Abstract
As the first line of defence against pathogens and endotoxins crossing the intestine-blood barrier, the intestinal epithelial barrier plays a determinant role in pigs' health and growth. 4-Phenylbutyric acid (4-PBA), an aromatic fatty acid, was reported to benefit homeostasis of endoplasmic reticulum and protein synthesis. However, whether 4-PBA affects intestinal epithelial barrier function in pigs is unknown. This study aimed to explore the effects of 4-PBA on the intestinal barrier function, using in vitro models of well-differentiated intestinal porcine epithelial cell (IPEC-J2) monolayers in the transwell plates. Cell monolayers with or without 4-PBA (1.0 mmol/L) treatment were challenged with physical scratch, deoxynivalenol (DON, 2.0 μg/mL, 48 h), and lipopolysaccharide (LPS, 5.0 μg/mL, 48 h), respectively. Transepithelial electrical resistance (TEER) and fluorescein isothiocyanate-dextran (FD-4) permeability were measured to indicate barrier integrity and permeability. Real-time PCR and Western blot were conducted to determine relative gene and protein expressions of tight junction proteins. As expected, physical scratch, DON, and LPS challenges decreased TEER and increased FD-4 permeability. 4-PBA treatment accelerated cell mitigation and rehabilitation of the physical scratch-damaged intestinal epithelial barrier but did not alleviate DON or LPS induced barrier damage. However, once 48-h DON and LPS challenges were removed, rehabilitation of the epithelial barrier function of IPEC-J2 monolayer was accelerated by the 4-PBA treatment. Also, the relative gene and protein expressions of zonula occludens-1 (ZO-1), occludin, and claudin-1 were further upregulated by the 4-PBA treatment during the barrier rehabilitation. Taken together, 4-PBA accelerated the IPEC-J2 cell monolayer barrier recovering from physical scratch, DON-, and LPS-induced damage, via enhancing cell mitigation and expressions of tight junction proteins.
Keywords: 4-Phenylbutyric acid, Intestinal barrier, Tight junction, Intestinal porcine epithelial cell, Deoxynivalenol, Lipopolysaccharide
1. Introduction
Weaning stress (Moeser et al., 2007), mycotoxin-contaminated feed ingestion (Pierron et al., 2016), and Escherichia coli infection (Ewaschuk et al., 2011) cause defective intestinal barrier functions in pigs. This subsequently increases intestinal permeability and endotoxins penetrating the intestine-blood barrier (Jiang et al., 2019), which triggers long-lasting inflammatory responses and reduces growth performance (Broom and Kogut, 2018). Feed additives supplements, such as polysaccharides, oligosaccharide, chitosan, and probiotics have been revealed to benefit intestinal barrier function and promote the growth performance of piglets (Hu et al., 2018; Wan et al., 2018; Wang et al., 2019a; Yi et al., 2018). It is noteworthy that the growth performance is closely and positively associated with the intestinal barrier functions in various cases (Huang et al., 2018; Park et al., 2020; Wang et al., 2019b), which affirms that intact intestinal barrier function is a crucial factor determining animals' health and growth performance.
The intestinal lumen is lined with a monolayer of conjunct epithelial cells, which separate the internal circulatory system from gut microbiota and endotoxins (Capaldo et al., 2017). The intestinal barrier maintenance largely depends on the homeostasis of intestinal epithelial cells (Peterson and Artis, 2014), which includes the integrity of tight junctions, immune responses, programmed cell death, and proliferation. Porcine intestinal epithelial cell line (IPEC-J2), which is originally from the jejunum of unsuckled piglets, is widely used for establishing in vitro models to mimic intestinal barrier function in piglets (Vergauwen, 2015). The decrease in transepithelial electrical resistance (TEER), increase in intestinal permeability, tight junction protein disruptions, and cell apoptosis disorder can be observed in mycotoxin or lipopolysaccharide (LPS) challenged IPEC-J2 monolayers (Devreese et al., 2013; He et al., 2019), in which the in vivo situations of mycotoxin-contaminated feed ingestion, or E. coli infection are simulated, respectively. An early-weaned piglet has an immature digestive system that provides low adaptability to the physical alteration of its diet, so can easily suffer from scratch damage of the intestinal barrier during a solid diet intake (Jayaraman and Nyachoti, 2017). The present study established in vitro damage models of intestinal barrier function using well-differentiated IPEC-J2 monolayers that were challenged with physical scratch, deoxynivalenol (DON), and E. coli-derived LPS, aiming to screen effective feed additives for protecting the intestinal barrier in pigs.
4-Phenylbutyric acid (4-PBA) is an aromatic fatty acid that has been reported to benefit endoplasmic reticulum homeostasis and protein synthesis in various stressed tissues, including the brain (Li et al., 2019), pancreas (Hong et al., 2018), lung (Zeng et al., 2017), liver (Shimizu et al., 2014), kidney (Carlisle et al., 2014), and intestines (You et al., 2019). 4-PBA, supplemented in the early stage, enhanced the intestinal barrier function (reflected by lower D-lactate and endotoxin levels in the blood) in the cecal ligation and puncture-induced septic rats (Liu et al., 2016). Due to the properties of high bioavailability, easy chemosynthesis, and nonpersistent residue, 4-PBA has the potential to be applied as a feed additive enhancing the intestinal barrier function in pigs. However, the influence of 4-PBA on pigs was unknown. The current study is the first one evaluating the effects of 4-PBA on the porcine intestinal barrier function using in vitro models, which may guide further 4-PBA application in the pig industry.
2. Materials and methods
2.1. Materials and reagents
4-Phenylbutyric acid (Cat. No. P21005, purity > 99%), E. coli O127:B8-derived LPS (Cat. No. L4516-1MG, quality level: 200), DON (Cat. No. SML1664-1mL, Quality Level: 200) and fluorescein isothiocyanate-dextran (FD-4, Cat. No. 46944-100MG-F, molar mass: 4,000) were purchased from Merck (Sigma Aldrich, Shanghai, China). Phosphate buffer solution (PBS, Cat. No. 20012027), Dulbecco's Modified Eagle's Medium/Ham's F-12 (DMEM/F12) cell culture medium (Cat. No. 11320082), 0.25% trypsin–EDTA–phenol red (Cat. No. 25200056), penicillin–streptomycin–glutamine (Cat. No. 10378016), and fetal bovine serum (Cat. No. 16140071) were purchased from Thermo Fisher Scientific (Shanghai, China). Six-well transwell plates (0.4-μm pore size, 24-mm diameter, Cat. No. 3491), 96-well, and 6-well cell culture plates were purchased from Corning (Shanghai, China). BeyoGold 96-well black opaque plates (Cat. No. FCP966-80pcs) were purchased from Beyotime (Shanghai, China). IPEC-J2 cells were provided by the Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture, Chinese Academy of Science (Changsha, Hunan, China).
2.2. Maintenance of IPEC-J2 monolayer in transwell plates
IPEC-J2 cells between 20th to 30th passages were seeded on the apical compartment of 6-well transwell plates at a density of 1 × 105 cells/well. Cells were cultured in a cell culture incubator, with DMEM/F12 medium supplemented with 10% fetal bovine serum, and 1× penicillin–streptomycin–glutamine. The culture medium was changed every 2 d before cell confluency and changed every day once the cells reached 100% confluency/differentiation. Cell monolayers that displayed a TEER greater than 880 Ω cm2 were used in the formal experiments.
2.3. Integrity determination via TEER measurement
The procedure for TEER measurement was described in our previous study (Jiang et al., 2019). Briefly, after equilibrating the cells with PBS for 10 min at room temperature, an electrical resistance monitor (ESR-2, Millipore, USA) was used for TEER measurement. TEER values of the transwell plates were calculated using the readout value multiplied by the basal area (4.52 cm2) of the apical inserts. The final calculated TEER values were used as an indicator reflecting the barrier integrity of IPEC-J2 monolayers in the transwell plates.
2.4. Permeability detection via FD-4 permeability experiment
To quantify the paracellular permeability of the cell monolayer, a final concentration of 1.0 mg/mL FD-4 was added to the apical compartment of transwell plates. The culture medium in the basolateral compartment was taken after 30-min incubation. Then, the medium was slightly mixed and transferred into 96-well black opaque plates (100 μL in each well, 3 replicates). A Spark multimode microplate reader (Tecan, Tecan Trading AG, Switzerland) was used for detecting the fluorescent intensity (excitation, 485 nm; emission, 528 nm). The measured fluorescent intensity was used as an indicator reflecting the paracellular permeability of the IPEC-J2 monolayer.
2.5. Barrier damages induced by physical scratch, DON, and LPS
A 10-μL pipette tip was used to linearly scratch the well-differentiated IPEC-J2 cells in both transwell plates and 6-well cell culture plates. The scratched cells from these plates were washed away with PBS 3 times. The cell confluency in 6-well plates was imaged with microscopy every 12 h, and relative wound closure ratios were analyzed. The barrier integrity of the cell monolayers in the transwell plates was evaluated by TEER measurement at −3, 0, 24, and 48 h after scratch. Paracellular permeability of the cell monolayers was quantified by FD-4 permeability experiments at −3, 24, and 48 h after scratch.
To induce barrier damage with optimal concentrations of DON and LPS, the dose-effects of DON and LPS on the IPEC-J2 monolayer were explored. A series of concentrations of DON (0, 0.5, 1.0, 2.0, 5.0, 10 μg/mL), and LPS (0, 0.5, 1.0, 2.0, 5.0, 10 μg/mL) were applied for 48 h, separately. The TEER measurement was conducted at 0, 12, 24, 36, and 48 h. The FD-4 permeability experiment was conducted at 48 h.
2.6. Determination of 4-PBA cytotoxicity on cell viability and barrier function of IPEC-J2
To evaluate the cytotoxicity of 4-PBA to IPEC-J2 cells, cells with 95% confluence in the 96-well cell culture plates, and well-differentiated IPEC-J2 monolayers in the 6-well transwell plates were treated with a series of concentrations (0, 0.5, 1.0, 5.0, 10, 50 mmol/L) of 4-PBA for 48 h. Cell-Counting Kit-8 (CCK-8, Sigma Aldrich, Shanghai, China) was used to determine the relative viability of the cells in the 96-well cell culture plates which were pre-seeded with cells at a density of 5 × 103 cells/well, following the manufacturer's instruction. TEER and FD-4 permeability were measured to evaluate the barrier function of the IPEC-J2 monolayer in transwell plates.
2.7. Experiment design
All the well-differentiated IPEC-J2 monolayers in the 6-well transwell plates or 6-well cell culture plates were treated with or without 1.0 mmol/L 4-PBA. Three replicates of cell monolayers were designed for each group.
For the scratch experiments, the cell monolayers with or without 4-PBA treatment (control, 4-PBA groups) were scratched with a pipette tip and cultured for 48 h. Cell confluency of the monolayers in 6-well cell culture plates was imaged with a microscopy-equipped camera at 0, 12, 24 h after scratch. For the cell monolayers in the 6-well transwell plates, TEER measurement and FD-4 permeability experiments were conducted at −3, 24, and 48 h after scratch.
For the DON-involving experiments, the IPEC-J2 monolayers with or without 1.0 mmol/L 4-PBA treatment were challenged with 2.0 μg/mL of DON (DON/control, 4-PBA + DON/4-PBA group) for 48 h. The culture medium was changed by a fresh medium with or without 4-PBA after the 48-h DON challenge, and the following observation experiments lasted for 48 h. During the formal experiment, the TEER measurement was conducted at initial (−52 h), DON added (−48 h), DON withdrawal (0, 24, and 48 h). FD-4 permeability experiments were conducted at 24 and 48 h after DON withdrawal. Cell monolayers without any treatments (blank control) received the same TEER measurement and FD-4 permeability experiments at the corresponding time points.
For the experiments involving LPS, the IPEC-J2 monolayers with or without 1.0 mmol/L 4-PBA treatment were challenged with 5.0 μg/mL of LPS (LPS/control, 4-PBA + LPS/4-PBA groups) for 48 h. The culture medium was changed by a fresh medium with or without 4-PBA after the 48-h LPS incubation, and the following observation experiments lasted for 48 h. During the formal experiment, the TEER measurement was conducted at initial (−52 h), LPS added (−48 h), and LPS withdrawal (0, 24, and 48 h). FD-4 permeability experiments were conducted at 24 and 48 h after LPS withdrawal. Cell monolayers without any treatments (blank control) received the same TEER measurement and FD-4 permeability experiments at the corresponding time points.
2.8. RNA extraction and real-time PCR
After these treatments, total RNA was extracted from IPEC-J2 cells using the SteadyPure RNA extract kit (Cat. No. AG21017, AG, Changsha, China) following the manufacturer's instruction. Evo M-MLV RT Kit with gDNA Clean (Cat. No. AG11705, AG, Changsha, China) was used for cDNA synthesis according to the manufacturer's protocol. RT-PCR was performed using the SYBR Green Premix Pro Tag HS qPCR kit (Cat. No. AG11701, AG, Changsha, China) in a LightCycler480 II system (Roche, Basel, Switzerland). The primers for RT-PCR were designed with Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) based on the published cDNA sequence in the Gene Bank. GAPDH was used as the internal reference gene to determine the relative expression of targeted genes. Information on the detected genes and primers is shown in Table 1.
Table 1.
Primers used in the present study.
| Gene | Primer sequences (5′-3′) | Length, bp | Access no. |
|---|---|---|---|
| GAPDH | F: GGGCATGAACCATGAGAAGT R: AAGCAGGGATGATGTTCTGG |
230 | XM_019925987.2 |
| ZO-1 | F: GGCCCTTACCTTTCGCCTGA R: GCCTCAGGGCTTGGTGTTCT |
158 | XM_003480423.4 |
| Claudin-1 | F: CCCGGTCAATGCCAGATATG R: CACCTCCCAGAAGGCAGAGA |
80 | XM_005666863.2 |
| Occludin | F: GCTGGAGGAAGACTGGAT R: ATCCGCAGATCCCTTAAC |
244 | NM_001163647 |
GAPDH = glyceraldehyde-3-phosphate dehydrogenase; ZO-1 = zonula occludens-1.
2.9. Protein extraction and Western blot
Cells were collected from the transwell plates after these treatments. The method for cell collection referred to the trypsinization procedure for transwell inserts that is provided on Corning's website. Total protein was extracted using a total protein extraction kit (Cat. No. SD-001/SN-002, INVENT, Beijing, China) according to the manufacturer's instruction. Protein concentrations of the samples were quantified using a BCA Protein Assay Kit (ZJ101L, EpiZyme, Shanghai, China). The proteins were adjusted into the same concentration at 5.0 μg/μL and then heat-denatured in a 5× loading buffer (P0015, Beyotime, Shanghai, China) at 99 °C. The following procedures of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis, membrane transferring, antibody incubation, and relative quantification of proteins are described in our previous study (Yang et al., 2019). In the present study, relative protein levels of zonula occludens-1 (ZO-1), occludin, and claudin-1 were determined, using β-actin as an internal-reference protein.
2.10. Statistical analysis
Data were presented as means ± standard deviations (SD). Statistical analysis was performed using GraphPad Prism 8.0 software (CA, USA). Differences between these treatments were evaluated using one-way ANOVA. Significance difference was considered when P < 0.05.
3. Results
3.1. Dose-effects of DON and LPS on the barrier function of IPEC-J2 monolayer
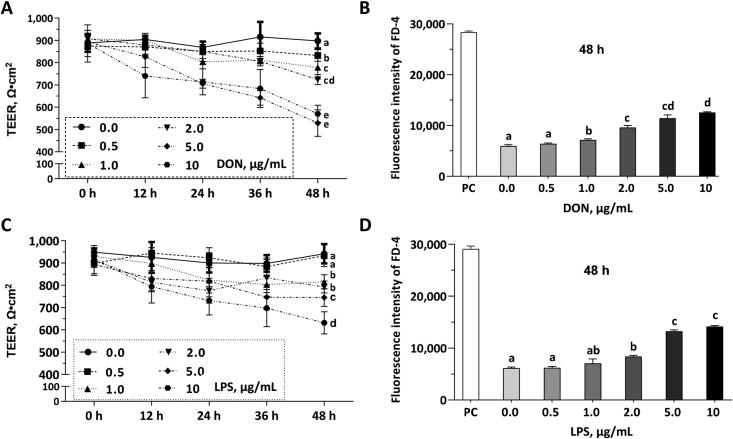
To induce barrier damage with optimal concentrations of DON and LPS, cell monolayers were challenged with DON (0, 0.5, 1.0, 2.0, 5.0, and 10 μg/mL) or LPS (0, 0.5, 1.0, 2.0, 5.0, and 10 μg/mL) for 48 h. DON concentrations from 0.5 to 2.0 μg/mL reduced the TEER values (Fig. 1A) and increased the FD-4 permeability (Fig. 1B) in a dose-dependent manner during the 48 h challenge; LPS concentrations from 2.0 to 5.0 μg/mL reduced the TEER values (Fig. 1C) and increased the FD-4 permeability (Fig. 1D) in a dose-dependent manner during the 48 h challenge. Final concentrations of 2.0 μg/mL DON and 5.0 μg/mL LPS were chosen to induce barrier damage in the following experiments.
Fig. 1.
Dose-effects of deoxynivalenol (DON) and lipopolysaccharide (LPS) on the barrier function of intestinal porcine epithelial cell (IPEC)-J2 monolayer. Cell monolayers in the 6-well transwell plates were challenged with different concentrations of DON or LPS for 48 h. The positive control (PC) group represents these wells in 6-well trans-well plate without cell seeding. (A) The transepithelial electrical resistance (TEER) values of the IPEC-J2 monolayers in each group were shown. (B) Fluorescein isothiocyanate-dextran (FD-4) intensities of the cell culture medium in the basal compartment were shown. (C) The TEER values of the IPEC-J2 monolayers in each group were shown. (D) FD-4 fluorescence intensities of the cell culture medium in the basal compartment were shown. Data were presented as means ± SD, n = 3. Data sets marked with different letters represent a significant difference (P < 0.05).
3.2. Dose-effects of 4-PBA on the viability and barrier function of IPEC-J2 monolayer
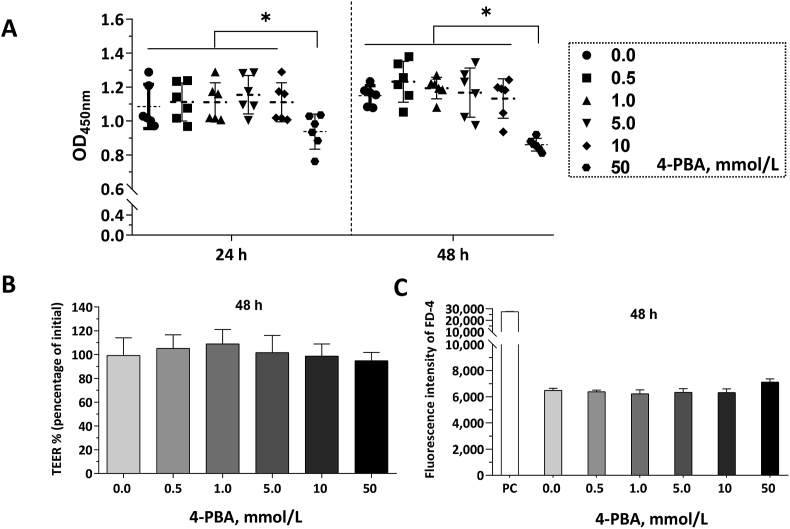
To investigate the toxicity of 4-PBA on IPEC-J2 cells and choose an optimal dosage of 4-PBA in our following experiment, cell monolayers were incubated with 4-PBA ranging from 0 to 50 mmol/L for 48 h. As shown in Fig. 2A, the 4-PBA dosages from 0 to 10 mmol/L 4-PBA did not affect the viability of IPEC-J2 cells, however, the 50 mmol/L 4-PBA decreased (P < 0.05) the viability of IPEC-J2 cells. The TEER values were not affected by the 4-PBA treatments, and the 1.0 mmol/L group showed the highest mean values (Fig. 2B). IPEC-J2 monolayers within 50 mmol/L 4-PBA treatment exhibited higher FD-4 permeability (Fig. 2C). A final concentration of 1.0 mmol/L for 4-PBA was chosen for the following experiments.
Fig. 2.
Dose-effects of 4-phenylbutyric acid (4-PBA) on cell viability and barrier function of intestinal porcine epithelial cell (IPEC)-J2 monolayer. Cell monolayers were treated with different concentrations of 4-PBA for 48 h. (A) The viability of cells in 96-well cell culture plates was determined by Cell-Counting Kit-8 (CCK-8) and presented as OD450nm values. The transepithelial electrical resistance (TEER) measurement and fluorescein isothiocyanate-dextran (FD-4) permeability experiments were conducted in 6-well transwell plates. The positive control (PC) group represents these wells in 6-well trans-well plate without cell seeding. (B) TEER values of the IPEC-J2 monolayers in each group were presented. (C) FD-4 fluorescence intensities of the culture medium in the basal compartment were shown. Data were presented as means ± SD, n = 6 for the cell viability determination, or n = 3 for the cell monolayers in the transwell plates. The data sets marked with ∗ represent a significant difference (P < 0.05).
3.3. Effects of 4-PBA on the IPEC-J2 monolayer during scratch damage and self-rehabilitation
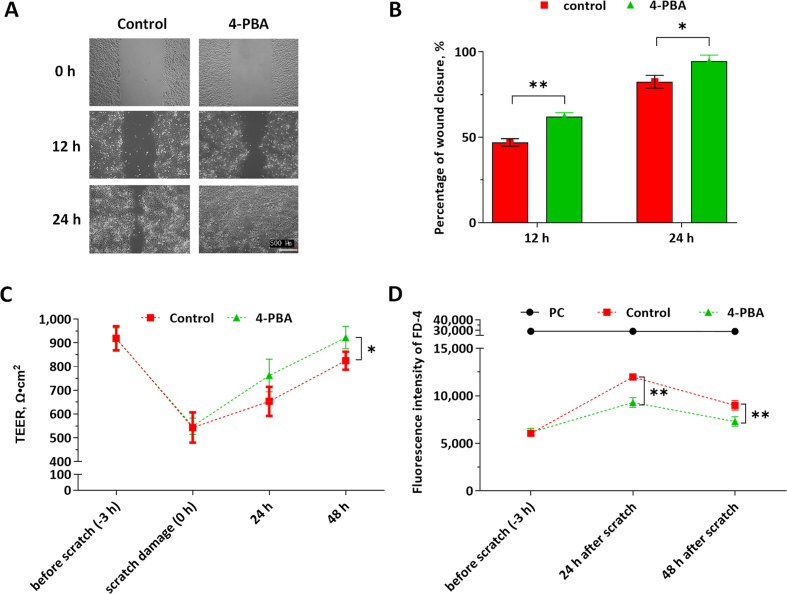
To investigate whether 4-PBA promotes cell migration of IPEC-J2, scratched cell monolayers in the 6-well cell culture plates were incubated with or without 4-PBA (1.0 mmol/L) for 24 h. As shown in Fig. 3A and B, the wound closure ratios were significantly higher in the 4-PBA supplemented group, which indicates that 4-PBA accelerated the migration of IPEC-J2 cells. As shown in Fig. 3C, physical scratch decreased the TEER values by 40.2%; 4-PBA accelerated the recovery of the barrier function of the IPEC-J2 monolayers during 48-h rehabilitation, which is indicated by higher TEER values and lower FD-4 permeability (at 24 and 48 h) in the 4-PBA group than the control (Fig. 3C, D).
Fig. 3.
Effects of 4-phenylbutyric acid (4-PBA) on cell migration, and barrier function of the scratched intestinal porcine epithelial cell (IPEC)-J2 monolayer. The cell monolayers were treated with or without 1.0 μmol/L 4-PBA (control, 4-PBA group) for 48 h. The cell confluency in 6-well cell culture plates was imaged with microscopy at 0, 12, and 24 h. (A) The representative figures were shown. (B) The relative wound closure of the IPEC-J2 monolayers in each group was shown. The transepithelial electrical resistance (TEER) measurement and fluorescein isothiocyanate-dextran (FD-4) permeability experiments were conducted in 6-well transwell plates. The positive control (PC) group represents these wells in 6-well trans-well plate without cell seeding. (C) TEER values of IPEC-J2 monolayers in each group were shown. (D) FD-4 fluorescence intensities of the culture medium in the basal compartment were presented. Data were presented as means ± SD, n = 3. Data sets marked with ∗ or ∗∗ represent a significant difference (P < 0.05 or < 0.01).
3.4. Effects of 4-PBA on the IPEC-J2 monolayer during DON-induced damage and self-rehabilitation
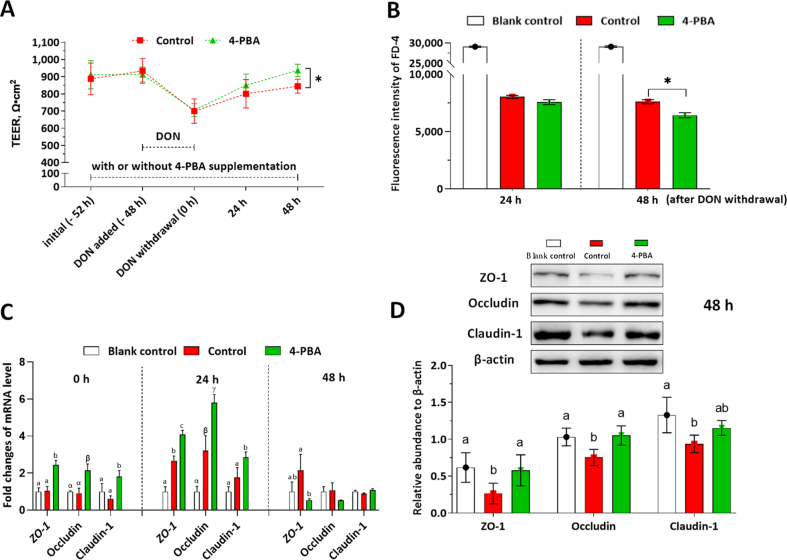
To investigate whether 4-PBA alleviates DON-induced barrier damage of the IPEC-J2 monolayer, TEER, FD-4 permeability, mRNA, and protein levels of the tight junction were determined. As shown in Fig. 4A, the TEER values were significantly decreased during the DON challenge, and the 4-PBA treatment did not alleviate the DON-induced decrease in TEER values. However, the 4-PBA accelerated the recovery of barrier integrity during the 48-h rehabilitation period, which was indicated by significantly higher TEER values at 48 h in the 4-PBA group when compared with the control. Likewise, the FD-4 permeability was significantly decreased by the 4-PBA treatment at 48 h after DON withdrawal (Fig. 4B). As shown in Fig. 4C, at 0 h after DON withdrawal, the relative mRNA levels of ZO-1, occludin, and claudin-1 in the 4-PBA group were significantly higher than the control; at 24 h after DON withdrawal, the mRNA levels of ZO-1, occludin, and claudin-1 were significantly upregulated in the 4-PBA group when compared with the blank control; at 48 h after DON withdrawal, the mRNA level of ZO-1 and occludin in the 4-PBA treated cells were significantly lower than the control group. As shown in Fig. 4D, the 4-PBA treatment improved the protein levels of ZO-1, occludin, and claudin-1 when compared with those in the control group.
Fig. 4.
Effects of 4-phenylbutyric acid (4-PBA) on the intestinal porcine epithelial cell (IPEC)-J2 monolayer during deoxynivalenol (DON)-induced damage and self-rehabilitation. The cell monolayers were treated with or without 1.0 μmol/L 4-PBA (control, 4-PBA group) during all the experimental periods. DON at 2.0 μg/mL was added in the medium at −48 h and withdrawn at 0 h. The positive control (PC) group represents these wells in 6-well trans-well plate without cell seeding. The blank control group represents cell monolayers without any treatment. The transepithelial electrical resistance (TEER) measurement and fluorescein isothiocyanate-dextran (FD-4) permeability experiments were conducted in 6-well transwell plates. (A) TEER values of the IPEC-J2 monolayers in each group were shown. (B) FD-4 fluorescence intensities of the culture medium in the basal compartment were presented. (C) The relative mRNA levels of ZO-1, occludin, and claudin-1 in each group were presented as the normalized ratio to the blank control. (D) The relative protein levels of ZO-1, occludin, and claudin-1 to β-actin were shown. Data were presented as means ± SD, n = 3. Data sets marked with ∗, or different letters represent a significant difference (P < 0.05).
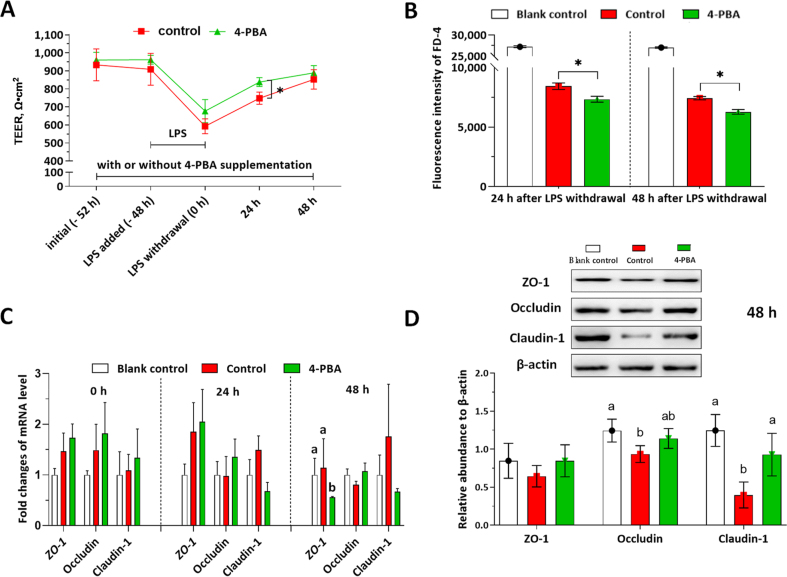
3.5. Effects of 4-PBA on IPEC-J2 monolayer during LPS-induced damage and self-rehabilitation
TEER, FD-4 permeability, mRNA, and protein levels of the tight junction were determined to investigate the effects of 4-PBA on the IPEC-J2 monolayer during LPS-induced barrier damage and self-rehabilitation (Fig. 5). As shown in Fig. 5A, the TEER values were significantly decreased by the LPS challenge. When compared with the control, numerically higher TEER values of IPEC-J2 monolayers were observed in the 4-PBA group at 0 h (after 48 h LPS exposure). Besides, the 4-PBA accelerated the recovery of barrier integrity (indicated by significantly higher TEER values at 24 h in the 4-PBA group when compared with the control) during the rehabilitation period. Likewise, FD-4 permeability was significantly decreased by the 4-PBA treatment at both 24 and 48 h after LPS withdrawal (Fig. 5B). As shown in Fig. 5C, at 0 h after LPS withdrawal, the relative mRNA levels of ZO-1, occludin, and claudin-1 in the control and 4-PBA group were numerically higher than the blank control; at 24 h after LPS withdrawal, the mRNA levels of ZO-1 and occludin in the 4-PBA group were numerically higher than the blank control; at 48 h after DON withdrawal, the mRNA level of ZO-1 in the 4-PBA treated cells were significantly lower than the control group. As shown in Fig. 5D, the 4-PBA treatment numerically improved the protein levels of ZO-1 and occludin and significantly improved the claudin-1 level when compared with those in the control group.
Fig. 5.
Effects of 4-phenylbutyric acid (4-PBA) on the intestinal porcine epithelial cell (IPEC)-J2 monolayer during lipopolysaccharide (LPS) challenge and self-rehabilitation. The cell monolayers were treated with or without 1.0 μmol/L 4-PBA (control, or 4-PBA group) during all the experimental periods. LPS at 5.0 μg/mL was added in the medium at −48 h and withdrawn at 0 h. The positive control (PC) group represents these wells in 6-well trans-well plate without cell seeding. The blank control group represents cell monolayers without any treatment. The transepithelial electrical resistance (TEER) measurement and fluorescein isothiocyanate-dextran (FD-4) permeability experiments were conducted in 6-well transwell plates. (A) TEER values of the IPEC-J2 monolayers in each group were shown. (B) FD-4 fluorescence intensities of the culture medium in the basal compartment were presented. (C) The relative mRNA levels of ZO-1, occludin, and claudin-1 in each group were presented as the normalized ratio to the blank control. (D) The relative protein levels of ZO-1, occludin, and claudin-1 to β-actin were shown. Data were presented as means ± SD, n = 3. Data sets marked with ∗ or different letters represent a significant difference (P < 0.05).
4. Discussion
The intestinal barrier acts as the first line of defence against bacteria and virus infections. Numerous studies in the livestock field have demonstrated that growth performance is closely and positively associated with intestinal barrier functions (Koo et al., 2020; Shang et al., 2020; Shi et al., 2020; Xiang et al., 2020), affirming intact intestinal barrier function as a crucial factor determining animals' health and growth performance. Under the severe challenges by in-feed antibiotic prohibition and African swine fever prevalence in China, it is crucial to effectively and at low cost, screen feed additives for protecting the porcine intestinal barrier against various damage induced by adverse factors, such as early weaning stress, mycotoxin-contaminated feed, and pathogens infection. In the current study, we established 3 in vitro models to mimic real porcine intestinal barrier damage induced by early weaning stress, mycotoxin-contaminated feed ingestion, and E. coli infection, respectively. Further, 4-PBA was validated as a potential feed additive to accelerate the rehabilitation of the barrier function in these in vitro models. This study is the first one demonstrating the stimulative effects of 4-PBA on the rehabilitation of porcine intestinal barrier function using different in vitro models, which may guide 4-PBA application in the pig industry.
Weaned piglets suffer from a “critical window period” of the intestinal epithelial barrier, which is characterized by deprivation of maternal immunity, unmatured development, and high permeability. Various adverse factors during early weaning are revealed to compromise the intestinal barrier and increase permeability (Wijtten et al., 2011). Oxidative dyshomeostasis is involved in weaning stress-induced intestinal barrier damage (Moeser et al., 2017). Several previous studies used the oxidative stress model to mimic the intestinal barrier damage in the weaned piglets (Cai et al., 2016; Wang et al., 2014). The general antioxidant N-acetyl cysteine (NAC) was revealed to alleviate intestinal barrier damage in weaned piglets (Zhu et al., 2013). However, one recent study revealed that vitamin E (a natural antioxidant) supplementation tended to decrease intestinal morphological development and inhibit the proliferation of intestinal epithelial cells (Chen et al., 2019), which decreased the feasibility of using the oxidative stress model to screen feed additives for protecting the intestine during early weaning. Given that the early-weaned piglet has an immature digestive system with a low adaptability to the physical alteration of the diet, it can easily suffer from physical intestinal barrier damage from a solid diet intake (Jayaraman and Nyachoti, 2017). IPEC-J2, a porcine intestinal epithelial cell line originally from the jejunum of unsuckled piglets, has been widely used for establishing in vitro models to mimic intestinal damage in piglets. The present study scratched the in vitro IPEC-J2 monolayer to mimic the early weaning stress-induced damage and the “critical window period” of the porcine intestinal epithelial barrier.
Mycotoxin contamination in the feed and feed ingredients is a global issue that has caused huge economic losses in livestock production (Cheli, 2020; Gruber-Dorninger et al., 2019). Concentrations of DON in the feed are closely associated with feed intake and growth performance of swine (Pasternak et al., 2018; Reddy et al., 2018). Evidence has indicated that DON easily disrupts the intestinal epithelial barrier in pigs (De Walle et al., 2010; Pinton et al., 2010), which can be reflected by an increase in paracellular permeability and decreases in the tight junction proteins. One previous study demonstrated that DON at 2.5 μmol/L (equals 0.85 μg/mL) increased LDH release and decreased the counts of adherent IPEC-J2 cells in the monolayer (Awad et al., 2012). Another one has shown that IPEC-J2 cells with 0.2 μg/mL DON exposure exhibited lower cell viability and mRNA levels of tight junction proteins, such as ZO-1, occludin, and claudin-1 (Liao et al., 2017). Transwell plates have been widely used for establishing the in vitro models of epithelial barriers to mimic the actual in vivo conditions (He et al., 2019; Jiang et al., 2019). In the present study, we firstly screened an optimal concentration of DON to induce intestinal barrier damage of the IPEC-J2 monolayer in the transwell plates. Results showed that 2.0 μg/mL DON significantly decreased the TEER values and increased permeability of the IPEC-J2 monolayer when compared with those in the 1.0 μg/mL DON group. Accordingly, we used 2.0 μg/mL DON to induce intestinal barrier damage in the experimental design. LPS, the cell wall component from Gram-negative bacteria, plays a key role in triggering intestinal inflammation. LPS has been widely used to induce barrier damage in different epithelial cell monolayers, such as IPEC-J2 (Yang et al., 2015) and colonic cancer cell line (Caco-2) (Bian et al., 2020). One previous study applied 10 μg/mL LPS for 24 h to induce barrier damage in the IPEC-J2 cell monolayer (Yan and Ajuwon, 2017). In our present study, we used the different concentrations of E. coli O127:B8-derived LPS to induce inflammatory damage in the IPEC-J2 monolayer and observed a numerical decrease in TEER values by LPS challenges (Fig. 1C). The IPEC-J2 monolayer challenged with 5.0 μg/mL LPS for 48 h exhibited significantly higher FD-4 permeability when compared with those challenged with lower concentrations of LPS (Fig. 1D). Thus, we used the LPS at 5.0 μg/mL to induce inflammatory damage of the IPEC-J2 monolayers.
4-PBA is an aromatic fatty acid that has been reported to benefit endoplasmic reticulum homeostasis and protein synthesis in different tissues, including intestines (You et al., 2019). For instance, 4-PBA was revealed to attenuate endoplasmic reticulum stress (ERS)-mediated apoptosis of the intestinal epithelial cells in rats and human hepatocytes (Liu et al., 2019; You et al., 2019). However, there is no evidence showing its influence on pigs. The results in the current study demonstrated that 4-PBA ranging from 0 to 10 mmol/L are not toxic to the viability of IPEC-J2 cells (Fig. 2A), indicating a high tolerance of the porcine intestine to 4-PBA supplementation. Besides, IPEC-J2 monolayers supplemented with 1.0 mmol/L 4-PBA showed the numerically highest TEER values (Fig. 2B) and lowest FD-4 permeability (not statistically significant) compared with the other groups (Fig. 2C), which prompted us to apply 1.0 mmol/L 4-PBA in the following experiments.
It is noteworthy that 4-PBA at 1.0 mmol/L exerted beneficial roles in rehabilitating the IPEC-J2 monolayers from scratch, DON, and LPS-induced damage, which was indicated by higher TEER values and lower permeability at 24 h or 48 h after scratch damage (Fig. 3C, D), DON withdrawal (Fig. 4A, B), and LPS withdrawal (Fig. 5A, B). The following results may support and explain these beneficial roles of 4-PBA. Firstly, the 4-PBA treatment significantly promoted the cell migration in the following 24 h after scratch damage, indicating a stronger self-repairing capability of the IPEC-J2 monolayer enhanced by the 4-PBA. A previous study reported that 4-PBA promotes cell migration of MGC-803 and MGC-823 cell lines via histone deacetylase inhibition (Shi et al., 2018). However, whether the 4-PBA exerts similar effects on histone deacetylase inhibition in the IPEC-J2 cells was unclear, which warrants continual study in our further work. Secondly, the tight junction proteins ZO-1, occludin, and claudin-1, which are highly expressed in the IPEC-J2 cells (He et al., 2019), are important for the small intestine to maintain the intestinal barrier function (Anderson and Van Itallie, 2009). In the present study, the 4-PBA significantly enhanced the mRNA transcription of ZO-1, occludin, and claudin-1 at 0 and 24 h after DON withdrawal (Fig. 4C), which contributed to higher levels of tight junction proteins (ZO-1 and occludin) at 48 h (Fig. 4D). Similarly, a previous study revealed that 4-PBA significantly ameliorated tight junction loss in an ischemia/reperfusion (I/R) injury model (Nan et al., 2019). Huang et al. verified the protective effects of 4-PBA on tight junction proteins in burn-induced intestinal barrier damage and attributed this effect to the ERS-autophagy axis (Huang et al., 2019). Finally, in the LPS-induced intestinal barrier damage of the IPEC-J2 monolayer, claudin-1 protein level at 48 h in the 4-PBA group was observed to be significantly higher than the control (Fig. 5D). Similarly, a previous study reported 4-PBA can inhibit LPS-induced inflammation in A549 alveolar epithelial cells via regulating ERS-autophagy pathways (Zeng et al., 2017). Nevertheless, whether the 4-PBA could exert anti-inflammatory or ERS-autophagy regulating roles in the porcine intestine is still unknown. The underlying mechanism of 4-PBA promotes the intestinal barrier functions' rehabilitation from these adverse factors-induced damage, which warrants further investigation. Although the present study on the IPEC-J2 monolayer models exhibits promising results, further in vivo studies are necessary to validate the beneficial effects of 4-PBA on the porcine intestinal barrier, and to explore the potential roles of 4-PBA in maintaining health and promoting the growth performance of weaning piglets.
5. Conclusion
In conclusion, for the first time, the present study demonstrated that 4-PBA accelerates the porcine intestinal epithelial barrier recovery from physical scratch, DON, and LPS induced damage via enhancing cell mitigation and expressions of tight junction proteins, which may guide the future application of 4-PBA in the pig industry.
Author contributions
Qian Jiang, Kang Yao, Bie Tan, and Yulong Yin conceptualized the project. Qian Jiang conducted the experiments and wrote the original draft. Jie Yin, Jiashun Chen, Xiaokang Ma, Miaomiao Wu, Xilong Li, Kang Yao, Bie Tan, and Yulong Yin revised and edited the manuscript. All authors proofread and approved the final version of the manuscript.
Conflict of interest
The authors declare the following competing financial interest(s): Qian Jiang, Bie Tan, and Yulong Yin are inventors of a Chinese patent application related to the initial evaluation of 4-phenylbutyric acid for the swine additive, which was filed on June 07, 2021.
Acknowledgments
This research was funded by the “Shennong Scholar funding of Hunan Agricultural University”, the “Changsha Municipal Natural Science Foundation (Grant No. kq2014068)”, and the “Open Project Program of Key Laboratory of Feed Biotechnology, the Ministry of Agriculture and Rural Affairs of the People's Republic of China”.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Qian Jiang, Email: jiangqian@hunau.edu.cn.
Kang Yao, Email: yaokang@isa.ac.cn.
References
- Anderson J.M., Van Itallie C.M. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a029314. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Aschenbach J.R., Zentek J. Cytotoxicity and metabolic stress induced by deoxynivalenol in the porcine intestinal IPEC-J2 cell line. J Anim Physiol Anim Nutr. 2012;96:709–716. doi: 10.1111/j.1439-0396.2011.01199.x. [DOI] [PubMed] [Google Scholar]
- Bian Y.F., Dong Y.Y., Sun J.J., Sun M., Hou Q.H., Lai Y.J. Protective effect of kaempferol on LPS-induced inflammation and barrier dysfunction in a coculture model of intestinal epithelial cells and intestinal microvascular endothelial cells. J Agric Food Chem. 2020;68:160–167. doi: 10.1021/acs.jafc.9b06294. [DOI] [PubMed] [Google Scholar]
- Broom L.J., Kogut M.H. Inflammation: friend or foe for animal production? Poult Sci. 2018;97:510–514. doi: 10.3382/ps/pex314. [DOI] [PubMed] [Google Scholar]
- Cai X., Zhu L., Chen X., Sheng Y., Guo Q., Bao J. X/XO or H2O2 induced IPEC-J2 cell as a new in vitro model for studying apoptosis in post-weaning piglets. Cytotechnology. 2016;68:713–724. doi: 10.1007/s10616-014-9823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo C.T., Powell D.N., Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med. 2017;95:927–934. doi: 10.1007/s00109-017-1557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle R.E., Brimble E., Werner K.E., Cruz G.L., Ask K., Ingram A.J. 4-Phenylbutyrate inhibits tunicamycin-induced acute kidney injury via CHOP/GADD153 repression. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli F. Mycotoxin contamination management tools and efficient strategies in feed industry. Toxins. 2020;12:480–483. doi: 10.3390/toxins12080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.C., Wang Z.B., Li J.Z., Li Y.L., Huang P.F., Ding X.Q. Dietary vitamin E affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets. J Anim Sci. 2019;97:1212–1221. doi: 10.1093/jas/skz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Walle J.V., Sergent T., Piront N., Toussaint O., Schneider Y.J., Larondelle Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol Appl Pharmacol. 2010;245:291–298. doi: 10.1016/j.taap.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Devreese M., Pasmans F., De Backer P., Croubels S. An in vitro model using the IPEC-J2 cell line for efficacy and drug interaction testing of mycotoxin detoxifying agents. Toxicol In Vitro. 2013;27:157–163. doi: 10.1016/j.tiv.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Ewaschuk J.B., Murdoch G.K., Johnson I.R., Madsen K.L., Field C.J. Glutamine supplementation improves intestinal barrier function in a weaned piglet model of Escherichia coli infection. Br J Nutr. 2011;106:870–877. doi: 10.1017/S0007114511001152. [DOI] [PubMed] [Google Scholar]
- Gruber-Dorninger C., Jenkins T., Schatzmayr G. Global mycotoxin occurrence in feed: a ten-year survey. Toxins. 2019;11:375–399. doi: 10.3390/toxins11070375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.M., Deng J., Hu X., Zhou S.C., Wu J.T., Xiao D. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019;10:1235–1242. doi: 10.1039/c8fo01123k. [DOI] [PubMed] [Google Scholar]
- Hong Y.P., Deng W.H., Guo W.Y., Shi Q., Zhao L., You Y.D. Inhibition of endoplasmic reticulum stress by 4-phenylbutyric acid prevents vital organ injury in rat acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G838–G847. doi: 10.1152/ajpgi.00102.2018. [DOI] [PubMed] [Google Scholar]
- Hu S., Wang Y., Wen X., Wang L., Jiang Z., Zheng C. Effects of low-molecular-weight chitosan on the growth performance, intestinal morphology, barrier function, cytokine expression and antioxidant system of weaned piglets. BMC Vet Res. 2018;14:215–221. doi: 10.1186/s12917-018-1543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wu P., Jiang W.D., Liu Y., Zeng Y.Y., Jiang J. Deoxynivalenol decreased the growth performance and impaired intestinal physical barrier in juvenile grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2018;80:376–391. doi: 10.1016/j.fsi.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Huang Y.L., Wang Y., Feng Y.H., Wang P., He X.C., Ren H. Role of endoplasmic reticulum stress-autophagy axis in severe burn-induced intestinal tight junction barrier dysfunction in mice. Front Physiol. 2019;10:606–617. doi: 10.3389/fphys.2019.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman B., Nyachoti C.M. Husbandry practices and gut health outcomes in weaned piglets: a review. Anim Nutr. 2017;3:205–211. doi: 10.1016/j.aninu.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Zhang H., Yang R., Hui Q., Chen Y., Mats L. Red-Osier dogwood extracts prevent inflammatory responses in Caco-2 cells and a Caco-2 BBe1/EA.hy926 cell co-culture model. Antioxidants. 2019;8:428–443. doi: 10.3390/antiox8100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B., Choi J., Yang C.B., Nyachoti C.M. Diet complexity and L-threonine supplementation: effects on growth performance, immune response, intestinal barrier function, and microbial metabolites in nursery pigs. J Anim Sci. 2020;98:125. doi: 10.1093/jas/skaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wen W., Xu H., Wu H., Xu M., Frank J.A. 4-Phenylbutyric acid protects against ethanol-induced damage in the developing mouse brain. Alcohol Clin Exp Res. 2019;43:69–78. doi: 10.1111/acer.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P., Liao M.F., Li L., Tan B., Yin Y.L. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol Res. 2017;6:866–877. doi: 10.1039/c7tx00202e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wu H., Zang J., Yang G., Zhu Y., Wu Y. 4-Phenylbutyric acid reveals good beneficial effects on vital organ function via anti-endoplasmic reticulum stress in septic rats. Crit Care Med. 2016;44:e689–e701. doi: 10.1097/CCM.0000000000001662. [DOI] [PubMed] [Google Scholar]
- Liu X., Wu F., Du T.T., Sun Z.M., Zhang Q. 4-Phenylbutyric acid attenuates endoplasmic reticulum stress-mediated apoptosis and protects the hepatocytes from intermittent hypoxia-induced injury. Sleep Breath. 2019;23:711–717. doi: 10.1007/s11325-018-1739-y. [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Klok C.V., Ryan K.A., Wooten J.G., Little D., Cook V.L. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G173–G181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Pohl C.S., Rajput M. Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim Nutr. 2017;3:313–321. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan D., Jin H.Q., Deng J.W., Yu W.W., Liu R., Sun W.P. Cilostazol ameliorates ischemia/reperfusion-induced tight junction disruption in brain endothelial cells by inhibiting endoplasmic reticulum stress. FASEB J. 2019;33:10152–10164. doi: 10.1096/fj.201900326R. [DOI] [PubMed] [Google Scholar]
- Park I., Lee Y., Goo D., Zimmerman N.P., Smith A.H., Rehberger T. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult Sci. 2020;99:725–733. doi: 10.1016/j.psj.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak J.A., Aiyer V.I.A., Hamonic G., Beaulieu A.D., Columbus D.A., Wilson H.L. Molecular and physiological effects on the small intestine of weaner pigs following feeding with deoxynivalenol-contaminated feed. Toxins. 2018;10:40–56. doi: 10.3390/toxins10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Pierron A., Alassane-Kpembi I., Oswald I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porcine Health Manag. 2016;2:21–28. doi: 10.1186/s40813-016-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Braicu C., Nougayrede J.P., Laffitte J., Taranu I., Oswald I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J Nutr. 2010;140:1956–1962. doi: 10.3945/jn.110.123919. [DOI] [PubMed] [Google Scholar]
- Reddy K.E., Song J., Lee H.J., Kim M., Kim D.W., Jung H.J. Effects of high levels of deoxynivalenol and zearalenone on growth performance, and hematological and immunological parameters in pigs. Toxins. 2018;10:114–128. doi: 10.3390/toxins10030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Q.H., Liu S.J., He T.F., Liu H.S., Mahfuz S., Piao X.S. Effects of wheat bran in comparison to antibiotics on growth performance, intestinal immunity, barrier function, and microbial composition in broiler chickens. Poult Sci. 2020;99:4929–4938. doi: 10.1016/j.psj.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.G., Xun W.J., Peng W.Q., Hu H.C., Cao T., Hou G.Y. Effect of the single and combined use of curcumin and piperine on growth performance, intestinal barrier function, and antioxidant capacity of weaned Wuzhishan piglets. Front Vet Sci. 2020;7:418–427. doi: 10.3389/fvets.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X.N., Zheng C.L., Li C., Hou K.Z., Wang X.X., Yang Z.C. 4-Phenybutyric acid promotes gastric cancer cell migration via histone deacetylase inhibition-mediated HER3/HER4 up-regulation. Cell Biol Int. 2018;42:53–62. doi: 10.1002/cbin.10866. [DOI] [PubMed] [Google Scholar]
- Shimizu D., Ishitsuka Y., Miyata K., Tomishima Y., Kondo Y., Irikura M. Protection afforded by pre- or post-treatment with 4-phenylbutyrate against liver injury induced by acetaminophen overdose in mice. Pharmacol Res. 2014;87:26–41. doi: 10.1016/j.phrs.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Vergauwen H. In: The impact of food bioactives on health: in vitro and ex vivo models. Verhoeckx K., Cotter P., Lopez-Exposito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. 2015. The IPEC-J2 cell line; pp. 125–134. Cham (CH) [PubMed] [Google Scholar]
- Wan J., Zhang J., Chen D.W., Yu B., Mao X.B., Zheng P. Alginate oligosaccharide-induced intestinal morphology, barrier function and epithelium apoptosis modifications have beneficial effects on the growth performance of weaned pigs. J Anim Sci Biotechnol. 2018;9:58–69. doi: 10.1186/s40104-018-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.W., Wu Z.L., Lin G., Hu S.D., Wang B., Dai Z.L. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J Nutr. 2014;144:1540–1548. doi: 10.3945/jn.114.194001. [DOI] [PubMed] [Google Scholar]
- Wang X.C., Zhang Y.F., Cao L., Zhu L., Huang Y.Y., Chen X.F. Deoxynivalenol induces intestinal damage and inflammatory response through the nuclear factor-kappaB signaling pathway in piglets. Toxins. 2019;11:663–676. doi: 10.3390/toxins11110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang B., Liu Q., Fan C., Li J., Zhou Y. Palygorskite supplementation improves growth performance, oxidative status, and intestinal barrier function in cherry valley ducks. J Poult Sci. 2019;56:186–194. doi: 10.2141/jpsa.0180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtten P.J., van der Meulen J., Verstegen M.W. Intestinal barrier function and absorption in pigs after weaning: a review. Br J Nutr. 2011;105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- Xiang Q.H., Wu X.Y., Pan Y., Wang L., Guo Y.W., Cui C.B. Early intervention using fecal microbiota transplantation combined with probiotics influence the growth performance, diarrhea, and intestinal barrier function of piglets. Appl Sci. 2020;10:568–579. [Google Scholar]
- Yan H., Ajuwon K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.J., Wang A.N., Zeng X.F., Hou C.L., Liu H., Qiao S.Y. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015;15:32–42. doi: 10.1186/s12866-015-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Hui Q., Jiang Q., Liu S., Zhang H., Wu J. Effect of Manitoba-grown red-osier dogwood extracts on recovering Caco-2 cells from H2O2-induced oxidative damage. Antioxidants. 2019;8:250–264. doi: 10.3390/antiox8080250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Wang L., Xiong Y., Wen X., Wang Z., Yang X. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J Anim Sci. 2018;96:2342–2351. doi: 10.1093/jas/sky129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y.D., Deng W.H., Guo W.Y., Zhao L., Mei F.C., Hong Y.P. 4-Phenylbutyric acid attenuates endoplasmic reticulum stress-mediated intestinal epithelial cell apoptosis in rats with severe acute pancreatitis. Dig Dis Sci. 2019;64:1535–1547. doi: 10.1007/s10620-018-5437-1. [DOI] [PubMed] [Google Scholar]
- Zeng M., Sang W., Chen S., Chen R., Zhang H., Xue F. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017;271:26–37. doi: 10.1016/j.toxlet.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Zhu L.H., Cai X., Guo Q., Chen X.L., Zhu S.W., Xu J.X. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways' response to oxidative stress in weaned piglets. Br J Nutr. 2013;110:1938–1947. doi: 10.1017/S0007114513001608. [DOI] [PubMed] [Google Scholar]