Abstract
Because of the rapid and serious nature of acute cardiovascular disease (CVD) especially ST segment elevation myocardial infarction (STEMI), a leading cause of death worldwide, prompt diagnosis and treatment is of crucial importance to reduce both mortality and morbidity. During a pandemic such as coronavirus disease-2019 (COVID-19), it is critical to balance cardiovascular emergencies with infectious risk. In this work, we recommend using wearable device based mobile health (mHealth) as an early screening and real-time monitoring tool to address this balance and facilitate remote monitoring to tackle this unprecedented challenge. This recommendation may help to improve the efficiency and effectiveness of acute CVD patient management while reducing infection risk.
Keywords: COVID-19, cardiovascular disease, wearables, mobile health, physiological monitoring
I. Introduction
As THE most serious form of cardiovascular disease (CVD), acute cardiovascular events, especially ST segment elevation myocardial infarction (STEMI) and other cardiovascular emergencies including non-STEMI (NSTEMI), arrhythmia, hypertensive crisis, acute heart failure (HF) and pulmonary embolism (PE), are life-threatening, time-sensitive emergencies that must be diagnosed and treated promptly. During the coronavirus disease-2019 (COVID-19) pandemic, some clinical issues should raise concern. Firstly, COVID-19 infection is associated with potentially severe cardiovascular complications. Secondly, there is evidence to suggest that during the pandemic, patients with acute cardiovascular events were not receiving appropriate treatment due to either fear of presenting to a hospital and/or a delay in diagnosis/treatment in the setting of an overwhelmed hospital system [1], [2]. Thirdly, those patients with chronic CVD for example late-stage HF are also at increased risk due to cross-infection when travelling to and attending hospital and community clinics. Under these circumstances, balancing cardiovascular emergencies with infectious risk has become a global challenge. Patient triage and identification based on symptoms before admission could be a possible solution. Wearable sensing and mHealth technologies, which can provide remote measurements of physiological parameters with molecular testing related to the COVID-19 and/or acute CVD symptoms in real world scenarios (see Fig. 1) [3], [4], has a huge potential to address this challenge and are expected to be better integrated in the inside/outside hospital workflow [5]. However, wearable sensors and mHealth have not been fully utilized in pandemic control to date. In this work, specific application scenarios encompassing the use of wearable sensors and mHealth in closed-loop management of acute CVD patients during the COVID pandemic are proposed. This could help to triage patients before hospitalization and provide opportunities to remotely monitor the health status of both patients and caregivers so as to limit virus spread and avoid cross-infections while assisting to provide emergency services timely to acute CVD patients.
Fig. 1.
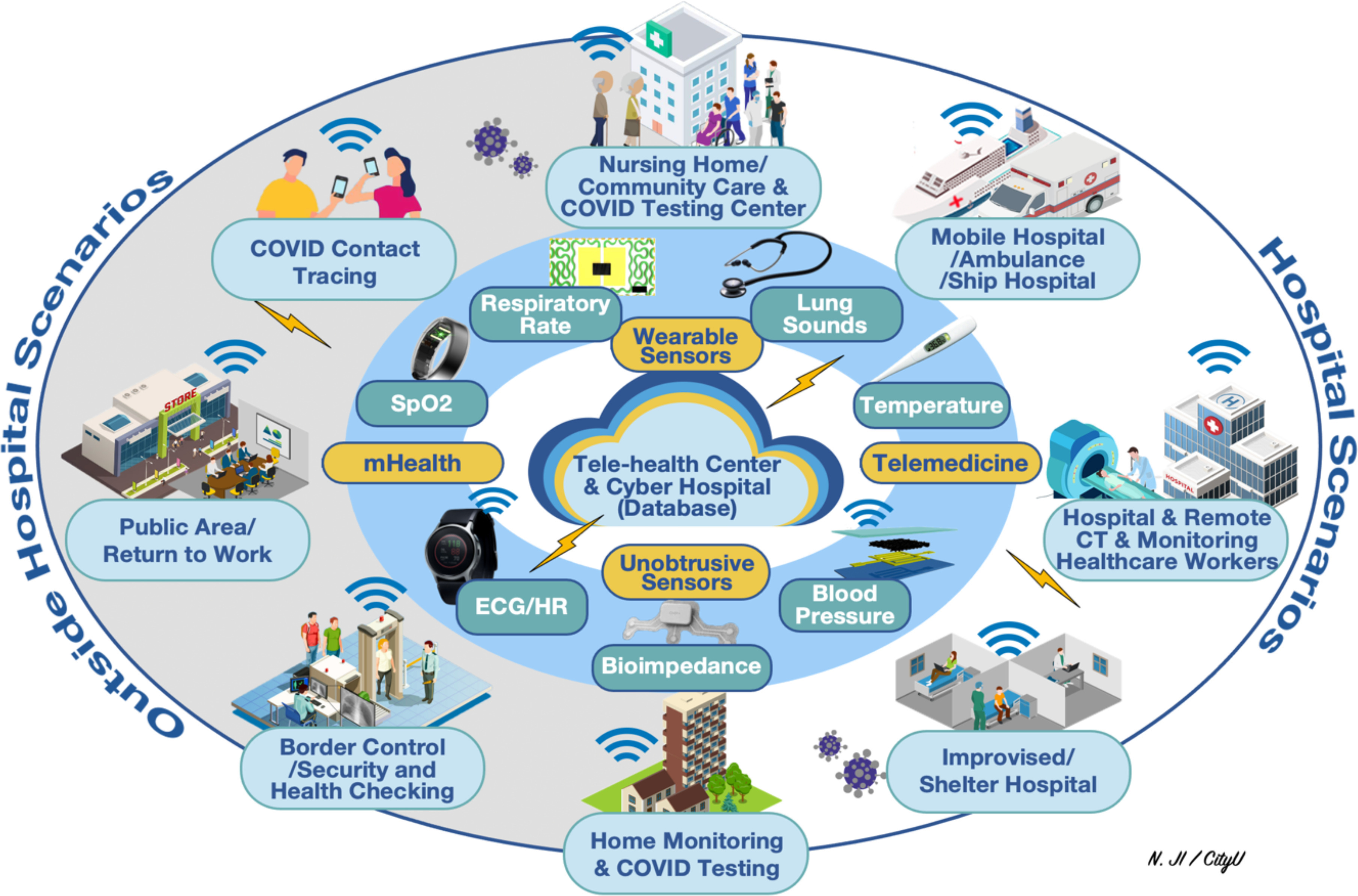
Application scenarios for wearable sensors during pandemics [3], [4].
In March 2020, based on its clinical experience in treating COVID-19 patients, Peking Union Medical College Hospital (PUMCH) published a recommendation of management procedures for acute myocardial infarction (AMI) with a focus on in-hospital scenarios [6]. Recently, the Society for Cardiovascular Angiography and Interventions (SCAI) and the Canadian Association of Interventional Cardiology (CAIC) in conjunction with the American College of Cardiology Interventional Council have collaborated to create a multi-center observational registry, NACMI, aimed at developing data-driven guidelines and therapies [7]. The proposed wearable-based mHealth workflow for acute CVD patients may further improve the efficiency and effectiveness of disease management from home, hospital to post-hospital at different stages in a closed-looped manner.
II. Closed-Loop Management of Acute CVD Patients During COVID-19 Using mHealth
According to the World Health Organization (WHO) report, the most common symptoms of COVID-19 are fever, tiredness, and dry cough [8]. Shortness of breath should also be considered in accordance with Centers for Disease Control and Prevention (CDC) [9]. Taking STEMI as an example of acute CVD, the proposed workflow suggests using wearable devices for real time monitoring of physiological parameters relevant to the COVID-19 symptoms such as body temperature, respiratory rate, lung sounds and SpO2, heart rate and ST segment elevation which could be extracted from electrocardiography (ECG) signals and blood pressure (BP) for STEMI diagnosis (see Table I), along with short message service and questionnaires, patients could be identified and classified before admission. Fig. 2 shows the wearable-based mHealth workflow, which summarizes the workflow of the recommended approach on how wearable sensor-based mHealth systems would be used for patient triaging outside hospital, real-time patient monitoring inside hospital and remote clinical follow-up post-hospital discharge. We will elaborate on this in more detail below.
TABLE I. Wearable-Based Parameters for Population Monitoring During COVID-19 Pandemic.
| Parameters | Units | Early warning for COVID-19 | Early warning for STEMI |
|---|---|---|---|
| Respiratory rate | bpm | ≥20bpm | — |
| Lung/heart sound | — | Crackles | — |
| Heart rate | bpm | ≥100bpm | ≥80bpm |
| Temperature | ℃ | ≥38℃ | — |
| Cough | — | Dry cough | — |
| SpO2 | % | ≤94% | ≤94% |
| ECG | mV | Arrhythmia | Arrhythmia/ST segment elevation |
| BP | mmHg | ≥140/90mmHg | Reduction in systolic BP [10] |
Fig. 2.
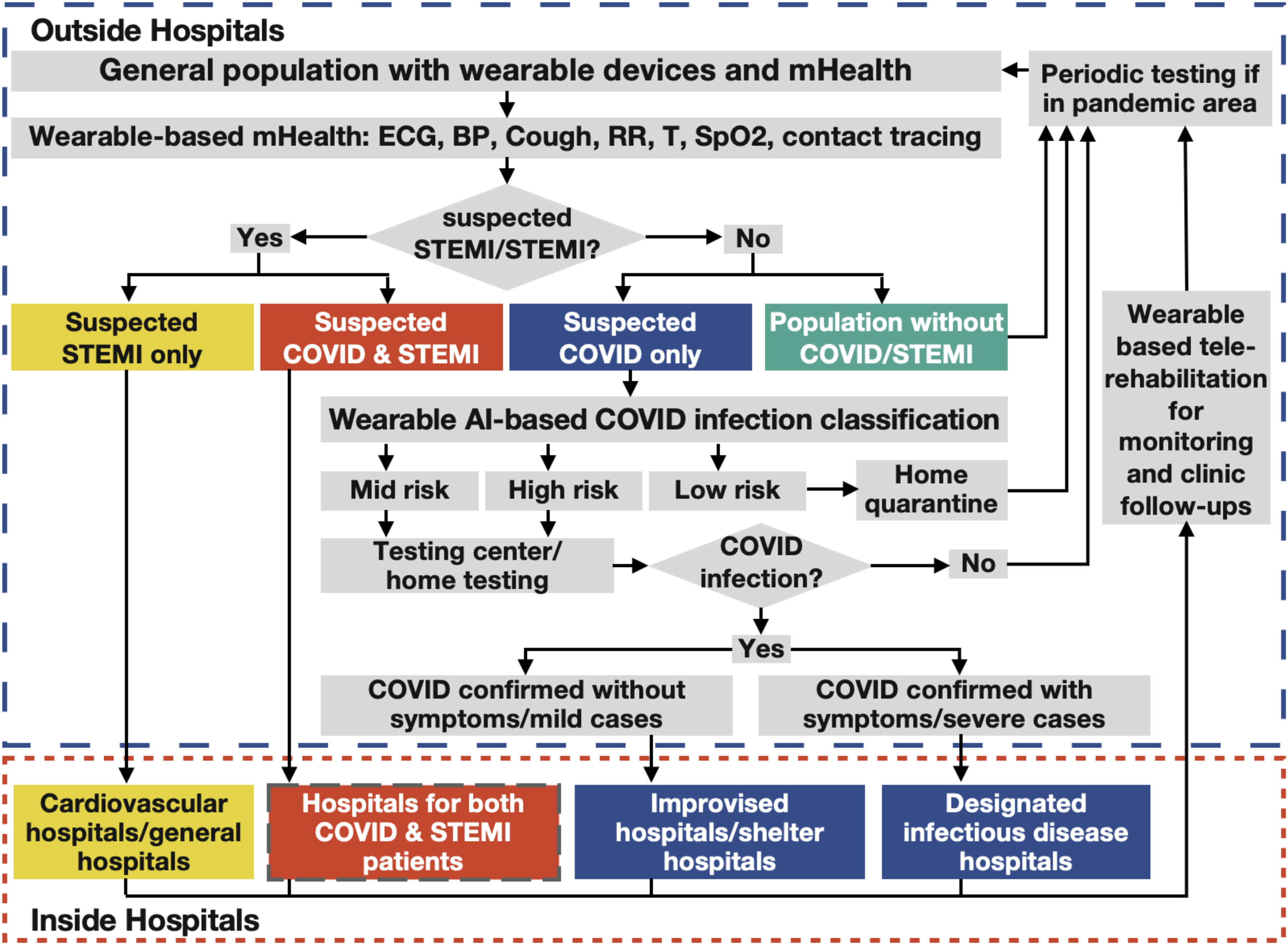
A flow chart of the applications of wearables and mHealth in closed-loop diagnosis and management of STEMI patients during COVID -19 pandemic.
Recommended wearable-based mHealth workflow for the closed-loop management of STEMI patients during the COVID-19 pandemic
A. Patient Triaging Before Admission
In the context of the proposed mHealth workflow, it is suggested that the vital sign data collected by wearable devices should be analyzed locally by artificial intelligence (AI) algorithms for prescreening and then transmitted to hospitals via mHealth systems for simultaneous diagnosis and risk stratification. AI-enabled wearables and mHealth will enable the triaging of patients in the community into four categories: patients with suspected STEMI only, patients with suspected COVID-19&STEMI, patients with suspected COVID-19 only and population without COVID-19/STEMI.
For STEMI patients without risk of COVID-19, procedures that follow the guidelines for management of suspected STEMI patients including systemic thrombolysis or primary percutaneous coronary revascularization should be performed immediately after being transferred to hospital [6], [7]. Since asymptomatic COVID-19 cases may not be detected by wearables, a nucleic acid test should still be performed upon patients without risk for screening of COVID-19. All health care professionals should be protected and the patient should be managed and isolated as if he/she is infected with COVID-19 in this initial phase. Once a negative COVID-19 result is received, patients could be then managed in the usual cardiac care unit (CCU).
For STEMI patients with risk of COVID-19 as classified by vital signs, strict isolation should start immediately. Patients should be transferred to hospitals equipped to manage both COVID-19 patients and acute STEMI patients and follow the clinical guidelines [6], [7]. At the same time, nucleic acid testing should be performed for COVID-19 confirmation. If COVID-19 is confirmed after treatment, patients should be isolated strictly and transferred to the designated infectious disease hospitals for further treatment. If COVID-19 is excluded after treatment, patients could be transferred to a standard CCU.
For the population with low/mid/high risk of COVID-19 and no STEMI symptoms as classified by vital signs, it is recommended that the sub-population with low risk of COVID-19 should quarantine at home, while the sub-population with mid/high risk of COVID-19 should perform home testing if available or send pharyngeal swab/sputum specimen/blood samples to a testing center for detection of novel coronavirus nucleic acid. Confirmed patients with COVID-19 should be transferred to the designated infectious disease hospital/improvised hospital for further treatment.
For the population in high infection rate areas, periodic testing should be performed even if no symptoms presented/test negative/recovered/staying home for quarantine and wearable ECG should be monitored continuously for the patients who are at high cardiovascular risk (due to the presence of one or more risk factors such as hypertension, diabetes, hyperlipidaemia or already established disease) [11].
B. Physiological Monitoring During Patient Transfer
Intra- and inter-hospital patient transfer is an important issue of patient care. As the transfer of patients may induce various physiological alterations which may adversely affect the prognosis of the patient, it is proposed that wearable sensing techniques are utilized to maintain the continuity of medical care during transfer. The physiological data collected during transfer along with complete information from transferring facility should be kept as a reference for the receiving facility.
C. Physiological Monitoring for Caregivers
Until the prevalence of the disease in the general population of the country is firmly established, all patients with suspected STEMI should be considered as being potentially infected with COVID-19 [12]. Due to the inevitable contact with patients, health workers are at a high risk of exposures and infections. Thus, in addition to the patients’ care, physiological status monitoring for caregivers is of crucial importance. In this recommendation, it is proposed that wearable technology is utilized to address this issue whilst ensuring minimum interference to normal medical activities.
D. Tele-Rehabilitation
In addition, it is reported that 12.3% of individuals in hospital with COVID-19 died within 140 days following discharge, which indicated that recovered individuals from COVID-19 face higher mortality compared with the background levels [13]. After successful STEMI therapy and COVID-19 treatment, it is recommended that patients are continuously monitored with the use of a wearable device-based mHealth system during cardiac rehabilitation. Such technology has been shown in pre-pandemic times to significantly increase adherence rates to cardiac rehabilitation [14]. Remote follow-up of patients enrolled in home tele-rehabilitation programs can be performed using a wearable-based mHealth system to allow for the early detection of clinical deterioration and need for hospital readmission, to deliver cardiac rehabilitation in the home and to ensure the safety of patients and healthcare workers.
III. Remarks on Some Future Perspectives
According to the most recent Global Health Estimates from 2000 to 2019 released by the WHO on 9 December 2020, CVD has remained the global leading cause of death for the last 20 years. However, it is now killing more people than ever before [15]. In addition, the new variants of coronavirus seem to be more transmissible [16]. The steady increases in CVD and the evolving spread of COVID-19 are still the unprecedented public health crises of international concerns. It is projected that the total global deaths from CVDs and the total cases of COVID-19 infection will all continue to rise if no timely, effective and preventive measures are taken.
To call for urgent actions addressing the society's most pressing health challenges, IEEE-EMBS launched the COVID-19 initiative early this year after some extensive strategic discussions with different stakeholders at the society level. A framework on COVID-19 initiative was proposed with some specific areas including the development of sensors and mHealth for tracking and follow-up of elderly patients. It is clear that though COVID-19 and acute CVD (especially STEMI) are different diseases in nature, they both need a precise and rapid response system for their early diagnosis and intervention. The recommendation to use wearable sensors based mHealth discussed above should be helpful in this regard. mHealth has already been shown to be important in the management of CVD, through prevention of readmissions, promotion of self-care and adherence to cardiac rehabilitation programs [17]. Complementary to the tremendous efforts made by healthcare communities, medical device industries and governments, scientists and engineers are developing state-of-the art sensing technologies for tackling the challenges in combating COVID-19 and CVD. Here are some of examples of these initiatives.
Researchers at Massachusetts Institute of Technology (MIT) and Harvard University proposed a novel mask with wearable sensing function to reduce the risk of COVID-19 transmission [18]. The mask is designed with fluorescent material which would lights up when coronavirus in breathes, coughs, or sneezes is detected. If the technology proves successful, it could be integrated with mHealth contact tracing and other wearable physiological sensors stated above to identify COVID-patients at the early phases even before they become symptomatic, and be used for early quarantine while alerting potential contacts to keep a safe distance from infected ones.
A cough recording based AI model has been created by a research group from MIT, which could discriminate 98.5% COVID-19 positives [19]. Heart rate and heart rate variability obtained through smart watches have also been used to predict the infection more than one week before symptom onset [20], [21]. It is suggested that more parameters which could be monitored through wearable devices should be introduced into prediction models for pre-screening of COVID-19 and its variants and monitoring of cardiovascular status [22]. However, the acquisition of various parameters may raise the implications of having to equip several wearable devices. High compact multi-modal/multi-parameter wearable devices hold great promise to address this problem, which can be achieved unobtrusively by the integration and miniaturization of bioelectronics [3], [23], [24]. An alternative solution is to implement the flexible and stretchable devices in a body-adapted manner with minimum inference and discomfort induced to users in practical daily use [25]–[28].
Besides the use of wearable based mHealth for triaging COVID-19 and CVD discussed above, contact tracing is another important application where wearables and mHealth should be utilized for the improvement of both accuracy and responding time during pandemic. To date, the identification of contacts usually relies mostly on the memory of patient which can be highly unreliable due to the work, educational and social interactions of individuals. Contact tracing technology ensures no contacts are missed by integrating Bluetooth-based location systems with wearable sensors, providing a unique platform to perform widespread surveillance. Together with subsequent isolation and nucleic acid testing, contact tracing has been shown to reduce transmission of infectious diseases [29]. Ideally, precise and rapid contact tracing technology would allow one to identify asymptomatic patients accurately and in a timely manner, possibly avoiding the cumbersome large-scale testing and shutdown of entire communities or cities. To this end, several countries including Singapore and Australia rolled out COVID-19 tracing apps collecting proximity data based on Bluetooth connectivity, with the overall approaches based on voluntary uptake, protected by legislation [30]. These apps have helped to shape and guide public health responses to assist individuals and communities cope with local outbreaks of the virus. However, it has been postulated that successful tracing requires a widespread adoption with at least 80% of all smartphone users using the app, or 56% of the population overall [31]. Therefore, the support and promotion from government is essential to ensure the effectiveness of mHealth and contact tracing technology for the benefits of both acute CVD and COVID-19 patients. In addition, in order to widely deploy the wearable-based mHealth, joint efforts among scientists, physicians, industrialists, government officials and policy makers are needed to address the ethical, practical, legal, and technical issues to improve user acceptance and ensure privacy protection [32].
Based on the data from WHO, ischemic heart disease, responsible for 16% of total deaths, is the world's biggest killer by a single disease and the number of deaths is rising [33]. Therefore, even after the COVID-19 pandemic is over, the continuous monitoring of those with acute and chronic CVD remains of crucial importance for early prediction and the longer-term closed loop management of CVD patients. Traditionally the diagnosis of acute ischemia relies on a 12-lead ECG which suffers from many problems associated with surface electrodes and cables, making it unsuitable for continuous and long-term monitoring. Wearable devices with single or even 6-lead ECGs have limited ability to diagnose acute CVD, such as STEMI, which requires ST elevation in at least two consecutive limb or chest leads. A possible solution could be soft ECG devices using E-textile or flexible and stretchable materials [34], [35] with integrated mHealth systems.
Wearable and mHealth technology can also play an important role in the closed loop management of CVD patients post-pandemic especially during the whole process of vaccination. Several vaccines have been approved for limited/full use, including mainly four types, genetic vaccines [36], [37], viral vector vaccines [38], protein-based vaccines [39] and inactivated or attenuated coronavirus vaccines [40]. It is indeed remarkable that scientists could develop these many effective coronavirus vaccines in record time with only some general short-term side-effects such as fever, tiredness, headache, muscle pain, nausea, and vomiting [37]. As long-term safety of vaccines is still a subject of investigation, mHealth systems with wearable and flexible sensors can be used, together with nucleic acid testing, for the precise tracking of the long-term safety and efficacy of these pharmaceuticals. Thus, the extended use of wearable-based mHealth system has enormous and wide-ranging applications in the closed-loop management of CVD patients during the post-pandemic.
Funding Statement
This work was supported in part by the ITC and internal starting-up grant of City University of Hong Kong. The work of David A. Clifton was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC).
Contributor Information
Nan Ji, Email: nanji3-c@my.cityu.edu.hk.
Ting Xiang, Email: txiang@cityu.edu.hk.
Paolo Bonato, Email: pbonato@mgh.harvard.edu.
Nigel H. Lovell, Email: n.lovell@unsw.edu.au.
Sze-Yuan Ooi, Email: szeyuan@unsw.edu.au.
David A. Clifton, Email: david.clifton@eng.ox.ac.uk.
Metin Akay, Email: makay@uh.edu.
Xiao-Rong Ding, Email: xiaorong.ding@uestc.edu.cn.
Bryan P. Yan, Email: bryan.yan@cuhk.edu.hk.
Vincent Mok, Email: vctmok@cuhk.edu.hk.
Dimitrios I. Fotiadis, Email: fotiadis@cc.uoi.gr.
Yuan-Ting Zhang, Email: yt.zhang@cityu.edu.hk.
References
- [1].“Fear of COVID-19 leads other patients to decline critical treatment,” Accessed: Feb. 8, 2021. [Online]. Available: https://www.nytimes.com/2020/05/25/health/coronavirus-cancer-heart-treatment.html
- [2].“COVID-19 significantly impacts health services for noncommunicable diseases,” Accessed: Feb. 8, 2021. [Online]. Available: https://www.who.int/news/item/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases
- [3].Ding X. R., et al. “Wearable sensing and telehealth technology with potential applications in the coronavirus pandemic,” IEEE Rev. Biomed. Eng., vol. 14, pp. 48–70, 2021. [DOI] [PubMed] [Google Scholar]
- [4].Ji N., et al. “Potential applications of wearable sensors in closed-loop management of STEMI patients during pandemics,” presented at 42nd Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., 2020.
- [5].Adans-Dester C. P., et al. “Can mHealth technology help mitigate the effects of the COVID-19 pandemic?,” IEEE Open J. Eng. Med. Biol., vol. 1, pp. 243–248, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jing Z. C., et al. “Recommendations from the peking union medical college hospital for the management of acute myocardial infarction during the COVID-19 outbreak,” Eur. Heart J , vol. 41, no. 19, pp. 1791–1794, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dehghani P., et al. “North american COVID-19 ST-segment elevation myocardial infarction (NACMI) registry: Rationale, design, and implications,” Amer. Heart J., vol. 227, pp. 11–18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].”Coronavirus disease (COVID-19),” Accessed: Dec. 14, 2020. [Online]. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19
- [9].“Symptoms of Coronavirus,” Accessed: Dec. 12, 2020. [Online]. Available: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
- [10].Kannel W. B., et al. “Blood pressure and survival after myocardial infarction: The framingham study,” Amer. J. Cardiol., vol. 45, no. 2, pp. 326–330, 1980. [DOI] [PubMed] [Google Scholar]
- [11].“Cardiovascular diseases (CVDs),” Accessed: Jan. 25, 2021. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- [12].Mahmud E., et al. “Management of acute myocardial infarction during the COVID-19 pandemic,” J. Amer. College Cardiol. , 2020. [DOI] [PMC free article] [PubMed]
- [13].Ayoubkhani D., et al. “Epidemiology of post-COVID syndrome following hospitalisation with coronavirus: A retrospective cohort study,” Medrxiv, 2021, doi: 10.1101/2021.01.15.21249885. [DOI]
- [14].Del Rosario M. B., et al. “Evaluation of an mHealth-based adjunct to outpatient cardiac rehabilitation,” IEEE J. Biomed. Health Inform., vol. 22, no. 6, pp. 1938–1948, 2017. [DOI] [PubMed] [Google Scholar]
- [15].“WHO reveals leading causes of death and disability worldwide: 2000–2019,” Accessed: Dec. 22, 2020. [Online]. Available: -2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019. https://www.who.int/news/item/09-12 [Google Scholar]
- [16].“New coronavirus variant: What do we know?,” Accessed: Dec. 23, 2020. [Online]. Available: https://www.bbc.com/news/health-55388846
- [17].Indraratna P., et al. “mHealth interventions in the management of heart failure, ischaemic heart disease and hypertension: A systematic review and meta-analysis of randomised controlled trials,” Eur. Heart J., vol. 41, no. Supplement_2, 2020, Art. no. ehaa946. [Google Scholar]
- [18].“Harvard and MIT researchers are developing a face mask that lights up when it detects the coronavirus,” Accessed: Dec. 10, 2020. [Online]. Available: https://www.businessinsider.com/coronavirus-face-mask-light-up-screening-tool-test-2020-5
- [19].Laguarta J., et al. “COVID-19 artificial intelligence diagnosis using only cough recordings,” IEEE Open J. Eng. Med. Biol., vol. 1, pp. 275–281, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mishra T., et al. “Pre-symptomatic detection of COVID-19 from smartwatch data,” Nat. Biomed. Eng., vol. 4, no. 12, pp. 1208–1220, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hirten R. P., et al. “Longitudinal physiological data from a wearable device identifies SARS-CoV-2 infection and symptoms and predicts COVID-19 diagnosis,” medRxiv, 2020, doi: 10.1101/2020.11.06.20226803. [DOI] [PMC free article] [PubMed]
- [22].Jeong H., et al. “Continuous on-body sensing for the COVID-19 pandemic: Gaps and opportunities,” Sci. Adv., vol. 6, no. 36, 2020. Art. no. eabd4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Seshadri D. R., et al. “Wearable sensors for monitoring the physiological and biochemical profile of the athlete,” NPJ Digit. Med. , vol. 2, no. 1, pp. 1–16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Poon C. C., et al. “M-health: The development of cuff-less and wearable blood pressure meters for use in body sensor networks,” in Proc. IEEE/NLM Life Science Systems Applications Workshop, 2006, doi: 10.1109/LSSA.2006.250377. [DOI] [Google Scholar]
- [25].Rogers J., et al. “Long-term, continuous, and multimodal monitoring of respiratory digital biomarkers via wireless epidermal mechano-acoustic sensing in clinical and home settings for COVID-19 patients,” 2020. [Online]. Available: https://www.researchsquare.com/article/rs-102060/v1
- [26].Liu Y. M., et al. “Epidermal electronics for respiration monitoring via thermo-sensitive measuring,” Mater. Today Phys., vol. 13, 2020, Art. no. 100199. [Google Scholar]
- [27].Yoon S., et al. “A flexible and wearable human stress monitoring patch,” Sci. Reps., vol. 6, no. 1, pp. 1–11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang S., et al. “‘Cut-and-paste’ manufacture of multiparametric epidermal sensor systems,” Adv. Mater., vol. 27, no. 41, pp. 6423–6430, 2015. [DOI] [PubMed] [Google Scholar]
- [29].Faye O., et al. “Chains of transmission and control of ebola virus disease in conakry, guinea, in 2014: an observational study,” Lancet Infect. Dis., vol. 15, no. 3, pp. 320–326, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Goggin G., et al. “COVID-19 apps in singapore and australia: Reimagining healthy nations with digital technology,” Media Int. Aust. , vol. 177, no. 1, pp. 61–75, 2020. [Google Scholar]
- [31].Hinch R., et al. “Effective configurations of a digital contact tracing app: A report to NHSX,” 2020. [Online]. Available: https://cdn.theconversation.com/static_files/files/1009/Report_-_Effective_App_Configurations.pdf
- [32].Braithwaite I., et al. “Automated and partly automated contact tracing: A systematic review to inform the control of COVID-19,” in Proc. Lancet Digital Health , 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].“The top 10 causes of death,” Accessed: Dec. 22, 2020. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- [34].Kuroda T., et al. “Evaluation of NISHIJIN e-textile for 12-lead ECG measurement through automatic ECG analyzer,” in Proc. 39th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., 2017. [DOI] [PubMed] [Google Scholar]
- [35].Rao R. K., et al. “Electronic skin patch for real time monitoring of cardiac activity and personal health management,” U.S. Patent No. 8,734,339, May 2014.
- [36].“Pfizer-BioNTech COVID-19 Vaccine,” Accessed: Jan. 2, 2021. [Online]. Available: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
- [37].“Moderna COVID-19 Vaccine,” Accessed: Jan. 2, 2021. [Online]. Available: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
- [38].“The Sputnik V Vaccine's efficacy is confirmed at 91.4% based on data analysis of the final control point of clinical trials,” Accessed: Jan. 3, 2021. [Online]. Available: https://sputnikvaccine.com/newsroom/pressreleases/the-sputnik-v-vaccine-s-efficacy-is-confirmed-at-91-4-based-on-data-analysis-of-the-final-control-po/ [Google Scholar]
- [39].“Russia approves second covid-19 vaccine after preliminary trials,” Accessed: Jan. 3, 2021. [Online]. Available: https://www.cnbc.com/2020/10/14/russia-approves-second-covid-19-vaccine-after-preliminary-trials-.html
- [40].“China approves covid-19 vaccine as it moves to inoculate millions,” Accessed: Jan. 3, 2021. [Online]. Available: https://www.nytimes.com/2020/12/30/business/china-vaccine.html


