported through the biennial questionnaires and confirmed with a validated supplemental questionnaire based on National Diabetes Data Group criteria. This included ≥1 of the following: ≥1 classic symptom (excessive thirst, polyuria or frequent urination, weight loss, hunger), fasting plasma glucose concentrations ≥7.8 mmol/L, or random plasma glucose concentrations ≥11.1 mmol/L (19). In the case of a lack of symptoms, diabetes was considered confirmed with ≥2 elevated plasma glucose concentrations on different occasions (fasting plasma glucose concentrations ≥7.8 mmol/L, random concentrations ≥11.1 mmol/L, and/or 2-h blood glucose concentrations ≥11.1 mmol/L during oral-glucose-tolerance testing); or treatment with hypoglycemic medications (insulin or oral hypoglycemic agent). For cases reported after 1998, criteria from the American Diabetes Association were used in which the threshold for fasting plasma glucose changed from 7.8 mmol/L to 7.0 mmol/L (20). The supplemental questionnaire was validated by a review of medical reports (21). In a random sample of 62 cases in the Nurses’ Health Study that were confirmed by the supplementary questionnaire, 61 (98%) cases were reconfirmed after medical records were reviewed by an endocrinologist blinded to the supplementary questionnaire.
Covariate assessment
Information on age, race, and height was collected at cohort inception. Body weight, cigarette smoking (including the number of cigarettes per day), physical activity, menopausal status and postmenopausal hormone use, oral contraceptive use, family history of diabetes, history of hypercholesterolemia, and high blood pressure were collected in each biennial questionnaire. BMI (in kg/m2) was calculated using height collected at baseline and weight reported at each questionnaire cycle. Alcohol intake and supplemental vitamin and mineral use were collected with FFQs.
Statistical analysis
For this analysis, follow-up duration in person-years was calculated from the date of return of the 1991 questionnaire to the date of diabetes diagnosis, last questionnaire returned, or 30 June, 2017. We computed cumulative averages of diet quality scores to reduce within-person variation and represent long-term intake (22). We used time-dependent Cox proportional hazards regression models to compute HRs of type 2 diabetes for quintiles of the GDQS, GDQS+, and GDQS−. Eggs are included in the GDQS+ because of their protein and vitamin content, but they also contain substantial amounts of cholesterol. Hence, we in addition computed an alternate GDQS+ without the egg component for sensitivity analysis. We tested for the proportional hazards assumption by including an interaction term of GDQS and age (which reflects time) and used the likelihood ratio test. The P value for the chi-square distribution was >0.05, hence it did not show a violation of the proportional hazards assumption.
All models were adjusted by age (mo) at the start of follow-up for each woman and the calendar year of each questionnaire cycle. Multivariable models were adjusted for race (white/nonwhite), family history of diabetes, smoking (never, past, 1–14 cigarettes/d, 15–24 cigarettes/d, ≥25 cigarettes/d), alcohol intake (none, <5 g/d, 5 to <10 g/d, ≥10 g/d), energy intake (quintiles), coffee intake (continuous), physical activity [<3 metabolic equivalent hours (METs)/wk, 3 to <9 METs/wk, 9 to <18 METs/wk, 18 to <27 METs/wk, ≥27 METs/wk], BMI (<23, 23 to < 25, 25 to <30, 30 to <35, ≥35), multivitamin use (yes/no), menopausal status and menopausal hormone therapy (premenopausal, no hormone use, past use, current use), oral contraceptive use (never, past, current), history of hypertension at baseline, and history of hyperlipidemia at baseline. We used restricted cubic spline regression to assess potential nonlinear association. To access potential differential association of the GDQS with diabetes by age, we conducted analyses stratified by age. We also stratified the analysis by BMI status and physical activity. To examine the potential influence of pregnancy on the association between the GDQS and diabetes, we ran regression models separately for women based on pregnancy history, and among ever-pregnant women by history of gestational diabetes. Tests for 2-way interaction between the GDQS and each of the stratified factors were conducted using the likelihood ratio test comparing regression models with and without an interaction term. Analysis was conducted using SAS version 9.4 (SAS Institute Inc.).
To compare the strength of association between the GDQS and the AHEI-2010 and MDD-W, we standardized each score and modeled each 1 SD of the scores in the same model. Differences in the regression coefficients were compared using the Wald test.
Results
In ≤26 y of follow-up, we ascertained 6305 incident cases of type 2 diabetes, of which 2266 were women younger than 50 y old and 4039 were women ≥50 y old. Women with a higher GDQS tended to be leaner, more physically active, less likely to be current smokers, and consumed more alcohol and coffee (Table 1).
TABLE 1.
Age-standardized baseline characteristics by quintiles of GDQS in the Nurses’ Health Study II1
| Q1 | Q2 | Q3 | Q4 | Q5 | |
|---|---|---|---|---|---|
| BMI | 24.8 ± 5.8 | 24.6 ± 5.5 | 24.4 ± 5.1 | 24.3 ± 5.0 | 24.2 ± 4.8 |
| Physical activity, METs | 14.5 ± 21.2 | 17.8 ± 24.1 | 20.4 ± 26.2 | 23.5 ± 28.7 | 29.1 ± 34.0 |
| Current smoker, % | 18 | 14 | 12 | 11 | 9 |
| GDQS | 14.3 ± 2.2 | 18.7 ± 0.9 | 21.5 ± 0.8 | 24.4 ± 0.9 | 28.8 ± 2.2 |
| Unhealthy GDQS components | 7.1 ± 2.3 | 8.2 ± 2.4 | 8.6 ± 2.4 | 9.1 ± 2.4 | 10.1 ± 2.2 |
| Healthy GDQS components | 7.3 ± 2.8 | 10.7 ± 2.5 | 12.9 ± 2.5 | 15.3 ± 2.4 | 18.7 ± 2.7 |
| MDD-W | 3.0 ± 1.3 | 3.6 ± 1.3 | 4.1 ± 1.3 | 4.6 ± 1.2 | 5.4 ± 1.2 |
| AHEI-2010 | 37.8 ± 7.6 | 43.7 ± 7.7 | 47.8 ± 7.9 | 52.0 ± 8.3 | 58.8 ± 8.8 |
| Energy intake, kcal/d | 1641 ± 536 | 1689 ± 537 | 1743 ± 532 | 1831 ± 530 | 1990 ± 529 |
| Fiber, g/d | 14.3 ± 3.6 | 16.5 ± 4.0 | 18.2 ± 4.8 | 20.0 ± 5.2 | 22.7 ± 5.8 |
| Alcohol, g/d | 2.4 ± 5.7 | 3.0 ± 6.3 | 3.3 ± 6.2 | 3.5 ± 6.1 | 3.9 ± 6.5 |
| Processed meats, servings/d | 0.31 ± 0.33 | 0.26 ± 0.28 | 0.22 ± 0.25 | 0.19 ± 0.23 | 0.15 ± 0.20 |
| Red meats, servings/d | 0.67 ± 0.43 | 0.60 ± 0.41 | 0.55 ± 0.38 | 0.52 ± 0.37 | 0.44 ± 0.34 |
| Vegetables, servings/d | 1.8 ± 1.0 | 2.5 ± 1.3 | 3.0 ± 1.5 | 3.8 ± 1.7 | 5.1 ± 2.4 |
| Fruit, servings/d | 1.2 ± 1.0 | 1.5 ± 1.1 | 1.8 ± 1.2 | 2.1 ± 1.3 | 2.6 ± 1.6 |
| Nuts and seeds, servings/d | 0.04 ± 0.08 | 0.05 ± 0.10 | 0.06 ± 0.11 | 0.07 ± 0.16 | 0.11 ± 0.21 |
| Legumes, servings/d | 0.16 ± 0.16 | 0.20 ± 0.18 | 0.24 ± 0.23 | 0.29 ± 0.26 | 0.41 ± 0.35 |
| Coffee, servings/d | 1.4 ± 1.7 | 1.5 ± 1.7 | 1.6 ± 1.7 | 1.7 ± 1.7 | 1.8 ± 1.7 |
1 n = 88,520. Values are means ± SDs unless otherwise indicated. AHEI-2010, Alternate Healthy Eating Index-2010; GDQS, Global Diet Quality Score; MDD-W, Minimum Diet Diversity score for Women; MET, metabolic equivalent hour; Q, quintile.
We observed a lower risk of diabetes with higher GDQS (multivariable HR comparing extreme quintiles: 0.83; 95% CI: 0.76, 0.91; P-trend < 0.001) (Table 2). The association for women age <50 y was 0.85 (95% CI: 0.73, 0.98; P-trend < 0.001) and for age ≥50 y was 0.82 (95% CI: 0.74, 0.91, P-trend < 0.001) with no significant interaction. We also separately examined the submetrics of the GDQS representing healthy (GDQS+) and unhealthy (GDQS−) food components. These 2 submetrics were only weakly correlated (Spearman r = −0.06, P < 0.001). The healthy components of the GDQS (GDQS+) were not associated with diabetes risk (Table 3). On the other hand, higher GDQS−, which represents lower intake of the unhealthy components, showed an inverse association (multivariable HR comparing extreme quintiles: 0.76; 95% CI: 0.69, 0.84; P-trend < 0.001). There was no apparent difference in association by age. Spline regression did not detect significant departure from linearity for the overall GDQS, GDQS+, or GDQS− (data not shown). In the sensitivity analysis in which we excluded the egg component from the GDQS+, the null association persisted in the remaining portion of the GDQS+.
TABLE 2.
HRs (95% CI) for type 2 diabetes according to quintiles of the Global Diet Quality Score in the Nurses’ Health Study II1
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | |
|---|---|---|---|---|---|---|
| All women | ||||||
| Median score | 15.8 | 19.5 | 21.9 | 24.4 | 27.8 | |
| Cases, n | 1647 | 1309 | 1262 | 1112 | 975 | |
| Person-years | 365,779 | 364,667 | 365,382 | 373,363 | 364,174 | |
| Age- and kcal-adjusted | 1 | 0.76 (0.71, 0.82) | 0.71 (0.66, 0.76) | 0.59 (0.55, 0.64) | 0.48 (0.44, 0.52) | <0.001 |
| Multivariable2 | 1 | 0.91 (0.84, 0.97) | 0.94 (0.87, 1.01) | 0.87 (0.80, 0.94) | 0.83 (0.76, 0.91) | <0.001 |
| Women < age 50 y | ||||||
| Median score | 15.3 | 18.9 | 21.3 | 23.8 | 27.3 | |
| Cases, n | 634 | 456 | 459 | 395 | 322 | |
| Person-years | 210,566 | 202,881 | 198,185 | 201,222 | 184,898 | |
| Age- and kcal-adjusted | 1 | 0.72 (0.64, 0.82) | 0.73 (0.65, 0.83) | 0.61 (0.53, 0.69) | 0.50 (0.44, 0.58) | <0.001 |
| Multivariable2 | 1 | 0.86 (0.76, 0.98) | 1.00 (0.88, 1.13) | 0.90 (0.79, 1.02) | 0.85 (0.73, 0.98) | 0.02 |
| Women age ≥ 50 y | ||||||
| Median score | 16.7 | 20.3 | 22.8 | 25.0 | 28.1 | |
| Cases, n | 1013 | 853 | 803 | 717 | 653 | |
| Person-years | 155,214 | 161,786 | 167,196 | 172,140 | 179,276 | |
| Age- and kcal-adjusted | 1 | 0.79 (0.72, 0.86) | 0.70 (0.63, 0.76) | 0.58 (0.53, 0.64) | 0.47 (0.43, 0.52) | <0.001 |
| Multivariable2 | 1 | 0.93 (0.85, 1.02) | 0.91 (0.82, 1.00) | 0.85 (0.77, 0.94) | 0.82 (0.74, 0.91) | <0.001 |
n = 88,520. Q, quintile.
Adjusted for age, BMI, energy intake, smoking, family history of diabetes, oral contraceptive use, menopausal status and postmenopausal hormone use (“all women” analysis only), physical activity, alcohol intake, and multivitamin use.
TABLE 3.
HRs (95% CI) for type 2 diabetes according to quintiles of the healthy (GDQS+) and unhealthy (GDQS−) submetrics of the GDQS in the Nurses’ Health Study II1
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | |
|---|---|---|---|---|---|---|
| GDQS+ submetric (max = 32) | ||||||
| All women | ||||||
| Median score | 8.0 | 11.3 | 13.6 | 15.8 | 18.8 | |
| Cases, n | 1441 | 1290 | 1188 | 1232 | 1154 | |
| Person-years | 366,057 | 365,828 | 368,066 | 366,408 | 367,005 | |
| Age- and kcal-adjusted | 1 | 0.83 (0.77, 0.90) | 0.70 (0.64, 0.76) | 0.67 (0.62, 0.73) | 0.54 (0.49, 0.59) | <0.001 |
| Multivariable2 | 1 | 1.00 (0.92, 1.08) | 0.98 (0.90, 1.07) | 1.05 (0.96, 1.14) | 1.00 (0.91, 1.10) | 0.86 |
| Women < age 50 y | ||||||
| Median score | 7.5 | 10.8 | 13.2 | 15.4 | 18.5 | |
| Cases, n | 554 | 459 | 403 | 443 | 407 | |
| Person-years | 205,773 | 202,984 | 200,220 | 197,583 | 191,192 | |
| Age- and kcal-adjusted | 1 | 0.79 (0.69, 0.89) | 0.64 (0.56, 0.73) | 0.67 (0.58, 0.76) | 0.55 (0.47, 0.64) | <0.001 |
| Multivariable2 | 1 | 0.96 (0.84, 1.09) | 0.92 (0.80, 1.06) | 1.04 (0.90, 1.20) | 1.00 (0.85, 1.17) | 0.97 |
| Women age ≥ 50 y | ||||||
| Median score | 8.6 | 11.9 | 14.1 | 16.2 | 19.1 | |
| Cases, n | 887 | 831 | 785 | 789 | 747 | |
| Person-years | 160,284 | 162,845 | 167,846 | 168,825 | 175,813 | |
| Age- and kcal-adjusted | 1 | 0.86 (0.78, 0.94) | 0.74 (0.67, 0.82) | 0.68 (0.61, 0.76) | 0.54 (0.48, 0.60) | <0.001 |
| Multivariable2 | 1 | 1.02 (0.92, 1.13) | 1.03 (0.92, 1.14) | 1.05 (0.94, 1.18) | 1.01 (0.89, 1.14) | 0.77 |
| GDQS− submetric (max = 14) (high score = less unhealthy) | ||||||
| All women | ||||||
| Median score | 5.5 | 7.2 | 8.5 | 9.6 | 11.0 | |
| Cases, n | 1701 | 1446 | 1151 | 1050 | 957 | |
| Person-years | 374,851 | 354,527 | 367,116 | 359,807 | 377,063 | |
| Age- and kcal-adjusted | 1 | 0.84 (0.78, 0.90) | 0.66 (0.61, 0.71) | 0.56 (0.51, 0.61) | 0.47 (0.43, 0.51) | <0.001 |
| Multivariable2 | 1 | 0.96 (0.89, 1.04) | 0.85 (0.78, 0.92) | 0.80 (0.73, 0.88) | 0.76 (0.69, 0.84) | <0.001 |
| Women < age 50 y | ||||||
| Median score | 5.3 | 7.0 | 8.0 | 9.5 | 11.0 | |
| Cases, n | 661 | 517 | 394 | 375 | 319 | |
| Person-years | 219,316 | 190,611 | 202,692 | 190,611 | 194,521 | |
| Age- and kcal-adjusted | 1 | 0.86 (0.76, 0.97) | 0.69 (0.60, 0.79) | 0.61 (0.53, 0.70) | 0.52 (0.44, 0.61) | <0.001 |
| Multivariable2 | 1 | 0.96 (0.85, 1.09) | 0.87 (0.76, 1.00) | 0.83 (0.72, 0.97) | 0.81 (0.68, 0.95) | <0.001 |
| Women age ≥ 50 y | ||||||
| Median score | 5.8 | 7.5 | 8.7 | 9.8 | 11.2 | |
| Cases, n | 1040 | 929 | 757 | 675 | 638 | |
| Person-years | 155,535 | 163,915 | 164,424 | 169,196 | 182,542 | |
| Age- and kcal-adjusted | 1 | 0.82 (0.75, 0.90) | 0.64 (0.58, 0.71) | 0.54 (0.48, 0.60) | 0.45 (0.40, 0.50) | <0.001 |
| Multivariable2 | 1 | 0.96 (0.87, 1.05) | 0.83 (0.75, 0.92) | 0.78 (0.70, 0.88) | 0.74 (0.65, 0.83) | <0.001 |
n = 88,520. GDQS, Global Diet Quality Score; Q, quintile.
Adjusted for age, BMI, energy intake, smoking, family history of diabetes, oral contraceptive use, menopausal status and postmenopausal hormone use (“all women” analysis only), physical activity, alcohol intake, multivitamin use, and mutually adjusted for the other submetric.
The GDQS was inversely associated with diabetes in both women ever or never pregnant (Supplemental Table 2). Although the magnitude of association did not differ substantially for pregnancy history, the trend appeared to be more consistent for never-pregnant women (P-interaction = 0.06). Among women who had been pregnant, an inverse association with the GDQS was only observed for those without a history of gestational diabetes (multivariable HR comparing extreme quintiles: 0.83; 95% CI: 0.75, 0.91; P-trend < 0.001). We also stratified the analysis by BMI and physical activity (Supplemental Table 3). The inverse association was significant regardless of BMI status; however, it was stronger among leaner women (P-interaction < 0.001). On the other hand, although the association between the GDQS and diabetes appeared stronger among those with physical activity above the median, the P value for interaction did not reach statistical significance.
We also compared the magnitude of association of the GDQS with 2 other diet quality scores: the AHEI-2010 and MDD-W. The Spearman correlation coefficient between the GDQS and the AHEI-2010 was 0.74 (P < 0.001); it was 0.64 (P < 0.001) with the MDD-W. The AHEI-2010 was inversely associated with diabetes (multivariable HR comparing extreme quintiles: 0.62; 95% CI: 0.56, 0.68; P-trend < 0.001) and there was no appreciable difference by age (Supplemental Table 4). However, no association was observed with the MDD-W (Supplemental Table 5). When we compared the association of the GDQS with diabetes pairwise with the AHEI-2010 and the MDD-W, the association for each SD increase in the AHEI-2010 was slightly stronger than for the GDQS (HR: 0.91 compared with 0.93, P for difference = 0.03) (Figure 1). On the other hand, the association for the GDQS was clearly stronger than for the MDD-W (P for difference < 0.001).
FIGURE 1.
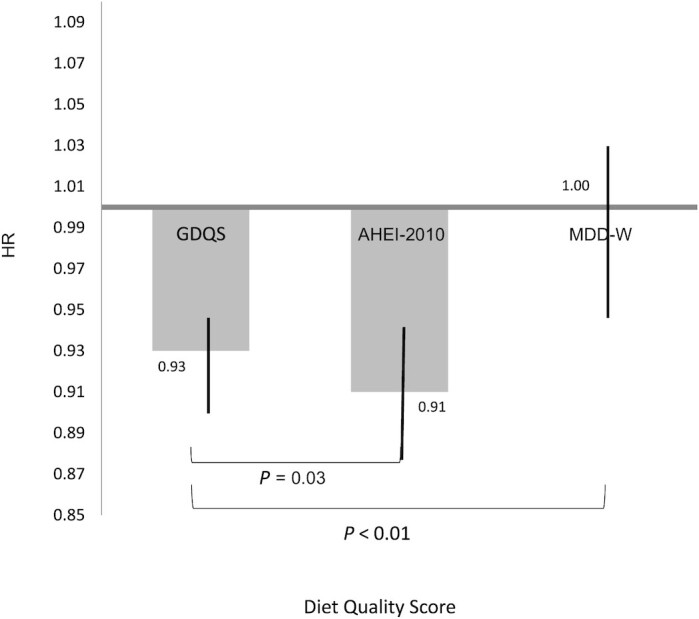
Multivariable HR for a 1-SD increase of the GDQS, AHEI-2010, and MDD-W. Models were adjusted for age, BMI, energy intake, smoking, family history of diabetes, oral contraceptive use, menopausal status and postmenopausal hormone use (“all women” analysis only), physical activity, alcohol intake, and multivitamin use. Vertical lines represent 95% CIs. Chi-square test P values tested for significant differences in HR between the GDQS and AHEI-2010, and GDQS and MDD-W. AHEI-2010, Alternate Healthy Eating Index-2010; GDQS, Global Diet Quality Score; MDD-W, Minimum Diet Diversity score for Women.
Discussion
In this analysis, we observed an inverse association between a diet quality score designed for global use and risk of type 2 diabetes among US women. The association appeared to be driven by lower intakes of unhealthy foods. The GDQS compared well with the AHEI-2010 which showed a strong inverse association with diabetes in a cohort of middle-aged nurses (23). The lower diabetes risk with a higher GDQS was similar between women of reproductive age and those who were older.
Prospective studies from the United States (24), Europe (6), and Asia (25, 26) have shown adherence to healthy eating guidelines, as reflected by higher diet quality indices, to be associated with lower risk of type 2 diabetes. Although different diet quality indices were used in these studies, such as the Healthy Diet Score, the Healthy Eating Index, the Alternate Healthy Eating Index, and some form of Mediterranean diet score, the common features among them were higher intakes of fruits, vegetables, whole grains, and lean protein and lower intakes of red and processed meats, added sugar, and refined grains. The number of components ranged from 6 in the Healthy Nordic Food Index (6) to 11 in the Alternate Healthy Eating Index (24). The GDQS features similar food groups, but in more refined categories and hence a total of 25 food groups. We have chosen the approach of using more specific food groups to better specify nutrients, such as vitamin C and provitamin A carotenoids that are nutrients of concern in some parts of the world.
In our analysis, lower intakes of foods in the unhealthy submetric of the GDQS (GDQS−) were more strongly associated with a lower diabetes risk than was the healthy submetric of the GDQS (GDQS+). Among the foods in the GDQS−, high intakes of red and processed meats (27), refined grains (28), sugar-sweetened beverages (28), and potatoes, especially as French fries (29), have previously been shown to be directly associated with higher risk of type 2 diabetes. In addition, fried foods have also been shown to increase risk of type 2 diabetes (30) or gestational diabetes (31) in US women. Fried foods may be a risk factor for diabetes owing to the high energy content or the increase in lipid oxidation products (32) and trans fat (33) created in the process of frying. Red and processed meat may be involved in the pathogenesis of type 2 diabetes through inducing proinflammatory advanced glycation end products (34) and pancreatic injury due to oxidative stress from heme iron (35). In addition, nitrites and nitrates in processed meats could be precursors for the pro-oxidant peroxynitrate (36). Refined grains and sugar-sweetened beverages may contribute to weight gain (37) and the high glycemic load has been associated with diabetes risk (38).
Healthy dietary patterns similar to the healthy submetric of the GDQS (GDQS+) are inversely associated with diabetes (39). However, a meta-analysis only found marginally significant inverse associations for individual food groups such as fruits, vegetables, and nuts (28). Our analysis also did not observe an inverse association of the GDQS+ with diabetes, even when the egg component, which has been associated with diabetes risk in US studies (40), was removed. Although the GDQS+ encompasses a number of healthy food groups and can potentially detect joint association of these food groups, each food group only has 3 levels of scoring. It is possible that only high intakes of specific foods or food groups are associated with lower risk of diabetes and our scoring could not differentiate these high intakes. On the other hand, the food groups in the unhealthy submetric might be more strongly associated with diabetes than our scoring method was sufficient to detect.
The strengths of this study include the large sample size and long follow-up which allowed us to accrue a sufficient number of cases to examine diabetes risk even among women of reproductive age. The detailed and repeated assessment of lifestyle and health information in the Nurses’ Health Study II allowed us to explore potential difference in risk by reproductive history. On the other hand, lifestyle and diet information was obtained from self-report. Although the validity of the dietary questionnaire has been well documented (41), some degree of misclassification is inevitable. And although we have adjusted for multiple confounders that were updated throughout follow-up, we cannot exclude the possibility of residual confounding.
In designing the GDQS, the metric has to be applicable to geographical regions with a wide range of economic resources and nutrition challenges. Therefore, the score was constructed to balance the needs to reflect nutrient adequacy and predict chronic disease risk. For that purpose, the red meat component which would normally be considered as unhealthy in high-income countries was given 1 point for moderate intake and 0 for low or high intake, to recognize its value as a protein and iron source in lower-resource regions. Similarly, points were given for moderate consumption of full-fat dairy to recognize its value as a protein, calcium, and energy source, but we did not award points for very high or no consumption. Also, the GDQS promotes moderate consumption of poultry, fish, eggs, and low fat dairy.
Because the GDQS was not designed specifically to predict the risk of diabetes, it does not include coffee (42) and moderate alcohol consumption in the metric score (43), both of which are inversely associated with type 2 diabetes risk. Nevertheless, we were still able to observe a strong association with type 2 diabetes risk, and the GDQS performed well against 2 other diet quality scores. In particular, the GDQS is easier to use than the AHEI-2010. The GDQS, however, reflects overall diet healthfulness and is not specifically aimed for the prevention of a specific disease. As a result, a high GDQS does not represent the optimal dietary characteristics for the prevention of diabetes.
In the current global drive to shift food consumption to be more plant focused for both human and planetary health (44), the food groups chosen for the GDQS have implicit concordance with this goal. Out of the 17 healthy food groups to emphasize in the diet, only 4 were from animal origin. And out of the 9 unhealthy food groups to minimize intake, 3 were animal protein, and 1 (sweets and ice cream) often has ingredients from animal origin. Therefore, a diet that scores high on the GDQS would tend to be correlated with diets that are relatively more plant-based.
Health metrics that have specific cutoffs are useful for risk assessment and setting treatment targets. Clinically relevant cutoffs can be identified if there are inflection points in the relation of the GDQS and risk of diabetes. Cutoffs can also be set by assigning a priori categories. However, this latter approach requires somewhat arbitrary decisions and also needs to consider other outcomes and diverse populations. In our results, there was no departure from linearity in the GDQS. Because our results point toward a progressively lower risk of diabetes with higher GDQS, there is no strong premise to support specific cutoffs for the GDQS in this cohort of US women.
In conclusion, the GDQS was inversely associated with type 2 diabetes in both reproductive-age and older women in a high-income country. It performed well compared with the AHEI-2010 in predicting diabetes risk and our results showed that lower intake of unhealthy foods appeared to be more important than higher intake of healthy foods. Further testing of the GDQS in other populations is needed to confirm its usefulness in a broad range of populations to predict noncommunicable diseases.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—TTF, SNB, and SB: designed the research; TTF: analyzed the data, wrote the paper, and had primary responsibility for the final content; WCW, MS, CB, MDH, FBH, YL, and MD: provided study oversight and advice; and all authors: read and approved the final manuscript.
Notes
Funding for the research was provided by FHI Solutions, recipient of a Bill & Melinda Gates Foundation grant to support Intake—Center for Dietary Assessment and by NIH grant U01 CA176726.
Author disclosures: TTF is an Associate Editor for the Journal of Nutrition and played no role in the Journal’s evaluation of the manuscript. All other authors report no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Published in a supplement to The Journal of Nutrition. Publication costs for this supplement were funded by the Bill & Melinda Gates Foundation in conjunction with FHI Solutions, recipient of a Bill & Melinda Gates Foundation grant to support Intake—Center for Dietary Assessment. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition. The Supplement Coordinators for the supplement publication were Megan Deitchler, Intake—Center for Dietary Assessment at FHI Solutions, Washington, DC; and Sabri Bromage, Harvard TH Chan School of Public Health, Boston, MA.
The GDQS research initiative was launched by Intake – Center for Dietary Assessment. The research was led by Harvard T.H. Chan School of Public Health, Department of Nutrition and carried out in collaboration with researchers at the National Public Health Institute (INSP), Mexico. Funding for the research was provided by FHI Solutions, recipient of a Bill & Melinda Gates Foundation grant to support Intake – Center for Dietary Assessment.
Abbreviations used: AHEI-2010, Alternate Healthy Eating Index-2010; GDQS, Global Diet Quality Score; MDD-W, Minimum Diet Diversity score for Women; MET, metabolic equivalent hour; PDQS, Prime Diet Quality Score.
Contributor Information
Teresa T Fung, Department of Nutrition, Simmons University, Boston, MA, USA; Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Yanping Li, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Shilpa N Bhupathiraju, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Sabri Bromage, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Carolina Batis, CONACYT—Health and Nutrition Research Center, National Institute of Public Health, Cuernavaca, Mexico.
Michelle D Holmes, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Meir Stampfer, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Frank B Hu, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Megan Deitchler, Intake—Center for Dietary Assessment, FHI Solutions, Washington, DC, USA.
Walter C Willett, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
References
- 1. Schulze MB, Martínez-González MA, Fung TT, Lichtenstein AH, Forouhi NG. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaluza J, Harris HR, Håkansson N, Wolk A. Adherence to the WCRF/AICR 2018 recommendations for cancer prevention and risk of cancer: prospective cohort studies of men and women. Br J Cancer. 2020;122:1562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirkpatrick SI, Reedy J, Krebs-Smith SM, Pannucci TE, Subar AF, Wilson MM, Lerman JL, Tooze JA. Applications of the healthy eating index for surveillance, epidemiology, and intervention research: considerations and caveats. J Acad Nutr Diet. 2018;118:1603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernández-Ruiz A, García-Villanova B, Guerra Hernández EJ, Amiano P, Azpiri M, Molina-Montes E. Description of indexes based on the adherence to the Mediterranean dietary pattern: a review. Nutr Hosp. 2015;32:1872–84. [DOI] [PubMed] [Google Scholar]
- 6. Lacoppidan SA, Kyrø C, Loft S, Helnæs A, Christensen J, Hansen CP, Dahm CC, Overvad K, Tjønneland A, Olsen A. Adherence to a healthy Nordic Food Index is associated with a lower risk of type-2 diabetes—the Danish Diet, Cancer and Health cohort study. Nutrients. 2015;7:8633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puaschitz NG, Assmus J, Strand E, Karlsson T, Vinknes KJ, Lysne V, Drevon CA, Tell GS, Dierkes J, Nygård O. Adherence to the Healthy Nordic Food Index and the incidence of acute myocardial infarction and mortality among patients with stable angina pectoris. J Hum Nutr Diet. 2019;32:86–97. [DOI] [PubMed] [Google Scholar]
- 8. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 9. Fung TT, Isanaka S, Hu FB, Willett WC. International food group–based diet quality and risk of coronary heart disease in men and women. Am J Clin Nutr. 2018;107:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gicevic S, Gaskins AJ, Fung TT, Rosner B, Tobias DK, Isanaka S, Willett WC. Evaluating pre-pregnancy dietary diversity vs. dietary quality scores as predictors of gestational diabetes and hypertensive disorders of pregnancy. PLoS One. 2018;13:e0195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bromage S, Batis C, Bhupathiraju SN, Fawzi WW, Fung TT, Li Y, Deitchler M, Angulo E, Birk N, Castellanos-Gutíerrez Aet al. Development and validation of a novel food-based Global Diet Quality Score (GDQS). J Nutr. 2021;151(Suppl 10):75S–92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova Ket al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 13. CDC. National Diabetes Statistics Report, 2020. Atlanta, GA: CDC, US Department of Health and Human Services; 2020. [Google Scholar]
- 14. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43:S98–S110. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association. 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43:S48–65. [DOI] [PubMed] [Google Scholar]
- 16. Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 18. Women's Dietary Diversity Project (WDDP) Study Group. Development of a dichotomous indicator for population-level assessment of dietary diversity in women of reproductive age. Curr Dev Nutr. 2017;1:cdn.117.001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–57. [DOI] [PubMed] [Google Scholar]
- 20. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. [DOI] [PubMed] [Google Scholar]
- 21. Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Hennekens CH, Speizer FE, Rimm EB, Krolewski AS. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–8. [DOI] [PubMed] [Google Scholar]
- 22. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 23. Fung TT, McCullough M, van Dam RM, Hu FB. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care. 2007;30:1753–7. [DOI] [PubMed] [Google Scholar]
- 24. Cespedes EM, Hu FB, Tinker L, Rosner B, Redline S, Garcia L, Hingle M, Van Horn L, Howard BV, Levitan EBet al. Multiple healthful dietary patterns and type 2 diabetes in the Women's Health Initiative. Am J Epidemiol. 2016;183:622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen G-C, Koh W-P, Neelakantan N, Yuan J-M, Qin L-Q, van Dam RM. Diet quality indices and risk of type 2 diabetes mellitus: the Singapore Chinese Health Study. Am J Epidemiol. 2018;187:2651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu D, Zheng W, Cai H, Xiang Y-B, Li H, Gao Y-T, Shu X-O. Long-term diet quality and risk of type 2 diabetes among urban Chinese adults. Diabetes Care. 2018;41:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes – an updated review of the evidence. Curr Atheroscler Rep. 2012;14:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwingshackl L, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwingshackl L, Schwedhelm C, Hoffmann G, Boeing H. Potatoes and risk of chronic disease: a systematic review and dose–response meta-analysis. Eur J Nutr. 2019;58:2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cahill LE, Pan A, Chiuve SE, Sun Q, Willett WC, Hu FB, Rimm EB. Fried-food consumption and risk of type 2 diabetes and coronary artery disease: a prospective study in 2 cohorts of US women and men. Am J Clin Nutr. 2014;100:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Osorio-Yáñez C, Gelaye B, Qiu C, Bao W, Cardenas A, Enquobahrie DA, Williams MA. Maternal intake of fried foods and risk of gestational diabetes mellitus. Ann Epidemiol. 2017;27:384–90.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta MK. Frying oils. In: Shahidi Feditor. Baileys' industrial oil and fat products. Hoboken, NJ: John Wiley & Sons; 2005. [Google Scholar]
- 33. Yang M, Yang Y, Nie S, Xie M, Chen F. Analysis and formation of trans fatty acids in corn oil during the heating process. J Am Oil Chem Soc. 2012;89:859–67. [Google Scholar]
- 34. Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–16.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta. 2009;1790:671–81. [DOI] [PubMed] [Google Scholar]
- 36. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malik VS, Hu FB. Sweeteners and risk of obesity and type 2 diabetes: the role of sugar-sweetened beverages. Curr Diab Rep. 2012;12:195–203. [DOI] [PubMed] [Google Scholar]
- 38. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–23. [DOI] [PubMed] [Google Scholar]
- 39. Jayedi A, Soltani S, Abdolshahi A, Shab-Bidar S. Healthy and unhealthy dietary patterns and the risk of chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Br J Nutr. 2020;124:1133–44. [DOI] [PubMed] [Google Scholar]
- 40. Djoussé L, Khawaja OA, Gaziano JM. Egg consumption and risk of type 2 diabetes: a meta-analysis of prospective studies. Am J Clin Nutr. 2016;103:474–80. [DOI] [PubMed] [Google Scholar]
- 41. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185:570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlström M, Larsson SC. Coffee consumption and reduced risk of developing type 2 diabetes: a systematic review with meta-analysis. Nutr Rev. 2018;76:395–417. [DOI] [PubMed] [Google Scholar]
- 43. Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care. 2015;38:1804–12. [DOI] [PubMed] [Google Scholar]
- 44. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, Garnett T, Tilman D, DeClerck F, Wood Aet al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


