Abstract
Not all plant-based and animal foods exert the same health effects due to their various nutrient compositions. We aimed to assess the quality of plant-based vs. animal foods in relation to mortality in a prospective cohort study. Using data collected from a nationally representative sample of 36,825 adults in the National Health and Nutrition Examination Survey 1999-2014, we developed a de novo Comprehensive Diet Quality Index (cDQI) that assesses the quality of 17 foods based on the healthfulness, and separately scored the quality of 11 plant-based foods in a plant-based Diet Quality Index (pDQI) and 6 animal foods in an animal-based Diet Quality index (aDQI), Mortality from all causes, heart disease, and cancer were obtained from linkage to the National Death Index through December 31, 2015. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) after multivariable adjustments. During a median follow-up of 8.3 years, 4,669 all-cause deaths occurred, including 798 deaths due to heart disease and 1,021 due to cancer. Compared to individuals in the lowest quartile, those in the highest quartile of cDQI had a lower risk of all-cause mortality (HR=0.75, 95% CI: 0.65, 0.86; P-trend<0,001), which largely reflected the inverse relationship between quality of plant-based foods (pDQI) and all-cause mortality (HR=0.66; 95% CI: 0.58, 0.74, P-trend<0.001). No independent association was found for the quality of animal-foods (aDQI) and mortality. Our results suggest that consuming healthy plant-based foods is associated with lower all-cause mortality among US adults.
Keywords: diet quality index, plant-based foods, animal foods, mortality, cancer, heart disease
INTRODUCTION
A plant-based diet has been recommended for preventing obesity, diabetes, cardiovascular disease, cancer, and other chronic diseases (1; 2; 3). While a plant-based diet refers to a diet rich in vegetables, fruits, and whole grains and low in red and processed meats in dietary recommendations (4), it may also be interpreted as the reduction or elimination of animal foods to various degrees. For example, a vegan diet refers to the complete elimination of animal foods from the diet, a lacto-ovo-vegetarian diet refers to the elimination of all animal foods except for dairy products and/or eggs, a pesco-vegetarian diet refers to the elimination of meat but not fish (e.g., fish eaters), and a semi-vegetarian diet refers to the reduction of meat consumption (e.g., occasional or low meat eaters) (5).
Whether eliminating or reducing animal foods from diet confers health benefits remains controversial. The Adventist Health Study 2 where over half of the participants had no or low consumption of animal foods from their diet (7.6% vegan, 28.9% lacto-ovo-vegetarian, 9.8% fish eaters, and 5.5% occasional meat eaters), found a 12% lower risk of all-cause mortality in those who had no or low consumption animal foods from their diet compared to those who consumed animal foods (6). The European Prospective Investigation into Cancer and Nutrition-Oxford (EPIC-Oxford) Cohort and the Oxford Vegetarian Study, however, found no difference in all-cause mortality between those who consumed no meat or fish and those who consumed meat and/or fish (7). When vegetarian diets were compared by subcategories, Adventists who were fish eaters had 19% lower all-cause mortality compared to non-vegetarian Adventists, and fish eaters in the two U.K. cohorts also had a 19% lower risk of cancer mortality (6). These findings suggest that the quality of animal food may play a more important role in health outcomes than simply reducing or eliminating animal foods from the diet.
Similarly, not all plant-based foods are equal in their nutrient contents and associations with health. For example, consumption of healthful plant-based foods (e.g., vegetables, fruits, whole grains, nuts/seeds, and legumes) has been associated with a lower risk of coronary heart disease (CHD), diabetes, and all-cause mortality, whereas consumption of less healthful plant-based foods (e.g., refined grains, white potatoes, and sugar-sweetened beverages) has been associated with a higher risk (8; 9; 10; 11; 12).
Previous studies that assessed the quality of plant-based foods did not jointly distinguish the quality of animal foods. Among a nationally representative sample of US adults, we evaluated whether the quality of plant-based foods, animal foods, or both is associated with mortality by using a Comprehensive Dietary Quality Index (cDQI) that distinguishes the quality of both plant-based and animal foods. We further explored whether associations between the cDQI and mortality differ by age, sex, income, weight status, levels of physical activity, and comorbidity conditions at baseline.
MATERIALS AND METHODS
Participants
This study utilized data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2014 and included 36,825 individuals aged 20+ years who completed at least 1 valid 24-hour diet recall. NHANES is conducted biannually by the National Center for Health Statistics (NCHS) and is designed to assess the health and nutritional status of adults and children in the United States. Written informed consent was obtained from all participants at the time of enrollment in the study to allow for their data to be used for research purposes. Ethical approval for NHANES was granted by the NCHS research ethic review board.
The average response rate of NHANES survey during the study period was 70.6%, ranging from 56.3% in the 2015-2016 cycle to 78.3% in the 2001-2002 cycle (13). Individuals with at least one valid diet recall were included in this study. For this analysis, we excluded pregnant and lactating women (n=1,550), individuals with potentially unreliable dietary intake, defined as total energy intake exceeding three standard deviations above and below the mean value of the natural log-transformed energy intake (n=209), individuals with no linked mortality data (n=48), and those who died within 12 months of dietary assessment (n=349). These exclusions left 36,825 individuals aged 20+ years as the final study population. We used NHANES sampling weights in all analyses, which account for the complex survey design, oversampling of minorities, and survey nonresponses. The dietary sampling weights additionally account for the dietary interview-specific nonresponse and day of the week for dietary intake interviews (14).
Dietary Intake
In-person 24-hour recalls conducted by trained interviewers at a Mobile Examination Center (MEC) are used to determine intake in NHANES participants. From 1999-2002, one dietary recall was conducted with participants (in-person at the MEC). From 2003 onwards, a second recall was included, which was carried out by telephone 3-10 days after the initial in-person recall. About 68% of the participants who provided the first diet recall also provided the second recall during the study period (1999-2014), with the percentage being 92%, 90%, 85%, 86%, 89% and 87% from 2003 and onwards. Both of these recalls employed the Automated Multiple Pass Method (AMPM) and used standard measuring guides to ensure that all food and beverage consumed on the previous day was recorded. These dietary records were then coded. The United States Department of Agriculture (USD A) Food Patterns Equivalents Database and MyPyramid Equivalents Database, which disaggregate mixed foods into their component parts, were harmonized and used to assess intake of major food groups. Food groups (e.g., vegetables were further disaggregated into subgroups (e.g., dark green vegetables vs white potatoes)) to evaluate subtype. Nutrients were estimated based on cycle-specific versions of the USDA Food and Nutrition Database for Dietary Studies (FNDDS) (15). To correct for measurement error associated with the use of one or two-day diet recalls to estimate usual intake, we used the National Cancer Institute (NCI) method to adjust for usual intake estimates. The method also uses regression calibration to correct for bias due to the measurement error in evaluating associations between usual intake and health outcomes (16; 17; 18).
Comprehensive Diet Quality Index (cDQI)
To assess the quality of both plant- and animal-based food components of the diet, we developed a de novo Comprehensive Diet Quality Index score (cDQI) based on a previously validated plant-based dietary index (PDI) (11; 19). The PDI distinguishes the quality of plant-based foods in its scoring but scores all animal foods reversely. The new cDQI additionally assesses the quality of animal foods by scoring positively for healthful animal foods and reversely for unhealthful animal foods. The selection and scoring of animal foods is based on meta-analyses of prospective cohort studies and randomized intervention trials with strong evidence-base, including the Third Expert Report of the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) (20) and the evidence review of the Global Burden of Disease (GBD) Nutrition and Chronic Disease Expert Group (NutriCoDE) (21). For example, processed meats and red meats were included as unhealthful animal foods and scored reversely whereas fish/seafood, dairy, and poultry are included as healthful animal foods and scored positively. Egg was included as an unhealthful animal food based on the most recent evidence (22).
The cDQI has 17 components, including 11 plant-based foods and 6 animal foods (Supplementary Table 1). Dietary intake of each food component was adjusted for total energy intake using the density method. For healthful plant-based foods (whole grains, vegetables excluding white potatoes, whole fruits, nuts/seeds/legumes, vegetable oils, and coffee/tea) or animal foods (fish/seafood, dairy, and poultry), a score of 0 is assigned for no intake or lowest quintile, and the scores increase proportionately as intakes increase. For unhealthful plant-based (refined grains, fruit juices, sugar-sweetened beverages, and sweets/desserts) or animal foods (processed meats, unprocessed red meats, and eggs), levels of intakes at the recommended level or lowest quintile are assigned the maximum score, and the scores decrease proportionally as intakes increase. The scoring standards were adapted from those used in the Health Eating Index (HEI)-2015 (23), Alternative Healthy Eating Index (AHEI),(24) and the American Heart Association (AHA) score based on the 2020 Strategic Impact Goals for Health. (25) For food components not included in HEI-2015, AHEI, and AHA scores, quintiles were used as the scorning standards, similar to those used to score the PDI (19). Separately, the total score for plant-based Diet Quality Index (pDQI) is the sum of the 11 plant-based food components, ranging from 0 to 55; and the total score for animal-based Diet Quality Index (aDQI) is the sum of the 6 animal components, ranging from 0 to 30. The total cDQI total score, combining both plant- and animal-based components, ranges from 0 to 85. A higher pDQI, aDQI, and cDQI score indicates a higher quality of plant-based foods, animal foods, and both foods, respectively (Table 1).
Table 1.
Components, Mean Intake, Scoring Standards, and Mean Score for the Comprehensive, Plant-Based, and Animal-Based Diet Quality Index among US Adults Aged 20+ Years, NHANES 1999-2014
| Components | Mean Intake (SD)1 | Max. Score | Standard for Max. Score | Standard for Min. Score of 0 | Mean Sore (95% CI) |
|---|---|---|---|---|---|
| Plant-based Components | |||||
| Healthful | |||||
| Whole grains2 | 0.77 (0.55) | 5 | ≥ 1.5 oz. equiv./1,000 kcal | No whole grains | 1.27 (1.25, 1.28) |
| Vegetables excluding white potatoes3 | 1.16 (0.43) | 5 | ≥ 1.25 cup equiv./1,000 kcal | No vegetables excluding white potatoes | 2.30 (2.29, 2.32) |
| Whole fruits2 | 0.71 (0.55) | 5 | ≥ 0.4 cup equiv./1,000 kcal | No whole fruit | 3.20 (3.16,3.23) |
| Nuts/seeds/legumes3 | 0.68 (0.43) | 5 | ≥ 0.5 oz. equiv./1,000 kcal | No nuts, seeds or legumes | 3.00 (2.97, 3.02) |
| Vegetable oils4 | 17.9 (4.94) | 5 | Highest quintile | Lowest quintile | 2.59 (2.56, 2.63) |
| Coffee/tea4 | 2.01 (1.74) | 5 | Highest quintile | Lowest quintile | 2.58 (2.53, 2.62) |
| Unhealthful | |||||
| Fruit juices5 | 0.29 (0.26) | 5 | No fruit juices | ≥ 0.35 cup equiv./1,000 kcal | 3.18 (3.15, 3.20) |
| Refined grains2 | 5.63 (1.12) | 5 | ≤ 1.8 oz. equiv./1,000 kcal | ≥ 4.3 oz. equiv./1,000 kcal | 3.06 (3.04, 3.08) |
| White potatoes5 | 0.34 (0.11) | 5 | No white potatoes | ⩾0.35 cup equiv./1,000 kcal | 2.57 (2.56, 2.59) |
| Sugar-sweetened beverages (SSB)3 | 1.43 (1.25) | 5 | No SSB | ≥ 1 cup equiv./day | 1.34 (1.30, 1.37) |
| Sweets and desserts4 | 1.77 (9.75) | 5 | Highest quintile | Lowest quintile | 2.49 (2.45, 2.52) |
|
|
|
||||
| Plant-based diet quality index (pDQI) | 55 | range: 0-55 | 27.6 (27.4, 27.7) | ||
| Animal-based Components | |||||
| Healthful | |||||
| Fish/seafood3 | 0.64 (0.32) | 5 | ≥ 0.5 oz./1,000 kcal | No fish or shellfish | 2.94 (2.92, 2.97) |
| Daily2 | 1.41 (0.51) | 5 | ≥ 1.3 cup equiv./1,000 kcal | No dairy | 2.76 (2.74, 2.78) |
| Poultry4 | 1.48 (0.45) | 5 | Highest quintile | Lowest quintile | 2.48 (2.44, 2.53) |
| Unhealthy | |||||
| Processed meats5 | 0.85 (0.35) | 5 | No processed meats | ≥ 1 oz. equiv./1,000 kcal | 2.87 (2.85, 2.89) |
| Red meats5 | 1.66 (0.48) | 5 | No red meats | ≥ 1.6 oz. equiv./1,000 kcal | 2.46 (2.44, 2.47) |
| Egg4 | 0.60 (0.28) | 5 | Lowest quintile | Highest quintile | 2.72 (2.69, 2.75) |
|
|
|
||||
| Animal-based diet quality index (aDQI) | 30 | range: 0-30 | 16.2 (16.2, 16.3) | ||
| Comprehensive Diet Quality Index (cDQI) | 85 | range: 0-85 | 43.8 (43.6, 44.0) |
Abbreviations: NHANES, National Health and Nutrition Examination Survey; SD, Standard Deviation; CI, confident intervals; SSB, sugar-sweetened beverages; pDQI, plant-based Diet Quality Index; aDQI, animal-based Diet Quality Index; cDQI, comprehensive Diet Quality Index.
Units are oz. equiv. for mean intake of whole grains, nuts/seeds/legumes, refined grains, fish/seafoods, poultry, processed meats, red meats, and eggs; cup equiv. for mean intake of whole fruits , fruit juices, vegetables excluding white potatoes, white potatoes, and dairy; 8-fluid oz. cup for mean intake of coffee/tea and SSB; and serving for mean intake of sweets and desserts; gram for vegetable oils. The conversion factors for conventional unit to metric unit vary by foods and food groups.(43) The approximate conversion factors are 1 cup equivalent fruits or vegetables is 100 grams; 1 cup equivalent legumes is 175 grams; 1 oz. equivalent whole or refine grains is 30 grams; 1 oz. equivalent fish/seafood, poultry, processed meat, unprocessed red meat, or nuts/seeds is 28.35 grams; 1 cup 8-fluid oz sugar-sweetened beverages, coffee, or tea is 226.8 grams, and 1 serving of sweets and desserts is 30 grams.
Scoring is based on scoring standards used in the Healthy Eating Index (HEI)-2015. (23)
Scoring is based on scoring standards using in the Alternative Healthy Eating Index (AHEI) adjusted to per 1,000 kcal (24).
Scoring is based on the scoring standards used in the Plant-Based Diet Index (PDI) by Satija et al. (19) The quintiles were Q1=13.0, Q2=15.5, Q3=17.6, Q4=19.8, and Q5=22.6 for vegetable oils (grams per 2,000 kcal); Q1=0.38, Q2=0.93, Q3=1.68, Q4=2.38, and Q5=3.43 for tea/coffee (cup equiv. per 2,000 kcal); Q1=6.8, Q2=2.5, Q3=2.0, Q4=1.65, and Q5=1.34 for sweets/desserts (serving per 2,000 kcal); Q1=1.05, Q2=1.22, Q3=1.40, Q4=1.61, and Q5=1.90 for poultry (oz. equiv. per 2,000kcal); and Q1=2.63, Q2=0.85, Q3=0.65, Q4=0.53, and Q5=0.43 for eggs (oz. equiv. per 2,000kcal).
Scoring is based on the scoring standards used in the American Heart Association (AHA) diet score based on the AHA 2020 Strategic Goals for Diet,(25) corresponding to 80th percentile of intake among U.S. adults in NHANES 1999-2014.
Mortality
The primary outcome was mortality from all causes and the secondary outcomes were mortality from heart disease and cancer. Mortality outcomes were identified through linkage to the National Death Index through December 31, 2015 (26). Death from heart disease was defined as I00 to I09, I11, I13, and I20 to I51 being the underlying cause of death using the International Statistical Classification of Disease, 10th revision (ICD-10), and death from cancer was defined as C00-C97 being the underlying cause of deaths. Other cause-specific mortality was not assessed due to the small number of deaths due to other specific causes. Follow-up length was defined as the interval of time from the 24-hour recall interview to the date of death for those individuals who had died or to the 31st December 2015 for those participants who were censored.
Demographic, Lifestyle, and Comorbidity Conditions
Demographic and lifestyle factors including age, sex, race/ethnicity, education, and income were collected during household interview. Alcohol intake, smoking, physical activity, body weight, and height were obtained among participants who received physical examinations in a MEC. Race/ethnicity were categorized as non-Hispanic whites, non-Hispanic blacks, Hispanic, and other racial/ethnic groups. Family income was classified as poverty-to-family income ratio (PIR) and were categorized as low-income (PIR<1.85) and higher-income (PIR ≥1.85). Smokers were defined as individuals who reported smoking at least 100 cigarettes during their lifetime, with former smokers defined as not currently smoking and current smokers defined as currently smoking. Participants who drank a minimum of 12 drinks in any given year were classed as drinkers with moderate drinkers defined as those who consumed <1 drink/day for women and <2 drinks/day for men and heavy drinkers being defined as those who consumed ≥1 drink/day for women and ≥2 drinks/day for men. Metabolic equivalent (MET)-hours of moderate-to-vigorous leisure-time physical activity (MVPA) was calculated by summarizing minutes of reported activity per week with the metabolic equivalent of physical activities with different intensities. Body mass index (BMI) was calculated using the formula weight (kg)/height (m)2 Co-morbidity conditions (cancer, congestive heart failure, coronary heart disease, myocardial infarction, stroke, high cholesterol, hypertension, diabetes) were defined if participants reported that they have ever been told by a healthcare professional that they had such conditions and/or to take prescribed medications because of these conditions, or they are currently taking medication for such a condition.
Statistical analysis
We first categorized the quality of plant-based, animal, and both foods based on the sex-specific quartiles of the total score of cDQI, pDQI, and aDQI, and compared the distribution of demographic, lifestyle factors, and comorbidity conditions across quartiles of pDQI, aDQI, and cDQI, using analysis of variance (ANOVA) for continuous variables and Chi-square test for categorical variables.
To examine the association between the quality of plant-based, animal, and both foods and mortality, we first evaluated pDQI, aDQI, and cDQI individually in association with mortality using Cox proportional hazard models with multivariable adjustments. The proportional hazard (PH) assumption was evaluated by comparing the log-log survival curves by quartiles of cDQI, pDQI, and aDQI. The parallel survival curves suggested that the PH assumption was met. To evaluate the relative importance of the quality of plant-based vs. animal foods, we included pDQI and aDQI simultaneously in the same model. We also evaluated each component of cDQI in association with mortality outcomes. All analyses were adjusted for age, sex, race/ethnicity, total energy intake, education, physical activity, cigarette smoking, alcohol drinking, BMI, and comorbidity conditions. We further investigated whether the associations between diet quality and mortality differed by age, sex, income, weight status, levels of physical activity, and presence of comorbidity conditions at baseline. Sensitivity analyses were conducted by restricting the analyses to participants who participated in the first (1999-2006) versus the last four cycles (2007-2014) and by scoring eggs positively.
Sampling weights were incorporated in all analyses to account for unequal probabilities of sample selection due to complex sample design and oversampling of certain subgroups. All analyses were conducted using SAS version 9.4 (SAS Institute). P <0.05 was considered statistically significant.
RESULTS
The mean total score of the cDQI among US adults was 43.8 (out of maximum score 85), among which the mean score for plant-based components was 27.6 (out of maximum score 55) and the mean score for animal-based components was 16.2 (out of maximum score 30) (Table 1). Among the 17 food components, 3 plant-based food components had mean score below 50% of the maximum score: whole grains (25.4%), sugar-sweetened beverages (26.8%), and vegetables excluding white potatoes (46.0%), suggesting that US adults had particularly poor adherence to the recommended intake of these foods.
Compared to individuals in the lowest quartile of cDQI, those in the highest quartile were older and more likely to be non-Hispanic white, college graduates with a higher income, physically active, and moderate drinkers, and report comorbidity conditions at baseline, and were less likely to be heavy smokers or obese (Table 2). Individuals with a higher pDQI score were older and more likely to be non-Hispanic whites, overweight, and report comorbidity conditions compared to those with a lower aDQI score, whereas individuals with a higher score of aDQI were younger and more likely to be non-Hispanic blacks and have a healthy weight and less likely to report comorbidity conditions compared to those with a lower aDQI score.
Table 2.
Characteristics of US Adults Aged ≥20 Years by Plant- and Animal-Based Diet Quality Scores, NHANES 1999-2014
| Total Adult Population (n=36,825) | Comprehensive Diet Quality Index (cDQI) |
Plant-based Diet Quality Index (pDQI) |
Animal-based Diet Quality Index (aDQI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (Male: <37.0; Female: <40.8) (n=9205) | Q4 (Male: ≥45.1; Female: ≥49.5 (n=9206) | P-value1 | Q1 (Male: <22.4; Female: <24.3) (n=9205) | Q4 (Male: ≥29.7; Female: ≥32.2) (n=9206) | P-value | Q1 (Male: <13.1; Female :<15.0) (n=9205) | Q4 (Male: ≥17.1; Female: ≥18.9 (n=9206) | P-value1 | ||
| Age, years 2 | 47.1 (0.2) | 40.6 (0.2) | 53.5 (0.3) | <0.001 | 36.9 (0.2) | 56.3 (0.3) | <0.001 | 52.3 (0.3) | 43.0 (0.3) | <0.001 |
| Sex, 3 | 0.4 | 0.03 | 0.09 | |||||||
| Male | 18443 (49.1) | 4610 (50.1) | 4611 (48.3) | 4610 (50.7) | 4611 (47.7) | 4610 (48.3) | 4611 (49.5) | |||
| Female | 18382 (50.9) | 4595 (49.9) | 4595 (51.7) | 4595 (49.3) | 4595 (52.3) | 4595 (51.7) | 4595 (50.5) | |||
| Race/ethnicity, 3 | <0.001 | <0.001 | <0.001 | |||||||
| Non-Hispanic White | 17494 (70.2) | 3368 (59.3) | 5385 (79.3) | 3309 (58.0) | 5569 (81.7) | 4360 (71.4) | 4238 (68.1) | |||
| Non-Hispanic Black | 7614 (11.1) | 2409 (16.7) | 1357 (6.0) | 2757 (18.9) | 1163 (5.0) | 1434 (8.7) | 2299 (12.9) | |||
| Hispanic | 9278 (12.9) | 3123 (20.3) | 1408 (6.5) | 2695 (18.0) | 1624 (6.8) | 3165 (17.3) | 1592 (9.2) | |||
| Other | 2439 (5.8) | 305 (3.7) | 1056 (8.1) | 444 (5.1) | 850 (6.5) | 246 (2.7) | 1077 (9.7) | |||
| Education, 3 | <0.001 | <0.001 | <0.001 | |||||||
| Grades 0-12 | 10362 (18.4) | 3188 (24.9) | 1706 (11.1) | 2885 (22.5) | 1896 (11.9) | 3160 (22.7) | 1976 (14.3) | |||
| Some college | 8613 (24.2) | 2448 (28.8) | 1827 (19.0) | 2398 (28.2) | 1879 (20.5) | 2314 (27.5) | 1991 (21.3) | |||
| College graduate and above | 17803 (57.5) | 3553 (46.3) | 5663 (69.9) | 3909 (49.3) | 5423 (67.6) | 3712 (49.7) | 5233 (64.3) | |||
| Poverty to income ratio (PIR), 3,4 | <0.001 | <0.001 | 0.01 | |||||||
| < 1.85 | 14729 (30.2) | 4549 (41.0) | 2597 (19.0) | 4535 (41.8) | 2636 (19.0) | 3918 (31.5) | 3413 (28.3) | |||
| ≥ 1.85 | 19175 (63.3) | 3952 (52.4) | 5876 (74.6) | 3993 (52.1) | 5825 (74.4) | 4554 (61.9) | 5089 (65.5) | |||
| Missing | 2921 (6.5) | 704 (6.6) | 733 (6.4) | 677 (6.1) | 745 (6.6) | 733 (6.6) | 704 (6.3) | |||
| Smoking, 3,5 | <0.001 | <0.001 | <0.001 | |||||||
| Nonsmokers | 19440 (52.2) | 4871 (51.9) | 4977 (53.4) | 5107 (54.4) | 4702 (50.7) | 4359 (47.3) | 5450 (57.7) | |||
| Former smokers | 9315 (24.9) | 1690 (17.1) | 3028 (33.1) | 1405 (14.5) | 3320 (36.1) | 2710 (28.7) | 1909 (21.3) | |||
| Current smokers | ||||||||||
| <15 cigarettes/day | 4701 (12.0) | 1615 (16.8) | 686 (7.3) | 1705 (17.6) | 656 (6.8) | 1188 (11.6) | 1167 (12.2) | |||
| 15-24.9 cigarettes/day | 2401 (7.6) | 754 (10.1) | 365 (4.0) | 726 (9.7) | 362 (4.2) | 663 (8.6) | 513 (6.5) | |||
| ≥25 cigarettes /day | 879 (3.2) | 253 (4.0) | 135 (2.1) | 238 (3.7) | 147 (2.1) | 261 (3.7) | 147 (2.1) | |||
| Alcohol,3,6 drink/week, | 0.02 | 0.05 | <0.001 | |||||||
| Nondrinkers | 13957 (34.0) | 3373 (35.2) | 3478 (32.0) | 3249 (33.9) | 3562 (32.6) | 3650 (36.1) | 3301 (32.3) | |||
| Moderate drinker | 18130 (57.0) | 4551 (56.1) | 4688 (59.6) | 4652 (57.7) | 4611 (58.4) | 4366 (54.4) | 4765 (60.1) | |||
| Heavy drinker | 2515 (8.9) | 683 (8.7) | 528 (8.4) | 667 (8.5) | 579 (9.0) | 693 (9.4) | 532 (7.6) | |||
| Physical activity, 2,7 | ||||||||||
| MET-hours/week, | 17.2 (0.4) | 16.4 (0.6) | 18.4 (0.5) | <0.001 | 17.7(0.7) | 17.6(0.5) | 0.006 | 14.2 (0.6) | 19.0 (0.5) | <0.001 |
| BMI, kg/m2, | 28.5 (0.1) | 28.8 (0.1) | 28.2 (0.1) | <0.001 | 28.6(0.1) | 28.4(0.1) | 0.12 | 29.2 (0.1) | 28.1 (0.1) | <0.001 |
| Weight status, 3,8 | <0.001 | <0.001 | <0.001 | |||||||
| BMI < 18.5 kg/m2 | 571(1.7) | 178 (2.2) | 112 (1.3) | 206 (2.5) | 100 (1.1) | 112 (1.2) | 176 (2.2) | |||
| BMI = 18.5-25 kg/m2 | 10377(30.6) | 2575 (31.3) | 2706 (31.2) | 2742 (32.8) | 2551 (29.7) | 2312 (27.2) | 2843 (33.2) | |||
| BMI = 25-29.9 kg/m2 | 12422 (33.7) | 2889 (30.0) | 3298 (36.4) | 2843 (29.8) | 3326 (36.3) | 3089 (32.8) | 3052 (33.5) | |||
| BMI ≥30 kg/m2f | 12869 (34.0) | 3438 (36.6) | 2947 (31.2) | 3302 (35.0) | 3085 (32.9) | 3544 (38.8) | 3008 (31.1) | |||
| Co-morbidities, 3 | ||||||||||
| Cancer | 3408 (9.3) | 537 (5.9) | 1224 (13.2) | <0.001 | 414 (4.3) | 1384 (15.1) | <0.001 | 1024 (11.9) | 683 (7.2) | <0.001 |
| Congestive heart failure | 1162 (2.4) | 213 (1.9) | 315 (2.4) | 0.2 | 165 (1.3) | 390 (3.0) | <0.001 | 388 (3.5) | 178 (1.3) | <0.001 |
| Coronary heart disease | 1546 (3.4) | 238 (2.3) | 534 (4.6) | <0.001 | 160 (1.5) | 621 (5.4) | <0.001 | 499 (4.6) | 259 (2.5) | <0.001 |
| Myocardial infarction | 1603 (3.5) | 283 (2.7) | 469 (4.0) | 0.002 | 199 (1.8) | 547 (4.8) | <0.001 | 510 (4.6) | 248 (2.3) | <0.001 |
| Stroke | 1360 (2.7) | 278 (2.5) | 340 (2.7) | 0.44 | 232 (2.0) | 409 (3.2) | <0.001 | 465 (4.0) | 246 (2.0) | <0.001 |
| High Cholesterol | 11759 (31.9) | 2158 (23.4) | 3857 (41.1) | <0.001 | 1814 (30.1) | 4111 (47.7) | <0.001 | 3356 (38.2) | 2569 (27.3) | <0.001 |
| Hypertension | 12832 (30.8) | 2517 (24.6) | 3748 (35.5) | <0.001 | 2161 (21.3) | 4092 (39.2) | <0.001 | 3743 (37.7) | 2706 (26.3) | <0.001 |
| Diabetes | 4422 (8.6) | 700 (5.4) | 1550 (12.2) | <0.001 | 492 (4.0) | 1787 (13.9) | <0.001 | 1439 (11.8) | 851 (6.7) | <0.001 |
Abbreviations: NHANES, National Health and Nutrition Examination Survey; cDQI, comprehensive Diet Quality Index; pDQI, plant-based Diet Quality Index; aDQI, animal-based Diet Quality Index; Q, quartile; PIR, poverty-to-income ratio; MET: metabolic equivalents; BMI, body mass index.
Analysis of variance was used to assess the difference in the distribution of continuous characteristic variables and chi-square test was used to assess the difference in the distribution of categorical variables by quartiles of cDQI, pDQI, and aDQI scores.
Values are mean (SD).
Values are n (%).
Poverty-to-income ratio (PIR) represents ratio of family income to the federal poverty threshold, adjusting for household size. For reference, the federal poverty threshold in 2014 for a family of 4 was $23,850 per year. A family of 4 earning $44,123 per year would have a ratio of 1.85. A lower ratio indicates a lower level of income.
Smokers were defined as individuals who reported smoking at least 100 cigarettes during their lifetime, with former smokers defined as not currently smoking and current smokers defined as currently smoking.
Participants who drank a minimum of 12 drinks in any given year were classed as drinkers with moderate drinkers defined as those who consumed <1 drink/day for women and <2 drinks/day for men and heavy drinkers being defined as those who consumed ≥1 drink/day for women and ≥2 drinks/day for men.
MET-hours of moderate-to-vigorous physical activity per week was by summarizing minutes of reported activity with the metabolic equivalent of physical activities with different intensities.
BMI was calculated by dividing weight in kilograms (kg) by height in meters squared (m2).
During a median 8.3 years of follow-up, 4,669 total deaths occurred, including 798 deaths due to heart disease and 1,021 deaths due to cancer. Compared to individuals in the lowest quartile, those in the highest quartile of cDQI had 25% lower all-cause mortality (Q4 vs. Q1: HR=0.75, 95% CI: 0.65, 0.86; P-trend<0.001) (Table 3 and Figure 1). After controlling for aDQI, individuals in the highest quartile of pDQI had 34% lower all-cause mortality (Q4 vs. Q1: HR=0.66; 95% CI: 0.58, 0.74; P-trend<0.001) compared to those in the lowest quartile. The aDQI was not associated with any of the mortality outcomes after controlling for pDQI. When each component of cDQI was evaluated individually, lower all-cause mortality was associated with higher intake of vegetables (excluding white potatoes) (HR=0.75, 95% CI: 0.64, 0.88), whole fruits (HR=0.72, 95% CI: 0.57, 0.91), nuts/seeds/legumes (HR=0.77, 95% CI: 0.67, 0.89), vegetable oils (HR=0.82, 95% CI: 0.71, 0.94), and coffee/tea (HR=0.81, 95% CI: 0.70, 0.94). No associations were found for other plant-based food components or any animal food components (Supplementary Table 2). Scoring eggs positively did not change the results. No associations were found between cDQI, pDQI, and aDQI and heart disease- or cancer-specific mortality. Similar associations were found among individuals who participated in the earlier (1999-2006) vs. later NHANES (2007-2014) cycles, although the associations were slightly stronger among those who participated in the later cycles (Supplementary Table 3).
Table 3.
Plant- and Animal-Based Diet Quality and All-Cause, Heart Disease, Cancer, and Other Mortality Among US Adults, NHANES 1999-2014
| Person-Years | All-Cause Mortality | Heart Disease Mortality | Cancer Mortality | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| N | HR (95% CI)1 | N | HR (95% CI)1 | N | HR (95% CI)1 | ||
| Comprehensive Diet Quality Index (cDQI) 2 | |||||||
| Q1 (male:<37.0; female:<40.8) | 78959 | 906 | Ref | 134 | Ref | 206 | Ref |
| Q2 (male: 37.0-40.7; female: 40.8-44.9) | 77336 | 1200 | 0.91 (0.81, 1.03) | 198 | 1.05 (0.74, 1.50) | 273 | 1.05 (0.87, 1.27) |
| Q3 (male:40.8-45.0; female: 45.0-49.4) | 72943 | 1351 | 0.83 (0.74, 0.95) | 237 | 1.03 (0.73, 1.45) | 289 | 0.92 (0.70, 1.23) |
| Q4 (male: ≥45.1; female: ≥49.5). | 65075 | 1212 | 0.75 (0.65, 0.86) | 229 | 1.05 (0.72, 1.53) | 253 | 0.88 (0.66, 1.18) |
| P-trend<0.001 | P-trend=0.82 | P-trend=0.29 | |||||
| Plant-Based Diet Quality Index (pDQI) 2 | |||||||
| Q1 (male:<22.4; female:<24.3) | 80595 | 690 | Ref | 94 | Ref | 155 | Ref |
| Q2 (male: 22.4-25.7; female: 24.3-28.0) | 76709 | 1049 | 0.78 (0.69, 0.89) | 165 | 0.85 (0.59, 1.22) | 245 | 0.97 (0.71, 1.33) |
| Q3 (male: 25.8-29.6; female: 28.1-32.1) | 72215 | 1502 | 0.82 (0.73, 0.94) | 279 | 1.11 (0.78, 1.60) | 325 | 0.99 (0.74, 1.33) |
| Q4 (male: ≥29.7; female: ≥32.2) | 64795 | 1428 | 0.66 (0.58, 0.74) | 260 | 0.95 (0.67, 1.35) | 296 | 0.80 (0.59, 1.10) |
| P-trend<0.001 | P-trend=0.80 | P-trend=0.12 | |||||
| Animal-Based Diet Quality Index (aDQI) 2 | |||||||
| Q1 (male:<13.1; female:<15.0) | 72069 | 1513 | Ref | 275 | Ref | 319 | Ref |
| Q2 (male: 13.1-14.9; female: 15.0-16.9) | 74897 | 1321 | 1.01 (0.92, 1.12) | 219 | 1.01 (0.74, 1.39) | 310 | 1.04 (0.83, 1.29) |
| Q3 (male: 15.0-17.0; female: 17.0-18.8) | 75050 | 1085 | 1.04 (0.92, 1.18) | 182 | 1.02 (0.78, 1.32) | 234 | 1.02 (0.77, 1.35) |
| Q4 (male: ≥17.1; female: ≥18.9). | 72298 | 750 | 0.99 (0.87, 1.13) | 122 | 0.93 (0.65, 1.31) | 158 | 1.02 (0.78, 1.33) |
| P-trend=0.81 | P-trend=0.78 | P-trend=0.90 | |||||
Abbreviations: NHANES, National Health and Nutrition Examination Survey; HR, hazard ratio; CI, confident intervals; cDQI, comprehensive Diet Quality Index; pDQI, plant-based Diet Quality Index; aDQI, animal-based Diet Quality Index.
Cox proportional hazard models were used to evaluate the associations between diet quality indices and mortality. HRs and 95% CIs were adjusted for age, sex, race/ethnicity, education, total energy intake, physical activity, cigarette smoking, alcohol consumption, BMI, comorbidities and accounted for NHANES survey weights. pDQI and aDQI were simultaneously adjusted in the same model.
The median scores in each quartile of cDQI were 33.8, 38.8, 43.0, and 48.9 among males and 37.4, 43.0, 47.6, and 53.5 among females; the median scores in each quartile of pDQI were 20.3, 24.1, 27.6, and 32.7 among males and 21.8, 26.2, 30.0, and 35.2 among females; and the median scores in each quartile of aDQI were 11.4, 14.0, 16.0, and 18.4 among males and 13.6, 16.1, 18.0, and 20.5 among females.
Figure 1.
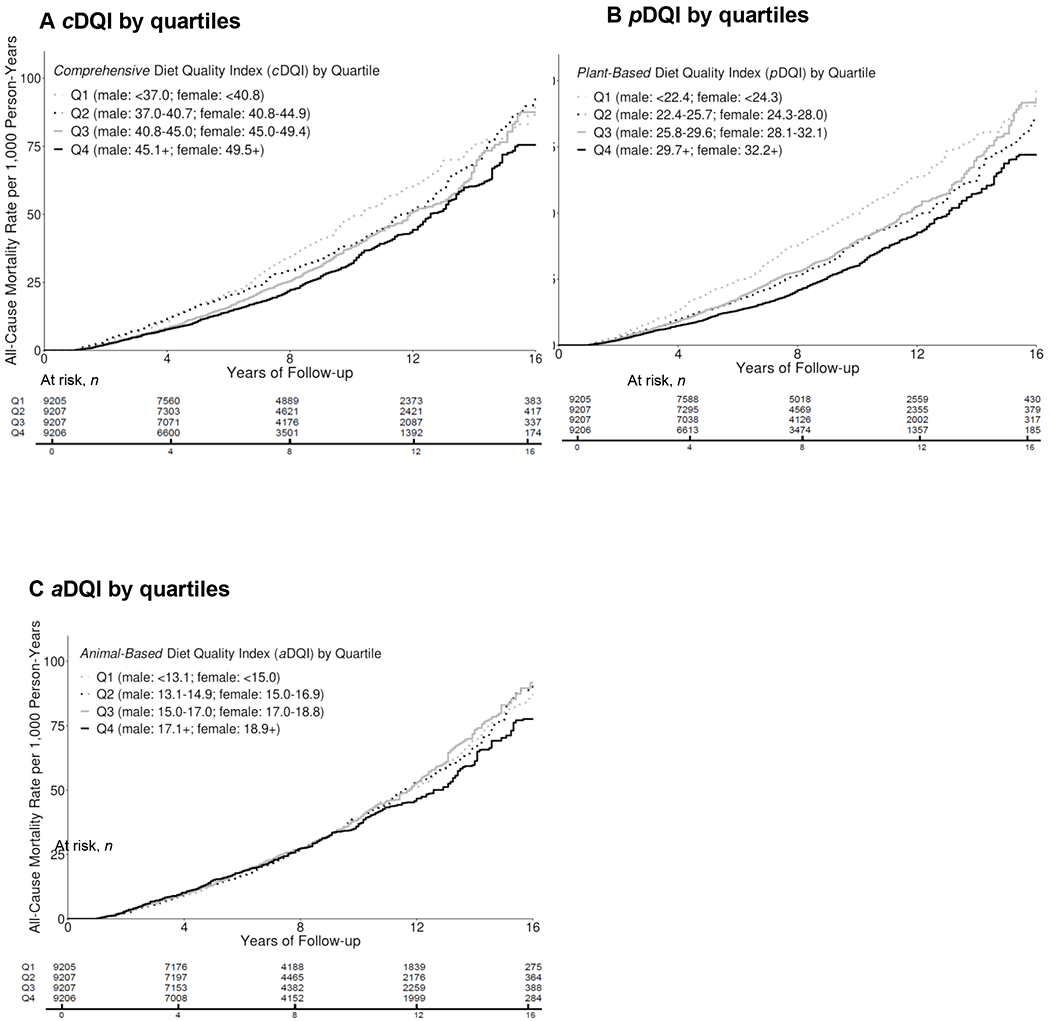
Cumulative Incidence of All-Cause Mortality by Quartiles of cDQI, pDQI, and aDQI Among US Adults, NHANES 1999-2014
Subgroup analyses revealed that among individuals with comorbidity conditions at baseline, those in the highest quartile of cDQI (HR=0.72, 95% CI: 0.64, 0.82, P-trend<0.001) or pDQI (HR=0.66, 95% CI: 0.58, 0.75, P-trend<0.001) had a lower risk of all-cause mortality compared to those in the lowest quartile. In contrast, no associations were found among individuals without comorbidity conditions at baseline. The inverse association between pDQI and all-cause mortality was slightly stronger among individuals who were overweight (Q4 vs. Q1: HR=0.59, 95% CI: 0.48, 0.73, P-trend<0.001) compared to those with a healthy weight (HR=0.69, 95% CI: 0.52, 0.91, P-trend=0.007) or obese individuals (HR=0.68, 95% CI: 0.51, 0.89, P-trend=0.02). The inverse association between cDQI and all-cause mortality was slightly stronger among individuals who were physically active (HR=0.62, 0.42-0.93, P-trend=0.008) compared to those physically inactive (HR=0.80, 0.68-0.94, P-trend=0.003). Although no association was found for aDQI and all-cause mortality among older individuals, younger individuals (20-44 years old) in the highest quartile of aDQI had a lower risk of all-cause mortality compared to those in the lowest quartile (Q4 vs. Q1: HR=0.65, 95% CI: 0.43, 0.97, P-trend=0.09). In contrary, the association between pDQI and all-cause mortality was only found among older individuals (45-59 years old: HR=0.67, 95% CI: 0.47, 0.96, P-trend=0.05; 60+ years old: HR=0.73, 95% CI: 0.61, 0.88, P-trend<0.001) but not younger ones. Similar associations between cDQI, pDQI, aDQI, and mortality were observed between men and women and by income.
DISCUSSION
In a nationally representative sample of US adults, we found that eating a diet with both high-quality plant-based and animal foods (i.e. scoring high in both the pDQI and the aDQI) was associated with a lower risk of all-cause mortality. This association largely reflects the inverse relationship between quality of plant-based foods and all-cause mortality. No independent associations were found for the quality of animal foods with mortality.
The public health and environmental impact of a plant-based diet has gained increased attention in recent years.(27) It remains debatable whether Americans shall eat more plant-based foods and less animal foods, and if so, whether eating a plant-based diet is affordable.(28; 29) Earlier studies reporting potential health benefits of eating plant-based foods were largely conducted among specific populations such as the Seventh Day Adventists (6; 7; 30; 31) who have demonstrated a wide range of healthy behaviors such as higher levels of physical activity and lower BMI than the general population regardless of dietary choices (32; 33). More recently, studies among the healthcare professionals (e.g., nurses from the Nurses’ Health Study and physicians from the Health Professionals Follow-up Study) reported that high intakes of healthy plant-based foods (e.g., whole grains, fruits/vegetables, nuts/legumes, oils, tea/coffee) were associated with a lower risk of coronary heart disease (CHD) whereas consumption of unhealthy plant-based foods (e.g., juices/sweetened beverages, refined grains, potatoes/fries, sweets) was associated with a higher CHD risk. Similar findings were observed for all-cause and cardiovascular disease (CVD) mortality.(34; 35)
Among the general US population, we found that consuming high-quality plant-based foods (pDQI) was associated with a lower risk of all-cause mortality. Although the associations with cancer and heart diseases-specific mortality were not statistically significant, the hazard ratios were below one, suggesting a non-significant inverse association. The bioactive components in healthy plant-based foods such as fiber and phytochemicals have been shown to decrease oxidative stress, reduce inflammation, and inhibit cell proliferation (36), which can confer a protective effect on chronic diseases (37; 38). Our findings support the dietary recommendations (4) that promote the consumption of high-quality plant-based foods for improving health. In particular, the inverse association between high-quality plant-based foods and all-cause mortality was observed among individuals with comorbidity conditions at baseline but not among those without comorbidity conditions. It is reasonable to suspect that individuals with comorbidity conditions might change their diet due to diet therapy and consequently their prognosis could be improved, contributing to reduced mortality.
We extended the diet quality index used in the previous studies by scoring the quality of animal foods based on the healthfulness of animal foods. However, we did not find that high-quality mortality. animal foods were associated with all-cause mortality after controlling for the quality of plant-based foods. Americans are experiencing improving trends in animal-based components of their diet. For example, there was a decreasing trend in red meat consumption among US adults in the past 10-15 years (39). Meanwhile, Americans fall significantly short for several healthy plant-based foods such as whole grains, fruits, and vegetables and have excess intake of unhealthy plant-based foods such as those high in added sugars (25). These trends may have made it more difficult to detect associations with animal-based components compared to plant-based ones. These results may suggest that the relationship between the quality of animal foods and all-cause mortality is not as strong as that for plant-based components. Indeed, when each plant-based and animal food component was evaluated individually, several plant components (non-starchy vegetables, whole fruits, nuts/seeds/legumes) were associated with lower all-cause mortality but none of the animal components had a significant association with all-cause mortality. Thus, the public health efforts to improve population health may be more effective to focus on increasing the consumption of healthful plant-based foods such as fruits, vegetables, whole grains, nuts/seeds and legumes. Our findings also support the recommendations made by the EAT-Lancet initiative to eat a diet rich in healthful plant-based foods with fewer animal foods for achieving both health and environment benefits.(27) Importantly, future dietary recommendations shall address not only the health aspects of a diet but also the sustainability of the diet through its environmental, economic, and social influences.
Interestingly, eating high-quality animal foods was associated with lower all-cause mortality among younger individuals and yet such an association was not found among older individuals. In contrast, eating high-quality plant-based foods was associated with lower all-cause mortality among older individuals but not younger individuals. This may reflect the different trends of eating high-quality animal vs. plant-based foods by age: younger individuals consumed a higher quality of animal foods than older individuals whereas older individuals had a better quality of plant-based foods than younger individuals. The heterogeneous results among young vs. old individuals may also reflect the difference in mortality risk and relative contribution of causes of death by age. Further investigations are needed to understand the potentially different roles of animal vs. plant-based foods in chronic disease prevention by age and other lifestyle factors.
Our study has several strengths. First, assessing diet quality as a whole is a more powerful approach to evaluate the impact of humans’ diet on health than studying individual foods/nutrients because humans do not consume foods/nutrients in isolation.(40) Dietary guidelines are also moving away from a single food/nutrient approach to focusing on the overall diet quality and eating patterns.(4) We assessed the quality of several key plant- and animal-based components in the context of dietary recommendations, which allows for more relevant and translatable findings. Second, we constructed a comprehensive diet quality index that scores the quality of animal foods along with plant-based foods. Different from previous diet quality indices that are also constructed to take healthy and unhealthy types of foods into account, the cDQI facilitates the evaluation of the relative importance of plant vs. animal foods in association with health outcomes. Indeed, our results suggested that while eating a diet with both high-quality plant-based and animal foods contributes to a lower risk of all-cause mortality, this association is largely driven by consuming high-quality plant-based foods not animal foods. Animal foods constitute a large proportion of our daily food intake. It is important to understand the different roles that animal vs. plant-based foods may play in health. Third, our study included the use of a large-scale nationally representative sample of US adults and the results can be more readily applied to the general population in the US. The longitudinal design minimizes selection bias and recall bias. We also used dietary data collected using validated measures (i.e. 24-hour diet recalls).(41; 42)
There are some limitations that need to be considered. First, distribution of diet was estimated based on self-reported dietary intake subject to measurement error. The NHANES used one or two days of 24-hour diet recalls as the primary source to measure dietary intake, which does not well capture usual intake due to large day-to-day variations in food intake. To improve the estimation of usual intake, we applied the NCI method to reduce the measure errors associated with usual intake estimation. Adjusting for energy intake also reduces measurement error (41). However, measurement error cannot be ruled out and is likely to be non-differential by mortality, which attenuates the associations. Second, a few food components included in cDQI were scored based on quintiles of consumption. Thus, the cut-offs may differ between studies where study participants have different consumption levels, which affects the comparability of study findings across studies. Third, diet quality is correlated with participants’ socioeconomic status and lifestyle factors such as education, cigarette smoking, body mass index, alcohol drinking, and physical activity. Having chronic health conditions such as cancer, cardiovascular disease, or diabetes may also change one’s dietary intake patterns. We excluded individuals who died within 12 months of dietary assessment to minimize the chance of reverse causation. To reduce the chance of residual confounding, we carefully adjusted for all these factors in the multivariable models. In addition, we stratified the association by presence or absence of comorbidity conditions. However, dietary intake patterns may be associated with factors that we have not identified or adjusted for, and residual confounding may still be present. Fourth, mortality was determined through probabilistic matching with the National Death Index. Although probabilistic matching is subject to misclassification, a prior validation study has shown that the accuracy of the method was high, with 96.1% of the decedents and 99.4% of the living participants classified correctly (26). Fifth, our sample size is limited to evaluate cause-specific mortality such as deaths due to cancer or heart disease. Thus, we treated cause-specific mortality analyses as secondary and the results should be interpreted with caution. Sixth, repeated assessment on dietary intake for the same individual were not available in NHANES. We were unable to evaluate how potential changes in dietary intake are associated with mortality outcomes.
Despite these limitations, our study is among the first to evaluate the relative importance of the quality of plant-based vs. animal foods in association with mortality outcomes among a nationally representative sample of US adults. Our results suggest that eating better-quality plant-based foods is associated with a lower risk of all-cause mortality among US adults. Conversely, the quality of animal foods does not independently contribute to mortality. Findings support the current dietary recommendations that promote high-quality plant-based diet for chronic disease prevention.
Supplementary Material
ACKNOWLEDGMENT
Funding: This study was supported by NIH/NIMHD 1R01MD011501 (FFZ).
Role of Funder: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Access to Data and Data Analysis: Dr. Zhang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest (COI) Statement: None of the authors has conflicts of interest to report.
REFERENCES
- 1.Fraser GE (2009) Vegetarian diets: what do we know of their effects on common chronic diseases? Am J Clin Nutr 89, 1607s–1612s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEvoy CT, Temple N, Woodside JV (2012) Vegetarian diets, low-meat diets and health: a review. Public health nutrition 15, 2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Gonzalez MA, Sanchez-Tainta A, Corella D et al. (2014) A provegetarian food pattern and reduction in total mortality in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr 100 Suppl 1, 320s–328s. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans 2015-2020. https://health.gov/dietaryguidelines/2015/guidelines (accessed June 15, 2019
- 5.Clarys P, Deliens T, Huybrechts I et al. (2014) Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 6, 1318–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orlich MJ, Singh PN, Sabate J et al. (2013) Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA internal medicine 173, 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appleby PN, Crowe FL, Bradbury KE et al. (2016) Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am J Clin Nutr 103, 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins DJ, Kendall CW, Marchie A et al. (2003) Type 2 diabetes and the vegetarian diet. Am J Clin Nutr 78, 610s–616s. [DOI] [PubMed] [Google Scholar]
- 9.Lattimer JM, Haub MD (2010) Effects of dietary fiber and its components on metabolic health. Nutrients 2, 1266–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daoud E, Scheede-Bergdahl C, Bergdahl A (2014) Effects of Dietary Macronutrients on Plasma Lipid Levels and the Consequence for Cardiovascular Disease. Journal of Cardiovascular Development and Disease 1. [Google Scholar]
- 11.Satija A, Bhupathiraju SN, Rimm EB et al. (2016) Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med 13, e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik VS, Popkin BM, Bray GA et al. (2010) Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 33, 2477–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. NHANES Response Rates and Population Totals. https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx (accessed June 15, 2019
- 14.Centers for Disease Control and Prevention. Key Concepts About the NHANES Sample Weights. https://www.cdc.gov/nchs/tutorials/dietary/SurveyOrientation/SurveyDesign/Info2.htm (accessed June 15, 2019
- 15.U.S. Department of Agriculture Food Surveys Research Group. Food and Nutrient Database for Dietary Studies, pp. Accessed at https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/ on December 8, 2018. Beltsville, MD. [Google Scholar]
- 16.Dodd KW, Guenther PM, Freedman LS et al. (2006) Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. Journal of the American Dietetic Association 106, 1640–1650. [DOI] [PubMed] [Google Scholar]
- 17.Freedman LS, Midthune D, Carroll RJ et al. (2004) Adjustments to improve the estimation of usual dietary intake distributions in the population. The Journal of nutrition 134, 1836–1843. [DOI] [PubMed] [Google Scholar]
- 18.Herrick KA, Rossen LM, Parsons R et al. (2018) Estimating Usual Dietary In take From National Health and Nut rition Examination Survey Data Using the National Cancer Institute Method. Vital and health statistics Series 2, Data evaluation and methods research, 1–63. [PubMed] [Google Scholar]
- 19.Satija A, Bhupathiraju SN, Spiegelman D et al. (2017) Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. Journal of the American College of Cardiology 70, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Cancer Research Fund/ American Institute for Cancer Research. Diet, Nutrition, Physical Activity, and Cancer: a Global Perspective. Contiuous Update Project Expert Report; 2018. https://www.wcrf.org/dietandcancer (accessed March 2, 2019 [Google Scholar]
- 21.Micha R, Shulkin ML, Penalvo JL et al. (2017) Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PloS One 12, e0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong VW, Van Horn L, Cornelis MC et al. (2019) Associations of Dietary Cholesterol or Egg Consumption With Incident Cardiovascular Disease and Mortality. Jama 321, 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs-Smith SM, Pannucci TE, Subar AF et al. (2018) Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet 118, 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiuve SE, Fung TT, Rimm EB et al. (2012) Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition 142, 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehm CD, Penalvo JL, Afshin A et al. (2016) Dietary Intake Among US Adults, 1999–2012. JAMA 315, 2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menke A, Muntner P, Batuman V et al. (2006) Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation 114, 1388–1394. [DOI] [PubMed] [Google Scholar]
- 27.Willett W, Rockstrom J, Loken B et al. (2019) Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet (London, England) 393, 447–492. [DOI] [PubMed] [Google Scholar]
- 28.Forouhi NG, Krauss RM, Taubes G et al. (2018) Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. Bmj 361, k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirvonen K, Bai Y, Headey D et al. (2019) Affordability of the EAT-Lancet reference diet: a global analysis. The Lancet Global health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowe FL, Appleby PN, Travis RC et al. (2013) Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr 97, 597–603. [DOI] [PubMed] [Google Scholar]
- 31.Chang-Claude J, Frentzel-Beyme R, Eilber U (1992) Mortality pattern of German vegetarians after 11 years of follow-up. Epidemiology (Cambridge, Mass) 3, 395–401. [DOI] [PubMed] [Google Scholar]
- 32.Key TJ, Appleby PN, Spencer EA et al. (2009) Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 89, 1613s–1619s. [DOI] [PubMed] [Google Scholar]
- 33.Waldmann A, Koschizke JW, Leitzmann C et al. (2003) Dietary intakes and lifestyle factors of a vegan population in Germany: results from the German Vegan Study. European journal of clinical nutrition 57, 947–955. [DOI] [PubMed] [Google Scholar]
- 34.Baden MY, Liu G, Satija A et al. (2019) Changes in Plant-Based Diet Quality and Total and Cause-Specific Mortality. Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Caulfield LE, Garcia-Larsen V et al. (2019) Plant-Based Diets Are Associated With a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J Am Heart Assoc 8, e012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Khor TO, Shu L et al. (2012) Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem 12, 1281–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu RH (2003) Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 78, 517s–520s. [DOI] [PubMed] [Google Scholar]
- 38.Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. The Journal of nutrition 134, 3479s–3485s. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Department of Agriculture (2018) USDA Agricultural Projections to 2027. Long-term Projections Report
- 40.Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Current Opinion in Lipidology 13, 3–9. [DOI] [PubMed] [Google Scholar]
- 41.Ahluwalia N, Dwyer J, Terry A et al. (2016) Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv Nutr 7, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moshfegh AJ, Rhodes DG, Baer DJ et al. (2008) The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 88, 324–332. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Department of Agriculture Food Surveys Research Group. Food Patterns Equivalents Database. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fped-databases/ (accessed June 15, 2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


