Abstract
The discovery of interleukin (IL)-10 more than 30 years ago marked the beginning of our understanding of how cytokines regulate immune responses, based on cross-regulation between T helper (Th)1 and Th2 cytokines. Although multiple cell types were shown to produce IL-10, its identity as a Th2 cytokine remained strong since it was rigidly associated with Th2 clones in mice, whereas both Th1 and Th2 clones could secrete IL-10 in humans. However, as new Th1/Th2 cell functionalities emerged, anti-inflammatory action of IL-10 gained more attention than its inhibitory effect on Th1 cells, which may occur as an indirect consequence of suppression of antigen-presenting cells. This notion is also supported by the discovery of Treg cells whose suppressor functions involve the mediation of IL-10, among other molecules. From this perspective, we discuss the functionalities of IL-10 by highlighting important differences between mice and humans with an emphasis on Th1 and Th2 paradigm.
Keywords: IL-10, IL-10R, Th2 cytokine
Introduction
The paradigm of T helper (Th)1 and Th2 cytokines has been one of the major landmarks in immunology since the discipline’s inception in 1986 (1). The idea of cytokines secreted by Th1 and Th2 clones with distinct functions, as well as their cross-regulation, offered an excellent framework for understanding the pathogenesis of infections caused by a broad spectrum of microbes. For example, interferon (IFN)-γ producing Th1 cells are critical for eliminating intracellular pathogens via cell-mediated immunity (2). On the other hand, B cell stimulating factor 1 [now called interleukin (IL)-4] produced by Th2 cells is critical for antibody production needed to eliminate extracellular pathogens. Furthermore, the discovery that cytokine synthesis inhibitory factor (now called IL-10) in Th2 clones was a key molecule in inhibiting cytokine production by Th1 clones led to the phenomenon of cross-regulation between Th1 and Th2 subsets (3, 4). While this paradigm is still relevant today, the discovery of additional subsets and the growing list of cytokines, combined with the use of modern molecular tools, has made us realize the complexity of cytokine networks and the need to reinterpret the literature from time to time.
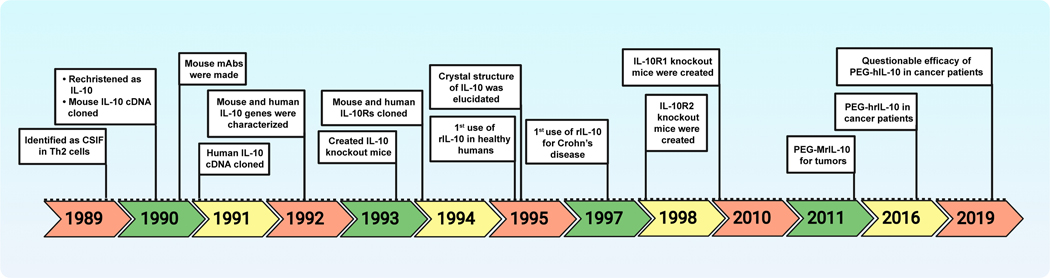
Cytokines are classified as families of interleukins, interferons, tumor necrosis factors (TNFs), growth factors, and chemokines based on their cellular sources, receptor elements, biological function, sequence homology, and common structural motifs. The IL-10 cytokine family consists of nine members categorized into three groups: IL-10 (standalone, group I); IL-19, IL-20, IL-22, IL-24 and IL-26 (IL-20 subfamily, group II); and IL-28A, IL-28B, and IL-29 (group III) (5, 6). The immunomodulatory effects of IL-10 have been extensively studied in various mouse models, which may have translational significance in humans. However, a certain degree of confusion continued to exist regarding the functional identities of IL-10 in these two species. For example, IL-10 was considered a Th2 cytokine in mice (3), whereas both Th1 and Th2 were known to secrete IL-10 in humans (7, 8). In this review, by accepting the limitation that it is difficult to make a head-to-head comparison between mice and humans for every known property of IL-10, we have made efforts to identify major differences between the two species regarding the properties and functions of IL-10 with an emphasis on its relationship to Th1 and Th2 subsets. Such a comparison may be helpful to better understand the role of IL-10 in infections and to refine therapeutic strategies involving IL-10. Their salient features are also discussed in this review. To illustrate the significance of IL-10 in health and disease, advancements made on the biology of IL-10 and its clinical applications are highlighted in Fig 1. However, for a more comprehensive understanding of IL-10 family cytokines, readers are encouraged to consult excellent reviews published on this topic (9–11).
Fig 1: Major advancements made on the biology of IL-10 and its clinical applications.
cDNA, complementary DNA; CSIF, cytokine synthesis inhibition factor; hIL-10, human IL-10; IL-10, Interleukin-10; IL-10R, IL-10 receptor; Mabs, monoclonal antibodies; mIL-10, murine IL-10; PEG-IL-10, pegylated IL-10; rIL-10, recombinant IL-10; Th, T helper.
Biology of IL-10
After the functionality of IL-10 was identified as an inhibitory molecule of cytokine synthesis (1), cDNA clones encoding mouse and human IL-10 were generated in 1990 and 1991, respectively (12, 13). In the following year, IL-10 genes were characterized in both species (Fig 1); the IL-10 gene in mice consists of five exons, spans 5.1 kb, and is located on chromosome 1E4, whereas the human gene spans ~4.7 kb and is located on chromosome 1q21–32 (14). The IL-10 protein comprises 160 amino acids that form a non-covalently linked homodimer of two interpenetrating alpha-helical bundles similar to IFN-γ, with a 73% identity between humans and mice (15). Recently, by linking IL-10 monomer subunits in a head-to-tail fashion with a flexible linker, murine IL-10 (mIL-10) has been engineered to form a dimer that showed enhanced biological activity and improved stability of IL-10 protein (16). Similarly, human IL-10 (hIL-10) has been engineered to create a monomer that can still bind to the IL-10 receptor and retain its biological activity, albeit with ~60-fold less affinity and ~10-fold lower specific activity than the IL-10 protein (17). Furthermore, synthetic peptides derived from the protein sequence of hIL-10 appear to mimic specific properties of IL-10, such as downregulation of expression of major histocompatibility complex (MHC) class I in antigen-presenting cells (APCs) and inhibition of IFN-γ-mediated induction of transporter associated with antigen processing (TAP)1/TAP2 in vitro (18). Since these mimics were shown to bind IL-10 receptor, they may have therapeutic benefits.
The IL-10 receptor (IL-10R), a member of the interferon receptor family, comprises two chains, IL-10R1 and IL-10R2, the homodimers of which combine to form heterodimers (19). Of these two components, the specificity of IL-10 binding is defined by IL-10R1, whereas IL-10R2 is a component of other cytokines, namely IL-22, IL-26, IL-29, IL-28A, and IL-28B (6). While mIL-10R1 binds to both mIL-10 and hIL-10, hIL-10R1 binds only to hIL-10 but not to mIL-10 (20). Furthermore, IL-10R1, although low in density, is expressed by most hematopoietic cells (21). Non-hematopoietic cells can also express IL-10R1 but not constitutively, and immune stimuli such as lipopolysaccharide (LPS) can upregulate IL-10R1 expression (21, 22). On the other hand, IL-10R2 is expressed constitutively (23). Furthermore, upregulation of IL-10R1 was found to be sufficient to activate the expression of IL-10R2 (24). Notably, the affinity of IL-10 to IL-10R1 is higher than its affinity to IL-10R2 (25). While IL-10R1 is indispensable for binding to IL-10, IL-10R2 does not bind to IL-10 directly (19). Instead, IL-10R2 is responsible for signaling events involving the participation of Janus Kinase 1/Tyrosine Kinase 2/Signal Transducing and Activator of Transcription (STAT) 3, although STAT1 and STAT5 can also be involved (26). Nonetheless, the finding that loss of IL-10R2 leads to loss of responsiveness to IL-10 implies that activation through both receptor complexes may be critical for IL-10 to mediate complete functional activation (27). Additionally, homologs of IL-10 have been discovered in ‒ and favor the survival of ‒ viruses such as Epstein Barr virus, Poxvirus, and Cytomegalovirus (28–30). Although the binding affinity of viral IL-10 with hIL-10R is ~1000-fold lower than the binding of native hIL-10, viruses can establish persistent/latent infection in the host by enhancing hIL-10 production (31, 32). These observations suggest that the IL-10 receptor system is finely regulated to respond to IL-10 depending on the context, raising questions about the sources and functionalities of IL-10 in health and disease.
Cellular sources of IL-10
Historically, the inclusion of IL-10 in the Th1 and Th2 paradigm identified IL-10 as a T cell cytokine. In fact, four years after its discovery, IL-10 was reviewed as a cytokine with multiple sources, with an expression pattern resembling that of IL-6 (33). However, the inclusion of IL-10 in the Th2 subset was held for a long time, based on the concept of cross-regulation between Th1 and Th2 cells that offered a valuable framework for understanding the outcomes of infections. For example, in mouse models of leishmaniasis, while the cutaneous form is associated with IFN-γ-secretion from Th1 cells in C57Bl/6 mice, Th2 cytokines dominated the visceral form by inhibiting IFN-γ-producing CD4+T cells in Balb/c mice (34). Although the concept of Th1-Th2 cross-regulation is still valid, several factors might have contributed to the exclusion of IL-10 as specific to the Th2 subset, as described below.
IFN-γ produced by Th1 cells is critical in eliminating intracellular pathogens that can also influence the production of antibodies [IgG2a in mice (35) and IgG1 (36) in humans]. Molecularly, IFN-γ suppresses the activation of STAT3 by shifting STAT activation from STAT3 to STAT1 (37), but the reverse is not true because the signaling molecules of IL-10 have not been known to suppress the signaling events of IFN-γ. Conversely, IL-4 produced by Th2 cells is needed for IgG1 and IgE production, whereas IL-5 can promote the formation of plasma cells and is a well-known growth factor of eosinophils in both mice and humans (38, 39). IL-13 could influence IgE secretion and facilitate barrier immunity by mucus production in epithelial cells that promotes gastrointestinal motility (40, 41). Thus, if the definition of Th2 cytokines promotes the expulsion of extracellular pathogens such as helminths, then the combination of IL-4, IL-5, and IL-13 fulfills the requirement. In this scenario, IL-10 can forcibly be included as a Th2 cytokine as IL-10 can enhance the survival of B cells in the germinal center of the spleen and stimulate the synthesis of IgA and IgG (42, 43). However, IL-10 also plays a role in downregulating IgE production (44). Such effects can be expected from any cytokine because of their pleiotropic, redundant, and synergistic effects, as long as the responding cells express relevant cytokine receptors. This argument can be made because of an elegant study involving conditional knockout (KO) mice, in which IL-10R was deleted specifically in B cells (45). In this system, antibody production was surprisingly increased, leading to the proposition that the IL-10R pathway may negatively regulate antibody production in response to microbial infections.
The production of IL-10 is not limited to Th2 cells alone, especially in humans (33). To identify cellular sources of IL-10 with certainty, IL-10GFP (VeRT-X) reporter mice were created where B cells of lymphoid origin and myeloid cells of blood and liver were found to be the major producers of IL-10 (46). Similar observations were also made in IL-10BiT mice (47). However, through the creation of a more sensitive IL-10–β-lactamase reporter mouse, major sources of IL-10 were found to be F4/80+ macrophages in melanoma and CD11b+Ly6G+ neutrophils during infection, suggesting that non-T cells may be the major producers of IL-10 (48). A recent report demonstrated that B cells from dominant-negative IL-10 receptor-expressing mice, in which IL-10 signaling is specifically blocked in T cells, produced lower amounts of antibodies than B cells from wild-type mice in the presence of CCR6+IL-10eGFP+T cells, but produced similar levels of antibodies in the presence of Th17 cells (49). Importantly, by using reporter knockin tiger mice, where GFP was integrated into the IL-10 locus, it was demonstrated that strong IL-10 expression occurred in intraepithelial lymphocytes in the small intestine and colonic lamina propria lymphocytes (50). Furthermore, by using a double knockin reporter mouse that expresses IL-10 and forkhead box P3 simultaneously, a distinct population of renal regulatory T cells (Tregs) was found as the source of IL-10 (51).
Molecularly, IL-10 expression involves transcription factors (TFs) ‒ cMaf, GATA3, E4 promoter-binding protein 4, STAT3, STAT4, and Jun independent of the expression pattern of well-known TFs of Th1 and Th2 subsets (52, 53). For example, T-box transcription factor TBX21 (T-bet), and GATA3 respectively, promote Th1 and Th2 responses, and these TFs cross-regulate each other in both mice and humans (54, 55). By this definition, IL-10 should be downregulated by T-bet, but this is not the case; instead, T-bet promotes IL-10 secretion (56). Similar effects have been noted with the RAR-related orphan receptor gamma T (57). It may be that the TFs needed for IL-10 production could be expressed in multiple Th subsets. For example, Th17, Th22, and T-Cytotoxic 1 cells can produce IL-10 in humans (58–60), but limited data are available in mice regarding IL-10 secretion in these subsets. Thus, it is possible that promiscuous expression of TFs, combined with potential functional plasticity of T cells responding to multiple cytokines in the microenvironment, may restrict the expression of IL-10 to any given cell type.
In some experimental infections in mice, such as Toxoplasma gondii (61) and cutaneous form of Leishmania major infection (62), Th1 cells were identified as the major producers of IL-10, while in visceral form of Leishmania major infection, Th2 cells were reported as the dominant producers (63). Regardless of sources (Th1 or Th2 cells), IL-10 is still a key molecule to prevent immune pathology (61–63). Conversely, regulatory B cells (Bregs) were identified as a major source of IL-10 in chronic schistosomiasis (64), while B cell-derived IL-10 suppressed Th1/Th17 responses in Pneumocystis murine infection (65). IL-10 produced by Bregs has also been critical for Th2 cell development in Leishmania major infection (66). Recent reports also suggest that IL-10 produced by plasmablasts and not splenic B cells in the draining lymph nodes is essential for the recovery process in the mouse model of experimental autoimmune encephalomyelitis (67). From studies in 10BiT reporter mice with persistent Lymphocytic choriomeningitis virus infections, follicular T helper cells (Tfh) producing IL-10 were critical for promoting antibody response (68). In contrast, IL-10 secreted by tonsillar follicular T cells in humans suppressed the class switching of B cells to IgE (69). Likewise, monocyte-derived IL-10 can suppress Th2 polarization to control allergic reactions in the nasal mucosa of mice, and peritoneal macrophages were found to be the major source of IL-10 in mice infected with Mycobacterium bovis or Escherichia coli (70, 71). Thus, IL-10 production by diverse cell types may be functionally different, that may vary by stimuli.
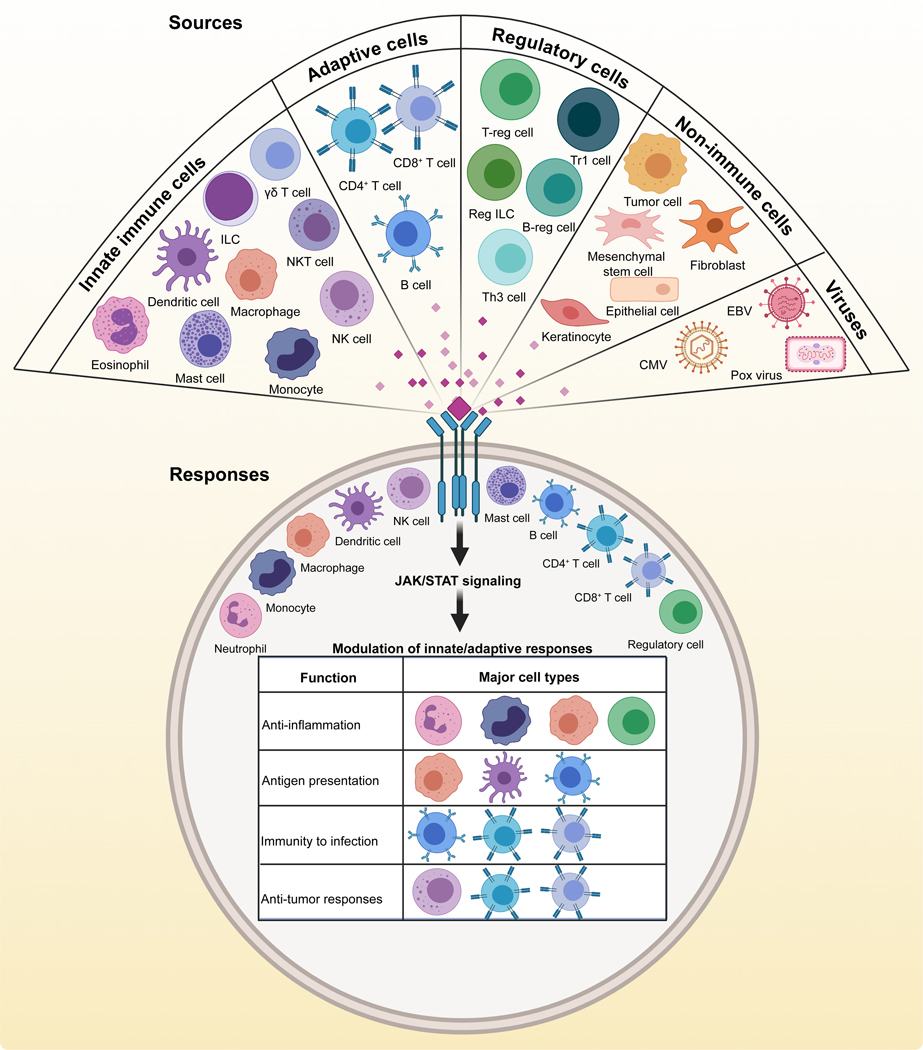
Overall, IL-10 appears to be produced by many types of immune cells (Fig 2). For simplicity, we have categorized these into innate immune cells, including eosinophils, monocytes, macrophages, dendritic cells (DCs), natural killer (NK) cells, NK-T cells, innate lymphoid cells (ILCs), mast cells, and γδT cells. Likewise, all adaptive immune cells (CD4+T cells, CD8+T cells, and B cells) can produce IL-10 (33). Additionally, various subsets possessing regulatory functions [Tregs, regulatory type 1 cells, Regulatory ILCs, Bregs, and Th3 cells] can produce IL-10 (66, 72–75). Notably, however, an unexpected phenotype has been observed with murine alveolar macrophages. Unlike humans (76), murine alveolar macrophages do not produce IL-10, even upon stimulation with LPS, but they retain their capacity to produce TNF-α (77). This observation has important implications for studying the pathogenesis of infectious and non-infectious triggers of lung disease. Because, in the absence of anti-inflammatory effects of IL-10, the macrophage response may be skewed toward inflammatory cytokines that may not be translationally relevant to humans. Similarly, human neutrophils appear not to secrete IL-10 in response to molecules associated with inflammation, such as LPS, Serum Amyloid A-1 protein (78, 79). However, Tregs treated with LPS can stimulate neutrophils to produce IL-10 (80). These observations may also have implications for immunotherapies. Nevertheless, IL-10 can also be produced by non-immune cells in both mice and humans that include mesenchymal stem cells, epithelial cells, and tumor cells (81–86). In mice, hepatic stellate cells can produce IL-10 to potentially overcome the effects of inflammatory cytokines (87, 88), but comparable studies are lacking in humans. Likewise, an unusual property is seen in a specific subset of taste cell receptors in mice, which secrete IL-10 to maintain the structural integrity of the peripheral gustatory system (89). Although such isolated observations may have translational significance, a deeper understanding is critical. For example, loss of taste has been identified as one symptom of coronavirus disease (COVID-19), and severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) may infect taste buds (90). By establishing a positive correlation between SARS CoV-2 infection of taste cells and IL-10 production, it may be possible to use blunt tongue scrapings from patients as a non-invasive modality to evaluate IL-10 as a prognostic marker. Furthermore, severely affected COVID-19 patients experiencing a cytokine storm can have elevated levels of serum IL-10, in addition to various inflammatory cytokines (91, 92). Reports also suggest that detection of IL-10 can be used as a biomarker of COVID-19 (91, 93). It appears that IL-10 may have a pathogenic role by enhancing the production of pro-inflammatory cytokines and activating CD8+T cells resulting potentially from hypo-responsiveness to IL-10 (93, 94). Taken together, IL-10 appears to be produced by both hematopoietic and non-hematopoietic cells, but the question arises whether such a broad spectrum of cell types can also respond to IL-10 to mediate its functions.
Fig 2: Major sources of IL-10 and its responses, common to both mice and humans.
Various immune and non-immune cells, as indicated in the top panel, are known to produce IL-10 in mice and humans. Additionally, viruses also carry the homolog of IL-10. Regardless of cellular sources, IL-10 can act only on cells expressing the IL-10 receptor consisting of both IL-10R1 and IL-10R2 components, as shown in the bottom panel. The table within the circle shows major functions of IL-10 mediated by the cell types shown for each function.
Responses to IL-10
Cytokines are bestowed with unique properties in that they can act on their producers (autocrine), influence neighbors (paracrine), and even work distantly (endocrine). Nonetheless, cytokines cannot cross the lipid bilayers of cells or diffuse into the cytoplasm; rather, cytokines must interact with their specific receptors to enter cells. Such a restriction is advantageous to the host since only the receptor-bearing responding cells react to cytokines and produce defined outcomes. IL-10 is no exception to this rule. Unlike the vast array of producers of IL-10, the range of responders may be limited to a few cell types. These include innate (NK cells, DCs, monocytes, macrophages, and neutrophils) and adaptive immune cells (B cells, CD4+ T cells, and CD8+ T cells), in addition to mast cells (a component of both systems) and regulatory cells. Functionally, it is well established that IL-10 limits tissue damage by suppressing inflammatory responses of innate immune cells (monocytes/macrophages and DCs among others) (95). Furthermore, IL-10 is also known to suppress antigen-presentation functions such as expression of MHC class II and costimulatory molecules e.g., B7 family (Fig 2) (96, 97), and effective cell-mediated immune responses continue to develop in healthy mice and humans, suggesting that the timing of IL-10 production may determine the outcomes of the immune responses. This can be best exemplified by the discovery that classically activated (M1) and alternatively activated (M2) macrophages mediate opposing functions; M2 cells producing IL-10 appear later in the innate response and suppress inflammatory cytokine production by M1 cells (98). Additionally, among various professional APCs, DCs are critical to present antigens to naïve T cells, but they can partially escape from the inhibitory effects of IL-10 by downregulating the expression of IL-10R1 as shown in the human studies (99). Nevertheless, determination of disease phenotypes under the conditions of IL-10 deficiency or genetic defects in IL-10 and IL-10Rs has enabled us to better understand the immunoregulatory roles of IL-10 that we have summarized below with examples.
Mice.
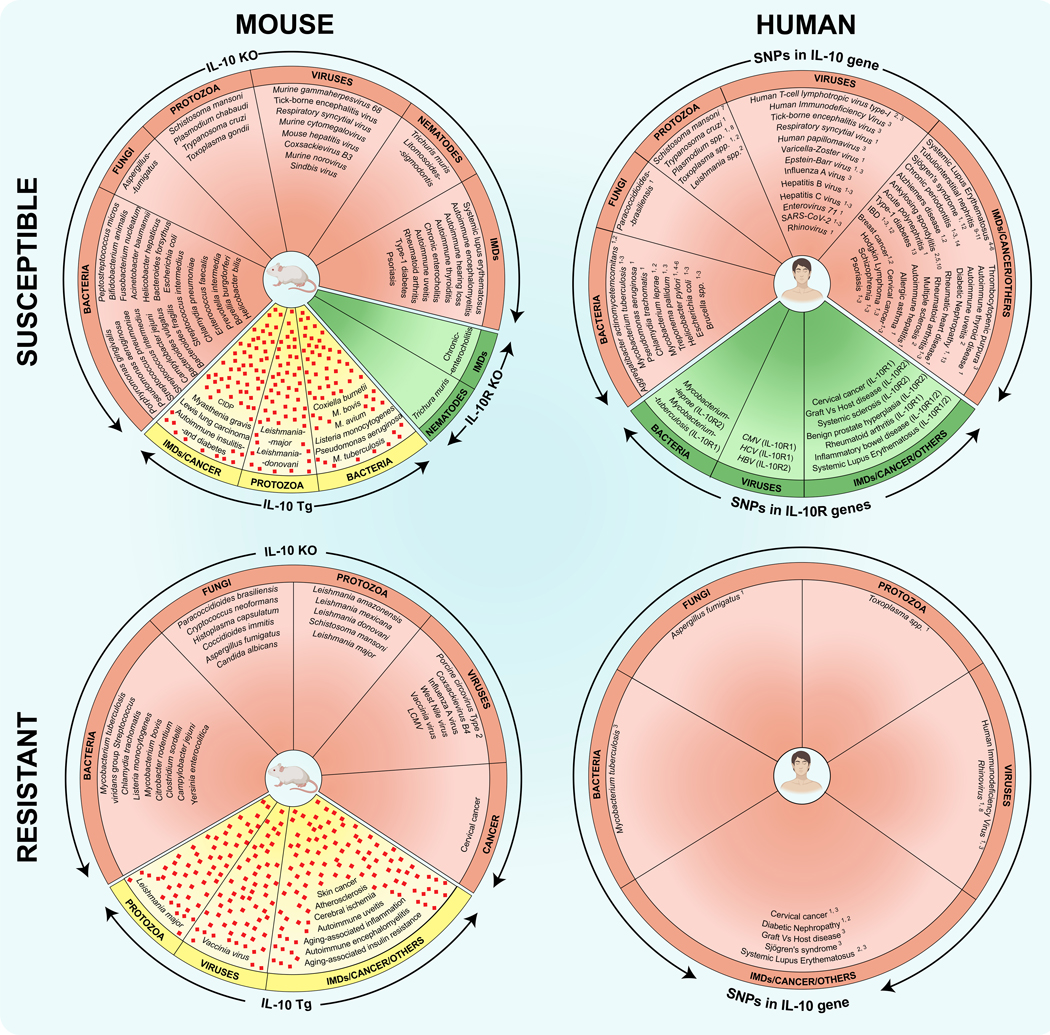
With the availability of genetically altered mice, it has become relatively easy to interpret the role of IL-10 in various infections. By using IL-10 knockout mice or its receptors (IL-10R1 or IL-10R2), IL-10 was found both beneficial and detrimental in a wide range of microbial infections that include intracellular and extracellular bacteria fungi, helminths, protozoa, and viruses (Fig 3). For example, IL-10KO mice infected with intracellular microbes such as Trypanosoma cruzi and Porphyromonas gingivalis were susceptible to infection with increased mortalities (100, 101). Similarly, in the case of classic extracellular pathogens such as helminths (Litomosoides sigmodontis and Trichuris muris), IL-10KO mice failed to expel the parasites and had increased mortality (102, 103). Expectedly, the absence of IL-10 aggravated the disease phenotype by promoting the production of pro-inflammatory cytokines such as IFN-γ (100–103). These observations suggest that IL-10 may be beneficial to control both intracellular and extracellular pathogens. However, in the case of a few other intracellular pathogens (e.g., Leishmania major, Leishmania donovoni, and Mycobacterium tuberculosis) and extracellular pathogens (e.g., Streptococcus spp.), IL-10KO mice were found to be resistant (104–106), indicating that IL-10 can contribute to their disease pathogenesis. While these findings suggest that immunomodulatory effects may vary by infection, it is possible that the cell type that produces IL-10 may dictate the disease outcome. For example, while both macrophages and T cells produce IL-10 during Leishmania major infection (107, 108), conditional deletion of IL-10 in T cells exacerbated the disease (104). In contrast, no phenotypic changes were observed in mice having macrophage-specific IL-10 deletion (104). But, the beneficial effects of IL-10 have been well documented in most immune-mediated/autoimmune diseases (Fig 3), and the best-characterized example is enterocolitis (109).
Fig 3: An overview of disease phenotypes (susceptible and resistant) observed in various mouse models (IL-10 KO, IL-10R KO, and IL-10 transgenic) and humans bearing SNPs in IL-10 or IL-10R genes.
The left panels indicate the utility of IL-10 KO, IL-10R KO, and IL-10 transgenic mice to study disease phenotypes for various microbial infections (bacteria, fungi, viruses, protozoa, and helminths) and immune-mediated diseases (IMDs)/cancers in mice. Pink diamonds denote IL-10 protein. Similar studies were performed in humans bearing SNPs in IL-10 and IL-10R genes, and the major findings are indicated in the right panels. The numbers shown with the superscripts correspond to the reference SNP IDs (rs) are as follows: 1, rs1800896; 2, rs180087; 3, rs1800872; 4, rs3024491; 5, rs1878672; 6, rs3024496; 7, rs1800870; 8, rs1800890; 9, rs2222202; 10, rs3024490; 11, rs6703630; 12, rs3024505; 13, rs1518111; 14, −597 C/A. See the Supplementary Table 1 for references.
Humans.
Translationally, a similar picture has emerged for many of the disease conditions described above in humans (Fig 3). For example, IL-10 and IL-10R deficiencies were associated with ulcerative colitis, Crohn’s disease, and celiac disease (110). Similarly, various single nucleotide polymorphisms (SNPs) have been identified in IL-10 or IL-10R genes, resulting in altered production of IL-10 or its functions (111, 112). These dysfunctionalities were associated with various microbial and immune-mediated diseases, including cancers, transplant rejections, and degenerative disease (Fig 3). The most common IL-10 polymorphisms are located in the promoter region upstream of the IL-10 gene that includes mainly rs1800871, rs1800872, and rs1800896 (113–116). Most SNPs are associated with an increased risk of infections/inflammation that could be influenced by ethnicity. In some cases, SNPs that increase susceptibility to one disease can decrease the risk for others. For example, SNPs rs1800871 (−819C/T) and rs1800872 (−592C/A) were associated with increased susceptibility to inflammatory bowel disease (IBD) in overall populations (117), but the same polymorphisms have decreased susceptibility to systemic lupus erythematosus (SLE) in Asian populations and hospital-based subgroups, respectively (118). However, it is to be noted that disease associations with SNPs in the IL-10 gene do not necessarily prove their causative role, and better interpretations can be made in studies involving large sample sizes, and ethnically diverse populations (116). Likewise, it is unknown whether the SNPs alter the production of IL-10 in different cell types. Although IL-10 polymorphisms may represent potential genetic biomarkers (113), it is possible that other cytokines can substitute the functions of IL-10. For example, STAT3-activating cytokines other than IL-10 (eg., IL-21) can substitute the functions of IL-10 to induce differentiation of naïve B cells to plasma cells (119, 120), suggesting that the immunomodulatory effects of IL-10 are complex in nature.
Role of IL-10 in inflammatory conditions.
The anti-inflammatory effects of IL-10 have been proposed as a major mechanism for suppressing excessive immune responses, the lack of which may lead to the occurrence of autoimmune diseases such as IBD, and Multiple Sclerosis, among others (109, 121). Conversely, IL-10 has been shown to play a pathogenic role in SLE by demonstrating that monocytes and B cells contributed to the overproduction of IL-10 in SLE patients, and also in the mouse model (122). Recently, IL-10-producing, CCR6+ T cells located in the extrafollicular areas, distinguishable from Tfh cells were found to promote autoantibody production in an IL-10-dependent manner (123). Systemic accumulation of CCR6+IL-10+ T cells was also noted in mice with lupus-like disease (123). Indeed, treatment of lupus-prone NZB/W F1 mice with IL-10 antibody led to a reduction in the levels of serum anti-dsDNA IgG autoantibodies in turn delaying the onset of disease, whereas administration of IL-10 accelerated autoimmunity (124). It may be that SLE patients may have a genetic predisposition to produce high levels of IL-10. Mechanistically, the ability of IL-10 to enhance survival and differentiation of B cells in conjunction with inhibition of apoptosis of autoreactive B cells may lead to the production of elevated levels of anti-dsDNA IgG titers in SLE patients (125). Similarly, by using the IL-10 transgenic mouse model involving the expression of IL-10 under the control of IL-2 promoter, IL-10 facilitated the development of experimental myasthenia gravis by increasing anti-acetylcholine receptor antibodies corresponding to reduced IFN-γ production (126). These observations suggest that functions of IL-10 may vary by disease condition and antigen.
As to allergy, IL-10 plays a beneficial role as IL-10 KO mice develop enhanced allergic reactions with increased eosinophilic airway inflammation (127, 128). Various polymorphisms in the IL-10 gene were shown to be associated with severe asthma, and decreased levels of IL-10 were noted in the bronchoalveolar lavage fluid from asthmatic patients (129). Successful immune therapies against allergy were also correlated with IL-10-secreting, antigen-specific T cells (125, 130). By using T cells deficient for IL-10R, it was demonstrated that IL-10 promotes Th2 cell death via granzyme B production (130). Although IL-10 produced by various cell types including regulatory cells can suppress type 2 responses and IgE production (131), the detrimental role of IL-10 cannot be discounted in allergic reactions. For example, IL-10 can promote the development of eosinophilia, airway hyper-responsiveness, mucus metaplasia and IL-5 production that culminate into proliferation and activation of mast cells as shown in the experimental food allergy model (132). Whether such a dual action of IL-10 is allergen-specific remains to be determined.
However, IL-10 has been shown to mediate a protective role in cardiovascular and metabolic disorders. For example, IL-10 can promote plaque healing by inhibiting IL-12 production in atherosclerosis patients (133). By using the chimeric low-density lipoprotein R KO and IL-10KO models, it was demonstrated that leukocytes play a critical role in the prevention of atherosclerosis through modulation of the composition of cellular and collagen plaques (134). Additionally, IL-10 can suppress inflammation during post-myocardial infarction by promoting M2 polarization of macrophages that suppress inflammation, and indirectly stimulate proliferative cardiac fibroblasts and collagen production (135). Likewise, IL-10 may act as a positive regulator of insulin sensitivity by influencing peripheral glucose metabolism, and cotreatment with IL-10 attenuates insulin resistance as noted in the acute lipid infusion model in mice (136). This observation is consistent with reports showing the association of polymorphisms in IL-10 promoter with obesity and insulin resistance (137). Similarly, IL-10 produced by placental villous trophoblasts and maternal immune cells (Treg cells, uterine NK cells and monocytes) has been proposed as one of the mechanisms for maintenance of fetal tolerance (138). IL-10 produced at the maternal-fetal interface during pregnancy may be critical for crosstalk between placental and decidual tissue (138). By acting on trophoblasts, IL-10 can regulate the expression of matrix metalloproteinase-9 that can cause hypertension leading to preeclampsia (139, 140). Thus, IL-10 can be considered to be a pregnancy-compatible cytokine that favors fetal tolerance. But a question may arise whether the immune-suppressive properties of IL-10 can be exploited in clinical settings.
IL-10 in therapy
Accumulated literature suggests that IL-10 functions similarly in both mice and humans. For example, the primary immunodeficiency syndrome characterized by IBD resulting from the loss of IL-10 function due to a mutation in IL-10R is very similar to the enterocolitis phenotype noted in mice deficient for IL-10 or IL-10Rs (141, 142). These models have proved beneficial in understanding the mechanisms of colitis and Crohn’s disease in which multihit hypotheses have been tested, leading to the development of therapies for IBD (143) (Fig 1). The recombinant hIL-10 has been tested for treatment of Crohn’s disease and acute pancreatitis (144). Similarly, the human recombinant fusion IL-10 has shown some degree of success against rheumatoid arthritis (145). However, these clinical applications of IL-10 to mitigate inflammatory conditions have yielded mixed successes that could have been influenced by other environmental factors such as gut flora, nutrition, pollution, and toxins (146) pointing to a possibility that IL-10 could be beneficial in other disease conditions.
Tumors.
It was believed for a long time that IL-10 produced by tumor cells in the tumor microenvironment was an escape mechanism because its production was proportional to the extent of metastasis, as demonstrated in melanoma patients (147). But the discovery that adenocarcinoma cells engineered to express mIL-10 in the mouse model led to regression of tumors was a contrasting finding (148). In this setting, tumor-specific CD8+ T cells producing IL-10 can acquire memory phenotype, and their adoptive transfers into syngeneic recipients resulted in the rejection of tumors. Mechanistically, the terminally exhausted T cells exposed to IL-10-Fc fusion protein displayed better anti-tumor activity in solid tumors by undergoing metabolic reprogramming events (149). In combination with similar successes with mIL-10 as an anti-tumor agent in various mouse models, a pegylated form of IL-10 (PEG-IL-10) was created to prolong the half-life of IL-10 (150). Expectedly, PEG-IL-10 induced long-term CD8+ T cell memory, leading to shrinkage of immune-resistant tumors in various mouse models (150), and showed promise in regulating plasma cholesterol levels in hypercholesteremic cancer patients (151). Phase 1/1b clinical trials with PEG-IL-10 as monotherapy (152) or in combination with chemotherapy were also successful in other tumor settings where IL-10 expression was correlated with infiltration of CD8+ T cells and survival rates (153). Therapeutic benefits of PEG-IL-10 have been ascribed to activation of CD8+ T cells leading to upregulation of IFN-γ and granzyme-B (152). Unfortunately, however, phase 2 and phase 3 clinical trials for metastatic pancreatic cancer and non-small cell lung carcinoma (NSCLC), respectively, with PEG-IL10 combined with a chemotherapeutic drug cocktail and a checkpoint inhibitor [programmed cell death protein 1 antibodies (anti-PD-1)], did not show significant clinical benefits (Fig 1) (154, 155). Nonetheless, a few other recent clinical trials for melanoma, renal cell carcinoma, and NSCLC with PEG-IL-10 in conjunction with checkpoint inhibitors (anti-PD-1) appear promising (156). While these outcomes reinforce the notion that anti-tumor drugs proven to be successful in mouse models may fail in human settings, the use of polytherapy as exemplified above can continue to be explored since universal recipes cannot be developed for all tumors. Such a notion may also be relevant for other disease conditions.
Virus infections.
Consistent with this theme, IL-10 may mediate protective functions in virus infections but can act as a double-edged sword. On the one hand, early production of IL-10 by innate immune cells may favor viral persistence and chronicity by impairing anti-viral innate and CD8+ T cell responses leading to T cell exhaustion. On the other hand, late production of IL-10 can limit excessive inflammation through feedback regulatory mechanisms (157). Additionally, IL-10 can promote anti-viral response by activating CD8+ T cells and NK cells (158, 159). In fact, in the mouse model of Corona virus-induced encephalitis, highly activated cytotoxic T lymphocytes (CTLs) in the brain have been shown to produce IL-10, and the cytolytic property was more pronounced in the IL-10+CTLs than IL-10-CTLs (160). Similarly, NK cells from chronically infected HCV patients were shown to secrete IL-10 in the presence of melanoma cells. In these circumstances, preservation of cytolytic properties of CD8+ T cells and NK cells has been ascribed to IFN-γ, whose secretion remained intact (161). Although anti-inflammatory effects of IL-10 are well documented, the use of IL-10 in the face of a cytokine storm in disease conditions such as COVID-19 may not be a viable option since excess production of IL-10 itself is considered a biomarker of severe disease that may also have a pathogenic role (91, 93).
Overall, clinical use of IL-10 still remains an enigma with a major challenge is to be able to optimize therapeutic doses for each disease condition since lower doses may fail to elicit a response, whereas higher doses may lead to detrimental effects (162, 163). For example, at higher doses of IL-10 in Crohn’s disease and psoriatic patients, several unexpected effects such as fatigue, headache, anemia and thrombocytopenia were observed in addition to the production of the pro-inflammatory cytokine, IFN-γ (10). Other contributing factors include heterogeneity in the selection of patient populations as might occur in Crohn’s disease (10). Likewise, IL-10 therapy may be less effective in ameliorating the established disease (162) and clinical success may depend on the stage of disease for each condition. It is also possible that IL-10 alone may not be enough to suppress all pro-inflammatory reactions, and the immunosuppressive effects of IL-10 may be counterbalanced by its immune-stimulatory effects (162). Furthermore, the dual effects of IL-10 add another layer of complexity as shown in transplantations. While IL-10 can stimulate the expansion of CD4+ and CD8+ T cells leading to exacerbation of graft vs. host disease in humanized mice (164), IL-10 can suppress allograft rejection in pancreatic islet transplantation when administered with rapamycin (165). Clinical challenges continue to hamper cytokine therapy, and IL-10 is no exception because of its wide range of producers and responders, a limitation to delineate the molecular mechanisms at specific cell types. More importantly, it is difficult to control cytokine actions because multiple cytokines can exert similar effects by displaying synergistic, agonistic, or antagonistic properties. However, when recombinant IL-10 therapies have failed, other strategies were explored. For example, a Cyclic adenosine monophosphate phosphodiesterase-4 inhibitor induces Bregs to produce IL-10 in patients with psoriasis and atopic dermatitis (166, 167). Such options may be better in patients if their ability to produce IL-10 remains intact. Likewise, naturally derived alternatives (e.g., curcumin) have been demonstrated to increase IL-10 production in the mouse models of bowel inflammation, pain, and allergies, among others (168). Such natural substitutes may have the potential to be translated for use in humans.
Conclusions
IL-10 is a multifunctional cytokine produced by multiple cell types. The innate immune cells, mainly macrophages, due to their inherent ability to rapidly respond to pathogens and initiate a broad range of cellular responses, have a higher capacity for producing cytokines than adaptive immune cells. This also may be true for tumors that are infiltrated with myeloid-derived suppressor cells (47). Conversely, adaptive immune cells (T cells and B cells) respond to pathogens antigen-specifically, but the frequencies of antigen-specific lymphocytes range from 1 in 1×106 to 1×107 cells (169). Even with the expansion of their effector populations, as might occur to the largest proportion with CD8+ T cells (∼10,000-fold) (170), not all T cells produce a set of cytokines in real-life situations. Although IL-10 was known to be secreted by both Th1 and Th2 cell types in humans (171), the identity of IL-10 as a Th2 cytokine is also lost in mice because Th1 cells can secrete IL-10 (172), in addition to various other Th subsets that are not discussed here [e.g., Th9, regulatory Tfh cells (173, 174). Nonetheless, functionalities of IL-10 have remained intact in both mice and humans, but no function can be singled out as unique to each species. Thus, observations made in mouse models may be translationally relevant, but outcomes should be viewed with caution, as noted with the failed PEG-IL-10 trials in cancer patients described above. Furthermore, reports indicate that the proven anti-cancerous drugs in mouse models are only about 8% effective in human settings (175, 176). Therefore, setbacks are expected because efficacies of therapeutics are tested under highly defined conditions in experimental models involving inbred mouse strains, as opposed to natural settings in the outbred human population.
Supplementary Material
Acknowledgments
We thank Dr. Scott McVey for critically reading this review and providing his input.
Funding:
This work was supported by the American Heart Association (AHA)18TPA34170206, HHS | National Institutes of Health (NIH) 5R21AI142281, and the University of Nebraska-Lincoln Institutional funds
Footnotes
Conflict of interest
None
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, and Coffman RL. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348–2357. [PubMed] [Google Scholar]
- 2.Cope A, Le Friec G, Cardone J, and Kemper C. 2011. The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol 32: 278–286. [DOI] [PubMed] [Google Scholar]
- 3.Fiorentino DF, Bond MW, and Mosmann TR. 1989. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 170: 2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Botran R, Sanders VM, Mosmann TR, and Vitetta ES. 1988. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med 168: 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fickenscher H, Hor S, Kupers H, Knappe A, Wittmann S, and Sticht H. 2002. The interleukin-10 family of cytokines. Trends Immunol 23: 89–96. [DOI] [PubMed] [Google Scholar]
- 6.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, and Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4: 63–68. [DOI] [PubMed] [Google Scholar]
- 7.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, and Romagnani S. 1993. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol 150: 353–360. [PubMed] [Google Scholar]
- 8.Mestas J, and Hughes CC. 2004. Of mice and not men: differences between mouse and human immunology. J Immunol 172: 2731–2738. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang W, and O’Garra A. 2019. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity 50: 871–891. [DOI] [PubMed] [Google Scholar]
- 10.Saraiva M, Vieira P, and O’Garra A. 2020. Biology and therapeutic potential of interleukin-10. J Exp Med 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saraiva M, and O’Garra A. 2010. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181. [DOI] [PubMed] [Google Scholar]
- 12.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, and Mosmann TR. 1990. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 248: 1230–1234. [DOI] [PubMed] [Google Scholar]
- 13.Vieira P, de Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, deVries JE, Roncarolo MG, Mosmann TR, and Moore KW. 1991. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A 88: 1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, and Moore KW. 1992. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol 148: 3618–3623. [PubMed] [Google Scholar]
- 15.Windsor WT, Syto R, Tsarbopoulos A, Zhang R, Durkin J, Baldwin S, Paliwal S, Mui PW, Pramanik B, Trotta PP, and et al. 1993. Disulfide bond assignments and secondary structure analysis of human and murine interleukin 10. Biochemistry 32: 8807–8815. [DOI] [PubMed] [Google Scholar]
- 16.Minshawi F, Lanvermann S, McKenzie E, Jeffery R, Couper K, Papoutsopoulou S, Roers A, and Muller W. 2020. The Generation of an Engineered Interleukin-10 Protein With Improved Stability and Biological Function. Front Immunol 11: 1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josephson K, DiGiacomo R, Indelicato SR, Iyo AH, Nagabhushan TL, Parker MH, and Walter MR. 2000. Design and analysis of an engineered human interleukin-10 monomer. J Biol Chem 275: 13552–13557. [DOI] [PubMed] [Google Scholar]
- 18.Kurte M, Lopez M, Aguirre A, Escobar A, Aguillon JC, Charo J, Larsen CG, Kiessling R, and Salazar-Onfray F. 2004. A synthetic peptide homologous to functional domain of human IL-10 down-regulates expression of MHC class I and Transporter associated with Antigen Processing 1/2 in human melanoma cells. J Immunol 173: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 19.Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, and Pestka S. 1997. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J 16: 5894–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan JC, Indelicato SR, Narula SK, Zavodny PJ, and Chou CC. 1993. Characterization of interleukin-10 receptors on human and mouse cells. J Biol Chem 268: 21053–21059. [PubMed] [Google Scholar]
- 21.Liu Y, Wei SH, Ho AS, de Waal Malefyt R, and Moore KW. 1994. Expression cloning and characterization of a human IL-10 receptor. J Immunol 152: 1821–1829. [PubMed] [Google Scholar]
- 22.Weber-Nordt RM, Meraz MA, and Schreiber RD. 1994. Lipopolysaccharide-dependent induction of IL-10 receptor expression on murine fibroblasts. J Immunol 153: 3734–3744. [PubMed] [Google Scholar]
- 23.Gibbs VC, and Pennica D. 1997. CRF2–4: isolation of cDNA clones encoding the human and mouse proteins. Gene 186: 97–101. [DOI] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal Malefyt R, Coffman RL, and O’Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765. [DOI] [PubMed] [Google Scholar]
- 25.Mosser DM, and Zhang X. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol Rev 226: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehinger J, Gouilleux F, Groner B, Finke J, Mertelsmann R, and Weber-Nordt RM. 1996. IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett 394: 365–370. [DOI] [PubMed] [Google Scholar]
- 27.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, and Aguet M. 1998. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. J Exp Med 187: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young VP, Mariano MC, Tu CC, Allaire KM, Avdic S, Slobedman B, and Spencer JV. 2017. Modulation of the Host Environment by Human Cytomegalovirus with Viral Interleukin 10 in Peripheral Blood. J Infect Dis 215: 874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jog NR, Chakravarty EF, Guthridge JM, and James JA. 2018. Epstein Barr Virus Interleukin 10 Suppresses Anti-inflammatory Phenotype in Human Monocytes. Front Immunol 9: 2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleming SB, McCaughan CA, Andrews AE, Nash AD, and Mercer AA. 1997. A homolog of interleukin-10 is encoded by the poxvirus orf virus. J Virol 71: 4857–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avdic S, McSharry BP, Steain M, Poole E, Sinclair J, Abendroth A, and Slobedman B. 2016. Human Cytomegalovirus-Encoded Human Interleukin-10 (IL-10) Homolog Amplifies Its Immunomodulatory Potential by Upregulating Human IL-10 in Monocytes. J Virol 90: 3819–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, de Waal Malefyt R, Briere F, Parham C, Bridon JM, Banchereau J, Moore KW, and Xu J. 1997. The EBV IL-10 homologue is a selective agonist with impaired binding to the IL-10 receptor. J Immunol 158: 604–613. [PubMed] [Google Scholar]
- 33.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, and Mosmann TR. 1993. Interleukin-10. Annu Rev Immunol 11: 165–190. [DOI] [PubMed] [Google Scholar]
- 34.Kedzierski L, and Evans KJ. 2014. Immune responses during cutaneous and visceral leishmaniasis. Parasitology: 1–19. [DOI] [PubMed] [Google Scholar]
- 35.Bretscher PA, Wei G, Menon JN, and Bielefeldt-Ohmann H. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257: 539–542. [DOI] [PubMed] [Google Scholar]
- 36.Hailu A, Menon JN, Berhe N, Gedamu L, Hassard TH, Kager PA, Olobo J, and Bretscher PA. 2001. Distinct immunity in patients with visceral leishmaniasis from that in subclinically infected and drug-cured people: implications for the mechanism underlying drug cure. J Infect Dis 184: 112–115. [DOI] [PubMed] [Google Scholar]
- 37.Herrero C, Hu X, Li WP, Samuels S, Sharif MN, Kotenko S, and Ivashkiv LB. 2003. Reprogramming of IL-10 activity and signaling by IFN-gamma. J Immunol 171: 5034–5041. [DOI] [PubMed] [Google Scholar]
- 38.Snapper CM, Finkelman FD, and Paul WE. 1988. Regulation of IgG1 and IgE production by interleukin 4. Immunol Rev 102: 51–75. [DOI] [PubMed] [Google Scholar]
- 39.Kouro T, and Takatsu K. 2009. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol 21: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 40.McKenzie GJ, Bancroft A, Grencis RK, and McKenzie AN. 1998. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol 8: 339–342. [DOI] [PubMed] [Google Scholar]
- 41.Bednarz-Misa I, Diakowska D, Szczuka I, Fortuna P, Kubiak A, Rosinczuk J, and Krzystek-Korpacka M. 2020. Interleukins 4 and 13 and Their Receptors Are Differently Expressed in Gastrointestinal Tract Cancers, Depending on the Anatomical Site and Disease Advancement, and Improve Colon Cancer Cell Viability and Motility. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hummelshoj L, Ryder LP, and Poulsen LK. 2006. The role of the interleukin-10 subfamily members in immunoglobulin production by human B cells. Scand J Immunol 64: 40–47. [DOI] [PubMed] [Google Scholar]
- 43.Levy Y, and Brouet JC. 1994. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest 93: 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larson D, Hubner MP, Torrero MN, Morris CP, Brankin A, Swierczewski BE, Davies SJ, Vonakis BM, and Mitre E. 2012. Chronic helminth infection reduces basophil responsiveness in an IL-10-dependent manner. J Immunol 188: 4188–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dooley A, Quintana M, Cheung M, Sun LL, and Gupta N. 2018. The B cell IL-10 receptor suppresses antibody production. Journal of Immunology 200. [Google Scholar]
- 46.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, and Karp CL. 2009. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol 183: 2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart KM, Byrne KT, Molloy MJ, Usherwood EM, and Berwin B. 2011. IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front Immunol 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouabe H, Liu Y, Moser M, Bosl MR, and Heesemann J. 2011. Novel highly sensitive IL-10-beta-lactamase reporter mouse reveals cells of the innate immune system as a substantial source of IL-10 in vivo. J Immunol 187: 3165–3176. [DOI] [PubMed] [Google Scholar]
- 49.Facciotti F, Larghi P, Bosotti R, Vasco C, Gagliani N, Cordiglieri C, Mazzara S, Ranzani V, Rottoli E, Curti S, Penatti A, Karnani B, Kobayashi Y, Crosti M, Bombaci M, van Hamburg JP, Rossetti G, Gualtierotti R, Gerosa M, Gatti S, Torretta S, Pignataro L, Tas SW, Abrignani S, Pagani M, Grassi F, Meroni PL, Flavell RA, and Geginat J. 2020. Evidence for a pathogenic role of extrafollicular, IL-10–producing CCR6+B helper T cells in systemic lupus erythematosus. Proceedings of the National Academy of Sciences: 201917834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, and Flavell RA. 2006. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 25: 941–952. [DOI] [PubMed] [Google Scholar]
- 51.Ostmann A, Paust HJ, Panzer U, Wegscheid C, Kapffer S, Huber S, Flavell RA, Erhardt A, and Tiegs G. 2013. Regulatory T cell-derived IL-10 ameliorates crescentic GN. J Am Soc Nephrol 24: 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, Atarashi K, Hori S, Watarai H, Zhu J, Taniguchi M, and Kubo M. 2011. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol 12: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, and Kuchroo V. 2019. Epigenetic and transcriptional mechanisms for the regulation of IL-10. Semin Immunol 44: 101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, and Sallusto F. 2003. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol 4: 78–86. [DOI] [PubMed] [Google Scholar]
- 55.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, and Murphy KM. 1998. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity 9: 745–755. [DOI] [PubMed] [Google Scholar]
- 56.Zhu C, Sakuishi K, Xiao S, Sun Z, Zaghouani S, Gu G, Wang C, Tan DJ, Wu C, Rangachari M, Pertel T, Jin HT, Ahmed R, Anderson AC, and Kuchroo VK. 2015. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun 6: 6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C, Yosef N, Gaublomme J, Wu C, Lee Y, Clish CB, Kaminski J, Xiao S, Meyer Zu Horste G, Pawlak M, Kishi Y, Joller N, Karwacz K, Zhu C, Ordovas-Montanes M, Madi A, Wortman I, Miyazaki T, Sobel RA, Park H, Regev A, and Kuchroo VK. 2015. CD5L/AIM Regulates Lipid Biosynthesis and Restrains Th17 Cell Pathogenicity. Cell 163: 1413–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Tian J, and Wang S. 2018. Insight Into Non-Pathogenic Th17 Cells in Autoimmune Diseases. Front Immunol 9: 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, and Cavani A. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119: 3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun J, Madan R, Karp CL, and Braciale TJ. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 15: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, and Sher A. 2007. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 204: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson CF, Oukka M, Kuchroo VJ, and Sacks D. 2007. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med 204: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chatelain R, Mauze S, and Coffman RL. 1999. Experimental Leishmania major infection in mice: role of IL-10. Parasite Immunol 21: 211–218. [DOI] [PubMed] [Google Scholar]
- 64.van der Vlugt LE, Labuda LA, Ozir-Fazalalikhan A, Lievers E, Gloudemans AK, Liu KY, Barr TA, Sparwasser T, Boon L, Ngoa UA, Feugap EN, Adegnika AA, Kremsner PG, Gray D, Yazdanbakhsh M, and Smits HH. 2012. Schistosomes induce regulatory features in human and mouse CD1d(hi) B cells: inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS One 7: e30883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rong HM, Li T, Zhang C, Wang D, Hu Y, Zhai K, Shi HZ, and Tong ZH. 2019. IL-10-producing B cells regulate Th1/Th17-cell immune responses in Pneumocystis pneumonia. Am J Physiol Lung Cell Mol Physiol 316: L291–L301. [DOI] [PubMed] [Google Scholar]
- 66.Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, and Launois P. 2010. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol 184: 886–894. [DOI] [PubMed] [Google Scholar]
- 67.Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, Kallies A, Nutt SL, Sakaguchi S, Takeda K, Kurosaki T, and Baba Y. 2014. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 41: 1040–1051. [DOI] [PubMed] [Google Scholar]
- 68.Xin G, Zander R, Schauder DM, Chen Y, Weinstein JS, Drobyski WR, Tarakanova V, Craft J, and Cui W. 2018. Single-cell RNA sequencing unveils an IL-10-producing helper subset that sustains humoral immunity during persistent infection. Nat Commun 9: 5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canete PF, Sweet RA, Gonzalez-Figueroa P, Papa I, Ohkura N, Bolton H, Roco JA, Cuenca M, Bassett KJ, Sayin I, Barry E, Lopez A, Canaday DH, Meyer-Hermann M, Doglioni C, Fazekas B. de St Groth, Sakaguchi S, Cook MC, and Vinuesa CG. 2019. Regulatory roles of IL-10-producing human follicular T cells. J Exp Med 216: 1843–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, and Hasko G. 2007. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood 110: 2685–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo Y, Han R, Evanoff DP, and Chen X. 2010. Interleukin-10 inhibits Mycobacterium bovis bacillus Calmette-Guerin (BCG)-induced macrophage cytotoxicity against bladder cancer cells. Clin Exp Immunol 160: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Garra A, Vieira PL, Vieira P, and Goldfeld AE. 2004. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest 114: 1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim JY, Im KI, Lee ES, Kim N, Nam YS, Jeon YW, and Cho SG. 2016. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep 6: 26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nidetz NF, McGee MC, Limper CB, Ye KX, Islam R, August A, and Huang WS. 2020. Development of regulatory IL-10-producing ILCs during type 2 inflammation. Journal of Immunology 204. [Google Scholar]
- 75.Doetze A, Satoguina J, Burchard G, Rau T, Loliger C, Fleischer B, and Hoerauf A. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol 12: 623–630. [DOI] [PubMed] [Google Scholar]
- 76.Chanteux H, Guisset AC, Pilette C, and Sibille Y. 2007. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir Res 8: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salez L, Singer M, Balloy V, Creminon C, and Chignard M. 2000. Lack of IL-10 synthesis by murine alveolar macrophages upon lipopolysaccharide exposure. Comparison with peritoneal macrophages. J Leukoc Biol 67: 545–552. [DOI] [PubMed] [Google Scholar]
- 78.Davey MS, Tamassia N, Rossato M, Bazzoni F, Calzetti F, Bruderek K, Sironi M, Zimmer L, Bottazzi B, Mantovani A, Brandau S, Moser B, Eberl M, and Cassatella MA. 2011. Failure to detect production of IL-10 by activated human neutrophils. Nat Immunol 12: 1017–1018; author reply 1018–1020. [DOI] [PubMed] [Google Scholar]
- 79.Tamassia N, Bianchetto-Aguilera F, Arruda-Silva F, Gardiman E, Gasperini S, Calzetti F, and Cassatella MA. 2018. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur J Clin Invest 48 Suppl 2: e12952. [DOI] [PubMed] [Google Scholar]
- 80.Lewkowicz N, Mycko MP, Przygodzka P, Cwiklinska H, Cichalewska M, Matysiak M, Selmaj K, and Lewkowicz P. 2016. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol 9: 364–378. [DOI] [PubMed] [Google Scholar]
- 81.Jerkic M, Masterson C, Ormesher L, Gagnon S, Goyal S, Rabani R, Otulakowski G, Zhang H, Kavanagh BP, and Laffey JG. 2019. Overexpression of IL-10 Enhances the Efficacy of Human Umbilical-Cord-Derived Mesenchymal Stromal Cells in E. coli Pneumosepsis. J Clin Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Ren H, Yuan X, Ma H, Shi X, and Ding Y. 2018. Interleukin-10 secreted by mesenchymal stem cells attenuates acute liver failure through inhibiting pyroptosis. Hepatol Res 48: E194–E202. [DOI] [PubMed] [Google Scholar]
- 83.Hyun J, Romero L, Riveron R, Flores C, Kanagavelu S, Chung KD, Alonso A, Sotolongo J, Ruiz J, Manukyan A, Chun S, Singh G, Salas P, Targan SR, and Fukata M. 2015. Human intestinal epithelial cells express interleukin-10 through Toll-like receptor 4-mediated epithelial-macrophage crosstalk. J Innate Immun 7: 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hokenson MA, Wang Y, Hawwa RL, Huang Z, Sharma S, and Sanchez-Esteban J. 2013. Reduced IL-10 production in fetal type II epithelial cells exposed to mechanical stretch is mediated via activation of IL-6-SOCS3 signaling pathway. PLoS One 8: e59598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gastl GA, Abrams JS, Nanus DM, Oosterkamp R, Silver J, Liu F, Chen M, Albino AP, and Bander NH. 1993. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer 55: 96–101. [DOI] [PubMed] [Google Scholar]
- 86.Ogarra A, Stapleton G, Dhar V, Pearce M, Schumacher J, Rugo H, Barbis D, Stall A, Cupp J, Moore K, Vieira P, Mosmann T, Whitmore A, Arnold L, Haughton G, and Howard M. 1990. Production of Cytokines by Mouse B-Cells - B-Lymphomas and Normal B-Cells Produce Interleukin-10. International Immunology 2: 821–832. [DOI] [PubMed] [Google Scholar]
- 87.Byun JS, Suh YG, Yi HS, Lee YS, and Jeong WI. 2013. Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J Hepatol 58: 342–349. [DOI] [PubMed] [Google Scholar]
- 88.Lee YS, Amadi-Obi A, Yu CR, and Egwuagu CE. 2011. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology 132: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng P, Chai J, Zhou M, Simon N, Huang L, and Wang H. 2014. Interleukin-10 is produced by a specific subset of taste receptor cells and critical for maintaining structural integrity of mouse taste buds. J Neurosci 34: 2689–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meunier N, Briand L, Jacquin-Piques A, Brondel L, and Penicaud L. 2020. COVID 19-Induced Smell and Taste Impairments: Putative Impact on Physiology. Front Physiol 11: 625110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J, Xu B, Dai Y, Li X, Zhang C, Peng Y, Feng Y, Li A, Hu Z, Xiang H, Ogg G, Ho LP, McMichael A, Jin R, Knight JC, Dong T, and Zhang Y. 2020. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song P, Li W, Xie J, Hou Y, and You C. 2020. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta 509: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu L, Zhang H, Dauphars DJ, and He YW. 2021. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol 42: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Islam H, Chamberlain TC, Mui AL, and Little JP. 2021. Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front Immunol 12: 677008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Couper KN, Blount DG, and Riley EM. 2008. IL-10: the master regulator of immunity to infection. J Immunol 180: 5771–5777. [DOI] [PubMed] [Google Scholar]
- 96.Chang CH, Furue M, and Tamaki K. 1995. B7–1 expression of Langerhans cells is up-regulated by proinflammatory cytokines, and is down-regulated by interferon-gamma or by interleukin-10. Eur J Immunol 25: 394–398. [DOI] [PubMed] [Google Scholar]
- 97.Willems F, Marchant A, Delville JP, Gerard C, Delvaux A, Velu T, de Boer M, and Goldman M. 1994. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol 24: 1007–1009. [DOI] [PubMed] [Google Scholar]
- 98.Atri C, Guerfali FZ, and Laouini D. 2018. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.von Haehling S, Wolk K, Hoflich C, Kunz S, Grunberg BH, Docke WD, Reineke U, Asadullah K, Sterry W, Volk HD, and Sabat R. 2015. Interleukin-10 receptor-1 expression in monocyte-derived antigen-presenting cell populations: dendritic cells partially escape from IL-10’s inhibitory mechanisms. Genes Immun 16: 8–14. [DOI] [PubMed] [Google Scholar]
- 100.Pino-Martinez AM, Miranda CG, Batalla EI, Gonzalez-Cappa SM, and Alba Soto CD. 2019. IL-10 participates in the expansion and functional activation of CD8(+) T cells during acute infection with Trypanosoma cruzi. J Leukoc Biol 105: 163–175. [DOI] [PubMed] [Google Scholar]
- 101.Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, and Stashenko P. 2004. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res 39: 432–441. [DOI] [PubMed] [Google Scholar]
- 102.Haben I, Hartmann W, Specht S, Hoerauf A, Roers A, Muller W, and Breloer M. 2013. T-cell-derived, but not B-cell-derived, IL-10 suppresses antigen-specific T-cell responses in Litomosoides sigmodontis-infected mice. Eur J Immunol 43: 1799–1805. [DOI] [PubMed] [Google Scholar]
- 103.Schopf LR, Hoffmann KF, Cheever AW, Urban JF Jr., and Wynn TA. 2002. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol 168: 2383–2392. [DOI] [PubMed] [Google Scholar]
- 104.Schwarz T, Remer KA, Nahrendorf W, Masic A, Siewe L, Muller W, Roers A, and Moll H. 2013. T cell-derived IL-10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell based vaccine. PLoS Pathog 9: e1003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphy ML, Wille U, Villegas EN, Hunter CA, and Farrell JP. 2001. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol 31: 2848–2856. [DOI] [PubMed] [Google Scholar]
- 106.Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft GJ, and O’Garra A. 2010. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol 40: 2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kane MM, and Mosser DM. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol 166: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 108.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, and Sacks DL. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420: 502–507. [DOI] [PubMed] [Google Scholar]
- 109.Beebe AM, Cua DJ, and de Waal Malefyt R. 2002. The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev 13: 403–412. [DOI] [PubMed] [Google Scholar]
- 110.Glocker EO, Kotlarz D, Klein C, Shah N, and Grimbacher B. 2011. IL-10 and IL-10 receptor defects in humans. Ann N Y Acad Sci 1246: 102–107. [DOI] [PubMed] [Google Scholar]
- 111.Iyer SS, and Cheng G. 2012. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 32: 23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eskdale J, Gallagher G, Verweij CL, Keijsers V, Westendorp RG, and Huizinga TW. 1998. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci U S A 95: 9465–9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang SL, and Huang SJ. 2019. Interleukin-10 polymorphisms (rs1800871, rs1800872 and rs1800896) and periodontitis risk: A meta-analysis. Arch Oral Biol 97: 59–66. [DOI] [PubMed] [Google Scholar]
- 114.Gao J, Wei L, Fu R, Wei J, Niu D, Wang L, Ge H, Yu Q, Wang M, Liu X, and Zhang W. 2017. Association of Interleukin-10 Polymorphisms (rs1800872, rs1800871, and rs1800896) with Predisposition to IgA Nephropathy in a Chinese Han Population: A Case-Control Study. Kidney Blood Press Res 42: 89–98. [DOI] [PubMed] [Google Scholar]
- 115.Holster A, Nuolivirta K, Tormanen S, Lauhkonen E, Terasjarvi J, Vuononvirta J, Koponen P, Helminen M, He Q, and Korppi M. 2019. Interleukin-10 gene polymorphism rs1800896 is associated with post-bronchiolitis asthma at 11–13 years of age. Acta Paediatr 108: 2064–2069. [DOI] [PubMed] [Google Scholar]
- 116.Zhu X, Hou C, Tu M, Shi C, Yin L, Peng Y, Li Q, and Miao Y. 2019. Gene polymorphisms in the interleukins gene and the risk of acute pancreatitis: A meta-analysis. Cytokine 115: 50–59. [DOI] [PubMed] [Google Scholar]
- 117.Su Y, and Zhao H. 2020. Predisposition of Inflammatory Bowel Disease Is Influenced by IL-8, IL-10, and IL-18 Polymorphisms: A Meta-Analysis. Int Arch Allergy Immunol 181: 799–806. [DOI] [PubMed] [Google Scholar]
- 118.Yuan Y, Wang X, Ren L, Kong Y, Bai J, and Yan Y. 2019. Associations between interleukin-10 gene polymorphisms and systemic lupus erythematosus risk: a meta-analysis with trial sequential analysis. Clin Exp Rheumatol 37: 242–253. [PubMed] [Google Scholar]
- 119.Moens L, and Tangye SG. 2014. Cytokine-Mediated Regulation of Plasma Cell Generation: IL-21 Takes Center Stage. Front Immunol 5: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoon SO, Zhang X, Berner P, and Choi YS. 2009. IL-21 and IL-10 have redundant roles but differential capacities at different stages of Plasma Cell generation from human Germinal Center B cells. J Leukoc Biol 86: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 121.Leach MW, Davidson NJ, Fort MM, Powrie F, and Rennick DM. 1999. The role of IL-10 in inflammatory bowel disease: “of mice and men”. Toxicol Pathol 27: 123–133. [DOI] [PubMed] [Google Scholar]
- 122.Llorente L, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Maillot MC, Durand-Gasselin I, Fourrier BM, Galanaud P, and Emilie D. 1993. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur Cytokine Netw 4: 421–427. [PubMed] [Google Scholar]
- 123.Facciotti F, Larghi P, Bosotti R, Vasco C, Gagliani N, Cordiglieri C, Mazzara S, Ranzani V, Rottoli E, Curti S, Penatti A, Karnani B, Kobayashi Y, Crosti M, Bombaci M, van Hamburg JP, Rossetti G, Gualtierotti R, Gerosa M, Gatti S, Torretta S, Pignataro L, Tas SW, Abrignani S, Pagani M, Grassi F, Meroni PL, Flavell RA, and Geginat J. 2020. Evidence for a pathogenic role of extrafollicular, IL-10-producing CCR6(+)B helper T cells in systemic lupus erythematosus. Proc Natl Acad Sci U S A 117: 7305–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, and Howard M. 1994. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med 179: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Godsell J, Rudloff I, Kandane-Rathnayake R, Hoi A, Nold MF, Morand EF, and Harris J. 2016. Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep 6: 34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ostlie NS, Karachunski PI, Wang W, Monfardini C, Kronenberg M, and Conti-Fine BM. 2001. Transgenic expression of IL-10 in T cells facilitates development of experimental myasthenia gravis. J Immunol 166: 4853–4862. [DOI] [PubMed] [Google Scholar]
- 127.Wilson MS, Elnekave E, Mentink-Kane MM, Hodges MG, Pesce JT, Ramalingam TR, Thompson RW, Kamanaka M, Flavell RA, Keane-Myers A, Cheever AW, and Wynn TA. 2007. IL-13Ralpha2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J Clin Invest 117: 2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grunig G, Corry DB, Leach MW, Seymour BW, Kurup VP, and Rennick DM. 1997. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J Exp Med 185: 1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim S, Crawley E, Woo P, and Barnes PJ. 1998. Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet 352: 113. [DOI] [PubMed] [Google Scholar]
- 130.Coomes SM, Kannan Y, Pelly VS, Entwistle LJ, Guidi R, Perez-Lloret J, Nikolov N, Muller W, and Wilson MS. 2017. CD4(+) Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol 10: 150–161. [DOI] [PubMed] [Google Scholar]
- 131.Akuffo HO, Fehniger TE, and Britton S. 1988. Differential recognition of Leishmania aethiopica antigens by lymphocytes from patients with local and diffuse cutaneous leishmaniasis. Evidence for antigen-induced immune suppression. J Immunol 141: 2461–2466. [PubMed] [Google Scholar]
- 132.Polukort SH, Rovatti J, Carlson L, Thompson C, Ser-Dolansky J, Kinney SR, Schneider SS, and Mathias CB. 2016. IL-10 Enhances IgE-Mediated Mast Cell Responses and Is Essential for the Development of Experimental Food Allergy in IL-10-Deficient Mice. J Immunol 196: 4865–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uyemura K, Demer LL, Castle SC, Jullien D, Berliner JA, Gately MK, Warrier RR, Pham N, Fogelman AM, and Modlin RL. 1996. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest 97: 2130–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, Groux H, Tedgui A, and Mallat Z. 2004. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 24: 1474–1478. [DOI] [PubMed] [Google Scholar]
- 135.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, and Lindsey ML. 2017. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, and Kim JK. 2004. Differential effects of interleukin-6 and −10 on skeletal muscle and liver insulin action in vivo. Diabetes 53: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 137.Scarpelli D, Cardellini M, Andreozzi F, Laratta E, Hribal ML, Marini MA, Tassi V, Lauro R, Perticone F, and Sesti G. 2006. Variants of the interleukin-10 promoter gene are associated with obesity and insulin resistance but not type 2 diabetes in caucasian italian subjects. Diabetes 55: 1529–1533. [DOI] [PubMed] [Google Scholar]
- 138.Thaxton JE, and Sharma S. 2010. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol 63: 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalkunte S, Nevers T, Norris WE, and Sharma S. 2011. Vascular IL-10: a protective role in preeclampsia. J Reprod Immunol 88: 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Espino YSS, Flores-Pliego A, Espejel-Nunez A, Medina-Bastidas D, Vadillo-Ortega F, Zaga-Clavellina V, and Estrada-Gutierrez G. 2017. New Insights into the Role of Matrix Metalloproteinases in Preeclampsia. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Klein C, Muise AM, and Snapper SB. 2014. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol 122: 177–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuhn R, Lohler J, Rennick D, Rajewsky K, and Muller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274. [DOI] [PubMed] [Google Scholar]
- 143.Keubler LM, Buettner M, Hager C, and Bleich A. 2015. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm Bowel Dis 21: 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, Van Deventer S, Ferguson A, Desreumaux P, Forbes A, Geboes K, Melani L, and Cohard M. 2001. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut 49: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schwager K, Kaspar M, Bootz F, Marcolongo R, Paresce E, Neri D, and Trachsel E. 2009. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther 11: R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bernstein CN 2017. Review article: changes in the epidemiology of inflammatory bowel disease-clues for aetiology. Aliment Pharmacol Ther 46: 911–919. [DOI] [PubMed] [Google Scholar]
- 147.Sato T, McCue P, Masuoka K, Salwen S, Lattime EC, Mastrangelo MJ, and Berd D. 1996. Interleukin 10 production by human melanoma. Clin Cancer Res 2: 1383–1390. [PubMed] [Google Scholar]
- 148.Giovarelli M, Musiani P, Modesti A, Dellabona P, Casorati G, Allione A, Consalvo M, Cavallo F, di Pierro F, De Giovanni C, and et al. 1995. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol 155: 3112–3123. [PubMed] [Google Scholar]
- 149.Guo Y, Xie YQ, Gao M, Zhao Y, Franco F, Wenes M, Siddiqui I, Bevilacqua A, Wang H, Yang H, Feng B, Xie X, Sabatel CM, Tschumi B, Chaiboonchoe A, Wang Y, Li W, Xiao W, Held W, Romero P, Ho PC, and Tang L. 2021. Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol 22: 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, Sheppard C, Hong K, Cutler C, Turner S, LaFace D, Kleinschek M, Judo M, Ayanoglu G, Langowski J, Gu D, Paporello B, Murphy E, Sriram V, Naravula S, Desai B, Medicherla S, Seghezzi W, McClanahan T, Cannon-Carlson S, Beebe AM, and Oft M. 2011. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 20: 781–796. [DOI] [PubMed] [Google Scholar]
- 151.Chan IH, Van Hoof D, Abramova M, Bilardello M, Mar E, Jorgensen B, McCauley S, Bal H, Oft M, Van Vlasselaer P, and Mumm JB. 2016. PEGylated IL-10 Activates Kupffer Cells to Control Hypercholesterolemia. PLoS One 11: e0156229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Naing A, Infante JR, Papadopoulos KP, Chan IH, Shen C, Ratti NP, Rojo B, Autio KA, Wong DJ, Patel MR, Ott PA, Falchook GS, Pant S, Hung A, Pekarek KL, Wu V, Adamow M, McCauley S, Mumm JB, Wong P, Van Vlasselaer P, Leveque J, Tannir NM, and Oft M. 2018. PEGylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, CD8(+) T Cell Invigoration and Polyclonal T Cell Expansion in Cancer Patients. Cancer Cell 34: 775–791 e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Autio K, and Oft M. 2019. Pegylated Interleukin-10: Clinical Development of an Immunoregulatory Cytokine for Use in Cancer Therapeutics. Curr Oncol Rep 21: 19. [DOI] [PubMed] [Google Scholar]
- 154.Hecht JR, Lonardi S, Bendell J, Sim HW, Macarulla T, Lopez CD, Van Cutsem E, Munoz Martin AJ, Park JO, Greil R, Wang H, Hozak RR, Gueorguieva I, Lin Y, Rao S, and Ryoo BY. 2021. Randomized Phase III Study of FOLFOX Alone or With Pegilodecakin as Second-Line Therapy in Patients With Metastatic Pancreatic Cancer That Progressed After Gemcitabine (SEQUOIA). J Clin Oncol 39: 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spigel D, Jotte R, Nemunaitis J, Shum M, Schneider J, Goldschmidt J, Eisenstein J, Berz D, Seneviratne L, Socoteanu M, Bhanderi V, Konduri K, Xia M, Wang H, Hozak RR, Gueorguieva I, Ferry D, Gandhi L, Chao BH, and Rybkin I. 2021. Randomized Phase 2 Studies of Checkpoint Inhibitors Alone or in Combination With Pegilodecakin in Patients With Metastatic NSCLC (CYPRESS 1 and CYPRESS 2). J Thorac Oncol 16: 327–333. [DOI] [PubMed] [Google Scholar]
- 156.Naing A, Wong DJ, Infante JR, Korn WM, Aljumaily R, Papadopoulos KP, Autio KA, Pant S, Bauer TM, Drakaki A, Daver NG, Hung A, Ratti N, McCauley S, Van Vlasselaer P, Verma R, Ferry D, Oft M, Diab A, Garon EB, and Tannir NM. 2019. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol 20: 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rojas JM, Avia M, Martin V, and Sevilla N. 2017. IL-10: A Multifunctional Cytokine in Viral Infections. J Immunol Res 2017: 6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, and Kaech SM. 2015. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol 16: 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stacey MA, Marsden M, Wang EC, Wilkinson GW, and Humphreys IR. 2011. IL-10 restricts activation-induced death of NK cells during acute murine cytomegalovirus infection. J Immunol 187: 2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Trandem K, Zhao J, Fleming E, and Perlman S. 2011. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J Immunol 186: 3642–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, and Moretta L. 2007. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol 37: 445–455. [DOI] [PubMed] [Google Scholar]
- 162.Marlow GJ, van Gent D, and Ferguson LR. 2013. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J Gastroenterol 19: 3931–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li MC, and He SH. 2004. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J Gastroenterol 10: 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abraham S, Choi JG, Ye C, Manjunath N, and Shankar P. 2015. IL-10 exacerbates xenogeneic GVHD by inducing massive human T cell expansion. Clin Immunol 156: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, and Roncarolo MG. 2006. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 55: 40–49. [PubMed] [Google Scholar]
- 166.Mavropoulos A, Zafiriou E, Simopoulou T, Brotis AG, Liaskos C, Roussaki-Schulze A, Katsiari CG, Bogdanos DP, and Sakkas LI. 2019. Apremilast increases IL-10-producing regulatory B cells and decreases proinflammatory T cells and innate cells in psoriatic arthritis and psoriasis. Rheumatology (Oxford) 58: 2240–2250. [DOI] [PubMed] [Google Scholar]
- 167.Saporito RC, and Cohen DJ. 2016. Apremilast Use for Moderate-to-Severe Atopic Dermatitis in Pediatric Patients. Case Rep Dermatol 8: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, and Sahebkar A. 2019. Immune modulation by curcumin: The role of interleukin-10. Crit Rev Food Sci Nutr 59: 89–101. [DOI] [PubMed] [Google Scholar]
- 169.Alanio C, Lemaitre F, Law HK, Hasan M, and Albert ML. 2010. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood 115: 3718–3725. [DOI] [PubMed] [Google Scholar]
- 170.Cui W, and Kaech SM. 2010. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev 236: 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yssel H, De Waal Malefyt R, Roncarolo MG, Abrams JS, Lahesmaa R, Spits H, and de Vries JE. 1992. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol 149: 2378–2384. [PubMed] [Google Scholar]
- 172.Trinchieri G. 2007. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med 204: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jager A, Dardalhon V, Sobel RA, Bettelli E, and Kuchroo VK. 2009. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol 183: 7169–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Laidlaw BJ, Lu Y, Amezquita RA, Weinstein JS, Vander Heiden JA, Gupta NT, Kleinstein SH, Kaech SM, and Craft J. 2017. Interleukin-10 from CD4(+) follicular regulatory T cells promotes the germinal center response. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stagnation IO 2004. Innovation or Stagnation : Challenge and Opportunity on the Critical Path to New Medical Products. [Google Scholar]
- 176.Mak IW, Evaniew N, and Ghert M. 2014. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 6: 114–118. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.