ABSTRACT
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is leading to public health crises worldwide. An understanding of the pathogenesis and the development of treatment strategies is of high interest. Recently, neutrophil extracellular traps (NETs) have been identified as a potential driver of severe SARS-CoV-2 infections in humans. NETs are extracellular DNA fibers released by neutrophils after contact with various stimuli and accumulate antimicrobial substances or host defense peptides. When massively released, NETs are described to contribute to immunothrombosis in acute respiratory distress syndrome and in vascular occlusions. Based on the increasing evidence that NETs contribute to severe COVID-19 cases, DNase treatment of COVID-19 patients to degrade NETs is widely discussed as a potential therapeutic strategy. Here, we discuss potential detrimental effects of NETs and their nuclease degradation, since NET fragments can boost certain bacterial coinfections and thereby increase the severity of the disease.
KEYWORDS: COVID-19, NETs, neutrophils
PERSPECTIVE
NETs AND COVID-19
The increasing severity of coronavirus disease 2019 (COVID-19) worldwide has caused an immense pressure on the health care system and the scientific community to find new intervention strategies. Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has proven to initiate an exacerbated host response in patients with severe COVID-19, which involves the massive infiltration of dysfunctional mature neutrophils into the lung as a potential risk factor (1). Generally, infiltrating neutrophils have been shown to release neutrophil extracellular traps (NETs) as a defense mechanism against invading pathogens. During the last decades, evidence showing that NETs play a crucial role in the defense mechanisms of various vertebrates, invertebrates, and plants has accumulated (2). NETs are extracellular fibers of DNA with associated histones, granule proteins (e.g., myeloperoxidase or elastase), and cationic antimicrobial peptides (3–9). They are released by activated neutrophils and were first described as an innate immune response to entrap and kill invading bacteria (7). Increasing knowledge demonstrates that NETs are built during various infectious diseases, including viral infections with influenza A virus or human immunodeficiency virus 1 (HIV-1) (10–13). In addition to having protective antimicrobial effects, aggregated NETs are able to degrade proinflammatory cytokines and thus have been shown to resolve the inflammation in gout patients (14). However, besides having protective effects, NETs have been shown to initiate several detrimental effects directly on the host, as described for thrombosis (15, 16), autoimmune diseases (17), acute respiratory distress syndrome (ARDS) (18, 19), stroke (20, 21), and other diseases (22, 23), especially when the efficient elimination of NETs is impaired (24). Furthermore, some bacteria are able to escape from NET structures via enhanced spreading inside the body (25), and some bacteria are able to use products of degraded NETs as growth factors (26). The effect of NETs on viruses is still not completely understood. However, especially for enveloped viruses, an antiviral effect of NETs was identified (12, 27, 28). Viruses can be bound and immobilized in NETs via electrostatic interactions of the positively charged molecules (e.g., histones or cathelicidins) and can attach to the negatively charged viral envelope, as was shown, for example, for influenza A virus and HIV-1 (10–12, 27, 29). In 2016, Schönrich and Raftery reviewed the mechanisms by which NETs are produced in the context of viral infection and how this may contribute to both antiviral immunity and immunopathology (10). Direct as well as indirect ways of NET induction by viral infections via antiviral pattern recognition receptors (PRRs), soluble proNET mediators, or the platelet/neutrophil axis are known. Finally, virus-induced NETs have the ability to control the virus but also damage the host (30).
Regarding COVID-19, there is clear evidence that NETs contribute to the severity of pathogenesis by damaging lung epithelial cells (31–33). Specific NET markers, like cell-free DNA, myeloperoxidase DNA (MPO-DNA), and citrullinated histone H3 (Cit-H3), are increased in sera from patients with COVID-19 compared to levels in uninfected controls (32, 34, 35). Several studies detected NETs in vivo in COVID-19 patients in lung tissue, blood (31, 32, 36), and tracheal aspirate fluid (31). Additionally, Mikacenic et al. have shown that soluble NET markers are increased in the bronchoalveolar lavage fluid (BALF) and alveolar spaces of patients with ventilator-associated pneumonia (37). The first studies demonstrated mechanistic explanations of how NETs are induced and contribute to COVID-19 pathogenesis. It was demonstrated that viable SARS-CoV-2 induces NETs in human neutrophils. This NET induction depends on three pathways: (i) the ROS-dependent protein arginine deiminase 4 (PAD-4) pathway, (ii) the angiotensin-converting enzyme 2 (ACE2)–serine protease axis, and (iii) virus replication (31). However, the complete mechanism of how SARS-CoV-2 induces NETs has not been known until now.
Nevertheless, NETs are strongly discussed as a potential driver of ARDS and the associated immunothrombosis (32) of COVID-19-patients (38–40). Therefore, new treatment strategies are being discussed to inhibit or destroy NETs in severe COVID-19 patients.
THERAPEUTIC DNase TREATMENT OF COVID-19 PATIENTS
The reasons why some patients exhibit severe symptoms in COVID-19 is not well understood, and several ideas are widely discussed, including age, gender, hormones, genetic background, or immunodeficiencies (41). In this regard, it is of interest that detrimental effects of uncontrolled NET formation have been demonstrated by several authors to play a role in certain diseases, e.g., lupus nephritis (42). It is well known that the host produces DNases to keep a balance between detrimental and beneficial effects of NETs (42). As an example, patients with DNase deficiencies are more susceptible to detrimental effects of NETs in the case of lupus nephritis (42, 43).
It seems that during severe SARS-CoV-2 infections, a balanced and immune-protective NET formation is out of control. Reasons for this may be (i) noneffective or deficient host nuclease activity, (ii) massive overwhelming induction of NET formation, or (iii) a combination of both reasons.
Several studies have focused on DNase treatments for severe COVID-19 to degrade NETs. Based on the described NET formation in COVID-19 patients, it is logical to consider dornase alfa (Pulmozyme) for the treatment of severe COVID-19 ARDS (44). Interestingly, local dornase alfa treatment was tested in a calf model after infection with bovine respiratory syncytial virus (bRSV). Infections with bRSV lead to airway obstruction, as in infections with RSV in humans, and NET formation was detected in the BALF of RSV-infected infants (45). Dornase alfa treatment reduced the NET formation in the lungs; in addition, fewer airway occlusions were detected (46). Dornase alfa is a recombinant human DNase I that is able to degrade NETs and cell-free DNA and thereby act mucolytic. It is commonly used in cystic fibrosis (CF) patients, which has led to a reduced demand for antibiotics, a reduced frequency of CF-related symptoms, and improved lung function (47–51).
Currently, there are ongoing clinical trials with dornase in COVID-19 patients that intend to define the impact of aerosolized intratracheal dornase alfa administration on the severity and progression of ARDS in COVID-19 patients (52, 53). It is speculated that dornase alfa treatment of patients might promote an improved clearance of secretions and reduce extracellular double-stranded DNA-induced hyperinflammation in alveoli, preventing further damage to the lungs. Weber et al. (54) recently reported a single-center case series where dornase alfa was administered through an in-line nebulizer system to five COVID-19 patients. Data on tolerability and responses, including longitudinal values capturing respiratory function and inflammatory status, were analyzed. Following nebulized in-line administration of dornase alfa with albuterol, the fraction of inspired oxygen requirements was reduced for all five patients. Albuterol is a bronchodilator that relaxes muscles in the airways and increases airflow to the lungs and, thus, was used to increase the delivery of dornase alfa to the alveoli. Overall, no drug-associated toxicities were identified in the five patients. The results presented in this case series suggest that dornase alfa will be well tolerated by critically ill patients with COVID-19. In an experimental study, recombinant DNase I-coated polydopamine-poly(ethylene glycol) nanoparticulates (named long-acting DNase I) were generated, and the authors hypothesized that exogenous administration of long-acting DNase I may suppress SARS-CoV-2-mediated neutrophil activities and the cytokine storm (55). However, detailed clinical trials are required to formally test the dosing, safety, and efficacy of dornase alfa in COVID-19 patients. Especially, it needs to be considered that some authors describe, on the basis of in vitro-observed phenomena, that the degradation product of NETs might be even more cytotoxic than the intact NETs themselves (56–58).
COINFECTIONS AS TRIGGERS OF SEVERE COVID-19 DISEASE?
Bacteria, e.g., Staphylococcus, Streptococcus, Haemophilus, Pseudomonas, and many more, are well known to induce NETs (59). Thus, coinfecting agents may also contribute to massive NET induction and associated detrimental effects. The complex influence of NETs in primary viral infections with influenza A virus and secondary bacterial coinfection with Streptococcus pneumoniae inside the ear has already been demonstrated (60).
In this context, it has also recently been discussed if early bacterial coinfections have an undefined impact during SARS-CoV-2 infections (61, 62). The study by Kreitmann et al. (61) demonstrated a higher prevalence of bacterial coinfections than of other viral infections. The main isolated pathogens were Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae. On the other hand, a recent systematic review and meta-analysis revealed that a low proportion of COVID-19 patients have a bacterial coinfection compared to proportions in previous influenza pandemics (63). However, the commonest bacteria were Mycoplasma pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae. It was discussed by other authors that a coinfection diagnostic is complex and that antibiotic use in COVID-19 patients is high in intensive care units (64). Therefore, the authors concluded that coinfections in COVID-19 patients need good management and treatment, as well as a characterization of the coinfecting agents. Table 1 summarizes a list of bacterial pathogens found as coinfecting agents in COVID-19 patients.
TABLE 1.
Overview of bacteria found as coinfecting agents in COVID-19 patients and their NAD biosynthesis
| Bacterial coinfection identified in COVID-19 patients | Family | Gram positive or negative | Presence of bacteria lacking de novo NAD biosynthesis (reference) | Reference(s) |
|---|---|---|---|---|
| Acinetobacter baumannii | Moraxellaceae | Negative | No | 62, 63 |
| Chlamydia spp. | Chlamydiaceae | Negative | Yes (69) | 62, 63 |
| Enterobacter spp. | Enterobacteriaceae | Negative | No | 62, 63 |
| Enterococcus faecium | Enterococcaceae | Positive | No | 62, 63 |
| Haemophilus influenzae | Pasteurellaceae | Negative | Yes (68) | 61, 63 |
| Klebsiella pneumoniae | Enterobacteriaceae | Negative | No | 62, 63 |
| Mycoplasma pneumoniae | Mycoplasmataceae | Lack of a cell wall | No | 62, 63 |
| Pseudomonas aeruginosa | Pseudomonadaceae | Negative | No | 63 |
| Serratia marcescens | Yersiniaceae | Negative | No | 62, 63 |
| Staphylococcus aureus | Staphylococcaceae | Positive | No | 61 – 63 |
| Streptococcus pneumoniae | Streptococcaceae | Positive | No | 61 |
Coinfections have been described in other pandemics in the world (like the Spanish flu) as one reason for high numbers of deaths (65, 66), and it is very important to investigate coinfections during SARS-CoV-2 infections. Compared to 1918 to 1919, the time of the Spanish flu, nowadays antibiotic treatments are widely used in intensive care units. However, having in mind that we live in a time of increasing numbers of antibiotic-resistant bacteria, preventive antibiotic treatment of COVID-19 patients without antibiograms should be avoided.
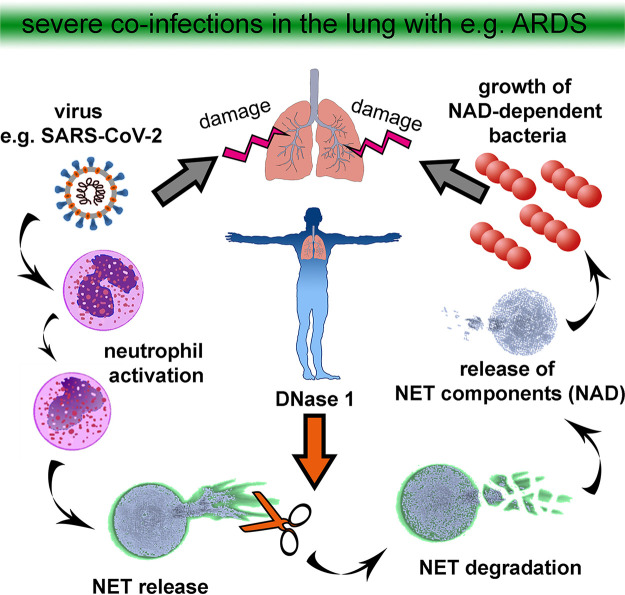
In this regard, it is of high interest that we have shown in our own recent publication that nuclease-mediated degradation of NETs promotes the growth of certain bacteria (e.g., Actinobacillus pleuropneumoniae or Haemophilus influenzae) that use degraded NET products as an efficient source for NAD or adenosine (26). As Haemophilus influenzae was found in different studies as a coinfecting agent in COVID-19 patients, this phenomenon is of high interest. These bacteria can enhance their growth rate in the presence of NETs that have been efficiently degraded by the host or bacterial nucleases (DNase I and micrococcal nuclease) (Fig. 1). This effect can be diminished by inhibiting bacterial adenosine synthase, indicating that degraded NETs serve as a source for NAD.
FIG 1.
Degradation of NETs as a risk factor for severe coinfections and damage of the lung. In the case of a severe lung infection, e.g., with SARS-CoV-2, neutrophils are activated and release NETs. The host itself produces nucleases to eliminate and recycle NET products. Importantly, nuclease-mediated degradation of NETs may promote the growth of certain bacteria that use degraded NET products as an efficient source for NAD or adenosine.
NAD is an essential coenzyme for redox reactions and a substrate of NAD-consuming enzymes, including ADP-ribose transferases, Sir2-related protein lysine deacetylases, and bacterial DNA ligases. Therefore, targeting NAD biosynthesis in bacterial pathogens has been discussed for the development of antibacterial agents with potential broad-spectrum activity (67). However, some bacteria have evolved to depend entirely on the salvage of NAD precursors from other cells; Haemophilus influenzae and Actinobacillus pleuropneumoniae do not carry genes for a de novo pathway of NAD (68) and belong to the group of NAD-dependent Pasteurellaceae.
Interestingly, an in vivo infection study with Actinobacillus pleuropneumoniae demonstrated a significant influence of host DNase I inside the lung on the patho-histological severity of infected pigs (26). In pigs with a high number of lung lesions, a significantly larger amount of DNase I and a smaller amount of free DNA than in infected pigs with a low number of lung lesions were detected. These data shed light on the detrimental effects of degraded NETs during the host immune response to certain bacterial species that require and/or efficiently take advantage of degraded DNA material, which has been provided by the host nucleases. As SARS-CoV-2 does not depend on NAD and therefore does not benefit from degraded NETs, it may be hypothesized based on the findings in COVID-19 patients that underestimated bacterial infections (61) are somehow part of the severity of pathogenesis. Indeed, it may be hypothesized that an acute COVID-19 infection induces NETs and subsequently provides nutrients for sleeping Haemophilus influenzae cells, a starting point of a fatal lung infection.
Whether degraded NETs also promote the growth of other bacterial pathogens, e.g., Mycoplasma pneumoniae, Pseudomonas aeruginosa, or Chlamydiaceae, is still not known and needs further investigations, especially for coinfections with SARS-CoV-2.
CONCLUSION: URGENT NEED FOR ADDITIONAL RESEARCH ON NETs AND COINFECTIONS IN COVID-19 PATHOGENESIS
On one hand, it is considered that a therapeutic nuclease treatment might be helpful to prevent the detrimental effects of massive NET formation during COVID-19. However, on the other hand, a nuclease treatment can impact the growth of certain NAD-dependent pathogenic bacteria, e.g., the lung pathogen Haemophilus influenzae, which can efficiently use degraded NETs as a growth factor (26). Therefore, it is necessary to include a systematic bacterial diagnostic followed by an adjusted antibiotic treatment in clinical trials with dornase alpha. As some bacteria identified in COVID-19 patients are not easy to cultivate from swap and organ samples, they may indeed be underestimated, as mentioned above. Therefore, there is an urgent need not only for additional clinical but also for experimental in vitro research studies focusing on bacterial coinfections in COVID-19 patients. Follow-up problems in patients may also occur with colonizing bacteria like Haemophilus influenzae if they benefit from degraded NETs and at the same time develop antibiotic resistance.
Another upcoming question is “At which time point is a DNase treatment beneficial or detrimental?” It is completely unknown if NET formation in early stages of COVID-19 may be antiviral, as enveloped viruses like SARS-CoV-2 are described to be vulnerable through NETs (10). Therefore, the effect of NETs on SARS-CoV-2 in vitro and in vivo should be investigated more in detail, as NET induction in the early phase of COVID-19 may prevent severe cases or help in specific groups, depending on age, general health status, host DNase activity, and further individual characteristics of infected people. Understanding the role of NETs in the pathogenesis of COVID-19 seems to be a key element for identifying new treatment strategies for severe and mild cases. More investigations of the complex host-pathogen interaction during SARS-CoV-2 infections are needed to clarify the influence of conceivable bacterial coinfections, NET formation, and DNase activity.
ACKNOWLEDGMENTS
Both authors wrote the paper and performed research.
This study was in part supported by the Ministry of Science and Culture of Lower Saxony in Germany (14 - 76103-184 CORONA-15/20).
Neither author has a relevant conflict of interest.
Footnotes
Citation de Buhr N, von Köckritz-Blickwede M. 2021. The balance of neutrophil extracellular trap formation and nuclease degradation: an unknown role of bacterial coinfections in COVID-19 patients? mBio 12:e03304-20. https://doi.org/10.1128/mBio.03304-20.
Contributor Information
Maren von Köckritz-Blickwede, Email: mkoeckbl@tiho-hannover.de.
Scot P. Ouellette, University of Nebraska Medical Center
REFERENCES
- 1.Wang J, Jiang M, Chen X, Montaner LJ. 2020. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumann A, Brogden G, von Köckritz-Blickwede M. 2020. Extracellular traps: an ancient weapon of multiple kingdoms. Biology (Basel) 9:34. doi: 10.3390/biology9020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann A, Völlger L, Berends ETM, Molhoek EM, Stapels DAC, Midon M, Friães A, Pingoud A, Rooijakkers SHM, Gallo RL, Mörgelin M, Nizet V, Naim HY, von Köckritz-Blickwede M. 2014. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun 6:860–868. doi: 10.1159/000363699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Buhr N, Reuner F, Neumann A, Stump-Guthier C, Tenenbaum T, Schroten H, Ishikawa H, Müller K, Beineke A, Hennig-Pauka I, Gutsmann T, Valentin-Weigand P, Baums CG, von Köckritz-Blickwede M. 2017. Neutrophil extracellular trap formation in the Streptococcus suis-infected cerebrospinal fluid compartment. Cell Microbiol 19:e12649. doi: 10.1111/cmi.12649. [DOI] [PubMed] [Google Scholar]
- 5.Meurer M, Öhlmann S, Bonilla MC, Valentin-Weigand P, Beineke A, Hennig-Pauka I, Schwerk C, Schroten H, Baums CG, von Köckritz-Blickwede M, de Buhr N. 2020. Role of bacterial and host DNases on host-pathogen interaction during Streptococcus suis meningitis. Int J Mol Sci 21:5289. doi: 10.3390/ijms21155289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petretto A, Bruschi M, Pratesi F, Croia C, Candiano G, Ghiggeri G, Migliorini P. 2019. Neutrophil extracellular traps (NET) induced by different stimuli: a comparative proteomic analysis. PLoS One 14:e0218946. doi: 10.1371/journal.pone.0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss D, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 8.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. 2011. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. 2012. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol 91:369–376. doi: 10.1189/jlb.0711387. [DOI] [PubMed] [Google Scholar]
- 10.Schönrich G, Raftery MJ. 2016. Neutrophil extracellular traps go viral. Front Immunol 7:366. doi: 10.3389/fimmu.2016.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew A-A, Phoon MC, van Rooijen N, Chow VT. 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto N, Akira S. 2012. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Chan LLY, Nicholls JM, Peiris JSM, Lau YL, Chan MCW, Chan RWY. 2020. Host DNA released by NETosis in neutrophils exposed to seasonal H1N1 and highly pathogenic H5N1 influenza viruses. Respir Res 21:160. doi: 10.1186/s12931-020-01425-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, Holmdahl R, Krenn V, Harrer T, Jeremic I, Bilyy R, Schett G, Hoffmann M, Herrmann M. 2014. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 15.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, DeVinney R, Doig CJ, Green FHY, Kubes P. 2007. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. 2010. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pieterse E, van der Vlag J. 2014. Breaking immunological tolerance in systemic lupus erythematosus. Front Immunol 5:164. doi: 10.3389/fimmu.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Su X, Pan P, Zhang L, Hu Y, Tan H, Wu D, Liu B, Li H, Li H, Li Y, Dai M, Li Y, Hu C, Tsung A. 2016. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep 6:37252. doi: 10.1038/srep37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. 2012. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallés J, Lago A, Santos MT, Latorre AM, Tembl J, Salom J, Nieves C, Moscardó A. 2017. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb Haemost 117:1919–1929. doi: 10.1160/TH17-02-0130. [DOI] [PubMed] [Google Scholar]
- 21.Thålin C, Demers M, Blomgren B, Wong SL, von Arbin M, von Heijne A, Laska AC, Wallén H, Wagner DD, Aspberg S. 2016. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res 139:56–64. doi: 10.1016/j.thromres.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Söderberg D, Segelmark M. 2016. Neutrophil extracellular traps in ANCA-associated vasculitis. Front Immunol 7:256. doi: 10.3389/fimmu.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meegan JE, Yang X, Coleman DC, Jannaway M, Yuan SY. 2017. Neutrophil-mediated vascular barrier injury: role of neutrophil extracellular traps. Microcirculation 24:e12352. doi: 10.1111/micc.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayadas TN, Cullere X, Lowell CA. 2014. The multifaceted functions of neutrophils. Annu Rev Pathol 9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. 2006. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 26.de Buhr N, Bonilla MC, Pfeiffer J, Akhdar S, Schwennen C, Kahl BC, Waldmann K, Valentin-Weigand P, Hennig-Pauka I, von Köckritz-Blickwede M. 2019. Degraded neutrophil extracellular traps promote the growth of Actinobacillus pleuropneumoniae. Cell Death Dis 10:657. doi: 10.1038/s41419-019-1895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeksema M, Tripathi S, White M, Qi L, Taubenberger J, van Eijk M, Haagsman H, Hartshorn KL. 2015. Arginine-rich histones have strong antiviral activity for influenza A viruses. Innate Immun 21:736–745. doi: 10.1177/1753425915593794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galani IE, Andreakos E. 2015. Neutrophils in viral infections: current concepts and caveats. J Leukoc Biol 98:557–564. doi: 10.1189/jlb.4VMR1114-555R. [DOI] [PubMed] [Google Scholar]
- 29.Wardini AB, Guimaraes-Costa AB, Nascimento MTC, Nadaes NR, Danelli MGM, Mazur C, Benjamim CF, Saraiva EM, Pinto-da-Silva LH. 2010. Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J Gen Virol 91:259–264. doi: 10.1099/vir.0.014613-0. [DOI] [PubMed] [Google Scholar]
- 30.Jenne CN, Kubes P. 2015. Virus-induced NETs—critical component of host defense or pathogenic mediator? PLoS Pathog 11:e1004546. doi: 10.1371/journal.ppat.1004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetité D, Tavares LA, Paiva IM, Rosales R, Colón D, Martins R, Castro IA, Almeida GM, Lopes MIF, Benatti MN, Bonjorno LP, Giannini MC, Luppino-Assad R, Almeida SL, Vilar F, Santana R, Bollela VR, Auxiliadora-Martins M, Borges M, Miranda CH, Pazin-Filho A, da Silva LLP, Cunha LD, Zamboni DS, Dal-Pizzol F, Leiria LO, Siyuan L, Batah S, Fabro A, Mauad T, Dolhnikoff M, Duarte-Neto A, Saldiva P, Cunha TM, Alves-Filho JC, Arruda E, Louzada-Junior P, Oliveira RD, Cunha FQ. 2020. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med 217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middleton EA, He X-Y, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, Cody MJ, Manne BK, Portier I, Harris ES, Petrey AC, Beswick EJ, Caulin AF, Iovino A, Abegglen LM, Weyrich AS, Rondina MT, Egeblad M, Schiffman JD, Yost CC. 2020. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, Looney MR, McAllister F, Rayes R, Renaud S, Rousseau S, Salvatore S, Schwartz RE, Spicer JD, Yost CC, Weber A, Zuo Y, Egeblad M. 2020. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med 217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair CN, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. 2020. Neutrophil extracellular traps in COVID-19. JCI Insight 5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. 2020. Neutrophil extracellular traps (NETs) as markers of disease severity in COVID-19. medRxiv doi: 10.1101/2020.04.09.20059626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, D’Emal C, Vanwinge C, Cataldo D, Oury C, Delvenne P, Marichal T. 2020. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med 217:e20201012. doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikacenic C, Moore R, Dmyterko V, West TE, Altemeier WA, Liles WC, Lood C. 2018. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit Care 22:358. doi: 10.1186/s13054-018-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker RC. 2020. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis 50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuo Y, Zuo M, Yalavarthi S, Gockman K, Madison JA, Shi H, Woodard W, Lezak SP, Lugogo NL, Knight JS, Kanthi Y. 5 November 2020. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis doi: 10.1007/s11239-020-02324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makatsariya A, Slukhanchuk E, Bitsadze V, Khizroeva J, Tretyakova M, Tsibizova V, Dobryakov A, Elalamy I, Gris JC. 2020. COVID-19, neutrophil extracellular traps and vascular complications in obstetric practice. J Perinat Med 48:985–994. doi: 10.1515/jpm-2020-0280. [DOI] [PubMed] [Google Scholar]
- 41.Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, Fox JM, Chen RE, Earnest JT, Keeler SP, Ritter JH, Kang L-I, Dort S, Robichaud A, Head R, Holtzman MJ, Diamond MS. 2020. Publisher Correction: SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21:1470–1470. doi: 10.1038/s41590-020-0794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. 2010. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonawane S, Khanolkar V, Namavari A, Chaudhary S, Gandhi S, Tibrewal S, Jassim SH, Shaheen B, Hallak J, Horner JH, Newcomb M, Sarkar J, Jain S. 2012. Ocular surface extracellular DNA and nuclease activity imbalance: a new paradigm for inflammation in dry eye disease. Invest Ophthalmol Vis Sci 53:8253–8263. doi: 10.1167/iovs.12-10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Earhart AP, Holliday ZM, Hofmann HV, Schrum AG. 2020. Consideration of dornase alfa for the treatment of severe COVID-19 acute respiratory distress syndrome. New Microbes New Infect 35:100689. doi: 10.1016/j.nmni.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortjens B, de Boer OJ, de Jong R, Antonis AF, Sabogal Piñeros YS, Lutter R, van Woensel JB, Bem RA. 2016. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J Pathol 238:401–411. doi: 10.1002/path.4660. [DOI] [PubMed] [Google Scholar]
- 46.Cortjens B, de Jong R, Bonsing JG, van Woensel JBM, Antonis AFG, Bem RA. 2018. Local dornase alfa treatment reduces NETs-induced airway obstruction during severe RSV infection. Thorax 73:578–580. doi: 10.1136/thoraxjnl-2017-210289. [DOI] [PubMed] [Google Scholar]
- 47.Frederiksen B, Pressler T, Hansen A, Koch C, Høiby N. 2006. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatr 95:1070–1074. doi: 10.1080/08035250600752466. [DOI] [PubMed] [Google Scholar]
- 48.Papayannopoulos V, Staab D, Zychlinsky A. 2011. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One 6:e28526. doi: 10.1371/journal.pone.0028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martínez-Alemán SR, Campos-García L, Palma-Nicolas JP, Hernández-Bello R, González GM, Sánchez-González A. 2017. Understanding the entanglement: neutrophil extracellular traps (NETs) in cystic fibrosis. Front Cell Infect Microbiol 7:104. doi: 10.3389/fcimb.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ten Berge M, van der Wiel E, Tiddens HAWM, Merkus PJFM, Hop WCJ, de Jongste JC. 2003. DNase in stable cystic fibrosis infants: a pilot study. J Cyst Fibros 2:183–188. doi: 10.1016/S1569-1993(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 51.Hodson ME. 1995. Aerosolized dornase Alfa (rhDNase) for therapy of cystic fibrosis. Am J Respir Crit Care Med 151:S70–S74. doi: 10.1164/ajrccm/151.3_Pt_2.S70. [DOI] [PubMed] [Google Scholar]
- 52.Okur HK, Yalcin K, Tastan C, Demir S, Yurtsever B, Karakus GS, Kancagi DD, Abanuz S, Seyis U, Zengin R, Hemsinlioglu C, Kara M, Yildiz ME, Deliceo E, Birgen N, Pelit NB, Cuhadaroglu C, Kocagoz AS, Ovali E. 2020. Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection. New Microbes New Infect 37:100756. doi: 10.1016/j.nmni.2020.100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desilles JP, Gregoire C, Le Cossec C, Lambert J, Mophawe O, Losser MR, Lambiotte F, Le Tacon S, Cantier M, Engrand N, Trouiller P, Pottecher J. 2020. Efficacy and safety of aerosolized intra-tracheal dornase alfa administration in patients with SARS-CoV-2-induced acute respiratory distress syndrome (ARDS): a structured summary of a study protocol for a randomised controlled trial. Trials 21:548. doi: 10.1186/s13063-020-04488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber AG, Chau AS, Egeblad M, Barnes BJ, Janowitz T. 2020. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: a case series. medRxiv doi: 10.1101/2020.05.13.20087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee YY, Park HH, Park W, Kim H, Jang JG, Hong KS, Lee J-Y, Seo HS, Na DH, Kim T-H, Bin Choy Y, Ahn JH, Lee W, Park CG. 2021. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials 267:120389. doi: 10.1016/j.biomaterials.2020.120389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marsman G, Zeerleder S, Luken BM. 2016. Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis 7:e2518. doi: 10.1038/cddis.2016.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marsman G, Von Richthofen H, Bulder I, Lupu F, Hazelzet J, Luken BM, Zeerleder S. 2017. DNA and factor VII-activating protease protect against the cytotoxicity of histones. Blood Adv 1:2491–2502. doi: 10.1182/bloodadvances.2017010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu T, Kobayashi SD, Quinn MT, DeLeo FR. 2012. A NET outcome. Front Immunol 3:365. doi: 10.3389/fimmu.2012.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Short KR, von Köckritz-Blickwede M, Langereis JD, Chew KY, Job ER, Armitage CW, Hatcher B, Fujihashi K, Reading PC, Hermans PW, Wijburg OL, Diavatopoulos DA. 2014. Antibodies mediate formation of neutrophil extracellular traps in the middle ear and facilitate secondary pneumococcal otitis media. Infect Immun 82:364–370. doi: 10.1128/IAI.01104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kreitmann L, Monard C, Dauwalder O, Simon M, Argaud L. 2020. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med 46:1787–1789. doi: 10.1007/s00134-020-06165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai C-C, Wang C-Y, Hsueh P-R. 2020. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect 53:505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lansbury L, Lim B, Baskaran V, Lim WS. 2020. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox MJ, Loman N, Bogaert D, O'Grady J. 2020. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe 1:e11. doi: 10.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morens DM, Taubenberger JK, Fauci AS. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCullers JA. 2014. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 12:252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 67.Sorci L, Pan Y, Eyobo Y, Rodionova I, Huang N, Kurnasov O, Zhong S, MacKerell AD, Zhang H, Osterman AL. 2009. Targeting NAD biosynthesis in bacterial pathogens: structure-based development of inhibitors of nicotinate mononucleotide adenylyltransferase NadD. Chem Biol 16:849–861. doi: 10.1016/j.chembiol.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gazzaniga F, Stebbins R, Chang SZ, McPeek MA, Brenner C. 2009. Microbial NAD metabolism: lessons from comparative genomics. Microbiol Mol Biol Rev 73:529–541. doi: 10.1128/MMBR.00042-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Omsland A, Sixt BS, Horn M, Hackstadt T. 2014. Chlamydial metabolism revisited: interspecies metabolic variability and developmental stage-specific physiologic activities. FEMS Microbiol Rev 38:779–801. doi: 10.1111/1574-6976.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]