Abstract
Cells have different sets of molecules for performing an array of physiological functions. Nucleic acids have stored and carried the information throughout evolution, whereas proteins have been attributed to performing most of the cellular functions. To perform these functions, proteins need to have a unique conformation and a definite lifespan. These attributes are achieved by a highly coordinated protein quality control (PQC) system comprising chaperones to fold the proteins in a proper three-dimensional structure, ubiquitin-proteasome system for selective degradation of proteins, and autophagy for bulk clearance of cell debris. Many kinds of stresses and perturbations may lead to the weakening of these protective cellular machinery, leading to the unfolding and aggregation of cellular proteins and the occurrence of numerous pathological conditions. However, modulating the expression and functional efficiency of molecular chaperones, E3 ubiquitin ligases, and autophagic proteins may diminish cellular proteotoxic load and mitigate various pathological effects. Natural medicine and small molecule-based therapies have been well-documented for their effectiveness in modulating these pathways and reestablishing the lost proteostasis inside the cells to combat disease conditions. The present article summarizes various similar reports and highlights the importance of the molecules obtained from natural sources in disease therapeutics.
KEY WORDS: Proteostasis, Proteinopathies, Chaperones, Ubiquitin proteasome system, Autophagy, Cancer, Neurodegeneration, Ageing, Natural molecules, Drug discovery
Abbreviations: 17-AAG, 17-allylamino-geldanamycin; APC, anaphase-promoting complex; BAG, BCL2-associated athanogene; CAP, chaperone-assisted proteasomal degradation; CASA, chaperone-assisted selective autophagy; CMA, chaperone-mediated autophagy; CHIP, carboxy-terminus of HSC70 interacting protein; DUBs, deubiquitinases; EGCG, epigallocatechin-3-gallate; ESCRT, endosomal sorting complexes required for transport; HECT, homologous to the E6-AP carboxyl terminus; HSC70, heat shock cognate 70; HSF1, heat shock factor 1; HSP, heat shock protein; KFERQ, lysine-phenylalanine-glutamate-arginine-glutamine; LAMP2a, lysosome-associated membrane protein 2a; LC3, light chain 3; NBR1, next to BRCA1 gene 1; PQC, protein quality control; RING, really interesting new gene; Ub, ubiquitin; UPS, ubiquitin–proteasome system
Graphical abstract
Extra-/intracellular stresses challenge cellular health throughout life. Small natural molecules modulate the functioning of protein quality control components to maintain proteins' native structures and preserve cells’ proteome under healthy and stressful conditions.
1. Introduction
A eukaryotic cell represents a well-evolved architecture, made up of many small components working independently and coherently. A highly efficient and coordinated way of functioning of multiple subsystems towards the fitness and survival of individual cells and the organism is a highly complex biological phenomenon. It remains a great challenge to understand the intricacies and complexities of the living systems. For a very long period, the central dogma, i.e., the idea of sequential flow of information from DNA to RNA, followed by its retrieval into the form of proteins, remained a mystery for the scientists. However, the improvements in the techniques and adaptations of the newer approaches to decipher the molecular arrangements have led to a higher understanding of the fine details of the cells’ structure and functional arrangements. The involvement of biochemical and molecular biology approaches has led to the deduction of most of the metabolic pathways and their subsequent impact on the cellular physiology. At the same time, structural and computational biologists have played a critical role in providing crucial insights about the mysteries of the genetic codes, amino acid sequences, and structural plans of the proteins. Many other tools have also helped in devising various ways of visualizing and interpreting the intermolecular interactions involved in different cellular pathways and mechanisms.
With all the advancements and our current understanding of cellular architecture, we can believe that a functional proteome is a prerequisite for regulating the essential physiological pathways and maintaining good cellular health1. To preserve an advantageous proteome, the cells have a well-developed protein quality control (PQC) machinery that ensures a healthy set of protein repertoires to execute all the necessary cellular tasks2,3. First, it helps the nascent polypeptide chains to attain their native conformations. Second, it identifies any dysfunctional or aberrant protein that remains present into our cellular milieu; and third, it removes all such unwanted proteins from the system. However, under certain kinds of stress conditions, their inefficiency may lead to the generation of unwanted proteinaceous species inside the cytoplasm, leading to misfolding and aggregation4. These events of failure of proteostasis and formation of large molecular weight aggregates or cytoplasmic inclusion bodies underlie the causal mechanism behind several systemic and non-systemic diseases, as summarized in Table 1.
Table 1.
Major amyloidosis and the associated aggregatory proteins. The table summarizes major systemic and non-systemic diseases associated with misfolding and aggregation of certain proteins.
| Disease/pathological condition | Proteins misfolded/aggregated | Ref. |
|---|---|---|
| Alzheimer's disease | Amyloid β42, Tau protein | 1,2 |
| Amyotrophic lateral sclerosis | SOD1, TDP43, | 3,4 |
| Atrial amyloidosis | Atrial natriuretic factor | 5 |
| BriPP amyloidosis | Amyloid-Bri | 6 |
| Cancer | Tumor protein 53 | 7 |
| Cataracts | Crystallins | 8 |
| Creutzfeldt Jakob disease | Prion | 9 |
| Cystic fibrosis | CFTR | 10 |
| Diabetes | Amylin, IAPP | 11,12 |
| Familial amyloid polyneuropathy I | Transthyretin | 13 |
| Familial amyloid polyneuropathy III | Apolipoprotein AI | 14 |
| Familial hypercholesterolemia | LDL receptor | 15 |
| Fibrinogen α-chain amyloidosis | Fibrinogen α-chain variants | 16 |
| Finnish hereditary systemic amyloidosis | Gelsolin | 17 |
| Fronto-temporal dementias | Tau protein | 18 |
| Haemodialysis-related amyloidosis | β2-Microglobulin | 19 |
| Hereditary cerebral amyloid angiopathy | Cystatin C | 20 |
| Lysozyme-related amyloidosis | Lysozyme | 21 |
| Hereditary renal amyloidosis | Fibrinogen α-A chain, lysozyme | 16,22 |
| Huntington's disease | Huntingtin | 23 |
| IBMPFD | Valosin-containing protein | 24 |
| Insulin-related amyloidopathy | Insulin | 25 |
| Leprechaunism | Insulin receptor | 26 |
| Marfan syndrome | Fibrillin | 27 |
| Medullary carcinoma of the thyroid | Calcitonin | 28 |
| Osteogenesis imperfecta | Type I procollagen pro α | 29 |
| Parkinson's disease/Lewy body dementia | α-Synuclein | 30 |
| Primary systemic amyloidosis | Ig light chains | 31 |
| Retinitis pigmentosa | Rhodopsin | 32 |
| Scrapie | Prion protein | 33 |
| Secondary systemic amyloidosis | Serum amyloid A | 34 |
| Senile systemic amyloidosis | Transthyretin | 35 |
| Spinal and bulbar muscular atrophy | Androgen receptor | 36 |
| Spinocerebellar ataxias | Ataxin proteins | 37 |
| Spinocerebellar ataxia 17 | TATA box-binding protein | 38 |
| Spongiform encephalopathies | Prion protein | 39 |
| Tay-Sachs disease | β-Hexosaminidase | 40 |
| α1-Antitrypsin deficiency | α1-Antitrypsin | 41 |
CFTR, cystic fibrosis transmembrane receptor; IBMPFD, inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia; IAPP, islet amyloid polypeptide.
In the past many years, substantial efforts have been made to affect the functional efficiency of many components of the PQC systems to establish and maintain the homeostatic conditions inside cells5,6. Small molecules obtained from plants and other natural sources may have diverse medicinal values, as they can modulate many cellular proteins and affect several associated signaling pathways7,8. The primary sources of these molecules of immense medicinal values include bacterial or fungal isolates, extracts of marine animals or plant sources, and few specific mammalian tissue secretions, etc. The upcoming sections describe the significance of these crucial cellular subsystems in regulating multiple molecular networks. The article further provides a brief overview of the available reports describing various naturally-derived molecules with the proposed medicinal values.
2. Cellular protein quality control system
A battery of multifaceted enzymes is involved in the replication and transcriptional processes, exhibiting highly efficient proof-reading activity to preserve the genomic contents of the cell9, 10, 11. Similarly, in association with an array of extremely proficient molecular chaperones, a well-organized ribosomal quality control (RQC) machinery maintains the robustness of the cellular proteome12, 13, 14. Additionally, a specialized pathway of quality assurance of newly synthesized polypeptides (called ERAD) operates inside the endoplasmic reticulum and associated secretory pathways15. Several molecular chaperones and additional proteins get involved in these QC pathways, regulating the folding and degradation processes inside the cells and maintain a healthy and functional cellular proteome16,17. All the cellular proteins have their unique turnover rate regulated by the ubiquitin–proteasome system (UPS) that involves a few hundred E3 ubiquitin ligase enzymes to provide the substrate specificity18,19.
Under some physiological conditions, the E3 ubiquitin ligases, along with few other adapter proteins, may take part in identifying and redirecting aberrant or aggregated forms of intracellular proteins to another proteolytic pathway, called autophagy, which is not as specific as UPS and is chiefly take part in the degradation of the bulk of cellular debris20,21. Similarly, heat shock proteins (HSPs) or molecular chaperones also play crucial roles in the triage of polypeptides inside the cytoplasm by switching among different quality control pathways. Here, we are providing a very brief outline of these major QC pathways in this section. An intracellular overview of these significant components of the cellular QC pathways is presented in Fig. 1.
Figure 1.
A eukaryotic cell showing the major cellular components constituting the cellular protein quality control machinery. Molecular chaperones are the immediate interactors of the newly synthesized proteins, which help them to achieve their natural active conformation and provide additional opportunities during lifetime to attain the same if they lose their native conformation. The UPS is comprised of three primary classes of enzymes: E1 ubiquitin-activating enzymes, in an ATP-dependent manner, activate small ubiquitin molecules that are later conjugated to E3 ubiquitin ligases by E3 conjugating enzymes. E3 ligases provide substrate specificity to transfer these conjugated ubiquitin molecules to the aberrant proteins, unfolded, misfolded, or aggregated inside the cytoplasm and other cell compartments. The ubiquitinated proteins are subjected to proteasomal degradation. Autophagy is a bulk degradation system in which a large amount of cytoplasmic waste material is packaged in membranous structures. The membrane-bound waste material is subjected to degradation by lysosomal proteases, which is accomplished by the fusion of the membranous vesicles to the lysosomes.
2.1. Molecular chaperones
Proteins are large (macro-) molecules inside the cells, which orchestrate most of the physiological and metabolic tasks and are inclusively involved in the structural organization of the cellular components. Therefore, the maintenance of their native conformations is a prerequisite for the cells to be healthy. Such a condition of a stable and healthy set of proteins is called proteostasis22,23. Chaperones are the first line of molecules that start their work immediately after the newly synthesized peptide exits from the ribosome24. Different classes of molecular chaperones have already been reported in various forms of life across different kingdoms, including prokaryotes and eukaryotes25,26. The de novo folding of nascent polypeptides is orchestrated by family chaperones and is accomplished by multiple cycles of ‘binding and release’ in an energy-dependent manner27,28. Folding of a proportion of proteins is governed by HSP70 and HSP40, whereas the rests of the proteins are transferred to HSP90 proteins29.
These chaperones are also implicated in refolding and disaggregating aberrantly folded polypeptides, or unfolding and degrading aggregated proteins30,31. In fact, a large number of chaperones and chaperonins are coherently involved in the folding, refolding, and disaggregation processes of all the cellular proteins32,33. Chaperones can guide the substrate proteins towards two well-established systems of proteins degradation, i.e., UPS and autophagy34. They may interact with crucial proteins implicated in these two pathways, e.g., sequestosome-1 (SQSTM1/P62), BCL2 associated athanogene 1 or 3 (BAG1/3), carboxy-terminus of heat shock cognate 70 (HSC70) interacting protein (CHIP), next to BRCA1 gene 1 (NBR1), and several E3 ubiquitin ligases35,36. The mechanisms that are driven by chaperones in concerted action with the other pathways are chaperone-mediated autophagy (CMA), chaperone-assisted selective autophagy (CASA), and chaperone-assisted proteasomal degradation (CAP)37,38.
2.2. Autophagy
The idea of autophagy originated in the 1960s when Christian de Duve identified lysosome, an organelle that contains hydrolytic enzymes, and got involved in removing cytoplasmic waste materials39,40. Nobel Prize in Medicine to Christian De Duve in 1974, and Yoshinori Ohsumi in 2014 for the discovery of the lysosome and detailed investigation of this degradation pathway confirm the importance of the autophagy machinery for the cells. This lysosomal degradation process targets not only the damaged organelles but also different forms of cellular proteins, either ubiquitylated or non-ubiquitylated41,42. Multiple lysosomal degradation pathways have been identified in the past with different roles and specificities; for example, the formation of a double-membrane bound structure, called the autophagosome, is a characteristic of macroautophagy that engulfs a large amount of cellular debris along with bulky proteinaceous inclusions43,44.
Aggrephagy is often used to describe selective targeting of bulky protein aggregates or inclusion bodies for degradation through macroautophagy in a process facilitated by adapter proteins, like P62 and NBR1 and light chain 3 (LC3), a phagophore membrane receptor45,46. A double-membrane structure called autophagosome is formed as a result of the closure of phagophore, which is followed by fusion with late endosomal vesicle or lysosomal sacs47,48. The contents within this newly formed structure, referred to as amphisome, are degraded by various lysosomal enzymes49,50. Similar to aggrephagy, few other pathways of selective degradation of cytoplasmic proteins are orchestrated by cytosolic chaperones HSC70 along with its regulatory co-chaperones51,52. For example, microautophagy involves selective transport of cytosolic proteins to vesicles using endosomal sorting complexes required for transport (ESCRT I and III) in the HSC70-dependent manner53,54. However, the microautophagy pathway involves invagination and tube formation, followed by vesicle expansion and degradation55,56.
Another highly selective proteolytic pathway is CMA that could be defined as a process of selective identification of the KFERQ motif-containing cellular proteins by HSC70 and co-chaperones57,58. The HSC70-conjugated substrates are internalized after binding to LAMP-2a (a lysosome-associated membrane protein) and later degraded by membrane-bound proteases59,60. BAG3-mediated selective degradation pathway, CASA is also governed by chaperones HSC70 and HSPB8, in concerted action with CHIP (an E3 ligase) that mediates the ubiquitination of the proteins before their disposal to the lysosomal compartment in a P62-dependent manner61,62.
2.3. Ubiquitin–proteasome system (UPS)
The ubiquitin–proteasomal pathway is a multistep process of protein degradation, in which a series of enzymes sequentially catalyze the substrate proteolysis inside a large barrel-shaped, cylindrical protein complex called proteasome63,64. The 26S proteasome is a multi-subunit complex containing a 20S core particle and one or two regulatory 19S sub-particles to regulate the entry of the ubiquitylated chains into the core65,66. The 20S core proteasome subunit contains three types of protease activities governing the cleavage of incoming polypeptides into smaller fragments67,68. Out of four heptameric rings forming the core, two inner rings, termed β-rings, contain the proteolytic activities of different types: post-glutamyl peptide hydrolase (β1), trypsin (β2), and chymotrypsin (β5)69,70. In the first ATP-dependent step, an E1 ubiquitin-activating enzyme activates the small 8 kDa ubiquitin molecule (Ub) and forms a thioester bond71,72. A transacylation reaction transfers this ubiquitin to the thiol group present on another class of enzymes called E2 ubiquitin-conjugating enzyme73,74. These thiol esters (ubiquitin-E2 conjugates) provide ubiquitin molecules to the third class of enzymes called E3 ubiquitin ligases for tagging the substrate proteins73,75. The C-terminus glycine of the ubiquitin polypeptide forms an isopeptide bond with one of the lysine residues present on the cellular proteins76.
According to the long-standing notion, attachment of single ubiquitin (monoubiquitination) generally does not target substrate proteins for proteolytic pathways; however, recent advancements also oppose this belief77. In addition, more than one ubiquitin molecules might get attached to the substrate proteins, independently (multi-monoubiquitination) or one over the other (polyubiquitination) through lysine residues present in the already conjugated ubiquitin or the N terminal methionine residue of the ubiquitin78. This may result in an array of signals, and ubiquitin codes interpreted and dealt in different manners by cellular subsystems79,80. The patterns of attachment of subsequent ubiquitin moieties may govern differential fates of the targeted proteins. For example, a Lys-63 linked ubiquitin chain preferably directs the proteins towards autophagic degradation81,82. Contrarily, highly abundant K-48 linked polyubiquitin chains are majorly targeted for proteasomal degradation83. Other ubiquitin chains formed with K6, K11, K27, K29, and K33 linkages form different kinds of signals and regulate multiple physiological processes, including cell cycle control, cellular transport, and DNA repair84, 85, 86.
Altogether, the involvement of the UPS has been reported in immune pathways, hormonal signaling, cellular metabolism, apoptosis, etc.19,87. Considering the coexistence of all these proteolytic processes inside the eukaryotic cells, we can assume that maintenance of proteostasis requires a very tightly regulated coordination between different components and arms of the cellular protein quality control88,89. Their involvement in the pathologies of cancer, neurodegeneration, and aging processes has led scientists to identify their therapeutic potential and devise methods or ways to modulate them for exploitation for remedial purposes. Natural molecules have remained a primary therapeutic tool over the years showing enormous potential to modulate crucial regulatory proteins inside the cells. Several reports over the past few years, as shown in Table 2, have been published describing various kinds of possible regulation of different UPS components, which ultimately govern many disease-associated pathways.
Table 2.
Small natural compounds having chaperone-modulating activities. A broad array of natural molecules have been identified over the years, which can enhance or suppress the cellular chaperoning activity by elevating the expression or interfering with the functioning of major chaperones belonging to HSP70, HSP90, small HSPs or co-chaperones.
| Compound | Source | Target protein | Target disease | Model system | Ref. |
|---|---|---|---|---|---|
| Inducers of chaperone machinery | |||||
| Actinomycin D | Streptomyces parvullus | HSP70 | Huntington's disease | S. cerevisiae | 42 |
| Celastrol | Tripterygium wilfordii | HSF1, SSA3/4 | Stress response | S. cerevisiae | 43 |
| Compound A | Salsola tuberculatiformis | HSP70 | Inflammation | A549 cells | 44 |
| Curcumin | Curcuma longa | HSF1, HSP70 | Stress response | C6 cells, rats | 45 |
| Geldanamycin | Streptomyces spp. | HSP70 | Neurodegeneration | H4 cells | 46 |
| Glycyrrhizin | Glycyrrhiza glabra | HSP70 | Stress response | HeLa cells | 47 |
| Handelin | Handelia trichophylla | HSP70 | Neuroinflammation | BV2, HEK293T | 48 |
| Lanosterol | Metabolic intermediate | CHIP | Neurodegeneration | Cos7 cells | 49 |
| Myricetin | Fruits and berries | HSP70, HSF1 | Neurodegeneration | Cos7 cells | 50 |
| Paeoniflorin | Paeonia lactiflora | HSF1, HSP70 | Stress response | HeLa cells | 47 |
| Prostaglandins | Human | HSF1, HSP70 | Stress response | C6 cells | 51 |
| Withaferin A | Withania somnifera | HSP25, HSP70 | ALS | Mice | 52 |
| HSP90 inhibitors | |||||
| Argenteoside A | Tabebuia argentea | HSP90 | Epithelial carcinoma | HeLa cells | 53 |
| Celastrol | Tripterygium wilfordii | HSP90 | Prostate cancer | LNCaP cells | 54 |
| Clorobiocin | Streptomyces spp. | HSP90 | Breast cancer | SKBR3, MCF7 | 55 |
| Coumermycin A1 | Streptomyces spp. | HSP90 | Breast cancer | SKBR3, MCF7 | 55 |
| Cruentaran A | Byssovorax cruenta | HSP90 | Lung, breast cancer | A549, MCF-7 | 56 |
| Curcumin | Curcuma longa | HSP90 | Viral infection | HELF cells | 57 |
| Deguelin | Derris trifoliata | HSP90 | Cancer | Mice | 58 |
| Derrubone | Derris robusta | HSP90 | Breast cancer | SKBR3, MCF-7 | 59 |
| EGCG | Camellia sinensis | HSP90 | Hepatoma | HePa, HspG2 | 60 |
| Gambogic acid | Garcinia harburyi | HSP90 | Cancer | SKBR3, MCF7 | 61 |
| Gedunin | Azadirachta indica | HSP90 | Prostate cancer | LNCaP cells | 54 |
| Geldanamycin | Streptomyces spp. | HSP90, HSF1 | Cancer | 3T3 cells | 62 |
| Herbimycin A | Streptomyces spp. | HSP90 | Cancer | 3T3 cells | 62 |
| Hypericin | Hypericum spp. | HSP90 | Squamous carcinoma | SQ2 cells | 63 |
| Kotschyn D | Pseudrocedrela kotschyi | HSP90 | Prostate cancer | PC-3 cells | 64 |
| Lentiginosine | Astragalus lentiginosus | HSP90 | Cancer | In silico | 65 |
| Macbecin | Actinomyces spp. | HSP90 | Prostate, lung cancer | DU145, H460 | 66 |
| Monocillin I | Monocillium nordinii | HSP90 | Breast cancer | MCF-7 cells | 67 |
| Novobiocin | Streptomyces niveus | HSP90 | Breast cancer | SKBR3, MCF-7 | 55 |
| Pochonins | Pochonia chlamydosporia | HSP90 | Cancer | In vitro | 68 |
| Radicicol | Monosporium bonorden | HSP90 | Cancer | NIH3T3 cells | 69 |
| Sansalvamide A | Fusarium spp. | HSP90 | Colon cancer | HCT-116 | 70 |
| Withanolides | Withania somnifera | HSP90, HSF1 | Thyroid cancer | DRO, NPA cells | 71 |
| Quercetin | Fruits and berries | HSP90, HSF1 | Breast cancer | HeLa | 72 |
| Triptolide | Triptergium wilfordii | HSP90 | Cancers | HeLa cells | 73 |
| HSP70 inhibitors | |||||
| Apidaecin | Insect peptides | DNAK, GROEL | Microbial infection | E. coli | 74 |
| Cantharidin | Epicauta funebris | HSP70 | Colorectal cancer | HCT-116 cells | 75 |
| Drosocin | Insect peptides | DNAK, GROEL | Microbial infection | E. coli | 74 |
| Fisetin | Fruits and berries | HSP70, HSF1 | Colorectal cancer | HCT-116 cells | 76 |
| Myricetin | Fruits and berries | DNAK | Proteostasis | E. coli | 77 |
| Novolactone | Fungal metabolites | HSP70 | Proteostasis | HCT-116 cells | 78 |
| Pyrrhocoricin | Insect peptides | DNAK, GROEL | Microbial infection | E. coli | 74 |
| Quercetin | Fruits and berries | HSP70 | Lung cancer | A549, H460 cells | 79 |
| adaSGC | Human | HSP70 | Proteostasis | BHK cells | 80 |
| Spergualin | Bacillus subtilis | HSC70 | Immune reaction | Jurkat cells | 81 |
| Triptolide | Triptergium wilfordii | HSF1, HSP70 | Cancers | HeLa, HEK293T | 82 |
| Tubocapsenolide A | Tubocapsicum anomalum | HSP90-HSP70 | Breast cancer | MDA-MB-231 | 83 |
3. Pathological conditions affected by altered protein quality control
Aging, neurodegeneration, and cancer have always remained significant challenges before the scientific community. Many theories and hypotheses have been formulated and postulated to explain these pathologies, but none has succeeded in understanding why these pathological changes occur. Genetic, environmental, infections and metabolic alterations are among the many possible reasons behind most proteopathies90,91. However, none of these could solely be held responsible for pathological conditions; instead, a blend of multiple factors contribute towards a highly diverse disease condition. This diversity among the individual cases of these pathologies further complicates the research processes and leads to failure of treatment options92, 93, 94. However, in the past few decades, tremendous progress is observed in our understanding of many of these pathologies. At the same time, these advancements have led to the evolution of multiple lines of research methodologies and approaches to understand a given problem. This has given rise to speculations and multiple lines of theories behind the origin, development, sustenance, and progression of these pathologies.
The declined competence of cellular defense mechanisms and pathways are suggested to be one such notion that has attained wide acceptance in recent decades4,5. Inefficient functions of quality control systems that regularly monitor the well-being of the genomic and proteomic repertoire of the cells could be a possible cause of instigating multiple pathways leading towards aging1. The compromised capacity of molecular chaperones to fold the nascent polypeptides into the proper three-dimensional shape and deficient functioning of autophagy and the proteasomal system could be credited for over-burdening the cytoplasmic milieu with misfolded proteins95,96. Aggregation of multiple types of aberrant proteins could lead to the formation of large perinuclear/cytoplasmic inclusion bodies that may further mount a heightened reaction by initiating immunological responses97. The increased burden of the aggregates may lead to increased neuronal deaths, as observed in many disease models of neurodegeneration98,99.
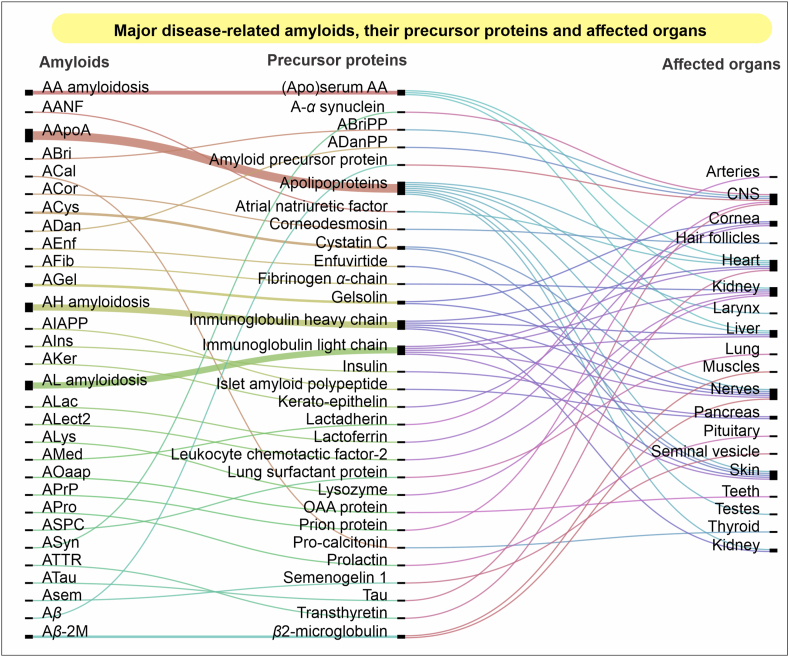
Aging encompasses several other attributes or hallmarks, which may include but is not limited to the genomic instability, mitochondrial loss, telomere shortening, metabolic alterations, etc.91,100. These pathways and alterations in their physiological conditions are also among the crucial factors responsible for most types of cancers101,102. Altogether, the conditions discussed above have many common features. One of the similarities is the compromised proteostasis caused due to the inefficient protein folding and degradation in cells103,104. Many other diseases, like diabetes, cataract, cystic fibrosis, myopathies, etc. are directly affected by the aggregation of one or more proteins22,105. An imbalanced proteostasis may directly or indirectly link with many other life-threatening diseases associated with lungs, heart, liver, kidneys, etc.19,106. Based on the recommendations made by the International Society of Amyloidosis, a depiction of various amyloidogenic proteins, their aggregatory forms, and the affected organs in many associated diseases is presented in Fig. 2107, 108, 109. However, drawing a common line across all these diseases would be difficult at the present state of our understanding of these intracellular systems. Based on their common connecting links, i.e., perturbed proteostasis and the cellular PQC machinery, various strategies have been postulated in the past, while some are currently under trial.
Figure 2.
An overview of amyloidosis. Various amyloid-forming proteins (left), their normal precursor protein forms (middle), and tissues or organs affected in one or more similar diseases caused by individual proteins106,107. The left column shows a list of amyloidic forms of various proteins shown in the center as precursors. These proteins may aggregate in such amyloidic structures in their full, cleaved, or modified forms, while several mutations contribute to their amyloidogenicity. The structural modification of these proteins may lead to abnormal metabolic or signaling alterations at the molecular level in different tissues and organs. These changes may lead to a possible functional loss or decline, causing multiple pathological conditions. Many proteins are found to be involved in multiple diseases of different organs, whereas some diseases may have several proteins involved together in the pathogenesis. The figure was prepared using RAWGraphs, an open source platform for data representation (http://rawgraphs.io)108.
4. Small natural molecules: An effective therapeutic armory targeting severe pathological conditions
Humans have learned the art of extraction and effectively utilizing naturally occurring bioactive components and chemical molecules towards medical purposes for centuries. Many groundbreaking discoveries about the inherent medicinal properties of natural compounds against numerous life-threatening diseases have been awarded Nobel Prizes in the past. The mid-nineteenth century discoveries of antibiotics penicillin and streptomycin have thoroughly changed the idea of drug discovery and accelerated the pursuit of more such compounds for other medicinal purposes in the following decades. Technological advancements, the inclusion of computational approaches, and the reincarnation of the vast literature of ancient Indian and Chinese medicine have substantially assisted and overwhelmed the field of drug discovery. The recent Nobel Prize for recognizing the medical importance of avermectins and artemisinin has again pressed upon the hidden potential of the small molecule-based drug substances.
In previous sections, we have discussed how the formation of inclusion bodies follows an aberrant protein aggregation. The inefficiency of cellular QC mechanisms to fight back and address the loss of proteostasis-like conditions may lead to an array of systemic and non-systemic diseases103. With the available knowledge and experimental evidence, it could be understood that reestablishing the lost activities of these pathways, by inducing chaperone function or enhancing the activities of proteolytic machinery (proteasome and autophagy), etc., may exhibit the tremendous potential to delay the onset of pathologies or aging-associated changes inside the organisms110. Results from many studies converge towards the consensus advocating for small molecule-based therapies as beneficial and low-cost strategic tools to suppress the aggregation of most kinds of disease-associated amyloidogenic aggregates111. Notably, many recently recognized molecules, termed as pharmacological chaperones, have a strong potential of precisely facilitating the folding and stabilization of aberrant proteins, thereby assist in restoring their native functions112,113.
The bulk degradation pathway of the cells, termed as ‘autophagy’ soon after its discovery by Christian de Duve, derived its name from Greek words meaning self-eating114. In later years, it was found that the autophagic degradation, which was initially considered a non-specific degradation system of the cells, could also be a part of much-targeted protein degradation pathways in association with chaperones or UPS components115,116. It joins hands with the UPS and plays a balancing act of degradation with the protein synthesis and folding machinery concurrently working in the cells89,117. Many studies also suggest that autophagy and proteasome pathways may also compensate each other under different stress conditions; therefore, a few drugs suppressing UPS activity, e.g., MG132 and lactacystin, may lead to activation of autophagic responses118, 119, 120. Contrarily, inhibition of autophagy overwhelms the cells with accumulating protein inclusions causing impairment of proteasomal degradation121. In truth, a clear understanding of how the two systems balance each other while protecting the cells from proteotoxic stresses is not known. Here, a comprehensive overview is provided for those molecules or drug candidates, which can bind and modulate the activity or functions of one or multiple cellular PQC machinery components.
4.1. Modulating cellular chaperoning potential: Provides additional buffer against stress conditions
Chaperones are essential regulators of cell homeostasis, and their anomalous functioning can lead to perturbance of many normal and stress-related pathways. The primary functions that the HSP family proteins perform inside the cells are recognizing any unusual change in the cellular homeostasis, encountering spontaneous stress condition, and providing a piece of machinery to monitor and establish the structures and functioning of other cellular proteins. The term ‘chemical chaperone’ has been widely used in the past decade for a group of potentially active molecules that can stabilize cellular proteins in a non-specific way and help in reversing the mislocalization or aggregation122,123. These molecules mostly act on the proteins' active domains or sites, providing them an increased opportunity to form hydrogen, electrostatic, and van der Waals interactions and potentially stabilize the overall structure of the proteins124. Additionally, an array of naturally occurring substances and their derivatives have shown modulatory potential over inherent chaperoning capacity inside the cells125.
These bioactive chemical molecules can bind and alter the structure, activity, and overall functions of the most active HSP70 and HSP90 chaperone complexes, along with many of their co-chaperones and accessory factors126,127. They also provide cushion for structural rearrangements of unfolded or misfolded proteins inside the cytosol, thus help in ameliorating the accumulation of aberrant proteins. However, the initial attempts to exploit chaperones for therapeutic purposes started with identifying the inhibitory activity of radicicol against HSP90 ATP-binding pockets128,129. It was initially used against malignant fibroblasts. Although promising, the drug failed in delivering the promises because of several pharmacokinetic challenges. The other prominent molecule in this category is a bacterial isolate geldanamycin that was later proved to be toxic to the liver130. In later years, advancements in the medicinal chemistry tools have led to the synthesis of many derivatives of these less successful drug candidates, e.g., monocillin I, pochonins, 17-allylamino-geldanamycin (17-AAG), etc.131, 132, 133.
Small molecules can shatter the interaction of major chaperones with their co-chaperones, thereby affecting chaperoning activities. For example, celestrol, a triterpene, and gambogic acid, a xanthonoid, can interfere with the interaction of HSP90 with its co-chaperone CDC37; while curcumin blocks HSP90–P23 binding, leading to the induction of cell death signaling pathways134,135. Other drug candidates with similar cell death-inducing effects are herbimycin A and derrubone130,136. Quercetin, one of the most studied flavonoids, shows an upstream regulation of heat-shock response inside the cells by suppressing the heat shock factor (HSF1), the major transcription factor that regulates the intracellular levels of most of the chaperones137. A green tree extracted molecule, epigallocatechin-3-gallate (EGCG), can also inhibit multiple chaperones, including HSP90, HSP70, and ER-resident GRP78, and suppress the growth of cancer cells138,139. Interestingly, other mechanisms of functional suppression of HSP90 are increased ubiquitination (by hypericin), destruction of chaperone cycle (by sansalvamide A), and oxidation (by tubocapsenolide A) of HSP90 itself140, 141, 142. All these can interfere with the turnover of the substrate proteins of the chaperones, thus deregulating the proteostasis balance of the cell.
Modulation of HSP70 functions by myricetin and spergualin may also help suppress cancerous cells’ growth, possibly by inhibiting the ATPase activity of the chaperone143,144. Few reports further suggest the possible activation of upstream regulator HSF1 in response to drug-mediated suppression of one or the other molecular chaperones; however, more work is required to understand the feedback mechanisms involved in this mechanism145. A few studies have shown that geldanamycin-mediated HSP90 inhibition may, in turn, upregulate the activities of HSP70 and HSP40, which could be helpful and may benefit the neuronal cells under different stress or pathology conditions, e.g., HD, ALS, cerebral ischemia, etc.146, 147, 148, 149, 150. Similarly, treatment of curcumin and withaferin A may also exert neuroprotective effects on the cells and mouse models; the effects could be due to improved activities of HSP70, HSP27, and α-crystallin chaperones151,152. A summarized overview of various such kinds of molecules of natural origin that can help in reestablishing the proteostasis inside the cells by modulating the inherent chaperoning capacity of the cell has been presented in Table 3.
Table 3.
Natural molecules affecting the activities and functions of the proteasome. These molecules have been identified as the putative modulators, including inducers and inhibitors, of the three known protease activities, including chymotrypsin, trypsin, and caspase-like specificity of the proteasome subunits. The associated diseases and studied model systems are also presented in adjacent columns.
| Compound | Source | Subunit | Pathway/disease | Model system | Ref. |
|---|---|---|---|---|---|
| Proteasomal inducers | |||||
| Betulinic acid | Betula sp. | β5 | Neurodegeneration | MT4 cells | 84 |
| Canthin-6-one | Ailanthus altissima | β5 | Parkinson's disease | Mice | 85 |
| Fatty acids | Animal sources | β1 | Ageing | Rats | 86 |
| Harmine | Peganum harmala | β1, β2, β5 | Parkinson's disease | Mice | 87 |
| Heparin | Animal sources | β2 | Ageing | Human erythrocytes | 88 |
| Lysophospholipids | Animal sources | β5 | Acrosome formation | Sea urchin sperm | 89 |
| Oleuropein | Olea europea | β1, β2, β5 | Ageing | IMR90, WI38 cells | 90 |
| Oxyphylla A | Alpinia oxyphylla | β5 | Parkinson's disease | Mice | 91 |
| Sulfatides | Animal sources | β5 | Ageing | Human erythrocytes | 88 |
| Sulforaphane | Brassica oleracea | β1, β2, β5 | Neurodegeneration | Mice | 92 |
| Zerumbone | Zingiber zerumbet | β5 | Neurodegeneration | Hepa1c1c7 cells | 93 |
| Proteasomal inhibitors | |||||
| Microbial sources | |||||
| Aaptamine | Aaptos suberitoides | β1, β5 | Cancer | HeLa cells | 94 |
| Aclarubicin | Streptomyces galilaeus | β5 | Cancer | Bovine pituitary | 95 |
| Agosterol C | Spongia sp. | β5 | Cervical carcinoma | HeLa cells | 96 |
| Antiprotealide | Salinispora tropica | β5 | Multiple myeloma | RPMI 8226 cells | 97 |
| Argyrin A | Archangium gephyra | β1, β2, β5 | Cancer | HeLa, SW480 cells, mice | 98 |
| Belactosin A/C | Streptomyces sp. | β5 | Muscle wasting | Rats | 99 |
| Carmaphycin-17 | Symploca sp. | β1, β5 | Trichomoniasis | Trichomonas vaginalis | 100 |
| Ciclosporine A | Tolypocladium inflatum | β5 | Inflammation | RAW, murine brain | 101 |
| Cinnabaramides | Streptomyces sp. | β5 | Cancer | PBMC cells | 102 |
| Cystargolide A | Kitasatospora cystarginea | β5 | Cancer | Purified 20S proteasome | 103 |
| Dibromophakellin | Phakellia flabellata | β1, β5 | Cancer | HeLa cells | 104 |
| Eponemycin | Streptomyces sp. | β5 | Murine thymoma | EL4 cells | 105 |
| Epoximycin | Actinomycetes sp. | β2, β5 | Inflammation | HUVEC cells | 106 |
| Fellutamide B | Penicillium fellutanum | β5 | Nerve injury | Mouse fibroblasts | 107 |
| Glidobactins | Polyangium brachysporum | β2, β5 | Cancer | Phaseolus vulgaris | 108 |
| Gliotoxin | Aspergillus fumigatus | β5 | Cancer | HeLa cells | 109 |
| Halicyclamine B | Haliclona sp. | β1, β2, β5 | Cancer | HeLa cells | 110 |
| Heteronemin | Hyrtios sp. | β2, β5 | Leukemia | K562, Jurkat T cells | 111 |
| Lactacystin | Streptomyces lactacystinicus | β1, β2, β5 | Neuroblastoma | Neuro2a | 112 |
| Lovastatin | Pleurotus ostreatus | β5 | Breast cancer | MDA-MB-157 cells | 113 |
| Marizomib | Salinospora sp. | β5 | Colon carcinoma | HCT-116 | 114 |
| Mevastatin | Penicillium citinium | β5 | Neuroblastoma | NBP2 cells | 115 |
| Mycalolides | Mycale sp. | β5 | Melanoma | B-16 cells | 116 |
| Omuralide | Streptomyces sp. | β5 | Neuroblastoma | Neuro2a | 112 |
| Palau'amine | Stylotella agminata | β5 | Cancer | HeLa cells | 104 |
| Petrosaspongiolide M | Petrosaspongia nigra | β1, β5 | Inflammation | THP cells | 117 |
| Rhabdastrellic acid-A | Rhabdastrella globostellata | β2, β5 | Leukemia | HL-60 cells | 118 |
| Syringolins | Pseudomonas syringae | β1, β2, β5 | Cancer | Phaseolus vulgaris | 108 |
| TMC-95 | Apiospora montagnei | β1, β2, β5 | Cancer | HCT-116, HL-60 cells | 119 |
| Tetradehydrohalicyclamine B | Acanthostrongylophora ingens | β1, β2, β5 | Cancer | HeLa cells | 110 |
| Tyropeptin A | Kitasatospora sp. | β2, β5 | Cancer | PC-12 cells | 120 |
| Plant products | |||||
| Ajoene | Allium sativum | β2, β5 | Leukemia | HL-60 cells | 121 |
| Apigenin | Portulaca oleracea | β5 | Breast cancer | MDA-MB-231, mice | 122 |
| Bisbibenzyls | Bryophytes | β5 | Prostate cancer | LNCaP cells | 123 |
| Capsaicin | Capsicum annuum | β1, β2, β5 | Prostate cancer | PC-3 cells | 124 |
| Celestrol | Tripterygium wilfordii | β5 | Prostate cancer | PC-3 cells, mice | 125 |
| Chrysin | Passiflora caerulea | β2, β5 | Cancer | HepG2, HL-60, A549 | 126 |
| Curcumin | Curcuma longa | β1, β2, β5 | Cancer | Neuro 2a cells | 127 |
| Catechin-gallate | Camellia sinensis | β5 | Cancer | Jurkat T cells | 128 |
| Emodin | Rheum palmatum | β1, β2, β5 | Cancer | HeLa cells, mice | 129 |
| Fangchinoline | Stephania tetrandra | β1 | Prostate cancer | LNCaP, PC-3 cells | 130 |
| Genistein | Glycine max | β5 | Cancer | LNCaP, MCF-7 cells | 131 |
| Ginsenosides | Panax ginseng | β5 | Cancer | Pig RBCs | 132 |
| Isoginkgetin | Ginkgo biloba | β1, β2, β5 | Cancer | HeLa cells | 133 |
| Kaempferol | Fruits and vegetables | β5 | Leukemia | Jurkat T cells | 134 |
| Luteolin | Cichorium endivia | β2, β5 | Cancer | HepG2, HL-60, A549 | 126 |
| Marchantin M | Marchantia sp. | β1, β5 | Prostate cancer | PC-3 cells | 135 |
| Myricetin | Fruits and vegetables | β5 | Leukemia | Jurkat T cells | 134 |
| Pectolinarin | Cirsium chanroenicum | β1, β5 | Tuberculosis | M. tuberculosis | 136 |
| Physalin B | Physalis angulata | β1, β2, β5 | Colon cancer | DLD-1 cells | 137 |
| PMI5011 | Artemisia dracunculus | β1, β5 | Diabetes | C2C12 cells, mice | 138 |
| Pristimerin | Maytenus ilicifolia | β5 | Prostate cancer | PC-3 cells, mice | 139 |
| Quercetin | Aesculus indica | β1, β2, β5 | Atherosclerosis | Rabbits | 140 |
| Resveratrol | Vitis viniferae | β5 | Neurodegeneration | N27 cells | 141 |
| Tannic acid | Caesalpinia spinosa | β5 | Cancer | Jurkat T cells | 142 |
| Vinblastine | Vinca rosea | β1, β2, β5 | Leukemia | HL-60 cells | 143 |
| Withaferin A | Withania somnifera | β5 | Prostate cancer | LNCaP cells, mice | 144 |
| Other natural compounds | |||||
| Arenobufagin | Toad venom | β1, β2, β5 | Cervical carcinoma | HeLa cells | 145 |
4.2. Regulating the UPS components: Playing with the fine balance
UPS is the next line of defense in most subcellular compartments and works continuously to regulate the proteostasis inside these organelles75. As described previously, ubiquitination and proteasomal degradation are a kind of intracellular regulatory mechanisms that often is crucial for many cellular pathways. Therefore, any disturbances in these systems may have deleterious effects on cellular health153. The proteasomal system comprises several components that could be regulated by different mechanisms and may exert varying effects on cellular physiology. For example, regulating the activities of proteasomal subunits has been shown to have a direct effect on the overall cellular protein degradation scheme and the overall proteostasis19. Many proteasome modulators have been proposed, and a few of them are under clinical trials for diseases like cancer and neurodegeneration154,155. A plethora of naturally-derived chemicals has been reported over the years, which have shown a substantial modulation of the activities of various enzymes of the pathway. Thus, their use may enhance or suppress the proteostasis provided by these enzymes19,156.
The proteasomal system is very specific in its activity and takes part in the precise regulation of the majority of physiological pathways; therefore, very tightly-controlled modulation is needed in order to exploit it for therapeutic purposes157,158. Bortezomib was the initial drug having the proteasomal inhibitory potential and has been widely used as an anticancer drug for long159. Later, another synthetic molecule, carfilzomib, was also approved by the U.S. Food and Drug Administration (FDA) for anti-cancer therapy160. Following the identification of these two FDA approved drugs, many other drugs with similar inhibitory activity against different proteolytic subunits (β1, β2, and β5) of 20S proteasome have been identified and thoroughly investigated for their therapeutic applications in many diseases161,162. Lactacystin is the most well-known natural molecule of this class that was initially reported to be effective against neuroblastoma cells and is currently one of the widely used drugs in the research163. Eponemycin and epoximycin specifically target chymotrypsin-like activity containing β5 subunits of the 20S core and help in suppressing the inflammation in cancer cells164,165. Mevastatin, belactosin A, and fellutamide B are other similar bacterial isolates that have been presented with the anti-protease activity of the proteasome in different experimental model systems166, 167, 168.
Fungi and marine animals are other prominent sources of many biologically active molecules having critical therapeutic properties. Many proteasomal inhibitors have been isolated from these animals also. For example, gliotoxin and cyclosporine A from fungal sources and agosterol C and aaptamine from sponges are prominent inhibitors of 20S proteases169, 170, 171, 172. These molecules could affect one or the multiple protease subunits of the 20S core particle of the proteasome. Interestingly, the toad venom contains a compound called arenobufagin that has the potential to inhibit all three activities simultaneously173. An exhaustive list of such natural molecules obtained from various biological sources has been presented in the form of Table 3. Plant-based molecules have specifically dragged lots of attention for their proteasome-modulatory activity and have been widely covered in other descriptive reviews156,174.
Flavonoids make the most comprehensively explored class and have shown tremendous potential to be used in therapeutics against many diverse kinds of diseases. For example, genistein, EGCG, and physalin B have anti-cancerous roles, while pectolinarin has positive effects on tuberculosis due to its anti-inflammatory potential175, 176, 177, 178. Apigenin, myricetin, quercetin, and luteolin are anti-atherogenic and may also help suppress tumor growth179, 180, 181, 182. PMI5011 is an ethanolic preparation obtained from a herb, Artemisia dracunculus, and shows pathological improvements in diabetes mice183. Polyphenols like vinblastine, capsaicin, resveratrol, tannic acid, and curcumin184, 185, 186, 187, 188, along with some well-known terpenoids, e.g., celestrol, pristimerin, etc., further adds up to the list189,190. The compounds like anthraquinones, saponins, sulfur-derivatives, and plant-derived lactones come next into this long list (Table 3) of compounds with different types of inhibitory potential against β1, β2, or β5 activities of proteasome.
Contrary to proteasomal suppression, which is widely exploited in cancer therapeutics, enhancing the proteasomal activities could be useful in many stressful conditions and in the diseases associated with protein misfolding and aggregation. Two widely explored terpenoids, zerumbone and betullinic acid, have activated the β5 activities and thus presented neuroprotective effects191,192. Myricetin, oleuropein, and sulforaphane are other plant-derived molecules representing the proteasomal activators that may upregulate one or multiple 20S core subunits193,194. Few other molecules were identified that might delay the aging and neurodegeneration processes by increasing proteasomal degradation of the substrate proteins. These are heparin, sulfatides, and lysophospholipids, a few metabolic byproducts or those obtained from other animal sources195,196. Unlike proteasome inhibition, the effects of proteasome activation are not widely explored and need a more rigorous investigation to identify new molecules with a positive effect on proteasome functioning and their downstream impact on protein clearance.
A few recent studies have given clear insights into Parkinson's disease models that activation of proteasome function by hermine, oxyphylla A, and canthin-6-one can significantly upregulate the clearance of alpha-synuclein, the major constituent of the Lewy bodies formed in the substantia nigra197, 198, 199. Apart from protease subunits of 20S particle, many other components involved in protein ubiquitination have been looked for their applicability as a possible drug target in aging, neurodegeneration, and many other diseases. Modulation of the major enzymes involved in the ubiquitination process, e.g., E1, E2s, E3s, and deubiquitinases (DUBs), could be a vital strategy to regulating several critical signaling and metabolism pathways19,200. E1 ubiquitin-activating enzyme is a unique protein required for the ubiquitination of all the possible cellular substrate proteins. Therefore, interfering with its activity may compromise the whole UPS and may have devastating effects71. However, this observation can be utilized in anticancer therapeutics as previously exemplified by hyrtioreticulins largazole, himeic acid A and panepophenanthrin201, 202, 203, 204.
The next line of drug targets is E2 ubiquitin-conjugating enzymes, which transfer ubiquitin molecules from the E1 enzymes to the E3 ligases. Not too many drugs have been identified, which can interfere with the enzymatic activities of E2; however, a few known naturally-occurring compounds are vitexin, a polyphenolic extract from Byrsonima crassifolia, and a few poriferan-derived leucettamol A, manadosterols A and B, etc.205, 206, 207. Deubiquitinases (DUBs) are a group of enzymes that are crucial for breaking down the ubiquitin chains, replenishing the ubiquitin pool of the cells, and playing regulatory roles in many biological pathways208,209. Betulinic acid and one curcumin analog are a few known inhibitors of this class of enzymes, which have shown tremendous promises as anti-cancer molecules210,211. Cruciferous vegetables have a group of compounds called isothiocyanates, which are prominent inhibitors of DUBs, and have shown significant anti-tumor properties212. A diterpenoid candidate, 15-oxospiramilactone, is another DUB inhibiting molecule that has a positive effect on the restoration of the mitochondrial network213.
Interestingly, the molecules that have the potency to modulate the most diverse class of enzymes of this pathway, the E3 ubiquitin ligases, has widely been explored for specific regulation of substrates and related pathways214. However, some molecules may inhibit multiple E3s simultaneously. Heclin is a recently developed molecule that can suppress many HECT domain-containing E3 ligases. Additionally, a few ubiquitin variants were prepared, which have shown tremendous inhibitory potential against RING and U-box domains of the E3 ligases215, 216, 217. A line of studies proposes several natural molecules as probable drug candidates against many life-threatening diseases. Inhibition of Mdm2 by matrine at the RNA level and by berberine via self-ubiquitination mechanism are prominent examples of regulating the turnover of P53, the primary tumor suppressor protein218,219. Oroxylin-A, apigenin, and genistein are plant flavonoids that may initiate a high apoptotic response in cancerous cells220, 221, 222. Many terpenoids (e.g., triptolide, inulanolide, etc.), saponins, chalcones, and polyphenols extracted from plants and other natural sources have also shown promising effects against cancerous cells by inhibiting the MDM2–P53 interaction and degradation of the tumor suppressor191,223,224.
Enhancing the functions of the anaphase-promoting complex (APC) by crosslinking CDC27 also exerts a similar effect by acting at the spindle assembly checkpoint of the proliferating cells225. Similarly, inducing the functioning of crucial E3 ligases like CHIP by lanosterol, a sterol molecule, as we found in our previous study, may help ameliorate the wide-spread proteotoxicity and related cellular deaths226. Enhancing the E3 ligase activities may elevate the clearance of accumulated proteins inside the cells, which could be a promising strategy against neurodegeneration. Recently, we found a similar effect of myricetin on the E6-AP and HSP70-mediated clearance of the substrate proteins227. Trehalose, an autophagy inducer, has also been shown to improve the clinical deficits caused by mutated CHIP in ataxia patient-derived fibroblasts228. Other studies from our group and possibly from many other labs are undergoing to identify other similar molecules with potency to modulate different E3 ubiquitin ligases so that specific molecular pathways could be targeted for disease therapeutics and drug development.
4.3. Natural modulators of autophagic pathway: Boosting the cellular stress response
The autophagic pathway was initially identified as an intracellular lysosomal degradation mechanism that targets consumed, unusable, or toxic cell material using protease enzymes present within membrane-bound organelles114. Autophagic clearance pathways may have many variants that select and degrade cellular proteins and debris differentially through varying mechanisms using multiple selections and targeting mechanisms using several adapters and membrane-bound receptor proteins55,229. In a way, this leads to a variety of opportunities to regulate these pathways of degradation at various points. An array of reports has shown that autophagy regulation using small natural molecules could also be achieved and used for drug discovery purposes229,230. Modulation of autophagic pathways is proposed for therapeutics against cancer and neurodegeneration in a large number of studies231,232. As shown in Table 4, different types of proteinopathies, neurodegenerative disorders, cancers, and several systemic diseases could be targeted by derivatives of natural molecules with modulatory effects on various effectors of the autophagy pathway. Several reports could still not be included in the present article due to space restrictions. The most prominent members of this class of natural autophagy inducers are resveratrol and trehalose233,234. Both these inducers have shown the tremendous potential of relieving neurons from various stresses by reducing free radicals and degrading protein aggregates234,235.
Table 4.
Small natural molecules affecting the cellular autophagy pathway. A concise representation of the potential candidates that can alter the autophagic flux, increase the protein degradation or interfere with different steps of autophagosome biogenesis or lysosome fusion, therefore can target specific molecular targets and pathways that are involved in many harmful diseases.
| Compound | Source | Target pathway | Physiological condition | Model system | Ref. |
|---|---|---|---|---|---|
| Autophagy inducers | |||||
| Marine/microbial products | |||||
| Actinonin | Streptomyces sp. | AMPK, mtRNA | Cancers | HeLa cells | 146 |
| Araguspongine C | Xestospongia sp. | PI3K/AKT/mTOR | Breast cancer | BT-474 cells | 147 |
| Chromomycin A2 | Streptomyces sp. | LC3 | Melanoma | MALME-3M cells | 148 |
| Clionamine B | Cliona celata | LC3 | Breast cancer | MCF-7 cells | 149 |
| Coibamide A | Leptolyngbya sp. | LC3 | Glioblastoma | U87-MG cells | 150 |
| Hirsutanol A | Chondrostereum sp. | LC3 | Hepatic carcinoma | Hep3B cells | 151 |
| Ilimaquinone | Hippospongia sp. | p53 | Colon cancer | RKO cells | 152 |
| Isoaaptamine | Aaptos sp. | LC3 | Breast cancer | T-47D cells | 153 |
| Monanchocin D | Monanhora pulchra | P38, ERK | Germ cell tumors | NCCIT cells | 154 |
| Ovothiol A | Paracentrotus lividus | Beclin-1, LC3 | Hepatic carcinoma | HepG2 cells | 155 |
| Papuamine | Haliclona sp. | LC3, JNK | Breast cancer | MCF-7 cells | 156 |
| Psammaplin A | Psammaplysilla sp. | P73 | Glioblastoma | U87-MG cells | 157 |
| Rapamycin | Streptomyces hygropicus | mTOR | Polyglutamine diseases | PC12, Cos7 cells | 158 |
| Rhabdastrellic acid A | Rhabdastrella sp. | AKT | Various human cancers | Hep3B, A549 cells | 159 |
| Salinosporamide A | Salinospora tropica | eIF2α | Prostate cancer | LNCaP-Pro5 | 160 |
| Stellettin B | Jaspis stellifera | PI3K/AKT/mTOR | Lung cancer | A549 cells | 161 |
| SD118-xanthocilin-X | Penicillium commune | MEK/ERK | Hepatic carcinoma | HepG2 cells | 162 |
| Trehalose | Streptomyces cerevisiae | mTOR | Neurodegeneration | SK-N-SH, PC12 cells | 163 |
| Urolithin A | Gut microbiome | AMPK | Ageing | C. elegans | 164 |
| Xestospongin B | Xestospongia exigua | IP3R | Cervical adenocarcin | HeLa cells | 165 |
| Plant products | |||||
| Terpenes | |||||
| Bigelovin | Inula helianthus | AKT/mTOR/S6K | Liver cancer | HepG2, mice | 166 |
| Eriocalyxin B | Isodon eriocalyx | AKT/mTOR/S6K | Breast cancer | MCF-7, MDA-MB-231 | 167 |
| Gossypol | Gossypium sp. | Beclin-1, ATG5, | Breast adenocarcinoma | MCF-7, HeLa cells | 168 |
| Grifolin | Albatrellus confluence | AKT/mTOR/S6K | Ovarian cancer | A2780, SKOV3 cells | 169 |
| Oridonin | Rabdosia rubescens | P21 | Prostate cancer | PC-3, LNCaP cells | 170 |
| Platycodin-D | Platycodon grandiflorum | PI3K/AKT/mTOR | Lung cancer | NCI–H460, A549 cells | 171 |
| Triptolide | Tripterygium wilfordii | SQSTM1, LC3 | Parkinson's disease | MN9D cells, rats | 172 |
| Ursolic acid | Ocimum sanctum | JNK, BCL-2 | Colorectal carcinomas | HCT-15 cells, mice | 173 |
| Flavonoids | |||||
| Ampelopsin | Ampelopsis sp. | AKT/mTOR/S6K | Breast cancer | MDA-MB-231, MCF-7 | 174 |
| Apigenin | Fruits, vegetables | mTOR, S6 | Leukemia | HL60, TF1 cells | 175 |
| Curcumin | Curcuma longa | FOXO1, beclin-1 | Oxidative stress | HUVEC cells | 176 |
| Delicaflavone | Selaginella doederleinii | AKT/mTOR/S6K | Lung cancer | A549, PC-9 | 177 |
| 5-Demethylnobiletin | Sideritis tragoriganum | JNK | Lung cancer | A549 and CL1-5 cells | 178 |
| Galangin | Alpinia officinarum | P53 | Hepatic carcinoma | HepG2 cells | 179 |
| Glabridin | Glycyrrhiza glabra | JNK1/2, P38, ERK | Hepatoma | Huh7 cells | 180 |
| Juglanin | Juglans mandshurica | JNK | Breast cancer | MCF-7 cells, mice | 181 |
| Kaempferol | Fruits and berries | AMPK, AKT | Hepatic cancer | SK-HEP-1 cells | 182 |
| Licochalcone A | Glycyrrhiza sp. | PI3K/AKT/mTOR | Cervical cancer | SiHa cells | 183 |
| Luteoloside | Gentiana macrophylla | AKT/mTOR/S6K | Lung cancer | A549, H292 cells | 184 |
| Myricetin | Fruits, vegetables | mTOR | Hepatic carcinoma | HepG2 cells | 185 |
| Quercetin | Fruits and berries | PI3K, beclin-1 | Leukemia | P39 cells, mice | 186 |
| Resveratrol | Vitis viniferae | SIRT1, RAB7 | Oxidative stress | Mice | 187 |
| Alkaloids | |||||
| Berberine | Coptidis Rhizoma | AKT/mTOR, beclin-1 | Hepatic carcinoma | HepG2, MHCC97-L cells | 188 |
| Capsaicin | Capsicum annuum | Beclin-1, LC3 | Hepatic carcinoma | HepG2 cells | 189 |
| Corynoxine B | Uncaria rhynchophylla | Beclin-1 | Parkinson's disease | N2a,SHSY-5Y cells | 190 |
| Fangchinoline | Stephania tetrandra | Sestrin2 | Hepatic carcinoma | HepG2 cells | 191 |
| Harmol | Peganum harmala | Survivin | Glioma | U251MG cells | 192 |
| Isorhynchophylline | Uncaria rhynchophylla | Beclin-1 | Parkinson's disease | N2a, PC12, SH-SY5Y | 193 |
| Matrine | Sophora flavescens | mTOR, P53 | Hepatic carcinoma | HepG2, SMMC-7721 | 194 |
| Piperlongumine | Piper longum | AKT/mTOR | Various cancers | 786-O, PC-3, MCF7 | 195 |
| Vinblastine | Vinca rosea | Cathepsin D | Stress conditions | Rat hepatocytes | 196 |
| Other natural molecules | |||||
| Arenobufagin | Toad venom | PI3K/AKT/mTOR | Hepatic carcinoma | HepG2 cells | 197 |
| Benzyl isothiocynate | Lepidium sativum | AKT, mTOR | Prostate cancer | Rv-1, PC-12 cells | 198 |
| Bisbibenzyls | Bryophytes | LC-3 | Prostate cancer | LNCaP cells | 123 |
| Bufalin | Bufo gargarizans | JNK, ATG5, beclin-1 | Colorectal cancer | HT-29 and Caco-2 cells | 199 |
| Cinobufagin | Bufo gargarizans | PARP, JNK/P38 | Osteosarcoma | U2OS cells | 200 |
| Concanavalin A | Canavalia ensiformis | LC3, BNIP3, AKT | Hepatoma | ML-1 cells | 201 |
| Daucosterol | Smilax glabra Roxb. | Beclin-1, LC-3 | Breast cancer | MCF-7 cells | 202 |
| Docosahexaenoic acid | Metabolic intermediate | NFE2L2 | Neurodegeneration | ARPE-19 | 203 |
| Embelin | Embelia ribes | ATG-5, ATG-12 | Oral cancer | Ca9-22 cells | 204 |
| Lanosterol | Metabolic intermediate | CHIP | Neurodegeneration | Cos-7 | 49 |
| Noggin | Xenopus | LC3, beclin-1 | Acute pancreatitis | AR42J cells, mice | 205 |
| Ophiopogonin B | Radix ophiopogon var. | PI3K/AKT/mTOR | Lung cancer | NCI-H157, NCI-H460 | 206 |
| Polyphyllin G | Paris yunnanensis | AKT, MAPK | Nasopharyngeal carcinoma | HONE-1 and NPC-039 | 207 |
| Rottlerin | Mallotus philippinensis | PI3K/AKT/mTOR | Pancreatic cancer | Cancer stem cells | 208 |
| 6-Shogaol | Zingiber officinale | AKT/mTOR | Lung cancer | A549 | 209 |
| Sitosterol | Plant sterols | P38 | Sitosterolemia | Mice macrophages | 210 |
| Spermidine | Natural polyamine | ATG7 | Ageing | Yeast, fly, worm, PBMC | 211 |
| Sulforaphane | Brassica oleracea | ERK | Huntington's disease | Mice | 212 |
| Autophagy inhibitors | |||||
| Aspargine | Natural amino acid | Lysosome fusion | Proteopathies | Rat hepatocytes | 213 |
| Cytochalasins | Aspergillus sp. | Microfilaments | Proteopathies | Rat kidney cells | 214 |
| Emodin | Fallopia japonica | LC3, beclin-1 | Acute pancreatitis | Rats | 215 |
| Estrogen | Natural hormone | CXCL12 | Endometriosis | Endometrial stromal cells | 216 |
| Leupeptin | Streptomyces sp. | Serine proteases | Proteopathies | Rat hepatocytes | 217 |
| 3-Methyladenine | Metabolic intermediate | PI3K | Proteopathies | Hepatocytes | 218 |
| Pepstatin A | Streptomyces sp. | Aspartyl peptidases | Proteopathies | Rat livers, hearts | 219 |
| Vinblastine | Catharanthus rosea | Microtubules | Proteopathies | Rat fibroblasts | 220 |
| Vincristine | Catharanthus rosea | Microtubules | Proteopathies | Rat fibroblasts | 220 |
| Wortmannin | Penicillium sp. | PI3K | Acute pancreatitis | Rats | 221 |
Interestingly, autophagy plays very crucial roles in the clearance of many infectious agents, including HIV, Mycobacterium, or other parasites236. Triggering this pathway by vitamin D or starvation mechanisms have shown improvements in various pathological conditions, ranging from viral/bacterial infections to tuberculosis and malaria237, 238, 239, 240. Autophagy also performs vital roles in cell metabolism and signaling, as evidenced by multiple lines of studies, which are covered in detail in several previous articles241,242. The influence of autophagy induction has been investigated in many metabolism-related disorders, including diabetes, glucose intolerance, obesity, and atherosclerosis115. It was evident from the past studies that modulation of autophagy may have enormous potential to counter the stress conditions and protect from several incurable diseases243, 244, 245. Likewise, the autophagy inducers, e.g., bigelovin, oridonin, and stellettin B may accelerate the apoptotic pathways in various types of cancer cells246, 247, 248. The majority of molecules (e.g., cinobufagin, juglanin, ursolic acid, ampelopsin, etc.) act on the target proteins, like PI3K, AKT, mTOR, S6K, MAPK, JNK, P38, ERK, etc., which are explicitly involved in the autophagy regulation249, 250, 251, 252. For the past many decades attempts to upregulate the autophagic degradation of large aggregates of proteins have been made, and considerable success has been achieved.
The research on exogenous autophagy induction using exercise/starvation like lifestyle changes or natural molecule-based food habits has shown enormous promises to deliver in many stress-related changes like neurodegeneration and aging253,254. Use of curcumin and triptolide in oxidative stress conditions in cells and Parkinson's disease animal models have shown neuroprotective effects of these drugs via the upregulation of autophagy255,256. Docosahexaenoic acid, sulforaphane, and lanosterol are other natural inducers of autophagy, which have shown multifactorial effects in ameliorating the stress conditions of the cells and alleviate the degenerative conditions in the brain194,226,257. Although a vast literature is available on the induction of autophagy by small molecules, there are limited reports of inhibitors that can demonstrate beneficial effects on disease conditions. Emodin, wortmannin, and 3-methyladenine are few known autophagy suppressors with disease modulating potential258, 259, 260. A comprehensive list of naturally derived inducers and inhibitors of the autophagy pathway is prepared in Table 4.
5. Conclusions and future perspectives
Aberrant protein's accumulation inside cells is very well-described as a leading factor of aging, neurodegeneration, and multiple other pathologies, including cancer, diabetes, cystic fibrosis, etc. Researchers and clinicians have made multiple efforts to understand the underlying causes and mechanisms for these diseases. The molecular mechanisms whose failures can lead to inappropriate protein folding events and the common features across all these pathologies are still unclear. Some unique features across all these diseases and a noticeable genetic diversity in various conditions have prevented the scientific community from reaching a common conclusion and devising possible solutions for these life-threatening diseases93,261. However, continuous efforts are made worldwide to identify underlying causes, including the most common genetic mutations and contributing environmental or lifestyle associated factors. A comprehensive picture of all these factors, associated changes, and the pathological conditions caused by them is presented in Fig. 3. Unfortunately, none of the hypotheses and explanations addressing the mechanisms and causes behind such detrimental changes leading to the age-associated decline in the efficiency of physiological systems have led us to develop a proper understanding and possible solutions to these conditions.
Figure 3.
An overview of intricate association of natural compounds and proteostasis machinery. (A) A summary of important natural sources of bioactive compounds that can affect the activities and functioning of chief players of protein quality control machinery inside the cells; examples of small molecules from each source are also presented in respective boxes. (B) A central block shows how crucial is the precise balance of protein synthesis, maintenance, trafficking and degradation of cellular proteins for the maintenance of healthy proteome. Some internal or external perturbance factors may alter the homeostasis of the cells, while multiple key actors of cellular proteostasis machinery continuously maintain the health of the proteome. (C) Multiple kinds of intracellular regulatory subsystems are identified, which are required to synthesize and maintain the healthy set of proteins in various cellular compartments to perform all kinds of cellular functions. The key proteins of each subcellular pathway are mentioned in each box. (D) Many intra- and extracellular factors and stressors could be responsible for generating different kinds of stresses during the lifetime of the organisms, which are counter-balanced by factors described in (C). (E) A number of proteinopathies, which are directly and indirectly linked with perturbance in cellular proteostasis machinery, have been reported. These diseases could be associated with one or multiple pathways, tissues, and organs specified in each box.
In the past, many attempts, both successful and unsuccessful, have been made for devising novel therapeutic approaches against various diseases. Numerous molecules have been proposed for their efficacy for mitigating the proteotoxicity generated by intracellular protein aggregates or inclusion bodies106,110,111. One consensus that most of the studies meet is that natural products could be medicinally very active and useful. They were used for centuries in ancient traditional natural medicinal approaches in old-world countries. Based on all the observations mentioned above, it could be stated that targeting cellular PQC machinery by modulating their activities using small molecules may have vast potential. Plant extracts were used by ancient researchers and physicians to cure deadly infections and diseases, described in many primitive Indian, Unani, and Chinese literature262,263. Most natural molecules posit lesser toxicity and side effects than synthetic chemicals when administered to cells or animals in laboratory tests264. This makes them preferred choices over costly synthetic chemicals in experimental studies. Many less-invaded human territories, like the Himalayas, are the homeland of such medicinally rich natural resources and are yet to be explored and utilized for treatments of life-threatening diseases in these regions. A thorough and well-managed exploration could be done in order to identify and delve into more effective and easily derived drugs.
Several naturally extracted drugs obtained from microbial and fungal isolates, marine and land animals, many aquatic and terrestrial plants, are currently in research allowing us to identify and investigate more such drugs. These small natural molecules may have several unexplored applications that need further studies. The primary benefit of these naturally derived molecules is a low-cost therapeutic alternative to many treatment strategies, which are in the pipeline against these diseases and may need a much higher cost, although many challenges remain unaddressed265. The identification, isolation, purification, and characterization of new molecules are a highly tedious and lengthy process, requiring lots of hard work, funds, and time19,266. Many times, designing or synthesizing some derivatives of already known drugs seems a more straightforward and cost-effective strategy in comparison to looking for new molecules. Repurposing older drugs could also be a beneficial drug discovery model to save much time, effort, and cost. Additionally, these small plant-based molecules could be used as food supplements to reduce the overall risk of many diseases.
Acknowledgments
The author apologizes to various groups and scientists whose work could not have been cited due to space and time constraints. Author thanks the Central University of Rajasthan for providing space and resources while the manuscript was prepared. The author also appreciates Servier (http://smart.servier.com/) for providing templates for the preparation of medical illustrations under Creative Commons Attribution 3.0 Unported License.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.01.006.
Author contributions
Arun Upadhyay is responsible for all work of this review.
Conflicts of interest
The author has no conflict of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Morimoto R.I., Cuervo A.M. Proteostasis and the aging proteome in health and disease. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:S33–S38. doi: 10.1093/gerona/glu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balchin D., Hayer-Hartl M., Hartl F.U. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 3.Chen B., Retzlaff M., Roos T., Frydman J. Cellular strategies of protein quality control. Cold Spring Harb Perspect Biol. 2011;3:a004374. doi: 10.1101/cshperspect.a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider K., Bertolotti A. Surviving protein quality control catastrophes–from cells to organisms. J Cell Sci. 2015;128:3861–3869. doi: 10.1242/jcs.173047. [DOI] [PubMed] [Google Scholar]
- 5.Dubnikov T., Ben-Gedalya T., Cohen E. Protein quality control in health and disease. Cold Spring Harb Perspect Biol. 2017;9:a023523. doi: 10.1101/cshperspect.a023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cromm P.M., Crews C.M. Targeted protein degradation: from chemical biology to drug discovery. Cell Chem Biol. 2017;24:1181–1190. doi: 10.1016/j.chembiol.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G., Lou H.X. Strategies to diversify natural products for drug discovery. Med Res Rev. 2018;38:1255–1294. doi: 10.1002/med.21474. [DOI] [PubMed] [Google Scholar]
- 8.Balunas M.J., Kinghorn A.D. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl T., Wood R.D. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 10.Isken O., Maquat L.E. vol. 21. 2007. pp. 1833–1856. (Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function). [DOI] [PubMed] [Google Scholar]
- 11.Maquat L.E., Carmichael G.G. Quality control of mRNA function. Cell. 2001;104:173–176. doi: 10.1016/s0092-8674(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 12.Ibba M., Söll D. Quality control mechanisms during translation. Science. 1999;286:1893–1897. doi: 10.1126/science.286.5446.1893. [DOI] [PubMed] [Google Scholar]
- 13.Bukau B., Weissman J., Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Bengtson M.H., Joazeiro C.A.P. Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature. 2010;467:470. doi: 10.1038/nature09371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellgaard L., Molinari M., Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 16.Wickner S., Maurizi M.R., Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science. 1999;286:1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- 17.Amm I., Sommer T., Wolf D.H. Protein quality control and elimination of protein waste: the role of the ubiquitin–proteasome system. Biochim Biophys Acta. 2014;1843:182–196. doi: 10.1016/j.bbamcr.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Lenk U., Sommer T. Ubiquitin-mediated proteolysis of a short-lived regulatory protein depends on its cellular localization. J Biol Chem. 2000;275:39403–39410. doi: 10.1074/jbc.M006949200. [DOI] [PubMed] [Google Scholar]
- 19.Mishra R., Upadhyay A., Prajapati V.K., Mishra A. Proteasome-mediated proteostasis: novel medicinal and pharmacological strategies for diseases. Med Res Rev. 2018;38:1916–1973. doi: 10.1002/med.21502. [DOI] [PubMed] [Google Scholar]
- 20.Klionsky D.J., Emr S.D. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amanullah A., Upadhyay A., Joshi V., Mishra R., Jana N.R., Mishra A. Progressing neurobiological strategies against proteostasis failure: challenges in neurodegeneration. Prog Neurobiol. 2017;159:1–38. doi: 10.1016/j.pneurobio.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Díaz-Villanueva J.F., Díaz-Molina R., García-González V. Protein folding and mechanisms of proteostasis. Int J Mol Sci. 2015;16:17193–17230. doi: 10.3390/ijms160817193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig E.A., Eisenman H.C., Hundley H.A. Ribosome-tethered molecular chaperones: the first line of defense against protein misfolding?. Curr Opin Microbiol. 2003;6:157–162. doi: 10.1016/s1369-5274(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 25.Wegele H., Müller L., Buchner J. Reviews of physiology, biochemistry and pharmacology. Springer; Berlin Heidelberg: 2004. Hsp70 and Hsp90—a relay team for protein folding; pp. p1–p44. [DOI] [PubMed] [Google Scholar]
- 26.Freeman B.C., Morimoto R.I. The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J. 1996;15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- 27.Hartl F.U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 28.Mayer M.P., Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartl F.U., Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 30.Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 31.Lee S., Tsai F.T. Molecular chaperones in protein quality control. J Biochem Mol Biol. 2005;38:259–265. doi: 10.5483/bmbrep.2005.38.3.259. [DOI] [PubMed] [Google Scholar]
- 32.Ellis R.J., Hemmingsen S.M. Molecular chaperones: proteins essential for the biogenesis of some macromolecular structures. Trends Biochem Sci. 1989;14:339–342. doi: 10.1016/0968-0004(89)90168-0. [DOI] [PubMed] [Google Scholar]
- 33.Walter S., Buchner J. Molecular chaperones—cellular machines for protein folding. Angew Chem Int Ed Engl. 2002;41:1098–1113. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Shiber A., Ravid T. Chaperoning proteins for destruction: diverse roles of Hsp70 chaperones and their co-chaperones in targeting misfolded proteins to the proteasome. Biomolecules. 2014;4:704–724. doi: 10.3390/biom4030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demand J., Alberti S., Patterson C., Höhfeld J. Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr Biol. 2001;11:1569–1577. doi: 10.1016/s0960-9822(01)00487-0. [DOI] [PubMed] [Google Scholar]
- 36.Wong E., Cuervo A.M. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb Perspect Biol. 2010;2:a006734. doi: 10.1101/cshperspect.a006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kettern N., Dreiseidler M., Tawo R., Hohfeld J. Chaperone-assisted degradation: multiple paths to destruction. Biol Chem. 2010;391:481–489. doi: 10.1515/BC.2010.058. [DOI] [PubMed] [Google Scholar]
- 38.Upadhyay A., Amanullah A., Chhangani D., Mishra R., Prasad A., Mishra A. Mahogunin ring finger-1 (MGRN1), a multifaceted ubiquitin ligase: recent unraveling of neurobiological mechanisms. Mol Neurobiol. 2016;53:4484–4496. doi: 10.1007/s12035-015-9379-8. [DOI] [PubMed] [Google Scholar]
- 39.de Duve C. The separation and characterization of subcellular particles. Harvey Lect. 1965;59:49. [PubMed] [Google Scholar]
- 40.de Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 41.Kim P.K., Hailey D.W., Mullen R.T., Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci U S A. 2008;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe Y., Tanaka M. p62/SQSTM1 in autophagic clearance of a non-ubiquitylated substrate. J Cell Sci. 2011;124:2692–2701. doi: 10.1242/jcs.081232. [DOI] [PubMed] [Google Scholar]
- 43.Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12:1535. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- 44.Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2013;24:24. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamark T., Johansen T. Aggrephagy: selective disposal of protein aggregates by macroautophagy. Int J Cell Biol. 2012;2012:736905. doi: 10.1155/2012/736905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overbye A., Fengsrud M., Seglen P.O. Proteomic analysis of membrane-associated proteins from rat liver autophagosomes. Autophagy. 2007;3:300–322. doi: 10.4161/auto.3910. [DOI] [PubMed] [Google Scholar]
- 47.Liou W., Geuze H.J., Geelen M.J., Slot J.W. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon P.B., Hoyvik H., Seglen P.O. Prelysosomal and lysosomal connections between autophagy and endocytosis. Biochem J. 1992;283 Pt 2:361–369. doi: 10.1042/bj2830361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berg T.O., Fengsrud M., Stromhaug P.E., Berg T., Seglen P.O. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 50.Lawrence B.P., Brown W.J. Autophagic vacuoles rapidly fuse with pre-existing lysosomes in cultured hepatocytes. J Cell Sci. 1992;102(Pt 3):515–526. doi: 10.1242/jcs.102.3.515. [DOI] [PubMed] [Google Scholar]
- 51.Agarraberes F.A., Dice J.F. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114:2491–2499. doi: 10.1242/jcs.114.13.2491. [DOI] [PubMed] [Google Scholar]
- 52.Chhangani D., Mishra A. Protein quality control system in neurodegeneration: a healing company hard to beat but failure is fatal. Mol Neurobiol. 2013;48:141–156. doi: 10.1007/s12035-013-8411-0. [DOI] [PubMed] [Google Scholar]
- 53.Shpilka T., Elazar Z. Shedding light on mammalian microautophagy. Dev Cell. 2011;20:1–2. doi: 10.1016/j.devcel.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Sahu R., Kaushik S., Clement C.C., Cannizzo E.S., Scharf B., Follenzi A. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mijaljica D., Prescott M., Devenish R.J. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7:673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 56.Li W.W., Li J., Bao J.K. Microautophagy: lesser-known self-eating. Cell Mol Life Sci. 2012;69:1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaushik S., Cuervo A.M. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dice J.F., Chiang H.L., Spencer E.P., Backer J.M. Regulation of catabolism of microinjected ribonuclease A. Identification of residues 7-11 as the essential pentapeptide. J Biol Chem. 1986;261:6853–6859. [PubMed] [Google Scholar]
- 59.Bandyopadhyay U., Kaushik S., Varticovski L., Cuervo A.M. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol. 2008;28:5747–5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dice J.F. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- 61.Arndt V., Dick N., Tawo R., Dreiseidler M., Wenzel D., Hesse M. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol. 2010;20:143–148. doi: 10.1016/j.cub.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 62.Gamerdinger M., Hajieva P., Kaya A.M., Wolfrum U., Hartl F.U., Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28:889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glickman M.H., Ciechanover A. The ubiquitin–proteasome proteolytic pathway. Destruct Sake Construct. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 64.Liu C.W., Corboy M.J., DeMartino G.N., Thomas P.J. Endoproteolytic activity of the proteasome. Science. 2003;299:408–411. doi: 10.1126/science.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coux O., Tanaka K., Goldberg A.L. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 66.Lupas A., Koster A.J., Baumeister W. Structural features of 26S and 20S proteasomes. Enzyme Protein. 1993;47:252–273. doi: 10.1159/000468684. [DOI] [PubMed] [Google Scholar]
- 67.Wilk S., Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J Neurochem. 1983;40:842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]
- 68.Bochtler M., Ditzel L., Groll M., Hartmann C., Huber R. The proteasome. Annu Rev Biophys Biomol Struct. 1999;28:295–317. doi: 10.1146/annurev.biophys.28.1.295. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka K. The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:12–36. doi: 10.2183/pjab.85.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orlowski M., Wilk S. Catalytic activities of the 20 S proteasome, a multicatalytic proteinase complex. Arch Biochem Biophys. 2000;383:1–16. doi: 10.1006/abbi.2000.2036. [DOI] [PubMed] [Google Scholar]
- 71.Ciechanover A., Heller H., Katz-Etzion R., Hershko A. Activation of the heat-stable polypeptide of the ATP-dependent proteolytic system. Proc Natl Acad Sci U S A. 1981;78:761–765. doi: 10.1073/pnas.78.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haas A.L., Warms J.V., Hershko A., Rose I.A. Ubiquitin-activating enzyme. Mechanism and role in protein–ubiquitin conjugation. J Biol Chem. 1982;257:2543–2548. [PubMed] [Google Scholar]
- 73.Hershko A., Heller H., Elias S., Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983;258:8206–8214. [PubMed] [Google Scholar]
- 74.Jentsch S. The ubiquitin-conjugation system. Annu Rev Genet. 1992;26:179–207. doi: 10.1146/annurev.ge.26.120192.001143. [DOI] [PubMed] [Google Scholar]
- 75.Ciechanover A. The ubiquitin–proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ciechanover A. The ubiquitin–proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 77.Kwon Y.T., Ciechanover A. The ubiquitin code in the ubiquitin–proteasome system and autophagy. Trends Biochem Sci. 2017;42:873–886. doi: 10.1016/j.tibs.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Dittmar G., Selbach M. Deciphering the ubiquitin code. Mol Cell. 2017;65:779–780. doi: 10.1016/j.molcel.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Komander D., Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 80.Thrower J.S., Hoffman L., Rechsteiner M., Pickart C.M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clague M.J., Urbé S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143:682–685. doi: 10.1016/j.cell.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 82.Wong E.S.P., Ho M.W.L., Tay S.P., Tan J.M.M., Lim K.L., Ko H.S. Lysine 63-linked ubiquitination promotes the formation and autophagic clearance of protein inclusions associated with neurodegenerative diseases. Hum Mol Genet. 2007;17:431–439. doi: 10.1093/hmg/ddm320. [DOI] [PubMed] [Google Scholar]
- 83.Chau V., Tobias J.W., Bachmair A., Marriott D., Ecker D.J., Gonda D.K. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 84.Ikeda F., Dikic I. Atypical ubiquitin chains: new molecular signals. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z.J., Sun L.J. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 86.Trempe J.F. Reading the ubiquitin postal code. Curr Opin Struct Biol. 2011;21:792–801. doi: 10.1016/j.sbi.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 87.Ciechanover A. The ubiquitin proteolytic system: from a vague idea, through basic mechanisms, and onto human diseases and drug targeting. Neurology. 2006;66:S7–S19. doi: 10.1212/01.wnl.0000192261.02023.b8. [DOI] [PubMed] [Google Scholar]
- 88.Lilienbaum A. Relationship between the proteasomal system and autophagy. Int J Biochem Mol Biol. 2013;4:1–26. [PMC free article] [PubMed] [Google Scholar]
- 89.Chhangani D., Chinchwadkar S., Mishra A. vol. 49. 2014. pp. 1270–1281. (Autophagy coupling interplay: can improve cellular repair and aging?). [DOI] [PubMed] [Google Scholar]
- 90.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Orme T., Guerreiro R., Bras J. The genetics of dementia with Lewy bodies: current understanding and future directions. Curr Neurol Neurosci Rep. 2018;18:a67. doi: 10.1007/s11910-018-0874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mroz E.A., Rocco J.W. The challenges of tumor genetic diversity. Cancer. 2017;123:917–927. doi: 10.1002/cncr.30430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Upadhyay A. Cancer: an unknown territory; rethinking before going ahead. Gene Dis. 2020 doi: 10.1016/j.gendis.2020.09.002. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koga H., Kaushik S., Cuervo A.M. Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev. 2011;10:205–215. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arslan M.A., Csermely P., Soti C. Protein homeostasis and molecular chaperones in aging. Biogerontology. 2006;7:383–389. doi: 10.1007/s10522-006-9053-7. [DOI] [PubMed] [Google Scholar]
- 97.Currais A., Fischer W., Maher P., Schubert D. Intraneuronal protein aggregation as a trigger for inflammation and neurodegeneration in the aging brain. FASEB J. 2017;31:5–10. doi: 10.1096/fj.201601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beyer K., Domingo-Sàbat M., Ariza A. Molecular pathology of Lewy body diseases. Int J Mol Sci. 2009;10:724–745. doi: 10.3390/ijms10030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bucciantini M., Giannoni E., Chiti F., Baroni F., Formigli L., Zurdo J. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 100.Niccoli T., Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 101.Jiang X., Overholtzer M., Thompson C.B. Autophagy in cellular metabolism and cancer. J Clin Invest. 2015;125:47–54. doi: 10.1172/JCI73942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellisdon A.M., Bottomley S.P. The role of protein misfolding in the pathogenesis of human diseases. IUBMB Life. 2004;56:119–123. doi: 10.1080/15216540410001674003. [DOI] [PubMed] [Google Scholar]
- 103.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 104.Dobson C.M. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 105.Chaudhuri T.K., Paul S. Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J. 2006;273:1331–1349. doi: 10.1111/j.1742-4658.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- 106.Cohen F.E., Kelly J.W. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 107.Rambaran R.N., Serpell L.C. Amyloid fibrils: abnormal protein assembly. Prion. 2008;2:112–117. doi: 10.4161/pri.2.3.7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sipe J.D., Benson M.D., Buxbaum J.N., Ikeda S.I., Merlini G., Saraiva M.J. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification international society of amyloidosis 2016 nomenclature guidelines. Amyloid. 2016;23:209–213. doi: 10.1080/13506129.2016.1257986. [DOI] [PubMed] [Google Scholar]
- 109.Mauri M., Elli T., Caviglia G., Uboldi G., Azzi M. ACM press; Cagliari, Italy: 2017. Proceedings of the 12th biannual conference on Italian SIGCHI chapter; p. 28. [Google Scholar]
- 110.Loo T.W., Clarke D.M. Chemical and pharmacological chaperones as new therapeutic agents. Expet Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- 111.Dey A., Bhattacharya R., Mukherjee A., Pandey D.K. Natural products against Alzheimer's disease: pharmaco-therapeutics and biotechnological interventions. Biotechnol Adv. 2017;35:178–216. doi: 10.1016/j.biotechadv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 112.Bernier V., Lagace M., Bichet D.G., Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metabol. 2004;15:222–228. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 113.Morello J.P., Petaja-Repo U.E., Bichet D.G., Bouvier M. Pharmacological chaperones: a new twist on receptor folding. Trends Pharmacol Sci. 2000;21:466–469. doi: 10.1016/s0165-6147(00)01575-3. [DOI] [PubMed] [Google Scholar]
- 114.de Duve C. Lysosomes ciba foundation symposium. Postgrad Med. 1964;40:557. [Google Scholar]
- 115.Joshi V., Upadhyay A., Prajapati V.K., Mishra A. How autophagy can restore proteostasis defects in multiple diseases?. Med Res Rev. 2020;40:1385–1439. doi: 10.1002/med.21662. [DOI] [PubMed] [Google Scholar]
- 116.Upadhyay A., Amanullah A., Chhangani D., Mishra R., Mishra A. Selective multifaceted E3 ubiquitin ligases barricade extreme defense: potential therapeutic targets for neurodegeneration and ageing. Ageing Res Rev. 2015;24:138–159. doi: 10.1016/j.arr.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 117.Upadhyay A. Structure of proteins: evolution with unsolved mysteries. Prog Biophys Mol Biol. 2019;149:160–172. doi: 10.1016/j.pbiomolbio.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 118.Ding W.X., Ni H.M., Gao W., Yoshimori T., Stolz D.B., Ron D. Linking of autophagy to ubiquitin–proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol. 2007;171:513–524. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang B., Cai J., Sun L., Li Y., Qu J., Snider B.J. Proteasome inhibitors activate autophagy involving inhibition of PI3K-Akt-mTOR pathway as an anti-oxidation defense in human RPE cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang D., Xu Q., Yuan Q., Jia M., Niu H., Liu X. Proteasome inhibition boosts autophagic degradation of ubiquitinated-AGR2 and enhances the antitumor efficiency of bevacizumab. Oncogene. 2019;38:3458–3474. doi: 10.1038/s41388-019-0675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Korolchuk V.I., Mansilla A., Menzies F.M., Rubinsztein D.C. Autophagy inhibition compromises degradation of ubiquitin–proteasome pathway substrates. Mol Cell. 2009;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dandage R., Bandyopadhyay A., Jayaraj G.G., Saxena K., Dalal V., Das A. Classification of chemical chaperones based on their effect on protein folding landscapes. ACS Chem Biol. 2015;10:813–820. doi: 10.1021/cb500798y. [DOI] [PubMed] [Google Scholar]
- 123.Perlmutter D.H. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res. 2002;52:832–836. doi: 10.1203/00006450-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 124.Ringe D., Petsko G.A. What are pharmacological chaperones and why are they interesting?. J Biol. 2009;8:a80. doi: 10.1186/jbiol186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pereira David M., Valentão P., Andrade P.B. Tuning protein folding in lysosomal storage diseases: the chemistry behind pharmacological chaperones. Chem Sci. 2018;9:1740–1752. doi: 10.1039/c7sc04712f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Amolins M.W., Blagg B.S.J. Natural product inhibitors of Hsp90: potential leads for drug discovery. Mini Rev Med Chem. 2009;9:140–152. doi: 10.2174/138955709787316056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jinwal U.K., Miyata Y., Koren J., 3rd, Jones J.R., Trotter J.H., Chang L. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29:12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kwon H.J., Yoshida M., Abe K., Horinouchi S., Beppu T. Radicicol, an agent inducing the reversal of transformed phenotypes of src-transformed fibroblasts. Biosci Biotechnol Biochem. 1992;56:538–539. doi: 10.1271/bbb.56.538. [DOI] [PubMed] [Google Scholar]
- 129.Sharma S.V., Agatsuma T., Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16:2639–2645. doi: 10.1038/sj.onc.1201790. [DOI] [PubMed] [Google Scholar]
- 130.Whitesell L., Mimnaugh E.G., de Costa B., Myers C.E., Neckers L.M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Münster P.N., Srethapakdi M., Moasser M.M., Rosen N. Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res. 2001;61:2945. [PubMed] [Google Scholar]
- 132.Moulin E., Zoete V., Barluenga S., Karplus M., Winssinger N. Design, synthesis, and biological evaluation of HSP90 inhibitors based on conformational analysis of radicicol and its analogues. J Am Chem Soc. 2005;127:6999–7004. doi: 10.1021/ja043101w. [DOI] [PubMed] [Google Scholar]
- 133.Turbyville T.J., Wijeratne E.M., Liu M.X., Burns A.M., Seliga C.J., Luevano L.A. Search for Hsp90 inhibitors with potential anticancer activity: isolation and SAR studies of radicicol and monocillin I from two plant-associated fungi of the Sonoran desert. J Nat Prod. 2006;69:178–184. doi: 10.1021/np058095b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sreeramulu S., Gande S.L., Göbel M., Schwalbe H. Molecular mechanism of inhibition of the human protein complex Hsp90–Cdc37, a kinome chaperone–cochaperone, by triterpene celastrol. Angew Chem Int Ed Engl. 2009;48:5853–5855. doi: 10.1002/anie.200900929. [DOI] [PubMed] [Google Scholar]
- 135.Davenport J., Manjarrez J.R., Peterson L., Krumm B., Blagg B.S., Matts R.L. Gambogic acid, a natural product inhibitor of Hsp90. J Nat Prod. 2011;74:1085–1092. doi: 10.1021/np200029q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hadden M.K., Galam L., Gestwicki J.E., Matts R.L., Blagg B.S. Derrubone, an inhibitor of the Hsp90 protein folding machinery. J Nat Prod. 2007;70:2014–2018. doi: 10.1021/np070190s. [DOI] [PubMed] [Google Scholar]
- 137.Nagai N., Nakai A., Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun. 1995;208:1099–1105. doi: 10.1006/bbrc.1995.1447. [DOI] [PubMed] [Google Scholar]
- 138.Palermo C.M., Westlake C.A., Gasiewicz T.A. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry. 2005;44:5041–5052. doi: 10.1021/bi047433p. [DOI] [PubMed] [Google Scholar]
- 139.Tran P.L., Kim S.A., Choi H.S., Yoon J.H., Ahn S.G. Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer. 2010;10:276. doi: 10.1186/1471-2407-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Blank M., Mandel M., Keisari Y., Meruelo D., Lavie G. Enhanced ubiquitinylation of heat shock protein 90 as a potential mechanism for mitotic cell death in cancer cells induced with hypericin. Cancer Res. 2003;63:8241–8247. [PubMed] [Google Scholar]
- 141.Vasko R.C., Rodriguez R.A., Cunningham C.N., Ardi V.C., Agard D.A., McAlpine S.R. Mechanistic studies of sansalvamide A-amide: an allosteric modulator of Hsp90. ACS Med Chem Lett. 2010;1:4–8. doi: 10.1021/ml900003t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen W.Y., Chang F.R., Huang Z.Y., Chen J.H., Wu Y.C., Wu C.C. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J Biol Chem. 2008;283:17184–17193. doi: 10.1074/jbc.M709447200. [DOI] [PubMed] [Google Scholar]
- 143.Chang L., Miyata Y., Ung P.M., Bertelsen E.B., McQuade T.J., Carlson H.A. Chemical screens against a reconstituted multiprotein complex: myricetin blocks DnaJ regulation of DnaK through an allosteric mechanism. Chem Biol. 2011;18:210–221. doi: 10.1016/j.chembiol.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nadler S.G., Tepper M.A., Schacter B., Mazzucco C.E. Interaction of the immunosuppressant deoxyspergualin with a member of the Hsp70 family of heat shock proteins. Science. 1992;258:484–486. doi: 10.1126/science.1411548. [DOI] [PubMed] [Google Scholar]
- 145.Bagatell R., Paine-Murrieta G.D., Taylor C.W., Pulcini E.J., Akinaga S., Benjamin I.J. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of Hsp90-binding agents. Clin Cancer Res. 2000;6:3312–3318. [PubMed] [Google Scholar]
- 146.Kim H.R., Kang H.S., Kim H.D. Geldanamycin induces heat shock protein expression through activation of HSF1 in K562 erythroleukemic cells. IUBMB Life. 1999;48:429–433. doi: 10.1080/713803536. [DOI] [PubMed] [Google Scholar]
- 147.Lu A., Ran R., Parmentier-Batteur S., Nee A., Sharp F.R. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81:355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 148.Batulan Z., Taylor D.M., Aarons R.J., Minotti S., Doroudchi M.M., Nalbantoglu J. Induction of multiple heat shock proteins and neuroprotection in a primary culture model of familial amyotrophic lateral sclerosis. Neurobiol Dis. 2006;24:213–225. doi: 10.1016/j.nbd.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 149.Shen H.Y., He J.C., Wang Y., Huang Q.Y., Chen J.F. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005;280:39962–39969. doi: 10.1074/jbc.M505524200. [DOI] [PubMed] [Google Scholar]
- 150.Sittler A., Lurz R., Lueder G., Priller J., Lehrach H., Hayer-Hartl M.K. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington's disease. Hum Mol Genet. 2001;10:1307–1315. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- 151.Kato K., Ito H., Kamei K., Iwamoto I. Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaperones. 1998;3:152–160. doi: 10.1379/1466-1268(1998)003<0152:sotsie>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Patel P., Julien J.P., Kriz J. Early-stage treatment with withaferin A reduces levels of misfolded superoxide dismutase 1 and extends lifespan in a mouse model of amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12:217–233. doi: 10.1007/s13311-014-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bence N.F., Sampat R.M., Kopito R.R. Impairment of the ubiquitin–proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 154.Leestemaker Y., de Jong A., Witting K.F., Penning R., Schuurman K., Rodenko B. Proteasome activation by small molecules. Cell Chem Biol. 2017;24:725–736. doi: 10.1016/j.chembiol.2017.05.010. e7. [DOI] [PubMed] [Google Scholar]
- 155.Orlowski R.Z., Kuhn D.J. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 156.Bonfili L., Cecarini V., Amici M., Cuccioloni M., Angeletti M., Keller J.N. Natural polyphenols as proteasome modulators and their role as anti-cancer compounds. FEBS J. 2008;275:5512–5526. doi: 10.1111/j.1742-4658.2008.06696.x. [DOI] [PubMed] [Google Scholar]
- 157.Harris J.L., Alper P.B., Li J., Rechsteiner M., Backes B.J. Substrate specificity of the human proteasome. Chem Biol. 2001;8:1131–1141. doi: 10.1016/s1074-5521(01)00080-1. [DOI] [PubMed] [Google Scholar]
- 158.Kisselev A.F., Callard A., Goldberg A.L. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 159.Hideshima T., Richardson P., Chauhan D., Palombella V.J., Elliott P.J., Adams J. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 160.Kuhn D.J., Chen Q., Voorhees P.M., Strader J.S., Shenk K.D., Sun C.M. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin–proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 162.Chitra S., Nalini G., Rajasekhar G. The ubiquitin proteasome system and efficacy of proteasome inhibitors in diseases. Int J Rheum Dis. 2012;15:249–260. doi: 10.1111/j.1756-185X.2012.01737.x. [DOI] [PubMed] [Google Scholar]
- 163.Fenteany G., Standaert R.F., Lane W.S., Choi S., Corey E.J., Schreiber S.L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 164.Meng L., Kwok B.H.B., Sin N., Crews C.M. Eponemycin exerts its antitumor effect through the inhibition of proteasome function. Cancer Res. 1999;59:2798–2801. [PubMed] [Google Scholar]
- 165.Meng L., Mohan R., Kwok B.H., Elofsson M., Sin N., Crews C.M. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kumar B., Andreatta C., Koustas W.T., Cole W.C., Edwards-Prasad J., Prasad K.N. Mevastatin induces degeneration and decreases viability of cAMP-induced differentiated neuroblastoma cells in culture by inhibiting proteasome activity, and mevalonic acid lactone prevents these effects. J Neurosci Res. 2002;68:627–635. doi: 10.1002/jnr.10241. [DOI] [PubMed] [Google Scholar]
- 167.Muthny T., Kovarik M., Sispera L., De Meijere A., Larionov O., Tilser I. The effect of new proteasome inhibitors, belactosin A and C, on protein metabolism in isolated rat skeletal muscle. J Physiol Biochem. 2009;65:137–146. doi: 10.1007/BF03179064. [DOI] [PubMed] [Google Scholar]
- 168.Hines J., Groll M., Fahnestock M., Crews C.M. Proteasome inhibition by fellutamide B induces nerve growth factor synthesis. Chem Biol. 2008;15:501–512. doi: 10.1016/j.chembiol.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kroll M., Arenzana-Seisdedos F., Bachelerie F., Thomas D., Friguet B., Conconi M. The secondary fungal metabolite gliotoxin targets proteolytic activities of the proteasome. Chem Biol. 1999;6:689–698. doi: 10.1016/s1074-5521(00)80016-2. [DOI] [PubMed] [Google Scholar]
- 170.Meyer S., Kohler N.G., Joly A. Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-κB activation. FEBS Lett. 1997;413:354–358. doi: 10.1016/s0014-5793(97)00930-7. [DOI] [PubMed] [Google Scholar]
- 171.Tsukamoto S., Tatsuno M., van Soest R.W., Yokosawa H., Ohta T. New polyhydroxy sterols: proteasome inhibitors from a marine sponge Acanthodendrilla sp. J Nat Prod. 2003;66:1181–1185. doi: 10.1021/np030120v. [DOI] [PubMed] [Google Scholar]
- 172.Tsukamoto S., Yamanokuchi R., Yoshitomi M., Sato K., Ikeda T., Rotinsulu H. Aaptamine, an alkaloid from the sponge Aaptos suberitoides, functions as a proteasome inhibitor. Bioorg Med Chem Lett. 2010;20:3341–3343. doi: 10.1016/j.bmcl.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 173.Yue Q., Zhen H., Huang M., Zheng X., Feng L., Jiang B. Proteasome inhibition contributed to the cytotoxicity of arenobufagin after its binding with Na, K-ATPase in human cervical carcinoma HeLa cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang H., Landis-Piwowar K., Chen D., Milacic V., Dou Q. Natural compounds with proteasome inhibitory activity for cancer prevention and treatment. Curr Protein Pept Sci. 2008;9:227–239. doi: 10.2174/138920308784533998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wan S.B., Chen D., Dou Q.P., Chan T.H. Study of the green tea polyphenols catechin-3-gallate (CG) and epicatechin-3-gallate (ECG) as proteasome inhibitors. Bioorg Med Chem. 2004;12:3521–3527. doi: 10.1016/j.bmc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 176.Kazi A., Daniel K.G., Smith D.M., Kumar N.B., Dou Q.P. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem Pharmacol. 2003;66:965–976. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 177.Vandenberghe I., Créancier L., Vispé S., Annereau J.-P., Barret J.-M., Pouny I. Physalin B, a novel inhibitor of the ubiquitin–proteasome pathway, triggers NOXA-associated apoptosis. Biochem Pharmacol. 2008;76:453–462. doi: 10.1016/j.bcp.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 178.Zheng Y., Jiang X., Gao F., Song J., Sun J., Wang L. Identification of plant-derived natural products as potential inhibitors of the Mycobacterium tuberculosis proteasome. BMC Compl Alternative Med. 2014;14:400. doi: 10.1186/1472-6882-14-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen D., Daniel K.G., Chen M.S., Kuhn D.J., Landis-Piwowar K.R., Dou Q.P. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 180.Chen D., Landis-Piwowar K.R., Chen M.S., Dou Q.P. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007;9:R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pashevin D.A., Tumanovska L.V., Dosenko V.E., Nagibin V.S., Gurianova V.L., Moibenko A.A. Antiatherogenic effect of quercetin is mediated by proteasome inhibition in the aorta and circulating leukocytes. Pharmacol Rep. 2011;63:1009–1018. doi: 10.1016/s1734-1140(11)70617-x. [DOI] [PubMed] [Google Scholar]
- 182.Wu Y.X., Fang X. Apigenin, chrysin, and luteolin selectively inhibit chymotrypsin-like and trypsin-like proteasome catalytic activities in tumor cells. Planta Med. 2010;76:128–132. doi: 10.1055/s-0029-1186004. [DOI] [PubMed] [Google Scholar]
- 183.Kirk-Ballard H., Wang Z.Q., Acharya P., Zhang X.H., Yu Y., Kilroy G. An extract of Artemisia dracunculus L. inhibits ubiquitin–proteasome activity and preserves skeletal muscle mass in a murine model of diabetes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mori A., Lehmann S., O'Kelly J., Kumagai T., Desmond J.C., Pervan M. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, P53 mutant prostate cancer cells. Cancer Res. 2006;66:3222–3229. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- 185.Chinta S.J., Poksay K.S., Kaundinya G., Hart M., Bredesen D.E., Andersen J.K. Endoplasmic reticulum stress-induced cell death in dopaminergic cells: effect of resveratrol. J Mol Neurosci. 2009;39:157–168. doi: 10.1007/s12031-008-9170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nam S., Smith D.M., Dou Q.P. Tannic acid potently inhibits tumor cell proteasome activity, increases p27 and Bax expression, and induces G1 arrest and apoptosis. Cancer Epidemiol Biomarkers Prev. 2001;10:1083–1088. [PubMed] [Google Scholar]
- 187.Piccinini M., Tazartes O., Mezzatesta C., Ricotti E., Bedino S., Grosso F. Proteasomes are a target of the anti-tumour drug vinblastine. Biochem J. 2001;356:835–841. doi: 10.1042/0264-6021:3560835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jana N.R., Dikshit P., Goswami A., Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J Biol Chem. 2004;279:11680–11685. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 189.Yang H., Chen D., Cui Q.C., Yuan X., Dou Q.P. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 190.Yang H., Landis-Piwowar K.R., Lu D., Yuan P., Li L., Reddy G.P.V. Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J Cell Biochem. 2008;103:234–244. doi: 10.1002/jcb.21399. [DOI] [PubMed] [Google Scholar]
- 191.Huang M., Zhang H., Liu T., Tian D., Gu L., Zhou M. Triptolide inhibits MDM2 and induces apoptosis in acute lymphoblastic leukemia cells through a p53-independent pathway. Mol Cancer Therapeut. 2013;12:184–194. doi: 10.1158/1535-7163.MCT-12-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ohnishi K., Nakahata E., Irie K., Murakami A. Zerumbone, an electrophilic sesquiterpene, induces cellular proteo-stress leading to activation of ubiquitin–proteasome system and autophagy. Biochem Biophys Res Commun. 2013;430:616–622. doi: 10.1016/j.bbrc.2012.11.104. [DOI] [PubMed] [Google Scholar]
- 193.Katsiki M., Chondrogianni N., Chinou I., Rivett A.J., Gonos E.S. The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res. 2007;10:157–172. doi: 10.1089/rej.2006.0513. [DOI] [PubMed] [Google Scholar]
- 194.Liu Y., Hettinger C.L., Zhang D., Rezvani K., Wang X., Wang H. Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential therapeutic reagent for Huntington's disease. J Neurochem. 2014;129:539–547. doi: 10.1111/jnc.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ohkubo I., Gasa S., Namikawa C., Makita A., Sasaki M. Human erythrocyte multicatalytic proteinase: activation and binding to sulfated galacto- and lactosylceramides. Biochem Biophys Res Commun. 1991;174:1133–1140. doi: 10.1016/0006-291x(91)91538-n. [DOI] [PubMed] [Google Scholar]
- 196.Matsumura K., Aketa K. Activation of proteasome in sea urchin sperm by lysophosphatidylinositol and by sperm lipids: (proteasome/sea urchin/sperm/acrosome reaction/lysophospholipid) Dev Growth Differ. 1991;33:259–266. doi: 10.1111/j.1440-169X.1991.00259.x. [DOI] [PubMed] [Google Scholar]
- 197.Cai C.Z., Zhou H.F., Yuan N.N., Wu M.Y., Lee S.M.Y., Ren J.Y. Natural alkaloid harmine promotes degradation of alpha-synuclein via PKA-mediated ubiquitin-proteasome system activation. Phytomedicine. 2019;61:152842. doi: 10.1016/j.phymed.2019.152842. [DOI] [PubMed] [Google Scholar]
- 198.Yuan N.N., Cai C.Z., Wu M.Y., Zhu Q., Su H., Li M. Canthin-6-One accelerates alpha-synuclein degradation by enhancing UPS activity: drug target identification by crispr-cas9 whole genome-wide screening technology. Front Pharmacol. 2019;10:16. doi: 10.3389/fphar.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhou H., Li S., Li C., Yang X., Li H., Zhong H. Oxyphylla A promotes degradation of α-synuclein for neuroprotection via activation of immunoproteasome. Aging Dis. 2020;11:559. doi: 10.14336/AD.2019.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Buckley D.L., Crews C.M. Small-molecule control of intracellular protein levels through modulation of the ubiquitin proteasome system. Angew Chem Int Ed. 2014;53:2312–2330. doi: 10.1002/anie.201307761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sekizawa R., Ikeno S., Nakamura H., Naganawa H., Matsui S., Iinuma H. Panepophenanthrin, from a mushroom strain, a novel inhibitor of the ubiquitin-activating enzyme. J Nat Prod. 2002;65:1491–1493. doi: 10.1021/np020098q. [DOI] [PubMed] [Google Scholar]
- 202.Tsukamoto S., Hirota H., Imachi M., Fujimuro M., Onuki H., Ohta T. Himeic acid A: a new ubiquitin-activating enzyme inhibitor isolated from a marine-derived fungus, Aspergillus sp. Bioorg Med Chem Lett. 2005;15:191–194. doi: 10.1016/j.bmcl.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 203.Ungermannova D., Parker S.J., Nasveschuk C.G., Wang W., Quade B., Zhang G. Largazole and its derivatives selectively inhibit ubiquitin activating enzyme (E1) PLoS One. 2012;7 doi: 10.1371/journal.pone.0029208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yamanokuchi R., Imada K., Miyazaki M., Kato H., Watanabe T., Fujimuro M. Hyrtioreticulins A–E, Indole alkaloids inhibiting the ubiquitin-activating enzyme, from the marine sponge Hyrtios reticulatus. Bioorg Med Chem. 2012;20:4437–4442. doi: 10.1016/j.bmc.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 205.Helms K.M., Wilson R.C., Ogungbe I.V., Setzer W.N., Twigg P.D. Vitexin inhibits polyubiquitin synthesis by the ubiquitin-conjugating enzyme E2-25K. Nat Prod Commun. 2011;6:1411–1416. [PubMed] [Google Scholar]
- 206.Tsukamoto S., Takeuchi T., Rotinsulu H., Mangindaan R.E., van Soest R.W., Ukai K. Leucettamol A: A new inhibitor of Ubc13-Uev1A interaction isolated from a marine sponge. Leucetta aff Micro Bioorg Med Chem Lett. 2008;18:6319–6320. doi: 10.1016/j.bmcl.2008.10.110. [DOI] [PubMed] [Google Scholar]
- 207.Ushiyama S., Umaoka H., Kato H., Suwa Y., Morioka H., Rotinsulu H. Manadosterols A and B, sulfonated sterol dimers inhibiting the Ubc13–Uev1A interaction, isolated from the marine sponge Lissodendryx fibrosa. J Nat Prod. 2012;75:1495–1499. doi: 10.1021/np300352u. [DOI] [PubMed] [Google Scholar]
- 208.Komander D., Clague M.J., Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 209.Pfoh R., Lacdao I.K., Saridakis V. Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocr Relat Cancer. 2015;22:T35–T54. doi: 10.1530/ERC-14-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Reiner T., Parrondo R., De Las Pozas A., Palenzuela D., Perez-Stable C. Betulinic acid selectively increases protein degradation and enhances prostate cancer-specific apoptosis: possible role for inhibition of deubiquitinase activity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou B., Zuo Y., Li B., Wang H., Liu H., Wang X. Deubiquitinase inhibition of 19S regulatory particles by 4-arylidene curcumin analogue AC17 causes NF-κB inhibition and p53 reactivation in human lung cancer cells. Mol Cancer Therapeut. 2013;12:1381–1392. doi: 10.1158/1535-7163.MCT-12-1057. [DOI] [PubMed] [Google Scholar]
- 212.Lawson A.P., Long M.J.C., Coffey R.T., Qian Y., Weerapana E., El Oualid F. Naturally occurring isothiocyanates exert anticancer effects by inhibiting deubiquitinating enzymes. Cancer Res. 2015;75:5130–5142. doi: 10.1158/0008-5472.CAN-15-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yue W., Chen Z., Liu H., Yan C., Chen M., Feng D. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014;24:482–496. doi: 10.1038/cr.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Upadhyay A., Joshi V., Amanullah A., Mishra R., Arora N., Prasad A. E3 ubiquitin ligases neurobiological mechanisms: development to degeneration. Front Mol Neurosci. 2017;10:151. doi: 10.3389/fnmol.2017.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen D., Gehringer M., Lorenz S. Developing small-molecule inhibitors of HECT-type ubiquitin ligases for therapeutic applications: challenges and opportunities. Chembiochem. 2018;19:2123–2135. doi: 10.1002/cbic.201800321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gabrielsen M., Buetow L., Nakasone M.A., Ahmed S.F., Sibbet G.J., Smith B.O. A general strategy for discovery of inhibitors and activators of RING and U-box E3 ligases with ubiquitin variants. Mol Cell. 2017;68:456–470. doi: 10.1016/j.molcel.2017.09.027. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mund T., Lewis M.J., Maslen S., Pelham H.R. Peptide and small molecule inhibitors of HECT-type ubiquitin ligases. Proc Nat Acad U S A. 2014;111:16736–16741. doi: 10.1073/pnas.1412152111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhou N., Li J., Li T., Chen G., Zhang Z., Si Z. Matrine-induced apoptosis in Hep3B cells via the inhibition of MDM2. Mol Med Rep. 2017;15:442–450. doi: 10.3892/mmr.2016.5999. [DOI] [PubMed] [Google Scholar]
- 219.Zhang X., Gu L., Li J., Shah N., He J., Yang L. Degradation of MDM2 by the interaction between berberine and DAXX leads to potent apoptosis in MDM2-overexpressing cancer cells. Cancer Res. 2010;70:9895–9904. doi: 10.1158/0008-5472.CAN-10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mu R., Qi Q., Gu H., Wang J., Yang Y., Rong J. Involvement of p53 in oroxylin A-induced apoptosis in cancer cells. Mol Carcinog. 2009;48:1159–1169. doi: 10.1002/mc.20570. [DOI] [PubMed] [Google Scholar]
- 221.Fang J., Xia C., Cao Z., Zheng J.Z., Reed E., Jiang B.H. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 222.Li M., Zhang Z., Hill D.L., Chen X., Wang H., Zhang R. Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res. 2005;65:8200–8208. doi: 10.1158/0008-5472.CAN-05-1302. [DOI] [PubMed] [Google Scholar]
- 223.Qin J.J., Wang W., Sarkar S., Voruganti S., Agarwal R., Zhang R. Inulanolide A as a new dual inhibitor of NFAT1-MDM2 pathway for breast cancer therapy. Oncotarget. 2016;7:32566–32578. doi: 10.18632/oncotarget.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Qin J.J., Li X., Hunt C., Wang W., Wang H., Zhang R. Natural products targeting the p53–MDM2 pathway and mutant p53: recent advances and implications in cancer medicine. Gene Dis. 2018;5:204–219. doi: 10.1016/j.gendis.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee S.J., Langhans S.A. Anaphase-promoting complex/cyclosome protein Cdc27 is a target for curcumin-induced cell cycle arrest and apoptosis. BMC Cancer. 2012;12:44. doi: 10.1186/1471-2407-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Upadhyay A., Amanullah A., Mishra R., Kumar A., Mishra A. Lanosterol suppresses the aggregation and cytotoxicity of misfolded proteins linked with neurodegenerative diseases. Mol Neurobiol. 2018;55:1169–1182. doi: 10.1007/s12035-016-0377-2. [DOI] [PubMed] [Google Scholar]
- 227.Joshi V., Mishra R., Upadhyay A., Amanullah A., Poluri K.M., Singh S. Polyphenolic flavonoid (myricetin) upregulated proteasomal degradation mechanisms: eliminates neurodegenerative proteins aggregation. J Cell Physiol. 2019;234:20900–20914. doi: 10.1002/jcp.28695. [DOI] [PubMed] [Google Scholar]
- 228.Casarejos M.J., Perucho J., Lopez-Sendon J.L., Garcia de Yebenes J., Bettencourt C., Gomez A. Trehalose improves human fibroblast deficits in a new CHIP-mutation related ataxia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Law B.Y.K., Chan W.K., Xu S.W., Wang J.R., Bai L.P., Liu L. Natural small-molecule enhancers of autophagy induce autophagic cell death in apoptosis-defective cells. Sci Rep. 2014;4:a5510. doi: 10.1038/srep05510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lin S.R., Fu Y.S., Tsai M.J., Cheng H., Weng C.F. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int J Mol Sci. 2017;18:1412. doi: 10.3390/ijms18071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Marinković M., Šprung M., Buljubašić M., Novak I. Autophagy modulation in cancer: current knowledge on action and therapy. Oxid Med Cell Longev. 2018;2018:a8023821. doi: 10.1155/2018/8023821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rahman M.A., Rhim H. Therapeutic implication of autophagy in neurodegenerative diseases. BMB Rep. 2017;50:345–354. doi: 10.5483/BMBRep.2017.50.7.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sarkar S., Davies J.E., Huang Z., Tunnacliffe A., Rubinsztein D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J Biol Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 234.Wang B., Yang Q., Sun Y.Y., Xing Y.F., Wang Y.B., Lu X.T. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J Cell Mol Med. 2014;18:1599–1611. doi: 10.1111/jcmm.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tanaka M., Machida Y., Niu S., Ikeda T., Jana N.R., Doi H. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med. 2004;10:148–154. doi: 10.1038/nm985. [DOI] [PubMed] [Google Scholar]
- 236.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Campbell G.R., Spector S.A. Hormonally active vitamin D3 (1α,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011;286:18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Campbell G.R., Spector S.A. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Deretic V. Autophagy in tuberculosis. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a018481. a018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Joy S., Thirunavukkarasu L., Agrawal P., Singh A., Sagar B.K.C., Manjithaya R. Basal and starvation-induced autophagy mediates parasite survival during intraerythrocytic stages of Plasmodium falciparum. Cell Death Disc. 2018;4:43. doi: 10.1038/s41420-018-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Codogno P., Meijer A.J. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12:1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 242.Rabinowitz J.D., White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brophy M.L., Dong Y., Wu H., Rahman H.N.A., Song K., Chen H. Eating the dead to keep atherosclerosis at bay. Front Cardiovasc Med. 2017;4:a2. doi: 10.3389/fcvm.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bugliani M., Mossuto S., Grano F., Suleiman M., Marselli L., Boggi U. Modulation of autophagy influences the function and survival of human pancreatic beta cells under endoplasmic reticulum stress conditions and in type 2 diabetes. Front Endocrinol. 2019;10:a52. doi: 10.3389/fendo.2019.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.He C., Bassik M.C., Moresi V., Sun K., Wei Y., Zou Z. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang B., Zhou T.Y., Nie C.H., Wan D.L., Zheng S.S. Bigelovin, a sesquiterpene lactone, suppresses tumor growth through inducing apoptosis and autophagy via the inhibition of mTOR pathway regulated by ROS generation in liver cancer. Biochem Biophys Res Commun. 2018;499:156–163. doi: 10.1016/j.bbrc.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 247.Li X., Li X., Wang J., Ye Z., Li J.C. Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells. Int J Biol Sci. 2012;8:901–912. doi: 10.7150/ijbs.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang R., Zhang Q., Peng X., Zhou C., Zhong Y., Chen X. Stellettin B induces G1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep. 2016;6:27071. doi: 10.1038/srep27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhou Y., Liang X., Chang H., Shu F., Wu Y., Zhang T. Ampelopsin-induced autophagy protects breast cancer cells from apoptosis through Akt–mTOR pathway via endoplasmic reticulum stress. Cancer Sci. 2014;105:1279–1287. doi: 10.1111/cas.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ma K., Zhang C., Huang M.Y., Li W.Y., Hu G.Q. Cinobufagin induces autophagy-mediated cell death in human osteosarcoma U2OS cells through the ROS/JNK/p38 signaling pathway. Oncol Rep. 2016;36:90–98. doi: 10.3892/or.2016.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sun Z.L., Dong J.L., Wu J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed Pharmacother. 2017;85:303–312. doi: 10.1016/j.biopha.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 252.Xavier C.P., Lima C.F., Pedro D.F., Wilson J.M., Kristiansen K., Pereira-Wilson C. Ursolic acid induces cell death and modulates autophagy through JNK pathway in apoptosis-resistant colorectal cancer cells. J Nutr Biochem. 2013;24:706–712. doi: 10.1016/j.jnutbio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 253.Rubinsztein D.C., Mariño G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 254.Frake R.A., Ricketts T., Menzies F.M., Rubinsztein D.C. Autophagy and neurodegeneration. J Clin Invest. 2015;125:65–74. doi: 10.1172/JCI73944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hu G., Gong X., Wang L., Liu M., Liu Y., Fu X. Triptolide promotes the clearance of α-synuclein by enhancing autophagy in neuronal cells. Mol Neurobiol. 2017;54:2361–2372. doi: 10.1007/s12035-016-9808-3. [DOI] [PubMed] [Google Scholar]
- 256.Han J., Pan X.Y., Xu Y., Xiao Y., An Y., Tie L. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8:812–825. doi: 10.4161/auto.19471. [DOI] [PubMed] [Google Scholar]
- 257.Johansson I., Monsen V.T., Pettersen K., Mildenberger J., Misund K., Kaarniranta K. The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells. Autophagy. 2015;11:1636–1651. doi: 10.1080/15548627.2015.1061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yu X., Li C., Song H., Wang X., Guo Y., Cui L. Emodin attenuates autophagy response to protect the pancreas from acute pancreatitis failure. Pancreas. 2018;47:892–897. doi: 10.1097/MPA.0000000000001080. [DOI] [PubMed] [Google Scholar]
- 259.Seglen P.O., Gordon P.B. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Abliz A., Deng W., Sun R., Guo W., Zhao L., Wang W. Wortmannin, PI3K/Akt signaling pathway inhibitor, attenuates thyroid injury associated with severe acute pancreatitis in rats. Int J Clin Exp Pathol. 2015;8:13821–13833. [PMC free article] [PubMed] [Google Scholar]
- 261.Wang Y.A., Kammenga J.E., Harvey S.C. Genetic variation in neurodegenerative diseases and its accessibility in the model organism Caenorhabditis elegans. Hum Genom. 2017;11:12. doi: 10.1186/s40246-017-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mohanraj K., Karthikeyan B.S., Vivek-Ananth R.P., Chand R.P.B., Aparna S.R., Mangalapandi P. IMPPAT: a curated database of Indian medicinal plants, phytochemistry and therapeutics. Sci Rep. 2018;8:a4329. doi: 10.1038/s41598-018-22631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lu K., Bhat M., Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287–295. doi: 10.1007/s10456-016-9512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Scannell J.W., Bosley J. When quality beats quantity: decision theory, drug discovery, and the reproducibility crisis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jachak S.M., Saklani A. Challenges and opportunities in drug discovery from plants. Curr Sci. 2007;92:1251–1257. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.