Significance
Although emerging evidence suggests that the STING-mediated immune response pathway plays a crucial role in microbial pathogen infection, few bacterial effectors have been reported to target this pathway. Here, we identified a T6SS-secreted micropeptide, TssS, which is crucial for the pathogenesis of Yptb. Distinct from traditional bacterial effectors that target host proteins or other macromolecules, TssS inhibits STING oligomerization and downstream signaling pathways by chelating Mn2+. Thus, TssS mediates a previously unrecognized immune evasion mechanism by modulating the availability of immunostimulatory Mn2+ in host cells. This finding reveals a strategy to modulate the STING pathway by microbial pathogens, provides a new perspective on the role of T6SS in pathogenesis, and highlights the importance of micropeptides in pathogen–host interactions.
Keywords: type VI secretion system (T6SS), micropeptide, manganese, innate immunity, STING
Abstract
Cellular ionic concentrations are a central factor orchestrating host innate immunity, but no pathogenic mechanism that perturbs host innate immunity by directly targeting metal ions has yet been described. Here, we report a unique virulence strategy of Yersinia pseudotuberculosis (Yptb) involving modulation of the availability of Mn2+, an immunostimulatory metal ion in host cells. We showed that the Yptb type VI secretion system (T6SS) delivered a micropeptide, TssS, into host cells to enhance its virulence. The mutant strain lacking TssS (ΔtssS) showed substantially reduced virulence but induced a significantly stronger host innate immune response, indicating an antagonistic role of this effector in host antimicrobial immunity. Subsequent studies revealed that TssS is a Mn2+-chelating protein and that its Mn2+-chelating ability is essential for the disruption of host innate immunity. Moreover, we showed that Mn2+ enhances the host innate immune response to Yptb infection by activating the stimulator of interferon genes (STING)-mediated immune response. Furthermore, we demonstrated that TssS counteracted the cytoplasmic Mn2+ increase to inhibit the STING-mediated innate immune response by sequestering Mn2+. Finally, TssS-mediated STING inhibition sabotaged bacterial clearance in vivo. These results reveal a previously unrecognized bacterial immune evasion strategy involving modulation of the bioavailability of intracellular metal ions and provide a perspective on the role of the T6SS in pathogenesis.
The type VI secretion system (T6SS) is a widely distributed phage tail-like protein export apparatus that delivers effectors into neighboring cells (1, 2). While primarily recognized as a bacterial weapon for delivering a vast array of enzymatic effectors into targeted prokaryotic cells (3, 4), some T6SSs associated with pathogens are necessary for full virulence, as they inject anti-eukaryotic effectors into host cells that interact with the microtubule network, promote bacterial escape into the cytoplasm, and modulate host inflammation (5–9). To expand our understanding of the versatile T6SS nanomachine, the identification of more anti-eukaryotic T6SS effectors that promote bacterial virulence in eukaryotic cells is needed.
The T6SS is also involved in the acquisition of metal ions such as manganese (Mn2+) through the release of metal-chelating effectors (10). Mn2+ is an essential micronutrient that serves as a cofactor for enzymes involved in intermediary metabolism and adaptation to oxidative stress (11). Mn2+ is also required by bacterial pathogens to establish an infective lifestyle within the host through the detoxification of reactive oxygen species released by immune cells (12). Recently, Wang et al. identified a novel role of Mn2+ in orchestrating host innate immune responses by acting as an activator of the cyclic GMP-AMP (cGAMP) synthase (cGAS)-stimulator of interferon genes (STING) pathway (13). Upon viral infection, Mn2+ is released from membrane-enclosed organelles into the cytosol, where it binds to cGAS to increase its enzymatic activity and sensitivity to double-stranded DNA. In addition, Mn2+ promotes STING activity by enhancing cGAMP–STING-binding affinity (13). This finding raises the question of whether metal-chelating T6SS effectors can be translocated into host cells to modulate the STING-mediated innate immune response by sequestering Mn2+, thus acting as anti-eukaryotic effectors.
As a cytosolic pattern recognition receptor (PRR), cGAS is thought to be the main receptor that senses cytosolic DNA (14) and subsequently produces the second messenger 2′3′-cGAMP (15), which binds STING with high affinity and triggers its oligomerization and translocation (16). The activation of STING activates the kinase TBK1 that leads to phosphorylation of the transcription factor IRF3, which is dimerized and translocated to the nucleus to induce the expression of type I interferons (IFNs) and IFN-stimulated genes (ISGs) (17). In addition to 2′3′-cGAMP, STING can also recognize bacterial cyclic dinucleotides such as cyclic dimeric guanosine monophosphate (c-di-GMP) (18), cyclic dimeric adenosine monophosphate (c-di-AMP) (19), and 3′3′-cGAMP (20), ubiquitous bacterial second messengers. Primarily identified as a direct sensor of cyclic dinucleotides (18, 19), the function of the STING pathway has been extensively studied in the context of microbial infections, autoinflammatory diseases, cancer, and other sterile inflammations (21–23). However, the role of STING in host immune response against bacterial infection remains controversial (24).
While studies on Yersinia outer proteins (Yops) secreted by the type III secretion system (T3SS) have provided tremendous information about bacterial pathogenic strategies (25–27), the role of Yersinia T6SS effectors in regulating the host cellular response remains largely unknown, despite the identification of four T6SS gene clusters in Yersinia pseudotuberculosis (Yptb) (28, 29). Here, we identified a T6SS-secreted micropeptide, TssS (T6SS-secreted micropeptide suppressing STING), which plays crucial roles in the pathogenesis of Yptb. Mechanistically, TssS deploys a unique virulence strategy by sequestering immunologically active Mn2+ in the host cell, antagonizing the STING-mediated innate immune response.
Results
A Micropeptide Translocated by the T6SS Contributes to Yptb Virulence.
Previously, it was reported that the Yptb T6SS4 functions in resistance to oxidative stress and host nutritional immunity by secreting a zinc-binding effector, YezP (YPK_3549), which is located near the end of the T6SS4 gene cluster (30). Interestingly, immediately downstream of the YPK_3549 ORF is a 48-residue micropeptide encoding ORF (YPK_3548, hereafter referred to as TssS) that is transcribed in the same direction as those of the T6SS4 genes, suggesting that this gene is part of the T6SS4 operon (Fig. 1A and SI Appendix, Fig. S1A).
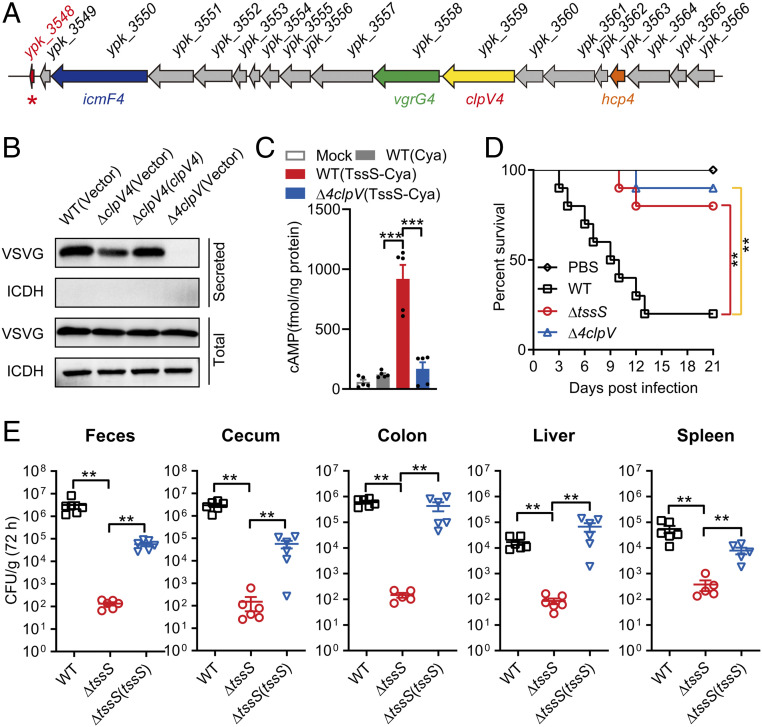
Fig. 1.
A T6SS-secreted micropeptide contributes to Yptb virulence. (A) Structure of the Yptb T6SS4 gene cluster. The tssS gene (ypk_3548) is indicated with a red asterisk. (B) TssS is a T6SS4 effector. An immunoblot analysis of vesicular stomatitis viral glycoprotein (VSVG)-tagged TssS protein levels in culture supernatant of the relevant Yptb strains is shown. The cytoplasmic protein isocitrate dehydrogenase (ICDH) was used as a loading control and lysis control for the total and secreted fractions. (C) Raw264.7 cells were mock infected or infected with indicated Yptb strains expressing Cya-fused TssS at a multiplicity of infection of 20 for 2 h. Cyclic AMP present in lysates was measured. (D) C57BL/6 mice were intragastrically inoculated with Yptb WT, Δ4clpV, or ΔtssS. The survival rate of the mice was determined. (E) C57BL/6 mice were intragastrically inoculated with Yptb WT, ΔtssS, or ΔtssS(tssS). Homogenates of different tissues were plated to determine the bacterial CFU count per gram of organs at 72 h postinfection. Error bars represent ± SEM; **P < 0.01; ***P < 0.001.
We first examined whether the TssS micropeptide is a T6SS-secreted effector like YezP. As shown in Fig. 1B, while significant amounts of TssS could be readily detected in the culture supernatant of Yptb wild-type (WT) culture, its secretion was substantially abrogated in the ΔclpV4 mutant and could be rescued to the WT level through complementation. The residual secretion exhibited by the ΔclpV4 mutant indicates potential cross-recognition of this effector by other T6SSs, as TssS secretion was completely abrogated in the Δ4clpV mutant (a mutant defective in all four T6SSs, SI Appendix, Fig. S1B), and complementation with any clpV gene only partially restored its secretion (SI Appendix, Fig. S1B). Notably, complementation of the clpV4 gene rescued TssS secretion to a large extent, suggesting that TssS is a substrate mainly secreted by T6SS4, although it can also be secreted by other T6SSs. This notion was further substantiated by the finding that TssS binds directly to Hcp4 (SI Appendix, Fig. S1C), the T6SS component that acts as a carrier protein for the secretion of multiple T6SS effectors (31).
To examine whether TssS is translocated into the cytosol of mammalian cells by the T6SS, the Bordetella pertussis cyclic AMP (cAMP) synthetase (Cya) reporter assay was employed in which the TssS-Cya fusion protein requires the cofactor calmodulin, which is only present in the cytosol of eukaryotic cells (32). Thus, intracellular cAMP levels could reflect intracellular translocation of the TssS-Cya fusion protein. As expected, high levels of intracellular cAMP were detected only in cells infected with the Yptb WT strain expressing TssS-Cya but not in those infected with the Δ4clpV mutant strain that expresses TssS-Cya (Fig. 1C), indicating that TssS is translocated into host cells during Yptb infection in a T6SS-dependent manner. The translocation of TssS into mammalian cells was confirmed using the TEM1 (β-lactamase) assay by fusing the TEM1 reporter protein to the C terminus of TssS. The T6SS-dependent translocation of TssS into HeLa cells was determined by fluorescence resonance energy transfer using the β-lactamase cleavable substrate CCF2/AM, which, upon excitation at 409 nm, emits green fluorescence (520 nm) in the absence of β-lactamase and emits blue fluorescence (447 nm) in the presence of β-lactamase (9). Consistently, fluorescence microscopy revealed that the cells showed blue fluorescence upon infection with the WT strain expressing TssS-TEM1 but exhibited green fluorescence after infection with the Δ4clpV mutant expressing TssS-TEM1 (SI Appendix, Fig. S1D).
The finding that TssS is translocated into mammalian cells indicated the role of TssS in Yptb virulence. To explore this possible association, Yptb WT, ΔtssS, and Δ4clpV were intragastrically inoculated into C57BL/6 mice, and the survival rates of each group were analyzed. The results showed that infection with the WT strain led to more than 80% death within 2 wk of infection. Surprisingly, significantly fewer mice were dead in the ΔtssS mutant-infected group (Fig. 1D). These results indicated that the TssS micropeptide is vital to the virulence of Yptb. A slightly higher survival rate was observed in the Δ4clpV mutant-infected mice, suggesting that other T6SS effectors contribute to the virulence of Yptb (30). Next, the bacterial loads recovered from the cecum, feces, colon, liver, and spleen were counted at 72 h postinfection with Yptb. Consistently, mice infected with ΔtssS had significantly fewer colony-forming units (CFU) per gram of tissue compared to WT-infected mice. Moreover, the complementation of tssS significantly restored the bacterial loads of the ΔtssS mutant in various tissues (Fig. 1E). Together, we characterized a T6SS-secreted micropeptide that plays crucial roles in the virulence of Yptb.
TssS Inhibits the Host Innate Immune Response.
To the best of our knowledge, no small bacterial effector (less than 48 amino acid [aa]) has been reported to play an important role in bacterial virulence. Therefore, we performed transcriptomic analysis using RNA sequencing (RNA-seq) to identify differentially expressed host genes upon WT and ΔtssS infection. We found that immune system and signal transduction pathways were differentially induced between ΔtssS and WT infection in mouse peritoneal macrophages (PMs) (SI Appendix, Fig. S2A). Gene set enrichment analysis results showed that many of these differentially induced genes belong to IFN- and STING-related signaling pathways (SI Appendix, Fig. S2B). Specifically, many ISGs were induced preferentially by ΔtssS in PMs (Fig. 2A). To validate the RNA-seq results, the expression of many ISGs including type I IFN Ifnb1 and the IRF3-responsive genes Rsad2, Isg15, Ifit1, Irf7, and Ifit2 was measured (13). Consistently, compared to infection with the WT strain, ΔtssS infection provoked higher expression levels of these genes in mouse PMs (Fig. 2 B and C). In particular, the expression of Ifnb1 and IRF3-responsive genes was elevated in ΔtssS-infected cells (Fig. 2B). Moreover, the phosphorylation of IRF3 and TBK1, hallmarks of IFN-related innate immune activation, and the protein expression of the IRF3-responsive gene ISG15 were more strongly induced by ΔtssS infection (Fig. 2C), suggesting that TssS inhibits the Yptb-induced IFN-related host innate immune response. Notably, ISG expression was comparable upon infection with the WT and the complemented strain ΔtssS(tssS), further corroborating the role of TssS in regulating host innate immunity (Fig. 2 B and C). Similarly, qRT-PCR results obtained from the cecum of infected mice confirmed that numerous ISGs were elicited at higher levels in ΔtssS-infected mice (Fig. 2D), despite fewer bacteria being recovered from their cecum (Fig. 1E). Together, these results indicated that TssS is important for inhibiting Yptb-elicited innate immunity.
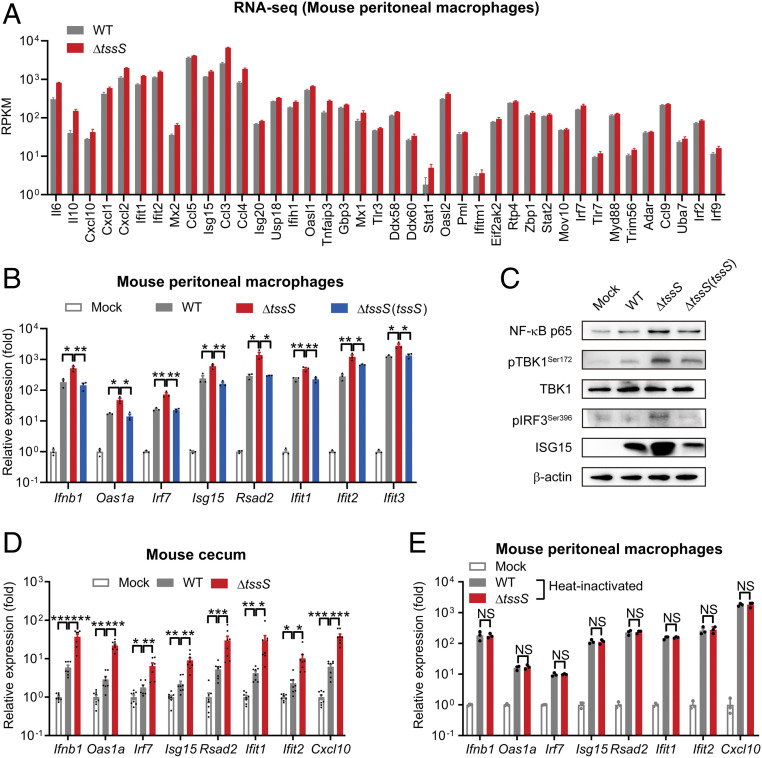
Fig. 2.
TssS inhibits the host innate immune response. (A) RNA-seq results of different ISGs of mouse PMs infected with Yptb WT or ΔtssS. RPKM: reads per kilobase of exon model per million mapped reads. (B) qRT-PCR analysis of the gene expression in PMs infected with Yptb WT, ΔtssS, or ΔtssS(tssS). (C) Immunoblot analysis of the protein expression in PMs infected with Yptb WT, ΔtssS, or ΔtssS(tssS). (D) qRT-PCR analysis of gene expression in the cecum of C57BL/6 mice mock infected or infected intragastrically with Yptb WT or ΔtssS for 72 h. (E) qRT-PCR analysis of gene expression in PMs infected with heat-inactivated strains. Data in B, D, and E were normalized to mock-infected control (Mock, set as 1), and Gapdh was used as the housekeeping gene. Error bars represent ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
Notably, the lipopolysaccharide (LPS) content in the ΔtssS mutant was the same as that in the WT strain (SI Appendix, Fig. S2C), indicating that different innate immune responses induced by WT and ΔtssS were not due to different LPS content. In addition, the difference in capacity to induce ISGs was not due to the growth advantage of ΔtssS, as it had a similar growth curve to WT in Yersinia lysogeny broth (YLB) medium (SI Appendix, Fig. S2D) and attenuated growth ability in Raw264.7 cells (SI Appendix, Fig. S2E). To further explore the role of TssS in the Yptb-induced innate immune response, we inactivated both the WT and ΔtssS strains through heating or ultraviolet (UV ) irradiation to eliminate the active secretion of bacterial effectors and used the inactivated bacterial cells to treat PMs. Similar ISG expression patterns were elicited by these inactivated strains, indicating that a secreted and functional TssS protein is essential to the observed inhibition (Fig. 2E and SI Appendix, Fig. S2F). Together, these results demonstrated that TssS plays crucial roles in suppressing the Yptb-induced host innate immune response.
TssS Is a Mn2+-Binding Effector, and Its Mn2+-Binding Activity Is Required for Yptb Virulence.
Recently, the T6SS4 of Burkholderia thailandensis was reported to be critical for combating oxidative stress by importing Mn2+ (10). Given the high similarity of their operon structures (Fig. 1A and SI Appendix, Fig. S3), we speculated that the T6SS4 of Yptb might also be involved in Mn2+ acquisition. Indeed, the survival of bacteria under oxidative stress and intracellular Mn2+ levels were both significantly reduced in the ΔclpV4 mutant (SI Appendix, Fig. S4 A and B), indicating that Yptb T6SS4 has the capacity for Mn2+ uptake to combat oxidative stress.
Previous research showed that B. thailandensis T6SS4 secreted a Mn2+-binding effector TseM (BTH_II1883, 141 aa), which is located at the end of the T6SS4 gene cluster, for importing Mn2+ (10). Speculatively, it is highly likely that TssS, which is located at the end of the T6SS4 gene cluster in Yptb, is also a Mn2+-binding protein, although no sequence similarity was found between these proteins. To experimentally assess the Mn2+-binding potential of TssS, atomic absorption spectrometry analysis was employed, which showed that purified recombinant TssS could specifically bind Mn2+ but not Ca2+, Mg2+, or Zn2+ (Fig. 3A). A binding Kd (6.83 μM) comparable to that of TseM (2.87 μM) was obtained through isothermal titration calorimetry (ITC) (Fig. 3B). The mutation of Glu30, Glu36, His43, and Gln44 (TssSE30A/E36A/H43A/Q44A, TssS*) (SI Appendix, Fig. S4C), residues predicted to be crucial for Mn2+-binding proteins (33–35), dramatically reduced the binding affinity of TssS for Mn2+ (Kd = 165.2 μM) (Fig. 3B). Notably, the TssS* protein exhibited similar properties to TssS in protein expression, secretion, and stability (SI Appendix, Fig. S4 D–G). Consistent with the report that TseM is required for T6SS-dependent Mn2+ acquisition under oxidative stress in B. thailandensis, deletion of tssS in Yptb markedly reduced intracellular Mn2+ accumulation under oxidative stress conditions, and complementation of the tssS gene restored intracellular Mn2+ to the WT level (Fig. 3C). However, deletion of tssS did not affect the accumulation of Fe, Mg, or Ca ions under the same conditions (SI Appendix, Fig. S4H). These results confirmed that TssS is an Mn2+-binding protein involved in Mn2+ acquisition.
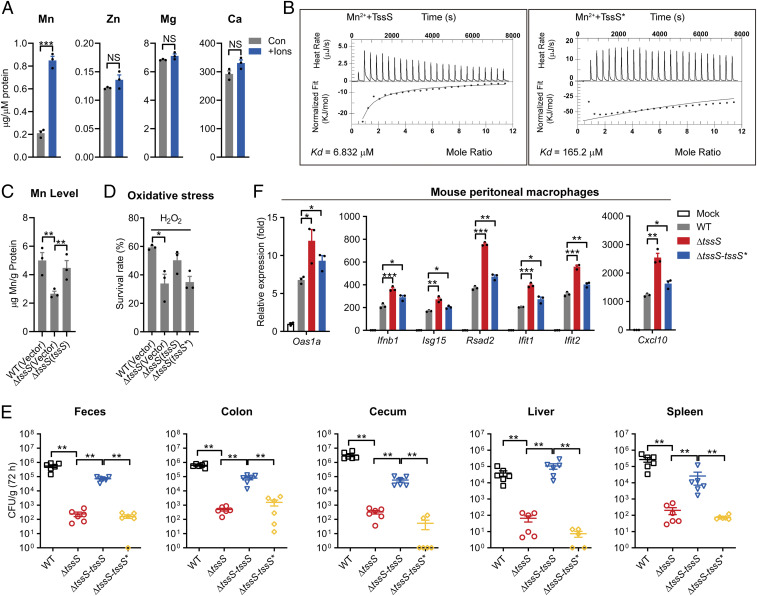
Fig. 3.
TssS is a Mn2+-binding micropeptide, and its Mn2+-binding activity is required for Yptb virulence. (A) The binding of divalent ions by TssS was detected by atomic adsorption spectrometry. (B) The binding of Mn2+ with Mn2+-free TssS protein or TssS* (TssSE30A/E36A/H43A/Q44A mutated protein) was determined via ITC. (C) Stationary-phase Yptb WT, ΔtssS, mutant and ΔtssS(tssS) were exposed to 2.5 mM H2O2 for 40 min in M9 medium with 1 μΜ MnSO4. Mn2+ associated with bacterial cells was measured by ICP-MS. (D) The viability of Yptb strains under oxidative stress was determined. Yptb WT, ΔtssS, ΔtssS(tssS), and ΔtssS(tssS*) were exposed to H2O2 (1 mM) for 40 min in M9 medium. (E) C57BL/6 mice were intragastrically inoculated with the Yptb WT, ΔtssS, ΔtssS-tssS, or ΔtssS-tssS*, respectively. Homogenates of different tissues were plated to determine the bacterial CFU counts per gram of organs at 72 h postinfection. (F) qRT-PCR analysis of the gene expression in PMs infected with Yptb WT, ΔtssS, ΔtssS-tssS, or ΔtssS-tssS*, respectively. Data in F were normalized to mock-infected control (Mock, set as 1), and Gapdh was used as the housekeeping gene. Error bars represent ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
As an Mn-binding effector of oxidative stress-resistant T6SS4, TssS is expected to be necessary for maximizing bacterial survival under conditions of oxidative stress. Indeed, ΔtssS exhibited a significantly lower survival rate under oxidative stress, and this sensitivity could be restored to the WT level through complementation with the tssS gene but not with the Mn2+-binding–defective tssS* mutant gene (Fig. 3D). To further explore whether the Mn2+-binding potential is required for TssS to exert its immunoregulatory function, we generated two complemented strains (ΔtssS-tssS and ΔtssS-tssS*) by knock-in of the WT tssS or the Mn2+-binding–defective mutant gene tssS*, respectively, at the original site on the ΔtssS chromosome. As shown in Fig. 3E, significantly fewer bacteria were recovered from mice infected with the ΔtssS-tssS* strain than from mice infected with ΔtssS-tssS, indicating that the Mn2+-binding ability of TssS is crucial for its function in bacterial virulence. Indeed, the bacterial burdens were comparable between ΔtssS-infected and ΔtssS-tssS*–infected mice. Notably, the growth ability of the ΔtssS-tssS* strain in PMs was similar to that of the ΔtssS mutant (SI Appendix, Fig. S4I). In addition, the ISG expression induced by ΔtssS-tssS* infection was also comparable to that induced by the ΔtssS strain (Fig. 3F). These results indicated that infection with the Mn2+-binding–defective strain ΔtssS-tssS* phenocopied ΔtssS mutant infection, suggesting that TssS requires its Mn2+-binding ability to function in bacterial virulence and innate immune regulation.
Mn2+ Primes the Host Defense against Yptb Infection via the STING Pathway.
Mn2+ enhances antiviral innate immunity by potentiating the cGAS-STING pathway (13). However, the protective role of Mn2+ against bacterial infection remains unknown. Therefore, we pretreated mice with MnCl2 (25 mg ⋅ kg−1) and infected them intragastrically with the Yptb WT strain. The results showed that Mn2+ pretreatment significantly increased the survival of mice infected with Yptb WT (Fig. 4A). Correspondingly, mice pretreated with Mn2+ had significantly fewer CFU per gram of tissue (cecum, feces, colon, liver, and spleen) compared to untreated mice (Fig. 4B). In addition, in Yptb-infected PMs, Mn2+ pretreatment significantly enhanced the gene expression of type I IFN Ifnb1 and IRF3-responsive genes Rsad2, Isg15, and Ifit1 (Fig. 4C). Consistently, Mn2+ pretreatment significantly increased Yptb-activated ISG15 and IFIT2 expression at the protein level (Fig. 4D). In particular, Yptb infection–induced phosphorylation of IRF3 and TBK1, hallmarks of STING-mediated immune response activation, was strongly potentiated by Mn2+ pretreatment (Fig. 4D), suggesting that Mn2+ enhances the STING-mediated host innate immune response. Moreover, ΔtssS infection–induced higher ISG expression was further enhanced with Mn2+ pretreatment (SI Appendix, Fig. S5 A and B). Previous research characterized c-di-GMP as a pathogen-associated molecular pattern that could activate the host immune response through direct binding to STING (18). Interestingly, the c-di-GMP–induced ISG expression in bone marrow–derived macrophages (BMDMs) was further potentiated by Mn2+ treatment (SI Appendix, Fig. S5C).
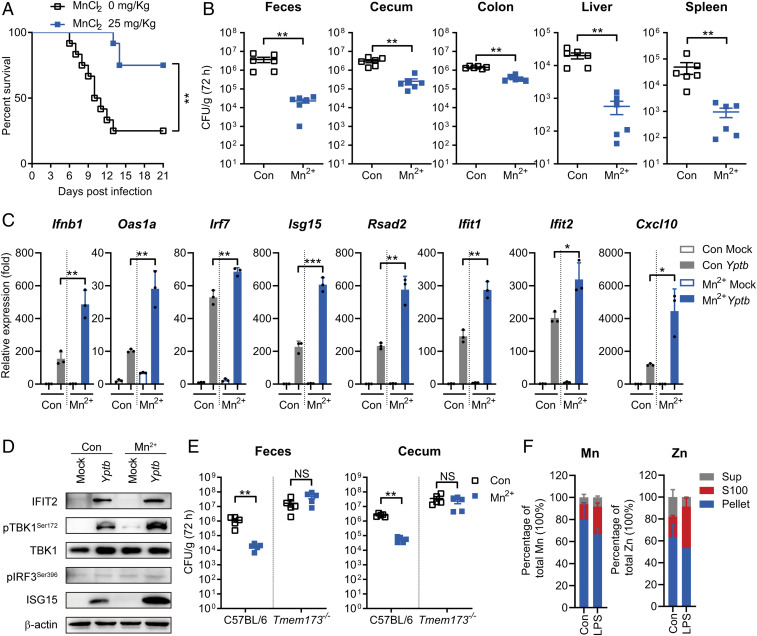
Fig. 4.
Mn2+ primes the antimicrobial immune response via STING. (A and B) C57BL/6 mice were pretreated with or without MnCl2 (25 mg ⋅ kg−1) intraperitoneally for 24 h and were intragastrically inoculated with Yptb WT. (A) The survival rate of the mice was determined. (B) Homogenates of different tissues were plated to determine the bacterial CFU counts per gram in the indicated organs of the untreated (Con) or Mn2+-treated (Mn2+) mice at 72 h postinfection. (C and D) qRT-PCR analysis of the gene expression (C) and immunoblot analysis of the protein (D) in PMs untreated (Con) or pretreated with 100 μM MnCl2 (Mn2+) for 24 h and mock infected (Mock) or infected with Yptb WT. (E) C57BL/6 or Tmem173−/− mice were untreated (Con) or pretreated with MnCl2 (25 mg ⋅ kg−1) (Mn2+) intravenously for 24 h and intragastrically inoculated with Yptb WT. Homogenates of feces and cecum were plated to determine the bacterial CFU numbers per gram of organs at 72 h postinfection. (F) Mn and Zn concentrations in the indicated cellular components and culture medium (Sup). Raw264.7 cells were untreated (Con) or treated with LPS (4 μg ⋅ mL−1). Data in C were normalized to untreated, mock-infected control (Con Mock, set as 1). Gapdh was used as the housekeeping gene. Error bars represent ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
To further assess the physiological significance of Mn2+ in defending against Yptb infection, weaned mice were fed ad libitum with an Mn-deficient diet for 6 wk to generate Mn-deficient mice as described (13). Mn-deficient mice were intragastrically infected with the Yptb WT strain. The bacterial loads of Mn-deficient mice were significantly higher than those of control mice at 72 h postinfection (SI Appendix, Fig. S5D). We next evaluated the cellular immune response to bacterial infection under Mn2+-deficient conditions. PMs from Mn2+-deficient mice were cultured in serum-free optimized minimal essential medium, which contains an untraceable level of Mn2+. The ISG expression in these Mn-deficient macrophages against Yptb infection was significantly attenuated (SI Appendix, Fig. S5E). Consistently, the cellular responses to c-di-GMP were also significantly attenuated in Mn2+-deficient macrophages (SI Appendix, Fig. S5F). Together, these results indicated that Mn2+ is important for host defense against Yptb infection.
To test directly whether Mn2+ protected the host from Yptb infection via STING, C57BL/6 or STING-deficient (Tmem173−/−) mice were pretreated with MnCl2, and bacterial burdens in mice were measured 72 h postinfection. Although Mn2+ pretreatment significantly reduced the bacterial burdens in the feces and cecum of C57BL/6 mice, this effect was not observed in Tmem173−/− mice (Fig. 4E). Similar results were obtained by infection of Mn2+-deficient C57BL/6 or Tmem173−/− mice. Although Mn2+-deficient C57BL/6 mice showed dramatically increased bacterial burdens compared to control C57BL/6 mice, no increase in CFU count was observed in the feces and cecum of Mn2+-deficient Tmem173−/− mice compared to control Tmem173−/− mice (SI Appendix, Fig. S5G). Therefore, consistent with its role in antiviral innate immunity, Mn2+ protected the host from Yptb infection in a manner involving STING.
In mammalian cells, most cellular Mn2+ is found in membrane-enclosed organelles. Upon viral infection, Mn2+ is released into the cytosol to enhance antiviral innate immunity (13, 36). As Mn2+ is also required for defense against Yptb infection, we explored whether bacterial infection could also induce Mn2+ release into the cytosol. Direct measurement of intracellular Mn2+ concentrations in the host cytosol upon Yptb infection is impractical due to technical challenges, and therefore, we measured intracellular Mn2+ concentrations following treatment of Raw264.7 cells with LPS, the major innate immune activator in gram-negative bacteria. We found that cells stimulated with LPS exhibited much higher cytosolic (S100) Mn levels compared to untreated cells, probably due to LPS-induced mitochondrial membrane potential disruption (37–39), which causes Mn to escape from mitochondria (13, 40). Correspondingly, the Mn level in the membrane fraction (pellet) was reduced in LPS-treated cells (Fig. 4F). Consistent with previous reports, the cytoplasmic Zn level was also sharply elevated upon LPS stimulation (41, 42), while the Ca and Mg levels were not affected (SI Appendix, Fig. S5H). These results indicated that, similar to viral infection, bacterial infection could induce Mn2+ release into the cytosol to enhance the STING-mediated immune response, which is important for host defense against Yptb infection.
TssS Inhibits STING Activity to Regulate Host Innate Immune Responses.
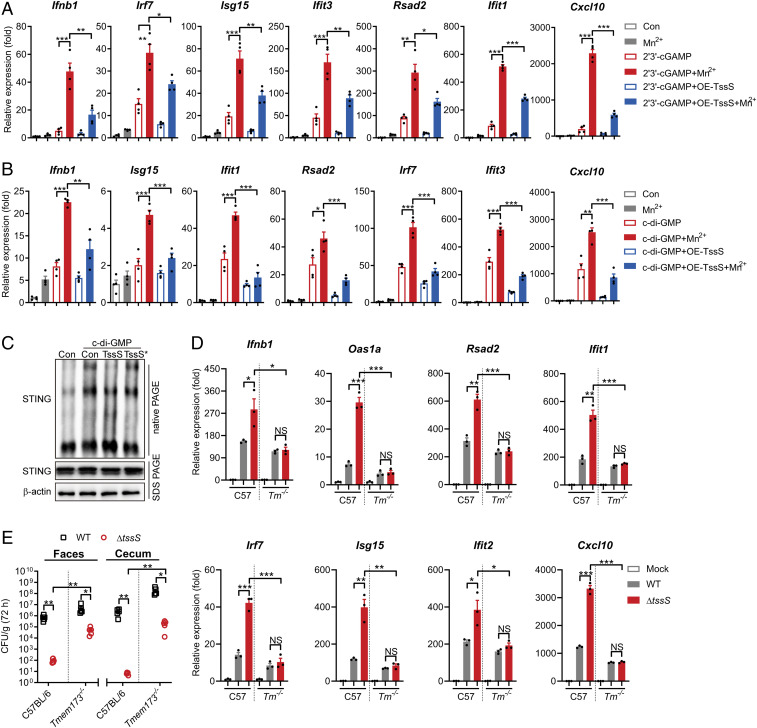
We have shown that TssS is a Mn2+-chelating anti-eukaryotic T6SS effector that inhibits host innate immunity (Figs. 1–3) and that Mn2+ strongly potentiates the innate immune response via the STING-mediated pathway (Fig. 4). These findings prompted us to further investigate whether the Mn2+-chelating TssS inhibits Mn2+-enhanced STING activation. First, we employed 2′3′-cGAMP and c-di-GMP to activate STING and its downstream pathways in PMs. Both 2′3′-cGAMP and c-di-GMP induced expression of a wide range of ISGs, and Mn2+ strongly potentiated their expression levels. Interestingly, the overexpression of TssS in macrophages significantly blocked the 2′3′-cGAMP– and c-di-GMP–induced Mn2+-enhanced expression of ISGs, suggesting that TssS inhibits Mn2+-enhanced STING activation (Fig. 5A and B). Similar results were obtained by the addition of purified TssS proteins into the medium (SI Appendix, Fig. S6 A and B). Next, we explored whether TssS could inhibit STING activity by testing STING oligomerization, the hallmark of STING activation (16). As shown in Fig. 5C, STING oligomerization in 293T cells induced by c-di-GMP and Mn2+ was greatly reduced by the addition of TssS protein but not the Mn2+-binding–defective TssS* protein.
Fig. 5.
TssS inhibits STING activity to suppress host innate immune responses. (A and B) PMs were transduced with the pSIN-IRES-Puro-4×tssS overexpression vector. At 24 h post-transduction, the cells were pretreated with or without 100 μM MnCl2 for 24 h. At 48 h post-transduction, cells were lipofectamine transfected with 4 μM 2′3′-cGAMP (A) or 20 μM c-di-GMP (B). Gene expression of ISGs were determined at 4 h post c-di-GMP or 2′3′-cGAMP transfection. (C) 293T cells were transfected with STING-Flag plasmid. Cells were further untreated (Con) or pretreated with 100 μM MnCl2 and along with or without 10 μΜ TssS or TssS* protein for 24 h and then lipofectamine transfected with 20 μM c-di-GMP. Cell lysates were immunoblotted with an antibody against STING after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (PAGE) and native PAGE. (D) qRT-PCR analysis of gene expression in C57BL/6 or Tmem173−/− PMs mock infected or infected with the Yptb WT or ΔtssS strain. (E) C57BL/6 or Tmem173−/− mice were intragastrically inoculated with Yptb WT or ΔtssS. Homogenates of different tissues were plated to determine the bacterial CFU numbers per gram of organs at 72 h postinfection. Data in A and B were normalized to untreated control (Con, set as 1). Data in D were normalized to mock-infected C57BL/6 PMs and mock-infected Tmem173−/− PMs, respectively (Mock, both set as 1). Gapdh was used as the housekeeping gene. Error bars represent ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
To further elucidate the role of STING in TssS-mediated innate immune suppression, BMDMs derived from C57BL/6 or STING-deficient Tmem173−/− mice were infected with the WT or ΔtssS mutant. As shown in Fig. 5D, the ΔtssS mutant induced significantly higher levels of ISG expression than the Yptb WT strain did in C57BL/6 cells, while no significant differences were detected in Tmem173−/− cells infected with Yptb WT and ΔtssS. Notably, a slightly but significantly decreased level of ISG expression was detected in Yptb WT-infected STING-deficient cells compared to that in C57BL/6-derived BMDMs. These results indicated that TssS inhibits host innate immune response via STING.
To directly confirm that TssS suppresses the host innate immune response through STING, we used the Yptb WT and ΔtssS strains to infect C57BL/6 or Tmem173−/− mice. In C57BL/6 mice, the bacterial burden of the WT strain was significantly higher (three logs in feces and five logs in cecum) than that of the ΔtssS mutant in the feces and cecum (Fig. 1E and 5E). In contrast, the differences in bacterial burden between WT and ΔtssS were significantly attenuated in Tmem173−/− mice (two logs in feces and three logs in cecum) (Fig. 5E). Of note, higher bacterial burdens were obtained from the feces and cecum in ΔtssS-infected Tmem173−/− mice than in those of ΔtssS-infected C57BL/6 mice (Fig. 5E). To exclude the role of TssS-mediated interbacterial competition in possibly affecting the Yersinia bacterial burden in mouse gut, we treated C57BL/6 and Tmem173−/− mice with an antibiotics mixture to eliminate the gut microbiome followed by intragastrical infection with Yptb WT or ΔtssS. As shown in SI Appendix, Fig. S6C, bacterial burden in ΔtssS-infected C57BL/6 mice is significantly lower than that in Yptb WT-infected mice. However, the differences in bacterial burden between WT and ΔtssS were strongly attenuated in Tmem173−/− mice. Similar results were obtained by intraperitoneal infection of C57BL/6 or Tmem173−/− mice with Yptb WT or ΔtssS (SI Appendix, Fig. S6D). Taken together, these results demonstrated that the T6SS effector TssS robustly inhibits STING activation, thereby inhibiting its downstream pathway and subverting the host innate immune response.
TssS Suppresses the STING-Mediated Innate Immune Response by Sequestering Mn2+.
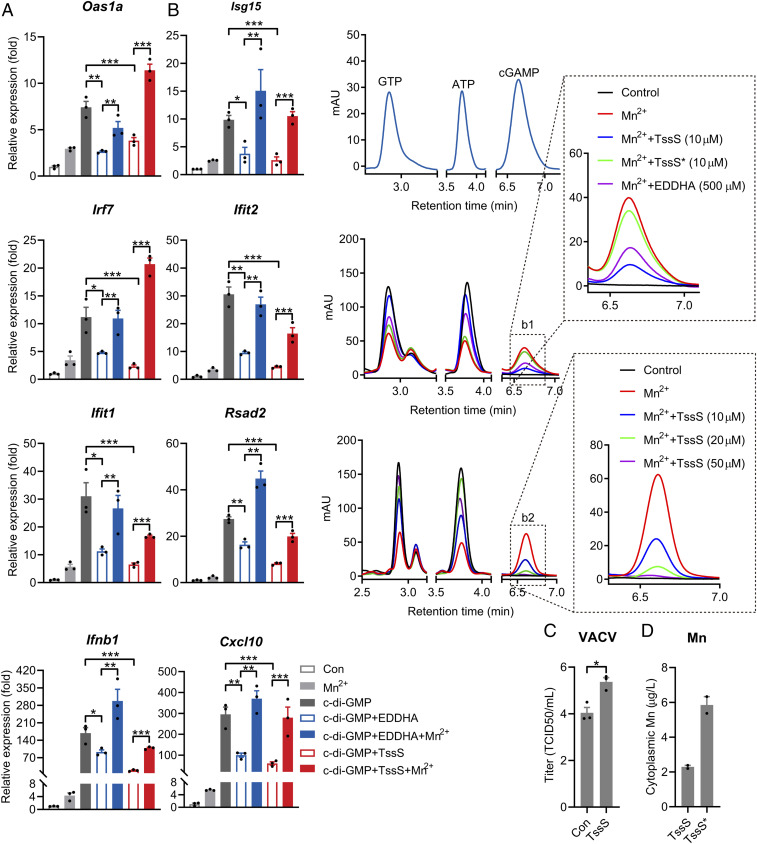
Given that Mn2+ potentiates the antimicrobial innate immune response and that the Mn2+-binding effector TssS was delivered into the cytosol of host cells via T6SS, we postulated that TssS could reduce free Mn2+ levels in the cytosol of host cells as a Mn2+ chelator, thereby suppressing host innate immunity. To test this hypothesis, we first treated PMs with TssS or the Mn2+ chelator ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) (EDDHA). As expected, the addition of EDDHA to the culture medium dramatically reduced c-di-GMP–stimulated ISG expression (Fig. 6A). More importantly, defective ISG expression was strongly reversed with the addition of Mn2+, confirming the notion that the host innate immune response could be suppressed by Mn2+ chelation. Similarly, the addition of apo-TssS protein to the culture medium substantially reduced c-di-GMP–stimulated ISG expression, and supplementation with Mn2+ effectively restored ISG expression (Fig. 6A). A recent study showed that Mn2+ promotes STING activation by enhancing 2′3′-cGAMP–STING affinity (13). Consistent with this, we found that Mn2+ enhanced the affinity of STING for c-di-GMP by 2.43-fold. As anticipated, the effect of Mn2+ on c-di-GMP/STING affinity was abrogated by supplementation of the apo-TssS protein but not TssS* protein (SI Appendix, Fig. S7A).
Fig. 6.
TssS acts as a Mn2+ chelator to suppress STING-mediated innate immune response. (A) qRT-PCR analysis of gene expression in PMs untreated (Con) or pretreated with 10 μΜ TssS and 50 μM EDDHA along with or without 100 μΜ Mn2+ for 24 h and then transfected with 20 μM c-di-GMP for 4 h. (B) 2′3′-cGAMP production was measured by high-performance liquid chromatography. 2′3′-cGAMP was produced in the presence of 0.2 mM ATP and 0.2 mM GTP by cGAS under 10−2 mg ⋅ mL−1 double-stranded DNA. (Middle) Added without (Con) or with 1 mM Mn2+, 10 μM TssS protein, 10 μM TssS* protein, or 500 μM EDDHA for 2 h. (Bottom) Added without (Con) or with 1 mM Mn2+ or the indicated concentrations of TssS protein for 2 h (as described in Materials and Methods). (Insets) Expanded views of the indicated products. (C) Viral titers in HeLa cells transduced with the pSIN-IRES-Puro-4×tssS overexpression vector. At 24 h post-transduction, the cells were infected with virus vaccinia at a multiplicity of infection of 0.01. Viral titers were determined at 24 h postinfection. (D) Mn concentrations in Raw264.7 cytoplasm. Raw264.7 was coincubated with TssS or TssS* protein (10 μΜ) for 30 min. The cytoplasmic Mn2+ concentration was measured by ICP-MS after removing His6-tagged TssS or TssS* proteins with His•Bind Ni-NTA resin. Data in A were normalized to untreated, nontransfected control (Con, set as 1), and Gapdh was used as the housekeeping gene. Error bars represent ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
The role of TssS as a Mn2+ chelator was confirmed by testing its ability to inhibit cGAS activity. Consistent with previous reports (13, 43), the addition of Mn2+ strongly increased cGAS-mediated 2′3′-cGAMP production, and this effect was abrogated by the addition of EDDHA or other chelators such as ethylenediaminetetraacetic acid and diethylenetriaminepentaacetic acid (SI Appendix, Fig. S7B). Similarly, the addition of 10 μM TssS protein potently inhibited Mn2+-activated cGAS enzymatic activity, but the TssS* mutant protein did not have this effect (Fig. 6 B, Middle). Moreover, TssS inhibits cGAS activity in a dose-dependent manner (Fig. 6 B, Bottom). As reported (13), the presence of Mg2+ supported cGAS activation in vitro, albeit less effectively than Mn2+ (SI Appendix, Fig. S7C). However, the TssS protein did not affect Mg2+-induced cGAS activation but strongly inhibited Mn2+-induced 2′3′-cGAMP production (SI Appendix, Fig. S7C), further confirming that TssS inhibits cGAS activity by sequestering Mn2+. It is noteworthy that TssS did not interact with cGAS in an in vitro–binding assay (SI Appendix, Fig. S7D), thus ruling out the possibility that TssS inhibits cGAS activity by direct physical contact. Together, these results demonstrated that TssS inhibits cGAS-STING activity by chelating Mn2+, an activator of the cGAS-STING pathway (13). Consistently, suppressing the cGAS-STING pathway by overexpressing TssS resulted in increased DNA virus infection as manifested by the higher titer of DNA virus vaccinia in HeLa cells (Fig. 6C and SI Appendix, Fig. S7E).
Finally, to verify whether TssS reduces the cytosolic free Mn2+ level in host cells by acting as an Mn2+ chelator, we measured the intracellular Mn2+ concentration after the addition of apo-TssS to the culture medium. The cytoplasmic Mn2+ concentration was markedly reduced by the addition of the apo-TssS protein but not the TssS* mutant protein (Fig. 6D and SI Appendix, Fig. S7F). Therefore, the Mn2+-chelating micropeptide TssS inhibits cGAS-STING activation by sequestering immunostimulatory Mn2+.
Discussion
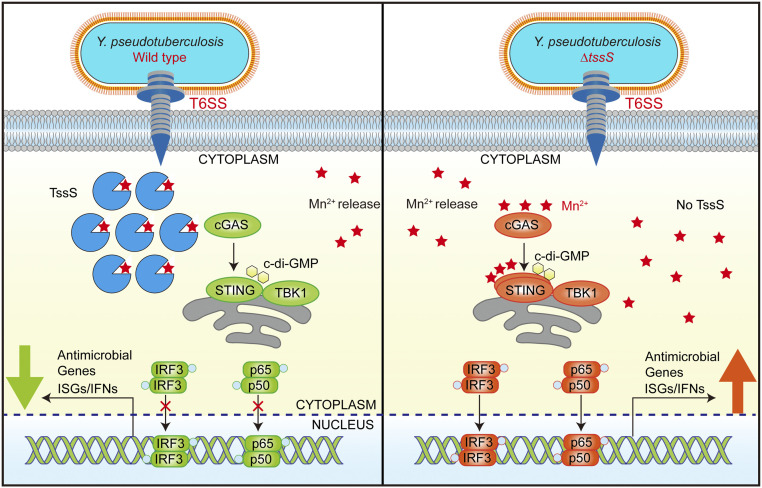
Here, we show that a T6SS-secreted anti-eukaryotic effector, TssS, deploys a unique virulence strategy by sequestering immunostimulatory Mn2+ in the host cell to antagonize the STING-mediated innate immune response (Fig. 7). To the best of our knowledge, TssS is the smallest bacterial effector reported to date that is involved in bacterial pathogen–host interactions. Although TssS is a micropeptide of only 48 amino acids, it uses a multifaceted mechanism to promote bacterial virulence, involving competition for essential Mn2+, resistance to oxidative stress, and suppression of the host immune response. Distinct from traditional bacterial effectors that are generally large proteins acting as enzymes, adaptors, and transcriptional activators (44–46), the micropeptide TssS conducts its functions simply but effectively by acting as a specific Mn2+ chelator.
Fig. 7.
Model of TssS-induced suppression of STING-mediated innate immunity. Yptb infection induces Mn2+ release from membrane-enclosed organelles into the cytosol of host cells, boosting activation of the cGAS-STING pathway. In host cells infected with Yptb WT (Left), the Mn2+-chelating micropeptide TssS was translocated into the cytosol via its T6SS. TssS suppresses the Mn2+-enhanced STING-mediated innate immune response by chelating Mn2+, thus reducing bacterial clearance. When host cells were infected with the ΔtssS mutant (Right), no Mn2+-chelating TssS was translocated, and the bioavailability of Mn2+ was higher. Consequently, STING and its downstream pathways were activated to a greater extent, restricting infection by the ΔtssS mutant.
Cellular ionic concentrations are central factors for orchestrating host innate immunity. For instance, intracellular potassium leakage inhibits the cGAS-STING-IFNβ cascade but triggers NLRP3 activation (47), abnormal intracellular calcium levels suppress the STING signaling pathway (48, 49), and an accumulation of Zn2+ in cytoplasm activates innate immunity by enhancing the enzymatic activity of cGAS (41, 50). We recently revealed that Mn2+ is vital to the innate immune response against DNA virus infection, as it potentiates the cGAS-STING pathway (13). Importantly, Mn2+ directly activates cGAS to produce 2′3′-cGAMP in a ligand binding–independent manner, and it also increases cGAMP-STING–binding affinity to potentiate STING activity, which are critical for antiviral and antitumor host responses (43, 51–53). In conjunction with these studies, we show here that Mn2+ enhanced the antimicrobial innate immune response and dramatically increased the survival rate of mice after Yptb infection (Fig. 4). Similar to that of Zn (41, 42), we found that the cytoplasmic level of Mn is strongly increased by LPS stimulation, indicating that Yptb infection may drive Mn release from membrane-enclosed organelles and thereby potentiate the cGAS-STING pathway (Fig. 4) (13, 36).
The finding that Mn2+ primes the STING-mediated innate immune response against Yptb infection is consistent with a previous report that in addition to the TLR-dependent innate immune response, a TLR-independent innate immune response was also induced against Yptb infection (54). When comparing the WT-infected C57BL/6 and STING-deficient mice, higher bacterial loads were obtained from the feces and cecum of STING-deficient mice (Fig. 4E), which signifies that the STING pathway plays weak but unambiguous roles in defending against Yptb infection. Thus, we speculated that the TLR-dependent pathway plays the major role, while the TLR-independent Mn2+-STING pathway plays an auxiliary role in the stimulation of innate immunity upon sensing Yptb infection. Although the spectrum of its antimicrobial activity requires further investigation, we envision that the Mn2+-mediated innate immune system activation may be involved in diverse biological processes and could represent a new strategy for the development of therapeutic approaches to combat microbial infection.
Given the crucial roles of metal ions in regulating host innate immunity, it is reasonable to speculate that bacterial pathogens may manipulate the bioavailability of these ions to mediate host innate responses. As anticipated, we found that Yptb T6SS translocated TssS into host cells to inhibit the STING-mediated innate immune response by sequestering immunostimulatory Mn2+. This finding is supported by at least four lines of evidence: 1) the transcriptomic analysis showed that the ΔtssS mutant elicited a significantly more intense innate immune response compared to Yptb WT infection, and many of the differentially expressed genes belonged to IFN- and STING-related signaling pathways (Fig. 2A); 2) in vitro experiments showed that TssS inhibited the Mn2+-enhanced cGAS catalytic activity, STING oligomerization, and activation of downstream signaling pathways (Figs. 5C and 6B); 3) the bioavailability of Mn2+ in the cytosol of host cells was reduced by TssS (Fig. 6D); and 4) the function of STING was inhibited by TssS in vivo as illustrated by the lower bacterial burdens of the ΔtssS mutant potently increasing in STING-deficient mice (Fig. 5E and SI Appendix, Fig. S6 C and D). Indeed, the addition of apo-TssS (50 μM) potently reduced free Mn2+ concentration from 1 to 0.4 mM in the Tris HCl buffer, indicating that one TssS molecule can bind roughly 12 Mn2+ ions (SI Appendix, Fig. S7G). Overall, we demonstrated an immune evasion strategy employed by Yptb, which modulates the cellular bioavailability of immunomodulatory metal ions using a metal-chelating T6SS effector. Interestingly, similar T6SS clusters containing putative Mn2+-binding effectors have been identified in diverse bacteria strains (SI Appendix, Fig. S3), suggesting this T6SS-mediated Mn2+ sequestration mechanism might be employed by a broad range of pathogenic bacteria to suppress host innate immune responses.
Indeed, modulation of the bioavailability of metal ions is a successful host strategy to prevent infection, which is termed as nutritional immunity (55). To prevent bacterial access to essential metal ions, vertebrate hosts use a number of metal-chelating proteins such as ferritin, transferrin, and siderocalin to sequester Fe and calprotectin to sequester Mn and Zn (55). Remarkably, the 24-meric heteropolymers of ferritin have the ability to store up to several thousand iron atoms per protein (56). To battle for essential micronutrients, bacterial pathogens have developed sophisticated transport systems to uptake specific nutrient metals (54). Although it remains unclear whether the Mn2+-chelating effector TssS is involved in combating calprotectin-mediated host nutritional immunity, we believe that reduction of the bioavailability of free metal ions in host cells by bacterial effectors may represent a universal immune evasion strategy employed by diverse pathogenic bacteria.
In summary, our results reveal a previously unrecognized mechanism of immune suppression involving sequestration of immunologically active metal ions (Fig. 7) and highlight the importance of micropeptides, which have been largely unexplored, in pathogen–host interactions. Considering that multiple T6SS effectors involved in the chelation of metal ions such as Mn, Zn, Fe, and Cu have been identified recently in various bacterial pathogens (10, 30, 57–59), the sequestration of metal ions by metal-chelating effectors may represent a unique and universal virulence strategy to perturb host immunity. Moreover, our identification of a STING-suppressing micropeptide may represent a novel strategy that can be applied to the development of treatments that prevent excessive innate immune activation as therapy for autoinflammatory diseases.
Materials and Methods
Bacterial Strains and Growth Conditions.
Bacterial strains and plasmids used in this study are listed in SI Appendix, Table S1. Details are in SI Appendix.
Plasmid Construction.
Primers used in this study are listed in SI Appendix, Table S2. Details are in SI Appendix.
Mouse Infection.
Mice used in this study were on a C57BL/6 background. Tmem173−/− mice were generated in a previous work (13). All mouse experimental procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of People’s Republic of China. For survival assays, 1 × 109 bacteria of each strain were applied to different groups of mice, and the survival rate of the mice was determined by monitoring the survival daily for 21 d (60). For the analysis of the bacterial load in the feces, the feces were sampled from individual living mice at specific time points, weighed, and homogenized in phosphate-buffered saline (PBS ). For the analysis of the bacterial load in the cecum, colon, spleen, and liver, mice were euthanized by carbon dioxide asphyxiation followed with cervical dislocation at specific time points after infection, the tissue were weighed and homogenized in PBS, and serial dilutions of the homogenates were plated on YLB plates with 20 μg ⋅ mL−1 nalidixic acid. Details are in SI Appendix.
Cell Culture.
Details are in SI Appendix.
Macrophage Infection.
The cells were infected by Yptb strains at a multiplicity of infection of 20 or otherwise indicated (61). The plates were centrifuged at 500 g for 5 min to improve contact of bacteria with cells and subsequently incubated infected cells at 37 °C for 2 h, and antibiotics and isopropylthio-β-galactoside (IPTG) (1 mM) need to be added during this time when necessary. Then, the cells were washed by PBS two times and resuspended in medium with fetal bovine serum and penicillin/streptomycin and subsequently incubated at 37 °C with 5% CO2 for 4 h. Cells were collected at 6 h postinfection. Then, the cells were collected for RNA isolation and immunoblotting. Details are in SI Appendix. All reagents and resources used in this study are listed in SI Appendix, Table S3.
Overexpression and Purification of Recombinant Protein.
Details are in SI Appendix.
GST Pull-down Assay.
The GST pull-down assay was performed as previously described with minor modifications (10, 62). Details are in SI Appendix.
Protein Secretion Assay.
A secretion assay for TssS was performed according to described methods (63). Details are in SI Appendix.
Quantitative Real-Time PCR.
qRT-PCR was performed in a CFX96 Real-Time PCR Detection System (Bio-Rad) with TransStart Green qPCR SuperMix (TransGen Biotech). The relative abundance of 16S ribosomal RNA was used as the internal standard in bacterial cells, and Gapdh was used as internal standard in mammalian cells. All samples were analyzed in triplicate, and the expression of target genes was calculated as relative fold values using the 2-ΔΔCt method. Details are in SI Appendix. All primers for qRT-PCR were listed in SI Appendix, Table S2.
Western Blot Analysis.
Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore). The membrane was blocked with QuickBlock Blocking Buffer (Beyotime Biotechnology) for 30 min at room temperature and incubated with primary antibodies at 4 °C overnight. The membrane was washed three times in Tris buffered saline with Tween 20 (TBST) buffer (50 mM Tris, 150 mM NaCl, and 0.05% Tween 20, pH 7.4) and incubated with horseradish peroxidase–conjugated secondary antibodies for 1 h. Signals were detected using the ECL Kit (Invitrogen) following the manufacturer′s specified protocol. Antibodies used are listed in SI Appendix, Table S3.
Bacterial Survival Assay.
Details are in SI Appendix.
Measurement of Microelement by Inductively Coupled Plasma Mass Spectrometry.
Bacterial intracellular ion content was determined as described previously (10, 30). Samples were analyzed by inductively coupled plasma mass spectrometry (ICP-MS, Varian 802-MS), and the results were corrected using the appropriate buffers for reference and dilution factors. For the intracellular ion in mammalian cells, the measurement of microelement by ICP-MS was performed as described with minor modifications (13). Details are in SI Appendix.
Metal-Free TssS Preparation and Metal Ion–Binding Assays.
Details are in SI Appendix.
ITC.
Mn2+ binding was measured using ITC at 25 °C as previously described with minor modifications with a NANO-ITC 2G microcalorimeter (TA Instruments) (64). Details are in SI Appendix.
Cya Assay.
The Cya assay was performed as previously described with minor modifications (32). Details are in SI Appendix.
Translocation Assay for TssS::TEM1 Fusions.
The translocation assay was performed as previously described (65). Details are in SI Appendix.
cGAS-cGAMP Activity Assay.
The cGAS-cGAMP activity assay was analyzed as described previously with minor modifications (13). High-performance liquid chromatography was performed at a constant flow rate of 1.0 mL ⋅ min−1 with 98% (vol/vol) phase A and 2% (vol/vol) phase C. The UV absorption was measured at 254 nm, and the column temperature was maintained at 27 °C. Various nucleotide states, including ATP, GTP, and 2′3′-cGAMP, were used as standards to determine the retention times and amounts of the various ingredients in the reaction mixture. Details are in SI Appendix.
Transfection.
Details are in SI Appendix.
Analyses of STING Oligomerization by Native Gels.
The assay of the STING oligomers was analyzed as described previously with minor modifications (66). Details are in SI Appendix.
RNA-Seq Experiment.
Details are in SI Appendix.
Diet-Induced Manganese Deficiency.
Diet-induced manganese deficiency mice models were generated as described (13). Details are in SI Appendix.
Statistical Analysis.
Experimental data analyzed for significance were performed by using GraphPad Prism 6 (GraphPad Software). P values for mice survival were calculated using log-rank (Mantel–Cox) test. P values for bacterial CFU in mouse tissues were calculated using Mann–Whitney U test (I). Statistical analyses for the rest of the assays were performed using paired two-tailed Student’s t test. Error bars represent ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
Acknowledgments
This work was supported by grants of the National Natural Science Foundation of China (Grants 31725003 and 31670053 to X.S., Grants 31970114 and 32170130 to Y.W., and Grant 31800113 to L.X.), National Key R&D Program of China (Grant 2018YFA0901200 to X.S.), the Open Project Program of the State Key Laboratory of Pathogen and Biosecurity (Grant SKLPBS1825 to X.S.), China Postdoctoral Science Foundation (Grant 2018M631201, to L.X. and Grant 2020M673501, to L.Z.), and Chinese Universities Scientific Fund (the Starting Research Fund from the Northwest A&F University, Grant 2452018045, to L.X.). L.X. is supported by the Shaanxi Postdoctoral Science Foundation (Grant 2018BSHTDZZ20) and the Natural Science Basis Research Plan in Shaanxi Province of China (Grant 2020JQ-245). We thank the Teaching and Research Core Facility at College of Life Science (Min Duan, Ningjuan Fan, Xiyan Chen, and Hui Duan) and Life Science Research Core Services, Northwest A&F University for the technical support.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2103526118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix. Raw FASTQ files for the RNA-seq libraries are deposited in the National Center for Biotechnology Information Sequence Read Archive and have been assigned BioProject accession PRJNA635733.
References
- 1.Russell A. B., Peterson S. B., Mougous J. D., Type VI secretion system effectors: Poisons with a purpose. Nat. Rev. Microbiol. 12, 137–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi A., et al., Rules of engagement: The type VI secretion system in Vibrio cholerae. Trends Microbiol. 25, 267–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood R. D., et al., A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7, 25–37 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ting S. Y., et al., Bifunctional immunity proteins protect bacteria against FtsZ-targeting ADP-ribosylating toxins. Cell 175, 1380–1392.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao W., Caro F., Robins W., Mekalanos J. J., Antagonism toward the intestinal microbiota and its effect on Vibrio cholerae virulence. Science 359, 210–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledvina H. E., et al., A phosphatidylinositol 3-kinase effector alters phagosomal maturation to promote intracellular growth of Francisella. Cell Host Microbe 24, 285–295.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aubert D. F., et al., A Burkholderia type VI effector deamidates Rho GTPases to activate the Pyrin inflammasome and trigger inflammation. Cell Host Microbe 19, 664–674 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Ma A. T., Mekalanos J. J., In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proc. Natl. Acad. Sci. U.S.A. 107, 4365–4370 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H., et al., The bacterial T6SS effector EvpP prevents NLRP3 inflammasome activation by inhibiting the Ca2+-dependent MAPK-Jnk pathway. Cell Host Microbe 21, 47–58 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Si M., et al., Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl. Acad. Sci. U.S.A. 114, E2233–E2242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguirre J. D., Culotta V. C., Battles with iron: Manganese in oxidative stress protection. J. Biol. Chem. 287, 13541–13548 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Ochoa V. E., et al., Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe 19, 814–825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C., et al., Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 48, 675–687.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Sun L., Wu J., Du F., Chen X., Chen Z. J., Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao D., et al., Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao T. S., Fitzgerald K. A., The cGAS-STING pathway for DNA sensing. Mol. Cell 51, 135–139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q., Sun L., Chen Z. J., Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 17, 1142–1149 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Burdette D. L., et al., STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer J. D., et al., The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 79, 688–694 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., et al., Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51, 226–235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopfner K. P., Hornung V., Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Saeed A. F. U. H., Ruan X., Guan H., Su J., Ouyang S., Regulation of cGAS-mediated immune responses and immunotherapy. Adv. Sci. (Weinh.) 7, 1902599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon J., Bakhoum S. F., The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 10, 26–39 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marinho F. V., Benmerzoug S., Oliveira S. C., Ryffel B., Quesniaux V. F. J., The emerging roles of STING in bacterial infections. Trends Microbiol. 25, 906–918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung L. K., et al., The Yersinia virulence factor YopM Hijacks host kinases to inhibit type III effector-triggered activation of the Pyrin inflammasome. Cell Host Microbe 20, 296–306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meinzer U., et al., Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe 11, 337–351 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Viboud G. I., Bliska J. B., Yersinia outer proteins: Role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59, 69–89 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Song L., et al., Contact-independent killing mediated by a T6SS effector with intrinsic cell-entry properties. Nat. Commun. 12, 423 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., et al., A type VI secretion system regulated by OmpR in Yersinia pseudotuberculosis functions to maintain intracellular pH homeostasis. Environ. Microbiol. 15, 557–569 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Wang T., et al., Type VI secretion system transports Zn2+ to combat multiple stresses and host immunity. PLoS Pathog. 11, e1005020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman J. M., et al., Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol. Cell 51, 584–593 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai H., et al., A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. U.S.A. 102, 826–831 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigonsky E., et al., Metal binding spectrum and model structure of the Bacillus anthracis virulence determinant MntA. Metallomics 7, 1407–1419 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Lu M., Fu D., Structure of the zinc transporter YiiP. Science 317, 1746–1748 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Martin J. E., Giedroc D. P., Functional determinants of metal ion transport and selectivity in paralogous cation diffusion facilitator transporters CzcD and MntE in Streptococcus pneumoniae. J. Bacteriol. 198, 1066–1076 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmona A., Roudeau S., Perrin L., Veronesi G., Ortega R., Environmental manganese compounds accumulate as Mn(II) within the Golgi apparatus of dopamine cells: Relationship between speciation, subcellular distribution, and cytotoxicity. Metallomics 6, 822–832 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Aude-Garcia C., et al., Different in vitro exposure regimens of murine primary macrophages to silver nanoparticles induce different fates of nanoparticles and different toxicological and functional consequences. Nanotoxicology 10, 586–596 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Kim Y. C., et al., Simvastatin induces caspase-independent apoptosis in LPS-activated RAW264.7 macrophage cells. Biochem. Biophys. Res. Commun. 339, 1007–1014 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Yadav U. C., Kalariya N. M., Srivastava S. K., Ramana K. V., Protective role of benfotiamine, a fat-soluble vitamin B1 analogue, in lipopolysaccharide-induced cytotoxic signals in murine macrophages. Free Radic. Biol. Med. 48, 1423–1434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunter T. E., Gunter K. K., Puskin J. S., Russell P. R., Efflux of Ca2+ and Mn2+ from rat liver mitochondria. Biochemistry 17, 339–345 (1978). [DOI] [PubMed] [Google Scholar]
- 41.Kapetanovic R., et al., Salmonella employs multiple mechanisms to subvert the TLR-inducible zinc-mediated antimicrobial response of human macrophages. FASEB J. 30, 1901–1912 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Haase H., et al., Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J. Immunol. 181, 6491–6502 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Hooy R. M., Massaccesi G., Rousseau K. E., Chattergoon M. A., Sohn J., Allosteric coupling between Mn2+ and dsDNA controls the catalytic efficiency and fidelity of cGAS. Nucleic Acids Res. 48, 4435–4447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kay S., Hahn S., Marois E., Hause G., Bonas U., A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 318, 648–651 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Müller M. P., et al., The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science 329, 946–949 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Xu Y., et al., A bacterial effector reveals the V-ATPase-ATG16L1 axis that initiates xenophagy. Cell 178, 552–566.e20 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Banerjee I., et al., Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity 49, 413–426.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon D., Sesaki H., Kang S. J., Intracellular calcium is a rheostat for the STING signaling pathway. Biochem. Biophys. Res. Commun. 500, 497–503 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Srikanth S., et al., The Ca2+ sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat. Immunol. 20, 152–162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du M., Chen Z. J., DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361, 704–709 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou L., et al., Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate Immunity. ACS Nano 14, 3927–3940 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Zhao Z., et al., Mn(2+) directly activates cGAS and structural analysis suggests Mn(2+) induces a noncanonical catalytic synthesis of 2′3′-cGAMP. Cell Rep. 32, 108053 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Lv M., et al., Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 30, 966–979 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Auerbuch V., Golenbock D. T., Isberg R. R., Innate immune recognition of Yersinia pseudotuberculosis type III secretion. PLoS Pathog. 5, e1000686 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hood M. I., Skaar E. P., Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10, 525–537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradley J. M., et al., Bacterial iron detoxification at the molecular level. J. Biol. Chem. 295, 17602–17623 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin J., et al., A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 8, 14888 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Y., et al., A Pseudomonas aeruginosa type VI secretion system regulated by CueR facilitates copper acquisition. PLoS Pathog. 15, e1008198 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C., et al., T6SS secretes an LPS-binding effector to recruit OMVs for exploitative competition and horizontal gene transfer. ISME J. 10.1038/s41396-021-01093-8. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schweer J., et al., The cytotoxic necrotizing factor of Yersinia pseudotuberculosis (CNFY) enhances inflammation and Yop delivery during infection by activation of Rho GTPases. PLoS Pathog. 9, e1003746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schoberle T. J., Chung L. K., McPhee J. B., Bogin B., Bliska J. B., Uncovering an important role for YopJ in the inhibition of caspase-1 in activated macrophages and promoting Yersinia pseudotuberculosis virulence. Infect. Immun. 84, 1062–1072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen X., et al., Targeting eEF1A by a Legionella pneumophila effector leads to inhibition of protein synthesis and induction of host stress response. Cell. Microbiol. 11, 911–926 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu S., et al., FliS modulates FlgM activity by acting as a non-canonical chaperone to control late flagellar gene expression, motility and biofilm formation in Yersinia pseudotuberculosis. Environ. Microbiol. 16, 1090–1104 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., et al., Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat. Commun. 11, 5371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang F., Waterfield N. R., Yang J., Yang G., Jin Q., A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe 15, 600–610 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Zhang C., et al., Structural basis of STING binding with and phosphorylation by TBK1. Nature 567, 394–398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article and SI Appendix. Raw FASTQ files for the RNA-seq libraries are deposited in the National Center for Biotechnology Information Sequence Read Archive and have been assigned BioProject accession PRJNA635733.