Abstract
The phosphorylation of IκB by the multiprotein IκB kinase complex (IKC) precedes the activation of transcription factor NF-κB, a key regulator of the inflammatory response. Here we identified the mixed-lineage group kinase 3 (MLK3) as an activator of NF-κB. Expression of the wild-type form of this mitogen-activated protein kinase kinase kinase (MAPKKK) induced nuclear immigration, DNA binding, and transcriptional activity of NF-κB. MLK3 directly phosphorylated and thus activated IκB kinase alpha (IKKα) and IKKβ, revealing its function as an IκB kinase kinase (IKKK). MLK3 cooperated with the other two IKKKs, MEKK1 and NF-κB-inducing kinase, in the induction of IKK activity. MLK3 bound to components of the IKC in vivo. This protein-protein interaction was dependent on the central leucine zipper region of MLK3. A kinase-deficient version of MLK3 strongly impaired NF-κB-dependent transcription induced by T-cell costimulation but not in response to tumor necrosis factor alpha or interleukin-1. Accordingly, endogenous MLK3 was phosphorylated and activated by T-cell costimulation but not by treatment of cells with tumor necrosis factor alpha or interleukin-1. A dominant negative version of MLK3 inhibited NF-κB- and CD28RE/AP-dependent transcription elicited by the Rho family GTPases Rac and Cdc42, thereby providing a novel link between these GTPases and the IKC.
In mammals, the inducible transcription factor NF-κB is composed of a homo- or heterodimer of various DNA-binding subunits. The most frequently detected form of NF-κB is a p50-p65 dimer which is retained in the cytoplasm of most cell types by an inhibitory subunit, called IκB. A wide variety of stimuli including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), lipopolysaccharide, phorbol-12-myristate 13-acetate (PMA), and T-cell costimulation and more general stimuli such as γ radiation lead to the induced phosphorylation of the two major forms of IκB proteins, termed IκB-α and IκB-β (2, 67). The inducible phosphorylation of IκB-α at serines 32 and 36 and of IκB-β at serines 19 and 23 facilitates their interaction with β-TrCP (47) and its subsequent ubiquitinylation and proteolytic degradation (71). NF-κB migrates to the nucleus and activates the expression of numerous target genes which are important not only for the immune and inflammatory response (2) but also for other functions such as the regulation of apoptosis (1) and cell cycle progression (18, 22, 30). The inducible phosphorylation of IκB-α is mediated by kinases contained within a high-molecular-weight complex (relative molecular mass, 700 to 900 kDa), the multisubunit IκB kinase complex (IKC) (6, 46). The IKC contains two homologous kinases, termed IKKα and IKKβ (13, 50, 60, 69, 73). The leucine zipper domains contained in both IKKs allow the formation of homo- or heterodimers in vitro, although the heterodimeric form predominates in vivo (48, 69). Gene disruption experiments revealed an important function of IKKβ for TNF-α- and IL-1-induced phosphorylation and degradation of IκB-α (40, 64). In contrast, the IKKα protein is involved in multiple morphogenic events such as skeletal development and the proliferation of epidermal keratinocytes (23, 41, 64). Further evidence against the involvement of IKKα in the cytokine-mediated activation of NF-κB is provided by experiments showing that the mutation of two serine residues (position 177 and 181) in the activation loop of IKKβ prevents cytokine and NF-κB-inducing kinase (NIK)-triggered IKK activation, whereas the elimination of the equivalent sites in IKKα has no effect (12). IKKβ binds to NEMO/IKK-γ (also called IKKAP), a protein composed of several coiled-coil motifs (51, 61, 70). Both IKKs are found in association with the scaffold protein IKAP, which was found to associate with NIK (7). The MAPKKK NIK was identified as a direct activator of both IKKs (44, 45), but recent results point to the existence of further IKK-activating kinases (32, 48). Since a variety of unrelated stimuli can activate NF-κB—and thus presumably the IKC—it seems unlikely that all of these inducers rely on the activity of one single IKKK. It is therefore reasonable to assume that different cell types and signaling pathways utilize distinct IKC activating kinases. Along this line, it was shown that the proto-oncogene product Cot kinase participates in the CD3/CD28 but not TNF-α-mediated activation of NF-κB (43). Triggering of the IL-1 receptor activates the transforming growth factor β-activated kinase 1 (TAK1) kinase, which phosphorylates NIK and thereby stimulates both IKKs (56).
Mixed-lineage kinases (MLKs) form a family of serine/threonine kinases with the features of mitogen-activated protein kinase kinase kinase (MAPKKKs) (16). The MLK family consists of two subgroups, one containing the highly related MLK1 (14), MLK2/MST (15, 33), and MLK3/SPRK/PTK (16, 17, 26) kinases. MLK2 and MLK3 share a number of structural motifs, including an N-terminal SH3 domain, followed by the catalytic domain and two tandem leucine/isoleucine zippers, a basic region, a Cdc42/Rac binding motif, and a proline-rich C terminus. MLK3 can be activated by interaction with the GTP-bound forms of the Rho family members Cdc42 and Rac (65). MLK3 is able to form dimers via its tandem leucine zipper. This dimerization can be induced by activated forms of the GTPase Cdc42 and leads to transphosphorylation and subsequent activation of MLK3 (36). Activated MLK3 then strongly stimulates the SAPK/JNK pathways but has no effect on the activation of ERKs (65, 66). The impact of MLK3 on the activation of p38/HOG1 is a matter of debate (65, 66). MLK3 exerts its function presumably by directly phosphorylating JNKK/SEK1 (for the JNK pathway) and MKK6 (for the p38 pathway) (59, 66). Dominant negative versions of MLK3 prevent JNK activation induced by Rac and Cdc42 but not JNK activation induced by MEKK1 (65).
In this study, we identified MLK3 as an activator of NF-κB. MLK3 was selectively involved in the T-cell costimulation-induced and Rac/Cdc42-mediated NF-κB activation. Only the wild-type form of MLK3, not a MLK3 mutant lacking its tandem leucine zipper, was constitutively attached to the IKC. Phosphorylation and thus activation of endogenous MLK3 was seen after CD3-CD28 costimulation of T cells but not upon activation with TNF-α or IL-1. Accordingly, a dominant negative version of MLK3 selectively prevented CD3-CD28-induced transcription of reporter genes controlled either by NF-κB or the CD28RE/AP-1 enhancer element contained within the IL-2 promoter, suggesting that this MAPKKK feeds pathway-specific signals into the IKC. Its ability to block Rac/Cdc42-mediated activation of NF-κB places MLK3 downstream from these GTPases, thus constituting a novel IKC activation pathway in T cells.
MATERIALS AND METHODS
Cell culture and transient transfections.
HeLa, 293T, and 293 cells were grown in Dulbecco's modified Eagle medium supplemented with 10% (vol/vol) fetal calf serum and 1% (vol/vol) penicillin-streptomycin (all from Life Technologies). These cell lines were transfected using the Superfect reagent (Qiagen Inc.) according to the instructions of the manufacturer. Jurkat T-leukemia cells expressing the large T antigen were grown at 37°C in RPMI 1640 medium containing 10% (vol/vol) heat-inactivated fetal calf serum, 10 mM HEPES, 2 mM glutamine, 1% (vol/vol) penicillin-streptomycin (all from Life Technologies), and 2 mg of G418 per ml. Transfection of Jurkat cells was performed by electroporation using a gene pulser (Bio-Rad) at 250 V and 950 μF.
Expression vectors and reporters.
The reporter plasmids (κB)3-luc (11) and 4×RE/AP-Luc (62), expression vectors for MLK3 and derivatives thereof (36), constitutively active variants of Cdc42 and Rac (8), ASK1 (25), and glutathione S-transferase (GST)–IKK plasmids (55) were previously described. The wild-type and mutant forms of IKKα and -β (44, 51) and the bacterial expression vectors for GST-IκB (1-54) (69), MEKK1 and MEKK1Δ (37), and NIK (45) have been published.
Antisera and reagents.
The MLK3-specific (αMLK3) (sc-536) and αIKKβ (H-4) antibodies and supershifting antibodies recognizing NF-κB p50 (sc-1190X) and p65 (sc-372X) were from Santa Cruz Biotechnology Inc., αFlag antibody M2 was from Sigma, hemagglutinin-specific (αHA) antibody 12CA5 was from Roche Molecular Biochemicals, αMyc antibody 9E10 was from Santa Cruz, and αIKKα antibody B7.1 was from Pharmingen. αTCR(CD3) (OKT3) and αCD28 antibodies were kindly provided by R. Breitkreuz. All other reagents were from Sigma or Roche Molecular Biochemicals.
Electrophoretic mobility shift assays (EMSAs).
HeLa cells (2 × 106) were washed twice with cold phosphate-buffered saline and harvested by scraping with a rubber policeman. The pellet was resuspended in TOTEX buffer, and equal amounts of protein contained in the supernatant were tested for DNA binding essentially as described elsewhere (21). The supershift experiments were performed by preincubating the total cell extracts with 2 μg of the relevant antibodies for 15 min at 4°C.
Immunoprecipitation experiments and Western blotting.
Cell extracts contained in NP-40 lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, 0.5 mM sodium vanadate, leupeptin [10 μg/ml], aprotinin [10 μg/ml], 1% [vol/vol] NP-40, 10% [vol/vol] glycerol) were either directly analyzed by Western blotting or immunoprecipitated following preclearance by adding 1 to 2 μg of antibody and 25 μl of protein A/G-Sepharose. After rotation for at least 4 h on a spinning wheel at 4°C, the immunoprecipitates were washed five times in lysis buffer. Phosphatase treatment was performed by incubating the cell extract with 400 U of λ phosphatase for 2 h at 30°C according to the instructions of the manufacturer (Biolabs). Immunoprecipitates were boiled in 1× sodium dodecyl sulfate (SDS) sample buffer and separated by SDS-polyacrylamide gel electrophoresis (PAGE) prior to blotting to a polyvinylidene difluoride membrane (Millipore). The membrane was then incubated in a small volume of TBST (25 mM Tris-HCl [pH 7.4], 137 mM NaCl, 5 mM KCl, 0.7 mM CaCl2, 0.1 mM MgCl2, 0.1% [vol/vol] Tween 20) containing various dilutions of the primary antibodies. The proteins were detected with an appropriate secondary antibody coupled to horseradish peroxidase and visualized by enhanced chemiluminescence according to the instructions of the manufacturer (Amersham Life Science).
Luciferase assays.
Harvested cells were lysed in reporter lysis buffer (25 mM Tris-phosphate, 2 mM dithiothreitol, [DTT], 2 mM trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 10% [vol/vol] glycerol, 1% [vol/vol] Triton X-100). Luciferase activity was determined in a luminometer (Duo Lumat LB 9507; Berthold) by injecting 50 μl of assay buffer [40 mM Tricine, 2.14 mM (MgCO3)4Mg(OH)2 · 5H2O, 5.34 mM MgSO4, 0.2 mM EDTA, 66.6 mM dithiothreitol, DTT, 540 μM coenzyme A, 940 μM luciferin, 1.06 mM ATP] and measuring light emission for 10 s. The results were normalized to the activity of β-galactosidase expressed by a cotransfected lacZ gene under the control of a constitutive Rous sarcoma virus promoter.
Immunofluorescence.
The p65 and MLK3 proteins were detected by immunostaining essentially as described elsewhere (73). Briefly, HeLa cells were grown on coverslips and analyzed by immunofluorescence 24 h after transfection. Cells were fixed with 3.7% (wt/vol) paraformaldehyde for 15 min at room temperature. After permeabilization with 0.02% (vol/vol) NP-40 in phosphate-buffered saline for 1 min, the cells were incubated 2 h with 50% (vol/vol) goat serum. The primary antibodies (αp65 and fluorescein isothiocyanate [FITC]-conjugated αFlag, respectively) were diluted 1:100 and added for 2 h at 37°C. After further washing steps, the p65-stained cells were incubated with a secondary Cy3-coupled antibody. The αp65- and αFlag-stained cells were photographed with a Zeiss Axiophot microscope and further analyzed using Axiovision software.
In vitro kinase assays.
Cells were lysed in NP-40 lysis buffer, and the IKK proteins contained in the cell lysate were immunoprecipitated. The precipitate was washed three times in lysis buffer and two times in kinase buffer (20 mM HEPES/KOH [pH 7.4], 25 mM β-glycerophosphate, 2 mM DTT, 20 mM MgCl2). The kinase assay was performed in a final volume of 20 μl of kinase buffer containing 40 μM ATP, 5 μCi of [γ-32P]ATP, and the purified substrate proteins. GST–IκB-α (1-54) was expressed and purified from bacteria; GST-IKKα KM and GST-IKKβ KA proteins were purified from 293T cells. After incubation for 20 min at 30°C, the reaction was stopped by the addition of 5× SDS loading buffer. After separation by SDS-PAGE, the gel was fixed, dried, and quantified with a phosphorimager.
RESULTS
Ectopic expression of MLK3 activates NF-κB.
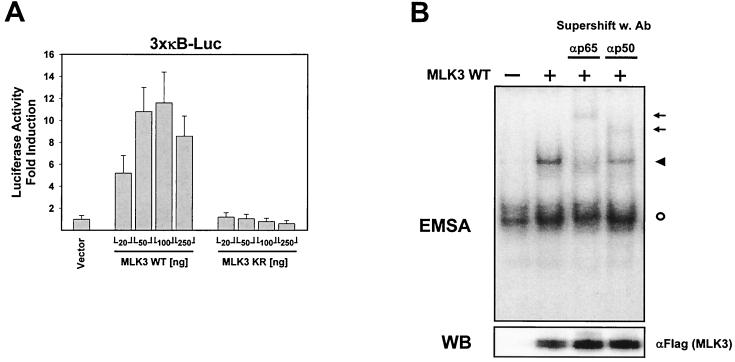
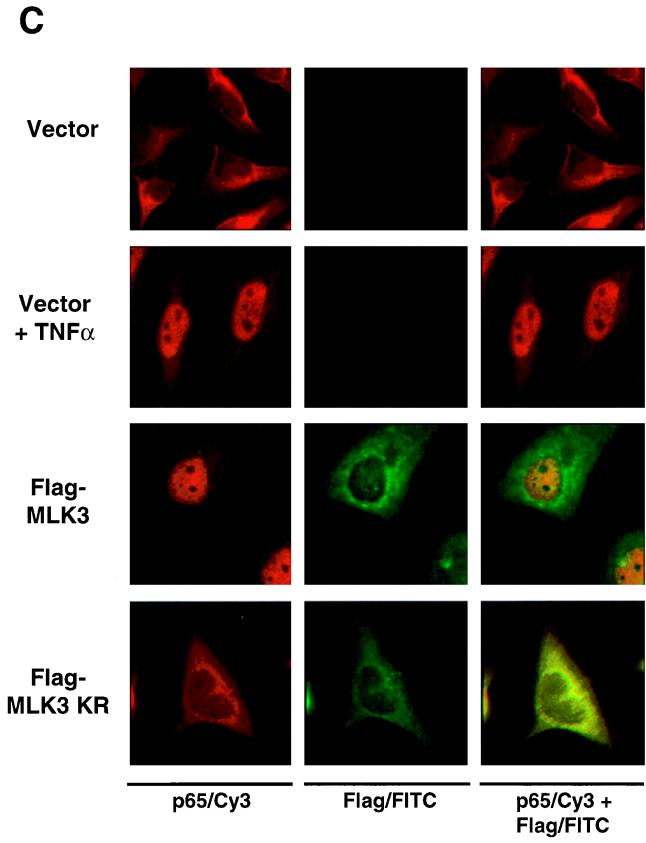
In the course of experiments on kinases of the JNK activation cascade, we noticed an activation of NF-κB-dependent transcription upon overexpression of MLK3 but not by any other of the numerous kinases tested (data not shown). To examine this finding in more detail, we cotransfected an NF-κB-dependent luciferase reporter gene with increasing amounts of expression vectors encoding either wild-type MLK3 or an inactive MLK3 variant (MLK3 KR) which carries a point mutation in the ATP binding loop of the kinase domain. The ectopic expression of MLK3 activated NF-κB-driven transcription in a dose-dependent manner (Fig. 1A). Maximal NF-κB induction was achieved with intermediate amounts of MLK3 expression vector, whereas a further increase in the amount of plasmid resulted in a markedly diminished NF-κB activation. In contrast, expression of the kinase-dead form of MLK3 was unable to activate NF-κB, revealing the importance of the kinase function for NF-κB triggering. We then asked whether ectopic expression of MLK3 would induce DNA-binding activity of NF-κB. To address this question, we transiently transfected HeLa cells with an expression vector for MLK3 and assayed the DNA-binding activity of NF-κB by EMSA. MLK3 expression resulted in the induction of NF-κB DNA binding (Fig. 1B). The subunit composition of the induced DNA-protein complex was further characterized with supershifting antibodies recognizing either the NF-κB p50 or p65 subunits. The NF-κB–DNA complex was supershifted by each of the two antibodies (Fig. 1B), indicating that the MLK3-induced NF-κB dimer contains the typical p50 and p65 subunits. However, expression of MLK3 KR failed to generate the DNA-binding activity of NF-κB (data not shown). MLK3-induced NF-κB activation was further investigated by examining the subcellular distribution of p65 and MLK3 by indirect immunofluorescence. HeLa cells were transfected with vectors encoding Flag-tagged MLK3, MLK3 KR, or the empty expression vector as a control. Double staining with αp65 and αFlag-FITC was used to identify transfected cells. As shown in Fig. 1C, the MLK3-induced nuclear translocation of p65 was as complete as the nuclear translocation triggered by TNF-α treatment. In contrast, ectopic expression of MLK3 KR failed to induce nuclear translocation of NF-κB p65, thereby leaving all of the p65 protein in the cytoplasm. Double staining for NF-κB p65 and MLK3 KR revealed areas of overlapping localization.
FIG. 1.
Ectopic expression of MLK3 activates NF-κB. (A) HeLa cells were transiently transfected with an NF-κB-dependent luciferase reporter gene together with increasing amounts of an expression vector for either wild-type (WT) MLK3 or a kinase inactive version of MLK3 (MLK3 KR) or an empty expression vector as a control. Luciferase activity was determined 20 h posttransfection. Results are expressed as average fold induction relative to vector-transfected cells. Mean values of three independent experiments performed in duplicate are shown; bars indicate standard errors of the mean. (B) HeLa cells were transfected with an MLK3 expression vector as shown; 24 h posttransfection, total cell extracts were prepared, supershifting antibodies (Ab) were added as indicated, and the DNA-binding activity of NF-κB was assayed by EMSA (upper). An autoradiogram showing the shifted DNA-protein complexes is displayed. Arrows point to supershifted complexes, the filled arrowhead indicates the location of the DNA-NF-κB complex, and the circle indicates the position of a constitutively DNA-binding protein. A sample of each lysate was analyzed by Western blotting (WB) for protein expression of MLK3 (lower). (C) HeLa cells were transiently transfected as indicated with a plasmid encoding either MLK3 or MLK3 KR or an empty expression vector as a control. One day after transfection, the intracellular localization of p65 was investigated by indirect immunofluorescence using an αp65 antibody and a Cy3-conjugated secondary antibody (left row). The cells that served as a positive control received 1000 U of TNF-α for 25 min. MLK3 distribution was analyzed by direct immunofluorescence using an αFlag antibody coupled to FITC (middle row). An overlay of both stainings is shown at the right. Yellow-stained areas indicate colocalization of MLK3 and p65.
MLK3 activates both IKKα and IKKβ.
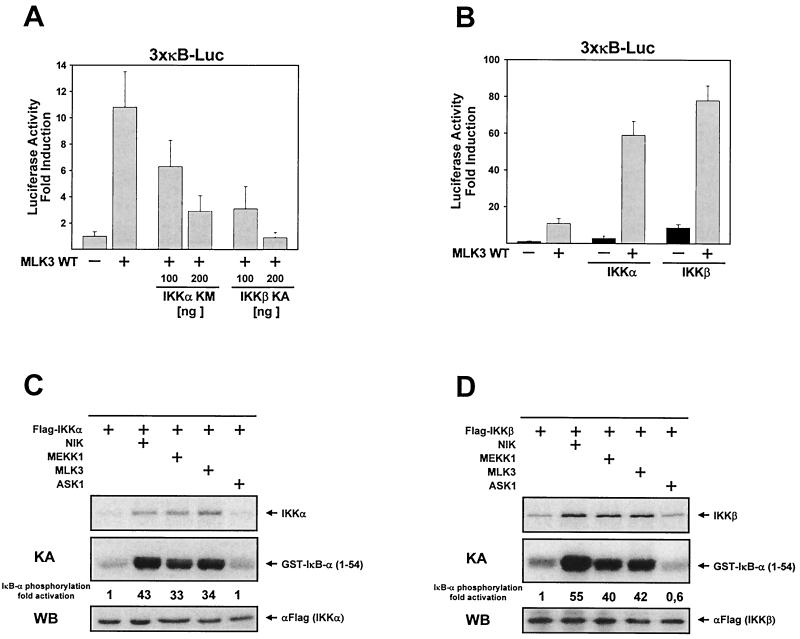
There is recent evidence for the existence of alternative NF-κB activation pathways. Reoxygenation-induced tyrosine phosphorylation of IκB-α allows the association of the regulatory subunit of phosphatidylinositol 3-kinase to IκB, thereby sequestering the inhibitory subunit from NF-κB (4), whereas UV radiation leads to phosphorylation-independent degradation of IκB-α (3, 39). Therefore, we examined whether MLK3-induced NF-κB activation is mediated via activation of the IKKs by transiently transfecting HeLa cells with an NF-κB-dependent reporter gene along with various combinations of expression vectors for MLK3 and dominant negative forms of IKKα and IKKβ. Under these conditions, MLK3-induced activation of NF-κB-dependent transcription was dose dependently impaired upon coexpression of either IKKα KM or IKKβ KA (Fig. 2A). The dominant negative form of IKKβ inhibited the MLK3-submitted signal more completely than IKKα KM, suggesting that IKKβ is more important for this activation process. This observation is in good accordance with the predominant role of IKKβ for the stimulus-induced phosphorylation of IκB as revealed by gene disruption experiments (40, 64). To obtain further evidence for the role of IKKs, we expressed the wild-type forms of MLK3, IKKα, and IKKβ and investigated the effects on activation of a NF-κB-dependent reporter gene. Expression of suboptimal amounts of IKKα and IKKβ, which by themselves only moderately activated NF-κB-dependent transcription, strongly synergized with coexpressed MLK3 to activate the κB-dependent reporter gene (Fig. 2B). It was then reasonable to examine whether ectopic expression of MLK3 induces the enzymatic activity of both IKKs. The transfection of moderate amounts of epitope-tagged IKKs in HeLa cells allows their incorporation into functional cytokine-responsive high-molecular-weight IKCs (32, 38; S. P. Hehner, unpublished observation), making this cellular system ideally suited for functional studies on IKKs. Accordingly, HeLa cells were transiently transfected with expression vectors for Flag-tagged IKKα either alone or in combination with vectors encoding MLK3 and the kinases NIK, MEKK1, and ASK1 as a control. We immunoprecipitated the tagged IKKα protein and examined its activity by measuring the phosphorylation of the exogenously added substrate protein [GST–IκB-α (1-54)] in immune complex kinase assays (Fig. 2C). MLK3 expression activated the kinase activity of IKKα as strong as the known IKK activator MEKK1, whereas the control MAPKKK ASK1 did not affect IKKα activity. In agreement with previous reports (43, 54), NIK was even more potent in the activation of IKKα than MEKK1. The phosphorylation of IKKα was induced by MLK3, MEKK1, and NIK to comparable extents (Fig. 2C). We assayed the impact of MLK3 expression on IKKβ activity by an analogous experimental approach by ectopically expressing IKKβ instead of IKKα. As seen for IKKα, the expression of MLK3 induced IKKβ activity as efficiently as MEKK1, but again expression of NIK induced the IKKβ activity to an even greater extent (Fig. 2D). All three kinases also induced the phosphorylation of IKKβ, clearly indicating that MLK3 activates NF-κB on the classical pathway employing IKKα and IKKβ.
FIG. 2.
MLK3 activates NF-κB via IKKα and IKKβ. (A) HeLa cells were cotransfected with a κB-dependent reporter gene and the indicated combinations of expression vectors encoding MLK3, IKKα KM, and IKKβ KA. Luciferase activity was determined 20 h posttransfection. Mean values from two independent experiments performed in duplicate are shown; bars indicate standard errors of the mean. WT, wild type. (B) Experiment performed as for panel A except that vectors encoding wild-type IKKα and IKKβ were used. (C) Flag-tagged IKKα was expressed either alone or in combination with NIK, MEKK1, MLK3, or ASK1 in HeLa cells; 24 h posttransfection, cell lysates were prepared and IKKα was immunoprecipitated. Kinase activity was determined by immune complex kinase assays (KA) using purified GST–IκB-α (1-54) as substrate. An autoradiogram from a reducing SDS-gel shows IKKα phosphorylation (upper) and phosphorylation of the recombinant substrate protein and a quantitative evaluation obtained by phosphorimaging (middle). A fraction of the immunoprecipitate was analyzed by Western blotting (WB) for IKKα expression (lower). (D) Experiment performed as for panel C except with an expression vector encoding IKKβ instead of IKKα. Representative results are shown.
The leucine zipper of MLK3 is required for IKK activation.
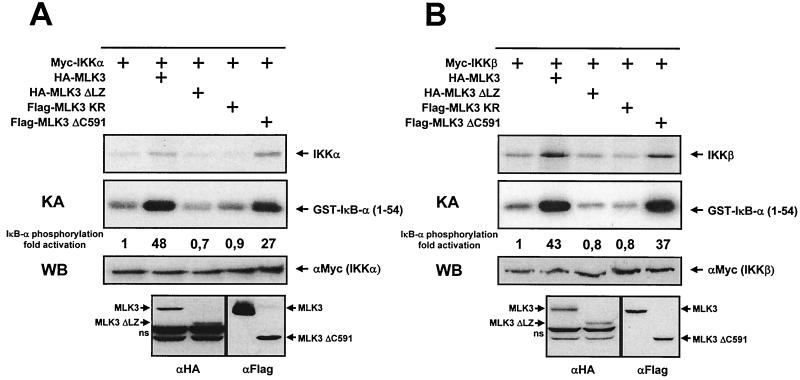
We then determined the domains within the MLK3 protein that are important for IKK activation. HeLa cells were transiently transfected with an expression vector for IKKα either alone or along with MLK3 and various derivatives thereof. The activity of immunoprecipitated IKKα was determined by immune complex kinase assays. Here the wild-type form of MLK3 and MLK3ΔC591, a variant which lacks the C-terminal 256 amino acids including the proline-rich region, efficiently activated IKKα, whereas MLK3ΔLZ, a deletion mutant that lacks the tandem leucine zippers, was as inactive as the kinase-dead mutant MLK3 KR (Fig. 3A). These two versions of MLK3 also failed to induce the phosphorylation of IKKα. In an analogous experimental approach, we tested the impact of these MLK3 variants on IKKβ activity. Kinase assays using immunoprecipitated epitope-tagged IKKβ showed the functionality of MLK3 and MLK3ΔC591 but revealed the inactivity of MLK3ΔLZ and MLK3 KR (Fig. 3B). Both inactive mutants were unable to induce the phosphorylation of IKKβ, showing that the leucine zipper is required for activation of the two IKKs. Similarly, the expression of MLK3ΔLZ did not induce the expression of an NF-κB-dependent luciferase gene (data not shown).
FIG. 3.
Mapping of the MLK3 domains required for NF-κB activation. (A) HeLa cells were transfected with expression vectors for Myc-tagged IKKα either alone or together with the indicated MLK3 variants as shown. After 24 h, in vitro kinase assays (KA) were performed. An autoradiogram showing the phosphorylation of IKKα (upper) and GST-IκB-α (lower) is displayed. Control Western blots (WB) ensuring the correct expression of the differentially tagged proteins are shown in the lower part. (B) Experiment performed as for panel A except that an expression vector for IKKβ was transfected. Representative results are displayed. ns, nonspecific band.
MLK3 is an IKKK.
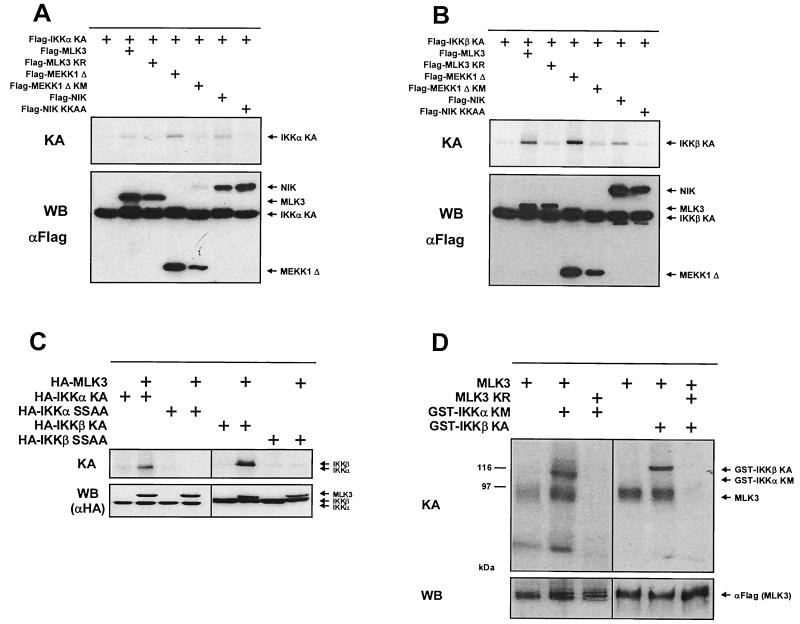
Both IKKs are activated upon transphosphorylation on serines contained in the activation loop and subsequently autophosphorylate themselves (12, 44). To explore whether MLK3 is able to transphosphorylate IKKα, a vector coding for a kinase-dead version of IKKα (to exclude autophosphorylation) was coexpressed in HeLa cells with active and inactive forms of MLK3, NIK, and the catalytic domain of MEKK1 (MEKK1Δ), respectively. All coexpressed proteins carried identical Flag tags to allow copurification of proteins by immunoprecipitation. Subsequent in vitro immune complex assays revealed that all three kinases were able to transphosphorylate IKKα KA (Fig. 4A). The inactive versions of the three kinases failed to induce IKKα KA phosphorylation, thereby making IKKα phosphorylation by coprecipitating kinases unlikely. We also investigated the transphosphorylation of IKKβ by coexpression of Flag-tagged IKKβ KA together with the various active and inactive forms of MLK3, NIK, and MEKK1Δ. The IKKβ KA protein was transphosphorylated upon expression of MLK3 and the other two control kinases, showing that MLK3 can induce the IKK-activating phosphorylation as efficiently as the other two IKKKs tested (Fig. 4B). Activation of IKKs relies on transphosphorylation of conserved serines contained in the T loop. Whereas IKKα becomes phosphorylated at serine residue 176 (45), IKKβ activation necessitates phosphorylation of serines 177 and 181 (12). We therefore tested whether MLK3 activates both IKKs by phosphorylating them at the described serine residues or whether MLK3 activation employs an alternative activation pathway. IKKα KA and IKKα KA (SA), a mutant where serine 176 was mutated to alanine, were coexpressed with MLK3 in HeLa cells. MLK3 and IKKα proteins were isolated by coimmunoprecipitation with αHA antibodies, and phosphorylation of IKKα was determined by immune complex kinase assays (Fig. 4C). IKKα KA but not IKKα KA (SA) was phosphorylated by MLK3. In parallel, we determined phosphorylation of either IKKβ KM or the double-point mutant IKKβ KM (SSAA) after coexpression and coimmunoprecipitation with MLK3. The IKKβ form with mutated serines 177 and 181 was not phosphorylated by MLK3 (Fig. 4C), showing that MLK3 activates both IKKs via phosphorylation of the serines contained in the activation loop. These findings suggest a direct phosphorylation of both IKKs by MLK3. This question was directly addressed by in vitro kinase assays using purified GST-IKKs as substrate proteins. Flag-tagged MLK3 and the MLK3 kinase-dead version as a control were expressed in HeLa cells, immunoprecipitated, and incubated with purified GST-IKK proteins in the presence of [γ-32P]ATP. Both, the GST-IKKα KM and GST-IKKβ KA proteins were phosphorylated in the presence of the wild-type but not the inactive form of MLK3 (Fig. 4D). These experiments also revealed a constitutive phosphorylation of overexpressed MLK3. Since both substrate proteins have no intrinsic kinase activity, this experiment suggests that the IKKs can serve as direct substrates for MLK3 at least in vitro.
FIG. 4.
MLK3 directly phosphorylates IKKα and IKKβ. (A) A plasmid encoding Flag-tagged IKKα KA was transfected either alone or along with Flag-tagged expression vectors for the wild-type and kinase-dead forms of MLK3, MEKK1Δ, and NIK into HeLa cells. After 24 h, the proteins were purified by immunoprecipitation and phosphorylation of IKKα KA was determined by in vitro kinase assays (KA) (upper). Aliquots of the extracts were analyzed for expression of the transfected proteins by Western blotting (WB) (lower). (B) HeLa cells were transfected with an expression vector for Flag-tagged IKKβ KA either alone or in combination with the MAPKKKs used for panel A. The cells were further analyzed for IKKβ KA phosphorylation and protein expression. (C) HeLa cells were transfected with expression vectors encoding HA-tagged versions of MLK, IKKα KA, IKKα KA (SA), IKKβ KM, and IKKβ KM (SSAA) as indicated. One day later, proteins were isolated by coimmunoprecipitation with αHA antibodies, and phosphorylation of IKKα and IKKβ was determined by in vitro kinase assays (upper). Western blots demonstrating comparable expression of transfected proteins are displayed (lower). (D) HeLa cells were transiently transfected with expression vectors for either the wild-type or the kinase-deficient form of MLK3. MLK3 was immunoprecipitated from cell lysates, and its activity was determined by immune complex kinase assays using 1 μg of purified GST-IKKα KM or GST-IKKβ KA as substrate. An autoradiogram from a reducing SDS-gel is shown (upper). A sample of each lysate was analyzed by immunoblotting for protein expression of MLK3 (lower). Sizes are indicated in kilodaltons.
Cooperative activation of IKKs by the IKKKs MLK3, NIK, and MEKK1.
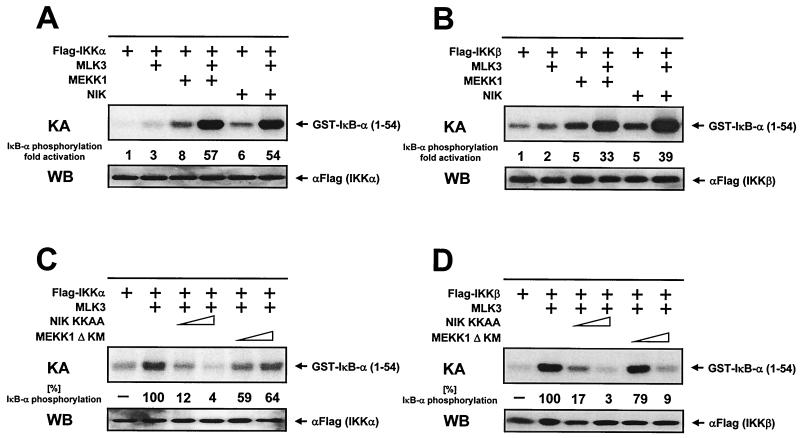
The existence of several IKK kinases raises the possibility of functional cross talk or cooperativity between these kinases. Therefore, it was of interest to study the functional interaction between the various IKKKs in nonstimulated cells, since the various signaling pathways (e.g., TNF-α, IL-1β, or T-cell costimulation) affect many different downstream targets and exert pleiotropic effects. To address this issue, HeLa cells were transfected with IKKα in the absence or presence of various combinations of expression vectors encoding MLK3, MEKK1, and NIK. To allow the detection of costimulatory effects, only low amounts of IKKK expression vectors were transfected, resulting only in suboptimal IKK activation. IKKα was immunoprecipitated, and its kinase activity was determined. The weak induction of IKKα activity induced by suboptimal amounts of MLK3 was synergistically augmented upon coexpression of MEKK1 (Fig. 5A). Similarly, the MLK3-derived signal was further boosted upon coexpression of NIK, showing a functional cooperation between MLK3 and the two other IKKKs. Subsequently, we tested whether the three IKKKs would also cooperate for the activation of IKKβ. Here again, the MLK3-induced IKKβ activity was synergistically activated by coexpression of either NIK or MEKK1 (Fig. 5B). Assuming that the different signaling cascades differentially employ the various IKKKs, these findings might help to explain the synergistic NF-κB activation occurring after the simultaneous administration of different NF-κB inducers. To further evaluate the relative contribution of NIK and MEKK1 for the transmission of the MLK3-derived signals, we investigated the impact of coexpressed dominant negative versions of NIK and MEKK1Δ on IKK activity induced by MLK3. The expression of dominant negative NIK (NIK KKAA) strongly reduced the MLK3-induced IKKα activity in a dose-dependent manner (Fig. 5C). In contrast, the overexpression of MEKK1Δ KM only marginally impaired the activity of IKKα. We then performed an analogous set of experiments to test the effects of dominant negative versions of NIK and MEKK1 on MLK3-induced activation of IKKβ. The kinase-dead versions of NIK and MEKK1 prevented the MLK3-induced activation of IKKβ in a dose-dependent fashion (Fig. 5D). The preferential inhibition of IKKβ activity by dominant negative MEKK1 is in line with previous findings showing that MEKK1 acts preferentially on IKKβ (34, 54).
FIG. 5.
MLK3, NIK, and MEKK1 synergize in IKK activation. (A) Flag-tagged IKKα was expressed either alone or together with various combinations of MLK3 (150 ng), MEKK1 (250 ng), and NIK (150 ng), as indicated, in HeLa cells. Cells were lysed 24 h later; IKKα was immunoprecipitated and its kinase activity (KA) was determined using GST–IκB-α (1-54) as substrate. An autoradiogram from a reducing SDS-gel and its quantitative evaluation are shown. WB, Western blot. (B) Experiment performed as for panel A except with IKKβ instead of IKKα. (C) HeLa cells were transfected with an expression vector for IKKα alone or together with vectors encoding MLK3 (500 ng) and 1 or 2 μg of dominant negative forms of NIK and MEKK1. IKKα kinase activity was determined by immune complex assays. Full kinase activation seen upon coexpression of MLK3 and IKKα was arbitrarily set as 100%. (D) IKKβ was coexpressed with MLK3 and the dominant negative forms of NIK and MEKK1 as shown. The experiment and its analysis were carried out as for panel C. Representative results from kinase assays and control Western blots are shown.
The leucine zipper of MLK3 is required for binding to IKKα and IKKβ in vivo.
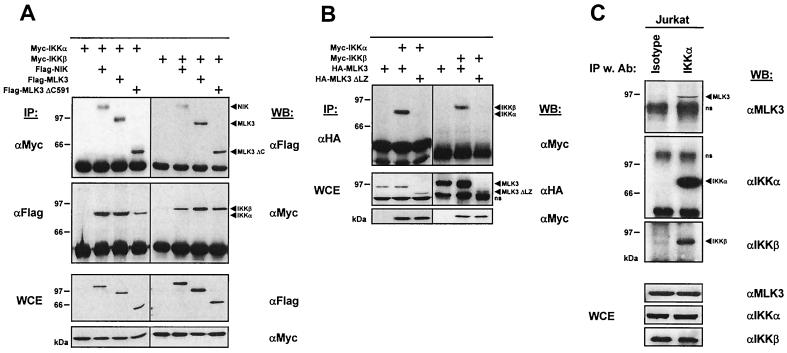
The overlapping subcellular localization of MLK3 KR and NF-κB p65 raises the possibility for a physical association between this MAPKKK and the IKC. To address this question, we examined whether MLK3 coprecipitates with its substrate proteins IKKα and IKKβ. 293 cells were transfected with epitope-tagged versions of IKKα and IKKβ either alone or together with a vector encoding MLK3, MLK3ΔC, or NIK as a positive control. The IKKs were immunoprecipitated from an aliquot of the cell lysates, and the associated proteins were detected by immunoblotting. Both IKKs were found to coprecipitate with MLK3, MLK3ΔC, and NIK (Fig. 6A). In a complementary experimental approach, the MAPKKKs were immunoprecipitated from another aliquot of the cell lysate, followed by the detection of associated IKK proteins by Western blotting. Also, these experiments revealed the mutual binding of MLK3 and MLK3ΔC to IKKα and IKKβ. To explore the possible function of the MLK3 leucine zipper region for binding to components of the IKC, we transfected 293 cells with expression vectors for both IKKs either alone or along with plasmids encoding the wild-type or leucine zipper-deficient version of MLK3. The wild-type form of MLK3 coprecipitated with IKKα and IKKβ, whereas the MLK3ΔLZ version failed to bind (Fig. 6B). We then investigated whether MLK3 is also found in association with the IKC without ectopic expression of the various binding partners. The IKC was isolated from Jurkat cell extracts by immunoprecipitation using a monoclonal αIKKα antibody (61). Subsequent immunoblotting revealed that MLK3 coimmunoprecipitated only with αIKKα antibodies, not with isotype-matched control antibodies, demonstrating the physical association of MLK3 with the IKC in vivo (Fig. 6C). The association of MLK3 with the IKC was also seen in extracts from CD3-CD28-PMA-stimulated Jurkat cells (data not shown), showing the constitutive association with the IKC. In the absence of antibodies clearly recognizing endogenous NIK, we could demonstrate the presence of this protein neither in the immunoprecipitated IKC nor in Jurkat cell lysates (data not shown). Taken together, these data show that MLK3 not only acts as a IKKK in vitro but also can be coprecipitated with the IKC.
FIG. 6.
MLK3 is constitutively associated with the IKC via its leucine zipper. (A) Expression vectors for IKKα and IKKβ were cotransfected into 293 cells with plasmids encoding a Flag-tagged version of either MLK3, MLK3ΔC, or NIK. One day later, cells were lysed and the IKK proteins were immunoprecipitated (IP) from an aliquot of the lysates with αMyc antibodies. The coprecipitating proteins were detected by immunoblotting using αFlag antibodies (upper). In a complementary experiment, the MAPKKKs were immunoprecipitated with αFlag antibodies, followed by the detection of coprecipitating IKK proteins by Western blotting (WB) with αMyc antibodies (middle). The correct expression of the ectopically expressed proteins in whole cell extracts (WCE) was ensured by immunoblotting (lower). (B) Expression vectors for IKK were expressed in 293 cells either alone or together with MLK3 or MLK3ΔLZ as indicated. MLK3 and MLK3ΔLZ were immunoprecipitated from cell lysates with αHA antibodies, and the coprecipitating IKK proteins were detected with αMyc antibodies (upper). Aliquots of the total cell extracts were tested by immunoblotting for the expression of the ectopically expressed proteins (lower). Positions of molecular weight markers are indicated at the left in kilodaltons. A representative result is shown. (C) Jurkat cells were lysed, and the IKC was immunoprecipitated from an aliquot of the cell lysates with αIKKα antibodies. Isotype-matched control antibodies (Ab) were added to another aliquot of the cell extract. Immunoprecipitates were washed five times; the proteins were eluted with 1× SDS sample buffer and analyzed by Western blotting. Blots showing the IKKα, IKKβ, and MLK3 proteins are shown; molecular masses of a protein marker are given at the left. Samples of the total cell extracts were also tested by immunoblotting for the expression of IKKα, IKKβ and MLK3 (lower).
MLK3 sends CD3-CD28-derived signals to the IKC.
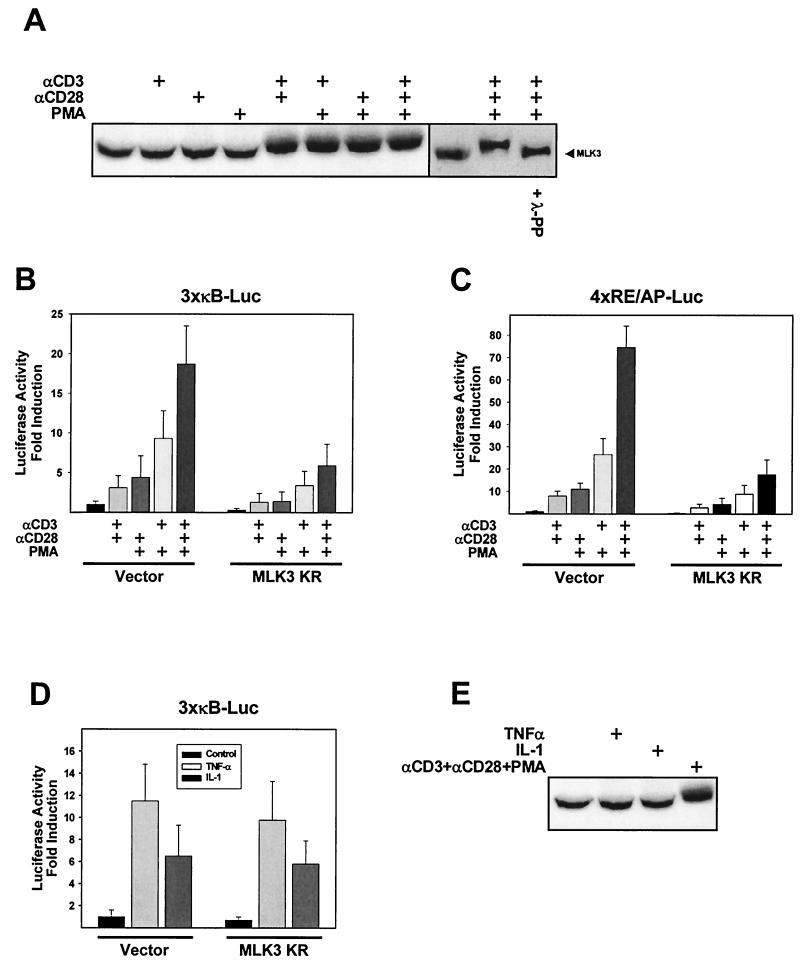
The MLK3-activating signals have been only incompletely described (36). Therefore, we tested various well-characterized NF-κB-activating signals for the ability to induce phosphorylation and subsequent activation of MLK3. Jurkat T cells were either left untreated or stimulated with different combinations of PMA or agonistic αCD3 and αCD28 antibodies. Treatment of cells with αCD3-αCD28, αCD3-PMA, αCD28-PMA, or αCD3-CD28-PMA resulted in the occurrence of an MLK3 form with a slightly decreased mobility in SDS-polyacrylamide gels as detected by immunoblotting (Fig. 7A). In contrast, the individual stimuli failed to induce this upshift. To test whether the upshifted band represents the phosphorylated form of the MLK3 protein, we incubated extracts from activated T cells with λ phosphatase. This treatment completely converted the slower-migrating form of MLK3 to the faster-migrating version that is found in unstimulated T cells (Fig. 7A). To evaluate the relative contribution of MLK3 for NF-κB-dependent transcription induced by T-cell stimulation, we cotransfected Jurkat cells with an NF-κB-dependent reporter gene along with either the empty expression vector or the kinase-deficient mutant of MLK3. Distinct combinations of αCD3 and αCD28 antibodies and PMA elicited NF-κB-dependent transcription to various degrees. Induced NF-κB activation was significantly impaired upon coexpression of MLK3 KR (Fig. 7B). A hallmark of costimulated T cells is the induced expression of the cytokine IL-2 (52). The IL-2 promoter harbors binding sites for numerous transcription factors including NF-AT, NF-κB, octamer factors, and the CD28RE/AP composite element (28). Knockout experiments showed the necessity of NF-κB for the induced IL-2 transcription (35). Since NF-κB is also important for gene expression triggered from the CD28RE/AP element (49), we examined the consequences of MLK3 KR expression on transcriptional activation from this element. Jurkat cells were transfected with a luciferase reporter construct controlled by four repeats of the CD28RE/AP element (4×RE/AP-Luc) along with either an empty expression vector or an expression vector for dominant negative MLK3. Various combinations of αCD3, αCD28, and PMA differentially activated CD28RE/AP-dependent transcription, but each of the activating signals was diminished upon coexpression of MLK3 KR (Fig. 7C). Also the T-cell costimulation-triggered transcription of a luciferase reporter construct fused to the human IL-2 promoter was dose dependently inhibited by coexpression of increasing amounts of MLK3 KR (data not shown). We next tested the influence of MLK3 KR expression on NF-κB activation induced by TNF-α or IL-1β. Transcription of an NF-κB-dependent reporter gene induced by TNF-α or IL-1β was not significantly changed in the presence of dominant negative MLK3 (Fig. 7D), showing the pathway preference of MLK3. Similarly, neither TNF-α nor IL-1β was able to generate the slower-migrating and phosphorylated form of MLK3, as revealed by Western blot experiments (Fig. 7E).
FIG. 7.
A role for MLK3 for T-cell activation-induced NF-κB activation. (A) Jurkat T cells were treated by the addition of agonistic αCD3 (5 μg/ml) and αCD28 (10 μg/ml) antibodies or with PMA (5 ng/ml) at the indicated combinations; 15 min later, total cell extracts were prepared and the electrophoretic mobility of MLK3 was determined by reducing SDS-PAGE followed by Western blotting. An aliquot of a protein extract from Jurkat cells that were stimulated for 15 min was preincubated with λ phosphatase. Typical results are displayed. (B) Jurkat cells were transfected with 5 μg of a NF-κB-dependent reporter construct together with 20 μg of the kinase inactive version of MLK3 as indicated; 16 h posttransfection, cells were stimulated by different combinations of αCD3, αCD28, and PMA for 8 h as indicated. Luciferase activity was determined, and results are expressed as average fold induction relative to unstimulated, vector-transfected cells. (C) Experiment was performed as for panel B except that a reporter plasmid containing four repeats of the composite CD28RE/AP element of the IL-2 promoter fused to luciferase (4×RE/AP-Luc) was used. (D) Jurkat cells were electroporated with a NF-κB-dependent reporter gene along with 20 μg of control vector or MLK3 KR. The next day, cells were treated for 8 h with TNF-α (2,000 U/ml) or IL-1β (10 ng/ml), and luciferase activity was determined. All results represent averages of three independent experiments; bars indicate standard errors. (E) Jurkat cells were left untreated or incubated for 15 min with TNF-α (2,000 U/ml), IL-1β (10 ng/ml), or αCD3-αCD28-PMA as indicated. The electrophoretic mobility of MLK3 was determined by Western blot analysis.
MLK3 is a downstream target of small GTPases.
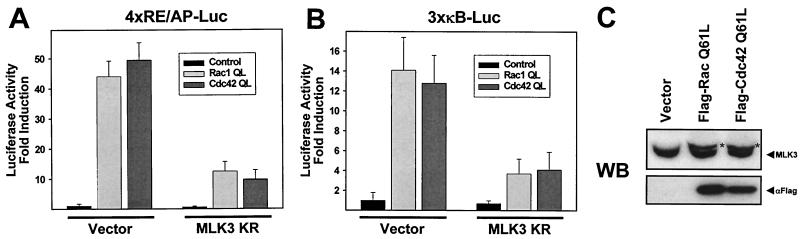
Since Rho family GTPases play an important role in the activation of T lymphocytes (27), we next determined the impact of MLK3 KR expression on transcription induced by Rac or Cdc42 in Jurkat cells. CD28RE/AP-dependent transcription induced by ectopic expression of constitutively active forms of Rac (Rac QL) or Cdc42 (Cdc42 QL) was significantly impaired in the presence of MLK3 KR (Fig. 8A). Similarly, this dominant negative form of MLK3 also efficiently antagonized NF-κB-dependent transcription triggered by Rac QL or Cdc42 QL (Fig. 8B). Subsequently we tested whether Rac and Cdc42 may influence the phosphorylation of MLK3. Expression of Rac QL and Cdc42 QL in Jurkat cells resulted in the occurrence of the slower-migrating form of MLK3 (Fig. 8C), showing that both GTPases act upstream from MLK3 and affect its phosphorylation. Not all MLK3 proteins were found in the upshifted form, which can be explained by the limited transfection efficiency of Jurkat cells. In summary, these data suggest that MLK3 is an integral component of the costimulation-induced, GTPase-mediated activation of NF-κB in T lymphocytes.
FIG. 8.
MLK3 is activated on a pathway employing Rac and Cdc42. (A) Expression vectors for constitutive active forms of Rac and Cdc42 and dominant negative MLK3 were transfected into Jurkat cells at the indicated combinations together with the 4×RE/AP-Luc reporter plasmid; 18 h posttransfection, cells were harvested and luciferase activity was determined. (B) Experiment performed as for panel A except that an NF-κB-dependent reporter gene was used. All results represent mean values from three independent experiments performed in duplicate; standard errors of the mean are shown by error bars. (C) Jurkat cells were transfected with an expression vector for a Flag-tagged versions of Rac QL or Cdc42 QL or an empty expression vector. One day later, cell extracts were analyzed by Western blotting for MLK3 phosphorylation (upper) and expression of Flag-tagged Rac and Cdc42 (lower). The asterisk indicates the position of the phosphorylated MLK3 protein.
DISCUSSION
T-cell costimulation by simultaneously triggering of the T-cell receptor (TCR) and the auxiliary receptor CD28 leads to the synergistic activation of JNK (63) and NF-κB (20, 29). Here we show that the serine/threonine kinase MLK3 plays an important role in the T-cell costimulation-induced activation of NF-κB. Since MLK3 is known to activate the JNK pathway (65, 66), this finding contributes to our understanding of how a given stimulus can simultaneously activate JNK as well as NF-κB. The behavior of MLK3 as a dual activator of JNK and NF-κB is reminiscent of MEKK1, which activates JNK via phosphorylation of JNKK1 (MKK4/SEK) (42) and triggers NF-κB by direct phosphorylation of both IKKs (38). In the absence of specificity constants of MLK3 for its substrates JNKK1 (66) and IKKα and IKKβ (this study), it is impossible to estimate whether MLK3 has a bias for either of the two pathways. The activation of a given pathway by more than one upstream activator is not without precedent. An example is provided by MEKK1-deficient embryonic stem cells, which are unable to activate JNK upon exposure to cold stress and microtubule disruption but display normal JNK activation in response to heat shock, UV irradiation, and anisomycin (72). The maintenance of JNK activation in response to certain stimuli points to the compensatory action of other JNKK kinases such as MLK3.
Here we identify T-cell costimulation as a physiological inducer of MLK3 activity. We suggest a scenario in which activated MLK3 promotes IL-2 transcription in two ways: first, it contributes to the activation of NF-κB, a transcription factor transactivating from the NF-κB binding site and the CD28RE/AP element contained in the IL-2 promoter. Second, triggering of MLK3 results in the activation of JNK, a kinase that is necessary for transactivation from the CD28RE/AP element (34) and the AP-1 sites (31) contained within the regulatory region upstream from the IL-2 gene. The T-cell costimulation-induced NF-κB-dependent transcription could not be absolutely blocked by dominant negative MLK3, raising the possibility for the existence of further kinases feeding costimulatory signals into the IKC. A candidate is the MAPKKK Cot, which was recently shown to participate in TCR-CD28-induced NF-κB activation (43). However, the pathway triggered by Cot is distinct from that activated by MLK3. Whereas Cot leads to the activation of NIK (43), MLK3 does not act upstream from NIK and directly phosphorylates both IKKs. Since the MEKK1-homologous proteins MEKK2 and MEKK3 were recently identified as NF-κB activators (74), it may well be that MLK3- and Cot-homologous proteins also contribute to the TCR-CD28-mediated activation of NF-κB. However, it remains to be seen whether endogenous Cot and MEKK1 are activated by T-cell costimulation.
This study identifies MLK3 as another IKKK, as judged by two criteria. First, MLK3 expression induced the phosphorylation of kinase-dead versions of IKKα and IKKβ to a comparable extent as did the expression of NIK and MEKK1. Second, immunoprecipitated MLK3 phosphorylated purified GST-IKKα KM and GST-IKKβ KA proteins. All IKKKs identified so far belong to the group of MAPKKKs and are constitutively attached to the IKC (38, 43, 45). A previous study showed the functional cooperation between NIK and MEKK1 for the activation of IKK activity (55). Our data indicate that also the MLK3-induced activation of both IKKs is further potentiated by the coexpression of MEKK1 or NIK. In contrast to the MAPKK TAK1, which links TRAF6-derived signals to its downstream target NIK, it seems that MEKK1, NIK, and MLK3 are direct activators of IKKs. Nevertheless, there is a mutual interference between (at least some) of the IKKKs. The MEKK1-induced NF-κB activation was impaired in the presence of dominant negative NIK (54), and the MLK3-induced activation of IKKs was reduced by kinase-inactive variants of NIK and MEKK1. From a molecular point of view this could be explained by competition of the IKKKs for a common activation site, which would be compatible with the finding that various IKKKs use identical IKK phosphorylation sites. On the other hand, this competition model does not explain the synergistic activation pattern of IKKKs, which suggests that these kinases act in parallel. The simultaneous activation of several IKKKs may help to explain the previous finding that one IKKK alone is not sufficient for full activation of NF-κB. For example, TNF-α-induced NF-κB activation cannot be completely inhibited by dominant negative forms of MEKK1 (37). It was suggested that MEKK1 has a predilection for IKKβ (54) and that NIK preferentially phosphorylates IKKα over IKKβ (44). IKKβ was more efficiently phosphorylated by MLK3 than IKKα. This might be explained by the phosphorylation of IKKα at only one serine, in comparison to IKKβ, which is phosphorylated at two serine residues. Since IKKβ KA repressed MLK3-elicited NF-κB activation more strongly than IKKα KM, it may also be possible that MLK3 preferentially phosphorylates IKKβ. All experiments discussed here used IKKs that were purified by immunoprecipitation, leaving the formal possibility that the phosphorylation might have been caused by a coprecipitating kinase. The tandem leucine zipper of MLK3 is essential for its binding to the IKC in vivo and in vitro. It remains to be seen whether the contact to both IKKs is direct or mediated by binding to intermediate proteins such as NIK, NEMO/IKKγ, or IKAP. MLK3 is also found in association with the scaffold protein JIP, along with other components of a JNK signaling pathway (68). It was proposed that the simultaneous binding of HPK1, MLK3, MKK7, and JNK to JIP may mediate the coordinate sequential interaction of these kinases (68), but the ratios between scaffold-attached and free MLK3 are not known. The ectopic expression of MLK3 potently activated NF-κB at low input levels, whereas high doses were ineffective. This may be taken as an indication that only MLK3 that is properly complexed and incorporated into the IKC (and/or the JIP complex) is competent for NF-κB activation. This also might explain the lack of NF-κB induction by ectopic expression of MLK3 described in a recent study (74), since the authors used rather high amounts of MLK3 expression vector (6 μg).
Since MLK3 does not play an important role in NF-κB activation by TNF-α or IL-1, this study corroborates the concept that different NF-κB-activating stimuli use different MAPKKKs. Along this line, IKKβ−/− fibroblasts show no TNF-α-induced degradation of IκB-α and activation of NF-κB, but the IL-1-induced IκB phosphorylation remains essentially unaffected (40, 64). The differential activation of distinct IKKKs would explain earlier findings describing the synergistic activation of NF-κB by the simultaneous addition of distinct NF-κB activators (2). It will be exciting to learn whether the cell contains distinct sets of differentially composed IKCs, or whether the distinct signaling cascades deliver their signals to uniformly built IKCs, thereby differentially affecting IKKKs.
Earlier studies revealed the activation of NF-κB by Rac/Cdc42-derived signals (58). Therefore, the identification of MLK3 as an activator of NF-κB uncovers one of the components of the signaling pathway between Rac and NF-κB. Activation of Rac by T-cell costimulation involves Vav family proteins, which act as a GTP/GDP exchange factor for the Rho family of GTPases (5, 10, 19). The analysis of Vav1−/− mice revealed that Vav1 is dispensable for CD28 costimulation (57) but necessary for the CD3/CD28-induced activation of NF-κB (9). Since MLK3 is widely expressed in a variety of tissues, it is reasonable to assume that MLK3 can be activated by further Rac/Cdc42-dependent stimuli (53), which await their identification in future studies.
ACKNOWLEDGMENTS
We thank Sandra Grunau for technical assistance, Susanne Bacher and Ingrid Fryson for helpful comments on the manuscript, and the following colleagues who generously provided plasmids and reagents, which made this work possible: D. Goeddel, S. Gutkind, T. Maniatis, F. Mercurio, E. Nishida, D. Wallach, and A. Weiss.
This work was supported by the Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum and Israeli's Ministry of Science (to W.D. and S.P.H.).
REFERENCES
- 1.Baichwal V R, Baeuerle P A. Activate NF-κB or die? Curr Biol. 1997;7:R94–R96. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S Jr. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Bender K, Göttlicher M, Whiteside S, Rahmsdorf H J, Herrlich P. Sequential DNA damage-independent and -dependent activation of NF-κB by UV. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beraud C, Henzel W J, Baeuerle P A. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-κB activation. Proc Natl Acad Sci USA. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantrell D. Lymphocyte signalling: a coordinating role for Vav? Curr Biol. 1998;8:R535–R538. doi: 10.1016/s0960-9822(07)00341-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 7.Cohen L, Henzel W J, Baeuerle P A. IKAP is a scaffold protein of the IκB kinase complex. Nature. 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 8.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 9.Costello P S, Walters A E, Mee P J, Turner M, Reynolds L F, Prisco A, Sarner N, Zamoyska R, Tybulewicz V L J. The Rho family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK and NF-κB pathways. Proc Natl Acad Sci USA. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 11.De Bosscher K, Schmitz M L, Plaisance S, Vanden Berghe W, Fiers W, Haegeman G. Glucocorticoid-mediated repression of NF-κB dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA. 1997;94:13504–13509. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 13.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 14.Dorow D S, Devereux L, Dietzsch E, DeKretser T. Identification of a new family of human epithelial protein kinases containing two leucine/isoleucine-zipper domains. Eur J Biochem. 1993;213:701–710. doi: 10.1111/j.1432-1033.1993.tb17810.x. [DOI] [PubMed] [Google Scholar]
- 15.Dorow D S, Devereux L, Tu G F, Price G, Nicholl J K, Sutherland G R, Simpson R J. Complete nucleotide sequence, expression, and chromosomal localisation of human mixed-lineage kinase 2. Eur J Biochem. 1995;234:492–500. doi: 10.1111/j.1432-1033.1995.492_b.x. [DOI] [PubMed] [Google Scholar]
- 16.Ezoe K, Lee S T, Strunk K M, Spritz R A. PTK1, a novel protein kinase required for proliferation of human melanocytes. Oncogene. 1994;9:935–938. [PubMed] [Google Scholar]
- 17.Gallo K A, Mark M R, Scadden D T, Wang Z, Gu Q, Godowski P J. Identification and characterization of SPRK, a novel src-homology 3 domain-containing proline-rich kinase with serine/threonine kinase activity. J Biol Chem. 1994;269:15092–15100. [PubMed] [Google Scholar]
- 18.Guttridge D C, Albanese C, Reuther J Y, Pestell R G, Baldwin A S., Jr NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han J, Luby Phelps K, Das B, Shu X, Xia Y, Mosteller R D, Krishna U M, Falck J R, White M A, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 20.Harhaj E W, Sun S C. IκB kinases serve as a target of CD28 signaling. J Biol Chem. 1998;273:25185–25190. doi: 10.1074/jbc.273.39.25185. [DOI] [PubMed] [Google Scholar]
- 21.Hehner S P, Heinrich M, Bork P M, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Dröge W, Schmitz M L. Sesquiterpene lactones specifically inhibit activation of NF-κB by preventing the degradation of IκB-α and IκB-β. J Biol Chem. 1998;273:1288–1298. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 22.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 24.Huxford T, Huang D B, Malek S, Ghosh G. The crystal structure of the IκBα/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 25.Ichijo H, Nishida E, Irie K, Ten-Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 26.Ing Y L, Leung I W, Heng H H, Tsui L C, Lassam N J. MLK-3: identification of a widely-expressed protein kinase bearing an SH3 domain and a leucine zipper-basic region domain. Oncogene. 1994;9:1745–1750. [PubMed] [Google Scholar]
- 27.Jacinto E, Werlen G, Karin M. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity. 1998;8:31–41. doi: 10.1016/s1074-7613(00)80456-2. [DOI] [PubMed] [Google Scholar]
- 28.Jain J, Loh C, Rao A. Transcriptional regulation of the IL2 gene. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 29.Jung S, Yaron A, Alkalay I, Hatzubai A, Avraham A, Ben-Neriah Y. Costimulation requirement for AP-1 and NF-κB transcription factor activation in T cells. Ann N Y Acad Sci. 1995;766:245–252. doi: 10.1111/j.1749-6632.1995.tb26672.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaltschmidt B, Kaltschmidt C, Hehner S P, Dröge W, Schmitz M L. Repression of NF-κB impairs HeLa cell proliferation by functional interference with cell cycle checkpoint regulators. Oncogene. 1999;18:3213–3225. doi: 10.1038/sj.onc.1202657. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 32.Karin M, Delhase M. JNK or IKK, AP-1 or NF-κB, which are the targets for MEK kinase 1 action? Proc Natl Acad Sci USA. 1998;95:9067–9069. doi: 10.1073/pnas.95.16.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katoh M, Hirai M, Sugimura T, Terada M. Cloning and characterization of MST, a novel (putative) serine/threonine kinase with SH3 domain. Oncogene. 1995;10:1447–1451. [PubMed] [Google Scholar]
- 34.Kempiak S J, Hiura T S, Nel A E. The Jun kinase cascade is responsible for activating the CD28 response element of the IL-2 promoter: proof of cross-talk with the IκB kinase cascade. J Immunol. 1999;162:3176–3187. [PubMed] [Google Scholar]
- 35.Köntgen F, Grumont R J, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 36.Leung I W, Lassam N. Dimerization via tandem leucine zippers is essential for the activation of the mitogen-activated protein kinase kinase kinase, MLK-3. J Biol Chem. 1998;273:32408–32415. doi: 10.1074/jbc.273.49.32408. [DOI] [PubMed] [Google Scholar]
- 37.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 38.Lee F S, Peters R T, Dang L C, Maniatis T. MEKK1 activates both IκB kinase α and IκB kinase β. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Van Antwerp D, Mercurio F, Lee K F, Verma I M. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999a;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Lu Q, Hwang J Y, Buscher D, Lee K F, Izpisua-Belmonte J C, Verma I M. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999b;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin A, Minden A, Martinetto H, Claret F X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 43.Lin X, Cunningham Jr E T, Mu Y, Geleziunas R, Greene W C. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- 44.Ling L, Cao Z, Goeddel D V. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 46.Maniatis T. Catalysis by a multiprotein IκB kinase complex. Science. 1997;278:818–819. doi: 10.1126/science.278.5339.818. [DOI] [PubMed] [Google Scholar]
- 47.Maniatis T. A ubiquitin ligase complex essential for the NF-κB, Wnt/Wingless and Hedgehog signaling pathways. Genes Dev. 1999;13:505–510. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- 48.May M J, Ghosh S. IκB kinases: kinsmen with different crafts. Science. 1999;284:271–273. doi: 10.1126/science.284.5412.271. [DOI] [PubMed] [Google Scholar]
- 49.McGuire K L, Iacobelli M. Involvement of Rel, Fos and Jun proteins in binding activity to the IL2 promoter CD28 response element/AP-1 sequence in human T cells. J Immunol. 1997;159:1319–1327. [PubMed] [Google Scholar]
- 50.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 51.Mercurio F, Murray B W, Shevchenko A, Bennett B L, Young D B, Li J W, Pascual G, Motiwala A, Zhu H, Mann M, Manning A M. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyazaki T, Liu Z J, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian E L, Permutter R M, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 53.Montaner S, Perona R, Saniger L, Lacal J C. Activation of serum response factor by RhoA is mediated by the nuclear factor-κB and C/EBP transcription factors. J Biol Chem. 1999;274:8506–8515. doi: 10.1074/jbc.274.13.8506. [DOI] [PubMed] [Google Scholar]
- 54.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IκB kinase α and β by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nemoto S, DiDonato J A, Lin A. Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-inducing kinase. Mol Cell Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 57.Penninger J M, Fischer K D, Sasaki T, Kozieradzki I, Le J, Tedford K, Bachmaier K, Ohashi P S, Bachmann M F. The oncogene product Vav is a crucial regulator of primary cytotoxic T cell responses but has no apparent role in CD28-mediated co-stimulation. Eur J Immunol. 1999;29:1709–1718. doi: 10.1002/(SICI)1521-4141(199905)29:05<1709::AID-IMMU1709>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 58.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal J C. Activation of the nuclear factor-κB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 59.Rana A, Gallo K, Godowski P, Hirai S, Ohno S, Zon L, Kyriakis J M, Avruch J. The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. J Biol Chem. 1996;271:19025–19028. doi: 10.1074/jbc.271.32.19025. [DOI] [PubMed] [Google Scholar]
- 60.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 61.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro V S, Truitt K E, Imboden J B, Weiss A. CD28 mediates transcriptional upregulation of the interleukin-2 (IL2) promoter through a composite element containing the CD28RE and NF-IL2B AP-1 sites. Mol Cell Biol. 1997;17:4051–4058. doi: 10.1128/mcb.17.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka M, Fuentes M E, Yamaguchi K, Durnin M H, Dalrymple S A, Hardy K L, Goeddel D V. Embryonic lethality, liver degeneration and impaired NF-κB activation in IKK-β-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 65.Teramoto H, Coso O A, Miyata H, Igishi T, Miki T, Gutkind J S. Signaling from the small GTP-binding proteins Rac and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem. 1996;271:27225–27228. doi: 10.1074/jbc.271.44.27225. [DOI] [PubMed] [Google Scholar]
- 66.Tibbles L A, Ing Y L, Kiefer F, Chan J, Iscove N, Woodgett J R, Lassam N J. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 1996;15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- 67.Verma I M, Stevenson J. IκB kinase: beginning, not the end. Proc Natl Acad Sci USA. 1997;94:11758–11760. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitmarsh A J, Cavanagh J, Tournier C, Yasuda J, Davis R J. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 69.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 70.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 71.Yaron A, Hatzubai A, Davis M, Lavon I, Amit S, Manning A M, Andersen J S, Mann M, Mercurio F, Ben-Neriah Y. Identification of the receptor component of the IκBα-ubiquitin ligase. Nature. 1998;396:590–594. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- 72.Yujiri T, Sather S, Fanger G R, Johnson G L. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 73.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Q, Lee F S. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-κB through IκB kinase-α and IκB kinase-β. J Biol Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]