Abstract
Background
Atopic diseases are the most common chronic conditions of childhood. The apparent rise in food anaphylaxis in young children over the past three decades is of particular concern, owing to the lack of proven prevention strategies other than the timely introduction of peanut and egg. Due to reported in vitro differences in the immune response of young infants primed with whole‐cell pertussis (wP) versus acellular pertussis (aP) vaccine, we systematically appraised and synthesised evidence on the safety and the potential allergy preventive benefits of wP, to inform recommendation for future practice and research.
Objectives
To assess the efficacy and safety of wP vaccinations in comparison to aP vaccinations in early infancy for the prevention of atopic diseases in children.
Search methods
We searched the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and grey literature. The date of the search was 7 September 2020.
Selection criteria
We included randomised controlled trials (RCTs) and non‐randomised studies of interventions (NRSIs) that reported the occurrence of atopic diseases, and RCTs only to assess safety outcomes. To be included studies had to have at least six months follow‐up, and involve children under 18 years old, who received a first dose of either wP (experimental intervention) or aP (comparator) before six months of age.
Data collection and analysis
Two review authors independently screened studies for eligibility, extracted the data, and assessed risk of bias using standard Cochrane methods. We assessed the certainty of the evidence using GRADE. Our primary outcomes were diagnosis of IgE‐mediated food allergy and all‐cause serious adverse events (SAEs). Secondary outcomes included: diagnosis of not vaccine‐associated anaphylaxis or urticaria, diagnosis of asthma, diagnosis of allergic rhinitis, diagnosis of atopic dermatitis and diagnosis of encephalopathy. Due to paucity of RCTs reporting on the atopic outcomes of interest, we assessed a broader outcome domain (cumulative incidence of atopic disease) as specified in our protocol. We summarised effect estimates as risk ratios (RR) and 95% confidence intervals (CI). Where appropriate, we pooled safety data in meta‐analyses using fixed‐effect Mantel‐Haenszel methods, without zero‐cell corrections for dichotomous outcomes.
Main results
We identified four eligible studies reporting on atopic outcomes, representing 7333 children. Based on a single trial, there was uncertain evidence on whether wP vaccines affected the risk of overall atopic disease (RR 0.85, 95% CI 0.62 to 1.17) or asthma only (RR 1.04, 95% CI 0.59 to 1.82; 497 children) by 2.5 years old.Three NRSIs were judged to be at serious or critical risk of bias due to confounding, missing data, or both, and were ineligible for inclusion in a narrative synthesis.
We identified 21 eligible studies (137,281 children) that reported the safety outcomes of interest. We judged seven studies to be at high risk of bias and those remaining, at unclear risk.
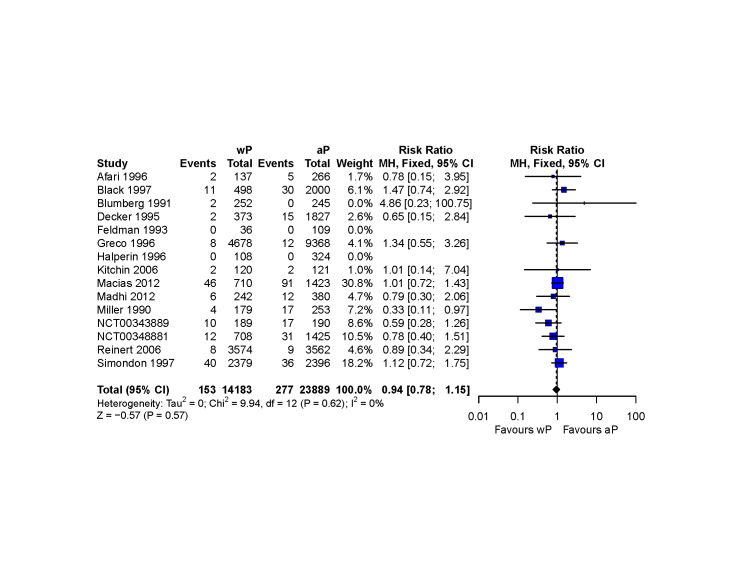
The pooled RR was 0.94 for all‐cause SAEs (95% CI 0.78 to 1.15; I2 = 0%; 15 studies, 38,072 children). For every 1000 children primed with a first dose of wP, 11 had an SAE. The corresponding risk with aP was 12 children (95% CI 9 to 13). The 95% CI around the risk difference ranged from three fewer to two more events per 1000 children, and the certainty of the evidence was judged as moderate (downgraded one level for imprecision).
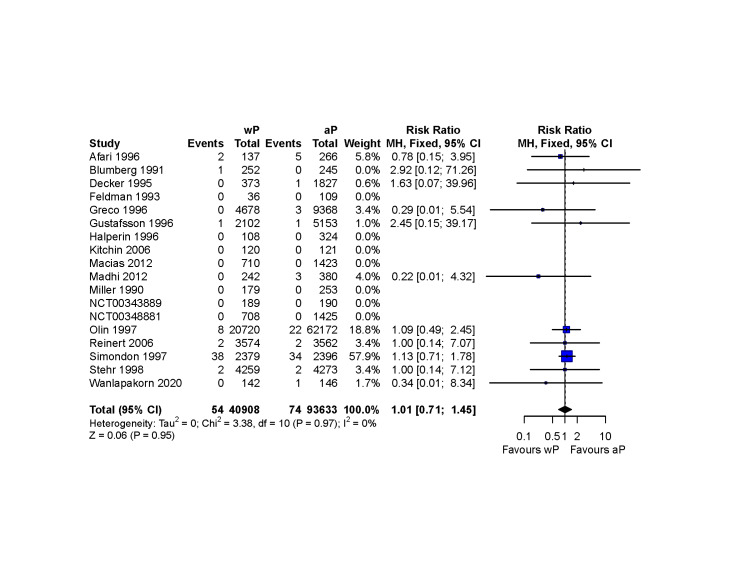
No diagnoses of encephalopathy following vaccination were reported (95% CI around the risk difference ‐ 5 to 12 per 100,000 children; seven primary series studies; 115,271 children). The certainty of the evidence was judged as low, since this is a serious condition, and we could not exclude a clinically meaningful difference.
Authors' conclusions
There is very low‐certainty evidence that a first dose of wP given early in infancy, compared to a first dose of aP, affects the risk of atopic diseases in children. The incidence of all‐cause SAEs in wP and aP vaccinees was low, and no cases of encephalopathy were reported. The certainty of the evidence was judged as moderate for all‐cause SAEs, and low for encephalopathy.
Future studies should use sensitive and specific endpoints of clinical relevance, and should be conducted in settings with high prevalence of IgE‐mediated food allergy. Safety endpoints should prioritise common vaccine reactions, parental acceptability, SAEs and their potential relatedness to the dose administered.
Plain language summary
Can a first dose of whole‐cell whooping cough vaccine given before six months old prevent allergic diseases in childhood?
What are allergic diseases?
Allergic diseases are among the most common persistent illnesses in children. They are caused by the immune system reacting abnormally to otherwise harmless substances such as foods and pollens. Food allergies are of increasing concern as the number of cases reported in a number of high‐income countries over the past 30 years appears to have increased.
Why we did this Cochrane Review?
The only proven preventive strategy against food allergies is early introduction of peanut and egg into the infant diet. However, a recent study found that food allergies appeared less common in children who had received one or more doses of whole‐cell (wP) whooping cough vaccine in early infancy than in those who had received acellular (aP) whooping cough vaccines only. That study could not determine whether the apparently lower risk of allergy was because of the wP vaccine, or whether it was because of other potential differences between wP and aP‐vaccinated children, as the vaccines were not randomly assigned. Therefore, a Cochrane Review was required to identify any evidence of wP as a food allergy prevention strategy.
What did we do?
We searched for studies that compared wP versus aP vaccination in babies younger than six months. We were interested in comparing babies vaccinated with wP vaccines and those vaccinated with aP vaccines, with respect to:
1. how many went on to develop food allergy, asthma or serious (and potentially life‐threatening) allergic reactions;
2. how many had serious unwanted events following vaccination; and,
3. how many had encephalopathy, a serious yet uncommon condition affecting the brain.
To compare rates of encephalopathy and other serious unwanted events, we looked for studies in which babies were given wP or aP vaccines at random (randomised controlled trials (RCTs)). To compare rates of allergic diseases, we also looked for studies where wP or aP vaccines were not given at random (non‐randomised studies of interventions (NRSIs)). In either case, studies lasted for at least six months.
Search date
We included evidence published up to September 2020.
What we found
Investigation 1
We found four studies (7333 children) carried out in Sweden (one), Australia (two) and the UK (one) that looked at the effect of whooping cough vaccines on allergic diseases. As we found little reliable data about the risk of food allergy after whooping cough vaccine, we decided to look at the risk of any allergic disease. Within 2.5 years of receiving a whooping cough vaccine (one RCT), 37/137 children vaccinated with wP, and 114/360 vaccinated with aP were diagnosed with at least one allergic disease. During the same period 15/137 vaccinated with wP and 38/360 vaccinated with aP were diagnosed with asthma specifically. No studies assessed serious or potentially life‐threatening allergic reactions.
Investigations 2 & 3
Low numbers of serious unwanted effects were reported for all groups (15 studies, 38,072 children). For every 1000 babies vaccinated with a first dose of wP, 11 had at least one serious unwanted effect. The risk for those who received aP vaccines was 12 children. No cases of encephalopathy were identified in either group (seven studies, 115,271 children).
How reliable are these findings?
One RCT reporting on whooping cough vaccines and allergic diseases included few children, and was carried out in a country with low levels of allergic disease. Therefore, it remains very uncertain whether a first dose of wP does or does not decrease the risk of allergic diseases.
Very few children experienced serious unwanted effects. We are uncertain whether there is a difference in the risk of serious unwanted effects in children vaccinated with a first dose of wP, compared with aP, but any difference is likely to be small. No cases of encephalopathy following vaccination were reported. Because this is a serious outcome, the certainty of the evidence was judged to be low.
Key messages
Ongoing and future studies may change our conclusions and provide more definitive evidence. The data reviewed suggest that wP is safe and support its continued use in countries where it is still recommended for preventing whooping cough.
Summary of findings
Summary of findings 1. Efficacy and safety of a first dose of whole‐cell pertussis vaccine compared to a first dose of acellular pertussis vaccine for the prevention of atopic diseases in children.
| Efficacy and safety of a first dose of whole‐cell pertussis vaccine compared to a first dose of acellular pertussis vaccine for the prevention of atopic diseases in children | |||||
| Patient or population: infants younger than six months of age Setting: paediatric and immunisation clinics, vaccine treatment units attached to academic institutions and healthcare centres. The trials were carried out in 11 countries across North and South America, Europe, Sub‐Saharan Africa and South East Asia Intervention: first dose of whole‐cell pertussis vaccine (wP) Comparison: first dose of acellular pertussis vaccine (aP) | |||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with first dose of acellular pertussis vaccine (aP) | Risk difference with first dose of whole‐cell pertussis vaccine (wP) | ||||
| Cumulative incidence of atopic disease at 2.5 years old | 497 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ‐ | It remains uncertain whether a first dose of wP, compared to aP, may prevent atopic diseases (RR 0.85, 95% CI 0.62 to 1.17). | |
| Diagnosis of IgE‐mediated food allergy | 0 (studies) | ‐ | ‐ | One study reported this (Nilsson 1998) as part of a broader outcome domain (i.e. cumulative incidence of atopic disease at 2.5 years old; reported in row above). However, it was not possible to obtain the data on incidence of IgE‐mediated food allergy specifically by study arm. | |
| Diagnosis of asthma | 497 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 | ‐ | It remains uncertain whether a first dose of wP, compared to aP, may prevent asthma (RR 1.04, 95% CI 0.59 to 1.82). | |
| Diagnosis of anaphylaxis (not vaccine associated) | 0 (0 studies) | ‐ | ‐ | ||
| All‐cause serious adverse events | 38,072 (15 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | RR 0.94 (0.78 to 1.15) | Study population | |
| 12 per 1,000 | 1 fewer per 1,000 (3 fewer to 2 more) | ||||
| Diagnosis of encephalopathy | 115,271 (7 RCTs) | ⊕⊕⊝⊝ LOW 5 | not estimable | Study population | |
| 0 per 100,000 | 0 fewer per 100,000 (5 fewer to 12 more)6 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval;RCT: randomised controlled trial; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
1 We downgraded the evidence by one level due to indirectness (study carried out in the late 1990s, in a country with low prevalence of IgE‐mediated food allergy)
2 We downgraded the evidence by two levels due to imprecision (single study, statistically underpowered to detect a reduction in its chosen endpoints, except for a very large reduction > 50%)
3 We downgraded by one level for indirectness, as it is plausible that children diagnosed with 'asthma' by 2.5 years old, may have been 'transient (episodic) wheezers'. We believe that the risk of developing transient wheeze in early childhood is unlikely to be affected by wP priming
4 We downgraded the certainty of the evidence by one due to imprecision (the 95% CI ranges from a potential decreased to a potential increased risk, unlikely to be clinically meaningful)
5 Although the 95% CI around the absolute difference is narrow, we could not rule out a clinically meaningful difference and therefore, we rated down two levels for imprecision
6 95% CI calculated using the score method (Newcombe 1998)
Background
See Appendix 1 for a glossary defining some of the scientific terms used throughout this review.
Description of the condition
Allergic (atopic) diseases are the most common non‐communicable diseases of childhood (Prescott 2013). The 'atopic march' is typically described as commencing in early childhood with the development of eczema (atopic dermatitis), followed by immunoglobulin E (IgE)‐mediated food allergy and later, asthma and hay fever (allergic rhinitis/allergic rhino‐conjunctivitis) (Hill 2018). The main mechanistic features of the atopic march are thought to be epidermal barrier disruption, pathologically skewed T helper (Th)2 immune responses, and chronic inflammation. This model has been challenged by cohort studies describing different disease trajectories (Illi 2004; Punekar 2009; Simpson 2010), and more recently by the characterisation of distinct atopic dermatitis phenotypes, according to age of onset, presence of sensitisation to food and aero‐allergens, family history of atopic diseases and subsequent development of asthma or other atopic comorbidities (Amat 2015; Roduit 2017).
Data from the Global Burden of Disease Study estimate that at least 6% of children aged between five and nine years old have a history of asthma; 3% of children aged between one and four years old have a history of urticaria, and 8% within the same age range have a history of atopic dermatitis (Global Burden of Disease 2018). For urticaria, the estimated prevalence is at least 2.5 times higher in countries with high socioeconomic indices than in less economically developed countries (Global Burden of Disease 2018).
The prevalence of asthma has levelled off in countries with the most affluent economies; by contrast, it has continued to increase in low‐to middle‐income countries with increasing urbanisation and the adoption of a Western lifestyle (Bousquet 2005; Holgate 2015). The true prevalence of IgE‐mediated food allergy, as well as its apparent increase, is difficult to determine using population‐based data (Dunlop 2018; NAS 2017). This is because a reproducible immune response following the consumption of the suspected food allergen, can only be assessed through a formal oral food challenge (Dunlop 2018). This medical procedure is expensive and time consuming, and as noted in a previous systematic review, over the last decade few epidemiological studies have used it to define IgE‐mediated food allergy in paediatric populations (Nwaru 2014). In that regard, the estimated overall prevalence of challenge‐confirmed IgE‐mediated food allergy in Australia during the first year of life was 10.4% (95% confidence interval (CI) 9.3% to 11.5%; 9.0% for raw egg allergy, 95% CI 7.8% to 10.0%) (HealthNuts Study 2011). On the other hand, findings from a large multinational birth cohort study carried out in Europe showed that the mean incidence of hen's egg allergy by two years old was estimated at 0.84%, (95% CI 0.67 to 1.03), and varied across countries with Greece reporting the lowest (0.07%; 95% CI 0.00 to 0.37%), and the UK the highest incidence (2.18%, 95% CI 1.27 to 3.47) (EuroPrevall 2016). In this study, double‐blinded placebo‐controlled oral food challenges were carried out with pasteurised raw hen egg powder, which is reported by the authors as having an analogous allergenicity to raw egg (EuroPrevall 2016).
Description of the intervention
Whole‐cell pertussis‐ (whooping cough) containing vaccines (wP) are suspensions of killed Bordetellapertussis bacteria, the causative agent of pertussis. These vaccines were introduced in the 1940s and implemented by the World Health Organization (WHO) in 1974 for the primary prevention of pertussis through the 'Expanded Programme on Immunization' (EPI) (Keja 1988). By 2015, 64% of countries worldwide had wP‐based national immunisation schedules (WHO 2015). wP vaccines are safe and mainly available as a multivalent co‐formulation with diphtheria (D) and tetanus (T) toxoids, Haemophilusinfluenzae type b (Hib) and hepatitis B (HepB) antigens (WHO 2015a). This combination vaccine is available in 73 of the lowest‐income economies via the support of Gavi, the Vaccine Alliance (Gavi 2020), as well as in self‐financed lower‐middle income countries non‐eligible for Gavi's funding programmes (UNICEF 2017). The inception of Gavi's support for wP‐based '5‐in‐1' (pentavalent) formulations commenced in 2001 and by the end of 2018, at least 467 million children living in eligible countries had been vaccinated (Gavi 2020). This has contributed to the marked reduction in the global burden of pertussis and pertussis‐related deaths (Chow 2016), and has had accompanying economic and social benefits.
Fever, irritability and local injection site reactions (such as pain, redness and swelling) are expected adverse events that arise following immunisation with wP‐based vaccines. Although these events are self‐limiting, the development of less reactogenic subunit acellular pertussis‐containing vaccines (aP) in the 1970s (Sato 1984), instigated a changeover from wP‐ to aP‐based schedules in high‐income countries from the 1980s to the early 2000s.
The tolerability profile of aP versus wP has been reviewed systematically elsewhere and favours aP formulations (Patterson 2018; Zhang 2014); nonetheless, priming with wP is safe, and may result in longer lasting protection against pertussis than priming with aP vaccines (CDC 2012; Liko 2013; Sheridan 2012; van der Lee 2018). A potential causal relationship between wP and rare neurological outcomes (i.e. encephalopathy) was proposed, but could not be confirmed by detailed examination in the UK National Childhood Encephalopathy Study (Miller 1993), as well as other epidemiological and genomic analyses (Berkovic 2006; McIntosh 2010; Ray 2006). The WHO have advised that countries using wP should continue using wP‐based primary vaccination courses (WHO 2015a).
How the intervention might work
During the neonatal and early infancy periods, a diversity of stimuli, including infections and vaccines, might determine future functional adaptations of the immune system (Olin 2018). In that regard, differential immune profiles elicited by B.pertussis and pertussis‐containing vaccines have been described in human (de Graaf 2020), non‐human primate (Warfel 2014), and other animal models (Mills 1998).
Priming with aP vaccines induces Th2‐dominated immune responses (Ausiello 1997; Rowe 2000), with transient enhanced production of diphtheria, tetanus toxoid and pertussis toxin IgE (Aalberse 2019; Hedenskog 1989; Holt 2016). Furthermore, Th2‐skewed responses observed with aP vaccines appear to extend beyond vaccine antigens, as evidenced by a transiently increased egg‐ and milk‐specific IgE in early infancy (Holt 2016), as well as the induction of type 2 cytokines to the food antigen beta‐lactoglobulin at six months old (Mascart 2007). In contrast, infection with Bordetellapertussis and wP vaccines induce Th1/Th17 polarisation with minimal expression of type 2 immunity (Ausiello 1997; Higgs 2012; Mascart 2007; Warfel 2014). This effect has been hypothesised to facilitate the healthy transition from the Th2‐dominant immunophenotype seen in early infancy, to a more balanced Th1/Th17/Th2 immunophenotype that may be necessary for the development of oral tolerance to foods, and allergy protective immune responses (Estcourt 2020). Therefore, vaccine schedules using wP as the first infant pertussis vaccine might overcome the persistent Th2‐skewed immunophenotype observed in some infants (Holt 2016), and thereby protect against IgE‐mediated food allergy and other atopic outcomes.
Although mechanistic studies have found a propensity to type 1 T‐cell differentiation and possible development of an 'allergy protective immunophenotype' following early priming with wP (Ausiello 1997; Mascart 2007), three studies found no association between the type of pertussis vaccine received and subsequent risk of atopic diseases among European (Nilsson 1998; Venter 2016), and Australian children (Toelle 2020).
Why it is important to do this review
Allergic diseases have a significant economic, healthcare and quality‐of‐life impact. To date, the timely introduction of peanut and egg into the infant diet are the only evidence‐based prevention approaches against egg and peanut allergy (Ierodiakonou 2016), and therefore, it is imperative to identify additional measures to avoid food sensitisation, and further development of food allergic reactions. Systematic reviews on the safety of pertussis‐containing vaccines have not addressed whether wP plays a role in the protection against food allergy or other atopic outcomes (Patterson 2018; Zhang 2014). Therefore, this review will provide a critical appraisal of the relevant evidence as well as directions for the future research.
Objectives
To assess the efficacy and safety of wP vaccinations in comparison to aP vaccinations in early infancy for the prevention of atopic diseases in children.
Methods
Criteria for considering studies for this review
Types of studies
Eligibility was restricted to studies with at least six months of follow‐up and the following designs, irrespective of publication status, date of publication, publication type or language.
Randomised controlled trials (RCTs) and cluster‐RCTs.
Controlled clinical trials (CCTs) or trials in which it was not clearly stated that the intervention or comparison was allocated at random, but in which it is not possible to exclude randomisation (Lefebvre 2021a). We classified quasi‐randomised studies as CCTs.
For atopic outcomes, we assessed case‐control and cohort studies (hereafter referred as non‐randomised studies of interventions (NRSIs)) in which the individual vaccine status of the child was known.
We did not include cross‐over trials since any differential immunological effects induced by pertussis vaccination are likely to be long term, and may still be patent in adulthood, irrespective of subsequent booster doses of aP during or after adolescence (Bancroft 2016; da Silva Antunes 2018).
Types of participants
Children aged less than 18 years old, who received their first dose of wP‐ or aP‐containing vaccines before the age of six months, irrespective of any subsequent pertussis vaccinations (wP, aP or none).
Types of interventions
We included studies where:
the experimental intervention was vaccination with any vaccine formulation that contained wP;
the comparator was vaccination with any vaccine formulation that contained aP.
Placebo vaccination or no intervention were not accepted as comparators, as they do not represent the standard of care for the primary prevention of pertussis.
The first dose of the wP‐ or aP‐containing vaccines was required to have been administered before participants reached six months old, irrespective of any subsequent vaccinations. This is because early infancy is thought to be the critical period for maturation from a Th2‐dominant to a balanced Th1/Th2/Th17 immunophenotype, and therefore, where immunisation might affect this process. Booster dose studies were only eligible if they met the following criteria:
the comparison was between recipients of one or more doses of wP versus aP;
children received a randomly allocated first dose of wP or aP before six months of age;
information on the type of first dose of pertussis‐containing vaccine was available.
We accepted co‐administered vaccines in either the experimental and control group. Matching between groups was not required for randomised studies; for NRSIs, we assessed co‐interventions as recommended by the ROBINS‐I tool (Sterne 2016a).
Types of outcome measures
We analysed the outcomes listed below. Studies that did not assess any of the outcomes of interest were excluded.
Primary outcomes
Diagnosis of IgE‐mediated food allergy.
Cumulative incidence of atopic diseases. As planned and prespecified in our protocol (Data Synthesis section), we added this outcome as only one study systematically assessed the atopic outcomes of interest, and 'diagnosis of IgE‐mediated food allergy' outcome data were not available by study arm.
-
All‐cause serious adverse events (SAEs) following immunisation with wP or aP (safety). This outcome was defined as any adverse event that resulted in death, was life‐threatening, required hospitalisation or prolongation of existing hospitalisation, or resulted in persistent or significant disability or incapacity (ICH 1997). Because this standard definition has not been universally applied in trials until recently, we also accepted adverse events that met the above‐mentioned criteria, irrespective of whether the report refers to ICH 1997. The following outcome domains were extracted from the definition and included in the review:
death (all‐cause mortality);
events leading to admission to hospital;
events described as 'life‐threatening';
events leading to persistent or significant disability or incapacity.
Secondary outcomes
Diagnosis of anaphylaxis (not vaccine‐associated).
Diagnosis of asthma.
Diagnosis of allergic rhinitis or allergic rhino‐conjunctivitis.
Diagnosis of eczema or atopic dermatitis.
Diagnosis of urticaria (not vaccine‐associated).
Diagnosis of encephalopathy (safety).
Primary and secondary atopic outcomes could be diagnosed at any point after enrolment by any of the following (any item listed under number 1 +/‐ any item listed under number 2 or where applicable, any item listed under number 3):
-
a positive history of that outcome ascertained via:
parental report (whether using validated questionnaires or not);
clinician diagnosis;
parental report and clinician diagnosis;
-
evidence of IgE‐mediated sensitisation via:
a positive skin prick test;
elevated total or specific elevated IgE;
-
one or both (where applicable) of:
evidence of a formal positive oral food challenge to the implicated food;
confirmed expiratory airflow limitation (i.e. spirometrically confirmed asthma).
If eligible studies reported atopic outcomes using more than one method, we used the following hierarchy of diagnoses: clinician‐diagnosed allergic disease with evidence of IgE‐mediated sensitisation, over clinician diagnosis without confirmed IgE‐mediated sensitisation. However, clinician diagnosis without confirmed IgE‐mediated sensitisation was used over parental report using validated questionnaires or not. Where applicable, we used formal challenge confirmed IgE‐mediated food allergy or evidence of variable expiratory airflow limitation over clinician‐diagnosed allergic disease with evidence of IgE‐mediated sensitisation.
As the efficacy of wP and aP for preventing pertussis has been summarised by a Cochrane Review (Zhang 2014), and solicited systemic and local adverse events have been reviewed separately (Patterson 2018), these were not included as outcomes.
Search methods for identification of studies
We conducted systematic searches following the recommendations provided in Chapter 4/Technical Supplement (Lefebvre 2021a; Lefebvre 2021b) and Chapter 24 (Reeves 2021) of the CochraneHandbookofSystematicReviewsofInterventions for the identification and selection of eligible studies. There were no language restrictions, but the electronic searches were limited from 1970 to present, as aP vaccines were developed in the late 1970s, and used for the first time in Japan for mass‐immunisation in 1981 (Sato 1984). The date of the search was 7 September 2020.
To maximise the sensitivity of the search strategies for the identification of controlled NRSIs, we applied a filter to the electronic searches in Ovid MEDLINE and Embase. This filter was developed by Waffenschmidt 2020, and at the time of the search had only been validated for Ovid MEDLINE and Pubmed.
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, issue 9; searched via the Cochrane Register of Studies Web): we modified the CENTRAL search strategy of Zhang 2014 by using free‐text words for subject‐specific aspects and by incorporating the study population into the search fields (Appendix 2). This strategy was adapted for the searches of other electronic databases.
Ovid MEDLINE (R) All (Appendix 3).
Embase (Appendix 4).
Searching other resources
We also searched the following resources from inception to 7 September 2020 (Appendix 5):
US National Library of Medicine's trial registry (clinicaltrials.gov/).
WHO International Clinical Trials Registry Platform (ICTRP) portal (www.who.int/clinical-trials-registry-platform/the-ictrp-search-portal).
US Food and Drug Administration (www.fda.gov/).
European Medicines Agency (www.ema.europa.eu/en).
Pharmaceutical companies: GSK trial registry (www.gsk-studyregister.com/en/), Sanofi (www.sanofi.com/en/science-and-innovation/clinical-trials-and-results/our-disclosure-commitments/pasteur#para_4), and Pfizer (www.pfizer.com/) websites.
Reference list and citations of eligible studies.
Additional grey literature (Open Grey; www.opengrey.eu/).
Data collection and analysis
Selection of studies
Two review authors (GPC and JR) independently screened the titles and abstracts of search results against the prespecified eligibility criteria (see Criteria for considering studies for this review). Disagreements were resolved through discussion with a third review author (TS). For potentially eligible references or where eligibility was unclear, we retrieved the full‐text reports.
Two review authors (GPC and JR) independently appraised the full‐text reports against the eligibility criteria. Similarly, disagreements were resolved through discussion with a third review author (of MJE and TS). We documented the selection process, and where applicable, collated multiple references of studies under the same identifier (so that the study, rather than the reference, was the unit of interest). Where a booster dose study enrolled children primed with wP or aP in a single RCT (i.e. a single cohort), we linked it to the primary series trial. However, if the population of a booster dose study included children from different cohorts, the booster dose study was presented separately.
Data extraction and management
Randomised controlled trials
Two review authors (GPC and JR) independently extracted data from the eligible studies using a customised data collection form, following the recommendations provided in Chapter 5 of the CochraneHandbookofSystematicReviewsofInterventions (Li 2021). We resolved discrepancies through discussion or through the arbitration of a third review author (of MJE and TS). Where available, we extracted the following information and where required, we attempted to contact authors of the original reports for clarification or to request missing data.
Initials of data extractors, date of data extraction and citation.
Study characteristics: study design, recruitment and sampling procedures, start and end dates of the trial, and length of follow‐up.
Population (P): study setting and country and World Bank income level of country, ethnicity, eligibility criteria, unit of analysis, number of children in each study group, withdrawals/loss to follow‐up, mean age, age range, sex, and comorbidities (if any).
Intervention (I) and comparator (C): type of pertussis‐containing vaccine administered (generic name), manufacturer, route of delivery, dose, and schedule.
Vaccines co‐administered: generic name, manufacturer, route of delivery, dose, and schedule.
Vaccination with Bacille‐Calmette‐Guérin (BCG or vaccine against tuberculosis): manufacturer and dose timing.
Antipyretic/analgesic use.
Outcomes (O): primary and secondary outcomes and their definition, evidence of assessment and whether they were collected systematically, time points reported and method of aggregation.
Risk of bias (Assessment of risk of bias in included studies).
Source(s) of funding.
Authors' conflicts of interest.
Miscellaneous: correspondence required, comments from the reviewers or study authors.
The data extracted on population, intervention, comparison and outcomes were used to systematically grade the directness of the evidence. This was described in the protocol of this review as "judgement of directness of each one of the PICO elements using Schünemann 2013 checklist" (Perez Chacon 2020).
Non‐randomised studies of interventions
For NRSIs, we extracted the information as for RCTs, as well as potential confounding factors (and any attempt to adjust for these). MJE, PR, PH and TS were not involved in any step regarding the assessment of their case‐control study (Estcourt 2020).
Assessment of risk of bias in included studies
Randomised controlled trials
Two review authors (GPC and JR) independently assessed the risk of bias using Cochrane's 'Risk of bias' tool version 1, following the guidance set out in the CochraneHandbookofSystematicReviewsofInterventions to evaluate the appropriate domains (Higgins 2011). These are sequence generation, allocation concealment, blinding of participants, personnel and outcome assessment, incomplete outcome data, and selective reporting, as well as other sources of bias (Higgins 2011). In this case, each domain was assessed as having low, unclear or high risk of bias. We resolved disagreements by discussion and where required, through the arbitration of a third review author (of MJE, CBJ, PR, PH or TS).
Non‐randomised studies of interventions
Two review authors (GPC and JR) independently assessed the risk of bias using the ROBINS‐I tool (version 1, August 2016; Sterne 2016a), following the tool's detailed guidance (Sterne 2016b), the CochraneHandbookofSystematicReviewsofInterventions (Sterne 2021), the author guidance (Cochrane Methods 2020), and the target trial methodology. The outcomes assessed were: diagnosis of IgE‐mediated food allergy, diagnosis of anaphylaxis (not vaccine‐associated) and diagnosis of asthma. We judged the bias arising pre‐intervention (bias due to confounding and the process of selection of children in the study); at‐intervention (bias in classification of interventions) and post‐intervention domains (bias due to deviations from intended interventions, due to missing data, in measurement of the outcome and in selection of the reported result), by answering 'signalling questions' with further risk of bias judgement, guided by the tool algorithms. We resolved disagreements through discussion, and where required, through the arbitration of a third review author (TS). Judgements were documented in free‐text boxes and incorporated into the ROBINS‐I tables; no further tools (i.e. computer programmes) were used to manage these assessments.
In our protocol (Perez Chacon 2020), we noted the following confounders: year of birth, birth order, family history of allergic diseases, socioeconomic status, vaccination with BCG, prematurity and breastfeeding status. BCG meets the definition of co‐intervention specified in the detailed guidance of the ROBINS‐I tool, and therefore, we used this term in the risk of bias assessments. We also considered whether the study involved unmatched co‐administration of vaccines between groups.
We classified the overall risk of bias judgement for a specific outcome within each NRSI as: low risk of bias (if we judged all the domains at low risk of bias); moderate risk of bias (if we judged all the domains at low or moderate risk of bias, and the study provided good‐quality evidence for an RCT, but not comparable to a well‐conducted RCT); serious risk of bias (if we judged at least one domain at serious risk of bias, but no domain as having a critical risk of bias); critical risk of bias (if we judged at least one domain at critical risk of bias) or no information (if data were insufficient and a judgement could not be made) (Sterne 2016a).
Measures of treatment effect
We summarised and reported the number and proportion of children who experienced primary and secondary outcomes at least once (rather than as a count of outcomes per child). For each outcome, we quantified the effect of wP versus aP as a ratio of the risk (using risk ratios (RRs) and 95% CIs) or ratio of the odds for case‐control studies (odds ratio (ORs) and 95% CIs).
Unit of analysis issues
If a study had multiple comparison groups, we omitted any groups that did not meet our inclusion criteria, but listed them in the Characteristics of included studies table. Where appropriate, we used one of the following strategies:
where more than one relevant group was reported, we combined them to create a single pairwise comparison or;
we included the intervention groups separately in the analysis and split the control group.
Dealing with missing data
We dealt with missing data as advised in Chapter 10 of the CochraneHandbookofSystematicReviews (Deeks 2019); where possible, we analysed primary and secondary outcomes as intention‐to‐treat (randomised studies). Irrespective of study design, we attempted to contact the study investigators or sponsors to obtain missing outcome data. If the report presented the outcome data in a figure and the raw values were not described or not feasible to obtain from the investigators of the study, we extracted the relevant information from a screenshot of the figure of interest using a web‐based data extraction tool (WebPlotDigitizer 2020).
Assessment of heterogeneity
We analysed the data in RCTs and NRSIs separately. We examined the clinical and methodological diversity between studies and used this information to decide whether studies were similar enough to be pooled meaningfully. The presence of statistical heterogeneity of intervention effects across studies included in meta‐analyses was assessed by inspecting the point estimates and CIs of forest plots. We assessed the results of the Chi2 test for each meta‐analysis (with significance at the 0.1 level) and quantified heterogeneity using the I2 statistic. We used the thresholds recommended in Chapter 10 of the CochraneHandbookofSystematicReviewsofInterventions (Deeks 2019), with considerable heterogeneity defined as an I2 greater than 75%.
We investigated potential causes of any detected heterogeneity through the analyses described in Sensitivity analysis below.
Assessment of reporting biases
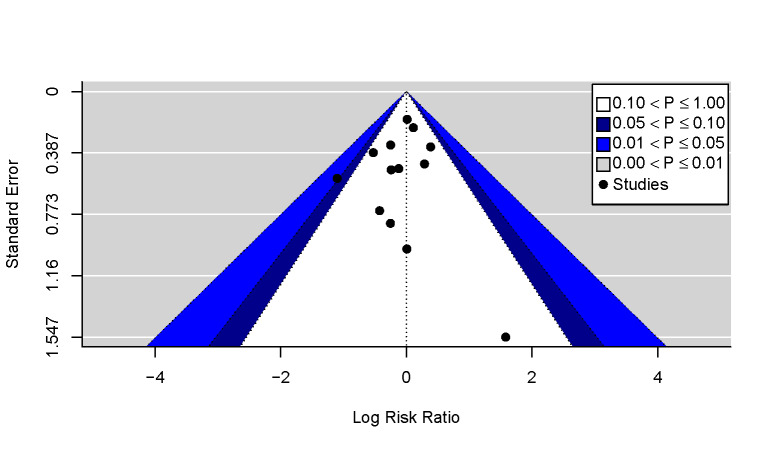
Where 10 or more studies were included in a meta‐analysis (see Data synthesis), we used contour‐enhanced funnel plots to distinguish non‐reporting bias from other sources of asymmetry (Page 2021; Peters 2008). These plots were generated in R using the 'metafor' package (R; Viechtbauer 2010).
Data synthesis
IgE‐mediated food allergy and other atopic outcomes
Randomised controlled trials
Only one study systematically assessed the atopic outcomes of interest (Nilsson 1998). Since IgE‐mediated food allergy was not reported by study arm, we extracted data on a broader outcome domain (i.e. cumulative incidence of atopic disease), as prespecified in our protocol (Perez Chacon 2020). The source of information was a bar plot, and we used WebPlotDigitizer 2020 to obtain the raw numbers. Analogous methods were implemented to obtain the relevant data points for asthma and atopic dermatitis.
We carried out narrative synthesis following the protocol of this review, and the SWiMReportingGuideline (Campbell 2020). We used RRs with 95% CIs as the standard metric. Data were pooled using random‐effects inverse‐variance method. This study is presented in forest plots generated in RevMan Web suppressing the summary estimate. Additional details are described in the Effects of interventions section based on GRADE (Campbell 2020; Reeves 2021).
Non‐randomised studies of interventions
Quantitative and narrative syntheses of NRSI reporting on atopic outcomes were not feasible due to the paucity of studies, the diversity of designs, and the risk of bias judgements (i.e. none were deemed at low or moderate risk of bias). However, these studies are described in the Characteristics of included studies table and Effects of interventions section.
Safety outcomes: serious adverse events and encephalopathy
We pooled RCTs and grouped them by safety endpoints. Data curation and meta‐analyses were performed in R, using the 'dplyr' (Wickham 2020) and 'meta' packages, respectively (Balduzzi 2019).
We used the Mantel‐Haenszel method assuming fixed‐effect, to summarise the RR and 95% CIs, without zero‐cell corrections for dichotomous outcomes, instead of stratified meta‐analyses using random‐effects inverse variance methods as initially proposed in our protocol (Perez Chacon 2020), because the safety outcomes of interest were rare (Deeks 2019; Efthimiou 2018).
Subgroup analysis and investigation of heterogeneity
We planned to undertake the following subgroup analyses:
grouped by age at first dose of pertussis‐containing vaccine: less than three months versus three months or greater;
grouped by BCG‐vaccinated versus not BCG‐vaccinated, since the Th1‐polarising properties of BCG may prevent atopic dermatitis and other atopic diseases in childhood (Steenhuis 2008; Thøstesen 2018); this in turn could reduce the benefits of priming with wP;
grouped by World Bank income level, for studies reporting on atopic outcomes; and,
grouped by family history of asthma, atopic dermatitis, food allergy, allergic rhinitis/rhino‐conjunctivitis, or a combination of these in first degree relatives, for studies reporting on atopic outcomes.
Due to a paucity of eligible studies in which the first dose of wP/aP was administered at or after three months old, as well as the small number of RCTs assessing the atopic outcomes of interest, we were unable to carry out subgroup analyses.
Sensitivity analysis
We carried out prespecified sensitivity analyses by removing studies judged as high risk of bias and those studies funded by pharmaceutical companies from any meta‐analyses pooling RCTs. Due to a paucity of studies that assessed the atopic outcomes of interest, it was not possible to conduct the prespecified analysis restricted to studies in which 'asthma' or 'current asthma' had been diagnosed after five years of age.
Summary of findings and assessment of the certainty of the evidence
Two review authors (of GPC, MJE and JR) independently assessed the certainty of the evidence as high, moderate, low or very low, using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) and standard Cochrane methods (Guyatt 2008; Ryan 2016; Schünemann 2021). The comparison of interest was the first dose of wP versus aP before the age of six months, and the following outcomes were assessed: cumulative incidence of atopic disease, diagnosis of IgE‐mediated food allergy, diagnosis of asthma, diagnosis of anaphylaxis (not vaccine‐associated), all‐cause SAEs following immunisation with wP or aP, and diagnosis of encephalopathy. We generated the summary of findings table using GRADEpro GDT software (GRADEpro GDT), and where synthesis without meta‐analysis was appropriate, we used narrative outcomes. Where justified, we downgraded or upgraded the level of evidence and documented all judgements clearly using written explanations. We prioritised the reporting of the assessments carried out in early infancy (i.e. primary series studies), or at the earliest time point of follow‐up. We resolved discrepancies by discussion or through the arbitration of a third review author (of CBJ, PR, PH or TS).
Results
Description of studies
Results of the search
Our database searches retrieved 13,999 references (CENTRAL, n = 1758; Ovid MEDLINE, n = 6825; Embase, n = 5416); and we identified further 982 references from the following sources: WHO trial registry (n = 608), clinicaltrials.gov (n = 310), and the website of Sanofi (n = 64). Together they represented 14,981 records that were subsequently managed through EndNote X9 and Covidence, where we removed duplicates.
We also screened titles and summaries from Open Grey (n = 16), the GSK trial registry (n = 763), the websites of Pfizer (n = 0), and regulatory assessments completed by the FDA (n = 340) and EMA (n = 36). We found 11 relevant titles in the references from eligible studies, and one study reported by the EMA that was not retrieved by the relevant trial registry. Together these represented 1166 records, labelled as 'other sources' in the PRISMA flow diagram (Figure 1).
1.
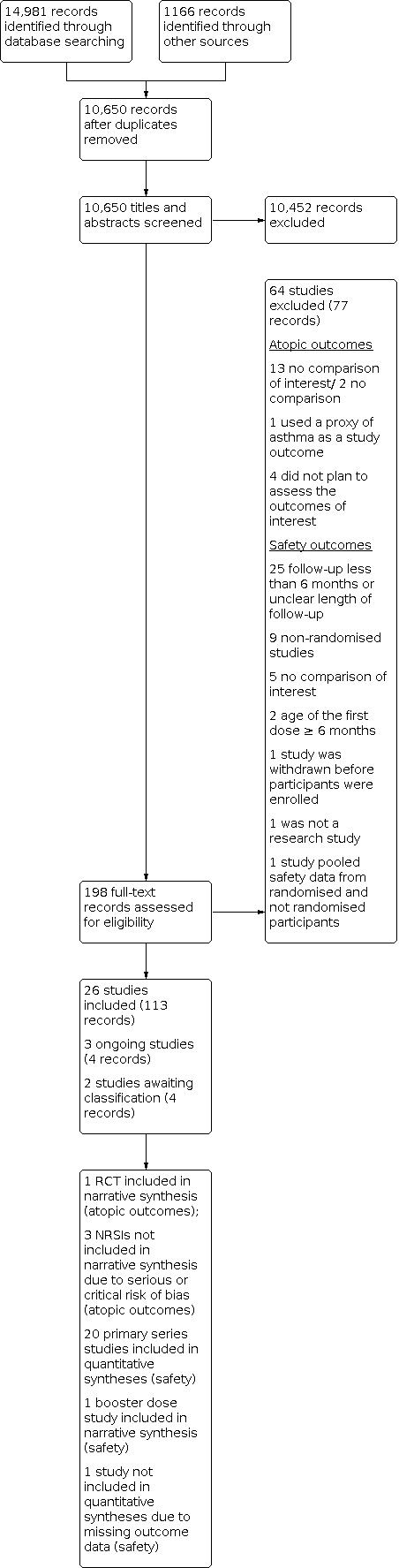
PRISMA flow diagram
After removing duplicates, we assessed a total of 10,650 titles, abstracts and regulatory data, and mapped records related to the same study, with further removal of 10,452 citations. Except for 'other sources', titles and abstracts were screened in Covidence.
We assessed 95 studies for eligibility (198 records), of which we included 26 (see the Characteristics of included studies table). Three studies were judged to be ongoing, two were awaiting classification, and 64 were excluded (see the Characteristics of excluded studies table for examples of these).
We depicted the flow of information through the different phases of the review using a PRISMA flow chart provided in Figure 1.
Included studies
Studies included in the review for IgE‐mediated food allergy and other atopic outcomes
Study design
This section of the review includes one randomised controlled trial (RCT) (Nilsson 1998), a cohort study (Venter 2016), a case‐control study (Estcourt 2020), and a post‐hoc analysis of an RCT, where data were treated as an observational longitudinal study to assess whether wP‐, compared to aP‐containing vaccines was associated with a decreased risk of atopic outcomes (Toelle 2020). A further trial ascertained symptoms consistent with early development of atopic diseases (i.e. wheezing, itchy rash, or sneezing) by 2.5 years in 97.8% of the children enrolled, but not the outcomes of interest (Gustafsson 1996).
Recruitment
Children living in the region of Linköping, who were recruited into the Swedish I efficacy, safety and immunogenicity of pertussis vaccines trial (Gustafsson 1996), were also offered enrolment in the allergy sub‐study of Nilsson 1998. Two follow‐up assessments were scheduled at 2.5 and seven years old. We prioritised the earlier point of follow‐up, because the corresponding report explicitly described IgE‐mediated food allergy as one of the outcomes of interest.
In non‐randomised studies of interventions (NRSIs), children were recruited antenatally in six hospitals in Sydney, Australia (Toelle 2020) or at birth on the Isle of Wight, UK (Venter 2016). For the case‐control study (Estcourt 2020), children with a diagnosis of IgE‐mediated food allergy were identified through medical records by specialist allergists from private and tertiary hospital allergy clinics in four out of nine states or territory jurisdictions in Australia.
Sample size
This section of the review includes 7333 children across three high‐income countries (Australia, Sweden and the UK), who were followed up between 2.5 and 15 years.
Setting
The trial of Nilsson 1998 was carried out in paediatric clinics and primary care centres. In the remaining studies, allergy assessments were undertaken in private and tertiary hospital allergy clinics in four out of nine states or territory jurisdictions in Australia (Estcourt 2020); in two metropolitan hospitals in New South Wales, Australia (Toelle 2020); and in a dedicated specialist allergy research unit on the Isle of Wight, UK (Venter 2016).
Intervention/exposure
Details of the combination vaccines used as intervention or comparator are provided in the Characteristics of included studies table. In the trial of Nilsson 1998, children received a first dose of diphtheria‐tetanus‐whole‐cell pertussis vaccine (DTwP) or diphtheria‐ tetanus‐acellular pertussis vaccine (DTaP) between 56 and 92 days old. In the study of Estcourt 2020, cases and controls were vaccinated with a first dose of DTwP or aP, with or without hepatitis B vaccine before 16 weeks old (DTaP‐HepB or DTaP). The children included in the cohorts of Venter 2016 and Toelle 2020 received a first dose of wP‐ or aP‐based formulations between six and 18 weeks of age.
Co‐interventions and BCG
In the trial of Nilsson 1998 the co‐administration of inactivated polio vaccine (IPV), Hib vaccine or both was scheduled with the first dose of DTwP or DTaP. Details of concurrent vaccination were not provided in the reports of NRSIs (Estcourt 2020; Toelle 2020; Venter 2016).
BCG was not included in the relevant national immunisation programmes at the time in which these children were enrolled in these studies, so it is unlikely that they received it before the first dose of pertussis‐containing vaccine.
Outcomes
Studies that reported on IgE‐mediated food allergy used confirmation via oral food challenge as the outcome measure (Estcourt 2020: sensitivity analysis) or defined it on the basis of a history of clinical symptoms and proven IgE‐mediated sensitisation via skin‐prick test (SPT) or serum specific IgE (Estcourt 2020; Nilsson 1998). In the study of Venter 2016, IgE‐mediated food allergy was diagnosed on the basis of either a compatible clinical history or oral food challenge. We followed a prespecified hierarchy of diagnosis and where both were reported separately (Estcourt 2020), we described the results of the association between a first dose of pertussis‐containing vaccine and challenge‐proven IgE‐mediated food allergy.
Studies that reported diagnoses of asthma, atopic dermatitis and allergic rhino‐conjunctivitis, provided different and in some cases several time points in their outcome definition (Nilsson 1998; Toelle 2020; Venter 2016). These are detailed in the Characteristics of included studies tables. No study assessed anaphylaxis (not vaccine‐associated) as an outcome of interest. Other trials described admissions to hospital for asthma or egg anaphylaxis as serious adverse events (SAEs) (Black 1997; Decker 1995; Kitchin 2006). None of these were their prespecified outcomes of interest, nor were their data collected systematically.
The overall numbers of children experiencing IgE‐mediated food allergy, allergic rhino‐conjunctivitis, and not‐vaccine associated urticaria by 2.5 years were included in the main report of the trial of Nilsson 1998; however, it was not possible to obtain a breakdown of these data by trial arm. Therefore, we decided to use a broader outcome domain (i.e. cumulative incidence of atopic disease) as prespecified in our protocol, as data were provided as required for this outcome. We estimated these data, and data on the diagnoses of asthma and atopic dermatitis from a bar chart using WebPlotDigitizer 2020.
Studies included in the review for safety
Study design
Primary series studies
Fourteen primary series studies looked at our prespecified safety outcomes, and were double‐blind, parallel, RCTs (Afari 1996; Black 1997; Blumberg 1991; Decker 1995; Feldman 1993; Greco 1996; Gustafsson 1996; Halperin 1996; Miller 1990; Miller 1997 ("trial 2"); NCT00348881; Olin 1997; Simondon 1997; Stehr 1998). Two used single masking (Macías 2012; NCT00343889), and five were open‐label (Dagan 1997; Kitchin 2006; Madhi 2011; Reinert 2006; Wanlapakorn 2020). Where aP‐based vaccine formulations were allocated to more than one study arm (Afari 1996; Decker 1995; Feldman 1993; Greco 1996; Gustafsson 1996; Halperin 1996; Macías 2012; Madhi 2011; Miller 1990; Miller 1997; Olin 1997), we combined these data to create a single pairwise comparison.
Booster dose studies
Children enrolled in the booster dose study of Edwards 1991 received a primary series with wP or aP in a double‐blind, parallel, randomised fashion; however, the primary series study published in 1989 has an unclear length of follow‐up, and therefore, we are not including its data for synthesis.
A subset of participants enrolled in the primary series trials of Decker 1995; Kitchin 2006; Madhi 2011; NCT00343889 and NCT00348881, completed an additional period of follow‐up after the administration of one or more booster doses of wP‐ or aP‐based formulations. The booster dose studies were reported in separate publications (Decker 1995; Kitchin 2006; Madhi 2011;) or under a different identifier on clinicaltrials.gov (NCT00343889; NCT00348881). In either case, we linked them to their corresponding primary series study and prioritised the earlier point of safety follow‐up to avoid double counting.
Studies with one or more arms that did not meet prespecified eligibility criteria
Four of the included studies that investigated relevant comparisons and outcomes in this review also included non‐relevant trial arms: Greco 1996, Gustafsson 1996 and Stehr 1998 included diphtheria and tetanus toxoids vaccine (DT) as a control arm, and Wanlapakorn 2020 co‐enrolled a non‐randomised group of infants and allocated them to wP, in accordance with the Thai 'Expanded Programme on Immunization' (EPI) The data from these specific trial arms were not included in this review as they did not meet prespecified inclusion criteria. Further details can be found in the Characteristics of included studies table.
Recruitment
Infants were recruited in paediatric practices (Decker 1995; Feldman 1993), from paediatric outpatient clinics attached to academic institutions (Feldman 1993), via letters to the parents of newborns living in the study catchment area (Gustafsson 1996; Olin 1997), from immunisation clinics (Miller 1990; Miller 1997; Simondon 1997), in maternal and child health centres (Afari 1996), or approached antenatally (Wanlapakorn 2020).
Sample size
A total of 137,281 children contributed data in 21 studies that reported our safety outcomes. Sample sizes ranged from 41 to 82,892 children per trial. As detailed in the PRISMA flowchart (Figure 1), one study did not contribute data to our quantitative syntheses (Miller 1997, "trial 2"). However, because it met the eligibility criteria for inclusion in this review, we summarise its characteristics below.
Location and World Bank income level of country
The studies assessing safety were carried out in Europe (Greco 1996; Gustafsson 1996; Kitchin 2006; Miller 1990; Miller 1997; Olin 1997; Reinert 2006; Stehr 1998), North America (Black 1997; Blumberg 1991; Decker 1995; Edwards 1991; Feldman 1993; Halperin 1996; Macías 2012), Sub‐Saharan Africa (Afari 1996; Madhi 2011; Simondon 1997), South East Asia (NCT00343889; NCT00348881; Wanlapakorn 2020), South America (Macías 2012) and the Middle East region (Dagan 1997). Studies included economies of all‐level income groups, according to the historical classification of the World Bank (World Bank 2021).
Setting
Community‐based studies in paediatric clinics, general practices, maternal and child health centres or public health units (Afari 1996; Dagan 1997; Greco 1996; Gustafsson 1996; Miller 1990; Miller 1997; Olin 1997; Reinert 2006; Simondon 1997) predominated over trials carried out in clinics attached to academic institutions (Decker 1995; Edwards 1991; Wanlapakorn 2020), or other healthcare facilities (Black 1997; Macías 2012; Madhi 2011). The trial of Feldman 1993 was carried out in private paediatric practices and outpatient clinics attached to a local university.
Population
Participants were typically healthy infants with an average age of approximately 9.8 weeks on the day of the administration of the first dose of pertussis‐containing vaccine (Dagan 1997; Feldman 1993; Greco 1996; Kitchin 2006; Macías 2012; Miller 1990; Miller 1997; NCT00343889; NCT00348881; Reinert 2006; Wanlapakorn 2020).
Only one study stratified randomisation by age at enrolment quote: "to minimise bias due to possible age‐associated safety outcomes" (Reinert 2006). The first dose of pertussis‐containing vaccine was given at three months old in the studies carried out by Miller 1990, and in a subset of infants enrolled in the trial of Olin 1997. In the trials of Halperin 1996 and Stehr 1998, the first dose of pertussis‐containing vaccine was given to healthy infants aged between two and three months old, and two to four months old, respectively. In the remaining studies, infants received their first dose before three months of age.
The proportion of children who were male ranged between 49% and 53% across studies (Afari 1996; Feldman 1993; Greco 1996; Gustafsson 1996; Halperin 1996; Kitchin 2006; Macías 2012; Madhi 2011; NCT00343889; NCT00348881; Olin 1997; Reinert 2006; Wanlapakorn 2020). Racial and ethnic categories (described in the Characteristics of included studies table as quote: "cultural and ethnic groups") were only reported by three studies (Feldman 1993; Decker 1995; Madhi 2011). Black 1997 used a qualitative statement to describe the infants enrolled in that trial as quote: "ethnically diverse" and "generally similar to the US census population in this region".
Intervention
A first dose of wP or aP was administered as a combination vaccine including diphtheria and tetanus toxoids (i.e. DTwP or DTaP; Afari 1996; Black 1997; Blumberg 1991; Decker 1995; Feldman 1993; Greco 1996; Gustafsson 1996; Halperin 1996; Miller 1990; Miller 1997; Olin 1997; Simondon 1997; Stehr 1998). Children enrolled in the studies of Edwards 1991 were primed with DTwP or DTaP in a previous study published in 1989.
DTwP‐HepB‐Hib (Macías 2012; NCT00343889; NCT00348881; Wanlapakorn 2020), DTwP‐Hib‐IPV (Dagan 1997; Reinert 2006) and DTwP‐Hib combination vaccines were used in the remaining eligible studies (Kitchin 2006; Madhi 2011). Similarly, DTaP‐based vaccine formulations administered in these trials included DTaP‐Hib‐IPV (Dagan 1997; Kitchin 2006), and DTaP‐HepB‐Hib‐IPV (Macías 2012; Madhi 2011; NCT00343889; NCT00348881; Reinert 2006; Wanlapakorn 2020). Additional details are provided in the Characteristics of included studies table.
Co‐interventions and BCG
Fifteen primary series studies reported the type of vaccines co‐administered with the first dose of pertussis‐containing vaccine. The regimens included Hib vaccine (Miller 1997), oral poliovirus vaccine (OPV) only (Feldman 1993; Halperin 1996; NCT00343889; NCT00348881), OPV and Hib vaccine (Black 1997; Decker 1995), OPV and hepatitis B vaccine (Greco 1996), OPV and meningococcal C conjugate vaccine (Kitchin 2006), BCG and IPV (Simondon 1997), IPV with or without Hib vaccine (Gustafsson 1996; Olin 1997). Whereas infants primed with a first dose of aP received a concomitant dose of OPV‐placebo (Macías 2012), or no concomitant vaccine (Madhi 2011; Reinert 2006; Wanlapakorn 2020), wP vaccinees were immunised with OPV (Macías 2012; Wanlapakorn 2020), OPV and hepatitis B vaccine (Madhi 2011), or hepatitis B vaccine (Reinert 2006).
Five trials did not provide any statement regarding co‐interventions (Afari 1996; Blumberg 1991; Dagan 1997; Miller 1990; Stehr 1998). Children enrolled in the trials of Macías 2012; Madhi 2011 and Wanlapakorn 2020 received BCG at birth in accordance with their local EPI.
Outcomes and endpoints (outcome domains)
Serious adverse events (SAEs)
All‐cause SAEs
The number of children experiencing at least one SAE could only be extracted from 15 primary series studies (Afari 1996; Black 1997; Blumberg 1991; Decker 1995; Feldman 1993; Greco 1996; Halperin 1996; Kitchin 2006; Macías 2012; Madhi 2011; Miller 1990; NCT00343889; NCT00348881; Reinert 2006; Simondon 1997), and the booster dose study of Edwards 1991. The timing of assessment differed across these studies and is summarised in the Characteristics of included studies table.
In the primary series trial of Decker 1995, data on events meeting the review definition of SAE were systematically collected from enrolment and reported at five and 18 months after randomisation. Because it is unclear whether any events reported at the 18‐month assessment occurred in children who had previously experienced an SAE, we only included data on the initial five months of follow‐up to avoid double counting.
A similar approach was undertaken to analyse the data from the primary series study, Kitchin 2006. In the initial stage of this open‐label trial, SAEs were defined as admissions to hospital (all‐cause) occurring within 10 months from enrolment. The investigators of this trial reported the safety data at two time points and it was not possible to determine if the infants with any SAE occurring before the age of five months also had an admission to hospital after this period. To avoid double counting, we only considered the outcomes reported at the earlier time point.
In the trial of Greco 1996, we assumed that children were censored after their first SAE, and calculated the total experiencing this outcome from the events reported per 1000 enrolled (i.e. deaths, quote: "other life‐threatening diseases," onset of chronic illness as a proxy of disability, and invasive bacterial infections; the latter were assumed to have led to hospital admission). We attempted to contact the authors of the trial to confirm this assumption, but were unsuccessful.
Gustafsson 1996 systematically collected the hospital records of all the infants admitted at any time from enrolment until two months after the third dose of pertussis‐containing vaccine (or eight months old, if series not completed). The FDA summarised the first admission to hospital of the infants enrolled in this trial according to the study arm and dose. Due to probable overlaps between admissions to hospital and other outcome domains, we were unable to extract the total number of infants who experienced any SAE.
The trial of Halperin 1996 monitored quote: "contacts with the healthcare system for any reason". Although no data regarding these events were included in the peer‐reviewed manuscript, the assessment completed by the FDA does report SAEs following the infant series.
Four trials published their safety data on clinicaltrials.gov (Macías 2012; Madhi 2011; NCT00343889; NCT00348881). We assumed that discrepancies between the number of children experiencing SAEs reported by study arm on the trial registry (Macías 2012; Madhi 2011; NCT00343889; NCT00348881), and the number affected by specific diagnoses could be explained by the presence of multiple conditions in the same infant at the time of the outcome assessment, or infants that experienced more than one SAE throughout the course of these trials. For clinicaltrials.gov, the definition of SAE not only includes the outcome domains extracted from ICH 1997, but also events that put the child in danger or required medical or surgical intervention to prevent any of the primary safety endpoints of interest for this review. Due to the discrepancies between the definition of SAE used in this review and the one included in the trial registry, these studies were included in the synthesis for 'all‐cause SAE' and where applicable, in the synthesis for 'all‐cause mortality'. Further disagreements between the number of children experiencing SAEs reported by peer‐reviewed articles arising from the studies of Macías 2012 and Madhi 2011 and their corresponding trial registries, are described in the Characteristics of included studies table.
"Trial 2" published by Miller 1997 warranted additional consideration. Following the introduction of an accelerated two‐, three‐ and 4‐month pertussis immunisation schedule in England in June 1990, the trial of Miller 1990 that compared the safety and immunogenicity of DTwP versus DTaP‐based formulations using a three‐, five‐ and eight‐ to 10‐month schedule, had to be repeated using the new regimen (Miller 1997). We confirmed with the corresponding author that the records of this study are unavailable, and therefore, we declared the outcome data as missing.
In the trial of Reinert 2006, children that experienced life‐threatening events (such as post‐vaccination anaphylaxis), were reported as withdrawn due to a definite medical contraindication to pertussis‐containing vaccines, but not counted among those who experienced SAEs. Similarly, deaths during the course of this trial were reported separately. For synthesis purposes, cases of post‐vaccination anaphylaxis and deaths were counted as SAEs.
Wanlapakorn 2020 reported the progress of the children enrolled throughout the study in a CONSORT diagram. Additional information on SAEs was not published on clinicaltrials.gov by 13 March 2021. Because it remains unclear whether SAEs other than deaths occurred during the course of this trial, this study was only included for synthesis on 'all‐cause mortality'.
All‐cause mortality
Eighteen primary series trials (Afari 1996; Blumberg 1991; Decker 1995; Feldman 1993; Greco 1996; Gustafsson 1996; Halperin 1996; Kitchin 2006; Macías 2012; Madhi 2011; Miller 1990; NCT00343889; NCT00348881; Olin 1997; Reinert 2006; Simondon 1997; Stehr 1998; Wanlapakorn 2020) and one booster dose study (Edwards 1991) provided information allowing us to extract data on deaths. Because the study of Black 1997 only planned to report data on sudden infant death syndrome (SIDS), we did not include it in the related meta‐analysis.
Events leading to admission to hospital (all‐cause)
Nine primary series trials (Black 1997; Blumberg 1991; Decker 1995; Edwards 1991; Gustafsson 1996; Halperin 1996; Kitchin 2006; Miller 1990; Simondon 1997) and one booster dose study (Edwards 1991) reported information on hospital admissions. The timing of assessment differed across these studies and is summarised in the Characteristics of included studies table.
In a personal communication, the corresponding author of the trial of Dagan 1997 confirmed that serious adverse reactions following immunisation did not lead to admission to hospital, and therefore, these events do not meet the regulatory definition of SAE considered in this review. It is unclear whether any SAEs unrelated to the study vaccines resulted in hospitalisation.
The trial of Olin 1997 only collected data on admissions to hospital for events contraindicating further vaccination with pertussis‐containing vaccines or for events that met their protocol definition of serious. The FDA assessment summarises the number of admissions to hospital per study arm occurring within 30 days of vaccination; nevertheless, we could not conclude from the report whether children were censored for this outcome domain after their first hospitalisation.
Stehr 1998 collected data on events requiring admission to hospital, but details are only provided for children hospitalised for serious infections.
Events described as life‐threatening
Here we included trials that provided information on this specific outcome domain (Greco 1996; Gustafsson 1996; Halperin 1996; Olin 1997; Stehr 1998), those which did not include life‐threatening events in their methods section but systematically collected data on post‐vaccination anaphylaxis (Dagan 1997; Simondon 1997), and those that reported the occurrence of anaphylaxis after any dose without further details (Reinert 2006).
The trial of Stehr 1998 assessed post‐vaccination anaphylaxis and events described as life‐threatening as separate study outcomes; however, the investigators only reported on vaccine‐associated anaphylaxis. The corresponding author estimated the number of children with other life‐threatening events in a personal communication.
The EMA reported adverse life‐threatening events for a single arm of the comparison of interest (i.e. infants vaccinated with an aP‐based vaccine formulation in the trials of Macías 2012 and Madhi 2011). Peer‐reviewed publications arising from these trials do not describe whether these data were systematically collected.
Events leading to persistent or significant disability or incapacity
Four studies contributed data to this outcome domain. One collected data on quote: "any illness resulting in sequelae" (Kitchin 2006), and three reported on the onset of chronic illnesses, a proxy of disability (Greco 1996; Gustafsson 1996; Halperin 1996). Stehr 1998 collected data on events defined as "permanently disabling", but results were not included in the publication assessed in this review. An author of the trial provided an estimate of the number of children who met this study endpoint through personal correspondence.
Diagnosis of encephalopathy
Seven primary‐series RCTs (Dagan 1997; Decker 1995; Feldman 1993; Greco 1996; Gustafsson 1996; Olin 1997; Stehr 1998) and one booster dose study (Edwards 1991) contributed safety data regarding encephalopathy for both relevant arms of the comparison. The timing of assessment differed across these trials and is summarised in the Characteristics of included studies table.
The EMA reported encephalopathy for a single arm of the comparison of interest (i.e. infants vaccinated with an aP‐based vaccine formulation in the trials of Macías 2012 and Madhi 2011). Peer‐reviewed publications arising from these trials do not describe whether these data were systematically collected.
Ongoing studies and studies awaiting classification
We identified three studies that are ongoing that may be eligible for inclusion in this review when complete (ACTRN12617000065392; ISRCTN17271364; NCT03606096). Further details are included in Characteristics of ongoing studies.
We also identified two studies where we were unable to make a judgment on eligibility. We were unable to source the report for 217744/025 (DTPa‐HBV‐IPV‐025), as this was no longer available through the GSK trial registry. For Mrozek‐Budzyn 2018, the age of the first dose of wP/aP was not stated in the report. In either case, our attempts to contact the sponsor of 217744/025 (DTPa‐HBV‐IPV‐025), or the lead and senior authors of the study of Mrozek‐Budzyn 2018 were unsuccessful. Further details are available in Characteristics of studies awaiting classification.
Excluded studies
Sixty‐four studies were excluded from this review at the full‐text screening stage. Thirty‐three examples of these are listed with reasons for exclusion in the Characteristics of excluded studies table.
The reasons for exclusion of studies reporting on atopy or atopic outcomes were: no comparison of interest (Bernsen 2006; Farooqi 1998; Grüber 2003; Grüber 2008; Henderson 1999; Kummeling 2007; Maitra 2004; Matheson 2010; McDonald 2008; McKeever 2004; Mullooly 2007; Swartz 2018; Thomson 2010), and no comparison (Wang 2012; Yamamoto‐Hanada 2020).
The study of Vogt 2014 warranted additional consideration. This observational study compared a cohort of children enrolled in the trial of Olin 1997, with children unvaccinated with pertussis antigens who were born five months before the start date of the RCT, or seven months after its end date, using "dispensed prescribed asthma medication" as a proxy of asthma. Therefore, it was classified as ineligible.
The reasons for exclusion for safety studies were length of follow‐up shorter than six months (Anderson 1988; Anderson 1994; Gylca 2000; Halperin 1994; Halperin 1995; Halperin 1999; Halperin 2003; Pichichero 1992; Pichichero 1993; Pichichero 1994; Pichichero 1996; Podda 1994; Simondon 1996; Vanura 1994; Wiersbitzky 1996); age at the first dose of pertussis‐containing vaccine (Blennow 1988); and study design (household contact study; Schmitt 1996).
Risk of bias in included studies
Studies included in the review for atopic outcomes
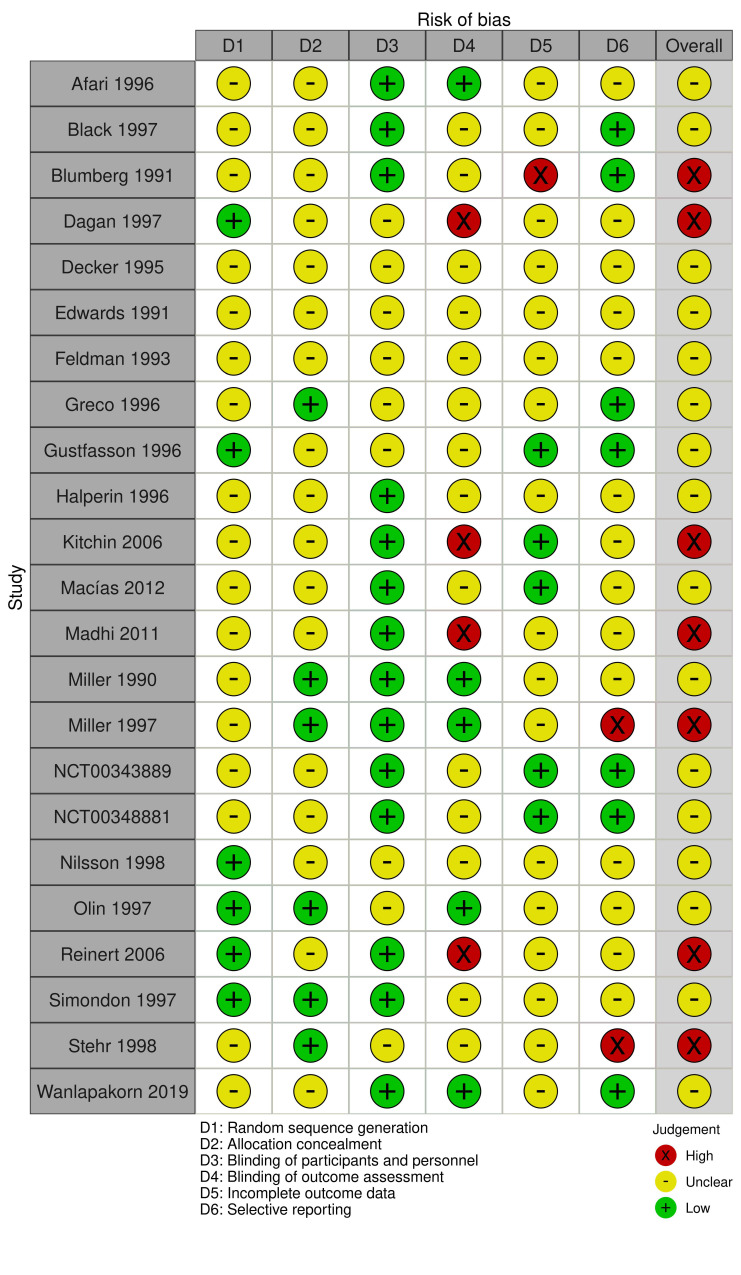
Randomised controlled trials
In Figure 2 we provide our judgement for each risk of bias category for the study of Nilsson 1998 (figure generated using robvis; McGuinness 2020).
2.
Risk of bias summary: judgement of the review authors about each risk of bias item for each included randomised controlled trial
Allocation (blinding)
The study of Nilsson 1998 enrolled a subset of children randomised in the trial of Gustafsson 1996. Therefore, the two trials each share the same judgements on the risk of bias arising from random sequence generation (low) and allocation concealment (unclear).
Blinding (performance bias and detection bias)
Because partial unblinding of the wP arm, but not the aP/DT arms occurred in the trial of Gustafsson 1996, we judged the study of Nilsson 1998 at unclear risk of bias for this domain. Additional information is provided in the Characteristics of included studies table.
Incomplete outcome data (attrition bias)
In the trial of Nilsson 1998, children who were not fully immunised, or who had incomplete follow‐up data were not included in the analyses. Other reasons for non‐completion include withdrawal of consent, and moving house away from the study area. The dropout rates cannot be calculated by study arm because the numbers randomised into each intervention group are not reported. This study was judged to be at unclear risk of bias due to incomplete outcome data.
Selective reporting (reporting bias)
The study of Nilsson 1998 was judged to be at unclear risk of bias since the data on IgE‐mediated food allergy was not made available by study group.
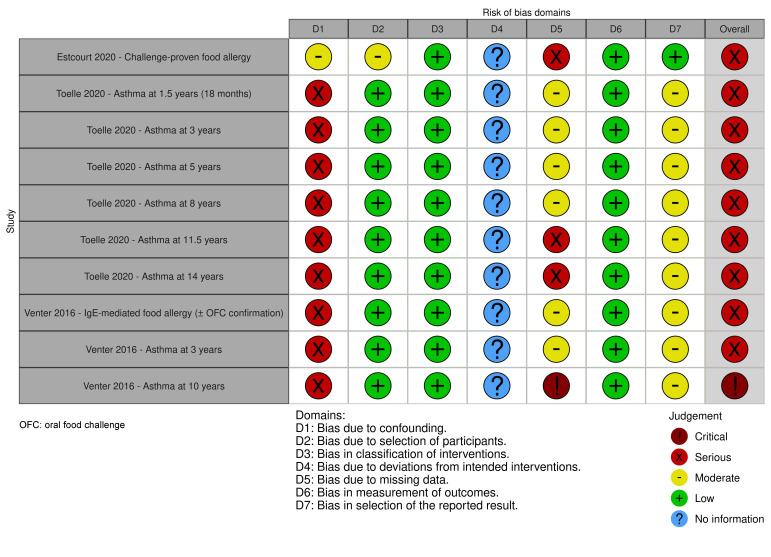
Non‐randomised studies of interventions
See Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8 and Figure 3 (figure generated using robvis; McGuinness 2020) for our risk of bias assessments for Estcourt 2020; Toelle 2020; and Venter 2016.
1. ROBINS‐I assessment for: first dose of whole‐cell versus acellular pertussis vaccine before 6 months of age. Outcome: diagnosis of challenge‐proven IgE‐mediated food allergy before 15 years old.
| Study | Bias due to confounding | Bias in selection of study participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall risk of bias |
| Estcourt 2020 | Moderate | Moderate | Low | No information | Serious | Low | Low | Serious |
| Rationale for judgement | Appropriately adjusted for surrogates of vaccine availability (date of birth and jurisdiction at vaccination) Potentially insufficient adjustment for socioeconomic status. Family history of atopy and breastfeeding could not have influenced the allocation to the intervention as this was largely dependent on vaccine availability. The method used to minimise confounding was direct matching |
This is a retrospective case‐control study. The selection of cases was based on the outcome of interest. The exposure distribution in the controls is likely to represent the exposure status of the overall cohort, as controls were selected from the Australian Immunisation Register irrespective of their past or future case status | Intervention status was well‐defined and based on information collected at the time of the intervention | There is no information reported | The association between vaccination status and challenge‐proven IgE‐mediated food allergy was tested through a sensitivity analysis, carried out according to the study protocol. Therefore, outcome data were only available for a non‐random subset of cases with a history of food allergic reaction coupled with IgE‐mediated sensitisation to the food of interest and a positive oral food challenge. A small number of children were excluded due to missing data on the exposure status. These data were likely to be missing at random |
The outcome assessors reviewed the medical records while blinded to the vaccination status. They determined whether a child met the primary outcome (clinical criteria of food allergy and evidence of sensitisation to the food that may have caused the allergic reaction), and whether there was a clinical record of a positive oral food challenge to that food at any time. During the follow‐up period, vaccination status would not have influenced the decision to challenge a child with the food of interest |
The study data were analysed according to a prespecified sensitivity analysis. There is no evidence of selective reporting. |
This risk of bias assessment was based on the data included in a sensitivity analysis (i.e. a non‐random subset of cases). There are some concerns regarding missing outcome data, insufficient adjustment for socioeconomic status and lack of adjustment by birth order |
2. ROBINS‐I assessment for: first dose of wP versus aP vaccine before 6 months of age. Outcome: diagnosis of asthma (current asthma) at five yearsa.
| Study | Bias due to confounding | Bias in selection of study participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall risk of bias |
| Toelle 2020 | Serious | Low | Low | No information | Moderate | Low | Moderate | Serious |
| Rationale for judgement | Allocation to the intervention was largely dependent on vaccine availability, as described in Estcourt 2020. The authors adjusted for breastfeeding status, sex, house dust mite avoidance and omega‐3 supplementation; however, these variables were unlikely to have influenced the assignment of the study vaccines | Children who would have been eligible for the target trial were included in the study. The start of the follow‐up period coincides with the start of intervention |
The intervention status was well‐defined and based on information collected at the time of the administration of the study vaccines | There is no information available |
Outcome data were available for nearly all children.bA small proportion of children was excluded due to incomplete information on the exposure of interest | The methods of outcome assessment were comparable across the intervention groups and unlikely to have been influenced by knowledge of the intervention received | This analysis was declared post‐hoc. However, there is no evidence of selective reporting | This study cannot be considered comparable to a well‐performed RCT, as there is potential for confounding |
aRisk of bias judgements are also applicable for the outcomes: diagnosis of asthma (current asthma) at 18 months and 3 years
bProportion of missing outcome data
- Current asthma at 18 months: wP = 0/293; 0.00%. aP = 1/204; 0.49%
- Current asthma at 3 years: wP = 9/293; 3.07%. aP= 8/204; 3.92%
- Current asthma at 5 years: wP = 15/293; 5.12%. aP = 14/204; 6.86%
3. ROBINS‐I assessment for: first dose of wP versus aP vaccine before 6 months of age. Outcome: diagnosis of asthma (current asthma) at eight years.
| Study | Bias due to confounding | Bias in selection of study participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing dataa | Bias in measurement of outcomes | Bias in selection of the reported results | Overall risk of bias |
| Toelle 2020 | Serious | Low | Low | No information | Moderate | Low | Moderate | Serious |
| Rationale for judgement | Allocation to the intervention was largely dependent on vaccine availability, as described in Estcourt 2020. The authors adjusted for breastfeeding status, sex, house dust mite avoidance and omega‐3 supplementation; however, these were unlikely to have influenced the assignment of the study vaccines | Children who would have been eligible for the target trial were included in the study. The start of the follow‐up period coincides with the start of intervention |
The intervention status was well‐defined and based on information collected at the time of the administration of the study vaccines | There is no information available | The reported analysis was unlikely to have removed the risk of bias arising from missing data | The methods of outcome assessment were comparable across the intervention groups and unlikely to be influenced by knowledge of the intervention received | This analysis was declared post‐hoc. However, there is no evidence of selective reporting | This study cannot be considered comparable to a well‐performed RCT, as there is potential for confounding |
aProportion of missing outcome data
- Current asthma by 8 years: wP = 59/293; 20.14%. aP = 30/204; 14.71%
4. ROBINS‐I assessment for: first dose of wP versus aP vaccine before 6 months of age. Outcome: diagnosis of asthma (current asthma) at 11.5 years.
| Study | Bias due to confounding | Bias in selection of study participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall risk of bias |
| Toelle 2020 | Serious | Low | Low | No information | Serious | Low | Moderate | Serious |
| Rationale for judgement | Allocation to the intervention was largely dependent on vaccine availability, as described in Estcourt 2020. The authors adjusted for breastfeeding status, sex, house dust mite avoidance and omega‐3 supplementation; however, these variables were unlikely to have influenced the assignment of the study vaccines | Children who would have been eligible for the target trial were included in the study. The start of the follow‐up period coincides with the start of intervention |
The intervention status is well‐defined and based on information collected at the time of the administration of the study vaccines | There is no information available | The proportion of missing data is higher in aP vaccinees than in recipients of wP. a However, this is unlikely to be related to the exposure status. The reported analysis was unlikely to have removed the risk of bias arising from missing data |
The methods of outcome assessment were comparable across the intervention groups and unlikely to be influenced by knowledge of the intervention received | This analysis was declared post hoc. However, there is no evidence of selective reporting | This study cannot be considered comparable to a well‐performed RCT, as there is potential for confounding |
aProportion of missing outcome data
- Current asthma by 11.5 years: wP = 83/293; 28.3%. aP = 76/204; 37.25%
5. ROBINS‐I assessment for: first dose of wP versus aP vaccine before 6 months of age. Outcome: diagnosis of asthma (current asthma) at 14 years.
| Study | Bias due to confounding | Bias in selection of study participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall risk of bias |
| Toelle 2020 | Serious | Low | Low | No information | Serious | Low | Moderate | Serious |
| Rationale for judgement | Allocation to the intervention was largely dependent on vaccine availability, as described in Estcourt 2020. The authors adjusted for breastfeeding status, sex, house dust mite avoidance and omega‐3 supplementation; however, these variables were unlikely to have influenced the assignment of the study vaccines | Children who would have been eligible for the target trial were included in the study. The start of the follow‐up period coincides with the start of intervention |
The intervention status was well‐defined and based on information collected at the time of the administration of the study vaccines | There is no information available | The proportion of missing data is high but balanced across the study groups.a The reported analysis was unlikely to have removed the risk of bias arising from missing data | The methods of outcome assessment were comparable across the intervention groups and unlikely to be influenced by knowledge of the intervention received | This analysis was declared post‐hoc. However, there is no evidence of selective reporting | This study cannot be considered comparable to a well‐performed RCT, as there is potential for confounding |
aProportion of missing outcome data
- Current asthma by 14 years: wP = 105/293; 35,84%. aP= 72/204; 35.29%
6. ROBINS‐I assessment for: first dose of wP versus aP vaccine before 6 months of age. Outcome: diagnosis of IgE‐mediated food allergy (on the basis of either a compatible clinical history or oral food challenge) by 10 yearsa.
| Study | Bias due to confounding | Bias in selection of study participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall risk of bias |
| Venter 2016 | Serious | Low | Low | No information | Moderate | Low | Moderate | Serious |
| Rationale for judgement | Vaccine allocation is described as 'almost at random, depending on the supply of the particular vaccine, avoiding potential biases due to secular trends in the risk of developing atopic disease'. The authors defined family history of asthma/hay fever, breastfeeding and sex as potential confounders; however, they were unlikely to have influenced the assignment of the study vaccines. Adjustment was made via multivariable binomial regression. No adjustment by date of birth, birth order and socioeconomic status was performed, 'as the number of cases was not sufficiently robust' |
Children who would have been eligible for the target trial were included in the study. The start of the follow‐up period coincides with the start of intervention |
The intervention status was well‐defined and based on information collected at the time of the administration of the study vaccines | There is no information available | Outcome data were available for nearly all children.b A small proportion of children was excluded due to incomplete information on the exposure of interest | The methods of outcome assessment were comparable across the intervention groups and unlikely to be influenced by knowledge of the intervention received | We did not find the protocol of this study. However, there is no evidence of selective reporting | This study cannot be considered comparable to a well‐performed RCT, as there is potential for confounding |
aAlso applicable for the outcome: diagnosis of asthma by 3 years
bProportion of missing outcome data
- Diagnosis of challenge proven IgE‐mediated food allergy: wP = 4/595; 0.67%. aP = 1/224; 0.45%
- Diagnosis of asthma by 3 years: wP = 35/595; 5.88%. aP = 20/224; 8.93%
7. ROBINS‐I assessment for: first dose of whole‐cell versus acellular pertussis vaccine before 6 months of age. Outcome: diagnosis of asthma by 10 years of age.
| Study | Bias due to confounding | Bias in selection of study participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall risk of bias |
| Venter 2016 | Serious | Low | Low | No information | Critical | Low | Moderate | Critical |
| Rationale for judgement | Vaccine allocation is described as 'almost at random, depending on the supply of the particular vaccine, avoiding potential biases due to secular trends in the risk of developing atopic disease'. The authors defined family history of asthma/hay fever, breastfeeding and sex as potential confounders; however, they were unlikely to have influenced the assignment of the study vaccines. Adjustment was made via multivariable binomial regression. No adjustment by date of birth, birth order and socioeconomic status was performed, 'as the number of cases was not sufficiently robust' |
Children who would have been eligible for the target trial were included in this cohort study. The start of the follow‐up period coincides with the start of intervention |
The intervention status was well‐defined and based on information collected at the time of the administration of the study vaccines | There is no information available | The proportion of missing data is high, but balanced across the study groups.a The reported analysis was unlikely to have removed the risk of bias arising from missing data | The methods of outcome assessment were comparable across the intervention groups and unlikely to be influenced by knowledge of the intervention received. | We did not find the study protocol or statistical analysis plan of this study. However, there is no evidence of selective reporting | This study cannot be considered comparable to a well‐performed RCT, as there is potential for confounding, and concerns abound missing data |
aProportion of missing outcome data
- Diagnosis of asthma by 10 years: wP = 259/595; 43.53%. aP = 112/224; 50.00%
3.
Risk of bias: 'traffic light' plot of the domain‐level judgements for each individual result of non‐randomised studies of interventions according to the ROBINS‐tool
The following risk of bias assessments examined the effect of assignment of the intervention/exposure at baseline. The consensus decisions for the signalling questions are available as Supplementary material 1.
Bias due to confounding
Ecological analyses of publicly available data have shown an increase in the number of admissions to hospital ICD‐coded as anaphylaxis following the transition from wP to aP vaccine schedules in Australia, between 1997 and 1999. There is little reason to expect that the receipt of wP or aP was influenced by factors other than calendar time, and chance during the switchover from wP to aP formulations in Australia, and also on the Isle of Wight (UK) when a period of shortage of wP meant that some children received aP instead. Family history of allergic diseases, gestational age at delivery and breastfeeding status are unlikely to have influenced the allocation of the intervention/exposure which was largely driven by the availability of the vaccine in the surgeries of general practitioners or immunisation clinics on the day of vaccination. Therefore, we only included in our assessments confounding domains relevant for these settings (i.e. availability of the vaccine, using date of birth as a proxy; socioeconomic status and birth order). One study was judged as moderate risk of bias for confounding (Estcourt 2020; diagnosis of challenge‐proven IgE‐mediated food allergy) and two at serious risk (Toelle 2020; diagnosis of asthma and Venter 2016; diagnoses of challenge‐proven IgE‐mediated food allergy and asthma).
Bias in selection of participants into the study
We judged two studies to be at low risk of bias (Toelle 2020; Venter 2016); this is because the selection of children into the analyses was not dependent on characteristics observed after the first dose of pertussis‐containing vaccine. In the study of Estcourt 2020, cases were identified from among children diagnosed by specialist allergists with a case‐based sampling approach used to mitigate any selection bias. Using the ROBINS‐I tool (version 1, August 2016; Sterne 2016a), the risk of bias for this domain was deemed to be moderate.
Bias in the classification of pertussis‐containing vaccines
We judged all of the studies reporting on primary and secondary atopic outcomes as low risk of bias. The exposure groups were clearly defined and their classification is unlikely to have been influenced by knowledge of the outcome status.
Bias due to deviations from intended pertussis‐containing vaccine
This domain of the ROBINS‐I tool refers to the biases that occur as a result of quote: "systematic differences between the care provided to experimental intervention and comparator groups, beyond the assigned interventions" (Sterne 2016b). There is no information available to judge whether there was bias due to deviations from intended intervention for any of the relevant studies.
Bias due to missing data
The definition of complete dataset for diagnosis of IgE‐mediated food allergy varies according to the outcome measure chosen. In this case, decisions were supported by a prespecified hierarchy of diagnosis described in the protocol of this review. In the study of Venter 2016 the diagnosis was on the basis of either a compatible clinical history or oral food challenge. In this case, outcome data were available for nearly all children.
In the study of Estcourt 2020, challenge‐proven IgE‐mediated food allergy was described in a pre‐planned sensitivity analysis of a non‐random subset of cases with a history of food hypersensitivity coupled with IgE‐mediated sensitisation to the food of interest. In both studies, a small number of children were excluded due to missing data on the exposure status. Although these data were likely to be missing at random, ROBINS‐I states that this is a marker of potential bias. Therefore, for the primary outcome of this review, the study of Venter 2016 was judged as moderate risk bias due to missing data. Restricting the analysis to those cases confirmed through oral food challenge, the study of Estcourt 2020 was judged to be at serious risk of bias.
For the secondary outcome diagnosis of asthma, data were available for nearly all children at the follow‐up assessments completed at 18 months, three and five years old, and we rated them as moderate risk of bias due to missing data. In contrast, decreasing completeness was noted in the assessments scheduled at 10, 11.5 and 14 years old, with analyses unlikely to have addressed the impact of missing data on the validity of the results. Therefore, we rated these studies to be at serious or critical risk for this domain.
Bias in measurement of outcomes
We judged all studies to be at low risk of bias (Estcourt 2020; Toelle 2020; Venter 2016), as the methods of outcome measurement were considered comparable across the study groups, outcome measures were unlikely to be influenced by knowledge of the intervention received, and errors in their measurement were unlikely to be related to intervention status.
Bias in selection of the reported result
The study of Estcourt 2020 was pre‐registered on clinicaltrials.gov and its prespecified analysis plan is also available as a peer‐reviewed publication; thus, it was judged to be at low risk of bias for this domain. We did not find the study protocol or statistical analysis plan of the study of Venter 2016, and the analyses presented in the study of Toelle 2020 were declared post hoc. In each case, outcome measures were clearly defined and there is no evidence of selective reporting; therefore, we judged them at moderate risk of bias for selection of the reported result.
Overall risk of bias assessment
We rated all of the NRSIs to be at serious or critical risk of bias, and therefore, not eligible for quantitative or narrative synthesis. The domains contributing to this judgment were 'confounding' and 'missing data'.
Other potential sources of bias
Funding
The study of Nilsson 1998 received funding from public and private agencies. NRSIs received funding from the National Health and Medical Research Council of Australia (NHMRC) and Public Health England, as well as other government funding agencies and academic institutions. Details are provided in the Characteristics of included studies table.
Declarations of interest
Investigators for three of the NRSIs declared conflicts of interest. Details are provided in the Characteristics of included studies table.
Randomised controlled trials included in the review for safety
See Figure 2 for the risk of bias summary for the RCTs that assessed safety outcomes, where we provide our judgement for each risk of bias category (figure generated using robvis; McGuinness 2020). We judged seven studies to be at high risk of bias (Blumberg 1991; Dagan 1997; Kitchin 2006; Madhi 2011; Miller 1997; Reinert 2006; Stehr 1998), and the remaining at unclear risk.
Allocation
We judged five studies to be at low risk of bias for sequence generation (Dagan 1997; Gustafsson 1996; Olin 1997; Simondon 1997) and the remaining at unclear risk. For allocation concealment, the studies of Greco 1996; Miller 1990; Miller 1997; Olin 1997; Reinert 2006; Simondon 1997 and Stehr 1998 were deemed at low risk of bias, and the remaining trials at unclear risk.
Blinding
Where encephalopathy was assessed as an outcome of interest, the studies were judged to be at unclear (Decker 1995; Edwards 1991; Feldman 1993; Greco 1996; Gustafsson 1996; Olin 1997; Stehr 1998), or high risk of performance bias (Dagan 1997). We considered that the primary safety outcome and associated endpoints were unlikely to be influenced by knowledge of the intervention received, and therefore, we judged all the studies reporting on them (but not on encephalopathy) at low risk of performance bias.
We assessed five studies as being at low risk for detection bias due to a detailed explanation of the strategies implemented to keep the outcome assessors blinded to the vaccine allocation (Miller 1990; Miller 1997; Olin 1997), or because the assessment of the outcome domain of interest (i.e. deaths from any cause) was unlikely to be influenced by knowledge of the intervention received (Afari 1996; Wanlapakorn 2020). In contrast, we judged open‐label trials at high risk for detection bias (Dagan 1997; Kitchin 2006; Madhi 2011; Reinert 2006).
Incomplete outcome data
One trial was judged as high risk of bias due to low retention rates (Blumberg 1991), and four at low risk of bias owing to low rates of dropout (Gustafsson 1996; Macías 2012; NCT00343889; NCT00348881); the remaining were assessed as unclear risk.
Selective reporting
Two trials were judged as high risk of bias for selective reporting (Miller 1997; Stehr 1998). The trial of Miller 1997 reported on reactogenicity of pertussis‐containing vaccines, but not on SAEs following immunisation. Similarly, the trial of Stehr 1998 only described admissions to hospital due to serious infections, in spite of the methods specifying that events requiring hospitalisation were going to be systematically reported irrespective to their relatedness to the study vaccines.
Among the trials pre‐registered on clinicaltrials.gov., three were judged as low risk of bias (NCT00343889; NCT00348881; Wanlapakorn 2020), and two as unclear risk due to apparent inconsistencies between the trial registry and peer‐reviewed publications (Macías 2012; Madhi 2011). Although we could not source the extended technical reports and pre‐planned statistical analysis plan of the trial of Gustafsson 1996, this study was judged as low risk of bias, because the data on the outcomes prespecified in the methods of this study were systematically collected and reported. The remaining trials were judged to be at unclear risk (see Characteristics of included studies table).
Other potential sources of bias
Funding
Eight trials received funding from vaccine manufacturers (Feldman 1993; Kitchin 2006; Macías 2012; Madhi 2011; NCT00343889; NCT00348881; Reinert 2006; Stehr 1998); four were carried out through research grants from the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institute of Health (NIH) in the USA (Decker 1995; Edwards 1991; Greco 1996; Gustafsson 1996); two were funded by the UK Medical Research Council (Miller 1990; Miller 1997); one by multiple academic and research institutions (Wanlapakorn 2020) and three were supported by public and private funding schemes (i.e. the trial of Afari 1996 was sponsored by the Government of Ghana and the Research Foundation for Microbial Diseases of Osaka University, a bio‐pharmaceutical group; the study of Olin 1997 by the NIAID NIH and three manufacturers which also provided the DTaP formulations; and the study of Simondon 1997 was sponsored by a pharmaceutical company and the Office de la Recherche Scientifique et Technique Outre‐mer, a French public research institution today known as Institut de Recherche Pour le Développement). In four trials, funding was not disclosed (Black 1997; Blumberg 1991; Dagan 1997; Halperin 1996).
Declarations of interest
Only four trials declared conflicts of interest (Kitchin 2006; Macías 2012; Madhi 2011; Wanlapakorn 2020). Among the remaining studies where no disclosure was made, one or more investigators were employees of a vaccine manufacturer (Afari 1996; Black 1997; Blumberg 1991; Dagan 1997; Feldman 1993; Halperin 1996; Reinert 2006; Simondon 1997; Stehr 1998), one received DTwP manufactured by Wellcome as a donation (Miller 1990) and one a pertussis vaccine antigen (69 kDa or pertactin) from Connaught Laboratories (Miller 1997).
Effects of interventions
See: Table 1
We report atopic and safety outcomes for the comparison: first dose as wP versus first dose aP before the age of six months.
Atopic outcomes
Cumulative incidence of atopic disease
One RCT was suitable for narrative synthesis (Nilsson 1998). This study was carried out in Sweden in the early 1990s, in a setting with lower prevalence of IgE‐mediated food allergy than identified in more recent cohorts. Because data on (our prespecified primary outcome (IgE‐mediated food allergy) could not be sourced by study arm (Nilsson 1998), we chose to report on the cumulative incidence of atopic disease outcome and calculated a risk ratio (RR) and 95% confidence interval (CI), as prespecified in our protocol (Perez Chacon 2020). This broader outcome domain encompasses children who were diagnosed with at least one of the following atopic diseases: IgE‐mediated food allergy, asthma, atopic dermatitis, urticaria and allergic rhino‐conjunctivitis by 2.5 years old.
This small study was statistically underpowered to detect a reduction in their chosen endpoints (except for a large reduction > 50%). Using GRADE, we assessed the evidence as of very low certainty. We downgraded by one level for indirectness, and two for imprecision, since the existence of effect in either direction remains plausible (RR 0.85, 95%CI 0.62 to 1.17; 497 children; Analysis 1.1).
1.1. Analysis.

Comparison 1: First dose of wP versus first dose of aP, Outcome 1: Cumulative incidence of atopic disease at 2.5 years
Diagnosis of IgE‐mediated food allergy
NRSIs that reported diagnosis of challenge‐proven IgE‐mediated food allergy (Estcourt 2020; Venter 2016) were not eligible for narrative synthesis due to serious risk of bias (Figure 3). This decision was made according to the protocol of this review (Perez Chacon 2020). The details of the risk of bias assessments are provided in Supplementary material 1.
Diagnosis of anaphylaxis
None of the included studies investigated our prespecified outcome of diagnosis of anaphylaxis (not vaccine‐associated).
Diagnosis of asthma
In Nilsson 1998, asthma was diagnosed in 15 of 137 children vaccinated with wP (10.95%), and 38 of 360 children vaccinated with aP (10.56%) by 2.5 years of age. There was insufficient evidence to determine whether wP may change the risk of asthma diagnosis by 2.5 years (RR 1.04; 95% CI 0.59 to 1.82; 497 children; very low certainty; Analysis 1.2). Although the investigators of this study argue that most of the study participants diagnosed with asthma had a previous history of atopic dermatitis, with or without evidence of IgE‐mediated sensitisation, it is plausible that some of them may have been 'transient (episodic) wheezers'. These children have been reported to mainly wheeze in the context of upper respiratory viral infections, with no or minimal symptoms between episodes, and lack of eosinophilic inflammation (Pavord 2018). This wheezing phenotype explains a large proportion of wheezing episodes in preschool‐aged children, and we believe that is unlikely to be affected by wP priming. Therefore, we downgraded by one level for indirectness, and by two levels for imprecision, as the CI of the effect size is wide, and includes the null effect.
1.2. Analysis.

Comparison 1: First dose of wP versus first dose of aP, Outcome 2: Diagnosis of asthma by 2.5 years
NRSI that reported diagnosis of asthma (Toelle 2020; Venter 2016) were not eligible for narrative synthesis due to serious or critical risk of bias (Figure 3).
Diagnosis of atopic dermatitis
In the trial of Nilsson 1998, atopic dermatitis was diagnosed by 2.5 years old in 23 of 137 (16.79%) children vaccinated with wP and 81 out of 360 vaccinated with aP (22.5%). There was insufficient evidence to determine whether wP may affect the risk of atopic dermatitis (RR 0.75; 95% CI 0.49 to 1.13, 497 children; low‐certainty evidence; Analysis 1.3). Therefore we downgraded the evidence by two levels for imprecision.
1.3. Analysis.

Comparison 1: First dose of wP versus first dose of aP, Outcome 3: Diagnosis of atopic dermatitis by 2.5 years
Other atopic outcomes
Data on diagnoses of urticaria and allergic rhino‐conjunctivitis could not be sourced by study arm (Nilsson 1998).
Safety outcomes
Primary series studies
All‐cause serious adverse events
One or more SAEs occurred in 153 of 14,183 children allocated at random to a first dose of wP (1.09%), and in 277 out of 23,889 recipients allocated to a first dose of aP (1.16%). The Mantel‐Haenszel RR without continuity correction was 0.94 (95% CI 0.78 to 1.15; I2 = 0%; 15 primary series studies, 38,072 children; Figure 4). For every 1000 infants primed with a first dose of aP before six months old, 12 experienced an SAE; the corresponding risk for wP was 11 (95% CI 9 to 13). Compared to aP, the 95% CI around the absolute risk difference of SAE for children receiving a first dose of wP ranged from three fewer to two more SAEs per 1000 vaccinees.
4.
Forest plot of comparison: first dose of wP versus first dose of aP before 6 months of age. Outcome: all‐cause serious adverse events
Removing studies at high risk of bias left 11 studies in the analysis (RR 0.94, 95% CI 0.77 to 1.16; I2 = 8%; 29,576 children), without changes to the interpretation of the result. The exclusion of studies funded by pharmaceutical companies resulted in moderate heterogeneity (RR 0.98, 95% CI 0.63 to 1.51; I2 = 44%; six studies, 20,105 children), but no change in the conclusion.
Using GRADE, we assessed the evidence as moderate certainty (downgraded one level for imprecision).
All‐cause mortality
We included 18 studies, involving 134,541 children, in the analysis. Deaths were reported in 54 out of 40,908 children vaccinated with a first dose of wP before 6 months (0.13%), and in 74 of 93,633 aP‐vaccinees (0.08%). Therefore, the proportion of children who died during the follow‐up period was greater in wP compared to aP‐vaccinees, but the confidence interval was wide around the RR of 1.01 (95% CI 0.71 to 1.45; I2 = 0%; Figure 5), indicating substantial imprecision.
5.
Forest plot of comparison: first dose of wP versus first dose of aP before 6 months of age. Outcome: all‐cause mortality
We tested the robustness of these findings through prespecified sensitivity analyses, removing studies at high risk of bias (RR 1.04, 95% CI 0.71 to 1.52; I2 = 0%; 13 studies, 117,513 children), and by excluding trials that were funded by pharmaceutical companies. For the latter, we removed the studies that had the greatest contribution to the weighted average, including Simondon 1997, which was carried out in a rural area of Senegal, with an infant mortality rate of 85 per 1000 live births. In this case, we observed a decrease in both the Mantel‐Haenszel RR and the precision of the estimate (RR 0.62, 95% CI 0.12 to 3.30; I2 = 0%; seven studies, 25,150 children); however, there was no resulting change in the interpretation of the results.
Using GRADE, we assessed the evidence as low certainty (downgraded two levels for imprecision).
Events leading to admission to hospital
We included eight studies in this analysis. At least one admission to hospital was reported in 122 out of 6011 children vaccinated with a first dose of wP before six months (2.03%), and in 306 out of 12,319 aP‐vaccinees (2.48%). The Mantel‐Haenszel RR without continuity correction for all‐cause admission to hospital was 0.98 (95% CI 0.80 to 1.21; I2 = 2%; 18,330 children; Supplementary material 2).
We carried out prespecified sensitivity analyses to assess the robustness of the main result, by removing studies at high risk of bias (RR 0.98, 95% CI 0.80 to 1.20; I2 = 30%; six studies, 17,592 children) and by excluding studies funded by vaccine manufacturers (RR 0.99, 95% CI 0.80 to 1.22; I2 = 32%; six studies, 13,314 children) without meaningful changes in the point estimates, CIs or interpretation of the findings.
Using GRADE, we assessed the evidence as low certainty (downgraded two levels for imprecision).
Events described as life‐threatening
Eight studies were pooled for this meta‐analysis; four contributed no events. One or more events described as life‐threatening were reported in four out of 37,376 children vaccinated with a first dose of wP before the age of six months (0.01%), and in nine out of 87,353 aP‐vaccinees (0.01%). The Mantel‐Haenszel RR with no continuity correction was 1.08 (95% CI 0.32 to 3.64; I2 = 0%; 124,729 children, Supplementary material 2). The reported number of children with this outcome was few and the confidence interval around the RR was wide.
Exclusion of studies at high risk of bias (RR 0.88, 95% CI 0.25 to 3.17; I2 = 0%; five studies, 108,860 children) did not cause any major changes in the CI. A decrease in the Mantel‐Haenszel RR and greater statistical heterogeneity were detected after removing studies funded by vaccine manufacturers (RR 0.55, 95% CI 0.07 to 4.62; I2 = 23%; four studies, 21,934 children), however this did not result in meaningful changes in the interpretation of the main findings.
Using GRADE, we assessed the evidence as of low certainty (downgraded two levels for imprecision).
Events leading to persistent or significant disability or incapacity
Four studies were pooled for this meta‐analysis; two contributed no events. At least one event leading to disability was reported in six out of 7008 children vaccinated with a first dose of wP before six months of age (0.09%), and in 9 out of 14,966 aP‐vaccinees (0.06%). The CI was wide around the RR of 1.45 (95% CI 0.51 to 4.16; I2 = 39%; 21,974 children, Supplementary material 2). No changes were observed after excluding one study judged to be at high risk of bias and funded by a pharmaceutical company (RR of 1.45, 95% CI 0.51 to 4.16; I2 = 39%; 21,733 children).
Using GRADE, we assessed the evidence as of low certainty (rated down two levels for imprecision).
Diagnosis of encephalopathy
Seven primary series studies systematically collected data on encephalopathy, but no events were reported in 32,268 recipients of wP and 83,003 aP‐vaccinees (Analysis 1.4). A 95% CI was calculated using the score method (Newcombe 1998). This is a serious outcome, and although the 95% CI around the absolute difference is narrow (‐5 per 100,000 to 12 per 100,000), we could not rule out a clinically meaningful difference. Using GRADE we assessed the evidence as low certainty (downgraded two levels for imprecision).
1.4. Analysis.

Comparison 1: First dose of wP versus first dose of aP, Outcome 4: Diagnosis of encephalopathy
Booster dose study
Within two years of follow‐up after a booster dose of DTaP, no SAEs (deaths or events leading to hospitalisation), or diagnoses of encephalopathy were reported in children who were randomly allocated to a primary series of DTwP (n = 23) or DTaP (n = 18) (Edwards 1991; Analysis 1.5; Analysis 1.6). In either case, using GRADE, we assessed the evidence as being of low certainty (downgraded two levels for imprecision).
1.5. Analysis.

Comparison 1: First dose of wP versus first dose of aP, Outcome 5: All‐cause serious adverse events (following a booster dose of aP)
1.6. Analysis.

Comparison 1: First dose of wP versus first dose of aP, Outcome 6: Diagnosis of encephalopathy (following a booster dose of aP)
Discussion
Summary of main results
This review includes four studies that reported on our primary and secondary atopic outcomes of interest, and 21 trials reporting on serious adverse events (SAEs) and/or encephalopathy for our comparison of interest (first dose of whole‐cell pertussis (wP) vaccine versus acellular pertussis (aP) vaccine in infants younger than six months).
Evidence on atopic outcomes was of very low certainty and we were unable to draw any conclusions on the relative effects of wP versus aP vaccines on atopic diseases (Table 1. Meta‐analyses of the allergy outcome data were not feasible due to the paucity of randomised controlled trials (RCTs) and high‐quality non‐randomised studies of interventions (NRSIs) assessing IgE‐mediated food allergy and/or asthma as study outcomes, and heterogeneity in the designs of existing studies. In addition, serious or critical risk of bias across the outcomes reported by three NRSIs precluded their inclusion in a narrative synthesis. As prespecified in the study protocol, we grouped the atopic outcomes reported by the trial of Nilsson 1998 using a broader outcome domain (i.e. cumulative incidence of atopic diseases at 2.5 years). We also synthesised narratively, the evidence regarding diagnoses of asthma and atopic dermatitis, arising from the same study. No study planned to assess non vaccine‐associated anaphylaxis as an outcome of interest, yet one ongoing study considers clinician‐diagnosed food anaphylaxis as evidence of IgE‐mediated food allergy (ACTRN12617000065392). This RCT is expected to provide more definitive evidence on protection against early onset of IgE‐mediated food allergy in a setting with high prevalence.
The incidence of all‐cause SAEs was within the expected range for otherwise healthy infants, and similar for wP and aP. The 95% confidence interval (CI around the absolute difference ranged from a potential decreased to an increased risk (Table 1), unlikely to be clinically meaningful.
The evidence regarding risk of encephalopathy was obtained only through studies that identified no events (Table 1). The absolute difference between wP and aP was 0%, with a 95% CI ranging from ‐0.005% to 0.012%. Although the CI is narrow, encephalopathy is a serious condition and therefore, we could not rule out a clinically meaningful difference.
Overall completeness and applicability of evidence
Completeness of evidence
We undertook a comprehensive review process involving the assessment of RCTs, trial registries and regulatory reports for atopic and safety outcomes, as well as NRSIs investigating the association between pertussis immunisation and atopic diseases. Although there is no consensus regarding suitable search strategies for controlled NRSIs, we decided to incorporate a filter developed by Waffenschmidt 2020 with the purpose to maximise sensitivity in the database searches. Through the review of the list of references of eligible studies, we found extended reports of the trials of Gustafsson 1996 and Olin 1997, a conference proceeding with detailed safety data (Miller 1990), cohort profiles and detailed descriptions of the outcome definitions (Mrozek‐Budzyn 2018; Toelle 2020), and the primary report of an ineligible study (Grüber 2008). A study awaiting classification was cited in an assessment of safety data completed by the EMA (217744/025 (DTPa‐HBV‐IPV‐025)). The final report of this trial was not available in the GSK trial registry when the searches were conducted, nor before the submission of this manuscript. We contacted authors of 12 studies requesting demographics, details on the interventions administered, or additional information regarding the study outcomes. However, it was not always possible to obtain the relevant data due to authors being seconded to work on coronavirus disease‐19 (COVID‐19) pandemic‐related roles, investigators being unable to access historical data (records unavailable), or due to non‐response to our requests. Nonetheless, we consider that our review process was robust.
Applicability of evidence
One trial was included in our narrative synthesis of atopic outcomes (Nilsson 1998). This study was unable to detect a true difference in the cumulative incidence of atopic diseases, asthma or atopic dermatitis by 2.5 years old in children primed with wP, compared to those receiving aP‐only schedules. The diagnoses were made based on parental report using questionnaires, physical examination, medical records and/or evidence of IgE‐mediated sensitisation, However, it is plausible that some children labelled as 'asthmatic' by this trial, may have been transient wheezers in retrospect, and we believe that this phenotype is unlikely to be affected by wP priming.
The incidence of SAEs following immunisation in infants primed with a first dose of either wP or aP was low. Although these findings support the safety of these vaccines, implementation of wP in countries where it is no longer the standard of care for preventing pertussis might be hindered by non‐serious adverse reactions which are more frequent after wP than aP vaccines, as described by a previous Cochrane Review (Zhang 2014), and more recently, by the systematic review of Patterson 2018.
Quality of the evidence
Atopic outcomes
We found four eligible studies reporting on atopic outcomes (one RCT and three NRSIs); however, pooling was not possible. The RCT was carried out in a country with low prevalence of IgE‐mediated food allergy (Nilsson 1998), and was statistically underpowered to detect a reduction in their chosen endpoints, except for a large reduction > 50%. Therefore, the evidence for cumulative incidence of atopic disease at 2.5 years of age was downgraded by one level for indirectness, and two levels for imprecision. Similarly, it is uncertain whether wP may change the risk of atopic dermatitis or asthma by 2.5 years old, as the 95% CI around the point estimates were wide, and include the null effect. NRSIs were judged as serious or critical risk of bias due to confounding, missing data or both, and thus, were ineligible for a narrative synthesis.
Safety outcomes
Except for four studies judged to be at high risk of bias (Blumberg 1991; Kitchin 2006; Madhi 2011; Reinert 2006), all the trials pooled for meta‐analysis of SAEs were assessed to be at unclear risk of bias. Overall, randomisation, allocation concealment and detection bias were poorly reported; however, most of these historical data were made available before the publication of the revised version of the CONSORT statement (Moher 2001). This is perhaps why the minimally required reporting standards arising from stages where bias was likely to occur remained unmet. Therefore, omissions are likely to be a result of a lack of reporting, rather than that bias is actually present.
The evidence regarding all‐cause SAE was judged as moderate (downgraded one level for imprecision), since a potential decrease or increase in the risk difference remains plausible, but unlikely to be clinically meaningful. No cases of encephalopathy were detected by seven primary series trials. Because this is a serious condition, we could not rule out a clinically meaningful difference and therefore, we judged the quality of the evidence as low (downgraded two levels for imprecision). Irrespective of these methodological caveats, our results support the safety of wP‐containing vaccines.
Potential biases in the review process
We are confident that we have identified all the studies that compared wP versus aP in regards to the development of atopic diseases. We followed standard Cochrane methods to select studies for inclusion, data extraction, assessment of the risk of bias and used GRADE to determine the certainty of the evidence.
We chose a follow‐up period of at least six months as an eligibility criterion, since the assessment of atopic conditions often requires confirmatory investigations. Furthermore, food sensitisation is likely to occur in the first months of life (possibly through low‐dose of cutaneous sensitisation (Du Toit 2018), and food allergic reactions usually require introduction of solid foods, which generally only occurs after six months old (especially for peanuts and tree nuts). Although we did not exclude any study reporting on atopic outcomes on the basis of the follow‐up period, some trials comparing the safety of primary vaccination series using wP versus aP did not meet the minimum follow‐up criterion and were therefore not included. The exclusion of these studies was unlikely to change the results or the certainty of the evidence, since these trials were small and unlikely to detect a true difference in the occurrence of SAEs with frequencies less than 2%. One out of three ongoing RCTs is systematically collecting data on our primary atopic and safety outcomes (ACTRN12617000065392), and the remaining studies are expected to report SAEs where occurring. Two studies 'awaiting classification' have not been included in our synthesis, creating a source of potential bias. The reasons for non‐inclusion are listed in the Characteristics of studies awaiting classification table, and encompass not being able to find the final report of an industry‐funded immunogenicity trial (217744/025 (DTPa‐HBV‐IPV‐025)), or to confirm the age at which the first dose of pertussis‐containing vaccine was administered (Mrozek‐Budzyn 2018).
Outcome data from Nilsson 1998 were synthesised narratively using a broader outcome domain (i.e. cumulative incidence of atopic disease at 2.5 years). The data were extracted from a bar chart using a web‐based data extraction tool (WebPlotDigitizer 2020). The same methods were implemented to extract the relevant data for diagnoses of asthma and atopic dermatitis. In spite of being more accurate compared to manual estimations, this method was not prespecified in our protocol, yet implemented as these critical data were not reported elsewhere.
We generated contour‐enhanced funnel plots for the outcomes 'all‐cause mortality' and 'all‐cause SAEs'. In the first plot we did not detect additional sources of bias or asymmetry (Supplementary material 3). In the second plot (Figure 6), the trial of Blumberg 1991 , which was judged to be at high risk of bias due to high attrition rates, is represented as an outlier at the bottom right‐hand side. Overall, the plot suggests a lack of smaller studies reporting on SAEs. However, as larger studies did not find a difference regarding the occurrence of this outcome and the type of priming schedule, it appears unlikely that the inclusion of smaller studies would change the effect estimate.
6.
Contour‐enhanced funnel plot of comparison: first dose of wP versus first dose of aP before 6 months of age. Outcome: all‐cause serious adverse events
Due in part to the limitations posed by the COVID‐19 pandemic, we were unable to source the extended technical reports and analysis plans of the Sweden I and II efficacy, immunogenicity and safety trials (Gustafsson 1996; Olin 1997). These documents are cited by the scholarly work of pertussis vaccine researchers, and likely to include additional details on the safety data that had otherwise been summarised by the FDA in their regulatory report, as well as in peer‐reviewed publications.
The number of SAEs in the trial of Olin 1997 was provided in both, regulatory data and peer‐reviewed publications. One of the peer‐reviewed articles included the number of children experiencing these events, but the data were not broken down by study arm. However, in some circumstances it was possible to match the number of children who met a specific endpoint (i.e. events described as life‐threatening or deaths), with their vaccination status as indicated in the FDA assessment of the safety data of this trial. These data points were included for synthesis.
Due to paucity of studies, we could only undertake two out of three prespecified sensitivity analyses (i.e. excluding trials at high risk of bias, or those sponsored by pharmaceutical companies). Subgroup analyses were not possible for similar reasons.
Four of our review authors were investigators of an included NRSI (Estcourt 2020) and five, are currently involved in ACTRN12617000065392, an ongoing study. The authors listed in the study of Estcourt 2020 were not involved in the extraction of these data, nor did they participate in their risk of bias assessment.
Agreements and disagreements with other studies or reviews
A review investigating associations between childhood vaccination and allergy has been recently published (Navaratna 2021). In contrast with our review, RCTs and studies comparing pertussis immunisation with no vaccination or placebo were deemed eligible. Similarly, based on the trial of Nilsson 1998, the review of Navaratna 2021 did not find an association between the type of pertussis‐containing vaccine and atopic outcomes.
As part of the development of the WHO pertussis vaccine position paper, a Strategic Advisory Group of Experts (SAGE) on Immunisation summarised the certainty of the evidence on the safety of wP and aP vaccine formulations in immunocompetent infants and children under seven years old (WHO 2015b; WHO 2015c). Where wP was assessed as the intervention, the comparison was no vaccine or "control"; similarly, where aP vaccine formulations were included as the reference, no vaccine or "control" were chosen as the comparator. In either case, the evidence was gathered using an inclusive principle. The assessments of the SAGE are available as qualitative statements using GRADE, and subsequently summarised as recommendations using standard decision domains (i.e. the certainty of the evidence; balance of clinically important outcomes and harms; values and preferences; and resource implications). The incidence of SAEs following vaccination with wP versus comparator, or aP versus comparator was described as "low"; for each comparison, the risk of this outcome was reported as "not significant", and the certainty of the evidence as "high" (WHO 2015b). This is in contrast with the certainty of our findings, which was judged to be moderate, and restricted to the comparison of a first dose of wP versus aP in infants younger than six months, who were followed up for at least six months. Despite these differences, the findings of our review are consistent with the current recommendations of the WHO, which upholds the continued use of wP‐based primary series as part of national immunisation programmes.
A previous Cochrane Review compared encephalopathy and mortality due to any cause in recipients of aP versus wP vaccine formulations using a Mantel‐Haenszel random‐effects model (Zhang 2014). In spite of eligibility being restricted to double‐blind RCTs irrespective of the length of follow‐up, we did not find a meaningful difference between the interpretation of their meta‐analysis for all‐cause mortality (risk ratio (RR) 1.14, 95% CI 0.82 to 1.60; I2 = 0%; 122,451 children, 16 studies; RR and CI calculated using a first dose of wP as the intervention versus a first dose of aP as the comparator) and ours (Mantel‐Haenszel fixed‐effect model without continuity correction; 1.01 (95% CI 0.71 to 1.45; I2 = 0%; 134,541, children, 18 studies; Figure 5). The review of Zhang 2014 did not find any cases of encephalopathy following a primary series of wP (32,161 children) versus aP (81,601). Similarly, no cases of encephalopathy were recorded among the children included in our review of seven primary series RCTs (nwP= 32,268; naP = 83,003), and a booster dose study with 2 years of safety follow‐up (nwP= 23; naP =18).
Authors' conclusions
Implications for practice.
The evidence on the effect of a first dose of whole‐cell pertussis (wP) vaccine on the cumulative incidence of atopic diseases at 2.5 years old is very uncertain. However, an ongoing randomised controlled trial (RCT) could change this conclusion, at least for the prevention of early onset IgE‐mediated food allergy in settings with high prevalence.
The incidence of serious adverse events (SAEs) following immunisation in infants primed with a first dose of wP versus acellular pertussis (aP) vaccine is low. There is moderate‐certainty evidence that a first or subsequent doses of wP do not reduce/increase the risk of SAEs. Therefore, there is no evidence to suggest that they are not safe for the prevention of pertussis in countries where they are currently recommended.
Implications for research.
Large, well‐conducted RCTs are needed to investigate the possible allergy protective benefits of a first dose of wP given before six months old, ideally in populations with high prevalence of IgE‐mediated food allergy.
Non‐randomised studies of interventions (NRSIs) using a target trial method may still be valuable. While confounding by targeted intervention is unlikely in NRSIs of historical cohorts, investigators still need to pay close attention to mitigating the risk of confounding as well as selection of specific endpoints.
Future allergy trials should not only assess reactogenicity, tolerability and parental acceptability of a first dose of wP compared to aP, but also the relative frequency of SAEs and in particular, the potential relatedness to the dose administered.
The selection of endpoints in future RCTs conceived to assess whether a first dose of wP may decrease the risk of IgE‐mediated food allergy, should not only prioritise the performance of oral food challenge with standardised stopping criteria, but also include diagnostic approaches in which IgE‐mediated food allergy is highly probable, based on a combination of parental reported, clinician‐diagnosed food allergic reaction coupled with evidence of IgE‐mediated sensitisation.
History
Protocol first published: Issue 7, 2020
Acknowledgements
We acknowledge Aboriginal and Torres Strait Islander People as the Traditional Custodians of the land and waters of Australia. We also acknowledge the Nyoongar Wadjuk, Yawuru, Kariyarra and Kaurna Elders, their people and their land upon which the Telethon Kids Institute is located, as well as the Gadigal people of the Eora Nation and their ancestral lands upon which the University of Sydney is built. We seek their wisdom in our work to improve the health and development of all children.
We would like to express our gratitude to Dr Nicola Lindson and Dr Jonathan Livingstone‐Banks (managing editors of the Cochrane Tobacco Addiction Group) for their insightful editorial advice, helpful comments and encouragement during the preparation of this review.
We would also like to thank Dr Linjie Zhang (Universidade Federal do Rio Grande, Brazil) and Abhijit Dutta for performing peer review and consumer review of this manuscript, respectively.
We thank Diana Blackwell and Vanessa Varis, librarians from the Faculty of Health Science at Curtin University for their advice regarding electronic searches. We are also very grateful to Bronwyn Bandy, Mariannne Hall and Tram Do from the Document Delivery Team, who made possible to obtain historical data across different sources. We would also like to thank Daniel Norman from the Wesfarmers Centre of Vaccines and Infectious Diseases (WCVID) at the Telethon Kids Institute, who helped us to find full‐text articles through the library of the University of Western Australia.
We thank Professor Bengt Bjökrstén, Professor Stephen B. Black, Professor James D. Cherry, Professor Ronald Dagan, Adjunct Professor Michael D. Decker, Professor Kathryn M. Edwards, Professor Janet A. Englund, Professor Beate Kampmann, Professor Liz Miller, Professor Lennart Nilsson, Professor Paul J.Turner and Professor I‐Jen Wang for their rapid responses to our queries regarding outcome data and study‐specific features.
We would like to sincerely thank Sarah Brazier (WCVID) for her guidance on the preparation of the Plain language summary of this review. We thank Michael Dymock (WCVID) for providing statistical advice, as well as Grahame Bowland (WCVID), Ana Paula Motta and Liam Scarlett for their technical assistance.
Appendices
Appendix 1. Glossary
| Acellular pertussis (whooping cough) vaccine | A whooping cough vaccine prepared from the purified antigenic components of the bacterium Bordetellapertussis. This type of vaccine is usually available in combination with diphtheria and tetanus toxoids |
| Allergen | Antigen that can cause an allergic reaction |
| Allergic rhinitis or allergic rhino‐conjunctivitis (hay fever) | Allergic reaction to aero‐allergens (e.g. house dust mite, pollens, etc.) that causes itchy nose, nasal congestion, sneezing, itchy/watery eyes, or a combination of these symptoms |
| Anaphylaxis | A serious allergic reaction that is rapid in onset and may cause death |
| Antibody | See immunoglobulin |
| Antigen | Any substance that is recognised by the immune system. Antigens normally trigger a reaction by the immune system |
| Asthma | A long‐term condition of the lungs where inflammation causes narrowing and swelling of the airways and increased production of mucous. It commonly manifests as persistent cough, wheezing and difficulty breathing |
| Atopic dermatitis (atopic eczema) | A long‐tem non‐infectious skin disease that often starts before the age of 12 months, and commonly causes dry, hot, itchy and red skin |
| Atopic march | Proposed theory of the disease progression of some allergic illness. The theory suggests disease starts in early infancy with eczema, followed by IgE‐mediated food allergy, hay fever and asthma |
| Bacillus Calmette‐Guérin (BCG) vaccine | Suspension of a live but weakened strain of the bacterium Mycobacteriumbovis, used as a vaccine against tuberculosis |
| Beta‐lactoglobulin | The major whey protein of cow's milk. Beta‐lactoglobulin is absent in human's milk |
| Case‐control study | A study that compares people with a specific disease or outcome of interest (cases) to people from the same population without that disease or outcome (controls), and which seeks to find associations between the outcome and prior exposure to particular risk factors. This design is particularly useful where the outcome is rare and past exposure can be reliably measured |
| Challenge‐confirmed (proven) IgE‐mediated food allergy | Diagnosis of IgE‐mediated food allergy confirmed with a medically supervised oral food challenge |
| Chronic inflammation (chronic allergic inflammation) | Long‐term inflammatory response caused by repetitive exposure to a particular allergen. Its main features are the presence of many immune cells at the affected site, as well as changes in the function and external characteristic of the cells within the affected tissue |
| Cluster randomised trial | A trial in which clusters of individuals (e.g. clinics, families, geographical areas), rather than individuals themselves, are randomised to different groups |
| Cohort study | An observational study in which a defined group of people (the cohort) is followed over time. The outcomes of people in subsets of this cohort are compared, to examine people who were exposed or not exposed (or exposed at different levels) to a particular intervention or other factor of interest. A prospective cohort study assembles participants and follows them into the future. A retrospective (or historical) cohort study identifies participants from past records and follows them from the time of those records to the present |
| Comorbidity | The presence of one or more diseases or conditions other than those of primary interest |
| Cytokines | Proteins secreted by a variety of cells, including the immune cells. Cytokines are important regulators of the intensity and duration of the immune response. They can act on the same cell that secreted them, on nearby cells, and more rarely, on distant cells |
| Encephalopathy | An uncommon but potentially serious condition affecting the brain |
| Epidermal barrier | See epidermis |
| Epidermal barrier disruption | Damage of the external layer of the skin. In people with atopic dermatitis, this may lead to ongoing exposure to allergens (e.g. peanut) and further allergic (IgE‐mediated) sensitisation |
| Epidermis | The external (non‐vascular) layer of the skin |
| Genomic analysis | Analysis of genomic content using next or third‐generation sequencing technologies |
| Immune response | The body's reaction of cells and fluid to a substance that is recognised by the immune system as foreign |
| Immunoglobulin | A protein produced by some immune cells that helps the body fight disease. In some cases (e.g. atopic diseases), immunoglobulins may be directly involved in the disease mechanism |
| Immunoglobulin E (IgE) | The class of antibody involved in allergic reactions (allergic immune responses) |
| Immunoglobulin E (IgE)‐mediated hypersensitivity reaction | Immune response to a specific allergen (e.g. peanut) that is mediated by immunoglobulin E |
| Immunoglobulin E (IgE)‐mediated sensitisation | Development of Ig‐E against a specific allergen (e.g. house dust mite) |
| Immunophenotype | Phenotypic features (types of antigens or markers) of the immune cells |
| Lung (pulmonary) function tests | A group of tests that measure how well the lungs work |
| Morbidity | Illness or harm |
| Non‐communicable diseases | Diseases that are usually long in duration and are not contagious |
| Oral food challenge | Medically supervised procedure where small and increasing amounts of a food are fed to a patient, to confirm if the food being tested causes an allergic reaction |
| Pathologically skewed Th2 immune responses | Abnormally biased (polarised) Th2 immune response (see T‐cell polarisation and type 2 immune response for further explanation) |
| Pentavalent vaccine (pentavalent formulation) | In the background section, this term alludes a '5‐in‐1' vaccine that provides protection against diphtheria, tetanus, whooping cough (pertussis), hepatitis B and Haemophilusinfluenzae type b disease. The '5‐in‐1' combination vaccine that this text refers to contains wP |
| Phenotype | Observable characteristics of an organism |
| Priming | A key process for the generation of vaccine‐specific immune cells |
| Randomised controlled trial | An experiment in which two or more interventions, possibly including a control intervention or no intervention, are compared by being randomly allocated to participants |
| Reactogenicity | Local (e.g. injection site redness) and systemic (e.g. fever, diarrhoea) expected reactions that occur following vaccination and are usually mild and self‐limiting |
| Spirometrically confirmed asthma | Diagnosis of asthma confirmed by lung function testing |
| T‐cell polarisation | Biased or skewed immune response for a T‐cell type(s). This occurs due to the release of cytokines triggered by antigen presenting cells |
| Th1 cells | Subset of CD4+ T cells that enhances the immune response against intracellular (within the cell) pathogens (microorganisms that can cause disease) |
| Th17 cells | Subset of CD4+ T cells that enhances the immune response against some fungi and bacteria |
| Th2 cells | Subset of CD4+ T cells that enhances the production of IgE and the immune response to helminths (worms) and allergens |
| Toxoid | A toxin that has been altered or inactivated and cannot cause disease. Toxoids are used in some combination vaccines (e.g. diphtheria and tetanus toxoids) as they can elicit immune responses |
| Type 1 immune response | Immune response to intracellular (within the cell) pathogens (microorganisms that can cause disease) |
| Type 2 cytokines | Cytokines involved in type 2 immune responses (e.g. interleukin‐4, interleukin‐5 and interleukin‐13) |
| Urticaria | A type of vascular reaction of the skin, characterised by a red or pink itchy rash and the presence of blotches (wheals). Some common conditions that may present with urticaria are infection, stress and allergy |
| Variable expiratory airflow limitation | More variation in how much air is blown out (exhaled) than would be expected in a healthy person |
| Whole‐cell (whooping cough) pertussis vaccine | A whooping cough vaccine prepared from inactivated Bordetellapertussis. This type of vaccine is mainly available as a pentavalent formulation with diphtheria and tetanus toxoids, Haemophilusinfluenzae type b and hepatitis B antigens |
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
MeSH descriptor: [Pertussis Vaccine] explode all trees
(pertussis vaccin*):ti,ab,kw
(Whooping cough vaccin*):ti,ab,kw
MeSH descriptor: [Diphtheria‐Tetanus‐Pertussis Vaccine] explode all trees
(whole‐cell OR 'whole cell' OR wP OR DTwP OR DTPw OR DTP OR DTwcP OR DTPwc) NEAR/5 vaccine
MeSH descriptor: [Whooping Cough] explode all trees
(whoop*):ti,ab,kw
MeSH descriptor: [Bordetella pertussis] explode all trees
(pertuss*):ti,ab,kw
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#10 with Publication Year from 1970 to present, in Trials
child* OR preschool* OR school* OR young OR infant* OR toddler* OR pediatric* OR paediatric*
#11 AND #12
Appendix 3. Ovid MEDLINE (R) search strategy
1. exp Pertussis Vaccine/
2. 'pertussis vaccin*'.ti,ab.
3. 'whooping cough vaccin*'.ti,ab.
4. exp Diphtheria‐Tetanus‐Pertussis Vaccine/
5. ((whole‐cell or wP or DTwP or DTPw or DTP or DTwcP or DTPwc) adj5 vaccine).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
6. 'whoop*'.ti,ab.
7. exp Bordetella pertussis/
8. 'pertuss*'.ti,ab.
9. (child* or preschool* or school* or young or infant* or toddler* or pediatric* or paediatric*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
10. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
11. 9 and 10
12. exp cohort studies/ or exp epidemiologic studies/ or exp clinical trial/ or exp evaluation studies as topic/ or exp statistics as topic/
13. ((control and (group* or study)) or (time and factors) or program or survey* or ci or cohort or comparative stud* or evaluation studies or follow‐up*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
14. or/12‐13
15. (animals/ not humans/) or comment/ or editorial/ or exp review/ or meta analysis/ or consensus/ or exp guideline/
16. hi.fs. or case report.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
17. or/15‐16
18. 14 not 17
19. 11 and 18
20. limit 19 to yr='1970 ‐Current'
Appendix 4. Embase search strategy
1. exp pertussis vaccine/
2. 'pertussis vaccin*'.ti,ab.
3. 'whooping cough vaccin*'.ti,ab.
4. exp diphtheria pertussis tetanus vaccine/
5. exp diphtheria pertussis tetanus Haemophilus influenzae type b hepatitis B vaccine/
6. ((whole‐cell or wP or DTwP or DTPw or DTP or DTwcP or DTPwc) adj5 vaccine).mp.
7. 'whoop*'.ti,ab.
8. exp Bordetella pertussis/
9. 'pertuss*'.ti,ab.
10. (child* or preschool* or school* or young or infant* or toddler* or pediatric* or paediatric*).mp.
11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
12. 10 and 11
13. exp cohort studies/ or exp epidemiologic studies/ or exp clinical trial/ or exp evaluation studies as topic/ or exp statistics as topic/
14. ((control and (group* or study)) or (time and factors) or program or survey* or ci or cohort or comparative stud* or evaluation studies or follow‐up*).mp.
15. or/13‐14
16. (animals/ not humans/) or comment/ or editorial/ or exp review/ or meta analysis/ or consensus/ or exp guideline/
17. hi.fs. or case report.mp.
18. or/16‐17
19. 15 not 18
20. 12 and 19
21. limit 20 to (embase and yr='1970 ‐Current')
Appendix 5. Search strategies (other resources)
ClinicalTrials.gov
Pertussis (condition or disease)
Pertussis vaccine (other terms)
EMA
Search terms: Pertussis
-
Filters:
Human
European public assessment reports (EPAR),
Paediatric investigation plans
Authorised
Withdrawn
FDA
Search terms: Pertussis vaccine
GSKtrialregistry
Advanced search
Keyword search: pertussis vaccine
-
Filters
Age: birth‐17 years
Vaccine studies: vaccine studies only
OpenGrey
Search terms: Pertussis vaccine
Pfizer
-
Find a trial
Condition, keyword or NCT number: pertussis
SanofiPasteur
Search term: pertussis
Clinical trials and results
WHO ICTRP (790 records for 608 trials found):
Pertussis vaccine
Data and analyses
Comparison 1. First dose of wP versus first dose of aP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Cumulative incidence of atopic disease at 2.5 years | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.2 Diagnosis of asthma by 2.5 years | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Diagnosis of atopic dermatitis by 2.5 years | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Diagnosis of encephalopathy | 7 | 115271 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 All‐cause serious adverse events (following a booster dose of aP) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Diagnosis of encephalopathy (following a booster dose of aP) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afari 1996.
| Study characteristics | ||
| Methods | Study design: 3‐arm, double‐blind, parallel‐group RCT Relative arm proportion:a 1 wP: 1 aP freeze‐dried (heat‐stable): 1 aP (liquid formulation) Duration of follow‐up: 14 months after the first dose of wP or aP Study setting and country: Ashaiman, a periurban community of southern Ghana World Bank income level of country: low Recruitment and sampling: infants aged between 0 and 6 weeks were recruited at the Maternal and Child Health Centre Study dates: September 1992 to unknown (enrolment completed by September 1993) |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children baseline characteristics
|
|
| Interventions |
Intervention: DTwP (Connaught Laboratory Limited): nwP = 137 Comparator: DTaP freeze‐dried and liquid formulations (Biken): naP = 266 Dose and route of administration: 0.5 mL SC; schedule: 3‐dose‐series (6, 10 and 14 weeks of ageb) Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes
|
|
| Funding |
|
|
| Conflicts of interest | Not stated | |
| Notes |
aIn this review, the DTaP study arms were combined to create a single pairwise comparison bAntipyretic/analgesic use: not stated cNot prespecified in the methods section of the available report dCauses of death
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: (children) "were randomly allocated to one of the treatment groups by means of a computer programme (EPI Info) as they attended the clinic" Comment: the method used to generate the random sequence was stated, but additional details were not provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method used to conceal the allocation was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the study was single‐blinded but double‐blinded for the field workers who followed up participants to record adverse reactions" Comment: children/carers and outcome assessors were blinded; the outcome of interest was unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the study was single‐blinded but double‐blinded for the field workers who followed up participants to record adverse reactions" Comment: the assessment of the outcome of interest was unlikely to have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the dropout rates were higher in recipients of DTwP, compared to DTaP‐vaccinees (nwP = 24/137; 17.5% and naP = 32/266; 12.0%). Reasons for no completion of the primary series include parental refusal for the collection of blood samples or moving out from the study area (nwP = 9/137, 6.6%; naP = 9/266, 3.4%). For the follow‐up phase of this trial, other than deaths (nwP = 2/137, 1.5%; naP = 5/266, 1.9%), the reasons for withdrawal were not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not find the study protocol. Although all‐cause mortality was not a prespecified outcome domain, deaths were likely to have been reported when they occurred |
Black 1997.
| Study characteristics | ||
| Methods | Study design: 2‐arm, double‐blind, parallel‐group RCT Duration of follow‐up:a 10 months after the first dose of wP or aP Relative arm proportion: 1 wP: 4 aP Study setting and country: 8 medical centres in Northern California, USA World Bank income level of country: high Recruitment and sampling: not stated Study dates: October 1992 to unknown (enrolment completed by November 1993) |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children baselinecharacteristics
|
|
| Interventions |
Intervention: DTwP (Connaught): nwP = 498 Comparator: DTaP (Chiron/Biocinea) : naP = 2000 Dose and route of administration: 0.5 mL IM; schedule: 3‐dose‐series (2, 4 and 6 months of ageb) Vaccine(s) co‐administered:
|
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes/outcomedomains
Secondaryoutcomes
|
|
| Funding | Not stated | |
| Conflicts of interest | CD, DG, AI and AP reported affiliations with Chiron Corporation, Emeryville, CA | |
| Notes |
aToddlers primed with DTaP were offered a booster dose of a DTaP‐based formulation between 15 and 18 months of age. These data are not reported in this review bAntipyretic/analgesic use: not stated cThe number of children experiencing any SAEs includes those admitted to hospital within 60 days of each dose and those diagnosed with SIDS dPrespecified in the methods section of the available report eCorrespondence: SBB confirmed that each child could only contribute once to the admissions to hospital analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "phase II, double‐blind, randomized trial. [...] After informed consent was obtained infants were randomly assigned in a 4:1 ratio to receive either three doses of the C‐aPDT vaccine (80% of infants) or three doses of Connaught wDPT (20%)" Comment: the method used to generate the random sequence was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method used to conceal the allocation was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding is mentioned, but details were not provided. The outcome of interest (all‐cause SAEs) was unlikely to have been influenced by knowledge of the intervention received, as events leading to admission to hospital were identified via computer databases containing records of all hospitalisations, and SIDS until the first year of life were identified in collaboration with the county‐SIDS reporting departments |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding is mentioned, but details were not provided (i.e. how likely it was to be broken). The assessment of events leading to hospital admission, could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout rates were low and balanced (nwP = 43/498; 8.6% and naP = 164/2000; 8.2%). The reasons for withdrawals/loss to follow‐up were not stated |
| Selective reporting (reporting bias) | Low risk | Comment: we did not find the study protocol. Events leading to admission to hospital within 60 days of each dose, were prespecified in the methods section as a study outcome. Data were systematically collected and reported by study arm |
Blumberg 1991.
| Study characteristics | ||
| Methods | Study design: 2‐arm, double‐blind, parallel‐group RCT Relative arm proportion: 1 wP: 1 aP (primary series) Duration of follow‐up: 17 months after the first dose of wP or aP Study setting and country: 10 study sites in the USA; no additional information is provided World Bank income level of country: high Recruitment and sampling: not stated Study dates: May 1987 to July 1989 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series
Dose and route of administration: 0.5 mL IM; schedule: 3‐dose‐series (2, 4 and 6 months of agea) Vaccine(s) co‐administered: not stated Booster dose
Dose and route of administration: 0.5 mL IM; schedule: 1 dose (18 months of agea) Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes/outcomedomains
Secondaryoutcomes
|
|
| Funding | Not stated | |
| Conflicts of interest | Not stated. JVS, MGS, JRM, JFG, GH (members of the APDT Vaccine Study Group), reported affiliations with Lederle Biologicals | |
| Notes |
aAntipyretic/analgesic use: recommended for rectal temperature ≥ 39∘ C bNot prespecified in the methods section of the available report cCauses of death:
dPrespecified in the methods section of the available report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in a double‐blind manner, 252 children were randomly selected to receive DTP vaccine at 2, 4, and 6 months of age, and 245 children were randomly selected to receive APDT vaccine at the same ages" Comment: the method used to generate the random sequence was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method used to conceal the allocation was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding is mentioned, but details were not provided; however, SAEs were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding is mentioned, but details were not provided. The assessment of events leading to admission to hospital, but no deaths, could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropout rates were high and similar for both groups (nwP = 53/252; 21% and naP = 47/245; 19.2%). Reasons for withdrawal/loss to follow‐up were provided and include one accidental death in the DTwP arm (a child strangled by a pacifier), but no events leading to hospital admission |
| Selective reporting (reporting bias) | Low risk | Comment: we did not find the study protocol. Prespecified and expected outcomes of interest (i.e. deaths) were all reported |
Dagan 1997.
| Study characteristics | ||
| Methods | Study design: 2‐arm, open‐label, parallel‐group RCT Relative arm proportion: 1 wP: 1 aP (primary series) Duration of follow‐up: between 9 and 11 months after the first dose of wP or aP Study setting and country:a maternal and child health units in the community, Israel World Bank income level of country: high Recruitment and sampling: not stated Study dates: not stated |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series
Dose and route of administration: 0.5 mL IM; schedule: 3‐dose‐series (2, 4 and 6 months of ageb) Vaccine(s) co‐administered: not statedb Booster dose
Dose and route of administration: 0.5 mL IM; schedule: one dose (12 months +/‐ 4 weeksb) Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes/outcomedomains
Secondaryoutcomesa
|
|
| Funding | Not stated | |
| Conflicts of interest | Not stated. PW, AG and AK reported affiliations with SmithKline Beecham Biologicals, Rixensart, Belgium | |
| Notes |
aCorrespondence: we contacted RD to determine the country where this study was conducted, the characteristics of the study setting, whether any child experienced encephalopathy and if any of the children with SAEs were admitted to hospital or were diagnosed with any of the atopic outcomes of interest bAntipyretic/analgesic use: prophylactic and reactive use allowed cUnable to calculate the number of children experiencing SAEs with the information provided in the report dUnable to determine the number of children that met this endpoint with the information provided in the report eSystematically assessed as a contraindication to DTP vaccines (i.e. any hypersensitivity reaction to the study vaccines) fPrespecified in the methods section of the relevant report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the study was conducted in an open, randomized manner. The randomization was made using an algorithm of pseudorandom numbers (given by RS/1 from BBN Inc.)" Comment: the random component of the sequence generation was described |
| Allocation concealment (selection bias) | Unclear risk | Comment: details about the allocation sequence concealment was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the different forms of presentation of the two vaccines, DTPa‐IPV in a vial and DTPw‐IPV in a prefilled syringe, precluded a blinded study..." Comment: there is no blinding in this study. In this case, the assessment of the outcome of interest could have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no blinding in this study; the assessment of the outcome of interest was likely to be influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: of the 201 children enrolled, 179 (89.1%) agreed to continue in the booster dose phase of this study. Reasons for withdrawal/loss to follow‐up were not provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not find the protocol of this study. Principal investigator (RD) confirmed that no child experienced encephalopathy during the study period, and no child with a serious adverse reaction was admitted to hospital. It is unclear whether events judged as 'serious' and unrelated to the study vaccines led to hospitalisation |
Decker 1995.
| Study characteristics | ||
| Methods | Study design:a double‐blind, parallel RCT of 13 aP‐based vaccine formulations and 2 wP‐based vaccine formulations (primary series). Duration of follow‐up (primary series study): 16 months after the first dose. Study setting and country: six university‐based vaccine and treatment evaluation units across the USA, sponsored by the National Institute of Allergy and Infectious Diseases World Bank income level of country: high Recruitment and sampling: carried out at suburban, middle‐to‐upper‐middle‐class private paediatric offices as well as suburban practices serving families of low to moderate incomes Study dates: 27 March 1990 until 1993/1994 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: wP group
Comparator: aP group
Dose and route of administration: 0.5 mL IM; schedule: 3‐dose‐series (2, 4 and 6 months of agec) Vaccine(s) co‐administered:
First booster (fourth dose): included all the pertussis vaccine formulations used in the primary series study, except for the DTaP vaccine manufactured by Lederle Praxis Biologicals (Pearl River, New York, USA)
Dose: 0.5 mL IM; one dose (15 to 20 months of agec) Concomitant vaccine(s): OPV (manufacturer and dose: not stated); per oral Second booster (fifth dose):
Dose: 0.5 mL IM; 1 dose (4 to 6 years of agec) Concomitant vaccine(s): OPV (manufacturer and dose: not stated); per oral |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes/outcomedomains
Secondaryoutcomesg
|
|
| Funding | National Institute of Allergy and Infectious Diseases, National Institute of Health | |
| Conflicts of interest | Not stated | |
| Notes |
aThe safety report of the trial compares 13 aP formulations with each other and with a conventional type of wP (Lederle). In this review, the DTaP study arms were combined to create a single pairwise comparison. A subset of children received a fourth dose between 15 and 20 months of age (N = 1374), and a fifth dose, between 4 and 6 years old (N = 351). bNot including a 14th type of DTaP formulation (n = 23), withdrawn from the study by the manufacturer due to demonstrated low immunogenicity in another trial cAntipyretic/analgesic use: reactive use allowed. dNot prespecified in the methods section of the relevant reports eCauses of death
fPrespecified in the methods section of the relevant reports gCorrespondence: JAE, KME, MDD confirmed that they did not systematically collect data on the atopic outcomes of interest for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "we conducted a randomized, double‐blinded, multicenter clinical trial of 13 DTaP vaccines to compare their safety and immunogenicity with each other and with a conventional whole‐cell pertussis vaccine [...] Blocking was used to ensure that each VTEU enrolled a roughly equal proportion of children to each study arm" Comment: the method to generate the random component of the sequence generation was described; however, the size of the blocks was not provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "parents, patient care nurses, participating clinicians, and laboratory personnel were blinded to the vaccine assignment. Vaccines were not identified by type of manufacturer; vials were labelled with letter codes. A separate cadre of nurses administered the vaccines but had no other contact with patients or parents" Comment: details regarding the concealment of the allocation sequence were not provided. It remains unclear if vaccinators had access to unblinding information (i.e. meaning of the letter codes) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding was mentioned, but details were not provided. Safety outcomes other than encephalopathy were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding is mentioned, but details were not provided. Except for deaths, the assessment of the outcomes of interest could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "twenty‐three infants were withdrawn from the study because of adverse reactions (most for prolonged, inconsolable, or high‐pitched cry), representing 2.5% and 0.8% of wP‐Lederle and DTaP recipients respectively (p = 0.02)'...'rates of withdrawal because of intercurrent illness or failure to return were not significantly associated with vaccine assignment" Comment: of 2342 children randomised, 2264 (96.7%) completed the trial (Pichichero 1997). The reasons for no completion were provided across different reports from the same study. The investigators described that 6 DTaP recipients and 1 child vaccinated with wP‐Lederle were withdrawn due to adverse reactions following the first immunisation; 39 recipients of DTaP and 11 of wP‐Lederle were withdrawn after the first immunisation due to other reasons.These were presumably included in the above‐mentioned reasons for no completion, although this is not clearly stated in the reports |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not find the protocol of this study. The safety report of the trial compares 13 aP formulations with each other, and with a conventional type of wP (Lederle). Prespecified and expected outcome domains of interest were all reported |
Edwards 1991.
| Study characteristics | ||
| Methods | Study design: a subset of children who had been randomly allocated to a 3‐dose priming schedule with either wP or aP (1:1), received a booster dose of aP at 19 months of age. The primary series trial was published in 1989, and their references are linked to this studya Duration of follow‐up: 2 years after the booster dose Study setting and country: Vanderbilt University clinical research centre, USA World Bank income level of country: high Recruitment and sampling: not stated Study dates: not stated |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series (Edwards 1989)
Dose and route of administration: 0.5 mL; route: not stated; schedule: 3‐dose‐series (2, 4 and 6 months of ageb) Vaccine(s) co‐administered: not stated Booster dose
Dose and route of administration: 0.5 mL; route: not stated; schedule: 1 dose (~ 19 months of age) Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes
|
|
| Funding |
|
|
| Conflicts of interest | Not stated | |
| Notes |
aThe primary vaccination study had an unclear length of follow‐up bAntipyretic/analgesic use: reactive use allowed cNot prespecified in the methods section of the available report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "infants [...] were randomly assigned to receive either conventional diphtheria‐tetanus‐pertussis vaccine (DTP) or acellular DTP in a double‐blind manner..." Comment: the quote refers to the primary series study (Edwards 1989). The random component of the sequence generation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method to conceal the allocation for the primary series was not provided (Edwards 1989) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding was mentioned (Edwards 1989), but details were not provided. Except for encephalopathy, the outcome/outcome domains of interest were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding was mentioned, but details were not provided (Edwards 1989). Except for deaths, the assessment of the outcomes/outcome domains of interest could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: of the 50 children enrolled in the primary series trial (Edwards 1989), 41 (82%) received a booster dose of DTaP at approximately 19 months of age. Reasons for loss to follow‐up after the third dose of the priming schedule were not stated. The length of follow‐up for the primary series study remains unclear, but presumably was longer than 5 months. During that period, no child developed the outcomes of interest |
| Selective reporting (reporting bias) | Unclear risk | Comments: we did not find the study protocol. Although the outcomes/outcome domains of interest were not prespecified in the methods section, they were likely to have been reported when they occurred |
Estcourt 2020.
| Study characteristics | ||
| Methods | Study design: retrospective cohort‐nested case‐control study Study setting and country: private and tertiary hospital allergy clinics in New South Wales, Victoria, South Australia and Western Australia (Australia) World Bank income level of country: high Recruitment and sampling:
Study dates: October 2015 to December 2018 |
|
| Participants |
Inclusioncriteria Cases and controls:
Cases only:
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
Confoundingdomainsidentifiedbytheinvestigatorsofthisstudyb
|
|
| Interventions |
Cases
Controls
Dose and route of administration: not stated Schedule:c first dose before 16 weeks of age Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Primaryoutcomes/outcomedomains
Secondaryoutcomes
|
|
| Funding | National Health and Medical Research Council of Australia | |
| Conflicts of interest |
|
|
| Notes |
a2out of 5020 controls had both wP and aP entered as first dose, and therefore, were excluded by the authors of this study bRisk of bias assessment available in Table 2 cAntipyretic/analgesic use: not stated |
|
Feldman 1993.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT Relative arm proportion:a 1 wP: 1 aP (lot 4547): 1 aP (lot 4548): 1 aP (lot 4549) Duration of follow‐up: 10 months after the first dose of wP or aP Study setting and country: community‐based private paediatric practices (> 90%) and paediatric outpatient clinics (University of Mississippi Medical Centre), Mississippi, southern USA World Bank income level of country: high Recruitment and sampling: children were recruited at the study sites Study dates: not stated |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: DTwP (Connaught Laboratories Inc, a Pasteur/Merieux company, Swiftwater, Pennsylvania, USA): nwP = 36 Comparator: DTaP (Biken Inc, the Research Foundation for Microbial Diseases of Osaka University; the components were combined at Connaught Laboratories): naP = 109 Dose and route of administration: 0.5 mL IM; schedule: 3‐dose‐series (2, 4 and 6 months of ageb) Vaccine(s) co‐administered:
|
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes
|
|
| Funding | Connaught Laboratories Inc (a Pasteur Merieux company, Swiftwater, Pennsylvania, USA) | |
| Conflicts of interest | Not stated. DL and CM reported affiliations with Connaught Laboratories Inc (a Pasteur Merieux company, Swiftwater, Pennsylvania, USA) | |
| Notes |
aIn this review, the DTaP study arms were combined to create a single pairwise comparison bAntipyretic/analgesic use: reactive use allowed cNot prespecified in the methods section of the available report dDeaths were not specifically reported; however, the reasons for no completion are clearly described and do not include this outcome domain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "infants were randomized at 2 months of age in a double‐blind fashion to receive either standard (whole‐cell) pertussis vaccine (DTP‐Wc) or one of three lots of acellular pertussis vaccine (DTP‐Ac)" Comment: the random component of the sequence generation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding was mentioned, but details were not provided. Safety outcomes other than encephalopathy were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding was mentioned, but details were not provided. The assessment of encephalopathy could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout rates were low and balanced (nwP = 3/36; 8.3% and naP = 10/109; 9.1%). Reasons for no completion were described as quote: "proportionally divided" between DTaP and DTwP vaccinees; however, these were not provided by study arm |
| Selective reporting (reporting bias) | Unclear risk | Quote: "the medical records of participants were reviewed at 1 year of age for intervening illnesses and vaccine related events" Comment: we did not find the protocol of this study. Diagnosis of encephalopathy was not an outcome clearly specified in the methods, however it is discussed in the results section Although all‐cause mortality was not a prespecified outcome domain, deaths were likely to have been reported when they occurred |
Greco 1996.
| Study characteristics | ||
| Methods | Study design: 4‐arm, double‐blind, parallel‐group RCT Relative arm proportion:a 3 wP: 3 aP (with genetically detoxified pertussis toxin): 3 aP (with pertussis toxin inactivated with formalin): 1 DT Duration of follow‐up (primary series): 16 months after the first dose of wP or aP Study setting and country: 62 public health (primary care) units in four out of 21 regions of Italy World Bank income level of country: high Recruitment and sampling: recruitment for this study was carried out in the postnatal period (quote "parents of each eligible newborn were invited to enter the trial"), however no further details were provided Study dates: 21 September 1992 to April 1995 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention:b DTwP (Connaught Laboratories, Swiftwater, Pennsylvania, USA), n = 4678 Comparator: DTaP (Chiron Biocine, Siena, Italy and SmithKline Beecham Biologicals, Rixensart, Belgium), n = 9368 Dose and route of administration: 0.5 mL IM; schedule: 3‐dose schedule (6 to 12, 13 to 20 and 21 to 28 weeks of agec) Vaccine(s) co‐administered:
|
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes/outcomedomains
Secondaryoutcomes
|
|
| Funding | National Institute of Allergy and Infectious Diseases, National Institute of Health | |
| Conflicts of interest | Not stated | |
| Notes |
aIn this review, the DTaP study arms were combined to create a single pairwise comparison. We omitted further information on the DT study arm, as it does not meet our inclusion criteria bRecipients of DTwP were unblinded in July 1995 and offered a booster dose of a DTaP vaccine formulation with no further follow‐up cAntipyretic/analgesic use: not stated dThese vaccines formulations were used interchangeably and according to the site availability eCorrespondence: we attempted to contact DG and MLCdA to confirm that children enrolled in this trial could only contribute once for the primary safety outcome in the specified time window; however, we were unsuccessful fPrespecified in the methods section gAll deaths were due to SIDS:
hDiagnosis of serious chronic illnesses within 60 days of the last vaccination (~ within 6 months of the first dose; prespecified in the methods section) was assessed as a proxy of events leading to persistent or significant disability or incapacity |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in 1992, we initiated the present randomized, double‐blind, controlled clinical trial of three pertussis vaccines" Comments: details on the sequence generation were not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "ten sets of three doses each of vaccine were boxed together (three sets of each of the three DTP vaccines and one set of the DT vaccine, all in identical vials); the sets were consecutively numbered according to randomization lists provided by the (National Institute of Allergy and Infectious Diseases) NIAID" Comments: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "neither parents nor investigators knew the infants' vaccine assignments" Comment: children/carers and personnel were unaware of the intervention received. Although partial unblinding of the vaccinator and parents/carers was possible (see Gustafsson 1996), safety outcomes other than encephalopathy were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding is mentioned, but details were not provided. Except for deaths, the assessment of the remaining outcomes/outcome domains of interest could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "all data regarding children who received at least one trial vaccine dose were included in the analysis" Comment: 769 children did not receive three doses of DTwP/DTaP. Dropout rates due to side effects were very low and described as 'more frequent after receipt DTwP' (nwP = 135/4678, 2.9%; naP = 31/9368; 0.33%); other causes of failure to administer three doses of the study vaccines were described as 'similar between the study groups' |
| Selective reporting (reporting bias) | Low risk | Comment: we did not find the protocol of this study. Primary and secondary outcomes/outcome domains were prespecified in the methods section and reported by study arm |
Gustafsson 1996.
| Study characteristics | ||
| Methods | Study design: 4‐arm,a double‐blind parallel‐group RCT Relative arm proportion: 1 wP: 1 aP (2c): 1 aP (5c): 1 DT. Due to availability issues, during the first two months of this trial, children were not randomised to DTwP Duration of follow‐up (primary series): 2 to 3 years Study setting and country: 14 study areas distributed across Sweden, with 3 to 4 study nurses and 1 or 2 part‐time paediatricians World Bank income level of country: high Recruitment and sampling: parents living in the study areas were informed about this trial through a letter. Research nurses followed up their expressions of interest and recruited them. Children recruited in Linköping were also offered to be enrolled in an allergy sub‐study, which is reported separately (Nilsson 1998) Study dates: March 1992 to January 1995 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series
Dose and route of administration: dose: 0.5 mL (not stated for DTwP); route: IM; schedule: 3‐dose‐series (2, 4 and 6 months of ageb) Vaccine(s) co‐administered:
|
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes/outcomedomains
Secondaryoutcomesh
|
|
| Funding |
|
|
| Conflicts of interest | Not stated | |
| Notes |
a In this review, the DTaP study arms were combined to create a single pairwise comparison. We omitted further information on the DT study arm, as it does not meet our inclusion criteria bAntipyretic/analgesic use: reactive use allowed cWe could not determine the number of children experiencing any SAEs due to overlaps between the data reported across some of the outcome domains: dPrespecified in the methods section of the relevant reports eAll deaths were due to SIDS:
fData systematically collected and reported gWe assumed that serious chronic illnesses within 60 days of the last vaccination, a safety endpoint prespecified in the methods section in the relevant reports, were a proxy of events leading to persistent or significant disability or incapacity hSymptoms consistent with atopic diseases were assessed at 2.5 years in 97.8% of the cohort. These symptoms were wheezing at any time during the last 12 months, itchy rash during at least 3 months behind the knees or runny nose when in contact with a dog or a cat. No definite diagnosis of atopic diseases were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the vaccines were supplied in identical vials, each of which was labelled with a unique computer‐generated randomization number. Twelve‐unit blocks were used to ensure balanced assignment of infants to the three groups randomized during the first two months of the trial, and thereafter, 16‐unit blocks were used for randomization to the four groups. The block sizes were not revealed to the investigators" Comment: adequate methods |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the whole cell vaccine (Connaught Laboratories Inc., Swiftwater, USA), required vigorous shaking to suspend the sediment and differed markedly in appearance from the other preparations. A majority of the study nurses engaged in that trial could readily identify the whole cell vaccine by appearance and reactogenicity. The whole cell vaccine arm is thus unblinded in this ongoing Swedish placebo‐controlled trial. However, randomization was not compromised..." Comment: more than half of the research nurses were unblinded to DTwP, but they could not distinguish between DTaP or DT formulations |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: safety outcomes other than encephalopathy were unlikely to have been influenced by knowledge of the intervention received, or unblinding of DTwP |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "fourteen days after the third dose, the study nurses could identify 53.5 percent of the recipients of whole cell vaccine but could not distinguish between recipients of the acellular vaccines and the DT vaccine'[...] 'In cases where a severe event occurred, the nurses immediately contacted the paediatricians of the studies, who reviewed the clinical history with the parents/or the treating physician. In case of hospitalization, the clinical record was obtained" Comment: except for deaths, the assessment of the outcomes/outcome domains of interest could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all data regarding children who received at least one trial vaccine dose were included in the analysis" Comment: of 9829 study children randomised, 199 did not complete the primary vaccination series (these include DT‐vaccinees). Dropout rates due to contraindicating events were generally low, but greater in recipients of DTwP, compared to DTaP (nwP = 67/2102; 3.2% and naP = 29/5153; 0.6%). Other reasons for no completion were not broken down by study group and include culture‐confirmed pertussis (n = 47/199) and withdrawal from the study (n = 40/199) |
| Selective reporting (reporting bias) | Low risk | Comments: data on the outcomes/outcome domains of interest were systematically collected and reported by study arm |
Halperin 1996.
| Study characteristics | ||
| Methods | Study design: 4‐arm,a double‐blind, parallel‐group RCT Relative arm proportion: 1 wP: 3 aP (equal allocation to 1 of 3 lots). The same vaccine lot assignment was kept for all doses Duration of follow‐up: 16‐to‐18‐month duration of follow‐up after the first dose of wP or aP Study setting and country: 3 study sites in Calgary, Alberta (1) and the Fraser Valley (2), Canada World Bank income level of country: high Recruitment and sampling: not stated Study dates: November 1990 until 1993/1994 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series
Dose and route of administration: 0.5 mL IM; 3‐dose schedule (2 to 3, 4 and 6 months of ageb) Vaccine(s) co‐administered: OPV (Connaught Laboratories Limited); dose not stated; route: per oral; 2‐dose schedule (2 and 4 months of age) First booster:
Dose and route of administration: 0.5 mL IM; 1 dose between 17 and 19 months of age Vaccine(s) co‐administered: OPV (Connaught Laboratories Limited); dose not stated; per oral; 1‐dose schedule Second boosterd:
Dose and route of administration: 0.5 mL IM; 1 dose between 4 and 6 years of age. Vaccine(s) co‐administered: presumably nil |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes
|
|
| Funding | Not stated | |
| Conflicts of interest | LB reported affiliations with Connaught Laboratories Limited, North York, Ontario, Canada | |
| Notes |
aThe DTaP study arms were combined to create a single pairwise comparison bAntipyretic/analgesic use: allowed cThis outcome was included in the assessment of the safety data from this trial, prepared by the FDA, but not in the relevant peer‐reviewed article dThe Hib vaccine was manufactured by Pasteur Mérieux Connaught, North York, Canada. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ..."randomized, double‐blind, multicentered clinical trial conducted at three sites[...] Vaccine allocation was via computer generated table of random numbers within each center; a balanced block containing an equal allocation of each of the three APDT lots and the DTP lot resulted in a 3:1 APDT: DTP ratio" Comment: the random component of the sequence generation was described, but the size of the block was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details were provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding is mentioned, but details were not provided. The outcome of interest was unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding was mentioned, but details were not provided. The assessment of this outcome could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: of the 432 children randomised, 398 (91%) completed the four immunisation series as detailed below The primary schedule was completed by 424 out of 432 study children (98%). Dropout rates were low across the study groups (nwP = 3/108; 2.7% and naP = 5/324; 1.5%). Reasons for withdrawal were stated and do not include the outcome of interest; however, except for one episode of high‐pitch crying following vaccination with wP, these reasons are not broken down by study arm Of the 423 children who completed the primary series and had bloods taken at the 7‐month study visit, 398 received a booster dose of DTwP/DTaP. Reasons for loss to follow‐up after the third dose were not stated |
| Selective reporting (reporting bias) | Unclear risk | Quote: "there were no serious adverse events, seizures or HHE reported following the infant series. A recipient of the whole‐cell vaccine was reported to have had a seizure and HHE episode in the first 48 hours after vaccination following receipt of the fourth dose" Comment: outcome data following the primary series were summarised in the assessment of the safety data carried out by the FDA. It remains unclear whether the events described following the fourth dose of DTwP resulted in hospitalisation (i.e. whether they met the review definition of SAE) |
Kitchin 2006.
| Study characteristics | ||
| Methods | Study design: 3‐stage, stratified, 2‐arm, open, parallel‐group RCT Relative arm proportion: 1 wP: 1 aP (primary series) Duration of follow‐up: 10 months after the first dose (primary series study) Study setting and country: 5 study centres in the UK World Bank income level of country: high Recruitment and sampling: not stated Study dates: November 2001 to unknown |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
BCGhistory: not stated |
|
| Interventions | Primary series
Dose and route of administration: 0.5 mL IM; 3‐dose‐series (2, 3 and 4 months of agea) Vaccine(s) co‐administered
Booster dose
Vaccine(s) co‐administered
|
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes
Secondaryoutcomes
|
|
| Funding |
|
|
| Conflicts of interest |
|
|
| Notes |
aAntipyretic/analgesic use: reactive use allowed bDeaths were not specifically reported; however, the reasons for no completion are clearly described and do not include this outcome domain cPrespecified in the methods section of the relevant report dPrespecified in the methods section of the relevant report as 'any illness resulting in sequelae' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "this was an open, randomised, controlled study performed in healthy infants [...] Subjects were randomised evenly to one of two groups, each containing two strata as follows..." Comment: information about the random component of the sequence generation is not provided. The randomisation must have been stratified by MCC‐TT and MCC‐CRM, although this is not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details are provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: no blinding of children/carers or personnel unlikely to have influenced the outcome of interest |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the assessment of the outcome of interest was likely to be influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: for the primary series phase of this study, dropout rates were low and similar (nwP: 3/120; 2.5% and naP: 2/121 (1.6%). Reasons for no completion are provided in a CONSORT flow diagram and are unrelated to the outcomes of interest |
| Selective reporting (reporting bias) | Unclear risk | Quote: "serious adverse events (e.g. those resulting in hospital admission) were recorded throughout the study" Comment: events leading to admission to hospital occurring within 10 months of the first dose were reported. It is not possible to determine whether children who had SAEs before 5 months of age, had a subsequent one afterwards. |
Macías 2012.
| Study characteristics | ||
| Methods | Study design: 4‐arm,a single‐blind, parallel‐RCT Relative arm proportion: 1 wP: 2 aP. Children allocated to DTaP were randomised into 3 subgroups of different vaccine batches Duration of follow‐up: 10 months after the first dose of wP or aP Study setting and country: clinical centres in Mexico and Peru World Bank income level of country:
Recruitment and sampling: not stated Study dates: July 2006 to February 2008 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention(wPgroup): DTwP‐HepB‐Hib (GlaxoSmithKline): nwP = 711 Comparator(aPgroup): DTaP‐HepB‐Hib‐IPV (Sanofi Pasteur, Argentina): naP = 1422 Dose and route of administration: 0.5 mL IM, schedule: 3‐dose series (2, 4 and 6 months of ageb) Vaccine(s) co‐administered:
Route of administration:b per oral; schedule: 3‐dose series (2, 4 and 6 months of age) |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes
Secondaryoutcomes
|
|
| Funding | Sanofi Pasteur, a Sanofi Company | |
| Conflicts of interest |
|
|
| Notes |
aIn the relevant reports of this trial and in this review, the DTaP study arms were combined to create a single pairwise comparison bAntipyretic/analgesic use: not stated cPrespecified as an outcome of interest dData extracted from clinicaltrials.gov. From the trial registry it is only possible to ascertain the number of children experiencing any SAEs as well as the number of children with a specific diagnosis eDeaths were not specifically reported; however, the reasons for no completion are clearly described and do not include this outcome domain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "permuted block randomisation was used in the primary series studies" Comment: details regarding the block size were not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "allocation: randomized; intervention model: parallel assignment; masking: single (outcomes assessor)..." Comment: details regarding the concealment of the allocation were not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "masking: single (outcomes assessor)" Comment: no blinding of children/carers or personnel unlikely to have influenced the outcome/outcome domain of interest |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "masking: single (outcomes assessor)" Comment: blinding is mentioned but additional details were not provided. The assessment of SAEs other than deaths, may have influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "total number of participants in each group adjusted for the participant that got a vaccine assigned for the other group" Comment: as‐treated analysis does not show substantial departure from allocation (nwP = 710 (as‐treated) versus nwP = 709 (ITT); naP = 1423 (as‐treated) versus naP = 1422 (ITT). Dropout rates were low and balanced (nwP = 41/711, 5.8%; naP = 94/1422, 6.6%). Reasons for non completion were stated and include SAEs (nwP = 1/41, 2.4%; naP: 6/94, 6.4%) |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol of this trial was identified through clinicaltrials.gov. Whereas the trial registry included outcome data as from the day of the first dose, until 6 months after the third dose of DTwP/DTaP, a peer‐reviewed article arising from this trial only included the number of children that experienced the outcome of interest within one month after the third dose |
Madhi 2011.
| Study characteristics | ||
| Methods | Study design:a 3‐arm,b open‐label, parallel‐group RCT Relative arm proportion: 2 wP (no hepatitis B vaccine at birth): 2 aP (no hepatitis B vaccine at birth): 1 aP (hepatitis B vaccine at birth) Duration of follow‐up: approximately 14 to 17 month after the first dose of wP or aP Study setting and country: two trial centres in Johannesburg, South Africa World Bank income level of country: upper‐middle Recruitment and sampling: not stated Study dates: August 2006 to August 2009 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series
Dose and route of administration: 0.5 mL IM; 3‐dose schedule (6, 10 and 14 weeks oldc) Vaccine(s) co‐administered:
Booster dose:
Dose and route of administration:c as above; 1 dose (15 to 18 months old) Vaccine(s) co‐administered:
|
|
| Outcomes |
Outcomesofinterestforthereview: Primaryoutcomes:
Secondaryoutcomes:
|
|
| Funding | Sanofi Pasteur, a Sanofi Company | |
| Conflicts of interest |
|
|
| Notes |
aImmunogenicity follow‐up at 3.5 and 4.5 years of age was carried out under a different trial registry. No safety data were recorded except for long‐term monitoring of ongoing SAEs after the primary series bIn this review, the DTaP study arms were combined to create a single pairwise comparison cAntipyretic/analgesic use: not stated dData extracted from clinicaltrials.gov. From the trial registry it is only possible to ascertain the number of children experiencing any SAEs as well as the number of children with a specific diagnosis. eAlthough 4 deaths were reported in a peer‐reviewed publication arising for this trial, no events were recorded in the section of the trial registry where all‐cause mortality is reported. 1 of the deaths occurred before the first dose of pertussis‐containing vaccine) fCauses of death
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..phase III, open‐label, randomized, controlled 2‐center trial...A 2‐step randomization procedure created by Sanofi Pasteur’s statistics department was used to assign participants to 1 of 3 groups [...] Those who did not receive hepatitis B vaccination at birth were further randomized at 6 weeks of age to receive the investigational (Group 1) or control (Group 2) vaccines"‐=] "Permuted block randomisation was used in the primary series studies" Comment: the size of the blocks was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details were provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: no blinding of children/carers or personnel unlikely to have influenced the outcome of interest |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no blinding in this study; the assessment of the outcome of interest was likely to have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: for the primary series phase of this study, dropout rates were low and balanced (nwP = 7/242, 2.9%; naP = 13/380, 3.4%). Reasons for non completion are stated and include SAEs (nwP = 0/7, 0.0%; naP = 2/13, 15,4%). Of the 602 children who completed the primary series, 567 (91%) returned for a fourth dose (nwP = 219/235, 93%; naP: 348/367,94.5%) at 15 to 18 months of age. Reasons for loss to follow‐up between the third and fourth dose were not provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: we identified the protocol of this study through clinicaltrials.gov. We noted discrepancies between the number of SAEs reported on the trial registry and a peer‐reviewed journal article arising from this study |
Miller 1990.
| Study characteristics | ||
| Methods | Study design: 2‐stage, parallel‐group RCT Relative arm proportion:a
Duration of follow‐up: 6 months after the first dose of wP or aP Study setting and country: immunisation clinics in North Hertfordshire District Health Authority, UK World Bank income level of country: high Recruitment and sampling: infants attending the above‐mentioned clinics for primary immunisation with DTwP, were offered to be involved in this study Study dates: March 1988 until unknown |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: DTwP (Wellcome Research Laboratories, Beckenham, England): nwP = 179 Comparator: DTaP: naP = 253
Dose and route of administration: 0.5 mL; deep SC Schedule: 3‐dose series (3, 5 and 8 to 10 months of ageb) Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Outcomesofinterestinthereview Primaryoutcomes/outcomedomains
Secondaryoutcomes
|
|
| Funding | UK Medical Research Council | |
| Conflicts of interest | Not stated | |
| Notes |
aIn this review, the DTaP study arms were combined to create a single pairwise comparison bAntipyretic/analgesic use: not stated cAlthough deaths were not specifically reported, the number of children who did not complete the primary series and their reasons are clearly described and do not include this outcome dNot prespecified in the methods section of the relevant reports |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in each stage, vaccines were randomly allocated to sequential study numbers in equal proportions, and infants were allocated to study numbers in order of attendance at the clinics. Block randomization of vaccines to study numbers was performed by a computer program. The vaccine code was not disclosed to parents or field, laboratory or coordinating staff until the analysis was completed" Comment: the size of the block was not described |
| Allocation concealment (selection bias) | Low risk | Quote: "the vaccine code was not disclosed to parents or field, laboratory or coordinating staff until the analysis was completed...[...] All four vaccines were dispensed in identical single‐dose 0.5 ml ampules..." Comment: adequate methods |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "in preparation for the phase III‐trial, a double ‐blind randomized phase II study was carried out with three candidate acellular vaccines..." Comment: blinding was described (see also random sequence generation). The outcomes of interest were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the assessment of the outcome of interest were unlikely to have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout rates were low (stage 1: nwP = 6/94, 6.4% and naP = 3/94, 3.2%; stage 2, nwP = 3/85, 3.5%; naP = 12/159, 7.5%) and include contraindications to DTP vaccines ( nwP = 3/179, 1.7%; naP = 8/253, 3.2%). Other reasons for no completion of the primary series were moving out from the study area, receipt ordinary vaccine in error, and intercurrent infection; however, these were not described by study arm, and include children enrolled in the trial of Miller 1997 |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not find the study protocol. There is no evidence of selective reporting |
Miller 1997.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Relative arm proportion:a 1.5 wP: 1 aP (4c): 1 aP (5c) Duration of follow‐up: 10 to 16 months after the first dose of wP or aP Study setting and country: immunisation clinics in North Hertfordshire District Health Authority, UK World Bank income level of country: high Recruitment and sampling: infants attending the above‐mentioned clinics for primary immunisation with DTwP, were offered to be involved in this study Study dates: June 1990 to January 1994 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: DTwP (Wellcome Research Laboratories, Beckenham, England): nwP = 139 Comparator: DTaP: naP = 266
Dose and route of administration: not stated Schedule: 3‐dose‐series (2, 3 and 4 monthsb) Vaccine(s) co‐administered: Hib vaccine; dose and route: not stated; schedule: 3‐dose‐series (2, 3 and 4 months) |
|
| Outcomes |
Outcomesofinterestinthereview Primaryoutcomes/outcomedomains
Secondaryoutcomes
|
|
| Funding |
|
|
| Conflicts of interest | No stated. Connaught Laboratories donated the aP vaccine | |
| Notes |
aIn this review, the DTaP study arms were combined to create a single pairwise comparison bAntipyretic/analgesic use: not stated cLead author of this trial (EM) confirmed that the records of this study are no longer available dTiming of assessment of the primary safety outcome, or specific length of follow‐up for these events are not stated, as the peer‐reviewed report arising from this trial did not include SAEs as an outcome of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "vaccines were randomly allocated to sequential study numbers by computer program and infants were assigned a study number in order of their attendance at clinics. Parents of all study subjects and field, laboratory and coordinating staff were ignorant of the vaccine codes until completion of data analysis" Comment: this study presumably used 'blocked randomisation' as described in Miller 1990. Additional details on the sequence generation were not provided |
| Allocation concealment (selection bias) | Low risk | Quote: "all vaccines were dispensed in identical single dose ampoules indistinguishable by eye from each other" Comment: adequate methods |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "parents of all study subjects and field, laboratory and coordinating staff were ignorant of the vaccine codes until completion of data analysis" Comment: blinding is described. SAEs were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding is described. The assessment of the outcome of interest was unlikely to have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 11 out of 405 children did not complete the primary series. Reasons for no completion include adverse events contraindicating further doses (nwP = 2/139, 0.7% and naP = 2/266, 1.4%), moving out from the study area and receipt of non‐trial vaccine by mistake. Other reasons for withdrawal were moving out from the study area, receipt ordinary vaccine in error, and intercurrent infection; however, these were not described by study arm, and include children enrolled in the trial of Miller 1990 |
| Selective reporting (reporting bias) | High risk | Comment: we did not find the study protocol of this trial. Reactogenicity reported; SAEs not an outcome |
NCT00343889.
| Study characteristics | ||
| Methods | Study design: 2‐arm, single‐blind, parallel RCT Relative arm proportion 1 wP: 1 aP (primary series study) Duration of follow‐up (primary series study): 238 days (8 months) after the first dose of wP or aP. Booster dose administered according to the schedule specified below Study setting and country: 2 clinical centres in the Philippines World Bank income level of country: lower‐middle Recruitment and sampling: not stated Study dates: July 2006 to April 2008 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series:
Dose and route of administration: 0.5 mL IM; schedule: 3‐dose primary series (6, 10 and 14 weeks of agea) Vaccine(s) co‐administered: OPV (manufacturer and dose: not stated); route: per oral Booster dose:
Dose and route of administration: 0.5 mL IM; schedule: 1‐dose (between 15 and 18 months of agea) Vaccine(s) co‐administered: OPV (manufacturer: not stated; dose: "0.5 mL"); route: per oral |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes:
|
|
| Funding | Sanofi Pasteur, a Sanofi Company | |
| Conflicts of interest | Industry‐funded study. Additional information is unavailable | |
| Notes |
aAntipyretic/analgesic use: not stated bAlthough all‐cause mortality was not a prespecified outcome domain, they were likely to have been reported when they occurred cData extracted from clinicaltrials.gov. No peer‐reviewed publication associated with this study. From the trial registry it is only possible to ascertain the number of children experiencing any SAEs as well as the number of children with a specific diagnosis dDeaths were not specifically reported; however, the reasons for no completion are clearly described and do not include this outcome domain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocation: randomized; intervention model: parallel assignment; masking: single (outcomes assessor)..." Comment: details on the random component of the sequence generation were not provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "allocation: randomized; intervention model: parallel assignment; masking: single (outcomes assessor)..." Comment: methods to conceal the allocation were not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the outcome/outcome domain of interest was unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: outcome assessors were blinded, but additional details were not provided. The assessment of SAEs other than deaths, could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropout rates were low in both study groups (nwP = 1/189; 0.5% and naP = 3/190; 1.6%) and unrelated to the outcome/outcome domain of interest |
| Selective reporting (reporting bias) | Low risk | Comment: we identified the protocol of this trial through clinicaltrials.gov. Although SAEs were not a prespecified study outcome, they were likely to have been reported when they occurred |
NCT00348881.
| Study characteristics | ||
| Methods | Study design: two‐arm, double‐blind, parallel RCT Relative arm proportion: 1 wP: 2 aP (primary series study) Duration of follow‐up (primary series study): 238 days (8 months) after the first dose of wP or aP. Booster dose administered according to the schedule specified below Study setting and country: 1 clinic centre in Manila, the Philippines World Bank income level of country: lower‐middle Recruitment and sampling: not stated Study dates: June 2006 to June 2008 |
|
| Participants |
Inclusioncriteria At screening:
At inclusion:
Exclusioncriteria At screening:
At screening and at inclusion:
At inclusion:
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series:
Dose and route of administration: 0.5 mL IM; 3‐dose schedule (6, 10 and 14 weeks of agea) Vaccine(s) co‐administered: OPV (manufacturer and dose not stated); route: per oral; 3‐dose schedule (6, 10 and 14 weeks of age) Booster dose:
Dose and route of administration: 0.5 mL IM; 1 dose (between 12 and 18 months olda) Vaccine(s) co‐administered: OPV (manufacturer and dose not stated); route: per oral |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes
Secondaryoutcomes
|
|
| Funding | Sanofi Pasteur, a Sanofi Company | |
| Conflicts of interest | Industry‐funded study. Additional information is unavailable. | |
| Notes |
aAntipyretic/analgesic use: not stated bAlthough this was not a prespecified study outcome, SAEs were likely to have been reported when they occurred cData extracted from clinicaltrials.gov. There is no peer‐reviewed publication associated with this study. From the trial registry it is only possible to ascertain the number of children experiencing any SAEs as well as the number of children with a specific diagnosis. dDeaths were not specifically reported; however, the reasons for no completion are clearly described and do not include this outcome domain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocation: randomized; intervention model: parallel assignment; masking: quadruple (participant, care provider, investigator, outcomes assessor)..." Comments: details on the random component of the sequence generation were not provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: the methods to conceal the allocation were not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding is mentioned, but details were not provided. The outcome/outcome domain of interest was unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding is mentioned, but details were not provided. The assessment SAEs other than deaths, could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the (safety) data were analyzed and presented according to the actual treatment received" Comment: dropouts were low and balanced across the study groups (nwP = 12/709; 1.7% and naP = 17/1407; 1.2%). Reasons for no completion include SAEs (nwP: n = 2/12; 16.7% and naP = 0/17). As treated analysis does not show substantial departure from allocation (nwP = 1425 (as‐treated) versus nwP = 1424 (ITT); naP = 708 (as‐treated) versus naP= 709 (ITT)) |
| Selective reporting (reporting bias) | Low risk | Comment: we identified the protocol of this study through clinicaltrials.gov. Although "all‐cause SAEs" was not a prespecified study outcome, SAEs were likely to have been reported when they occurred |
Nilsson 1998.
| Study characteristics | ||
| Methods | Study design: 4‐arm,a double‐blind parallel‐group RCT Relative arm proportion:1 wP: 1 aP (2c): 1 aP (5c): 1 DT Duration of follow‐up: ~ 2.5 years after the administration of a first dose of wP or aPb Study setting and country: primary care centres and the paediatric clinic in Linköping, Sweden World Bank income level of country: high Recruitment and sampling: children recruited in this region for the Swedish I efficacy, safety and immunogenicity trial (Gustafsson 1996c), were also offered to be enrolled in the allergy sub‐study reported below Study dates: March 1992 to unknown |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: DTwP (Connaught Laboratories Incorporated Swiftwater, Pennsylvania, USA): nwP = 137 Comparison: DTaP (2c: SmithKline Beecham, Rixensart, Belgium and 5c: Connaught Laboratories, Toronto Canada): naP = 360 Dose and route of administration:d as described in Gustafsson 1996 Vaccine(s) co‐administered: as described in Gustafsson 1996 |
|
| Outcomes |
Outcomesofinterestforthereview The following atopic outcomes were diagnosed through the combination of questionnaires, clinical findings, medical records and IgE‐mediated sensitisation (i.e. IgE‐mediated food allergy and urticaria) by the age of 2.5 years. Questions regarding skin, nose and bronchi symptoms were modified from the International Study of Asthma and Allergies (ISAAC) questionnaires. Physical examination and additional tests (were required) were completed at 2.5 years Primaryoutcomes/outcomedomains
Secondaryoutcomes
|
|
| Funding |
|
|
| Conflicts of interest | Not stated | |
| Notes |
aIn this review, the DTaP study arms were combined to create a single pairwise comparison. Where applicable, we omitted further information on the DT study arm, as it does not meet the inclusion criteria of this review bA subset of children enrolled in this trial were also followed‐up at 7 years old cSince the studies of Gustafsson 1996 and Nilsson 1998 addressed different research questions, they are reported separately dAntipyretic/analgesic use: as described in Gustafsson 1996 eThis broader outcome domain was used for synthesis purposes, as specified in the protocol of this review fCorrespondence: LN and BB were contacted regarding the priming schedule received by the children who experienced the outcomes of interest. We were unable to source these data before the submission of this manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | As described in Gustafsson 1996 |
| Allocation concealment (selection bias) | Unclear risk | As described in Gustafsson 1996 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "as part of a study of the efficacy of 3 pertussis vaccines, we prospectively studied the development of atopic disease and sensitization during the first 21/2 years of life in relation to type of vaccine and possible confounders, including the effect of pertussis infection[...] 'The investigation was blinded to the families, nurses and investigating physicians through the use of coded bottles until the diagnoses were established in all the children" Comment: the authors of this study described that the main purpose of the trial was to detect considerable increases in the risk of atopic disease by pertussis vaccination. Partial unblinding of the vaccinator and parents/carers was possible (see Gustafsson 1996), and except for IgE‐mediated food allergy and urticaria (where evidence of IgE‐mediated sensitisation to the food/allergen that may have triggered the allergic reaction was required), the outcomes could have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: except for IgE‐mediated food allergy and urticaria (where evidence of IgE‐mediated sensitisation to the food/allergen that may have triggered the allergic reaction was required), the assessment of the outcomes of interest could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 711children (including DT vaccinees) were enrolled in this study. The number of infants assigned to each study vaccine at enrolment is not provided. Five‐hundred and fifteen (excluding recipients of DT) completed a 3‐dose priming schedule and of them, 497 were followed up until 2.5 years. Withdrawals were not broken down by study arm. Reasons for no completion were described (30/699, includes DT vaccinees) and do not include the outcomes of interest |
| Selective reporting (reporting bias) | Unclear risk | Quote: "the cumulative incidence of atopic diseases at 2 1/2 years of age, as well as the individual manifestations, were similar in the 3 pertussis vaccine groups and the DT group" Comment: the authors of this study described the number of children experiencing the outcomes of interest. These results were not made available by study group in the text or tables, but in a bar chart that only includes atopic dermatitis, asthma and all‐cause atopic disease by 2.5 years of age |
Olin 1997.
| Study characteristics | ||
| Methods | Study design: 4‐arm,a double‐blinded parallel‐group RCT Relative arm proportion: 1 wP: 1 aP (2c): 1 aP (3c, with genetically detoxified pertussis toxin), 1: aP (5c) Duration of follow‐up: 22 months after the first dose of wP or aP Study setting and country: child‐health centres across Sweden, except Göteborg and surrounding counties World Bank income level of country: high Recruitment and sampling: direct contact with parents during visits to clinics and/or by letter through the child‐health or district‐health nurse when the child was one to three weeks old Study dates: September 1993 to October 1996 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: DTwP (Evans Medical [ex Wellcome] Leatherhead, United Kingdom): nwP = 20,720 Comparator: DTaP (2c, SmithKline Beecham, Rixensart, Belgium; 3c, Chiron Biocine; 5c Pasteur‐Merieux‐Connaught, Toronto, Canada): naP = 62,172 Dose and route of administration: 0.5 mL IM Schedule: 3‐dose‐series
Vaccine(s) co‐administered:
|
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes
|
|
| Funding |
|
|
| Conflicts of interest | Not stated | |
| Notes |
aIn this review, the DTaP study arms were combined to create a single pairwise comparison bAntipyretic/analgesic use: not stated cIt was not possible to determine the total number of children experiencing any SAEs due to overlaps between the data reported across some of the outcome domains; dThese outcome domains have been prespecified in the methods section of the relevant reports eCauses of death not reported by study arm. These include SIDS (n = 13), injuries (n = 5), infections (n = 4), congenital heart disease (n = 3), hepatic disease (n = 2), metabolic diseases (n = 3) fAdmissions to hospital were only captured through passive follow‐up and only if they were related to contraindicating events or adverse events described as serious, according to the definition of SAE provided in the methods section of the relevant reports. The number of events (but not the number of children experiencing hospitalisations) was reported by he FDA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "all vaccines were easily resuspended to homogenous opaque suspensions [...] Each vial was labelled with a unique computer‐generated randomisation number (SAS, version 6) provided by the Swedish Medical products Agency. We used eight‐unit blocks to ensure balanced assignment to the four treatment groups. The investigators were unaware of the block size. We randomly assigned babies to a vaccine group at the time of the first dose. After parental consent, nurses sequentially assigned babies the next available randomisation number at each child‐health centre" Comment: adequate methods |
| Allocation concealment (selection bias) | Low risk | Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "masking was maintained for all vaccine groups until the safety dataset was locked for analysis in August 1995. The treatment status of the two‐component vaccine group was made known at that time to allow boosting with a three‐component monovalent pertussis vaccine (SmithKlineBeecham), but the other three groups remained coded until the datasets were locked for analysis in April, 1997. Inadvertent unmasking for individual children due to differences in immediate reactogenicity between vaccine groups has not been reported, but the possibility cannot be excluded" Comment: adequate blinding. Other than encephalopathy, the outcomes of interest were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: adequate blinding; outcome assessors were unaware of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: reasons for withdrawals/loss to follow‐up were provided, but not broken down by the type of pertussis vaccine assigned at enrolment |
| Selective reporting (reporting bias) | Unclear risk | Comment: a technical report based on a pre‐planned statistical analysis plan was published, but it was not possible to source it before the submission of this manuscript. The number of SAEs was provided in both regulatory data and peer‐reviewed publications arising from this trial. A peer‐reviewed article includes the number of children experiencing these events, but not by study arm; however, in some circumstances it was possible to match the number of children who met a specific endpoint (i.e. events described as life‐threatening/deaths), with their vaccination status included in the FDA assessment of the safety data of this trial. In those cases, the data were included for synthesis |
Reinert 2006.
| Study characteristics | ||
| Methods | Study design: 2‐arm, open‐label, parallel‐RCT Relative arm proportion: 1 wP: 1 aP Duration of follow‐up: 17 months after the first dose of wP or aP Study setting and country: 388 surgeries of paediatricians in France World Bank income level of country: high Recruitment and sampling: not stated Study dates: 2001 to unknown |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesizea
Children's baselinecharacteristics
|
|
| Interventions | Primary series
Dose and route of administration: 0.5 mL IM; schedule: 3‐dose‐series (2, 3 and 4 months of ageb) Vaccine(s) co‐administered:
Booster dose:b
Dose and route of administration: 0.5 mL IM; schedule: 1 dose, between 12 to 18 months old Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes
|
|
| Funding | Sanofi Pasteur MSD | |
| Conflicts of interest | Not stated. AF, ST, AS, MW reported affiliations with Sanofi Pasteur MSD, Lyon, France | |
| Notes |
aNumber randomised and not vaccinated: 15 (excluded) bAntipyretic/analgesic use: reactive use of antipyretics allowed with temperature ≥ 38∘ C cNot prespecified in the methods section of the available report as an outcome domain of interest dCauses of death not stated, but described as not attributable to the study vaccines eHere we included an adverse event recorded by the investigators of this trial as a definite medical contraindication to DTP‐containing vaccines (anaphylactic reaction: "cutaneous eruption on face" in an infant vaccinated with DTwP) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from report): "open, large‐scale, pragmatic, randomized controlled clinical trial" [...] "The randomization was stratified by age (four age groups: 7–8 weeks; 8–10 weeks; 10–12 weeks; 12–14 weeks) and study center, to minimize bias due to centre effects or possible age‐associated safety outcomes". [...] "Six subjects were vaccinated but not randomized. All six non randomized subjects were vaccinated with HEXAVAC® and were analysed in Group 1 in the full analysis population" Comment: the random component of the sequence generation was described. A subset of children was vaccinated with aP, but not randomised (naP = 6/3562). This was unlikely to influence the results of this trial |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details were provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: no blinding of children/carers or personnel unlikely to have influenced the outcome domains of interest |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there was no blinding in this study. Except for deaths, the assessment of the remaining SAEs was likely to have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout rates were higher in the wP study group, compared with aP (nwP = 429/3574; 12% and naP = 290/3562; 8.1%) |
| Selective reporting (reporting bias) | Unclear risk | Comment: this trial was conducted upon a post‐licensure request from the Committee for Medicinal Products for Human Use at the EMA; however, we were unable to source the study protocol. The definition of SAEs provided in the manuscript only includes those adverse events judged as serious, resulting in withdrawal from the study. It remains unclear whether data on SAEs that did not result in study withdrawal were systematically collected |
Simondon 1997.
| Study characteristics | ||
| Methods | Study design: two‐arm, double‐blind, parallel‐RCT Relative arm proportion: 1: wP, 1: aP Durtion of follow‐up: ~ 22 months after the first dose of wP or aP Study setting and country: one centre study in Niakhar, a rural area of East Senegal World Bank income level of country: Senegal was classified as a lower‐middle income country between 1990 and 1993, and as a lower income country in 1994 Recruitment and sampling: children due to be vaccinated according to a central database were visited by a field worker the week before a monthly vaccination session. Transportation to the study site was offered Study dates: May 1990 to June 1995 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: DTwP (Pasteur Mérieux Sérums and Vaccins): nwP = 2379 Comparator: DTaP (Pasteur Mérieux Sérums and Vaccins): naP = 2396 Dose and route of administration: 0.5 mL IM; schedule: 3‐dose‐series (2, 4 and 6 months of ageb) Vaccine(s) co‐administered:
|
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes
|
|
| Funding |
|
|
| Conflicts of interest | Not stated. MC reported affiliations with Pasteur Merieux Serums et Vaccins, Marnes La Coquette, France | |
| Notes |
aNumber of infants screened that me at least one exclusion criterion: 37/4973. It remains unclear whether the remaining 4936 were randomised bAntipyretic/analgesic use: not stated cPrespecified in the methods section of the relevant reports dCauses of death were not reported by study arm. These include: gastroenteritis (39%), pneumonia (21%), malaria (11%), meningitis (7%), others (11%) and unknown aetiologies (11%) eSystematically assessed as a contraindication to DTP vaccines (i.e. anaphylactic reaction within 48 hours of DTP vaccination') |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from report): "before the first dose, enrolled infants were randomly assigned to one of the two vaccine groups based on consecutive numbers randomized by computer at the National Institute of Health (Bethesda, MD, USA) and balanced in blocks of ten" Comment: the random component of the sequence generation was described in the methods |
| Allocation concealment (selection bias) | Low risk | Quote: "the two vaccines, identical in appearance..." Comment: the allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "prospective, cohort, double‐blind, randomized in two arms..." Comment: blinding was mentioned, but details were not provided. The outcome domains of interest were unlikely to be influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "prospective, cohort, double‐blind, randomized in two arms..." Comment: blinding was mentioned, but details were not provided. Except for deaths, the assessment of the remaining outcome domains could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (from report): "surveillance [of SAEs] was completed after each dose in 97% of children" Comment: reasons for incomplete outcome data were not provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not find the protocol of this study. There is no evidence of selective reporting |
Stehr 1998.
| Study characteristics | ||
| Methods | Study design: 2‐arm, double‐blind parallel‐group RCT, with an open control group Relative arm proportion: 1.5 wP (blinded): 1.5 aP (blinded): 1 DT (opena) Duration of follow‐up: 26 months after the first dose of wP or aP Study setting and country: 227 private medical practices across Germany World Bank income level of country: high Recruitment and sampling: by the participating physicians in their practices Study dates: May 1991 to December 1994 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions | Primary series
Dose and route of administration: 0.5 mL IM; schedule: 3‐dose‐series (dose 1: 2 to 4 months of age; dose 2: ≥ 6 weeks after the first dose; dose 3: ≥ 6 weeks after the second doseb) Vaccine(s) co‐administered: not stated Booster dose
Dose and route of administration: 0.5 mL IM; schedule: 1 dose (15 to 18 months of ageb) Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcomes/outcomedomains
Secondaryoutcomes
|
|
| Funding | Wyeth‐Lederle Vaccines and Pediatrics, Pearl River, New York, USA | |
| Conflicts of interest | Not stated. SL and TE reported affiliations with Wyeth‐Lederle Vaccines and Pediatrics, Pearl River, New York, USA | |
| Notes |
aWe omitted further information on the DT study arm, as it does not meet our inclusion criteria bAntipyretic/analgesic use: reactive use reported cCorrespondence: missing outcome data requested to JDC dThe available data did not allow us to determine the total number of children experiencing this outcome ePrespecified in the methods section of the available reports fCauses of death
gThe available data did not allow us to determine the total number of children experiencing this event at least once |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "DTaP and DTP were administered in a double‐blind randomized manner [...]. Study vaccines (DTaP and DTP) were supplied in single dose vials in groups of 10. Each vial contained a subject number and the vaccines were assigned in numerical sequence to enrollees" Comment: details on the random sequence generation were not stated |
| Allocation concealment (selection bias) | Low risk | Comment: adequate methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "DTaP and DTP vaccines were administered in a double‐blind, randomized manner..." Comment: blinding was mentioned, but details were not provided. Safety outcomes other than encephalopathy were unlikely to have been influenced by knowledge of the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding is mentioned, but details were not provided. Except for deaths, the remaining SAEs and other outcomes of interest could have been influenced by knowledge of the intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout rates were low and balanced (nwP = 335/4259; 7.4% and naP = 283/4273; 6.62%). No completion due to 'adverse experiences' was more common in children vaccinated with DTwP (nwP = 94/4259; 2.2%), compared to DTaP (naP = 34/4273; 0.8%) |
| Selective reporting (reporting bias) | High risk | Comment: we did not find the protocol of this study. Presumably, no events described as life‐threatening or leading to persistent or significant disability or incapacity were experienced by the study children; however, this was not clearly stated in the report |
Toelle 2020.
| Study characteristics | ||
| Methods | Study design: post‐hoc analysis of an RCT that enrolled a birth cohort of children born in Australia, between October 1997 and January 2000, with 14‐year duration of follow‐up. The period of enrolment coincided with the switchover from DTwP to DTaP‐only schedules in this country Study setting: two tertiary hospitals in New South Wales, Australia World Bank income level of country: high Recruitment and sampling: infants were originally recruited into the Childhood Asthma Prevention Study (CAPS), an RCT that tested the effectiveness of house‐dust mite avoidance and dietary fatty acid modification in the first five years of life, for the primary prevention of asthma and other atopic conditions in high‐risk children (first degree relative with 'current asthma' or 'frequent wheeze') Study dates: October 1997 to unknown |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: wP (manufacturer no stated): nwP = 293 Comparator: aP (manufacturer no stated): naP = 204 Dose and route of administration: not stated Schedule:b first dose between 6 and 18 weeks of age Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Primaryoutcomes/outcomedomains
Secondaryoutcomesc
Timing for the assessment of the outcomes: 18 months, 3, 5, 11.5 and 14 years |
|
| Funding |
|
|
| Conflicts of interest | The authors declared no competing interests | |
| Notes |
aRisk of bias assessments are available in Table 3; Table 4; Table 5; and Table 6 bAntipyretic/analgesic use: not stated cAssessed as part of the CAPS study |
|
Venter 2016.
| Study characteristics | ||
| Methods | Study design: population‐based birth cohort study of infants born in the Isle of Wight between September 2001 and August 2002; this birth cohort was established to study the prevalence of food hypersensitivity in children Length of follow‐up: 10 years Study setting and country:a allergy assessments were performed at a dedicated specialist allergy research unit in the Isle of Wight, UK World Bank income level of country: high Recruitment and sampling: all infants born in the Isle of Wright between September 2001 and August 2002 were included in the Food Allergy and Intolerance Research (FAIR) birth cohort; of them 91% of the parents consented to scheduled allergy assessments Study dates: September 2001 to August 2012 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize
Children's baselinecharacteristics
|
|
| Interventions |
Intervention: first dose of wP (manufacturer: not stated): nwP = 595 Comparator: first dose of aP (manufacturer: not stated): naP = 224 Dose and route of administration: not stated Schedule:c first dose between 6 and 18 weeks of age Vaccine(s) co‐administered: not stated |
|
| Outcomes |
Outcomesofinterestforthisreview Primaryoutcome/outcomedomainsc
Secondaryoutcomesd
|
|
| Funding | Public Health England, an executive agency of the Department of Health | |
| Conflicts of interest | All authors declared no conflicts for this report. Other conflicts of interest were stated as follows:
|
|
| Notes |
aCorrespondence: PJT and CV were contacted via email in regards to the study setting, confounders and BCG vaccination bRisk of bias assessments are available in Table 7 and Table 8 cAntipyretic/analgesic use: not stated dAllergy assessments were prespecified |
|
Wanlapakorn 2020.
| Study characteristics | ||
| Methods | Study design: two‐arm, double‐blind parallel‐group RCT, with a simultaneous non‐randomised study group Relative arm proportion: 2 wP (randomised): 2 aP (randomised): 1 wP (non‐randomised EPI groupa) Duration of follow‐up: 17 months after the first dose of wP or aP Study setting and country: King Chulalongkorn Memorial Hospital, University Chulalongkorn, Thailand World Bank income level of country: upper‐middle Recruitment and sampling: antenatal recruitment of pregnant women was carried out at King Chulalongkorn Memorial Hospital. Those who consented for Tdap vaccination and delivered infants who met the study eligibility criteria, were approached for a second informed consent (follow‐up phase of the trial) Study dates: April 2015 to August 2018 |
|
| Participants |
Inclusioncriteria
Exclusioncriteria
Samplesize Number randomised at birth: 315; number vaccinated at 2 months of age: 288 Children's baselinecharacteristics
|
|
| Interventions | Primary series
Dose and route of administration: IM; schedule: 3‐dose‐series (2, 4 and 6 months of ageb) Vaccine(s) co‐administered: see notesc
Booster dose:
Dose and route of administration: IM; schedule: 1 dose (18 months of age) Vaccine(s) co‐administered:c see notes |
|
| Outcomes |
Outcomesofinterestforthereview Primaryoutcome/outcomedomains
Secondaryoutcomes
|
|
| Funding |
|
|
| Conflicts of interest | PVD reported grants to the University of Antwerp from GSK Biologicals, Merck, SP, MSD, Pfizer, Sanofi, Takeda, Baxter, CanSino China, Themis, Johnson & Johnson, the Bill & Melinda Gates Foundation, the Flemish government, the European Union, and Abbott. All other authors reported no potential conflicts of interest | |
| Notes |
aWe omitted further information on the DTwP‐EPI study arm, as it does not meet the inclusion criteria of this review bAntipyretic/analgesic use: not stated cSome infants received non‐EPI vaccines concomitantly (i.e. rotavirus, pneumococcal, varicella, or rabies vaccines). These vaccines were purchased by their parents dCorrespondence: we attempted to contact EL requesting further information about the safety data of this trial; however, we were unsuccessful eNot prespecified as an outcome domain of interest fCauses of death
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "healthy full‐term and late preterm infants, born at the gestational age of 36 weeks with birth weights > 2,500 grams, were randomized to receive either the hexavalent aP‐containing vaccine (Infanrix hexa, GlaxoSmithKline Biologicals, Rixensart, Belgium; hexavalent group) or the pentavalent wP‐containing vaccine (Quinvaxem, Crucell‐Janssen, Incheon, South Korea; pentavalent group)" Comment: the random component of the sequence generation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details were provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from report): "this study was not blinded as wP‐vaccinated infants received oral poliovirus vaccine (OPV) whereas aP‐vaccinated infants received inactivated polio (IPV) vaccine (hexavalent vaccine)" Comment: no blinding of children/carers or personnel unlikely to have influenced the outcome domain of interest |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no blinding of children/carers or personnel unlikely to have influenced the assessment of the outcome domain of interest |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout rates were low but higher in the DTwP study group, compared with DTaP (nwP = 14/142; 9.9% and naP = 10/146; 6.8%). Reasons for no completion were not stated. A child was not given a booster dose of wP because of previous 'side‐effects' to this study vaccine. No additional information was provided |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was identified through clinicaltrials.gov, but safety data are yet to be posted on this website. Deaths were not a prespecified outcome domain, however they were reported in a CONSORT diagram |
2c: two‐component acellular pertussis vaccine; 3c: three‐component acellular pertussis vaccine; 4c: four‐component acellular pertussis vaccine; 5c: five‐component acellular pertussis vaccine. aP: acellular pertussis vaccine; DTP: diphtheria‐tetanus‐pertussis vaccine; DTaP: diphtheria‐ tetanus‐acellular pertussis vaccine; DTwP: diphtheria‐tetanus‐whole‐cell pertussis vaccine; EPI: 'Expanded Programme on Immunization'; FDA: US Food and Drug Administrstion; HepB: hepatitis B vaccine; HHE: hypotonic hyporesponsive episode; Hib:Haemophilusinfluenzae type b; IM: intramuscular;ITT: intention‐to‐treat; HIV: human immunodeficiency virus; IgE: immunoglobulin E; IPV: inactivated poliovirus vaccine; MCC‐TT: meningitis C conjugate vaccine with tetanus toxoid; MCC‐CRM: meningitis C conjugate vaccine with genetically modified cross‐reacting material of diphtheria toxin;MMR: measles, mumps and rubella vaccine; NRSIs: non‐randomised studies of interventions; OPV: oral poliovirus vaccine.;RCT: randomised controlled trial;SAEs: serious adverse events; SC: subcutaneous; SIDS: sudden infant death syndrome; Tdap: low‐dose diphtheria‐tetanus‐acellular pertussis vaccine; wP: whole‐cell pertussis vaccine;
Where a dose/route of administration is not stated, it was assumed that was administered as per the local standard of practice/recommendations of the manufacturer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 1988 | Length of follow‐up < 6 months |
| Anderson 1994 | Length of follow‐up < 6 months |
| Bernsen 2006 | Cross‐sectional study; no comparison of interest |
| Blennow 1988 | First dose of pertussis‐containing vaccine given at 6 months of age |
| Farooqi 1998 | No comparison of interest |
| Grüber 2003 | No comparison of interest |
| Grüber 2008 | Cross‐sectional study; cannot determine the type of pertussis‐containing vaccine administered, nor the age at the first dose |
| Gylca 2000 | Length of follow‐up < 6 months |
| Halperin 1994 | Length of follow‐up < 6 months |
| Halperin 1995 | Length of follow‐up < 6 months |
| Halperin 1999 | Length of follow‐up |
| Halperin 2003 | Booster dose study with length of follow‐up < 6 months |
| Henderson 1999 | No comparison of interest |
| Kummeling 2007 | No comparison of interest |
| Maitra 2004 | No comparison of interest |
| Matheson 2010 | No comparison of interest |
| McDonald 2008 | No comparison of interest |
| McKeever 2004 | No comparison of interest |
| Mullooly 2007 | No comparison of interest |
| Pichichero 1992 | Length of follow‐up < 6 months |
| Pichichero 1993 | Length of follow‐up < 6 months |
| Pichichero 1994 | Length of follow‐up < 6 months |
| Pichichero 1996 | Length of follow‐up < 6 months |
| Podda 1994 | Length of follow‐up < 6 months |
| Schmitt 1996 | Household contact study |
| Simondon 1996 | Follow‐up period < 6 months |
| Swartz 2018 | No comparison of interest |
| Thomson 2010 | No comparison of interest |
| Vanura 1994 | Follow‐up period < 6 months |
| Vogt 2014 | No comparison of interest; the authors of this study used quote "dispensed prescribed asthma medication for each individual in the cohort during 2008–2010" as a proxy of diagnosis of asthma |
| Wang 2012 | No comparison |
| Wiersbitzky 1996 | Follow‐up period < 6 months |
| Yamamoto‐Hanada 2020 | No comparison |
Characteristics of studies awaiting classification [ordered by study ID]
217744/025 (DTPa‐HBV‐IPV‐025).
| Methods | Open‐label randomised controlled triala |
| Participants | 2‐month old healthy infants |
| Interventions |
Intervention(wPgroup): DTwP‐Hib‐IPV (Pasteur‐Mérieux‐Connaught) Comparator(aPgroup): DTPa‐HepB‐Hib‐IPV or DTPa‐HepB‐IPV (SB Biologicals) Schedule: 3‐dose primary series (2, 3 and 4 months of age) Vaccine(s) co‐administered:
|
| Outcomes | Unclear |
| Funding | GlaxoSmithKline |
| Conflicts of interest | Not stated |
| Notes | We were unable to locate the report. We attempted to contact the sponsor; however, we were unsuccessful |
Mrozek‐Budzyn 2018.
| Methods | Prospective birth cohort study originally designed to describe whether polycyclic aromatic hydrocarbons have a negative impact on fetal growth and early child development. A subset of these children that received a 3‐dose priming schedule with pertussis‐containing vaccines, were studied for atopic outcomes. Study setting and country: Krakow, Poland WorldBankincomelevelofcountry: upper‐middle |
| Participants |
Recruitmentandsampling: pregnant women visiting antenatal clinics in high0 and low‐pollution areas in Krakow were recruited towards the end of the first trimester of pregnancy Studydates: 3 November 2000 to unknown (enrolment was completed by 22 August 2003) Inclusioncriteria:
Exclusioncriteria:
Samplesize
Children's baselinecharacteristics
Confoundingdomainsidentifiedbytheinvestigatorsofthisstudy:
|
| Interventions |
Intervention:a,b DTwP‐only priming schedule (Biomed Krakow, Poland): nwP = 142 Comparator: DTaP‐only priming schedule (Sanofi Pasteur or GlaxoSmithKline): naP = 77 Dose and route of administration: not stated Schedule: 3‐dose priming schedule. The age at the first dose is not stated, but presumably given before the age of 6 months as per local immunisation guidelines Vaccine(s) co‐administered: poliomyelitis vaccine (formulation not described) and hepatitis B vaccine. Manufacturer, dose and schedule: not stated |
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
| Funding |
|
| Conflicts of interest |
|
| Notes |
aWe attempted to contact the lead and senior author of this study regarding the age of first dose of the exposure; however, we were unsuccessful b15 out of 234 children received a mixed DTwP/DTaP schedule. The type of the first dose it is not stated cPrespecified in the methods section and assessed from the 1st until the 6th year of life |
aP: acellular pertussis vaccine; DTwP: diptheria, tetanus and whole‐cell pertussis vaccine; DTaP: diptheria, tetanus and acellular pertussis vaccine; HepB: hepatitis B vaccine; Hib:Haemophilus influenzae type b vaccine. IPV: inactivated poliovirus vaccine; NRSIS: non‐randomised studies of interventions; wP: whole‐cell pertussis vaccine.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617000065392.
| Study name | Optimising immunisation using mixed schedules (OPTIMUM) |
| Methods |
|
| Participants |
Recruitmentandsampling: currently carried out by trained staff during the antenatal and immediate postnatal periods in private hospitals in the metropolitan area of Perth, Western Australia, as well as through expressions of interest received via email Inclusioncriteria:
Exclusioncriteria:
Samplesize: up to 3000 study children
|
| Interventions | Primary series:
Dose and route of administration: 0.5 mL, IM Schedule:
Concomitant vaccines: as recommended in the National Immunisation Program Booster dose:
Concomitant vaccines: as recommended in the National Immunisation Program |
| Outcomes | Primary:
Secondary
|
| Starting date | January 2018 |
| Contact information | Dr Marie J Estcourt; optimum.project@sydney.edu.au |
| Funding |
|
| Notes |
aAntipyretic/analgesic use: prophylactic paracetamol given before the first dose of the study vaccines. Reactive use: allowed bSAEs deemed related to the study vaccines or procedures will be captured throughout the entire study period; if unrelated, they will only be reported from the first dose of the study vaccines until 6 months post‐randomisation |
ISRCTN17271364.
| Study name | Pertussis acellular whole cell advanced research (AWARE) study |
| Methods |
|
| Participants |
Recruitmentandsampling:notstated Inclusioncriteria:
Exclusioncriteria:
Samplesize:
|
| Interventions | Primary series
Schedule:
Vaccine(s) co‐administered:
|
| Outcomes | SAEs not prespecified as outcomes of interest, but likely to be recorded when occurring |
| Starting date | August 2019 |
| Contact information | Ms Nelly Owino; nelly.owino@paediatrics.ox.ac.uk |
| Funding | University of Oxford |
| Notes |
NCT03606096.
| Study name | Gambia pertussis study (GaPs) |
| Methods |
|
| Participants |
Recruitmentandsampling: not stated Inclusioncriteria: infants born to women enrolled in the maternal immunisation phase of this trial Samplesize:
|
| Interventions |
Schedule: 3‐dose‐series (2, 3 and 4 months of age); dose and route of administration and concomitant vaccines: as per the local EPI |
| Outcomes | SAEs not prespecified as outcomes of interest, but likely to be recorded when occurring |
| Starting date | January 2019 |
| Contact information | Dr Beate Kampmann (bkampmann@mrc.gm) and Dr Michael E Okoye (mokoye@mrc.gm) |
| Funding | Sponsors and collaborators
|
| Notes | BK was contacted regarding the manufacturer of DTwP‐containing vaccine |
aP: acellular pertussis vaccine; DTaP: diphtheria, tetanus, whole‐cell pertussis vaccine; DTwP: diphtheria, tetanus, whole‐cell pertussis vaccine; HepB: hepatitis B vaccine;Hib:Haemophilus influenzae type b vaccine; G1P[8]: human group A rotavirus genotype 1P[8; EPI: 'Expanded Programme on Immunization';.IgE: immunoglobulin E; IM: intramuscular; IPV: inactivated poliovirus vaccine;PCV‐13: 13‐valent pneumococcal conjugate vaccine. RCT: randomised controlled trial; SAE: serious adverse event; Tdap: tetanus, diptheria and acellular pertussis vaccine;TT: tetanus toxoid;wP: whole‐cell pertussis vaccine.
Differences between protocol and review
-
The primary safety outcome (SAEs following immunisation) was defined in our protocol according to the International Conference of Harmonisation (ICH 1997). In this review, we also deemed it appropriate to report data on the following outcome domains/endpoints extracted from the SAE definition:
death;
events leading to admission to hospital;
events described as life‐threatening; and
events leading to persistent disability or incapacity.
We originally stated in our protocol that "diagnosis of anaphylaxis" and "diagnosis of urticaria" were secondary outcomes of interest. To avoid confusion with vaccine‐associated anaphylaxis and vaccine‐associated urticaria, these outcomes were listed as: "diagnosis of anaphylaxis (not vaccine‐associated)" and "diagnosis of urticaria (not vaccine‐associated)".
In this review we provide clarification regarding the diagnosis of primary and secondary atopic outcomes, for data extraction purposes.
This review summarises the methods implemented to extract data from figures. These have been included in the section Dealing with missing data.
The section Assessment of reporting biases was updated following the advice provided in the ROBINS‐I resources and reporting guidance (Cochrane Methods 2020). This guidance advises authors to include the consensus decisions for the signalling questions as supplemental data or files.
We specified in our protocol that stratified meta‐analyses would be carried out using random‐effects inverse variance methods with RRs and 95% CIs for dichotomous outcomes. Because the safety outcome/endpoints of interest were uncommon, we used the Mantel‐Haenszel method to summarise the RR and 95% CIs without zero‐cell corrections, as specified in Data synthesis section.
Contributions of authors
GPC and JR screened the studies in duplicate and independently, extracted the data and completed the risk of bias assessments. The assessments of the certainty of the evidence were completed by GPC, MJE and JR. TS provided statistical expertise. All authors read, provided feedback and approved the final version of this manuscript.
Sources of support
Internal sources
-
Wesfarmers Centre of Vaccines and Infectious Diseases (Telethon Kids Institute, Perth, Western Australia) and Forrest Research Foundation (Perth, Western Australia), Australia
Infrastructure and equipment used by GPC to carry out this review
External sources
-
Australian Department of Education and Training Endeavour Scholarship, Wesfarmers Centre of Vaccine and Infectious Diseases (Telethon Kids Institute) top‐up scholarship, and Forrest Research Foundation supplementary scholarship, Australia
Funding for the PhD of GPC
-
National Health and Medical Research Council, Australia
CBJ is supported by an Early Career Fellowship (GNT1142897)
-
Medical Research Future Fund, Australian Government, Department of Health, Australia
TS is supported by a Medical Research Future Fund Investigator Grant (MRF1195153)
Declarations of interest
GPC: has received travel support from Seqirus to attend a conference (June 2018). Seqirus solely manufacture influenza vaccines and, therefore, are not associated with pertussis vaccines. GPC did not benefit personally from the grant.
MJE: no conflict of interest.
JR: no conflict of interest.
CBJ: no conflict of interest.
PR: his institution (University of Western Australia) received a grant from GlaxoSmithKline. PR did not benefit financially. The research funding was controlled by the University of Western Australia. He served on advisory panels for GlaxoSmithKline (2019) and Sanofi (2019) with no remuneration.
PH: no conflict of interest.
TS: no conflict of interest.
New
References
References to studies included in this review
Afari 1996 {published data only}
- Afari EA, Kamiya Y, Nkrumah FK, Dunyo SK, Akpedonu P, Kamiya H, et al. Randomized controlled trial of acellular diphtheria, pertussis and tetanus vaccines in southern Ghana. Annals of Tropical Paediatrics 1996;16(1):39–48. [DOI: 10.1080/02724936.1996.11747802] [DOI] [PubMed] [Google Scholar]
Black 1997 {published and unpublished data}
- Black SB, Shinefield HR, Bergen R, Hart C, Kremers R, Lavetter A, et al. Safety and immunogenicity of Chiron/Biocine recombinant acellular pertussis-diphtheria-tetanus vaccine in infants and toddlers. Pediatric Infectious Disease Journal 1997;16(1):53–8. [DOI: 10.1097/00006454-199701000-00012] [DOI] [PubMed] [Google Scholar]
- Perez Chacon G. Query re: whole-cell versus acellular pertussis vaccine study published in PIDJ, 1997 [personal communication]. Email to: SB Black 21 January 2021.
Blumberg 1991 {published data only}
- Blumberg DA, Mink CM, Cherry JD, Johnson C, Garber R, Plotkin SA, et al. Comparison of acellular and whole-cell pertussis-component diphtheria-tetanus-pertussis vaccines in infants. Journal of Pediatrics 1991;119(2):194–204. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Blumberg DA, Pineda E, Cherry JD, Caruso A, Scott JV. The agglutinin response to whole-cell and acellular pertussis vaccines is Bordetella pertussis--strain dependent. American Journal of Diseases of Children 1992;146(10):1148–50. [DOI: 10.1001/archpedi.1992.02160220034016] [DOI] [PubMed] [Google Scholar]
- Cherry JD, Mortimer EA, Hackell JG, Scott JV. Clinical trials in the United States and Japan with the Lederle-Takeda and Takeda acellular pertussis-diphtheria-tetanus (APDT) vaccines. The Multicenter APDT Vaccine Study Groups. Developments in Biological Standardization 1991;73:51–8. [PMID: ] [PubMed] [Google Scholar]
- Stout MG. U.S. clinical trials with Takeda acellular pertussis vaccine. Tokai Journal of Experimental and Clinical Medicine 1988;13:21–8. [PMID: ] [PubMed] [Google Scholar]
Dagan 1997 {published and unpublished data}
- Dagan R, Igbaria K, Piglansky L, Melamed R, Willems P, Grossi A, et al. Safety and immunogenicity of a combined pentavalent diphtheria, tetanus, acellular pertussis, inactivated poliovirus and Haemophilus influenzae type b-tetanus conjugate vaccine in infants, compared with a whole cell pertussis pentavalent vaccine. Pediatric Infectious Disease Journal 1997;16(12):1113–21. [DOI: 10.1097/00006454-199712000-00004] [DOI] [PubMed] [Google Scholar]
- Perez Chacon G. Queries re: whole-cell versus acellular pertussis vaccine study published in PIDJ, 1997 [personal communication]. Email to R Dagan 21 January 2021.
Decker 1995 {published and unpublished data}
- Christy C, Pichichero ME, Reed GF, Decker MD, Anderson EL, Rennels MB, et al. Effect of gender, race, and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics 1995;96(3):584–7. [PMID: ] [PubMed] [Google Scholar]
- Decker MD, Edwards KM, Steinhoff MC, Rennels MB, Pichichero ME, Englund JA, et al. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics 1995;96(3):557–66. [PMID: ] [PubMed] [Google Scholar]
- Decker MD, Edwards KM. The multicenter acellular pertussis trial: an overview. Journal of Infectious Diseases 1996;174(Suppl 3):S270–5. [DOI: 10.1093/infdis/174.supplement_3.s270] [DOI] [PubMed] [Google Scholar]
- Deloria MA, Blackwelder WC, Decker MD, Englund JA, Steinhoff MC, Pichichero ME, et al. Association of reactions after consecutive acellular or whole-cell pertussis vaccine immunizations. Pediatrics 1995;96(3):592–4. [PMID: ] [PubMed] [Google Scholar]
- Edwards KM, Meade BD, Decker MD, Reed GF, Rennels MB, Steinhoff MC, et al. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics 1995;96(3 Pt 2):548–57. [PMID: ] [PubMed] [Google Scholar]
- Englund JA, Anderson EL, Reed GF, Decker MD, Edwards KM, Pichichero ME, et al. The effect of maternal antibody on the serologic response and the incidence of adverse reactions after primary immunization with acellular and whole-cell pertussis vaccines combined with diphtheria and tetanus toxoids. Pediatrics 1995;96(3):580–4. [PMID: ] [PubMed] [Google Scholar]
- Klein DL. A phase II multicenter evaluation of several candidate pertussis vaccines in infants. Developments in Biological Standardization 1991;73:305–9. [PMID: ] [PubMed] [Google Scholar]
- Meade BD, Deforest A, Edwards KM, Romani TA, Lynn F, O'Brien CH, et al. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 1995;96(3):570–5. [PMID: ] [PubMed] [Google Scholar]
- Perez Chacon G. Multicenter acellular pertussis trial - data request [personal communication]. Email to: JA Englund 19 April 2020.
- Pichichero ME, Anderson EL, Rennels MB, Edwards KM, Englund JA. Fifth vaccination with diphtheria, tetanus and acellular pertussis is beneficial in four- to six-year-olds. Pediatric Infectious Disease Journal 2001;20(4):427–33. [DOI: 10.1097/00006454-200104000-00011] [DOI] [PubMed] [Google Scholar]
- Pichichero ME, Christy C, Decker MD, Steinhoff MC, Edwards KM, Rennels MB, et al. Defining the key parameters for comparing reactions among acellular and whole-cell pertussis vaccines. Pediatrics 1995;96(3):588–92. [DOI: ] [PubMed] [Google Scholar]
- Pichichero ME, Deloria MA, Rennels MB, Anderson EL, Edwards KM, Decker MD, et al. A safety and immunogenicity comparison of 12 acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fourth dose in 15- to 20-month-old children. Pediatrics 1997;100(5):772–8. [DOI: 10.1542/peds.100.5.772] [DOI] [PubMed] [Google Scholar]
- Pichichero ME, Edwards KM, Anderson EL, Rennels MB, Englund JA, Yerg DE, et al. Safety and immunogenicity of six acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fifth dose in four- to six-year-old children. Pediatrics 2000;105(1):e11. [DOI: doi: 10.1542/peds.105.1.e11] [DOI] [PubMed] [Google Scholar]
- Rennels MB, Reed GF, Decker MD, Edwards KM, Pichichero ME, Deloria MA, et al. Simultaneous administration of Haemophilus influenzae type b vaccine with acellular or whole-cell pertussis vaccine: effects on reactogenicity and immune responses to pertussis vaccines. Pediatrics 1995;96(3):576–9. [PMID: ] [PubMed] [Google Scholar]
- Steinhoff MC, Reed GF, Decker MD, Edwards KM, Englund JA, Pichichero ME, et al. A randomized comparison of reactogenicity and immunogenicity of two whole-cell pertussis vaccines. Pediatrics 1995;96(3):567–70. [PMID: ] [PubMed] [Google Scholar]
- US Food & Drug Administration. Clinical review - Infanrix; May 2003 [Clinical review of supplement to license application for Infanrix®(diphtheria and tetanus toxoids and acellular pertussis vaccine, adsorbed) to include fifth dose indication]. Available at www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm103006.htm Accessed on 25 January 2021.
- US Food & Drug Administration. Clinical review of biological license application - DAPTACEL (CPDT); April 2002. Available at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM103057.pdf.
Edwards 1991 {published data only}
- Edwards KM, Bradley RB, Decker MD, Palmer PS, Van Savage J, Taylor JC, et al. Evaluation of a new highly purified pertussis vaccine in infants and children. Journal of Infectious Diseases 1989;160(5):832–7. [DOI: 10.1093/infdis/160.5.832] [DOI] [PubMed] [Google Scholar]
- Edwards KM, Decker MD, Bradley RB, Taylor JC, Hager CC. Booster response to acellular pertussis vaccine in children primed with acellular or whole cell vaccines. Pediatric Infectious Disease Journal 1991;10(4):315–8. [DOI: 10.1097/00006454-199104000-00010] [DOI] [PubMed] [Google Scholar]
Estcourt 2020 {published data only}
- Estcourt MJ, Campbell DE, Gold MS, Richmond P, Allen KJ, Quinn HE, et al. Whole-cell pertussis vaccination and decreased risk of IgE-mediated food allergy: a nested case-control study. Journal of Allergy and Clinical Immunology. In practice 2020;8(6):2004–14. [DOI: 10.1016/j.jaip.2019.12.020] [DOI] [PubMed] [Google Scholar]
- Estcourt MJ, Marsh JA, Campbell DE, Gold MS, Allen KJ, Richmond P, et al. Protocol for Pertussis Immunisation and Food Allergy (PIFA): a case-control study of the association between pertussis vaccination in infancy and the risk of IgE-mediated food allergy among Australian children. BMJ Open 2018;8(1):e020232. [DOI: 10.1136/bmjopen-2017-020232] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02490007. Case-control study of the association between pertussis vaccination in infancy and the risk of IgE-mediated food allergy. clinicalTrials.gov/show/NCT02490007 (first posted 3 July 2015).
Feldman 1993 {published data only}
- Feldman S, Perry CS, Andrew M, Jones L, Moffitt JE, Lamb D, et al. Primary immunization series for infants: comparison of two-component acellular and standard whole-cell pertussis vaccines combined with diphtheria-tetanus toxoids. Southern Medical Journal 1993;86(3):269–75, 284. [PMID: ] [PubMed] [Google Scholar]
Greco 1996 {published data only}
- Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infection and Immunity 1997;65(6):2168–74. [DOI: 10.1128/IAI.65.6.2168-2174.1997] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausiello CM, Urbani F, La Sala A, Lande R, Piscitelli A, Cassone A. Bordetella pertussis antigens in infants undergoing primary vaccination against pertussis. Developments in Biological Standardization 1997;89:315–20. [PMID: ] [PubMed] [Google Scholar]
- Ciofi degli Atti ML, Olin P. Severe adverse events in the Italian and Stockholm I pertussis vaccine clinical trials. Developments in Biological Standardization 1997;89:77–81. [PMID: ] [PubMed] [Google Scholar]
- Cossone A, Ausiello CM, Urbani F, Lande R, Giuliano M, Sala AL, et al. Cell-mediated and antibody responses to Bordetella pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. Archives of Pediatrics & Adolescent Medicine 1997;151(3):283–9. [DOI: 10.1001/archpedi.1997.02170400069013] [DOI] [PubMed] [Google Scholar]
- Giuliano M, Mastrantonio P, Giammanco A, Bottone M, Piscitelli A, Di Tommaso S, et al. Antibody kinetics and long-term sero-prevalence in the Italian clinical trial of acellular pertussis vaccines. Developments in Biological Standardization 1997;89:315–20. [PMID: ] [PubMed] [Google Scholar]
- Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak SG. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. Journal of Pediatrics 1998;132(6):938–8. [DOI: 10.1016/s0022-3476(98)70395-6] [DOI] [PubMed] [Google Scholar]
- Greco D, Salmaso S, Mastrantonio P, Giuliano M, The Italian Pertussis Working Group. Clinical trial of acellular pertussis vaccines in Italy - Study design. Biologicals 1993;21(1):38. [DOI: 10.1006/biol.1993.1036] [DOI] [Google Scholar]
- Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. New England Journal of Medicine 1996;334(6):341–8. [DOI: 10.1056/NEJM199602083340601] [DOI] [PubMed] [Google Scholar]
- Heijbel H, Ciofi degli Atti M, Harzer E, Liese J, Preziosi MP, Rasmussen F, et al. Hypotonic hyporesponsive episodes in eight pertussis vaccine studies. Developments in Biological Standardization 1997;89:101–3. [PMID: ] [PubMed] [Google Scholar]
- Salmaso S, Anemona A, Mastrantonio P, Stefanelli P, Tozzi AE, Ciofi degli Atti ML. Long-term efficacy of pertussis vaccines in Italy. PROPER Study Working Group. Developments in Biological Standardization 1998;95:189–94. [PMID: ] [PubMed] [Google Scholar]
- Salmaso S, Moiraghi A, Barale A, Ferraro P, Dal Lago P, Coppola N, et al. Case definitions. Developments in Biological Standardization 1997;89:135–42. [PubMed] [Google Scholar]
- Salmaso S, Piscitelli A, Rapicetta M, Chionne P, Madonna E, Argentini C. Immunogenicity of hepatitis B vaccines among infant recipients of acellular and whole cell pertussis DTP vaccines. Vaccine 1998;16(6):643–6. [DOI: 10.1016/s0264-410x(97)00231-4] [DOI] [PubMed] [Google Scholar]
- Tozzi AE, Ciofi degli Atti ML, Wassilak SG, Salmaso S, Panei P, Anemona A, et al. Predictors of adverse events after the administration of acellular and whole-cell diphtheria-tetanus-pertussis vaccines. Vaccine 1998;16(2):320–2. [DOI: 10.1016/s0264-410x(97)00163-1] [DOI] [PubMed] [Google Scholar]
- Tozzi A E, Greco D, Salmaso S, Wassilak SG. Pertussis immunization: results of new trials. Pediatric Pulmonology Supplement 1997;16:282–3. [DOI: 10.1002/ppul.19502308146] [DOI] [PubMed] [Google Scholar]
- Tozzi A E, Olin P. Common side effects in the Italian and Stockholm I trials. Developments in Biological Standardization 1997;89:105–8. [PMID: ] [PubMed] [Google Scholar]
- US Federal Drug & Administration. Summary basis of approval. Diphtheria and tetanus toxoids, and acellular pertussis vaccine adsorbed. Available at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM244609.pdf.
Gustafsson 1996 {published data only}
- Ciofi degli Atti ML, Olin P. Severe adverse events in the Italian and Stockholm I pertussis vaccine clinical trials. Developments in Biological Standardization 1997;89:77–81. [PMID: ] [PubMed] [Google Scholar]
- Gustaffson L, Hallander H, Olin P, Reizenstein E, Storsaeter, J. Efficacy trial of acellular pertussis vaccines. Technical report Trial I with results of preplanned analysis of safety, efficacy and immunogenicity. Swedish Institute for Infectious Disease Control, 1995. [Google Scholar]
- Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. New England Journal of Medicine 1996;334(6):349–55. [DOI: 10.1056/NEJM199602083340602] [DOI] [PubMed] [Google Scholar]
- Hallander H, Advani A, Alexander F, Gustafsson L, Ljungman M, Pratt C, et al. Antibody responses to Bordetella pertussis Fim2 or Fim3 following immunization with a whole-cell, two-component, or five-component acellular pertussis vaccine and following pertussis disease in children in Sweden in 1997 and 2007. Clinical and Vaccine Immunology 2014;21(2):165–73. [DOI: 10.1128/CVI.00641-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallander HO, Gustafsson L, Ljungman M, Storsaeter J. Pertussis antitoxin decay after vaccination with DTPa. Response to a first booster dose 3 1/2-6 1/2 years after the third vaccine dose. Vaccine 2005;23(46):5359–64. [DOI: 10.1016/j.vaccine.2005.06.009] [DOI] [PubMed] [Google Scholar]
- Heijbel H, Ciofi degli Atti M, Harzer E, Liese J, Preziosi MP, Rasmussen F, et al. Hypotonic hyporesponsive episodes in eight pertussis vaccine studies. Developments in Biological Standardization 1997;89:101–3. [PMID: ] [PubMed] [Google Scholar]
- NCT00004799. Phase III randomized, double-blind, placebo-controlled study of acellular and whole-cell pertussis vaccines. clinicaltrials.gov/ct2/show/record/NCT00004799 (first posted 25 February 2000).
- Nilsson L, Kjellman NI, Storsaeter J, Gustafsson L, Olin P. Lack of association between pertussis vaccination and symptoms of asthma and allergy. JAMA 1996;275(10):760. [PMID: ] [PubMed] [Google Scholar]
- Olin P, Rasmussen F, Gottfarb P. Schedules and protection, simultaneous vaccination and safety: experiences from recent controlled trials. International Journal of Infectious Diseases 1997;1(3):143–7. [Google Scholar]
- Olin P. Acellular pertussis vaccines - A question of efficacy. Journal of Hospital Infection 1995;30(Suppl):503–7. [DOI: 10.1016/0195-6701(95)90055-1] [DOI] [PubMed] [Google Scholar]
- Olin P. Efficacy trial of acellular pertussis vaccines; trial I. Developments in Biological Standardization 1997;89:52–4. [PMID: ] [PubMed] [Google Scholar]
- Storsaeter J, Hallnder HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998;16(20):1907–16. [DOI: 10.1016/s0264-410x(98)00227-8] [DOI] [PubMed] [Google Scholar]
- Tiru M, Hallander HO, Gustafsson L, Storsaeter J, Olin P. Diphtheria antitoxin response to DTP vaccines used in Swedish pertussis vaccine trials, persistence and projection for timing of booster. Vaccine 2000;18(21):2295–306. [DOI: 10.1016/s0264-410x(99)00539-3] [DOI] [PubMed] [Google Scholar]
- US Food & Drug Administration. Clinical review of biological license application - DAPTACEL (CPDT); April 2002. Available at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM103057.pdf.
Halperin 1996 {published data only}
- Halperin SA, Eastwood BJ, Barreto L, Friesen B, Medd L, Meekison W, et al. Adverse reactions and antibody response to four doses of acellular or whole cell pertussis vaccine combined with diphtheria and tetanus toxoids in the first 19 months of life. Vaccine 1996;14(8):767–72. [DOI: 10.1016/0264-410x(95)00250-5] [DOI] [PubMed] [Google Scholar]
- US Food & Drug Administration. Clinical review of biological license application - DAPTACEL (CPDT); April 2002. Available at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM103057.pdf.
Kitchin 2006 {published data only}
- Kitchin N, Southern J, Morris R, Borrow R, Fiquet A, Boisnard F, et al. Antibody persistence in UK pre-school children following primary series with an acellular pertussis-containing pentavalent vaccine given concomitantly with meningococcal group C conjugate vaccine, and response to a booster dose of an acellular pertussis-containing quadrivalent vaccine. Vaccine 2009;27(3):5096–102. [DOI: 10.1016/j.vaccine.2009.06.049] [DOI] [PubMed] [Google Scholar]
- Kitchin N, Southern J, Morris R, Hemme F, Cartwright K, Watson M, et al. A randomised controlled study of the reactogenicity of an acellular pertussis-containing pentavalent infant vaccine compared to a quadrivalent whole cell pertussis-containing vaccine and oral poliomyelitis vaccine, when given concurrently with meningococcal group C conjugate vaccine to healthy UK infants at 2, 3 and 4 months of age. Vaccine 2006;24(18):3964–70, Figure Flow diagram of subjects’ progress through the study; p 3966. [DOI: 10.1016/j.vaccine.2006.02.018] [DOI] [PubMed]
- Kitchin N, Southern J, Morris R, Hemme F, Cartwright K, Watson M, et al. A randomised controlled study of the reactogenicity of an acellular pertussis-containing pentavalent infant vaccine compared to a quadrivalent whole cell pertussis-containing vaccine and oral poliomyelitis vaccine, when given concurrently with meningococcal group C conjugate vaccine to healthy UK infants at 2, 3 and 4 months of age. Vaccine 2006;24(18):3964–70. [DOI: 10.1016/j.vaccine.2006.02.018] [DOI] [PubMed] [Google Scholar]
- Kitchin NR, Southern J, Morris R, Hemme F, Thomas S, Watson MW, et al. Evaluation of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b vaccine given concurrently with meningococcal group C conjugate vaccine at 2, 3 and 4 months of age. Archives of Disease in Childhood 2007;92(1):11–6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macías 2012 {published data only}
- European Medicines Agency. EMA/373968/2013 Hexyon assessment report; March 2013. Available at www.ema.europa.eu/en/documents/assessment-report/hexyon-epar-public-assessment-report_en.pdf.
- European Medicines Agency EMA/373868/2013. Hexacima assessment report; March 2013. Available at www.ema.europa.eu/en/documents/assessment-report/hexacima-epar-public-assessment-report_en.pdf.
- Macías M, Lanata CF, Zambrano B, Gil AI, Amemiya I, Mispireta M, et al. Safety and immunogenicity of an investigational fully liquid hexavalent DTaP-IPV-Hep B-PRP-T vaccine at two, four and six months of age compared with licensed vaccines in Latin America. Pediatric Infectious Disease Journal 2012;31(8):e126–32. [DOI: 10.1097/INF.0b013e318258400d] [DOI] [PubMed] [Google Scholar]
- NCT00313911. Safety of DTaP-IPV-Hep B-PRP~T combined vaccine compared to Tritanrix-HepB/Hib™ and OPV given at age 2, 4, and 6 months [Large scale safety study of a DTaP-IPV-Hep B-PRP~T combined vaccine, in comparison to Tritanrix-Hep B/Hib™ and OPV administered at 2, 4, and 6 months of age in Latin American infants]. clinicaltrials.gov/ct2/show/NCT00313911 (first posted 19 November 2012).
Madhi 2011 {published data only}
- EMA/373868/2013. European Medicines Agency Hexacima assessment report; March 2013. Available at www.ema.europa.eu/en/documents/assessment-report/hexacima-epar-public-assessment-report_en.pdf.
- EMA/373968/2013. European Medicines Agency Hexyon assessment report; March 2013. Available at www.ema.europa.eu/en/documents/assessment-report/hexyon-epar-public-assessment-report_en.pdf.
- Madhi SA, Koen A, Cutl, C, Groome M, Santos-Lima E. Antibody persistence and booster vaccination of a fully liquid hexavalent vaccine coadministered with measles/mumps/rubella and varicella vaccines at 15-18 months of age in healthy South African infants. Pediatric Infectious Disease Journal 2013;32(8):889–97. [DOI: 10.1097/INF.0b013e318292f7b1] [DOI] [PubMed] [Google Scholar]
- Madhi SA, Lopez P, Zambrano B, Jordanov E, B'Chir S, Noriega F, et al. Antibody persistence in pre-school children after hexavalent vaccine infant primary and booster administration. Human Vaccines & Immunotherapeutics 2019;15(3):658–68. [DOI: 10.1080/21645515.2018.1546524] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi SA, Mitha I, Cutl, C, Groome M, Santos-Lima E. Immunogenicity and safety of an investigational fully liquid hexavalent combination vaccine versus licensed combination vaccines at 6, 10, and 14 weeks of age in healthy South African infants. Pediatric Infectious Disease Journal 2011;30(4):e68–e74. [DOI: 10.1097/INF.0b013e31820b93d2] [DOI] [PubMed] [Google Scholar]
- NCT00362336. Comparison of DTaP-IPV-Hep B-PRP~T combined vaccine to CombAct-HIB® concomitantly given with Engerix B® paediatric and OPV [Immunogenicity study of a DTaP-IPV-Hep B-PRP~T combined vaccine in comparison to CombAct-HIB® concomitantly administered with Engerix B® paediatric and OPV at 6, 10, and 14 Weeks of age in South African infants]. clinicaltrials.gov/ct2/show/results/NCT00362336 (first posted 10 August 2006).
- NCT01105559. Antibody persistence in healthy children after primary and booster DTaP-IPV-Hep B-PRP-T vaccine or control vaccine [Antibody persistence in healthy South African children after primary series and booster vaccination with an investigational (DTaP-IPV-Hep B-PRP-T) or control vaccines]. clinicaltrials.gov/ct2/show/NCT01105559 (first posted 16 April 2010).
Miller 1990 {published data only}
- Miller E, Ashworth LA, Robinson A, Waight PA, Irons LI. Phase II trial of whole-cell pertussis vaccine vs an acellular vaccine containing agglutinogens. Lancet 1991;337(8733):70–3. [DOI: 10.1016/0140-6736(91)90735-8] [DOI] [PubMed] [Google Scholar]
- Miller E, Ashworth L A E, Redhead K, Thornton C, Waight PA, Coleman T. Effect of schedule on reactogenicity and antibody persistence of acellular and whole-cell pertussis vaccines: value of laboratory tests as predictors of clinical performance. Vaccine 1997;15(1):51–60. [DOI: doi: 10.1016/s0264-410x(96)00112-0] [DOI] [PubMed] [Google Scholar]
- Miller E, Miller DL, Ashworth LA, Waight PA, Harbert KA. Preliminary comparison of antibody responses and symptoms following primary immunization with British whole-cell and three acellular DTP vaccines. Proceedings of the Sixth International Symposium on Pertussis; 1990 Sep 26–28; Bethesda (MD) 1990:303-9.
Miller 1997 {published data only}
- Miller E, Ashworth LA, Redhead K, Thornton C, Waight PA, Coleman T. Effect of schedule on reactogenicity and antibody persistence of acellular and whole-cell pertussis vaccines: value of laboratory tests as predictors of clinical performance. Vaccine 1997;15(1):51–60. [DOI: 10.1016/s0264-410x(96)00112-0] [DOI] [PubMed] [Google Scholar]
- Perez Chacon G. Queries re: "trial II" Effect of schedule on reactogenicity and whole-cell pertussis vaccine. Email to E Miller 05 May 2021.
NCT00343889 {published data only}
- NCT00343889. Comparison of DTaP-HB-PRP~T combined vaccine to Tritanrix-HepB/Hib™, both given concomitantly with oral polio vaccine [Immunogenicity study of a DTaP-Hep B-PRP-T combined vaccine compared to Tritanrix-HepB/Hib™, both given concomitantly with the oral polio vaccine at 6, 10, and 14 weeks of age in healthy infants in the Philippines]. clinicaltrials.gov/ct2/show/record/NCT00343889?view=record (first posted 10 December 2013).
- NCT00534833. Immunogenicity study of antibody persistence and booster effect of DTaP-HB-PRP~T combined vaccine or Tritanrix-HepB/Hib™ [Immunogenicity study of the antibody persistence and booster effect of DTaP-Hep B-PRP-T combined vaccine or Tritanrix HepB/Hib™ at 15 to 18 months of age following a primary series at 6, 10 and 14 weeks of age in healthy Filipino infants]. clinicaltrials.gov/ct2/show/record/NCT00534833 (first posted 26 September 2007).
NCT00348881 {published data only}
- NCT00348881. Study comparing a DTaP-HB-PRP~T combined vaccine with Tritanrix HepB/Hib™, concomitantly with OPV in healthy infants [Large scale safety and immunogenicity study of a DTaP-Hep B-PRP-T combined vaccine compared to Tritanrix HepB/Hib™, both given concomitantly with OPV at 6, 10, and 14 weeks of age in healthy Filipino infants]. clinicaltrials.gov/ct2/show/record/NCT00348881 (first posted 22 October 2013).
- NCT00514709. Immunogenicity study of antibody persistence and booster effect of DTaP-HB PRP~T combined vaccine in Filipino infants [Immunogenicity study of the antibody persistence and booster effect of DTaP-Hep B-PRP-T combined vaccine at 12 to 18 months of age following a primary series at 6, 10 and 14 weeks of age in healthy Filipino infants having received hepatitis B vaccine at birth]. clinicaltrials.gov/ct2/show/record/NCT00514709 (first posted 2 August 2016).
Nilsson 1998 {published data only}
- Aalberse RC, Grüber C, Ljungman M, Kakat S, Wahn U, Niggemann B, et al. Further investigations of the IgE response to tetanus and diphtheria following covaccination with acellular rather than cellular Bordetella pertussis. Pediatric Allergy and Immunology 2019;30(8):841–7. [DOI: 10.1111/pai.13113] [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. New England Journal of Medicine 1996;334(6):349–55. [DOI: 10.1056/NEJM199602083340602] [DOI] [PubMed] [Google Scholar]
- Nilsson L, Grüber C, Granström M, Björkstén B, Kjellman N-I. Pertussis IgE and atopic disease. Allergy 1998;53(12):1195–201. [DOI: 10.1111/j.1398-9995.1998.tb03841.x] [DOI] [PubMed] [Google Scholar]
- Nilsson L, Kjellman NI, Björkstén B. A randomized controlled trial of the effect of pertussis vaccines on atopic disease. Archives of Pediatrics & Adolescent Medicine 1998;152(8):734–8. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Nilsson L, Kjellman NI, Björkstén B. A randomized controlled trial of the effect of pertussis vaccines on atopic disease. Archives of Pediatrics & Adolescent Medicine 1998;152(8):Figure, Atopic manifestations in 669 children during the first 2 1/2 years of age in relation to type of pertussis vaccine; p. 736. [DOI] [PubMed]
- Nilsson L, Kjellman N-I, Bjorksten B. Allergic disease at the age of 7 years after pertussis vaccination in infancy: results from the follow-up of a randomized controlled trial of 3 vaccines. Archives of Pediatrics & Adolescent Medicine 2003;157(12):1184–9. [PMID: 10.1001/archpedi.157.12.1184] [DOI] [PubMed] [Google Scholar]
Olin 1997 {published data only}
- Anonymous. Whooping cough vaccine trial compares British whole cell vaccine and acellular vaccines. Communicable Disease Report 1997;7(21):181. [PMID: ] [PubMed] [Google Scholar]
- Heijbel H, Ciofi degli Atti M, Harzer E, Liese J, Preziosi MP, Rasmussen F, et al. Hypotonic hyporesponsive episodes in eight pertussis vaccine studies. Developments in Biological Standardization 1997;89:101–3. [PMID: ] [PubMed] [Google Scholar]
- Heijbel H, Rasmussen F, Olin P. Safety evaluation of one whole-cell and three acellular pertussis vaccines in Stockholm trial II. Developments in Biological Standardization 1997;89:99–100. [PMID: ] [PubMed] [Google Scholar]
- Olin P, Gustafsson L, Rasmussen F, Hallander H, Heijbel H, Gottfarb P. Efficacy trial of acellular pertussis vaccines: technical report trial II with preplanned analyses of efficacy, immunogenicity and safety. Swedish Institute for Infectious Disease Control (SMI-tryck) 1997;116:1–70.
- Olin P, Hallander HO, Gustafsson L, Barreto L, Podda A. Measuring protection; a case study of pertussis vaccines--Swedish Trial II: secondary non-randomized comparisons between two schedules of infant vaccination. Developments in Biological Standardization 1998;95:211–20. [PMID: ] [PubMed] [Google Scholar]
- Olin P, Rasmussen F, Gottfarb P. Schedules and protection, simultaneous vaccination and safety: experiences from recent controlled trials. International Journal of Infectious Diseases 1997;1(3):143–7. [Google Scholar]
- Olin P, Rasmussen F, Gustafsson L, Hallander HO, Heijbel H. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Ad Hoc Group for the Study of Pertussis Vaccines. Lancet 1997;350(9091):1569–77. [DOI: 10.1016/s0140-6736(97)06508-2] [DOI] [PubMed] [Google Scholar]
- Olin P. Acellular pertussis vaccines - A question of efficacy. Journal of Hospital Infection 1995;30(Suppl):503–7. [DOI: 10.1016/0195-6701(95)90055-1] [DOI] [PubMed] [Google Scholar]
- Tiru M, Hallander HO, Gustafsson L, Storsaeter J, Olin P. Diphtheria antitoxin response to DTP vaccines used in Swedish pertussis vaccine trials, persistence and projection for timing of booster [Vaccine]. 2000 18;21:2295–306. [DOI: 10.1016/s0264-410x(99)00539-3] [DOI] [PubMed] [Google Scholar]
- US Food & Drug Administration. Clinical review of biological license application - DAPTACEL (CPDT); April 2002. Available at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM103057.pdf.
Reinert 2006 {published data only}
- Reinert P, Fiquet A, Thomas S, Schuyleman A, Watson M. Fever as a marker of reactogenicity of an acellular pertussis-containing hexavalent vaccine (HEXAVAC) in a large-scale, open, randomized safety study in healthy French infants. Human Vaccines 2006;2(5):215–21. [DOI: 10.4161/hv.2.5.3220] [DOI] [PubMed] [Google Scholar]
Simondon 1997 {published data only}
- Heijbel H, Ciofi degli Atti M, Harzer E, Liese J, Preziosi MP, Rasmussen F, et al. Hypotonic hyporesponsive episodes in eight pertussis vaccine studies. Developments in Biological Standardization 1997;89:101–3. [PMID: ] [PubMed] [Google Scholar]
- Lacombe K, Yam A, Simondon K, Pinchinat S, Simondon F. Risk factors for acellular and whole-cell pertussis vaccine failure in Senegalese children. Vaccine 2004;23(5):623–8. [DOI: 10.1016/j.vaccine.2004.07.007] [DOI] [PubMed] [Google Scholar]
- Preziosi MP, Ndiaye M, Coll-Seck A, Simondon F. The Senegal pertussis trial: safety and surveillance of adverse reactions. Developments in Biological Standardization 1997;89:91–7. [PMID: ] [PubMed] [Google Scholar]
- Simondon F, Preziosi MP, Yam A, Kane CT, Chabirand L, Iteman I, et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 1997;15(15):1606–12. [DOI: 10.1016/s0264-410x(97)00100-x] [DOI] [PubMed] [Google Scholar]
- Simondon F. Senegal pertussis trial. Developments in Biological Standardization 1997;89:63–6. [PMID: ] [PubMed] [Google Scholar]
- Vose JR, Hoffenbach A. Safety, immunogenicity and efficacy results of a 2-component DT acellular pertussis paediatric vaccine and its combinations with IPV and HIB antigens. Biologicals 1999;27(2):97–8. [DOI: 10.1006/biol.1999.0188] [DOI] [PubMed] [Google Scholar]
Stehr 1998 {published and unpublished data}
- Cherry JD, Heininger U, Stehr K, Christenson P. The effect of investigator compliance (observer bias) on calculated efficacy in a pertussis vaccine trial. Pediatrics 1998;102(4):909–12. [DOI: 10.1542/peds.102.4.909] [DOI] [PubMed] [Google Scholar]
- Heijbel H, Ciofi degli Atti M, Harzer E, Liese J, Preziosi MP, Rasmussen F, et al. Hypotonic hyporesponsive episodes in eight pertussis vaccine studies. Developments in Biological Standardization 1997;89:101–3. [PMID: ] [PubMed] [Google Scholar]
- Heininger U, Cherry JD, Stehr K, Schmitt-Grohé S, Uberall M, Laussucq S, et al. Comparative efficacy of the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine and Lederle whole-cell component DTP vaccine in German children after household exposure. Pertussis Vaccine Study Group. Pediatrics 1998;102(3):546–53. [DOI: 10.1542/peds.102.3.546] [DOI] [PubMed] [Google Scholar]
- Heininger U, Stehr K, Christenson P, Cherry JD. Evidence of efficacy of the Lederle/Takeda acellular pertussis component diphtheria and tetanus toxoids and pertussis vaccine but not the Lederle whole-cell component diphtheria and tetanus toxoids and pertussis vaccine against Bordetella parapertussis infection. Clinical Infectious Diseases 1999;28(3):602–4. [DOI: 10.1086/515154] [DOI] [PubMed] [Google Scholar]
- Lugauer S, Heininger U, Cherry JD, Stehr K. Long-term clinical effectiveness of an acellular pertussis component vaccine and a whole cell pertussis component vaccine. European Journal of Pediatrics 2002;161(3):142–6. [DOI: 10.1007/s00431-001-0893-5] [DOI] [PubMed] [Google Scholar]
- Perez Chacon G. RE: efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine [personal communication]. Email to: JD Cherry 10 March 2021.
- Schlapfer G, Cherry JD, Heininger U, Uberall M, Schmitt-Grohe S, Laussucq S, et al. Polymerase chain reaction identification of Bordetella pertussis infections in vaccinees and family members in a pertussis vaccine efficacy trial in Germany. Pediatric Infectious Disease Journal 1995;14(3):209–14. [DOI: 10.1097/00006454-199503000-00008] [DOI] [PubMed] [Google Scholar]
- Schmitt-Grohé S, Stehr K, Cherry JD, Heininger U, Uberall MA, Laussucq S, et al. Minor adverse events in a comparative efficacy trial in Germany in infants receiving either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP (DTP) or DT vaccine. The Pertussis Vaccine Study Group. Developments in Biological Standardization 1997;89:113–8. [PMID: ] [PubMed] [Google Scholar]
- Stehr K, Cherry JD, Heininger U, Schmitt-Grohé S, Uberall M, Laussucq S, et al. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics 1998;101(1):1–11. [DOI: 10.1542/peds.101.1.1] [DOI] [PubMed] [Google Scholar]
- Stehr K, Cherry JD. A comparative efficacy trial in Germany in which infants received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP (DTP) vaccine or DT vaccine. Developments in Biological Standardization 1997;89:58–62. [PMID: ] [PubMed] [Google Scholar]
- Tapiainen T, Cherry J D, Heininger U. Effect of injection site on reactogenicity and immunogenicity of acellular and whole-cell pertussis component diphtheria-tetanus-pertussis vaccines in infants. Vaccine 2005;23(43):5106–12. [DOI: 10.1016/j.vaccine.2005.05.039] [DOI] [PubMed] [Google Scholar]
- Uberall MA, Stehr K, Cherry JD, Heininger U, Schmitt-Grohé S, Laussucq S, et al. Severe adverse events in a comparative efficacy trial in Germany in infants receiving either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP (DTP) or DT vaccine. The Pertussis Vaccine Study Group. Developments in Biological Standardization 1997;89:83–9. [PMID: ] [PubMed] [Google Scholar]
Toelle 2020 {published data only}
- Garden FL, Toelle BG, Mihrshahi S, Webb KL, Almqvist C, Tovey ER, et al. Cohort profile: the Childhood Asthma Prevention Study (CAPS). International Journal of Epidemiology 2018;47(6):1736–6k. [DOI: 10.1093/ije/dyy078] [DOI] [PubMed] [Google Scholar]
- Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2006;118(1):53–61. [PMID: 10.1016/j.jaci.2006.04.004] [DOI] [PubMed] [Google Scholar]
- Toelle BG, Garden FL, McIntyre PB, Wood N, Marks GB. Pertussis vaccination and allergic illness in Australian children. Pediatric Allergy and Immunology 2020;31(7):857–61. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Toelle BG, Garden FL, McIntyre PB, Wood N, Marks GB. Pertussis vaccination and allergic illness in Australian children. Pediatric Allergy and Immunology 2020;31(7):857–61. Supplementary material. Table 3. Comparison of atopy and allergic illness in children who received first dose of wP vs aP. Outcomes for years 1.5, 3 and 14 only. [DOI] [PubMed]
- Toelle BG, Garden FL, Ng KK, Belousova EG, Almqvist C, Cowell CT, et al. Outcomes of the childhood asthma prevention study at 11.5 years. Journal of Allergy and Clinical Immunology 2013;132(5):1220–2.e3. [DOI: 10.1016/j.jaci.2013.06.005] [DOI] [PubMed] [Google Scholar]
- Toelle BG, Ng KK, Crisafulli D, Belousova EG, Almqvist C, Webb K, et al. Eight-year outcomes of the childhood asthma prevention study. Journal of Allergy and Clininical Immunology 2010;126(2):388–9.e1–3. [DOI: 10.1016/j.jaci.2010.04.031] [DOI] [PubMed] [Google Scholar]
Venter 2016 {published and unpublished data}
- Perez Chacon G. Re: Query re: No association between atopic outcomes and type of pertussis vaccine given in children born on the Isle of Wight 2001–2002 [personal communication]. Email to PJ Turner and C Venter 31 January 2021.
- Venter C, Stowe J, Andrews N, Miller E, Turner PJ. No association between atopic outcomes and pertussis vaccine given in children born on the Isle of Wight 2001–2002. Journal of Allergy and Clinical Immunology. In Practice 2016;4(6):1248–50. [DOI: 10.1016/j.jaip.2016.06.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wanlapakorn 2020 {published data only}
- NCT02408926. Vaccine responses in infants after acellular pertussis vaccination during pregnancy in Thailand [Vaccine responses in infants after acellular pertussis vaccination during pregnancy in Thailand]. clinicaltrials.gov/ct2/show/NCT02408926 (first posted 6 April 2015).
- Posuwan N, Wanlapakorn N, Vongpunsawad S, Sintusek P, Leuridan E, Van Damme P, et al. Comparison of hepatitis B surface antibody levels induced by the pentavalent DTwP-HB-Hib versus the hexavalent DTaP-HB-Hib-IPV vaccine, administered to infants at 2, 4, 6, and 18 months of age, following monovalent hepatitis B vaccination at birth. Vaccine 2020;38(7):1643–51. [DOI: 10.1016/j.vaccine.2019.12.065] [DOI] [PubMed] [Google Scholar]
- Wanlapakorn N, Maertens K, Thongmee T, Srimuan D, Thatsanathorn T, Van Damme P, et al. Levels of antibodies specific to diphtheria toxoid, tetanus toxoid, and Haemophilus influenzae type b in healthy children born to Tdap-vaccinated mothers. Vaccine 2020;38(44):6914–21. [DOI: 10.1016/j.vaccine.2020.08.058] [DOI] [PubMed] [Google Scholar]
- Wanlapakorn N, Maertens K, Vongpunsawad S, Puenpa J, Tran TM, Hens N, et al. Quantity and quality of antibodies after acellular versus whole cell pertussis vaccines in infants born to mothers who received Tdap during pregnancy: a randomised trial. Clinical Infectious Diseases 2020;71(1):72–80. [DOI: 10.1093/cid/ciz778] [DOI] [PubMed] [Google Scholar]
- Wanlapakorn N, Maertens K, Vongpunsawad S, Puenpa J, Tran TM, Hens N, et al. Quantity and quality of antibodies after acellular versus whole-cell pertussis vaccines in infants born to mothers who received tetanus, diphtheria, and acellular pertussis vaccine during pregnancy: a randomized trial. Clinical Infectious Diseases 2020;71(1):72–80. Figure, The Consolidated Standards for Reporting Trials flow diagram; p. 71. [DOI: 10.1093/cid/ciz778] [DOI] [PubMed]
References to studies excluded from this review
Anderson 1988 {published data only}
- Anderson EL, Belshe RB, Bartram J. Differences in reactogenicity and antigenicity of acellular and standard pertussis vaccines combined with diphtheria and tetanus in infants. Journal of Infectious Diseases 1988;157(4):731–7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Anderson 1994 {published data only}
- Anderson EL, Mink CM, Berlin BS, Shih CN, Tung FF, Belshe RB. Acellular pertussis vaccines in infants: evaluation of single component and two-component products. Vaccine 1994;12(1):28–31. [PMID: 10.1016/0264-410x(94)90007-8] [DOI] [PubMed] [Google Scholar]
Bernsen 2006 {published data only}
- Bernsen RM, Koes BW, Jongste JC, Wouden JC. Haemophilus influenzae type b vaccination and reported atopic disorders in 8-12-year-old children. Pediatric Pulmonology 2006;41(5):463–9. [DOI: 10.1002/ppul.20397] [DOI] [PubMed] [Google Scholar]
Blennow 1988 {published data only}
- Blennow M, Granström M, Jäätmaa E, Olin P. Primary immunization of infants with an acellular pertussis vaccine in a double-blind randomized clinical trial. Pediatrics 1988;82(3):293–9. [PubMed] [Google Scholar]
- Blennow M, Granström M, Olin P, Tiru M, Jäätmaa E, Askelöf P, et al. Preliminary data from a clinical trial (phase 2) of an acellular pertussis vaccine, J-NIH-6. Developments in Biological Standardization 1986;65:185–90. [PubMed] [Google Scholar]
- Blennow M, Granstrom M. Sixteen-month follow-up of antibodies to pertussis toxin after primary immunization with acellular or whole cell vaccine. Pediatric Infectious Disease Journal 1989;8(9):621–5. [DOI] [PubMed] [Google Scholar]
- Tindberg Y, Blennow M, Granstrom M. A ten year follow-up after immunization with a two component acellular pertussis vaccine. Pediatric Infectious Disease Journal 1999;18(4):361–5. [DOI: 10.1097/00006454-199904000-00011] [DOI] [PubMed] [Google Scholar]
Farooqi 1998 {published data only}
- Farooqi IS, Hopkin JM. Early childhood infection and atopic disorder. Thorax 1998;53(11):927–32. [DOI: 10.1136/thx.53.11.927] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grüber 2003 {published data only}
- Grüber C, Illi S, Lau S, Nickel R, Forster J, Kamin W, et al. Transient suppression of atopy in early childhood is associated with high vaccination coverage. Pediatrics 2003;111(3):e282–8. [DOI: 10.1542/peds.111.3.e282] [DOI] [PubMed] [Google Scholar]
- Nickel R, Lau S, Niggemann B, Gruber C, Von Mutius E, Illi S, et al. Messages from the German Multicentre Allergy Study. Pediatric Allergy and Immunology 2002;13(15):7–10. [DOI] [PubMed] [Google Scholar]
Grüber 2008 {published data only}
- Grüber C, Warner J, Hill D, Bauchau V. Early atopic disease and early childhood immunization--is there a link? Allergy 2008;63(11):1464–72. [DOI: 10.1111/j.1398-9995.2008.01696.x] [DOI] [PubMed] [Google Scholar]
Gylca 2000 {published data only}
- Ball, L. Clinical Review of BLA STN 103907: InfanrixDTPaHepB-IPVTM. Available at www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM106897.pdf 2001.
- Gylca R, Gylca V, Benes O, Melnic A, Chicu V, Weisbecker C, et al. A new DTPa-HBV-IPV vaccine co-administered with Hib, compared to a commercially available DTPw-IPV/Hib vaccine co-administered with HBV, given at 6, 10 and 14 weeks following HBV at birth. Vaccine 2000;19(7):825–33. [DOI] [PubMed] [Google Scholar]
Halperin 1994 {published data only}
- Halperin SA, Barreto L, Eastwood BJ, Law B, Roberts EA. Safety and immunogenicity of a five-component acellular pertussis vaccine with varying antigen quantities. Archives of Pediatrics & Adolescent Medicine 1994;148(11):1220–4. [DOI: 10.1001/archpedi.1994.02170110106025] [DOI] [PubMed] [Google Scholar]
Halperin 1995 {published data only}
- Halperin SA, Mills E, Barreto L, Pim C, Eastwood BJ. Acellular pertussis vaccine as a booster dose for seventeen- to nineteen-month-old children immunized with either whole cell or acellular pertussis vaccine at two, four and six months of age. Pediatric Infectious Disease Journal 1995;14(9):792-7. [DOI: 10.1097/00006454-199509000-00012] [DOI] [PubMed] [Google Scholar]
Halperin 1999 {published data only}
- Halperin SA, Scheifele D, Barreto L, Pim C, Guasparini R, Medd L, et al. Comparison of a fifth dose of a five-component acellular or a whole cell pertussis vaccine in children four to six years of age. Pediatric Infectious Disease Journal 1999;18(9):772-9. [DOI: 10.1097/00006454-199909000-00006] [DOI] [PubMed] [Google Scholar]
Halperin 2003 {published data only}
- Halperin SA, Scheifele D, Mills E, Guasparini R, Humphreys G, Barreto L, et al. Nature, evolution, and appraisal of adverse events and antibody response associated with the fifth consecutive dose of a five-component acellular pertussis-based combination vaccine. Vaccine 2003;21(19):2298–306. [DOI: 10.1016/s0264-410x(03)00173-7] [DOI] [PubMed] [Google Scholar]
Henderson 1999 {published data only}
- Henderson J, North K, Griffiths M, Harvey I, Golding J. Pertussis vaccination and wheezing illnesses in young children: prospective cohort study. The Longitudinal Study of Pregnancy and Childhood Team. BMJ 1999;318(7192):1173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kummeling 2007 {published data only}
- Kummeling I, Thijs C, Stelma F, Huber M, den Brandt PA, Dagnelie PC. Diphtheria, pertussis, poliomyelitis, tetanus, and Haemophilus influenzae type b vaccinations and risk of eczema and recurrent wheeze in the first year of life: the KOALA birth cohort study. Pediatrics 2007;119(2):e367–73. [DOI: 10.1542/peds.2006-1479] [DOI] [PubMed] [Google Scholar]
Maitra 2004 {published data only}
- Maitra A, Sherriff A, Griffiths M, Henderson J, Avon Longitudinal Study of Parents, Children Study Team. Pertussis vaccination in infancy and asthma or allergy in later childhood: birth cohort study. BMJ 2004;328(7445):925–6. [DOI: 10.1136/bmj.38045.858889] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipatanakul W. Pertussis vaccination in infancy and asthma or allergy in later childhood: birth cohort study. Pediatrics 2005;116(2):541–2. [DOI: 10.1542/peds.2005-0698M] [DOI] [Google Scholar]
Matheson 2010 {published data only}
- Matheson MC, Haydn Walters E, Burgess JA, Jenkins MA, Giles GG, et al. Childhood immunization and atopic disease into middle-age--a prospective cohort study. Pediatric Allergy and Immunology 2010;21(2):301–6. [DOI: 10.1111/j.1399-3038.2009.00950.x] [DOI] [PubMed] [Google Scholar]
McDonald 2008 {published data only}
- McDonald KL, Huq SI, Lix LM, Becker AB, Kozyrskyj AL. Delay in diphtheria, pertussis, tetanus vaccination is associated with a reduced risk of childhood asthma. Journal of Allergy and Clinical Immunology 2008;121(3):626–31. [DOI: 10.1016/j.jaci.2007.11.034] [DOI] [PubMed] [Google Scholar]
McKeever 2004 {published data only}
- McKeever T M, Lewis S A, Smith C, Hubbard R. Vaccination and allergic disease: a birth cohort study. American Journal of Public Health 2004;94(6):985–9. [DOI: 10.2105/ajph.94.6.985] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullooly 2007 {published data only}
- Mullooly JP, Schuler R, Barrett M, Maher JE. Vaccines, antibiotics, and atopy. Pharmacoepidemiology and Drug Safety 2007;16(3):275–88. [DOI: ] [DOI] [PubMed] [Google Scholar]
Pichichero 1992 {published data only}
- Pichichero ME, Francis AB, Blatter MM, Reisinger KS, Green JL, Marsocci SM, et al. Acellular pertussis vaccination of 2-month-old infants in the United States. Pediatrics 1992;89(5):882–7. [PMID: ] [PubMed] [Google Scholar]
- Pichichero ME, Francis AB, Marsocci SM, Green JL, Disney FA, Meschievitz C. Safety and immunogenicity of an acellular pertussis vaccine booster in 15- to 20-month-old children previously immunized with acellular or whole-cell pertussis vaccine as infants. Pediatrics 1993;91(4):756–60. [PMID: ] [PubMed] [Google Scholar]
Pichichero 1993 {published data only}
- Pichichero ME, Francis AB, Marsocci SM, Green JL, Disney FA. Comparison of a diphtheria and tetanus toxoids and bicomponent acellular pertussis vaccine with diphtheria and tetanus toxoids and whole-cell pertussis vaccine in infants. American Journal of Diseases of Children 1993;147(3):295–9. [DOI: 10.1001/archpedi.1993.02160270057018] [DOI] [PubMed] [Google Scholar]
Pichichero 1994 {published data only}
- Pichichero ME, Green JL, Francis AB, Marsocci SM, Lynd AM, Litteer T. Comparison of a three-component acellular pertussis vaccine with whole cell pertussis vaccine in two-month-old children. Pediatric Infectious Disease Journal 1994;13(3):193–6. [DOI: 10.1097/00006454-199403000-00005] [DOI] [PubMed] [Google Scholar]
Pichichero 1996 {published data only}
- Pichichero ME, Green JL, Francis AB, Marsocci SM, Murphy AM, Buscarino C. Antibody response and reactions to completion of a four-dose series with a two- or three-component acellular pertussis vaccine compared to whole cell pertussis vaccine. Scandinavian Journal of Infectious Diseases 1996;28(2):159–63. [DOI: 10.3109/00365549609049068] [DOI] [PubMed] [Google Scholar]
Podda 1994 {published data only}
- Podda A, Bona G, Canciani G, Pistilli AM, Contu B, Furlan R, et al. Effect of priming with diphtheria and tetanus toxoids combined with whole-cell pertussis vaccine or with acellular pertussis vaccine on the safety and immunogenicity of a booster dose of an acellular pertussis vaccine containing a genetically inactivated pertussis toxin in fifteen- to twenty-one-month-old children. Italian Multicenter Group for the Study of Recombinant Acellular Pertussis Vaccine. Journal of Pediatrics 1995;127(2):238–43. [DOI: 10.1016/s0022-3476(95)70301-2] [DOI] [PubMed] [Google Scholar]
- Podda A, De La Luca EC, Contu B, Furlan R, Maida A, Moiraghi A, et al. Comparative study of a whole-cell pertussis vaccine and a recombinant acellular pertussis vaccine. Journal of Pediatrics 1994;124(6):921–6. [DOI: 10.1016/s0022-3476(05)83181-6] [DOI] [PubMed] [Google Scholar]
Schmitt 1996 {published data only}
- Schmitt HJ, Konig CH, Neiss A, Bogaerts H, Bock HL, Schulte-Wissermann H, et al. Efficacy of acellular pertussis vaccine in early childhood after household exposure. JAMA 1996;275(1):37–41. [PMID: ] [PubMed] [Google Scholar]
Simondon 1996 {published data only}
- Simondon F, Yam A, Gagnepain JY, Wassilak S, Danve B, Cadoz M. Comparative safety and immunogenicity of an acellular versus whole-cell pertussis component of diphtheria-tetanus-pertussis vaccines in Senegalese infants. European Journal of Clinical Microbiology & Infectious Diseases 1996;15(12):927–32. [DOI] [PubMed] [Google Scholar]
Swartz 2018 {published data only}
- Swartz J, Aronsson B, Lindblad F, Järnbert-Pettersson H, Scheynius A, Pershagen G, et al. Vaccination and allergic sensitization in early childhood - The ALADDIN birth cohort. EClinicalMedicine 2018;4-5:92–8. [DOI: 10.1016/j.eclinm.2018.10.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomson 2010 {published data only}
- Thomson JA, Widjaja C, Darmaputra AA, Lowe A, Matheson MC, Bennett CM, et al. Early childhood infections and immunisation and the development of allergic disease in particular asthma in a high-risk cohort: a prospective study of allergy-prone children from birth to six years. Pediatric Allergy and Immunology 2010;21(7):1076–85. [DOI] [PubMed] [Google Scholar]
Vanura 1994 {published data only}
- Vanura H, Just M, Ambrosch F, Berger RM, Bogaerts H, Wynen J, et al. Study of pertussis vaccines in infants: comparison of response to acellular pertussis DTP vaccines containing 25 micrograms of FHA and either 25 or 8 micrograms of PT with response to whole-cell pertussis DTP vaccine. Vaccine 1994;12(3):210–4. [DOI: 10.1016/0264-410x(94)90196-1] [DOI] [PubMed] [Google Scholar]
Vogt 2014 {published data only}
- Vogt H, Braback L, Kling AM, Grunewald M, Nilsson L. Pertussis immunization in infancy and adolescent asthma medication. Pediatrics 2014;134(4):721–8. [PMID: 10.1542/peds.2014-0723] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2012 {published and unpublished data}
- Perez Chacon G. Re: query: Hib combination vaccine and atopic disorders: a prospective cohort study [personal communication]. Email to P Chen 12 December 2020.
- Wang IJ, Huang LM, Guo YL, Hsieh WS, Lin TJ, Chen PC. Haemophilus influenzae type b combination vaccines and atopic disorders: a prospective cohort study. Journal of the Formosan Medical Association 2012;111(12):711–8. [DOI: 10.1016/j.jfma.2011.09.022] [DOI] [PubMed] [Google Scholar]
Wiersbitzky 1996 {published data only}
- Wiersbitzky S, Bock HL, Bruns R, Clemens R, Graul G, Koditz H, et al. Reactogenicity and immunogenicity of a three-component acellular pertussis DTPa vaccine compared to the conventional whole-cell DTPg vaccine. Monatsschr Kinderheilkd 1996;144(2):159–66. [Google Scholar]
Yamamoto‐Hanada 2020 {published data only}
- Yamamoto-Hanada K, Pak K, Saito-Abe M, Yang L, Sato M, Mezawa H, et al. Cumulative inactivated vaccine exposure and allergy development among children: a birth cohort from Japan. Environmental Health and Preventive Medicine 2020;25(1):27. [DOI: 10.1186/s12199-020-00864-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
217744/025 (DTPa‐HBV‐IPV‐025) {published data only}
- European Medicines Agency. Infanrix Hexa - EPAR- scientific discussion; 2005. Available at www.ema.europa.eu/en/documents/scientific-discussion/infanrix-hexa-epar-scientific-discussion_en.pdf.
- GlaxoSmithKline. A phase III, open randomised clinical study to assess the immunogenicity and reactogenicity of SBBiologicals’ Haemophilus influenzae type b conjugate vaccine co-administered with SB Biologicals’ combined diphtheria tetanus-acellular pertussis-hepatitis B-inactivated polio vaccine(DTPa-HBV-IPV) in one single injection or as two separate injections, in comparison with Pasteur-Mérieux-Connaugh't’s combined diphtheria-tetanus-whole-cell pertussis-inactivated polio Haemophilus influenzae type b vaccine (Pentacoq™) coadministered with SB Biologicals’ hepatitis B vaccine as two concomitant injections in opposite limbs, given to healthy infants as a primary vaccination course at 2, 3 and 4 months of age. No longer available.
Mrozek‐Budzyn 2018 {published data only}
- Jedrychowski W, Whyatt RM, Camann DE, Bawle UV, Peki K, Spengler JD, et al. Effect of prenatal PAH exposure on birth outcomes and neurocognitive development in a cohort of newborns in Poland. Study design and preliminary ambient data. International Journal of Occupational Medicine and Environmental Health 2003;16(1):21–9. [PMID: ] [PubMed] [Google Scholar]
- Mrozek-Budzyn D, Majewska R, Kieltyka A, Augustyniak M. Whole-cell pertussis vaccine (DTwP) has no influence on allergic diseases and atopic sensitization in children. Postepy Dermatologii i Alergologii 2018;35(4):381–6. [DOI: 10.5114/ada.2018.77668] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12617000065392 {published data only}
- ACTRN12617000065392. Optimising immunisation using mixed schedules (OPTIMUM): comparing allergic outcomes in infants following pertussis vaccination [A double-blind, randomised, controlled trial to compare allergic outcomes in children following vaccination with acellular pertussis antigen given at 2 months of age versus whole cell pertussis in the infant vaccine schedule]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000065392 (date submitted 15 December 2016).
ISRCTN17271364 {published data only}
- ISRCTN17271364. A study exploring whooping cough protection in infants following vaccination [A randomised, open label study, exploring the differences in immunogenicity and reactogenicity of infants after immunisation with either an acellular (aP) or whole cell pertussis (wP) vaccine]. isrctn.com/ISRCTN17271364 (date applied 27 January 2020). [DOI: ]
- University of Oxford. A randomised, open label study, exploring the differences in immunogenicity and reactogenicity of infants after immunisation with either an acellular (aP) or whole cell pertussis (wP) vaccine ‐ Pertussis acellular whole cell advanced research (AWARE) study. Available at: www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-001789-13-GB, 2019 2019. [EUCTR-001789-13-GB,]
NCT03606096 {published and unpublished data}
- NCT03606096. Gambia pertussis study (GaPs) [Randomised, double-blinded, open label study in pregnant women, exploring the impact of acellular pertussis vaccination in pregnancy on the immunogenicity in infants randomized to receive either an acellular (aP) or whole cell pertussis (wP) vaccine subsequently]. clinicaltrials.gov/ct2/show/study/NCT03606096 (first posted 30 July 2018).
- Perez Chacon G. Re: GaPs study query! [personal communication]. Email to: B Kampmann 20 March 2019.
Additional references
Aalberse 2019
- Aalberse RC, Grüber C, Ljungman M, Kakat S, Wahn U, Niggemann B, et al. Further investigations of the IgE response to tetanus and diphtheria following covaccination with acellular rather than cellular Bordetella pertussis. Pediatric Allergy and Immunology 2019;30(8):841–7. [DOI: 10.1111/pai.13113] [DOI] [PubMed] [Google Scholar]
Amat 2015
- Amat F, Saint-Pierre P, Bourrat E, Nemni A, Couderc R, Boutmy-Deslandes E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? Results from the ORCA cohort. PLoS One 2015;10(6):e0131369. [DOI: 10.1371/journal.pone.0131369] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ausiello 1997
- Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infection and Immunity 1997;65(6):2168–74. [PMID: 9169747] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balduzzi 2019
- Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Mental Health 2019;22(4):153–60. [DOI: 10.1136/ebmental-2019-300117] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bancroft 2016
- Bancroft T, Dillon MB, da Silva Antunes R, Paul S, Peters B, Crotty S, et al. Th1 versus Th2 T cell polarization by whole-cell and acellular childhood pertussis vaccines persists upon re-immunization in adolescence and adulthood. Cellular Immunology 2016;304-305:35–43. [DOI: 10.1016/j.cellimm.2016.05.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkovic 2006
- Berkovic SF, Harkin L, McMahon JM, Pelekanos JT, Zuberi SM, Wirrell EC, et al. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurology 2006;5(6):488–92. [DOI: 10.1016/S1474-4422(06)70446-X] [DOI] [PubMed] [Google Scholar]
Bousquet 2005
- Bousquet J, Bousquet PJ, Godard P, Daures JP. The public health implications of asthma. Bulletin of the World Health Organization 2005;83(7):548–54. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [DOI: 10.1136/bmj.l6890] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2012
- Centers for Disease Control and Prevention (CDC). Pertussis epidemic – Washington, 2012. MMWR. Morbidity and Mortality Weekly Report 2012;61(28):517–22. [PMID: ] [PubMed] [Google Scholar]
Chow 2016
- Chow MY, Khandaker G, McIntyre P. Global childhood deaths from pertussis: a historical review. Clinical Infectious Diseases 2016;63 Suppl 4:S134–41. [DOI: 10.1093/cid/ciw529] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Methods 2020
- Cochrane Methods. Risk Of Bias In Non-Randomized Studies - of Interventions (ROBINS-I). ROBINS-I Resources and Reporting Guidance 2020:1-14.
Covidence [Computer program]
- Covidence. Version accessed 9 September 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
da Silva Antunes 2018
- da Silva Antunes R, Babor M, Carpenter C, Khalil N, Cortese M, Mentzer AJ, et al. Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters. Journal of Clinical Investigation 2018;128(9):3853–65. [DOI: 10.1172/JCI121309] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
de Graaf 2020
- Graaf H, Ibrahim M, Hill AR, Gbesemete D, Vaughan AT, Gorringe A, et al. Controlled human infection with Bordetella pertussis induces asymptomatic, immunising colonisation. Clinical Infectious Diseases 2020;71(2):403–11. [DOI: 10.1093/cid/ciz840] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunlop 2018
- Dunlop JH, Keet CA. Epidemiology of food allergy. Immunology and Allergy Clinics of North America 2018;38(1):13–25. [PMID: 10.1016/j.iac.2017.09.002.] [DOI] [PubMed] [Google Scholar]
Du Toit 2018
- Du Toit G, Sampson HA, Plaut M, Burks AW, Akdis CA, Lack G. Food allergy: update on prevention and tolerance. Journal of Allergy Clinical Immunology 2018;141(1):30–40. [PMID: 10.1016/j.jaci.2017.11.010] [DOI] [PubMed] [Google Scholar]
Efthimiou 2018
- Efthimiou O. Practical guide to the meta-analysis of rare events. Evidence-Based Mental Health 2018;21(2):72–6. [DOI: 10.1136/eb-2018-102911] [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote X9 [Computer program]
- EndNote. The EndNote Team, Version EndNote X9. Philadelphia, PA: Clarivate, 2013.
EuroPrevall 2016
- Xepapadaki P, Fiocchi A, Grabenhenrich L, Roberts G, Grimshaw KE, Fiandor A, et al. Incidence and natural history of hen's egg allergy in the first 2 years of life-the EuroPrevall birth cohort study. Allergy 2016;71(3):350-7. [DOI: 10.1111/all.12801] [DOI] [PubMed] [Google Scholar]
Gavi 2020
- Gavi, the Vaccine Alliance. Pentavalent vaccine support. www.gavi.org/types-support/vaccine-support/pentavalent (accessed 11 March 2020).
Global Burden of Disease 2018
- Global Burden of Disease Collaborative Network. Global Burden of Disease study 2017 (GBD 2017) results, 2018. ghdx.healthdata.org/gbd-results-tool (accessed 25 March 2020).
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
HealthNuts Study 2011
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. Journal of Allergy and Clinical Immunology 2011;127(3):668–76.e1–2. [DOI: 10.1016/j.jaci.2011.01.039] [DOI] [PubMed] [Google Scholar]
Hedenskog 1989
- Hedenskog S, Björkstén B, Blennow M, Granström G, Granström M. Immunoglobulin E response to pertussis toxin in whooping cough and after immunization with a whole-cell and an acellular pertussis vaccine. International Archives of Allergy and Applied Immunology 1989;89(2-3):156–61. [DOI: 10.1159/000234939] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org 2011.
Higgs 2012
- Higgs R, Higgins SC, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunology 2012;5(5):485–500. [DOI: 10.1038/mi.2012.54] [DOI] [PubMed] [Google Scholar]
Hill 2018
- Hill DA, Spergel JM. The atopic march: critical evidence and clinical relevance. Annals of Allergy, Asthma & Immunology 2018;120(2):131–7. [DOI: 10.1016/j.anai.2017.10.037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holgate 2015
- Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nature Reviews. Disease Primers 2015;1:15025. [DOI: 10.1038/nrdp.2015.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holt 2016
- Holt PG, Snelling T, White OJ, Sly PD, DeKlerk N, Carapetis J, et al. Transiently increased IgE responses in infants and pre-schoolers receiving only acellular Diphtheria-Pertussis-Tetanus (DTaP) vaccines compared to those initially receiving at least one dose of cellular vaccine (DTwP) – immunological curiosity or canary in the mine? Vaccine 2016;34(35):4257–62. [DOI: ] [DOI] [PubMed] [Google Scholar]
ICH 1997
- International Conference on Harmonisation. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) adopts consolidated guideline on good clinical practice in the conduct of clinical trials on medicinal products for human use. International Digest of Health Legislation 1997;48(2):231–4. [PMID: ] [PubMed]
Ierodiakonou 2016
- Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA 2016;316(11):1181–92. [DOI: 10.1001/jama.2016.12623] [DOI] [PubMed] [Google Scholar]
Illi 2004
- Illi S, Mutius E, Lau S, Nickel R, Grüber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. Journal of Allergy and Clinical Immunology 2004;113(5):925–31. [DOI: 10.1016/j.jaci.2004.01.778] [DOI] [PubMed] [Google Scholar]
Keja 1988
- Keja K, Chan C, Hayden G, Henderson RH. Expanded programme on immunization. World Health Statistics Quarterly. Rapport Trimestriel de Statistiques Sanitaires Mondiales 1988;41(2):59–63. [PMID: ] [PubMed] [Google Scholar]
Lefebvre 2021a
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al, on behalf of the Cochrane Information Retrieval Methods Group. Technical supplement to chapter 4: searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Lefebvre 2021b
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Li 2021
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Liko 2013
- Liko J, Robison SG, Cieslak PR. Priming with whole-cell versus acellular pertussis vaccine. New England Journal of Medicine 2013;368(6):581–2. [DOI: 10.1056/NEJMc1212006] [DOI] [PubMed] [Google Scholar]
Mascart 2007
- Mascart F, Hainaut M, Peltier A, Verscheure V, Levy J, Locht C. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine 2007;25(2):391–8. [DOI: 10.1016/j.vaccine.2006.06.046] [DOI] [PubMed] [Google Scholar]
McGuinness 2020
- McGuinness, LA, Higgins, JP. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 2021;12(1):55–61. [DOI: ] [DOI] [PubMed] [Google Scholar]
McIntosh 2010
- McIntosh AM, McMahon J, Dibbens LM, Iona X, Mulley JC, Scheffer IE, et al. Effects of vaccination on onset and outcome of Dravet syndrome: a retrospective study. Lancet Neurology 2010;9(6):592–8. [DOI: 10.1016/S1474-4422(10)70107-1] [DOI] [PubMed] [Google Scholar]
Miller 1993
- Miller D, Madge N, Diamond J, Wadsworth J, Ross E. Pertussis immunisation and serious acute neurological illnesses in children. BMJ 1993;307(6913):1171–6. [DOI: 10.1136/bmj.307.6913.1171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 1998
- Mills KH, Ryan M, Ryan E, Mahon BP. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infection and Immunity 1998;66(2):594–602. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357(9263):1191–4. [PMID: ] [PubMed] [Google Scholar]
NAS 2017
- National Academies of Sciences, Engineering, and Medicine. Finding a path to safety in food allergy: assessment of the global burden, causes, prevention, management, and public policy. Washington, DC: The National Academies Press 2017. [DOI: ] [PubMed]
Navaratna 2021
Newcombe 1998
- Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Statistics in medicine 1998;17(8):873-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nwaru 2014
- Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy 2014;69(1):62-75. [DOI: 10.1111/all.12305] [DOI] [PubMed] [Google Scholar]
Olin 2018
- Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, et al. Stereotypic immune system development in newborn children. Cell 2018;174(5):1277–92.e14. [DOI: 10.1016/j.cell.2018.06.045] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Patterson 2018
- Patterson J, Kagina BM, Gold M, Hussey GD, Muloiwa R. Comparison of adverse events following immunisation with acellular and whole-cell pertussis vaccines: a systematic review. Vaccine 2018;36(40):6007–16. [DOI: 10.1016/j.vaccine.2018.08.022] [DOI] [PubMed] [Google Scholar]
Pavord 2018
- Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al . After asthma: redefining airways diseases. Lancet 2018;391(10118):350-400. [DOI: 10.1016/S0140-6736(17)30879-6] [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991–6. [PMID: 18538991] [DOI] [PubMed] [Google Scholar]
Prescott 2013
- Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. Journal of Allergy and Clinical Immunology 2013;131(1):23–30. [DOI: 10.1016/j.jaci.2012.11.019] [DOI] [PubMed] [Google Scholar]
Punekar 2009
- Punekar YS, Sheikh A. Establishing the sequential progression of multiple allergic diagnoses in a UK birth cohort using the general practice research database. Clinical & Experimental Allergy 2009;39(12):1889–95. [DOI: 10.1111/j.1365-2222.2009.03366.x] [DOI] [PubMed] [Google Scholar]
R [Computer program]
- R: A language and environment for statistical computing. Version 4.0.2. Vienna, Austria: R Foundation for Statistical Computing, 2020. Available at www.R-project.org.
Ray 2006
- Ray P, Hayward J, Michelson D, Lewis E, Schwalbe J, Black S, et al. Encephalopathy after whole-cell pertussis or measles vaccination: lack of evidence for a causal association in a retrospective case-control study. Pediatric Infectious Disease Journal 2006;25(9):768–73. [PMID: 16940831] [DOI] [PubMed] [Google Scholar]
Reeves 2021
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA, on behalf of the Cochrane Non-Randomized Studies of Interventions Methods Group. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
RevMan Web [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Roduit 2017
- Roduit C, Frei R, Depner M, Karvonen AM, Renz H, Braun-Fahrländer C, et al. Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatrics 2017;17(7):655–62. [DOI: 10.1001/jamapediatrics.2017.0556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2000
- Rowe J, Macaubas C, Monger TM, Holt BJ, Harvey J, Poolman JT, et al. Antigen-specific responses to diphtheria-tetanus-acellular pertussis vaccine in human infants are initially Th2 polarized. Infection and Immunity 2000;68(7):3873–7. [DOI: 10.1128/iai.68.7.3873-3877.2000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of evidence. Cochrane Consumers and Communication Group, available at http://cccrg.cochrane.org/author-resources. Version 3.0, December 2016.
Sato 1984
- Sato Y, Kimura M, Fukumi H. Development of a pertussis component vaccine in Japan. Lancet 1984;1(8369):122–6. [PMID: 6140441] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013;4:49–62. [DOI: 10.1002/jrsm.1078 ] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al, on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Sheridan 2012
- Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 2012;308(5):454–6. [DOI: 10.1001/jama.2012.6364] [DOI] [PubMed] [Google Scholar]
Simpson 2010
- Simpson A, Tan VY, Winn J, Svensén M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. American Journal of Respiratory and Critical Care Medicine 2010;181(11):1200–6. [DOI: 10.1164/rccm.200907-1101OC] [DOI] [PubMed] [Google Scholar]
Steenhuis 2008
- Steenhuis TJ, Aalderen WM, Bloksma N, Nijkamp FP, vander Laag J, Loveren H, et al. Bacille-Calmette-Guerinvaccination and the development of allergic disease in children: a randomized, prospective, single-blind study. Clinical and Experimental Allergy 2008;38(1):79–85. [DOI: 10.1111/ j.1365-2222.2007.02859.x] [DOI] [PubMed] [Google Scholar]
Sterne 2016a
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI: 10.1136/bmj.i4919 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016b
- Sterne JA, Higgins JP, Elbers RG, Reeves BC and the development group for ROBINS-I. Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I): detailed guidance, updated 12 October 2016. Available from http://www.riskofbias.info [accessed before April 2021].
Sterne 2021
- Sterne JA, Hernán MA, McAleenan A, Reeves BC, Higgins JP. Chapter 25: Assessing risk of bias in a non-randomized study. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Thøstesen 2018
- Thøstesen LM, KjaergaardJ, Pihl GT, Birk NM, NissenTN, Aaby P, et al. Neonatal BCG vaccination and atopic dermatitis before 13 months of age: a randomized clinical trial. Allergy 2018;73(2):498–504. [DOI: 10.1111/all.13314] [DOI] [PubMed] [Google Scholar]
UNICEF 2017
- United Nations Children's Fund Supply Division. Pentavalent vaccine (DTwP-HepB-Hib): market and supply update. www.unicef.org/supply/reports/pentavalent-vaccinedtwp-hepb-hib-market-and-supply-update (accessed 13 July 2020).
van der Lee 2018
- Lee S, Hendrikx LH, Sanders EA, Berbers GA, Buisman A-M. Whole-cell or acellular pertussis primary immunizations in infancy determines adolescent cellular immune profiles. Frontiers in Immunology 2018;9:51. [DOI: 10.3389/fimmu.2018.00051] [DOI] [PMC free article] [PubMed] [Google Scholar]
Viechtbauer 2010
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 2010;36(3):1–48. [Google Scholar]
Waffenschmidt 2020
- Waffenschmidt S, Navarro-Ruan T, Hobson N, Hausner E, Sauerland S, Haynes RB. Development and validation of study filters for identifying controlled non-randomized studies in PubMed and Ovid MEDLINE. Research Synthesis Methods 2020;11(5):617–26. [DOI: 10.1002/jrsm.1425] [DOI] [PubMed] [Google Scholar]
Warfel 2014
- Warfel JM, Zimmerman LI, Merkel, TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proceedings of the National Academy of Sciences of the United States of America 2014;111(2):787–92. [DOI: 10.1073/pnas.1314688110] [DOI] [PMC free article] [PubMed] [Google Scholar]
WebPlotDigitizer 2020 [Computer program]
- WebPlotDigitizer. Version 4.4. Rohatgi A, 2020. Available at automeris.io/WebPlotDigitizer.
WHO 2015
- World Health Organization. Pertussis vaccine position paper. Whole cell pertussis vaccines: summary of evidence relevant to schedules. www.who.int/immunization/sage/meetings/2015/april/2_wP_summary_WG_23Mar2015_submitted.pdf?ua= (accessed 13 July 2020).
WHO 2015a
- World Health Organization. Pertussis vaccines: WHO position paper – September 2015. Weekly Epidemiological Record 2015;90(35):433–58. [PMID: ] [PubMed]
WHO 2015b
- World Health Organization. Pertussis vaccines position paper. GRADE Table 2. Safety of pertussis vaccines in immunocompetent infants and children. www.who.int/immunization/position_papers/PertussisGradeTable2.pdf?ua=1 (accessed 11 April 2021).
WHO 2015c
- World Health Organization. Pertussis vaccines position paper. Table III: Pertussis vaccine evidence to recommendations table. www.who.int/immunization/position_papers/PertussisGradeTable3.pdf?ua=1 (accessed 11 April 2021).
Wickham 2020
- Wickham H, François R, Henry L, Müller K. dplyr: a grammar of data manipulation. R package version 1.0.2. Available at: CRAN.R-project.org/package=dplyr 2020.
World Bank 2021
- Historical classification by income in XLS format. Available at http://databank.worldbank.org/data/download/site-content/OGHIST.xls (accessed before 21 March 2020).
Zhang 2014
- Zhang L, Prietsch SO, Axelsson I, Halperin, SA. Acellular vaccines for preventing whooping cough in children. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD001478. [DOI: 10.1002/14651858.CD001478.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Perez Chacon 2020
- Perez Chacon G, Estcourt M, Ramsay J, Brennan-Jones C, Holt P, Snelling T. Whole-cell pertussis vaccine in infancy for the prevention of allergy. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013682. [DOI: 10.1002/14651858.CD013682] [DOI] [PMC free article] [PubMed] [Google Scholar]