Abstract
Introduction
We evaluated the association between carotid compliance, a measure of arterial stiffness, to parahippocampal volume (PHV) and hippocampal volume (HV) over 20 years later in the Atherosclerosis Risk in the Community study.
Methods
We included participants with common carotid compliance measurements at visit 1 (1987–1989) and volumetric brain MRI at visit 5 (2011–2013). The primary outcomes are pooled bilateral PHV and HV. We performed linear regression models adjusting for age, sex, vascular risk factors, and total brain volume.
Results
Of the 614 participants, higher compliance was correlated with higher PHV (R = 0.218[0.144–0.291], p < 0.001) and HV (R = 0.181 [0.105–0.255, p < 0.001]). The association was linear and significant after adjusting for confounders. At follow-up MRI, 30 patients with dementia had lower PHV and HV than patients without dementia (p < 0.001 and p < 0.001, respectively).
Conclusion
Carotid compliance is associated with higher PHV and HV when measured 20 years later, further supporting the link between arterial stiffness and cognitive decline.
Keywords: Brain imaging, Cerebrovascular diseases, Vascular causes of cognitive impairment
Introduction
Given the increasing prevalence and economic impact of cognitive decline and dementia, there have been intensified efforts in recognizing targets for prevention or treatment [1]. Recently, the link between vascular disease and cognitive impairment and dementia has become more apparent with more evidence of an association with all-cause dementia and Alzheimer's disease [2, 3]. Arterial stiffness, loosely defined as the rigidity of arterial walls, can be measured using a variety of different techniques in different vascular beds but is most commonly measured centrally [4]. Arterial stiffness is an established marker for cerebrovascular disease and can be caused by hypertension, advanced age, smoking, diabetes, and genetic predisposition [5, 6]. Sonographically measured carotid artery stiffness is also an independent risk factor for cerebrovascular disease, cognitive impairment, and mortality [6, 7, 8].
In addition, MR markers of brain aging, specifically, low parahippocampal volume (PHV), and hippocampal volume (HV) are associated with general dementia and cognitive decline [9, 10, 11, 12, 13]. Decreased PHV and HV are validated early biomarkers for Alzheimer's disease [14, 15, 16]. While carotid artery disease and PHV/HV appear to be independently associated with cognitive decline, the relationship between carotid artery disease and PHV/HV is less established. Though there is some evidence that central arterial stiffness is associated with lower volumes in the HV and PHV regions [17], the association between carotid stiffness measures and brain volumes in the HV and PHV regions is unclear. Given the known deleterious downstream effects of arterial stiffness [7, 18], it is biologically plausible that carotid stiffness could lead to measurable brain atrophy, which could in turn cause cognitive impairment [19, 20]. In order to further elucidate the contribution of carotid vascular disease to cognitive decline and dementia, we sought to examine the association between carotid artery compliance, a measure of arterial stiffness, and PHV and HV. We evaluated the association between carotid compliance measured on carotid ultrasound (US) to PHV and HV measured on brain MRI approximately 20 years later in the community-based Atherosclerosis Risk in the Community (ARIC) study.
Materials and Methods
Subjects
This study is a secondary analysis of the ARIC study, which is a large prospective epidemiological study performed in 4 communities in the US [21]. Participants were 45–64 years old at enrollment in 1987–1989 and were followed periodically to identify vascular health status. We performed a secondary analysis using the anonymized ARIC dataset from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. The Institutional Review Board at the University of Utah provided a waiver to evaluate this anonymized dataset. All participants provided informed consent. We included 614 participants (Fig. 1) who had common carotid artery compliance measurements on US at visit 1 (1987–1989) and who also had brain MR imaging at visit 5 (2011–2013).
Fig. 1.
Flowchart of participant inclusion from the total Atherosclerosis Risk in Communities cohort.
Imaging Technique
The detailed methodology for obtaining carotid sonographical measurements has been described previously [22, 23]. Briefly, B-mode US was performed to measure the continuous variation of arterial diameter throughout the cardiac cycle with echo tracking techniques. Immediately before, after, and during the US examination, supine blood pressure was measured automatically from the right brachial artery in 5-min intervals using Dinamap equipment. Systole, mean arterial pressure, diastole, and pulse rate were measured by the Dinamap cuff [22]. Based on the US measurements and concomitant supine blood pressure measurements, arterial compliance was calculated. Carotid compliance is defined as the absolute volume increase within the carotid artery segment during the cardiac cycle divided by the arterial pulse pressure (π × [Peak systolic arterial diameter2-end diastolic-arterial diameter2]/[4 × Pulse pressure]). Higher values of compliance indicate less carotid stiffness. Methodology for the MR imaging protocols and measurement of PHV and HV has also been previously described [24]. Briefly, MR imaging was performed using a standardized protocol at 3T field strength. The PHV and HV were measured on sagittal T1-weighted 3D volumetric MPRAGE sequences and reported as a pooled bilateral volume in mL. Freesurfer (version 5.1; [http://surfer.nmr.mgh.harvard.edu]) was used with an ARIC-specific algorithm. The ARIC MRI reading center (Mayo Clinic, Rochester, MN, USA) was responsible for quality control and interpretation of the imaging studies.
Statistical Analysis
We report the mean values for carotid compliance with the correlation coefficients for the association with pooled bilateral PHV and HV measured on brain MR. To assess the association of carotid compliance with PHV and HV, we utilized the mean value of each of the measured vascular risk factors over the course of their follow-up for each subject. We also adjusted for baseline vascular risk factors for each subject. Vascular risk factors include current and former cigarette smoking, current and former alcohol use, presence of diabetes mellitus, systolic blood pressure, body mass index, cholesterol levels, aspirin use, history of myocardial infarction, and history of hypertension. We fit multivariate linear regression models adjusted for total brain volume, patient age, sex, race, and vascular risk factors. We first fit a saturated model controlled for confounders determined a priori. Then, we performed a second model adjusting for variables with p < 0.1 in stepwise backward elimination. This model resulted in total brain volume, patient age, mean blood glucose, and smoking status being added as covariates in the model. Carotid compliance was treated as a continuous variable and split into quartiles for the statistical analyses. We performed an additional analysis, and we derived inverse probability weighting based on baseline measurements on all participants who had carotid compliance measures. We weighted the same regression models as reported above.
The assumptions of linear regression were met. A linear relationship between dependent and independent variables was assessed graphically via scatterplots and correlation between the 2 variables. Normality of residuals was assessed graphical and statistically via the Shapiro-Wilk test for normality. The Shapiro-Wilk test resulted in p values greater than the 0.05 threshold indicating evidence for normality of residuals. Homoscedasticity of residuals was checked visually and statistically via the Breusch-Pagan/Cook-Weisberg test for heteroskedasticity at the 0.05 significance threshold. Multicollinearity was assessed using the variance inflation factor. No concerning variance inflation factor values were observed in relation to either set of models.
Results
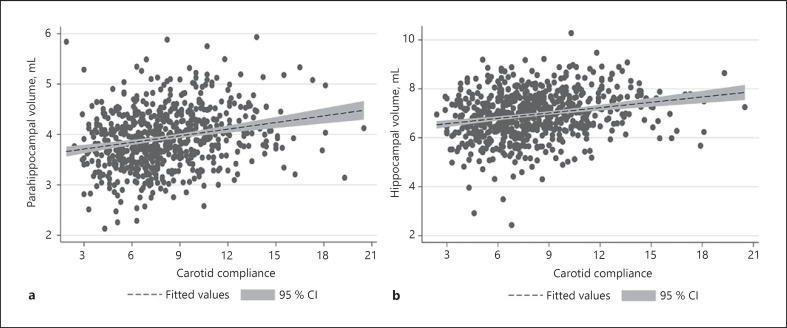
We included a total of 614 participants with a mean (standard deviation [SD]) age of 52.9 (2.3) years (59% female) at baseline. Demographics for the included participants adjusted for mean values of confounders are shown in Table 1 and for baseline covariates in Table 2. Mean (SD) carotid compliance was 8.0 (3.0) mm3/kPa. The mean (SD) values for PHV and HV at visit 5 were 3.9 (0.6) mL and 6.9 (1.0) mL, respectively. Carotid compliance, for which higher values indicate lower carotid stiffness, was positively correlated with PHV (R = 0.218 [0.144–0.291], p < 0.001) and HV (R = 0.181 [0.105–0.255], p < 0.001). The associations were linear (Fig. 2) and nearly all remained significant after adjusting for confounders including patient age, race, sex, cigarette smoking, and follow-up total brain volume (Table 3). We also derived inverse probability weighting based on baseline carotid compliance measurements and received similar results for both HV and PHV. Furthermore, at the follow-up MR, 30 (4.7%) participants had an adjudicated diagnosis of dementia with lower PHV (3.4 ± 0.6 vs. 4.0 ± 0.6 mL, p < 0.001) and lower HV (5.8 ± 1.4 vs. 7.0 ± 0.9 mL, p < 0.001) than participants without dementia, further validating the significance of PHV and HV. Nearly all associations remained significant after excluding participants with a diagnosis of dementia and controlling for total brain volume and vascular risk factors (Table 4).
Table 1.
Demographics for included participants
| Variable | Value across cohort (n = 614) | Association with HV, p value | Association with PHV, p value |
|---|---|---|---|
| Baseline age | 52.87 (2.25) | <0.001 | <0.001 |
| Race, n (%) | |||
| White | 538 (83.9) | 0.08 | <0.001 |
| Nonwhite | 103 (16.1) | ||
| Sex, n (%) | |||
| Male | 264 (41.2) | <0.001 | <0.001 |
| Female | 377 (58.8) | ||
| Carotid compliance in, mm3/kPa | 8.00 (2.96) | <0.001 | <0.001 |
| HV, mL | 6.94 (0.95) | N/A | N/A |
| PHV, mL | 3.92 (0.57) | N/A | N/A |
| Total intracranial volume, mL | 1,444.75 (154.68) | <0.001 | <0.001 |
| Mean, BMI | 27.48 (4.46) | 0.30 | 0.07 |
| Mean fasting blood glucose, mmol/L | 105.68 (20.38) | 0.88 | 0.52 |
| Mean HDL, mg/dL | 52.32 (15.47) | 0.004 | 0.05 |
| Mean LDL, mg/dL | 124.26 (24.83) | 0.09 | 0.09 |
| Mean systolic blood pressure | 138.87 (12.05) | 0.001 | 0.006 |
| Mean proportion of current drinking status | 0.57 (0.43) | 0.006 | <0.001 |
| Mean proportion of current smoking status | 0.12 (0.27) | 0.54 | 0.87 |
| Mean proportion of diabetes status | 0.10 (0.22) | 0.34 | 0.53 |
| Mean proportion of hypertension status | 0.37 (0.35) | 0.016 | 0.007 |
| Mean proportion of prevalent CVD | 0.04 (0.14) | 0.41 | 0.02 |
| Mean proportion of statin status | 0.14 (0.16) | 0.02 | 0.10 |
| Mean proportion of prevalent stroke status | 0.02 (0.10) | 0.46 | 0.59 |
HV, hippocampal volume; PHV, parahippocampal volume; BMI, body mass index; CVD, cerebrovascular disease.
Table 2.
Demographics from baseline visit (visit 1) at the time of carotid US
| Variable | Value across cohort (n = 614) | p value for association with PHV | p value for association with HV |
|---|---|---|---|
| Age (mean) | 52.78±5.28 | <0.001 | <0.001 |
| White, n (%) | 563 (83.2) | 0.001 | 0.083 |
| Female, n (%) | 394 (58.2) | <0.001 | <0.001 |
| Diabetes, n (%) | 25 (3.7) | 0.799 | 0.944 |
| Hypertension, n (%) | 119 (17.8) | 0.003 | 0.132 |
| Heart failure, n (%) | 11 (1.7) | 0.111 | 0.186 |
| History of myocardial infarction, n (%) | 8 (1.2) | 0.945 | 0.630 |
| Current cigarette smoking, n (%) | 120 (17.9) | 0.956 | 0.779 |
| Ever cigarette smoker, n (%) | 341 (50.8) | 0.513 | 0.210 |
| Current alcohol consumption, n (%) | 411 (61.3) | 0.004 | 0.158 |
| Ever alcohol drinker, n (%) | 506 (75.5) | <0.001 | 0.004 |
| Antihypertensive use, n (%) | 99 (14.8) | 0.005 | 0.07 |
| Aspirin use, n (%) | 316 (47.2) | 0.002 | 0.05 |
| Cholesterol medication use, n (%) | 6 (0.9) | 0.362 | 0.413 |
| Mean systolic blood pressure, mm Hg | 133.6±12.0 | 0.002 | <0.001 |
| BMI | 26.4±4.1 | 0.092 | 0.681 |
| LDL cholesterol (mean), mg/dL | 134.1±35.2 | 0.06 | 0.024 |
| HDL cholesterol (mean), mg/dL | 53.1±17.5 | 0.027 | 0.007 |
| Fasting blood glucose (mean), mmol/L | 100.8±20.5 | 0.116 | 0.250 |
HV, hippocampal volume; PHV, parahippocampal volume; BMI, body mass index; US, ultrasound.
Fig. 2.
Positive linear correlation between increasing carotid compliance and (a) PHV and (b) HV. CI, confidence interval; HV, hippocampal volume; PHV, parahippocampal volume.
Table 3.
Linear regression model fit to the PHV and HV
| β coefficient for continuous carotid compliance (95% CI) (p value) | β coefficient for quartile carotid compliance (95% CI) (p value) | |
|---|---|---|
| PHVs | ||
| Model 1* (n = 614) | 0.022 (0.008–0.359) (0.002) | 0.078 (0.042, 0.114) (<0.001) |
| Model 2† (n = 614) | 0.026 (0.012, 0.041) (<0.001) | 0.089 (0.051–0.127) (<0.001) |
|
| ||
| HVs | ||
| Model 1* (n = 614) | 0.024 (0.002–0.046) (0.03) | 0.074 (0.017–0.132) (0.01) |
| Model 2† (n = 614) | 0.024 (0.001–0.048) (0.049) | 0.076 (0.014–0.138) (0.02) |
Adjusted for total brain volume, age, mean glucose level, current or former cigarette smoking.
Adjusted for patient age, race, sex, BMI, current or former cigarette smoking, current or former alcohol use, total brain volume, history of hypertension, diabetes, heart failure, or myocardial infarction, the mean systolic blood pressure, mean LDL, mean HDL, and mean glucose levels, and use of aspirin, antihypertensive medication, or cholesterol medications. HV, hippocampal volume; PHV, parahippocampal volume; BMI, body mass index; CI, confidence interval.
Table 4.
Linear regression model fit to the PHV and HV with participants with dementia excluded
| β coefficient for continuous carotid compliance (95% CI) (p value) | β coefficient for quartile carotid compliance (95% CI) (p value) | |
|---|---|---|
| PHVs | ||
| Model 1* (n = 602) | 0.021 (0.007–0.347) (0.003) | 0.073 (0.037, 0.109) (<0.001) |
| Model 2† (n = 602) | 0.026 (0.012, 0.041) (<0.001) | 0.087 (0.049–0.125) (<0.001) |
|
| ||
| HVs | ||
| Model 1* (n = 602) | 0.019 (−0.002 to 0.040) (0.07) | 0.066 (0.011–0.121) (0.02) |
| Model 2† (n = 602) | 0.020 (−0.003 to 0.043) (0.08) | 0.072 (0.012–0.131) (0.02) |
Adjusted for total brain volume, age, mean glucose level, current or former cigarette smoking.
Adjusted for patient age, race, sex, BMI, current or former cigarette smoking, current or former alcohol use, total brain volume, history of hypertension, diabetes, heart failure, or myocardial infarction, the mean systolic blood pressure, mean LDL, mean HDL, and mean glucose levels, and use of aspirin, antihypertensive medication, or cholesterol medications. HV, hippocampal volume; PHV, parahippocampal volume; BMI, body mass index; CI, confidence interval.
Discussion
We found that higher carotid compliance is associated with higher PHV and HV measured 20 years after the baseline carotid artery measurements. This association is independent of potential confounders including basic demographics, vascular risk factors, and follow-up total brain volume. Notably, at the 20-year follow-up, those with dementia were more likely to have lower PHV and HV than those without dementia, further validating the significance of PHV and HV in this cohort. Our findings further support the link between medium artery vascular findings, as measured by carotid compliance, and cognition [2, 3, 18, 25].
Our study is unique because it relates baseline carotid compliance to future PHV and HV. There is evidence of a link between carotid stiffness and cognitive decline and dementia, although the mechanism is unclear. While some studies have not shown an association between arterial stiffness and cognitive decline and dementia [26], other studies, including a systematic review and meta-analysis, found that arterial stiffness was predictive of cognitive decline [25, 27]. Another recent study showed no association between arterial stiffness and HV [20]. Many of these studies used other methods for measuring arterial stiffness, including carotid-femoral pulse wave velocity, rather than measuring carotid stiffness more directly with carotid compliance [20, 25, 26, 27]. Furthermore, many studies have shown that cerebral volume loss, particularly lower HV, is associated with cognitive impairment [9, 12, 13]. Although the exact pathophysiological mechanism underpinning the association between carotid stiffness and lower PHV and HV is still unclear, some studies have shown that arterial stiffness may lead to cerebral gliosis mediated by oxidative stress [28], microvascular injury and early brain capillary damage, or that increased arterial stiffness, and cerebral hypoperfusion may interfere with β-amyloid clearance from the brain [29, 30, 31, 32]. Further evaluation of how carotid stiffness relates to HV and PHV is warranted.
The strengths of our study include the long-term 20-year follow-up of a cohort of asymptomatic participants to assess for lower PHVs. Despite the fact that the included participants had a generally younger age and lower vascular risk profile at baseline, we still found a robust association between carotid stiffness and PHV, independent of potential confounders. Furthermore, having a large number of participants with volumetric measures of PHV allowed for the power to detect significant associations.
Our study is limited by the lack of baseline brain MRI to evaluate for changes in PHV and HV over time. An additional limitation is the inability to rigorously control for other potential confounders specifically antihypertensive use, as some studies have shown that the use of antihypertensive may blunt the association between carotid artery disease and cognitive function and HVs [25]. We have attempted to adjust for potential confounders by adjusting for both mean and baseline values for included confounders, though there is still some residual confounding due to the time-variation of exposure to vascular risk factors and other unmeasured confounders. Another limitation is the inherent variability in defining and measuring arterial stiffness which can be expressed using multiple methods and measured in different vascular beds [4]. In this study, we evaluated a single measure of stiffness, which is carotid compliance, but many of the other cited studies used other methods, most commonly pulse wave velocity. Although the hippocampus receives its blood supply predominantly from the posterior circulation [33], carotid stiffness is thought to represent a surrogate biomarker for general vascular health [4, 7, 8, 34, 35, 36, 37]. Last, another major limitation is inherent selection bias as we did not account for participants who were lost to follow-up and who did not undergo a follow-up brain MR. To mitigate this limitation, we performed an additional analysis and we derived inverse probability weighting based on baseline measures from all participants with carotid compliance measures and found similar results to our original analysis.
Conclusions
We have found a strong association between elevated carotid compliance on baseline US and higher PHV and HV on MR imaging performed 20 years later. This association was seen in a relatively young population and persisted even when accounting for potential confounders. Since lower HVs are thought to precede the development of clinically apparent cognitive decline and clinical dementia [14, 15, 16], carotid stiffness may be a preclinical target for preventative efforts. Though carotid stiffness is not routinely measured in clinical practice, our findings suggest further investigation of the role of carotid stiffness measurement in the evaluation of dementia-risk may be warranted. Our findings further support the link between medium artery vascular disease and cognitive decline which may have implications for the prevention of cognitive impairment and dementia.
Statement of Ethics
Research complied with the guidelines for human studies and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. This article is exempt from local Ethical Committee approval due to use of completely anonymized data.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Association of University Radiologists-GE Radiology Research Academic Fellowship; the National Institutes of Health (Grant Numbers NIH/NINDS U24NS107228, R01 HL127582, R01CA224141, R01 EB028316), and American Heart Association (Grant Number 17SDG33460420).
Author Contributions
Hediyeh Baradaran and Adam deHavenon gave substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; Final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Alen Delic; J. Scott McNally; Matthew Alexander; Jennifer Majersik; and Dennis Parker gave substantial contributions to the conception or design of the work; revising the work critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability Statement
The data that support the findings of this study are openly available from ARIC at the NHLBI by request at https://sites.cscc.unc.edu/aric/distribution-agreements.
References
- 1.International AsD . World Alzheimer report 2010: the global economic impact of dementia. Alzheimer's Disease International; 2010. [Google Scholar]
- 2.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61((5)):403–10. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42((9)):2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackenzie IS, Wilkinson IB, Cockcroft JR. Assessment of arterial stiffness in clinical practice. QJM. 2002;95((2)):67–74. doi: 10.1093/qjmed/95.2.67. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham heart study. Circulation. 2007;115:2628–36. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 6.Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E. Arterial stiffness, the brain and cognition: a systematic review. Ageing Res Rev. 2014;15:16–27. doi: 10.1016/j.arr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Dijk JM, van der Graaf Y, Grobbee DE, Bots ML. Carotid stiffness indicates risk of ischemic stroke and TIA in patients with internal carotid artery stenosis: the SMART study. Stroke. 2004;35((10)):2258–62. doi: 10.1161/01.STR.0000141702.26898.e9. [DOI] [PubMed] [Google Scholar]
- 8.van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;53:121–30. doi: 10.1016/j.neubiorev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55((4)):484–9. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72((11)):999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Holland D, et al. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73((6)):457–65. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez A, et al. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol Aging. 2010;31((7)):1077–88. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao LL, Mueller SG, Buckley ST, Peek K, Raptentsetseng S, Elman J, et al. Evidence of neurodegeneration in brains of older adults who do not yet fulfill MCI criteria. Neurobiol Aging. 2010;31((3)):368–77. doi: 10.1016/j.neurobiolaging.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology. 1991;41((1)):51–4. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- 15.Echávarri C, Aalten P, Uylings HB, Jacobs HI, Visser PJ, Gronenschild EH, et al. Atrophy in the parahippocampal gyrus as an early biomarker of Alzheimer's disease. Brain Struct Funct. 2011;215((3–4)):265–71. doi: 10.1007/s00429-010-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z. Characterizing early Alzheimer's disease and disease progression using hippocampal volume and arterial spin labeling perfusion MRI. J Alzheimers Dis. 2014;42((Suppl 4)):S495–502. doi: 10.3233/JAD-141419. [DOI] [PubMed] [Google Scholar]
- 17.Palta P, Sharrett AR, Wei J, Meyer ML, Kucharska-Newton A, Power MC, et al. Central arterial stiffness is associated with structural brain damage and poorer cognitive performance: the ARIC study. J Am Heart Assoc. 2019;8((2)):e011045. doi: 10.1161/JAHA.118.011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes TM, Wagenknecht LE, Craft S, Mintz A, Heiss G, Palta P, et al. Arterial stiffness and dementia pathology: atherosclerosis risk in communities (ARIC)-PET study. Neurology. 2018;90((14)):e1248–56. doi: 10.1212/WNL.0000000000005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, et al. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens. 2009;22((5)):525–30. doi: 10.1038/ajh.2009.35. [DOI] [PubMed] [Google Scholar]
- 20.Triantafyllou A, Ferreira JP, Kobayashi M, Micard E, Xie Y, Kearney-Schwartz A, et al. Longer duration of hypertension and MRI microvascular brain alterations are associated with lower hippocampal volumes in older individuals with hypertension. J Alzheimers Dis. 2020;74:227–35. doi: 10.3233/JAD-190842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ARIC-Investigators The atherosclerosis risk in communit (ARIC) study: design and objectives. Am J Epidemiol. 1989;129((4)):687–702. [PubMed] [Google Scholar]
- 22.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC study. Atherosclerosis risk in communities study. Circulation. 1995;91((5)):1432–43. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 23.Yang EY, Chambless L, Sharrett AR, Virani SS, Liu X, Tang Z, et al. Carotid arterial wall characteristics are associated with incident ischemic stroke but not coronary heart disease in the Atherosclerosis risk in communities (ARIC) study. Stroke. 2012;43((1)):103–8. doi: 10.1161/STROKEAHA.111.626200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power MC, Lamichhane AP, Liao D, Xu X, Jack CR, Gottesman RF, et al. The association of long-term exposure to particulate matter air pollution with brain MRI findings: the ARIC study. Environ Health Perspect. 2018;126((2)):027009. doi: 10.1289/EHP2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. J Alzheimers Dis. 2012;32((3)):541–9. doi: 10.3233/JAD-2012-120757. [DOI] [PubMed] [Google Scholar]
- 26.Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, et al. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke. 2007;38((3)):888–92. doi: 10.1161/01.STR.0000257998.33768.87. [DOI] [PubMed] [Google Scholar]
- 27.Pase MP, Herbert A, Grima N, Pipingas A, O'Rourke MF. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta-analysis. Intern Med J. 2012;42((7)):808–15. doi: 10.1111/j.1445-5994.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 28.Sadekova N, Iulita MF, Vallerand D, Muhire G, Bourmoum M, Claing A, et al. Arterial stiffness induced by carotid calcification leads to cerebral gliosis mediated by oxidative stress. J Hypertens. 2018;36((2)):286–98. doi: 10.1097/HJH.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 29.de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109((23 Suppl 1)):III33–8. doi: 10.1161/01.CIR.0000131516.65699.ba. [DOI] [PubMed] [Google Scholar]
- 30.Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bønaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the tromsø study. Neurology. 2004;62((5)):695–701. doi: 10.1212/01.wnl.0000113759.80877.1f. [DOI] [PubMed] [Google Scholar]
- 31.Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol. 2005;57((6)):789–94. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 32.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330((6012)):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens RB, Stilwell DL. Arteries and veins of the human brain. Thomas; 1969. [Google Scholar]
- 34.Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens. 2005;23((6)):1211–6. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- 35.Anderson TJ. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can J Cardiol. 2006;22((Suppl B)):72B–80. doi: 10.1016/s0828-282x(06)70990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, et al. Arterial stiffness and cerebral small vessel disease: the Rotterdam scan study. Stroke. 2012;43((10)):2637–42. doi: 10.1161/STROKEAHA.111.642264. [DOI] [PubMed] [Google Scholar]
- 37.Ding J, Mitchell GF, Bots ML, Sigurdsson S, Harris TB, Garcia M, et al. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: the age, gene/environment susceptibility (AGES)-Reykjavik study. Arterioscler Thromb Vasc Biol. 2015;35((8)):1889–95. doi: 10.1161/ATVBAHA.115.305451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available from ARIC at the NHLBI by request at https://sites.cscc.unc.edu/aric/distribution-agreements.