Abstract
Background
GATA2 deficiency is a genetic disorder of hematopoiesis, lymphatics, and immunity caused by autosomal dominant or sporadic mutations in GATA2. The disease has a broad phenotype encompassing immunodeficiency, myelodysplasia, leukemia, and vascular or lymphatic dysfunction as well as prominent pulmonary manifestations.
Research Question
What are the pulmonary manifestations of GATA2 deficiency?
Study Design and Methods
A retrospective review was conducted of clinical medical records, diagnostic imaging, pulmonary pathologic specimens, and tests of pulmonary function.
Results
Of 124 patients (95 probands and 29 ascertained), the lung was affected in 56%. In addition to chronic infections, pulmonary alveolar proteinosis (11 probands) and pulmonary arterial hypertension (nine probands) were present. Thoracic CT imaging found small nodules in 54% (54 probands and 12 relatives), reticular infiltrates in 40% (45 probands and four relatives), paraseptal emphysema in 25% (30 probands and one relative), ground-glass opacities in 35% (41 probands and two relatives), consolidation in 21% (23 probands and two relatives), and a typical crazy-paving pattern in 7% (eight probands and no relatives). Nontuberculous mycobacteria were the most frequent organisms associated with chronic infection. Allogeneic hematopoietic stem cell transplantation successfully reversed myelodysplasia and immune deficiency and also improved pulmonary hypertension and pulmonary alveolar proteinosis in most patients.
Interpretation
GATA2 deficiency has prominent pulmonary manifestations. These clinical observations confirm the essential role of hematopoietic cells in many aspects of pulmonary function, including infections, alveolar proteinosis, and pulmonary hypertension, many of which precede the formal diagnosis, and many of which respond to stem cell transplantation.
Key Words: GATA2 deficiency, pulmonary alveolar proteinosis, pulmonary hypertension
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; HSCT, hematopoietic stem cell transplantation; NK, natural killer; PAH, pulmonary arterial hypertension; PAP, pulmonary alveolar proteinosis
GATA2 deficiency is a recently described primary immune deficiency that affects hematopoiesis, lymphatics, and immunity and is transmitted as an autosomal dominant or sporadic disease. Heterozygous mutations in GATA2 lead to haploinsufficiency, which typically presents clinically in late childhood or early adulthood.1,2 The condition often is recognized clinically by severe or recurrent infections, lymphedema, monocytopenia, natural killer (NK) cell and dendritic cell cytopenias, and a high rate of myelodysplastic syndrome and acute myeloid leukemia.3, 4, 5 GATA2 is a zinc finger transcription factor essential for differentiation of endothelial and immature hematopoietic cells.6 Among many other functions, GATA2 is involved in phagocytosis by alveolar macrophages.7,8
The respiratory tract in GATA2 deficiency frequently is affected by viral, fungal, or mycobacterial infections.9 Pulmonary alveolar proteinosis (PAP) develops, sometimes complicated by pulmonary arterial hypertension (PAH), as well.10, 11, 12 Importantly, allogeneic hematopoietic stem cell transplantation (HSCT) successfully reverses the myelodysplasia, monocytopenia, and B-cell and NK cell deficiencies and often ameliorates some of the pulmonary manifestations of this disease, including PAP and PAH.13,14 Therefore, GATA2 deficiency is critical to identify early to prevent infections and end-organ damage,15 especially to the lung.16
Over the last 25 years, we have collected data on patients with GATA2 deficiency and their relatives, some of whom were followed up syndromically, before genetic diagnosis. We have been impressed with the early onset and broad range of presentations of GATA2 lung disease, including subpleural and paraseptal emphysema, PAP, and PAH. We describe herein the pulmonary manifestations, treatments, and transplantation responses of GATA2 deficiency in a large cohort of patients followed up at a single center.
Methods
A retrospective review was conducted of clinical medical records, diagnostic imaging, pulmonary pathologic specimens, and tests of pulmonary function in 124 patients with mutation-proven GATA2 deficiency seen at the National Institutes of Health between 1992 and 2020. All patients were enrolled on approved protocols of the National Institute of Allergy and Infectious Diseases, and all participants or their guardians gave written informed consent. Included patients were both those referred for evaluation and treatment of active disease (probands) as well as those recruited from kindreds with proven GATA2 mutations (ascertained relatives). Pulmonary function and echocardiography findings were evaluated before and after HSCT, as available.
Pulmonary function and 6-min walk test results were reviewed and assessed independently in accordance with 2005 American Thoracic Society and European Respiratory Society guidelines.17, 18, 19 Additionally, chest CT scans selected from time points unassociated with acute infections, as determined by clinical documentation, negative culture results, or both, were submitted for independent review by a radiologist (L. R. F.) with more than 30 years of experience. Each CT scan was evaluated for nodular opacities, reticular opacities, paraseptal emphysema (peripheral cysts along the pleura), ground-glass opacities, consolidations, and crazy-paving pattern.20 Ground-glass opacities were defined as areas of increased attenuation in which bronchial walls and vessels remained visible. The crazy-paving pattern previously described in association with PAP refers to the appearance of ground-glass opacities with superimposed interlobular septal thickening and intralobular reticular thickening.
Mutation groups were categorized as follows: missense included all missense mutations and in-frame deletions in the C-terminal zinc finger, null included nonsense and frameshift mutations and large gene deletions, and regulatory included mutations in the intronic enhancer region with demonstrated reduced expression of wild-type protein.1,21
Statistical Analysis
Continuous variables with nonnormal distribution were reported as median (interquartile range). For comparison of probands’ and relatives’ variables, Mann-Whitney U test findings were reported. The Wilcoxon test was used for data before and after bone marrow transplantation. For comparison of categorical data, such as the radiographic findings on CT imaging, we used either the χ 2 or Fisher exact test. A P value of < .05 was defined as significant. All statistical analyses and graphs were performed with the aid of Prism8 software (GraphPad Software) for macOS.
Results
Disease Presentation
Initially, probands were referred to the National Institutes of Health Clinical Center predominantly for evaluation of disseminated or pulmonary mycobacterial infections, which tended to occur in late childhood or adulthood. Most probands sought treatment with nonspecific symptoms of recurrent fever, night sweats, weight loss, fatigue, and persistent hematologic anomalies, including low absolute monocytes, NK cells, and B cells.5 Cough, dyspnea with or without exertion, decreased exercise tolerance, and recurrent upper and lower airway infections in late childhood to early adulthood also were noted. After identifying probands, family members were screened genetically and family members with GATA2 mutations were offered evaluation. For those seen at the National Institutes of Health, routine laboratory, CT imaging, and pulmonary function testing were carried out under the same protocol. The frequency distribution of clinical manifestations was similar to that reported by previous studies.1 Thirty-five probands and 13 relatives were reported previously.1 Population characteristics are listed in Table 1.
Table 1.
Characteristics of Patients With GATA2 Deficiency (N = 124)
| Characteristic | Probands (n = 95) | Relatives (n = 29) |
|---|---|---|
| Sex | ||
| Male | 34 (36) | 13 (44) |
| Female | 61 (64) | 16 (55) |
| Age, y | ||
| Mediana | 33 | 36 |
| Range | 8-86 | 6-86 |
| IQR | 22-40 | 18-59 |
| Race | ||
| White | 73 (76) (n = 46 female) | 23 (80) (n = 13 female) |
| Black | 3 (3) (n = 3 female) | |
| Asian | 2 (2) (n = 2 male) | |
| Unknown or multiracial | 15 (15) (n = 11 female) | 3 (10) (n = 2 female) |
| American native | 2 (n = 1 female) | 3 (10) (n = 1 female) |
| Pulmonary alveolar proteinosis | 11 | 0 |
| Pulmonary function tests | ||
| FEV1, % predicted | 82 (24-121) | 94 (60-113) |
| FVC, % predicted | 92 (37-129) | 96 (83-120) |
| Dlco adjusted for hemoglobin, % predicted | 63 (31-111) | 76 (54-115) |
| Myelodysplasia or monocytopenia | 42 (44) | 4 (14) |
| Nontuberculous mycobacteria | 42(44) | 0 |
| Viral infection (including warts) | 70 (73) | 16 (55) |
| Fungal infection | 24 (25) | 3 (10) |
| Malignancy (other than myelodysplasia or leukemia) | 11 (11) | 1 (0.3) |
| Bone marrow transplantation | 58 (61) | 7 (24) |
Data are presented as No. (%), No., or median (range), unless otherwise indicated. Dlco = diffusing capacity for carbon monoxide; IQR = interquartile range.
Median age reflects all patients alive at time of report. Those who underwent transplantation were censored at the time of hematopoietic stem cell transplantation.
Pulmonary infections predominantly were the result of nontuberculous mycobacteria (commonly Mycobacterium avium complex or Mycobacterium kansasii), but some patients showed fungal infections as well. Most patients demonstrated early-onset persistent disseminated human papillomavirus verrucae or condylomata of the skin, genitals, and anus, which were usually refractory to multiple treatment methods (e-Table 1).
Of the total of 124 patients identified, 95 were probands and 29 were ascertained through family screening (relatives). Probands demonstrated a variable spectrum of clinical manifestations of GATA2 deficiency; of the 29 family members recruited, nine were entirely asymptomatic.
At the time of pulmonary function testing, proband and relative immunologic profiles were significantly different (P ≤ .001 for all variables analyzed). NK cells were low in 86% of the probands, but also in 52% of the relatives; B cells were low in 90% of the probands, but also in 26% of the relatives; monocytes were low in 88% of probands, but also in 48% of relatives (e-Fig 1).
Overall, 56% (n = 70) of the total cohort of probands (n = 64) and relatives (n = 6) had histories of clinical pulmonary disease, most (n = 32) being infections, such as pneumonia or recurrent bronchitis. At the time of proband identification, four of the 29 ascertained relatives already had clinically apparent pulmonary symptoms. Four probands had lung infections only early in life without later infections, whereas six had only a single pneumonia episode. About 50% of those with pulmonary manifestations demonstrated chronic and progressive symptoms; 8% (n = 11) had PAP, which was diagnosed only in probands, one of whom had anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibodies. Overall, 36% of the combined proband and relative groups had a history of lung infections; nontuberculous mycobacteria were the most frequently associated pathogens, also reported only in probands (Table 1). The overall age of the living patients was not significantly different between the two groups.
Pathologic Findings
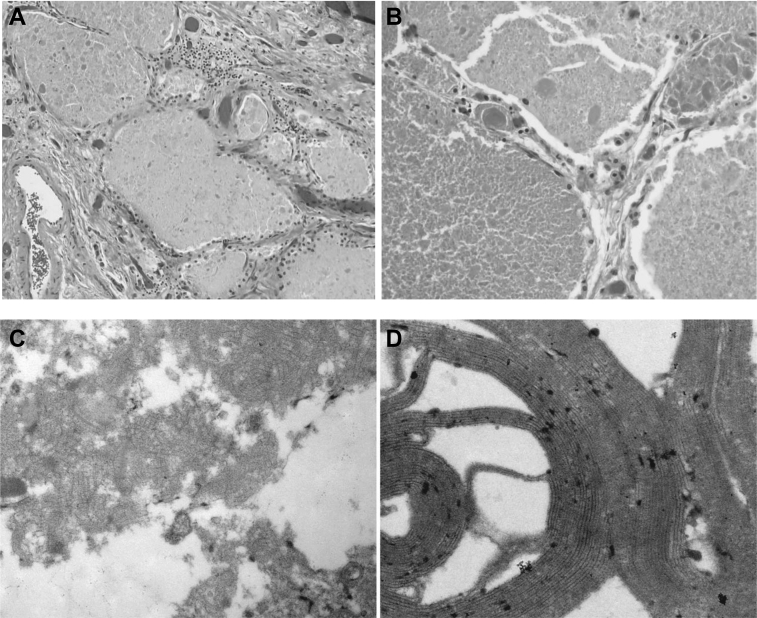
Bronchoscopy, lung biopsy, or both were performed to diagnose possible infectious, malignant, or infiltrative processes. Pathologic specimens from BAL fluid (38 probands), lung biopsies (five probands), or autopsy specimens (seven probands) were available. The BAL fluid showed lymphocytic (mean, 21 ± 23%), monocytic (52 ± 40%), and neutrophilic (40 ± 38%) predominances. Lung biopsies showed chronic lymphohistiocytic inflammation with scattered eosinophils, but no granuloma formation. Eight patients showed histopathologic findings consistent with PAP, whereas three patients had received a diagnosis of PAP from a referring institution (Fig 1A, 1B).22 Electron microscopic evaluation of selected pathologic specimens confirmed whorled membranous structures and amorphous, concentric, osmiophilic membranes resembling tubular myelin, consistent with the diagnosis of PAP (Fig 1C, 1D).23 The proteinaceous background material harbored amorphous globules that stained positive for periodic acid-Schiff and were diastase resistant.24 Lung specimens demonstrated an abundance of alveolar macrophages, despite the absence of peripheral blood monocytes.
Figure 1.
A, B, Photomicrographs showing lipoproteinaceous material filling alveolar spaces with positive periodic acid-Schiff results (A, ×200; B, ×400). C, Electron microscopic image showing BAL fluid exhibiting whorled membranous structures and amorphous osmiophilic particles. D, Higher magnification electron microscopic image showing a whorled membranous body with concentric osmiophilic membranes resembling tubular myelin.
Diagnostic Imaging
All but three young asymptomatic patients underwent chest CT scan examinations. Disease-defining images were selected in the absence of active pulmonary exacerbations, negative respiratory culture findings, or both so as to more likely capture the radiographic features intrinsic to GATA2 deficiency, as opposed to those resulting from intercurrent infection. The analysis was carried out with first imaging available.
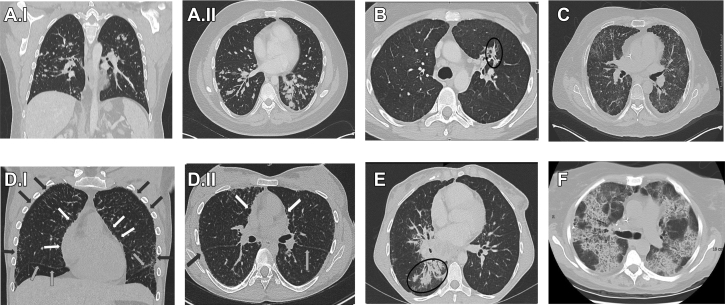
Apical predominant reticular opacities, central pulmonary vascular prominence, and paraseptal emphysema were common. In particular, the paraseptal emphysema, representing peripheral destruction of subpleural lung, seemed to progress over time and could be extensive. Seven relatives had chest CT scans with completely normal findings, whereas only four probands did. Nonspecific bronchiectasis was recorded in 10% of the probands. We recognized small nodules in 54% (n = 66), (Fig 2A I,II) reticular infiltrates in 40% (n = 49) (Fig 2B), ground-glass opacities in 36% (n = 44) (Fig 2C), paraseptal emphysema in 25% (n = 31) (Fig 2D I,II), and consolidations in 20% (n = 25) (Fig 2E, e-Table 2). Typical radiographic crazy-paving pattern, as defined by networks of smoothly thickened septal lines superimposed on areas of ground-glass opacity (Fig 2E), was identified in eight patients (all probands). Five patients with PAP showed typical crazy-paving pattern on chest CT scans at the first encounter. No patterns of pulmonary radiographic findings showed statistically significant correlation with mutation type, but radiographic findings were more common in probands than in relatives (e-Table 2).
Figure 2.
CT scans with the characteristics described in GATA2 deficiency. A, Nodules: I, coronal and II, axial on 20 mm maximum intensity projection show numerous diffuse small nodules bilaterally (male patient, aged 29 years). B, Reticular infiltrate: Axial CT scan reticular shows infiltrate in left upper lobe (female patient, aged 31 years). C, Ground-glass opacities: Axial CT scan (male patient, aged 16 years). D, Paraseptal emphysema: I, Coronal multiplanar reformation 0.5 x 0.3 mm CT scan shows striking diffuse paraseptal emphysema (subpleural blebs) along all pleural surfaces (black arrows peripherally) including fissures and mediastinum (white arrows); II, Axial 0.5 x 0.3 mm CT scan diffuse paraseptal emphysema (subpleural blebs) along all pleural surfaces (black arrows peripherally) including fissures and mediastinum (white arrows) (male patient, aged 21 years). E, Consolidation: Axial CT scan (female patient, aged 21 years). F, Crazy paving: Axial CT scan shows extensive areas of ground-glass opacity within regions of interstitial markings resembling a “crazy paving” pattern (female patient, aged 37 years).
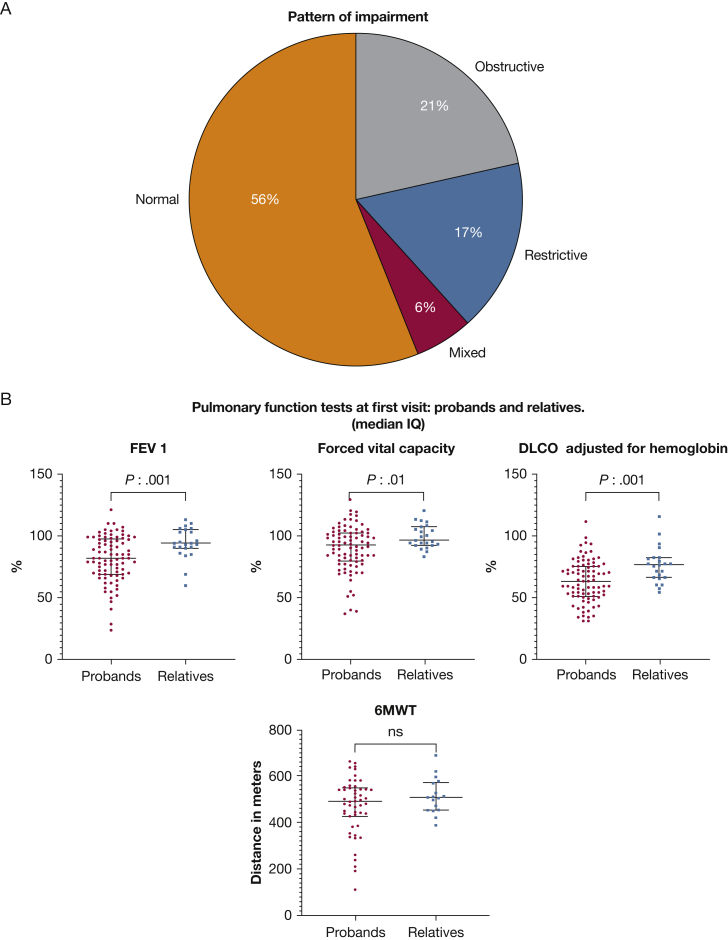
Pulmonary Function
Pulmonary function data were available for 109 patients from the initial visit (Fig 3). At the initial visit, 70% already showed diffusion defects (less 75% diffusing capacity for carbon monoxide). Thirty-five percent of patients showed reductions in FEV1 % predicted (< 80%) and 44% overall showed an obstructive or restrictive patterns on pulmonary function tests (Fig 3A). One patient from the ascertained relative group showed an obstructive pattern on initial spirometry. All patients with PAP had mild to severe ventilatory defects. At the first visit, 13% of evaluable patients demonstrated decreased distance on the 6-min walk test. All these parameters (FEV1, diffusing capacity for carbon monoxide, and FVC) were significantly worse in probands, as expected (Fig 3B). Apparently, age may not be a relevant factor in the difference between probands and relatives and pulmonary function test abnormalities (e-Table 3).
Figure 3.
A, B, Distribution of pulmonary function test patterns at first visit. A, Pie chart showing pattern of impairment. B, Scatterplots showing pulmonary function test findings at first visit in probands and relatives (median and interquartile range). 6MWT = 6-min walk test; Dlco = diffusing capacity for carbon monoxide.
Pulmonary Hypertension
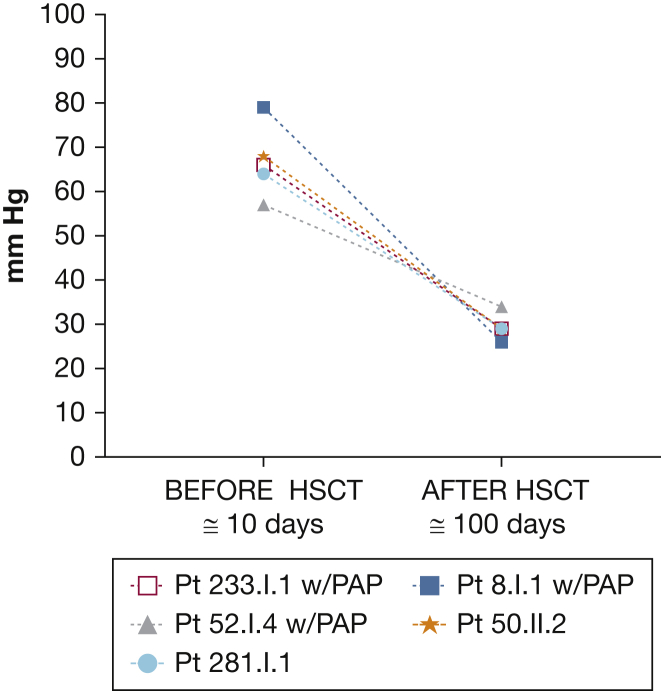
Echocardiography was performed on 87% of the overall cohort. PAH was diagnosed in nine patients (8%), all probands, by echocardiographic measurements, based principally on right ventricular systolic pressure of > 35 mm Hg, elevation of tricuspid regurgitation velocity to > 3.4 m/s, increased right ventricular size or dysfunction, or a combination thereof.25 The findings of one autopsy were consistent with PAH. Six patients with PAH had received a diagnosis of concomitant myelodysplastic syndrome.1,13 At least three patients demonstrated PAH during follow-up. In three patients with PAP and PAH who had undergone HSCT, pulmonary arterial pressures returned to normal as determined by echocardiography and confirmed in one patient by right heart catheterization (Fig 4). Two patients had PAH without PAP, and the pulmonary hypertension improved in them after HSCT, as well.
Figure 4.
Graph showing pulmonary arterial hypertension findings: right ventricular systolic pressure measured by echocardiography before and after bone marrow transplantation. HSCT = hematopoietic stem cell transplantation; PAP = pulmonary alveolar proteinosis.
Outcome: Treatment and Response
Disseminated nontuberculous mycobacteria were treated according to the 2007 American Thoracic Society and Infectious Diseases Society of America recommendations.17 GM-CSF therapy (aerosolized and subcutaneous) was tried in four patients with GATA2-associated PAP without appreciable response. Whole lung lavage, performed on nine patients, was moderately effective for PAP with recovery of typical milky lavage fluid. After whole lung lavage, patients showed transient improvement in oxygenation and restrictive ventilatory defects as well as improved radiographic appearance.
As of this report, 64 patients had undergone HSCT since 2006, with an overall survival of 80%. Median time after transplantation for this cohort was 2.7 years (range, 6 months-16 years). Fifty-one patients (80%) received myeloablative conditioning before transplantation from 13 (25%) matched related donors, 22 (43%) unrelated donors, and 16 (31%) haploidentical donors. Of the 13 patients who received nonmyeloablative conditioning, three patients (23%) underwent HSCT with material from matched related donors, five patients (38%) underwent HSCT with material from matched unrelated donors, one patient underwent HSCT with material from a haploidentical donor, four patients (30%) underwent HSCT with material from umbilical cord blood, and one patient underwent HSCT with material from an haploidentical donor.
In contrast to the results with GM-CSF therapy or whole lung lavage, we saw rapid and remarkable clinical responses in PAP after HSCT. Five patients with PAP received nonmyeloablative conditioning, and two others received myeloablative conditioning with similar overall survival. Improvements in diffusing capacity for carbon monoxide, FVC, total lung capacity, and FEV1 were seen in five patients with PAP in whom pulmonary data from before and after transplantation were available.
One patient without PAP initially received a diagnosis of idiopathic PAH in childhood and received maintenance treatment with prostacyclin and sildenafil. She showed complete resolution of PAH and ceased all PAH medications after transplantation. One of the patients with PAP and PAH that resolved after HSCT went on to demonstrate progressive worsening pulmonary fibrosis that required bilateral lung transplantation (patient 1 in reference 13). Pulmonary function evaluation before and after HSCT was recorded in 48 patients (Table 2). The crude mortality rate was 19% overall, but 54% in those with PAP. Median survival was not significantly different between those with and without severe lung disease (infections, PAP, and so forth; P = .19, log-rank test). Importantly, 75% of those who died without having undergone HSCT showed severe lung compromise at initial presentation.
Table 2.
Pulmonary Function Test Results Before and After HSCT
| PFT (N = 48) | Before HSCT, % Predicted | After HSCT, % Predicted | > 10% Improvement | > 10% Decline |
|---|---|---|---|---|
| FEV1, % | 84 (24-110) | 78 (35-109) | 8 | 20 |
| Dlco, % adjusted for hemoglobin | 67 (34-111) | 60 (26-108) | 9 | 21 |
| FVC, % | 93 (40-129) | 91 (45-120) | 9 | 13 |
| 6MWT (n = 14), m | 456 (191-629) | 522 (361-655) | 9 | 2 |
Data are presented as No. or mean (range). Data are presented for 75% of the patients who underwent transplantation. Six-minute walk tests were carried out for only 20% of patients who underwent transplantation. PFTs after transplantation carried out after a median of 1 y (range, 6 mo-6 y) after transplantation. 6MWT = 6-min walk test; HSCT = hematopoietic stem cell transplantation; PFT = pulmonary function test.
Discussion
GATA2 deficiency has a high rate of lung involvement, even in the absence of overt hematopoietic dysfunction. Whether these manifestations are the result of abnormal alveolar macrophages, impaired pulmonary lymphatic circulation, impaired phagocytosis, or the lack of new monocytes repopulating the pulmonary compartment remains unclear.26
Recurrent infection and alveolar macrophage dysfunction in GATA2 deficiency may lead to damage of lung elastin, which also may contribute to the pulmonary changes seen in these patients.27,28 GATA2 plays a crucial role in hematopoiesis, but it is also involved in the development and function of macrophages. Impaired surfactant metabolism by dysfunctional alveolar macrophages also may underlie PAP. The rapid resolution of PAP after transplantation in GATA2 deficiency suggests that PAP is quite sensitive to the supply of new hematopoietic elements, although whether it is in fact the monocyte-derived component is not yet proven.13,29
The mechanisms by which GATA2 regulates phagocytosis are unclear, but likely are to be exacerbated by the lack of NK cells, a common feature of advanced GATA2 deficiency. GATA2 may interact with other hematopoietic transcription factors, including the transcription factor PU.1. Ets family.30 Numerous studies have demonstrated a central role for the ETS family transcription factor, PU.1, in GM-CSF mediated regulation of alveolar macrophage phagocytosis.31, 32, 33 Further studies are needed to elucidate better the molecular mechanisms underlying PAP in GATA2 deficiency, including the relationships among GATA2, the transcription factor PU.1 and GM-CSF. Cytopenias and dysfunction of other hematopoietic compartments, including NK cells, B cells, and dendritic cells, also may contribute to GATA2-mediated pulmonary disease.
Interestingly, at a point in the disease process when they typically have neither circulating monocytes nor marrow monocyte precursors, patients with GATA2 deficiency still have abundant macrophages in BAL fluid, as seen on cytopathologic examination of clinical aspirates and at autopsy in patients who have not undergone transplantation. This normal pulmonary alveolar macrophage abundance suggests persistence of macrophages from the period of normal hematopoiesis, which is seen early in life in GATA2 deficiency. However, these persisting pulmonary macrophages also may derive from tissue-resident macrophages, in which case, their dysfunction may be secondary to loss of other blood elements.34
Regardless of origin, these persistent alveolar macrophages eventually are incompetent regarding surfactant clearance, whether because of intrinsic GATA2 dysfunction or their failure to be stimulated properly by other cells. The rapid and definitive resolution of PAP in most patients after HSCT suggests that regardless of the initial origin of pulmonary alveolar macrophages, they are replaced rapidly or reinvigorated by elements transferred at HSCT. Although some patients in our study showed significant improvement in pulmonary function after HSCT, it should be noted that both transplant conditioning (particularly total body radiation and busulfan-based regimens) and pulmonary graft-vs-host disease also can impair pulmonary function, and these complications should be recognized and managed promptly.35
Dysfunction of the nitric oxide pathway in the pulmonary endothelium may lead to increased vascular smooth muscle tone and vascular remodeling, and thus may contribute to the development and progression of PAH.36 Cell-specific endothelial nitric oxide synthase expression in airway epithelium is dependent on the interaction of GATA2 with the endothelial nitric oxide synthase promoter.37 Hematopoietic element participation in the pulmonary vasculature also is possible and may explain the rapid resolution of PAH after HSCT. Further studies are required to understand better the possible roles of GATA2 in the pulmonary endothelium and in the development of pulmonary hypertension.38,39
Alveolar macrophage dysfunction and overall pulmonary compromise in GATA2 deficiency are temporized, reversed, or both by donor-derived cells, a process that occurs relatively rapidly after transplantation.40 Interestingly, HSCT has been shown to reverse PAH in both mice and rats,41 consistent with the identification of lung trafficking of cells arising from hematopoietic cell types.26 The latter study demonstrated reduced right ventricular pressures with reversal of right ventricular dysfunction and lung pathologic features in recipient rats. We saw reversal of hematologic, immunologic, and pulmonary manifestations, including PAP, PAH, and some parenchymal disease, after HSCT.42
Previously, we reported a prevalence of PAP of 31%, compared with the much lower rates in this report of 11%. Our previous report largely was based on patients referred for severe or chronic mycobacterial infections who typically had been sick for prolonged periods and had extensive marrow dysfunction. Most of those patients were referred before the identification of GATA2 as the genetic defect underlying this disease. In contrast, more recent patients have been detected much earlier in the course of the disease, already had undergone molecular diagnostic testing, and often had begun prophylactic antibacterial treatment. Other factors may be unaccounted for as well.
Interpretation
GATA2 deficiency is associated with characteristic, significant, and progressive pulmonary disease. More than half of the present cohort of patients showed lung abnormalities on pulmonary function testing or imaging, even in asymptomatic relatives identified through family screening. In addition to recurrent respiratory infections, patients demonstrated characteristic parenchymal changes, including paraseptal emphysema and PAP associated with progressive restrictive ventilatory defects and pulmonary hypertension. Remarkably, PAP and PAH improved rapidly and durably with HSCT. The reversal of PAH by HSCT suggests important and dynamic interactions of the hematopoietic and pulmonary vascular compartments. Many and complex pulmonary manifestations of GATA2 deficiency are detectable before hematopoietic failure. Earlier recognition may lead to earlier definitive therapy.
Take-home Points.
Study Question: What are the pulmonary manifestations of GATA2 deficiency?
Results: The lung was affected in 56% of patients with GATA2. Chronic nontuberculous mycobacterial infection was the most common, but pulmonary alveolar proteinosis and pulmonary hypertension were seen in 9% and 7%, respectively. Clinical presentations within families were variable. Allogeneic hematopoietic stem cell transplantation reversed infections, pulmonary alveolar proteinosis, and pulmonary arterial hypertension.
Interpretation: GATA2 deficiency has characteristic clinical and radiographic manifestations. Hematopoietic stem cell transplantation can reverse the major pulmonary manifestations of the disease.
Acknowledgments
Author contributions: B. E. M. collected and analyzed the data and drafted the manuscript. K. N. O. analyzed and interpreted the data and helped to write the manuscript. K. N.O. and S. M. H. designed the study and interpreted the data and revised and corrected the content of the manuscript. L. R. F. performed the radiologic analysis. L. R. F., A. F. F., A. P. H., A. C. F., C. S. Z., M. A. S., L. A. S., J. P. L., M. P., J. M.C.-R., and D. D. H. made substantial contributions to the generation and acquisition of the data and revised and corrected the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: L. R. F.: Patent (no royalties): “Radiographic marker that displays upright angle on portable x-rays.” US Patent 9,541,822 B2; Patent (no royalties): “Multigrayscale Universal CT Window.” US Patent 8,406,493 B2; Research agreement: Philips Healthcare; and author royalties, Springer. None declared (B. E. M., K. N. O., C. S. Z., A. P. H., A. F. F., A. C. F., M. A. S., L. A. S., J. P. L., M. P., J. M. C.-R., D. D. H., S. M. H.).
Additional information: The e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Marciano and Olivier contributed equally to this manuscript.
FUNDING/SUPPORT: Funding for this study was provided in part by the Divisions of Intramural Research, National Institute of Allergy and Infectious Diseases, National Heart, Lung and Blood Institute, National Cancer Institute (under contract nos. HHSN261200800001E and 75N910D00024 and task order no. 75N91019F00131) and the National Institutes of Health Clinical Center, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Supplementary Data
References
- 1.Spinner M.A., Sanchez L.A., Hsu A.P. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishida H., Honma K., Tamura S.-I., Imamura T., Ito M., Nonoyama S. GATA-2 anomaly and clinical phenotype of a sporadic case of lymphedema, dendritic cell, monocyte, B- and NK-cell (DCML) deficiency, and myelodysplasia. Eur J Pediatr. 2012;171(8):1273–1276. doi: 10.1007/s00431-012-1715-7. [DOI] [PubMed] [Google Scholar]
- 3.Wlodarski M.W., Collin M., Horwitz M.S. GATA2 deficiency and related myeloid neoplasms. Semin Hematol. 2017;54(2):81–86. doi: 10.1053/j.seminhematol.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collin M., Bigley V., McClain K.L., Allen C.E. Cell(s) of origin of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 2015;29(5):825–838. doi: 10.1016/j.hoc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostergaard P., Simpson M.A., Connell F.C. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43(10):929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- 6.Vicente C., Conchillo A., Garcia-Sanchez M.A., Odero M.D. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol. 2012;82(1):1–17. doi: 10.1016/j.critrevonc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Kitajima K., Tanaka M., Zheng J. Redirecting differentiation of hematopoietic progenitors by a transcription factor, GATA-2. Blood. 2006;107(5):1857–1863. doi: 10.1182/blood-2005-06-2527. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues N.P., Boyd A.S., Fugazza C. GATA-2 regulates granulocyte-macrophage progenitor cell function. Blood. 2008;112(13):4862–4873. doi: 10.1182/blood-2008-01-136564. [DOI] [PubMed] [Google Scholar]
- 9.Vinh D.C., Patel S.Y., Uzel G. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(9):1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griese M., Zarbock R., Costabel U. GATA2 deficiency in children and adults with severe pulmonary alveolar proteinosis and hematologic disorders. BMC Pulm Med. 2015;15:87. doi: 10.1186/s12890-015-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trapnell B.C., Carey B.C., Uchida K., Suzuki T. Pulmonary alveolar proteinosis, a primary immunodeficiency of impaired GM-CSF stimulation of macrophages. Curr Opin Immunol. 2009;21(5):514–521. doi: 10.1016/j.coi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jouneau S., Ballerie AA-Ohoo, Kerjouan M., Demant X., Blanchard E., Lederlin M. Haemodynamically proven pulmonary hypertension in a patient with GATA2 deficiency-associated pulmonary alveolar proteinosis and fibrosis. Eur Respir J. 2017;49(5):1700407. doi: 10.1183/13993003.00407-2017. [DOI] [PubMed] [Google Scholar]
- 13.Cuellar-Rodriguez J., Gea-Banacloche J., Freeman A.F. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118(3):3715–3720. doi: 10.1182/blood-2011-06-365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parta M., Shah N.N., Baird K. Allogeneic hematopoietic stem cell transplantation for GATA2 deficiency using a busulfan-based regimen. Biol Blood Marrow Transplant. 2018;24(6):1250–1259. doi: 10.1016/j.bbmt.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole K., Avila D., Parta M. GATA2 deficiency: early identification for improved clinical outcomes. Clin J Oncol Nurs. 2019;23(4):417–422. doi: 10.1188/19.CJON.417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svobodova T., Mejstrikova E., Salzer U. Diffuse parenchymal lung disease as first clinical manifestation of GATA-2 deficiency in childhood. BMC Pulm Med. 2015;15:8. doi: 10.1186/s12890-015-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffith De, Aksamit T., Brown-Elliott B.A. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 175(4):367-416. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino R., Viegi G., Brusasco V. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 19.Johnson J.D., Theurer W.M. A stepwise approach to the interpretation of pulmonary function tests. Am Fam Physician. 2014;89(5):359–366. [PubMed] [Google Scholar]
- 20.Lee K.N., Levin D.L., Webb W.R., Chen D., Storto M.L., Golden J.A. Pulmonary alveolar proteinosis: high-resolution CT, chest radiographic, and functional correlations. Chest. 1997;111(4):989–995. doi: 10.1378/chest.111.4.989. [DOI] [PubMed] [Google Scholar]
- 21.Hsu A.P., Johnson K.D., Falcone E.L. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood. 2013;121(19):3830–3837. doi: 10.1182/blood-2012-08-452763. s3831-s3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B.M., Stern E.J., Schmidt R.A., Pierson D.J. Diagnosing pulmonary alveolar proteinosis. A review and an update. Chest. 1997;111(2):460–466. doi: 10.1378/chest.111.2.460. [DOI] [PubMed] [Google Scholar]
- 23.Papiris S.A., Tsirigotis P., Kolilekas L. Pulmonary alveolar proteinosis: time to shift? Expert Rev Respir Med. 2015;9(3):337–349. doi: 10.1586/17476348.2015.1035259. [DOI] [PubMed] [Google Scholar]
- 24.Maygarden S.J., Iacocca M.V., Funkhouser W.K., Novotny D.B. Pulmonary alveolar proteinosis: a spectrum of cytologic, histochemical, and ultrastructural findings in bronchoalveolar lavage fluid. Diagn Cytopathol. 2001;24(6):389–395. doi: 10.1002/dc.1086. [DOI] [PubMed] [Google Scholar]
- 25.Galiè N., Hoeper M.M., Humbert M., Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC) European Respiratory Society (ERS) International Society of Heart and Lung Transplantation (ISHLT) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):1219–1263. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 26.Summer R., Kotton D.N., Sun X., Fitzsimmons K., Fine A. Translational physiology: origin and phenotype of lung side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;287(3) doi: 10.1152/ajplung.00020.2004. [DOI] [PubMed] [Google Scholar]
- 27.Tang X., Lasbury M.E., Davidson D.D., Bartlett M.S., Smith J.W., Lee C.H. Down-regulation of GATA-2 transcription during Pneumocystis carinii infection. Infect Immun. 2000;68(8):4720–4724. doi: 10.1128/iai.68.8.4720-4724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasbury M.E., Tang X., Durant P.J., Lee C.-H. Effect of transcription factor GATA-2 on phagocytic activity of alveolar macrophages from Pneumocystis carinii-infected hosts. Infect Immun. 2003;71(9):4943–4952. doi: 10.1128/IAI.71.9.4943-4952.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishinakamura R., Wiler R., Dirksen U. The pulmonary alveolar proteinosis in granulocyte macrophage colony-stimulating factor/interleukins 3/5 beta c receptor-deficient mice is reversed by bone marrow transplantation. J Exp Med. 1996;183(6):2657–2662. doi: 10.1084/jem.183.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P., Behre G., Pan J. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc Natl Acad Sci U S A. 1999;96(15):8705–8710. doi: 10.1073/pnas.96.15.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibata Y., Berclaz P.Y., Chroneos Z.C., Yoshida M., Whitsett J.A., Trapnell B.C. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15(4):557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 32.Bonfield T.L., Raychaudhuri B., Malur A. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2003;285(5):L1132–L1136. doi: 10.1152/ajplung.00216.2003. [DOI] [PubMed] [Google Scholar]
- 33.Berclaz P.Y., Shibata Y., Whitsett J.A., Trapnell B.C. GM-CSF, via PU.1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma-mediated molecular connection between innate and adaptive immunity in the lung. Blood. 2002;100(12):4193–4200. doi: 10.1182/blood-2002-04-1102. [DOI] [PubMed] [Google Scholar]
- 34.Perdiguero E.G., Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17(1):2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan A., Sunkara A., Mitchell W. Recovery of pulmonary function after allogeneic hematopoietic cell transplantation in children is associated with improved survival. Biol Blood Marrow Transplant. 2017;23(12):2102–2109. doi: 10.1016/j.bbmt.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lázár Z., Mészáros M., Bikov A. The nitric oxide pathway in pulmonary arterial hypertension: pathomechanism, biomarkers and drug targets. Curr Med Chem. 2020;27(42):7168–7188. doi: 10.2174/0929867327666200522215047. [DOI] [PubMed] [Google Scholar]
- 37.German Z., Chambliss K.L., Pace M.C., Arnet U.A., Lowenstein C.J., Shaul P.W. Molecular basis of cell-specific endothelial nitric-oxide synthase expression in airway epithelium. J Biol Chem. 2000 doi: 10.1074/jbc.275.11.8183. 275(11):8183-8189. [DOI] [PubMed] [Google Scholar]
- 38.Lanzola E., Farha S., Erzurum S.C., Asosingh K. Bone marrow-derived vascular modulatory cells in pulmonary arterial hypertension. Pulm Circ. 2013;3(4):781–791. doi: 10.1086/674769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pu X., Du L., Hu Y., Fan Y., Xu Q. Stem/progenitor cells and pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2021;41(1):167–178. doi: 10.1161/ATVBAHA.120.315052. [DOI] [PubMed] [Google Scholar]
- 40.Faiz S.A., Iliescu C., Lopez-Mattei J., Patel B., Bashoura L., Popat U. Resolution of myelofibrosis-associated pulmonary arterial hypertension following allogeneic hematopoietic stem cell transplantation. Pulm Circ. 2016;6(4):611–613. doi: 10.1086/687291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umar S., de Visser Y.P., Steendijk P. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297(5):H1606–H1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 42.Pittman C., Hsieh M.M., Coles W., Tisdale J.F., Weir N.A., Fitzhugh C.D. Reversal of pre-capillary pulmonary hypertension in a patient with sickle cell anemia who underwent haploidentical peripheral blood stem cell transplantation. Bone Marrow Transplant. 2017;52(4):641–642. doi: 10.1038/bmt.2016.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.