Abstract
v-Jun accelerates G1 progression and shares the capacity of the Myc, E2F, and E1A oncoproteins to sustain S-phase entry in the absence of mitogens; however, how it does so is unknown. To gain insight into the mechanism, we investigated how v-Jun affects mitogen-dependent processes which control the G1/S transition. We show that v-Jun enables cells to express cyclin A and cyclin A-cdk2 kinase activity in the absence of growth factors and that deregulation of cdk2 is required for S-phase entry. Cyclin A expression is repressed in quiescent cells by E2F acting in conjunction with its pocket protein partners Rb, p107, and p130; however, v-Jun overrides this control, causing phosphorylated Rb and proliferation-specific E2F-p107 complexes to persist after mitogen withdrawal. Dephosphorylation of Rb and destruction of cyclin A nevertheless occur normally at mitosis, indicating that v-Jun enables cells to rephosphorylate Rb and reaccumulate cyclin A without exogenous mitogenic stimulation each time the mitotic “clock” is reset. D-cyclin–cdk activity is required for Rb phosphorylation in v-Jun-transformed cells, since ectopic expression of the cdk4- and cdk6-specific inhibitor p16INK4A inhibits both DNA synthesis and cell proliferation. Despite this, v-Jun does not stimulate D-cyclin–cdk activity but does induce a marked deregulation of cyclin E-cdk2. In particular, hormonal activation of a conditional v-Jun–estrogen receptor fusion protein in quiescent, growth factor-deprived cells stimulates cyclin E-cdk2 activity and triggers Rb phosphorylation and DNA synthesis. Thus, v-Jun overrides the mitogen dependence of S-phase entry by deregulating Rb phosphorylation, E2F-pocket protein interactions, and ultimately cyclin A-cdk2 activity. This is the first report, however, that cyclin E-cdk2, rather than D-cyclin–cdk, is likely to be the critical Rb kinase target of v-Jun.
The vertebrate cell division cycle is regulated primarily at the transition between the G1 and S phases of the cell cycle, also known as the restriction point, beyond which cells become committed to mitosis (49, 51). Normal cells require mitogenic signals in the form of soluble growth factors and substrate attachment in order to make this transition, while oncogenic lesions frequently deregulate cell proliferation by mimicking or circumventing the need for such signals (43).
The retinoblastoma (Rb) tumor suppressor protein and the related p107 and p130 “pocket proteins” are negative growth regulators which play a pivotal role in controlling the G1/S transition through their association with the E2F and DP-1 families of transcription factors (15, 49). E2F and DP-1 proteins form heterodimers which bind to specific DNA recognition sequences either alone as “free” E2F–DP-1 or as complexes with Rb, p107, or p130 (6). Although the functional consequences of E2F-pocket protein interactions are incompletely understood, free E2F has the potential to activate, whereas E2F-pocket protein complexes repress, target gene transcription (6, 15).
A critical feature of the pocket proteins is that their growth-inhibitory function is inactivated through the action of cyclin-dependent kinases (cyclin-cdk's), a process best understood in the case of Rb. Rb is phosphorylated at multiple sites during G1 by D-cyclin–cdk complexes acting in concert with cyclin E-cdk2 (collectively referred to as G1/S cyclin cdk's) (34). One important consequence of this phosphorylation is to nullify the capacity of Rb to bind E2F, thus dissociating E2F-Rb repressor complexes in favor of free, transcriptionally active E2F (15, 34).
Many E2F-regulated genes are expressed periodically during the cell cycle and encode products required for DNA replication or metabolism, such as cyclin A, DNA polymerase α, dihydrofolate reductase, and ORC1 (15), and a variety of evidence indicates that recurrent activation of E2F via Rb phosphorylation is required for S-phase entry in each cell cycle. For example, ectopic overexpression of hypophosphorylated Rb arrests proliferating cells in G1 (17), while inhibition of D-cyclin–cdk activity using the cyclin-cdk inhibitor p16INK4A blocks S-phase entry, providing that the cells express wild-type Rb (33). Conversely, forced expression of E2F is sufficient to promote S-phase entry in quiescent cells (20) and can circumvent a p16INK4A-induced growth arrest (29).
An additional level of regulation occurs when cells withdraw from the cycle. Whereas Rb is ubiquitous, p130 is found preferentially in quiescent or terminally differentiated cells, while p107 is associated with proliferation (6). Cyclin-cdk-mediated phosphorylation promotes p130 degradation, thus explaining why the protein accumulates in nonproliferating cells where these activities are low (6). The resulting E2F-p130 complexes distinguish cells in G0 from cells in G1 (46) and are thought to play a role in stabilizing the quiescent or differentiated state by repressing E2F-dependent gene transcription and preventing inappropriate S-phase entry (6, 46).
As one might expect from the central importance of E2F for G1/S control, a number of oncogenes which promote DNA synthesis do so by targeting E2F-pocket protein interactions. Thus, adenovirus E1A and human papillomavirus E7 trigger S-phase entry by binding to Rb and dissociating it from E2F-Rb complexes, thereby mimicking the effect of regulatory phosphorylation and releasing active E2F (19). Alternatively, activation of c-Myc in quiescent cells stimulates cyclin E-cdk2 activity (and possibly D-cyclin–cdk activity [see reference 1 for discussion]), leading to Rb phosphorylation, E2F activation, expression of cyclin A, and DNA synthesis (41, 48).
The c-jun proto-oncogene encodes a second cellular transcription factor implicated in cell cycle control. Inhibition of c-Jun function by microinjection of neutralizing antibodies (23) or antisense RNA (47) or through c-jun gene disruption (21) indicates an essential role for c-Jun in cell cycle progression. Additional evidence comes from the finding that the oncogenic form of c-Jun, v-Jun (31), shares the capacity of the Myc, E1A, and E2F oncoproteins to promote S-phase entry. This was evident from the phenotype of chicken embryo fibroblasts (CEFs) transformed by v-Jun, which exhibited a shorter G1 phase under optimal growth conditions and failed to exit the cell cycle after mitogen deprivation (4). As with Myc, however, v-Jun does not enable cells to multiply without growth factors, since cell cycle progression in the absence of serum is limited by apoptosis (4).
Although the role of c-Jun in cell cycle control is not yet understood, most attention has focused on the hypothesis that c-Jun positively regulates expression of genes which promote the G1/S transition. For example, c-Jun can stimulate transcription of the cyclin D1 promoter in transient-transfection assays (16), and consistent with this, cyclin D1 levels and D-cyclin–cdk kinase activity are reduced in c-jun-deficient cells (50). More recently, however, this model has been challenged by the discovery that the proliferative defect in c-jun-deficient cells is dependent on p53 function and that c-Jun may repress, rather than activate, p53 gene transcription (42).
Since v-Jun can also both activate and repress gene transcription (10, 12, 13, 14, 18, 32), it seems likely that the loss of cell cycle control in v-Jun-expressing cells stems from deregulation of genes involved directly in DNA replication (36), mitogenic signaling, or modulating the cyclin-cdk activities which control the G1/S transition. It should be borne in mind, however, that the possibility that v-Jun might act through a nontranscriptional mechanism cannot be formally excluded at this point, particularly since c-Jun has recently been shown to bind to Rb (37).
To identify the point at which v-Jun acts on the cell cycle machinery, we therefore considered it essential to first establish whether v-Jun overrides the mitogen dependence of Rb phosphorylation and associated downstream events or bypasses these processes by promoting S-phase entry directly. Each of these potential mechanisms makes testable predictions, and to distinguish between them, we have documented how v-Jun affects the mitogen-dependent processes which in normal cells control the G1/S transition.
MATERIALS AND METHODS
Cell culture and retroviral vectors.
Primary and secondary CEFs were cultured in Dulbecco modified Eagle medium-based growth medium (GM) containing 10% newborn calf serum and 2% heat-inactivated chicken serum or in low-serum (LS) medium containing 0.2% newborn calf serum, as described previously (4). The cultures were routinely fed with fresh medium every 24 h.
To generate cultures of retrovirus-infected CEFs, primary CEFs were transfected with the appropriate retroviral DNA, or combination of DNAs, and the cultures were passaged until uniform infection was achieved. ASV17 was regenerated using RCAS (replication competent, subgroup A, spliced) as a helper virus to generate v-Jun-transformed CEFs, while control CEFs were infected with RCAS alone (4). CEFs expressing Δv-JunER (estrogen receptor) or human ER (hER) were generated by infection with previously described RCAS vectors encoding the appropriate proteins (24). Human p16INK4A (38) was excised from pRcCMV-p16 as a HindIII/EcoRI (end-filled) fragment and cloned into the neomycin-selectable vector SFCV between the HindIII and ApaI (end-filled) sites to generate SFCV-p16. SFCV-p16, or SFCV alone, was regenerated using RCAS, or RCAS-v-Jun, as a helper virus as described previously (26). Forty-eight hours after transfection, the cultures were trypsinized, seeded at a range of dilutions, and selected with G418. Five days after selection, the cultures were fixed in methanol and stained with Giemsa stain to visualize colony growth. Control experiments have shown that selection with G418 results in cultures of CEFs efficiently infected with both retroviruses by this approach (26).
Microinjection.
CEFs were microinjected with a Zeiss Axiovert 100 microscope and an Eppendorf Micromanipulator 5171. The cells were plated for microinjection onto Cellocate coverslips (Eppendorf). All plasmids were microinjected into the nuclei of the cells in 10 mM potassium phosphate (pH 7.4)–130 mM potassium chloride at a concentration of 50 to 100 μg/ml. The plasmids used were pcDNA3.1-EGFP, pCMV-cdk2D145N, pRcCMV, and pRcCMV-p16. The cells were grown in GM or LS medium for 24 h prior to injection. Twenty-four hours after injection, bromodeoxyuridine (BrdU) was added to 25 μM, and the cells were incubated in the relevant medium for a further 24 h.
Injected cells were washed in phosphate-buffered saline, fixed in acetone-methanol (1:1), and stained for incorporation of BrdU and expression of green fluorescent protein (GFP) using anti-BrdU monoclonal antibody (catalog no. M0744; Dako) and anti-GFP polyclonal antibody (catalog no. 598; MBL). Anti-BrdU and anti-GFP antibodies were detected with Texas red-conjugated sheep anti-mouse (catalog no. 515-075-003; Immunoresearch Laboratories) and fluorescein-conjugated goat anti-rabbit (catalog no. 111-095-144; Immunoresearch Laboratories) antibodies.
E2F gel retardation analysis.
Cell extracts and E2F band shift assays were performed using an oligonucleotide spanning the adenovirus E2A-E2F site essentially as described previously (11). Where indicated, antisera specific for DP-1; E2F-1, -4, and -5; Rb; or p107 were included in the binding reactions prior to addition of the oligonucleotide probe and electrophoresis (3, 11).
Centrifugal elutriation.
Elutriations were performed using a Beckman J2-21 centrifuge and JE-6B rotor at 4°C. Ten 150-mm-diameter dishes of v-Jun CEFs which had been maintained in LS medium for 48 h were trypsinized and suspended in 10 ml of phosphate-buffered saline containing 2% newborn calf serum. This suspension (0.5 ml) was retained on ice throughout the elutriation procedure (unfractionated [U]), while the remainder was fractionated into nine 150-ml fractions at 3,000 rpm by progressively increasing the flow rate. The cells were recovered by centrifugation, and a portion was fixed with 70% ethanol for flow cytometry while whole-cell extracts were prepared from the remainder for Western blotting. The fixed cells were stained with propidium iodide and analyzed by flow cytometry as described previously (4).
Preparation of cell extracts, Western blotting, immunoprecipitation, and kinase assays.
Whole-cell extracts were prepared from CEF cultures, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and analyzed by Western blotting as described previously (4, 22). The antisera used for Western blotting, and in some cases for immunoprecipitation, were as follows: anti-cdk2 (rabbit polyclonal; sc-163G; Santa Cruz), anti-cyc A (rabbit polyclonal; R28; kind gift of E. Nigg), cdk6 (anti-cdk6 Ab-1; Neomarkers), anti-cyc D1 (DCS-6), anti-cyc D2 (anti-cyclin D2 Ab-3; Neomarkers), anti-p27 KIP1 (PC52; Calbiochem), anti-Rb (14441A; Pharmingen), anti-p107 (sc318; Santa Cruz), and anti-p130 (sc31; Santa Cruz). A rabbit antiserum specific for avian cyclin E was generated by immunizing rabbits with a synthetic peptide corresponding to the C-terminal 13 amino acids of chicken cyclin E conjugated to keyhole limpet hemocyanin. The resulting antiserum was affinity purified with a column of the immunogenic peptide and used in both Western blot and immunoprecipitation assays. The preparation and characterization of this antiserum will be described in detail elsewhere.
For immunoprecipitation kinase assays, cell extracts were prepared in lysis buffer consisting of 50 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 1 mM dithiothreitol, 0.1% Tween 20, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM Na orthovanadate, 2 μg of aprotinin and leupeptin per ml, and 0.1 mM phenylmethylsulfonyl fluoride (28). Portions of the extract were incubated with the appropriate precipitating antibodies for 4 h at 4°C, and the immune complexes were captured on Sepharose beads coated with protein A (for rabbit antibodies) or protein G (for monoclonal antibodies). The precipitates were washed three times with lysis buffer and twice with kinase buffer consisting of 50 mM HEPES (pH 7.5), 10 mM MgCl2, 10 mM MnCl2, and 1 mM dithiothreitol. Kinase reactions were performed in a final volume of 20 μl of kinase buffer containing 20 μM ATP, 0.1 mM protein kinase A inhibitor (PO300; Sigma), 10 μC of [γ-32P]ATP, and 1 μg of either histone H1 (U.S. Biochemical) (for anti-Cdk2, -cyclin A, and -cyclin E kinase assays) or glutathione S-transferase–Rb (Rb amino acids 379 to 928; for anti-cyclin D1 kinase assays) as substrates (28). Kinase assays were performed at 30°C for 30 min, after which the reactions were resolved by electrophoresis on sodium dodecyl sulfate–10% polyacrylamide gels and either dried or, in some cases, transferred to nitrocellulose prior to autoradiography to permit subsequent Western blotting analysis of the immunoprecipitates. Kinase activity was quantitated by laser densitometry of the autoradiograms.
RESULTS
v-Jun enables cells to sustain cdk2 kinase activity and cyclin A expression after prolonged mitogen deprivation.
Previously, we showed that v-Jun-transformed CEFs continue to synthesize DNA and divide in the absence of serum growth factors when control cells arrest in G0 (4). Since cdk2 is a principal regulator of S-phase entry and progression, we investigated the effect of v-Jun on cdk2 kinase activity as a first step towards establishing the mechanism of cell cycle deregulation.
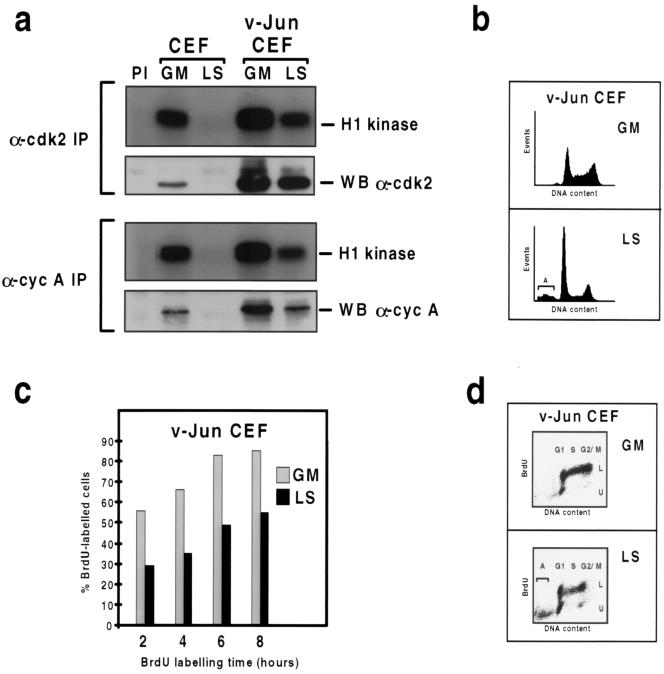
As shown in Fig. 1a (top), both cdk2 protein and kinase activity were readily detectable in growing normal control CEFs (lane GM); however, neither persisted in quiescent cells which had been maintained in LS medium (lane LS) for 48 h when DNA synthesis became negligible (4). Most of the active cdk2 in growing control cells was associated with cyclin A, since quantitatively similar levels of kinase activity were recovered in cyclin A precipitates (Fig. 1a, bottom). As with cdk2, cyclin A was not expressed in quiescent cells (Fig. 1a).
FIG. 1.
v-Jun enables cells to sustain cdk2 kinase activity and cyclin A expression after prolonged mitogen deprivation. (a) Immunoprecipitation (IP) kinase assays of total cdk2 and cyclin (cyc) A-cdk2 kinase activity in cultures of control and v-Jun-transformed CEFs growing in GM or after incubation in LS medium for 48 h. Cell extracts were immunoprecipitated with antibodies specific for cdk2 (top) and cyclin A (bottom), and the precipitates were analyzed for kinase activity using histone H1 as a substrate. Subsequently, the precipitates were analyzed for cdk2 or cyclin A protein expression by Western blotting (WB). (b) DNA content cytograms of cultures of v-Jun-transformed CEFs growing in GM or after incubation in LS medium for 48 h. The plots correspond to the samples shown in panel d. (c) Cultures of v-Jun-transformed CEFs growing in GM or after incubation in LS medium for 48 h were labeled with BrdU for 2, 4, 6, or 8 h. The culture medium was changed immediately before BrdU addition to remove from the LS culture apoptotic cells which had died and detached prior to the start of the experiment. After being labeled, both adherent and detached cells were harvested, and the percentage of labeled cells was determined by flow cytometry. (d) Plots of DNA content versus BrdU incorporation for 8-h GM and LS medium samples shown in panel c. Labeled (L) and unlabeled (U) cell populations are indicated, as are the positions of G1, S, G2/M, and apoptotic (A) cells.
In comparison, v-Jun-transformed CEFs expressed more cdk2 and cyclin A than control CEFs in GM and exhibited higher levels of both total and cyclin A-associated cdk2 kinase activity (Fig. 1a). We have previously shown that v-Jun-transformed cultures contain a substantial (several-fold) excess of S-phase cells compared to control cultures under optimal growth conditions owing to shortening of the G1 phase (4). Since cyclin A expression is normally maximal during S phase, we believe the upregulation of cyclin A in v-Jun-transformed CEFs in GM is due primarily to this excess rather than being a direct effect of v-Jun on the cyclin A gene promoter. Additional observations which support this interpretation are described in detail below. Whether the upregulation of cdk2 in v-Jun CEFs is also a consequence of altered cell cycle distribution or is a direct effect of v-Jun on cdk2 gene expression remains to be determined.
Strikingly, both cyclin A expression and cdk2 kinase activity persisted in v-Jun CEFs after 48 h in LS medium, although at reduced levels (Fig. 1a). As in GM, most of the active cdk2 which persisted in LS medium was evidently associated with cyclin A, since similar levels of kinase activity were obtained in cdk2 and cyclin A precipitates (Fig. 1a) and the decline in cdk2 kinase activity which occurred after mitogen deprivation was associated with a similar decline in the amount of cyclin A. In contrast the level of cdk2, although elevated, did not vary (Fig. 1a).
Prolonged growth factor deprivation also led to a pronounced decrease in the proportion of S- and G2/M-phase cells in the v-Jun CEF cultures (Fig. 1b). This was mainly due to a slowing in the rate of S-phase entry rather than selective depletion of S and G2/M cells by v-Jun-induced apoptosis (4), since the percentage of cells incorporating BrdU was lower and increased more slowly in LS medium than in GM (Fig. 1c), yet few, if any, of the labeled cells appeared in the apoptotic population (Fig. 1d).
These experiments demonstrate that v-Jun renders cells competent to express cyclin A and cdk2 kinase activity after prolonged mitogen deprivation. In addition, they reveal a close correlation between the lower levels of cyclin A and cdk2 kinase activity which persist in the mitogen-deprived cultures and a reduction in the rate of S-phase entry. Taken together, these results are consistent with the hypothesis that v-Jun overrides the mitogen dependence of DNA synthesis by deregulating cyclin A expression and cdk2 kinase activity.
Deregulation of cdk2 is required for v-Jun-induced S-phase entry.
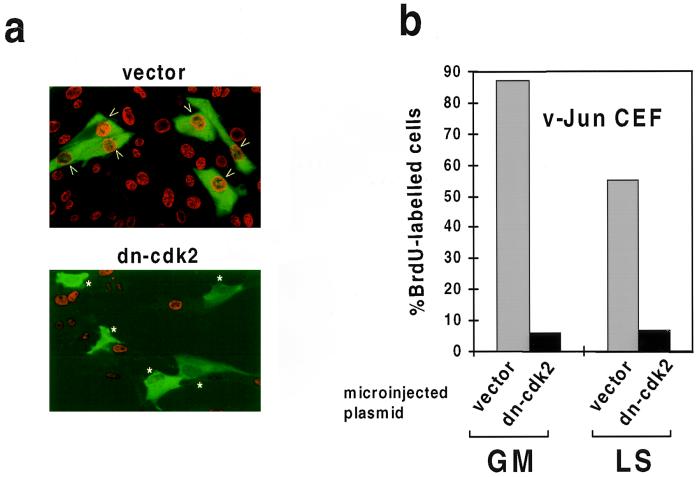
To confirm that cdk2 activity was required for DNA synthesis in mitogen-deprived v-Jun CEFs, we microinjected an expression plasmid encoding a catalytically inactive cdk2 (dn-cdk2) together with a plasmid encoding GFP as an injection marker. After allowing sufficient time for expression, the percentage of control and dn-cdk2-injected cells which synthesized DNA in 24 h was determined by BrdU labeling. An example of this analysis is shown for illustration in Fig. 2a.
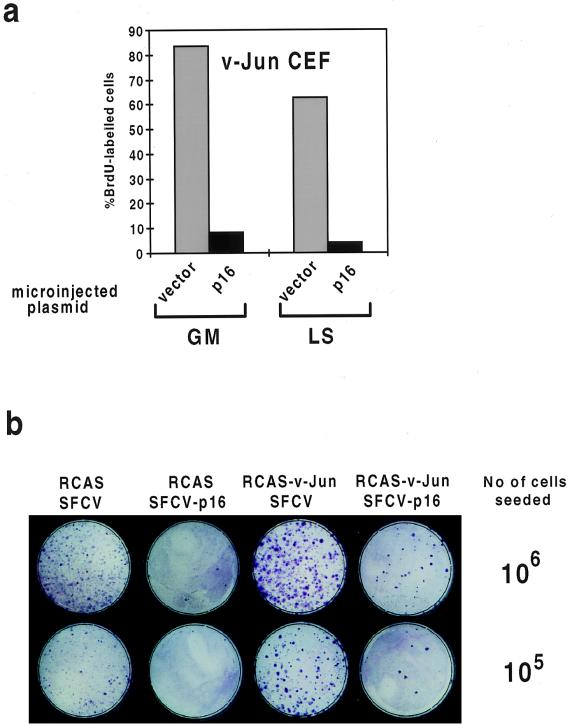
FIG. 2.
Deregulation of cdk2 is required for v-Jun-induced S-phase entry. v-Jun-transformed CEFs cultured in GM or LS medium were microinjected with control (vector) or expression plasmid encoding catalytically inactive cdk2 protein (dn-cdk2) together with a plasmid encoding GFP to identify injected cells. After sufficient time was allowed for expression, the percentage of injected cells which synthesized DNA in 24 h was determined by BrdU labeling and immunocytochemistry. (a) A representative example of this analysis (LS medium). Injected cells staining positive for BrdU incorporation are indicated with arrows, while negative cells are indicated by asterisks. (b) At least 100 surviving injected cells were analyzed for each growth condition. The experiment was repeated three times with similar results.
Quantitation of these data showed that dn-cdk2 strongly inhibited DNA synthesis in v-Jun-transformed CEFs both in the presence and in the absence of growth factors (Fig. 2b). Similar inhibition was observed in control CEFs growing in GM, although since no significant DNA synthesis occurred in quiescent cells, the effect of dn-cdk2 could not be evaluated in LS medium (data not shown).
Thus, the residual cdk2 activity which persists in v-Jun CEFs after mitogen deprivation is essential for S-phase entry and/or progression. Although it was not feasible to inhibit cyclin A-associated cdk2 selectively using this approach, it seems likely that cyclin A is also required and is potentially rate limiting for DNA synthesis under these conditions for two reasons. Firstly, most of the active cdk2 is associated with cyclin A (Fig. 1b), and secondly, there is a close association between cyclin A expression and DNA synthesis when individual cells in the population are separated according to cell cycle position by centrifugal elutriation (see below).
v-Jun overrides mitogen regulation of E2F-pocket protein interactions.
Cyclin A expression is repressed in quiescent cells by E2F-p130 or E2F-Rb repressor complexes via an E2F site in the cyclin A gene promoter (6, 15). The cyclin A promoter, however, also contains an activating transcription factor site which is a potential target site for activation by v-Jun (45). We therefore considered that v-Jun could deregulate cyclin A expression by one of two possible mechanisms: indirectly, by overriding the normal mitogen regulation of E2F, or directly, by binding to the cyclin A promoter, in which case global E2F regulation would be undisturbed.
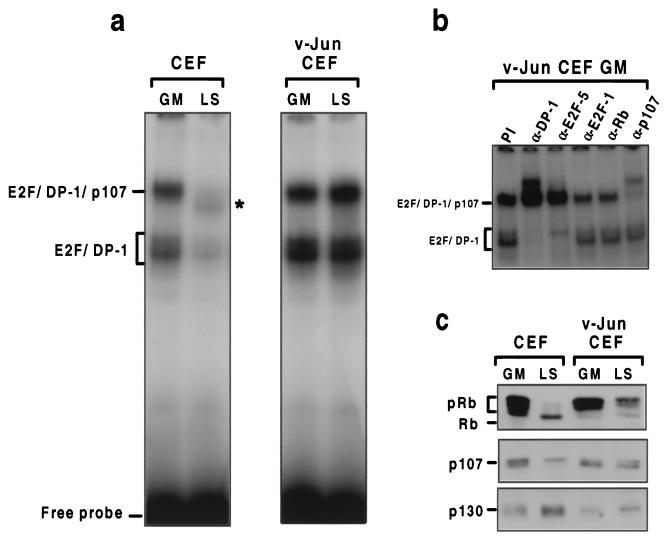
To evaluate the effect of v-Jun on E2F regulation, we compared E2F binding complexes in normal and v-Jun-transformed fibroblasts by band shift analysis. As shown in Fig. 3a, two electrophoretically distinct complexes formed on an oligonucleotide spanning the E2F site from the E2A gene promoter (11) in extracts from control CEFs growing in GM. An identical pattern was observed in v-Jun-transformed cells in GM, although the overall level of binding activity was greater (Fig. 3a). In each case, binding of both complexes was eliminated by addition of excess specific, but not nonspecific, cold competitor DNA (data not shown).
FIG. 3.
v-Jun overrides mitogen regulation of E2F-pocket protein interactions. (a) Cell extracts prepared from control or v-Jun-transformed CEFs growing in GM or after 48 h in LS medium were analyzed for E2F binding activity by band shift assay using an oligonucleotide spanning the E2F site from the E2A gene promoter as a probe. Complexes corresponding to free E2F–DP-1 and E2F-p107 complexes on the basis of antibody addition experiments (b) are indicated. The putative E2F-p130 detected in quiescent CEFs (LS) is indicated with an asterisk. (b) Analysis of E2F complexes in growing v-Jun CEFs using antibodies specific for DP-1, E2F family members, and individual pocket proteins. DNA binding reactions prepared as described for panel a were preincubated with the indicated antisera prior to addition of the oligonucleotide probe. (c) Western blotting analysis of Rb, p107, and p130 expression in the cell extracts shown in panel a.
To further characterize these complexes, we included antibodies specific for E2F, DP-1, Rb, and p107 in the binding reactions. Since identical results were obtained for both extracts, only representative results for v-Jun CEFs are shown (Fig. 3b and data not shown). In each case the more rapidly migrating complex was almost completely shifted and/or disrupted by anti-E2F-5 and anti-DP-1 antibodies but was unaffected by antiserum specific for p107 or Rb. In contrast, most, although not all, of the more slowly migrating complex was shifted by anti-p107 antibody and by anti-DP-1 (Fig. 3b). These results indicate that the most rapidly migrating complex consists of “free” E2F-5 in association with DP-1, while the slower-migrating species represents an E2F-p107 pocket protein complex (6, 15). The identity of the E2F family member(s) associated with p107 could not be established, since none of the E2F antibodies tested (E2F-1, -4, and -5) reacted with this complex. This could be because another E2F family protein associates specifically with p107 in these cells or because the epitope(s) recognized by the test antibodies is concealed within the p107-containing complex.
A very different pattern of binding was observed in control cells which had been rendered quiescent by incubation in LS medium for 48 h (Fig. 3a, lane LS). In addition to a substantial reduction in the amount of free E2F–DP-1, the E2F-p107 species was lost and replaced by a complex with distinct electrophoretic mobility (Fig. 3a) which was not reactive with antibodies specific for p107 or Rb (data not shown). By analogy with other well-characterized systems, this complex most likely corresponds to E2F-p130, which accumulates in quiescent fibroblasts (6, 46). Consistent with this interpretation, Western blotting revealed that cell cycle exit was also associated with changes in the phosphorylation state and/or abundance of the pocket proteins. Thus, whereas hyperphosphorylated Rb predominated in growing cells, only the hypophosphorylated, growth-inhibitory form was retained in quiescent cells. Similarly, while the abundance of p130 increased on cell cycle exit, the amount of both Rb and p107 declined (Fig. 3c).
In striking contrast, prolonged mitogen deprivation had no effect on the amount of free E2F-5–DP-1 or of the E2F-p107 pocket protein complex in v-Jun-transformed cells (Fig. 3a), nor was there any change in the phosphorylation state of Rb, reduction in p107, or increase in p130 levels (Fig. 3c). Thus, v-Jun overrides the mitogen dependence of Rb phosphorylation and E2F-pocket protein interactions.
v-Jun promotes mitogen-independent Rb phosphorylation and reaccumulation of cyclin A after mitosis.
The persistence of phosphorylated Rb and proliferation-specific E2F-p107 complexes provided a potential explanation for the continued expression of cyclin A in mitogen-deprived v-Jun CEFs. During the course of a normal cell cycle, however, cyclin A is degraded and Rb is dephosphorylated at the end of mitosis (27, 35). It therefore became important to determine whether v-Jun circumvented this resetting of the mitotic “clock” by blocking these normal processes or enabled cells to rephosphorylate Rb and reaccumulate cyclin A after mitosis in the absence of exogenous mitogenic stimulation.
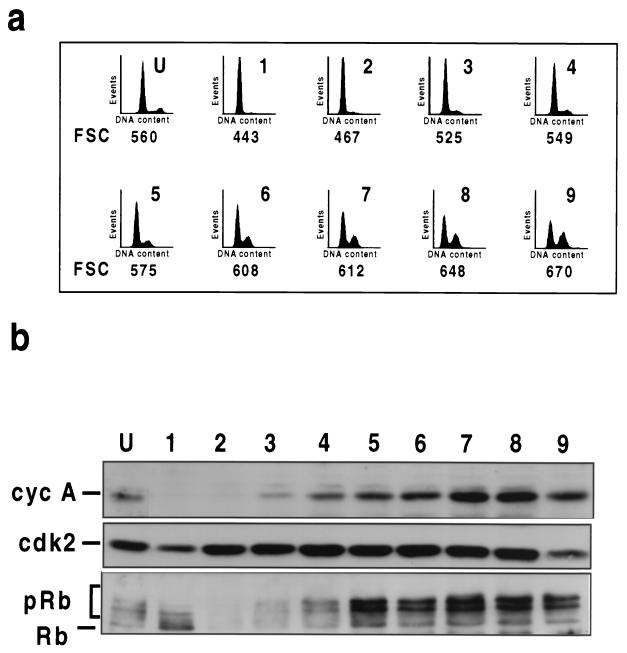
To this end, we used centrifugal elutriation to separate cells according to size. Since cell mass increases in parallel with the cell division cycle, this has the effect of separating cells according to their positions in the cell cycle, from the smallest G1 cells, which are the products of mitosis, to the largest G2/M cells, which are about to divide. A culture of v-Jun-transformed CEFs which had been maintained in LS medium for 48 h (equivalent to that shown in Fig. 1a) was separated into nine fractions by elutriation. The cell cycle distribution and average cell size as determined by mean forward scatter (FSC) of the initial population and each fraction were then determined by flow cytometry. Cell extracts were also prepared and analyzed for cyclin A expression and Rb phosphorylation by Western blotting.
Fraction 1 consisted almost exclusively of small G1 cells, which are the products of mitosis, while fraction 9 contained the largest cells and was substantially enriched in the S- and G2/M-phase cells which otherwise constituted a relatively small proportion of the total population (Fig. 4a, graph U). Remarkably, the level of cyclin A varied enormously among these fractions, from undetectable in the small G1 cells to abundant in the largest cells (Fig. 4b). Analysis of the intervening fractions (2 to 8) revealed a progressive accumulation of cyclin A as cell size and the proportion of S- and G2/M-phase cells increased. In comparison, similar levels of cdk2 were present in all fractions (Fig. 4b).
FIG. 4.
v-Jun promotes mitogen-independent Rb phosphorylation and reaccumulation of cyclin A after mitosis. (a) Flow cytometry analysis of mitogen-deprived v-Jun CEFs separated by centrifugal elutriation. The starting population was separated into nine fractions (F1 to -9) of increasing size as determined by FSC. DNA content cytograms and FSC values of the unfractionated population (U) and successive fractions are shown. (b) Western blotting analysis of cyclin (cyc) A, cdk2, and Rb expression in the elutriated fractions shown in panel a.
The phosphorylation state of Rb also varied among the elutriated fractions. This was most evident in the small G1 cells, which contained predominantly hypophosphorylated Rb, even though this was only a minor component in the unfractionated population (Fig. 4b). In contrast, hyperphosphorylated Rb predominated in the largest cells and in most of the intervening fractions (Fig. 4b). For unknown reasons the absolute amount of Rb diminished transiently (fractions 2 and 3) and then increased steadily with increasing cell size (Fig. 4b).
We draw two conclusions from this experiment. Firstly, cyclin A is degraded at mitosis in the mitogen-deprived cells and therefore reaccumulation of the protein is likely to be necessary for subsequent S-phase entry. Secondly, since Rb is dephosphorylated normally at mitosis, v-Jun must enable cells to rephosphorylate Rb without the requirement for exogenous mitogenic stimulation.
p16INK4A inhibits DNA synthesis and cell proliferation in v-Jun-transformed cells.
Phosphorylation of Rb in G1 is catalyzed by the D-cyclin–cdk and cyclin E-cdk2 kinase complexes (34). D-cyclins associate specifically with cdk4 and cdk6, kinases which can in turn be selectively inhibited by the p16INK4A cyclin-dependent kinase inhibitor (44). To evaluate the requirement for D-cyclin–cdk activity in cells transformed by v-Jun, we first adopted a microinjection approach analogous to that utilized for the analysis shown in Fig. 2. An expression plasmid encoding p16INK4A, or vector control, was microinjected into v-Jun-transformed CEFs in GM or LS medium. After allowing sufficient time for expression, the percentage of cells incorporating BrdU in 24 h was determined. This analysis (Fig. 5a) revealed that p16INK4A effectively blocked DNA synthesis under both growth conditions. Similar results were obtained with growing control cells (data not shown).
FIG. 5.
p16INK4A inhibits DNA synthesis and cell proliferation in v-Jun-transformed CEFs. (a) v-Jun-transformed CEFs cultured in GM or LS medium were microinjected with control or expression plasmid encoding human p16INK4A together with a plasmid encoding GFP to identify injected cells. After time was allowed for expression, the percentage of injected cells which synthesized DNA in 24 h was determined by BrdU labeling and immunocytochemistry. At least 100 surviving injected cells were analyzed in each case. The experiment was repeated three times with similar results. (b) CEFs doubly infected with the indicated combinations of retroviruses were seeded at two dilutions (105 or 106 cells/dish) and selected with G418 for 5 days after being plated. The cultures were fixed in ethanol and stained with Giemsa stain to visualize colony growth.
As an alternative approach to this issue, we used retroviral gene transfer to stably express p16INK4A in CEFs either with or without v-Jun. To this end, p16INK4A was expressed using the neomycin-selectable, replication-defective retroviral vector SFCV (9). SFCV-p16 was regenerated by transfection using the replication-competent vector RCAS, or a derivative encoding v-Jun (RCAS-v-Jun), as a helper virus (26). Control experiments have shown that drug selection under these conditions efficiently generates populations of cells doubly infected with both retroviruses (26). The CEFs were transfected with the appropriate combinations of plasmids, and after allowing sufficient time for retrovirus regeneration and spread, the cultures were plated at two dilutions and drug-resistant colonies were selected.
As shown in Fig. 5b, ectopic expression of p16INK4A strongly inhibited cell proliferation in both control and v-Jun-transformed CEFs, as indicated by a large decrease in both the number and average size of drug-resistant colonies. Although this approach was only feasible under normal growth conditions, since neither cell type will multiply in LS medium (4), the results are consistent with the microinjection analysis and show that v-Jun-transformed CEFs depend on D-cyclin–cdk kinase activity for long-term cell proliferation as well as DNA synthesis.
v-Jun stimulates cyclin E-cdk2 but not D-cyclin–cdk kinase activity.
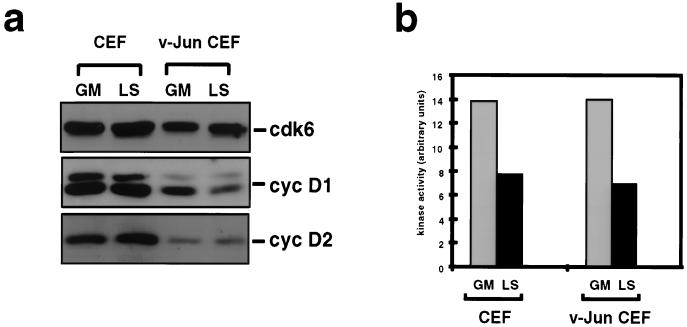
The finding that D-cyclin–cdk kinase activity was required for DNA synthesis and cell proliferation in v-Jun-transformed CEFs raised the possibility that deregulation of D-cyclin–cdk kinase activity might account for the mitogen-independent phosphorylation of Rb. To evaluate this possibility, we first examined the effect of v-Jun on the expression of D-cyclin–cdk components. Systematic attempts to identify the avian homologue of cdk 4 have been unsuccessful (unpublished results); however, the avian homologue of cdk6 has been isolated and appears to be the predominant D-cyclin-associated and p16INK4A-sensitive kinase in CEFs (Fig. 6a and data not shown). Similarly, although cyclins D1 and D2 are expressed in CEFs, cyclin D3 is not thought to exist in avian cells (25). Interestingly, Western blotting showed that the levels of cyclin D1 and cyclin D2 were not significantly growth regulated in control CEFs (Fig. 6a). Furthermore, the expression of both cyclins was if anything slightly reduced, rather than increased, as a consequence of cell transformation by v-Jun.
FIG. 6.
v-Jun does not stimulate D-cyclin expression or cyclin D1-cdk kinase activity. (a) Western blotting analysis of cyclin (cyc) D1 and D2 and cdk6 expression in control and v-Jun CEFs growing in GM or after 48 h in LS medium. (b) Determination of cyclin D1-cdk kinase activity in normal and v-Jun-transformed CEFs growing in GM or after 48 h in LS medium using recombinant Rb as a substrate. Cell extracts prepared from the indicated cultures were immunoprecipitated with a matched pair of anti-cyclin D1 monoclonal antibodies: DCS-6, which precipitates only free cyclin D1, and DCS-11, which precipitates cyclin D1-cdk holoenzyme complexes. After quantitation of kinase activity, the DCS-6 value for each sample was subtracted from the DCS-11 value to correct for background.
To rule out the possibility that v-Jun might nevertheless stimulate D-cyclin–cdk catalytic activity by a mechanism independent of cyclin expression, cyclin D1-associated kinase activity was determined by an immunoprecipitation kinase assay using recombinant glutathione S-transferase–Rb as a substrate (Fig. 6b). Cell extracts were precipitated with a matched pair of anti-cyclin D1 monoclonal antibodies: DCS-6, which precipitates only free cyclin D1, and DCS-11, which precipitates cyclin D1-cdk holoenzyme complexes (28). After quantitation of kinase activity in the precipitates, the DCS-6 value was subtracted from the DCS-11 value to correct for background in each sample.
Several conclusions can be drawn from the results of this analysis. Firstly, the level of cyclin D1-associated kinase activity in growing control cells declines only modestly when these cells are rendered quiescent by growth factor deprivation (Fig. 6b). Although unusual, this is consistent with the continued presence of cyclins D1 and D2 and cdk6 under these conditions (Fig. 6a). Secondly, transformation by v-Jun does not affect cyclin D1-associated kinase activity under either growth condition. Taken together, these results argue that while D-cyclin–cdk activity is required for cell cycle progression in cells transformed by v-Jun, deregulation of this activity per se is unlikely to account for the persistence of Rb phosphorylation induced by v-Jun.
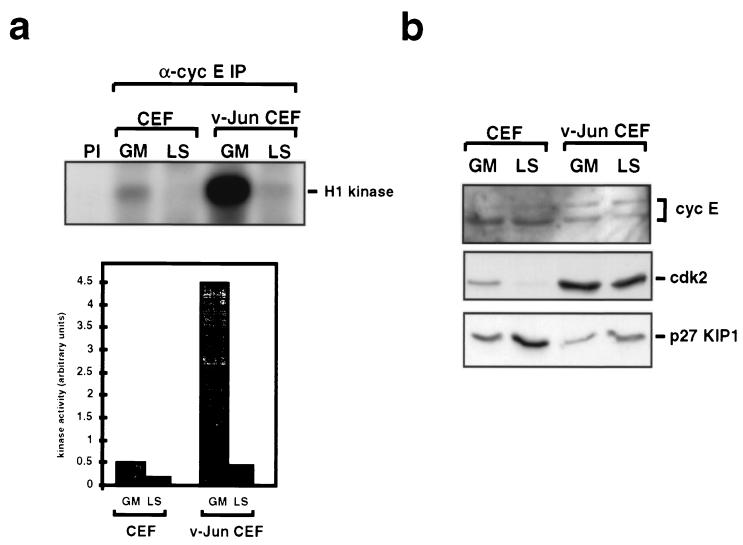
Cyclin E-cdk2 is the other principal kinase considered to act on Rb during G1 phase (34). Immunoprecipitation kinase assays using an antiserum specific for avian cyclin E and histone H1 as a substrate (Fig. 7a) revealed that cyclin E-cdk2 kinase activity was detectable in growing control CEFs (lane GM). Activity declined in quiescent cells (lane LS), although as with cyclins D1 and D2, the levels of cyclin E protein were similar under both growth conditions, as determined by Western blotting (Fig. 7b).
FIG. 7.
v-Jun stimulates cyclin E-cdk2 catalytic activity. (a) Levels of cyclin (cyc) E-cdk2 kinase activity in normal and v-Jun-transformed CEFs growing in GM or after 48 h in LS medium. Extracts prepared from the indicated cell cultures were immunoprecipitated using a cyclin E-specific antiserum, and the immunoprecipitates were assayed for kinase activity using histone H1 as a substrate. Quantitation of these data (bottom) indicated a ninefold amplification of cyclin E-cdk2 kinase activity in growing v-Jun-transformed cells compared to that in the control. (b) Western blotting analysis of cyclin E, cdk2, and p27KIP1 expression in normal and v-Jun-transformed CEFs maintained in GM and LS medium.
Remarkably, there was a massive (ninefold) increase in cyclin E-cdk2 kinase activity in growing v-Jun-transformed CEFs (Fig. 7a), which was not associated with any corresponding increase in the level of cyclin E as determined by Western blotting (Fig. 7b). The increase in kinase activity was associated with a modest reduction in the level of p27KIP1 protein (Fig. 7b); however, p27KIP1 mRNA levels were not altered (data not shown), suggesting that this may reflect posttranslational regulation rather than an effect of v-Jun on p27KIP1 gene expression.
Cyclin E-cdk2 kinase activity declined dramatically in v-Jun CEFs after mitogen deprivation; however, the residual activity which persisted was comparable to that seen in growing control cells (Fig. 7a). We conclude that cell transformation by v-Jun results in a dramatic upregulation of cyclin E-cdk2 kinase activity under optimal growth conditions which persists at lower, but nevertheless potentially significant, levels after mitogens are withdrawn.
Activation of Δv-JunER in quiescent, growth factor-deprived fibroblasts stimulates cyclin E-cdk2 kinase activity, Rb phosphorylation, and S-phase entry.
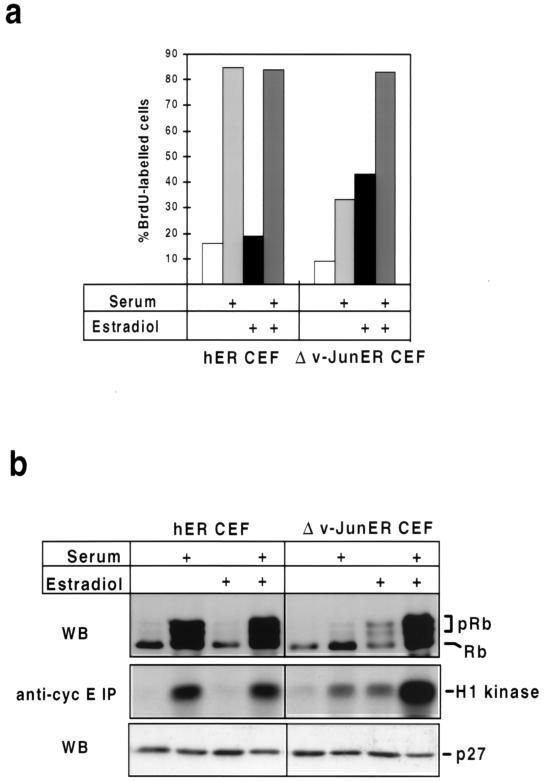
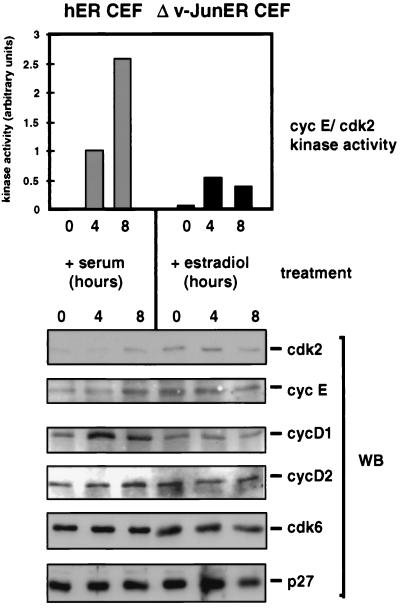
To investigate whether activation of v-Jun in quiescent cells could trigger DNA synthesis, we exploited a conditional v-Jun–estrogen receptor fusion protein, Δv-JunER, which elicits estradiol-dependent cell transformation (24). CEFs expressing Δv-JunER, or hER alone, were rendered quiescent by growth factor deprivation in the absence of hormone. The effect of adding serum, estradiol, or both, on DNA synthesis was then determined by BrdU labeling and flow cytometry. The effect of these manipulations on cyclin E-cdk2 kinase activity and Rb phosphorylation was also monitored by Western blotting and kinase assay.
As shown in Fig. 8, both control and Δv-JunER-expressing CEFs were effectively quiescent after 48 h in LS medium in the absence of hormone, as judged by very low levels of DNA synthesis, persistence of only hypophosphorylated Rb, and absence of cyclin E-cdk2 kinase activity. As expected, serum was an effective mitogen in control cells, triggering a large increase in BrdU labeling, cyclin E-cdk2 kinase activity, and Rb phosphorylation. In comparison, estradiol was ineffective as a mitogen, although it did not antagonize the effects of serum (Fig. 8). Mitogenic stimulation did not result in rapid downregulation of p27KIP1, indicating that in contrast to some other cell types (5) this is not obligatory for cell cycle reentry in CEFs (Fig. 8b).
FIG. 8.
Activation of Δv-JunER stimulates cyclin E-cdk2 kinase activity, Rb phosphorylation, and S-phase entry in quiescent, growth factor-deprived CEFs. (a) Cultures of CEFs expressing Δv-JunER, or hER as a control, were rendered quiescent by culture in LS medium for 48 h in the absence of estradiol. Newborn calf serum (10%), estradiol (2 μM), or both were then added, and the cultures were labeled with BrdU for 20 h. After being labeled, the cultures were harvested and the percentage of labeled cells was determined by flow cytometry. (b) Cell extracts prepared from replicate cultures of those shown in panel a were analyzed for Rb phosphorylation and p27KIP1 expression by Western blotting (WB) and cyclin (cyc) E-cdk2 kinase activity by immunoprecipitation (IP) using histone H1 as a substrate.
In contrast, addition of estradiol alone elicited a significant increase in cyclin E-cdk2 kinase activity and DNA synthesis in cells expressing Δv-JunER, although these responses were of smaller magnitude than those induced by serum in control cells (Fig. 8). Estradiol also induced efficient phosphorylation of Rb, although this was not accompanied by the increase in the amount of Rb protein associated with cell cycle reentry in normal cells (Fig. 8b).
Unexpectedly, the mitogenic potency of serum was substantially blunted in Δv-JunER cells in the absence of hormone, and this was associated with impaired induction of cyclin E-cdk2 kinase activity and almost complete inhibition of Rb phosphorylation (Fig. 8). This is consistent with previous work, which documented growth inhibition by Δv-JunER in the absence of estradiol (24). We assume that represents a dominant-negative effect of the chimeric protein in its inactive state, perhaps by antagonizing the function of the endogenous c-Jun protein; however, the molecular mechanism remains to be elucidated.
A maximal mitogenic response in terms of DNA synthesis, cyclin E-cdk2 kinase activation, and Rb phosphorylation in Δv-JunER cells was obtained by simultaneous addition of serum and estradiol. In fact, the level of cyclin E-cdk2 kinase activity achieved by the combination of serum and estradiol greatly exceeded that induced by serum in control cells (Fig. 8b), indicating that in the presence of activating ligand, Δv-JunER shares the capacity of the native v-Jun oncoprotein to stimulate this activity (Fig. 6c). As in control cells, there was little evidence that variations in the level of p27KIP1 were a significant factor in determining the mitogenic response to these treatments in the short term (Fig. 8b).
Activation of Δv-JunER does not stimulate D-cyclin expression.
Hormonal activation of conditional MycER alleles in quiescent rodent fibroblasts is sufficient to stimulate cyclin E-cdk2 kinase activity and trigger DNA synthesis (7, 41, 48). Interestingly, recent reports have demonstrated that MycER promotes cyclin E-cdk2 activation indirectly by upregulating cyclins D1 and D2 via a combination of transcriptional and posttranscriptional mechanisms (2, 39). This leads to a transient increase in the level of D-cyclin–cdk complexes which, by sequestering p27KIP1 and p21CIP1, release existing or newly synthesized cyclin E-cdk2 complexes from inhibition (2, 39).
To determine whether Δv-JunER might act by a similar mechanism, we examined the effect of estradiol on the expression of D-cyclins and cyclin E-cdk2 kinase activity in quiescent Δv-JunER CEFs. For comparison, quiescent control CEFs were stimulated with serum. As expected, serum stimulation induced a robust activation of cyclin E-cdk2 in control cells which was readily detectable at 4 h and maximal at 8 h (Fig. 9, upper panel), thus correlating well with the onset of Rb phosphorylation, which occurs at around 5 h in these cells (4). The initial increase in cyclin E-cdk2 kinase activity at 4 h was not associated with any upregulation of cyclin E or cdk2, which is in any case very low in quiescent cells (4), but was preceded by a modest increase in the level of cyclin D1 expression (Fig. 9, lower panel). Since the steady-state levels of cyclin D1 are similar in growing and quiescent cells (Fig. 6), we assume that this transient increase is specifically associated with cell cycle reentry after mitogenic stimulation. In comparison, the levels of cyclin D2, cdk6, and p27KIP1 did not vary (Fig. 9).
FIG. 9.
Activation of Δv-JunER does not enhance D-cyclin expression. Cultures of CEFs expressing Δv-JunER or hER were rendered quiescent by culture in LS medium for 48 h in the absence of estradiol. Newborn calf serum (10%) or estradiol (2 μM) was then added as indicated, and replicate cultures were assayed for cyclin (cyc) E-cdk2 kinase activity by immunoprecipitation kinase assay (upper panel) and for expression of cyclin E, cdk2, cyclin D1, cyclin D2, cdk6, and p27KIP1 by Western blotting (WB) (lower panel).
Estradiol also stimulated cyclin E-cdk2 kinase activity in Δv-JunER cells at 4 h, although as expected from previous experiments (Fig. 8), the scale of the increase was much smaller than that induced by serum in control cells and there was no further increase at 8 h. In contrast to control cells, it was clear that estradiol did not stimulate expression of cyclin D1, nor was there any obvious effect on the level of cyclin E, cdk2, cyclin D2, cdk6, or p27KIP1 (Fig. 9). Thus, although these experiments do not reveal the molecular mechanism through which Δv-JunER stimulates cyclin E-cdk2 activity, we conclude that it does not require upregulation of D-cyclin expression and therefore must be distinct from that elicited by activation of MycER (2, 39).
DISCUSSION
Previously, we found that cells transformed by v-Jun continued to cycle actively after mitogen withdrawal (4). Although these experiments demonstrated that v-Jun shared the capacity of the Myc, E1A, and E2F oncoproteins (1, 19), to override the mitogen dependence of DNA synthesis, they did not reveal the mechanism by which or the point(s) at which v-Jun acts on the cell cycle machinery. To clarify this issue, we have now investigated the effect of v-Jun on mitogen-regulated processes which control the G1/S transition and, where possible, intervened to evaluate the functional significance of activities which are deregulated by v-Jun.
We began by investigating cdk2 and cyclin A, since cyclin A-cdk2 activity is essential for DNA synthesis, and under normal circumstances, cyclin A expression is strictly dependent on mitogens. This analysis revealed that v-Jun-transformed CEFs were able to sustain cyclin A expression and cdk2 kinase activity after mitogen withdrawal and that the levels correlated closely with the rate of S-phase entry. Importantly, the residual cdk2 activity which persisted was essential for S phase, since ectopic expression of dn-cdk2 blocked DNA synthesis.
Although it was not feasible to inhibit cyclin A-associated cdk2 activity selectively, most of the active cdk2 in the mitogen-deprived cells was associated with cyclin A, and we found a close association between cyclin A expression and DNA synthesis when individual cells in the population were separated according to cell cycle position by centrifugal elutriation. Since elutriation also revealed that cyclin A was degraded normally at mitosis, these observations suggest that v-Jun confers competence for cyclin A expression in the absence of mitogens but that accumulation of cyclin A after mitosis is nevertheless rate limiting for S-phase entry or progression. Additional work will be required to determine whether v-Jun also uncouples the timing of cyclin A expression from cell growth, as has been shown for Myc (40), in addition to relieving the requirement for mitogens.
Cyclin A expression is actively repressed in quiescent cells by E2F-p130 or E2F-Rb repressor complexes via an E2F site in the gene promoter (15); however, the cyclin A promoter also contains an ATF site which has been implicated in growth regulation (45) and is a potential target site for v-Jun. We therefore reasoned that v-Jun could override the mitogen dependence of cyclin A expression either by preventing the formation of E2F repressor complexes or through a direct effect on the cyclin A gene promoter, in which case global E2F regulation would be undisturbed.
Comparison of the effect of mitogen withdrawal on E2F DNA binding complexes in control and v-Jun CEFs provided strong support for the first of these two mechanisms. Thus, whereas E2F-p107 complexes predominated in proliferating cells, they were replaced by a putative E2F-p130 complex after mitogen deprivation. Quiescence also resulted in accumulation of hypophosphorylated Rb, a decline in the amount of p107, and an increase in the level of p130. Similar coordinated changes in Rb phosphorylation and E2F-pocket protein interactions have been documented in a variety of other cell systems and are considered diagnostic of quiescence (46). In marked contrast, E2F-p107 complexes persisted unchanged after prolonged mitogen deprivation in v-Jun CEFs with no evidence for formation of the putative E2F-p130 complex, dephosphorylation of Rb, or change in the relative levels of p107 or p130.
The finding that v-Jun abolished the mitogen regulation of E2F-pocket protein interactions argues strongly that v-Jun deregulates cyclin A expression by preventing the E2F-mediated repression which normally extinguishes expression in quiescent cells. Since E2F controls the expression of many other genes required for DNA synthesis or for subsequent cell cycle progression, such as cdc2 (15), this may explain why v-Jun-transformed cells are able not only to enter S phase in the absence of mitogens but also to successfully complete the cell cycle and divide (4).
Although phosphorylated isoforms of Rb continued to predominate in v-Jun CEFs after mitogen deprivation, elutriation analysis revealed that the protein was transiently dephosphorylated after mitosis, implying that rephosphorylation of Rb occurs continuously in the absence of exogenous mitogenic stimulation and that v-Jun might stimulate one or more Rb kinases. Rb phosphorylation in G1 is catalyzed by complexes of D-cyclins and cdk4 and -6 and cyclin E-cdk2 (34, 49). Since D-cyclin–cdk kinases in particular have been widely invoked as links between mitogenic signaling and Rb phosphorylation (43), we evaluated the functional requirement for D-cyclin–cdk activity in v-Jun CEFs using the cdk4- and -6-specific inhibitor p16INK4A.
The results of this analysis were very clear: ectopic expression of p16INK4A blocked DNA synthesis in v-Jun CEFs both in the presence and in the absence of growth factors, while retrovirus-mediated expression of p16INK4A also inhibited cell proliferation. Taken together, these observations argue strongly that D-cyclin–cdk activity is required for Rb phosphorylation in v-Jun CEFs, since the ability of p16INK4A to block DNA synthesis is dependent on Rb function (33). Despite this, we found no increase in the expression of the rate-limiting cyclin D1 or D2 subunit as a result of cell transformation by v-Jun, nor was there any increase in cyclin D1-associated kinase activity. Thus, although D-cyclin–cdk kinase activity is required for Rb phosphorylation in v-Jun CEFs, deregulation of this activity seems unlikely to account for the persistence of Rb phosphorylation after mitogen withdrawal.
In comparison, cyclin E-cdk2 kinase activity was greatly increased in growing v-Jun CEFs, and although activity declined substantially after mitogen withdrawal, it persisted at a level comparable to that seen in proliferating control cells. In addition, activation of Δv-JunER with estradiol in quiescent, growth factor-deprived cells was sufficient to stimulate cyclin E-cdk2 kinase activity and trigger Rb phosphorylation and DNA synthesis. Remarkably, in the absence of estradiol the converse was true; Δv-JunER actively antagonized serum-induced cell cycle reentry and inhibited activation of cyclin E-cdk2 and Rb phosphorylation. Although the molecular mechanism of this dominant-negative effect is unknown, these experiments provide clear evidence that v-Jun, or its conditional surrogate Δv-JunER, controls processes which modulate the activity of cyclin E-cdk2. Interestingly, cyclin E-cdk2 is also thought to be the principal cell cycle target of Myc (1), suggesting that it may represent a common target for cell transformation by these oncogenes.
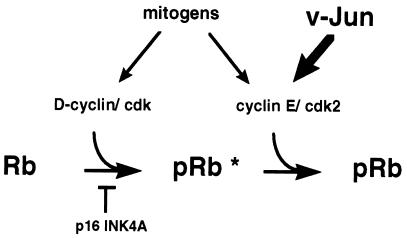
A model summarizing our observations and illustrating the proposed point of action of v-Jun on the cell cycle machinery is shown in Fig. 10. In normal CEFs, Rb phosphorylation is catalyzed by the sequential action of D-cyclin–cdk's and cyclin E-cdk2. Both of these activities are mitogen regulated; however, a significant basal level of cyclin D1-associated kinase activity persists in quiescent cells (Fig. 6b). In line with current thinking (30), we assume that complete inactivation of the growth-inhibitory function of Rb requires prior partial modification of the protein by D-cyclin–cdk's (Fig. 10) before cyclin E-cdk2 can complete the process.
FIG. 10.
Model illustrating the proposed point of action of v-Jun on the cell cycle machinery. In normal CEFs, Rb phosphorylation is catalyzed by the sequential action of D-cyclin-cdks and cyclin E-cdk2. Both are mitogen regulated; however, a basal level of cyclin D1-associated kinase activity persists in quiescent cells. v-Jun stimulates cyclin E-cdk2 kinase activity, accelerating G1 progression under optimal growth conditions and sustaining Rb phosphorylation after mitogen deprivation. p16INK4A inhibits DNA synthesis by preventing prior modification of Rb (pRb∗) by D-cyclin–cdk's, which is required for subsequent phosphorylation by cyclin E-cdk2.
Under optimal growth conditions, v-Jun induces a very large amplification of cyclin E-cdk2 kinase activity, which accelerates G1 progression (3). Although this abnormally high level declines on mitogen withdrawal, sufficient cyclin E-cdk2 kinase activity persists to sustain Rb phosphorylation and thus prevent cell cycle exit. In each case, p16INK4A inhibits DNA synthesis by preventing prior Rb phosphorylation by D-cyclin–cdk's. An interesting feature of this model is that it predicts that cyclin E-cdk2, rather than D-cyclin–cdk activity, is the principal rate-limiting Rb kinase regulated by mitogens in primary CEFs. Further work will be required to test this hypothesis explicitly.
This model is provisional and makes no specific assumptions regarding the molecular mechanism through which v-Jun stimulates cyclin E-cdk2 activity. The simplest possibility, namely, upregulation of the rate-limiting cyclin E subunit, can be discounted, since we show that cyclin E protein expression is not increased as a result of cell transformation by v-Jun. In addition, we found no evidence that v-Jun, or its conditional derivative Δv-JunER, stimulated the expression of cyclin D1 or D2. It seems very unlikely, therefore, that sequestration of p27KIP1 or p21CIP1 by D-cyclin–cdk complexes mediates the effect of v-Jun or Δv-JunER on cyclin E-cdk2 kinase activity, as has been proposed for MycER (2, 39).
Since v-Jun can repress gene transcription, an alternative possibility is that v-Jun downregulates a cyclin-dependent kinase inhibitor involved in setting the threshold of mitogenic signals needed to generate cyclin E-cdk2 kinase activity and initiate Rb phosphorylation. We considered p27KIP1 as a candidate for this role (5); however, although the level of p27KIP1 protein was modestly reduced in v-Jun-transformed cells, p27KIP1 mRNA levels were unaffected, suggesting that this is unlikely to be a direct effect of v-Jun. In the future it will be important to determine whether v-Jun antagonizes p27KIP1 function rather than expression and to evaluate the possible role of other candidates, such as p21CIP1, particularly in view of the genetic interaction between c-Jun and p53 (42).
Finally, the possibility that v-Jun activates growth-stimulatory genes should also be explored. Recently v-Jun has been found to stimulate the expression of a heparin-binding EGF-like growth factor (8), which induces cell transformation when ectopically expressed in CEFs. It is therefore possible that an autocrine loop involving this or some other factor contributes to cell cycle deregulation by v-Jun. Future work will seek to distinguish between these possibilities.
ACKNOWLEDGMENTS
We thank the following people for generous provision of reagents and technical advice: G. McLean, Sybille Mittnacht, E. Nigg, K. Parkinson, D. Johnson, J. Bartek, and Max Walker. We thank J. A. Wyke and I. Morgan for comments on the manuscript.
This work was supported by the Cancer Research Campaign (CRC) of the United Kingdom.
REFERENCES
- 1.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:D250–D268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, Reed S, Sicinski P, Bartek J, Eilers M. Direct induction of cyclin D2 by myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck V, Allen K E, Sorensen T, Bybee A, Hijmans E M, Voorhoeve P M, Bernards R, La Thangue N B. Molecular and functional characterisation of E2F-5, a new member of the E2F family. Oncogene. 1995;11:31–38. [PubMed] [Google Scholar]
- 4.Clark W, Gillespie D A. Transformation by v-Jun prevents cell cycle exit and promotes apoptosis in the absence of serum growth factors. Cell Growth Differ. 1997;8:371–380. [PubMed] [Google Scholar]
- 5.Coats S, Flanagan W M, Nourse J, Roberts J M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 6.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 7.Eilers M, Picard D, Yamamoto K R, Bishop J M. Chimaeras of Myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989;340:66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- 8.Fu S, Bottoli I, Goller M, Vogt P K. Heparin-binding epidermal growth factor-like growth factor, a v-Jun target gene, induces oncogenic transformation. Proc Natl Acad Sci USA. 1999;96:5716–5721. doi: 10.1073/pnas.96.10.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuerstenberg S, Beug H, Introna M, Khazaie K, Munoz A, Ness S, Nordstrom K, Sap J, Stanley I, Zenke M, et al. Ectopic expression of the erythrocyte band 3 anion exchange protein, using a new avian retrovirus vector. J Virol. 1990;64:5891–5902. doi: 10.1128/jvi.64.12.5891-5902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao M, Morgan I, Vogt P K. Differential and antagonistic effects of v-Jun and c-Jun. Cancer Res. 1996;56:4229–4235. [PubMed] [Google Scholar]
- 11.Girling R, Partridge J F, Bandara L R, Burden N, Totty N F, Hsuan J J, La Thangue N B. A new component of the transcription factor DRTF1/E2F. Nature. 1993;362:83–87. doi: 10.1038/362083a0. [DOI] [PubMed] [Google Scholar]
- 12.Hadman M, Gabos L, Loo M, Sehgal A, Bos T J. Isolation and cloning of JTAP-1: a cathepsin like gene upregulated in response to V-Jun induced cell transformation. Oncogene. 1996;12:135–142. [PubMed] [Google Scholar]
- 13.Hadman M, Lin W, Bush L, Bos T J. Apolipoprotein A-1 is a negative target of v-Jun overexpression. Oncogene. 1998;16:655–660. doi: 10.1038/sj.onc.1201574. [DOI] [PubMed] [Google Scholar]
- 14.Hartl M, Bister K. Structure and transcriptional regulation of BKJ, a novel AP-1 target gene activated during jun- or fos-induced fibroblast transformation. Oncogene. 1998;17:2901–2913. doi: 10.1038/sj.onc.1202219. [DOI] [PubMed] [Google Scholar]
- 15.Helin K. Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genet Dev. 1998;8:28–35. doi: 10.1016/s0959-437x(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 16.Herber B, Truss M, Beato M, Muller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994;9:1295–1304. [PubMed] [Google Scholar]
- 17.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 18.Hussain S, Kilbey A, Gillespie D A. v-Jun represses c-jun proto-oncogene expression in vivo through a 12-O-tetradecanoylphorbol-13-acetate-responsive element in the proximal gene promoter. Cell Growth Differ. 1998;9:677–686. [PubMed] [Google Scholar]
- 19.Jansen-Durr P. How viral oncogenes make the cell cycle. Trends Genet. 1996;12:270–275. doi: 10.1016/0168-9525(96)81455-7. [DOI] [PubMed] [Google Scholar]
- 20.Johnson D G, Schwarz J K, Cress W D, Nevins J R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 21.Johnson R S, van Lingen B, Papaioannou V E, Spiegelman B M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- 22.Kilbey A, Black E J, Unlu M, Gillespie D A F. The v-Jun oncoprotein replaces p39 c-Jun as the predominant AP-1 constituent in ASV17-transformed fibroblasts: implications for SAPK/JNK-mediated signal transduction. Oncogene. 1996;12:2409–2418. [PubMed] [Google Scholar]
- 23.Kovary K, Bravo R. The Jun and Fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991;11:4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruse U, Iacovoni J S, Goller M E, Vogt P K. Hormone-regulatable neoplastic transformation induced by a Jun-estrogen receptor chimera. Proc Natl Acad Sci USA. 1997;94:12396–12400. doi: 10.1073/pnas.94.23.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahti J M, Li H, Kidd V J. Elimination of cyclin D1 in vertebrate cells leads to an altered cell cycle phenotype, which is rescued by overexpression of murine cyclins D1, D2, or D3 but not by a mutant cyclin D1. J Biol Chem. 1997;272:10859–10869. doi: 10.1074/jbc.272.16.10859. [DOI] [PubMed] [Google Scholar]
- 26.La Rocca S A, Crouch D H, Gillespie D A. c-Myc inhibits myogenic differentiation and myoD expression by a mechanism which can be dissociated from cell transformation. Oncogene. 1994;9:3499–3508. [PubMed] [Google Scholar]
- 27.Ludlow J W, Shon J, Pipas J M, Livingston D M, DeCaprio J A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990;60:387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- 28.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukas J, Petersen B O, Holm K, Bartek J, Helin K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol Cell Biol. 1996;16:1047–1057. doi: 10.1128/mcb.16.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maki Y, Bos T J, Davis C, Starbuck M, Vogt P K. Avian sarcoma virus 17 carries the jun oncogene. Proc Natl Acad Sci USA. 1987;84:2848–2852. doi: 10.1073/pnas.84.9.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May G H, Funk M, Black E J, Clark W, Hussain S, Woodgett J R, Gillespie D A. An oncogenic mutation uncouples the v-Jun oncoprotein from positive regulation by the SAPK/JNK pathway in vivo. Curr Biol. 1998;8:117–120. doi: 10.1016/s0960-9822(98)70043-0. [DOI] [PubMed] [Google Scholar]
- 33.Medema R H, Herrera R E, Lam F, Weinberg R A. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittnacht S. Control of pRB phosphorylation. Curr Opin Genet Dev. 1998;8:21–27. doi: 10.1016/s0959-437x(98)80057-9. [DOI] [PubMed] [Google Scholar]
- 35.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 36.Morgan I M, Asano M, Havarstein L S, Ishikawa H, Hiiragi T, Ito Y, Vogt P K. Amino acid substitutions modulate the effect of Jun on transformation, transcriptional activation and DNA replication. Oncogene. 1993;8:1135–1140. [PubMed] [Google Scholar]
- 37.Nead M A, Baglia L A, Antinore M J, Ludlow J W, McCance D J. Rb binds c-Jun and activates transcription. EMBO J. 1998;17:2342–2352. doi: 10.1093/emboj/17.8.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto A, Demetrick D J, Spillare E A, Hagiwara K, Hussain S P, Bennett W P, Forrester K, Gerwin B, Serrano M, Beach D H, et al. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci USA. 1994;91:11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Roger I, Kim S H, Griffiths B, Sewing A, Land H. Cyclins D1 and D2 mediate myc-induced proliferation via sequestration of p27(Kip1) and p21(Cip1) EMBO J. 1999;18:5310–5320. doi: 10.1093/emboj/18.19.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pusch O, Bernaschek G, Eilers M, Hengstschlager M. Activation of c-Myc uncouples DNA replication from activation of G1-cyclin-dependent kinases. Oncogene. 1997;15:649–656. doi: 10.1038/sj.onc.1201236. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph B, Saffrich R, Zwicker J, Henglein B, Muller R, Ansorge W, Eilers M. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J. 1996;15:3065–3076. [PMC free article] [PubMed] [Google Scholar]
- 42.Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J, Karin M, Angel P, Wagner E F. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 44.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu M, Nomura Y, Suzuki H, Ichikawa E, Takeuchi A, Suzuki M, Nakamura T, Nakajima T, Oda K. Activation of the rat cyclin A promoter by ATF2 and Jun family members and its suppression by ATF4. Exp Cell Res. 1998;239:93–103. doi: 10.1006/excr.1997.3884. [DOI] [PubMed] [Google Scholar]
- 46.Smith E J, Leone G, DeGregori J, Jakoi L, Nevins J R. The accumulation of an E2F-p130 transcriptional repressor distinguishes a G0 cell state from a G1 cell state. Mol Cell Biol. 1996;16:6965–6976. doi: 10.1128/mcb.16.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith M J, Prochownik E V. Inhibition of c-jun causes reversible proliferative arrest and withdrawal from the cell cycle. Blood. 1992;79:2107–2115. [PubMed] [Google Scholar]
- 48.Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1996;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 50.Wisdom R, Johnson R S, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zetterberg A, Larsson O, Wiman K G. What is the restriction point? Curr Opin Cell Biol. 1995;7:835–842. doi: 10.1016/0955-0674(95)80067-0. [DOI] [PubMed] [Google Scholar]