Abstract
BACKGROUND
Worldwide, the prevalence of obesity in women of reproductive age is increasing. Bariatric surgery is currently viewed as the most effective, long-term solution for this problem. Preconception bariatric surgery can reduce the prevalence of obesity-related subfertility and adverse maternal, pregnancy and birth outcomes. Maternal health during the periconception period is crucial for optimal gametogenesis and for embryonic and fetal development which also affects health in the later lives of both mother and offspring. Although preconception bariatric surgery improves several pregnancy outcomes, it can also increase the prevalence of pregnancy complications due to excessive and rapid weight loss. This can lead to iatrogenic malnutrition with vitamin deficiencies and derangements in metabolic and endocrine homeostasis. Thus, bariatric surgery can greatly influence periconception maternal health with consequences for reproduction, pregnancy and health in later life. However, its influence on periconception maternal health itself has never been reviewed systematically.
OBJECTIVE AND RATIONALE
The aim of this review was to investigate associations between bariatric surgery and determinants of periconception maternal health such as endocrine changes, fertility, vitamin status, irregular menstrual cycles, miscarriages and congenital malformations.
SEARCH METHODS
Medline, Embase, PubMed, Web of Science, Google Scholar and the Cochrane databases were used for the literature search until 1 November 2020. The search strategy terms included, among others, bariatric surgery, hormones, fertility, malformations, miscarriages and vitamin status. We searched for human studies that were written in English. Abstracts, reviews, meta-analyses and conference papers were excluded. The ErasmusAGE score was used to assess the quality of the included studies.
OUTCOMES
A total of 51 articles were analysed. The mean quality score was 5 (range 2–8). After bariatric surgery, hormonal axes normalized and menstrual cycle regularity was restored, resulting in increased fertility. Overall, there were no short-term risks for reproductive outcomes such as the increased risk of miscarriages or congenital malformations. However, the risk of vitamin deficiencies was generally increased after bariatric surgery. A meta-analysis of 20 studies showed a significant decrease in infertility (risk difference (RD) −0.24, 95% confidence interval (CI) −0.42, −0.05) and menstrual cycle irregularities (RD −0.24, 95% CI −0.34, −0.15) with no difference in rates of miscarriage (RD 0.00, 95% CI −0.09, 0.10) and congenital malformations (RD 0.01, 95% CI −0.02, 0.03).
WIDER IMPLICATIONS
The current systematic review and meta-analysis show associations between bariatric surgery and periconception maternal health and underlines the need for providing and personalizing preconception care for women after bariatric surgery. We recommend preconception care including the recommendation of postponing pregnancy until weight loss has stabilized, irrespective of the surgery-to-pregnancy interval, and until vitamin status is normalized. Therefore, regular monitoring of vitamin status and vitamin supplementation to restore deficiencies is recommended. Furthermore, this systematic review emphasizes the need for a long-term follow-up research of these women from the periconception period onwards as well as their pregnancies and offspring, to further improve care and outcomes of these mothers and children.
Keywords: female infertility, menstrual cycle, hormones, vitamins, periconception, pregnancy, congenital malformations, bariatric surgery, abortion, malnutrition
Introduction
The prevalence of obesity, defined as a BMI of ≥30 kg/m2, is increasing worldwide (Finucane et al., 2011). Globally, 34% of women of reproductive age are obese. The solution to counteract the obesity epidemic has been weight loss by adopting a healthy lifestyle including proper nutrition and regular exercise. Unfortunately, these behavioural changes have shown to have a limited impact since they tend to be unsustainable in the long term (Wadden et al., 2005, 2011; Appel et al., 2011).
Maternal obesity is associated with subfertility and severe adverse pregnancy outcomes, e.g. pre-eclampsia and preterm birth (Mission et al., 2015). Importantly, maternal obesity also increases the prevalence of childhood obesity in the offspring, which can increase the risk for cardiovascular diseases in later life (Yu et al., 2013). One of the factors that may reduce this risk is adopting a healthy maternal lifestyle before, during and after pregnancy (Dhana et al., 2018a,b). During this period, healthy nutrition, including appropriate vitamin levels, is vital for optimal fertilization and for preventing congenital malformations, miscarriage and fetuses which are small for gestational age (Boxmeer et al., 2008; Obermann‐Borst et al., 2011; Hovdenak and Haram, 2012; Parisi et al., 2017; Hoek et al., 2020). Moreover, vitamin deficiencies are associated with adverse periconception maternal health (de Weerd et al., 2003; Hague, 2003; Steegers-Theunissen and Steegers, 2003; Ebisch et al., 2007; Kloss et al., 2018). In addition, intra-uterine malnutrition, as investigated in the Dutch famine birth cohort during World War II, can increase the risk of cardiovascular diseases in later life (Heijmans et al., 2008; Roseboom et al., 2011).
Bariatric surgery, already introduced around the year 1950 to treat obesity, is currently envisioned as the only effective, long-term therapy for obesity. Worldwide, in order to qualify for bariatric surgery, a patient must have a BMI >40 kg/m2 or a BMI >35 kg/m2 with at least one obesity-related comorbidity, such as hypertension or diabetes mellitus (Fried et al., 2014). Between 2013 and 2018, almost 400 000 registered patients have undergone bariatric surgery in 51 different countries. Notably, the prevalence of these procedures is increasing in women of childbearing age: 73.7% of bariatric patients are female, with 83% of these patients undergoing bariatric surgery during their reproductive period (Maggard et al., 2008; Welbourn et al., 2019).
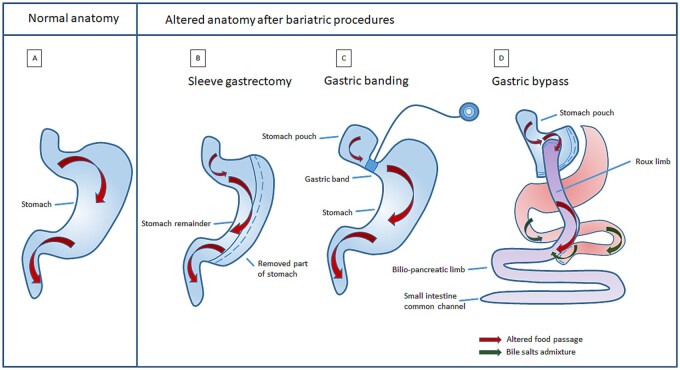
Over the years, different bariatric surgery procedures have been introduced, resulting in either restrictive and/or malabsorptive anatomical changes (Fig. 1). Chronologically, the following bariatric surgery procedures have been performed: jejuno-ileal bypass, Roux-en-Y gastric bypass (RYGB) (which started in the late seventies), vertical banded gastroplasty, biliopancreatic diversion (BPD), duodenal switch, (adjustable) gastric banding and sleeve gastrectomy (Buchwald, 2014). Due to high numbers of post-surgical complications and vitamin deficiencies, jejuno-ileal bypass has been replaced by RYGB.
Figure 1.
Normal anatomy versus situation after three different bariatric surgical procedures. (A) Normal anatomy. (B) Sleeve gastrectomy: restrictive procedure in which the main corpus of the stomach is resected, reducing gastric volume. (C) Gastric banding: restrictive procedure in which food intake is reduced by decreasing gastric volume and limiting the amount of food that can pass through the inflatable band restricted stomach. (D) Gastric bypass: combined procedure (both restrictive and malabsorptive) in which only a small stomach pouch remains. The food bypasses the rest of the stomach and the duodenum via the Roux limb and enters directly into the small intestines.
Currently, gastric bypass and sleeve gastrectomy are the most performed types of bariatric surgery. Table I shows an overview of bariatric surgery procedures performed worldwide between 2015 and 2018 (Ramos et al., 2019).
Table I.
Overview of the number of bariatric surgery procedures worldwide (Ramos et al., 2019 ).
| Total number per bariatric surgery procedure | Percentage of total number of bariatric surgery procedures (%) | |
|---|---|---|
| Sleeve gastrectomy (restrictive procedure) | 305 242 | 58.6 |
| Gastric banding (restrictive procedure) | 19 255 | 3.7 |
| Duodenal switch (malabsorptive procedure) | 2642 | 0.5 |
| Biliopancreatic diversion (malabsorptive procedure) | 190 | 0.0 |
| Gastric bypass (combined procedure) | 184 860 | 35.6 |
| Other or unspecified | 8794 | 1.7 |
| All | 520 983 |
Bariatric surgery procedures
Restrictive procedures
Sleeve gastrectomy (Fig. 1B) uses vertical staples to permanently reduce the stomach size and is often considered a restrictive procedure, although its metabolic effects have been well established (Benaiges Foix et al., 2015). Restrictive procedures can lead to vitamin deficiencies due to highly limited caloric intake and increased risk of vomiting (Xanthakos, 2009).
Gastric banding (Fig. 1C) (temporarily) reduces the stomach size, thereby restricting gastric volume and limiting the amount of food intake.
Malabsorptive procedures
Malabsorptive procedures such as BPD and the abandoned jejuno-ileal bypass partially bypass the small intestines. After breakdown in the stomach, fat is digested and absorbed in the small intestines. Since the small intestines are also responsible for 95% of the uptake of vitamins and minerals, intestinal uptake after the aforementioned bariatric techniques is severely compromised, especially concerning fat-soluble vitamins (Caspary, 1992).
Combined procedures
More recent procedures such as RYGB (Fig. 1D) are both restrictive, as a small stomach pouch is created, and malabsorptive. These procedures create a bypass to the duodenum and proximal jejunum, in which the majority of the absorption of micronutrients (minerals and vitamins) takes place (Andari Sawaya et al., 2012). Intrinsic factor (IF), produced by the parietal cells (located in the fundus and corpus of the stomach), binds to vitamin B12 in the duodenum and is essential for the uptake of vitamin B12 in the terminal ileum. By bypassing the duodenum, IF-binding is lacking and absorption of vitamin B12 is therefore impaired, leading to vitamin B12 deficiencies and an essential need for supplementation. The reduced gastric volume in combination with bypassed small intestines also results in suboptimal digestion of food and iron uptake. As iron is absorbed in the duodenum and upper jejunum, this can increase the risk of iron deficiency (Abbaspour et al., 2014).
Bariatric surgery and periconception health
The periconception period is defined as the period from 14 weeks before until 10 weeks after conception (Steegers-Theunissen et al., 2013). Although obesity negatively affects periconception maternal health, the therapeutic anti-obesity option of bariatric surgery itself may also have a negative impact on periconception maternal health. The altered intestinal anatomy impairs absorptive capacities resulting in iatrogenic malnutrition. In turn, this results in a different metabolic homeostasis postoperatively (Andari Sawaya et al., 2012).
Gametogenesis and embryonic, fetal and placental development take place during the periconception period, which has consequences for the course of pregnancy and for neonatal and offspring health outcomes. The influence of bariatric surgery during this critical period is essential to improve clinical care and prevention of diseases from early life onwards.
In this review, we focus on periconception outcome measures, as this is a new topic that has not been reviewed systematically before. Systematic reviews regarding the association between bariatric surgery and pregnancy complications have already been published (Maggard et al., 2008; Falcone et al., 2018; Kwong et al., 2018; Akhter et al., 2019; Al-Nimr et al., 2019; Shawe et al., 2019). We will provide an overview of the influence of bariatric surgery on different periconception maternal health parameters within the current systematic review. An additional meta-analysis was performed on the associations between bariatric surgery and fertility, menstrual cycle regularity, miscarriages and congenital malformations.
Methods
Protocol and registration
The protocol for this systematic review was designed and registered a priori at the PROSPERO registry (PROSPERO 2019: CRD42019130788).
Information sources and search terms
Searches were performed in the databases of Medline, Embase, PubMed, Web of Science, Google Scholar and the Cochrane databases. The search strategy terms used the following MeSH-terms including but not limited to: bariatric surgery, hormones, fertility, malformations, miscarriages and vitamin status (Supplementary File S1). These were combined using the Boolean operator ‘or’. The authors of the included articles were contacted if additional information was needed. These databases were used for the literature search until 1 November 2020.
Inclusion and exclusion criteria
Studies that investigated women undergoing bariatric surgery were included. Topics of interest during this period therefore included endocrine changes, fertility, vitamin status, irregular menstrual cycles, miscarriages and congenital malformations.
All articles discussing endocrine changes after bariatric surgery were included. Fertility was considered as the chance of becoming pregnant spontaneously or the need for and success of assisted reproductive technology (ART). Post-bariatric vitamin status before conception and during the first trimester was included. Irregular menstrual cycles before and after bariatric surgery were included. Studies investigating the association between bariatric surgery and a difference in the prevalence of only first-trimester miscarriage were included. As most congenital malformations develop within the first 10 weeks of fetal development (Polifka and Friedman, 2002), the association between bariatric surgery and the prevalence of congenital malformations was also studied. All types of bariatric surgery were included. Only human studies, written in English and articles that could be accessed in full text were included in this systematic review and meta-analysis. Moreover, systematic reviews, meta-analyses, expert opinions, conference meeting papers and abstracts were excluded. As polycystic ovary syndrome (PCOS) may influence the associations between bariatric surgery and the outcome parameters, we excluded articles that only included PCOS patients.
Study selection, full-text review and data extraction
All articles were independently assessed on subject, analysis and data extraction based on title, abstract and full text of the articles by K.M.S. and S.S. Full-text articles that were assessed were summarized in a template regarding country of research, year of publication, study design, study population, sample size, geographical background of study patients, type of bariatric surgery, topics of interest (i.e. endocrine changes, fertility, vitamin status, irregular menstrual cycles, miscarriages and congenital malformations), outcome data, exclusion criteria, statistical analysis, confounders, results, conclusion and ErasmusAGE score (National Collaborating Centre for Methods and Tools, 2008).
Differences in the inclusion or exclusion of articles were resolved by discussion between K.M.S. and S.S. The PRISMA guidelines for systematic reviews and meta-analysis protocols were followed (Shamseer et al., 2015).
Quality score and risk of bias
The ErasmusAGE quality score (specifically designed for systematic reviews) was used to score the quality of the articles that were selected based on the full text (National Collaborating Centre for Methods and Tools, 2008) (Supplementary File S2). These articles were scored on five items and each item was scored between 0 and 2 points resulting in a total score of minimum 0 and maximum 10 points (a score of 10 representing the highest quality). These items include study design (cross-sectional study = 0, longitudinal study = 1, intervention study = 2), study size (<50 patients = 0, 50–100 patients = 1, >100 patients = 2), quality of exposure measurement (no appropriate exposure (type of bariatric surgery not specified) = 0, moderate quality exposure (type of bariatric surgery described, but not in detail) = 1, adequate exposure (procedures of bariatric surgery described in detail) = 2), method of outcome measurement (no appropriate outcome i.e. outcomes not specified) = 0, moderate quality outcome (outcomes described, but not in detail) = 1, adequate outcome (outcome described in detail, with reference) = 2 and adjustments for confounders (no adjustment = 0, adjustment for key confounders (BMI, age) = 1, adjustment for additional covariates or extra confounders = 2). The studies were divided into low (ErasmusAGE quality score ≤5) and high (ErasmusAGE quality score ≥6) quality score articles.
Meta-analysis
To estimate the effect sizes of bariatric surgery on periconception maternal health, a meta-analysis was performed on the following: fertility, menstrual cycle, miscarriages and congenital malformations. Adverse outcomes were defined as infertility, irregular menstrual cycles, miscarriages and congenital malformations.
In case-control studies, the numbers of patients with and without an adverse outcome were collected as post-bariatric patients and controls without bariatric surgery.
In before-after studies, the numbers of pre-bariatric patients with and without an adverse outcome were collected from the articles. From the pre-bariatric group with an adverse outcome, the numbers of patients with and without an adverse outcome after surgery were collected.
Next, all of the above collected patient numbers from both study types were pooled using a random-effects model and used to calculate the risk difference (RD) associated with the effect of surgery and the standard error of the RD.
The estimate of the between-study variance was based on maximum-likelihood estimates. For the proportion of adverse outcomes, we used the estimation method of Stijnen et al. (201 (Stijnen et al., 2010) since it corrects for the correlation between the estimations and standard errors. We report the pooled effect, together with a 95% confidence interval and the estimated study heterogeneity (I2). The analyses were performed using R version 4.03 and the meta-package (version 4.15-1).
Concerning other periconception parameters considered in the current systematic review, not enough information was available to perform a meta-analysis or there was no proper control group.
Results
Study selection
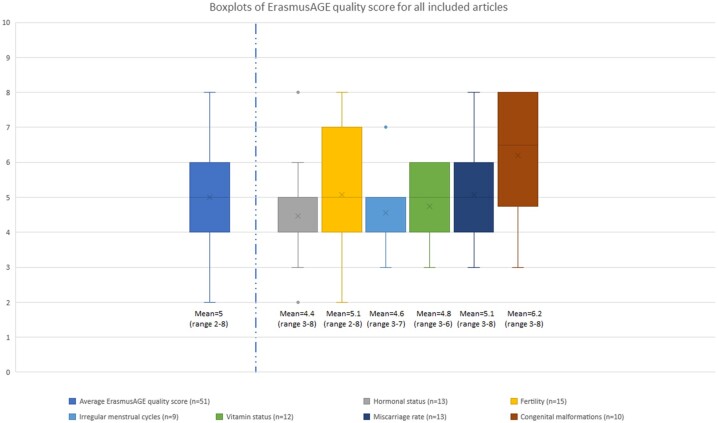
Figure 2 shows the ErasmusAGE score per periconception parameter. The median quality score of all included studies is 5 (range 2–8). The flowchart summarizes the process of literature search and selection of studies (Fig. 3).
Figure 2.
Boxplots of ErasmusAGE quality score for all included articles (n = 51) and divided per outcome. The score assesses included articles on quality, divided per periconception parameter: hormonal status, fertility, irregular menstrual cycles, vitamin status, miscarriage rate, congenital malformations. The boxplot shows median values for the ErasmusAGE score with minimum and maximum values.
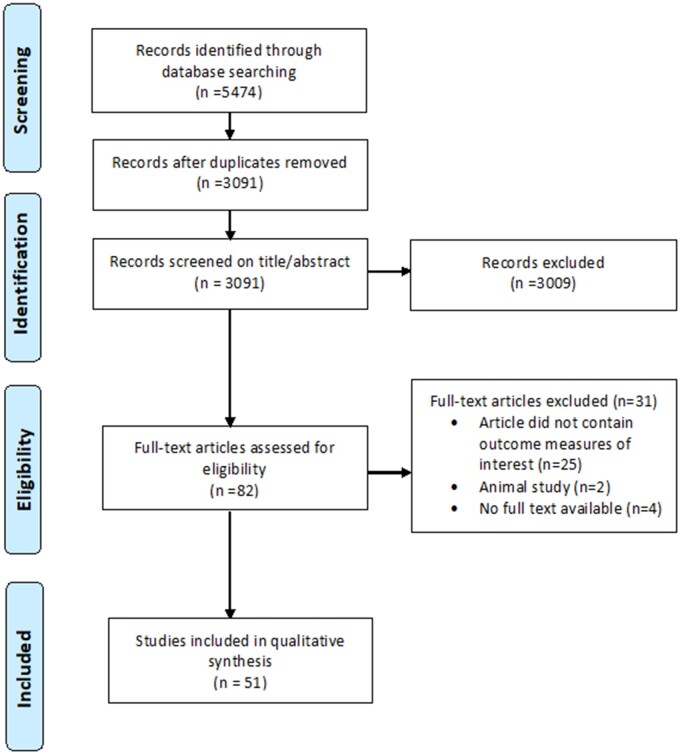
Figure 3.
Flowchart of included and excluded studies.
The initial search identified 5474 articles of which 3091 remained after removing duplicates. After this, 3009 articles were excluded based on the previously discussed selection criteria. The full text of 82 articles was read and 31 articles were excluded, resulting in 51 articles to be analysed.
The range of sample sizes varied widely, ranging from 7 to 2 194 348 study cases. There were 6 articles that addressed malabsorptive procedures, 11 focused on restrictive surgery and 37 investigated a combination of surgeries or did not specify the type of surgery. Three articles separately described outcomes for different types of surgery within one article and will therefore be discussed per type of surgery.
In order to weigh the data from the included articles and correctly interpret the available clinical evidence, we decided to divide the articles into high- and low-quality score articles, as assessed by the ErasmusAGE score (≥6 is regarded as high, ≤5 as low). High-quality score articles are discussed in more detail in the result sections. Table II describes the main characteristics of all included studies, ordered based on the ErasmusAGE score, while Tables III, IV and V each give an overview of the associations between malabsorptive, restrictive and combined surgery and the investigated periconception parameters, respectively. Finally, Supplementary Table SI shows a summary of the quantitative results of all included studies (n = 51), per individual study.
Table II.
Main characteristics of the included studies concerning different types of bariatric surgery and periconception outcomes of maternal health.
| First author (year) | Study type | Sample size | Cases | Controls | What surgery* | Type of surgery | Outcome(s) | ErasmusAGE quality score |
|---|---|---|---|---|---|---|---|---|
| Auger N (2019) | Case-control | 2 194 348 | Preconceptional bariatric surgery | Non-obese women with no surgery | Restrictive, malabsorptive, unspecified | Combined | Congenital malformations | 8 |
| Grzegorczyk-Martin V (2020) | Case-control | 332 | Group 1: Bariatric surgery before IVF | Group 1: No bariatric surgery before IVF, BMI-matched to Group 1. Group 2: No bariatric surgery before IVF, obese. | SG, gastric banding, gastric bypass | Combined | Miscarriages, fertility, hormonal levels | 8 |
| Josefsson A (2013) | Case-control study | 244 612 | Firstborns after maternal bariatric surgery | Firstborns without maternal bariatric surgery | Gastroplasty, gastric banding, gastric bypass | Restrictive, combined | Congenital malformations | 8 |
| Menke MN (2019) | Before-after study | 650 | Post-bariatric women with and without a history of infertility | The same women before surgery |
RYGB, AGB, SG, BPD |
Combined | Fertility | 8 |
| Parent B (2017) | Case-control study | 10 296 | All mothers with a history of a bariatric operation at any time before conception | Population-based random sample of Washington State mothers and their infants |
Banded gastroplasty, adjustable gastric banding, sleeve gastrectomy or Roux-en-Y gastric bypass |
Combined | Congenital malformations | 8 |
| Goldman RH (2016) | Case-control study | 219 | Women after bariatric surgery | Obese women without bariatric surgery | RYGB, AGB | Combined, restrictive | Fertility, irregular menstrual cycles, miscarriages | 7 |
| Neovius M (2019) | Case-control study | 33 494 | Infants born to women after bariatric surgery | Infants born to women without bariatric surgery |
RYGB |
Combined | Congenital malformations | 7 |
| Sheiner E (2004) | Case-control study | 298 | Pregnancies after bariatric surgery | Pregnancies without previous bariatric surgery | Not specified | Not specified | Fertility, congenital malformations | 7 |
| Christofolini J (2014) | Case-control study | 180 | Women after bariatric surgery | Group 1: Obese patients without bariatric surgery. Group 2: Women 18<BMI < 29.9 with infertility due to male factor | Restrictive and/or malabsorptive | Combined | Fertility | 6 |
| De Carolis S (2018) | Before-after study | 65 | Women who were both pregnant before and after bariatric surgery, aged ≥18 years | The same women before bariatric surgery | RYGB, BPD, AGB, SG | Combined, malabsorptive, restrictive | Miscarriage rate, congenital malformations | 6 |
| Devlieger R (2014) | Comparative study (restrictive vs malabsorptive/combined surgery) | 49 | Women aged >18 years after restrictive surgery | Women aged >18 years after malabsorptive/combined surgery | Restrictive, malabsorptive, combined (not further specified) | Restrictive, malabsorptive, combined | Vitamin status | 6 |
| Lapolla A (2010) | Case-control study and before-after study | 69 | Women after bariatric surgery | Group 1: The same women before bariatric surgery Group 2: Women BMI > 40 without bariatric surgery | LAGB | Restrictive | Miscarriages | 6 |
| Machado SN (2016) | Case-control study | 120 | Women after bariatric surgery | Women without bariatric surgery | RYGB | Combined | Vitamin status | 6 |
| Mead NC (2014) | Before-after study | 149 | Women after bariatric surgery | The same women before bariatric surgery | BPD, RYGB, SG | Malabsorptive, combined, restrictive | Vitamin status | 6 |
| Medeiros M (2016) | − (no control group, no before-after study) | 46 | Adult pregnant women after bariatric surgery | – | RYGB | Combined | Vitamin status | 6 |
| Nilsson-Condori E (2018) | Before-after study | 48 | Women aged 18–35 years, undergoing RYGB | The same women before bariatric surgery | RYGB | Combined | Endocrine changes | 6 |
| Sheiner E (2006) | Case-control study | 159 210 | Women after bariatric surgery | Women without bariatric surgery | Gastric banding, vertical gastroplasty, RYGB | Restrictive, combined | Fertility, miscarriages, congenital malformations | 6 |
| Bebber FE (2011) | − (no control group, no before-after study) | 39 | Women after bariatric surgery | – | RYGB | Combined | Vitamin status | 5 |
| Deitel M (1988) | Before-after study | 109 | Morbidly obese women with >50% excess weight loss after bariatric surgery | Same women before bariatric surgery | Jejuno-ileal bypass, gastroplasty | Malabsorptive | Irregular menstrual cycles, miscarriages | 5 |
| Di Carlo C (1999) | Case-control study | 8 | Women after bariatric surgery | Healthy normal-weight women | BPD | Malabsorptive | Endocrine changes | 5 |
| Edison E (2016) | Case-control study | 144,99 | Women after bariatric surgery, aged 18–45 years | Women without bariatric surgery, BMI > 40 | RYGB, gastric banding, SG, balloon, other, unspecified, BPD, duodenal switch | Combined, restrictive, malabsorptive | Irregular menstrual cycles | 5 |
| Gadgil MD (2014) | Before-after study | 794 | Women after bariatric surgery | Women before bariatric surgery | Gastric bypass, gastric banding, restrictive other, BPD, unknown | Restrictive, malabsorptive, combined | Vitamin status | 5 |
| Gerrits EG (2003) | Before-after study | 40 | Women aged 16–44 years after bariatric surgery | Same women before bariatric surgery | BPD | Malabsorptive | Endocrine changes, irregular menstrual cycles | 5 |
| Günakan E (2020) | Comparative study (early vs late post-bariatric pregnancy) | 23 | Pregnant <1 year after SG | Pregnant >1year after SG | SG | Restrictive | Vitamin status | 5 |
| Hazart J (2017) | − (no control group, no before-after study) | 57 | Women after bariatric surgery | – | SG, gastric bypass, gastric banding | Restrictive, combined | Vitamin status | 5 |
| Musella M (2011) | Before-after study | 27 | Women after bariatric surgery | The same women before bariatric surgery | Intragastric balloon | Restrictive | Fertility | 5 |
| Nørgaard LN (2014) | Case-control study | 57 755 | Women after bariatric surgery | General Danish population without bariatric surgery | Gastric bypass | Combined | Fertility | 5 |
| Musella M (2011) | Case-control study | 280 | Morbidly obese women after bariatric surgery | Women without bariatric surgery | RYGB | Combined | Miscarriages, congenital malformations | 5 |
| Pilone V (2014) | Before-after study | 53 | Women before SG | Women 6 months after SG | SG | Restrictive | Hormonal levels, irregular cycles | 5 |
| Alatishe A (2013) | Before-after study | 21 | Women aged 18–45 years after bariatric surgery | The same women before bariatric surgery | RYGB, gastric banding, SG, other (not specified) | Combined, restrictive | Miscarriages | 4 |
| Basbug A (2019) | Comparative study (early vs late pregnancy after bariatric surgery) | 23 | Women after LSG | Group 1: Pregnant <18 months after SG, Group 2: pregnant >18 months after SG | SG | Restrictive | Congenital malformations | 4 |
| Chagas C (2016) | − (no control group, no before-after study) | 30 | Women after bariatric surgery | – | RYGB | Combined | Vitamin status | 4 |
| Cruz S (2019) | Comparative study (early vs intermediate vs late pregnancy after bariatric surgery) | 42 | Pregnant <1 year after RYGB | Group 1: Pregnant >12 and <24 months after RYGB, Group 2: Pregnant >24 months after RYGB | RYGB | Malabsorptive | Miscarriages | 4 |
| Dao T (2006) | Comparative study (early vs late pregnancy after bariatric surgery) | 34 | Pregnancies <1 year after bariatric surgery | Pregnancies >1 year after bariatric surgery | RYBG | Combined | Fertility, vitamin status | 4 |
| Hey H (1981) | Before-after study | 38 | Women aged 21–39 years after bariatric surgery | Same women before bariatric surgery | Jejuno-ileostomy | Malabsorptive | Irregular menstrual cycles | 4 |
| Jans G (2014) | Comparative study (restrictive vs malabsorptive/combined surgery) | 49 | Women after restrictive surgery | Women after malabsorptive/combined surgery | Restrictive, malabsorptive, combined (not further specified) | Combined | Vitamin status | 4 |
| Kjær MM (2017) | Before-after study | 23 | Women after bariatric surgery | The same women before bariatric surgery | RYGB | Combined | Endocrine changes, irregular menstrual cycles | 4 |
| Kruchinin EV (2018) | Before-after study | 45 | Women before bariatric surgery | Women after bariatric surgery | BPD, SG, AGB | Combined | Irregular cycles, hormonal levels | 4 |
| Merhi ZO (2008) | Before-after study | 16 | Morbidly obese women after bariatric surgery | Women before bariatric surgery | RYGB | Combined | Endocrine changes | 4 |
| Dell'Agnolo CM (2015) | Before-after study | 32 | Women after bariatric surgery | The same women before bariatric surgery | Not specified | Not specified | Miscarriages | 4 |
| Milone M (2017) | Before-after study | 40 | Women after bariatric surgery | The same women before bariatric surgery | SG | Restrictive | Endocrine changes, fertility | 4 |
| Neto RML (2012) | Before-after study | 9 | Women after bariatric surgery | The same women before bariatric surgery | RYGB | Combined | Fertility | 4 |
| Rochester D (2009) | Case-control study and before-after study | 23 | Women after bariatric surgery |
|
Gastric banding, gastric bypass | Restrictive, combined | Endocrine changes | 4 |
| Sahab Al kabbi M (2018) | Before-after study | 60 | Women in reproductive age groups between 18 and 40 years old, with a BMI >36 kg/m2 without medical diseases or hormonal abnormality, after bariatric surgery | The same women before bariatric surgery | Gastric sleeve or gastric bypass | Combined | Endocrine changes, irregular menstrual cycles | 4 |
| Tsur A (2014) | Before-after study | 7 | Women after bariatric surgery | The same women before bariatric surgery | SG, gastric banding | Restrictive | Fertility | 4 |
| Karadağ C (2020) | Comparative study (early and late pregnancy after surgery) and case-control study | 144 | Pregnant <1 year after SG | Group 1: Pregnant >1year after SG Group 2: Non-bariatric, BMI >30 | SG | Restrictive | Congenital malformations | 3 |
| Khazraei H (2017) | Before-after study | 15 | Women after bariatric surgery | The same women before bariatric surgery | SG | Restrictive | Fertility, irregular menstrual cycles, miscarriages | 3 |
| Sapre N (2009) | − (no control group, no before-after study) | 17 | Women after bariatric surgery | – | RYGB | Combined | Fertility, vitamin status, miscarriages | 3 |
| Vincentelli C (2018) | Before-after study | 39 | Women of reproductive age (18–45 years) that underwent bariatric surgery | The same women before bariatric surgery | RYGB, SG | Combined | Endocrine changes | 3 |
| Nayak R (2020) | Comparative study (women with and without PCOS after surgery) | 28 | Women with PCOS after bariatric surgery | Women without PCOS after bariatric surgery | SG | Not described | Fertility, hormonal levels | 2 |
| Vieira APPS (2020) | Before-after study | 49 | Women before bariatric surgery | Women after bariatric surgery | SG, RYGB | Combined | Fertility | 2 |
AGB, adjustable gastric banding; BPD, biliopancreatic diversion; LAGB, laparoscopic adjustable gastric banding; PCOS, polycystic ovary syndrome; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Table III.
Description and summary of data from studies that investigated associations between malabsorptive surgery and periconception outcomes of maternal health.
| Author | Type of bariatric surgery | Studied periconception outcome | Fertility | Irregular menstrual cycles | Vitamin status | Endocrine changes | Miscarriages | Congenital malformations | ErasmusAGE quality score |
|---|---|---|---|---|---|---|---|---|---|
| De Carolis S (2018) | BPD* | Miscarriages, congenital malformations | = | = | 6 | ||||
| Mead NC (2014) | BPD | Vitamin status |
|
6 | |||||
| Deitel M (1988) | Jejuno-ileal bypass, gastroplasty | Irregular menstrual cycles, miscarriages | ↓ | ↑ | 5 | ||||
| Di Carlo C (1999) | BPD | Endocrine changes |
|
5 | |||||
| Gerrits EG (2003) | BPD | Endocrine changes, irregular menstrual cycles | = |
|
5 | ||||
| Hey H (1981) | Jejuno-ileostomy | Irregular menstrual cycles | ↑ | 4 |
*BPD, biliopancreatic diversion.
↓ indicates a decrease in the occurrence of the periconception parameter of interest, ↑ indicates an increase in the occurrence of the periconception parameter of interest and = indicates no change.
Table IV.
Description and summary of data from studies that investigated associations between restrictive surgery and periconception outcomes of maternal health.
| Author | Type of bariatric surgery | Studied periconception outcome | Fertility | Irregular menstrual cycles | Vitamin status | Endocrine changes | Miscarriages | Congenital malformations | ErasmusAGE quality score |
|---|---|---|---|---|---|---|---|---|---|
| Goldman RH (2016) | AGB* | Fertility, irregular menstrual cycles, miscarriages | ↑ | ↓ | = | 7 | |||
| Lapolla A (2010) | AGB | Miscarriage | ↑ | 6 | |||||
| Mead NC (2014) | SG* | Vitamin status |
|
6 | |||||
| Günakan E (2020) | SG | Vitamin status | = (Vitamin D) | 5 | |||||
| Musella M (2011) | Intragastric balloon | Fertility | ↑ | 5 | |||||
| Pilone V (2014) | SG | Hormonal levels, irregular menstrual cycles | ↓ | ↑ (AMH) | 5 | ||||
| Basbug A (2019) | SG | Congenital malformations | = | 4 | |||||
| Milone M (2017) | SG | Endocrine changes, fertility | ↑ | = (FSH, AMH) | 4 | ||||
| Tsur A (2014) | SG, gastric banding | Fertility | ↑/= | = (Oestradiol) | 4 | ||||
| Karadağ C (2020) | SG | Congenital malformations | = | 3 | |||||
| Khazraei H (2017) | SG | Fertility, irregular menstrual cycles, miscarriages | ↑ | ↓ | = | 3 |
AGB, adjustable gastric banding; SG, sleeve gastrectomy.
↓ indicates a decrease in the occurrence of the periconception parameter of interest, ↑ indicates an increase in the occurrence of the periconception parameter of interest and = indicates no change.
Table V.
Description and summary of data from studies that investigated the effect of combined or unspecified types of surgery on periconception outcomes and maternal health.
| Author | Type of bariatric surgery | Studied periconception outcome | Fertility | Irregular menstrual cycles | Vitamin status | Endocrine changes | Miscarriages | Congenital malformations | ErasmusAGE quality score |
|---|---|---|---|---|---|---|---|---|---|
| Auger N (2019) | Restrictive, malabsorptive, unspecified | Congenital malformations | = | 8 | |||||
| Grzegorczyk-Martin V (2020) | SG*, gastric banding, gastric bypass | Miscarriages, fertility, hormonal levels | = | = (AMH) | = | 8 | |||
| Josefsson A (2013) | Gastroplasty, gastric banding, gastric bypass | Congenital malformations | = | 8 | |||||
| Menke MN (2019) | RYGB*, AGB*, SG, BPD* | Fertility | ↑ | 8 | |||||
| Parent B (2017) | Banded gastroplasty, AGB, SG, RYGB | Congenital malformations | = | 8 | |||||
| Goldman RH (2016) | RYGB | Fertility, irregular menstrual cycles, miscarriages | =/↑ | ↓ | ↑ | 7 | |||
| Neovius M (2019) | RYGB | Congenital malformations | ↓ | 7 | |||||
| Sheiner E (2004) | Not specified | Fertility, congenital malformations | ↓ | = | 7 | ||||
| Christofolini J (2014) | Restrictive and/or malabsorptive (not further specified) | Fertility | ↓/= | 6 | |||||
| Devlieger R (2014) | Restrictive, malabsorptive, combined (not further specified) | Vitamin status | = (Vitamin B1, B12, folate, D, E) | 6 | |||||
| Machado SN (2016) | RYGB | Vitamin status | ↑ (more retinol and beta-carotene deficiencies) | 6 | |||||
| Medeiros M (2016) | RYGB | Vitamin status | ↑ (more vitamin D deficiencies) | 6 | |||||
| Mead NC (2014) | BPD, RYGB, SG | Vitamin status |
|
6 | |||||
| Nilsson-Condori E (2018) | RYGB | Endocrine changes |
|
6 | |||||
| Sheiner E (2006) | Gastric banding, vertical gastroplasty, RYGB | Fertility, miscarriages, congenital malformations | ↓ | = | = | 6 | |||
| Bebber FE (2011) | RYGB | Vitamin status | ↑ (more vitamin B12 and folate deficiencies) | 5 | |||||
| Edison E (2016) | RYGB, gastric banding, SG, balloon, other, unspecified, BPD, duodenal switch | Irregular menstrual cycles | ↓ | 5 | |||||
| Gadgil MD (2014) | Gastric bypass, gastric banding, restrictive other, BPD, unknown | Vitamin status | ↑ (more vitamin B1, B12, folate, D deficiencies) | 5 | |||||
| Hazart J (2017) | SG, gastric bypass, gastric banding | Vitamin status | ↑ (more vitamin A, B1, B6, B9, B12, C, D deficiencies) | 5 | |||||
| Nørgaard LN (2014) | Gastric bypass | Fertility | = | 5 | |||||
| Patel JA (2008) | RYGB | Miscarriages, congenital malformations | = | = | 5 | ||||
| Alatishe A (2013) | RYGB, gastric banding, SG, other (not specified) | Miscarriages | ↓ | 4 | |||||
| Chagas C (2016) | RYGB | Vitamin status | ↑ (more retinol, beta carotene deficiencies) | 4 | |||||
| Cruz S (2019) | RYGB | Miscarriages | = | 4 | |||||
| Dao T (2006) | RYBG | Fertility, vitamin status | ↑ | = (Vitamin B1 and B12) | 4 | ||||
| Jans G (2014) | Restrictive, malabsorptive, combined (not further specified) | Vitamin status | ↑ (more vitamin K deficiencies) | 4 | |||||
| Kjær MM (2017) | RYGB | Endocrine changes, irregular menstrual cycles |
|
4 | |||||
| Kruchinin EV (2018) |
BPD, AGB, SG |
Irregular cycles, hormonal levels |
↓ |
|
4 | ||||
| Merhi ZO (2008) | RYGB | Endocrine changes |
|
4 | |||||
| Dell'Agnolo CM (2015) | Not specified | Miscarriages | = | 4 | |||||
| Neto RML (2012) | RYGB | Fertility | ↑ | 4 | |||||
| Rochester D (2009) | Gastric banding, gastric bypass | Endocrine changes, irregular cycles | = |
|
4 | ||||
| Sahab Al kabbi M (2018) | SG, gastric bypass | Endocrine changes, irregular menstrual cycles | ↓ | ↓ (AMH) | 4 | ||||
| Sapre N (2009) | RYGB | Fertility, vitamin status, miscarriages | ↑ | ↑ (more vitamin B12, folate deficiencies) | ↓ | 3 | |||
| Vincentelli C (2018) | RYGB, SG | Endocrine changes | ↓ (AMH) | 3 | |||||
| Nayak R (2020) | SG |
Fertility, hormonal levels |
= (AMH) | 2 | |||||
| Vieira APPS (2020) | SG, RYGB | Fertility | ↑ | 2 |
SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; AGB, adjustable gastric banding; BPD, biliopancreatic diversion.
↓ indicates a decrease in the occurrence of the periconception parameter of interest, ↑ indicates an increase in the occurrence of the periconception parameter of interest and = indicates no change.
Malabsorptive procedures
BPD, jejuno-ileal bypass and jejuno-ileostomy are malabsorptive procedures of bariatric surgery.
Endocrine changes
High-quality studies
No high-quality studies investigated this outcome.
Low-quality studies
Hormonal levels decreased postoperatively at the onset of amenorrhoea regarding LH and oestradiol, whereas FSH, testosterone and dehydroepiandrosterone sulphate (DHEA-S) increased and androstenedione remained the same (Di Carlo et al., 1999). Seven days postoperatively, hormonal levels increased with respect to sex hormone-binding globulin (SHBG) serum levels and decreased concerning free testosterone and DHEA-S (Gerrits et al., 2003).
Summary
LH and oestradiol decreased, FSH and SHBG levels increased, testosterone and DHEA-S increased or decreased and androstenedione levels remained similar.
Fertility
The influence of malabsorptive procedures on fertility has not been studied by the included articles.
Vitamin status
High-quality studies
One article reported significantly higher post-bariatric folate levels following folic acid supplementation and lower vitamin B12 serum levels in the first trimester (mean ± SD 5.8 ± 3.0 vs 15.8 ± 7.7 ng/ml, P < 0.001 and 428 ± 273 vs 239 ± 134 pg/ml, P < 0.001, respectively) (Mead et al., 2014).
Low-quality studies
This outcome was not evaluated by low-quality studies.
Summary
Postoperatively vitamin B12 levels decreased, whereas folate levels increased.
Irregular menstrual cycles
High-quality studies
The change in menstrual cycle regularity was not investigated.
Low-quality studies
Irregular menstrual cycles became regular in one of the three included articles (Hey and Niebuhr-Jorgensen, 1981; Deitel et al., 1988; Gerrits et al., 2003).
Summary
There was no consistent effect on irregular menstrual cycles.
Miscarriages
High-quality studies
De Carolis et al. observed no significant differences between women who had undergone BPD compared to the same women before surgery regarding miscarriages (12.9% vs 15.7%, P = 0.730) (De Carolis et al., 2018).
Low-quality studies
Miscarriage rates increased (Deitel et al., 1988).
Summary
Generally, miscarriage rates either increased or remained similar.
Congenital malformations
High-quality studies
There was no association with congenital malformations (11.1% before surgery vs 30.2% after surgery, P = 0.064) (De Carolis et al., 2018).
Low-quality studies
The included low-quality studies did not research this outcome.
Summary
There was no significant influence of malabsorptive surgery on congenital malformations.
Restrictive procedures
Restrictive procedures of bariatric surgery include gastric banding, intragastric balloon and sleeve gastrectomy.
Endocrine changes
High-quality studies
Endocrine changes have not been examined by this group of studies.
Low-quality studies
FSH and oestradiol serum levels did not change after surgery (Tsur et al., 2014; Milone et al., 2017). There was either a positive or no association with anti-Müllerian hormone (AMH) serum levels (Milone et al., 2017; Pilone et al., 2019).
Summary
FSH and oestradiol levels remained the same and AMH either increased or did not change.
Fertility
High-quality studies
There was less use of infertility services after gastric banding (OR 0.08, 95% CI 0.01–0.84) (Goldman et al., 2016).
Low-quality studies
Fertility generally increased regarding spontaneous pregnancies and there was a decreased need for ART (Musella et al., 2011; Tsur et al., 2014; Khazraei et al., 2017; Milone et al., 2017).
Summary
There was a positive effect on fertility.
Vitamin status
High-quality studies
Folate serum levels were significantly higher after sleeve gastrectomy during pregnancy compared to before surgery following supplementation (mean ± SD 5.3 ± 2.4 vs 10.8 ± 5.4 ng/ml, P < 0.006), but there were no significant changes in vitamin B12 serum levels in the first trimester (mean ± SD 406 ± 138 vs 301 ± 183 pg/ml, P-value not significant) (Mead et al., 2014).
Low-quality studies
The incidence of low vitamin D serum levels remained unchanged (Günakan et al., 2020).
Summary
In general, folate levels increased and vitamin B12 and D levels remained the same.
Irregular menstrual cycles
High-quality studies
Goldman et al. (2016) investigated women after adjustable gastric banding compared to obese women without surgery and found a decrease in menstrual cycle irregularity (OR 0.23, 95% CI 0.06–0.96).
Low-quality studies
Menstrual cycles became more regular (Pilone et al., 2014; Khazraei et al., 2017).
Summary
There was a positive effect on menstrual cycle regularity.
Miscarriages
High-quality studies
There were no associations with the miscarriage rate in one study (OR 2.76, 95% CI 0.46–16.99) (Goldman et al., 2016). On the other hand, another article demonstrated an increased number of miscarriages in women who had undergone adjustable gastric banding compared to obese women without surgery (OR 2.45, 95% CI 1.02–6.57) (Lapolla et al., 2010).
Low-quality studies
There was no effect on the miscarriage rate (Khazraei et al., 2017).
Summary
In summary, there was either no effect or an increased rate of miscarriages.
Congenital malformations
High-quality studies
The association between restrictive procedures and congenital malformations has not been studied in the included articles.
Low-quality studies
The incidence of congenital malformations remained unchanged (Basbug et al., 2019; Karadağ et al., 2020).
Summary
There was no effect on congenital malformations.
Combined procedures
This category contains articles that described patients who had undergone combined procedures such as gastric bypass, articles that included both restrictive and/or malabsorptive procedures without specifying the results per type of surgery, and articles that did not specify the type of bariatric procedure at all. Most of the included articles (n = 37) are within this category.
Endocrine changes
High-quality studies
One article reported no significant difference in AMH between bariatric surgery patients and non-operated obese patients (mean ± SD 4.6 ± 5.4 vs 3.9 ± 4.0 ng/ml, P = 0.08) (Grzegorczyk-Martin et al., 2020). On the other hand, Nilsson-Condori et al. (2018) did find a significant decrease in AMH, free androgen index, androstenedione and testosterone 12 months postoperatively (P < 0.05), whereas SHBG and oestradiol increased significantly (P < 0.05) and LH and FSH did not change (P > 0.05).
Low-quality studies
While overall AMH, FSH, gonadotropin, oestrone and oestrone sulphate remained the same, when stratifying, Merhi et al. (2008) found a negative association with AMH in women under 35 years and a positive association in women above 35 years. LH remained unchanged (Nayak et al., 2020) or increased (Kruchinin et al., 2018), whereas overall progesterone increased after surgery (Rochester et al., 2009; Kruchinin et al., 2018).
Summary
The influence of bariatric surgery on AMH was inconsistent, while LH and FSH remained unchanged, the free androgen index, androstenedione and testosterone decreased and SHBG, progesterone and oestradiol generally increased.
Fertility
High-quality studies
The results regarding fertility were conflicting. Two articles reported a negative impact on fertility compared to a non-surgical group, which was demonstrated by higher odds ratios for fertility treatments after bariatric surgery compared to non-operated women (OR 2.3, 95% CI 1.6–3.8, P < 0.001 and OR 4.2, 95% CI 1.5–13.7, P < 0.001, respectively) (Sheiner et al., 2004, 2006). Christofolini et al. reported fewer follicles on ultrasound, fewer retrieved oocytes and metaphase II oocytes in post-bariatric patients. However, they showed no difference in metaphase I oocytes, prophase I oocytes, degenerated/abnormal oocytes or inseminated oocytes. Most importantly, clinical pregnancy rates after ART were not significantly different (Christofolini et al., 2014). Fecundity after bariatric surgery increased as compared to obese controls. Moreover, there was no significant difference in the need for ART between their post-bariatric patients and controls (Goldman et al., 2016). Live birth rate per transfer was higher in post-bariatric women than in non-operated obese controls (20.0% vs 9.3%, P = 0.017) (Grzegorczyk-Martin et al., 2020). Menke et al. (2019) reported that post-bariatric women with a history of infertility showed a higher conception rate (121.2, 95% CI 102.3–143.5 per 1000 woman-year) than women without previous infertility (47.0, 95% CI 34.2–62.9 per 1000 woman-year), P < 0.001.
Low-quality articles
Most included articles reported a positive effect on fertility regarding the need for ART or the incidence of infertility before and after surgery (Dao et al., 2006; Sapre et al., 2009; Neto et al., 2012; Nørgaard et al., 2014; Chagas et al., 2016; Vieira et al., 2020).
Summary
In summary, most articles found increased fertility after bariatric surgery.
Vitamin status
High-quality studies
The effect of bariatric surgery on vitamin status was inconsistent. Machado et al. (2016) reported that pregnant women after RYGB as compared to non-surgical pregnant patients had a 9-fold increased chance of developing a vitamin A deficiency at an average surgery-to-pregnancy interval of 21 months. Another study found significantly higher level folate serum levels after surgery and during pregnancy due to supplementation (Mead et al., 2014). Medeiros et al. (2016) found that 72% of women after RYGB had an inadequate vitamin D status during the first trimester despite daily vitamin D supplementation.
Devlieger et al. (2014) investigated first-trimester vitamin status (A, vitamin B1, B12, folate, D and E) in restrictive (gastric banding) versus malabsorptive/combined (BPD/RYGB) procedures and found no significant differences between the groups. Deficiency percentages of these vitamins were, independent of the type of bariatric surgery, between 0% and 37%.
Low-quality studies
Bariatric surgery was often negatively associated with vitamin status with regard to vitamin A, including its derivatives, and vitamin B1, B6, B12, folate, C, D and K (Sapre et al., 2009; Bebber et al., 2011; Gadgil et al., 2014; Jans et al., 2014; Chagas et al., 2016; Hazart et al., 2017). One study found no effect on vitamin B1 and B12 levels after bariatric surgery (Dao et al., 2006).
Summary
Bariatric surgery often led to an impaired vitamin status.
Irregular menstrual cycles
High-quality studies
There was no significant difference in irregular menstrual cycles between post-bariatric women and an obese non-surgical group (OR 1.04, 95% CI 0.31–3.56), however, when comparing the same group of women before and after surgery, there was a positive effect (OR 0.21, 95% CI 0.07–0.61) (Goldman et al., 2016).
Low-quality studies
Bariatric surgery often led to a return of regular menstrual cycles (Rochester et al., 2009; Edison et al., 2016; Kruchinin et al., 2018; Sahab Al Kabbi et al., 2018).
Summary
Generally, bariatric surgery had a beneficial effect on irregular menstrual cycles.
Miscarriages
High-quality studies
While Goldman et al. (2016) reported that miscarriage occurred more often after RYGB (OR 9.81, 95% CI 1.12–85.71), two other articles observed no change in either miscarriage rate (38.7% vs 56.5%, P = 0.256) or recurrent miscarriages (OR 1.8 95% CI 0.6–5.2, P = 0.208) (Sheiner et al., 2006; Grzegorczyk-Martin et al., 2020).
Low-quality studies
The included articles reported either no association between bariatric surgery and miscarriages (Patel et al., 2008; Dell'Agnolo et al., 2015; Cruz et al., 2019) or a beneficial effect (Sapre et al., 2009; Alatishe et al., 2013).
Summary
The relationship between combined bariatric procedures and miscarriages was inconsistent.
Congenital malformations
High-quality studies
After exact matching, Neovius et al. (2019) found a decreased relative risk for major congenital defects (RR 0.67, 95% CI 0.52–0.87, P = 0.002). The other articles found no significant association between bariatric surgery and the incidence of congenital malformations (Sheiner et al., 2004, 2006; Josefsson et al., 2013; Parent et al., 2017; Auger et al., 2019).
Low-quality studies
The incidence of congenital malformations remained the same (Patel et al., 2008).
Summary
The incidence of congenital malformations either remained unchanged or decreased after bariatric surgery.
Meta-analyses of the effect of bariatric surgery on fertility, irregular menstrual cycles, miscarriages and congenital malformations
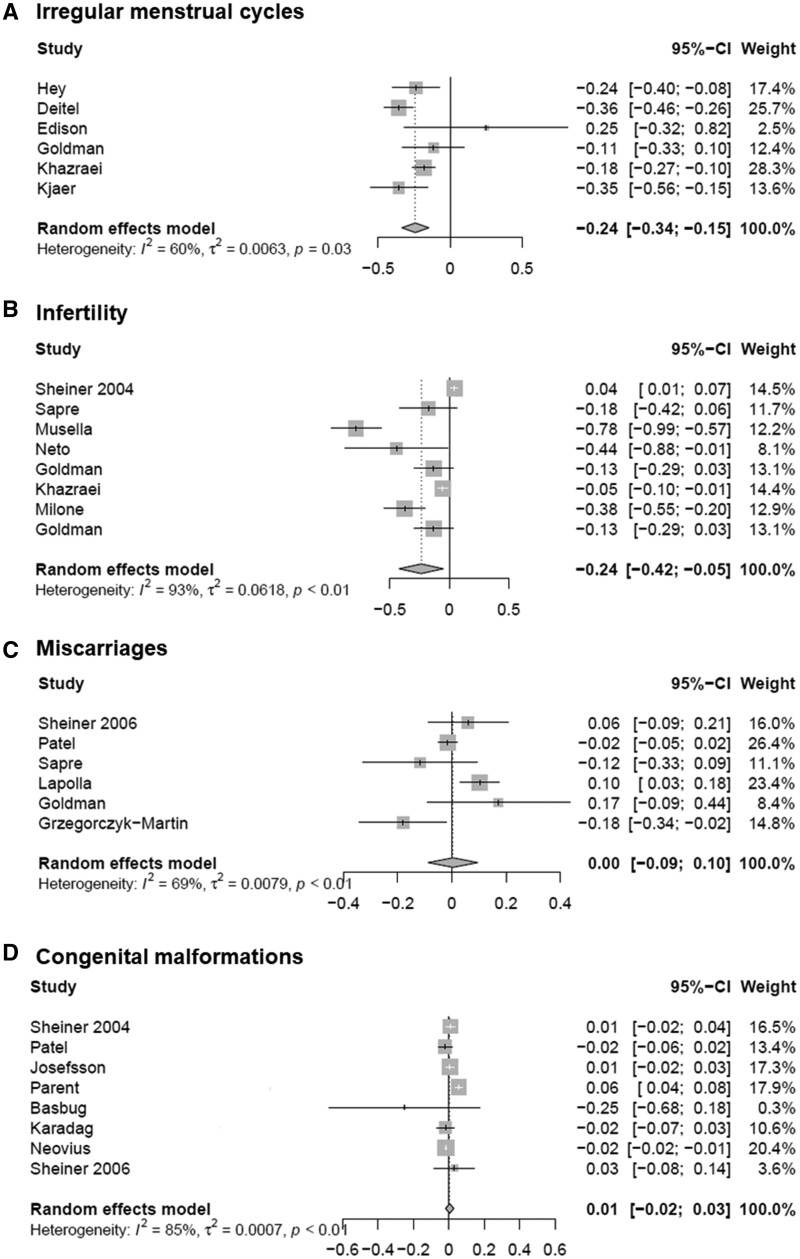
There were 20 studies focusing on the periconception outcomes of (in)fertility, menstrual cycle irregularities, miscarriages and congenital malformations that were eligible for meta-analyses (Hey and Niebuhr-Jorgensen, 1981; Deitel et al., 1988; Sheiner et al., 2004, 2006; Patel et al., 2008; Sapre et al., 2009; Lapolla et al., 2010; Musella et al., 2011; Neto et al., 2012; Josefsson et al., 2013; Edison et al., 2016; Goldman et al., 2016; Khazraei et al., 2017; Kjær et al., 2017; Milone et al., 2017; Parent et al., 2017; Basbug et al., 2019; Neovius et al., 2019; Grzegorczyk-Martin et al., 2020; Karadağ et al., 2020). The forest plots are shown in Fig. 4. We used a random-effects model to estimate the effect of bariatric surgery on the aforementioned periconception outcomes. We observed a significant RD of 24% for infertility (−0.24, 95% CI −0.42, −0.05) (Sheiner et al., 2004; Sapre et al., 2009; Musella et al., 2011; Neto et al., 2012; Goldman et al., 2016; Khazraei et al., 2017; Kjær et al., 2017; Milone et al., 2017). Menstrual cycle regularity restored after bariatric surgery (RD −0.24, 95% CI −0.34, −0.15) (Hey and Niebuhr-Jorgensen, 1981; Deitel et al., 1988; Edison et al., 2016; Goldman et al., 2016; Khazraei et al., 2017; Kjær et al., 2017). Bariatric surgery had no significant effect on miscarriage rate (RD 0.00, 95% CI −0.09, 0.10) (Sheiner et al., 2006; Patel et al., 2008; Sapre et al., 2009; Lapolla et al., 2010; Goldman et al., 2016; Grzegorczyk-Martin et al., 2020) or on congenital malformations (RD 0.01, 95% CI −0.02, 0.03) (Sheiner et al., 2004, 2006; Patel et al., 2008; Josefsson et al., 2013; Parent et al., 2017; Basbug et al., 2019; Neovius et al., 2019; Karadağ et al., 2020). We found significant heterogeneity in the study populations (P < 0.05). This indicates that there were differences in participants and interventions, study design and variation in effects. This can be explained by the inclusion of both case-control studies and before-after studies, differences in study sizes and differences in the type of bariatric surgery that was performed.
Figure 4.
Forest plots of the effect of bariatric surgery. (A) Irregular menstrual cycles; (B) infertility; (C) miscarriages; (D) congenital malformations.
Discussion
This systematic review and meta-analysis summarize the associations between different types of bariatric surgery and maternal periconception outcomes, including endocrine changes, fertility, vitamin status, irregular menstrual cycles, miscarriages and congenital malformations.
Before-after studies showed that hormonal serum levels normalized and menstrual cycles became more regular with an associated decrease in infertility (more natural conceptions and better results regarding ART). The studies also showed no short-term risks for reproductive periconception outcomes such as the increased risk of miscarriages or congenital malformations. On the other hand, vitamin deficiencies occurred regularly after bariatric surgery. The meta-analysis showed that fertility and menstrual cycle regularity improved whereas there was no association between bariatric surgery and congenital malformations and miscarriage rate, respectively.
For substantiated clinical implementation and applicability, we will elaborate on the results of the included studies based on a scientific quality score.
Endocrine changes
AMH was not higher in obese premenopausal women than in non-obese women, and another study found no relationship between AMH levels, obesity and fertility (Freeman et al., 2007; Sahmay et al., 2012). Importantly, the reported AMH decrease was not associated with subfertility in post-bariatric women. The post-bariatric decrease in AMH could be linked with malnutrition, however, no relationship between AMH and nutritional deficiencies was found (Vincentelli et al., 2018). The AMH decrease could reflect a decreased ovarian reserve, although it is unknown how bariatric surgery should affect the ovarian follicle pool. FSH and LH did not change significantly after bariatric surgery and the long-term effects of bariatric surgery on a fertility outcome such as the age of menopause have not been studied yet. The reported increase in SHBG, which tightly binds to androgens and oestrogens and inhibits their function (Gerrits et al., 2003; Kjær et al., 2017) leads to lower free, active testosterone levels, most likely contributing to the restoration of the menstrual cycle.
Fertility
Bariatric surgery has an overall positive effect on fertility, although an increased need for fertility treatments after bariatric surgery has been reported (Sheiner et al., 2004, 2006). However, the reason for fertility treatment was not specified in these articles and a comparison with women of the same BMI category as before bariatric surgery was not made. Most patients who could not conceive spontaneously before bariatric surgery had no difficulties after this surgery. The positive effect of bariatric surgery on sexual functioning could also play a role (Sarwer et al., 2014).
As fewer units of gonadotropins were needed after surgery, this indicates that less medication resulted in lower costs and fewer possible side effects with the same fertility outcomes (Tsur et al., 2014; Milone et al., 2017).
Obesity is associated with disorders such as diabetes mellitus, which can affect fertility and endocrine homeostasis. However, these associated disorders often resolve or diminish when body weight decreases (Skubleny et al., 2016; Ramos et al., 2019). It is unknown whether obesity and associated disorders aggravate each other or operate independently. Therefore, we recommend evaluating the individual effect of bariatric surgery on these outcomes and fertility.
A weight-loss trial in obese women with infertility showed that the study group that had undergone a 6-month lifestyle-intervention programme had more ongoing pregnancies from natural conception and a decreased need for ART, although the live birth rate was similar in both groups (Mutsaerts et al., 2016). Another randomized controlled trial investigated the effect of weight reduction on IVF outcomes, comparing a very low-calorie diet group with a control group without this diet (Einarsson et al., 2017). They found that the live birth rate was not significantly different between the two groups, nor in a subgroup analyses of women who lost at least 5 BMI units or reached a pre-pregnancy BMI <25 kg/m2. The positive effect of weight loss on fertility may not necessarily be a given, but major weight loss after surgery and before achieving pregnancy does seem to be beneficial. Natural conception after the restoration of menstrual cycle regularity improves pregnancy outcomes and health later in the life course of both mother and offspring (Gao et al., 2017).
Unplanned pregnancies occur more often in post-bariatric patients of reproductive age as they may not feel like contraception use is necessary due to previous infertility. Additionally, vomiting and diarrhoea often occur after bariatric surgery, which influences the bioavailability of oral contraceptives, and thereby also contribute to more unplanned pregnancies (Gerrits et al., 2003; Paulen et al., 2010). We therefore advise non-oral contraceptives such as intra-uterine devices to effectively postpone pregnancy until (relative) homeostasis is achieved.
Irregular menstrual cycles
Obesity is associated with irregular cycles which can be resolved by weight loss due to the restoration of hormonal axes.
Most studies that evaluated the effect of bariatric surgery concluded that fertility recovers due to restoring the normal, regular menstrual cycles. Malabsorption is associated with bariatric surgery and can lead to malnutrition and severe weight loss in a short period of time, which can result in hypothalamic dysfunction and amenorrhea (Di Carlo et al., 1999). As restrictive surgery often leads to less weight loss than malabsorptive surgery, the effect of restrictive surgery on irregular menstrual cycles is diminished (Miras and Le Roux, 2013). For this reason, we would recommend interventions with sufficient weight loss. We have found that in general, temporary restrictive surgery such as adjustable gastric banding, does not lead to sufficient long-term weight loss.
Since PCOS is another independent cause of irregular menstrual cycles, we excluded articles that focused on PCOS patients, but we cannot exclude that a portion of the included patients suffered from PCOS.
We conclude that weight loss that is induced by bariatric surgery is associated with the restoration of a regular menstrual cycle (Teitelman et al., 2006). Obesity causes a state of chronic oxidative stress and is associated with metabolic and endocrine process imbalances (Furukawa et al., 2004; Fernández-Sánchez et al., 2011). Bariatric surgery and its associated weight loss can result in a new, partially restored homeostatic equilibrium.
Vitamin status
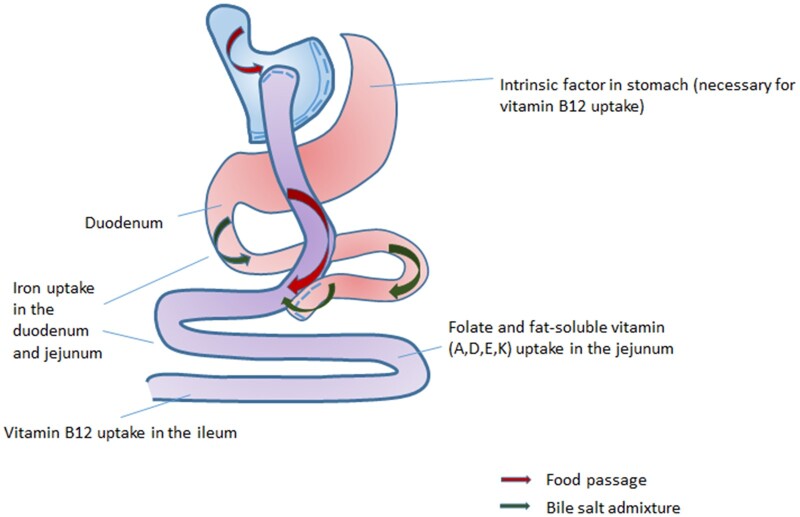
The anatomical sites of nutrient uptake in the gastro-intestinal tract after combined surgery are illustrated in Fig. 5. RYGB, the second most performed bariatric procedure, is a combined procedure that can lead to a deficiency in fat-soluble vitamins (A, D, E and K) due to the bypassing of the proximal intestine, which is the anatomical region where most of these vitamins are absorbed (Fig. 5). In addition, obesity in general is associated with chronic inflammation and oxidative stress, which often leads to increased consumption of vitamin A (Stephensen, 2001). The average BMI 1 year after bariatric surgery is still 33 kg/m2 despite an average BMI drop of 15 points (Varban et al., 2017). This could contribute to the vitamin A deficiencies reported in this specific group of patients. However, the included articles have not taken this into account.
Figure 5.
Anatomical changes and consequences for intestinal uptake of nutrients after gastric bypass surgery.
Vitamin K is essential for the adequate functioning of haemostasis, indicating that vitamin K deficiencies are associated with an increased risk of impaired coagulation (Bersani et al., 2011). Fetuses are dependent on maternal vitamin K serum levels and congenital vitamin K deficiencies can lead to neonatal bleeding (Pichler and Pichler, 2008; Takahashi et al., 2011).
Folate serum levels were usually lower, except for a group studied by Mead et al. (2014), who reported significantly fewer folate deficiencies after use of oral supplementation, in patients after RYGB, gastric sleeve and BPD. This shows that adequate oral folate supplementation is effective in reducing folate deficiencies after bariatric surgery.
The small intestines are responsible for vitamin C uptake and as such vitamin C deficiency is expected after malabsorptive surgery (Parrott et al., 2017). Moreover, malabsorptive procedures may physically impair the uptake of vitamin B12. Gastric restriction, which takes place after sleeve gastrectomy and RYGB, impairs digestion, acid secretion (which takes place in the proximal two-thirds of the stomach) and IF production, which are necessary for the absorption of vitamin B12. To understand the impact of RYGB, it is important to take the percentage of the bypassed small intestine into account as this influences the degree and kind of malabsorption (Schweiger and Keidar, 2010). However, the length of the surgically resected small intestine was not mentioned in all included articles discussing vitamin deficiencies.
Purely restrictive procedures such as sleeve gastrectomy and vertical banding gastroplasty can lead to vitamin deficiencies due to highly limited intake and increased vomiting (Xanthakos, 2009).
The included articles used different supplement treatments, which complicates the assessment of the impact of bariatric surgery on vitamin status. Therefore, it is a challenge to discriminate between the effects of bariatric surgery, vitamin supplementation and patient characteristics.
The take-home message is the need for worldwide, substantiated guidelines for post-bariatric patients regarding vitamin supplementation, before and during pregnancy. We recommend guidelines that enable a personalized approach, taking the length of the bypassed small intestines into account. Most included articles recommended the use of vitamin supplements after bariatric surgery. However, possible side or negative effects of over-supplementation due to iatrogenic supra-physiological vitamin serum levels need further investigation.
Another important concern is that follow-up and vitamin supplement adherence in post-bariatric patients are moderate, mostly due to patient-related factors and a lack of guidelines, which can lead to substandard care for these patients.
Overall, independent of the type of bariatric surgery, post-surgical patients are vulnerable to develop both fat- and water-soluble vitamin deficiencies.
We propose professional guidance by a multidisciplinary team with a gynaecologist or midwife, specialized dietitian, bariatric surgeon and internal medicine specialist. It is imperative that post-bariatric women should be closely monitored before and during pregnancy to prevent gestational vitamin deficiencies which affect maternal health and fetal development. We therefore advise support for these women in particular during the periconception period, use of evidence-based monitoring and effective digital coaching programmes.
Miscarriages
A recent meta-analysis showed that having a BMI ≥25kg/m2 is associated with increased risk of miscarriage (OR 1.67, CI 1.25–2.25) (Metwally et al., 2008). However, our systematic review and meta-analysis showed no association between bariatric surgery and miscarriages (RD 0.00, 95% CI −0.09, −0.10). Consequently, despite extensive weight loss after bariatric surgery, most patients still stabilize at a BMI >30 kg/m2 and as such bariatric surgery alone will not completely decrease the BMI-associated risk of miscarriages (Varban et al., 2017). Moreover, other causes of miscarriages such as chromosome anomalies are not changed by bariatric surgery (Kroon et al., 2011). The post-bariatric increase in vitamin deficiencies could counteract the expected positive effect of weight loss on miscarriages.
Congenital malformations
The included articles and meta-analyses did not show an increased risk for congenital malformations in offspring of women with bariatric surgery compared to women without preconception bariatric surgery (RD 0.01, 95% CI −0.02, −0.03).
Obesity itself is associated with an increased risk of congenital malformations, which progressively increases per higher BMI category (Persson et al., 2017). We hypothesize that a delicate balance exists between obesity and the side effects of bariatric surgery. Embryonic development may be disturbed as obesity can cause chronic inflammation and endothelial dysfunction. Moreover, nutritional deficiencies and lipid mobilization shortly after bariatric surgery may impair embryonic development (Jarvie et al., 2010).
The possible risk reduction due to decreased obesity could be counteracted by post-bariatric undernutrition and vitamin deficiencies. Some malformations in the included articles were caused by rare recessive mutations and therefore cannot be attributed to maternal BMI. Congenital malformations were generally not a primary outcome in most of the included articles and they are rare (3.5%). This could indicate that a lack of power was the reason why no effect was found. As most malformations are diagnosed during the first year of life, it is possible that subtle malformations that are diagnosed later in life were not included in the articles. Also, some articles reported congenital malformation as defined by the EUROCAT classification, which does not include minor defects.
Strengths and limitations
One of the strengths of the current systematic review is that it specifically investigates the effect of bariatric surgery on periconception maternal health. This is vital for successful implantation, placentation and embryonic development with long-lasting effects during the life course of the offspring. Different types of bariatric surgery have varying physiological mechanisms. Subsequently, more specific preconception advice and the prospects concerning potential effects can be given according to the type of surgery.
It is important to adequately discuss the advantages, disadvantages and risks of current bariatric procedures with obese women contemplating pregnancy, especially with the continuing increase in the number of post-bariatric patients. For example, malabsorptive procedures such as BPD make patients more prone to develop vitamin deficiencies, whereas purely restrictive bariatric procedures have a less prominent risk. Most systematic reviews in reproductive medicine, obstetrics and gynaecology investigate the influence of bariatric surgery in general on pregnancy outcome. The current systematic review shows that it is essential to report the outcomes per type of bariatric surgery, which will be of great value for the current practice of preconception care and counselling.
Furthermore, the preconception care consultations (including lifestyle counselling) need to be more individualized and patient-tailored. The results in this review show that the now abandoned, strictly malabsorptive procedures are not beneficial for women of reproductive age, as they featured a high number of post-surgical complications and vitamin deficiencies (Buchwald, 2014). Therefore, we do not recommend this type of surgery for these patients. Another strength of this review is that it did not exclude articles based on the year of publication, which decreases selection bias. This review also includes a meta-analysis on the association between bariatric surgery and fertility, irregular menstrual cycles, miscarriages and congenital malformations. As mentioned, the heterogeneity of the included studies was considerable, which we accounted for by using a random-effects model.
This review also has some limitations. The possibility of a reporting bias can never be ruled out, although articles without significant results were also included in this study. Some of the included articles reported on small study groups, which increased the risk for type II errors, meaning that the risk of false-negative findings was increased. By taking the ErasmusAGE quality score into consideration, we accounted for this when interpreting the results of the included studies. However, despite the above-mentioned extensive literature search, the overall amount of evidence and quality of the studies were moderate. We have tried to partially compensate for this by focusing on discussing and reporting on the results of high-quality score studies.
Implications for future research
Many studies did not investigate the different categories of bariatric surgery separately. Due to considerably differing mechanisms and anatomical and physiological consequences, we advise that future research should be divided per type of bariatric surgery. This makes the results applicable for the subgroups, while simultaneously maintaining the level of heterogeneity that is needed to extrapolate the findings.
When discussing case-control studies, the controls were not always comparable regarding BMI, which can influence the interpretation of the effect of bariatric surgery. We suggest the addition of BMI-matched controls and the use of before-after studies combined with a control group with a BMI similar to pre-bariatric patients. Depending on the primary objective of future studies, possible confounders that may influence the results must be taken into account. For example, a woman with a BMI between 35 and 40 kg/m2 only classifies for bariatric surgery if she also suffers from an obesity-related comorbidity such as diabetes, which can also influence the effect on the studied outcome (Fried et al., 2014).
As the long-term consequences of bariatric surgery on fertility are still unknown, we propose longer follow-up in women of reproductive age before and after surgery. We also recommend follow-up of their offspring along the entire life course.
Conclusion
The current systematic review reports that different types of bariatric surgery are associated with beneficial changes in maternal periconception outcomes, in terms of improvement of fertility and restoration of menstrual cycle irregularity. Although congenital malformations do not occur more often after bariatric surgery, the risk of long-term diverse vitamin deficiencies is increased.
This overview underlines the importance of adequate preconception care and counselling for women before and after bariatric surgery if they are contemplating pregnancy. The iatrogenic malnutrition with severe vitamin deficiencies is a compelling reason to advise postponement of pregnancy until a return to or maintenance of physiological vitamin concentrations, indicating specialized follow-up outpatient clinics.
We advise adequate, preferably patient-tailored, supplementation until appropriate vitamin serum levels are reached and weight has stabilized, before a patient tries to become pregnant. Bariatric surgery has proven to be effective in the achievement of weight reduction, but the risks of iatrogenic malnutrition can also influence fetal growth and development and offspring health, even though the effects may not be directly visible and are largely unknown (Hovdenak and Haram, 2012). Therefore, we advise extensive follow-up of both mother and her unborn offspring, from the preconception period onward, including regular assessment of maternal vitamin status, fetal growth and follow-up of the offspring.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
The authors would like to thank Wichor M. Bramer, biomedical information specialist, Erasmus MC, University Medical Center, for his assistance with the search strategy.
Authors’ roles
R.P.M.S.-T. initiated the review. K.M.S. and S.S. selected the articles and wrote the first version together with R.P.M.S.-T. K.M.S. extracted the data from the articles for the meta-analysis and S.P.W. performed the meta-analysis. R.P.M.S.-T., E.J.H., S.G. and J.S.E.L. provided input on the review and the revisions. All authors contributed to the writing of this article and approved the final version.
Funding
This research was funded by the Department of Obstetrics and Gynaecology, Erasmus MC, University Medical Centre, Rotterdam, The Netherlands.
Conflict of interest
J.S.E.L. reports grants and personal fees from Ansh Labs, Webster, TX, USA, grants from Astellas, Tokyo, Japan, grants from Dutch Heart Association, Utrecht, the Netherlands, grants and personal fees from Ferring, Hoofddorp, the Netherlands, personal fees from Titus Healthcare, Hoofddorp, the Netherlands, and grants from Zon MW, Amsterdam, Netherlands, outside the submitted work. The remaining authors have no conflicts of interest.
References
- Abbaspour N, Hurrell R, Kelishadi R.. Review on iron and its importance for human health. J Res Med Sci 2014;19:164. [PMC free article] [PubMed] [Google Scholar]
- Akhter Z, Rankin J, Ceulemans D, Ngongalah L, Ackroyd R, Devlieger R, Vieira R, Heslehurst N.. Pregnancy after bariatric surgery and adverse perinatal outcomes: a systematic review and meta-analysis. PLos Med 2019;16:e1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatishe A, Ammori BJ, New JP, Syed AA.. Bariatric surgery in women of childbearing age. QJM 2013;106:717–720. [DOI] [PubMed] [Google Scholar]
- Al-Nimr RI, Hakeem R, Moreschi JM, Gallo S, McDermid JM, Pari-Keener M, Stahnke B, Papoutsakis C, Handu D, Cheng FW.. Effects of bariatric surgery on maternal and infant outcomes of pregnancy—an evidence analysis center systematic review. J Acad Nutr Diet 2019;119:1921–1943. [DOI] [PubMed] [Google Scholar]
- Andari SR, Jaffe J, Friedenberg L, Friedenberg FK.. Vitamin, mineral, and drug absorption following bariatric surgery. Curr Drug Metab2012;13:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, Miller ER 3rd, Dalcin A, Jerome GJ, Geller S.. et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365:1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger N, Bilodeau-Bertrand M, Tith RM, Arbour L.. Bariatric surgery and the risk of congenital anomalies in subsequent pregnancies. Am J Clin Nutr 2019;110:1168–1174. [DOI] [PubMed] [Google Scholar]
- Basbug A, Kaya AE, Dogan S, Pehlivan M, Goynumer G.. Does pregnancy interval after laparoscopic sleeve gastrectomy affect maternal and perinatal outcomes? J Matern Fetal Neonatal Med 2019;32:3764–3770. [DOI] [PubMed] [Google Scholar]
- Bebber FE, Rizzolli J, Casagrande DS, Rodrigues MT, Padoin AV, Mottin CC, Repetto G.. Pregnancy after bariatric surgery: 39 pregnancies follow-up in a multidisciplinary team. Obes Surg 2011;21:1546–1551. [DOI] [PubMed] [Google Scholar]
- Benaiges D, Más-Lorenzo A, Goday A, Ramon JM, Chillarón JJ, Pedro-Botet J, Flores-Le Roux JA.. Laparoscopic sleeve gastrectomy: More than a restrictive bariatric surgery procedure?. World J Gastroenterol 2015;21:11804–11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani I, Carolis MPD, Salvi S, Zecca E, Romagnoli C, De Carolis S.. Maternal-neonatal vitamin K deficiency secondary to maternal biliopancreatic diversion. Blood Coagul Fibrinolysis 2011;22:334–336. [DOI] [PubMed] [Google Scholar]
- Boxmeer JC, Brouns RM, Lindemans J, Steegers EAP, Martini E, Macklon NS, Steegers-Theunissen RPM.. Preconception folic acid treatment affects the microenvironment of the maturing oocyte in humans. Fertil Steril 2008;89:1766–1770. [DOI] [PubMed] [Google Scholar]
- Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg 2014;24:1126–1135. [DOI] [PubMed] [Google Scholar]
- Caspary WF. Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr 1992;55:299S–308S. [DOI] [PubMed] [Google Scholar]
- Chagas C, Saunders C, Pereira S, Silva J, Saboya C, Ramalho A.. Vitamin A status and its relationship with serum zinc concentrations among pregnant women who have previously undergone Roux-en-Y gastric bypass. Int J Gynecol Obstet 2016;133:94–97. [DOI] [PubMed] [Google Scholar]
- Christofolini J, Bianco B, Santos G, Adami F, Christofolini D, Barbosa CP.. Bariatric surgery influences the number and quality of oocytes in patients submitted to assisted reproduction techniques. Obesity 2014;22:939–942. [DOI] [PubMed] [Google Scholar]
- Cruz S, Matos A, Cruz S, Pereira S, Saboya C, Ramalho A.. Pregnancy after 24 postoperative months of Roux-En-Y gastric bypass presents risk of pregnancy complications similar to pregnancy within the first postoperative year. Ann Nutr Metab 2019;75:24–30. [DOI] [PubMed] [Google Scholar]
- Dao T, Kuhn J, Ehmer D, Fisher T, McCarty T.. Pregnancy outcomes after gastric-bypass surgery. Am J Surg 2006;192:762–766. [DOI] [PubMed] [Google Scholar]
- De Carolis S, Botta A, Del Sordo G, Guerrisi R, Salvi S, De Carolis MP, Iaconelli A, Giustacchini P, Raffaelli M, Lanzone A.. Influence of biliopancreatic diversion on pregnancy outcomes in comparison to other bariatric surgery procedures. Obes Surg 2018;28:3284–3292. [DOI] [PubMed] [Google Scholar]
- de Weerd S, Steegers-Theunissen RPM, De Boo TM, Thomas CMG, Steegers EAP.. Maternal periconceptional biochemical and hematological parameters, vitamin profiles and pregnancy outcome. Eur J Clin Nutr 2003;57:1128–1134. [DOI] [PubMed] [Google Scholar]
- Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ.. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147–153. [DOI] [PubMed] [Google Scholar]
- Dell’Agnolo CM, Cyr C, de Montigny F, de Barros Carvalho MD, Pelloso SM.. Pregnancy after Bariatric Surgery: Obstetric and Perinatal Outcomes and the Growth and Development of Children. Obes Surg 2015;25:2030–2039. [DOI] [PubMed] [Google Scholar]
- ΔελλΑγνoλo ΧΜ, Χαρϖαληo ΜΔ, Πελλoσo ΣΜ.. Pregnancy after bariatric surgery: implications for mother and newborn. Obes Surg 2011;21:699–706. [DOI] [PubMed] [Google Scholar]
- Devlieger R, Guelinckx I, Jans G, Voets W, Vanholsbeke C, Vansant G.. Micronutrient levels and supplement intake in pregnancy after bariatric surgery: a prospective cohort study. PLoS One 2014;9:e114192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhana K, Haines J, Liu G, Zhang C, Wang X, Field AE, Chavarro JE, Sun Q.. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: results from two prospective cohort studies of mother-child pairs in the United States. BMJ 2018a;362:k2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhana K, Zong G, Yuan C, Schernhammer E, Zhang C, Wang X, Hu FB, Chavarro JE, Field AE, Sun Q.. Lifestyle of women before pregnancy and the risk of offspring obesity during childhood through early adulthood. Int J Obes 2018b;42:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo C, Palomba S, De Fazio M, Gianturco M, Armellino M, Nappi C.. Hypogonadotropic hypogonadism in obese women after biliopancreatic diversion. Fertil Steril 1999;72:905–909. [DOI] [PubMed] [Google Scholar]
- Ebisch IMW, Thomas CMG, Peters WHM, Braat DDM, Steegers-Theunissen RPM.. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update 2007;13:163–174. [DOI] [PubMed] [Google Scholar]
- Edison E, Whyte M, van Vlymen J, Jones S, Gatenby P, de Lusignan S, Shawe J.. Bariatric surgery in obese women of reproductive age improves conditions that underlie fertility and pregnancy outcomes: retrospective cohort study of UK National Bariatric Surgery Registry (NBSR). Obes Surg 2016;26:2837–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlström P-O, Kluge L, Larsson I, Loft A, Mikkelsen-Englund A-L.. et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Hum Reprod 2017;32:1621–1630. [DOI] [PubMed] [Google Scholar]
- Falcone V, Stopp T, Feichtinger M, Kiss H, Eppel W, Husslein PW, Prager G, Göbl CS.. Pregnancy after bariatric surgery: a narrative literature review and discussion of impact on pregnancy management and outcome. BMC Pregnancy Childbirth 2018;18:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González Á, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA.. Inflammation, oxidative stress, and obesity. Int J Mol Sci 2011;12:3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN.. et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC-L, Strauss JF.. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril 2007;87:101–106. [DOI] [PubMed] [Google Scholar]
- Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres A, Weiner R, Yashkov Y, Frühbeck G; on behalf of International Federation for the Surgery of Obesity and Metabolic Disorders—European Chapter (IFSO-EC); European Association for the Study of Obesity (EASO); European Association for the Study of Obesity Obesity Management Task Force (EASO OMTF). Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg 2014;24:42–55. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I.. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil MD, Chang HY, Richards TM, Gudzune KA, Huizinga MM, Clark JM, Bennett WL.. Laboratory testing for and diagnosis of nutritional deficiencies in pregnancy before and after bariatric surgery. J Women's Health 2014;23:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Yan Y, Xiang S, Zeng G, Liu S, Sha T, He Q, Li H, Tan S, Chen C.. et al. The mutual effect of pre-pregnancy body mass index, waist circumference and gestational weight gain on obesity-related adverse pregnancy outcomes: a birth cohort study. PLoS One 2017;12:e0177418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits EG, Ceulemans R, Van Hee R, Hendrickx L, Totté E.. Contraceptive treatment after biliopancreatic diversion needs consensus. Obes Surg 2003;13:378–382. [DOI] [PubMed] [Google Scholar]
- Goldman RH, Missmer SA, Robinson MK, Farland LV, Ginsburg ES.. Reproductive outcomes differ following Roux-en-Y gastric bypass and adjustable gastric band compared with those of an obese non-surgical group. Obes Surg 2016;26:2581–2589. [DOI] [PubMed] [Google Scholar]
- Grzegorczyk-Martin V, Freour T, De Bantel Finet A, Bonnet E, Merzouk M, Roset J, Roger V, Cedrin-Durnerin I, Wainer R, Avril C.. et al. IVF outcomes in patients with a history of bariatric surgery: a multicenter retrospective cohort study. Hum Reprod2020;35:2755–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günakan E, Buluş H, Tohma YA.. Early pregnancy after bariatric surgery: a single-institute preliminary experience. Turk J Med Sci 2020;50:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague WM. Homocysteine and pregnancy. Best Pract Res Clin Obstet Gynaecol 2003;17:459–469. [DOI] [PubMed] [Google Scholar]
- Hazart J, Le Guennec D, Accoceberry M, Lemery D, Mulliez A, Farigon N, Lahaye C, Miolanne-Debouit M, Boirie Y.. Maternal nutritional deficiencies and small-for-gestational-age neonates at birth of women who have undergone bariatric surgery. J Pregnancy 2017;2017:4168541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH.. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 2008;105:17046–17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey H, Niebuhr-Jorgensen U.. Jejuno-ileal bypass surgery in obesity. Gynecological and obstetrical aspects. Acta Obstet Gynecol Scand 1981;60:135–140. [PubMed] [Google Scholar]
- Hoek J, Steegers-Theunissen R, Sinclair K, Schoenmakers S.. The Science of Preconception. A life course approach. Springer International Publishing, 2020, 21–34. [Google Scholar]
- Hovdenak N, Haram K.. Influence of mineral and vitamin supplements on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 2012;164:127–132. [DOI] [PubMed] [Google Scholar]
- Jans G, Guelinckx I, Voets W, Galjaard S, Van Haard PMM, Vansant GM, Devlieger R.. Vitamin K1 monitoring in pregnancies after bariatric surgery: a prospective cohort study. Surg Obes Relat Dis 2014;10:885–890. [DOI] [PubMed] [Google Scholar]
- Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ.. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 2010;119:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson A, Bladh M, Wiréhn AB, Sydsjö G.. Risk for congenital malformations in offspring of women who have undergone bariatric surgery. A national cohort. BJOG 2013;120:1477–1482. [DOI] [PubMed] [Google Scholar]
- Karadağ C, Demircan S, Çalışkan E.. Effects of laparoscopic sleeve gastrectomy on obstetric outcomes within 12 months after surgery. J Obstet Gynaecol Res 2020;46:266–271. [DOI] [PubMed] [Google Scholar]
- Khazraei H, Hosseini SV, Amini M, Bananzadeh A, Najibpour N, Ganji F, Sadeghi F, Vafa L.. Effect of weight loss after laparoscopic sleeve gastrectomy on infertility of women in shiraz. J Gynecol Surg 2017;33:43–46. [Google Scholar]
- Kjær MM, Madsbad S, Hougaard DM, Cohen AS, Nilas L.. The impact of gastric bypass surgery on sex hormones and menstrual cycles in premenopausal women. Gynecol Endocrinol 2017;33:160–163. [DOI] [PubMed] [Google Scholar]
- Kloss O, Eskin NAM, Suh M.. Thiamin deficiency on fetal brain development with and without prenatal alcohol exposure. Biochem Cell Biol 2018;96:169–177. [DOI] [PubMed] [Google Scholar]
- Kroon B, Harrison K, Martin N, Wong B, Yazdani A.. Miscarriage karyotype and its relationship with maternal body mass index, age, and mode of conception. Fertil Steril 2011;95:1827–1829. [DOI] [PubMed] [Google Scholar]
- Kruchinin EV, Autlev KM, Smetanin EI, Akhundova SAK, Stradchuk AA.. Research on reproductive function in patients with morbid obesity after bariatric surgeries. Int J Pharm Res 2018;10:665–668. [Google Scholar]
- Kwong W, Tomlinson G, Feig DS.. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am J Obstet Gynecol 2018;218:573–580. [DOI] [PubMed] [Google Scholar]
- Lapolla A, Marangon M, Dalfrà MG, Segato G, De Luca M, Fedele D, Favretti F, Enzi G, Busetto L.. Pregnancy outcome in morbidly obese women before and after laparoscopic gastric banding. Obes Surg 2010;20:1251–1257. [DOI] [PubMed] [Google Scholar]
- Machado SN, Pereira S, Saboya C, Saunders C, Ramalho A.. Influence of Roux-en-Y gastric bypass on the nutritional status of vitamin A in pregnant women: a comparative study. Obes Surg 2016;26:26–31. [DOI] [PubMed] [Google Scholar]
- Maggard MA, Yermilov I, Li Z, Maglione M, Newberry S, Suttorp M, Hilton L, Santry HP, Morton JM, Livingston EH.. et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA 2008;300:2286–2296. [DOI] [PubMed] [Google Scholar]
- Mead NC, Sakkatos P, Sakellaropoulos GC, Adonakis GL, Alexandrides TK, Kalfarentzos F.. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis 2014;10:1166–1175. [DOI] [PubMed] [Google Scholar]
- Medeiros M, Matos AC, Pereira SE, Saboya C, Ramalho A.. Vitamin D and its relation with ionic calcium, parathyroid hormone, maternal and neonatal characteristics in pregnancy after roux-en-Y gastric bypass. Arch Gynecol Obstet 2016;293:539–547. [DOI] [PubMed] [Google Scholar]
- Menke MN, King WC, White GE, Gosman GG, Courcoulas AP, Dakin GF, Flum DR, Orcutt MJ, Pomp A, Pories WJ.. et al. Conception rates and contraceptive use after bariatric surgery among women with infertility: evidence from a prospective multicenter cohort study. Surg Obes Relat Dis 2019;15:777–785. [DOI] [PubMed] [Google Scholar]
- Merhi ZO, Minkoff H, Feldman J, Macura J, Rodriguez C, Seifer DB.. Relationship of bariatric surgery to Müllerian-inhibiting substance levels. Fertil Steril 2008;90:221–224. [DOI] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC.. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril 2008;90:714–726. [DOI] [PubMed] [Google Scholar]
- Milone M, Sosa Fernandez LM, Sosa Fernandez LV, Manigrasso M, Elmore U, De Palma GD, Musella M, Milone F.. Does bariatric surgery improve assisted reproductive technology outcomes in obese infertile women? Obes Surg 2017;27:2106–2112. [DOI] [PubMed] [Google Scholar]
- Miras AD, Le Roux CW.. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 2013;10:575–584. [DOI] [PubMed] [Google Scholar]
- Mission JF, Marshall NE, Caughey AB.. Pregnancy risks associated with obesity. Obstet Gynecol Clin N Am 2015;42:335–353. [DOI] [PubMed] [Google Scholar]
- Musella M, Milone M, Bellini M, Sosa Fernandez ME, Sosa Fernandez LM, Leongito M, Milone F.. The potential role of intragastric balloon in the treatment of obese-related infertility: personal experience. Obes Surg 2011;21:426–430. [DOI] [PubMed] [Google Scholar]
- Mutsaerts MAQ, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WKH, Perquin DAM, Koks CAM, van Golde R, Kaaijk EM, Schierbeek JM.. et al. Randomized trial of a lifestyle program in obese infertile women. N Engl J Med 2016;374:1942–1953. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Methods and Tools. Quality Assessment Tool for Quantitative Studies. Hamilton, ON: McMaster University, 2008. [Google Scholar]
- Nayak R, Gunasheela D, Kumar V, Rafi F.. Effectiveness of bariatric surgery-induced weight loss on infertility among PCOS and non-PCOS women: experience of a maternity hospital and in vitro fertilization (IVF) center in India. Indian J Surg 2020; [Google Scholar]
- Neovius M, Pasternak B, Näslund I, Söderling J, Johansson K, Stephansson O.. Association of maternal gastric bypass surgery with offspring birth defects. JAMA 2019;322:1515–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto RML, Herbella FAM, Tauil RM, Silva FS, De Lima SE Jr. Comorbidities remission after Roux-en-Y gastric bypass for morbid obesity is sustained in a long-term follow-up and correlates with weight regain. Obes Surg 2012;22:1580–1585. [DOI] [PubMed] [Google Scholar]
- Nilsson-Condori E, Hedenbro JL, Thurin-Kjellberg A, Giwercman A, Friberg B.. Impact of diet and bariatric surgery on anti-Müllerian hormone levels. Hum Reprod 2018;33:690–693. [DOI] [PubMed] [Google Scholar]
- Nørgaard LN, Gjerris ACR, Kirkegaard I, Berlac JF, Tabor A, Hoseth E, Jørgensen FS, Zingenberg H, Sperling L, Skibsted L; Danish Fetal Medicine Research Group. Fetal growth in pregnancies conceived after gastric bypass surgery in relation to surgery-to-conception interval: a Danish national cohort study. PLoS One 2014;9:e90317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann‐Borst SA, Vujkovic M, De Vries JH, Wildhagen MF, Looman CW, de Jonge R, Steegers EAP, Steegers‐Theunissen RPM.. A maternal dietary pattern characterised by fish and seafood in association with the risk of congenital heart defects in the offspring. BJOG 2011;118:1205–1215 [DOI] [PubMed] [Google Scholar]
- Parent B, Martopullo I, Weiss NS, Khandelwal S, Fay EE, Rowhani-Rahbar A.. Bariatric surgery in women of childbearing age, timing between an operation and birth, and associated perinatal complications. JAMA Surg 2017;152:128–135. [DOI] [PubMed] [Google Scholar]
- Parisi F, Rousian M, Koning AHJ, Willemsen SP, Cetin I, Steegers EAP, Steegers-Theunissen RPM.. Periconceptional maternal biomarkers of one-carbon metabolism and embryonic growth trajectories: the Rotterdam Periconceptional Cohort (Predict Study). Fertil Steril 2017;107:691–698.e691. [DOI] [PubMed] [Google Scholar]
- Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L.. American Society for Metabolic and Bariatric Surgery integrated health nutritional guidelines for the surgical weight loss patient 2016 update: micronutrients. Surg Obes Relat Dis 2017;13:727–741. [DOI] [PubMed] [Google Scholar]
- Patel JA, Patel NA, Thomas RL, Nelms JK, Colella JJ.. Pregnancy outcomes after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2008;4:39–45. [DOI] [PubMed] [Google Scholar]
- Paulen ME, Zapata LB, Cansino C, Curtis KM, Jamieson DJ.. Contraceptive use among women with a history of bariatric surgery: a systematic review. Contraception 2010;82:86–94. [DOI] [PubMed] [Google Scholar]
- Persson M, Cnattingius S, Villamor E, Söderling J, Pasternak B, Stephansson O, Neovius M.. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ 2017;357:j2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler E, Pichler L.. The neonatal coagulation system and the vitamin K deficiency bleeding–a mini review. Wien Med Wochenschr 2008;158:385–395. [DOI] [PubMed] [Google Scholar]
- Pilone V, Hasani A, Di Micco R, Vitiello A, Monda A, Izzo G, Iacobelli L, Villamaina E, Forestieri P.. Pregnancy after laparoscopic gastric banding: maternal and neonatal outcomes. Int J Surg 2014;12:136–139. [DOI] [PubMed] [Google Scholar]
- Pilone V, Tramontano S, Renzulli M, Monda A, Cutolo C, Romano M, Schiavo L.. Evaluation of anti-Müller hormone AMH levels in obese women after sleeve gastrectomy. Gynecol Endocrinol 2019;35:548–551. [DOI] [PubMed] [Google Scholar]
- Polifka JE, Friedman JM.. Medical genetics: 1. Clinical teratology in the age of genomics. Can Med Assoc J 2002;167:265–273. [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Kow L, Brown W, Welbourn R, Dixon J, Kinsman R, Walton P.. 5th IFSO Global Registry Report. The International Federation for the Surgery of Obesity and Metabolic Disorders 2019;3:782-795. [Google Scholar]
- Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, Zeitlian G, Hickmon C, Feng S, Santoro N.. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril 2009;92:1410–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom TJ, Painter RC, van Abeelen AFM, Veenendaal MVE, de Rooij SR.. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas 2011;70:141–145. [DOI] [PubMed] [Google Scholar]
- Sahab Al Kabbi M, Al-Taee HA, Kareem Al Hussaini S.. Impact of Bariatric surgery on antimularian hormone in reproductive age women. Middle East Fertility Society Journal 2018;23:273–277. [Google Scholar]
- Sahmay S, Usta T, Erel CT, İmamoğlu M, Küçük M, Atakul N, Seyisoğlu H.. Is there any correlation between amh and obesity in premenopausal women? Arch Gynecol Obstet 2012;286:661–665. [DOI] [PubMed] [Google Scholar]
- Sapre N, Munting K, Pandita A, Stubbs R.. Pregnancy following gastric bypass surgery: what is the expected course and outcome? N Z Med J 2009;122:33–42. [PubMed] [Google Scholar]
- Sarwer DB, Spitzer JC, Wadden TA, Mitchell JE, Lancaster K, Courcoulas A, Gourash W, Rosen RC, Christian NJ.. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surg 2014;149:26–33. [DOI] [PubMed] [Google Scholar]
- Schweiger C, Keidar A. [Nutritional deficiencies in bariatric surgery patients: prevention, diagnosis and treatment]. Harefuah 2010;149:715–720,. [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; the PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- Shawe J, Ceulemans D, Akhter Z, Neff K, Hart K, Heslehurst N, Štotl I, Agrawal S, Steegers‐Theunissen R, Taheri S.. et al. Pregnancy after bariatric surgery: consensus recommendations for periconception, antenatal and postnatal care. Obes Rev 2019;20:1507–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiner E, Levy A, Silverberg D, Menes TS, Levy I, Katz M, Mazor M.. Pregnancy after bariatric surgery is not associated with adverse perinatal outcome. Am J Obstet Gynecol 2004;190:1335–1340. [DOI] [PubMed] [Google Scholar]
- Sheiner E, Menes TS, Silverberg D, Abramowicz JS, Levy I, Katz M, Mazor M, Levy A.. Pregnancy outcome of patients with gestational diabetes mellitus following bariatric surgery. Am J Obstet Gynecol 2006;194:431–435. [DOI] [PubMed] [Google Scholar]
- Skubleny D, Switzer NJ, Gill RS, Dykstra M, Shi X, Sagle MA, de Gara C, Birch DW, Karmali S.. The impact of bariatric surgery on polycystic ovary syndrome: a systematic review and meta-analysis. 2016;26:169–176. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen R, Steegers E.. Nutrient-gene interactions in early pregnancy: a vascular hypothesis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;106:115–117. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RPM, Twigt J, Pestinger V, Sinclair KD.. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update 2013;19:640–655. [DOI] [PubMed] [Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001;21:167–192. [DOI] [PubMed] [Google Scholar]
- Stijnen T, , HamzaTH, , Özdemir P.. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Statist Med 2010;29:3046–3067. 10.1002/sim.4040 [DOI] [PubMed] [Google Scholar]
- Takahashi D, Shirahata A, Itoh S, Takahashi Y, Nishiguchi T, Matsuda Y.. Vitamin K prophylaxis and late vitamin K deficiency bleeding in infants: fifth nationwide survey in Japan. Pediatr Int 2011;53:897–901. [DOI] [PubMed] [Google Scholar]
- Teitelman M, Grotegut CA, Williams NN, Lewis JD.. The impact of bariatric surgery on menstrual patterns. Obes Surg 2006;16:1457–1463. [DOI] [PubMed] [Google Scholar]
- Tsur A, Orvieto R, Haas J, Kedem A, Machtinger R.. Does bariatric surgery improve ovarian stimulation characteristics, oocyte yield, or embryo quality? J Ovarian Res 2014;7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varban OA, Cassidy RB, Bonham A, Carlin AM, Ghaferi A, Finks JF; for the Michigan Bariatric Surgery Collaborative. Factors associated with achieving a body mass index of less than 30 after bariatric surgery. JAMA Surg 2017;152:1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira APPS, Santa Cruz F, Machado Júnior AS, Ferraz ÁAB, Kreimer F.. Evaluation of the quality of life in women of reproductive age after bariatric surgery. Bariatr Surg Pract Patient Care 2020;15:88–92. [Google Scholar]
- Vincentelli C, Maraninchi M, Valéro R, Béliard S, Maurice F, Emungania O, Berthet B, Lombard E, Dutour A, Gaborit B.. et al. One-year impact of bariatric surgery on serum anti-Mullerian-hormone levels in severely obese women. J Assist Reprod Genet 2018;35:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ.. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005;353:2111–2120. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R.. et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011;365:1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, Ramos A, Våge V, Al-Sabah S, Brown W.. et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO Global Registry Report 2018. Obes Surg 2019;29:782–795. [DOI] [PubMed] [Google Scholar]
- Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am 2009;56:1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X.. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 2013;8:e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.