Abstract
Background
Effective and applicable predictors of non-alcoholic fatty liver disease (NAFLD) are needed for the non-obese Chinese population. This study was undertaken to investigate: whether serum gamma-glutamyl transferase (GGT) was associated with incident NAFLD in the non-obese Chinese population.
Methods
This was a retrospective cohort study that enrolled 33,153 initially NAFLD-free individuals who underwent a health examination in Wenzhou Medical Center of Wenzhou People’s Hospital from January 2010 to December 2014.
Serum GGT levels at the time of enrollment were evaluated in 11,906 persons who follow-up. The relationship between GGT levels and incident NAFLD was analyzed using Cox regression and generalized additive models after adjusting for demographic and clinical variables. In addition, Subgroup analysis was conducted, which was explored by Cox proportional hazard models. It was stated that the data had been downloaded from the DATADRYAD website.
Results
Multivariable Cox regression models were used to estimate the hazard ratio (HR) for GGT with incident NAFLD after adjusted demographic and clinical variables (HR, 1.010; 95% CI, 1.007–1.012; P < 0.001). The incident NAFLD in the highest quartile of GGT levels was 3.653 times as high (95% confidence interval, 2.915 to 4.579) as that the lowest quartile. A non-linear relationship was firstly detected between GGT and incidence of NAFLD, which had an inflection point of GGT was 26 U/L. The effect sizes and the confidence intervals on the left and right sides of the inflection point were 1.104 (1.089–1.120) and 1.001 (0.999–1.004), respectively. In subgroup analyses, the hazard ratio for incident NAFLD remained consistent across subgroups.
Conclusions
In conclusion, the GGT level in the non-obese Chinese population was statistically significantly associated with incident NAFLD. The relationship between GGT level and incident NAFLD is non-linear. When GGT level is less than 26 U/L, GGT was strong positively with incident NAFLD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-021-01577-8.
Keywords: Gamma-Glutamyl transferase, Incident non-alcoholic fatty liver disease, Nonlinearity, Inflection point
Background
Non-alcoholic fatty liver disease (NAFLD) is the excessive deposition of non-alcohol fat in the liver, leading to non-alcohol steatohepatitis [1, 2]. It is a growing public health burden affecting approximately one-quarter of adults worldwide [3, 4] and is considered to be the main cause of liver-related morbidity and mortality [2, 5]. Several diseases including insulin resistance further contribute to the progression of NAFLD, including type2 diabetes and metabolic syndrome.
Gamma-glutamyltransferase (GGT), which is secreted mainly by the liver, has been regarded as a biomarker of liver disease [6]. Decreased serum GGT levels are associated with the improvement of liver histology [7]. Weight loss reduces the GGT level in patients with NAFLD [8]. In Chinese, recent studies explore the decreased GGT levels associated with the improvement of metabolic disturbances after the routine treatment of NAFLD [3]. Previous research reported that the GGT level is a marker of NAFLD in patients with metabolic syndrome [9]. Up to 80% of NAFLD patients were caused by obese [10]. However, the percentage of NAFLD in lean or non-obese patients is increasing. A better biomarker to predict the progression of NAFLD is needed to be explored. The relationship between the baseline GGT and incident NAFLD should be elucidated.
In the present research, we postulate that GGT may serve as an early predictor for the incident NAFLD in the non-obese Chinese population. To test the hypothesis, we used the previously published data for secondary data analysis. We explored the relationship between GGT levels and the risk of NAFLD in the non-obese Chinese population. In addition, a generalized additive model (GAM) is further applied to study the curve relationship between GGT level and NAFLD.
Methods
Study design and participants
The original data is obtained free of charge from the “DATADRYAD” database. The rationale and design have been described in detail previously [11]. This was a longitudinal study conducted from January 2010 to December 2014, which enrolled 33,153 NAFLD-free individuals who underwent a health examination in Wenzhou Medical Center of Wenzhou People’s Hospital. Individuals were excluded if they reported excess alcohol consumption (> 140 g/week for men and > 70 g/week for women) [11, 12], or had a history of viral hepatitis, autoimmune hepatitis, or other known causes of chronic liver disease; a body mass index (BMI) of ≥25 kg/m2; a low-density lipoprotein cholesterol (LDL-C) of > 3.12 mmol/L; were taking antihypertensive agents, anti-diabetic agents or lipid-lowing agents; and were lost to follow-up or their data were missing.
According to this study, of these 16,173 participants, 4267 were excluded according to the exclusion criteria. Individuals were excluded if the GGT value was missing or an extreme value (the extreme value is defined as the mean plus/minus three standard deviations) [13]. This study included 11,906 non-obese individuals without NAFLD. Approximately 2044 participants developed NAFLD during follow-up.
Diagnosis of NAFLD
The ultrasound diagnostic criteria for NAFLD have been described in detail previously [14].
Data collection
Baseline clinical examinations were performed as described previously [11]. The biochemical measurements were measured by an automated analyzer (Abbott AxSYM) using standard methods.
Follow-up and outcome definitions
During the observation period, a follow-up evaluation will be carried out annually. The outcome was the incident NAFLD, diagnosed by ultrasonic examination.
Statistical analysis
The purpose of multiple imputations was to generate possible values for missing values, which had been used in multivariate data sets. The participants were stratified by quartiles of GGT. Continuous variables were expressed as the means ± standard deviations or as medians and interquartile ranges, and categorical variables were expressed as proportions. Categorical variables were compared by chi-square test, and continuous variables were compared by one-way analysis of variance or Kruskal-Wallis.
The Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of the NAFLD risk associated with GGT levels, without and with controlling for the impact of key clinical confounding variables. A sensitivity analysis was conducted by converted the GGT into a categorical variable. A smooth curve fitting (penalty curve method) and a GAM were applied to address the relationship of GGT and the incident NAFLD. As the non-linearity relationship was observed, a two-piecewise linear regression model was performed to calculate the threshold effect of the GGT on the incident NAFLD in terms of the smoothing plot. The best-fitting model was determined based on the P value of the log of the likelihood ratio. A subgroup analysis was conducted, which was explored by Cox proportional hazard models with adjustment. NAFLD-free survival curves were used by the Kaplan-Meier method. The log-rank test was used to compare the Kaplan–Meier hazard ratios. The analyses were performed by package R (http://www.R-project.org, The R Foundation) and Empower-Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). Two-tailed P values of less than 0.05 were considered to indicate statistical significance.
Results
Study participants and the baseline characteristics
11,906 participates (54.69% male and 45.31% female) who met the inclusion standards were enrolled (Fig.1). Participant’s age varied from 14 to 95 years old, with an average of 43.26 years old. 2044 participants developed NAFLD during follow-up. The baseline characteristics were stratified by the quartiles of the GGT (<16 U/L, 16-21 U/L, 21-31 U/L, > 31 U/L), which were shown in Table 1. Participants with the highest GGT had higher BMI, SBP, DBP, FPG, UA, TC, LDL-C, Cr, ALT and AST.
Fig. 1.
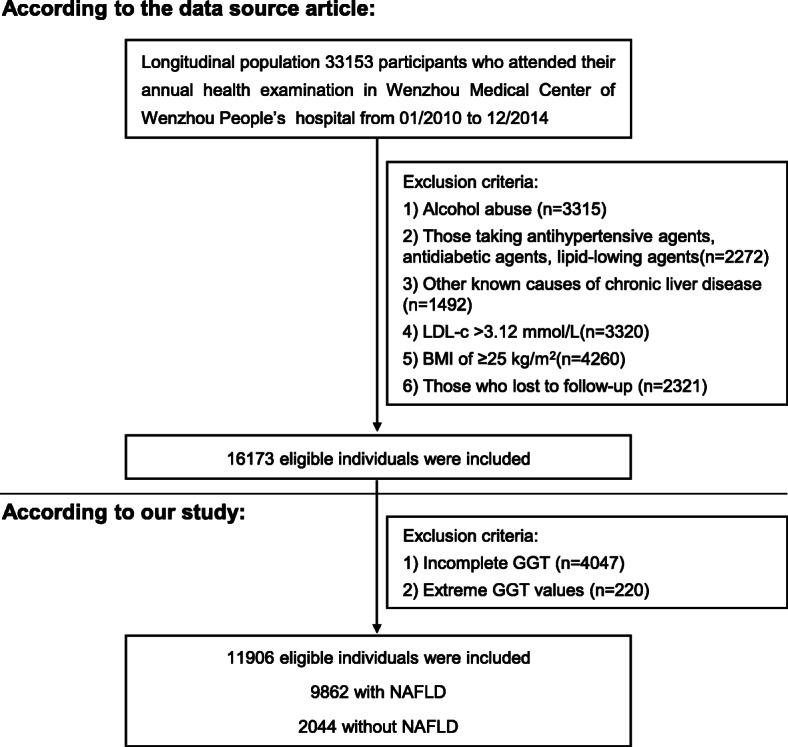
Study design and participant flow
Table 1.
The baseline characteristics of participants
| GGT (U/L) | Q1 (<16) | Q2 (16 to<21) | Q3 (21 to<31) | Q4 (≥31) | P-value |
|---|---|---|---|---|---|
| Participants | 2401 | 3077 | 3448 | 2980 | |
| Age, years | 42.63 ± 14.96 | 43.06 ± 15.01 | 43.39 ± 15.12 | 43.81 ± 14.63 | 0.028 |
| Gender -n (%) | < 0.001 | ||||
| Female | 1231 (51.27%) | 1416 (46.02%) | 1524 (44.20%) | 1224 (41.07%) | |
| Male | 1170 (48.73%) | 1661 (53.98%) | 1924 (55.80%) | 1756 (58.93%) | |
| BMI (kg/m2) | 20.76 ± 1.93 | 21.17 ± 2.01 | 21.84 ± 2.01 | 22.42 ± 1.81 | < 0.001 |
| SBP (mmHg) | 115.36 ± 15.70 | 119.93 ± 16.52 | 124.32 ± 16.26 | 127.66 ± 16.55 | < 0.001 |
| DBP (mmHg) | 69.48 ± 9.39 | 72.20 ± 9.75 | 74.92 ± 10.14 | 77.11 ± 10.38 | < 0.001 |
| TC (mmol/L) | 4.42 ± 0.70 | 4.54 ± 0.72 | 4.62 ± 0.72 | 4.79 ± 0.77 | < 0.001 |
| TG (mmol/L) | 0.89 (0.70–1.14) | 1.01 (0.79–1.33) | 1.22 (0.92–1.65) | 1.52 (1.11–2.16) | < 0.001 |
| HDL-c (mmol/L) | 1.55 ± 0.35 | 1.49 ± 0.35 | 1.41 ± 0.35 | 1.36 ± 0.35 | < 0.001 |
| LDL-c (mmol/L) | 2.13 ± 0.45 | 2.24 ± 0.47 | 2.32 ± 0.47 | 2.36 ± 0.46 | < 0.001 |
| ALT (U/L) | 12.00 (10.00–16.00) | 14.00 (11.00–18.00) | 17.00 (14.00–23.00) | 23.00 (17.00–32.00) | < 0.001 |
| AST (U/L) | 19.00 (17.00–22.00) | 20.00 (18.00–23.00) | 21.50 (19.00–25.00) | 24.00 (21.00–29.00) | < 0.001 |
| ALP (mmol/L) | 62.52 ± 17.67 | 68.98 ± 19.42 | 74.28 ± 21.08 | 79.31 ± 25.74 | < 0.001 |
| ALB (U/L) | 44.15 ± 2.75 | 44.49 ± 2.73 | 44.70 ± 2.78 | 44.71 ± 2.79 | < 0.001 |
| GLB (g/L) | 29.01 ± 3.99 | 29.35 ± 3.97 | 29.39 ± 3.82 | 29.45 ± 4.07 | < 0.001 |
| TB (umol/L) | 10.90 (8.30–14.10) | 11.02 (8.40–14.30) | 11.70 (8.91–14.90) | 11.80 (9.20–15.20) | < 0.001 |
| DBIL (umol/L) | 2.00 (1.40–2.75) | 2.02 (1.44–2.78) | 2.10 (1.50–2.80) | 2.08 (1.44–2.83) | 0.211 |
| BUN (mmol/L) | 4.34 ± 1.39 | 4.51 ± 1.33 | 4.67 ± 1.33 | 4.77 ± 1.55 | < 0.001 |
| Cr (umol/L) | 74.30 ± 18.51 | 80.15 ± 19.96 | 86.74 ± 20.85 | 90.12 ± 34.70 | < 0.001 |
| UA (umol//L) | 240.63 ± 78.66 | 273.12 ± 83.65 | 303.91 ± 79.35 | 339.09 ± 84.52 | < 0.001 |
| FBG (mmol/L) | 5.02 ± 0.54 | 5.13 ± 0.68 | 5.24 ± 0.83 | 5.39 ± 1.06 | < 0.001 |
ALB albumin, ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, BUN blood urea nitrogen, Cr creatinine, DBIL Direct Bilirubin, DBP diastolic blood pressure, FPG fasting plasma glucose, GGT γ-glutamyl transpeptidase, GLB globulin, HDL-c high-density lipoprotein cholesterol, LDL-c low-density lipoprotein cholesterol, SBP systolic blood pressure, TB total bilirubin, TC total cholesterol, TG triglyceride, UA uric acid
Univariate analyses
Perform univariate Cox proportional hazards analysis to compare the role of GGT and other variables in predicting NAFLD (Table 2). In univariate analysis, the results showed that age, BMI, TC, TG, LDL, SBP, DBP, AST, ALT, ALP, BUN, Cr, UA and FBG were positively correlated with the probability of NAFLD.
Table 2.
The results of univariate COX regression
| Statistics | HR(95%CI) | P-value | |
|---|---|---|---|
| Age, years | 43.26 ± 14.94 | 1.01 (1.00, 1.01) | < 0.001 |
| Gender -n (%) | 0.2083 | ||
| Female | 5395 (45.31%) | Ref | |
| Male | 6511 (54.69%) | 1.06 (0.97, 1.15) | |
| BMI (kg/m2) | 21.60 ± 2.04 | 1.68 (1.63, 1.73) | < 0.001 |
| SBP (mmHg) | 122.22 ± 16.88 | 1.01 (1.01, 1.02) | < 0.001 |
| DBP (mmHg) | 73.67 ± 10.33 | 1.03 (1.03, 1.04) | < 0.001 |
| TC (mmol/L) | 4.60 ± 0.74 | 1.28 (1.22, 1.35) | < 0.001 |
| TG (mmol/L) | 1.36 ± 0.92 | 1.19 (1.17, 1.20) | < 0.001 |
| HDL-c (mmol/L) | 1.45 ± 0.36 | 0.27 (0.23, 0.31) | < 0.001 |
| LDL-c (mmol/L) | 2.27 ± 0.47 | 1.78 (1.61, 1.97) | < 0.001 |
| ALT (U/L) | 19.59 ± 15.49 | 1.01 (1.01, 1.01) | < 0.001 |
| AST (U/L) | 22.72 ± 8.78 | 1.01 (1.01, 1.01) | < 0.001 |
| ALP (mmol/L) | 71.80 ± 22.14 | 1.01 (1.01, 1.01) | < 0.001 |
| ALB (U/L) | 44.54 ± 2.77 | 0.99 (0.98, 1.01) | 0.4209 |
| GLB (g/L) | 29.32 ± 3.96 | 1.02 (1.01, 1.03) | < 0.001 |
| TB (umol/L) | 12.04 ± 5.07 | 0.99 (0.99, 1.00) | 0.1830 |
| DBIL (umol/L) | 2.22 ± 1.17 | 0.68 (0.65, 0.71) | 0.437 |
| BUN (mmol/L) | 4.58 ± 1.41 | 0.88 (0.85, 0.91) | < 0.001 |
| Cr (umol/L) | 83.37 ± 25.16 | 1.00 (1.00, 1.00) | < 0.001 |
| UA (umol//L) | 292.00 ± 88.80 | 1.00 (1.00, 1.00) | < 0.001 |
| FBG (mmol/L) | 5.21 ± 0.82 | 1.22 (1.19, 1.25) | < 0.001 |
Relationship between GGT levels and incident NAFLD during follow-up
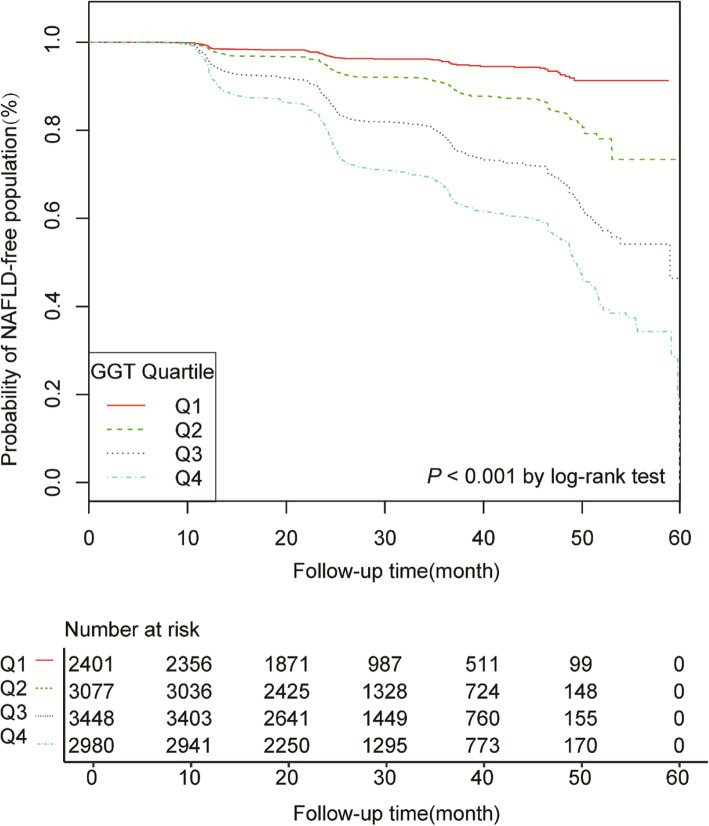
Among 11,906 participants of the study cohort who had a median follow-up period of 24.97 (21.88–38.10) months. NAFLD developed in 2044 participants (17.17%) during follow-up. Higher GGT levels at baseline are associated with higher incidence of NAFLD (P < 0.001) (Fig.2). The rate of NAFLD was 35.22% at 2 years among participants with a GGT level of at least 21 U/L (third and fourth quartiles), compared with 8.82% among participants with a GGT level of less than 21 U/L (first and second quartiles).
Fig. 2.
Kaplan–Meier analysis of NAFLD-free population based on GGT quartiles
Participants with a GGT level in the third quartile had a risk of incident NAFLD that was 2.88 times (HR 2.88, 95%CI 2.310, 3.609) that in the first quartile, and participants in the fourth quartile had a risk that was 3.653 times (HR 3.653, 95%CI 2.915, 4.579) that in the first quartile (Table 3). This association of GGT and NAFLD persisted despite adjustment for age, gender, BMI, SBP and DBP (HR 1.013, 95%CI 1.011, 1.015) (model I) or inclusion of the baseline mentioned above characteristics and TC, TG, HDL-C, LDL-C, ALT, AST, ALP, ALB, GLB, TB, DBIL, BUN, Cr, UA and FBG (model II) (HR 1.010, 95%CI 1.007, 1.012) (Table 3). Then use GAM to insert the continuity covariate as a curve into the equation, which is basically consistent with Model II (HR 1.004; 95% CI 1.004, 1.007), which proves the robustness of the results (Table 3).
Table 3.
Relationship between GGT and the incident NAFLD in different models
| Variable | Crude model (HR, 95%CI, P) |
Model I (HR, 95% CI, P) |
Model II (HR, 95% CI, P) |
GAM (HR, 95% CI, P) |
|---|---|---|---|---|
| GGT | 1.020 (1.018–1.022)<0.001 | 1.013 (1.011, 1.015) <0.001 | 1.010 (1.007, 1.012)<0.001 | 1.004 (1.002, 1.007) <0.001 |
| GGT (quartile) | ||||
| Q1 | Ref | Ref | Ref | Ref |
| Q2 | 2.176 (1.717, 2.757)<0.001 | 1.809 (1.427, 2.293)<0.001 | 1.661 (1.308, 2.108)<0.001 | 1.402 (1.100, 1.785) <0.001 |
| Q3 | 5.066 (4.075, 6.298)<0.001 | 3.335 (2.678, 4.154)<0.001 | 2.888 (2.310, 3.609)<0.001 | 2.103 (1.671, 2.648) <0.001 |
| Q4 | 8.202 (6.625, 10.156)<0.001 | 4.548 (3.661, 5.650)<0.001 | 3.653 (2.915, 4.579)<0.001 | 2.347 (1.853, 2.973)<0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
The analyses of the non-liner relationship
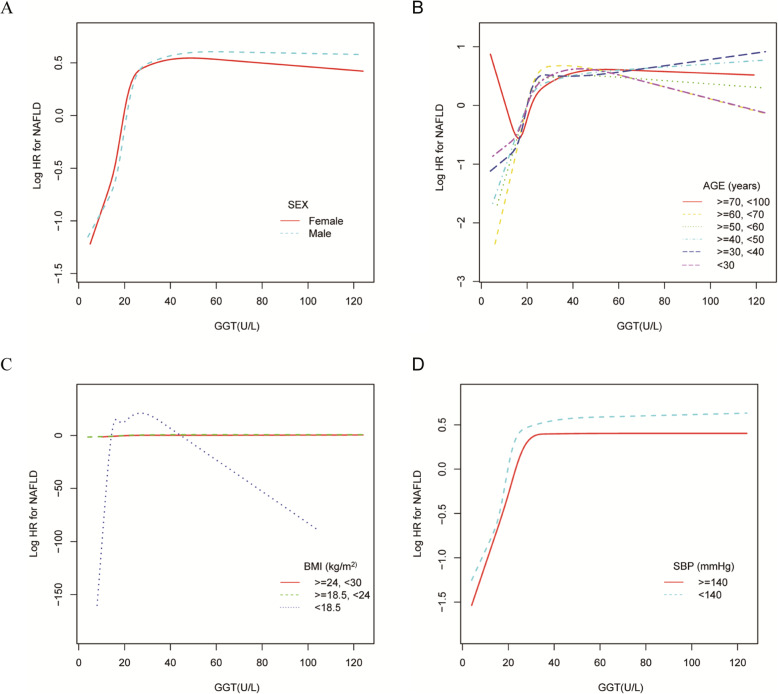
The GGM and smooth curve fitting were applied to study the relationship between GGT level and the incidence NAFLD. Therefore, a non-linear relationship of GGT level with incidence NAFLD was detected adjusting for confounding variables, which has not been detected in previous studies. (Fig.3). Meanwhile, the curve fitting graphs were made in the subgroup analysis (Fig.4 and Supplemental Fig. 2). The two-piecewise linear regression model and recursive algorithm calculated the inflection point of GGT (Log-likelihood ratio test P < 0.001). When GGT levels were ≤ 26 U/L, a 1 U/L increase in GGT level is accompanied by a 10.4% increase in HR for NAFLD (HR 1.104; 95% CI 1.089, 1.120) and when GGT levels were >26 U/L, GGT have not been observed to be associated with incident NAFLD (HR 1.001; 95% CI 0.999, 1.004, P: 0.364) (Table 4). There was a threshold effect between GGT and incident NAFLD.
Fig. 3.
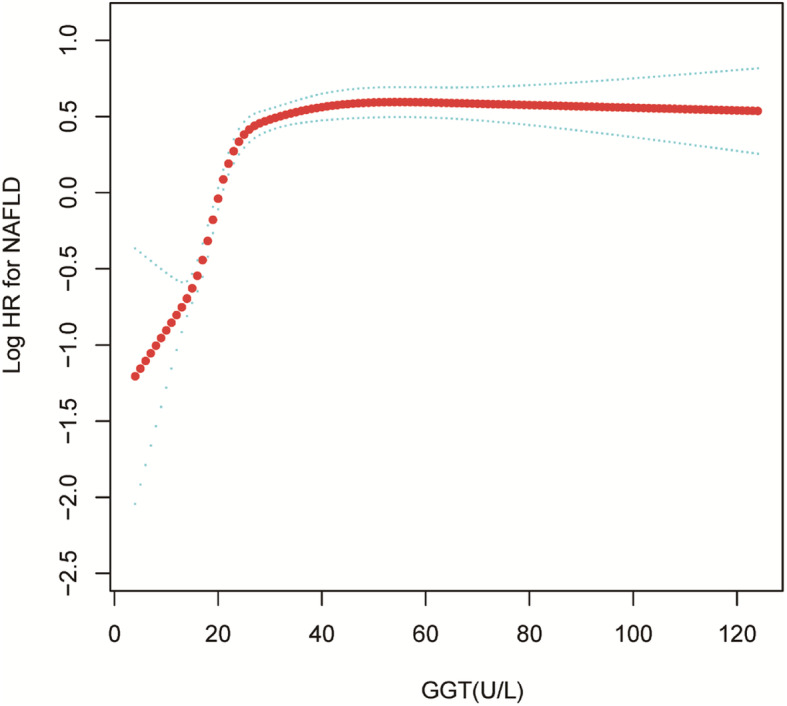
Association of GGT with the risk of NAFLD. The non-linear relationship between GGT and incident of NAFLD. A nonlinear relationship between them was detected after adjusting for age, gender, BMI, SBP, DBP, TC, TG, HDL-C, LDL-C, ALT, AST, ALP, ALB, GLB, TB, DBIL, BUN, Cr, UA, FBG
Fig. 4.
Association of GGT with the risk of NAFLD in subgroups analysis The non-linear relationship between GGT and incident of NAFLD according to subgroups in the adjusted analysis. Note 1: Above model adjusted for age, gender, BMI, SBP, DBP,, TC, TG, HDL-C, LDL-C, ALT, AST, ALP, ALB, GLB, TB, DBIL, BUN
Table 4.
The result of two-piecewise linear regression model
| Incident NAFLD | HR (95% CI) | P |
|---|---|---|
| Fitting model by standard linear regression | 1.010 (1.007–1.012) | <0.001 |
| Fitting model by two-piecewise linear regression | ||
| Inflection point of GGT | ||
| ≤26 U/L | 1.104 (1.089–1.120) | <0.001 |
| > 26 U/L | 1.001 (0.999–1.004) | 0.3640 |
| P for log likelihood ratio test | <0.001 | |
We adjusted age, gender, BMI, SBP, DBP, TC, TG, HDL-C, LDL-C, ALT, AST, ALP, ALB, GLB, TB, DBIL, BUN, Cr, UA, FBG
CI Confidence interval, HR hazard ratio, NAFLD non-alcoholic fatty liver disease, GGT γ-glutamyl transpeptidase
The results of subgroup analyses
Sensitivity analyses were also performed to determine whether age, gender, BMI, SBP, DBP, HDL-C, ALT, Cr, UA, and FBG influenced the relationship between the GGT level and the incidence of NAFLD. In subgroup analyses, the hazard ratio for incident NAFLD remained consistent across subgroups (Table 5). These findings suggest that GGT is independently associated with the incidence of NAFLD.
Table 5.
Effect size of GGT on NAFLD in prespecified and exploratory subgroups
| Characteristic | No of participants | HR (95%CI) | P value | P for interacion |
|---|---|---|---|---|
| Age, years | 0.5082 | |||
| < 30 | 2251 | 1.005 (0.999, 1.010) | 0.1221 | |
| 30 to < 40 | 3549 | 1.010 (1.006, 1.014) | < 0.001 | |
| 40 to < 50 | 2700 | 1.011 (1.006, 1.016) | < 0.001 | |
| 50 to < 60 | 1570 | 1.009 (1.003, 1.015) | 0.003 | |
| 60 to < 70 | 826 | 1.005 (0.996, 1.014) | 0.272 | |
| ≥ 70 | 1010 | 1.010 (1.003, 1.018) | 0.009 | |
| Gender | 0.2162 | |||
| Female | 5395 | 1.008 (1.005–1.011) | < 0.001 | |
| Male | 6511 | 1.011 (1.008–1.013) | < 0.001 | |
| BMI (kg/m2) | 0.1886 | |||
| < 18.5 | 942 | 1.038 (0.932, 1.155) | 0.499 | |
| ≥ 18.5, < 24 | 9377 | 1.013 (1.010, 1.015) | < 0.001 | |
| ≥ 24 | 1587 | 1.009 (1.005, 1.012) | < 0.001 | |
| SBP (mmHg) | 0.9399 | |||
| < 140 | 10,188 | 1.009 (1.007–1.012) | < 0.001 | |
| ≥ 140 | 1718 | 1.010 (1.005–1.014) | < 0.001 | |
| DBP (mmHg) | 0.7567 | |||
| < 90 | 10,941 | 1.010 (1.007–1.012) | < 0.001 | |
| ≥ 90 | 965 | 1.010 (1.005–1.016) | < 0.001 | |
| HDL-c (mmol/L) | 0.6828 | |||
| <1 | 1014 | 1.007 (1.001, 1.013) | 0.0179 | |
| ≥ 1 | 10,892 | 1.009 (1.006, 1.011) | < 0.001 | |
| ALT (U/L) | 0.0011 | |||
| ≤ 40 | 11,314 | 1.012 (1.010, 1.015) | < 0.001 | |
| >40 | 592 | 1.003 (0.998, 1.008) | 0.1828 | |
| Cr (mmol/L) | 0.0769 | |||
| < 82 | 5919 | 1.007 (1.003, 1.010) | < 0.001 | |
| ≥ 82 | 5987 | 1.011 (1.008, 1.014) | < 0.001 | |
| UA (umol//L) | 0.3937 | |||
| <420 | 10,962 | 1.010 (1.008, 1.012) | < 0.001 | |
| ≥ 420 | 944 | 1.007 (1.002, 1.013) | 0.007 | |
| FBG (mmol/L) | 0.2557 | |||
| <6.1 | 11,074 | 1.010 (1.008, 1.013) | < 0.001 | |
| ≥ 6.1 | 832 | 1.007 (1.002, 1.012) | 0.009 | |
Note 1: Above model adjusted for age, gender, BMI, SBP, DBP,, TC, TG, HDL-C, LDL-C, ALT, AST, ALP, ALB, GLB, TB, DBIL, BUN, Cr, UA, FBG.
Note 2: In each case, the model is not adjusted for the stratification variable
Discussion
This study in the non-obese Chinese population provided evidence that the baseline GGT level was associated with incident NAFLD during follow-up. Concurrently, a saturation effect was detected between the GGT level and incident NAFLD, with an inflection point at 26 U/L. Moreover, in multivariate analysis of the subgroup, GGT was also as an independent biomarker to predict the incidence of NAFLD.
NAFLD is a world public health problem [15], which is accompanied by liver dysfunction. The abnormal of liver enzymes are the markers of NAFLD in the general population [16]. As reports, the abnormal GGT is associated with the future development of the fatty liver [17]. This study showed that the baseline GGT predicted the incident NAFLD during follow in the non-obese Chinese population, which supports the previous similar studies. From a traditional perspective, central obesity and metabolic syndrome increase the risk of NAFLD [18, 19]. However, the percentage of non-obese patients with NAFLD is now growing [20]. This study first investigated the association between GGT and NAFLD in a large non-obese Chinese population. Previous studies used propensity scores to divide GGT into two categorical variables, which would greatly damage the variable’s information [9]. Moreover, previous studies did not further explore the possible curvilinear relationship between GGT and NAFLD. On the contrary, as a continuous variable, GGT (increase by 1 U/L) has a significant association with NAFLD after adjusting for confounding variables (HR = 1.010, 95% CI 1.007, 1.012). The highest quartile of GGT was associated with increased risk for NAFLD by 2.653-fold compared with the lowest quartile. Although the conclusions are consistent with previous studies, they are more ideal from the perspective of information preservation. Meanwhile, this study conducted in a large sample of the non-obese Chinese population, and the results of the study are expected to be successfully promoted among the Chinese population undergoing a physical examination. Elevated GGT reminds the population of the high risk of NAFLD during follow up, which will be an alarm for people to adjust their living habits in advance to reduce the incidence of NAFLD [21].
A GAM and smooth curve fitting were applied to study the nonlinear relation between GGT level and incident NAFLD. Two piecewise linear regressions were used to determine the relationship in detail. In the present study, the inflection point was 26 U/L after adjusting the confounding variables (including age, gender, BMI, SBP, DBP, TC, TG, HDL-C, LDL-C, ALT, AST, ALP, ALB, GLB, TB, DBIL, BUN, Cr, UA, FBG). When GGT levels were ≤ 26 U/L, a 1 U/L increase in the GGT level was associated with a 10.4% greater adjusted HR of incident NAFLD (HR 1.104; 95% CI 1.12, 5.87), and when GGT levels were >26 U/L, no relationship was observed with incident NAFLD.
In the highest baseline GGT quartile, many potentially alcohol-related variables were abnormal. The smooth curve fitting was used to show the relationship between GGT and liver examination, blood pressure and blood lipids (Supplemental Fig. 1). GGT level was positively correlated with the liver examination, blood pressure and blood lipids, the above indicators are potential alcohol-related variables, which are the highest among the highest baseline GGT quartiles. This may be related to excessive alcohol intake. However, previous research demonstrated that the GGT was strongly positively correlated to total and LDL cholesterol, triglycerides in young healthy adults [22]. The serum GGT was positively associated with uric acid, SBP, and DBP in normotensive Chinese adults. In middle-aged and elderly (females who were > 40 years old) Chinese females, the serum GGT level is associated with the UA level [23]. The above results suggest that the abnormality of these indicators (liver examination, blood pressure and blood lipids) may also be related to metabolism. The curve fitting graphs were applied in the subgroup analysis (Fig.4 and Supplemental Fig. 2). The results suggest that BMI may affect the relationship between GGT and NAFLD. However, in this study, multivariable Cox regression models were used to estimate the hazard ratio (HR) for GGT with incident NAFLD after adjusting for confounding variables (including SBP, DBP, TC, LDL, UA and drinking status), which showed GGT is an independent risk factor for NAFLD. Furthermore, the hazard ratio for incident NAFLD remained consistent across subgroups in sensitivity analysis.
Comparisons with other studies and what does the current work add to the existing knowledge
Previous studies have shown that elevated GGT levels are the risk of NAFLD in patients with metabolic syndrome or obesity [24, 25]. However, this study was conducted in a large cohort of non-obese Chinese, allowing for subgroup analysis and adjustment of clinical confounding variables. Meantime, the non-linear relationship between NAFLD and GGT levels was investigated in different populations (with GGT above or below the inflection point). The inflection point firstly provides evidence for the management of GGT in non-obese Chinese population for the first time. Therefore, this analysis has more excellent clinical value.
Study strength and limitations
The present study has some advantages. Firstly, GGT is used as a categorical variable and continuous variable for statistical analysis, which greatly protects the integrity of the data and enhances the robustness of the results. Secondly, we considered the possibility of the curve relationship and made a curve adjustment when adjusting confounding factors. The present study also has some limitations. Firstly, the cohort of this study included only the Chinese population, a validation study from multi-ethnic subjects was required. Secondly, the diagnosis of NAFLD was dependent on ultrasonography but not liver biopsy. Thirdly, the association between the GGT level and the different stages of NAFLD could not be done. Lastly, in this study, alcohol consumption data were obtained through questionnaires. Questionnaires have limitations, especially if patients find it embarrassing to admit that they drink too much. Since this present study is the second retrospective study, there were no data on breath, blood or urine alcohol and Big MCV. Therefore, the direct influence of excess alcohol intake on GGT levels remains unclear. At the same time, there was no data on the development of diabetes or metabolic syndrome after the follow-up period. Therefore, the influence of diabetes or metabolic syndrome on GGT levels remains unclear and needs further investigation.
Conclusions
This present study demonstrates a positive and non-linear relationship between GGT and incident NAFLD in the non-obese Chinese population. Elevated GGT levels are associated with incident NAFLD before ultrasound diagnosis. There is a threshold effect between the GGT level and NAFLD. When GGT is lower than 26 U/L, it is positively correlated with the occurrence of NAFLD. This result is expected to provide reference for the clinicians to control GGT. From a treatment perspective, it makes sense to reduce the GGT level below the inflection point. Reducing the GGT level can significantly reduce the risk of progression to NAFLD when the GGT level is below the inflection point. Thus, abnormal GGT supports identifying the non-obese Chinese population at high risk of NAFLD, which would help clinicians plan and initiate the appropriate management strategies in advance.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributors
LLW contributed to the conception and design of the study. MZ was responsible for data analysis, LLW and MZ were responsible for data interpretation. HFH wrote the original draft and QJW verified the data. All authors were involved in the reviewing and editing of the manuscript and approved the final version.
Abbreviations
- ALB
Albumin
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- AST
Aspartate aminotransferase
- BMI
Body mass index
- BUN
Blood urea nitrogen
- CIs
Confidence intervals
- Cr
Creatinine
- DBP
Diastolic blood pressure
- DBIL
Direct bilirubin
- FPG
Fasting plasma glucose
- GAM
Generalized additive model
- GLB
Globulin
- GGT
Gamma-glutamyltransferas
- HDL-C
High density liptein cholesterol
- LDL-C
Low density liptein cholesterol
- NAFLD
Non-alcoholic fatty liver disease
- SBP
Systolic blood pressure
- TB
Total bilirubin
- TC
Total cholesterol
- TG
Triglyceride
- UA
Uric acid
Funding
This study was supported by National Natural Science Foundation of China (grant number.8210031954), Guangdong Basic and Applied Basic Research Foundation (grant number.2020A1515110398), and Shenzhen Key Medical Discipline Construction Fund (grant number. SZXK009).
Availability of data and materials
Data can be downloaded from ‘DATADRYAD’ database (www.Datadryad.org).
Declarations
Ethics approval and consent to participate
In the previously published article [11], Dan-Qin Sun, et al. has clearly stated that: the study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all Participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liling Wu and Man Zhang contributed equally to this work.
Contributor Information
Haofei Hu, Email: huhaofei0319@126.com.
Qijun Wan, Email: wanqijun12345@126.com.
References
- 1.Lazo M, Rubin J, Clark JM, Coresh J, Schneider AL, et al. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. J Hepatol. 2015;62(4):841–847. doi: 10.1016/j.jhep.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain IA, Rahman Shah MM, Rahman MK, Ali L. Gamma glutamyl transferase is an independent determinant for the association of insulin resistance with nonalcoholic fatty liver disease in Bangladeshi adults: association of GGT and HOMA-IR with NAFLD. Diabetes Metab Syndr. 2016;10(1 Suppl 1):S25–S29. doi: 10.1016/j.dsx.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Bai L, Zhang XJ, Li H, Cai J. Nonalcoholic fatty liver disease and cardiac remodeling risk: pathophysiological mechanisms and clinical implications. Hepatology. 2021. 10.1002/hep.32072. [DOI] [PubMed]
- 4.Kumar S, Duan Q, Wu R, Harris EN, Su Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv Drug Deliv Rev. 2021;176:113869. doi: 10.1016/j.addr.2021.113869113869. [DOI] [PubMed] [Google Scholar]
- 5.Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza Babaei M, Khamseh ME. Effect of Empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, Placebo-Controlled Trial. Adv Ther. 2020;37(11):4697–4708. doi: 10.1007/s12325-020-01498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C-F, Yeh M-L, Tsai P-C, Hsieh M-H, Yang H-L, Hsieh MY, Yang JF, Lin ZY, Chen SC, Wang LY, Dai CY, Huang JF, Chuang WL, Yu ML. Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication. J Hepatol. 2014;61(1):67–74. doi: 10.1016/j.jhep.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Ma Q, Liao X, Shao C, Lin Y, Wu T, Sun Y, Feng ST, Ye J, Zhong B. Normalization of gamma-glutamyl transferase levels is associated with better metabolic control in individuals with nonalcoholic fatty liver disease. BMC Gastroenterol. 2021;21(1):215. doi: 10.1186/s12876-021-01790-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha GM, Guzman G, Correa De Mello LL, Trein B, Spina L, et al. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients With Obesity. Front Endocrinol. 2020;11:607. doi: 10.3389/fendo.2020.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banderas DZ, Escobedo J, Gonzalez E, Liceaga MG, Ramirez JC, et al. Gamma-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24(7):805–810. doi: 10.1097/MEG.0b013e328354044a. [DOI] [PubMed] [Google Scholar]
- 10.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:9282–9297. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Sun DQ, Wu SJ, Liu WY, Wang LR, Chen YR, Zhang DC, Braddock M, Shi KQ, Song D, Zheng MH. Association of low-density lipoprotein cholesterol within the normal range and NAFLD in the non-obese Chinese population: a cross-sectional and longitudinal study. BMJ Open. 2016;6(12):e013781. doi: 10.1136/bmjopen-2016-013781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Jiang CQ, Cheng KK, Au Yeung SL, Zhang WS, et al. Alcohol use and gamma-Glutamyltransferase using a Mendelian randomization Design in the Guangzhou Biobank Cohort Study. PLoS One. 2015;10(9):e0137790. doi: 10.1371/journal.pone.0137790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang N, Hu X, Zhang Q, Bai P, Cai M, Zeng TS, Zhang JY, Tian SH, Min J, Huang HT, Zheng J, Peng MM, Li MJ, Chen LL. Non-high-density lipoprotein cholesterol: high-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: results from a population-based cohort study. J Diabetes. 2018;10(9):708–714. doi: 10.1111/1753-0407.12650. [DOI] [PubMed] [Google Scholar]
- 14.Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, Mao YM, Chinese National Consensus Workshop on Nonalcoholic Fatty Liver Disease Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9(2):108–112. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 15.Bao L, Yin J, Gao W, Wang Q, Yao W, Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. 2018;175(16):3379–3393. doi: 10.1111/bph.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu L, Shao X, Qiu C, Shao X, Wang X, Niu R, Wang Y. Hepatic steatosis is associated with abnormal hepatic enzymes, visceral adiposity, altered myocardial glucose uptake measured by (18) F-FDG PET/CT. BMC Endocr Disord. 2020;20(1):75. doi: 10.1186/s12902-020-00556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni S, Naz N, Gu H, Stoll JM, Thompson MD, DeBosch B. A clinical model to predict fibrosis on liver biopsy in paediatric subjects with nonalcoholic fatty liver disease. Clin Obes. 2021;11(5):e12472. doi: 10.1111/cob.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oye-Somefun A, Kuk JL, Ardern CI. Associations between elevated kidney and liver biomarker ratios, metabolic syndrome and all-cause and coronary heart disease (CHD) mortality: analysis of the U.S. National Health and Nutrition Examination Survey (NHANES) BMC Cardiovasc Disord. 2021;21(1):352. doi: 10.1186/s12872-021-02160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LW, Huang MS, Shyu YC, Chien RN. Gamma-glutamyl transpeptidase elevation is associated with metabolic syndrome, hepatic steatosis, and fibrosis in patients with nonalcoholic fatty liver disease: a community-based cross-sectional study. Kaohsiung J Med Sci. 2021;37(9):819–827. doi: 10.1002/kjm2.12395. [DOI] [PubMed] [Google Scholar]
- 20.Sheng G, Peng N, Hu C, Zhong L, Zhong M, Zou Y. The albumin-to-alkaline phosphatase ratio as an independent predictor of future non-alcoholic fatty liver disease in a 5-year longitudinal cohort study of a non-obese Chinese population. Lipids Health Dis. 2021;20(1):50. doi: 10.1186/s12944-021-01479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asada F, Nomura T, Hosui A, Kubota M. Influence of increased physical activity without body weight loss on hepatic inflammation in patients with nonalcoholic fatty liver disease. Environ Health Prev Med. 2020;25(1):18. doi: 10.1186/s12199-020-00857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Hamnvik OP, Chamberland JP, Petrou M, Gong H, et al. Circulating alanine transaminase (ALT) and γ-glutamyl transferase (GGT), but not fetuin-a, are associated with metabolic risk factors, at baseline and at two-year follow-up: the prospective Cyprus metabolism study. Metabolism. 2014;63(6):773–782. doi: 10.1016/j.metabol.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ya Z, Fei L, Yue Z, Dan L, Neng-Bo L, et al. Association between serum gamma-glutamyl transferase and serum uric acid levels in Chinese females: a cross-sectional study. Endocr Res. 2017;42(4):296–301. doi: 10.1080/07435800.2017.1300809. [DOI] [PubMed] [Google Scholar]
- 24.Banderas DZ, Escobedo J, Gonzalez E, Liceaga MG, Ramírez JC, Castro MG. γ-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24(7):805–810. doi: 10.1097/MEG.0b013e328354044a. [DOI] [PubMed] [Google Scholar]
- 25.Pitisuttithum P, Chan WK, Goh GB, Fan JG, Song MJ, et al. Gamma-glutamyl transferase and cardiovascular risk in nonalcoholic fatty liver disease: the gut and obesity Asia initiative. World J Gastroenterol. 2020;26(19):2416–2426. doi: 10.3748/wjg.v26.i19.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be downloaded from ‘DATADRYAD’ database (www.Datadryad.org).